Abstract
Interleukin-17 (IL-17) family cytokines are potent drivers of inflammatory responses. Although IL-17 was originally identified as a cytokine that induces protective effects against bacterial and fungal infections, IL-17 can also promote chronic inflammation in a number of autoimmune diseases. Research in the last decade has also elucidated critical roles of IL-17 during cancer development and treatment. Intriguingly, IL-17 seems to play a role in the risk of cancers that are associated with metabolic disorders. In this review, we summarize our current knowledge on the biochemical basis of IL-17 signaling, IL-17′s involvement in cancers and metabolic disorders, and postulate how IL-17 family cytokines may serve as a bridge between these two types of diseases.
Keywords: interleukin-17, metabolic disorders, inflammation, cancer
1. IL-17 Family Cytokines and Their Receptors
Initially detected in murine T cell hybridoma clones and identified as cytotoxic T lymphocyte-associated antigen 8 (CTLA-8) in 1993, interleukin-17, also known as interleukin-17A (IL-17A), was the first member of the IL-17 family of cytokines to be discovered [1,2]. In mammals, there are currently six identified members of the IL-17 family: IL-17A, IL-17B, IL-17C, IL-17D, IL-17E, and IL-17F [1,2,3,4,5,6,7,8]. Each member of the family shares some degree of homology in amino acid sequence with IL-17A, ranging from just under 20% to around 55% in similarity [9,10]. IL-17F is located next to IL-17A on human chromosome 6 and mouse chromosome 1 but is transcribed independently. IL-17F is IL-17A’s closest homolog— it has an amino acid sequence similarity of around 40 or 55% depending on the IL-17F isoform [9,10,11,12]. The next closest homolog is IL-17B, which shares around 29% amino acid sequence similarity with IL-17A, followed by IL-17D at 25%, IL-17C at 23%, and the most divergent homolog IL-17E (also called IL-25) at roughly 17% [7,8,12]. The IL-17 family of cytokines are homodimeric glycoproteins, or heterodimeric in the case of the IL-17A/F cytokine, and these dimers range from around 35 to 50 kDa in size [4,10,12]. The IL-17 family of cytokines share a conserved C-terminus, which contains four cysteine and two serine residues, which are necessary to form the cysteine knot fold observed in the crystal structures of IL-17A and IL-17F [4,9,10,12,13]. The IL-17 family of cytokines diverge in their N-terminal segments [4,10,12]. What is known about the structural aspects of these molecules has been extensively described in reviews by Zhang et al. (2011) and Liu (2019) [14,15].
The six members of the IL-17 family also differ in receptor binding preferences. There are five currently identified members in the IL-17 receptor family: IL-17RA, IL-17RB, IL-17RC, IL-17RD, and IL-17RE [16]. Each receptor polypeptide shares sequence homology with IL-17RA, and many of the genes encoding the IL-17 receptors are clustered on human chromosome 3 or on mouse chromosomes 6 and 14 [14,16,17]. Except for IL-17RA, IL-17 receptors can undergo alternative splicing, sometimes leading to soluble secreted proteins that inhibit IL-17 signaling [12,17,18]. The IL-17 receptors are unique, in that, given the current structural knowledge, they do not fit into any of the six major families of cytokine receptors [14]. The receptors are single-pass, type I transmembrane domain-containing receptors, which share conserved structural motifs, such as a fibronectin III-like domain in the extracellular region and a SEF/IL-17R (SEFIR) domain in the intracellular region [5,16,19]. IL-17 receptors bind to IL-17 family cytokines as homo- or heterodimers and transduce signal through adaptor protein NF-κB activator 1 (ACT1) and E3 ubiquitin ligase tumor necrosis factor receptor-associated factor protein (TRAF) TRAF6, which leads to the activation of nuclear factor kappa B (NF-κB), mitogen-activated protein kinases (MAPK), and CCAAT/enhancer binding protein (C/EBP) pathways [20]. Detailed information on the signaling cascade of IL-17 family cytokines will be introduced in later sections.
IL-17RA was first identified as the receptor for IL-17A, but it also binds to IL-17F and the IL-17A/F heterodimer [2,9,16,21,22]. Alone, IL-17RA has a high affinity for IL-17A, a much weaker affinity for IL-17F, and an intermediate affinity for IL-17A/F, as well as an even weaker affinity for the other four cytokines in the IL-17 family [4,20,21,23]. The IL-17RC and IL-17RA heterodimer is the major receptor complex that mediates IL-17A and F signaling. IL-17RA has been shown to bind asymmetrically with IL-17A and IL-17F in a one receptor chain to one dimer manner (1:2 stoichiometry), whereas the IL-17A/F heterodimer binds to IL-17RA in a binding stoichiometry of one receptor chain per (hetero)dimer (1:1 stoichiometry) [4,9,20,22]. IL-17RA binds to either the ‘A’ face or ‘F’ face of the IL-17A/F heterodimer with similar affinity. While IL-17RA undergoes conformational changes when binding to the ‘F’ face, its structure is not altered upon binding to the ‘A’ face, as induced-fit changes to relieve steric hindrance would be needed [21,22].
IL-17RB (IL-17Rh1) has affinities for both IL-17B and IL-17E but exhibits a stronger affinity for IL-17E [3,6,8]. When binding IL-17B, IL-17RB forms a homodimer, whereas it forms a heteromeric receptor complex with IL-17RA to bind IL-17E [6,8,16,24,25,26].
IL-17RC exists in multiple splice forms. When forming a heterodimeric complex with IL-17RA, IL-17RC binds to IL-17A, IL-17F, and IL-17A/F heterodimer [21,23,27,28,29]. Both IL-17RC and IL-17RA can independently interact with IL-17A or IL-17F, but the complex of both receptors is required to carry out the biological activity of the cytokines, and the binding of a cytokine to one receptor encourages preference for the second receptor-binding site [20]. Recently, it has been shown that IL-17F forms a complex with homodimeric IL-17RC in solution [27]. This may represent how IL-17RC, in its alternatively spliced soluble form, may inhibit IL-17 signaling in the extracellular space [16,22,27]. The mechanism and function of the potential IL-17RC-only transduction of IL-17 signaling remains to be explored and may provide further insight into the development of therapeutic agents for autoimmune diseases, such as psoriasis, where a blockade of IL-17A and IL-17RA showed significant efficacy. From the structure data, we now know that IL-17RC binds to the IL-17A/F heterodimer on either the ‘A’ face or ‘F’ face equally, potentially inferring the formation of two topologically distinct binary complexes [22,27].
IL-17RD (Sef) forms a heterodimer with IL-17RA to selectively bind to IL-17A. It has also been shown to colocalize with IL-17RB [30,31,32]. IL-17RD shares overlapping functions with IL-17RC, such as mediating IL-17A signaling, albeit binding IL-17A at around 20-fold lower affinity than IL-17RC [30]. IL-17RE associates with IL-17RA to bind homodimeric IL-17C [33]. To date, no additional members of the IL-17 family of cytokines have been found to bind to IL-17RE. In addition to the canonical IL-17 receptors, CD93 acts as a receptor of IL-17D and promotes the production of IL-22 by type 3 innate lymphoid cells (iLC3) [34,35].
Overall, the slight differences in IL-17 cytokines and IL-17 receptor structures alter their function. Knowing these differences and their impact on biology will help when identifying therapeutic targets. However, understanding other factors, including how other cytokines affect IL-17-producing cells, is important for understanding IL-17′s role in diseases.
2. Multiple Cytokine Pathways Guide the Differentiation of IL-17-Producing Cells
While the inflammatory effects of IL-17 family cytokines are observed as a product of their downstream effects on target cells and induction of their target genes, the upstream pathways have a profound impact on the ability of cells to produce these cytokines and must be considered when trying to understand cellular immunity. Important pathways that have become a focus around the IL-17 family of cytokines include the IL-6/transforming growth factor (TGF) β, IL-23/T helper cell (Th) 17, and IL-1β pathways.
A stimulation of naïve CD4+ T cells with cytokines IL-6 and TGFβ in vitro produces a distinct class of T helper cells, Th17 (ThIL-17) cells, and results in a regulatory and protective phenotypic outcome when compared with the phenotypes produced from the IL-23/Th17 axis and the IL-1β axis, which is discussed below [36,37]. Stimulation of Th17 cells with TGFβ and IL-6 results in the production of IL-17A, and in some cases, IL-10. IL-10 mitigates the inflammatory activities of Th17 cells [38,39]. This pathway was initially observed in naïve T cells harboring an activated forkhead box p3 (Foxp3) locus, where IL-6 inhibited the induction of Tregs by TGFβ, resulting in their conversion into IL-17-producing cells [39]. Since its initial discovery, IL-6 has been found to be a potent activator of signal transducer and activator of transcription 3 (STAT3), which subsequently suppresses the differentiation of these naïve CD4+ T cells into Tregs by regulating the expression of transcription factor retinoic acid receptor-related orphan receptor-γt (RORγt), promoting differentiation into the Th17 subset and subsequent expression of Th17 genes [37,39,40].
The discovery of IL-23, which differentiates naïve CD4+ T cells into Th17 cells, expanded our understanding of cellular immunity beyond a dichotomous Th1- and Th2-centric view [37,41,42]. IL-23, which consists of two subunits—p19 and p40, the latter of which it shares with IL-12—is a critical facilitator in promoting inflammation through the induction of Th17 cells that produce IL-17 and additional inflammatory mediators [42,43,44]. Differentiation of CD4+ T cells into the inflammatory Th17 subset occurs when antigen-engaging T cells are simultaneously stimulated by IL-6 and TGF-β [39]. Subsequently, IL-23 activates STAT3, further inducing transcription factor RORγt, which is designated as the master transcription factor in IL-23-responsive cells [37,45,46,47,48,49,50,51]. Activation of RORγt results in the production of proinflammatory mediators, such as IL-17A, IL-17F, IL-6, IL-22, and TNFα, which are implicated in the development of multiple inflammatory diseases [42,43,44,51].
In certain inflammatory diseases, IL-23 has been found to promote RORγt expression and reduce Foxp3 expression in regulatory T cells (Tregs), resulting in ‘plastic’ Tregs that produce IL-17A [52,53]. Notably, IL-23 requires additional factors to promote Th17 differentiation, as it is primarily a maintenance factor for Th17 cells and, when acting alone, does not induce differentiation of the Th17 subset [37,42,54,55]. IL-23 functions to expand Th17 cells as well as maintain the cell population by upregulating IL-6, IL-1β, and TNFα through a positive feedback loop [42,53,55]. Th17 differentiation by IL-23 is inhibited by IFN-γ and IL-4 [42].
TGFβ and IL-6 have been predominantly implicated in Th17 differentiation in murine cells, whereas IL-1β has been identified as an important cytokine in human Th17 differentiation [36,37,56]. IL-1β functions alone or together with cytokines, such as IL-23 and IL-6, and regulates the expression of transcription factors interferon regulatory factor 4 (IRF4) and RORγt [36,37,42,55,56,57,58,59,60]. IL-1β promotes the excision of exon 7 of Foxp3 through alternative splicing, skewing differentiation of naïve T cells toward a Th17 phenotype and away from a Treg phenotype [56]. On their own, neither cytokine is sufficient, but when both IL-1β and IL-23 are present together, Th17 cell differentiation occurs. In addition to the IL-1β/IL-R1/myeloid differentiation primary response 88 (MyD88) signaling cascade, the activation of MyD88-mediated signaling by the engagement of Toll-like receptors (TLRs) also promotes the differentiation of CD4+ T cells toward a Th17 fate [57,58,59,60]. Further, IL-1β and IL-23 alter Th17 metabolism to increase aerobic glycolysis to produce sufficient energy for differentiation, which concordantly supports gene transcription of the Th17 subset [58].
When IL-1β acts together with IL-6, IL-6 upregulates the expression of IL-23 via STAT3, which induces IL-17 gene expression as well as enhancing the expression of RORγt [60]. Additionally, IL-6 upregulates the IL-1R1 receptor expression on Th17 cells, whose signaling promotes expression of transcription factors IRF4 and RORγt [57]. As observed with IL-23, IL-1β also works with IL-6 to alter cell metabolism, thereby allowing for Th17 expansion in inflammatory environments where resources are limited [37].
By comparison, much less is known about the regulation of the expression of other IL-17 family members. The current findings suggest that IL-17C is upregulated by IL-1β, IL-17A, and TNFα, as well as TLR-MyD88 signaling [61,62,63,64]. IL-17C expression is elevated upon activation of p38 MAPK and NF-κB [61,65,66]. IL-17D expression is promoted by transcription factor Nrf2 in response to oxidative stress [5,67]. In certain cell types, IL-17E expression is induced via IL-22 [68].
Studies into cytokine regulation of Th17 cell differentiation have uncovered many important transcriptional pathways. While these may all play specific roles in metabolic diseases and cancers, the RORγt transcription factor should be further investigated, as it is linked to promoting IL-17 induction in other cells as well.
3. Transcriptional Control of IL-17 Family Cytokines
The induction of IL-17 cytokines depends on the expression of RORγt transcription factor [46,47,48]. IL-17-producing cells, which will be introduced in the following sections and comprise both innate and adaptive cells, appear to share a dependence on this transcription factor, as well as on receptors to IL-23, IL-1β. They also share the expression of chemokine receptor CCR6 [47,48]. Several additional factors also play a role in inducing IL-17 cytokines through interaction with RORγt. The aryl hydrocarbon receptor (AHR), which is expressed in several IL-17-producing cells, such as those in the gut, skin, and spleen, interacts with RORγt, resulting in an increase in the production of IL-17 cytokines [48]. Additionally, the interaction of RORγt with runt-related transcription factor 1 (RUNX1) in subsets of IL-17-producing cells, such as those in the gut and skin, also results in an increase in the production of IL-17 cytokines, potentially through shifting differentiation toward IL-17-producing cell types [48,49,69]. Importantly, STAT3 is involved in the activation of RORγt upon IL-6 stimulation [48,49]. STAT3, in its active and phosphorylated form, regulates the differentiation and maturation of some subsets of IL-17-producing cells, which can lead to increases in the production of IL-17 when interacting with RORγt [48,50]. Other factors RORγt is known to interact with, resulting in IL-17 family cytokine production, include IRF4, NF-κB, basic leucin zipper transcription factor, ATF-like (BATF), rho-associated kinase (ROCK2), Ets-family transcription factor (Etv5), and sphingosine 1-phosphate-/type 1 S1P receptors (S1P1S) [46,70]. In summary, RORγt interacts with a number of transcription factors to induce IL-17 production in a variety of cell types.
4. Signaling Events following IL-17 Cytokine–Receptor Engagement
There are several known pathways that can be activated upon the engagement of an IL-17 family receptor by an IL-17 family cytokine. Currently, the known activated signal transduction pathways include MAPK, NF-κB, and C/EBP cascades [17,19,20]. The activation of these pathways involves adaptor proteins, such as Act1, and E3 ubiquitin ligases TRAF2, 5, and 6 [17,19,71]. The activation of intracellular signaling components by IL-17 frequently leads to the production of proinflammatory chemokines and cytokines that are typically associated with innate immune responses [16,17,19,71,72,73]. The following is a brief summary of the signaling events following IL-17 receptor engagement.
The binding of IL-17A or IL-17F homodimers, or the IL-17A/F heterodimer, to IL-17RA and IL-17RC results in the activation of TRAF6-dependent gene transcription [10,74,75]. This ‘canonical’ IL-17 responsive pathway depends on the recruitment of ACT1 adaptor protein that interacts with the SEFIR domain on IL-17 receptors [74,75,76,77,78]. ACT1 interacts with TRAF6 via its TB1 and TB2 domains and facilitates the ubiquitination of TRAF6, leading to TRAF6 autoubiquitination [74,75,76,77]. TRAF6 then ubiquitinates TGFβ-activated kinase 1 (TAK1), which subsequently phosphorylates IKK, leading to IkBα degradation and the activation of NF-κB, as well as MAPK signaling pathways [10,74,75]. In some systems, JAK1-associated PI3K signaling has been shown to mediate NF-κB activation [18,75,79]. Synergy with cytokines such as TNF-α increases the activation of NF-κB [10,18,74,75]. The MAPK signaling pathway has been shown to involve the following transcription modifiers: extracellular signal-regulated kinase (ERK) 1 and 2 (ERK1/ERK2), c-jun N-terminal kinase (JNK), and p38 [2,10,16,71,74,75,80]. Additionally, the C/EBP transcription factor pathway also falls downstream of ACT1 activation [74,81].
Binding of homodimers of IL-17A or IL-17F, or the IL-17A/F heterodimer, to the IL-17RA and IL-17RC receptor complex also increases the half-life of previously transcribed proinflammatory mRNAs via the ‘non-canonical’ signaling pathway [10,74,75,82]. The non-canonical pathway stabilizes these mRNAs through TRAF2- and TRAF5-mediated interactions with molecules controlling mRNA turnover [10,75,83]. This pathway also involves ACT1, but unlike the canonical pathway, it is dependent on TBK1 and IκB kinase (IKKi) phosphorylation of ACT1; it acts as a positive regulator toward the activation of proinflammatory gene transcription pathways [10,74,75,82,83,84].
The binding of IL-17C to the IL-17RA and IL-17RE complex induces signaling via ACT1 to induce NF-κB activation [33]. IL-17C signaling, by this complex, targets and induces IκBζ expression [33]. The recruitment of ACT1 and TRAF6 in IL-17RA and IL-17RB complex activation by IL-17E also results in NF-κB activation [18]. IL-17E also activates ERK, JNK, and p38 in a TRAF6-independent manner, as well as transcription factors JunB, nuclear factor of activated T cells 1 (NFATc1), GATA-binding factor 3 (GATA-3), and STAT6 [18,25,85,86]. Data detailing the IL-17B signaling pathway activated upon interaction with the homodimeric IL-17RB complex are limited. It is known that IL-17B activates NF-κB, which can occur independently of TRAF6 [87]. ERK, JNK, as well as p38 pathways are also activated by IL-17B [87]. IL-17RD has been shown to both regulate IL-17 pathway signaling, as well as negatively regulate NF-κB and IRF signaling pathways downstream of TLRs [31,88,89]. The domains of IL-17RD have been proposed to interact with ERK1/2 MAPK cascade to inhibit fibroblast growth factor signaling [89,90]. When the IL-17RA and IL-17-RD complex is stimulated by IL-17A, IL-17RD sequesters ACT1, disrupting the ACT1/TRAF6 signaling complex, leading to differential regulation of NF-κB and p38 MAPK pathways [89,91,92]. IL-17A signaling through the IL-17RA and IL-17RD complex regulates proinflammatory gene expression in cell-type-dependent manner. IL-17A signal transduction mediated by the IL-17RA and IL-17RD complex competes with IL-17A-induced signaling dependent on TRAF6 recruitment to ACT1, reducing the activation of NF-κB transcription factor and dampening upregulation of IL-6 [31,89,92].
Additional regulatory mechanisms exist in each signaling pathway of the IL-17 family [75]. For instance, inhibition can be achieved when ACT1 interacts with IL-17RD to prevent its interaction with IL-17RA [74]. Ubiquitin-specific peptidase 25 (USP25) and ubiquitin-editing enzyme A20 also deubiquitinate TRAF molecules as means of negative feedback to modulate NF-κB and MAPK activation [74,75]. TRAF3 and TRAF4, likewise, prevent downstream signaling; TRAF3 directly binds IL-17RA, preventing ACT1 recruitment and interaction with TRAF6, whereas TRAF4 competes with TRAF6 through binding at the same location on ACT1 [74]. Moreover, microRNA (miRNA) expression, such as miR-23b, or transcription factors, such as C/EBPδ, can prevent overactivation of proinflammatory pathways [74,75].
The pairing of IL-17 cytokines with their cognate receptors and downstream pathways determines the biological function of these signaling circuits. Further, connecting these IL-17 cytokine/receptor pairs with downstream effector molecules, such as cytokines and chemokines, improves our understanding of how their dysregulation can impact disease.
5. Transcriptional Targets of IL-17 Family Cytokines
IL-17 activates the transcription of molecules that are critical for host defense and potentiation of inflammatory responses that are observed in both innate and adaptive arms of the immune system [81,93,94]. While specific pathways activated by IL-17 family receptors upon the binding of an IL-17 family cytokine may differ depending upon cell source, and thereby result in production of different effector molecules, some of the common target genes include proinflammatory and hematopoietic cytokines, chemokines, antimicrobial peptides, and tissue-remodeling substances [74,75,94].
IL-17A is known to induce the expression of several proinflammatory cytokines. A canonical IL-17 target is IL-6, whose production is augmented when IL-17A acts synergistically with TNFα, which also leads to the production of IL-1β and IL-8 [81,94,95,96,97,98]. Additionally, IL-17A acts synergistically with IFNγ to increase the production of IL-8 and monocyte chemoattractant protein-1 (MCP-1) [96,97]. IL-17A also activates the production of IL-17C, IL-36γ, IL-19, and vascular endothelial growth factor (VEGF) [97,99,100]. Chemokines, including CXCL1, CXCL2, CXCL5, CXCL8, CXCL10, CCL2, and CCL20, are also downstream targets of IL-17A [21,81,93,94,95,97,99,100,101,102,103]. In addition, IL-17A induces the production of antimicrobial peptides (AMPs), such as β-defensin, and acute phase proteins, such as lipocalin [81,94]. Moreover, tissue remodeling matrix metalloproteinases (MMPs), which can lead to extracellular matrix destruction and tissue damage, are often induced by IL-17A signaling, notably including MMP1, MMP2, MMP3, MMP9, and MMP13 [98,100,104,105,106,107]. On the other hand, IL-17A downregulates tissue inhibitors of metalloproteinases (TIMP)1 and TIMP2 [106].
IL-17F, which, in addition to being the most homologous cytokine to IL-17A, also exhibits functional similarities with IL-17A. IL-17F stimulates the production of cytokines and chemokines that are proinflammatory, as well as induces tissue-remodeling MMPs and stimulates the production of AMPs [108]. IL-17F induces the production of TNF, IL-1β, IL-6, IL-8, CXCL1, CXCL8, CXCL10, ICAM1, and GM-CSF [21,108,109,110,111,112,113]. IL-17F, although less potently than IL-17A, also induces CCL2, CCL7, TSLP, and MMP13 [108]. MMP1, MMP3, and MCP1 production also results from stimulation with IL-17F [98,113]. Additionally, when combined with TNFα, IL-17F upregulates the expression of G-CSF, IL-6, and MMP3 [4,18,98,108,114]. IL-17F together with IL-22 also results in the production of mhBD2, as well as other antimicrobial peptides [108,115].
While much remains to be investigated regarding the heterodimer of IL-17A/F, this cytokine complex is known to induce proinflammatory molecules, such as IL-6 and CXCL1 [116,117]. IL-17A/F has also been shown to induce the secretion of chemokine CXCL1 [21]. Upregulation of MMP3 as well as podoplanin (PDPN) mRNA expression is also observed upon IL-17A/F stimulation [98]. Additionally, a synergistic effect on the expression of IL-6 and MMP3 mRNA is seen when IL-17A/F is combined with TNF-α [98].
Similarly, IL-17B, IL-17C, and IL-17D can also induce proinflammatory cytokines. IL-17B is known to target cytokine IL-8, as well as chemokines CXCL1, CCL20, and additionally, trefoil factor 1 (TFF1) [118]. Factors upregulated by IL-17B also include antiapoptotic factor BCL-2, stemness factors Oct4, Sox2, Sall4, and the AKT/β-catenin pathway components [119,120]. As with IL-17A, IL-17C induces IL-6, IL-8, and VEGF expression [61,121]. Interestingly, IL-17C also activates the transcription of IL-1β and TNFα [61,121]. Additional proinflammatory cytokines induced by IL-17C include IL-17A/F and IL-22, as well as chemokines CXCL1, CXCL2, CXCL3, and CCL20 [61,64,121]. Further, IL-17C plays a role in upregulating AMPs, such as human β-defensin 2 (hBD2), lipocalin 2 (LCN2), and granzyme B [121]. IL-17C also promotes the expression of anti-apoptotic factors BCL-2 and BCL-XL [61,62]. IL-17D promotes the production of proinflammatory cytokines, including IL-1β, TNFα, IL-6, and IL-8 [5,7,122]. IL-17D also enhances the expression of chemokines CXCL8 and CCL2 [5,122]. Recently, it has been found that IL-17D is important for the production of IL-22 [34]. Additionally, IL-17D targets IL-6 and GM-CSF, and it inhibits hemopoiesis by myeloid progenitor cells [7].
While most IL-17 family cytokines have proinflammatory effects, IL-17E—the least homologous IL-17 family cytokine—is known to induce the production of both pro- and anti-inflammatory cytokines. The cytokine and chemokine targets of IL-17E include IL-1β, TNFα, IL-6, IL-8, MCP-1, CCL5, CCL11, and GM-CSF [8,93,113,123,124,125]. The production of MMP-1 is also stimulated by IL-17E [113]. The synergism of IL-17E with TNFα results in the upregulation of GM-CSF and CXCL8 mRNA, while co-stimulation of IL-17E and TGFβ1 upregulates CXCL8 [125]. IL-17E has been found to promote anti-inflammatory effects associated with the expression of IL-4, IL-5, as well as IL-13, and thymic stromal protein (TSLP), while the expression of IL-13 has been associated with suppression of IL-1, IL-6, and IL-23 [93].
While understanding which cytokines IL-17 family members can induce is useful for understanding their general function, the cytokines they induce are strongly influenced by the source cell and their targets. Thus, it is important to provide this biological context to understand IL-17′s role in health and disease.
6. IL-17 Producers, Cellular Targets, and Biological Consequences
IL-17 family cytokines execute diverse functions in health and diseases. Such diversity is in part due to the complexity of cellular targets that these cytokines stimulate. Engagement of the same IL-17 ligand on different target cell types can result in the transcription of different genes, leading to tissue-specific functions. In addition, while the targets of IL-17 signaling determine the biological outcome, the sources of IL-17 cytokines also have a heavy hand in determining the immunologic response. Notably, the IL-17 family of cytokines play a vital role in fighting extracellular fungi, viruses, and bacteria—especially at cutaneous and mucosal surfaces. On the other hand, these proinflammatory molecules are also implicated in the pathogenesis of a variety of inflammatory diseases, which will be covered in greater detail later in this review [46,47,93,118,119].
As discussed previously, and shown in Table 1, IL-17A induces a host of pleiotropic effects, as observed from the induction of target genes that encode cytokines, chemokines, antimicrobial peptides, as well as tissue-remodeling substances. There are several sources of IL-17A, which include both innate and adaptive immune cells [126,127,128]. While notoriously associated with the Th17 adaptive immune cell subset, additional cell sources have a hand in producing this cytokine [127,128]. Other adaptive immune cells known to produce IL-17A include CD8+ T cells, such as Tc17 cells, as well as resident memory T cells (TRM) [126,128,129]. Cells involved in innate defense mechanisms, such as γδT cells, invariant natural killer T cells (iNKT), neutrophils, mucosal-associated invariant T cells (MAIT), as well as type 3 innate lymphoid cells (ILC3), all produce IL-17A [46,126,127,128,130].
Table 1.
IL-17 cytokine family producing cells, targets, and effector molecules.
| Cytokine | Cellular Sources | Target Cells | Downstream Effector Molecules | References |
|---|---|---|---|---|
| IL-17A | Th17, Tc17, TRM, γδT, iNKT, neutrophils, MAIT, ILC3 | Mesenchymal cells, myeloid cells, macrophages, epithelial cells, keratinocytes, intestinal and nasal epithelial cells, B cells, CD4+ T cells | G-CSF, MIP2, IL-8, MCP-1, IL-1β, TNFα, IL-6, Il-10, IL-2, PGE2, hBD-2, β-defensin, S100A9, S100A8, S100A7, calprotectin, lipocalin, MMP-9 | [46,72,94,96,104,115,126,127,128,132,134,135,136,137,138,139,140] |
| IL-17B | Neutrophils, chondrocytes, neurons, stromal cells, gut epithelium, B cells (germinal and memory) | ILC2, NKT, THP-1 monocytes, fibroblasts, Th2, PBMC, ILC2 | IL-5, IL-13, G-CSF, IL-6, CCL20, TNFα, IL-1β | [3,87,124,143,144,145,146,147,148] |
| IL-17C | Epithelial cells | Epithelial cells, Th17 | G-CSF, IL-6, IL-1β, TNFα, AMPs, occluding, claudin-1, claudin-4 | [64,66,124,151,152,153,155,156,157] |
| IL-17D | Fibroblasts, colonic epithelial cells, brain, skeletal muscle, adipose tissue, heart, lung, pancreas | Endothelial cells, ILC3 | IL-22, RegIIIβ/γ, MCP-1, IL-8, IL-6, GM-CSF | [7,34,67,159,160] |
| IL-17E (IL-25) | Mast cells, alveolar macrophages, eosinophils, basophils, ILC2, dendritic cells, stromal cell, epithelial cells, Th2 | Keratinocytes, lung epithelial cells, endothelial cells, Th2, Th9, macrophages, fibroblasts, ILC2, DCs | Keratin 6, keratin 16, keratin 17, IL-1β, IL-9, IL-4, IL-5, IL-6, IL-13, TNFα | [47,68,85,86,124,161,162,163,164,165,166,167,172,173] |
| IL-17F | Th17, LTi, NK, iNKT, neutrophils, ILC3, γδT | Epithelial cells, endothelial cells, stromal cells, eosinophils, fibroblasts, macrophages, primary keratinocytes | CXCL1, G-CSF, GM-CSF, ICAM-1, IL-6, IL-8, IL-1β, CXCL, hBD-2, S100A7, S100A8, S100A9 | [4,12,21,48,109,110,111,114,115,116,141,142] |
| IL-17A/F | Th17, γδT, iNKT, NKp46+ | Epithelial cells, fibroblasts | CXCL1, IL-6, IL-8 | [18,23,117] |
The receptor of IL-17 is found on a broad variety of tissues [131]. IL-17A contributes to the production of inflammatory mediators, often by targeting mesenchymal and myeloid cells, and promotes neutrophil-mediated immunity. IL-17 also induces an immune response against microbes. For example, iNKT IL-17A secretion has been found to recruit neutrophils in the respiratory tract [127], whereas in the lung, when secreted by neutrophils, IL-17A promotes the production of IFNγ by T cells [130]. During a bacterial pneumonia infection, IL-17A, produced by CD4+ and CD8+ T cells, is important for the production of G-CSF and MIP-2, as well as recruiting neutrophils to the site of infection to aid host defense [132].
The amplification of an inflammatory response in psoriatic skin is observed when IL-17A is secreted by neutrophils; however, this point remains controversial due to the phagocytic function of these innate cells [127,133]. Additionally, in the blood and synovia of spondylarthritis and psoriatic arthritis patients, secretion of IL-17A by ILC3s and γδT cells has been found to drive enthesitis and induce the production of cytokines by mesenchymal cells [127]. The inflammatory response in human pancreatic periacinar myofibroblasts due to IL-8 and MCP-1 chemokine secretion is induced by IL-17A from CD4+ T cells, which is further exacerbated by IFNγ [96]. Additionally, the expression of IL-1β, TNFα, IL-6, IL-10, IL-2, and PGE2 results from IL-17A stimulation on macrophages [72].
As discussed previously, IL-17A also induces the expression of antimicrobial peptides. The production of antimicrobial hBD-2 results from IL-17A stimulation of human airway epithelial cells, which further serves as a chemotaxin to recruit immune cells, such as monocytes, DCs, and memory T cells [134,135]. Innate immunity in the skin is impacted by IL-17A too; the synergism of IL-17A and IL-22 from Th17 cells on keratinocytes results in the expression of AMPs, including β-defensin 2, S100A9, S100A8, and S100A7 [115]. In the gut, where Th17 cells interact with resident gut microbiota, which influence their development, IL-17A induces a protective response against gut microbiota and pathogens through the induction of β-defensins, calprotectin, and lipocalin, as well as other AMPs by intestinal epithelial cells [136,137,138,139,140].
Tissue-remodeling substances can also be expressed as a product of IL-17A stimulation. Treatment with IL-17A on diseased tendon-derived fibroblasts induces MMP3 expression [98]. In the nasal tissue of patients with chronic rhinosinusitis with nasal polyps, CD8+ T cell production of IL-17A results in an increase in the expression of MMP-9 by nasal epithelial cells [104].
In lymphocytes, IL-17A secreted by CD4+ T cells represses Th1 polarization, but when acting on B cells, it promotes B cell survival, proliferation, and differentiation into plasma cells [94].
IL-17F shares a great amount of overlap with the function and cellular sources of IL-17A, its close homolog. Often inflammatory, IL-17F functions in promoting tissue-mediated innate and adaptive immune responses against microbial infections by bacteria and fungi. IL-17F-producing cells include Th17 cells, LTi, NK cells, iNKT cells, neutrophils, ILC3, and γδ T cells [21,48,141]. The target cells of IL-17F include epithelial cells, endothelial cells, and stromal cells [4,12].
Inflammation induced by IL-17F, as with IL-17A, harbors infiltrate predominantly comprising neutrophils. IL-17F activates the expression of GM-CSF, ICAM-1, IL-6, and IL-8 by bronchial epithelial cells, thus recruiting lymphocytes, neutrophils, and macrophages. This process has been implicated in allergic inflammatory responses in the lung [109,110,111,114]. Additionally, stimulation with IL-17F and TNFα induces chemokine CXCL1 and cytokine G-CSF production in human bronchial epithelial cells, while IL-17F and IL-23 enhance the production of IL-6 and IL-1β from eosinophils linked to allergic inflammation [114,142]. The upregulation of proinflammatory mediators IL-6 and CXCL1 has also been observed, resulting from IL-17F stimulation of fibroblasts and macrophages [116]. In addition to TNFα and IL-23, IL-17F also works with IL-22 to induce the expression of AMPs, such as hBD-2, S100A7, S100A8, and S100A9, from primary keratinocytes as a function of innate immunity in the skin [115].
IL-17A and IL-17F can also form a heterodimer (IL-17A/F) that signals through the same IL-17RA/RC complex [23]. IL-17A/F has been reported to be produced by the same cell types as those producing its homodimer counterparts, such as Th17 cells, γδT cells, and ILCs [18,23,117].
As with IL-17A and IL-17F, IL-17B is involved in fostering an inflammatory response to aid host defense. However, IL-17B signaling is restricted to certain target cells and has been associated with type 2 immunity [6,124,143]. Like IL-17A, IL-17B has been found to be produced by neutrophils. However, unlike IL-17A, IL-17B is not produced by Th17 cells; it is expressed in a wide variety of tissues, albeit not ubiquitously [6,87,124,144,145]. IL-17B is produced by chondrocytes, neurons, stromal cells, intestinal epithelial cells, as well as memory and germinal center B cells [87,124,144,146,147,148]. IL-17B is expressed in the spinal cord, and at lower levels, in the trachea, lung, stomach, small intestine, colon, prostate, testes, ovary, adrenal gland, and pancreas [3,6,144,148]. The known target cells of IL-17B include ILC2s, NKT cells, fibroblasts, and Th2 cells [3,143,145].
Induction of a type 2 immune response by IL-17B is observed when the cytokine acts on peripheral blood mononuclear cells (PBMCs), NKT cells, or ILC2s. Stimulation of PBMCs by IL-17B results in the production of IL-5 and IL-13. Similarly, IL-17B promotes the production of IL-5 by ILC2s [3]. Co-stimulation of NKT cells by IL-17B and IL-25 promotes the release of IL-5 [3]. On the other hand, anti-inflammatory functions of IL-17B are observed through its ability to inhibit the proinflammatory effects of IL-25 in the lung by restraining the Th2 response [149]. Protective effects of IL-17B through type 2 immunity are also observed through its inhibition of IL-6 production by IL-25 in models of colitis [149].
However, a more inflammatory phenotype induced by IL-17B is seen when the cytokine acts on fibroblasts co-stimulated by TNFα, resulting in expression of G-CSF, contributing to neutrophil migration, as well as expression of IL-6 and CCL20, which are Th17 chemotactic and induction factors [145]. Proinflammatory phenotypes are also observed when IL-17B, as well as IL-17C, stimulate THP-1 cells to release TNFα and IL-1β or stimulate 3T3 cell lines and peritoneal exudate cells to release IL-6 and IL-23 [3,150].
Akin to its homologs IL-17A and IL-17B, IL-17C shares a role in host defense by promoting inflammation. IL-17C is heavily involved in the innate immune response against bacteria, fungi, and viruses [61]. IL-17C is predominantly expressed by epithelial cells of the lung, skin, and colon, but it is not found to be expressed by lymphocytes [64,66,124,151,152,153]. Often, IL-17C stimulates an innate response of epithelial cells in an autocrine fashion. For this reason, the inflammation incurred by IL-17C is often linked to the skin [64,152,153]. Additionally, IL-17C acts on Th17 cells to aid their development, thus promoting the production of IL-17A and IL-17F [33,154].
In response to bacterial pathogens and other microbes in the respiratory tract, IL-17C promotes the release of proinflammatory IL-6 from bronchial epithelial cells [152]. Similarly, as a response to intestinal pathogens, IL-17C synergizes with IL-22 to induce the production of AMPs, chemokines, and proinflammatory cytokines from colon epithelial cells, where it is also involved in barrier stability via enhancement of occludin, claudin-1, and claudin-4 expression [155,156]. In mucosal and cutaneous epithelial cells, IL-17C is known to induce proinflammatory IL-1β, as well as TNF, in response to bacterial pathogens [64,153]. Additionally, IL-17C expression is observed in kidney epithelial cells after fungal infection, resulting in expression of proinflammatory cytokines TNFα, IL-1β, as well as IL-6, by renal epithelial cells [157]. IL-17C also has a hand in combating viral infections; when produced by keratinocytes as a response to herpes simplex virus-2, it functions as a neurotrophic factor exerting protective effect on peripheral nerve systems [61,158].
The functional importance of IL-17D has been slowly uncovered but still requires more investigation. Thus far, IL-17D has been implicated in stress response, intestinal homeostasis as well as anti-viral and anti-tumor responses [34,67,159,160]. Expression of IL-17D has been found in fibroblasts, skeletal muscle, brain, adipose tissue, tumor cell lines, heart, lung, pancreas, as well as colon epithelial cells [7,34,67,159,160]. The known target cells of IL-17D include endothelial cells and ILC3s [67]. IL-17D produced by colonic epithelial cells stimulates ILC3 for their production of IL-22 and antimicrobial peptides RegIIIβ and RegIIIγ, thus helping confer protection against bacterial infection, dysbiosis, and colonic inflammation [34]. Additionally, oxidative stress, due to tumor development or viral infection, induces transcription factor Nrf2 to promote IL-17D expression. IL-17D stimulates endothelial cells to express MCP-1 for the recruitment of NK cells to elicit an effective anti-tumor or anti-viral effect [67,159,160]. Further, IL-17D is implicated in inhibiting hemopoiesis and myeloid progenitor proliferation, which is thought to occur through its ability to stimulate the production of cytokines, such as IL-8, from endothelial cells or other target cells [7].
A wider variety of cells produce the pleiotropic IL-17E. IL-17E-producing cells include mast cells, alveolar macrophages, eosinophils, basophils, ILC2s, DCs, stromal and epithelial cells, and Th2 cells [47,68,85,86,124,161,162,163,164,165,166,167]. The target cells of IL-17E include keratinocytes, lung epithelial cells, endothelial cells, Th2 and Th9 cells, macrophages, fibroblasts, and ILC2s [68,163,165,168]. IL-17E protects the host against parasites, such as helminths, as it orchestrates type 2 immunity. IL-17E has also been implicated in allergic and skin inflammation, anti-fungal immunity, inhibition of Th17 development, as well as maintenance of intestinal homeostasis [85,86,151,163,165,167,169,170]. Keratinocyte-produced IL-17E promotes skin inflammation through the recruitment of neutrophils and macrophages [171]. IL-17E also directly targets keratinocytes in an autocrine manner to facilitate wound healing through the induction of keratins 6, 16, and 17. In addition, IL-17E increases the metabolic activity of keratinocytes by activating the mTOR pathway during skin inflammation [68]. Interestingly, IL-17E does not appear to induce the production of β-defensin 2, LL-37, or S100A7 when acting on keratinocytes [68,172,173]. The involvement of IL-17E in antifungal immunity is observed through the ILC2 inflammatory phenotype following IL-17E stimulation, which confers protection against Candida albicans [174]. IL-17E also induces Th2 differentiation and promotes the production of Th2 cytokines. For example, IL-17E stimulation of Th9 cells results in the production of IL-9, a cytokine implicated in allergic airway inflammation [168]. Stimulation of Th2 memory cells by IL-17E secreted by basophils and eosinophils, similarly, has been found to promote sustained Th2 transcription factor expression, leading to Th2 expansion and polarization, and allergic inflammation [86]. Additionally, IL-17E recruits macrophages and eosinophils to the lung, promotes Th2 cell differentiation, and stimulates release of Th2 cytokines, such as IL-4, IL-5, and IL-13, and by doing so, it initiates innate and adaptive proallergic responses by lung epithelial cells [85,161]. A type 2 anti-helminth immune response to maintain intestinal homeostasis is imparted through tuft cell-derived IL-17E, which targets ILC2 and Th2 cell-mediated release of IL-4, IL-5, and IL-13 [165,169]. In addition, IL-17E produced by mast cells and keratinocytes acts on dermal DCs to stimulate the production of IL-1β, IL-6, and TNFα, which subsequently activate Th17 cells [68,172,173].
7. Pathological Consequences of IL-17 Signaling
Despite their involvement in host protective immune responses, the IL-17 family of cytokines are also implicated in a variety of proinflammatory pathologies. Aberrant expression of the tissue-specific and context-dependent IL-17 family cytokines, or an inappropriate response of target cells to these cytokines, are often associated with autoimmune disorders and cancers. In certain cases, IL-17 family-dependent pathologies, or simply the dysregulation of IL-17 family cytokine production or synergism, are drivers for metabolic disorders. On the other hand, metabolic disorders can increase the severity of IL-17-induced pathology, representing a unique interplay between immunity and metabolism, which will be discussed later in this review.
While the production of IL-17 cytokines during infection occurs in a controlled manner, chronic production of and biological responses to IL-17 can lead to inflammatory disorders, such as psoriasis. Psoriasis is labeled as an IL-17A-driven disease; however, IL-17C, IL-17E as well as IL-17F also contribute to this pathology [63,93,124]. In healthy skin, the presence of IL-17A is beneficial—facilitating epithelial barrier protection against fungi and bacteria, as well as promoting tissue repair and wound healing [93,124]. The host protective responses driven by IL-17A and IL-17F include keratinocyte proliferation and differentiation, production of AMPs and inflammatory and chemoattractant molecules that recruit neutrophils, macrophages, and immune cells, such as Th17 or Tc17 cells [93,124,175]. Additionally, IL-17A and IL-17F can synergize with TNFα to increase the production of inflammatory mediators [63,124,175]. Similarly, IL-17C, which, like IL-17A and IL-17F, is upregulated in psoriatic skin, targets endothelial cells and keratinocytes and works with TNFα to induce inflammation [66,175,176,177]. IL-17E also contributes to this pathology by stimulating keratinocytes and macrophages to produce inflammatory cytokines and chemokines [123,124].
The inflammatory effects of IL-17A are also implicated in bone destruction observed in rheumatoid arthritis (RA) [178]. As seen in psoriasis, the synergy between IL-17A and TNFα is involved in the pathogenesis of RA. IL-17A and TNFα promote inflammation through the production of IL-6 and IL-8 cytokines released from fibroblast-like synoviocytes [95]. Additionally, IL-17A contributes to RA by promoting osteoclast differentiation and inducing chemokines that recruit neutrophils, macrophages, as well as other lymphocytes to the joint, eventually leading to the destruction of synovium [178,179,180,181]. Moreover, Th17 cells not only contribute to the inflammatory milieu observed in RA, but also induce MMPs that further the destruction of synovial tissues [179,180,181].
Inflammatory bowel disease (IBD) is another autoimmune condition where the IL-17 family of cytokines are involved. IBD encompasses both ulcerative colitis (UC) and Crohn’s disease (CD). Although complex, as Th17 cells function in maintaining gut homeostasis, the number of Th17 cells and the levels of their associated cytokines are increased in intestinal mucosa of IBD patients [182,183]. IL-17A, IL-17C, and IL-17F are all overexpressed in IBD [184,185,186]. Specifically, IL-17A is responsible for amplifying TNFα-induced IL-17C production by enteroendocrine and goblet cells. IL-17C subsequently upregulates CCL20 to allow for further influx of Th17 cells [186]. Increased expression of CXCL1 in CD and CXCL6 in UC by intestinal fibroblasts following IL-17A stimulation also contributes to IBD pathology [187].
Additional disorders are also linked to the IL-17 family cytokines. To highlight a few: asthma, Behçet’s disease, multiple sclerosis, systemic lupus erythematosus, ankylosing spondylarthritis, systemic sclerosis, and uveitis [128,188,189,190,191,192]. While IL-17 is linked to a variety of inflammatory disorders, more research into how IL-17 influences these disorders may uncover novel therapeutic targets.
8. IL-17 and Cancer—A Dangerous Liaison
The IL-17 family of cytokines have been associated with multiple types of cancers. IL-17A, IL-17B, IL-17C, as well as IL-17F have been shown to promote tumor development, whereas IL-17D and IL-17E play anti-cancer roles in a context-dependent manner by activating the Nrf2 stress surveillance pathway, recruiting innate immune cells, and activating leukocytes [67,159,193,194,195].
Breast cancer growth, migration, angiogenesis, and invasiveness are further enhanced by IL-17A [196,197,198]. IL-17A promotes ERK1/2 phosphorylation and p38 MAPK activation, which in turn promotes breast cancer cell proliferation, migration, and tissue invasion [196,197,198,199]. Additionally, IL-17A alters the tumor microenvironment through the induction of tissue-remodeling substances, such as MMPs, inhibition of apoptosis, promotion of angiogenesis as well as recruitment of immature myeloid cells, which can suppress the activity of CD8+ T cells [198,199,200,201,202]. While IL-17E has been shown to induce apoptosis in breast cancer cells, IL-17B as well as IL-17RB expression correlates with worse cancer outcomes [120,203,204]. Elevated levels of IL-17B/IL-17RB signaling is associated with enhanced tumorigenicity due to ERK1/2 pathway activation leading to upregulation of Bcl-2 expression, as well as HER2 amplification [120,203].
IL-17A and IL-17C both promote the development of colorectal cancer (CRC) [93,205,206]. IL-17A promotes CRC cell proliferation, tumor growth, and angiogenesis resulting from VEGF production in CRC. IL-17C, in response to stimulation by gut microbes, upregulates the expression of Bcl-2 and Bcl-xL in intestinal epithelial cells, promoting cell survival [62,202,207]. Moreover, IL-17C promotes tumor angiogenesis in CRC [208]. IL-17F is another player in CRC; in different contexts, IL-17F exerts a protective anti-angiogenic effect or pro-tumorigenic effect through promotion of the epithelial–mesenchymal transition [195,209,210]. The role of IL-17B in CRC requires additional investigation, but its overexpression could be linked to increased inflammatory cytokine expression [211]. A review by Razi et al. provides further detail on IL-17A in CRC [212]. The context-dependent malignant versus protective role of IL-17F is seen in a variety of cancer types and has been extensively investigated by Mikkola et al. [195].
Although not exhaustive, the IL-17 family of cytokines have also been implicated in cancers of the lung, prostate, cervix, ovary, bladder, and skin [213,214,215,216,217,218,219,220,221,222]. These areas have been extensively covered by a number of review articles and will not be elaborated here. Nonetheless, the tumor-promoting roles of IL-17 family cytokines share common mechanistic traits, such as promoting myeloid cell infiltration, enhancing cancer cell survival and growth, and inhibiting anti-cancer immune responses (Figure 1).
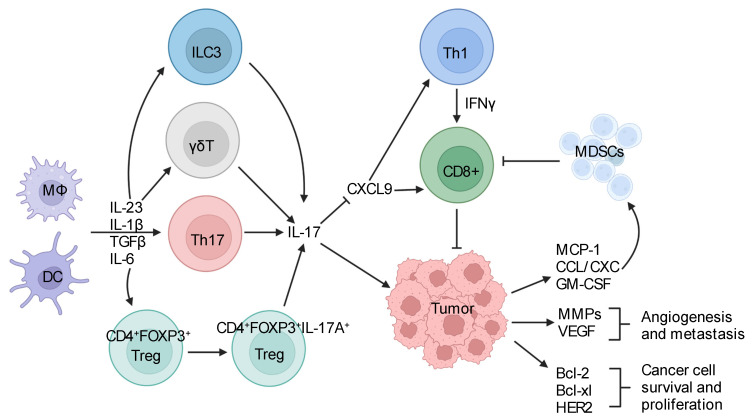
Figure 1.
How IL-17 promotes cancer development.
Tumor-infiltrating dendritic cells (DC) and other myeloid cells respond to stimuli present in the tumor microenvironment and produce multiple cytokines to drive the differentiation and activation of IL-17-producing Th17 cells and other immune cells. IL-17, in turn, signals to tumor cells and turns on genes that are responsible for tumor cell survival and proliferation, as well as factors that further exacerbate tumor-associated inflammation and angiogenesis. IL-17 also inhibits the activity of anti-tumor immune cells by recruiting myeloid-derived suppressor cells (MDSC) and inhibiting the production of T-cell-attracting chemokines. Overall, hijacking IL-17 signaling by tumors results in a pro-tumor inflammatory milieu that favors tumor growth and impedes tumor destruction.
9. IL-17 Family Cytokines That Fuel Metabolic Disorders
The IL-17 family of cytokines are involved in, and influence, metabolic processes. IL-17A has been shown to induce differential expression of genes in pre-adipocytes versus adipocytes [223]. In murine models, IL-17A negatively regulates adipogenesis and glucose metabolism [223,224]. This is mediated by IL-17′s function in downregulating genes that are important for both lipid and glucose metabolism [224,225]. In human bone marrow mesenchymal stem cells (hBM-MSC), IL-17A increases the expression of IL-17RC, which is associated with inhibition of adipocyte differentiation [225]. However, in differentiated adipocytes, IL-17A inhibits adipose tissue accumulation by preventing glucose uptake [224]. In fully differentiated adipocytes, IL-17A decreases the transcriptional activities of peroxisome proliferator activating receptor γ (PPARγ) and fatty acid binding protein 4 (FABP4), increases lipolysis, stimulates IL-6, TNFα, and IL-8 cytokine production, and upregulates COX2 gene expression, which consequently increases PGE2 levels [225,226]. Interestingly, previous reports have demonstrated the immunomodulatory effects of PGE2 produced by MSCs on fully differentiated Th17 cell cytokine production, where PGE2 production by MSCs is augmented upon cell–cell contact with Th17 cells, likely due to the presence of TNFα secreted by Th17 cells [227]. TNFα has been cited as important in lipid homeostasis, acting as an adipokine produced from adipose tissue that is important in inducing insulin resistance, lipolysis, and inhibiting adipocyte differentiation [228].
MSCs further suppress adipogenesis by inhibiting the differentiation of naïve T cells into Th17 cells [227]. In 3T3-L1 cells, IL-17A-mediated suppression of adipogenesis is observed through suppression of Kruppel-like family transcription factor (KLF) 15, PPARγ, as well as C/EBPα [229]. However, such effect was not observed in the adipose tissue of obese individuals, warranting further study to elucidate the context-dependent regulation of adipogenesis by IL-17 [230]. Of note, there is a high level of IL-6 expression in undifferentiated MSCs, which is necessary for undifferentiated MSC proliferation, as well as inhibition of the expression of FABP4 and LPL genes [226]. In MSCs, IL-6 activates the ERK1/2 pathway [226]. However, the overall contribution of IL-6 to metabolism is complex. Recent reports describe the contribution of IL-6 in metabolism as dependent upon the source, signaling pathway—canonical or noncanonical—and diet, which subsequently influences leukocyte accumulation and inflammation [231]. IL-6 secreted from adipocytes is also associated with adipose tissue macrophage accumulation [231].
In adipose tissue, leukocytes, such as γδ T cells, have been identified as the main IL-17A producers [224,232]. Recently, γδ T cells, which were previously linked to controlling body temperature via IL-17A expression, have been shown to promote adaptive thermogenesis through sympathetic innervation resulting from stimulation of IL-17F binding IL-17RC, driving parenchymal cell production of TGFβ1 in adipocytes [232].
Certain metabolic disorders have been linked with the IL-17 family of cytokines and their dysregulation. Alterations in the levels of cytokines important in the expression of the IL-17 family of cytokines or their targets have also been implicated. For example, IL-17A has been associated and studied for its contribution to obesity [223,230,233,234,235,236]. In investigating the differences relating to IL-17A observed between obese mice that were fed a high-fat diet (HFD) with lean mice on a regular diet (RD), Qu et al. observed elevated levels of IL-17A in subcutaneous adipose tissue (SAT) of obese mice [223]. Congruently, in a separate study, adipose tissue from obese human subjects was found to promote IL-17A release from CD4+ T cells, which was positively correlated with IL-1β concentrations in conditioned media from omental adipose tissue [233]. Additionally, Qu et al. reported that IL-17A significantly increases inflammatory cytokines in adipose tissue of obese mice. However, lean mouse visceral adipose tissue (VAT) expresses a greater level of IL-6 at baseline [223,230]. Human pre-adipocytes, in the presence of IL-17A or IL-1β, upregulate a host of genes related to fibrosis and inflammation [233]. In human pre-adipocytes, IL-17A, to a lesser extent than IL-1β, upregulates MMP1, MMP3, MMP8, IL-1β, CCL2, CEBPB, SERPINE1, and GREM1. In human adipocytes, IL-6, IL-8, CCL2, and CCL20 are induced by IL-17A, again, to a lesser extent than IL-1β [233]. Additionally, the differences in metabolic gene expression as well as signaling pathway activation in obese mouse adipose tissue versus lean mouse adipose tissue reveal that IL-17A in obese mice induces expression of genes associated with diet-induced obesity (DIO) and dampen glucose transport [223]. Concordantly, alterations in metabolic genes in human adipocytes were associated with IL-17A and IL-1β signaling on adipocytes [233]. Moreover, IL-17A increases STAT3 signaling in obese mouse adipose tissue [223].
Prevention of DIO and the associated metabolic and inflammatory disturbances is seen upon suppression of IL-17A expression via inhibition of RORγt by ubiquitous deletion of IL-17RA or use of IL-17A and IL-1β neutralizing antibodies in adipocytes [233,236]. Global inhibition of IL-17RA is associated with thermogenesis, as well as decreased leukocyte infiltration into adipose tissue [236]. Further, reduced obesity and improved metabolic markers are observed by IL-17RA deletion from adipocytes, where IL-17A phosphorylation of PPARγ is associated with the repression of browning and expression of diabetogenic and obese genes [236]. Further, IL-17A deficiency inhibits TANK-binding kinase 1 (TBK1) activation in adipocytes and is also associated with reduced inflammation in obese mice [234].
In addition to increased inflammation and alterations in metabolic markers, obesity is associated with other IL-17 family cytokine-related diseases. Obesity has been identified as a risk factor for psoriasis, and likewise, a trend in weight increase in patients with psoriasis has been observed [237,238,239]. The attribution of the bidirectional risk is speculated due to the overlap of inflammatory cytokines involved in both diseases, cellular sources of inflammatory cytokines, and alterations in oxidative stress levels [240,241]. A bidirectional association and risk of obesity are also observed with rheumatoid arthritis, which is similarly attributed to increased inflammatory cytokine levels, as well as leukocyte recruitment [242,243]. In addition, the altered abundances of metabolites in obesity and other metabolic disorders may function as immune regulators independent from their conventional role in metabolism [244]. It is also important to note that inflammation does not necessarily stem from host obesity status. For example, a diet high in fat, such as the standard Western diet, has been linked to inflammation due to the alteration in gut microbiota causing dysbiosis, barrier dysfunction, and inflammation [245].
Beyond, and including obesity, metabolic dysregulation linked with the IL-17 family of cytokines has been associated with certain cancers. A mechanistic consideration linking the involvement of the IL-17 family of cytokines with metabolic dysregulation leading to cancer will be discussed in the next section of this review.
10. IL-17 Bridges Metabolism and Cancer
Increasing evidence suggests that dysregulated metabolic pathways accompany the process of tumorigenesis. Metabolic syndrome (MetS), which is clinically characterized by the presence of at least three metabolic abnormalities, including insulin resistance and high fasting blood sugar levels, dyslipidemia and low fasting HDL cholesterol, obesity with an emphasis on abdominal obesity, and hypertension, has been clinically and experimentally associated as a risk factor for a variety of cancers and associated with a worse clinical outcome [246,247,248,249,250,251,252]. A meta-analysis has revealed gender- and population-specific differences in risks associated with developing certain cancer types in those with MetS [247]. MetS is associated with increased risks of liver and colorectal cancer in males, and pancreatic and rectal cancer in females [247]. The presence of MetS is also associated with breast cancer risk, as well as with total cancer mortality [248,249,250,251,252]. Individual components that characterize MetS have been associated with total cancer mortality risk and all-cause cancer mortality in men [249,252]. Although it is well established that MetS is associated with an increased risk of cancer, the mechanisms linking both pathologies require further investigation. When examining individual components of MetS, the IL-17 family of cytokines may provide a link between metabolic dysregulation and the path to developing cancer.
Recent advances in cancer biology point to a role of IL-17 in linking metabolic disorders and cancers (Figure 2). γδ T cells that produce IFN-γ and IL-17 exhibit distinct metabolic preferences [253]. While IFN-γ-producing γδ T cells rely on glycolysis, IL-17-producing γδ T cells undergo predominantly oxidative metabolism [253]. This selective preference of metabolism was imprinted in the thymus during T-cell development and maintained in the periphery. Tumor-promoting IL-17+ γδ T cells showed high lipid uptake and storage and were expanded in tumors of obese mice [253]. Therefore, obesity contributes to tumor development by upregulating the production of IL-17 by γδ T cells. On the contrary, IFNγ-secreting γδ T cells were associated with tumor regression and thrived in a glucose-rich environment [253,254]. In separate studies examining the impact of obesity on T-cell antiviral immunity, obesity was found to negatively impact the percentage of Vγ9Vδ2 T cells, an IFNγ-secreting γδ T-cell subset [255,256].
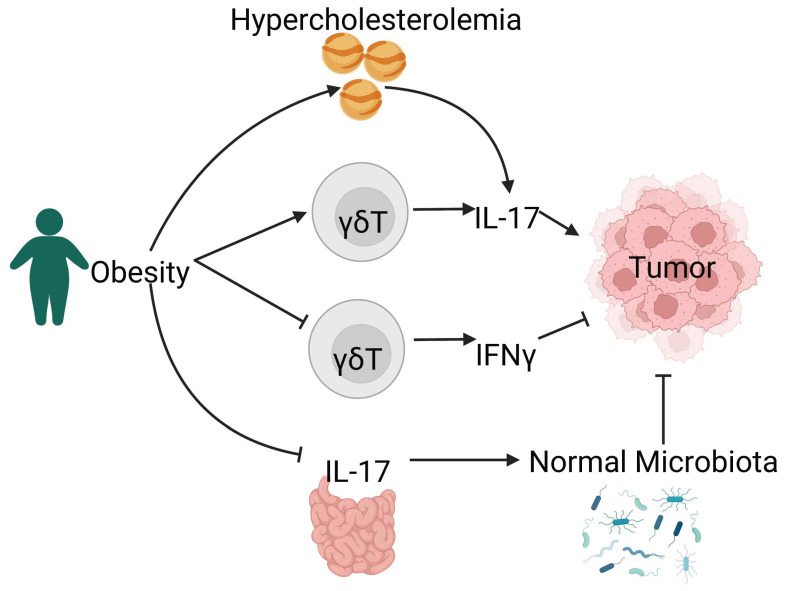
Figure 2.
IL-17 bridges metabolic disorders and cancer.
Recent studies on IL-17 have started to link its function with both metabolic disorders and cancer. IL-17 may promote tumor development following disrupted metabolism in one of the following three ways: (1) obesity enhances tumor development by promoting IL-17 production from γδ T cells within the tumor environment. At the same time, the production of anti-tumor IFN-γ by γδ T cells is reduced; (2) hypercholesteremia induces higher levels of IL-17 in the serum and promotes tumor growth; (3) obesity disrupts IL-17-mediated regulation on intestinal microbiota, which results in glucose intolerance and insulin resistance, which are also linked to increased risk in cancer development.
Hypercholesteremia, a metabolic abnormality and component of MetS, where serum levels of low-density lipoprotein cholesterol (LDL-C) are elevated, is associated with increased risk of atherosclerotic cardiovascular disease as well as cancer [257,258]. Interestingly, in a study by Roohi et al. involving serum collected from 120 healthy subjects, a statistically significant difference in serum IL-17A levels was found between those with LDL-C ≥ 130 mg/dL when compared with those with LDL-C < 130 mg/dL [259]. The positive association of serum IL-17A with LDL-C through the perturbation of IL-17A levels, as well as IL-23 levels, may pave the road for the development of MetS and cancer. In white males, low levels of high-density lipoprotein (HDL) and high levels of LDL are associated with an increased risk of prostate cancer [260]. Experimentally, LDL-C was found to induce phenotypic and genotypic changes in ER-positive breast cancer cells [261]. In vitro LDL-C was found to induce ER-positive breast cancer cell proliferation and reduce cell adhesion, which results from upregulation of genes related to cell survival and proliferation pathways as well as downregulation of adhesion molecules [261]. Additionally, in vivo, LDL-C is found to promote ER-positive breast cancer growth [261]. Hypercholesterolemia is also associated with increased incidence of CRC [262]. In a study by Tie et al., the presence of hypercholesteremia in mice increased the number of azoxymethane-induced colorectal tumors [262]. The authors found reduced differentiation of γδT and NKT thymic and colon submucosa populations via an increase in oxidative stress on hematopoietic stem cells (HSCs) due to oxidized LDL [262]. The skewing of lineage fate, controlled by downregulation of Tet1, also impacts the function of terminally differentiated γδT and NKT cells [262]. In ApoE-/- mice, γδT cells were found to produce less IL-17. However, γδT cells derived from Tet1-overexpressing HSCs produced greater amounts of IL-17 when compared with wild-type mice [262]. However, it is unknown whether the change in IL-17 level is responsible for the altered rate of CRC development.
Another way in which the IL-17 cytokine family may link metabolic dysregulation with cancer is through its impact on intestinal microbiota [263,264]. Th17 cells in the small intestine have been found to promote the expansion of gut microbiota associated with leanness and metabolic homeostasis in an IL-17-dependent manner [263]. HFD-fed obese mice have reduced Th17 cells in the small intestine as well as increased IFNγ+ Th1 cells and γδ T cells [263]. Similarly, in patients with MetS, serum levels of IL-17 are reduced in conjunction with the increasing presence of metabolic abnormalities [265]. IL-17 signaling to intestinal epithelial cells drives changes in the microbiota, and this mechanism is required for the resistance to metabolic disorders induced by a HFD [264]. This intestinal-specific IL-17–microbiota–metabolism axis is of special interest because it differs from the traditional view of obesity as a proinflammatory state, where researchers found increased levels of IL-17A in the serum [266,267] and visceral adipose tissues [268]. There has already been evidence linking disrupted glucose metabolism with cancer. Elevated levels of blood glucose, hyperinsulinemia, and insulin resistance are associated with increased risk of CRC mortality in both sexes [269]. Insulin resistance in postmenopausal women also increases both risk of and death from breast cancer [270,271].
11. Future Perspectives
The development of effective anticancer therapeutics and immunotherapies is exceptionally complex. Issues range from combating drug toxicity and adverse side effects, harnessing a drug or patient’s immune system to target malignant tumor cells effectively and selectively, to molecular resistance observed through the course of treatment. Additionally, the complications are compounded by the presence of metabolic diseases or when considering the bidirectional impact of anticancer therapies on metabolism.
As discussed, alterations in metabolism can support the development of tumors. The distinct metabolic demands of cancer cells have been harnessed in cancer therapy, with most research uncovering vulnerabilities of malignant and rapidly proliferating cancer cells. Additional research has been poured into understanding lymphocyte metabolism in the tumor microenvironment to further harness drug specificity and therapeutic potential.
IL-17, as a family of proinflammatory cytokines, has been implicated in both metabolic disorders as well as cancer development and resistance to therapy. Further investigation on the role of IL-17 as a link between disrupted metabolism and cancer is needed to elucidate the function and mechanism of action of such link, so as to validate the potential of the IL-17 pathway as a target for novel cancer therapy in humans. Pharmacological agents against different members of IL-17 and their receptors have been proven effective and safe for the treatment of autoimmune diseases in humans. Notable ones include Secukinumab and Ixekizumab targeting IL-17A, Bimekizumab targeting IL-17A and F, and Brodalumab targeting IL-17RA [272]. Inhibitors of IL-17E are also gaining traction for the treatment of asthma, and more recently, psoriasis [123,273,274]. These agents could be tested in a trial on IL-17 pathway blockade as adjuvant therapy against cancers in humans, especially those who also suffer from metabolic syndromes.
Acknowledgments
We thank Geneva Hargis at UConn School of Medicine for scientific editing. Figures were created with BioRender.com.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Institute of Health/National Cancer Institute (NIH/NCI R01CA262430) to K.W. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rouvier E., Luciani M.F., Mattei M.G., Denizot F., Golstein P. CTLA-8, cloned from an activated T cell, bearing AU-rich messenger RNA instability sequences, and homologous to a herpesvirus saimiri gene. J. Immunol. 1993;150:5445–5456. [PubMed] [Google Scholar]
- 2.Yao Z., Fanslow W.C., Seldin M.F., Rousseau A.M., Painter S.L., Comeau M.R., Cohen J.I., Spriggs M.K. Herpesvirus Saimiri encodes a new cytokine, IL-17, which binds to a novel cytokine receptor. Immunity. 1995;3:811–821. doi: 10.1016/1074-7613(95)90070-5. [DOI] [PubMed] [Google Scholar]
- 3.Li H., Chen J., Huang A., Stinson J., Heldens S., Foster J., Dowd P., Gurney A.L., Wood W.I. Cloning and characterization of IL-17B and IL-17C, two new members of the IL-17 cytokine family. Proc. Natl. Acad. Sci. USA. 2000;97:773–778. doi: 10.1073/pnas.97.2.773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hymowitz S.G., Filvaroff E.H., Yin J.P., Lee J., Cai L., Risser P., Maruoka M., Mao W., Foster J., Kelley R.F., et al. IL-17s adopt a cystine knot fold: Structure and activity of a novel cytokine, IL-17F, and implications for receptor binding. EMBO J. 2001;20:5332–5341. doi: 10.1093/emboj/20.19.5332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liu X., Sun S., Liu D. IL-17D: A Less Studied Cytokine of IL-17 Family. Int. Arch. Allergy Immunol. 2020;181:618–623. doi: 10.1159/000508255. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y., Ullrich S.J., Zhang J., Connolly K., Grzegorzewski K.J., Barber M.C., Wang W., Wathen K., Hodge V., Fisher C.L., et al. A novel cytokine receptor-ligand pair. Identification, molecular characterization, and in vivo immunomodulatory activity. J. Biol. Chem. 2000;275:19167–19176. doi: 10.1074/jbc.M910228199. [DOI] [PubMed] [Google Scholar]
- 7.Starnes T., Broxmeyer H.E., Robertson M.J., Hromas R. Cutting edge: IL-17D, a novel member of the IL-17 family, stimulates cytokine production and inhibits hemopoiesis. J. Immunol. 2002;169:642–646. doi: 10.4049/jimmunol.169.2.642. [DOI] [PubMed] [Google Scholar]
- 8.Lee J., Ho W.H., Maruoka M., Corpuz R.T., Baldwin D.T., Foster J.S., Goddard A.D., Yansura D.G., Vandlen R.L., Wood W.I., et al. IL-17E, a novel proinflammatory ligand for the IL-17 receptor homolog IL-17Rh1. J. Biol. Chem. 2001;276:1660–1664. doi: 10.1074/jbc.M008289200. [DOI] [PubMed] [Google Scholar]
- 9.Liu S., Song X., Chrunyk B.A., Shanker S., Hoth L.R., Marr E.S., Griffor M.C. Crystal structures of interleukin 17A and its complex with IL-17 receptor A. Nat. Commun. 2013;4:1888. doi: 10.1038/ncomms2880. [DOI] [PubMed] [Google Scholar]
- 10.Kostareva O.S., Gabdulkhakov A.G., Kolyadenko I.A., Garber M.B., Tishchenko S.V. Interleukin-17: Functional and Structural Features, Application as a Therapeutic Target. Biochemistry. 2019;84:S193–S205. doi: 10.1134/S0006297919140116. [DOI] [PubMed] [Google Scholar]
- 11.Akimzhanov A.M., Yang X.O., Dong C. Chromatin remodeling of interleukin-17 (IL-17)-IL-17F cytokine gene locus during inflammatory helper T cell differentiation. J. Biol. Chem. 2007;282:5969–5972. doi: 10.1074/jbc.C600322200. [DOI] [PubMed] [Google Scholar]
- 12.Kolls J.K., Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21:467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 13.Gerhardt S., Abbott W.M., Hargreaves D., Pauptit R.A., Davies R.A., Needham M.R., Langham C., Barker W., Aziz A., Snow M.J., et al. Structure of IL-17A in complex with a potent, fully human neutralizing antibody. J. Mol. Biol. 2009;394:905–921. doi: 10.1016/j.jmb.2009.10.008. [DOI] [PubMed] [Google Scholar]
- 14.Zhang X., Angkasekwinai P., Dong C., Tang H. Structure and function of interleukin-17 family cytokines. Protein Cell. 2011;2:26–40. doi: 10.1007/s13238-011-1006-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu S. Structural Insights into the Interleukin-17 Family Cytokines and Their Receptors. Adv. Exp. Med. Biol. 2019;1172:97–117. doi: 10.1007/978-981-13-9367-9_5. [DOI] [PubMed] [Google Scholar]
- 16.Gaffen S.L. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moseley T.A., Haudenschild D.R., Rose L., Reddi A.H. Interleukin-17 family and IL-17 receptors. Cytokine Growth Factor Rev. 2003;14:155–174. doi: 10.1016/S1359-6101(03)00002-9. [DOI] [PubMed] [Google Scholar]
- 18.Chang S.H., Dong C. Signaling of interleukin-17 family cytokines in immunity and inflammation. Cell. Signal. 2011;23:1069–1075. doi: 10.1016/j.cellsig.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Song X., Qian Y. The activation and regulation of IL-17 receptor mediated signaling. Cytokine. 2013;62:175–182. doi: 10.1016/j.cyto.2013.03.014. [DOI] [PubMed] [Google Scholar]
- 20.Ely L.K., Fischer S., Garcia K.C. Structural basis of receptor sharing by interleukin 17 cytokines. Nat. Immunol. 2009;10:1245–1251. doi: 10.1038/ni.1813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wright J.F., Guo Y., Quazi A., Luxenberg D.P., Bennett F., Ross J.F., Qiu Y., Whitters M.J., Tomkinson K.N., Dunussi-Joannopoulos K., et al. Identification of an interleukin 17F/17A heterodimer in activated human CD4+ T cells. J. Biol. Chem. 2007;282:13447–13455. doi: 10.1074/jbc.M700499200. [DOI] [PubMed] [Google Scholar]
- 22.Goepfert A., Lehmann S., Wirth E., Rondeau J.M. The human IL-17A/F heterodimer: A two-faced cytokine with unique receptor recognition properties. Sci. Rep. 2017;7:8906. doi: 10.1038/s41598-017-08360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright J.F., Bennett F., Li B., Brooks J., Luxenberg D.P., Whitters M.J., Tomkinson K.N., Fitz L.J., Wolfman N.M., Collins M., et al. The human IL-17F/IL-17A heterodimeric cytokine signals through the IL-17RA/IL-17RC receptor complex. J. Immunol. 2008;181:2799–2805. doi: 10.4049/jimmunol.181.4.2799. [DOI] [PubMed] [Google Scholar]
- 24.Nesmond S., Muller C., Le Naour R., Viguier M., Bernard P., Antonicelli F., Le Jan S. Characteristic Pattern of IL-17RA, IL-17RB, and IL-17RC in Monocytes/Macrophages and Mast Cells From Patients With Bullous Pemphigoid. Front. Immunol. 2019;10:2107. doi: 10.3389/fimmu.2019.02107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maezawa Y., Nakajima H., Suzuki K., Tamachi T., Ikeda K., Inoue J., Saito Y., Iwamoto I. Involvement of TNF receptor-associated factor 6 in IL-25 receptor signaling. J. Immunol. 2006;176:1013–1018. doi: 10.4049/jimmunol.176.2.1013. [DOI] [PubMed] [Google Scholar]
- 26.Rickel E.A., Siegel L.A., Yoon B.R., Rottman J.B., Kugler D.G., Swart D.A., Anders P.M., Tocker J.E., Comeau M.R., Budelsky A.L. Identification of functional roles for both IL-17RB and IL-17RA in mediating IL-25-induced activities. J. Immunol. 2008;181:4299–4310. doi: 10.4049/jimmunol.181.6.4299. [DOI] [PubMed] [Google Scholar]
- 27.Goepfert A., Lehmann S., Blank J., Kolbinger F., Rondeau J.M. Structural Analysis Reveals that the Cytokine IL-17F Forms a Homodimeric Complex with Receptor IL-17RC to Drive IL-17RA-Independent Signaling. Immunity. 2020;52 doi: 10.1016/j.immuni.2020.02.004. [DOI] [PubMed] [Google Scholar]
- 28.Toy D., Kugler D., Wolfson M., Vanden Bos T., Gurgel J., Derry J., Tocker J., Peschon J. Cutting edge: Interleukin 17 signals through a heteromeric receptor complex. J. Immunol. 2006;177:36–39. doi: 10.4049/jimmunol.177.1.36. [DOI] [PubMed] [Google Scholar]
- 29.Kuestner R.E., Taft D.W., Haran A., Brandt C.S., Brender T., Lum K., Harder B., Okada S., Ostrander C.D., Kreindler J.L., et al. Identification of the IL-17 receptor related molecule IL-17RC as the receptor for IL-17F. J. Immunol. 2007;179:5462–5473. doi: 10.4049/jimmunol.179.8.5462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Su Y., Huang J., Zhao X., Lu H., Wang W., Yang X.O., Shi Y., Wang X., Lai Y., Dong C. Interleukin-17 receptor D constitutes an alternative receptor for interleukin-17A important in psoriasis-like skin inflammation. Sci. Immunol. 2019;4:eaau9657. doi: 10.1126/sciimmunol.aau9657. [DOI] [PubMed] [Google Scholar]
- 31.Rong Z., Wang A., Li Z., Ren Y., Cheng L., Li Y., Wang Y., Ren F., Zhang X., Hu J., et al. IL-17RD (Sef or IL-17RLM) interacts with IL-17 receptor and mediates IL-17 signaling. Cell Res. 2009;19:208–215. doi: 10.1038/cr.2008.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pande S., Yang X., Friesel R. Interleukin-17 receptor D (Sef) is a multi-functional regulator of cell signaling. Cell Commun. Signal. 2021;19:6. doi: 10.1186/s12964-020-00695-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chang S.H., Reynolds J.M., Pappu B.P., Chen G., Martinez G.J., Dong C. Interleukin-17C promotes Th17 cell responses and autoimmune disease via interleukin-17 receptor E. Immunity. 2011;35:611–621. doi: 10.1016/j.immuni.2011.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang J., Lee H.Y., Zhao X., Han J., Su Y., Sun Q., Shao J., Ge J., Zhao Y., Bai X., et al. Interleukin-17D regulates group 3 innate lymphoid cell function through its receptor CD93. Immunity. 2021;54 doi: 10.1016/j.immuni.2021.03.018. [DOI] [PubMed] [Google Scholar]
- 35.Riether C., Radpour R., Kallen N.M., Burgin D.T., Bachmann C., Schurch C.M., Luthi U., Arambasic M., Hoppe S., Albers C.E., et al. Metoclopramide treatment blocks CD93-signaling-mediated self-renewal of chronic myeloid leukemia stem cells. Cell Rep. 2021;34:108663. doi: 10.1016/j.celrep.2020.108663. [DOI] [PubMed] [Google Scholar]
- 36.Lasiglie D., Traggiai E., Federici S., Alessio M., Buoncompagni A., Accogli A., Chiesa S., Penco F., Martini A., Gattorno M. Role of IL-1 beta in the development of human T(H)17 cells: Lesson from NLPR3 mutated patients. PLoS ONE. 2011;6:e20014. doi: 10.1371/journal.pone.0020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gaffen S.L., Jain R., Garg A.V., Cua D.J. The IL-23-IL-17 immune axis: From mechanisms to therapeutic testing. Nat. Rev. Immunol. 2014;14:585–600. doi: 10.1038/nri3707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGeachy M.J., Bak-Jensen K.S., Chen Y., Tato C.M., Blumenschein W., McClanahan T., Cua D.J. TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nat. Immunol. 2007;8:1390. doi: 10.1038/ni1539. [DOI] [PubMed] [Google Scholar]
- 39.Bettelli E., Carrier Y., Gao W., Korn T., Strom T.B., Oukka M., Weiner H.L., Kuchroo V.K. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441:235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 40.Yang X.O., Panopoulos A.D., Nurieva R., Chang S.H., Wang D., Watowich S.S., Dong C. STAT3 regulates cytokine-mediated generation of inflammatory helper T cells. J. Biol. Chem. 2007;282:9358–9363. doi: 10.1074/jbc.C600321200. [DOI] [PubMed] [Google Scholar]
- 41.Park H., Li Z., Yang X.O., Chang S.H., Nurieva R., Wang Y.H., Wang Y., Hood L., Zhu Z., Tian Q., et al. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Iwakura Y., Ishigame H. The IL-23/IL-17 axis in inflammation. J. Clin. Investig. 2006;116:1218–1222. doi: 10.1172/JCI28508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yen D., Cheung J., Scheerens H., Poulet F., McClanahan T., McKenzie B., Kleinschek M.A., Owyang A., Mattson J., Blumenschein W., et al. IL-23 is essential for T cell-mediated colitis and promotes inflammation via IL-17 and IL-6. J. Clin. Investig. 2006;116:1310–1316. doi: 10.1172/JCI21404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Langrish C.L., Chen Y., Blumenschein W.M., Mattson J., Basham B., Sedgwick J.D., McClanahan T., Kastelein R.A., Cua D.J. IL-23 drives a pathogenic T cell population that induces autoimmune inflammation. J. Exp. Med. 2005;201:233–240. doi: 10.1084/jem.20041257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ivanov I.I., McKenzie B.S., Zhou L., Tadokoro C.E., Lepelley A., Lafaille J.J., Cua D.J., Littman D.R. The orphan nuclear receptor RORgammat directs the differentiation program of proinflammatory IL-17+ T helper cells. Cell. 2006;126:1121–1133. doi: 10.1016/j.cell.2006.07.035. [DOI] [PubMed] [Google Scholar]
- 46.Kumar R., Theiss A.L., Venuprasad K. RORgammat protein modifications and IL-17-mediated inflammation. Trends Immunol. 2021;42:1037–1050. doi: 10.1016/j.it.2021.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Monin L., Gaffen S.L. Interleukin 17 Family Cytokines: Signaling Mechanisms, Biological Activities, and Therapeutic Implications. Cold Spring Harb. Perspect. Biol. 2018;10:a028522. doi: 10.1101/cshperspect.a028522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cua D.J., Tato C.M. Innate IL-17-producing cells: The sentinels of the immune system. Nat. Rev. Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 49.Zhou L., Littman D.R. Transcriptional regulatory networks in Th17 cell differentiation. Curr. Opin. Immunol. 2009;21:146–152. doi: 10.1016/j.coi.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chen X.W., Zhou S.F. Inflammation, cytokines, the IL-17/IL-6/STAT3/NF-kappaB axis, and tumorigenesis. Drug Des. Dev. Ther. 2015;9:2941–2946. doi: 10.2147/DDDT.S86396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu T., Li S., Ying S., Tang S., Ding Y., Li Y., Qiao J., Fang H. The IL-23/IL-17 Pathway in Inflammatory Skin Diseases: From Bench to Bedside. Front. Immunol. 2020;11:594735. doi: 10.3389/fimmu.2020.594735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bovenschen H.J., van de Kerkhof P.C., van Erp P.E., Woestenenk R., Joosten I., Koenen H.J. Foxp3+ regulatory T cells of psoriasis patients easily differentiate into IL-17A-producing cells and are found in lesional skin. J. Investig. Dermatol. 2011;131:1853–1860. doi: 10.1038/jid.2011.139. [DOI] [PubMed] [Google Scholar]
- 53.Girolomoni G., Strohal R., Puig L., Bachelez H., Barker J., Boehncke W.H., Prinz J.C. The role of IL-23 and the IL-23/TH 17 immune axis in the pathogenesis and treatment of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017;31:1616–1626. doi: 10.1111/jdv.14433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Stritesky G.L., Yeh N., Kaplan M.H. IL-23 promotes maintenance but not commitment to the Th17 lineage. J. Immunol. 2008;181:5948–5955. doi: 10.4049/jimmunol.181.9.5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Veldhoen M., Hocking R.J., Atkins C.J., Locksley R.M., Stockinger B. TGFbeta in the context of an inflammatory cytokine milieu supports de novo differentiation of IL-17-producing T cells. Immunity. 2006;24:179–189. doi: 10.1016/j.immuni.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Mailer R.K., Joly A.L., Liu S., Elias S., Tegner J., Andersson J. IL-1beta promotes Th17 differentiation by inducing alternative splicing of FOXP3. Sci. Rep. 2015;5:14674. doi: 10.1038/srep14674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung Y., Chang S.H., Martinez G.J., Yang X.O., Nurieva R., Kang H.S., Ma L., Watowich S.S., Jetten A.M., Tian Q., et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30:576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Revu S., Wu J., Henkel M., Rittenhouse N., Menk A., Delgoffe G.M., Poholek A.C., McGeachy M.J. IL-23 and IL-1beta Drive Human Th17 Cell Differentiation and Metabolic Reprogramming in Absence of CD28 Costimulation. Cell Rep. 2018;22:2642–2653. doi: 10.1016/j.celrep.2018.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shaw M.H., Kamada N., Kim Y.G., Nunez G. Microbiota-induced IL-1beta, but not IL-6, is critical for the development of steady-state TH17 cells in the intestine. J. Exp. Med. 2012;209:251–258. doi: 10.1084/jem.20111703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ghoreschi K., Laurence A., Yang X.P., Tato C.M., McGeachy M.J., Konkel J.E., Ramos H.L., Wei L., Davidson T.S., Bouladoux N., et al. Generation of pathogenic T(H)17 cells in the absence of TGF-beta signalling. Nature. 2010;467:967–971. doi: 10.1038/nature09447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nies J.F., Panzer U. IL-17C/IL-17RE: Emergence of a Unique Axis in TH17 Biology. Front. Immunol. 2020;11:341. doi: 10.3389/fimmu.2020.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Song X., Gao H., Lin Y., Yao Y., Zhu S., Wang J., Liu Y., Yao X., Meng G., Shen N., et al. Alterations in the microbiota drive interleukin-17C production from intestinal epithelial cells to promote tumorigenesis. Immunity. 2014;40:140–152. doi: 10.1016/j.immuni.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 63.Chiricozzi A., Guttman-Yassky E., Suarez-Farinas M., Nograles K.E., Tian S., Cardinale I., Chimenti S., Krueger J.G. Integrative responses to IL-17 and TNF-alpha in human keratinocytes account for key inflammatory pathogenic circuits in psoriasis. J. Investig. Dermatol. 2011;131:677–687. doi: 10.1038/jid.2010.340. [DOI] [PubMed] [Google Scholar]
- 64.Ramirez-Carrozzi V., Sambandam A., Luis E., Lin Z., Jeet S., Lesch J., Hackney J., Kim J., Zhou M., Lai J., et al. IL-17C regulates the innate immune function of epithelial cells in an autocrine manner. Nat. Immunol. 2011;12:1159–1166. doi: 10.1038/ni.2156. [DOI] [PubMed] [Google Scholar]
- 65.Johansen C., Vinter H., Soegaard-Madsen L., Olsen L.R., Steiniche T., Iversen L., Kragballe K. Preferential inhibition of the mRNA expression of p38 mitogen-activated protein kinase regulated cytokines in psoriatic skin by anti-TNFalpha therapy. Br. J. Dermatol. 2010;163:1194–1204. doi: 10.1111/j.1365-2133.2010.10036.x. [DOI] [PubMed] [Google Scholar]
- 66.Johansen C., Riis J.L., Gedebjerg A., Kragballe K., Iversen L. Tumor necrosis factor alpha-mediated induction of interleukin 17C in human keratinocytes is controlled by nuclear factor kappaB. J. Biol. Chem. 2011;286:25487–25494. doi: 10.1074/jbc.M111.240671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saddawi-Konefka R., Seelige R., Gross E.T., Levy E., Searles S.C., Washington A., Jr., Santosa E.K., Liu B., O’Sullivan T.E., Harismendy O., et al. Nrf2 Induces IL-17D to Mediate Tumor and Virus Surveillance. Cell Rep. 2016;16:2348–2358. doi: 10.1016/j.celrep.2016.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Borowczyk J., Buerger C., Tadjrischi N., Drukala J., Wolnicki M., Wnuk D., Modarressi A., Boehncke W.H., Brembilla N.C. IL-17E (IL-25) and IL-17A Differentially Affect the Functions of Human Keratinocytes. J. Investig. Dermatol. 2020;140 doi: 10.1016/j.jid.2019.12.013. [DOI] [PubMed] [Google Scholar]
- 69.Zhang F., Meng G., Strober W. Interactions among the transcription factors Runx1, RORgammat and Foxp3 regulate the differentiation of interleukin 17-producing T cells. Nat. Immunol. 2008;9:1297–1306. doi: 10.1038/ni.1663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Khan D., Ansar Ahmed S. Regulation of IL-17 in autoimmune diseases by transcriptional factors and microRNAs. Front Genet. 2015;6:236. doi: 10.3389/fgene.2015.00236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Shalom-Barak T., Quach J., Lotz M. Interleukin-17-induced gene expression in articular chondrocytes is associated with activation of mitogen-activated protein kinases and NF-kappaB. J. Biol. Chem. 1998;273:27467–27473. doi: 10.1074/jbc.273.42.27467. [DOI] [PubMed] [Google Scholar]
- 72.Jovanovic D.V., Di Battista J.A., Martel-Pelletier J., Jolicoeur F.C., He Y., Zhang M., Mineau F., Pelletier J.P. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J. Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 73.Fossiez F., Djossou O., Chomarat P., Flores-Romo L., Ait-Yahia S., Maat C., Pin J.J., Garrone P., Garcia E., Saeland S., et al. T cell interleukin-17 induces stromal cells to produce proinflammatory and hematopoietic cytokines. J. Exp. Med. 1996;183:2593–2603. doi: 10.1084/jem.183.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Gu C., Wu L., Li X. IL-17 family: Cytokines, receptors and signaling. Cytokine. 2013;64:477–485. doi: 10.1016/j.cyto.2013.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Swaidani S., Liu C., Zhao J., Bulek K., Li X. TRAF Regulation of IL-17 Cytokine Signaling. Front. Immunol. 2019;10:1293. doi: 10.3389/fimmu.2019.01293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu C., Qian W., Qian Y., Giltiay N.V., Lu Y., Swaidani S., Misra S., Deng L., Chen Z.J., Li X. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Sci. Signal. 2009;2:ra63. doi: 10.1126/scisignal.2000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Schwandner R., Yamaguchi K., Cao Z. Requirement of tumor necrosis factor receptor-associated factor (TRAF)6 in interleukin 17 signal transduction. J. Exp. Med. 2000;191:1233–1240. doi: 10.1084/jem.191.7.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maitra A., Shen F., Hanel W., Mossman K., Tocker J., Swart D., Gaffen S.L. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc. Natl. Acad. Sci. USA. 2007;104:7506–7511. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Huang F., Kao C.Y., Wachi S., Thai P., Ryu J., Wu R. Requirement for both JAK-mediated PI3K signaling and ACT1/TRAF6/TAK1-dependent NF-kappaB activation by IL-17A in enhancing cytokine expression in human airway epithelial cells. J. Immunol. 2007;179:6504–6513. doi: 10.4049/jimmunol.179.10.6504. [DOI] [PubMed] [Google Scholar]
- 80.Ruddy M.J., Wong G.C., Liu X.K., Yamamoto H., Kasayama S., Kirkwood K.L., Gaffen S.L. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J. Biol. Chem. 2004;279:2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 81.Onishi R.M., Gaffen S.L. Interleukin-17 and its target genes: Mechanisms of interleukin-17 function in disease. Immunology. 2010;129:311–321. doi: 10.1111/j.1365-2567.2009.03240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hartupee J., Liu C., Novotny M., Sun D., Li X., Hamilton T.A. IL-17 signaling for mRNA stabilization does not require TNF receptor-associated factor 6. J. Immunol. 2009;182:1660–1666. doi: 10.4049/jimmunol.182.3.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sun D., Novotny M., Bulek K., Liu C., Li X., Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulat.tory factor SF2 (ASF) Nat. Immunol. 2011;12:853–860. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Qu F., Gao H., Zhu S., Shi P., Zhang Y., Liu Y., Jallal B., Yao Y., Shi Y., Qian Y. TRAF6-dependent Act1 phosphorylation by the IkappaB kinase-related kinases suppresses interleukin-17-induced NF-kappaB activation. Mol. Cell Biol. 2012;32:3925–3937. doi: 10.1128/MCB.00268-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Angkasekwinai P., Park H., Wang Y.H., Wang Y.H., Chang S.H., Corry D.B., Liu Y.J., Zhu Z., Dong C. Interleukin 25 promotes the initiation of proallergic type 2 responses. J. Exp. Med. 2007;204:1509–1517. doi: 10.1084/jem.20061675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang Y.H., Angkasekwinai P., Lu N., Voo K.S., Arima K., Hanabuchi S., Hippe A., Corrigan C.J., Dong C., Homey B., et al. IL-25 augments type 2 immune responses by enhancing the expansion and functions of TSLP-DC-activated Th2 memory cells. J. Exp. Med. 2007;204:1837–1847. doi: 10.1084/jem.20070406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bastid J., Dejou C., Docquier A., Bonnefoy N. The Emerging Role of the IL-17B/IL-17RB Pathway in Cancer. Front. Immunol. 2020;11:718. doi: 10.3389/fimmu.2020.00718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Mellett M., Atzei P., Bergin R., Horgan A., Floss T., Wurst W., Callanan J.J., Moynagh P.N. Orphan receptor IL-17RD regulates Toll-like receptor signalling via SEFIR/TIR interactions. Nat. Commun. 2015;6:6669. doi: 10.1038/ncomms7669. [DOI] [PubMed] [Google Scholar]
- 89.Girondel C., Meloche S. Interleukin-17 Receptor D in Physiology, Inflammation and Cancer. Front. Oncol. 2021;11:656004. doi: 10.3389/fonc.2021.656004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kovalenko D., Yang X., Chen P.Y., Nadeau R.J., Zubanova O., Pigeon K., Friesel R. A role for extracellular and transmembrane domains of Sef in Sef-mediated inhibition of FGF signaling. Cell Signal. 2006;18:1958–1966. doi: 10.1016/j.cellsig.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 91.Ren Y., Cheng L., Rong Z., Li Z., Li Y., Zhang X., Xiong S., Hu J., Fu X.Y., Chang Z. hSef potentiates EGF-mediated MAPK signaling through affecting EGFR trafficking and degradation. Cell Signal. 2008;20:518–533. doi: 10.1016/j.cellsig.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Mellett M., Atzei P., Horgan A., Hams E., Floss T., Wurst W., Fallon P.G., Moynagh P.N. Orphan receptor IL-17RD tunes IL-17A signalling and is required for neutrophilia. Nat. Commun. 2012;3:1119. doi: 10.1038/ncomms2127. [DOI] [PubMed] [Google Scholar]
- 93.McGeachy M.J., Cua D.J., Gaffen S.L. The IL-17 Family of Cytokines in Health and Disease. Immunity. 2019;50:892–906. doi: 10.1016/j.immuni.2019.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Xu S., Cao X. Interleukin-17 and its expanding biological functions. Cell Mol. Immunol. 2010;7:164–174. doi: 10.1038/cmi.2010.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Katz Y., Nadiv O., Beer Y. Interleukin-17 enhances tumor necrosis factor alpha-induced synthesis of interleukins 1,6, and 8 in skin and synovial fibroblasts: A possible role as a "fine-tuning cytokine" in inflammation processes. Arthritis Rheumatol. 2001;44:2176–2184. doi: 10.1002/1529-0131(200109)44:9<2176::AID-ART371>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 96.Takaya H., Andoh A., Makino J., Shimada M., Tasaki K., Araki Y., Bamba S., Hata K., Fujiyama Y., Bamba T. Interleukin-17 stimulates chemokine (interleukin-8 and monocyte chemoattractant protein-1) secretion in human pancreatic periacinar myofibroblasts. Scand. J. Gastroenterol. 2002;37:239–245. doi: 10.1080/003655202753416948. [DOI] [PubMed] [Google Scholar]
- 97.Huang Q., Duan L., Qian X., Fan J., Lv Z., Zhang X., Han J., Wu F., Guo M., Hu G., et al. IL-17 Promotes Angiogenic Factors IL-6, IL-8, and Vegf Production via Stat1 in Lung Adenocarcinoma. Sci. Rep. 2016;6:36551. doi: 10.1038/srep36551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mimpen J.Y., Snelling S.J.B., Carr A.J., Dakin S.G. Interleukin-17 Cytokines and Receptors: Potential Amplifiers of Tendon Inflammation. Front. Bioeng. Biotechnol. 2021;9:795830. doi: 10.3389/fbioe.2021.795830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Muromoto R., Hirao T., Tawa K., Hirashima K., Kon S., Kitai Y., Matsuda T. IL-17A plays a central role in the expression of psoriasis signature genes through the induction of IkappaB-zeta in keratinocytes. Int. Immunol. 2016;28:443–452. doi: 10.1093/intimm/dxw011. [DOI] [PubMed] [Google Scholar]
- 100.Suryawanshi A., Veiga-Parga T., Reddy P.B., Rajasagi N.K., Rouse B.T. IL-17A differentially regulates corneal vascular endothelial growth factor (VEGF)-A and soluble VEGF receptor 1 expression and promotes corneal angiogenesis after herpes simplex virus infection. J. Immunol. 2012;188:3434–3446. doi: 10.4049/jimmunol.1102602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Homey B., Dieu-Nosjean M.C., Wiesenborn A., Massacrier C., Pin J.J., Oldham E., Catron D., Buchanan M.E., Muller A., deWaal Malefyt R., et al. Up-regulation of macrophage inflammatory protein-3 alpha/CCL20 and CC chemokine receptor 6 in psoriasis. J. Immunol. 2000;164:6621–6632. doi: 10.4049/jimmunol.164.12.6621. [DOI] [PubMed] [Google Scholar]
- 102.Ruddy M.J., Shen F., Smith J.B., Sharma A., Gaffen S.L. Interleukin-17 regulates expression of the CXC chemokine LIX/CXCL5 in osteoblasts: Implications for inflammation and neutrophil recruitment. J. Leukoc. Biol. 2004;76:135–144. doi: 10.1189/jlb.0204065. [DOI] [PubMed] [Google Scholar]
- 103.Kao C.Y., Huang F., Chen Y., Thai P., Wachi S., Kim C., Tam L., Wu R. Up-regulation of CC chemokine ligand 20 expression in human airway epithelium by IL-17 through a JAK-independent but MEK/NF-kappaB-dependent signaling pathway. J. Immunol. 2005;175:6676–6685. doi: 10.4049/jimmunol.175.10.6676. [DOI] [PubMed] [Google Scholar]
- 104.Chen X., Chang L., Li X., Huang J., Yang L., Lai X., Huang Z., Wang Z., Wu X., Zhao J., et al. Tc17/IL-17A Up-Regulated the Expression of MMP-9 via NF-kappaB Pathway in Nasal Epithelial Cells of Patients With Chronic Rhinosinusitis. Front. Immunol. 2018;9:2121. doi: 10.3389/fimmu.2018.02121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wu Z., He D., Zhao S., Wang H. IL-17A/IL-17RA promotes invasion and activates MMP-2 and MMP-9 expression via p38 MAPK signaling pathway in non-small cell lung cancer. Mol. Cell BioChem. 2019;455:195–206. doi: 10.1007/s11010-018-3483-9. [DOI] [PubMed] [Google Scholar]
- 106.Feng M., Wang Y., Chen K., Bian Z., Jinfang W., Gao Q. IL-17A promotes the migration and invasiveness of cervical cancer cells by coordinately activating MMPs expression via the p38/NF-kappaB signal pathway. PLoS ONE. 2014;9:e108502. doi: 10.1371/journal.pone.0108502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sakurai T., Yoshiga D., Ariyoshi W., Okinaga T., Kiyomiya H., Furuta J., Yoshioka I., Tominaga K., Nishihara T. Essential role of mitogen-activated protein kinases in IL-17A-induced MMP-3 expression in human synovial sarcoma cells. BMC Res. Notes. 2016;9:68. doi: 10.1186/s13104-016-1892-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Chang S.H., Dong C. IL-17F: Regulation, signaling and function in inflammation. Cytokine. 2009;46:7–11. doi: 10.1016/j.cyto.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kawaguchi M., Onuchic L.F., Li X.D., Essayan D.M., Schroeder J., Xiao H.Q., Liu M.C., Krishnaswamy G., Germino G., Huang S.K. Identification of a novel cytokine, ML-1, and its expression in subjects with asthma. J. Immunol. 2001;167:4430–4435. doi: 10.4049/jimmunol.167.8.4430. [DOI] [PubMed] [Google Scholar]
- 110.Kawaguchi M., Kokubu F., Odaka M., Watanabe S., Suzuki S., Ieki K., Matsukura S., Kurokawa M., Adachi M., Huang S.K. Induction of granulocyte-macrophage colony-stimulating factor by a new cytokine, ML-1 (IL-17F), via Raf I-MEK-ERK pathway. J. Allergy Clin. Immunol. 2004;114:444–450. doi: 10.1016/j.jaci.2004.03.047. [DOI] [PubMed] [Google Scholar]
- 111.Yang X.O., Chang S.H., Park H., Nurieva R., Shah B., Acero L., Wang Y.H., Schluns K.S., Broaddus R.R., Zhu Z., et al. Regulation of inflammatory responses by IL-17F. J. Exp. Med. 2008;205:1063–1075. doi: 10.1084/jem.20071978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Korn T., Bettelli E., Oukka M., Kuchroo V.K. IL-17 and Th17 Cells. Annu. Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 113.Lonati P.A., Brembilla N.C., Montanari E., Fontao L., Gabrielli A., Vettori S., Valentini G., Laffitte E., Kaya G., Meroni P.L., et al. High IL-17E and low IL-17C dermal expression identifies a fibrosis-specific motif common to morphea and systemic sclerosis. PLoS ONE. 2014;9:e105008. doi: 10.1371/journal.pone.0105008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.McAllister F., Henry A., Kreindler J.L., Dubin P.J., Ulrich L., Steele C., Finder J.D., Pilewski J.M., Carreno B.M., Goldman S.J., et al. Role of IL-17A, IL-17F, and the IL-17 receptor in regulating growth-related oncogene-alpha and granulocyte colony-stimulating factor in bronchial epithelium: Implications for airway inflammation in cystic fibrosis. J. Immunol. 2005;175:404–412. doi: 10.4049/jimmunol.175.1.404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Liang S.C., Tan X.Y., Luxenberg D.P., Karim R., Dunussi-Joannopoulos K., Collins M., Fouser L.A. Interleukin (IL)-22 and IL-17 are coexpressed by Th17 cells and cooperatively enhance expression of antimicrobial peptides. J. Exp. Med. 2006;203:2271–2279. doi: 10.1084/jem.20061308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Chang S.H., Dong C. A novel heterodimeric cytokine consisting of IL-17 and IL-17F regulates inflammatory responses. Cell Res. 2007;17:435–440. doi: 10.1038/cr.2007.35. [DOI] [PubMed] [Google Scholar]
- 117.Liang S.C., Long A.J., Bennett F., Whitters M.J., Karim R., Collins M., Goldman S.J., Dunussi-Joannopoulos K., Williams C.M., Wright J.F., et al. An IL-17F/A heterodimer protein is produced by mouse Th17 cells and induces airway neutrophil recruitment. J. Immunol. 2007;179:7791–7799. doi: 10.4049/jimmunol.179.11.7791. [DOI] [PubMed] [Google Scholar]
- 118.Wu H.H., Hwang-Verslues W.W., Lee W.H., Huang C.K., Wei P.C., Chen C.L., Shew J.Y., Lee E.Y., Jeng Y.M., Tien Y.W., et al. Targeting IL-17B-IL-17RB signaling with an anti-IL-17RB antibody blocks pancreatic cancer metastasis by silencing multiple chemokines. J. Exp. Med. 2015;212:333–349. doi: 10.1084/jem.20141702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bie Q., Sun C., Gong A., Li C., Su Z., Zheng D., Ji X., Wu Y., Guo Q., Wang S., et al. Non-tumor tissue derived interleukin-17B activates IL-17RB/AKT/beta-catenin pathway to enhance the stemness of gastric cancer. Sci. Rep. 2016;6:25447. doi: 10.1038/srep25447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang C.K., Yang C.Y., Jeng Y.M., Chen C.L., Wu H.H., Chang Y.C., Ma C., Kuo W.H., Chang K.J., Shew J.Y., et al. Autocrine/paracrine mechanism of interleukin-17B receptor promotes breast tumorigenesis through NF-kappaB-mediated antiapoptotic pathway. Oncogene. 2014;33:2968–2977. doi: 10.1038/onc.2013.268. [DOI] [PubMed] [Google Scholar]
- 121.Swedik S., Madola A., Levine A. IL-17C in human mucosal immunity: More than just a middle child. Cytokine. 2021;146:155641. doi: 10.1016/j.cyto.2021.155641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Du L., Qin L., Wang X., Zhang A., Wei H., Zhou H. Characterization of grass carp (Ctenopharyngodon idella) IL-17D: Molecular cloning, functional implication and signal transduction. Dev. Comp. Immunol. 2014;42:220–228. doi: 10.1016/j.dci.2013.09.015. [DOI] [PubMed] [Google Scholar]
- 123.Senra L., Stalder R., Alvarez Martinez D., Chizzolini C., Boehncke W.H., Brembilla N.C. Keratinocyte-Derived IL-17E Contributes to Inflammation in Psoriasis. J. Investig. Dermatol. 2016;136:1970–1980. doi: 10.1016/j.jid.2016.06.009. [DOI] [PubMed] [Google Scholar]
- 124.Brembilla N.C., Senra L., Boehncke W.H. The IL-17 Family of Cytokines in Psoriasis: IL-17A and Beyond. Front. Immunol. 2018;9:1682. doi: 10.3389/fimmu.2018.01682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Letuve S., Lajoie-Kadoch S., Audusseau S., Rothenberg M.E., Fiset P.O., Ludwig M.S., Hamid Q. IL-17E upregulates the expression of proinflammatory cytokines in lung fibroblasts. J. Allergy Clin. Immunol. 2006;117:590–596. doi: 10.1016/j.jaci.2005.10.025. [DOI] [PubMed] [Google Scholar]
- 126.McGonagle D.G., McInnes I.B., Kirkham B.W., Sherlock J., Moots R. The role of IL-17A in axial spondyloarthritis and psoriatic arthritis: Recent advances and controversies. Ann. Rheum. Dis. 2019;78:1167–1178. doi: 10.1136/annrheumdis-2019-215356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Lore N.I., Bragonzi A., Cigana C. The IL-17A/IL-17RA axis in pulmonary defence and immunopathology. Cytokine Growth Factor Rev. 2016;30:19–27. doi: 10.1016/j.cytogfr.2016.03.009. [DOI] [PubMed] [Google Scholar]
- 128.Taams L.S., Steel K.J.A., Srenathan U., Burns L.A., Kirkham B.W. IL-17 in the immunopathogenesis of spondyloarthritis. Nat. Rev. Rheumatol. 2018;14:453–466. doi: 10.1038/s41584-018-0044-2. [DOI] [PubMed] [Google Scholar]
- 129.Liang Y., Pan H.F., Ye D.Q. Tc17 Cells in Immunity and Systemic Autoimmunity. Int. Rev. Immunol. 2015;34:318–331. doi: 10.3109/08830185.2014.954698. [DOI] [PubMed] [Google Scholar]
- 130.Cai S., Batra S., Langohr I., Iwakura Y., Jeyaseelan S. IFN-gamma induction by neutrophil-derived IL-17A homodimer augments pulmonary antibacterial defense. Mucosal Immunol. 2016;9:718–729. doi: 10.1038/mi.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yao Z., Spriggs M.K., Derry J.M., Strockbine L., Park L.S., VandenBos T., Zappone J.D., Painter S.L., Armitage R.J. Molecular characterization of the human interleukin (IL)-17 receptor. Cytokine. 1997;9:794–800. doi: 10.1006/cyto.1997.0240. [DOI] [PubMed] [Google Scholar]
- 132.Ye P., Rodriguez F.H., Kanaly S., Stocking K.L., Schurr J., Schwarzenberger P., Oliver P., Huang W., Zhang P., Zhang J., et al. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. J. Exp. Med. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Tamassia N., Arruda-Silva F., Calzetti F., Lonardi S., Gasperini S., Gardiman E., Bianchetto-Aguilera F., Gatta L.B., Girolomoni G., Mantovani A., et al. A Reappraisal on the Potential Ability of Human Neutrophils to Express and Produce IL-17 Family Members In Vitro: Failure to Reproducibly Detect It. Front. Immunol. 2018;9:795. doi: 10.3389/fimmu.2018.00795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Kao C.Y., Chen Y., Thai P., Wachi S., Huang F., Kim C., Harper R.W., Wu R. IL-17 markedly up-regulates beta-defensin-2 expression in human airway epithelium via JAK and NF-kappaB signaling pathways. J. Immunol. 2004;173:3482–3491. doi: 10.4049/jimmunol.173.5.3482. [DOI] [PubMed] [Google Scholar]
- 135.Selsted M.E., Ouellette A.J. Mammalian defensins in the antimicrobial immune response. Nat. Immunol. 2005;6:551–557. doi: 10.1038/ni1206. [DOI] [PubMed] [Google Scholar]
- 136.Douzandeh-Mobarrez B., Kariminik A. Gut Microbiota and IL-17A: Physiological and Pathological Responses. Probiotics Antimicrob. Proteins. 2019;11:1–10. doi: 10.1007/s12602-017-9329-z. [DOI] [PubMed] [Google Scholar]
- 137.Kamiya T., Watanabe Y., Makino S., Kano H., Tsuji N.M. Improvement of Intestinal Immune Cell Function by Lactic Acid Bacteria for Dairy Products. Microorganisms. 2016;5:1. doi: 10.3390/microorganisms5010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Dixon B.R., Radin J.N., Piazuelo M.B., Contreras D.C., Algood H.M. IL-17a and IL-22 Induce Expression of Antimicrobials in Gastrointestinal Epithelial Cells and May Contribute to Epithelial Cell Defense against Helicobacter pylori. PLoS ONE. 2016;11:e0148514. doi: 10.1371/journal.pone.0148514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Crhanova M., Hradecka H., Faldynova M., Matulova M., Havlickova H., Sisak F., Rychlik I. Immune response of chicken gut to natural colonization by gut microflora and to Salmonella enterica serovar enteritidis infection. Infect. Immun. 2011;79:2755–2763. doi: 10.1128/IAI.01375-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Kumar P., Monin L., Castillo P., Elsegeiny W., Horne W., Eddens T., Vikram A., Good M., Schoenborn A.A., Bibby K., et al. Intestinal Interleukin-17 Receptor Signaling Mediates Reciprocal Control of the Gut Microbiota and Autoimmune Inflammation. Immunity. 2016;44:659–671. doi: 10.1016/j.immuni.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pappu R., Ramirez-Carrozzi V., Sambandam A. The interleukin-17 cytokine family: Critical players in host defence and inflammatory diseases. Immunology. 2011;134:8–16. doi: 10.1111/j.1365-2567.2011.03465.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cheung P.F., Wong C.K., Lam C.W. Molecular mechanisms of cytokine and chemokine release from eosinophils activated by IL-17A, IL-17F, and IL-23: Implication for Th17 lymphocytes-mediated allergic inflammation. J. Immunol. 2008;180:5625–5635. doi: 10.4049/jimmunol.180.8.5625. [DOI] [PubMed] [Google Scholar]
- 143.Ramirez-Carrozzi V., Ota N., Sambandam A., Wong K., Hackney J., Martinez-Martin N., Ouyang W., Pappu R. Cutting Edge: IL-17B Uses IL-17RA and IL-17RB to Induce Type 2 Inflammation from Human Lymphocytes. J. Immunol. 2019;202:1935–1941. doi: 10.4049/jimmunol.1800696. [DOI] [PubMed] [Google Scholar]
- 144.Bie Q., Jin C., Zhang B., Dong H. IL-17B: A new area of study in the IL-17 family. Mol. Immunol. 2017;90:50–56. doi: 10.1016/j.molimm.2017.07.004. [DOI] [PubMed] [Google Scholar]
- 145.Kouri V.P., Olkkonen J., Ainola M., Li T.F., Bjorkman L., Konttinen Y.T., Mandelin J. Neutrophils produce interleukin-17B in rheumatoid synovial tissue. Rheumatology. 2014;53:39–47. doi: 10.1093/rheumatology/ket309. [DOI] [PubMed] [Google Scholar]
- 146.Kokubu T., Haudenschild D.R., Moseley T.A., Rose L., Reddi A.H. Immunol.ocalization of IL-17A, IL-17B, and their receptors in chondrocytes during fracture healing. J. HistoChem. CytoChem. 2008;56:89–95. doi: 10.1369/jhc.7A7223.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ferretti E., Ponzoni M., Doglioni C., Pistoia V. IL-17 superfamily cytokines modulate normal germinal center B cell migration. J. Leukoc. Biol. 2016;100:913–918. doi: 10.1189/jlb.1VMR0216-096RR. [DOI] [PubMed] [Google Scholar]
- 148.Moore E.E., Presnell S., Garrigues U., Guilbot A., LeGuern E., Smith D., Yao L., Whitmore T.E., Gilbert T., Palmer T.D., et al. Expression of IL-17B in neurons and evaluation of its possible role in the chromosome 5q-linked form of Charcot-Marie-Tooth disease. Neuromuscul. Disord. 2002;12:141–150. doi: 10.1016/S0960-8966(01)00250-4. [DOI] [PubMed] [Google Scholar]
- 149.Reynolds J.M., Lee Y.H., Shi Y., Wang X., Angkasekwinai P., Nallaparaju K.C., Flaherty S., Chang S.H., Watarai H., Dong C. Interleukin-17B Antagonizes Interleukin-25-Mediated Mucosal Inflammation. Immunity. 2015;42:692–703. doi: 10.1016/j.immuni.2015.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Yamaguchi Y., Fujio K., Shoda H., Okamoto A., Tsuno N.H., Takahashi K., Yamamoto K. IL-17B and IL-17C are associated with TNF-alpha production and contribute to the exacerbation of inflammatory arthritis. J. Immunol. 2007;179:7128–7136. doi: 10.4049/jimmunol.179.10.7128. [DOI] [PubMed] [Google Scholar]
- 151.Matsuzaki G., Umemura M. Interleukin-17 family cytokines in protective immunity against infections: Role of hematopoietic cell-derived and non-hematopoietic cell-derived interleukin-17s. MicroBiol. Immunol. 2018;62:1–13. doi: 10.1111/1348-0421.12560. [DOI] [PubMed] [Google Scholar]
- 152.Pfeifer P., Voss M., Wonnenberg B., Hellberg J., Seiler F., Lepper P.M., Bischoff M., Langer F., Schafers H.J., Menger M.D., et al. IL-17C is a mediator of respiratory epithelial innate immune response. Am. J. Respir. Cell Mol. Biol. 2013;48:415–421. doi: 10.1165/rcmb.2012-0232OC. [DOI] [PubMed] [Google Scholar]
- 153.Yamaguchi S., Nambu A., Numata T., Yoshizaki T., Narushima S., Shimura E., Hiraishi Y., Arae K., Morita H., Matsumoto K., et al. The roles of IL-17C in T cell-dependent and -independent inflammatory diseases. Sci. Rep. 2018;8:15750. doi: 10.1038/s41598-018-34054-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Reynolds J.M., Martinez G.J., Nallaparaju K.C., Chang S.H., Wang Y.H., Dong C. Cutting edge: Regulation of intestinal inflammation and barrier function by IL-17C. J. Immunol. 2012;189:4226–4230. doi: 10.4049/jimmunol.1103014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Im E., Jung J., Rhee S.H. Toll-like receptor 5 engagement induces interleukin-17C expression in intestinal epithelial cells. J. Interferon Cytokine Res. 2012;32:583–591. doi: 10.1089/jir.2012.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Song X., Zhu S., Shi P., Liu Y., Shi Y., Levin S.D., Qian Y. IL-17RE is the functional receptor for IL-17C and mediates mucosal immunity to infection with intestinal pathogens. Nat. Immunol. 2011;12:1151–1158. doi: 10.1038/ni.2155. [DOI] [PubMed] [Google Scholar]
- 157.Huang J., Meng S., Hong S., Lin X., Jin W., Dong C. IL-17C is required for lethal inflammation during systemic fungal infection. Cell Mol. Immunol. 2016;13:474–483. doi: 10.1038/cmi.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Peng T., Chanthaphavong R.S., Sun S., Trigilio J.A., Phasouk K., Jin L., Layton E.D., Li A.Z., Correnti C.E., De van der Schueren W., et al. Keratinocytes produce IL-17c to protect peripheral nervous systems during human HSV-2 reactivation. J. Exp. Med. 2017;214:2315–2329. doi: 10.1084/jem.20160581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.O’Sullivan T., Saddawi-Konefka R., Gross E., Tran M., Mayfield S.P., Ikeda H., Bui J.D. Interleukin-17D mediates tumor rejection through recruitment of natural killer cells. Cell Rep. 2014;7:989–998. doi: 10.1016/j.celrep.2014.03.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Saddawi-Konefka R., O’Sullivan T., Gross E.T., Washington A., Jr., Bui J.D. Tumor-expressed IL-17D recruits NK cells to reject tumors. OncoImmunology. 2014;3:e954853. doi: 10.4161/21624011.2014.954853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Morita H., Arae K., Unno H., Toyama S., Motomura K., Matsuda A., Suto H., Okumura K., Sudo K., Takahashi T., et al. IL-25 and IL-33 Contribute to Development of Eosinophilic Airway Inflammation in Epicutaneously Antigen-Sensitized Mice. PLoS ONE. 2015;10:e0134226. doi: 10.1371/journal.pone.0134226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kouzaki H., Tojima I., Kita H., Shimizu T. Transcription of interleukin-25 and extracellular release of the protein is regulated by allergen proteases in airway epithelial cells. Am. J. Respir. Cell Mol. Biol. 2013;49:741–750. doi: 10.1165/rcmb.2012-0304OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Xu M., Dong C. IL-25 in allergic inflammation. Immunol. Rev. 2017;278:185–191. doi: 10.1111/imr.12558. [DOI] [PubMed] [Google Scholar]
- 164.Ikeda K., Nakajima H., Suzuki K., Kagami S., Hirose K., Suto A., Saito Y., Iwamoto I. Mast cells produce interleukin-25 upon Fc epsilon RI-mediated activation. Blood. 2003;101:3594–3596. doi: 10.1182/blood-2002-09-2817. [DOI] [PubMed] [Google Scholar]
- 165.Schneider C., O’Leary C.E., von Moltke J., Liang H.E., Ang Q.Y., Turnbaugh P.J., Radhakrishnan S., Pellizzon M., Ma A., Locksley R.M. A Metabolite-Triggered Tuft Cell-ILC2 Circuit Drives Small Intestinal Remodeling. Cell. 2018;174 doi: 10.1016/j.cell.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Fort M.M., Cheung J., Yen D., Li J., Zurawski S.M., Lo S., Menon S., Clifford T., Hunte B., Lesley R., et al. IL-25 induces IL-4, IL-5, and IL-13 and Th2-associated pathologies in vivo. Immunity. 2001;15:985–995. doi: 10.1016/S1074-7613(01)00243-6. [DOI] [PubMed] [Google Scholar]
- 167.Deleuran M., Hvid M., Kemp K., Christensen G.B., Deleuran B., Vestergaard C. IL-25 induces both inflammation and skin barrier dysfunction in atopic dermatitis. Chem. Immunol. Allergy. 2012;96:45–49. doi: 10.1159/000331871. [DOI] [PubMed] [Google Scholar]
- 168.Angkasekwinai P., Chang S.H., Thapa M., Watarai H., Dong C. Regulation of IL-9 expression by IL-25 signaling. Nat. Immunol. 2010;11:250–256. doi: 10.1038/ni.1846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Fallon P.G., Ballantyne S.J., Mangan N.E., Barlow J.L., Dasvarma A., Hewett D.R., McIlgorm A., Jolin H.E., McKenzie A.N. Identification of an interleukin (IL)-25-dependent cell population that provides IL-4, IL-5, and IL-13 at the onset of helminth expulsion. J. Exp. Med. 2006;203:1105–1116. doi: 10.1084/jem.20051615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Hvid M., Vestergaard C., Kemp K., Christensen G.B., Deleuran B., Deleuran M. IL-25 in atopic dermatitis: A possible link between inflammation and skin barrier dysfunction? J. Investig. Dermatol. 2011;131:150–157. doi: 10.1038/jid.2010.277. [DOI] [PubMed] [Google Scholar]
- 171.Deng C., Peng N., Tang Y., Yu N., Wang C., Cai X., Zhang L., Hu D., Ciccia F., Lu L. Roles of IL-25 in Type 2 Inflammation and Autoimmune Pathogenesis. Front. Immunol. 2021;12:691559. doi: 10.3389/fimmu.2021.691559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Suto H., Nambu A., Morita H., Yamaguchi S., Numata T., Yoshizaki T., Shimura E., Arae K., Asada Y., Motomura K., et al. IL-25 enhances TH17 cell-mediated contact dermatitis by promoting IL-1beta production by dermal dendritic cells. J. Allergy Clin. Immunol. 2018;142 doi: 10.1016/j.jaci.2017.12.1007. [DOI] [PubMed] [Google Scholar]
- 173.Borowczyk J., Shutova M., Brembilla N.C., Boehncke W.H. IL-25 (IL-17E) in epithelial Immunol.ogy and pathophysiology. J. Allergy Clin. Immunol. 2021;148:40–52. doi: 10.1016/j.jaci.2020.12.628. [DOI] [PubMed] [Google Scholar]
- 174.Huang Y., Guo L., Qiu J., Chen X., Hu-Li J., Siebenlist U., Williamson P.R., Urban J.F., Jr., Paul W.E. IL-25-responsive, lineage-negative KLRG1(hi) cells are multipotential “inflammatory” type 2 innate lymphoid cells. Nat. Immunol. 2015;16:161–169. doi: 10.1038/ni.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Martin D.A., Towne J.E., Kricorian G., Klekotka P., Gudjonsson J.E., Krueger J.G., Russell C.B. The emerging role of IL-17 in the pathogenesis of psoriasis: Preclinical and clinical findings. J. Investig. Dermatol. 2013;133:17–26. doi: 10.1038/jid.2012.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Johansen C., Usher P.A., Kjellerup R.B., Lundsgaard D., Iversen L., Kragballe K. Characterization of the interleukin-17 isoforms and receptors in lesional psoriatic skin. Br. J. Dermatol. 2009;160:319–324. doi: 10.1111/j.1365-2133.2008.08902.x. [DOI] [PubMed] [Google Scholar]
- 177.Johnston A., Fritz Y., Dawes S.M., Diaconu D., Al-Attar P.M., Guzman A.M., Chen C.S., Fu W., Gudjonsson J.E., McCormick T.S., et al. Keratinocyte overexpression of IL-17C promotes psoriasiform skin inflammation. J. Immunol. 2013;190:2252–2262. doi: 10.4049/jimmunol.1201505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Yago T., Nanke Y., Ichikawa N., Kobashigawa T., Mogi M., Kamatani N., Kotake S. IL-17 induces osteoclastogenesis from human monocytes alone in the absence of osteoblasts, which is potently inhibited by anti-TNF-alpha antibody: A novel mechanism of osteoclastogenesis by IL-17. J. Cell BioChem. 2009;108:947–955. doi: 10.1002/jcb.22326. [DOI] [PubMed] [Google Scholar]
- 179.Van Hamburg J.P., Asmawidjaja P.S., Davelaar N., Mus A.M., Colin E.M., Hazes J.M., Dolhain R.J., Lubberts E. Th17 cells, but not Th1 cells, from patients with early rheumatoid arthritis are potent inducers of matrix metalloproteinases and proinflammatory cytokines upon synovial fibroblast interaction, including autocrine interleukin-17A production. Arthritis Rheum. 2011;63:73–83. doi: 10.1002/art.30093. [DOI] [PubMed] [Google Scholar]
- 180.Chabaud M., Garnero P., Dayer J.M., Guerne P.A., Fossiez F., Miossec P. Contribution of interleukin 17 to synovium matrix destruction in rheumatoid arthritis. Cytokine. 2000;12:1092–1099. doi: 10.1006/cyto.2000.0681. [DOI] [PubMed] [Google Scholar]
- 181.Hwang S.Y., Kim J.Y., Kim K.W., Park M.K., Moon Y., Kim W.U., Kim H.Y. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-kappaB- and PI3-kinase/Akt-dependent pathways. Arthritis Res. Ther. 2004;6:R120–R128. doi: 10.1186/ar1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Dige A., Stoy S., Rasmussen T.K., Kelsen J., Hvas C.L., Sandahl T.D., Dahlerup J.F., Deleuran B., Agnholt J. Increased levels of circulating Th17 cells in quiescent versus active Crohn’s disease. J. Crohns Colitis. 2013;7:248–255. doi: 10.1016/j.crohns.2012.06.015. [DOI] [PubMed] [Google Scholar]
- 183.Jiang W., Su J., Zhang X., Cheng X., Zhou J., Shi R., Zhang H. Elevated levels of Th17 cells and Th17-related cytokines are associated with disease activity in patients with inflammatory bowel disease. Inflamm. Res. 2014;63:943–950. doi: 10.1007/s00011-014-0768-7. [DOI] [PubMed] [Google Scholar]
- 184.Fujino S., Andoh A., Bamba S., Ogawa A., Hata K., Araki Y., Bamba T., Fujiyama Y. Increased expression of interleukin 17 in inflammatory bowel disease. Gut. 2003;52:65–70. doi: 10.1136/gut.52.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Seiderer J., Elben I., Diegelmann J., Glas J., Stallhofer J., Tillack C., Pfennig S., Jurgens M., Schmechel S., Konrad A., et al. Role of the novel Th17 cytokine IL-17F in inflammatory bowel disease (IBD): Upregulated colonic IL-17F expression in active Crohn’s disease and analysis of the IL17F p.His161Arg polymorphism in IBD. Inflamm. Bowel Dis. 2008;14:437–445. doi: 10.1002/ibd.20339. [DOI] [PubMed] [Google Scholar]
- 186.Friedrich M., Diegelmann J., Schauber J., Auernhammer C.J., Brand S. Intestinal neuroendocrine cells and goblet cells are mediators of IL-17A-amplified epithelial IL-17C production in human inflammatory bowel disease. Mucosal Immunol. 2015;8:943–958. doi: 10.1038/mi.2014.124. [DOI] [PubMed] [Google Scholar]
- 187.Kerami Z., Duijvis N.W., Vogels E.W., van Dooren F.H., Moerland P.D., Te Velde A.A. Effect of interleukin-17 on gene expression profile of fibroblasts from Crohn’s disease patients. J. Crohns Colitis. 2014;8:1208–1216. doi: 10.1016/j.crohns.2014.02.009. [DOI] [PubMed] [Google Scholar]
- 188.Wang K., Song F., Fernandez-Escobar A., Luo G., Wang J.H., Sun Y. The Properties of Cytokines in Multiple Sclerosis: Pros and Cons. Am. J. Med. Sci. 2018;356:552–560. doi: 10.1016/j.amjms.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 189.Goncalves R.S.G., Pereira M.C., Dantas A.T., Almeida A.R., Marques C.D.L., Rego M., Pitta I.R., Duarte A., Pitta M.G.R. IL-17 and related cytokines involved in systemic sclerosis: Perspectives. Autoimmunity. 2018;51:1–9. doi: 10.1080/08916934.2017.1416467. [DOI] [PubMed] [Google Scholar]
- 190.Larosa M., Zen M., Gatto M., Jesus D., Zanatta E., Iaccarino L., Ines L., Doria A. IL-12 and IL-23/Th17 axis in systemic lupus erythematosus. Exp. Biol. Med. 2019;244:42–51. doi: 10.1177/1535370218824547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Nanke Y., Yago T., Kotake S. The Role of Th17 Cells in the Pathogenesis of Behcet’s Disease. J. Clin. Med. 2017;6:74. doi: 10.3390/jcm6070074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Zhong Z., Su G., Kijlstra A., Yang P. Activation of the interleukin-23/interleukin-17 signalling pathway in autoinflammatory and autoimmune uveitis. Prog. Retin. Eye Res. 2021;80:100866. doi: 10.1016/j.preteyeres.2020.100866. [DOI] [PubMed] [Google Scholar]
- 193.Brevi A., Cogrossi L.L., Grazia G., Masciovecchio D., Impellizzieri D., Lacanfora L., Grioni M., Bellone M. Much More Than IL-17A: Cytokines of the IL-17 Family Between Microbiota and Cancer. Front. Immunol. 2020;11:565470. doi: 10.3389/fimmu.2020.565470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Benatar T., Cao M.Y., Lee Y., Lightfoot J., Feng N., Gu X., Lee V., Jin H., Wang M., Wright J.A., et al. IL-17E, a proinflammatory cytokine, has antitumor efficacy against several tumor types in vivo. Cancer Immunol. ImmunoTher. 2010;59:805–817. doi: 10.1007/s00262-009-0802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Mikkola T., Almahmoudi R., Salo T., Al-Samadi A. Variable roles of interleukin-17F in different cancers. BMC Cancer. 2022;22:54. doi: 10.1186/s12885-021-08969-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhu X., Mulcahy L.A., Mohammed R.A., Lee A.H., Franks H.A., Kilpatrick L., Yilmazer A., Paish E.C., Ellis I.O., Patel P.M., et al. IL-17 expression by breast-cancer-associated macrophages: IL-17 promotes invasiveness of breast cancer cell lines. Breast Cancer Res. 2008;10:R95. doi: 10.1186/bcr2195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Cochaud S., Giustiniani J., Thomas C., Laprevotte E., Garbar C., Savoye A.M., Cure H., Mascaux C., Alberici G., Bonnefoy N., et al. IL-17A is produced by breast cancer TILs and promotes chemoresistance and proliferation through ERK1/2. Sci. Rep. 2013;3:3456. doi: 10.1038/srep03456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Fabre J.A.S., Giustinniani J., Garbar C., Merrouche Y., Antonicelli F., Bensussan A. The Interleukin-17 Family of Cytokines in Breast Cancer. Int. J. Mol. Sci. 2018;19:3880. doi: 10.3390/ijms19123880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Du J.W., Xu K.Y., Fang L.Y., Qi X.L. Interleukin-17, produced by lymphocytes, promotes tumor growth and angiogenesis in a mouse model of breast cancer. Mol. Med. Rep. 2012;6:1099–1102. doi: 10.3892/mmr.2012.1036. [DOI] [PubMed] [Google Scholar]
- 200.Coffelt S.B., Kersten K., Doornebal C.W., Weiden J., Vrijland K., Hau C.S., Verstegen N.J.M., Ciampricotti M., Hawinkels L., Jonkers J., et al. IL-17-producing gammadelta T cells and neutrophils conspire to promote breast cancer metastasis. Nature. 2015;522:345–348. doi: 10.1038/nature14282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Eiro N., Fernandez-Garcia B., Gonzalez L.O., Vizoso F.J. Cytokines related to MMP-11 expression by inflammatory cells and breast cancer metastasis. OncoImmunology. 2013;2:e24010. doi: 10.4161/onci.24010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wang L., Yi T., Kortylewski M., Pardoll D.M., Zeng D., Yu H. IL-17 can promote tumor growth through an IL-6-Stat3 signaling pathway. J. Exp. Med. 2009;206:1457–1464. doi: 10.1084/jem.20090207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Laprevotte E., Cochaud S., du Manoir S., Lapierre M., Dejou C., Philippe M., Giustiniani J., Frewer K.A., Sanders A.J., Jiang W.G., et al. The IL-17B-IL-17 receptor B pathway promotes resistance to paclitaxel in breast tumors through activation of the ERK1/2 pathway. Oncotarget. 2017;8:113360–113372. doi: 10.18632/oncotarget.23008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Furuta S., Jeng Y.M., Zhou L., Huang L., Kuhn I., Bissell M.J., Lee W.H. IL-25 causes apoptosis of IL-25R-expressing breast cancer cells without toxicity to nonmalignant cells. Sci. Transl. Med. 2011;3:78ra31. doi: 10.1126/scitranslmed.3001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Li L., Boussiotis V.A. The role of IL-17-producing Foxp3+ CD4+ T cells in inflammatory bowel disease and colon cancer. Clin. Immunol. 2013;148:246–253. doi: 10.1016/j.clim.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Wang K., Karin M. The IL-23 to IL-17 cascade inflammation-related cancers. Clin. Exp. Rheumatol. 2015;33:S87–S90. [PubMed] [Google Scholar]
- 207.Liu J., Duan Y., Cheng X., Chen X., Xie W., Long H., Lin Z., Zhu B. IL-17 is associated with poor prognosis and promotes angiogenesis via stimulating VEGF production of cancer cells in colorectal carcinoma. BioChem. Biophys. Res. Commun. 2011;407:348–354. doi: 10.1016/j.bbrc.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 208.Lee Y., Kim S.J., Choo J., Heo G., Yoo J.W., Jung Y., Rhee S.H., Im E. miR-23a-3p is a Key Regulator of IL-17C-Induced Tumor Angiogenesis in Colorectal Cancer. Cells. 2020;9:1363. doi: 10.3390/cells9061363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Chen Y., Yang Z., Wu D., Min Z., Quan Y. Upregulation of interleukin17F in colorectal cancer promotes tumor invasion by inducing epithelialmesenchymal transition. Oncol. Rep. 2019;42:1141–1148. doi: 10.3892/or.2019.7220. [DOI] [PubMed] [Google Scholar]
- 210.Tong Z., Yang X.O., Yan H., Liu W., Niu X., Shi Y., Fang W., Xiong B., Wan Y., Dong C. A protective role by interleukin-17F in colon tumorigenesis. PLoS ONE. 2012;7:e34959. doi: 10.1371/journal.pone.0034959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Al-Samadi A., Moossavi S., Salem A., Sotoudeh M., Tuovinen S.M., Konttinen Y.T., Salo T., Bishehsari F. Distinctive expression pattern of interleukin-17 cytokine family members in colorectal cancer. Tumour Biol. 2016;37:1609–1615. doi: 10.1007/s13277-015-3941-x. [DOI] [PubMed] [Google Scholar]
- 212.Razi S., Baradaran Noveiry B., Keshavarz-Fathi M., Rezaei N. IL-17 and colorectal cancer: From carcinogenesis to treatment. Cytokine. 2019;116:7–12. doi: 10.1016/j.cyto.2018.12.021. [DOI] [PubMed] [Google Scholar]
- 213.Jin C., Lagoudas G.K., Zhao C., Bullman S., Bhutkar A., Hu B., Ameh S., Sandel D., Liang X.S., Mazzilli S., et al. Commensal Microbiota Promote Lung Cancer Development via gammadelta T Cells. Cell. 2019;176 doi: 10.1016/j.cell.2018.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Nicola S., Ridolfi I., Rolla G., Filosso P., Giobbe R., Boita M., Culla B., Bucca C., Solidoro P., Brussino L. IL-17 Promotes Nitric Oxide Production in Non-Small-Cell Lung Cancer. J. Clin. Med. 2021;10:4572. doi: 10.3390/jcm10194572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Salazar Y., Zheng X., Brunn D., Raifer H., Picard F., Zhang Y., Winter H., Guenther S., Weigert A., Weigmann B., et al. Microenvironmental Th9 and Th17 lymphocytes induce metastatic spreading in lung cancer. J. Clin. Investig. 2020;130:3560–3575. doi: 10.1172/JCI124037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Charles K.A., Kulbe H., Soper R., Escorcio-Correia M., Lawrence T., Schultheis A., Chakravarty P., Thompson R.G., Kollias G., Smyth J.F., et al. The tumor-promoting actions of TNF-alpha involve TNFR1 and IL-17 in ovarian cancer in mice and humans. J. Clin. Investig. 2009;119:3011–3023. doi: 10.1172/JCI39065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Tartour E., Fossiez F., Joyeux I., Galinha A., Gey A., Claret E., Sastre-Garau X., Couturier J., Mosseri V., Vives V., et al. Interleukin 17, a T-cell-derived cytokine, promotes tumorigenicity of human cervical tumors in nude mice. Cancer Res. 1999;59:3698–3704. [PubMed] [Google Scholar]
- 218.Haudenschild D., Moseley T., Rose L., Reddi A.H. Soluble and transmembrane isoforms of novel interleukin-17 receptor-like protein by RNA splicing and expression in prostate cancer. J. Biol. Chem. 2002;277:4309–4316. doi: 10.1074/jbc.M109372200. [DOI] [PubMed] [Google Scholar]
- 219.Fattahi S., Karimi M., Ghatreh-Samani M., Taheri F., Shirzad H., Mohammad Alibeigi F., Anjomshoa M., Bagheri N. Correlation between aryl hydrocarbon receptor and IL-17(+) and Foxp3(+) T-cell infiltration in bladder cancer. Int. J. Exp. Pathol. 2021;102:249–259. doi: 10.1111/iep.12392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mukherjee N., Ji N., Tan X., Lin C.L., Rios E., Chen C.L., Huang T., Svatek R.S. Bladder tumor ILC1s undergo Th17-like differentiation in human bladder cancer. Cancer Med. 2021;10:7101–7110. doi: 10.1002/cam4.4243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Zhang Z.S., Gu Y., Liu B.G., Tang H., Hua Y., Wang J. Oncogenic role of Tc17 cells in cervical cancer development. World J. Clin. Cases. 2020;8:11–19. doi: 10.12998/wjcc.v8.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.You Z., Shi X.B., DuRaine G., Haudenschild D., Tepper C.G., Lo S.H., Gandour-Edwards R., de Vere White R.W., Reddi A.H. Interleukin-17 receptor-like gene is a novel antiapoptotic gene highly expressed in androgen-independent prostate cancer. Cancer Res. 2006;66:175–183. doi: 10.1158/0008-5472.CAN-05-1130. [DOI] [PubMed] [Google Scholar]
- 223.Qu Y., Zhang Q., Ma S., Liu S., Chen Z., Mo Z., You Z. Interleukin-17A Differentially Induces Inflammatory and Metabolic Gene Expression in the Adipose Tissues of Lean and Obese Mice. Int. J. Mol. Sci. 2016;17:522. doi: 10.3390/ijms17040522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Zuniga L.A., Shen W.J., Joyce-Shaikh B., Pyatnova E.A., Richards A.G., Thom C., Andrade S.M., Cua D.J., Kraemer F.B., Butcher E.C. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J. Immunol. 2010;185:6947–6959. doi: 10.4049/jimmunol.1001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Shin J.H., Shin D.W., Noh M. Interleukin-17A inhibits adipocyte differentiation in human mesenchymal stem cells and regulates pro-inflammatory responses in adipocytes. BioChem. Pharmacol. 2009;77:1835–1844. doi: 10.1016/j.bcp.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 226.Pricola K.L., Kuhn N.Z., Haleem-Smith H., Song Y., Tuan R.S. Interleukin-6 maintains bone marrow-derived mesenchymal stem cell stemness by an ERK1/2-dependent mechanism. J. Cell BioChem. 2009;108:577–588. doi: 10.1002/jcb.22289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Ghannam S., Pene J., Moquet-Torcy G., Jorgensen C., Yssel H. Mesenchymal stem cells inhibit human Th17 cell differentiation and function and induce a T regulatory cell phenotype. J. Immunol. 2010;185:302–312. doi: 10.4049/jimmunol.0902007. [DOI] [PubMed] [Google Scholar]
- 228.Sethi J.K., Hotamisligil G.S. Metabolic Messengers: Tumour necrosis factor. Nat. Metab. 2021;3:1302–1312. doi: 10.1038/s42255-021-00470-z. [DOI] [PubMed] [Google Scholar]
- 229.Ahmed M., Gaffen S.L. IL-17 inhibits adipogenesis in part via C/EBPalpha, PPARgamma and Kruppel-like factors. Cytokine. 2013;61:898–905. doi: 10.1016/j.cyto.2012.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Pestel J., Chehimi M., Bonhomme M., Robert M., Vidal H., Eljaafari A. IL-17A contributes to propagation of inflammation but does not impair adipogenesis and/or insulin response, in adipose tissue of obese individuals. Cytokine. 2020;126:154865. doi: 10.1016/j.cyto.2019.154865. [DOI] [PubMed] [Google Scholar]
- 231.Han M.S., White A., Perry R.J., Camporez J.P., Hidalgo J., Shulman G.I., Davis R.J. Regulation of adipose tissue inflammation by interleukin 6. Proc. Natl. Acad. Sci. USA. 2020;117:2751–2760. doi: 10.1073/pnas.1920004117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Hu B., Jin C., Zeng X., Resch J.M., Jedrychowski M.P., Yang Z., Desai B.N., Banks A.S., Lowell B.B., Mathis D., et al. gammadelta T cells and adipocyte IL-17RC control fat innervation and thermogenesis. Nature. 2020;578:610–614. doi: 10.1038/s41586-020-2028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Caer C., Rouault C., Le Roy T., Poitou C., Aron-Wisnewsky J., Torcivia A., Bichet J.C., Clement K., Guerre-Millo M., Andre S. Immune cell-derived cytokines contribute to obesity-related inflammation, fibrogenesis and metabolic deregulation in human adipose tissue. Sci. Rep. 2017;7:3000. doi: 10.1038/s41598-017-02660-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Lee S.H., Jhun J., Byun J.K., Kim E.K., Jung K., Lee J.E., Choi J.Y., Park S.H., Cho M.L. IL-17 axis accelerates the inflammatory progression of obese in mice via TBK1 and IKBKE pathway. Immunol. Lett. 2017;184:67–75. doi: 10.1016/j.imlet.2017.02.004. [DOI] [PubMed] [Google Scholar]
- 235.Brien A.O., Kedia-Mehta N., Tobin L., Veerapen N., Besra G.S., Shea D.O., Hogan A.E. Targeting mitochondrial dysfunction in MAIT cells limits IL-17 production in obesity. Cell Mol. Immunol. 2020;17:1193–1195. doi: 10.1038/s41423-020-0375-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Teijeiro A., Garrido A., Ferre A., Perna C., Djouder N. Inhibition of the IL-17A axis in adipocytes suppresses diet-induced obesity and metabolic disorders in mice. Nat. Metab. 2021;3:496–512. doi: 10.1038/s42255-021-00371-1. [DOI] [PubMed] [Google Scholar]
- 237.Snekvik I., Smith C.H., Nilsen T.I.L., Langan S.M., Modalsli E.H., Romundstad P.R., Saunes M. Obesity, Waist Circumference, Weight Change, and Risk of Incident Psoriasis: Prospective Data from the HUNT Study. J. Investig. Dermatol. 2017;137:2484–2490. doi: 10.1016/j.jid.2017.07.822. [DOI] [PubMed] [Google Scholar]
- 238.Kumar S., Han J., Li T., Qureshi A.A. Obesity, waist circumference, weight change and the risk of psoriasis in US women. J. Eur. Acad. Dermatol. Venereol. 2013;27:1293–1298. doi: 10.1111/jdv.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Setty A.R., Curhan G., Choi H.K. Obesity, waist circumference, weight change, and the risk of psoriasis in women: Nurses’ Health Study II. Arch. Intern. Med. 2007;167:1670–1675. doi: 10.1001/archinte.167.15.1670. [DOI] [PubMed] [Google Scholar]
- 240.Hao Y., Zhu Y.J., Zou S., Zhou P., Hu Y.W., Zhao Q.X., Gu L.N., Zhang H.Z., Wang Z., Li J. Metabolic Syndrome and Psoriasis: Mechanisms and Future Directions. Front. Immunol. 2021;12:711060. doi: 10.3389/fimmu.2021.711060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Hwang J., Yoo J.A., Yoon H., Han T., Yoon J., An S., Cho J.Y., Lee J. The Role of Leptin in the Association between Obesity and Psoriasis. BioMol. Ther. 2021;29:11–21. doi: 10.4062/biomolther.2020.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.De Resende Guimaraes M.F.B., Rodrigues C.E.M., Gomes K.W.P., Machado C.J., Brenol C.V., Krampe S.F., de Andrade N.P.B., Kakehasi A.M. High prevalence of obesity in rheumatoid arthritis patients: Association with disease activity, hypertension, dyslipidemia and diabetes, a multi-center study. Adv. Rheumatol. 2019;59:44. doi: 10.1186/s42358-019-0089-1. [DOI] [PubMed] [Google Scholar]
- 243.Jhun J.Y., Yoon B.Y., Park M.K., Oh H.J., Byun J.K., Lee S.Y., Min J.K., Park S.H., Kim H.Y., Cho M.L. Obesity aggravates the joint inflammation in a collagen-induced arthritis model through deviation to Th17 differentiation. Exp. Mol. Med. 2012;44:424–431. doi: 10.3858/emm.2012.44.7.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Zaslona Z., O’Neill L.A.J. Cytokine-like Roles for Metabolites in Immunity. Mol. Cell. 2020;78:814–823. doi: 10.1016/j.molcel.2020.04.002. [DOI] [PubMed] [Google Scholar]
- 245.Malesza I.J., Malesza M., Walkowiak J., Mussin N., Walkowiak D., Aringazina R., Bartkowiak-Wieczorek J., Madry E. High-Fat, Western-Style Diet, Systemic Inflammation, and Gut Microbiota: A Narrative Review. Cells. 2021;10:3164. doi: 10.3390/cells10113164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Fahed G., Aoun L., Bou Zerdan M., Allam S., Bou Zerdan M., Bouferraa Y., Assi H.I. Metabolic Syndrome: Updates on Pathophysiology and Management in 2021. Int. J. Mol. Sci. 2022;23:786. doi: 10.3390/ijms23020786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Esposito K., Chiodini P., Colao A., Lenzi A., Giugliano D. Metabolic syndrome and risk of cancer: A systematic review and meta-analysis. Diabetes Care. 2012;35:2402–2411. doi: 10.2337/dc12-0336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Park S.K., Oh C.M., Kim M.H., Ha E., Choi Y.S., Ryoo J.H. Metabolic syndrome, metabolic components, and their relation to the risk of pancreatic cancer. Cancer. 2020;126:1979–1986. doi: 10.1002/cncr.32737. [DOI] [PubMed] [Google Scholar]
- 249.Jaggers J.R., Sui X., Hooker S.P., LaMonte M.J., Matthews C.E., Hand G.A., Blair S.N. Metabolic syndrome and risk of cancer mortality in men. Eur. J. Cancer. 2009;45:1831–1838. doi: 10.1016/j.ejca.2009.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Choi I.Y., Chun S., Shin D.W., Han K., Jeon K.H., Yu J., Chae B.J., Suh M., Park Y.M. Changes in Metabolic Syndrome Status and Breast Cancer Risk: A Nationwide Cohort Study. Cancers. 2021;13:1177. doi: 10.3390/cancers13051177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Chen H., Zheng X., Zong X., Li Z., Li N., Hur J., Fritz C.D., Chapman W., Jr., Nickel K.B., Tipping A., et al. Metabolic syndrome, metabolic comorbid conditions and risk of early-onset colorectal cancer. Gut. 2021;70:1147–1154. doi: 10.1136/gutjnl-2020-321661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Gathirua-Mwangi W.G., Monahan P.O., Murage M.J., Zhang J. Metabolic syndrome and total cancer mortality in the Third National Health and Nutrition Examination Survey. Cancer Causes Control. 2017;28:127–136. doi: 10.1007/s10552-016-0843-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Lopes N., McIntyre C., Martin S., Raverdeau M., Sumaria N., Kohlgruber A.C., Fiala G.J., Agudelo L.Z., Dyck L., Kane H., et al. Distinct metabolic programs established in the thymus control effector functions of gammadelta T cell subsets in tumor microenvironments. Nat. Immunol. 2021;22:179–192. doi: 10.1038/s41590-020-00848-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Chen X., Morrissey S., Chen F., Yan J. Novel Insight Into the Molecular and Metabolic Mechanisms Orchestrating IL-17 Production in gammadelta T Cells. Front. Immunol. 2019;10:2828. doi: 10.3389/fimmu.2019.02828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Melo A.M., Mylod E., Fitzgerald V., Donlon N.E., Murphy D.M., Foley E.K., Bhardwaj A., Reynolds J.V., Doherty D.G., Lysaght J., et al. Tissue distribution of gammadelta T cell subsets in oesophageal adenocarcinoma. Clin. Immunol. 2021;229:108797. doi: 10.1016/j.clim.2021.108797. [DOI] [PubMed] [Google Scholar]
- 256.Xiang Z., Tu W. Dual Face of Vgamma9Vdelta2-T Cells in Tumor Immunol.ogy: Anti- versus Pro-Tumoral Activities. Front. Immunol. 2017;8:1041. doi: 10.3389/fimmu.2017.01041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Miname M.H., Santos R.D. Reducing cardiovascular risk in patients with familial hypercholesterolemia: Risk prediction and lipid management. Prog. Cardiovasc Dis. 2019;62:414–422. doi: 10.1016/j.pcad.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 258.Ding X., Zhang W., Li S., Yang H. The role of cholesterol metabolism in cancer. Am. J. Cancer Res. 2019;9:219–227. [PMC free article] [PubMed] [Google Scholar]
- 259.Roohi A., Tabrizi M., Yaseri M., Mohammadrezaei F.M., Nikbin B. Healthy Adult LDL-C Bears Reverse Association with Serum IL-17A Levels. Curr. Chem. Genom. Transl. Med. 2018;12:1–8. doi: 10.2174/2213988501812010001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Magura L., Blanchard R., Hope B., Beal J.R., Schwartz G.G., Sahmoun A.E. Hypercholesterolemia and prostate cancer: A hospital-based case-control study. Cancer Causes Control. 2008;19:1259–1266. doi: 10.1007/s10552-008-9197-7. [DOI] [PubMed] [Google Scholar]
- 261.Dos Santos C.R., Domingues G., Matias I., Matos J., Fonseca I., de Almeida J.M., Dias S. LDL-cholesterol signaling induces breast cancer proliferation and invasion. Lipids Health Dis. 2014;13:16. doi: 10.1186/1476-511X-13-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Tie G., Yan J., Khair L., Messina J.A., Deng A., Kang J., Fazzio T., Messina L.M. Hypercholesterolemia Increases Colorectal Cancer Incidence by Reducing Production of NKT and gammadelta T Cells from Hematopoietic Stem Cells. Cancer Res. 2017;77:2351–2362. doi: 10.1158/0008-5472.CAN-16-1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Hong C.P., Park A., Yang B.G., Yun C.H., Kwak M.J., Lee G.W., Kim J.H., Jang M.S., Lee E.J., Jeun E.J., et al. Gut-Specific Delivery of T-Helper 17 Cells Reduces Obesity and Insulin Resistance in Mice. Gastroenterology. 2017;152:1998–2010. doi: 10.1053/j.gastro.2017.02.016. [DOI] [PubMed] [Google Scholar]
- 264.Gaudino S.J., Huang H., Jean-Pierre M., Joshi P., Beaupre M., Kempen C., Wong H.T., Kumar P. Cutting Edge: Intestinal IL-17A Receptor Signaling Specifically Regulates High-Fat Diet-Mediated, Microbiota-Driven Metabolic Disorders. J. Immunol. 2021;207:1959–1963. doi: 10.4049/jimmunol.2000986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Surendar J., Aravindhan V., Rao M.M., Ganesan A., Mohan V. Decreased serum interleukin-17 and increased transforming growth factor-beta levels in subjects with metabolic syndrome (Chennai Urban Rural Epidemiology Study-95) Metabolism. 2011;60:586–590. doi: 10.1016/j.metabol.2010.06.003. [DOI] [PubMed] [Google Scholar]
- 266.Ahmed M., Gaffen S.L. IL-17 in obesity and adipogenesis. Cytokine Growth Factor Rev. 2010;21:449–453. doi: 10.1016/j.cytogfr.2010.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Sumarac-Dumanovic M., Stevanovic D., Ljubic A., Jorga J., Simic M., Stamenkovic-Pejkovic D., Starcevic V., Trajkovic V., Micic D. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int. J. Obes. 2009;33:151–156. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]
- 268.Zapata-Gonzalez F., Auguet T., Aragones G., Guiu-Jurado E., Berlanga A., Martinez S., Marti A., Sabench F., Hernandez M., Aguilar C., et al. Interleukin-17A Gene Expression in Morbidly Obese Women. Int. J. Mol. Sci. 2015;16:17469–17481. doi: 10.3390/ijms160817469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Trevisan M., Liu J., Muti P., Misciagna G., Menotti A., Fucci F., Risk F., Life Expectancy Research G. Markers of insulin resistance and colorectal cancer mortality. Cancer Epidemiol. Biomarkers Prev. 2001;10:937–941. [PubMed] [Google Scholar]
- 270.Pan K., Chlebowski R.T., Mortimer J.E., Gunter M.J., Rohan T., Vitolins M.Z., Adams-Campbell L.L., Ho G.Y.F., Cheng T.D., Nelson R.A. Insulin resistance and breast cancer incidence and mortality in postmenopausal women in the Women’s Health Initiative. Cancer. 2020;126:3638–3647. doi: 10.1002/cncr.33002. [DOI] [PubMed] [Google Scholar]
- 271.Gallagher E.J., Fei K., Feldman S.M., Port E., Friedman N.B., Boolbol S.K., Killelea B., Pilewskie M., Choi L., King T., et al. Insulin resistance contributes to racial disparities in breast cancer prognosis in US women. Breast Cancer Res. 2020;22:40. doi: 10.1186/s13058-020-01281-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Bugaut H., Aractingi S. Major Role of the IL17/23 Ax.xis in Psoriasis Supports the Development of New Targeted Therapies. Front. Immunol. 2021;12:621956. doi: 10.3389/fimmu.2021.621956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Ballantyne S.J., Barlow J.L., Jolin H.E., Nath P., Williams A.S., Chung K.F., Sturton G., Wong S.H., McKenzie A.N. Blocking IL-25 prevents airway hyperresponsiveness in allergic asthma. J. Allergy Clin. Immunol. 2007;120:1324–1331. doi: 10.1016/j.jaci.2007.07.051. [DOI] [PubMed] [Google Scholar]
- 274.Xu M., Lu H., Lee Y.H., Wu Y., Liu K., Shi Y., An H., Zhang J., Wang X., Lai Y., et al. An Interleukin-25-Mediated Autoregulatory Circuit in Keratinocytes Plays a Pivotal Role in Psoriatic Skin Inflammation. Immunity. 2018;48 doi: 10.1016/j.immuni.2018.03.019. [DOI] [PubMed] [Google Scholar]