Abstract
Estrogen circulating in blood has been proved to be a strong biomarker for breast cancer. A β-glucuronidase enzyme (GUS) from human gastrointestinal tract (GIT) microbiota including probiotics has significant involvement in enhancing the estrogen concentration in blood through deconjugation of glucuronidated estrogens. The present project has been designed to explore GIT microbiome-encoded GUS enzymes (GUSOME) repertoire in normal human and breast cancer patients. For this purpose, a total of nineteen GUS enzymes from human GIT microbes, i.e., seven from healthy and twelve from breast cancer patients have been focused on. Protein sequences of enzymes retrieved from UniProt database were subjected to ProtParam, CELLO2GO, SOPMA (secondary structure prediction method), PDBsum (Protein Database summaries), PHYRE2 (Protein Homology/AnalogY Recognition Engine), SAVES v6.0 (Structure Validation Server), MEME version 5.4.1 (Multiple Em for Motif Elicitation), Caver Web server v 1.1, Interproscan and Predicted Antigenic Peptides tool. Analysis revealed the number of amino acids, isoelectric point, extinction coefficient, instability index and aliphatic index of GUS enzymes in the range of 586–795, 4.91–8.92, 89,980–155,075, 25.88–40.93 and 71.01–88.10, respectively. Sub-cellular localization of enzyme was restricted to cytoplasm and inner-membrane in case of breast cancer patients’ bacteria as compared to periplasmic space, outer membrane and extracellular space in normal GIT bacteria. The 2-D structure analysis showed α helix, extended strand, β turn and random coil in the range of 27.42–22.66%, 22.04–25.91%, 5.39–8.30% and 41.75–47.70%, respectively. The druggability score was found to be 0.05–0.45 and 0.06–0.80 in normal and breast cancer patients GIT, respectively. The radius, length and curvature of catalytic sites were observed to be 1.1–2.8 Å, 1.4–15.9 Å and 0.65–1.4, respectively. Ten conserved protein motifs with p < 0.05 and width 25–50 were found. Antigenic propensity-associated sequences were 20–29. Present study findings hint about the use of the bacterial GUS enzymes against breast cancer tumors after modifications via site-directed mutagenesis of catalytic sites involved in the activation of estrogens and through destabilization of these enzymes.
Keywords: breast cancer, estrogen, probiotics, Lactobacillus, reactivation, deconjugation
1. Introduction
A variety of microbes exist in human gastrointestinal tract (GIT) which may have positive or negative impact on human body. Microbes with good or positive impact are usually known as probiotics. Probiotics are the living microorganisms which confer benefits to human health when administered in the body in sufficient concentrations [1]. They are available as live microbial feed supplements [2]. They exert positive effects on human health such as digestion of lactose, normalization of small bowel-associated microbes, conferring resistance to enteric pathogens, immune system regulation, anticancer by detoxification of carcinogenic metabolites, intestinal wall synthesis, short chain fatty acids and vitamin B and K synthesis, colonization resistance, antihypertensive action, reduction in detoxification, inhibition of H. pylori, stimulation of immune response against viruses and production of cofactors and vitamins etc. [3,4]. Generally, probiotics also play a positive role in cancer prevention due to anti-proliferative and apoptotic activities. These cancers include colorectal cancer, gastric cancer, bladder cancer, prostate cancer, breast cancer, skin cancer and leukemia. Incidence of breast cancer has been reported to be reduced in females with post puberty consumption of Lactobacillus [4]. Bacillus subtilis has also been found to produce a surfactant with anti-tumor effect with respect to breast cancer [5]. In addition to these beneficial microbes, some bacteria of GIT are also harmful to the human body in various ways. For example, some GIT microbiota enzymes have been found to enhance the estrogen level in blood circulation leading to breast cancer incidence which constitutes 30% of female cancers [6,7].
Due to health benefits, probiotics contribute to the development of commercially important functional foods and pharmaceutical formulations. For example, L. rhamnosus, L. acidophilus, L. casei, L. plantarum and Bifidobacterium species are commonly used in dairy food production including fermented milk, yogurt, cheese, and baby milk powder [8]. Probiotics including L. Rosell 52, Bifidobacterium, S. cerevisiae, L. salivarius, L. reuteri and L. acidophilus NCFB 1748 have potential applications in pharmacy due to their association with prevention of pathogens, travelers diarrhea and antibiotic associated diarrhea, reduction in upper respiratory tract infections and constipation improvement etc. [9,10].
In 2014, microbiota presence was verified for the first time in breast tissue [11]. Literature reports presence of unique microbiota in tumor breast tissue as compared to adjacent non-tumor tissue in breast cancer patient. Even the bacterial composition of tumor breast tissue is different from breast tissues of healthy woman [12]. Human mammary and GIT microbiome composition has been reported to show variation under normal and breast cancer states. Several studies reporting breast cancer dysbiosis are present in literature [13,14,15,16]. Both the GIT and mammary β-glucuronidase (GUS) enzyme exhibit high diversification with respect to structure, function, size, sub-cellular localization, substrate specificity and binding and biocatalytic activity. This diversity is contributed by environmental factors including pathological conditions, antibiotics and diet composition [17]. Initiation and progression of breast cancer has a strong association with microbial dysbiosis of breast tissue [12]. A comparison of healthy and breast cancer patient’s microbiota has been explored in human females. Under normal conditions, bacteria belonging to Roseburi species are reported to constitute 7% of healthy human microbiota [18]. Lactic acid bacteria possess anticancer potential. A number of these bacteria have been reported to be reduced in breast cancer patients [19]. Proprionibacterium acnes, Coprococcus spp., Faecalibacterium prausnitzii, Neisseria elongata, Propioni acnes, Allisonella sp., Megasphaera sp., Pedicoccus sp., Abiotrophia sp., Clostridium sensu and Variovorax sp. W03 have also been found in non-tumor tissues [20,21,22]. Bacteria Bacillus, Enterobacteriaceae, Staphylococcus and Escherichia coli have been reported in high abundance in breast cancer patients. Histone-2AX (H2AX) phosphorylation assay performed on HeLa cells showed the role of these bacteria in DNA double stranded breaks [19]. A study initiated to analyze breast cancer-associated dysbiosis in pre-menopausal women using targeted metabolomics, 16S rRNA sequencing and cell culture methods confirmed the presence of Fusobacterium nucleatum, Pediococcus, Salmonella enterica, Corynbacterium, Shewanella putrefaciens, Enterococcus gallinarum and Thermus scotoductus and Desulfovibrio [22]. Ralstonia genus has been found in breast cancer tissue through analysis of variable regions of 16S rRNA [23].
β-glucuronidase (GUS) is a glycosyl hydrolase enzyme which converts glycosides into aglycones by hydrolyzing O- or S-glycosidic moieties [24]. In bacteria, this enzyme is encoded by uidA gene. GIT microbiome-encoded GUS enzymes also known as GUSOME exhibit high levels of diversity. Approximately 279 GUS enzymes have been reported in Human Microbiome Project database [25]. The enzymes can be classified into seven groups i.e., Loop 1 (L1), Mini-Loop 1 (mL1), Loop2 (L2), Mini-Loop 2 (mL2), Mini-Loop 1, 2 (mL1, 2), No Loop (NL) and no coverage groups [25,26]. Three types of GUS i.e., BuGUS-1, BuGUS-2 and BuGUS-3 have been reported in Bacteroides uniformis [27].
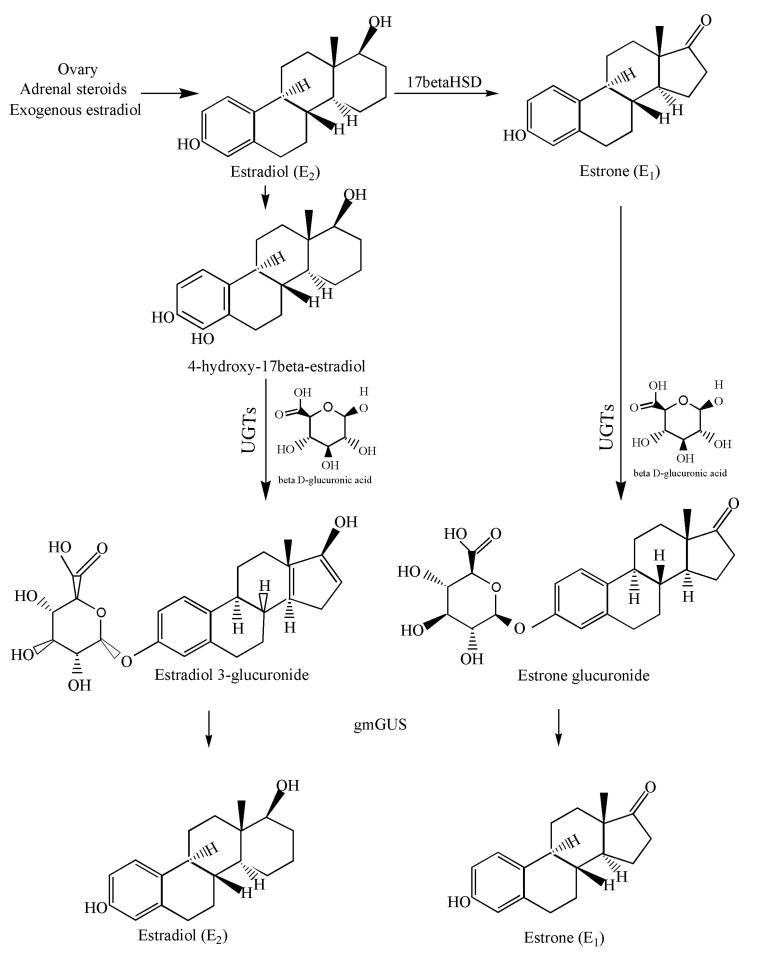
In 2019, the role of β-glucuronidase in reactivation of estrogen was proved experimentally [28]. Estrogen is found in two circulating forms i.e., estradiol and estrone in pre- and postmenopausal women, respectively [29]. During estrogen metabolism, both these forms are conjugated with glucuronic acid in the presence of UDP-glucuronosyl transferase enzymes (UGTs) leading to the formation of estrone 3-glucuronide and estradiol-17-glucuronide [30]. Due to high polarity and hydrophilicity, glucuronidated estrogens have more tendency to dissolve in blood and excrete via urine. However, a major proportion of conjugated forms enters the GI tract via bile and metabolized further [31]. Once in the intestine, glucuronidated estrogens are deconjugated in the presence of GUSOME into aglycones estrone and aglycones estradiol. The activated estrogen is absorbed in mucosa and re-enters blood circulation via a portal vein thus contributing to breast cancer (Figure 1) [32].
Figure 1.
Pathway involved in conjugation and deconjugation of estradiol and estrone by UGTs and gmGUS, respectively.
Breast cancer has been found to be reduced in human females on stopping estrogen replacement therapy (ERT) [33]. Estrogen has been reported to be a potential biomarker for breast cancer [34]. This is due to its contribution to enhanced proliferation of cancerous cells, angiogenesis and metastasis stimulation and resistance to chemotherapy [35,36,37,38]. Keeping in view the association of estrogen with breast cancer and the significant role of microbial GUS enzyme in reactivation of this hormone, we designed the present study. This study targets the GUS enzymes in bacteria inhabiting GIT of normal and breast cancer patients. Characterization of the GUS enzyme might help us in the manipulation of GIT-associated bacteria including probiotics to reduce estrogen-related cancer risk. Manipulation can be performed to reduce the stability of enzyme, to alter the 3-D configuration and catalytic site of enzymes, thus reducing the breast cancer risk associated with the activity of enzymes.
2. Methodology
2.1. Protein Sequences
To retrieve protein sequences of bacteria documented in present project, Uniprot database (https://www.uniprot.org, accessed on 21–24 July 2022) was explored [39]. Sequences retrieved, their accession numbers and bacterial species selected for this study are mentioned (Supplementary Data Table S1).
2.2. Phylogeny Analysis
To construct the phylogenetic tree initially, protein sequences of nineteen bacteria documented in present study were aligned using Clustal Omega Multiple Sequence Alignment Tool [40]. The aligned file was then subjected to MEGA version 7 [41]. The evolutionary history was inferred using the Neighbor-Joining method [42]. The evolutionary distances were computed using the Poisson correction method and are in the units of the number of amino acid substitutions per site [43]. All positions containing gaps and missing data were eliminated. There were a total of 586 positions in the final dataset.
2.3. Prediction of Physicochemical Properties
To explore the physicochemical properties of bacteria, ProtParam tool (https://web.expasy.org/protparam/, accessed on 24 July 2022) was employed. Computed attributes of bacterial proteins include molecular weight, theoretical isoelectric point (pI), half-life, instability index and aliphatic index.
2.4. Sub-Cellular Localization and Ontology Analysis
To predict the sub-cellular localization and ontology of uidA encoded GUS protein in the bacteria addressed in present study, CELLO2GO tool (cello.life.nctu.edu.tw/cello2go/, accessed on 25 July 2022) was employed.
2.5. 2D Structure
For secondary structure prediction, SOPMA tool from Network Protein Sequence Analysis (https://npsa-prabi.ibcp.fr/cgi-bin/npsa_automat.pI?page=npsa_sopma.html, accessed on 30 July 2022,) was employed [44]. To predict secondary motif map PDBsum tool (http://www.ebi.ac.uk/thornton-srv/databases/cgibin/pdbsum/GetPage.pl?pdbcode=index.html, accessed on 30 July 2022) was used. To predict the catalytic site of GUS enzyme Caver Web server v 1.1 (https://loschmidt.chemi.muni.cz/caverweb/, accessed on 4 August 2022) was used [45]. The 25 to 75% of protein is made up of secondary structure building blocks [46]. α helix, extended strand, β turn and bends are the basic elements of secondary conformation. It is important to analyze the impact of SNPs on these elements in order to gain an insight into the deleteriousness of SNPs.
2.6. 3D Structure
The three dimensional structures of GUS enzyme for all the microbes were explored using Phyre2 tool (www.sbg.bio.ic.ac.uk/~phyre2/html/page.cgi?id=index, accessed on 26–28 July 2022). The 3D models were visualized using PyMOL. To measure the accuracy of protein model and predict stereochemical characteristics of protein structures, Ramachandran plot was used [47]. To generate Ramachandran plot, SAVES v6.0 (https://saves.mbi.ucla.edu/, accessed on 30 July 2022) was used. Structure quality has been estimated on the basis of peptide bond planarity, hydrogen bonds energy backbone phi and psi angles. Analysis is based on 118 structures of resolution of at least 2.0 angstrom (Å) and R-factor no greater than 20%.
2.7. Conserved Protein Motifs Analysis
To predict the conserved motifs in protein sequences of probiotics, MEME version 5.4.1 (http://meme.sdsc.edu/meme/meme.html, accessed on 26 July 2022) was used. This tool usually finds three motifs by default however, in the present study we tried to find up to ten motifs. All other parameters were set according to default settings. To estimate the ontology of each individual conserved domain, Interproscan (http://www.ebi.ac.uk/interpro/search/sequence/, accessed on 4 August 2022) was employed.
2.8. Predicted Antigenic Peptides Tool
To predict the antigenic determinants of GUS enzymes of all the bacteria included in study, Kolaskar and Tongaonkar method i.e., Predicted Antigenic Peptides Tool was used (https://imed.med.ucm.es/Tools/antigenic.pl, accessed on 26 August 2022). This prediction algorithm depends on amino acids occurrence in experimentally determined epitopes.
3. Results
3.1. Phylogeny Prediction Based on uidA Gene Sequence
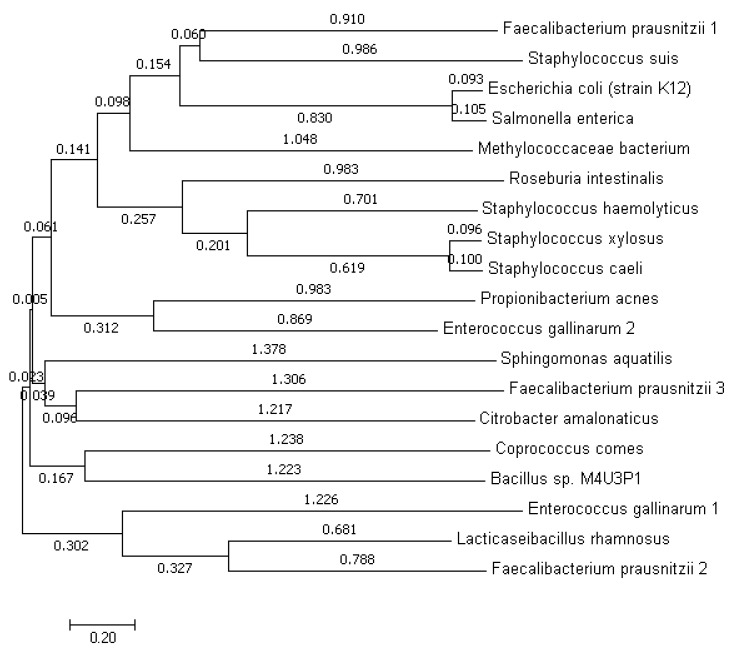
As we are trying to explore the GUS enzyme among the bacteria inhabiting the GIT of normal and breast cancer patients. Therefore, it is important to gain insight into the evolution of the GUS-coding uidA gene of these bacteria. For this purpose, the phylogenetic tree has been constructed using GUS enzymes sequences (Figure 2). According to this phylogeny study, F. prausnitzii 1 and S. suis are sharing the same clade so are closely related to each other. E. coli strain K12 and S. enterica are also originating from the same branch point. M. bacterium is not sharing closeness with any of the bacteria as it is not sharing clade. S. xylosus and S. caeli are closely related with each other and also shared clade with S. hemolyticus. These three bacteria are also related with R. intestinalis. P. acnes and E. gallinarum 2 are sharing closeness. S. aquatilis, F. prausnitzii 3 and C. amalonaticus are also related more with each other as compared to other bacteria. C. comes and Bacillus sp. are showing the common ancestry due to origin from common branch point. L. rhamnosus and F. prausnitzii 2 are sharing clade with each other. E. gallinarum 1 is also originating from the same branch point as that of L. rhamnosus and F. prausnitzii 2.
Figure 2.
Phylogenetic tree based on GUS protein sequences of present study bacteria constructed using neighbor-joining method The optimal tree with the sum of branch length = 19.62208961 is shown. The tree is drawn to scale, with branch lengths (next to the branches) in the same units as those of the evolutionary distances used to infer the phylogenetic tree.
3.2. Physicochemical Attributes
The GUS enzymes in most of the microbes comprise of different numbers of amino acids (Table 1). The highest number is 795 and the lowest is 586 in C. comes and M. bacterium, respectively. The half-life was observed to be the same in all the bacteria i.e., 30 h. The highest isoelectric point (8.92) was observed in the case of S. aquatilis NBRC 16722 while the lowest (4.91) was found in S. caeli. Extinction coefficient, instability index and aliphatic index also showed variability. Highest (155075) and the lowest values (89980) of extinction coefficient have been observed in cases of E. gallinarum 1 and S. xylosus, respectively. The instability index is observed to be highest (40.93) in C. amalonaticus and lowest (25.88) in E. gallinarum 1. As far as the aliphatic index is concerned, the largest value 88.10 was found in the case of S. suis and the smallest 71.01 in L. rhamnosus.
Table 1.
Physicochemical properties of GUS enzymes in bacteria found in normal and breast cancer patients.
| # | Probiotics | No. of Amino Acids |
Mol. Wt. | pI | Half-Life (Hours) |
Extinction Coefficient | Instability Index | Aliphatic Index |
|---|---|---|---|---|---|---|---|---|
| 1 | L. rhamnosus | 603 | 68,570.92 | 5.35 | 30 | 101,760 | 30.86 | 71.01 |
| 2 | R. intestinalis | 600 | 68,337.66 | 5.06 | 30 | 109,140 | 33.60 | 73.75 |
| 3 | C. comes | 795 | 87,709.17 | 5.85 | 30 | 126,920 | 26.87 | 78.06 |
| 4 |
F. prausnitzii 1 |
598 | 67,535.00 | 5.34 | 30 | 119,345 | 35.75 | 74.03 |
| 5 |
F. prausnitzii 2 |
608 | 69,345.36 | 6.13 | 30 | 118,970 | 33.77 | 75.41 |
| 6 |
F. prausnitzii 3 |
639 | 72,500.39 | 5.92 | 30 | 131,960 | 32.82 | 71.85 |
| 7 | P. acnes | 594 | 67,236.34 | 5.12 | 30 | 125,040 | 33.75 | 78.94 |
| 8 | M. bacterium | 586 | 66,604.06 | 5.91 | 30 | 131,125 | 36.17 | 81.98 |
| 9 | S. xylosus | 597 | 67,983.63 | 5.21 | 30 | 89,980 | 31.77 | 82.84 |
| 10 | S. suis | 599 | 68,912.07 | 5.11 | 30 | 95,355 | 29.20 | 88.10 |
| 11 |
Bacillus sp. M4U3P1 |
604 | 68,912.10 | 5.17 | 30 | 100,855 | 37.19 | 80.98 |
| 12 |
E. coli (strain K12) |
603 | 68,447.00 | 5.24 | 30 | 140,760 | 26.68 | 77.74 |
| 13 |
S. aquatilis NBRC 16722 |
631 | 69,237.47 | 8.92 | 30 | 124,455 | 37.69 | 83.88 |
| 14 |
C.
amalonaticus |
601 | 69,674.51 | 5.33 | 30 | 119,805 | 40.93 | 80.80 |
| 15 |
E. gallinarum 1 |
596 | 69,632.94 | 5.01 | 30 | 155,075 | 25.88 | 75.87 |
| 16 |
E. gallinarum 2 |
602 | 69,494.74 | 5.05 | 30 | 106,480 | 30.79 | 85.12 |
| 17 | S. enterica | 603 | 68,922.61 | 5.45 | 30 | 136,750 | 30.39 | 77.78 |
| 18 | S. caeli | 597 | 67,893.91 | 4.91 | 30 | 92,960 | 30.87 | 81.76 |
| 19 | S. haemolyticus | 601 | 68,750.54 | 5.46 | 30 | 92,835 | 32.41 | 81.20 |
3.3. Sub-Cellular Localization
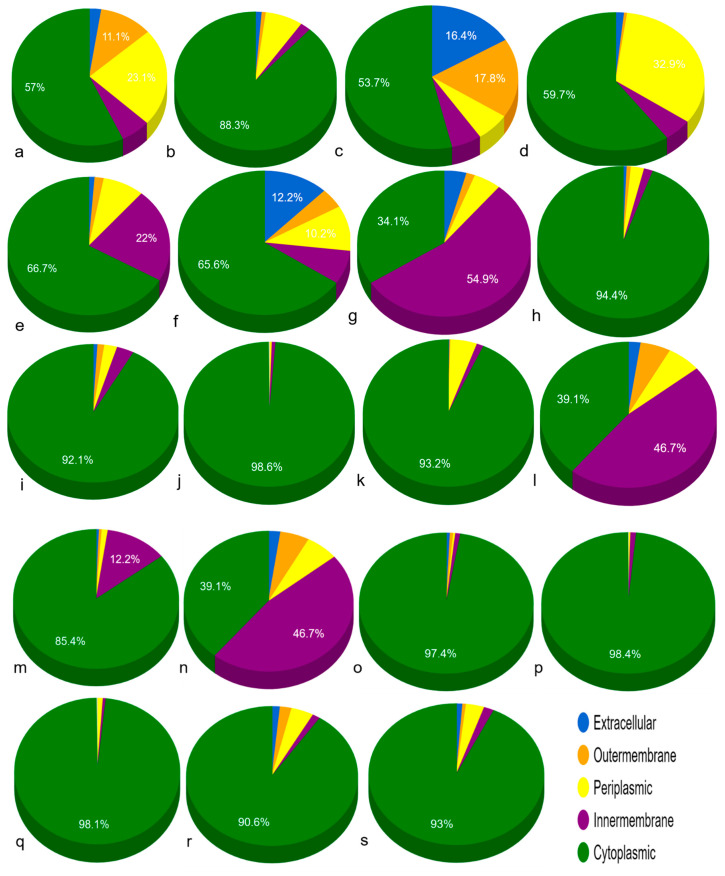
In normal tissue-associated bacteria, GUS was found to be present in cytoplasm (Table 2, Figure 3). Meanwhile, in C. comes, L. rhamnosus, F. prausnitzii 1 and F. prausnitzii 2, protein was additionally localized in extracellular, outer-membrane, periplasmic space and inner-membrane. The protein in majority of the breast cancer-associated bacteria was found to be localized in cytoplasm with the exception of M. bacterium and S. aquatilis NBRC 16722 in which it was additionally localized in inner-membrane.
Table 2.
Sub-cellular localization of GUS enzymes in the present study bacteria predicted on the basis of CELLU2GO tool.
| Bacteria | Sub-Cellular Localization | ||||
|---|---|---|---|---|---|
| Extracellular | Outer Membrane | Periplasmic Space | Inner Membrane | Cytoplasm | |
| Bacteria associated with normal individuals | |||||
| L. rhamnosus | 0.176 | 0.779 | 1.619 | 0.433 | 3.993 |
| R. intestinalis | 0.091 | 0.057 | 0.525 | 0.148 | 6.180 |
| C. comes | 1.146 | 1.249 | 0.448 | 0.396 | 3.761 |
|
F. prausnitzii 1 |
0.111 | 0.038 | 2.304 | 0.365 | 4.183 |
|
F. prausnitzii 2 |
0.084 | 0.126 | 0.579 | 1.541 | 4.669 |
|
F. prausnitzii 3 |
0.857 | 0.311 | 0.713 | 0.530 | 4.589 |
| P. acnes | 0.030 | 0.010 | 0.232 | 0.230 | 6.498 |
| Bacteria associated with breast cancer patients | |||||
| M. bacterium | 0.298 | 0.112 | 0.360 | 3.839 | 2.390 |
| S. xylosus | 0.050 | 0.054 | 0.174 | 0.117 | 6.604 |
| S. suis | 0.063 | 0.089 | 0.169 | 0.229 | 6.449 |
| Bacillus sp. M4U3P1 | 0.004 | 0.008 | 0.038 | 0.049 | 6.901 |
|
E. coli (strain K12) |
0.019 | 0.012 | 0.353 | 0.090 | 6.526 |
|
S. aquatilis NBRC 16722 |
0.161 | 0.389 | 0.440 | 3.271 | 2.738 |
|
C.
amalonaticus |
0.043 | 0.036 | 0.083 | 0.857 | 5.982 |
|
E. gallinarum 1 |
0.052 | 0.041 | 0.026 | 0.066 | 6.815 |
|
E. gallinarum 2 |
0.011 | 0.008 | 0.021 | 0.074 | 6.885 |
| S. enterica | 0.008 | 0.007 | 0.081 | 0.038 | 6.865 |
| S. caeli | 0.108 | 0.152 | 0.296 | 0.102 | 6.343 |
| S. haemolyticus | 0.082 | 0.044 | 0.236 | 0.131 | 6.508 |
Figure 3.
Sub-cellular localization of GUS proteins in bacteria found in normal human and breast cancer patients GIT (a) L. rhamnosus, (b) R. intestinalis, (c) C. comes, (d) F. prausnitzii 1, (e) F. prausnitzii 2, (f) F. prausnitzii 3, (g) P. acnes, (h) M. bacterium, (i) S. xylosus, (j) S. suis, (k) Bacillus sp. M4U3P1, (l) E. coli (strain K12), (m) S. aquatilis NBRC 16722, (n) C. amalonaticus, (o) E. gallinarum 1, (p) E. gallinarum 2, (q) S. enterica, (r) S. caeli, (s) S. haemolyticus.
3.4. 2D Structure Prediction
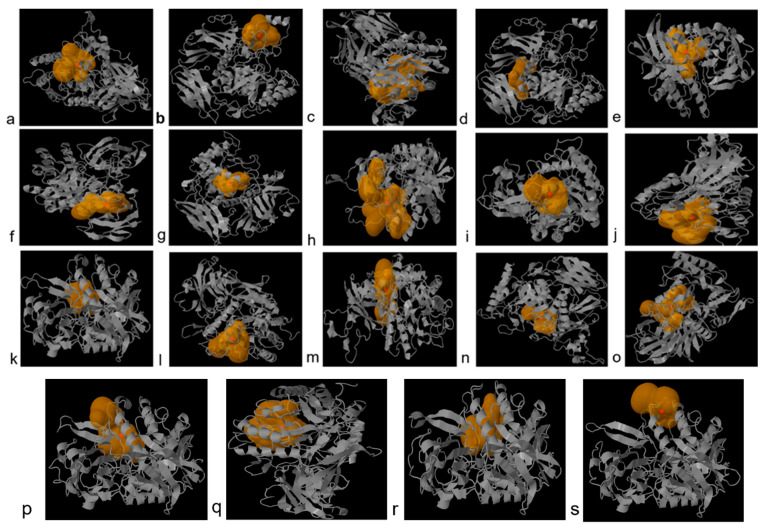
Secondary structure composition analysis based on SOPMA tool revealed that α helices, extended strand, β turn and random coil of GUS proteins in bacteria were comprised of amino acids in the range of 22.66 to 27.81%, 22.04 to 25.91%, 5.39 to 8.30% and 41.75 to 47.70%, respectively (Table 3). Secondary Motif Maps predicted using PDBsum tool are shown in Supplementary Data Figure S1. The catalytic site properties were predicted using Caver Web. The catalytic sites with most reliable starting points and 100% relative scores with tunnel druggability, bottleneck radius, length and curvature are shown in Figure 4 and Table 4. Druggability, bottle neck radius, length and curvature of predicted catalytic sites were found to be in the range of 0.052 to 0.80, 1.1–2.8 Å, 1.4–15.9 Å and 0.65–1.4, respectively, for GUS enzymes. Highest and lowest tunnel bottleneck radii were observed in E. gallinarum 2 and S. aquatilis, respectively. Highest and lowest tunnel lengths were found in cases of E. gallinarum and L. rhamnosus, respectively. Highest and lowest tunnel curvature was observed in F. prausnitzii and P. acnes and S. aquatilis, respectively.
Table 3.
Secondary structure for GUS proteins of bacteria predicted using SOPMA tool.
| Probiotics | α Helix (%) | Extended Strand (%) | β Turn (%) | Random Coil (%) |
|---|---|---|---|---|
| Normal tissue associated bacteria | ||||
| L. rhamnosus | 26.37 | 23.55 | 7.13 | 42.95 |
| R. intestinalis | 27.33 | 22.67 | 6.50 | 43.50 |
| C. comes | 23.02 | 25.91 | 8.30 | 42.77 |
| F. prausnitzii 1 | 27.42 | 22.74 | 6.69 | 43.14 |
| F. prausnitzii 2 | 26.15 | 22.04 | 6.25 | 45.56 |
| F. prausnitzii 3 | 23.32 | 25.82 | 8.29 | 42.57 |
| P. acnes | 26.60 | 24.75 | 6.90 | 41.75 |
| Breast cancer patients associated bacteria | ||||
| M. bacterium | 26.45 | 23.21 | 6.31 | 44.03 |
| S. xylosus | 26.80 | 23.95 | 6.87 | 42.38 |
| S. suis | 26.88 | 22.37 | 6.84 | 43.91 |
| Bacillus sp. M4U3P1 | 27.32 | 22.52 | 6.62 | 43.54 |
| E. coli (strain K12) | 25.70 | 23.88 | 7.13 | 43.28 |
| S. aquatilis NBRC 16722 | 22.66 | 24.25 | 5.39 | 47.70 |
| C. amalonaticus | 26.79 | 24.13 | 5.49 | 43.59 |
| E. gallinarum 1 | 25.17 | 23.49 | 6.21 | 45.13 |
| E. gallinarum 2 | 27.24 | 23.59 | 7.14 | 42.03 |
| S. enterica | 26.37 | 24.21 | 6.63 | 42.79 |
| S. caeli | 27.81 | 23.62 | 6.70 | 41.88 |
| S. haemolyticus | 26.46 | 24.13 | 6.99 | 42.43 |
Figure 4.
Catalytic sites predicted using Caver Web server in present study bacteria (a) L. rhamnosus, (b) R. intestinalis, (c) C. comes, (d) F. prausnitzii 1, (e) F. prausnitzii 2, (f) F. prausnitzii 3, (g) P. acnes, (h) M. bacterium, (i) S. xylosus, (j) S. suis, (k) Bacillus sp. M4U3P1, (l) E. coli (strain K12), (m) S. aquatilis NBRC 16722, (n) C. amalonaticus, (o) E. gallinarum 1, (p) E. gallinarum 2, (q) S. enterica, (r) S. caeli, (s) S. haemolyticus.
Table 4.
Relevance score, druggability, bottleneck radius, length and curvature of catalytic sites tunnels determined in present study bacteria using Caver Web tool.
| Bacteria | Relevance Score (%) |
Druggability | Bottleneck Radius (Angstrom) |
Length (Angstrom) | Curvature |
|---|---|---|---|---|---|
| Normal tissue associated microbiota | |||||
| L. rhamnosus | 100 | 0.14 | 2.3 | 1.4 | 1.1 |
| R. intestinalis | 100 | 0.17 | 1.6 | 4.3 | 1.2 |
| C. comes | 100 | 0.28 | 1.9 | 2.7 | 1.3 |
| F. prausnitzii 1 | 100 | 0.07 | 2.1 | 2.5 | 1.0 |
| F. prausnitzii 2 | 100 | 0.07 | 1.6 | 8.8 | 1.1 |
| F. prausnitzii 3 | 100 | 0.05 | 1.2 | 10.7 | 1.4 |
| P. acnes | 100 | 0.45 | 1.2 | 10.7 | 1.4 |
| Breast cancer patients associated bacteria | |||||
|
Methylococcaceae bacterium |
100 | 0.80 | 2.3 | 6.9 | 1.1 |
| S. xylosus | 100 | 0.60 | 2.0 | 5.7 | 1.1 |
| S. suis | 100 | 0.07 | 2.1 | 1.5 | 1.0 |
| Bacillus sp. M4U3P1 | 100 | 0.07 | 2.2 | 4.4 | 1.2 |
| E. coli (strain K12) | 100 | 0.06 | 2.2 | 1.9 | 1.2 |
|
S. aquatilis NBRC 16722 |
100 | 0.06 | 1.1 | 8.7 | 0.65 |
| C. amalonaticus | 100 | 0.14 | 1.3 | 14.6 | 1.2 |
| E. gallinarum 1 | 100 | 0.13 | 1.2 | 15.9 | 1.3 |
| E. gallinarum 2 | 100 | 0.23 | 2.8 | 2.0 | 1.2 |
| S. enterica | 100 | 0.51 | 2.5 | 1.5 | 1.0 |
| S. caeli | 100 | 0.39 | 2.3 | 4.0 | 1.1 |
| S. haemolyticus | 100 | 0.52 | 1.9 | 3.4 | 1.0 |
3.5. 3D Structure Prediction
Three dimensional configurations of GUS enzymes were obtained through PHYRE2 tool and visualized by PyMol (Figure 5). According to verification by Ramachandran plot, values of quality model were found to be closer to 90% in the most favored region which reflects the accuracy of GUS protein structures in the case of all bacteria (Supplementary Data Figure S2, Table 5).
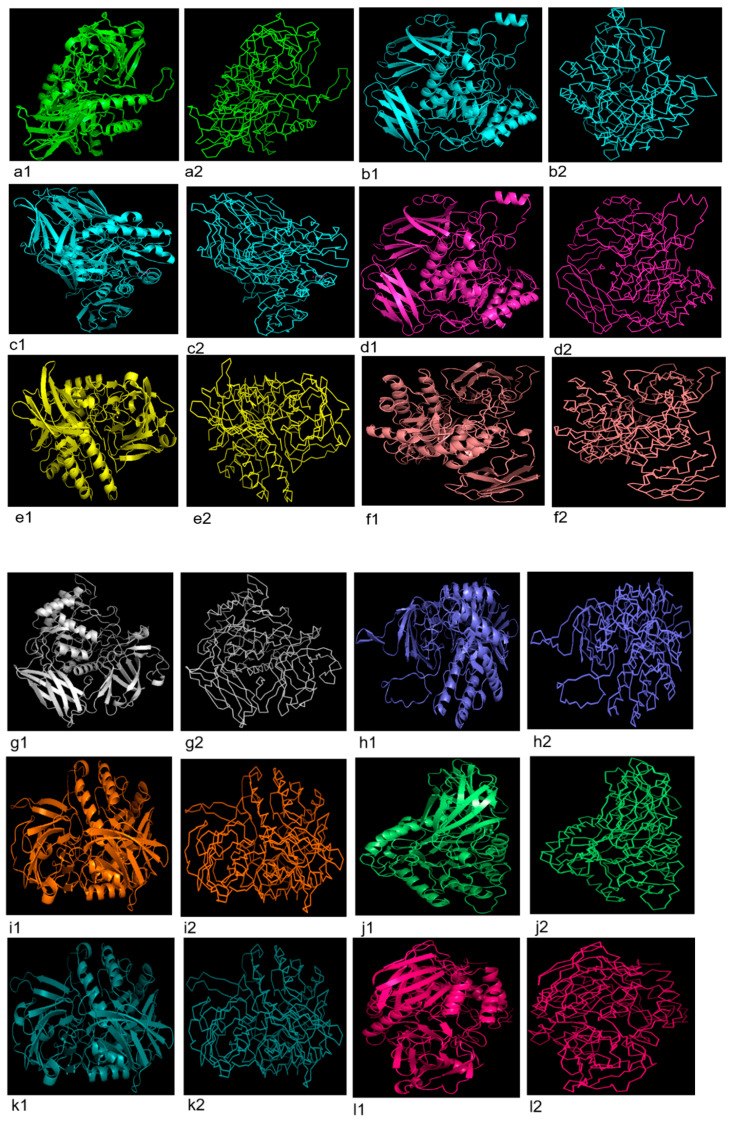
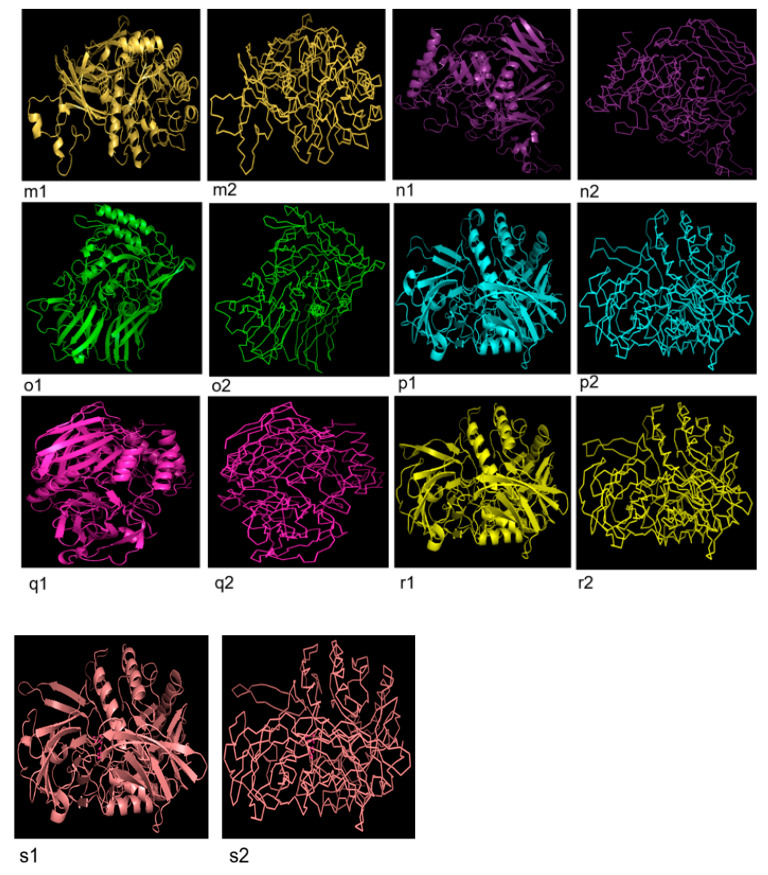
Figure 5.
Three dimensional configuration (cartoon and ribbon form) of uidA protein predicted using PHYRE2 tool and visualized by PyMOL, in probiotics documented in present study. (a1,a2) L. rhamnosus, (b1,b2) R. intestinalis, (c1,c2) C. comes, (d1,d2) F. prausnitzii 1, (e1, e2) F. prausnitzii 2, (f1,f2) F. prausnitzii 3, (g1,g2) P. acnes, (h1,h2) M. bacterium, (i1,i2) S. xylosus, (j1,j2) S. suis, (k1,k2) Bacillus sp. M4U3P1, (l1,l2) E. coli (strain K12), (m1,m2) S. aquatilis NBRC 16722, (n1,n2) C. amalonaticus, (o1,o2) E. gallinarum 1, (p1,p2) E. gallinarum 2, (q1,q2) S. enterica, (r1,r2) S. caeli, (s1,s2) S. haemolyticus.
Table 5.
Interpretation of Ramachandran plots for GUS proteins of bacteria.
| Bacteria | Residues in Most Favored Region (%) |
Residues in Additional Allowed Regions (%) |
Residues in Generously Allowed Regions (%) |
Residues in Disallowed Regions (%) | G-Factors | ||
|---|---|---|---|---|---|---|---|
| Dihedrals | Covalent | Overall | |||||
| Normal tissue associated bacteria | |||||||
| L. rhamnosus | 88.4 | 10.6 | 0.8 | 0.2 | −0.13 | 0.47 | 0.11 |
| R. intestinalis | 85.2 | 14 | 0.2 | 0.6 | 0.07 | 0.42 | 0.22 |
| C. comes | 88.2 | 10.0 | 1.1 | 0.6 | −0.11 | 0.28 | 0.05 |
| F. prausnitzii 1 | 86.3 | 13.3 | 0.2 | 0.2 | 0.07 | 0.48 | 0.24 |
| F. prausnitzii 2 | 91.3 | 7.9 | 0.6 | 0.2 | −0.10 | 0.49 | 0.14 |
| F. prausnitzii 3 | 81.7 | 16.7 | 0.9 | 0.7 | −0.26 | 0.37 | 0.00 |
| P. acnes | 82.1 | 16.9 | 0.4 | 0.6 | −0.23 | 0.42 | 0.03 |
| Breast cancer patients associated bacteria | |||||||
|
Methylococcaceae bacterium |
88.2 | 10 | 1.0 | 0.8 | −0.14 | 0.37 | 0.07 |
| S. xylosus | 86.8 | 11.5 | 0.9 | 0.8 | −0.05 | 0.57 | 0.20 |
| S. suis | 87.5 | 11.7 | 0.6 | 0.2 | −0.00 | 0.62 | 0.24 |
| Bacillus sp. M4U3P1 | 87.4 | 11.7 | 0.6 | 0.4 | −0.04 | 0.57 | 0.20 |
| E. coli (strain K12) | 87.3 | 11.3 | 0.9 | 0.4 | −0.11 | 0.24 | 0.04 |
|
S. aquatilis NBRC 16722 |
83.6 | 14.6 | 1.4 | 0.4 | −0.32 | −0.06 | −0.21 |
| C. amalonaticus | 86.4 | 12.0 | 1.1 | 0.4 | −0.14 | 0.47 | 0.10 |
| E. gallinarum 1 | 84.9 | 10.2 | 3.1 | 1.8 | −0.08 | 0.31 | 0.08 |
| E. gallinarum 2 | 86.5 | 11.3 | 1.3 | 0.9 | −0.02 | 0.57 | 0.21 |
| S. enterica | 86.6 | 11.3 | 1.3 | 0.9 | −0.02 | 0.57 | 0.21 |
| S. caeli | 86.6 | 11.7 | 1.1 | 0.6 | −0.12 | 0.25 | 0.04 |
| S. haemolyticus | 85.9 | 12.1 | 0.9 | 1.1 | −0.05 | 0.55 | 0.19 |
3.6. Conserved Protein Motifs Prediction
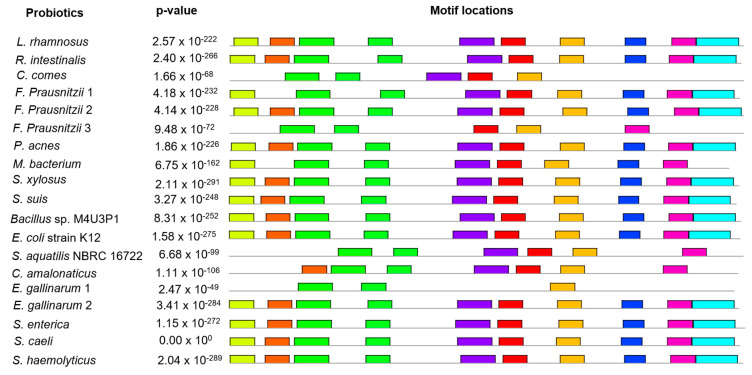
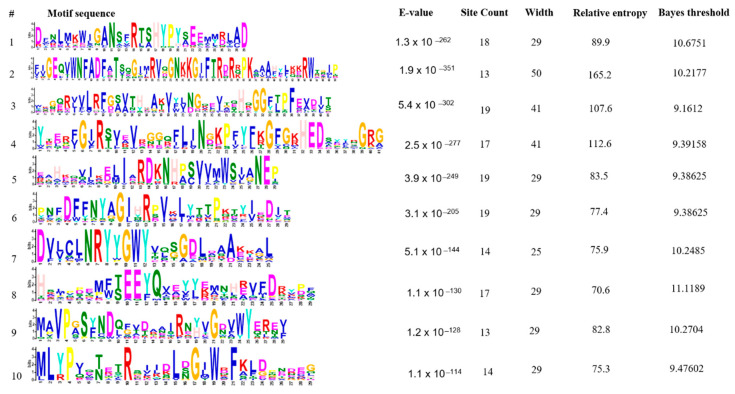
In total, ten conserved protein motifs were explored in GUS protein of nineteen bacteria through MEME. The number of amino acids were found to be 29 (motifs 1, 5, 6, 8, 9 and 10), 50 (motif 2), 41 (motif 3 and 4) and 25 (motif 7). The locations of these motifs with their p-values are shown (Figure 6). The motif results were found to be significant with p-value < 0.05 in the case of all bacteria except S. caeli. Sequences, E-values, site count, width, relative entropy and bayes threshold are also mentioned (Figure 7). E-value is an estimate of the expected number of motifs with the given log likelihood ratio (or higher) and with the same width and site count, that one would find in a similarly sized set of random sequences. The E-values for all the ten motifs were found to be significant i.e., <0.05. Site count is the number of sites contributing to the construction of motif. The maximum number of site count, i.e., 19, was observed in the case of third, fifth and sixth motif sequences. The lowest number of site count, i.e., 13, was observed in case of second and ninth motifs. The width of the motif describes a pattern of a fixed width as no gaps are allowed in MEME motifs. The width was observed in the range of 25–50. Maximum was predicted in second motif and minimum in the case of first, fifth, sixth, eighth, ninth and tenth motifs. The conserved proteins motifs explored via MEME were subjected to Interproscan to predict their molecular and biological functions. This revealed association of these motifs with carbohydrate metabolism and hydrolyses of O-glycosyl compounds.
Figure 6.
Location of motif sites with their corresponding combined match p-value for uidA protein of bacteria, predicted by MEME suite. Each block represents the position and strength of a motif site. The height of a block gives an indication of the significance of the site as taller blocks are more significant. The height is calculated to be proportional to the negative logarithm of the p-value of the site, truncated at the height for p-value of 1 × 10−10. Combined match p-value is defined as the probability that a random sequence (with the same length and conforming to the background) would have position p-values such that the product is smaller or equal to the value calculated for the sequence under test. Ten different colors are used to depict the ten different motifs.
Figure 7.
Sequences, E-values, site count, width, relative entropy and bayes threshold of conserved motifs of uidA protein predicted in probiotics documented in present study E-value show the statistical significance of the motif. It is an estimate of the expected number of motifs with the given log likelihood ratio (or higher) and with the same width and site count that one would find in a similarly sized set of random sequences. Site count is the number of sites contributing to the construction of the motif. The width of the motif describes a pattern of a fixed width as no gaps are allowed in MEME motifs.
3.7. Antigenic Peptide Prediction
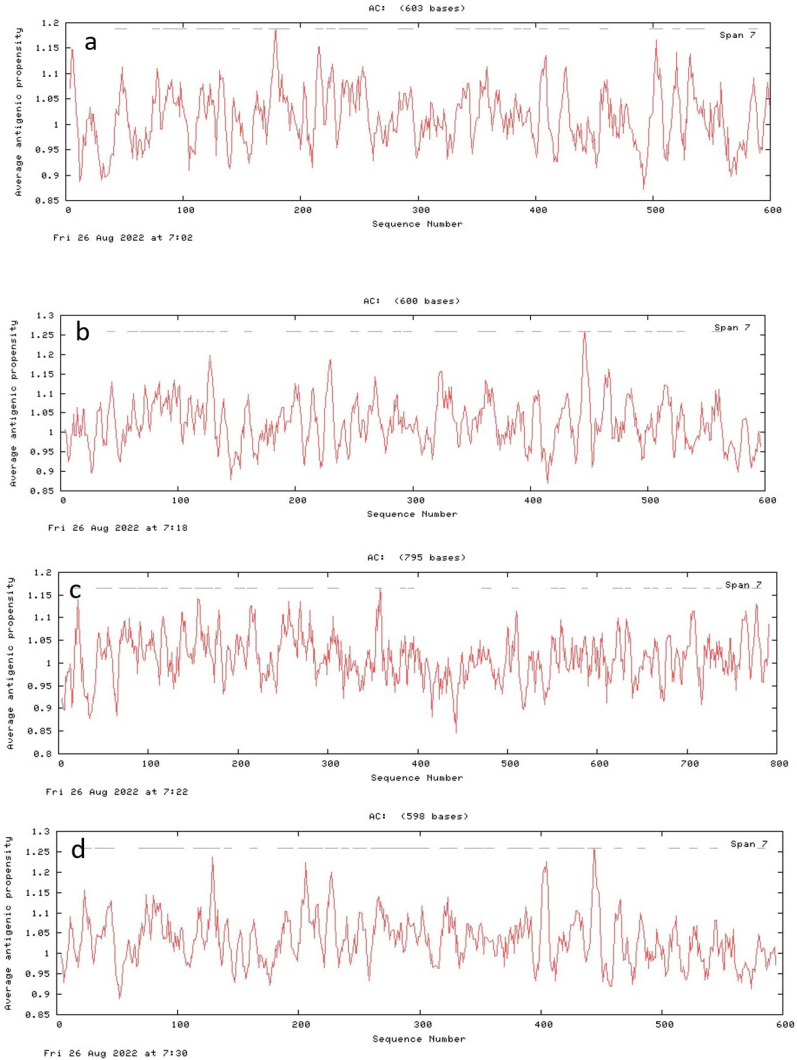
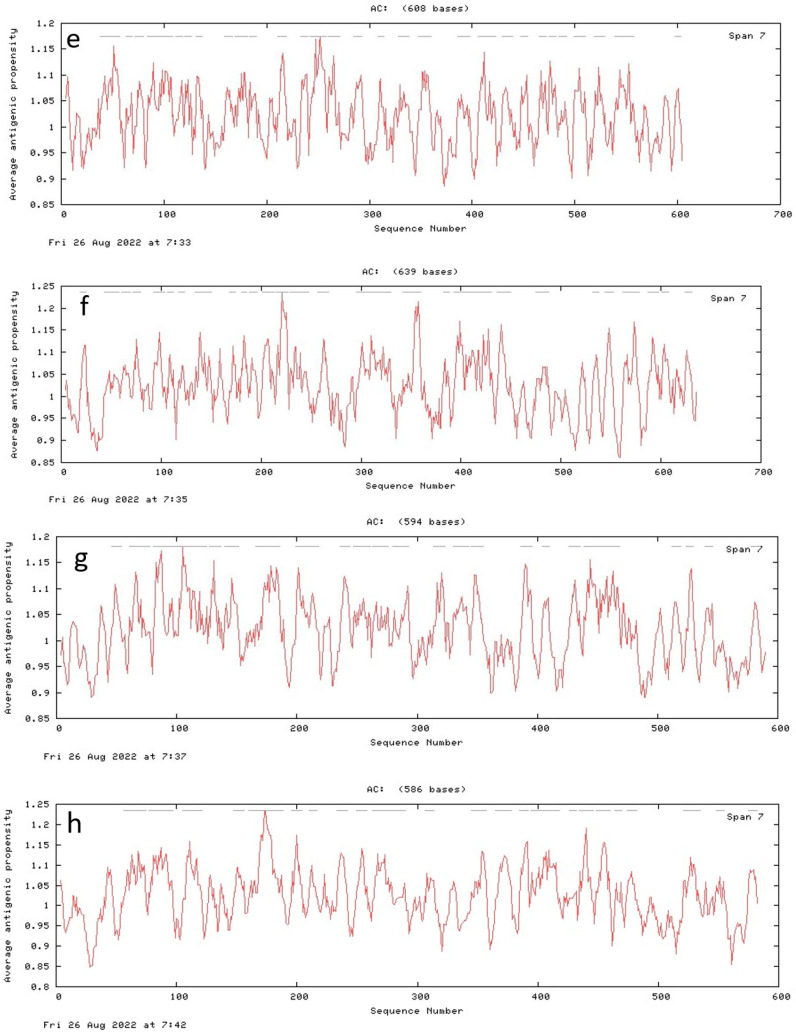
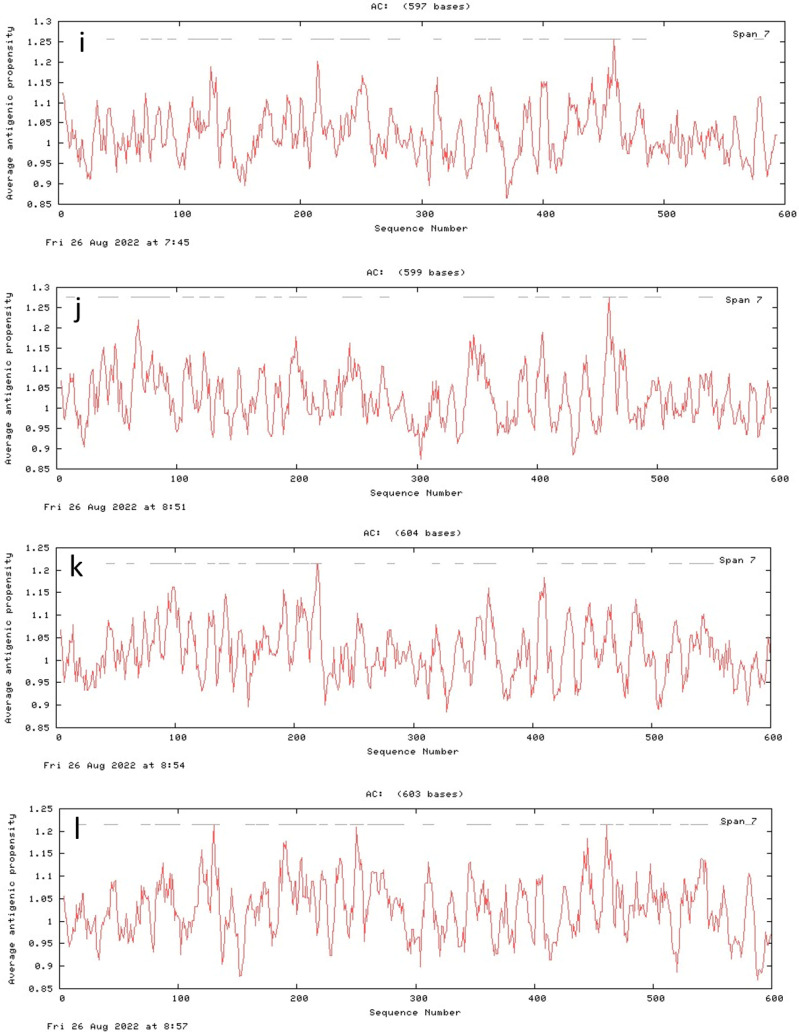
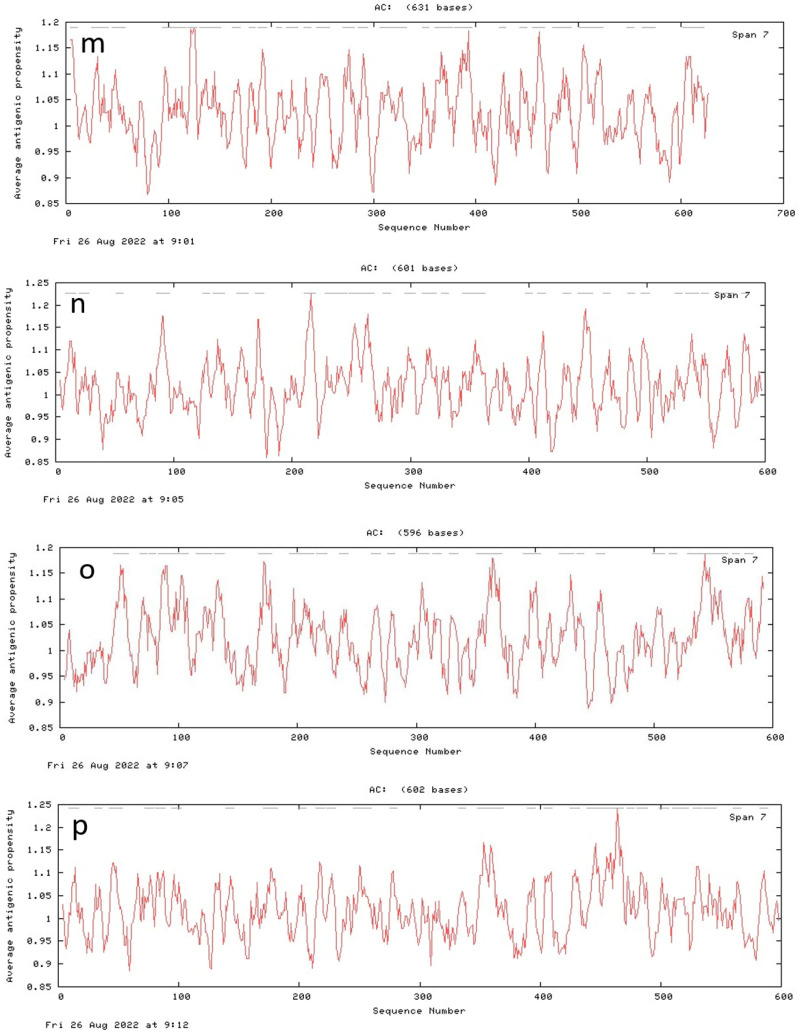
Positions and number of sequences that might be involved in antigenic propensity were different in all proteins i.e., Lacticaseibacillus rhamnosus (24), Roseburia intestinalis (27), Coprococcus comes (30), Faecalibacterium prausnitzii 1 (25), Faecalibacterium prausnitzii 2 (29), Faecalibacterium prausnitzii 3 (27), Propionibacterium acnes (22), Methylococcaceae bacterium (23), Staphylococcus xylosus (19), Streptococcus suis (20), Bacillus sp. M4U3P1 (22), Escherichia coli (strain K12) (26), Sphingomonas aquatilis NBRC 16722 (27), Citrobacter amalonaticus (28), Enterococcus gallinarum 1 (26), Enterococcus gallinarum 2 (27), Salmonella enterica (27), Staphylococcus caeli (22), Staphylococcus haemolyticus (23). The antigenicity plots of GUS proteins are indicated in Figure 8.
Figure 8.
Antigenecity plots for uidA proteins of probiotics (a) L. rhamnosus, (b) R. intestinalis, (c) C. comes, (d) F. prausnitzii 1, (e) F. prausnitzii 2, (f) F. prausnitzii 3, (g) P. acnes, (h) M. bacterium, (i) S. xylosus, (j) S. suis, (k) Bacillus sp. M4U3P1, (l) E. coli (strain K12), (m) S. aquatilis NBRC 16722, (n) C. amalonaticus, (o) E. gallinarum 1, (p) E. gallinarum 2, (q) S. enterica, (r) S. caeli, (s) S. haemolyticus.
4. Discussion
The first verification of microbiota presence and dysbiosis in breast cancer tissue has been reported through next generation sequencing (NGS) analysis of breast tumor tissue as well as the normal tissue adjacent to tumor. Qualitative analysis revealed the presence of Methylobacterium radiotolerans and Sphingomonas yanoikuyae in tumor and normal tissues, respectively. Quantitative PCR-based analysis showed reduced load of bacterial DNA in cancerous tissue proving the breast cancer association with dysbiosis [11]. Multiple experimental evidences of the role of microbial GUS enzyme in breast cancer has been reported in the literature. In a study, the potential of 35 GUS enzymes to reactivate glucuronidated estrogen was explored using in-vivo, in-vitro and in-fimo techniques. It was found that GUS enzymes belonging to classes L1, ML1 and FMN were very active in the activation of conjugated estrogens [28]. The association of gmGUS with estrobolome has also been studied via the inspection of estrogen replacement therapy impact on GUS enzymes of GIT microbiota and the microbial composition. Long-term exposure to ERT altered the microbial composition of GIT accompanied with reduced GUS enzyme activities. ERT induced dysbiosis by reducing the number of L. rhamnosus, F. prausnitzii and enhancing the R. gnavus [25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48].
The gmGUS play their role in estrobolome by reversing the glucuronidation process and catalyze estrogens activation by breaking glucuronic moiety. Estrogen and estrone glucuronides that might be the substrates for gmGUS include 17-α estradiol 17-O, 17-β estradiol 17-O, 17-β estradiol 3-O, 2-hydroxy-17-β estradiol 2-O, 2-hydroxy-17-β estradiol 3-O, 4-hydroxy 17-β estradiol-3-O, 4-hydroxy 17-β estradiol-4-O, estrone 3-O, 2-hydroxy estrone-2-O, 2-hydroxy estrone-3-O, 4-hydroxy estrone-3-O, 16-α-17-β estriol 3-O, 16-β-17-β estriol 3-O, 16-α-17-α estriol 3-O, 16-α-17-β estriol 16-O, 16-α-17-α estriol 16-O, 16-β-17-β estriol 16-O, 16-α-17-β estriol-16-O, 16-α-17-α estriol-17-O and 16-β-17-β estriol-17-O, respectively [30].
This reaction releases aglycones. The process of deconjugation occurs as estrogens after GIT via bile [24]. Estrogens without deconjugation, due to high polarity and hydrophilicity, are dissolved in blood and are removed through the kidneys as urine. However, on deconjugation, estrogens via reabsorption in mucosa enter the portal vein [32]. Due to the association of high estrogen concentration with breast cancer gut microbes and breast cancer axis has been as emerging research area.
With reference to sub-cellular localization and physicochemical properties, bacteria in breast cancer patients were found to be more diverse as compared to those reported in normal tissues. The diversity of GUS enzyme found in the present study is consistent with the literature [49]. This protein in Ruminococcus gnavus has been reported to show similarity of 69%, 61%, 59% and 58% with L. gasseri, E. coli, C. perfringens and S. aureus, respectively [50]. Therefore, this study has further strengthened the diversified nature of GUS enzyme.
The GUS protein in S. xylosus, S. suis, S. aquatilis NBRC 16722, C. amalonaticus, S. caeli and E. gallinarum 1 exhibited extreme values of pI, extinction coefficient, instability index and aliphatic index. Among the probiotics of normal tissue, only L. rhamnosus GUS protein showed extreme value for aliphatic index. The total number of amino acids was found to be in the range of 596–795 with variable molecular weights. The isoelectric point analysis revealed that GUS of S. aquatilis NBRC 16722 was alkaline with pI value 8.92 while in all other cases, GUS showed acidic pI. The pI value for S. caeli is consistent with earlier reported pI value for E. coli HGU-3 [51]. Literature reports that GUS enzyme activity increases at high pH and causes cancer [52]. According to this information, in the present study GUS enzyme of S. aquatilis showed alkaline nature as per its pI. While enzymes of all other bacteria except S. caeli exhibited low acidic pI, which might have some association with the cancer-causing potential of this enzyme.
Aliphatic index reflects the relative volume of protein occupied by aliphatic side chain containing amino acids which indicates increased thermostability [53]. As all the bacterial proteins in the present study showed the range of 71.01–88.10 so GUS protein is found to be highly thermostable. Instability index is a measure of protein stability in test tube [54]. The value below 40 indicates stability of protein, so GUS enzyme in the case of all present study bacteria except C. amalonaticus is considered to be highly stable.
The 3-D configuration was generated using PHYRE2 tool and further verified by plotting Ramachandran plot using SAVES server. Except in the R. intestinalis and F. prausnitzii 1, GUS enzyme in all other bacteria showed marked variation in 3D structures. GUS enzymes from nineteen microbiota showed high variation with regard to physicochemical properties, sub-cellular localization, 3D configuration and antigenic sites so this is a highly diverse protein.
The catalytic pockets were identified along with different parameters of tunnels. Druggability is the potential of a molecule of being controlled by therapeutic drugs [55]. It is the measurement of binding affinity of catalytic site to drug like organic molecule of host [56]. The druggability values for catalytic sites of GUS enzyme are reflecting their challenging nature and do not prove them as excellent drug targets. Bottle neck radius is the measure of maximum size of probe that can fit in the narrowest portion of tunnel. Length of tunnel measures the distance between starting point and protein surface. In the present study, tunnel length was measured in the range of 1.4 to 15.9. Curvature shows the shape of tunnel which is the ratio of tunnel length and shortest distance between the starting and ending points of tunnel. In GUS enzymes, tunnel curvature was measured in the range of 0.65 to 1.4. Length and curvature also gives an idea about substrate specificity of the enzyme. Tunnels geometry may affect catalysis via channeling of substrate [57]. However, these catalytic sites can be engineered for targeting by drugs to inhibit the estrogen reactivation in breast cancer patients. The attributes of tunnel can be useful in drug designing experiments in future.
Conserved protein motifs are those sequences of proteins that undergo small variations with time. The variations might involve substitutions of fewer amino acids and replacement with amino acid having similar biochemical properties. These motifs play crucial roles in the stability and formation of catalytic site of protein. Conserved protein motifs identified in present study bacteria also show their relatedness [58].
Antigenic peptides are the bacterial proteins which directly interact with host immune system; therefore, they can be good candidates for the development of vaccines [59]. GUS enzyme in all the nineteen bacteria showed antigenic peptides in the range of 22–29. The presence of these large numbers of antigenic peptides gives a clue about the possible immunomodulatory role of these bacteria. Due to the variety in antigenic peptides, catalytic sites and the adjacent loop structures, anti-cancer medication therapy can be managed via GUS enzyme inhibition also reported in literature [49]. High concentration and deglucuronidation potential of GUS enzyme in breast cancer patients can also be used for bioactivation of glucuronide anticancer prodrugs [60].
5. Conclusions
Identification of the structural properties and present study findings regarding estrogen-reactivating protein (GUS enzyme) in bacteria found in normal and breast cancer patients might provide us multiple directions for modification of this enzyme. As it is very easy to perform manipulations successfully at the level of probiotics as compared to human genes, the manipulation at probiotics level might be helpful in reducing breast cancer risk through inhibiting reactivation of this estrobolome-associated protein. Additionally, the active sites explored in the present study can be inspected further for their possible role in deglucuronidation of glucuronidated estrogens. Exploration of these catalytic sites might help in modification of GUS enzyme to prevent its estrogen deconjugation potential. Modifications may include alteration in the structure of active sites participating in deglucuronidation by inducing mutations at specific points in uidA gene and deletion of conserved protein motifs. For these modifications, site-directed mutagenesis can be performed which may lead to destabilization of protein, thus inhibiting its estrogen reactivation potential.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/genes13091545/s1, Figure S1: Secondary structure motif maps of GUS enzymes for probiotics predicted by PDBsum tool; Figure S2: Ramachandran plots predicting quality of GUS protein structures of bacteria discussed in present study; Table S1: Names, UniProt Accession IDs and sequences of probiotics documented in present study
Author Contributions
Methodology & Writing—original draft, F.M.; Formal analysis, W.A. & S.G.; Revision of manuscript, M.A.-Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The sequences of bacterial proteins documented in present study are available at Uniprot (https://www.uniprot.org) database.
Conflicts of Interest
The authors declare that they have no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ganguly N.K., Bhattacharya S.K., Sesikeran B., Nair G.B., Ramakrishna B.S., Sachdev H.P.S., Batish V.K., Kanagasabapathy A.S., Muthuswamy V., Kathuria S.C. ICMR-DBT guidelines for evaluation of probiotics in food. Indian J. Med. Res. 2011;134:22. [PMC free article] [PubMed] [Google Scholar]
- 2.Fuller R. Probiotics in man and animals. J. Appl. Bacteriol. 1989;66:365–378. [PubMed] [Google Scholar]
- 3.Nagpal R., Kumar A., Kumar M., Behare P.V., Jain S., Yadav H. Probiotics, their health benefits and applications for developing healthier foods: A review. FEMS Microbiol. Lett. 2012;334:1–15. doi: 10.1111/j.1574-6968.2012.02593.x. [DOI] [PubMed] [Google Scholar]
- 4.Şener D., Bulut H.N., Güneş Bayir A. Probiotics and Relationship between Probiotics and Cancer Types. Bezmialem Sci. 2021;9:490–497. doi: 10.14235/bas.galenos.2021.5375. [DOI] [Google Scholar]
- 5.Lee J.H., Nam S.H., Seo W.T., Yun H.D., Hong S.Y., Kim M.K., Cho K.M. The production of surfactin during the fermentation of cheonggukjang by potential probiotic Bacillus subtilis CSY191 and the resultant growth suppression of MCF-7 human breast cancer cells. Food Chem. 2012;131:1347–1354. doi: 10.1016/j.foodchem.2011.09.133. [DOI] [Google Scholar]
- 6.Bland K.I., Copeland E.M., Klimberg V.S., Gradishar W.J. The Breast E-Book: Comprehensive Management of Benign and Malignant Diseases. Elsevier Health Sciences; Amsterdam, The Netherlands: 2017. [Google Scholar]
- 7.Siegel R.L., Miller K.D., Jemal A. Cancer statistics, 2019. Cancer J. Clin. 2019;69:7–34. doi: 10.3322/caac.21551. [DOI] [PubMed] [Google Scholar]
- 8.Kumar V., Naik B., Kumar A., Khanduri N., Rustagi S., Kumar S. Probiotics media: Significance, challenges, and future perspective-a mini review. Food Prod. Process Nutr. 2022;4:1–13. [Google Scholar]
- 9.Doron S.I., Hibberd P.L., Gorbach S.L. Probiotics for prevention of antibiotic-associated diarrhea. J. Clin. Gastroenterol. 2008;42:S58–S63. doi: 10.1097/MCG.0b013e3181618ab7. [DOI] [PubMed] [Google Scholar]
- 10.Choi C.H., Jo S.Y., Park H.J., Chang S.K., Byeon J.S., Myung S.J. A randomized, double-blind, placebo-controlled multicenter trial of Saccharomyces boulardii in irritable bowel syndrome: Effect on quality of life. J. Clin. Gastroenterol. 2011;45:679–683. doi: 10.1097/MCG.0b013e318204593e. [DOI] [PubMed] [Google Scholar]
- 11.Xuan C., Shamonki J.M., Chung A., DiNome M.L., Chung M., Sieling P.A., Lee D.J. Microbial dysbiosis is associated with human breast cancer. PLoS ONE. 2014;9:e83744. doi: 10.1371/journal.pone.0083744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dieleman S., Aarnoutse R., Ziemons J., Kooreman L., Boleij A., Smidt M. Exploring the potential of breast microbiota as biomarker for breast cancer and therapeutic response. AME J. Pathol. 2021;191:968–982. doi: 10.1016/j.ajpath.2021.02.020. [DOI] [PubMed] [Google Scholar]
- 13.Wang H., Altemus J., Niazi F., Green H., Calhoun B.C., Sturgis C., Grobmyer S.R., Eng C. Breast tissue, oral and urinary microbiomes in breast cancer. Oncotarget. 2017;8:88122. doi: 10.18632/oncotarget.21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hieken T.J., Chen J., Hoskin T.L., Walther-Antonio M., Johnson S., Ramaker S., Xiao J., Radisky D.C., Knutson K.L., Kalari K.R. The microbiome of aseptically collected human breast tissue in benign and malignant disease. Sci. Rep. 2016;6:30751. doi: 10.1038/srep30751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Komorowski A.S., Pezo R.C. Untapped “-omics”: The microbial metagenome, estrobolome, and their influence on the development of breast cancer and response to treatment. Breast Cancer Res. Treat. 2020;179:287–300. doi: 10.1007/s10549-019-05472-w. [DOI] [PubMed] [Google Scholar]
- 16.Alizadehmohajer N., Shojaeifar S., Nedaeinia R., Esparvarinha M., Mohammadi F., Ferns G.A., Ghayour-Mobarhan M., Manian M., Balouchi A. Association between the microbiota and women’s cancers–Cause or consequences? Biomed. Pharm. 2020;127:110203. doi: 10.1016/j.biopha.2020.110203. [DOI] [PubMed] [Google Scholar]
- 17.Faith J.J., McNulty N.P., Rey F.E., Gordon J.I. Predicting a human gut microbiota’s response to diet in gnotobiotic mice. Science. 2011;333:101–104. doi: 10.1126/science.1206025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Aminov R.I., Walker A.W., Duncan S.H., Harmsen H.J.M., Welling G.W., Flint H.J. Molecular diversity, cultivation, and improved detection by fluorescent in situ hybridization of a dominant group of human gut bacteria related to Roseburia spp. or Eubacterium rectale. Appl. Environ. Microbiol. 2006;72:6371–6376. doi: 10.1128/AEM.00701-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Urbaniak C., Gloor G.B., Brackstone M., Scott L., Tangney M., Reid G. The microbiota of breast tissue and its association with breast cancer. Appl. Environ. Microbiol. 2016;82:5039–5048. doi: 10.1128/AEM.01235-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Esposito M.V., Fosso B., Nunziato M., Casaburi G., D’Argenio V., Calabrese A., D’Aiuto M., Botti G., Pesole G., Salvatore F. Microbiome composition indicate dysbiosis and lower richness in tumor breast tissues compared to healthy adjacent paired tissue, within the same women. BMC Cancer. 2022;22:30. doi: 10.1186/s12885-021-09074-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ma J., Sun L., Liu Y., Ren H., Shen Y., Bi F., Zhang T., Wang X. Alter between gut bacteria and blood metabolites and the anti-tumor effects of Faecalibacterium prausnitzii in breast cancer. BMC Microbiol. 2020;20:1–19. doi: 10.1186/s12866-020-01739-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.He C., Liu Y., Ye S., Yin S., Gu J. Changes of intestinal microflora of breast cancer in premenopausal women. Eur. J. Clin. Microbiol. Infect. Dis. 2021;40:503–513. doi: 10.1007/s10096-020-04036-x. [DOI] [PubMed] [Google Scholar]
- 23.Costantini L., Magno S., Albanese D., Donati C., Molinari R., Filippone A., Masetti R., Merendino N. Characterization of human breast tissue microbiota from core needle biopsies through the analysis of multi hypervariable 16S-rRNA gene regions. Sci. Rep. 2018;8:16893. doi: 10.1038/s41598-018-35329-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Awolade P., Cele N., Kerru N., Gummidi L., Oluwakemi E., Singh P. Therapeutic significance of β-glucuronidase activity and its inhibitors: A review. Eur. J. Med. Chem. 2020;187:111921. doi: 10.1016/j.ejmech.2019.111921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pollet R.M., D’Agostino E.H., Walton W.G., Xu Y., Little M.S., Biernat K.A., Pellock S.J., Patterson L.M., Creekmore B.C., Isenberg H.N. An atlas of β-glucuronidases in the human intestinal microbiome. Structure. 2017;25:967–977.e965. doi: 10.1016/j.str.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wallace B.D., Wang H., Lane K.T., Scott J.E., Orans J., Koo J.S., Venkatesh M., Jobin C., Yeh L.A., Mani S. Alleviating cancer drug toxicity by inhibiting a bacterial enzyme. Science. 2010;330:831–835. doi: 10.1126/science.1191175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pellock S.J., Walton W.G., Biernat K.A., Torres-Rivera D., Creekmore B.C., Xu Y., Liu J., Tripathy A., Stewart L.J., Redinbo M.R. Three structurally and functionally distinct β-glucuronidases from the human gut microbe Bacteroides uniformis. J. Biol. Chem. 2018;293:18559–18573. doi: 10.1074/jbc.RA118.005414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ervin S.M., Li H., Lim L., Roberts L.R., Liang X., Mani S., Redinbo M.R. Gut microbial β-glucuronidases reactivate estrogens as components of the estrobolome that reactivate estrogens. J. Biol. Chem. 2019;294:18586–18599. doi: 10.1074/jbc.RA119.010950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Travis R.C., Key T.J. Oestrogen exposure and breast cancer risk. Breast Cancer Res. 2003;5:239. doi: 10.1186/bcr628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schiffer L., Barnard L., Baranowski E.S., Gilligan L.C., Taylor A.E., Arlt W., Shackleton C.H.L., Storbeck K.H. Human steroid biosynthesis, metabolism and excretion are differentially reflected by serum and urine steroid metabolomes: A comprehensive review. J. Steroid Biochem. Mol. Biol. 2019;194:105439. doi: 10.1016/j.jsbmb.2019.105439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adlercreutz H., Martin F. Biliary excretion and intestinal metabolism of progesterone and estrogens in man. J. Steroid Biochem. 1980;13:231–244. doi: 10.1016/0022-4731(80)90196-X. [DOI] [PubMed] [Google Scholar]
- 32.Flores R., Shi J., Fuhrman B., Xu X., Veenstra T.D., Gail M.H., Gajer P., Ravel J., Goedert J.J. Fecal microbial determinants of fecal and systemic estrogens and estrogen metabolites: A cross-sectional study. J. Transl. Med. 2012;10:1–11. doi: 10.1186/1479-5876-10-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y.S., Zhao Z., Yang Z.N., Xu F., Lu H.J., Zhu Z.Y., Shi W., Jiang J., Yao P.P., Zhu H.P. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 2017;13:1387. doi: 10.7150/ijbs.21635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Godone R.L.N., Leitão G.M., Araújo N.B., Castelletti C.H.M., Lima-Filho J.L., Martins D.B.G. Clinical and molecular aspects of breast cancer: Targets and therapies. Biomed. Pharm. 2018;106:14–34. doi: 10.1016/j.biopha.2018.06.066. [DOI] [PubMed] [Google Scholar]
- 35.Kumar S., Srivastav R.K., Wilkes D.W., Ross T., Kim S., Kowalski J., Chatla S., Zhang Q., Nayak A., Guha M. Estrogen-dependent DLL1-mediated Notch signaling promotes luminal breast cancer. Oncogene. 2019;38:2092–2107. doi: 10.1038/s41388-018-0562-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gupta P.B., Proia D., Cingoz O., Weremowicz J., Naber S.P., Weinberg R.A., Kuperwasser C. Systemic stromal effects of estrogen promote the growth of estrogen receptor–negative cancers. Cancer Res. 2007;67:2062–2071. doi: 10.1158/0008-5472.CAN-06-3895. [DOI] [PubMed] [Google Scholar]
- 37.Jiang C.F., Shi Z.M., Li D.M., Qian Y.C., Ren Y., Bai X.M., Xie Y.X., Wang L., Ge X., Liu W.T. Estrogen-induced miR-196a elevation promotes tumor growth and metastasis via targeting SPRED1 in breast cancer. Mol. Cancer. 2018;17:1–18. doi: 10.1186/s12943-018-0830-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zeng L., Zielinska H.A., Arshad A., Shield J.P., Bahl A., Holly J.M.P., Perks C.M. Hyperglycaemia-induced chemoresistance in breast cancer cells: Role of the estrogen receptor. Endocr. Relat. Cancer. 2016;23:125–134. doi: 10.1530/ERC-15-0507. [DOI] [PubMed] [Google Scholar]
- 39.Consortium U. UniProt: A hub for protein information. Nucleic Acids Res. 2015;43:D204–D212. doi: 10.1093/nar/gku989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sievers F., Higgins D.G. Multiple Sequence Alignment. Springer; Berlin/Heidelberg, Germany: 2021. The clustal omega multiple alignment package; pp. 3–16. [DOI] [PubMed] [Google Scholar]
- 41.Kumar S., Stecher G., Tamura K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 2016;33:1870–1874. doi: 10.1093/molbev/msw054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saitou N., Nei M. The neighbor-joining method: A new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 1987;4:406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 43.Zuckerkandl E., Pauling L. Evolving Genes and Proteins. Elsevier; Amsterdam, The Netherlands: 1965. Evolutionary divergence and convergence in proteins; pp. 97–166. [Google Scholar]
- 44.Geourjon C., Deleage G. SOPMA: Significant improvements in protein secondary structure prediction by consensus prediction from multiple alignments. Bioinformatics. 1995;11:681–684. doi: 10.1093/bioinformatics/11.6.681. [DOI] [PubMed] [Google Scholar]
- 45.Stourac J., Vavra O., Kokkonen P., Filipovic J., Pinto G., Brezovsky J., Damborsky J., Bednar D. Caver Web 1.0: Identification of tunnels and channels in proteins and analysis of ligand transport. Nucleic Acids Res. 2019;47:W414–W422. doi: 10.1093/nar/gkz378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Khan S., Vihinen M. Spectrum of disease-causing mutations in protein secondary structures. BMC Struct. Biol. 2007;7:1–18. doi: 10.1186/1472-6807-7-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Agnihotry S., Pathak R.K., Singh D.B., Tiwari A., Hussain I. Bioinformatics. Elsevier; Amsterdam, The Netherlands: 2022. Protein structure prediction; pp. 177–188. [Google Scholar]
- 48.Kwa M., Plottel C.S., Blaser M.J., Adams S. The intestinal microbiome and estrogen receptor–positive female breast cancer. J. Natl. Cancer Inst. 2016;108 doi: 10.1093/jnci/djw029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wang P., Jia Y., Wu R., Chen Z., Yan R. Human gut bacterial β-glucuronidase inhibition: An emerging approach to manage medication therapy. Biochem. Pharm. 2021;190:114566. doi: 10.1016/j.bcp.2021.114566. [DOI] [PubMed] [Google Scholar]
- 50.Beaud D., Tailliez P., Anba-Mondoloni J. Genetic characterization of the β-glucuronidase enzyme from a human intestinal bacterium, Ruminococcus gnavus. Microbiology. 2005;151:2323–2330. doi: 10.1099/mic.0.27712-0. [DOI] [PubMed] [Google Scholar]
- 51.Kim D., Jin Y., Jung E., Han M., Kobashi K. Purification and characterization of β-glucuronidase from Escherichia coli HGU-3, a human intestinal bacterium. Biol. Pharm. Bull. 1995;18:1184–1188. doi: 10.1248/bpb.18.1184. [DOI] [PubMed] [Google Scholar]
- 52.Kim D., Kang H., Park S., Kobashi K. Characterization of β-glucosidase and β-glucuronidase of alkalotolerant intestinal bacteria. Biol Pharm. Bull. 1994;17:423–426. doi: 10.1248/bpb.17.423. [DOI] [PubMed] [Google Scholar]
- 53.Morya V.K., Yadav S., Kim E.K., Yadav D. In silico characterization of alkaline proteases from different species of Aspergillus. Appl. Biochem. Biotechnol. 2012;166:243–257. doi: 10.1007/s12010-011-9420-y. [DOI] [PubMed] [Google Scholar]
- 54.Gamage D.G., Gunaratne A., Periyannan G.R., Russell T.G. Applicability of instability index for in vitro protein stability prediction. Protein Pept. Lett. 2019;26:339–347. doi: 10.2174/0929866526666190228144219. [DOI] [PubMed] [Google Scholar]
- 55.Barril X. Druggability predictions: Methods, limitations, and applications. Wiley Interdiscip. Rev. Comput. Mol. Sci. 2013;3:327–338. doi: 10.1002/wcms.1134. [DOI] [Google Scholar]
- 56.Michel M., Homan E.J., Wiita E., Pedersen K., Almlöf I., Gustavsson A.L., Lundbäck T., Helleday T., Warpman Berglund U. In silico druggability assessment of the NUDIX hydrolase protein family as a workflow for target prioritization. Front. Chem. 2020;8:443. doi: 10.3389/fchem.2020.00443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kingsley L.J., Lill M.A. Substrate tunnels in enzymes: Structure–function relationships and computational methodology. Proteins Struct. Funct. Bioinform. 2015;83:599–611. doi: 10.1002/prot.24772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wong A., Gehring C., Irving H.R. Conserved functional motifs and homology modeling to predict hidden moonlighting functional sites. Front. Bioeng. Biotechnol. 2015;3:82. doi: 10.3389/fbioe.2015.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sun Z., Fu P., Wei K., Zhang H., Zhang Y., Xu J., Jiang F., Liu X., Xu W., Wu W. Identification of novel immunogenic proteins from Mycoplasma bovis and establishment of an indirect ELISA based on recombinant E1 β subunit of the pyruvate dehydrogenase complex. PLoS ONE. 2014;9:e88328. doi: 10.1371/journal.pone.0088328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sperker B., Backman J.T., Kroemer H.K. The role of β-glucuronidase in drug disposition and drug targeting in humans. Clin. Pharm. 1997;33:18–31. doi: 10.2165/00003088-199733010-00003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The sequences of bacterial proteins documented in present study are available at Uniprot (https://www.uniprot.org) database.