Abstract
Gut microbiota has emerged as an important key regulator of health and disease status. Indeed, gut microbial dysbiosis has been identified in an increasing number of diseases, including neurodegenerative disorders. Accordingly, microbial alterations have been reported also in Alzheimer’s disease (AD), suggesting possible pathogenetic mechanisms contributing to the development of specific AD hallmarks and exacerbating metabolic alterations and neuroinflammation. The identification of these mechanisms is crucial to develop novel, targeted therapies and identify potential biomarkers for diagnostic purposes. Thus, the possibility to have AD in vivo models to study this microbial ecosystem represents a great opportunity for translational applications. Here, we characterized both gut microbiome and mycobiome of 3xTg-AD mice, one of the most widely used AD models, to identify specific microbial alterations with respect to the wild-type counterpart. Interestingly, we found a significant reduction of the Coprococcus and an increased abundance of Escherichia_Shigella and Barnesiella genera in the AD mice compatible with a pro-inflammatory status and the development of AD-related pathogenetic features. Moreover, the fungal Dipodascaceae family was significantly increased, thus suggesting a possible contribution to the metabolic alterations found in AD. Our data point out the strict connection between bacterial dysbiosis and AD and, even if further studies are required to clarify the underlining mechanisms, it clearly indicates the need for extensive metagenomic studies over the bacterial counterpart.
Keywords: Alzheimer’s disease, microbiome, mycobiome, metagenomic studies, 3xTg-AD mice
1. Introduction
It has been well established that the gut microbiota plays an important role in health status acquisition and maintenance, and, consequently, its alterations can be associated with several diseases [1]. This statement is true not only for intestinal diseases but can be extended also to extra-intestinal disorders [1]. In particular, it is well known that a stable and complex communication system exists between gut and brain, and the gut microbiota has been claimed as a factor able to influence the gut–brain axis [2,3,4]. As a consequence, it has been proposed that a microbial dysbiosis at the gut level, by acting through different mechanisms such as membrane permeability modifications, inflammatory response induction or toxic metabolite production, may play a role also in neurodegenerative disorders such as Alzheimer’s disease (AD) [5]. Accordingly, several studies have shown a correlation between gut microbiota alterations and cognitive impairment and/or specific AD features [5,6,7]. These studies, even if often observatory and at a preliminary stage, have the overall merit of having highlighted an additional molecular mechanism involved in AD pathogenesis that has the potential advantage to be easily actionable for prevention or therapeutic purposes [8]. In this context, it has been reported that a high-fat diet is able to reduce the Lactobacillus, Bacteroides and Prevotella species and increases Bifidobacterium abundance in mice. Interestingly, this microbial dysbiosis is related to alterations of gut permeability, as assessed by zonulin and occludin proteins reduced expression, and to a consequent increase of circulating bacterial lipopolysaccharide (LPS) and other pro-inflammatory markers [9]. More recently, a high-fat diet was reported to induce metabolic alterations, cognitive impairment and tau hyperphosphorylation in transgenic mice [10]. These findings suggest that lifestyle changes (i.e., dietary modifications), by modifying the gut microbiota composition, may ameliorate some AD typical features. Indeed, probiotics administration has shown promising results. Kaur et al. administered a 2-month probiotic supplementation to an AD mouse model and reported an improvement of gut functions and reduced anxiety-like behavior [11]. Webberley et al. tested a probiotic consortium in 3xTg-AD mice and reported improved cognitive functions, reduced systemic inflammation and a lower Firmicutes:Bacteroidetes ratio in the treated group [12].
Using the same AD model (3xTg-AD mice), we reported that the oral administration of a lactic acid bacteria and Bifidobacteria formulation was able to reduce cognitive decline and Aβ aggregates deposition ameliorating also neuronal proteolytic pathways [13]. Moreover, the same formulation was found to reduce the oxidative stress by a sirtuin-1 (SIRT1)-dependent mechanism [14], to ameliorate glucose homeostasis and decrease phosphorylated tau aggregates [15], and to positively impact lipid metabolism [16]. Finally, we have recently evaluated the effects of a yeast-enriched beer on the gut microbiota and neurodegeneration of 3xTg-AD mice, showing a positive modulation in the treated group [17]. Since 3xTg-AD mice are a common model used for studying AD related-features and the underlying mechanisms, including gut microbiota modifications, the definition of the microbial differences between 3xTg-AD and wild-type (WT) mice may be important to design more targeted studies. Here, we report the bacterial and fungal microbiota composition of 3xTg-AD mice, showing specific features with respect to the WT group that will be useful for further investigations aimed to clarify their role in AD pathogenesis.
2. Materials and Methods
2.1. Animals and Study Design
AD triple-transgenic mice, B6;129-Psen1tm1Mpm Tg (APPSwe, tauP301L) 1Lfa/J (named 3xTg-AD) and the wild-type B6129SF2 mice were purchased from the Jackson Laboratory (Bar Harbor, ME, USA). 3xTg-AD mice contain three mutations associated with frontotemporal dementia or familial AD (amyloid precursor protein [APP] Swe, tau MAPT P301L and presenilin-1 M146V). This reliable model of human AD displays both plaque and tangle pathology, with Aβ intracellular immunoreactivity detectable at three months of age and hyperphosphorylation of tau protein occurring by 12 to 15 months of age [18], reliably reproducing traits similar to those observed in the entire life of Alzheimer’s disease patients.
Experiments complied with the ARRIVE guidelines, in accordance with the EU Directive 2010/63/EU for animal experiments, and with a protocol approved by the Italian Ministry of Health (518/2018-PR). Mice were housed in plastic cages (Makrolon, Covestro A.G., Filago, Italy) in a temperature-controlled room (21 ± 5 °C) and 60% humidity on 12-hour light/dark reversed cycle (light was switched on at 8:00 p.m.) and maintained on standard laboratory diet (Mucedola, Settimo Milanese (MI), Italy) and water ad libitum. Appropriate measures minimized pain and discomfort in experimental animals.
2.2. Fecal Samples Collection and DNA Extraction
Eleven 8-week-old mice, 5 AD and 6 WT mice, were analyzed. Fecal samples were collected from each mouse at the time of sacrifice and were immediately frozen at −80 °C until further processing. Genomic DNA was extracted from all stool samples by using the RSC Blood DNA kit and the Maxwell RSC instrument (both from Promega, Madison, WI, USA), as previously reported [19]. Specifically, 100 mg of each stool sample were lysed by adding 400 µL of Lysis Buffer, pestled and vortexed until reached a homogeneous status and incubated at 95 °C for 5 min at 800 rpm on thermomixer. A centrifugation step of 5 min at 13,000 rpm was carried out and 300 µL of the supernatant were transferred into a fresh tube. Thirty µL of Proteinase K solution were added to the samples that were vortexed and incubated at 56 °C for 20 min at 500 rpm on thermomixer. Next, the lysed samples were loaded onto the RSC cartridge and processed from the Maxwell RSC instrument. Final DNA samples were eluted in 100 µL of Elution Buffer and quantitatively assessed by using the Nanodrop spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA).
2.3. Bacterial 16S rRNA Analysis
Fecal bacterial community analysis was carried out using 16S rRNA custom primers designed to selectively amplify the V4-V6 hypervariable regions, as previously described [20]. PCR mix solutions and amplification conditions were optimized to guarantee the correct target amplification. In particular, AmpliTaq Gold polymerase, GC enhancer (both from Thermo Fisher Scientific, Waltham, MA, USA) and 20 µM of forward and reverse custom primers were used. After 2% agarose gel electrophoretic analysis, all the PCR products were purified by using the AMPure XP beads (Beckman Coulter, Brea, CA, USA) and quality-checked on the Tape Station System with the D1000 ScreenTapes (both from Agilent Technologies, Santa Clara, CA, USA). Next, the purified amplicons were quantified with Qubit HS (Qubit, dsDNA HS Assay, Life Technologies, Carlsbad, CA, USA) and diluted to 2 ng/µL in order to being processed with a second-round PCR, necessary to add specific indexes to each sample and also the universal adapters for the following NGS reactions. During this step, Nextera DNA CD Indexes (Illumina, San Diego, CA, USA) were used. Then, further AMpure XP beads-based purification and Tape Station qualitative analysis were carried out. Finally, 5 µL of each library were pooled to be sequenced in a single sequencing run. A total of six blank samples (2 for DNA extraction, 2 for the first PCR and 2 for the second PCR) was processed together with the others to check for any contaminants and environmental biases potentially occurring during the experimental procedure.
Sequencing reaction was carried out using the MiSeq reagent Kit V3 600 cycles on the MiSeq instrument (Illumina, San Diego, CA, USA). The library’s pool was loaded at a final concentration of 9 pM with a 30% PhiX.
2.4. Fungal ITS Analysis
Mycobiome analysis of fecal communities was carried out on the same samples to assess also fungal composition. Specific custom primers for ITS1 amplification were used as previously reported [17]. After this first-round PCR and a beads-based purification step (AMPure XP beads, Beckman Coulter, Brea, CA, USA), the amplicons were quality assessed by using a D1000 ScreenTape (Agilent Technologies, Santa Clara, CA, USA) and further amplified to allow samples indication and add the sequencing adapters. The obtained libraries were then purified and visualized on the Tape Station (Agilent Technologies, Santa Clara, CA, USA) before being pooled for sequencing. Blank samples corresponding to DNA extraction and the 2 PCR rounds (totally, n = 6) were also processed to avoid environmental contaminants. Next-generation sequencing was performed on the Illumina MiSeq system (V3, 300 × 2 PE) with the same conditions specified above.
2.5. Bioinformatics Analysis of Metagenomic Data
The FASTQ files generated by the sequencing run were processed by the CRG bioinformatic facility (https://biocore.crg.eu/wiki/Main_Page, last accession on 1 July 2022). In particular, sequencing reads quality was assessed by FastQC [21] and primary data analysis was carried out using the mothur tool (version 1.44.1) [22], according to its pipeline (https://mothur.org/wiki/miseq_sop/, last accession on 1 July 2022). Reference sequences for reads mapping were obtained (i) from the SILVA database, version 138 [23] for the bacterial 16S rRNA, and (ii) from the UNITE database, version 04.02.2020 [24] for the ITS. Next, the R packages Phyloseq v. 1.30.0 [25] and microbiome v. 1.8.0 [26] were used to estimate α- and β-diversity [27], while the identification of significantly enriched taxa in the 2 study groups, was evaluated by using the R package DESeq2 v. 1.26.0 [28].
The mothur output was further analyzed using the Microbiome Analyst tool [29]. In particular, samples richness and/or evenness were evaluated using the ANOVA test to assess any significant difference. β diversity was measured using the unweighted and weighted UniFrac distance measures coupled with the PERMANOVA test to verify the significance of samples grouping. Linear discriminant analysis (LDA) effect size (LEfSe) was used to detect significant differentially abundant taxa based on the Kruskal–Wallis rank sum test coupled with linear discriminant analysis to assess the relevance of any differential abundant taxa (p-value cutoff: 0.1, FDR adjusted. Log LDA score: 2).
3. Results
3.1. Microbiome Composition Analysis
All the collected fecal samples were analyzed as described under the Methods section to assess their bacterial composition and verify any significant feature able to discriminate between the two studied groups. An average of 29,030 reads/sample, corresponding to a total of 126 operational taxonomic units (OTUs), was obtained and used for subsequent analyses. It has to be underlined that the six negative samples, used as analytic controls for the experimental procedure, did not produce reads, assessing the absence of contaminants during samples preparation and were not further considered for the downstream analyses.
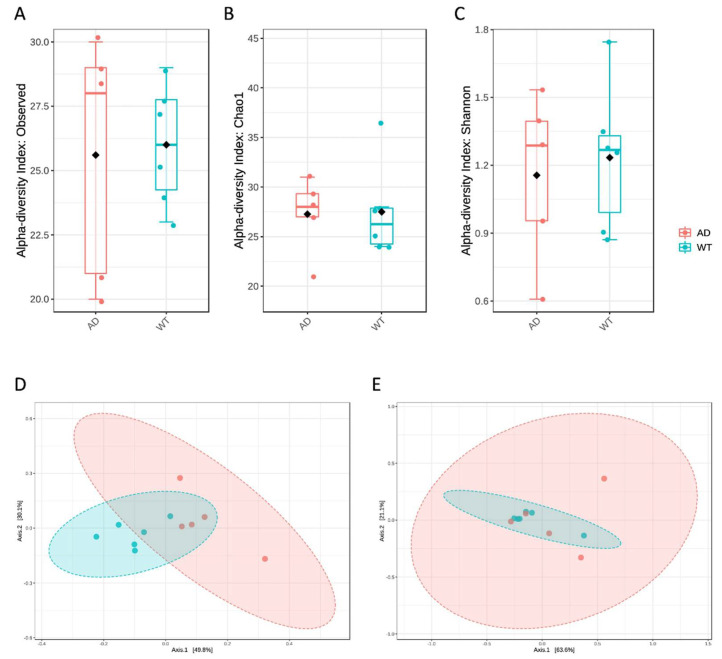
First, diversity measures were obtained to evaluate both the within- and between-group variabilities (Figure 1). In particular, both richness (Figure 1A,B) and evenness (Figure 1C) did not highlight significant differences between the tested conditions, also using different metrics. However, β diversity, as obtained by unweighted UniFrac distance measure, showed a significantly different microbial composition between AD and WT mice (p < 0.005, Figure 1D), even if not confirmed by using the weighted UniFrac distance metric (Figure 1E). As already reported elsewhere [17], unweighted and weighted UniFrac measures are respectively a quality-based and a quantity-based index. Thus, the result reported herein may indicate that the variation between the two compared groups is not due to the taxa abundances but rather to the type of taxa present in the microbiome.
Figure 1.
Diversity measures of the bacterial taxa identified in the AD and WT mice. Alpha diversity was calculated using several metrics to measure the within-group diversity and bacterial richness. In particular, observed species (A), Chao1 (B) and Shannon (C) indices were obtained showing no significant differences between the 2 tested conditions. β diversity was also measured using the unweighted (D) and weighted (E) UniFrac distance measures. Statistical significance was assessed by PERMANOVA test, resulting in significance (p < 0.005) only in the case of unweighted UniFrac.
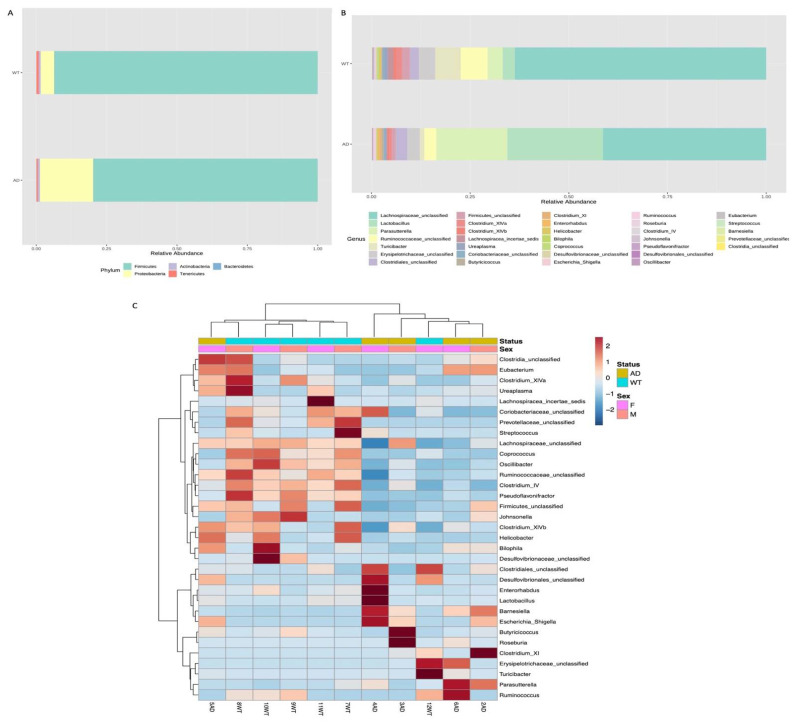
Taxonomic assignment highlighted the presence of a total of five phyla, Firmicutes and Proteobacteria being the most abundant in both groups. However, we observed a reduction of Firmicutes (from 93.8% to 79.8%) and an increase of Proteobacteria (from 4.7% to 18.8%) relative abundances in the AD respect to the WT mice (Figure 2A).
Figure 2.
Bacterial composition of AD and WT mice gut microbiome. Taxonomic assignment is shown at phylum (A) and genus (B) level. For each of these taxonomic levels, the barplots show the relative abundance (%) of each identified taxon/group. A different bacterial composition is highlighted both at phylum and at genus level. A heatmap of variance was obtained by grouping the reads based on the assigned taxa: a good cluster was obtained at genus level showing a clear separation between AD and WT mice (C).
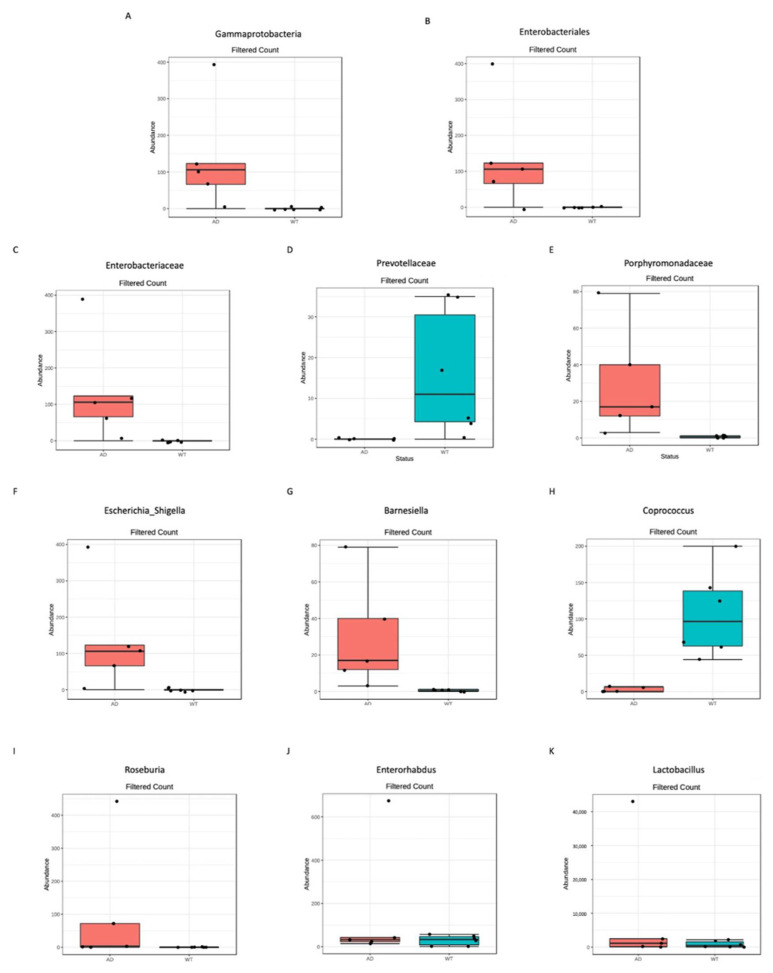
At the genus level, we found in the AD mice an increased abundance of Lactobacillus (from 0.3% to 24%) and Parasutterella (from 3.8% to 17.9%) and a reduced abundance of Lachnospiraceae_unclassified (from 63.7% to 41.3%), Ruminococcaceae_unclassified (from 6.8% to 3%) and Turicibacter (from 6.4% to 1%) genera (Figure 2B). Next, a clustering analysis was carried out to verify if these observed different taxonomic abundances are able to cluster the analyzed samples: as shown in Figure 2C, at the genus level, samples from the same group are more similar each to the others than samples from the other group, highlighting also differential abundance patterns between AD and WT mice. Thus, to verify the presence of statistically significant differences between the two study groups differential abundance analysis was performed. No significant taxa were identified at the phylum level; however, we found one class, one order, three families and six genera significantly different between AD and WT mice (Figure 3).
Figure 3.
Significantly different taxa identified between AD and WT mice. Differential abundance analysis was performed to highlight significantly different taxa using DESeq2, p-value < 0.05 after FDR correction; 1 class (A), 1 order (B), 3 families (C–E), and 6 genera (F–K) resulted in significant differences between the 2 tested conditions.
In particular, among the differentially abundant taxa, the Gammaproteobateria class (Figure 3A), the Enterobacteriales order (belonging to Gammaproteobateria class, Figure 3B), the Enterobacteriaceae family (belonging to Enterobacteriales order, Figure 3C) and the Escherichia_Shigella genus (belonging to Enterobacteriaceae family, Figure 3F) were significantly more abundant in the AD respect to the WT mice. Moreover, other two families, both belonging to the Bacteroides phylum, were significantly different, with the Prevotellaceae family being more abundant in the WT mice (Figure 3D) and the Porphiromonadaceae family in the AD (Figure 3E). Finally, at the genus level, the other five genera were significantly different between the two study groups: the Barnesiella (belonging to Bacteroides phylum), the Roseburia and the Lactobacillus (both belonging to Firmicutes phylum) and the Enterorhabdus (belonging to Actinobacteria phylum) genera being more abundant in the AD mice, while the Coprococcus genus (belonging to Firmicutes phylum) was less abundant in the AD mice with respect to the WT.
Finally, linear discriminant analysis (LDA) effect size (LEfSe) showed that at the genus level two taxa are most likely to explain the differences between the two study groups, i.e., the Barnesiella and the Coprococcus genera, respectively, were more abundant in the AD and in the WT mice.
3.2. Mycobiome Composition Analysis
The fungal contribution to gut microbiota was also investigated in the same study groups. A total of 469 OTUs was identified with and average reads/sample of 95,194.
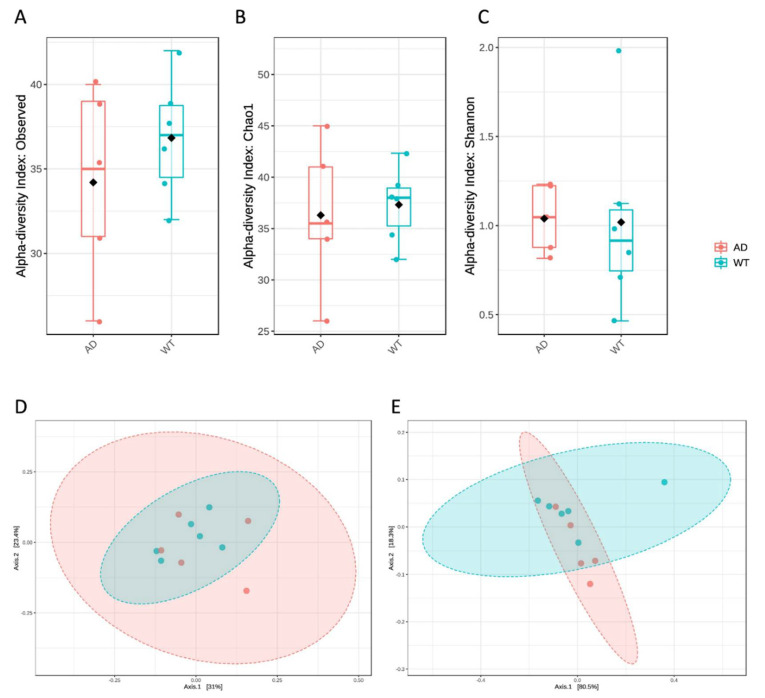
Diversity indices were measured showing no significant differences between AD and WT mice (Figure 4), even if it is possible to observe a trend in the reduction of richness in the AD mice (Figure 4A,B), while evenness seems to increase (Figure 4C).
Figure 4.
Diversity measures of the fungal taxa identified in the AD and WT mice. α diversity was calculated using observed species (A), Chao1 (B) and Shannon (C) indices. No significant differences were highlighted between the 2 tested conditions. β diversity was also measured using the unweighted (D) and weighted (E) UniFrac distance measures; in this case also, no significant differences were observed.
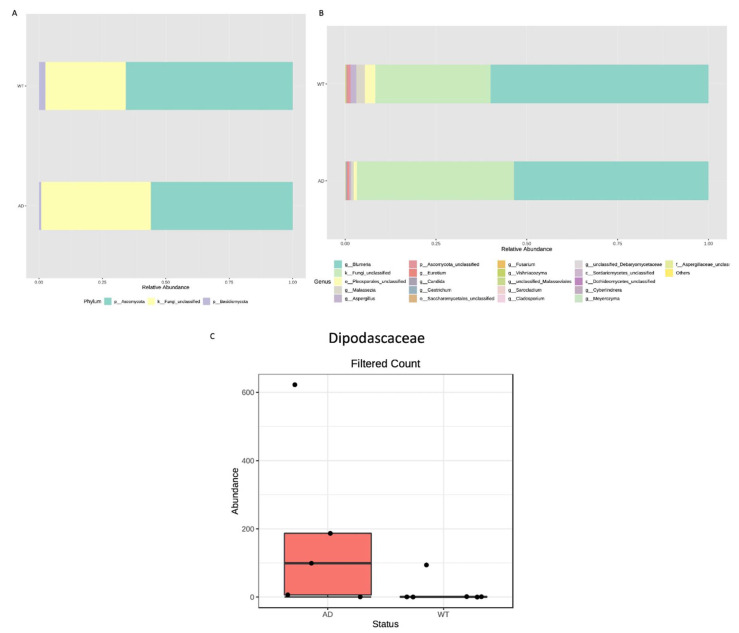
Taxonomic assignment allowed the identification of two phyla, Basidiomycota and Ascomycota, both being more abundant in the WT respect to the AD mice (Figure 5A). A different fungal composition is highlighted also in the other taxonomic ranks up to the genus level (Figure 5B). Thus, differential abundance analysis was performed to verify any significant difference. Only a family resulted in significant differences (p = 0.001): the Dipodascaceae, which appears more abundant in the AD group (Figure 5C).
Figure 5.
Fungal microbiota composition of AD and WT mice. Taxonomic assignment is shown at phylum (A) and genus (B) levels as relative abundance (%) of each identified taxon/group. Differential abundance analysis highlighted just one significantly different taxon, the Dipodascaceae family, which appears increased in the AD compared with the WT mice (C).
4. Discussion
Gut microbiota has emerged as an important factor contributing to AD pathogenesis [30,31]. This observation has prompted the research in the field due to the potential of using microbiota features for diagnostic purposes and, mainly, as actionable targets of specific therapies. In this context, the use of in vivo models resembling AD has shown its efficacy in evaluating the effects of different therapies on cognitive functions and AD pathogenesis. Here, we describe the bacterial and fungal gut microbiota content of 3xTg-AD mice with respect to WT mice in order to verify the presence of specific signatures related to the disease status. Interestingly, our data suggest that microbiota alterations are mainly related to specific taxa rather than quantitative differences. This is highlighted not only by diversity indices but also by clustering analysis showing bacterial communities more similar within- than between-group variation. Taxonomic analysis showed a reduction of Firmicutes phylum in the AD mice. Even if this difference was not statistically significant, the finding is also in line with previous report in humans [7]. In particular, the reduction of the Firmicutes phylum has been described in several diseases, including diabetes and obesity [32,33]. Since glucose metabolism alterations, such as insulin resistance, have been associated to an increased risk of developing AD, it has been hypothesized that gut dysbiosis may promote AD progression by inducing insulin resistance [7]. Interestingly, in our study, the Coprococcus genus, within the Firmicutes phylum, was significantly less abundant in the AD respect to the WT mice and this was one of the two bacterial features able to explain the difference between the two compared groups. Coprococcus is usually considered as an anti-inflammatory bacterium, and its abundance has been recently reported as reduced in seven different brain-related diseases, including AD (specifically, attention deficit hyperactivity disorder, autism spectrum disorder, schizophrenia, Alzheimer’s disease, Parkinson’s disease, major depressive disorder and bipolar disorder) [34]. In addition, the Coprococcus genus is involved in carbohydrate fermentation and short-chain fatty acid (SCFAs) production [35]. A reduction of SCFAs production is able to induce inflammation, leaky gut and microglial activation and has been consequently related to the pathogenesis of human diseases [31,36,37] Thus, the Coprococcus reduction we observed in the AD mice may play a role in disease-related dysbiosis and in the development of the typical pathogenetic clues.
On the contrary, we highlighted an increase of the phylum Proteobacteria in AD respect to WT mice with a significant increase, at the genus level, of the Escherichia_Shigella taxon. Similarly, we also reported significant changes of taxa belonging to the Bacteroidetes phylum including, in the AD mice, the increase of the Barnesiella genus. These genera, as the other members of both Proteobacteria and Bacteroidetes phyla, are Gram-negative bacteria having the lipopolysaccharide (LPS) as a major component of their outer membrane. LPS proinflammatory properties have been well established [38], and, accordingly, the increase of Bacteroidetes and/or Proteobacteria phyla has been found in different diseases, including AD [7,32,39]. It has to be mentioned that LPS has been reported as a factor able to promote amyloid deposition and tau-related pathology [40,41]. Moreover, LPS and Escherichia coli (E. coli) fragments have been found in post-mortem brain tissues from AD patients as co-localizers of amyloid plaques [42]. E. coli has been also reported as one of the bacterial strains able to produce amyloid [31,43]. This bacterial amyloid is able to trigger pro-inflammatory responses by recognizing the TLRs and by promoting additional amyloid and misfolded Aβ production, leaky gut and neuroinflammation [43,44,45]. Considering all the above, the significant increase of such Gram-negative genera we found in the gut microbiota of AD mice is compatible with increased LPS translocation into the blood and with pro-inflammatory activities able to promote AD.
In addition to bacterial content, we also investigated fungal fecal composition. Diversity analysis showed a reduction, even if not statistically significant, of fungal richness in AD respect to WT mice. Taxonomic assignment highlighted a different composition and just one significantly different taxon, i.e., the Dipodascaceae family, which is most represented in AD mice. Members of the Dipodascaceae family have been reported as potential colonizers of the human gut, even if their role is still to be clarified [46]. A study analyzing the gut mycobiome of obese subjects reported the increase of the Dipodascaceae family in the obese group and found also a positive correlation with adiposity, serum total cholesterol and fasting triglycerides [47]. Even if further studies are required to confirm this finding, since both glucose and lipids metabolisms are impaired in AD, it is conceivable to suppose the involvement of the Dipodascaceae family in these processes.
5. Conclusions
Taken together, our data show the presence of a different microbiome and mycobiome composition in 3xTg-AD respect to WT mice. In particular, significantly different taxa potentially contributing to AD pathogenetic clues were identified. Even if further studies are required to move towards a more mechanistic interpretation, our results suggest possible key microbial taxa of AD-related dysbiosis potentially contributing to the disease. In addition, most metagenomic studies to date focus just on bacterial content, while gut communities are more complex ecosystems. Here, we studied also the fungal composition highlighting a significantly different abundance of the Dipodascaceae family. This reinforces the hypothesis that several microbial alterations participate to metabolic and pro-inflammatory imbalances that, consequently, can enhance the development of pathological conditions. Finally, since the microbial alterations found in the 3xTg-AD model resemble those found also in humans, we assessed it as an effective model to study AD microbiome and to test possible therapeutic interventions based on microbiota manipulation.
Author Contributions
Conceptualization, A.M.E. and V.D.; methodology, V.D., I.V., C.G., V.C. and L.B.; formal analysis, V.D., I.V. and L.B.; data curation, V.D. and A.M.E.; writing—original draft preparation, V.D.; writing—review and editing, V.D., L.B., V.C. and A.M.E.; supervision and project administration, A.M.E. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Italian Ministry of Health (1D580.28, 625/2020-PR).
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.D’Argenio V. Human Microbiome Acquisition and Bioinformatic Challenges in Metagenomic Studies. Int. J. Mol. Sci. 2018;19:383. doi: 10.3390/ijms19020383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Erny D., de Angelis A.L.H., Jaitin D., Wieghofer P., Staszewski O., David E., Keren-Shaul H., Mahlakoiv T., Jakobshagen K., Buch T., et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015;18:965–977. doi: 10.1038/nn.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Braniste V., Al-Asmakh M., Kowal C., Anuar F., Abbaspour A., Tóth M., Korecka A., Bakocevic N., Ng L.G., Kundu P., et al. The gut microbiota influences blood-brain barrier permeability in mice. Sci. Transl. Med. 2014;6:263ra158. doi: 10.1126/scitranslmed.3009759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Strandwitz P. Neurotransmitter modulation by the gut microbiota. Brain Res. 2018;1693 Pt B:128–133. doi: 10.1016/j.brainres.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.D’Argenio V., Sarnataro D. Microbiome Influence in the Pathogenesis of Prion and Alzheimer’s Diseases. Int. J. Mol. Sci. 2019;20:4704. doi: 10.3390/ijms20194704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu P., Wu L., Peng G., Han Y., Tang R., Ge J., Zhang L., Jia L., Yue S., Zhou K., et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019;80:633–643. doi: 10.1016/j.bbi.2019.05.008. [DOI] [PubMed] [Google Scholar]
- 7.Vogt N.M., Kerby R.L., Dill-McFarland K.A., Harding S.J., Merluzzi A.P., Johnson S.C., Carlsson C.M., Asthana S., Zetterberg H., Blennow K., et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017;7:13537. doi: 10.1038/s41598-017-13601-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.D’Argenio V., Sarnataro D. Probiotics, prebiotics and their role in Alzheimer’s disease. Neural. Regen. Res. 2021;16:1768–1769. doi: 10.4103/1673-5374.306072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cani P.D., Bibiloni R., Knauf C., Waget A., Neyrinck A.M., Delzenne N.M., Burcelin R. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–1481. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 10.Xiong J., Deng I., Kelliny S., Lin L., Bobrovskaya L., Zhou X.F. Long term high fat diet induces metabolic disorders and aggravates behavioral disorders and cognitive deficits in MAPT P301L transgenic mice. Metab. Brain Dis. 2022;37:1941–1957. doi: 10.1007/s11011-022-01029-x. [DOI] [PubMed] [Google Scholar]
- 11.Kaur H., Golovko S., Golovko M.Y., Singh S., Darland D.C., Combs C.K. Effects of Probiotic Supplementation on Short Chain Fatty Acids in the AppNL-G-F Mouse Model of Alzheimer’s Disease. J. Alzheimers Dis. 2020;76:1083–1102. doi: 10.3233/JAD-200436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Webberley T.S., Masetti G., Bevan R.J., Kerry-Smith J., Jack A.A., Michael D.R., Thomas S., Glymenaki M., Li J., McDonald J.A.K., et al. The Impact of Probiotic Supplementation on Cognitive, Pathological and Metabolic Markers in a Transgenic Mouse Model of Alzheimer’s Disease. Front. Neurosci. 2022;16:843105. doi: 10.3389/fnins.2022.843105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bonfili L., Cecarini V., Berardi S., Scarpona S., Suchodolski J.S., Nasuti C., Fiorini D., Boarelli M.C., Rossi G., Eleuteri A.M. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 2017;7:2426. doi: 10.1038/s41598-017-02587-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bonfili L., Cecarini V., Cuccioloni M., Angeletti M., Berardi S., Scarpona S., Rossi G., Eleuteri A.M. SLAB51 probiotic formulation activates SIRT1 pathway promoting antioxidant and neuroprotective effects in an AD mouse model. Mol. Neurobiol. 2018;55:7987–8000. doi: 10.1007/s12035-018-0973-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bonfili L., Cecarini V., Gogoi O., Berardi S., Scarpona S., Angeletti M., Rossi G., Eleuteri A.M. Gut microbiota manipulation through probiotics oral administration restores glucose homeostasis in a mouse model of Alzheimer’s disease. Neurobiol. Aging. 2020;87:35–43. doi: 10.1016/j.neurobiolaging.2019.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Bonfili L., Cuccioloni M., Gong C., Cecarini V., Spina M., Zheng Y., Angeletti M., Eleuteri A.M. Gut microbiota modulation in Alzheimer’s disease: Focus on lipid metabolism. Clin. Nutr. 2022;41:698–708. doi: 10.1016/j.clnu.2022.01.025. [DOI] [PubMed] [Google Scholar]
- 17.Cecarini V., Gogoi O., Bonfili L., Veneruso I., Pacinelli G., De Carlo S., Benvenuti F., D’Argenio V., Angeletti M., Cannella N., et al. Modulation of Gut Microbiota and Neuroprotective Effect of a Yeast-Enriched Beer. Nutrients. 2022;14:2380. doi: 10.3390/nu14122380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oddo S., Caccamo A., Shepherd J.D., Murphy M.P., Golde T.E., Kayed R., Metherate R., Mattson M.P., Akbari Y., LaFerla F.M. Triple-transgenic model of Alzheimer’s disease with plaques and tangles: Intracellular Abeta and synaptic dysfunction. Neuron. 2003;39:409–421. doi: 10.1016/S0896-6273(03)00434-3. [DOI] [PubMed] [Google Scholar]
- 19.Cossiga V., Lembo V., Nigro C., Mirra P., Miele C., D’Argenio V., Leone A., Mazzone G., Veneruso I., Guido M., et al. The Combination of Berberine, Tocotrienols and Coffee Extracts Improves Metabolic Profile and Liver Steatosis by the Modulation of Gut Microbiota and Hepatic miR-122 and miR-34a Expression in Mice. Nutrients. 2021;13:1281. doi: 10.3390/nu13041281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nardelli C., Granata I., D’Argenio V., Tramontano S., Compare D., Guarracino M.R., Nardone G., Pilone V., Sacchetti L. Characterization of the Duodenal Mucosal Microbiome in Obese Adult Subjects by 16S rRNA Sequencing. Microorganisms. 2020;8:485. doi: 10.3390/microorganisms8040485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Andrews S. FastQC: A Quality Control Tool for High Throughput Sequence Data. 2019. [(accessed on 1 August 2022)]. Available online: https://www.bioinformatics.babraham.ac.uk/projects/fastqc/
- 22.Schloss P.D., Westcott S.L., Ryabin T., Hall J.R., Hartmann M., Hollister E.B., Lesniewski R.A., Oakley B.B., Parks D.H., Robinson C.J., et al. Introducing mothur: Open-source, platform-independent, community-supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 2009;75:7537–7541. doi: 10.1128/AEM.01541-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pruesse E., Quast C., Knittel K., Fuchs B.M., Ludwig W., Peplies J., Glockner F.O. SILVA: A comprehensive online resource for quality checked and aligned ribosomal RNA sequence data compatible with ARB. Nucleic Acids Res. 2007;35:7188–7196. doi: 10.1093/nar/gkm864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nilsson R.H., Larsson K.H., Taylor A.F.S., Bengtsson-Palme J., Jeppesen T.S., Schigel D., Kennedy P., Picard K., Glockner F.O., Tedersoo L., et al. The UNITE database for molecular identification of fungi: Handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 2019;47:D259–D264. doi: 10.1093/nar/gky1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McMurdie P.J., Holmes S. phyloseq: An R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE. 2013;8:e61217. doi: 10.1371/journal.pone.0061217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lahti L., Shetty S. Tools for Microbiome Analysis in R. 2017. [(accessed on 26 August 2022)]. Available online: https://bioconductor.org/packages/release/bioc/html/microbiome.html.
- 27.Callahan B.J., Sankaran K., Fukuyama J.A., McMurdie P.J., Holmes S.P. Bioconductor Workflow for Microbiome Data Analysis: From raw reads to community analyses. F1000Res. 2016;5:1492. doi: 10.12688/f1000research.8986.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McHardy A.C., McMurdie P.J., Holmes S. Waste Not, Want Not: Why Rarefying Microbiome Data Is Inadmissible. PLoS Computat. Biol. 2014;10:e1003531. doi: 10.1371/journal.pcbi.1003531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dhariwal A., Chong J., Habib S., King I.L., Agellon L.B., Xia J. MicrobiomeAnalyst: A web-based tool for comprehensive statistical, visual and meta-analysis of microbiome data. Nucleic Acids Res. 2017;45:W180–W188. doi: 10.1093/nar/gkx295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cryan J.F., O’Riordan K.J., Cowan C.S., Sandhu K.V., Bastiaanssen T.F., Boehme M., Codagnone M.G., Cussotto S., Fulling C., Golubeva A.V., et al. The Microbiota-Gut-Brain Axis. Physiol. Rev. 2019;99:1877–2013. doi: 10.1152/physrev.00018.2018. [DOI] [PubMed] [Google Scholar]
- 31.Tran S.M.-S., Mohajeri M.H. The Role of Gut Bacterial Metabolites in Brain Development, Aging and Disease. Nutrients. 2021;13:732. doi: 10.3390/nu13030732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsen N., Vogensen F.K., van den Berg F.W., Nielsen D.S., Andreasen A.S., Pedersen B.K., Al-Soud W.A., Sørensen S.J., Hansen L.H., Jakobsen M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwiertz A., Taras D., Schäfer K., Beijer S., Bos N.A., Donus C., Hardt P.D. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 34.Eicher T.P., Mohajeri M.H. Overlapping Mechanisms of Action of Brain-Active Bacteria and Bacterial Metabolites in the Pathogenesis of Common Brain Diseases. Nutrients. 2022;14:2661. doi: 10.3390/nu14132661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Peralta-Marzal L.N., Prince N., Bajic D., Roussin L., Naudon L., Rabot S., Garssen J., Kraneveld A.D., Perez-Pardo P. The Impact of Gut Microbiota-Derived Metabolites in Autism Spectrum Disorders. Int. J. Mol. Sci. 2021;22:10052. doi: 10.3390/ijms221810052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zheng S.Y., Li H.X., Xu R.C., Miao W.T., Dai M.Y., Ding S.T., Liu H.D. Potential roles of gut microbiota and microbial metabolites in Parkinson’s disease. Ageing Res. Rev. 2021;69:101347. doi: 10.1016/j.arr.2021.101347. [DOI] [PubMed] [Google Scholar]
- 37.Sochocka M., Donskow-Łysoniewska K., Diniz B.S., Kurpas D., Brzozowska E., Leszek J. The Gut Microbiome Alterations and Inflammation-Driven Pathogenesis of Alzheimer’s Disease—A Critical Review. Mol. Neurobiol. 2019;56:1841–1851. doi: 10.1007/s12035-018-1188-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cani P.D., Amar J., Iglesias M.A., Poggi M., Knauf C., Bastelica D., Neyrinck A.M., Fava F., Tuohy K.M., Chabo C., et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–1772. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 39.Keshavarzian A., Green S.J., Engen P.A., Voigt R.M., Naqib A., Forsyth C.B., Mutlu E., Shannon K.M. Colonic bacterial composition in Parkinson’s disease. Mov. Disord. 2015;30:1351–1360. doi: 10.1002/mds.26307. [DOI] [PubMed] [Google Scholar]
- 40.Kahn M.S., Kranjac D., Alonzo C.A., Haase J.H., Cedillos R.O., McLinden K.A., Boehm G.W., Chumley M.J. Prolonged elevation in hippocampal Aβ and cognitive deficits following repeated endotoxin exposure in the mouse. Behav. Brain Res. 2012;229:176–184. doi: 10.1016/j.bbr.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 41.Sy M., Kitazawa M., Medeiros R., Whitman L., Cheng D., Lane T.E., Laferla F.M. Inflammation induced by infection potentiates tau pathological features in transgenic mice. Am. J. Pathol. 2011;178:2811–2822. doi: 10.1016/j.ajpath.2011.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhan X., Stamova B., Jin L.W., DeCarli C., Phinney B., Sharp F.R. Gram-negative bacterial molecules associate with Alzheimer disease pathology. Neurology. 2016;87:2324–2332. doi: 10.1212/WNL.0000000000003391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Megur A., Baltriukienė D., Bukelskienė V., Burokas A. The Microbiota-Gut-Brain Axis and Alzheimer’s Disease: Neuroinflammation Is to Blame? Nutrients. 2020;13:37. doi: 10.3390/nu13010037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kesika P., Suganthy N., Sivamaruthi B.S., Chaiyasut C. Role of gut-brain axis, gut microbial composition, and probiotic intervention in Alzheimer’s disease. Life Sci. 2021;264:118627. doi: 10.1016/j.lfs.2020.118627. [DOI] [PubMed] [Google Scholar]
- 45.Jiang C., Li G., Huang P., Liu Z., Bin Zhao B. The Gut Microbiota and Alzheimer’s Disease. J. Alzheimer’s Dis. 2017;58:1–15. doi: 10.3233/JAD-161141. [DOI] [PubMed] [Google Scholar]
- 46.Hallen-Adams H.E., Suhr M.J. Fungi in the healthy human gastrointestinal tract. Virulence. 2017;8:352–358. doi: 10.1080/21505594.2016.1247140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mar Rodríguez M., Pérez D., Javier Chaves F., Esteve E., Marin-Garcia P., Xifra G., Vendrell J., Jové M., Pamplona R., Ricart W., et al. Obesity changes the human gut mycobiome. Sci. Rep. 2015;5:14600. doi: 10.1038/srep14600. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are contained within the article.