Abstract
Background: Alcohol use disorder (AUD) is a condition prevalent in many countries around the world, and the public burden of its treatment is close to $130 billion. mHealth offers several possible interventions to assist in the treatment of AUD. Objectives: To analyze the effectiveness of mHealth and wearable sensors to manage AUD from evidence published over the last 10 years. Methods: Following the Kruse Protocol and PRISMA 2020, four databases were queried (PubMed, CINAHL, Web of Science, and Science Direct) to identify studies with strong methodologies (n = 25). Results: Five interventions were identified, and 20/25 were effective at reducing alcohol consumption. Other interventions reported a decrease in depression and an increase in medication compliance. Primary barriers to the adoption of mHealth interventions are a requirement to train users, some are equally as effective as the traditional means of treatment, cost, and computer literacy. Conclusion: While not all mHealth interventions demonstrated statistically significant reduction in alcohol consumption, most are still clinically effective to treat AUD and provide a patient with their preference of a technologically inclined treatment Most interventions require training of users and some technology literacy, the barriers identified were very few compared with the litany of positive results.
Keywords: substance use disorder, alcohol use disorder, wearable sensors, mHealth, eHealth, telemedicine
1. Rationale
Alcohol use disorder (AUD) is characterized by the inability to stop or control alcohol use despite social, occupational, or health consequences [1]. Approximately 85.6% of people aged 18 years and older in the U.S. reported they drank alcohol, 69.5% reported they drank in the last year, and 54.9% reported they drank in the last month. In a survey of primary care providers in the European Union, AUDs were prevalent in 11.8% of the population, which is 1.6 times the population estimate [2]. AUD is specifically attributed to 735,153 deaths in 2019, but indirectly associated with 7,599,264 when alcohol-related deaths are taken into consideration [3]. In the U.S., AUD is associated with $120 billion per year in medical costs in the US, and $7.6 billion in the EU [3,4].
Telemedicine is defined by the World Health Organization (WHO) as healing at a distance through information and communication technologies (ICT) [5]. Telemedicine provides clinical support, overcomes geographical boundaries, involves ICT, and has a goal to improve health outcomes. Telemedicine comes in many forms, but wearable sensors can be connected to apps on mobile devices. When these wearable sensors provide clinical data to providers, this falls under the scope of mHealth.
Treatments for AUD can be both inpatient and outpatient, and they often must be tailored to the individual [1]. Wearable sensors have the ability to observe behavior and physiological constructs and combine them with location tracking. Tracking gait and sweat can provide feedback on abstinence and intoxication [6,7]. The geographic location can provide pre-programmed text messages to warn against danger areas (proximity to establishments that sell alcohol) [8].
In general, a systematic literature review is conducted to summarize recent science on a particular subject. A continuous growth of research combined with the rapid growth of technology compels scientists to systematically summarize available research and synthesize evidence. These products form the basis for funded research, and they can provide a foundation for modifying evidence based practice. As of the writing of this systematic review, 13 funded grant opportunities exist in the area of alcohol use disorder in the USA alone. Technology often serves as a fulcrum of change, and many mHealth solutions exist to help manage alcohol use disorder. A systematic literature review at the intersection of mHealth and the treatment of alcohol use disorder seemed timely. A systematic review in 2020 analyzed 32 articles over a 5-year period [9]. This study found half of the interventions reported improvements in at least one outcome (reduced cravings, or alcohol use). Only two of the interventions utilized wearable sensors. The remainder were feedback apps for craving management, coping assistance, and tailored feedback [9].
Another systematic review published in 2020 analyzed 22 articles over 10 years [10]. The study team found that most interventions resulted in a positive outcome (reduced depression, increased satisfaction, increased accessibility, increase quality of life, and decreased cost. Interventions included mobile health apps, eHealth (computer programs), telephone intervention, and 2-way video [10].
2. Objectives
The purpose of this review is to analyze the effectiveness of mHealth and wearable sensors to manage AUD, compared with the outcomes of the same conditions under traditional, face-to-face (in person) treatment, from evidence published in peer-reviewed and indexed journals over the last ten years. Effectiveness will be measured as improvements in AUD cravings, decrease in alcohol consumption, and a positive rating in patient satisfaction.
3. Methods
3.1. Eligibility Criteria
Articles for analysis were published in the last 10 years in peer-reviewed academic journals, and published in the English language. They must include participants who are adults (18 years of age or older). Preferred methods were true experiments (RCT, etc.), but quasi-experimental, non-experimental, and qualitative studies were also accepted. Other systematic reviews were not accepted so as not to confound the results. Works that did not mention wearable sensors or mHealth to treat AUD were excluded. Studies with participants under age 18 were excluded. Studies that did not report results were excluded.
3.2. Information Sources
Four data sources were queried: PubMed (MEDLINE), Cumulative Index of Nursing and Allied Health Literature (CINAHL), Web of Science, and Science Direct and a focused journal search in the Journal of Addictive Medicine. These databases were chosen because they are well known, exhaustive, and easily accessible by those who want to duplicate the research. MEDLINE was excluded from all searches except PubMed. Searches were conducted on 8 January 2022.
3.3. Search Strategy
Our study team used the Medical Subject Heading (MeSH) feature of the National Library of Medicine to create a Boolean search string that combined key index terms: (mhealth OR telemedicine OR “mobile app” OR biosensors) AND (“alcohol use disorder” OR “AUD”). We used the same search string in all databases and the focused journal search. As close as databases would allow, we used the same filter strategies.
3.4. Selection Process
We used the Boolean search string in all databases, filtered the results, and screened the abstracts for applicability, in accordance with the Kruse Protocol [11]. The Kruse Protocol defines a systematic methodology to conduct an exhaustive summary of evidence and report in accordance with the PRISMA standard. Studies were removed that did not address the objective statement.
3.5. Data Collection Process
We used a standardized Microsoft Excel spreadsheet as a data extraction tool collecting additional data fields at each step. The Kruse Protocol standardized the spreadsheet. We used a series of three consensus meetings to confirm the group of studies for analysis, conduct the thematic analysis, and perform additional analysis [12]. Abstracts were screened and studies were analyzed by at a minimum two reviewers.
3.6. Data Items
The Kruse Protocol dictated we collect the following fields of data at each step: DB Source, Date of publication, author names, title, participants, experimental intervention, results, medical outcomes, study design, sample size, bias within study, effect size (Cohen d), sensitivity, specificity, F1, country of origin, statistics uses, patient satisfaction, barriers to adoption, strength of evidence and quality of evidence.
3.7. Study Risk of Bias Assessment
Each reviewer noted observed bias and assessed the quality of each study using the Johns Hopkins Nursing Evidence Based Practice tool (JHNEBP) [13]. This was done because bias can limit the external validity of studies [14].
3.8. Effect Measures
This study included both qualitative and quantitative studies. Due to the fact that we accepted this range of methodology, we were unable to standardize summary measures, as would be performed in a meta-analysis. Measures of effect are summarized in tables for those studies in which it was reported.
3.9. Synthesis Methods
This subheading is for meta-analyses—NOT for systematic reviews. It will be removed by the editor prior to publication.
3.10. Reporting Bias Assessment
The overall ratings of strength and quality from the JHNEBP tool provided an assessment of the applicability of the cumulative evidence. Observations of bias were discussed for their implications on their reported results.
3.11. Additional Analyses and Certainty Assessment
We performed a narrative or thematic analysis of the observations to convert observations into themes (an observation that occurred multiple times became a theme) [12]. We calculated a frequency of occurrence and report this in an affinity matrix. Reporting the frequency provided confidence in the data analyzed.
4. Results
4.1. Study Selection
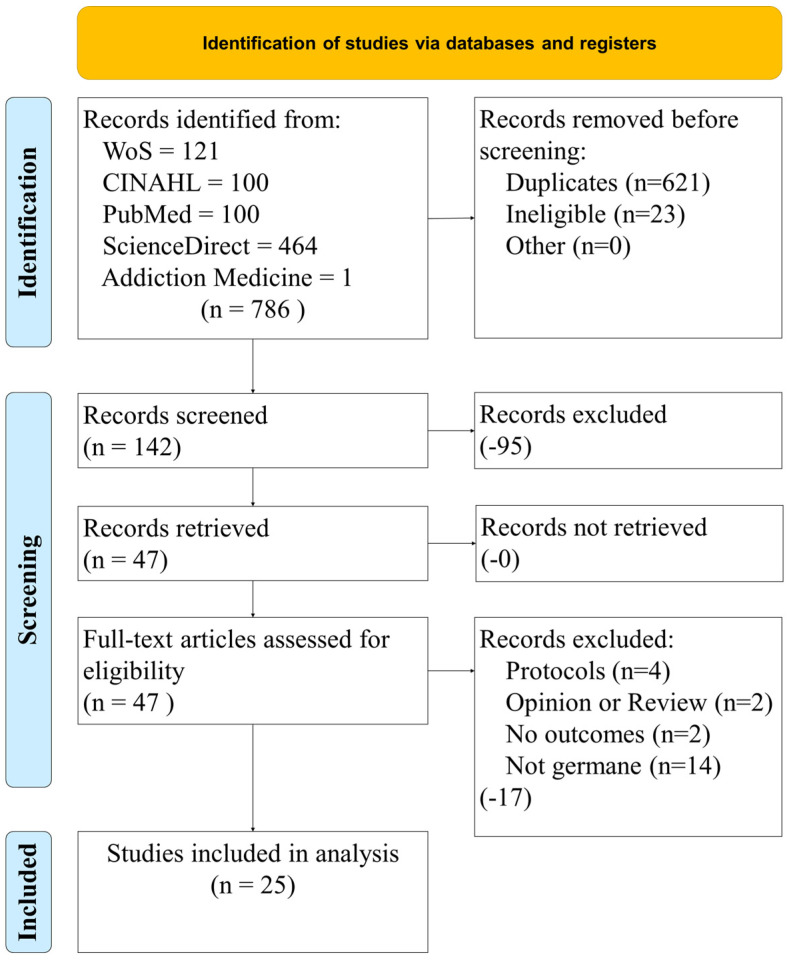
Figure 1 illustrates the study selection process from the four databases and one targeted journal search. Using established methods, we calculated a kappa statistic (k = 0.96, almost perfect agreement) [15,16]. Figure 1 illustrates the initial search results of 786 and how we filtered and screened these down to the group for analysis (n = 25).
Figure 1.
Study selection process.
4.2. Study Characteristics
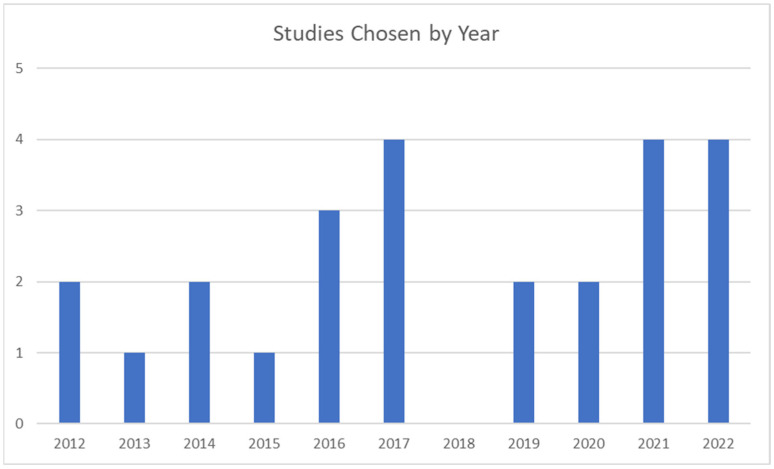
Following PRISMA 2020 guidance, we created a PICOS table to tabulate the participants, intervention, results, medical outcomes, and study design for each study analyzed. In the 25 studies analyzed, all used adults as participants, and the experimental intervention was some form of mHealth. Of the 25 studies, 14/25 (56%) used an mHealth app, 4/25 (16%) used telephone or interactive voice response, 3/25 (12%) used mHealth serious games or cognitive training delivered on mobile devices, and four studies used either mHealth SMS or mHealth + telephone (2/26 each, 8%). Of the 25 studies analyzed over a 10-year period, 2 were from 2012 [17,18], 1 was from 2013 [19], 2 were from 2014 [20,21], 1 was from 2015 [22], 3 were from 2016 [23,24,25], 4 were from 2017 [26,27,28,29], 2 were from 2019 [30,31], and 2020 [32,33], 4 were from 2021 [34,35,36,37], and 2022 [38,39,40,41]. Zero studies were from 2018. A graphical display of this evolution of studies is illustrated in Figure 2.
Figure 2.
Evolution of studies chosen for analysis.
Table 1 summarizes the study characteristics. From the 25 studies analyzed, 14/25 (56%) were randomized controlled trials, 4/26 (16%) were true experiments, 2/26 (8%) were non-experimental and 2 were mixed-methods, and 2 were observational, and 1/26 (4%) was qualitative. Results showed a reduction in consumption in 15/31 (45%) results themes, but also no significant difference in treatment outcomes in 5/31 (16%) results themes. For multiple interventions the no-difference variable brings into question whether organizations should expend the energy and expense to train users and implement the intervention.
Table 1.
PICOS.
| Authors | Participants | Experimental Intervention | Results | Medical Outcomes Reported | Study Design (See the List Below) |
|---|---|---|---|---|---|
| McTavish et al. [17] | Adults, average age 38.3, 60.6% male, 82.9% Caucasian | mHealth app to control SUD and AUD | 94% used the app 1st week, and 80% used continued to use at week 16 | Participants with AUD will use an app to manage their condition, App decreased cravings | True experiment |
| Murray et al. [18] | Adults ≥ 18, affluent area outside of London | mHealth app Down Your Drink (DYD) | No control group. Of those still using the app at 12 month, the reduction in drinking was 35 units | Reduction in consumption, reduction in AUD identification test, reduction in Leeds Dependence Questionnaire (LDQ, dependence), no significant change in Clinical Outcomes in Routine Evaluation (CORE-10, mental health) or EQ-5D (quality of life). | Mixed-Methods |
| Morgan et al. [19] | Adults ≥ 18 | mHealth Internet-based app | Internet-based recruitment to mental health interventions is feasible | Improved rates of depression using intervention | RCT |
| Chih et al. [20] | Adults ≥ 18, average age 38, 62% male, 83% Caucasian | mHealth (A-CHESS) and BN | No control group. Responses to weekly check-in on A-CHESS can be a predictor of relapse | The prediction of lapse in sobriety gives counselors the chance to intervene through text, email, or phone call | Qualitative |
| Kalapatapu et al. [21] | Adults ≥ 18, average age 43.7, 87% female | telephone | Face-to-face cognitive based therapy (CBT) and T-CBT groups were similar on all treatment adherence outcomes and depression outcomes at all time points | telehealth means of treating is equally as effective as traditional therapy | True experiment |
| Stoner et al. [22] | Adults ≥ 18 (22–55), average age 37.5, 34.5% female | mHealth SMS | Adequate adherence ≥80% at week 8, not statistically significant between groups | SMS messages do not improve medication adherence, but equally as effective as traditional treatment to reduce consumption | RCT |
| Bock et al. [23] | Adults ≥ 18, average age 22, 61.3% female | mHealth SMS, text-message alcohol program (TMAP) | At week 6–12, TMAP participants less likely to report heavy drinking and negative alcohol consequences. Increased self-efficacy to resist drinking. | SMS effective at reducing consumption and increasing self-efficacy | True experiment |
| Freyer-Adam et al. [24] | Adults ≥ 18 (18–64) | mHealth Internet-based app | reached individuals and helped retain them in AUD programs | Not reported | RCT |
| Gamito et al. [25] | Adults ≥ 18, average age 45.45, 90% male | mHealth cognitive stimulation program (CSP) serious games | Cognitive ability between groups not statistically significant, but frontal-lobe function (Frontal Lobe Assessment, FAB) was significantly improved in the intervention group | Improvement in FAB with mHealth intervention | RCT |
| Barrio et al. [26] | Adults ≥ 18, average age 48, 50% female | mHealth app, SIDEAL | Reduced binge drinking and mean daily consumption, participants achieved their self-imposed objectives | Significant reduction in alcohol consumption | Non-experimental (no randomization, no control) |
| Gajecki et al. [27] | Adults ≥ 18, students, 66.7% female | mHealth app (skills training) | Reduced binge drinking and mean daily consumption, participants achieved their self-imposed objectives | Reduced alcohol consumption | RCT |
| Glass et al. [28] | Adults ≥ 18 | mHealth app (A-CHESS) | Intervention showed increased odds of outpatient addiction treatment across follow-ups, but not mutual help | Reduced alcohol consumption, increased treatment | RCT |
| Rose et al. [29] | Adults ≥ 18 | interactive voice response (IVR) brief intervention (BI) | Reduced alcohol consumption, but not statistically different than control | Reduced alcohol consumption | RCT |
| Jo et al. [30] | Adults ≥ 18 | mHealth (online-based brief empowerment for alcohol-use monitor, on-BEAM) | Intervention group reported consuming less alcohol during the past week and lower AUDIT score | Reduced alcohol consumption | RCT |
| Mellentin et al. [31] | Adults ≥ 18 | mHealth (cue exposure) | No differences were detected between the two experimental CET groups on any outcomes | Reduced alcohol consumption | RCT |
| Harder et al. [32] | Adults ≥ 18 | mHealth (motivational interviewing) | Average AUDIT scores were lower for the intervention group | Reduced alcohol consumption, increased self-efficacy | RCT |
| Hendershot et al. [33] | Adults ≥ 18 (21–55) | mHealth feedback, opioid receptor gene (OPRM1) | OPRM1 genotype moderated the association of daily adherence with reduced same-day consumption (p = 0.007) and craving (p = 0.06), with these associations being stronger for participants with the 118 G variant. OPRM1 genotype did not moderate changes in craving and consumption over time | high-density assessments and person-centered analytic approaches, including modeling within-person variation in medication adherence, could be advantageous for pharmacogenetic studies | Non-experimental (no randomization, no control) |
| Constant et al. [34] | Adults ≥ 18 | telephone | Study group had better alcohol abstinence rates than control | Intervention improves patient coping skills and motivation to modify alcohol use behaviors | RCT |
| Graser et al. [35] | Adults ≥ 18 (69% male) | telephone and smartphone-based intervention | Telephone-based intervention was more effective than text-based intervention | Sustained abstinence from excessive drinking occurred in telephone intervention group | RCT |
| Hammond et al. [36] | Adults ≥ 18 (61% male) | mHealth app | Intervention group utilized mobile app more effectively than control group | Complemented community substance use intervention programs | True Experiment |
| Manning et al. [37] | Adults ≥ 18 (58% female) | mHealth app | Intervention group reduced alcohol consumption rates | Improved alcohol consumption rates | Observational |
| Howe et al. [38] | Adults ≥ 18 (85% female; 62% Caucasian) | mHealth app | Use of mobile app improved decision making of study group participants | Mobile data collection can positively influence drinking decisions | Observational |
| Leightley et al. [39] | Adults > 18 (95% male; 100% Veterans) | mHealth app | Use of mobile app reduced rate of alcohol consumption among Veterans in study group | Reduced rates of alcohol consumption | RCT |
| McKay et al. [40] | Adults ≥ 18 (71% male; 82% African American) | telephone and smartphone-based intervention | Use of telephone or smartphone was effective in treating AUD | Improved rates of alcohol dependent persons | RCT |
| O’Grady et al. [41] | Adults ≥ 18 (Quant = 87% male/13% female; Qual = 43% male/57% female) | mHealth app | Provider-facing technology is effective alcohol intervention services and increased access to care in low- and middle-income countries. | Improved rates in alcohol dependent persons | Mixed Methods |
4.3. Risk of Bias within and across Studies
Because of the high number of RCTs and true experiments in the group of articles analyzed, the JHNEBP quality assessment tool identified 18/25 (72%) as Strength of Evidence I. Only 7/25 (28%) were classified as Strength of Evidence III. Similarly, the strong methodology, large sample sizes, and consistency of results caused the JHNEBP tool to identify 23/25 (92%) as Quality of Evidence A. Only 2/26 (8%) were classified as Quality of Evidence B.
Reviewers also made note of internal and external bias in the studies. All articles were conducted in either one or multiple regions of only one country, which is an indication of selection bias. This threatens the internal validity of the study. Furthermore, 10/25 (40%) observations of sample bias were identified because the sample used a disproportionate percentage of one gender or race. This form of bias threatens the external validity of the results.
4.4. Results of Individual Studies
Following the Kruse Protocol, reviewers recorded independent observations during data extraction. These observations were discussed in Consensus Meeting number two. Through the discussion of observations, a thematic analysis was performed to make sense of the data [12]. Reviewers identified themes and performed a second data extraction to ensure no themes were omitted. Table 2 tabulates the themes identified in the literature. Appendix A and Appendix B provide an observation-to-theme match. While there is some overlap between Results, Medical Outcomes, and Effectiveness, reviewers felt it was necessary to report them separately in order to highlight both similarities and differences between the studies. Appendix C provides the other observations made by reviewers (sample size, bias, effect size, country of origin, statistics used, and the JHNEBP observations of strength and quality of evidence).
Table 2.
Summary of analysis, sorted most chronologically.
| Authors | Intervention Theme | Results Themes | Medical Outcomes Themes | Effectiveness Themes | Barrier Themes |
|---|---|---|---|---|---|
| McTavish et al. [17] | mHealth app | Good retention | Reduction in cravings | Low cost | Must train users |
| Good acceptance | |||||
| Decreased consumption/cravings | |||||
| Increased self-efficacy/self-determination | |||||
| Murray et al. [18] | mHealth app | Reduction in consumption | Reduction in consumption | Low cost | Cost |
| Improvement in dependence | Decreased consumption/cravings | Must train users | |||
| No significant difference in treatment outcomes | Equally as effective as traditional care (preference) | ||||
| Good acceptance | |||||
| Morgan et al. [19] | mHealth app | High rates of acceptance | Improved rates of depression | Decreased depression symptoms | Computer literacy/access to Internet |
| Cost | |||||
| Chih et al. [20] | mHealth app | Computer models can predict relapse | With prediction of relapse, providers can intervene | Can predict relapse and enable intervention | Must train users |
| Kalapatapu et al. [21] | Telephone/Interactive voice response | No significant difference in treatment outcomes | No significant difference in treatment outcomes | Decreased consumption/cravings | Equally as effective, so change may not be necessary |
| Equally as effective as traditional care (preference) | |||||
| Stoner et al. [22] | mHealth SMS | No significant difference in treatment outcomes | No significant difference in treatment outcomes | Decreased consumption/cravings | Equally as effective, so change may not be necessary |
| Equally as effective as traditional care (preference) | Cost | ||||
| Bock et al. [23] | mHealth SMS | Reduction in consumption | Reduction in consumption | Decreased consumption/cravings | Must train users |
| Increased self-efficacy | Increased self-efficacy | Increased self-efficacy/self-determination | |||
| Freyer-Adam et al. [24] | mHealth app | Good retention | Not reported | Educates | Must train users |
| Increased retention in treatment program | |||||
| Gamito et al. [25] | mHealth serious games/cognitive training | Positive frontal lobe function (FAB) | Increased frontal lobe function | Increased frontal lobe function | Must train users |
| Barrio et al. [26] | mHealth app | Decreased binge drinking | Reduction in consumption | Decreased consumption/cravings | Must train users |
| Reduction in consumption | Increased self-efficacy/self-determination | ||||
| Gajecki et al. [27] | mHealth serious games/cognitive training | Decreased binge drinking | Reduction in consumption | Decreased consumption/cravings | Must train users |
| Reduction in consumption | Increased self-efficacy/self-determination | ||||
| Glass et al. [28] | mHealth app | Good retention | Reduction in consumption | Decreased consumption/cravings | Must train users |
| Increased motivation to change | Increased retention in treatment program | ||||
| Rose et al. [29] | Telephone/Interactive voice response | No significant difference in treatment outcomes | Reduction in consumption | Decreased consumption/cravings | Cost |
| Equally as effective, so change may not be necessary | |||||
| Must train users | |||||
| Jo et al. [30] | mHealth app | Reduction in consumption | Reduction in consumption | Decreased consumption/cravings | Must train users |
| Increased self-efficacy | Increased self-efficacy | Increased self-efficacy/self-determination | |||
| Mellentin et al. [31] | mHealth serious games/cognitive training | No significant difference in treatment outcomes | Reduction in consumption | Decreased consumption/cravings | Equally as effective, so change may not be necessary |
| Must train users | |||||
| Harder et al. [32] | Telephone/Interactive voice response | Reduction in consumption | Reduction in consumption | Decreased consumption/cravings | Must train users |
| Increased self-efficacy | Increased self-efficacy | Increased self-efficacy/self-determination | |||
| Hendershot et al. [33] | mHealth app | Reduction in consumption | Reduction in consumption | Increased medication compliance | Must train users |
| No significant difference in treatment outcomes | Increased medication compliance | Equally as effective as traditional care (preference) | |||
| Decreased consumption/cravings | |||||
| Constant et al. [34] | Telephone/Interactive voice response | Reduction in consumption | Reduction in consumption | Decreased consumption/cravings | Must train users |
| Increased motivation to change | Increased self-efficacy/self-determination | Must sustain intervention for long-term results | |||
| Sustained abstinence from drinking | |||||
| Graser et al. [35] | mHealth + telephone | Reduction in consumption | Reduction in consumption | Decreased consumption/cravings | Must train users |
| Sustained abstinence from drinking | |||||
| Hammond et al. [36] | mHealth app | Reduction in consumption | Reduction in consumption | Increased self-efficacy/self-determination | Must train users |
| Manning et al. [37] | mHealth app | Reduction in consumption | Reduction in consumption | Decreased consumption/cravings | Must train users |
| Howe et al. [38] | mHealth app | Reduction in consumption | Reduction in consumption | Increased self-efficacy/self-determination | Must train users |
| Increased motivation to change | Decreased consumption/cravings | ||||
| Leightley et al. [39] | mHealth app | Reduction in consumption | Reduction in consumption | Decreased consumption/cravings | Must train users |
| McKay et al. [40] | mHealth + telephone | Reduction in consumption | Reduction in consumption | Increased self-efficacy/self-determination | Must train users |
| Decreased consumption/cravings | |||||
| O’Grady et al. [41] | mHealth app | Reduction in consumption | Reduction in consumption | Increased self-efficacy/self-determination | Computer literacy/access to Internet |
| Decreased consumption/cravings | Impacts provider workload | ||||
| Increased access to care | Must train users |
4.5. Results of Syntheses
This subheading is for a meta-analysis, not for a systematic review. This section will be removed by the editor before publishing.
4.6. Additional Analysis and Certainty of Evidence
Affinity matrices were created to summarize the frequency and probability of occurrence of each theme or observations. Frequency and probability do not imply importance: They only state the probability the theme or observation would be identified in the group for analysis. As part of the thematic analysis, observations that occurred more than once were identified as themes. All others are listed as individual observations.
4.6.1. Patient Satisfaction
Patient satisfaction was very positive for all studies. The reason for this may have been because participants had already presented themselves for treatment for AUD, therefore, they would be positively disposed toward most interventions. The exact modality may not have negatively affected patient satisfaction. This is a significant error of both internal and external validity, and this variable should not be used to form any conclusions about interventions.
4.6.2. Results of Studies
Table 3 summarizes the results of studies compared with a control group. Table 2 identifies which studies did not have a control group. Five themes and three individual observations were identified by the reviewers for a total of 68 occurrences in the literature. Reduction in consumption was identified in 15/31 (48%) of the occurrences [18,23,26,27,30,32,33,34,35,36,37,38,39,40,41]. In 10/31 (32%) of the occurrences, the reduction was statistically significant, but in 5/31 (15%) of the occurrences, it was not statistically significant [21,22,29,31,33]. Three of 25 (10%) occurrences mentioned the intervention caused positive retention in treatment programs [17,24,28]. Furthermore, in 3/25 occurrences, the participants increased self-efficacy and scored better on the AUDIT [23,30,32]. In 2/31 (6%) occurrences, the intervention decreased binge drinking [26,27]. The following are individual observations that could not fit into a theme. One intervention used a Bayesian Network Model to predict relapses. This enabled providers to intervene through text, email, or phone. One intervention highlighted a high rate of acceptance among participants, which may have been related to the fact that participants already volunteered for treatment—the modality may not have played a significant part. One intervention noted positive frontal lobe function which could lead to a decrease in addiction behaviors [19,20,25].
Table 3.
Results to the studies.
| Results Themes and Observations | Frequency |
|---|---|
| Reduction in consumption [18,23,26,27,30,32,33,34,35,36,37,38,39,40,41] | 15 |
| No significant difference in treatment outcomes [21,22,29,31,33] | 5 |
| Good retention in treatment [17,24,28] | 3 |
| Increased self-efficacy [23,30,32] | 3 |
| Decreased binge drinking [26,27] | 2 |
| Computer models can predict relapse [20] | 1 |
| High rates of acceptance [19] | 1 |
| Positive frontal lobe function (FAB) [25] | 1 |
| 31 |
Medical Outcome Commensurate with the Use of mHealth
Table 4 summarizes the medical outcomes observed. Ten themes and two individual observations were recorded commensurate with the adoption of (intervention) for a total of 34 occurrences. Many of these themes were like those highlighted in results. Only differences from results will be reported. Three interventions identified an increase motivation to change behavior as a result of the intervention. This occurred in 3/34 (9%) observations [28,34,38]. A high number of observations were unable to be fit into themes. One article mentioned a reduction in craving for alcohol. One mentioned an improved rate of depression indicators. One mentioned an improvement in dependence on alcohol. One highlighted an increase in medication compliance [17,18,19,33].
Table 4.
Medical outcomes commensurate with the adoption of mHealth.
| Medical Outcomes Themes and Observations | Frequency |
|---|---|
| Reduction in consumption [18,23,26,27,28,29,30,31,32,33,34,35,36,38,39,40,41] | 18 |
| Increased self-efficacy [23,30,32] | 3 |
| Increased motivation to change [28,34,38] | 3 |
| No significant difference in treatment outcomes [18,21,22] | 3 |
| Reduction in cravings [17] | 1 |
| With prediction of relapse, providers can intervene | 1 |
| Improved rates of depression [19] | 1 |
| Improvement in dependence [18] | 1 |
| Increased frontal lobe function [25] | 1 |
| Increased medication compliance [33] | 1 |
| Not reported [24] | 1 |
| 34 |
Effectiveness Themes and Observations
Table 5 summarizes the effectiveness themes and observations. Eight themes and six individual observations were recorded by reviewers for a total of 50 occurrences. Many of these themes overlapped with study results and medical outcomes. Only the differences will be reported. In four of the interventions, it was highlighted that these are equally as effective at treating AUD, so the decision to choose one method over the other could fulfil a patient’s preference, and this preference may increase the success of the intervention [18,21,22,33]. Two interventions were highlighted as low cost [17,18]. Two interventions resulted in sustained abstinence from drinking [34,35]. One intervention was noted as exceptionally good at providing education about AUD and healthy habits [24].
Table 5.
Effectiveness Themes and Observations.
| Effectiveness Themes and Observations. | Frequency |
|---|---|
| Decreased consumption/cravings [17,18,21,22,23,26,27,28,29,30,31,32,33,34,35,37,38,39,40,41] | 20 |
| Increased self-efficacy/self-determination [17,23,26,27,30,32,34,36,38,40,41] | 11 |
| Equally as effective as traditional care (preference) [18,21,22,33] | 4 |
| Increased retention in treatment program [17,24,28] | 3 |
| Low cost [17,18] | 2 |
| Good acceptance [17,18] | 2 |
| Sustained abstinence from drinking [34,35] | 2 |
| Increased frontal lobe function [25] | 1 |
| Increased access to care [41] | 1 |
| Decreased depression symptoms [19] | 1 |
| Increased medication compliance [33] | 1 |
| Educates [24] | 1 |
| Can predict relapse and enable intervention [20] | 1 |
| 50 |
Barriers to the Adoption of mHealth and Wearable Sensors to Manage AUD
Table 6 summarizes the barriers to the adoption of mHealth and wearable sensors to manage AUD. Four themes and two observations were reported for a total of 34 occurrences. Almost every intervention would require additional teaching of users and provider teams to work with it. This theme occurred 22/34 (65%) occurrences [17,18,20,23,24,25,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41]. In 4/34 (12%) occurrences, it was highlighted that the intervention was equally as effective at managing AUD as the traditional treatment methods, so change may not be necessary [21,22,29,31]. In four of the interventions, it was mentioned that cost could be a consideration in implementing it [22,29]. There were 2/34 (6%) occurrences of computer literacy or needed access to the Internet [19,41]. Finally, the two interventions that could not be fitted into themes: Must sustain the intervention for a long period to get positive results, and the intervention impacts the provider’s workload [34,41].
Table 6.
Barrier themes and observations.
| Barriers Themes and Observations | Frequency |
|---|---|
| Must train users [17,18,20,23,24,25,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41] | 22 |
| Equally as effective, so change may not be necessary [21,22,29,31] | 4 |
| Cost [22,29] | 4 |
| Computer literacy/access to Internet [19,41] | 2 |
| Must sustain intervention for long-term results [34] | 1 |
| Impacts provider workload [41] | 1 |
| Not reported | 0 |
| 34 |
Interactions between Observations
Overall, mHealth apps mostly resulted in a reduction in alcohol consumption or a reduction in cravings [17,18,19,20,24,26,28,30,33,36,37,38,39,41]. The mHealth + telephone interventions had the same effect [35,40]. The mHealth SMS interventions had mixed results: They both reduced consumption of alcohol, but only one was a statistically significant decrease [22,23]. The telephone interactive voice intervention also showed mixed results: They all decreased alcohol consumption, but not all were statistically significant [21,29,32,34]. Finally, the mHealth with serious games or cognitive training showed the most promise with a younger population. This intervention also showed a decrease in alcohol consumption, and one of them highlighted an increase in frontal lobe function, which is theorized will decrease addiction [25,27,31].
5. Discussion
5.1. Summary of Evidence
Twenty-five studies published in the last 10 years were analyzed for implications to the adoption of mHealth and wearable sensors for the treatment of AUD. Five intervention themes were identified in the literature. Twenty of the 25 studies analyzed reported effectivness at reducing alcohol consumption or cravings [17,18,21,22,23,26,27,28,29,30,31,32,33,34,35,37,38,39,40,41], but some improvements were not statistically significant [21,22,29,31,33]. Using mHealth or wearable sensors, even if not statistically significant, can fulfil the preference of a patient, and this preference may increase the success of the intervention [18,21,22,33]. mHealth is effective at educating patients [24], is inexpensive [17,18], and can increase self-efficacy or self-determination of AUD patients [17,23,26,27,30,32,34,36,38,40,41]. Overall, mHealth offers a viable alternative to traditional treatments, and in some cases, the results are stronger than traditional care.
Practitioners should be comfortable adopting this intervention for the treatment of AUD. Although some training will be necessary for most mHealth interventions [17,18,20,23,24,25,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41], the efficacy of the intervention is well supported by the literature. Providers should also be mindful that mHealth interventions could adversely affect their workload [41], and the intervention requires some computer literacy and access to the Internet or WiFi [19,41]. This intervention could be preferred by some patients and enabling their preference could positively affect outcomes.
Future research should explore why some of these interventions did not demonstrate a statistically significant reduction in alcohol consumption. There may have been customization of SMS messages or tailoring of the apps to cater to preferences of the patients. This may increase the efficacy of the intervention and decrease prevalence of AUD.
5.2. Limitations
Several reviewers were used in this study to control for confirmation bias. Unfortunately, a review is often limited to what can be found. To ensure studies were of high quality, we only accepted studies that had been published, however, this subjects the study to publication bias because we did not consider grey literature. The studies selected all exhibited small instances of selection and sample bias, which affect their internal and external validity, respectively. We only selected published articles from the last 10 years because technology advances so rapidly. Had we looked back 15 years, we may have identified additional themes in the literature.
6. Conclusions
mHealth and wearable sensors are effective tools to decrease alcohol consumption, increase self-efficacy and self-determination, and provide overall treatment of AUD. The evolution of studies on this topic has slowly grown over time. mHealth technology may require additional training of users at both ends, but its low cost and efficacy outweigh the disadvantages. Although some interventions are not statistically different from traditional care, the use of mHealth and wearable sensors may fulfill the preference of a patient and increase the success of treatment.
Appendix A
Table A1.
Observation-to-theme conversion (Intervention, Results, Medical Outcomes).
| Authors | Experimental Intervention | Intervention Theme | Results | Results Themes | Medical Outcomes Reported | Medical Outcomes Themes |
|---|---|---|---|---|---|---|
| McTavish et al. [17] | mHealth app to control SUD and AUD | mHealth app | 94% used the app 1st week, and 80% used continued to use at week 16 | Good retention | Participants with AUD will use an app to manage their condition, App decreased cravings | Reduction in cravings |
| Murray et al. [18] | mHealth app Down Your Drink (DYD) | mHealth app | No control group. Of those still using the app at 12 months, the reduction in drinking was 35 units |
Reduction in consumption | Reduction in consumption, reduction in AUD identification test, reduction in Leeds Dependence Questionnaire (LDQ, dependence), no significant change in Clinical Outcomes in Routine Evaluation (CORE-10, mental health) or EQ-5D (quality of life). | Reduction in consumption |
| Improvement in dependence | ||||||
| No significant difference in treatment outcomes | ||||||
| Morgan et al. [19] | mHealth Internet-based app | mHealth app | Internet-based recruitment to mental health interventions is feasible | High rates of acceptance | Improved rates of depression using intervention | Improved rates of depression |
| Chih et al. [20] | mHealth (A-CHESS) and BN | mHealth app | No control group. Responses to weekly check in on A-CHESS can be a predictor of relapse |
Computer models can predict relapse | The prediction of lapse in sobriety gives counselors the chance to intervene through text, email, or phone call | With prediction of relapse, providers can intervene |
| Kalapatapu et al. [21] | telephone | Telephone/Interactive voice response | Face-to-face cognitive based therapy (CBT) and T-CBT groups were similar on all treatment adherence outcomes and depression outcomes at all time points | No significant difference in treatment outcomes | telehealth means of treating is equally as effective as traditional therapy | No significatn difference in treatment outcomes |
| Stoner et al. [22] | mHealth SMS | mHealth SMS | Adequate adherence ≥80% at week 8, not statistically significant between groups | No significant difference in treatment outcomes | SMS messages do not improve medication adherence, but equally as effective as traditional treatment to reduce consumption | No significant difference in treatment outcomes |
| Bock et al. [23] | mHealth SMS, text-message alcohol program (TMAP) | mHealth SMS | At week 6–12, TMAP participants less likely to report heavy drinking and negative alcohol consequences. Increased self-efficacy to resist drinking. | Reduction in consumption | SMS effective at reducing consumption and increasing self-efficacy | Reduction in consumption |
| Increased self-efficacy | Increased self-efficacy | |||||
| Freyer-Adam et al. [24] | mHealth Internet-based app | mHealth app | reached individuals and helped retain them in AUD programs | Good retention | Not reported | Not reported |
| Gamito et al. [25] | mHealth cognitive stimulation program (CSP) serious games | mHealth serious games/cognitive training | Cognitive ability between groups not statistically significant, but frontal-lobe function (Frontal Lobe Assessment, FAB) was significantly improved in the intervention group | Positive frontal lobe function (FAB) | Improvement in FAB with mHealth intervention | Increased frontal lobe function |
| Barrio et al. [26] | mHealth app, SIDEAL | mHealth app | Reduced binge drinking and mean daily consumption, participants achieved their self-imposed objectives | Decreased binge drinking | Significant reduction in alcohol consumption` | Reduction in consumption |
| Reduction in consumption | ||||||
| Gajecki et al. [27] | mHealth app (skills training) | mHealth serious games/cognitive training | Reduced binge drinking and mean daily consumption, participants achieved their self-imposed objectives | Decreased binge drinking | Reduced alcohol consumption | Reduction in consumption |
| Reduction in consumption | ||||||
| Glass et al. [28] | mHealth app (A-CHESS) | mHealth app | Intervention showed increased odds of outpatient addiction treatment across follow-ups, but not mutual help | Good retention | Reduced alcohol consumption, increased treatment | Reduction in consumption |
| Increased motivation to change | ||||||
| Rose et al. [29] | interactive voice response (IVR) brief intervention (BI) | Telephone/Interactive voice response | Reduced alcohol consumption, but not statistically different than control | No significant difference in treatment outcomes | Reduced alcohol consumption | Reduction in consumption |
| Jo et al. [30] | mHealth (online-based brief empowerment for alcohol-use monitor, on-BEAM) | mHealth app | intervention group reported consuming less alcohol during the past week and lower AUDIT score | Reduction in consumption | Reduced alcohol consumption | Reduction in consumption |
| Increased self-efficacy | Increased self-efficacy | |||||
| Mellentin et al. [31] | mHealth (cue exposure) | mHealth serious games/cognitive training | No differences were detected between the two experimental CET groups on any outcomes | No significant difference in treatment outcomes | Reduced alcohol consumption | Reduction in consumption |
| Harder et al. [32] | mHealth (motivational interviewing) | Telephone/Interactive voice response | Average AUDIT scores were lower for the intervention group | Reduction in consumption | Reduced alcohol consumption, increased self-efficacy | Reduction in consumption |
| Increased self-efficacy | Increased self-efficacy | |||||
| Hendershot et al. [33] | mHealth feedback, opiod receptor gene (OPRM1) | mHealth app | OPRM1 genotype moderated the association of daily adherence with reduced same-day consumption (p = 0.007) and craving (p = 0.06), with these associations being stronger for participants with the 118 G variant. OPRM1 genotype did not moderate changes in craving and consumption over time. | Reduction in consumption | high-density assessments and person-centered analytic approaches, including modeling within-person variation in medication adherence, could be advantageous for pharmacogenetic studies. | Reduction in consumption |
| No significant difference in treatment outcomes | Increased medication compliance | |||||
| Constant et al. [34] | telephone | Telephone/Interactive voice response | Study group had better alcohol abstinence rates than control | Reduction in consumption | Intervention improves patient coping skills and motivation to modify alcohol use behaviors | Reduction in consumption |
| Increased motivation to change | ||||||
| Graser et al. [35] | telephone and smartphone-based intervention | mHealth + telephone | Telephone-based intervention was more effective than text-based intervention | Reduction in consumption | Sustained abstinence from excessive drinking occurred in telephone intervention group | Reduction in consumption |
| Hammond et al. [36] | mHealth app | mHealth app | Intervention group utilized mobile app more effectively than control group | Reduction in consumption | Complemented community substance use intervention programs | Reduction in consumption |
| Manning et al. [37] | mHealth app | mHealth app | No control group. Participants reduced alcohol consumption rates | Reduction in consumption | Improved alcohol consumption rates | Reduction in consumption |
| Howe et al. [38] | mHealth app | mHealth app | No control group. Use of mobile app improved decision making of study group participants | Reduction in consumption | Mobile data collection can positively influence drinking decisions | Reduction in consumption |
| Increased motivation to change | ||||||
| Leightley et al. [39] | mHealth app | mHealth app | Use of mobile app reduced rate of alcohol consumption among Veterans in study group | Reduction in consumption | Reduced rates of alcohol consumption | Reduction in consumption |
| McKay et al. [40] | telephone and smartphone-based intervention | mHealth + telephone | Use of telephone or smartphone was effective in treating AUD | Reduction in consumption | Improved rates of alcohol dependent persons | Reduction in consumption |
| O’Grady et al. [41] | mHealth app | mHealth app | Provider-facing technology is effective alcohol intervention services and increase access to care in low- and middle-income countries. | Reduction in consumption | Improved rates in alcohol dependent persons | Reduction in consumption |
Appendix B
Table A2.
Observation-to-theme conversion (Effectiveness, and Barriers).
| Authors | Measures of Effectiveness | Effectiveness Themes | Barriers to Adoption | Barrier Themes |
|---|---|---|---|---|
| McTavish et al. [17] | Low cost intervention, good acceptance, decreased cravings, increased autonomy and self-determination | Low cost | Must train users | Must train users |
| Good acceptance | ||||
| Decreased consumption/cravings | ||||
| Increased self-efficacy/self-determination | ||||
| Murray et al. [18] | Low operation cost (120/mo), effective at reducing consumption | Low cost | High setup cost (3200), must train users | Cost |
| Decreased consumption/cravings | Must train users | |||
| Equally as effective as traditional care (preference) | ||||
| Good acceptance | ||||
| Morgan et al. [19] | Improved rates of depression | Decreased depression symptoms | Must have access to internet, average cost of AUD $12 per participant | Computer literacy/access to Internet |
| Cost | ||||
| Chih et al. [20] | Effective at predicting relapse | Can predict relapse and enable intervention | Must train users | Must train users |
| Kalapatapu et al. [21] | Effective at treating | Decreased consumption/cravings | none | Equally as effective, so change may not be necessary |
| Equally as effective as traditional care (preference) | ||||
| Stoner et al. [22] | Equally as effective at reducing consumption | Decreased consumption/cravings | Equally as effective, but expensive (unnecessarily) | Equally as effective, so change may not be necessary |
| Equally as effective as traditional care (preference) | Cost | |||
| Bock et al. [23] | SMS effective at reducing consumption and increasing self-efficacy | Decreased consumption/cravings | Must train users | Must train users |
| Increased self-efficacy/self-determination | ||||
| Freyer-Adam et al. [24] | Educates participants and increases retention in programs | Educates | Must train users, computer literacy | Must train users |
| Increased retention in treatment program | ||||
| Gamito et al. [25] | Improved FAB indicates greater frontal-lobe activity, which could decrease alcohol addiction | Increased frontal lobe function | Must train users | Must train users |
| Barrio et al. [26] | Effective at reducing consumption | Decreased consumption/cravings | Must train users | Must train users |
| Increased self-efficacy/self-determination | ||||
| Gajecki et al. [27] | Effective at reducing consumption | Decreased consumption/cravings | Must train users | Must train users |
| Increased self-efficacy/self-determination | ||||
| Glass et al. [28] | Effective at reducing consumption, effective at increasing treatment participation | Decreased consumption/cravings | Must train users | Must train users |
| Increased retention in treatment program | ||||
| Rose et al. [29] | Equally as effective at reducing consumption | Decreased consumption/cravings | Equally as effective, but expensive (unnecessarily), must train users | Cost |
| Equally as effective, so change may not be necessary | ||||
| Must train users | ||||
| Jo et al. [30] | Reduced alcohol consumption, improved self-efficacy | Decreased consumption/cravings | Must train users | Must train users |
| Increased self-efficacy/self-determination | ||||
| Mellentin et al. [31] | Equally as effective at reducing consumption | Decreased consumption/cravings | Equally as effective, but expensive (unnecessarily), must train users | Equally as effective, so change may not be necessary |
| Must train users | ||||
| Harder et al. [32] | Reduced alcohol consumption, increased self-efficacy | Decreased consumption/cravings | Must train users | Must train users |
| Increased self-efficacy/self-determination | ||||
| Hendershot et al. [33] | increased medication adherence, decreased consumption, decreased cravings | Increased medication compliance | Must train users | Must train users |
| Equally as effective as traditional care (preference) | ||||
| Decreased consumption/cravings | ||||
| Constant et al. [34] | Sustained abstinence from excessive drinking | Decreased consumption/cravings | Must train users Must sustain intervention repeated for best results |
Must train users |
| Increased self-efficacy/self-determination | Must sustain intervention for long-term results | |||
| Sustained abstinence from drinking | ||||
| Graser et al. [35] | Sustained abstinence from excessive drinking | Decreased consumption/cravings | Must train users | Must train users |
| Sustained abstinence from drinking | ||||
| Hammond et al. [36] | Reinforced positive behaviors | Increased self-efficacy/self-determination | Must train users | Must train users |
| Manning et al. [37] | Reduced alcohol consumption | Decreased consumption/cravings | Must train users | Must train users |
| Howe et al. [38] | Improved decision making of alcohol users | Increased self-efficacy/self-determination | Must train users Must possess smartphone | Must train users |
| Decreased consumption/cravings | ||||
| Leightley et al. [39] | Reduced alcohol consumption rates | Decreased consumption/cravings | Must train users | Must train users |
| McKay et al. [40] | Improved rates of alcohol dependent persons | Increased self-efficacy/self-determination | Must train users | Must train users |
| Decreased consumption/cravings | ||||
| O’Grady et al. [41] | Improved rates of alcohol dependent persons, increased access | Increased self-efficacy/self-determination | Must have access to internet; time constraints on provider workload | Computer literacy/access to Internet |
| Decreased consumption/cravings | Impacts provider workload | |||
| Increased access to care | Must train users |
Appendix C
Table A3.
Other observations incident to review.
| Authors | Sample Size (#s Only) |
Bias within Study (See Article) Selection Bias, Sample Bias, etc. |
Effect Size (Small, Medium, or Large with Cohen’s d Statistic) Sensitivity, Specificity, F1 |
Country of Origin (Where Was the Study Conducted?) | Statistics Used | Strength of Evidence | Quality of Evidence |
|---|---|---|---|---|---|---|---|
| McTavish et al. [17] | 349 | One region of one country (selection bias), mostly Caucasian males (sample bias) | not reported | USA | Measures of central tendency, Descriptive Statistics | I | A |
| Murray et al. [18] | 19 | One region of one country (selection bias) | not reprted | UK | Measures of central tendency, Descriptive Statistics | III | B |
| Morgan et al. [19] | 1326 | One region of one country (selection bias) | small effect size | Australia | Descriptive statistics | I | A |
| Chih et al. [20] | 152 | One region of one country (selection bias), 81% Caucasian (sample bias) | sensitivity (prediction of true lapse) at >4–6% 75% (21/28), specificity (prediction of non-lapse) at >4–6% 88% (234/266) | USA | Bayesian Network Model | III | A |
| Kalapatapu et al. [21] | 103 | One region of one country (selection bias), 87% female (sample bias) | not reported | USA | Kolmogorov–Smirnov and Shapiro–Wilk tests (normality), Levene’s test (homogeneity of variance), non-parametric tests (continuous variables), chi-square and Fisher’s exact tests (categorical variables), Wilcoxon signed-rank test (change in AUDIT) score from baseline to end of treatment. |
I | A |
| Stoner et al. [22] | 76 | One region of one country (selection bias), mostly male (sample bias) | w = 0.32 | USA | t-test, chi-square test, and ANCOVA | I | A |
| Bock et al. [23] | 60 | One region of one country (selection bias), mostly female (sample bias) | Magnitude of effect ranged from small to large (d+ = 0.46–0.62; 12-week follow-up: d+ = 0.13–0.35). | USA | chi-square test and ANOVA | I | A |
| Freyer-Adam et al. [24] | 961 | One region of one country (selection bias) | not reported | Germany | Descriptive statistics, measures of central tendency, multivariate logistic regression analysis, t-test | I | A |
| Gamito et al. [25] | 42 | One region of one country (selection bias), mostly male (sample bias) | medium effect (0.30) | Portugal | ANCOVA, t-tests | I | A |
| Barrio et al. [26] | 24 | One region of one country (selection bias) | not reported | Spain | Measures of central tendency, Descriptive Statistics, paired t-tests and chi-square tests | III | B |
| Gajecki et al. [27] | 144 | One region of one country (selection bias), mostly female (sample bias) | not reported | Sweden | ANOVA | I | A |
| Glass et al. [28] | 349 | One region of one country (selection bias) | not reported | USA | Measures of central tendency, descriptive statistics, logistic models, mixed effects models, chi-square | I | A |
| Rose et al. [29] | 1855 | One region of one country (selection bias) | not reported | USA | chi square, t-tests and Wilcoxon Rank Sum tests | I | A |
| Jo et al. [30] | 1496 | One region of one country (selection bias) | alcohol consumption (d = 0.24), binge drinking (d = 0.29), total AUDIT-K score (d = 0.34) | Korea | t-test or chi-square test | I | A |
| Mellentin et al. [31] | 164 | One region of one country (selection bias) | not reported | Denmark | Generalized mixed models | I | A |
| Harder et al. [32] | 230 | One region of one country (selection bias) | sensitivity = 0.86; specificity = 0.72 | Kenya | Multiple linear regression | I | A |
| Hendershot et al. [33] | 76 | One region of one country (selection bias) | not reported | USA | Multi-level modeling and multi-level structural equation modeling | III | A |
| Constant et al. [34] | 799 | Multiple regions of one country (selection bias) | not reported | Brazil | logistic regression and chi-square | I | A |
| Graser et al. [35] | 240 | One region of one country (selection bias) | not reported | USA | Descriptive statistics | I | A |
| Hammond et al. [36] | 61 | One region of one country (selection bias) | d = 0.26 (small) | USA | Descriptive statistics, t-test | I | A |
| Manning et al. [37] | 1309 | One region of one country (selection bias) | not reported | Australia | Descriptive statistics | I | A |
| Howe et al. [38] | 104 | One region of one country (selection bias), mostly young adults median age 20 yrs old (sample bias) | not reported | USA | Descriptive statistics | I | A |
| Leightley et al. [39] | 123 | One region of one country (selection bias), mostly Veteran males (sample bias) | Cohen d = 0.5 (medium effect) | UK | Descriptive statistics | I | A |
| McKay et al. [40] | 262 | One region of one country (selection bias), mostly African American males (sample bias) | PDHD lower in TMC (d = 0.35, medium), A-CHESS (d = 0.31, medium), TMC + A-CHESS (d = 0.36, medium), differences between groups small (d ≤ 0.06, small) | USA | Descriptive statistics | I | A |
| O’Grady et al. [41] | 60 | Selection bias | not reported | USA | Descriptive statistics | I | A |
Author Contributions
Conceptualization C.S.K., methodology C.S.K., abstract screening C.S.K., J.A.B., S.M., C.W.L. and V.W. data extraction C.S.K., J.A.B., S.M., C.W.L. and V.W. thematic analysis C.S.K. and J.A.B., writing C.S.K., J.A.B., S.M., C.W.L. and V.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
This study used no human subjects, so it is IRB exempt.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data from this study can be obtained by asking the lead author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.National Institute on Alcohol Abuse and Alcoholisma Understanding Alcohol Abuse Disorder. [(accessed on 1 August 2022)]; Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/understanding-alcohol-use-disorder.
- 2.Manthey J., Gual A., Jakubczyk A., Pieper L., Probst C., Struzzo P., Trapencieris M., Wojnar M., Rehm J. Alcohol use disorders in Europe: A comparison of general population and primary health care prevalence rates. J. Subst. Use. 2016;21:478–484. doi: 10.3109/14659891.2015.1063719. [DOI] [Google Scholar]
- 3.World Health Organization . Status Report on Alcohol Consumption, Harm and Policy Responses in 30 European Countries 2019. World Health Organization Regional Office for Europe; København, Denmark: 2019. [Google Scholar]
- 4.Buono F.D., Gleed C., Boldin M., Aviles A., Wheeler N. Preliminary Effectiveness of a Remotely Monitored Blood Alcohol Concentration Device as Treatment Modality: Protocol for a Randomized Controlled Trial. JMIR Res. Protoc. 2022;11:e30186. doi: 10.2196/30186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.World Health Organization . Telemedicine: Opportunities and Developments in Member States. Report on the Second Global Survey on eHealth. World Health Organization; Geneva, Switzerland: 2010. [Google Scholar]
- 6.Davis-Martin R.E., Alessi S.M., Boudreaux E.D. Alcohol use disorder in the age of technology: A review of wearable biosensors in alcohol use disorder treatment. Front. Psychiatry. 2021;12:642813. doi: 10.3389/fpsyt.2021.642813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lansdorp B., Ramsay W., Hamid R., Strenk E. Wearable enzymatic alcohol biosensor. Sensors. 2019;19:2380. doi: 10.3390/s19102380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Goldfine C., Lai J.T., Lucey E., Newcomb M., Carreiro S. Wearable and wireless mHealth technologies for substance use disorder. Curr. Addict. Rep. 2020;7:291–300. doi: 10.1007/s40429-020-00318-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carreiro S., Newcomb M., Leach R., Ostrowski S., Boudreaux E.D., Amante D. Current reporting of usability and impact of mHealth interventions for substance use disorder: A systematic review. Drug Alcohol Depend. 2020;215:108201. doi: 10.1016/j.drugalcdep.2020.108201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kruse C.S., Lee K., Watson J.B., Lobo L.G., Stoppelmoor A.G., Oyibo S.E. Measures of effectiveness, efficiency, and quality of telemedicine in the management of alcohol abuse, addiction, and rehabilitation: Systematic review. J. Med. Internet Res. 2020;22:e13252. doi: 10.2196/13252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruse C.S. Writing a Systematic Review for Publication in a Health-Related Degree Program. JMIR Res. Protoc. 2019;8:e15490. doi: 10.2196/15490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braun V., Clarke V. Using thematic analysis in psychology. Qual. Res. Psychol. 2006;3:77–101. doi: 10.1191/1478088706qp063oa. [DOI] [Google Scholar]
- 13.Newhouse R., Dearholt S., Poe S., Pugh L., White K. The Johns Hopkins Nursing Evidence-Based Practice Rating Scale. The Johns Hopkins Hospital; Baltimore, MD, USA: 2005. [Google Scholar]
- 14.Pannucci C.J., Wilkins E.G. Identifying and avoiding bias in research. Plast. Reconstr. Surg. 2010;126:619. doi: 10.1097/PRS.0b013e3181de24bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Light R.J. Measures of response agreement for qualitative data: Some generalizations and alternatives. Psychol. Bull. 1971;76:365. doi: 10.1037/h0031643. [DOI] [Google Scholar]
- 16.McHugh M.L. Interrater reliability: The kappa statistic. Biochem. Med. 2012;22:276–282. doi: 10.11613/BM.2012.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McTavish F.M., Chih M.-Y., Shah D., Gustafson D.H. How Patients Recovering From Alcoholism Use a Smartphone Intervention. J. Dual Diagn. 2012;8:294–304. doi: 10.1080/15504263.2012.723312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Murray E., Linke S., Harwood E., Conroy S., Stevenson F., Godfrey C. Widening Access to Treatment for Alcohol Misuse: Description and Formative Evaluation of an Innovative Web-Based Service in One Primary Care Trust. Alcohol Alcohol. 2012;47:697–701. doi: 10.1093/alcalc/ags096. [DOI] [PubMed] [Google Scholar]
- 19.Morgan A.J., Jorm A.F., Mackinnon A.J. Internet-based recruitment to a depression prevention intervention: Lessons from the Mood Memos study. J. Med. Internet Res. 2013;15:e2262. doi: 10.2196/jmir.2262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chih M.-Y., Patton T., McTavish F.M., Isham A.J., Judkins-Fisher C.L., Atwood A.K., Gustafson D.H. Predictive modeling of addiction lapses in a mobile health application. J. Subst. Abus. Treat. 2014;46:29–35. doi: 10.1016/j.jsat.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kalapatapu R.K., Ho J., Cai X., Vinogradov S., Batki S.L., Mohr D.C. Cognitive-behavioral therapy in depressed primary care patients with co-occurring problematic alcohol use: Effect of telephone-administered vs. Face-to-face treatment—A secondary analysis. J. Psychoact. Drugs. 2014;46:85–92. doi: 10.1080/02791072.2013.876521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoner S.A., Arenella P.B., Hendershot C.S. Randomized controlled trial of a mobile phone intervention for improving adherence to naltrexone for alcohol use disorders. PLoS ONE. 2015;10:e0124613. doi: 10.1371/journal.pone.0124613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bock B.C., Barnett N.P., Thind H., Rosen R., Walaska K., Traficante R., Foster R., Deutsch C., Fava J.L., Scott-Sheldon L.A. A text message intervention for alcohol risk reduction among community college students: TMAP. Addict. Behav. 2016;63:107–113. doi: 10.1016/j.addbeh.2016.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Freyer-Adam J., Baumann S., Haberecht K., Tobschall S., Schnuerer I., Bruss K., Bandelin E., John U., Gaertner B. In-person and computer-based alcohol interventions at general hospitals: Reach and retention. Eur. J. Public Health. 2016;26:844–849. doi: 10.1093/eurpub/ckv238. [DOI] [PubMed] [Google Scholar]
- 25.Gamito P., Oliveira J., Lopes P., Brito R., Morais D., Rebelo S., Silva D., Caçôete C., Deus A. Cognitive stimulation through mHealth-based program for patients with alcohol dependence syndrome: A randomized controlled study. J. Pain Manag. 2016;9:235–241. [Google Scholar]
- 26.Barrio P., Ortega L., López H., Gual A. Self-management and Shared Decision-Making in Alcohol Dependence via a Mobile App: A Pilot Study. Int. J. Behav. Med. 2017;24:722–727. doi: 10.1007/s12529-017-9643-6. [DOI] [PubMed] [Google Scholar]
- 27.Gajecki M., Andersson C., Rosendahl I., Sinadinovic K., Fredriksson M., Berman A.H. Skills training via smartphone app for university students with excessive alcohol consumption: A randomized controlled trial. Int. J. Behav. Med. 2017;24:778–788. doi: 10.1007/s12529-016-9629-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glass J.E., McKay J.R., Gustafson D.H., Kornfield R., Rathouz P.J., McTavish F.M., Atwood A.K., Isham A., Quanbeck A., Shah D. Treatment seeking as a mechanism of change in a randomized controlled trial of a mobile health intervention to support recovery from alcohol use disorders. J. Subst. Abus. Treat. 2017;77:57–66. doi: 10.1016/j.jsat.2017.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rose G.L., Badger G.J., Skelly J.M., MacLean C.D., Ferraro T.A., Helzer J.E. A randomized controlled trial of brief intervention by interactive voice response. Alcohol Alcohol. 2017;52:335–343. doi: 10.1093/alcalc/agw102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jo S.J., Lee H.K., Kang K., Joe K.H., Lee S.B. Efficacy of a Web-Based Screening and Brief Intervention to Prevent Problematic Alcohol Use in Korea: Results of a Randomized Controlled Trial. Alcohol. Clin. Exp. Res. 2019;43:2196–2202. doi: 10.1111/acer.14169. [DOI] [PubMed] [Google Scholar]
- 31.Mellentin A.I., Nielsen B., Nielsen A.S., Yu F., Mejldal A., Nielsen D.G., Stenager E. A Mobile Phone App Featuring Cue Exposure Therapy As Aftercare for Alcohol Use Disorders: An Investigator-Blinded Randomized Controlled Trial. JMIR Mhealth Uhealth. 2019;7:e13793. doi: 10.2196/13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Harder V.S., Musau A.M., Musyimi C.W., Ndetei D.M., Mutiso V.N. A randomized clinical trial of mobile phone motivational interviewing for alcohol use problems in Kenya. Addiction. 2020;115:1050–1060. doi: 10.1111/add.14903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hendershot C.S., Dermody S.S., Wardell J.D., Zaso M.J., Kennedy J.L., Stoner S.A. OPRM1 Moderates Daily Associations of Naltrexone Adherence With Alcohol Consumption: Preliminary Evidence From a Mobile Health Trial. Alcohol. Clin. Exp. Res. 2020;44:983–991. doi: 10.1111/acer.14300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Constant H.M.R.M., Ferigolo M., Barros H.M.T., Moret-Tatay C. A clinical trial on a brief motivational intervention in reducing alcohol consumption under a telehealth supportive counseling. Psychiatry Res. 2021;303:114068. doi: 10.1016/j.psychres.2021.114068. [DOI] [PubMed] [Google Scholar]
- 35.Graser Y., Stutz S., Rösner S., Moggi F., Soravia L.M. Telephone-and Text Message–Based Continuing Care After Residential Treatment for Alcohol Use Disorder: A Randomized Clinical Multicenter Study. Alcohol. Clin. Exp. Res. 2021;45:224–233. doi: 10.1111/acer.14499. [DOI] [PubMed] [Google Scholar]
- 36.Hammond A.S., Sweeney M.M., Chikosi T.U., Stitzer M.L. Digital delivery of a contingency management intervention for substance use disorder: A feasibility study with DynamiCare Health. J. Subst. Abuse Treat. 2021;126:108425. doi: 10.1016/j.jsat.2021.108425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manning V., Piercy H., Garfield J.B.B., Clark S.G., Andrabi M.N., Lubman D.I. A Personalized Approach Bias Modification Smartphone App (“SWiPE”) to Reduce Alcohol Use: Open-Label Feasibility, Acceptability, and Preliminary Effectiveness Study. JMIR Mhealth Uhealth. 2021;9:e31353. doi: 10.2196/31353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Howe L.K., Copeland S., Fisher L., Farmer E., Nemes L., Finn P.R. Mobile assessment of decisions to drink in young adults: Examining the role of incentives and disincentives. Alcohol. Clin. Exp. Res. 2022;46:152–165. doi: 10.1111/acer.14746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Leightley D., Williamson C., Rona R.J., Carr E., Shearer J., Davis J.P., Simms A., Fear N.T., Goodwin L., Murphy D. Evaluating the Efficacy of the Drinks: Ration Mobile App to Reduce Alcohol Consumption in a Help-Seeking Military Veteran Population: Randomized Controlled Trial. JMIR Mhealth Uhealth. 2022;10:e38991. doi: 10.2196/38991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.McKay J.R., Gustafson D.H., Ivey M., Pe-Romashko K., Curtis B., Thomas T., Oslin D.W., Polsky D., Quanbeck A., Lynch K.G. Efficacy and comparative effectiveness of telephone and smartphone remote continuing care interventions for alcohol use disorder: A randomized controlled trial. Addiction. 2022;117:1326–1337. doi: 10.1111/add.15771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Grady M.A., Mootz J., Suleman A., Sweetland A., Teodoro E., Anube A., Feliciano P., Bezuidenhout C., Dos Santos P.F., Fumo W. Mobile technology and task shifting to improve access to alcohol treatment services in Mozambique. J. Subst. Abus. Treat. 2022;134:108549. doi: 10.1016/j.jsat.2021.108549. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this study can be obtained by asking the lead author.