Abstract
Members of the family Pasteurellaceae are classified in part by whether or not they require an NAD supplement for growth on laboratory media. In this study, we demonstrate that this phenotype can be determined by a single gene, nadV, whose presence allows NAD-independent growth of Haemophilus influenzae and Actinobacillus pleuropneumoniae. This gene was cloned from a 5.2-kb plasmid which was previously shown to be responsible for NAD independence in Haemophilus ducreyi. When transformed into A. pleuropneumoniae, this cloned gene allowed NAD-independent growth on complex media and allowed the utilization of nicotinamide in place of NAD on defined media. Sequence analysis revealed an open reading frame of 1,482 bp that is predicted to encode a protein with a molecular mass of 55,619 Da. Compared with the sequence databases, NadV was found to have significant sequence homology to the human pre-B-cell colony-enhancing factor PBEF and to predicted proteins of unknown function identified in the bacterial species Mycoplasma genitalium, Mycoplasma pneumoniae, Shewanella putrefaciens, Synechocystis sp., Deinococcus radiodurans, Pasteurella multocida, and Actinobacillus actinomycetemcomitans. P. multocida and A. actinomycetemcomitans are among the NAD-independent members of the Pasteurellaceae. Homologues of NadV were not found in the sequenced genome of H. influenzae, an NAD-dependent member of the Pasteurellaceae, or in species known to utilize a different pathway for synthesis of NAD, such as Escherichia coli. Sequence alignment of these nine homologues revealed regions and residues of complete conservation that may be directly involved in the enzymatic activity. Identification of a function for this gene in the Pasteurellaceae should help to elucidate the role of its homologues in other species.
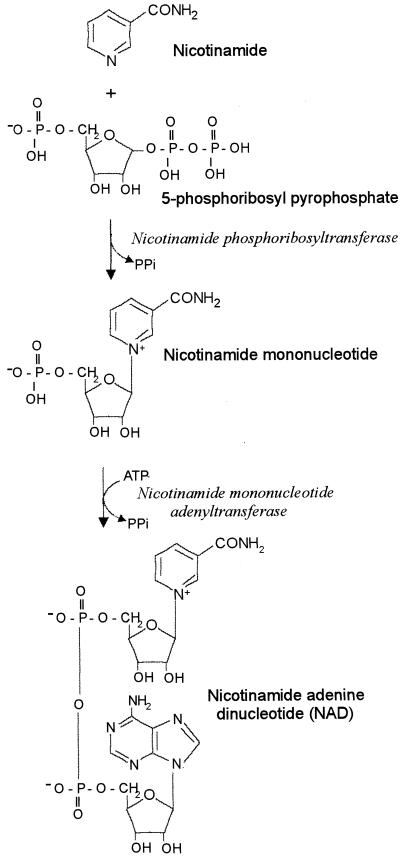
NAD is a critical cofactor required for energy metabolism and many oxidation-reduction reactions in both prokaryotic and eukaryotic cells. In many bacterial species, synthesis of NAD occurs de novo via quinolinic acid (3, 4). NAD can also be synthesized by a pyridine nucleotide salvage pathway via nicotinic acid (3, 4). Members of the family Pasteurellaceae do not possess either of these pathways for NAD biosynthesis. These bacterial species must acquire this essential nutrient from their environment either as NAD directly or from a limited number of precursors (18, 19). This pyridine nucleotide requirement has been historically important in the identification and classification of members of the Pasteurellaceae, with species requiring an NAD supplement for growth in vitro described as V-factor dependent (12, 13). In V-factor-dependent species, the pyridine nucleotide source must possess an intact pyridine-ribose bond and the pyridine-carbonyl group must be amidated; therefore, nicotinamide mononucleotide (NMN) and nicotinamide riboside (NR) can function as V-factors, but quinolinic acid, nicotinic acid, nicotinic acid mononucleotide, and nicotinamide (NAm) cannot (3, 19). The ability to use NAm as a precursor for NAD biosynthesis has been shown to differentiate V-factor-dependent from V-factor-independent strains among the Pasteurellaceae (20). Haemophilus haemoglobinophilus, which is V-factor independent, synthesizes the enzyme nicotinamide phosphoribosyltransferase, which converts NAm to NMN and allows the use of NAm as a source of pyridine nucleotide (11) (Fig. 1). Since NAm is available in most complex bacteriologic media, bacteria that can utilize NAm are V-factor independent.
FIG. 1.
Biochemical pathway for the biosynthesis of NAD as found in the family Pasteurellaceae. NAD-dependent species lack the ability to convert NAm to NMN.
For many species of Pasteurellaceae defined as V-factor dependent, V-factor-independent variants have been identified. These include strains of Actinobacillus pleuropneumoniae, which causes pleuropneumonia in swine (22); Haemophilus paragallinarum, which causes fowl choryza (1, 15); Haemophilus parainfluenzae, which can cause pneumonia and meningitis in humans (7); and Haemophilus ducreyi, which causes the sexually transmitted disease chancroid in humans (26, 27). In H. parainfluenzae, H. paragallinarum, and H. ducreyi, V-factor independence has been shown to be encoded on a plasmid (1, 27, 28). However, in A. pleuropneumoniae, it appears that V-factor independence may be conferred by a chromosomal gene (10).
In this paper, we report the cloning and sequence analysis of a plasmid-encoded gene in a V-factor-independent strain of H. ducreyi. This gene was capable of conferring NAD independence to a variety of Pasteurellaceae and appears to be widely distributed among both prokaryotic and eukaryotic organisms.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Escherichia coli XL1-Blue MRF′ (Stratagene, La Jolla, Calif.) was used for propagation of the plasmid vectors pUC18 (Gibco-BRL, Rockville, Md.) and pGZRS18 (25) as well as derivatives of these plasmids. E. coli strains were grown on Luria-Bertani (LB) medium supplemented with ampicillin (100 μg/ml) for plasmid selection. A. pleuropneumoniae ATCC 27088 and H. influenzae KW20 Rd- (2) strains were grown at 37°C under a 5% CO2 atmosphere on brain heart infusion (BHI) broth or agar (Difco Laboratories, Detroit, Mich.) supplemented with V-factor (NAD) and X-factor (hemin), both at 10 μg/ml, and ampicillin at 50 μg/ml as needed. NAD was omitted when selecting for V-factor-independent transformants. H. ducreyi ATCC 27722 was grown on chocolate agar (BHI agar base plus 5% boiled sheep blood plus 1% IsoVitaLex) at 35°C in a candle jar.
The defined medium used to grow A. pleuropneumoniae and H. influenzae was a modification of the recipe developed by Herriott for H. influenzae (9), with 10 mM glucose added and the amino acid stock solution from the Neisseria defined medium developed by Morse and Bartenstein (16) substituted for Herriott's amino acid solution. This medium was supplemented with hemin (10 μg/ml) and with 10 μg/ml NAD or NAm (Sigma Chemical Co., St. Louis, Mo.) as needed to determine specific nutritional requirements.
DNA manipulations.
Restriction enzymes, calf intestinal phosphatase, and DNA ligase were purchased from Boehringer Mannheim Biochemicals (Indianapolis, Ind.) and used according to the manufacturer's instructions. DNA fragments for subcloning were purified from agarose gels by excising the bands and isolating the DNA with Qiaex beads (Qiagen Inc., Valencia, Calif.). Plasmid DNA was isolated from E. coli, A. pleuropneumoniae, H. ducreyi, and H. influenzae using the QIAprep spin plasmid purification kit (Qiagen). E. coli was transformed with plasmids using the method of Hanahan (8). Plasmids were transformed into H. influenzae using methods described by Herriott (9). Plasmids were introduced into A. pleuropneumoniae by electroporation as previously described (5).
DNA sequencing.
Templates for DNA sequence analysis were constructed by subcloning fragments generated from defined restriction sites within pNAD1 into pUC18. Remaining gaps in the sequence were filled using synthetic oligonucleotide primers made at the Macromolecular Structural Facility at Michigan State University as primers for sequencing. DNA sequencing was performed using an ABI100 model 377 automated sequencer (Applied Biosystems, Foster City, Calif.). Sequence analysis was performed using the Web-based Genetics Computer Group package of programs (6). Database searches were performed using the Blast program provided by the National Center for Biotechnology Information (NCBI). Partially sequenced genomes were accessed and searched either from the NCBI genome database or from individual databases listed in and linked to the Institute for Genome Research website at http://www.tigr.org.
PCR product subcloning.
The open reading frame (ORF) predicted to encode the nadV gene was amplified using synthetic primers MM199 (5′-GCC TGC AGA AAA ATC TTT TGA ATT ATA TAA ACA AC-3′) and MM191 (5′-GCG TAT TAA CTG CAG ATA TCA TAG CGT AGT GCG-3′), which were designed to introduce unique PstI sites at either end of the ORF. The amplification product was digested with PstI and ligated into pUC18 to form pCNAD9. The insert was then cloned into pGZRS18 in both forward and reverse directions to form pGZNAD9 and pGZNAD10, respectively.
Enzyme assay.
The assay for synthesis of NAD from NAm was adapted from that of Kasarov and Moat (11). A. pleuropneumoniae serotype 1A containing either pGZNAD9 or pGZRS18 was grown overnight at 37°C in BHI broth containing NAD (10 μg/ml) and ampicillin (50 μg/ml). Cells were harvested by centrifugation, washed in sterile 0.9% saline, suspended in 0.1% of the original culture volume, and disrupted by sonication on ice. Cell debris was pelleted by centrifugation. Cell-free supernatants were combined in a reaction mix that contained 1 ml of supernatant sample, 80 mM potassium phosphate buffer (pH 7.4), 16 mM MgCl2, 1 mM ATP, 5 mM phosphoribosyl pyrophosphate (PRPP; Sigma), and 2 mM NAm, and the mix was incubated at 37°C in a waterbath shaker. At designated time points, 250-μl aliquots were removed and combined with 250 μl of saline and 500 μl of methanol to stop the reaction.
Analysis of products was performed by high-pressure liquid chromatography (HPLC) using a Hewlett-Packard model 1050 system with an Alltech LiChrosorb RP-18 column (10-μm particle size, 250 by 4 mm) equipped with a guard column (LiChrosorb RP-18; 5-μm particle size; EM Separations, Wakefield, R.I.). The mobile phase consisted of two elements, with an elution gradient as described in reference 14. Eluant A was 8 mM tetrabutylammonium bromide (HPLC grade; Sigma) in 0.1 M KH2PO4 (pH 6.0). Eluant B was 70% eluant A and 30% methanol. Absorbance was measured at 254 nm.
In assays containing radioactive substrate, assay conditions were identical except that 350 μM carbonyl-[14C]NAm (American Radiolabeled Chemicals, Inc., St. Louis, Mo.) was added in place of the 2 mM NAm. To assay for radioactive incorporation, column fractions were collected into 10 ml of Safety Solve scintillation cocktail, and radioactivity was counted on a Beckman LS 6500 scintillation counter.
Nucleotide sequence accession number.
The sequences reported in this paper have been submitted to GenBank and given accession number AF273842.
RESULTS
Isolation of the NAD independence plasmid from H. ducreyi.
H. ducreyi ATCC 27722 had previously been shown to contain a 5.25-kb plasmid which possessed the ability to confer NAD independence on H. influenzae (27). We corroborated this finding by purifying the plasmid DNA from H. ducreyi 27722, using this DNA to transform an NAD-dependent strain of H. influenzae, and selecting transformants able to grow on complex media in the absence of NAD. One of the NAD-independent colonies recovered was selected, and its plasmid content was analyzed. This transformant contained a single plasmid of approximately 5.2 kb. This plasmid was used to retransform H. influenzae, and NAD-independent colonies which carried the 5.2-kb plasmid were again recovered, confirming that the NAD independence phenotype was conferred by this plasmid. Thus, the H. ducreyi plasmid was designated pNAD1.
Localization of the NAD independence locus on pNAD1.
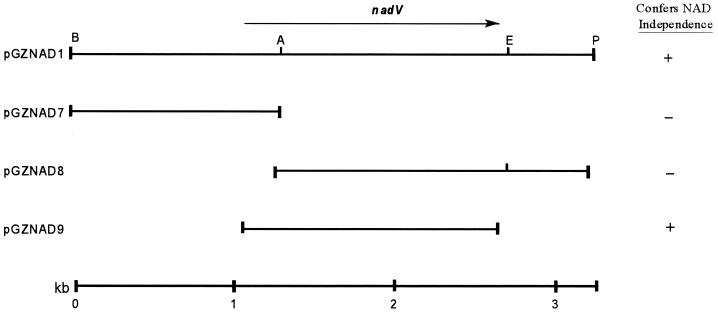
Plasmid pNAD1 was digested with a variety of restriction enzymes, and an initial restriction map of this plasmid was used to direct the subcloning of fragments of pNAD1 into the cloning vector pUC18. The largest, a 3.3-kb BamHI-PstI fragment, was subcloned into the shuttle vector pGZRS18 to determine whether it contained the NAD independence locus. This subclone, pGZNAD1, was electroporated into A. pleuropneumoniae, and transformants were plated onto BHI agar lacking NAD. Six of the colonies recovered were found to contain a plasmid with a restriction pattern identical to that of pGZNAD1. This revealed that the gene for NAD independence was functional in A. pleuropneumoniae and was located on the 3.3-kb BamHI-PstI fragment of pNAD1 (Fig. 2).
FIG. 2.
Subclones of pNAD1 constructed in the E. coli–A. pleuropneumoniae shuttle vector pGZRS18 (25). The location of the nadV gene is indicated with an arrow. Plasmids pGZNAD1, pGZNAD7, and pGZNAD8 were constructed using the restriction sites shown. Plasmid pGZNAD9 was constructed using synthetic primers to PCR amplify the nadV gene. The ability of these clones to confer NAD independence on A. pleuropneumoniae is indicated in the right-hand column. Restriction sites: A, AvaI; B, BamHI; E, EcoRI; and P, PstI.
Sequence analysis of pNAD1.
The complete insert of pGZNAD1 was sequenced. The insert was 3,307 bp in length and had a G+C content of 34%. This high A+T content resulted in a high frequency of stop codons in all three reading frames. One large ORF of 1,482 bp in length was predicted to encode a protein of 494 amino acids with a molecular mass of 55,619 Da. There was an AvaI site located 230 bp into the ORF. Deletions made from this site in pGZNAD1 resulted in the loss of ability to complement the NAD dependence of A. pleuropneumoniae (Fig. 2). Based on this genetic evidence linking this ORF to the ability to confer V-factor independence on A. pleuropneumoniae and H. influenzae, the gene encoding this ORF was designated nadV.
To confirm that the NadV ORF conferred NAD independence, synthetic primers were used to PCR amplify the region containing the ORF and 75 bp upstream of the start codon, and the PCR product was cloned into pGZRS18 in both orientations to form pGZNAD9 (Fig. 2) and pGZNAD10. Plasmid pGZNAD9 conferred NAD independence on A. pleuropneumoniae but pGZNAD10 did not, suggesting that the nadV gene was expressed from a promoter in the vector rather than from its native promoter.
The complete nucleotide sequence and predicted amino acid sequence of nadV have been submitted to GenBank and given accession number AF273842. A putative ribosome-binding site was found upstream of the start codon of nadV. No significant inverted repeat sequences characteristic of transcriptional terminators were found downstream of the stop codon of this gene.
The predicted amino acid sequence of NadV was analyzed for the presence of functionally conserved motifs. This protein did not contain a hydrophobic, N-terminal leader sequence characteristic of secreted proteins, nor did it contain any long stretches of internal hydrophobic residues which could serve as membrane anchors. Compared against a protein motif database (6), no significant matches were found to conserved regions of previously identified protein families.
Homologues of the nadV gene in other organisms.
The NadV sequence was used to search sequence databases. This identified one protein with a putative function and seven matches to proteins of unknown function from partially or completely sequenced microbial genomes. The protein with a putative function was the human pre-B-cell colony-enhancing factor (PBEF) protein (24). The homologues discovered in the bacterial genome databases were found in a diverse array of species, including the cyanobacterium Synechocystis; the radiation-resistant organism Deinococcus radiodurans; two Mycoplasma species, M. genitalium and M. pneumoniae; the gram-negative aquatic and soil organism Shewanella putrefaciens; and two NAD-independent members of the Pasteurellaceae, Pasteurella multocida and Actinobacillus actinomycetemcomitans. Pairwise comparisons of these sequences revealed that NadV had the highest similarity to the homologue from S. putrefaciens and that these two were more closely related to the Mycoplasma homologues than to the remaining sequences. All nine sequences were aligned (Fig. 3), and numerous regions were found which contained clusters of highly conserved amino acid residues. Also conspicuous were regions where the sequences or sequence gaps from A. actinomycetemcomitans, P. multocida, D. radiodurans, Synechocystis sp., and human PBEF were identical but different from sequences from M. genitalium, M. pneumoniae, S. putrefaciens, and H. ducreyi NadV. This clustering is indicative of two broad families among the homologues of NadV.
FIG. 3.
Alignment of the predicted NadV amino acid sequence with homologues found in other species. Black shaded regions indicate residues that are identical in the majority of species. Gray shaded regions indicate residues that are functionally conserved in the majority of species. Species abbreviations: aact, Actinobacillus actinomycetemcomitans; pmul, Pasteurella multocida; syn, Synechocystis; drad, Deinococcus radiodurans; mgen, Mycoplasma genitalium; mpne, M. pneumoniae; sput, Shewanella putrefaciens; hduc, Haemophilus ducreyi; hum, human PBEF. The alignment was obtained using the Pileup program from the Genetics Computer Group package (6).
Functional analysis of the NAD independence locus.
Previous studies have shown that NAD-independent members of the family Pasteurellaceae differ from the NAD-dependent members solely in their ability to utilize the NAD precursor NAm as V-factor (18, 20). To determine whether nadV was responsible for this difference, A. pleuropneumoniae strains containing pGZNAD1, pGZNAD9, or the pGZRS18 vector were plated onto defined media lacking V-factor and onto defined media containing either NAD or NAm. All three strains failed to grow in the absence of supplement and grew in the presence of NAD, but only the strains containing the cloned nadV gene could grow in the presence of NAm. This indicated that the presence of the nadV gene allowed A. pleuropneumoniae to utilize NAm as a precursor for NAD biosynthesis, as diagrammed in Fig. 1, and suggests that the enzyme encoded by this gene is a novel NAm phosphoribosyltransferase (NAm-PRTase).
Assay for NAm-PRTase activity.
Crude cell extracts were prepared from A. pleuropneumoniae strains containing either pGZNAD9 or the pGZRS18 vector and assayed for the ability to synthesize NMN and NAD from NAm plus PRPP. As shown in Table 1, NAm-PRTase assays performed with extracts of A. pleuropneumoniae containing pGZNAD9 showed a decrease in NAm and a concomitant increase in NAD as well as a slight but consistent increase in the levels of NMN. A. pleuropneumoniae containing the pGZRS18 vector alone did not show an equivalent increase in NAD or decrease in NAm, nor was this pattern seen when assays with A. pleuropneumoniae containing pGZNAD9 were performed without PRPP in the reaction mix.
TABLE 1.
Synthesis of NAD from NAm and PRPP by extracts of A. pleuropneumoniae containing nadVa
| Time (min) | NAm (μmol) | NMN (μmol) | NAD (μmol) |
|---|---|---|---|
| 0 | 360 | 25 | 25 |
| 30 | 220 | 40 | 123 |
Reaction mixture contained cell extract, 80 mM potassium phosphate buffer (pH 7.4), 16 mM MgCl2, 1 mM ATP, 5mM PRPP, and 2 mM NAm, incubated for 30 min at 37°C. Data are from a representative experiment. Trends were identical in all experiments.
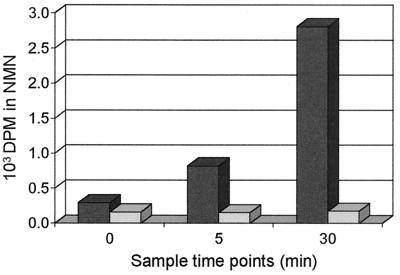
To confirm that NMN is indeed an intermediate in the biosynthesis of NAD from NAm as catalyzed by the NadV gene product, [14C] NAm was used as the substrate in the same assay system. As shown in Fig. 4, 14C label is incorporated into NMN by cell extracts from A. pleuropneumoniae containing pGZNAD9 but not in control reactions with extracts from A. pleuropneumoniae containing pGZRS18.
FIG. 4.
Incorporation of [14C]NAm into NMN. NAm-PRTase assays were performed with [14C]NAm as the substrate, and incorporation of radiolabel into NMN monitored over time. Dark bars, A. pleuropneumoniae/pGZNAD9; light bars, A. pleuropneumoniae/pGZRS18.
DISCUSSION
In this paper, we describe the cloning, sequence analysis, and characterization of a plasmid-encoded gene, nadV, from H. ducreyi which confers V-factor independence on several species of V-factor-dependent Pasteurellaceae. A 5.25-kb plasmid from H. ducreyi 27722 was previously described by Windsor et al. (26) and shown to confer V-factor independence on H. influenzae and H. parainfluenzae. Similar plasmids have been described in V-factor-independent strains of H. parainfluenzae (28) and H. paragallinarum (1). We isolated plasmid DNA from H. ducreyi 27722 and confirmed that a 5.2-kb plasmid could confer NAD independence on H. influenzae. We further demonstrated that subclones constructed from this plasmid in an appropriate shuttle vector could confer NAD independence on a different member of the family Pasteurellaceae, A. pleuropneumoniae, and that a single gene on this plasmid, nadV, was responsible for this phenotype.
Members of the family Pasteurellaceae are incapable of either de novo synthesis of NAD via quinolinic acid or recycling pyridine nucleotides via nicotinic acid (3, 4, 18), which leads to their requirement for an exogenous source of pyridine nucleotide, or V-factor. V-factor dependence in the Pasteurellaceae has been defined as the requirement for either NAD, NMN, or NR for growth on complex media (18). Using this definition, species such as H. influenzae, H. parainfluenzae, H. parasuis, and A. pleuropneumoniae are V-factor dependent, while P. multocida, P. haemolytica, H. haemoglobulinophilus, and A. actinomycetemcomitans are not. However, all of the members of the Pasteurellaceae require a pyridine nucleotide when grown on chemically defined media (18). In this case, the difference is that the V-factor-independent strains can utilize NAm as the pyridine nucleotide as well as NAD, NMN, and NR, but the V-factor-dependent strains cannot. This distinction between V-factor-dependent and -independent strains based on growth on complex media is somewhat artificial, since most complex media contain significant amounts of NAm (17, 18). Niven and O'Reilly (18) proposed that the distinction between V-factor-independent and -dependent strains in the family Pasteurellaceae may reflect the presence or absence of a single enzyme, NAm-PRTase, to convert NAm to NMN. Our results support this proposal. We used the ability to grow on a complex medium without added V-factor as our selection for nadV and demonstrated that the presence of nadV could also confer the ability to grow on a chemically defined medium to which NAm had been added, but not on the same medium with no exogenous source of pyridine nucleotide. We further demonstrated that NAD could be synthesized from NAm and PRPP via NMN in A. pleuropneumoniae strains containing the nadV gene, which supports the conclusion that this gene encodes an NAm-PRTase. In addition, in an analysis of currently available genomic databases, we found homologues of NadV in P. multocida and A. actinomycetemcomitans, two V-factor-independent species, but failed to find a homologue in H. influenzae, which is V-factor dependent.
We also identified homologues of NadV in genomes from a variety of highly diverse bacterial species, including two mycoplasmas, a cyanobacterium, a gram-negative aquatic and soil bacterium, and a gram-positive radiation-resistant coccus. The H. ducreyi nadV gene was more closely related to the homologues found in Shewanella and in Mycoplasma species than to either the P. multocida or A. actinomycetemcomitans homologue. This likely indicates that horizontal transfer of this gene has occurred. The nadV gene is located on a plasmid in H. ducreyi but in the chromosome of the other bacterial species. One possibility is that this gene moved into H. ducreyi from M. genitalium. A similar horizontal transfer has been proposed as the source of the tetM gene found in most urogenital pathogens of humans (23). We did not find NadV homologues in a wide variety of other species, including members of the Enterobacteriaceae and Bacillaceae, known to either synthesize NAD de novo or possess pyridine salvage pathways.
The only homologue of NadV with a proposed function to date is human PBEF (24). The human PBEF gene was transcribed mainly in human bone marrow, liver, and muscle cells as well as in activated human lymphocytes. It was proposed to encode a novel cytokine-like molecule that enhanced the effect of stem cell factor and interleukin-7 on B-cell development, but this has not been studied further. The function of NadV in the biosynthesis of NAD should provide an important clue to the role of PBEF in mammalian species.
To date, NadV homologues identified in microbial genome sequencing projects have been designated as homologues of PBEF. For M. genitalium, this similarity led to the hypothesis that this gene could be linked to pathogenicity via a potential immune regulatory function (21). Our discovery provides a more plausible explanation for the role of this gene in bacterial metabolism and will be useful in future microbial genome analyses as an indicator of the presence of an alternative NAD biosynthetic pathway.
The requirement for V-factor is a key taxonomic criterion for identification of members of the Pasteurellaceae. Our results suggest that the inability to utilize NAm to fill this requirement is due to the absence of a single gene, nadV. We have shown that two V-factor-independent species, P. multocida and A. actinomycetemcomitans, possess chromosomal copies of this gene, while H. influenzae, the only V-factor-dependent species for which a complete genome sequence is available, does not possess this gene. The location of the H. ducreyi nadV gene on a plasmid and its apparent mobility into other V-factor-dependent species of haemophili suggest that the use of NAD requirements in identification of individual members of the Pasteurellaceae could prove problematic in the future. However, at this time NAD independence is not widespread in H. ducreyi, H. paragallinarum, H. parainfluenzae, or A. pleuropneumoniae, and it seems feasible to continue to use this characteristic as a taxonomic criterion with the caveat that NAD-independent strains of these species do exist.
ACKNOWLEDGMENTS
This work was supported by USDA CSREES grants 96-01855 and 98-02202, as well as by funds from the State of Michigan Research Excellence Fund Center for Animal Production Enhancement.
We thank Julie Hotopp and Benjamin Griffin for assistance with the HLPC procedures; Robert Hausinger and James Tiedje for the use of their HPLC systems; Mary Tobin for the chemically defined medium; and Scott Doree, Trevor Wagner, and Amanda Goeman for their contributions to this project.
REFERENCES
- 1.Bragg R R, Coetzee L, Verschoor J A. Plasmid-encoded NAD independence in some South African isolates of Haemophilus paragallinarum. Onderstepoort J Vet Res. 1993;60:147–152. [PubMed] [Google Scholar]
- 2.Bricker J, Mulks M H, Plaut A G, Moxon E R, Wright A. IgA1 proteases of Haemophilus influenzae: cloning and characterization in Escherichia coli K-12. Proc Natl Acad Sci USA. 1983;80:2681–2685. doi: 10.1073/pnas.80.9.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cynamon M H, Sorg T B, Patapow A. Utilization and metabolism of NAD by Haemophilus parainfluenzae. J Gen Microbiol. 1988;134:2789–2799. doi: 10.1099/00221287-134-10-2789. [DOI] [PubMed] [Google Scholar]
- 4.Foster J W, Moat A G. Nicotinamide adenine dinucleotide biosynthesis and pyridine nucleotide cycle metabolism in microbial systems. Microbiol Rev. 1980;44:83–105. doi: 10.1128/mr.44.1.83-105.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fuller T E, Thacker B J, Mulks M H. A riboflavin auxotroph of Actinobacillus pleuropneumoniae is attenuated in swine. Infect Immun. 1996;64:4659–4664. doi: 10.1128/iai.64.11.4659-4664.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Genetics Computer Group. Program manual for the Wisconsin package. 10th ed. Madison, Wis: Genetics Computer Group; 1999. [Google Scholar]
- 7.Gromkova R, Koornhof H. Naturally occurring NAD-independent Haemophilus parainfluenzae. J Gen Microbiol. 1990;136:1031–1035. doi: 10.1099/00221287-136-6-1031. [DOI] [PubMed] [Google Scholar]
- 8.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 9.Herriott R M, Meyer E M, Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970;101:517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holloway de Corsier M A. Ph.D. thesis. Berne, Switzerland: University of Berne; 1994. [Google Scholar]
- 11.Kasarov L B, Moat A G. Biosynthesis of NAD in Haemophilus haemoglobinophilus. Biochim Biophys Acta. 1973;320:372–378. doi: 10.1016/0304-4165(73)90318-8. [DOI] [PubMed] [Google Scholar]
- 12.Kilian M. A taxonomic study of the genus Haemophilus, with the proposal of a new species. J Gen Microbiol. 1976;93:9–62. doi: 10.1099/00221287-93-1-9. [DOI] [PubMed] [Google Scholar]
- 13.Kilian M, Biberstein E L. Genus II: Haemophilus. In: Krieg N R, Holt J G, editors. Bergey's manual of systematic bacteriology. Vol. 1. Baltimore, Md: The Williams and Wilkins Co.; 1984. pp. 558–575. [Google Scholar]
- 14.Michelli V, Sestini S. Determining NAD synthesis in erythrocytes. Methods Enzymol. 1997;280:211–221. doi: 10.1016/s0076-6879(97)80112-7. [DOI] [PubMed] [Google Scholar]
- 15.Miflin J K, Horner R F, Blackall P J, Chen X, Bishop G C, Morrow C J, Yamaguchi T, Iritani Y. Phenotypic and molecular characterization of V-factor (NAD)-independent Haemophilus paragallinarum. Avian Dis. 1995;39:304–308. [PubMed] [Google Scholar]
- 16.Morse S A, Bartenstein L. Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can J Microbiol. 1980;26:13–20. doi: 10.1139/m80-003. [DOI] [PubMed] [Google Scholar]
- 17.Niven D, Levesque M. V-factor-dependent growth of Actinobacillus pleuropneumoniae biotype 2 (Bertschinger 2008/76) Int J Syst Bacteriol. 1988;38:319–320. [Google Scholar]
- 18.Niven D F, O'Reilly T. Significance of V-factor dependency in the taxonomy of Haemophilus species and related organisms. Int J Syst Bacteriol. 1990;40:1–4. doi: 10.1099/00207713-40-1-1. [DOI] [PubMed] [Google Scholar]
- 19.O'Reilly T, Niven D F. Defining the metabolic and growth responses of porcine haemophili to exogenous pyridine nucleotides and precursors. J Gen Microbiol. 1986;132:807–818. doi: 10.1099/00221287-132-3-807. [DOI] [PubMed] [Google Scholar]
- 20.O'Reilly T, Niven D F. Pyridine nucleotide metabolism by extracts derived from Haemophilus parasuis and H. pleuropneumoniae. Can J Microbiol. 1986;32:733–737. doi: 10.1139/m86-133. [DOI] [PubMed] [Google Scholar]
- 21.Ouzounis C, Casari G, Valencia A, Sander C. Novelties from the complete genome of Mycoplasma genitalium. Mol Microbiol. 1996;20:898–900. doi: 10.1111/j.1365-2958.1996.tb02529.x. [DOI] [PubMed] [Google Scholar]
- 22.Pohl S, Bertschinger H U, Frederiksen W, Mannheim W. Transfer of Haemophilus pleuropneumoniae and the Pasteurella haemolytica-like organism causing porcine necrotic pleuropneumonia to the genus Actinobacillus (Actinobacillus pleuropneumoniae comb. nov.) on the basis of phenotypic and deoxyribonucleic acid relatedness. Int J Syst Bacteriol. 1983;11:510–514. [Google Scholar]
- 23.Roberts M C, Hillier S L, Hale J, Holmes K K, Kenny G E. Tetracycline resistance and tetM in pathogenic urogenital bacteria. Antimicrob Agents Chemother. 1986;30:810–812. doi: 10.1128/aac.30.5.810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre-B-cell colony-enhancing factor. Mol Cell Biol. 1994;14:1431–1437. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.West S E, Romero M J, Regassa L B, Zielinski N A, Welch R A. Construction of Actinobacillus pleuropneumoniae-Escherichia coli shuttle vectors: expression of antibiotic-resistance genes. Gene. 1995;160:81–86. doi: 10.1016/0378-1119(95)00236-y. [DOI] [PubMed] [Google Scholar]
- 26.Windsor H M, Gromkova R C, Koornhof H J. Transformation of V-factor independence from Haemophilus ducreyi to Haemophilus influenzae and Haemophilus parainfluenzae. Med Microbiol Lett. 1993;2:159–167. [Google Scholar]
- 27.Windsor H M, Gromkova R C, Koornhof H J. Plasmid-mediated NAD independence in Haemophilus parainfluenzae. J Gen Microbiol. 1991;137:2415–2421. doi: 10.1099/00221287-137-10-2415. [DOI] [PubMed] [Google Scholar]
- 28.Windsor H M, Gromkova R C, Koornhof H J. Growth characteristics of V-factor-independent transformants of Haemophilus influenzae. Int J Syst Bacteriol. 1993;434:799–804. doi: 10.1099/00207713-43-4-799. [DOI] [PubMed] [Google Scholar]