Abstract
Stimuli-responsive hydrogel drug delivery systems are designed to release a payload when prompted by an external stimulus. These platforms have become prominent in the field of drug delivery due to their ability to provide spatial and temporal control for drug release. Among the different external triggers that have been used, ultrasound possesses several advantages: it is non-invasive, has deep tissue penetration, and can safely transmit acoustic energy to a localized area. This review summarizes the current state of understanding about ultrasound-responsive hydrogels used for drug delivery. The mechanisms of inducing payload release and activation using ultrasound are examined, along with the latest innovative formulations and hydrogel design strategies. We also report on the most recent applications leveraging ultrasound activation for both cancer treatment and tissue engineering. Finally, the future perspectives offered by ultrasound-sensitive hydrogels are discussed.
Keywords: hydrogels, polymers, stimuli-responsive, ultrasound, smart hydrogels, drug delivery, Tissue engineering, cancer therapy, controlled drug release, thermoresponsive materials
1. Introduction
Stimuli-responsive drug delivery systems enable the delivery of payloads on-demand, at a specific time, and at a specific location [1,2,3,4]. These platforms can be designed to respond to a variety of different stimuli, either internal such as redox, pH, or enzymes, or external physical triggers such as magnetic field, ultrasound, light, electricity, or temperature [5,6,7,8,9,10].
For the past 70 years, ultrasound has been extensively used as a diagnostic tool [11,12]. However, it has recently been applied to a broad range of therapeutic applications such as the treatment of vascular thrombosis by dissolving clots, the ablation of tumors, and the healing of bone fractures [12,13,14]. Ultrasound has proven to be both safe and ethical for in vivo use in a variety of applications [15,16]. Ultrasound also induces biological effects that are beneficial for therapeutic applications. It enhances transdermal drug delivery, enhances uptake in cells and tissues, and facilitates wound healing [13,17,18,19,20,21]. Ultrasound provides the capability for a wide variety of applications in the biomedical field including imaging [22], clinical diagnosis [23], therapeutics delivery [20,24,25], detection [26], sensing [27,28], the initiation of chemical and biological processes [29,30,31], and the release of signaling molecules [32].
Ultrasound also possesses several advantages as a stimulus for drug delivery platforms. It allows for the control of material properties and functions both easily and safely. It is non-ionizing, non-invasive, localized, and allows for deep tissue penetration and spatiotemporal control [33,34,35,36]. Ultrasound possesses the ability to be focused and localized to a small region of interest [15,37]. Acoustic energy can then be transferred by high or low intensity focused ultrasound either via thermal or non-thermal mechanisms [24]. A wide variety of polymeric carriers have been developed for ultrasound-responsive drug delivery. The possibilities offered by micelles, nanobubbles, nanodroplets, emulsions, and vesicles have already been thoroughly reviewed [38,39,40,41,42,43,44,45]. Consequently, we will only focus on the prospects offered by hydrogels as ultrasound-responsive delivery platforms. The aim of the present article is to highlight the mechanisms of inducing payload release via ultrasound, examine the latest innovative strategies employed to rationally design hydrogels, and describe their successful applications.
2. Acoustics
The developing field of responsive hydrogels is reaching new intersection points with external stimulus triggers. Recent developments have brought stimuli-responsive hydrogels into the field of acoustics and ultrasound. In this case, the acoustics field can be defined as the use of mechanical waves for energetic transfer in materials such as solids, liquids, or gases [37,46]. The transfer of energy into and through materials is then converted into specific acoustic responses for each hydrogel. These acoustic responses include payload delivery, modulation of material properties, initiation of biochemical processes, directed assembly, actuation, locomotion, or sensing [37,47,48,49,50].
The positive characteristics of ultrasound acoustics are frequency, wavelength, time, and transmission loss [51]. While acoustic frequencies range anywhere from 1 Hz to over 100 GHz, ultrasound frequencies only make up the range of 20 kHz to 50 MHz [37,46]. This range of frequencies is particularly interesting since it is outside of the range of human hearing [37]. Additionally, these ultrasound frequencies have generally small wavelengths in water, making them extremely compatible with responsive systems used within the human body [52]. The short time scales of ultrasound frequencies also make them extremely efficient in energy exchange [53]. Another positive characteristic is the low amount of transmission loss within the human body in this frequency range [37]. Due to these positive characteristics, ultrasound is an ideal external trigger for stimuli-responsive hydrogels.
3. Acoustic Mechanisms
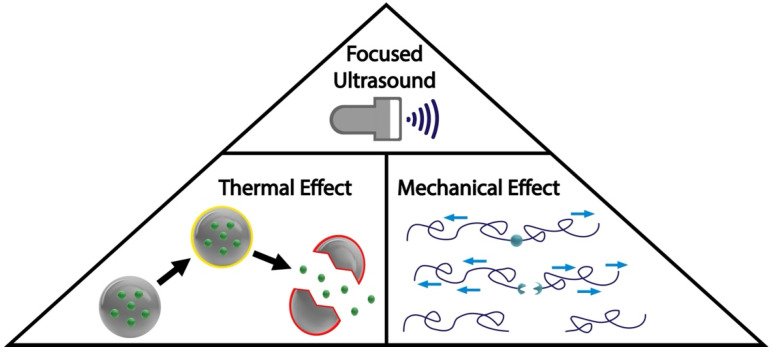
When using ultrasound acoustics on stimuli-responsive hydrogels, acoustic mechanisms are the pathway in which energy is transferred to induce a response. Acoustic responses typically involve work that is not directly correlated to acoustic waves. The acoustic waves are instead used for energetic transfer through both thermal and non-thermal mechanisms within a responsive hydrogel (Figure 1).
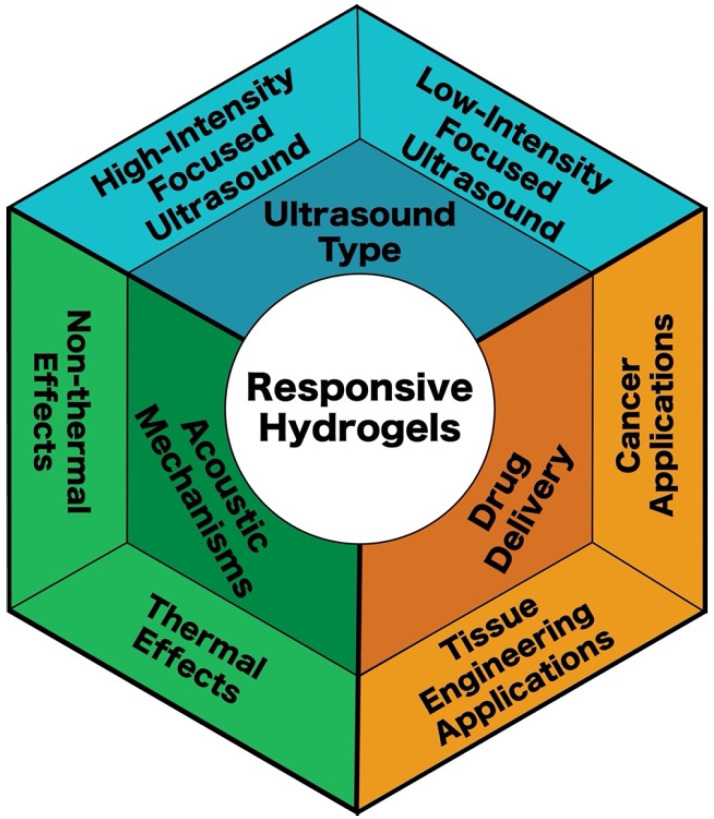
Figure 1.
Ultrasound-sensitive hydrogels are designed to respond to ultrasound (either low or high-intensity) via thermal or non-thermal effects. Applications for these drug delivery systems include cancer therapy and tissue engineering.
The thermal mechanism (Figure 2) is the pathway in which acoustic energy is transferred into thermal energy. The increase in temperature caused by ultrasound irradiation enhances drug diffusion and increases cell permeability [54]. Positive results have been observed with ultrasound-triggered drug release in thermosensitive hydrogels containing colloids such as nanoparticles [55], liposomes [56], and micelles [57]. While the power of high-intensity focused ultrasound is proven to be useful for drug delivery, damage to surrounding cells should be accounted for when considering long-term hyperthermia [33,58,59].
Figure 2.
Mechanisms of the ultrasound response of hydrogels. Acoustic energy can be transferred either via thermal or mechanical effects.
The non-thermal mechanism (Figure 2) is the pathway in which acoustic energy is transferred into mechanical energy in the form of oscillation and force [33]. This mechanical energy can take the form of acoustic cavitation. Cavitation is the formation of bubbles within a material, in which the bubble rapidly oscillates and then collapses within itself [60]. Cavitation has been used for drug delivery for chemotherapy [61] and bone regeneration [62,63]. Mechanical energy can also take the form of ultrasonic mechanical force. This mechanical force can be used to cleave unstable bonds [33]. Acoustic radiation force is another form of mechanical energy derived from ultrasound. The forces created by the acoustic waves act on the particles suspended within a fluid, these particles then move, cluster, and interact with one another [64]. The movement and interaction of these particles create acoustic radiation forces, which when paired with low-intensity focused ultrasound can be used for drug delivery and bone regeneration [65,66].
High-intensity focused ultrasound and low-intensity focused ultrasound prove to be effective in drug delivery using both thermal and non-thermal mechanisms in stimuli-responsive hydrogels. High-intensity focused ultrasound is extremely effective when inducing drug release, however possible damages and challenges may occur for sensitive biological systems [37,67]. While low-intensity focused ultrasound may be less powerful, it is at lower risk of damaging sensitive biological systems [68,69]. In scenarios using thermo-responsive hydrogels with hyperthermia as the thermal mechanism, high-intensity focused ultrasound would be ideal [33]. While both forms of focused ultrasound have respective challenges, it is seen that each can be useful for different applications.
Thermo-responsive and ultrasound-responsive hydrogels respond positively to ultrasound acoustics, making focused ultrasound an excellent external trigger for both systems. Both types of hydrogels prove to be responsive to ultrasound stimulation due to the combination of hyperthermia and sonoporation induced by focused ultrasound [33,58,70]. While different mechanisms exist for both types of hydrogels, each transfers acoustic energy into a form of work proven to be useful for drug delivery. Specifically, drug delivery for the purpose of cancer therapeutics and tissue engineering. Thermo-responsive materials paired with focused ultrasound have been used for both cancer treatments [42,71] and tissue repair [72]. Ultrasound-responsive materials paired with focused ultrasound have been used for both chemotherapy [73] and bone tissue engineering [63,74].
4. Designing Hydrogels for Drug Delivery
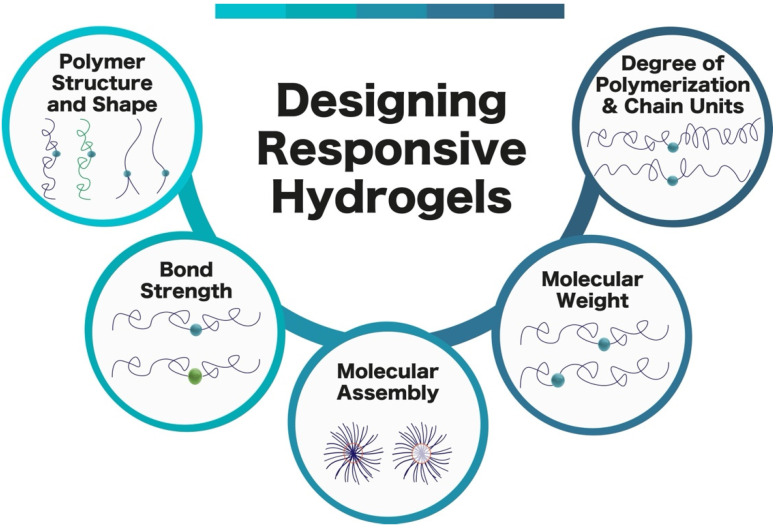
Rationally designing stimuli-responsive hydrogels to be used for ultrasound-triggered drug delivery requires a thorough understanding of the parameters that affect hydrogel response (Figure 3). These key factors are: bond strength, molecular weight, degree of polymerization, chain units, polymer structure, shape, and molecular assembly [33,75,76]. Rationally designing hydrogels to be as sensitive to ultrasound as possible is critical, as it will greatly decrease the chances of adverse biological effects [12,58].
Figure 3.
Overview of parameters influencing the design of ultrasound-responsive polymer-based hydrogels.
These parameters are crucial when rationally designing stimuli-responsive hydrogels. Drug release from polymer systems requires relatively low amounts of energy to break, when paired with weaker bonds [77,78,79]. Molecular weight distribution also affects the responsiveness and location of mechanical force acting along a polymer chain [80,81,82]. The degree of polymerization and chain units influence the mechanochemical activity of polymeric materials [83,84,85]. Polymer structure and shape both play a role in the sonomechanical effects of ultrasound on materials [86,87,88]. The designed molecular assembly can also influence the mechanochemical activity of the materials [89,90,91]. The amount of energy used will be lowered by implementing these factors into the design of hydrogel matrices, which will also decrease the chances of surrounding tissue damage.
The factors involving the structure of a stimuli-responsive hydrogel have large effects on drug delivery, but another important parameter is the embedded payload or carrier within the hydrogel matrix. Possible embedded nanocarriers include microbubbles [92], nanoparticles [93,94,95], liposomes [92], loaded nanodroplets [72,96], and micelles [97,98]. Cells can be placed into hydrogel matrices for direct diffusion into the surrounding area [65] or aided by nanocarriers for increased targeting specificity [72]. Proteins have been diffused from hydrogels without direct targeting [99,100,101], or aided by nanocarriers in drug delivery systems [102]. Payloads such as drugs can also be directly diffused from hydrogels [103], or aided by nanocarriers for targeted drug delivery [104]. The rational design of hydrogels for ultrasound-triggered drug release is dependent on both the structural factors of the matrix and the embedded materials within the hydrogel.
While hydrogel matrices affect the response to focused ultrasound, the specific parameters of the applied ultrasound also influence the outcome. Two types of ultrasound can be used, either High-Intensity Focused Ultrasound (HIFU) or Low-Intensity Focused Ultrasound (LIFU), each being beneficial for different applications [37,66,69]. LIFU is advantageous for applications involving reversible cellular effects [15] and increased tissue regeneration [105]. For instance, Kearney et al. [93] and Levingstone et al. [106] used LIFU at 2.5 min per hour for 5 h with an intensity of 9.6 mW/cm2 to induce bone regeneration aided by BMP-2 release. For applications involving irreversible cell death or tissue ablation, HIFU would most likely be preferred [107]. For example, HIFU was used by Meng et al. [108] and Zhu et al. [109] at a 50% duty cycle with intensities of 6 W/cm2 and 1 W/cm2, respectively, to promote release and uptake in tumor systems.
Ultrasound has proven to be both safe and ethical for in vivo use in a variety of applications [15,16]. The Food and Drug Administration (FDA) has defined safety guidelines for ultrasound exposure [15]. Criteria such as the mechanical index, thermal index, spatial peak pulse average intensity, and spatial peak temporal average intensity have been defined to stipulate the maximum allowed ultrasound exposure [58,110,111]. Adverse biological effects can be avoided during in vivo ultrasound studies when following these.
Drug delivery applications must be fully understood to rationally design hydrogels specific for each application. The two main applications for ultrasound drug delivery via hydrogel systems are tissue engineering and cancer therapy. Each application features a variety of hydrogel systems, ultrasound parameters, delivery methods, and drugs used.
5. Tissue Engineering Applications
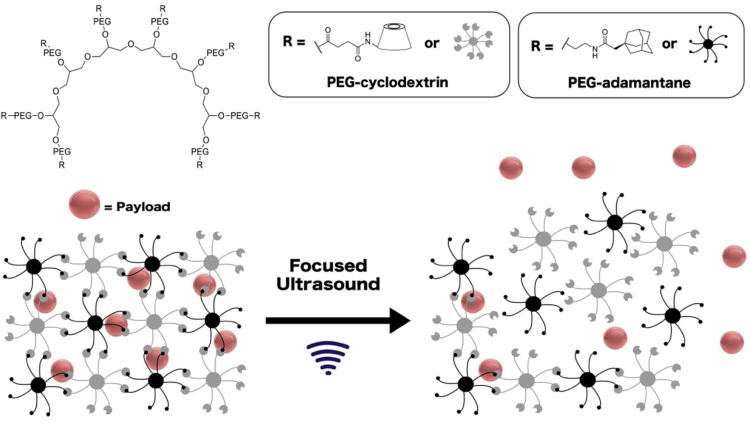
Ultrasound has traditionally been used for imaging tissue and bone defects, but is more recently being used to control drug release from responsive hydrogel systems with spatiotemporal control. Injectable hydrogels have been chosen as drug release systems due to their high capabilities of drug loading and biocompatibility [112,113]. More advantages of the hydrogel networks come from their ability to act as scaffolds and carry therapeutic materials for release [113,114]. For instance, Yamaguchi and al. [100] developed supramolecular PEG hydrogels crosslinked with a host-guest interaction between PEG-β-cyclodextrin and PEG-adamantane. Embedded protein payloads were released in a site-specific manner from these hydrogels during exposure to focused ultrasound (Figure 4).
Figure 4.
Design of ultrasound-responsive supramolecular PEG hydrogels crosslinked with a host-guest interaction between PEG-β-cyclodextrin and PEG-adamantane. This hydrogel matrix was developed by Yamaguchi et al. [100] and used for the controlled delivery of protein payloads.
Tissue engineering hydrogel systems are rationally designed for a specific use, meaning each system has its own application, polymeric backbone, and delivery method (Table 1). Some of the tissue engineering applications include bone regeneration [65,93,106], cartilage repair [72,115], and skin repair [116]. The polymeric backbone of responsive hydrogels includes materials such as alginate [93,106], chitosan [72,115], cellulose [116], fibrin [117,118,119], and collagen [65]. The loading of these systems is made up of cells, proteins, or drugs. These ultrasound-responsive hydrogels then release their embedded payloads when exposed to focused ultrasound. This release occurs both with and without nanocarriers to aid in targeting. Ultrasound also proved to be safe when used in vivo for the delivery of angiogenic growth factors [117,119,120].
Table 1.
Characteristics of ultrasound-responsive hydrogels for tissue engineering applications.
| Application | Hydrogel Polymer System | Payload | Ultrasound Parameters | Reference |
|---|---|---|---|---|
| Bone Regeneration |
Alginate Hydrogel |
BMP-2 | 2.5 min/h for 5 h Amplitude of 25% 9.6 mW/cm2 |
[106] |
| Cartilage Repair | Chitosan Hydrogel |
BMSCs aided by nanocarriers | 1 MHz 2–3 W/cm2 20–30% duty cycle |
[72] |
| Bone Regeneration |
Alginate Hydrogel |
BMP-2 conjugated gold nanoparticles | 2.5 min/h for 5 h Amplitude of 25% 9.6 mW/cm2 |
[93] |
| Cartilage Repair |
Chitosan Hydrogel |
Kartogenin on microparticles |
2 and 5 min intervals | [115] |
| Skin Repair | Cellulose Hydrogel Film |
Mimosa drug | LIFU 23, 43, and 96 kHz 5–30 W |
[116] |
| Vascularization | Fibrin Hydrogel | bFGF release | 100 Hz, 6.1 MPa 5.4 μs pulse |
[117] |
| Bone Regeneration |
Collagen Hydrogel |
Osteoblasts | LIPUS 1 MHz, 1 kHz, 1 Hz Duty cycle: 20%, 50% or 100% 30 and 150 mW/cm2 |
[65] |
6. Applications for Cancer Therapy
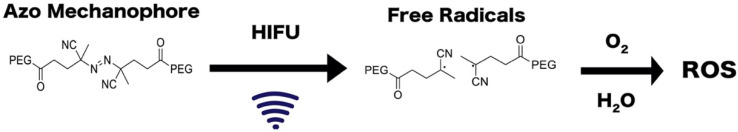
Ultrasound has successfully been used as a minimally invasive diagnostic tool for the detection and follow-up of cancer patients [26,107,121] and for analyte detection [122,123,124,125]. Ultrasound has also been used for cancer treatment due to its effective real-time capabilities in imaging and has more recently been used for drug delivery from responsive hydrogel systems [126,127,128,129,130,131]. Like the hydrogel systems used for drug delivery in tissue engineering, these hydrogels were chosen due to their high loading efficiencies, stability, and flexibility [17]. The hydrogel systems could be loaded with either therapeutic drugs [132] or contrast agents for cancer [133]. For instance, Kim and al. [134] embedded mechanophores into PEG hydrogels. When activated by ultrasound, the mechanophores generated free radicals that converted to free oxygen species effectively killing melanoma and breast cancer cells in vitro (Figure 5).
Figure 5.
Design of ultrasound-responsive mechanophores embedded into PEG hydrogels, generating reactive oxygen species (ROS) when activated by high-intensity focused ultrasound. This hydrogel matrix was developed by Kim et al. [134] and used for the selective elimination of cancer cells in vitro.
The hydrogel systems that are rationally designed for cancer therapeutics cover a wide variety of applications, polymer systems, materials delivered, and nanocarriers used. Some of these applications include breast cancer [134,135,136], melanoma [103,134], tumor systems [108,109,137], and general cancer therapy [104]. Hydrogel polymer systems include alginate [103,135], PEG [134], OEGMA [108], hyaluronic acid [104], polylysine [136], chitosan [109], and silk fibroin [137]. These systems are used to deliver a variety of drugs, proteins, cells, and therapeutics payloads (Table 2). This delivery is completed both with and without the aid of nanocarriers within the system to complete the task of drug delivery. Ultrasound also proved to be safe when used in vivo for the delivery of antitumor agents such as doxorubicin or mitoxantrone [99,108,135].
Table 2.
Characteristics of hydrogel polymer systems used for cancer therapy.
| Application | Hydrogel Polymer System | Payload | Ultrasound Parameters | Reference |
|---|---|---|---|---|
| Breast Cancer Treatment | Alginate Hydrogel |
Mitoxantrone | HIFU 9.6 mW/cm2 5 min pulses/h, /2 h, or /24 h |
[135] |
| Melanoma and Breast Cancer | PEG Hydrogel | AZO-Mechanophores for MDT | HIFU 550 kHz 115 W/cm2, 1.9 MPa 10 s on and 20 s off |
[134] |
| Tumor Systems | Nanocomposite Hydrogel | Nanovaccines (ORP nanoparticles) | HIFU 40 kHz 6 W/cm2, 50% duty cycle |
[108] |
| Cancer Therapy | Hyaluronic Acid Hydrogel | Doxorubicin loaded gold nanoparticles | HIFU 10, 20, 30, or 50 W 30 or 60 min 1.5 MHz 50% Duty cycle 1 Hz pulse frequency |
[104] |
| Melanoma | Alginate Hydrogel |
Mitoxantrone | HIFU 20% or 40% amplitude 1 or 5 min |
[103] |
| Breast Cancer | Polylysine Nanogel |
Epirubicin aided by ICAM-1 | HIFU 15 or 30 min 10 W |
[136] |
| Tumor Systems | Chitosan Hydrogel |
Piezoelectric Tetragonal BaTiO3 |
HIFU 1 MHz, 1 W/cm2 50% duty cycle 1, 2, 3, 4, 5, or 10 min |
[109] |
| Tumor Systems |
Silk Fibroin Hydrogel |
Vincristine | HIFU 1, 2, or 3 W 14.3, 28.5, or 42.8 W/cm2 20 s or 1 min |
[137] |
7. Conclusions
Ultrasound-responsive hydrogels have been developed using a wide range of methods for delivery applications ranging from cancer therapeutics to bone regeneration. Ultrasound offers great advantages as an external trigger. It is localized, non-invasive, has deep tissue penetration, and offers real-time feedback by sonography. Ultrasound can also be focused to a small region of space and transfer acoustic energy via different thermal or non-thermal mechanisms.
We envision that future hydrogel delivery platforms will be custom-tailored for the chosen embedded payload in order to create synergistic effects between the payload and the ultrasound application in a specific tissue. One promising area of development is the use of thermoresponsive Diels-Alder linkers to crosslink polymeric hydrogels. This Click Chemistry reaction presents several advantages, it can be conducted in aqueous solution, it is highly efficient, it does not require a catalyst, and it is thermally reversible. When triggered by heat, the retro Diels-Alder reaction yields the original reactants. The chemical composition of these linkers can be modified to adjust the forward and reverse energy barriers, allowing to fine-tune the associated payload release kinetics.
Drug delivery systems could also be designed to respond to a combination of external focused ultrasound and internal physiological trigger (pH, enzyme, redox, or temperature) to combine their benefits. Upcoming platforms might also try to leverage the ability of ultrasound to facilitate transdermal delivery and enhanced uptake in cells and tissues.
Focused ultrasound will continue to be used in all types of drug delivery applications due to its ability to deliver payloads on-demand with spatiotemporal control. The interactions between acoustic mechanisms and drug delivery mechanisms will be critical in defining a specific application for an ultrasound-responsive hydrogel delivery system. Acoustic energy can be transmitted either via thermal mechanisms or non-thermal mechanisms. Delivery platforms for cancer therapy will most likely be dependent on high-intensity focused ultrasound due to its ability to invoke a thermal mechanism in solid tumors. Low-intensity focused ultrasound will be used for tissue engineering thanks to its capability to enhance uptake in cells and tissue.
The main challenges for future ultrasound-responsive drug delivery systems are related to the safety of the focused ultrasound, especially for high-intensity focused ultrasound. Running a system with the lowest amount of energy required is always beneficial to mitigate any potential damage. Future ultrasound-responsive hydrogels will most likely be rationally designed to reduce the amount of energy required to trigger the release and minimize any risk of damage to the surrounding tissues. Guidance from regulating agencies such as the safe operating guidelines developed by the FDA will be helpful in the future to safely translate to the clinic the emerging early-stage strategies currently explored in vitro.
Overall, ultrasound has a tremendous potential to become increasingly popular as a stimulus for on-demand drug delivery platforms and to improve the clinical outcome of a variety of advanced drug delivery applications.
Author Contributions
Conceptualization, T.J.Y., J.H.A. and D.J.H.; writing—original draft preparation, T.J.Y., J.H.A. and D.J.H.; writing—review and editing, T.J.Y., J.H.A. and D.J.H.; supervision, D.J.H.; project administration, D.J.H.; funding acquisition, D.J.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported partially by the National Institute of Dental and Craniofacial Research of the National Institutes of Health under award number (RDE024790A), the Office of the Assistant Secretary of Defense for Health Affairs through the Peer Reviewed Medical Research Program under Award No. W81XWH2110052. The opinions, interpretations, conclusions, and recommendations are those of the authors and are not necessarily endorsed by the National Institutes of Health or the Department of Defense.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Spiller K.L., Vunjak-Novakovic G. Clinical translation of controlled protein delivery systems for tissue engineering. Drug Deliv. Transl. Res. 2015;5:101–115. doi: 10.1007/s13346-013-0135-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li J., Mooney D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016;1:16071. doi: 10.1038/natrevmats.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brudno Y., Mooney D.J. On-demand drug delivery from local depots. J. Control. Release. 2015;219:8–17. doi: 10.1016/j.jconrel.2015.09.011. [DOI] [PubMed] [Google Scholar]
- 4.Wells C.M., Harris M., Choi L., Murali V.P., Guerra F.D., Jennings J.A. Stimuli-Responsive Drug Release from Smart Polymers. J. Funct. Biomater. 2019;10:34. doi: 10.3390/jfb10030034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manouras T., Vamvakaki M. Field responsive materials: Photo-, electro-, magnetic- and ultrasound-sensitive polymers. Polym. Chem. 2017;8:74–96. doi: 10.1039/C6PY01455K. [DOI] [Google Scholar]
- 6.Lavrador P., Esteves M.R., Gaspar V.M., Mano J.F. Stimuli-Responsive Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2021;31:2005941. doi: 10.1002/adfm.202005941. [DOI] [Google Scholar]
- 7.El-Husseiny H.M., Mady E.A., Hamabe L., Abugomaa A., Shimada K., Yoshida T., Tanaka T., Yokoi A., Elbadawy M., Tanaka R. Smart/stimuli-responsive hydrogels: Cutting-edge platforms for tissue engineering and other biomedical applications. Mater. Today Bio. 2022;13:100186. doi: 10.1016/j.mtbio.2021.100186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sun Y., Davis E. Nanoplatforms for Targeted Stimuli-Responsive Drug Delivery: A Review of Platform Materials and Stimuli-Responsive Release and Targeting Mechanisms. Nanomaterials. 2021;11:746. doi: 10.3390/nano11030746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mirvakili S.M., Langer R. Wireless on-demand drug delivery. Nat. Electron. 2021;4:464–477. doi: 10.1038/s41928-021-00614-9. [DOI] [Google Scholar]
- 10.Yoshida R. Self-oscillating gels driven by the Belousov-Zhabotinsky reaction as novel smart materials. Adv. Mater. 2010;22:3463–3483. doi: 10.1002/adma.200904075. [DOI] [PubMed] [Google Scholar]
- 11.Nielsen M.B., Søgaard S.B., Bech Andersen S., Skjoldbye B., Hansen K.L., Rafaelsen S., Nørgaard N., Carlsen J.F. Highlights of the development in ultrasound during the last 70 years: A historical review. Acta Radiol. 2021;62:1499–1514. doi: 10.1177/02841851211050859. [DOI] [PubMed] [Google Scholar]
- 12.Dalecki D. Mechanical Bioeffects of Ultrasound. Annu. Rev. Biomed. Eng. 2004;6:229–248. doi: 10.1146/annurev.bioeng.6.040803.140126. [DOI] [PubMed] [Google Scholar]
- 13.Mitragotri S. Healing sound: The use of ultrasound in drug delivery and other therapeutic applications. Nat. Rev. Drug Discov. 2005;4:255–260. doi: 10.1038/nrd1662. [DOI] [PubMed] [Google Scholar]
- 14.Elhelf I.A.S., Albahar H., Shah U., Oto A., Cressman E., Almekkawy M. High intensity focused ultrasound: The fundamentals, clinical applications and research trends. Diagn. Interv. Imaging. 2018;99:349–359. doi: 10.1016/j.diii.2018.03.001. [DOI] [PubMed] [Google Scholar]
- 15.Baek H., Pahk K.J., Kim H. A review of low-intensity focused ultrasound for neuromodulation. Biomed. Eng. Lett. 2017;7:135–142. doi: 10.1007/s13534-016-0007-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller D.L., Smith N.B., Bailey M.R., Czarnota G.J., Hynynen K., Makin I.R. Overview of therapeutic ultrasound applications and safety considerations. J. Ultrasound Med. 2012;31:623–634. doi: 10.7863/jum.2012.31.4.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou Y., Liu G., Guo S. Advances in ultrasound-responsive hydrogels for biomedical applications. J. Mater. Chem. B. 2022 doi: 10.1039/D2TB00541G. [DOI] [PubMed] [Google Scholar]
- 18.Huang D., Sun M., Bu Y., Luo F., Lin C., Lin Z., Weng Z., Yang F., Wu D. Microcapsule-embedded hydrogel patches for ultrasound responsive and enhanced transdermal delivery of diclofenac sodium. J. Mater. Chem. B. 2019;7:2330–2337. doi: 10.1039/C8TB02928H. [DOI] [PubMed] [Google Scholar]
- 19.Yang C.D., Jessen J., Lin K.Y. Ultrasound-assisted ocular drug delivery: A review of current evidence. J. Clin. Ultrasound. 2022;50:685–693. doi: 10.1002/jcu.23214. [DOI] [PubMed] [Google Scholar]
- 20.Noble M.L., Mourad P.D., Ratner B.D. Digital drug delivery: On–off ultrasound controlled antibiotic release from coated matrices with negligible background leaching. Biomater. Sci. 2014;2:893–902. doi: 10.1039/C3BM60203F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang S., Zhang S., Luo S., Tang P., Wan M., Wu D., Gao W. Ultrasound-assisted brain delivery of nanomedicines for brain tumor therapy: Advance and prospect. J. Nanobiotechnol. 2022;20 doi: 10.1186/s12951-022-01464-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Carovac A., Smajlovic F., Junuzovic D. Application of ultrasound in medicine. Acta Inf. Med. 2011;19:168–171. doi: 10.5455/aim.2011.19.168-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fowler J.R., Gaughan J.P., Ilyas A.M. The Sensitivity and Specificity of Ultrasound for the Diagnosis of Carpal Tunnel Syndrome: A Meta-analysis. Clin. Orthop. Relat. Res. 2011;469:1089–1094. doi: 10.1007/s11999-010-1637-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ter Haar G. Therapeutic applications of ultrasound. Prog. Biophys. Mol. Biol. 2007;93:111–129. doi: 10.1016/j.pbiomolbio.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 25.Grimaudo M.A., Krishnakumar G.S., Giusto E., Furlani F., Bassi G., Rossi A., Molinari F., Lista F., Montesi M., Panseri S. Bioactive injectable hydrogels for on demand molecule/cell delivery and for tissue regeneration in the central nervous system. Acta Biomater. 2022;140:88–101. doi: 10.1016/j.actbio.2021.11.038. [DOI] [PubMed] [Google Scholar]
- 26.Voit C., Schoengen A., Schwürzer-Voit M., Weber L., Ulrich J., Sterry W., Proebstle T.M. The role of ultrasound in detection and management of regional disease in melanoma patients. Semin. Oncol. 2002;29:353–360. doi: 10.1053/sonc.2002.34113. [DOI] [PubMed] [Google Scholar]
- 27.Piech D.K., Johnson B.C., Shen K., Ghanbari M.M., Li K.Y., Neely R.M., Kay J.E., Carmena J.M., Maharbiz M.M., Muller R. A wireless millimetre-scale implantable neural stimulator with ultrasonically powered bidirectional communication. Nat. Biomed. Eng. 2020;4:207–222. doi: 10.1038/s41551-020-0518-9. [DOI] [PubMed] [Google Scholar]
- 28.Shi C., Andino-Pavlovsky V., Lee S.A., Costa T., Elloian J., Konofagou E.E., Shepard K.L. Application of a sub-0.1-mm(3) implantable mote for in vivo real-time wireless temperature sensing. Sci. Adv. 2021;7:eabf6312. doi: 10.1126/sciadv.abf6312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang X., Zhong X., Gong F., Chao Y., Cheng L. Newly developed strategies for improving sonodynamic therapy. Mater. Horiz. 2020;7:2028–2046. doi: 10.1039/D0MH00613K. [DOI] [Google Scholar]
- 30.Celli J.P., Spring B.Q., Rizvi I., Evans C.L., Samkoe K.S., Verma S., Pogue B.W., Hasan T. Imaging and photodynamic therapy: Mechanisms, monitoring, and optimization. Chem. Rev. 2010;110:2795–2838. doi: 10.1021/cr900300p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rosenthal I., Sostaric J.Z., Riesz P. Sonodynamic therapy––A review of the synergistic effects of drugs and ultrasound. Ultrason. Sonochem. 2004;11:349–363. doi: 10.1016/j.ultsonch.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Fang Y., Cheng J., Shen Z., You T., Ding S., Hu J. Ultrasound-Mediated Release of Gaseous Signaling Molecules for Biomedical Applications. Macromol. Rapid Commun. 2022;43:e2100814. doi: 10.1002/marc.202100814. [DOI] [PubMed] [Google Scholar]
- 33.Tu L., Liao Z., Luo Z., Wu Y.L., Herrmann A., Huo S. Ultrasound-controlled drug release and drug activation for cancer therapy. Exploration. 2021;1:20210023. doi: 10.1002/EXP.20210023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kost J., Leong K., Langer R. Ultrasound-enhanced polymer degradation and release of incorporated substances. Proc. Natl. Acad. Sci. USA. 1989;86:7663–7666. doi: 10.1073/pnas.86.20.7663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ferrara K.W. Driving delivery vehicles with ultrasound. Adv. Drug Deliv. Rev. 2008;60:1097–1102. doi: 10.1016/j.addr.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shin Low S., Nong Lim C., Yew M., Siong Chai W., Low L.E., Manickam S., Ti Tey B., Show P.L. Recent ultrasound advancements for the manipulation of nanobiomaterials and nanoformulations for drug delivery. Ultrason. Sonochem. 2021;80:105805. doi: 10.1016/j.ultsonch.2021.105805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Athanassiadis A.G., Ma Z., Moreno-Gomez N., Melde K., Choi E., Goyal R., Fischer P. Ultrasound-Responsive Systems as Components for Smart Materials. Chem. Rev. 2022;122:5165–5208. doi: 10.1021/acs.chemrev.1c00622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Boissenot T., Bordat A., Fattal E., Tsapis N. Ultrasound-triggered drug delivery for cancer treatment using drug delivery systems: From theoretical considerations to practical applications. J. Control. Release. 2016;241:144–163. doi: 10.1016/j.jconrel.2016.09.026. [DOI] [PubMed] [Google Scholar]
- 39.Sirsi S.R., Borden M.A. State-of-the-art materials for ultrasound-triggered drug delivery. Adv. Drug Deliv. Rev. 2014;72:3–14. doi: 10.1016/j.addr.2013.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ouyang J., Xie A., Zhou J., Liu R., Wang L., Liu H., Kong N., Tao W. Minimally invasive nanomedicine: Nanotechnology in photo-/ultrasound-/radiation-/magnetism-mediated therapy and imaging. Chem. Soc. Rev. 2022;51:4996–5041. doi: 10.1039/D1CS01148K. [DOI] [PubMed] [Google Scholar]
- 41.Husseini G.A., Pitt W.G. Micelles and nanoparticles for ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 2008;60:1137–1152. doi: 10.1016/j.addr.2008.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Frenkel V. Ultrasound mediated delivery of drugs and genes to solid tumors. Adv. Drug Deliv. Rev. 2008;60:1193–1208. doi: 10.1016/j.addr.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hernot S., Klibanov A.L. Microbubbles in ultrasound-triggered drug and gene delivery. Adv. Drug Deliv. Rev. 2008;60:1153–1166. doi: 10.1016/j.addr.2008.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Huang S.-L. Liposomes in ultrasonic drug and gene delivery. Adv. Drug Deliv. Rev. 2008;60:1167–1176. doi: 10.1016/j.addr.2008.03.003. [DOI] [PubMed] [Google Scholar]
- 45.Wei P., Cornel E.J., Du J. Ultrasound-responsive polymer-based drug delivery systems. Drug Deliv. Transl. Res. 2021;11:1323–1339. doi: 10.1007/s13346-021-00963-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Entzian K., Aigner A. Drug Delivery by Ultrasound-Responsive Nanocarriers for Cancer Treatment. Pharmaceutics. 2021;13:1135. doi: 10.3390/pharmaceutics13081135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Low S.S., Yew M., Lim C.N., Chai W.S., Low L.E., Manickam S., Tey B.T., Show P.L. Sonoproduction of nanobiomaterials—A critical review. Ultrason. Sonochem. 2022;82:105887. doi: 10.1016/j.ultsonch.2021.105887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Norris E.G., Dalecki D., Hocking D.C. Acoustic Fabrication of Collagen–Fibronectin Composite Gels Accelerates Microtissue Formation. Appl. Sci. 2020;10:2907. doi: 10.3390/app10082907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Norris E.G., Dalecki D., Hocking D.C. Using Acoustic Fields to Fabricate ECM-Based Biomaterials for Regenerative Medicine Applications. Recent Prog. Mater. 2020;2:1–24. doi: 10.21926/rpm.2003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Norris E.G., Majeski J., Wayson S.E., Coleman H., Choe R., Dalecki D., Hocking D.C. Non-invasive acoustic fabrication methods to enhance collagen hydrogel bioactivity. Mater. Res. Express. 2019;6:125410. doi: 10.1088/2053-1591/ab597a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kinsler L.E., Frey A.R., Coppens A.B., Sanders J.V. Fundamentals of Acoustics. John Wiley & Sons; Hoboken, NJ, USA: 2000. [Google Scholar]
- 52.Seidi F., Jenjob R., Crespy D. Designing Smart Polymer Conjugates for Controlled Release of Payloads. Chem. Rev. 2018;118:3965–4036. doi: 10.1021/acs.chemrev.8b00006. [DOI] [PubMed] [Google Scholar]
- 53.Zhao J., Lee V.E., Liu R., Priestley R.D. Responsive Polymers as Smart Nanomaterials Enable Diverse Applications. Annu. Rev. Chem. Biomol. Eng. 2019;10:361–382. doi: 10.1146/annurev-chembioeng-060718-030155. [DOI] [PubMed] [Google Scholar]
- 54.Rapoport N.Y., Kennedy A.M., Shea J.E., Scaife C.L., Nam K.-H. Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles. J. Control. Release. 2009;138:268–276. doi: 10.1016/j.jconrel.2009.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kaczmarek K., Hornowski T., Kubovčíková M., Timko M., Koralewski M., Józefczak A. Heating Induced by Therapeutic Ultrasound in the Presence of Magnetic Nanoparticles. ACS Appl. Mater. Interfaces. 2018;10:11554–11564. doi: 10.1021/acsami.8b02496. [DOI] [PubMed] [Google Scholar]
- 56.Grüll H., Langereis S. Hyperthermia-triggered drug delivery from temperature-sensitive liposomes using MRI-guided high intensity focused ultrasound. J. Control. Release. 2012;161:317–327. doi: 10.1016/j.jconrel.2012.04.041. [DOI] [PubMed] [Google Scholar]
- 57.Ahmed S.E., Martins A.M., Husseini G.A. The use of ultrasound to release chemotherapeutic drugs from micelles and liposomes. J. Drug Target. 2015;23:16–42. doi: 10.3109/1061186X.2014.954119. [DOI] [PubMed] [Google Scholar]
- 58.Wu J., Nyborg W.L. Ultrasound, cavitation bubbles and their interaction with cells. Adv. Drug Deliv. Rev. 2008;60:1103–1116. doi: 10.1016/j.addr.2008.03.009. [DOI] [PubMed] [Google Scholar]
- 59.Draper D.O., Castel J.C., Castel D. Rate of Temperature Increase in Human Muscle During 1 MHz and 3 MHz Continuous Ultrasound. J. Orthop. Sports Phys. Ther. 1995;22:142–150. doi: 10.2519/jospt.1995.22.4.142. [DOI] [PubMed] [Google Scholar]
- 60.Frohly J., Labouret S., Bruneel C., Looten-Baquet I., Torguet R. Ultrasonic cavitation monitoring by acoustic noise power measurement. J. Acoust. Soc. Am. 2000;108:2012–2020. doi: 10.1121/1.1312360. [DOI] [PubMed] [Google Scholar]
- 61.Coussios C.C., Farny C.H., Ter Haar G., Roy R.A. Role of acoustic cavitation in the delivery and monitoring of cancer treatment by high-intensity focused ultrasound (HIFU) Int. J. Hyperth. 2007;23:105–120. doi: 10.1080/02656730701194131. [DOI] [PubMed] [Google Scholar]
- 62.Reher P., Elbeshir E.-N.I., Harvey W., Meghji S., Harris M. The stimulation of bone formation in vitro by therapeutic ultrasound. Ultrasound Med. Biol. 1997;23:1251–1258. doi: 10.1016/S0301-5629(97)00031-8. [DOI] [PubMed] [Google Scholar]
- 63.El-Husseiny H.M., Mady E.A., El-Dakroury W.A., Zewail M.B., Noshy M., Abdelfatah A.M., Doghish A.S. Smart/stimuli-responsive hydrogels: State-of-the-art platforms for bone tissue engineering. Appl. Mater. Today. 2022;13:101560. doi: 10.1016/j.apmt.2022.101560. [DOI] [Google Scholar]
- 64.Doinikov A.A. Acoustic radiation forces: Classical theory and recent advances. Recent Res. Dev. Acoust. 2003;1:39–67. [Google Scholar]
- 65.Veronick J.A., Assanah F., Piscopo N., Kutes Y., Vyas V., Nair L.S., Huey B.D., Khan Y. Mechanically Loading Cell/Hydrogel Constructs with Low-Intensity Pulsed Ultrasound for Bone Repair. Tissue Eng. Part A. 2018;24:254–263. doi: 10.1089/ten.tea.2016.0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McCarthy C., Camci-Unal G. Low Intensity Pulsed Ultrasound for Bone Tissue Engineering. Micromachines. 2021;12:1488. doi: 10.3390/mi12121488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Copelan A., Hartman J., Chehab M., Venkatesan A. High-Intensity Focused Ultrasound: Current Status for Image-Guided Therapy. Semin. Interv. Radiol. 2015;32:398–415. doi: 10.1055/s-0035-1564793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bystritsky A., Korb A.S., Douglas P.K., Cohen M.S., Melega W.P., Mulgaonkar A.P., DeSalles A., Min B.-K., Yoo S.-S. A review of low-intensity focused ultrasound pulsation. Brain Stimul. 2011;4:125–136. doi: 10.1016/j.brs.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 69.Ogura M., Paliwal S., Mitragotri S. Low-frequency sonophoresis: Current status and future prospects. Adv. Drug Deliv. Rev. 2008;60:1218–1223. doi: 10.1016/j.addr.2008.03.006. [DOI] [PubMed] [Google Scholar]
- 70.Zardad A.Z., Choonara Y.E., Du Toit L.C., Kumar P., Mabrouk M., Kondiah P.P.D., Pillay V. A Review of Thermo- and Ultrasound-Responsive Polymeric Systems for Delivery of Chemotherapeutic Agents. Polymers. 2016;8:359. doi: 10.3390/polym8100359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yang F.-Y., Wong T.-T., Teng M.-C., Liu R.-S., Lu M., Liang H.-F., Wei M.-C. Focused ultrasound and interleukin-4 receptor-targeted liposomal doxorubicin for enhanced targeted drug delivery and antitumor effect in glioblastoma multiforme. J. Control. Release. 2012;160:652–658. doi: 10.1016/j.jconrel.2012.02.023. [DOI] [PubMed] [Google Scholar]
- 72.Liu H., Xiang X., Huang J., Zhu B., Wang L., Tang Y., Du F., Li L., Yan F., Ma L., et al. Ultrasound augmenting injectable chemotaxis hydrogel for articular cartilage repair in osteoarthritis. Chin. Chem. Lett. 2021;32:1759–1764. doi: 10.1016/j.cclet.2020.12.004. [DOI] [Google Scholar]
- 73.Fabiilli M.L., Haworth K.J., Sebastian I.E., Kripfgans O.D., Carson P.L., Fowlkes J.B. Delivery of Chlorambucil Using an Acoustically-Triggered Perfluoropentane Emulsion. Ultrasound Med. Biol. 2010;36:1364–1375. doi: 10.1016/j.ultrasmedbio.2010.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bez M., Sheyn D., Tawackoli W., Avalos P., Shapiro G., Giaconi J.C., Da X., David S.B., Gavrity J., Awad H.A., et al. In situ bone tissue engineering via ultrasound-mediated gene delivery to endogenous progenitor cells in mini-pigs. Sci. Transl. Med. 2017;9:eaal3128. doi: 10.1126/scitranslmed.aal3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chai Q., Jiao Y., Yu X. Hydrogels for Biomedical Applications: Their Characteristics and the Mechanisms behind Them. Gels. 2017;3:6. doi: 10.3390/gels3010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Echeverria C., Fernandes S., Godinho M., Borges J., Soares P. Functional Stimuli-Responsive Gels: Hydrogels and Microgels. Gels. 2018;4:54. doi: 10.3390/gels4020054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wang Z.J., Jiang J., Mu Q., Maeda S., Nakajima T., Gong J.P. Azo-Crosslinked Double-Network Hydrogels Enabling Highly Efficient Mechanoradical Generation. J. Am. Chem. Soc. 2022;144:3154–3161. doi: 10.1021/jacs.1c12539. [DOI] [PubMed] [Google Scholar]
- 78.Lee B., Niu Z., Wang J., Slebodnick C., Craig S.L. Relative Mechanical Strengths of Weak Bonds in Sonochemical Polymer Mechanochemistry. J. Am. Chem. Soc. 2015;137:10826–10832. doi: 10.1021/jacs.5b06937. [DOI] [PubMed] [Google Scholar]
- 79.Perera M.M., Ayres N. Dynamic covalent bonds in self-healing, shape memory, and controllable stiffness hydrogels. Polym. Chem. 2020;11:1410–1423. doi: 10.1039/C9PY01694E. [DOI] [Google Scholar]
- 80.Caruso M.M., Davis D.A., Shen Q., Odom S.A., Sottos N.R., White S.R., Moore J.S. Mechanically-Induced Chemical Changes in Polymeric Materials. Chem. Rev. 2009;109:5755–5798. doi: 10.1021/cr9001353. [DOI] [PubMed] [Google Scholar]
- 81.Davis D.A., Hamilton A., Yang J., Cremar L.D., Van Gough D., Potisek S.L., Ong M.T., Braun P.V., Martínez T.J., White S.R., et al. Force-induced activation of covalent bonds in mechanoresponsive polymeric materials. Nature. 2009;459:68–72. doi: 10.1038/nature07970. [DOI] [PubMed] [Google Scholar]
- 82.Gandhi A., Paul A., Sen S.O., Sen K.K. Studies on thermoresponsive polymers: Phase behaviour, drug delivery and biomedical applications. Asian J. Pharm. Sci. 2015;10:99–107. doi: 10.1016/j.ajps.2014.08.010. [DOI] [Google Scholar]
- 83.Cravotto G., Cintas P. Forcing and Controlling Chemical Reactions with Ultrasound. Angew. Chem. Int. Ed. 2007;46:5476–5478. doi: 10.1002/anie.200701567. [DOI] [PubMed] [Google Scholar]
- 84.May P.A., Munaretto N.F., Hamoy M.B., Robb M.J., Moore J.S. Is Molecular Weight or Degree of Polymerization a Better Descriptor of Ultrasound-Induced Mechanochemical Transduction? ACS Macro Lett. 2016;5:177–180. doi: 10.1021/acsmacrolett.5b00855. [DOI] [PubMed] [Google Scholar]
- 85.Potisek S.L., Davis D.A., Sottos N.R., White S.R., Moore J.S. Mechanophore-Linked Addition Polymers. J. Am. Chem. Soc. 2007;129:13808–13809. doi: 10.1021/ja076189x. [DOI] [PubMed] [Google Scholar]
- 86.Xue L., Agarwal U.S., Lemstra P.J. Shear Degradation Resistance of Star Polymers during Elongational Flow. Macromolecules. 2005;38:8825–8832. doi: 10.1021/ma0502811. [DOI] [Google Scholar]
- 87.Striegel A.M. Influence of chain architecture on the mechanochemical degradation of macromolecules. J. Biochem. Biophys. Methods. 2003;56:117–139. doi: 10.1016/S0165-022X(03)00054-X. [DOI] [PubMed] [Google Scholar]
- 88.Ribas-Arino J., Shiga M., Marx D. Mechanochemical Transduction of Externally Applied Forces to Mechanophores. J. Am. Chem. Soc. 2010;132:10609–10614. doi: 10.1021/ja104958e. [DOI] [PubMed] [Google Scholar]
- 89.Lee C.K., Diesendruck C.E., Lu E., Pickett A.N., May P.A., Moore J.S., Braun P.V. Solvent Swelling Activation of a Mechanophore in a Polymer Network. Macromolecules. 2014;47:2690–2694. doi: 10.1021/ma500195h. [DOI] [Google Scholar]
- 90.Min Y., Huang S., Wang Y., Zhang Z., Du B., Zhang X., Fan Z. Sonochemical Transformation of Epoxy–Amine Thermoset into Soluble and Reusable Polymers. Macromolecules. 2015;48:316–322. doi: 10.1021/ma501934p. [DOI] [Google Scholar]
- 91.Black A.L., Lenhardt J.M., Craig S.L. From molecular mechanochemistry to stress-responsive materials. J. Mater. Chem. 2011;21:1655–1663. doi: 10.1039/C0JM02636K. [DOI] [Google Scholar]
- 92.Epstein-Barash H., Orbey G., Polat B.E., Ewoldt R.H., Feshitan J., Langer R., Borden M.A., Kohane D.S. A microcomposite hydrogel for repeated on-demand ultrasound-triggered drug delivery. Biomaterials. 2010;31:5208–5217. doi: 10.1016/j.biomaterials.2010.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kearney C.J., Skaat H., Kennedy S.M., Hu J., Darnell M., Raimondo T.M., Mooney D.J. Switchable Release of Entrapped Nanoparticles from Alginate Hydrogels. Adv. Healthc. Mater. 2015;4:1634–1639. doi: 10.1002/adhm.201500254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Kubota T., Kurashina Y., Zhao J., Ando K., Onoe H. Ultrasound-triggered on-demand drug delivery using hydrogel microbeads with release enhancer. Mater. Des. 2021;203:109580. doi: 10.1016/j.matdes.2021.109580. [DOI] [Google Scholar]
- 95.Jang K.W., Seol D., Ding L., Heo D.N., Lee S.J., Martin J.A., Kwon I.K. Ultrasound-triggered PLGA microparticle destruction and degradation for controlled delivery of local cytotoxicity and drug release. Int. J. Biol. Macromol. 2018;106:1211–1217. doi: 10.1016/j.ijbiomac.2017.08.125. [DOI] [PubMed] [Google Scholar]
- 96.Li G., Wang Y., Wang S., Jiang J. A Tough Composite Hydrogel can Controllably Deliver Hydrophobic Drugs under Ultrasound. Macromol. Mater. Eng. 2018;303:1700483. doi: 10.1002/mame.201700483. [DOI] [Google Scholar]
- 97.Wei L., Cai C., Lin J., Chen T. Dual-drug delivery system based on hydrogel/micelle composites. Biomaterials. 2009;30:2606–2613. doi: 10.1016/j.biomaterials.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 98.De Graaf A.J., Azevedo Próspero dos Santos I.I., Pieters E.H.E., Rijkers D.T.S., van Nostrum C.F., Vermonden T., Kok R.J., Hennink W.E., Mastrobattista E. A micelle-shedding thermosensitive hydrogel as sustained release formulation. J. Control. Release. 2012;162:582–590. doi: 10.1016/j.jconrel.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 99.Wu C.-H., Sun M.-K., Shieh J., Chen C.-S., Huang C.-W., Dai C.-A., Chang S.-W., Chen W.-S., Young T.-H. Ultrasound-responsive NIPAM-based hydrogels with tunable profile of controlled release of large molecules. Ultrasonics. 2018;83:157–163. doi: 10.1016/j.ultras.2017.03.019. [DOI] [PubMed] [Google Scholar]
- 100.Yamaguchi S., Higashi K., Azuma T., Okamoto A. Supramolecular Polymeric Hydrogels for Ultrasound-Guided Protein Release. Biotechnol. J. 2019;14:1800530. doi: 10.1002/biot.201800530. [DOI] [PubMed] [Google Scholar]
- 101.Arrizabalaga J.H., Smallcomb M., Abu-Laban M., Liu Y., Yeingst T.J., Dhawan A., Simon J.C., Hayes D.J. Ultrasound-Responsive Hydrogels for On-Demand Protein Release. ACS Appl. Bio Mater. 2022;5:3212–3218. doi: 10.1021/acsabm.2c00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hamidi M., Azadi A., Rafiei P. Hydrogel nanoparticles in drug delivery. Adv. Drug Deliv. Rev. 2008;60:1638–1649. doi: 10.1016/j.addr.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 103.Emi T., Michaud K., Orton E., Santilli G., Linh C., O’Connell M., Issa F., Kennedy S. Ultrasonic Generation of Pulsatile and Sequential Therapeutic Delivery Profiles from Calcium-Crosslinked Alginate Hydrogels. Molecules. 2019;24:1048. doi: 10.3390/molecules24061048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.An J.Y., Um W., You D.G., Song Y., Lee J., Van Quy N., Joo H., Jeon J., Park J.H. Gold-installed hyaluronic acid hydrogel for ultrasound-triggered thermal elevation and on-demand cargo release. Int. J. Biol. Macromol. 2021;193:553–561. doi: 10.1016/j.ijbiomac.2021.10.071. [DOI] [PubMed] [Google Scholar]
- 105.Carvalho D.C., Cliquet Júnior A. The action of low-intensity pulsed ultrasound in bones of osteopenic rats. Artif. Organs. 2004;28:114–118. doi: 10.1111/j.1525-1594.2004.07091.x. [DOI] [PubMed] [Google Scholar]
- 106.Levingstone T., Ali B., Kearney C., Dunne N. Hydroxyapatite sonosensitization of ultrasound-triggered, thermally responsive hydrogels: An on-demand delivery system for bone repair applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021;109:1622–1633. doi: 10.1002/jbm.b.34820. [DOI] [PubMed] [Google Scholar]
- 107.Phenix C.P., Togtema M., Pichardo S., Zehbe I., Curiel L. High intensity focused ultrasound technology, its scope and applications in therapy and drug delivery. J. Pharm. Pharm. Sci. 2014;17:136–153. doi: 10.18433/J3ZP5F. [DOI] [PubMed] [Google Scholar]
- 108.Meng Z., Zhang Y., She J., Zhou X., Xu J., Han X., Wang C., Zhu M., Liu Z. Ultrasound-Mediated Remotely Controlled Nanovaccine Delivery for Tumor Vaccination and Individualized Cancer Immunotherapy. Nano Lett. 2021;21:1228–1237. doi: 10.1021/acs.nanolett.0c03646. [DOI] [PubMed] [Google Scholar]
- 109.Zhu P., Chen Y., Shi J. Piezocatalytic Tumor Therapy by Ultrasound-Triggered and BaTiO 3 -Mediated Piezoelectricity. Adv. Mater. 2020;32:2001976. doi: 10.1002/adma.202001976. [DOI] [PubMed] [Google Scholar]
- 110.Nelson T.R., Fowlkes J.B., Abramowicz J.S., Church C.C. Ultrasound biosafety considerations for the practicing sonographer and sonologist. J. Ultrasound Med. 2009;28:139–150. doi: 10.7863/jum.2009.28.2.139. [DOI] [PubMed] [Google Scholar]
- 111.Bigelow T.A., Church C.C., Sandstrom K., Abbott J.G., Ziskin M.C., Edmonds P.D., Herman B., Thomenius K.E., Teo T.J. The thermal index: Its strengths, weaknesses, and proposed improvements. J. Ultrasound Med. 2011;30:714–734. doi: 10.7863/jum.2011.30.5.714. [DOI] [PubMed] [Google Scholar]
- 112.Lee J.H. Injectable hydrogels delivering therapeutic agents for disease treatment and tissue engineering. Biomater. Res. 2018;22 doi: 10.1186/s40824-018-0138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Fakhari A., Anand Subramony J. Engineered in-situ depot-forming hydrogels for intratumoral drug delivery. J. Control. Release. 2015;220:465–475. doi: 10.1016/j.jconrel.2015.11.014. [DOI] [PubMed] [Google Scholar]
- 114.Wang C., Varshney R.R., Wang D.-A. Therapeutic cell delivery and fate control in hydrogels and hydrogel hybrids. Adv. Drug Deliv. Rev. 2010;62:699–710. doi: 10.1016/j.addr.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 115.Yuan F.-Z., Wang H.-F., Guan J., Fu J.-N., Yang M., Zhang J.-Y., Chen Y.-R., Wang X., Yu J.-K. Fabrication of Injectable Chitosan-Chondroitin Sulfate Hydrogel Embedding Kartogenin-Loaded Microspheres as an Ultrasound-Triggered Drug Delivery System for Cartilage Tissue Engineering. Pharmaceutics. 2021;13:1487. doi: 10.3390/pharmaceutics13091487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jiang H., Tovar-Carrillo K., Kobayashi T. Ultrasound stimulated release of mimosa medicine from cellulose hydrogel matrix. Ultrason. Sonochem. 2016;32:398–406. doi: 10.1016/j.ultsonch.2016.04.008. [DOI] [PubMed] [Google Scholar]
- 117.Huang L., Quesada C., Aliabouzar M., Fowlkes J.B., Franceschi R.T., Liu Z., Putnam A.J., Fabiilli M.L. Spatially-directed angiogenesis using ultrasound-controlled release of basic fibroblast growth factor from acoustically-responsive scaffolds. Acta Biomater. 2021;129:73–83. doi: 10.1016/j.actbio.2021.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Farrell E., Aliabouzar M., Quesada C., Baker B.M., Franceschi R.T., Putnam A.J., Fabiilli M.L. Spatiotemporal control of myofibroblast activation in acoustically-responsive scaffolds via ultrasound-induced matrix stiffening. Acta Biomater. 2022;138:133–143. doi: 10.1016/j.actbio.2021.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Moncion A., Arlotta K.J., O’Neill E.G., Lin M., Mohr L.A., Franceschi R.T., Kripfgans O.D., Putnam A.J., Fabiilli M.L. In vitro and in vivo assessment of controlled release and degradation of acoustically responsive scaffolds. Acta Biomater. 2016;46:221–233. doi: 10.1016/j.actbio.2016.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Moncion A., Lin M., O’Neill E.G., Franceschi R.T., Kripfgans O.D., Putnam A.J., Fabiilli M.L. Controlled release of basic fibroblast growth factor for angiogenesis using acoustically-responsive scaffolds. Biomaterials. 2017;140:26–36. doi: 10.1016/j.biomaterials.2017.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Narayanamurthy V., Padmapriya P., Noorasafrin A., Pooja B., Hema K., Firus Khan A.a.Y., Nithyakalyani K., Samsuri F. Skin cancer detection using non-invasive techniques. RSC Adv. 2018;8:28095–28130. doi: 10.1039/C8RA04164D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kost J., Mitragotri S., Gabbay R.A., Pishko M., Langer R. Transdermal monitoring of glucose and other analytes using ultrasound. Nat. Med. 2000;6:347–350. doi: 10.1038/73213. [DOI] [PubMed] [Google Scholar]
- 123.Schoellhammer C.M., Blankschtein D., Langer R. Skin permeabilization for transdermal drug delivery: Recent advances and future prospects. Expert Opin. Drug Deliv. 2014;11:393–407. doi: 10.1517/17425247.2014.875528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Szreniawa-Sztajnert A., Zabiegała B., Namieśnik J. Developments in ultrasound-assisted microextraction techniques for isolation and preconcentration of organic analytes from aqueous samples. TrAC Trends Anal. Chem. 2013;49:45–54. doi: 10.1016/j.trac.2013.02.015. [DOI] [Google Scholar]
- 125.Lavon I., Kost J. Ultrasound and transdermal drug delivery. Drug Discov. Today. 2004;9:670–676. doi: 10.1016/S1359-6446(04)03170-8. [DOI] [PubMed] [Google Scholar]
- 126.Díaz-Alejo J.F., González Gómez I., Earl J. Ultrasounds in cancer therapy: A summary of their use and unexplored potential. Oncol. Rev. 2022;16:531. doi: 10.4081/oncol.2022.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang N., Wang J., Foiret J., Dai Z., Ferrara K.W. Synergies between therapeutic ultrasound, gene therapy and immunotherapy in cancer treatment. Adv. Drug Deliv. Rev. 2021;178:113906. doi: 10.1016/j.addr.2021.113906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Awad N.S., Paul V., Alsawaftah N.M., Ter Haar G., Allen T.M., Pitt W.G., Husseini G.A. Ultrasound-Responsive Nanocarriers in Cancer Treatment: A Review. ACS Pharmacol. Transl. Sci. 2021;4:589–612. doi: 10.1021/acsptsci.0c00212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Tehrani Fateh S., Moradi L., Kohan E., Hamblin M.R., Shiralizadeh Dezfuli A. Comprehensive review on ultrasound-responsive theranostic nanomaterials: Mechanisms, structures and medical applications. Beilstein J. Nanotechnol. 2021;12:808–862. doi: 10.3762/bjnano.12.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lin X., Wu J., Liu Y., Lin N., Hu J., Zhang B. Stimuli-Responsive Drug Delivery Systems for the Diagnosis and Therapy of Lung Cancer. Molecules. 2022;27:948. doi: 10.3390/molecules27030948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wu C.H., Sun M.K., Kung Y., Wang Y.C., Chen S.L., Shen H.H., Chen W.S., Young T.H. One injection for one-week controlled release: In vitro and in vivo assessment of ultrasound-triggered drug release from injectable thermoresponsive biocompatible hydrogels. Ultrason. Sonochem. 2020;62:104875. doi: 10.1016/j.ultsonch.2019.104875. [DOI] [PubMed] [Google Scholar]
- 132.Wang X., Qiao L., Yu X., Wang X., Jiang L., Wang Q. Controllable Formation of Ternary Inorganic-Supramolecular-Polymeric Hydrogels by Amidation-Fueled Self-assembly and Enzymatic Post-cross-linking for Ultrasound Theranostic. ACS Biomater. Sci. Eng. 2019;5:5888–5896. doi: 10.1021/acsbiomaterials.9b01065. [DOI] [PubMed] [Google Scholar]
- 133.Wu Q., Zhang Q., Yu T., Wang X., Jia C., Zhao Z., Zhao J. Self-Assembled Hybrid Nanogel as a Multifunctional Theranostic Probe for Enzyme-Regulated Ultrasound Imaging and Tumor Therapy. ACS Appl. Bio Mater. 2021;4:4244–4253. doi: 10.1021/acsabm.1c00079. [DOI] [PubMed] [Google Scholar]
- 134.Kim G., Wu Q., Chu J.L., Smith E.J., Oelze M.L., Moore J.S., Li K.C. Ultrasound controlled mechanophore activation in hydrogels for cancer therapy. Proc. Natl. Acad. Sci. USA. 2022;119:e2109791119. doi: 10.1073/pnas.2109791119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Huebsch N., Kearney C.J., Zhao X., Kim J., Cezar C.A., Suo Z., Mooney D.J. Ultrasound-triggered disruption and self-healing of reversibly cross-linked hydrogels for drug delivery and enhanced chemotherapy. Proc. Natl. Acad. Sci. USA. 2014;111:9762–9767. doi: 10.1073/pnas.1405469111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sun M., Yue T., Wang C., Fan Z., Gazit E., Du J. Ultrasound-Responsive Peptide Nanogels to Balance Conflicting Requirements for Deep Tumor Penetration and Prolonged Blood Circulation. ACS Nano. 2022;16:9183–9194. doi: 10.1021/acsnano.2c01407. [DOI] [PubMed] [Google Scholar]
- 137.Gharehnazifam Z., Dolatabadi R., Baniassadi M., Shahsavari H., Kajbafzadeh A.-M., Abrinia K., Gharehnazifam K., Baghani M. Multiphysics modeling and experiments on ultrasound-triggered drug delivery from silk fibroin hydrogel for Wilms tumor. Int. J. Pharm. 2022;621:121787. doi: 10.1016/j.ijpharm.2022.121787. [DOI] [PubMed] [Google Scholar]