ABSTRACT
A range of antibiotic alternative products is increasingly studied and manufactured in the current animal agriculture, particularly in the poultry industry. Phytogenic feed additives are known for their remarkable ability to suppress pathogens such as Clostridium spp., Escherichia coli, and Salmonella. Other than enhancing biosecurity, improvements in productivity and performance were also observed. However, clear mechanisms for these improvements were not established. In this study, 20,000 Lohman-Brown layers were provided with phytogenic supplement from 16 to 40 weeks of age, and performance parameters were assessed against the same number of unsupplemented control birds. The performance results showed that the birds with phytogenic supplementation presented consistently reduced mortality, increased rate of lay, and increased average egg weight. Functional analysis through shotgun sequencing of cecal metagenomes confirmed a substantial functional shift in the microbial community, showing that phytogen significantly reduced the range of microbial functions, including the production of essential vitamins, cofactors, energy, and amino acids. Functional data showed that phytogen supplementation induced a phenotypic shift in intestinal bacteria LPS phenotype toward the less pathogenic form. The study corroborates the use of phytogenic products in antibiotic-free poultry production systems. The productivity improvements in the number and weight of eggs produced during Spotty Liver Disease justify further optimizing phytogenic alternatives for use in high-risk open and free-range poultry systems.
IMPORTANCE The present study establishes the beneficial effects of the continuous phytogenic supplementation reflected in reduced diarrhea and mortality and higher egg productivity under normal conditions and during a natural outbreak of Spotty Liver Disease. Our data points to the importance of phytogen-driven alteration of microbial pathogenicity and fitness-related functional capabilities revealed on the commercial layer farm. Phytogenic product showed an ability to improve the bird’s welfare and sustainability in free-range poultry production systems.
KEYWORDS: eggs, poultry, biosecurity, phytogen, intestinal microbiota, functional profile, metagenome
INTRODUCTION
Poultry production has an enormous contribution to global economics. Over the years, the exponential increase in chicken consumption resulted in an upsurge in Australian chicken meat production, from 3 million in 1950 to 1951 to around 653 million in 2016 to 2017 (1). The industry is facing challenges in controlling the health and welfare of chickens for optimum and cost-effective production. The increase of antimicrobial resistance (AMR) in humans and livestock adversely affects public health. Consumers are seeking high-quality meat or egg produced humanely under antibiotic-free production systems. The industry strives to keep up with rapidly changing consumer demands by investing in modern and highly automated production systems and research to achieve high-quality products while providing animal welfare and quality nutrition to ensure resilience to disease. In many countries, the layer industry does not use antibiotics as growth promoters; however, antibiotics can be used to treat sick birds, but often eggs produced by antibiotic-treated birds must be excluded from production. This is pushing the egg industry to depend more on alternatives to antibiotic products.
Alternative antibiotic products are actively studied and developed to reduce the use of antibiotics in the livestock industry. The trend is leaning toward organic sources to decrease possible adverse effects. The alternatives to antibiotics include enzymes, bacteriocins, antimicrobial peptides, organic acids, probiotics, prebiotics, natural antimicrobial products (bentonite, zeolite, charcoal, and biochar), and essential oils and their active antimicrobial phytochemicals such as carvacrol, thymol, cinnamaldehyde, eugenol, eucalyptol, and others (2–6). This brought a range of products onto the animal-food market, with phytogenic products currently dominating.
The phytogenic products can be used alone or with other additives such as prebiotics, probiotics, or organic acids. Well-established ability to control pathogens and enhance the performance in chickens (3, 5, 7–12) have brought phytogenic products into the spotlight. Several studies have reported a reduction in devastating pathogens such as the species of Clostridium (13), Escherichia (14), and Salmonella (15). Numerous studies have examined adding the phytogens as a supplement into feeds and showed improvements in critical production parameters such as quality of eggs and feed conversion ratio in layers (16, 17) and enhancement of performance in the broilers (18–21). Further studies have observed significant interactions between phytogens with levels of cholesterol (16, 22, 23), gut-brain axes (Bajagai et al. 2021b), sex hormones (4, 23) and immune systems (21, 24).
Despite the abundance of information on the benefits of phytogenic products, running free-range production systems remains a significant challenge for the poultry industry, which still struggles with disease outbreaks and high mortality. An immense number of phytogenic products are available on the market, and understanding how these products perform in different production setups, feed formulations, pathogens, and disease control spectrum is essential for choosing the best product to address the different issues each farm is facing. The phytogen used in this experiment is a mixture of essential oils, bitter substances, spices, and saponins. The effects of this product have been examined to investigate the health, performance, egg quality and intestinal microbiota composition, and changes in microbiota functional capability under a free-range system at the time of the year corresponding to the highest heat stress and disease outbreak peak.
RESULTS
Performance and health of birds.
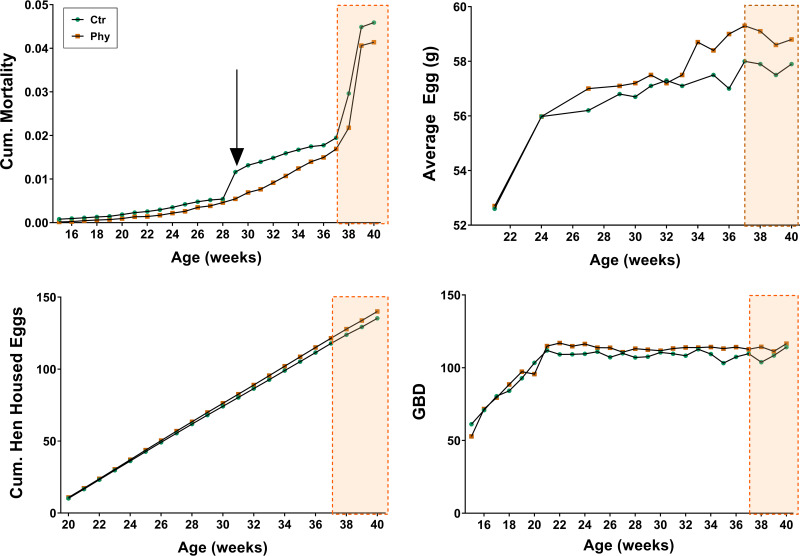
Phytogen was applied from the arrival to the shed at 19 to 40 weeks of age. The Spotty Liver Disease outbreak was apparent from week 37 when the mortality soared and birds were positively diagnosed with Campylobacter hepaticus. Fig. 1 shows some of the most affected performance parameters. The arrow in the Cumulative Mortality graph indicates the height of summer when daily temperatures exceed 40°C, inducing high heat stress conditions.
FIG 1.
Cumulative mortality, average egg mass, cumulative hen housed eggs, and average intake (GBD = Grams per Bird per Day) during the phytogen administration period. The onset and outbreak of the Spotty Liver Disease are outlined in the shaded rectangle.
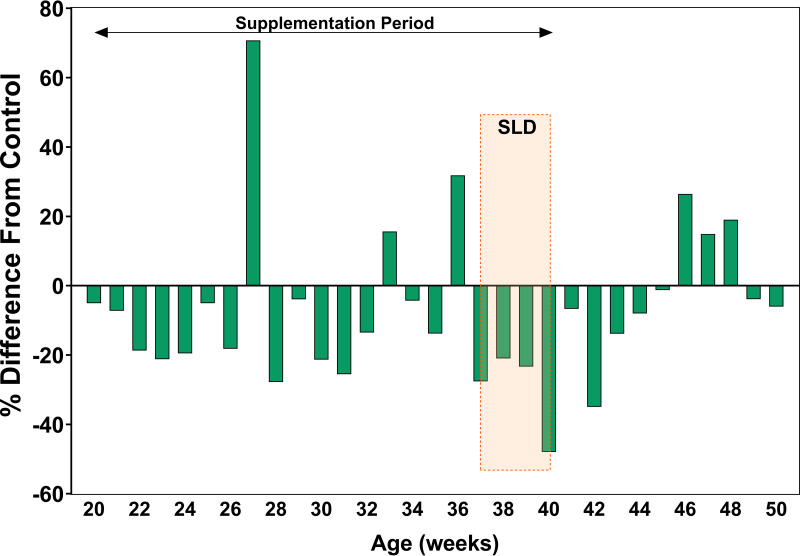
The performance data indicate that phytogen consistently reduced mortality, increased rate of lay, and average egg weight; however, there was no significant change in FCR. Cumulative hen housed eggs were higher throughout the phytogen (Phy) application achieving five extra eggs per bird at 40 weeks when the phytogen was removed. Not accounting for mortality, the phytogen supplemented shed produced approximately 8,333 additional cartons of dozen eggs, or looking at egg mass improvements, approximately extra 5.8 tons over the supplementation period. The phytogen showed protective effects during the Spotty Liver Disease outbreak via considerably lower mortality (Fig. 1) and larger eggs than control birds. Fig. 2 shows the number of dirty eggs in the phytogen supplemented group as % difference from the control. During 20 weeks of phytogen supplementation, only during 3 weeks were there more dirty eggs in the phytogen supplemented group than in the control.
FIG 2.
The number of dirty eggs in phytogen-supplemented birds is shown as a % difference from the control. Negative values are presenting % lower than control, and positive values indicate % higher than in control for that week. The graph shows that after removing phytogen supplementation at 40 weeks of age, these benefits were quickly lost.
Most prevalent bacteria in the cloacal microbiota.
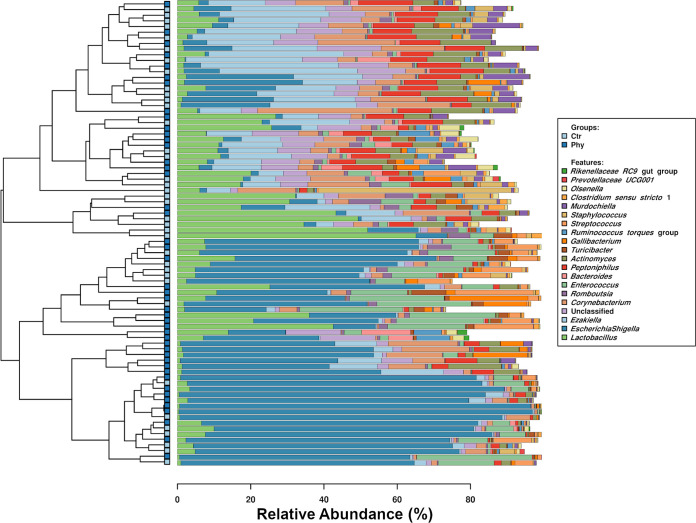
The microbial community of cloacal swabs is presented in a clustered bar chart (Fig. 3). Both the control and treatment birds had beneficial Bacteroides, Lactobacillus, unfavorable Escherichia-Shigella, Corynebacterium, Clostridium, Gallibacterium, Actinomyces, Streptococcus, and Staphylococcus. Other unique 13 genera presented in Fig. 3, Ezakiella, Romboustsia, Peptoniphilus, Turibacter, Ruminococcus torques group, Murdochiella, Olsenella, Prevotellaceae UCG001, and Rikenellaceae RC9 gut group were widely distributed in both control and treatment flocks.
FIG 3.
Barchart shows 20 most abundant genera obtained from the cloacal swab of 50 layer hens from birds with a control diet (Ctr) and groups supplemented with phytogenic product (Phy).
Effect of phytogenic products on bacterial abundance.
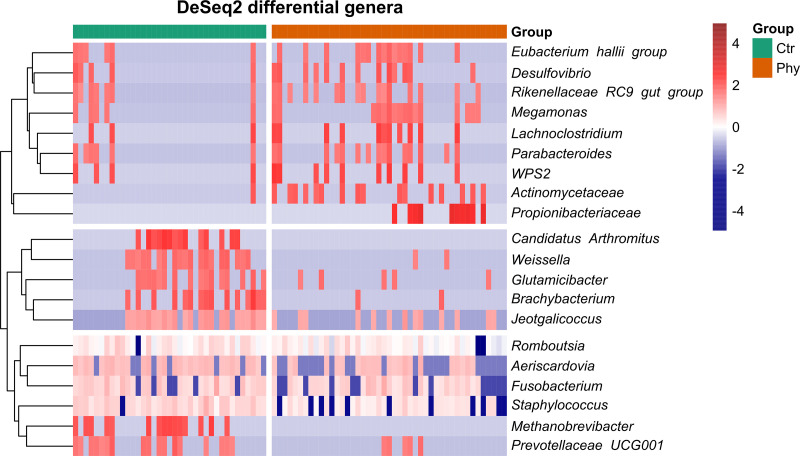
From the 20 identified genera obtained from the flocks, nine genera (Eubacterium hallii group, Desulfovibrio, Rikenellaceae RC9 gut group, Megamonas, Lachnoclostridium, Parabacteroides, WPS2, Un. Actinomycetaceae, and Un. Propionibacteriaceae) were increased in the birds fed with phytogenic supplements as shown in the first cluster of the heatmap (Fig. 4). Apart from increased genera, others were reduced in the treatment group (Candidatus Arthromitus, Weissella, Glutamicibacter, Brachybacterium, Jeotgalicoccus, Romboutsia, Aeriscardovia, Fusobacterium, Staphylococcus, Methanobrevibacter, and Prevotellaceae UCG001).
FIG 4.
Heatmap generated using the pheatmap R package. Data were transformed into mean scaled log2 with the abundance in the range shown in the scale bar blue (low) to red (high). Significant genera shown on the heatmap were selected using DeSeq2 analysis (P < 0.05).
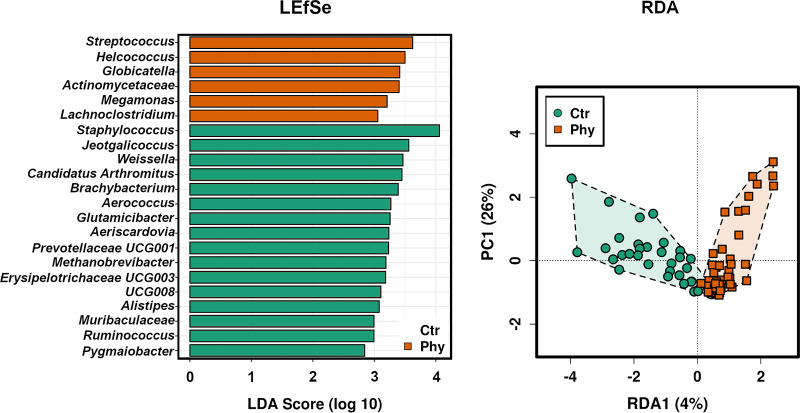
The Linear discriminant analysis Effect Size (LEfSe) in Fig. 5 represents significant genera (P < 0.05) at the biomarker level of the microbial communities in both control and phytogen. Six genera (Streptococcus, Helcococcus, Globicatella, Actinomycetaceae, Megamonas, and Lachnoclostridum) were highly enriched in the treatment flocks, and the remaining 16 genera (Staphylococcus, Jeotgalicoccus, Weisella, Candidatus, Brachybacterium, Aerococcus, Glutamicibacter, Aericardovia, Prevotellaceae UCG001, Methanobrevibacter, Erysipelotrichaceae UCG003, UCG008, Alistipes, Muribaculaceae, Ruminococcus, and Pygmaiobacter) were more abundant in the control birds. Redundancy Analysis (RDA) is presented in Fig. 5. The ordination plot distinctively separated control and treatment groups. There were no significant alterations (Wilcoxon test; P < 0.05) in any of the alpha diversity measures inspected, including Richness, Evenness, Shannon, and Simpson index.
FIG 5.
LEfSe (left) and RDA (right) plot comparing the chicken cloacal samples of the flocks fed with a control diet (Ctr) and groups supplemented with phytogenic treatment (Phy). Linear discriminant analysis (LDA) score was scaled into log10, and SILVA taxonomy was compared to allocate the specific genera.
Effects on the functional profiles.
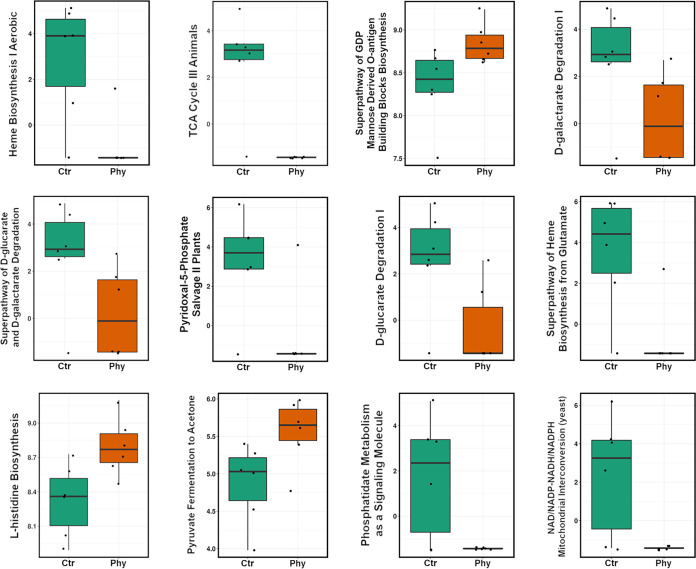
Twelve functional pathways such as Heme biosynthesis I aerobic, TCA cycle III animals, super pathway of GDP mannose derived O-antigen building blocks biosynthesis, d-galactarate degradation I, super pathway of d-glucarate and d-galactarate degradation, pyridoxal-5-phosphate salvage II plants, d-glucarate degradation I, super pathway of heme biosynthesis from glutamate, l-histidine biosynthesis, pyruvate fermentation to acetone, phosphatidate metabolism as a signaling molecule, and NAD/NADP-NADH/NADPH mitochondrial interconversion were selected using Wilcoxon ranked test, and they display significant (RDA P = 0.007) difference in the functional profiles between the two groups shown in Fig. 6.
FIG 6.
Differences in functional profiles of microbial communities between the control group and phytogenic supplemented group (P = 0.0096 to 0.05).
Histological and SCFA analysis.
Both histological analyses of the ileum and SCFA analysis of the cecum contents were within the expected range and did not show a significant difference (Wilcoxon test; P < 0.05) between the control and phytogen supplemented birds in any of the measured parameters.
DISCUSSION
Performance data demonstrated clear benefits of phytogen application in a large-scale commercial layer setup. Although the phytogen supplementation did not prevent the outbreak of the Spotty Liver Disease in supplemented birds, cumulative mortalities were consistently lower, and egg production was better than in the control birds during the experiment, including the disease outbreak period. Similarly, performance parameters, including the cumulative hen-housed eggs, and weight and number of individual eggs produced, were better in the phytogen supplemented flocks, demonstrating the improvements in the overall health and performance. This is of high significance for the layer industry, where the push for free range resulted in reemerging of Spotty Liver Disease because of the pathogen Campylobacter hepaticus, which is readily found in the free-range soil, insects, and native free-range animals such as rodents and wild birds (25). Reducing mortality is often not the top priority for the industry, but it is gaining more power with the rise of consumer interest in animal welfare issues. Dirty eggs are an indicator of diarrhea and intestinal issues in birds. Although these performance improvements may look small when given per bird, multiplying this by 20,000 birds in each group, the benefit of improved egg production accumulated to approximately 5,800 additional kg of eggs, or 8,333 additional cartons of dozen eggs, compared to control throughout the product administration.
Based on the above taxonomy results, the 16S amplicon data appears to be inconclusive; there is no desired reduction of pathogens and boost of beneficial species that would explain the performance benefits. However, after years of intensive poultry microbiota research, we now know that such scenarios are highly unlikely. Our data indicate that microbiota change affected fewer taxa, and that instead of targeting specific pathogens (taxa), the product is likely targeting the specific pathogenic and fitness functions across all bacteria, thus generating minor taxa abundance-based alterations but affecting overall community functional abilities. Indeed, our shotgun sequencing of cecal metagenomes shows that a range of essential vitamin, energy, and amino acid production functions were decimated across all taxonomy in supplemented birds. The metagenomic analysis showed a significant functional shift in the overall community (P = 0,007).
Heme biosynthesis pathway is an essential pathway for oxidative metabolism strongly reduced by phytogen supplementation. It functions as a regulatory molecule in protein stability, protein targeting, transcription, translation, and cellular differentiation (26). In humans, defects in this pathway can cause a metabolic disease known as porphyrias which express neurologic and photocutaneous symptoms (27). In bacteria, heme is an essential molecule to the function of hemoprotein. During the infection, bacterial pathogens must acquire heme to generate energy and detoxify immune effector cells from the host (28), which makes heme a vital element for bacterial pathogenicity.
The tricarboxylic acid (TCA), one of the most investigated pathways, also reduced by phytogen, produces precursors for biosynthesis and is a ubiquitous pathway in almost all aerobic living organisms (29). It creates energy and the reducing power in the mitochondria, strongly influencing overall metabolism. TCA cycle metabolites regulate essential roles such as controlling chromatin modifications, signaling molecules with functions, hypoxic response, immunity, and DNA methylation, which can alter the function and fate of the cells (30). Another reduced super pathway of d-galactarate and d-gulcarate are naturally occurring diacid sugars. Both compounds can be utilized as a sole source of carbon for E. coli (31). E.coli is a frequent problem on this farm and is often manifested with diarrhea and dirty eggs. Weakening the pathogen metabolically and depleting its energy to a level sufficing to keep it under control could be a possible mechanism of phytogenic action.
Supplementation of phytogen reduced Pyridoxal 5′-phosphate (PLP) pathway, involved in amino acid biosynthetic pathways and enzymatic reactions as an essential cofactor (32). Together with pyridoxin 5′-phosphate (PNP) and pyridoxamine 5’phosphate (PMP) it is considered the active form of vitamin B6. The production of vitamin B6 in plants, bacteria, and fungi occurs through de novo PLP biosynthesis with their own biosynthetic capabilities.
Vitamins are known for their potent antioxidant properties that effectively quench the form of reactive oxygen species, thus ameliorating the stress response pathways for cellular well-being (33). Super pathway of NAD/NADH–NADH/NADPH interconversion is another phytogen reduced pathway that has a vital role in all living cells for electron transfer, energy metabolism and signaling pathways (34). Interconvertibility of the various species of NAD+ supports redox balance in the cells that is of utmost importance to determine metabolite excretion and efficiency in the growth. In major pathogenic bacteria, the biosynthesis of NAD+ has developed toward an auxotrophic growth requirement; therefore, a rapid drop or failure in maintaining a certain level of NAD+ to activate metabolic sensors may cause massive cell death (35).
Super pathway of GDP-mannose-derived O-antigen building blocks biosynthesis exhibited a significant increase in the phytogen supplemented flock. O-antigen molecules are polysaccharide chains that extend away from the lipopolysaccharides (LPS) in Gram-negative bacteria and the S-layer in some Gram-positive bacteria, which protects the bacterial membrane from all kinds of chemical attacks and induces an immune response. The presence of O-antigen chains controls if the LPS is classified as rough or smooth. More O-chains would make the LPS smooth, whereas the lack of, or reduction in O-chains, would result in rough LPS. This indicates that phytogen supplementation likely resulted in a shift of LPS phenotype from rough to smooth LPS which would, in turn, alter host-pathogen interactions. According to Stranahan and Arenas-Gamboa (36), a rough phenotype is associated with an enhanced level of pathogenic stealth that could allow them to persist and avoid host defenses. The ability of phytogen products to induce the shift to a less virulent smooth LPS phenotype in bacteria could be another contributor to the improved gut health of the birds.
The Human Microbiome Project (HMP) showed that the microbiota community was highly variable and influenced by factors such as ethnicity, genetics, nutrition, external environment, and temporal and spatial elements (37, 38). This taxonomic instability is even more exaggerated in poultry (39). However, one of the major findings of HMP was that despite the variability in microbiota, the microbial functions were preserved, stable, and reproducible in the microbiota communities from different cultures, continents, and genetic backgrounds. This shifted the interest of researchers toward studying the functional profiles; however, this was hindered by the price of shotgun sequencing compared to 16S amplicon sequencing. Our data show that the functional and taxonomic analysis can present two sides of the coin, both equally important, compensating for high variability in the gut microbial community. The phytogenic feed supplements in chickens can target specific microbial functions rather than microbial taxa to give health and production benefits.
Conclusions.
There is abundant evidence that phytogenic products and their base ingredients, such as essential oils, if dosed correctly, can bring many health and performance benefits. This research area is centered on interpreting 16S amplicon microbiota data with the balance of good and bad bacteria in focus. However, beneficial and pathogenic effects are strain specific and beyond the resolution of the 16S methodology. Here, we suggest that the mechanisms of phytogen-derived functional alterations resulting in health and performance improvements are far more complex and likely different between the products and their main ingredients. This presents an opportunity for better evaluation of phytogen appropriateness to confront different health and production issues and presents a prospect to investigate the functional alterations in a range of phytogenic molecules to allow for future specific pathogen-tailored product development.
MATERIALS AND METHODS
Experimental design.
This experiment was completed at one of Australia’s leading layer farms, performed in the sheds specifically designed to investigate different products to study the productivity of the layer hens. Lohman-Brown day-old chicks were sourced from Specialised Breeders Australia P/L (Bendigo, Victoria) and reared in a separate farm location until they reached 16 weeks of age on concrete floors with ad libitum access to food and water. After the rearing stage, at 16 weeks of age, birds from the same raring flock were randomized and separated into two groups (control and treatment), with 20,000 birds in each group, with ad libitum access to food and water. Two sections of the shed were physically divided by a common egg collection room, and the range was separated and divided by mesh wires so that the birds did not have any contact. Feed and water originated from the same source/batch in both sections, with the phytogen treatment product being the only difference. Company nutritionists designed the feeds to fulfill the animal welfare and performance requirements, with the feed’s main composition being sorghum with the mix of lower inclusion of grains such as barley and wheat as an energy source. The protein sources were a mixture of soybean meal and a low level of meat and bone meal. Minerals and vitamins were supplemented with standard premix.
The phytogenic product used was a feed additive Biostrong Protect. The dosage recommendation is 400 to 600 mg/kg; here, it was dosed at a maximum recommended 600 mg/kg as we wanted to evaluate efficacy against Spotty Liver Disease, common in this region of Australia, especially in the hottest summer period. The supplementation period started just before the height of summer.
Performance of birds was measured regularly by recording the parameters such as rate of lay (ROL, measured daily as eggs produced over the number of birds in the shed), cumulative mortality, birds body weight (measured weekly on 100 randomly selected birds), feed conversion ratio (amount of feed consumed to produce one dozen of eggs), cumulative hen housed eggs (total number of eggs laid compared to the number of birds adjusted for mortality), GBD (grams of feed consumed per bird per day), egg weight, eggshell thickness, cumulative dirty eggs (rejected dirty eggs), yolk color (the color was scored according to the DSM yolk color palette from 1 to 16), and Haugh units (weekly measurement of the internal quality of eggs).
For microbiota analysis, cloacal swabs were taken from 50 randomly selected birds from both control and treatment sheds after they had passed the peak of lay at 30 weeks of age. Intestinal samples were taken from 10 randomly selected birds from each group. Cecal samples were also used to analyze the short-chain fatty acids (SCFA) and for metagenomics sequencing for functional analysis, and sections of ileum tissue were collected to evaluate organ health through histology.
Short-chain fatty acids.
Analysis of SCFAs was described previously (40), briefly, 0.2 g cecum contents were suspended in 1.5 mL 2.5% metaphosphoric acid solution, homogenized and centrifuged, mixed with 1 mL of 70% ethanol, and filtered through 0.45 μm cellulose syringe filter and analyzed using gas chromatography-mass spectrometer (GC-MS-QP2010 Ultra) fitted with Shimadzu AOC-20 robotic autosampler and autoinjector and Agilent J&W GC polar column (30m, 0.25 mm in diameters, 0.25 μm of film and 40°C to 260°C temperature limit). One microliter of the sample was injected at 250°C with a helium carrier (1.97 mL/min, 5.0, Coregas, Australia) with the injection mode of 5.0 split under helium flow at 103.4 mL/min and the pressure maintained at 143.3 kPa. The mass spectrometer was used in electron ionization mode, at 0.2 kV and temperature of 220°C, and scan mode between 33 to 150 m/z. Standards of acetic acid, butyric acid, isobutyric acid, propinoic acid, and valeric acid were used to calculate the concentrations of SCFA.
Histology.
The tissue sample in the midsection of the ileum was collected and fixed into a 10% buffered formalin solution, followed by fixation, paraffin embedding, deparaffinization, and rehydration that was outsourced to a commercial pathology company (QML Pathology, Australia). Leica RM2135 rotary microtome was used to cut the paraffin-embedded blocks and then stained using Hematoxylin and Eosin. The Dibutyl phthalate Polystyrene Xylene (DPX, Ajax Finechem Australia) mounting medium is then applied to prevent stain fading. Stained slides were then outsourced to the University of Queensland School of Biomedical Science histology scanning facility. QuPath v0.3.0 was used to perform morphometric analysis of the ileum. Clear, intact crypts and villi (n < 20) were positioned, and villus height and crypt depth were measured. Measurements were then imported into statistical software GraphPad Prism v9.2.0 (GraphPad, San Diego, CA, USA) for statistical analysis.
Sequencing and data analysis.
The swab DNA extraction was performed as previously described (40). Briefly, the cells were broken using the lysis protocol developed by Yu and Morrison (41) and purified using DNA mini spin (Enzymax LLC, CAT# EZC101, Kentucky, USA). After extraction, the quality and quantity of the DNA were measured using NanoDrop One UV-Vis spectrophotometer. The V3-V4 region of 16S rRNA gene was amplified using the following primers with spacers, barcodes, and Illumina sequencing linkers (42): The forward primer was 338F (5′-ACTCCTACGGGAGGCAGCAG-3′′) and the reverse primer was 806R (5′-GGACTACHVGGGTWTCTAAT-3′). The sequencing library was completed following the manufacturer’s protocol (Illumina Inc., San Diego, CA, USA), and the Illumina MiSeq platform was used for sequencing (2 × 300 bp paired-end). Cutadapt (43) was then used to demultiplex the data, followed by the microbiota analysis in Quantitative Insights into Microbial Ecology 2 (QIIME2) (44). Quality filtering, denoising, and chimera removal were done using Dada2 (45) plugin with all recommended parameters. SILVA v 138.1 database (46) was used as reference data to assign taxonomy. Data were analyzed at ASV and genus levels. The analysis and interpretation of the data were completed using the Hellinger transformed (47) data unless stated otherwise. For other presentation and visualization of the performance data, Calypso v8.72, and a range of R packages such as pheatmap, phyloseq, vegan, and DeSeq2 were used.
Metagenomic data were obtained with shotgun sequencing of genomic DNA using the Illumina NovaSeq sequencing platform, with an overall number of 409.8, million quality-filtered joined sequences (minimum 30.3, maximum 40.1, and average 34.1 million sequences per sample) with a minimum phred score of 22, zero ambiguous bases and raw sequences with less than 150 nt excluded. The functional analysis was done using the computational tool HUMAnN2 with UniRef90 databases.
Ethical statement.
The Animal Ethics Committee of Central Queensland University approved the study under approval number 0000022879. All applicable international, national, and institutional guidelines for the care and use of animals were followed.
Data availability.
All sequencing data are publicly available in NCBI SRA database under project number PRJNA821923.
ACKNOWLEDGMENTS
This research was conducted and funded by Central Queensland University Merit Grant. The data were analyzed using the Isaac Newton High-Performance Computing System at Central Queensland University. We acknowledge and appreciate Jason Bell’s help in all aspects of High-Performance Computing.
S.J.Y. analyzed and interpreted the data and wrote the paper. Y.S.B. performed the experiments and analyzed and interpreted the data. F.P. performed the experiments. D.S. planned and designed the experiments and analyzed and interpreted the data. All authors read and approved the manuscript.
We have no conflicts of interest to declare.
This research was conducted and funded by the Central Queensland University Merit Grant scheme.
Contributor Information
Dragana Stanley, Email: D.Stanley@cqu.edu.au.
Johanna Björkroth, University of Helsinki.
REFERENCES
- 1.Australian Chicken Meat Federation Inc. 2020. History of the chicken industry in Australia. Australian Chicken Meat Federation Inc. North Sydney, Australia. [Google Scholar]
- 2.Anonye BO. 2016. General commentary on: alternatives to antibiotic growth promoters in animals. Front Vet Sci 3:74. 10.3389/fvets.2016.00074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Attia YA, Alagawany MM, Farag MR, Alkhatib FM, Khafaga AF, Abdel-Moneim AE, Asiry KA, Mesalam NM, Shafi ME, Al-Harthi MA, Abd El-Hack ME. 2020. Phytogenic products and phytochemicals as a candidate strategy to improve tolerance to Coronavirus. Front Vet Sci 7:573159. 10.3389/fvets.2020.573159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bajagai YS, Radovanovic A, Steel JC, Stanley D. 2021. The effects of continual consumption of Origanum vulgare on liver transcriptomics. Animals (Basel) 11:398. 10.3390/ani11020398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Murugesan GR, Syed B, Haldar S, Pender C. 2015. phytogenic feed additives as an alternative to antibiotic growth promoters in broiler chickens. Front Vet Sci 2:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thacker PA. 2013. Alternatives to antibiotics as growth promoters for use in swine production: a review. J Anim Sci Biotechnol 4:35. 10.1186/2049-1891-4-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cazorla SI, Maldonado-Galdeano C, Weill R, De Paula J, Perdigon GDV. 2018. Oral administration of probiotics increases paneth cells and intestinal antimicrobial activity. Front Microbiol 9:736. 10.3389/fmicb.2018.00736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Flees JJ, Ganguly B, Dridi S. 2021. Phytogenic feed additives improve broiler feed efficiency via modulation of intermediary lipid and protein metabolism-related signaling pathways. Poult Sci 100:100963. 10.1016/j.psj.2020.12.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Galli GM, Petrolli TG, Aniecevski E, Santo AD, Leite F, Griss LG, Dazuk V, Boiago MM, Dos Santos HV, Simoes C, Wagner R, Bissacotti BF, Schentiger MR, Da Silva AS. 2021. Phytogenic blend protective effects against microbes but affects health and production in broilers. Microb Pathog 152:104590. 10.1016/j.micpath.2020.104590. [DOI] [PubMed] [Google Scholar]
- 10.Huang FC, Lu YT, Liao YH. 2020. Beneficial effect of probiotics on Pseudomonas aeruginosa-infected intestinal epithelial cells through inflammatory IL-8 and antimicrobial peptide human beta-defensin-2 modulation. Innate Immun 26:592–600. 10.1177/1753425920959410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sabo SDS, Mendes MA, Araujo EDS, Muradian LBA, Makiyama EN, LeBlanc JG, Borelli P, Fock RA, Knobl T, Oliveira RPS. 2020. Bioprospecting of probiotics with antimicrobial activities against Salmonella Heidelberg and that produce B-complex vitamins as potential supplements in poultry nutrition. Sci Rep 10:7235. 10.1038/s41598-020-64038-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Terada T, Nii T, Isobe N, Yoshimura Y. 2020. Effects of probiotics Lactobacillus reuteri and Clostridium butyricum on the expression of Toll-like receptors, pro- and anti-inflammatory cytokines, and antimicrobial peptides in broiler chick intestine. J Poult Sci 57:310–318. 10.2141/jpsa.0190098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bajagai YS, Alsemgeest J, Moore RJ, Van TTH, Stanley D. 2020. Phytogenic products, used as alternatives to antibiotic growth promoters, modify the intestinal microbiota derived from a range of production systems: an in vitro model. Appl Microbiol Biotechnol 104:10631–10640. 10.1007/s00253-020-10998-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zou Y, Xiang Q, Wang J, Peng J, Wei H. 2016. Oregano essential oil improves intestinal morphology and expression of tight junction proteins associated with modulation of selected intestinal bacteria and immune status in a pig model. Biomed Res Int 2016:1–11. 10.1155/2016/5436738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abudabos AM, Alyemni AH, Dafalla YM, Khan RU. 2016. The effect of phytogenic feed additives to substitute in-feed antibiotics on growth traits and blood biochemical parameters in broiler chicks challenged with Salmonella typhimurium. Environ Sci Pollut Res 23:24151–24157. 10.1007/s11356-016-7665-2. [DOI] [PubMed] [Google Scholar]
- 16.Abou-Elkhair R, Selim S, Hussein E. 2018. Effect of supplementing layer hen diet with phytogenic feed additives on laying performance, egg quality, egg lipid peroxidation and blood biochemical constituents. Anim Nutr 4:394–400. 10.1016/j.aninu.2018.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saki AA, Aliarabi H, Hosseini Siyar SA, Salari J, Hashemi M. 2014. Effect of a phytogenic feed additive on performance, ovarian morphology, serum lipid parameters and egg sensory quality in laying hen. Vet Res Forum 5:287–293. [PMC free article] [PubMed] [Google Scholar]
- 18.Khalaji S, Zaghari M, Hatami K, Hatami KH, Hedari-Dastjerdi S, Lotfi L, Nazarian H. 2011. Black cumin seeds, Artemisia leaves (Artemisia sieberi), and Camellia L. plant extract as phytogenic products in broiler diets and their effects on performance, blood constituents, immunity, and cecal microbial population. Poult Sci 90:2500–2510. 10.3382/ps.2011-01393. [DOI] [PubMed] [Google Scholar]
- 19.Paraskeuas V, Fegeros K, Palamidi I, Theodoropoulos G, Mountzouris KC. 2016. Phytogenic Administration and reduction of dietary energy and protein levels affects growth performance, nutrient digestibility and antioxidant status of broilers. Jpn Poult Sci 53:264–273. 10.2141/jpsa.0150113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pirgozliev V, Mansbridge SC, Rose SP, Lillehoj HS, Bravo D. 2019. Immune modulation, growth performance, and nutrient retention in broiler chickens fed a blend of phytogenic feed additives. Poult Sci 98:3443–3449. 10.3382/ps/pey472. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Su S, Pender C, Murugesan R, Syed B, Kim WK. 2021. Effect of a phytogenic feed additive on growth performance, nutrient digestion, and immune response in broiler-fed diets with two different levels of crude protein. Animals (Basel) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ahmadipour B, Pat S, Abaszadeh S, Hassanpour H, Khajali F. 2021. Pomegranate peel as a phytogenic in broiler chickens: influence upon antioxidant, lipogenesis and hypotensive response. Vet Med Sci 7:1907–1913. 10.1002/vms3.556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bajagai YS, Steel JC, Radovanovic A, Stanley D. 2021. Prolonged continual consumption of oregano herb interferes with the action of steroid hormones and several drugs, and effects signaling across the brain-gut axis. Food Funct 12:726–738. 10.1039/d0fo02988b. [DOI] [PubMed] [Google Scholar]
- 24.Moon SG, Lee SK, Lee WD, Niu KM, Hwang WU, Oh JS, Kothari D, Kim SK. 2021. Effect of dietary supplementation of a phytogenic blend containing Schisandra chinensis, Pinus densiflora, and Allium tuberosum on productivity, egg quality, and health parameters in laying hens. Anim Biosci 34:285–294. 10.5713/ajas.20.0552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Phung C, Vezina B, Anwar A, Wilson T, Scott PC, Moore RJ, Van TTH. 2019. Campylobacter hepaticus, the cause of spotty liver disease in chickens: transmission and routes of infection. Front Vet Sci 6:505. 10.3389/fvets.2019.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Panek H, O'Brian MR. 2002. A whole genome view of prokaryotic haem biosynthesis. Microbiology (Reading) 148:2273–2282. 10.1099/00221287-148-8-2273. [DOI] [PubMed] [Google Scholar]
- 27.Eales L. 1961. The prophyrins and the porphyrias. Annu Rev Med 12:251–270. 10.1146/annurev.me.12.020161.001343. [DOI] [PubMed] [Google Scholar]
- 28.Choby JE, Skaar EP. 2016. heme synthesis and acquisition in bacterial pathogens. J Mol Biol 428:3408–3428. 10.1016/j.jmb.2016.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Krebs HA, Eggleston LV. 1945. Metabolism of acetoacetate in animal tissues. 1. Biochem J 39:408–419. 10.1042/bj0390408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chakraborty AA, Laukka T, Myllykoski M, Ringel AE, Booker MA, Tolstorukov MY, Meng YJ, Meier SR, Jennings RB, Creech AL, Herbert ZT, McBrayer SK, Olenchock BA, Jaffe JD, Haigis MC, Beroukhim R, Signoretti S, Koivunen P, Kaelin WG, Jr, 2019. Histone demethylase KDM6A directly senses oxygen to control chromatin and cell fate. Science 363:1217–1222. 10.1126/science.aaw1026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hubbard BK, Koch M, Palmer DR, Babbitt PC, Gerlt JA. 1998. Evolution of enzymatic activities in the enolase superfamily: characterization of the (D)-glucarate/galactarate catabolic pathway in Escherichia coli. Biochemistry 37:14369–14375. 10.1021/bi981124f. [DOI] [PubMed] [Google Scholar]
- 32.Mooney S, Leuendorf JE, Hendrickson C, Hellmann H. 2009. Vitamin B6: a long known compound of surprising complexity. Molecules 14:329–351. 10.3390/molecules14010329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bilski P, Li MY, Ehrenshaft M, Daub ME, Chignell CF. 2000. Vitamin B6 (pyridoxine) and its derivatives are efficient singlet oxygen quenchers and potential fungal antioxidants. Photochem Photobiol 71:129–134. . [DOI] [PubMed] [Google Scholar]
- 34.Koch-Nolte F, Haag F, Guse AH, Lund F, Ziegler M. 2009. Emerging roles of NAD+ and its metabolites in cell signaling. Sci Signal 2:mr1. 10.1126/scisignal.257mr1. [DOI] [PubMed] [Google Scholar]
- 35.Mesquita I, Varela P, Belinha A, Gaifem J, Laforge M, Vergnes B, Estaquier J, Silvestre R. 2016. Exploring NAD+ metabolism in host-pathogen interactions. Cell Mol Life Sci 73:1225–1236. 10.1007/s00018-015-2119-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stranahan LW, Arenas-Gamboa AM. 2021. When the going gets rough: the significance of Brucella lipopolysaccharide phenotype in host-pathogen interactions. Front Microbiol 12:713157. 10.3389/fmicb.2021.713157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kolde R, Franzosa EA, Rahnavard G, Hall AB, Vlamakis H, Stevens C, Daly MJ, Xavier RJ, Huttenhower C. 2018. Host genetic variation and its microbiome interactions within the Human Microbiome Project. Genome Med 10:6. 10.1186/s13073-018-0515-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Human Microbiome Project C. 2012. Structure, function and diversity of the healthy human microbiome. Nature 486:207–214. 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stanley D, Geier MS, Hughes RJ, Denman SE, Moore RJ. 2013. Highly variable microbiota development in the chicken gastrointestinal tract. PLoS One 8:e84290. 10.1371/journal.pone.0084290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bauer BW, Gangadoo S, Bajagai YS, Van TTH, Moore RJ, Stanley D. 2019. Oregano powder reduces Streptococcus and increases SCFA concentration in a mixed bacterial culture assay. PLoS One 14:e0216853. 10.1371/journal.pone.0216853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu Z, Morrison M. 2004. Improved extraction of PCR-quality community DNA from digesta and fecal samples. Biotechniques 36:808–812. 10.2144/04365ST04. [DOI] [PubMed] [Google Scholar]
- 42.Fadrosh DW, Ma B, Gajer P, Sengamalay N, Ott S, Brotman RM, Ravel J. 2014. An improved dual-indexing approach for multiplexed 16S rRNA gene sequencing on the Illumina MiSeq platform. Microbiome 2:6. 10.1186/2049-2618-2-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Martin M. 2011. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnet J 17. 10.14806/ej.17.1.200. [DOI] [Google Scholar]
- 44.Bolyen E, Rideout JR, Dillon MR, Bokulich NA, Abnet CC, Al-Ghalith GA, Alexander H, Alm EJ, Arumugam M, Asnicar F, Bai Y, Bisanz JE, Bittinger K, Brejnrod A, Brislawn CJ, Brown CT, Callahan BJ, Caraballo-Rodríguez AM, Chase J, Cope EK, Da Silva R, Diener C, Dorrestein PC, Douglas GM, Durall DM, Duvallet C, Edwardson CF, Ernst M, Estaki M, Fouquier J, Gauglitz JM, Gibbons SM, Gibson DL, Gonzalez A, Gorlick K, Guo J, Hillmann B, Holmes S, Holste H, Huttenhower C, Huttley GA, Janssen S, Jarmusch AK, Jiang L, Kaehler BD, Kang KB, Keefe CR, Keim P, Kelley ST, Knights D, et al. 2019. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat Biotechnol 37:852–857. 10.1038/s41587-019-0209-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Callahan BJ, McMurdie PJ, Rosen MJ, Han AW, Johnson AJ, Holmes SP. 2016. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods 13:581–583. 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Quast C, Pruesse E, Yilmaz P, Gerken J, Schweer T, Yarza P, Peplies J, Glockner FO. 2013. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res 41:D590–6. 10.1093/nar/gks1219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Legendre P, Gallagher ED. 2001. Ecologically meaningful transformations for ordination of species data. Oecologia 129:271–280. 10.1007/s004420100716. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All sequencing data are publicly available in NCBI SRA database under project number PRJNA821923.