Abstract
Mining the key genes involved in the balance of rice salt tolerance is extremely important for developing salt-tolerant rice varieties. A library of japonica mutants was screened under salinity conditions to identify putative salt stress-responsive genes. We identified a highly salt-sensitive mutant ss3 and used a map-based cloning approach to isolate the gene SS3, which encodes mannose-1-phosphate guanylyltransferase. Under salt treatment, ss3 mutants have decreased ascorbic acid (AsA) content and increased reactive oxygen species (ROS) levels compared with the wild type (WT). Exogenous AsA restored the salt tolerance of ss3 plants, indicating that inhibition of AsA synthesis was an important factor in the salt sensitivity of the mutant. Functional complementation using the WT allele rescued the mutation, and transcription of SS3 was induced by salt stress. Vector SS3p:SS3 was constructed containing the 1086 bp coding sequence of SS3. Under salinity conditions, transgenic seedlings expressing SS3p:SS3 had improved salt tolerance relative to WT, as demonstrated by better growth status, higher chlorophyll content, a lower level of Na+, and a reduced Na+/K+ ratio. Further investigation revealed that several senescence- and autophagy-related genes were expressed at lower levels in salt-stressed transgenic lines compared to WT. These results demonstrate the positive impact of SS3 on salt tolerance in rice through the regulation of AsA synthesis and ROS accumulation, and indicate that SS3 is a valuable target for genetic manipulation.
Keywords: rice, salt stress, mannose-1-phosphate guanylyltransferase, ascorbic acid, reactive oxygen species
1. Introduction
Rice is a dietary staple for much of the world’s population. To ensure food security, it is essential to increase production by utilizing marginal land including extensive saline areas where the yields of current elite varieties are reduced [1]. In the presence of salt, growth is inhibited throughout development [2] but particularly at the early seedling stage [3]. Normal progression of rice plants here is a strong indicator of overall tolerance to salinity.
Plants grown under salt stress exhibit many adverse effects. In particular, damage to membranes can lead to lipid peroxidation and increased formation of ROS above normal homeostasis levels [4] with the concomitant accumulation of malondialdehyde (MDA) [5], which may further disrupt the fundamental components of the cell. Thus, plants have developed mechanisms to control undesirable oxidation, including antioxidant enzymes and low-molecular-weight compounds such as carotenoids, glutathione, flavonoids, and ascorbic acid (AsA) [6].
AsA is a ubiquitous scavenger of ROS [7] and, in tomatoes, increased levels have been associated with resistance to salt [8]. Its synthesis in higher plants is controlled by the D-mannose/L-galactose pathway, all the components of which have been characterized [9,10]. The importance of the L-galactose pathway in AsA synthesis has been demonstrated by studying multiple mutants. In Arabidopsis, the VTC2 gene encodes a GDP-L-galactose phosphorylase. The AsA content in vtc2-1 and vtc2-2 mutants is only ~20% of that in the wild type and is sensitive to ozone [11]. In the T-DNA knockout vtc4 (L-galactose-1-phosphate phosphatase) Arabidopsis mutant, AsA content and seed germination rate decreased [12]. vtc1 is a knock-down mutant of mannose-1-phosphate guanylate transferase (GDP-mannose pyrophosphorylase, EC 2.7.7.13, MPGase) gene that to contains only 25% of the AsA level compared with the wild type [13]. OsVTC1 in the rice genome consists of three homologous genes. Among these homologs, OsVTC1-1 is closely related to the formation of AsA in rice leaves, while OsVTC1-3 plays an important role in the synthesis of AsA in roots [14]. Enhancing VTC1 expression increases the production of AsA [15,16,17]. Tobacco overexpressing MPGase shows greater tolerance to temperature extremes [18].
Increased synthesis of AsA under the control of MPGase is a key response to environmental stress as can be demonstrated in several examples. The MPGase gene VTC1 may be induced under oxidative stress, increasing the formation of AsA [19], while Arabidopsis mutant vtc1 exhibits increased sensitivity to salt, arising from reduced synthesis of AsA with a corresponding lower capacity for the removal of ROS [20]. The salt tolerance of Arabidopsis is increased by enhanced transcription of VTC1 under the control of factor AtERF98 [21], and the production of AsA in rice root under salt stress is increased via the OsVTC1-3 MPGase gene, leading to improved growth [16].
Dissecting the key genes involved in rice salt tolerance is an important objective for accelerating rice breeding [22]. To identify putative salinity tolerance genes, a library of ethane methyl sulfonate (EMS)-induced japonica rice cv. Nipponbare (NPB) mutants was screened under salinity conditions. We isolated a highly salt stress-sensitive mutant ss3 and cloned the gene responsible, which encodes MPGase. Genetic, phenotypic, and biochemical analyses revealed that SS3 modulates AsA synthesis and ROS scavenging in rice.
2. Results
2.1. Isolation of a Rice Mutant Sensitive to Salt Stress
A library of EMS-induced japonica rice mutants was screened under salinity stress conditions using 100 mM NaCl. We identified a mutant that is highly sensitive to salt stress, designated ss3. As shown in Figure 1A,B, no differences were detected between the growth of wild type (WT) and ss3 under normal conditions. Upon exposure to salinity, ss3 plants wilted severely and foliar chlorosis was observed, whereas WT showed only slight wilting and withered blade tips (Figure 1A,B). The stress treatment led to suppression of growth in both WT and ss3, but there was no significant difference in shoot height between WT and ss3 (Figure 1C). However, the shoot and root biomasses of ss3 seedlings were significantly reduced to 81% and 87% of those of WT under equal salt stress, respectively (Figure 1D,E).
Figure 1.
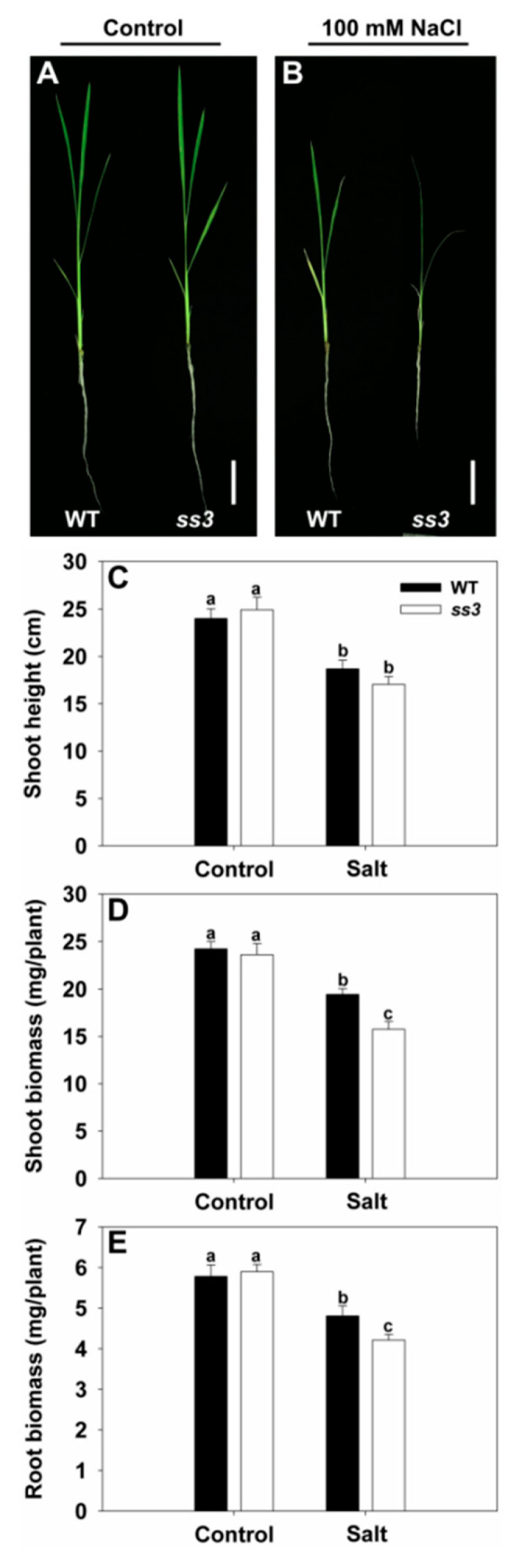
Growth performance in response to salinity stress of ss3 mutant compared with WT. Growth performance of WT and ss3 mutants under normal (A) or 100 mM NaCl treatment (B). Ten-day-old seedlings were treated for four days with or without the addition of 100 mM NaCl. Shoot height (C), shoot biomass (D), and root biomass (E) of the seedlings under normal and salt stress conditions. Bars = 4 cm. The values are means ± SE of five replicates. Significant differences at p < 0.05 are indicated with different letters.
2.2. Map-Based Cloning of SS3
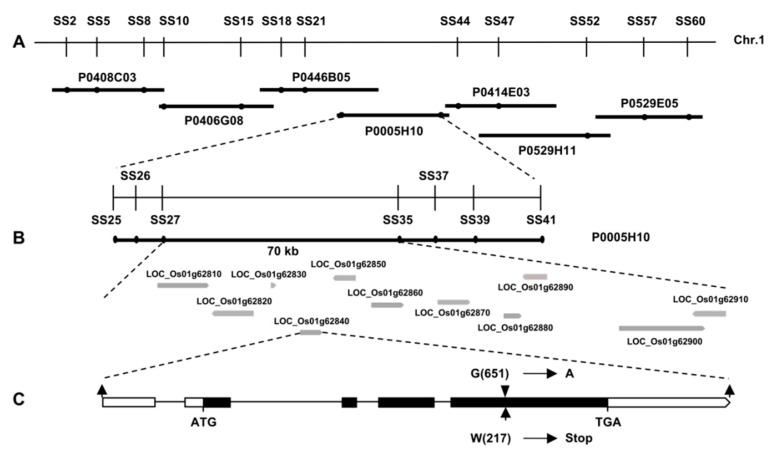
Positional cloning was employed to identify the SS3 gene and characterize the ss3 phenotype. The mutant was crossed with indica variety TN1 to obtain the F2 population for mapping. The SS3 gene occurs on chromosome 1 between SSR markers SS2 and SS60 (Figure 2A). Fine mapping via additional PCR-based markers localized SS3 to a 70 kb segment on BAC clone P0005H10 between SS27 and SS35 (Figure 2B). Eleven open reading frames were identified in this interval by database searching (https://ensembl.gramene.org/Oryza_sativa/Location/View?db=core;r=1:36373818–36443332 accessed on 10 June 2021) and the corresponding sequences in the ss3 and WT parents were compared. The ss3 genome was found to contain a G to A substitution at position 651 in exon 4 of the predicted MPGase gene LOC_Os01g62840. This results in a change from a tryptophan codon to a stop codon, with truncation of the protein (Figure 2C).
Figure 2.
Map-based cloning of SS3. (A) SS3 was initially mapped to chromosome 1 (Chr.1) and fine mapping was carried out with markers developed based on the sequence of BAC clone P0005H10. The SS3 locus was narrowed to a 70 kb genomic DNA region between markers SS27 and SS35. (B) Eleven open reading frames were located in the region. (C) Gene structure of the SS3 candidate LOC_Os01g62840. Start codon (ATG) and the stop codon (TGA) are indicated. Blank boxes indicate coding sequence. Arrows show mutation site of ss3.
2.3. SS3 Is Involved in AsA Production and ROS Scavenging in Rice
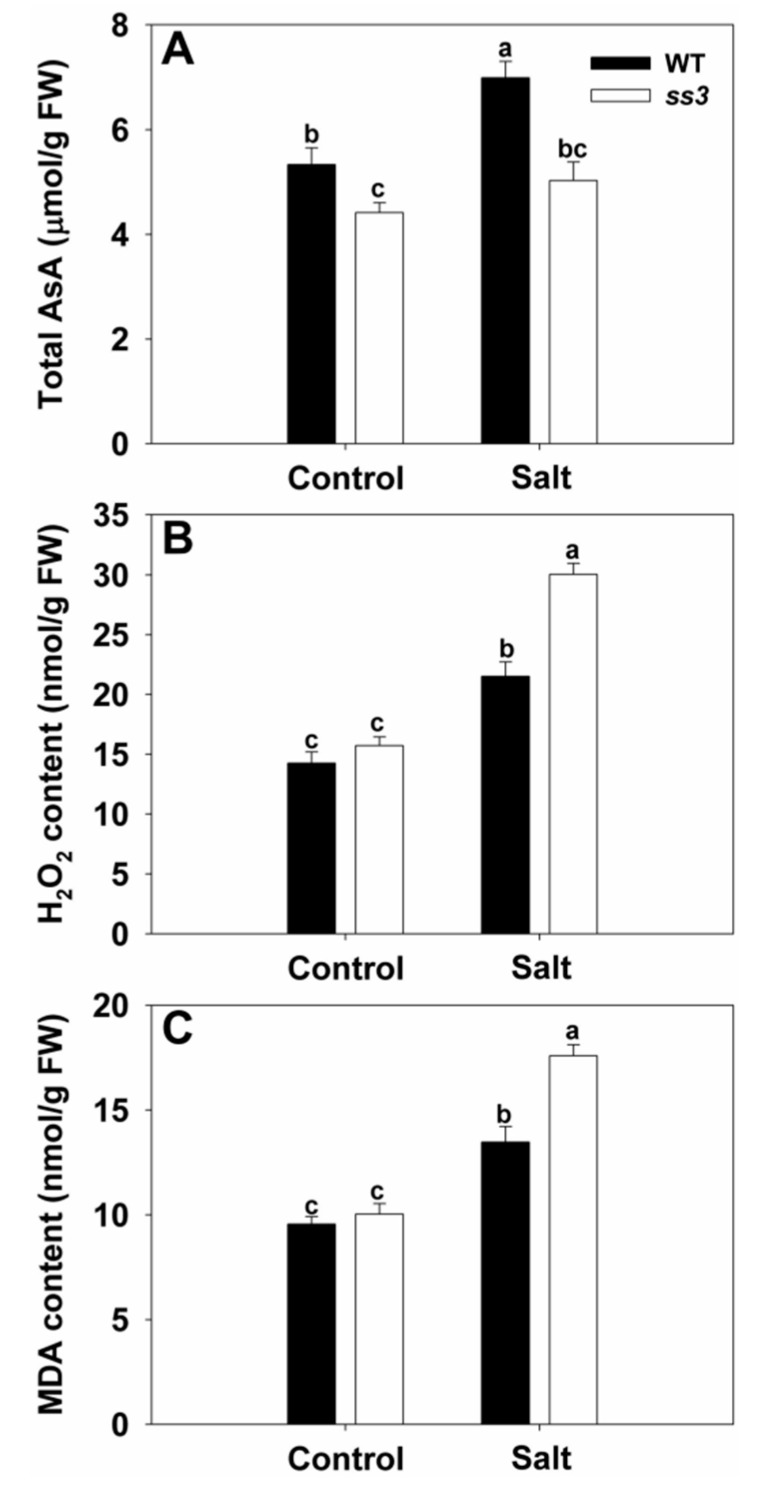
Since MPGase catalyzes the formation of GDP-D-mannose, the precursor of AsA [23], we determined the AsA contents in WT and ss3 mutants grown normally or under salt stress. Under control conditions, AsA in the leaves of ss3 was significantly lower compared to WT, while NaCl treatment markedly increased AsA in WT but not in ss3 (Figure 3A). Since AsA plays an important role in ROS scavenging to protect rice against oxidant-induced damage under environmental stress [15,16], we also monitored H2O2 and MDA. WT and ss3 had similar levels of both under normal conditions, but when grown with added NaCl, the H2O2 and MDA contents of ss3 were 40% and 31% higher than those of WT, respectively (Figure 3B,C), indicating that functional SS3 is important for ROS scavenging in rice under salinity conditions.
Figure 3.
ROS accumulation in response to salinity stress in ss3 mutant compared with WT. Growth conditions and treatments were the same as described in Figure 1. The AsA content (A), H2O2 content (B), and MDA content (C) are shown. The values are means ± SE of five replicates. Significant differences at p < 0.05 are indicated with different letters. FW—fresh weight.
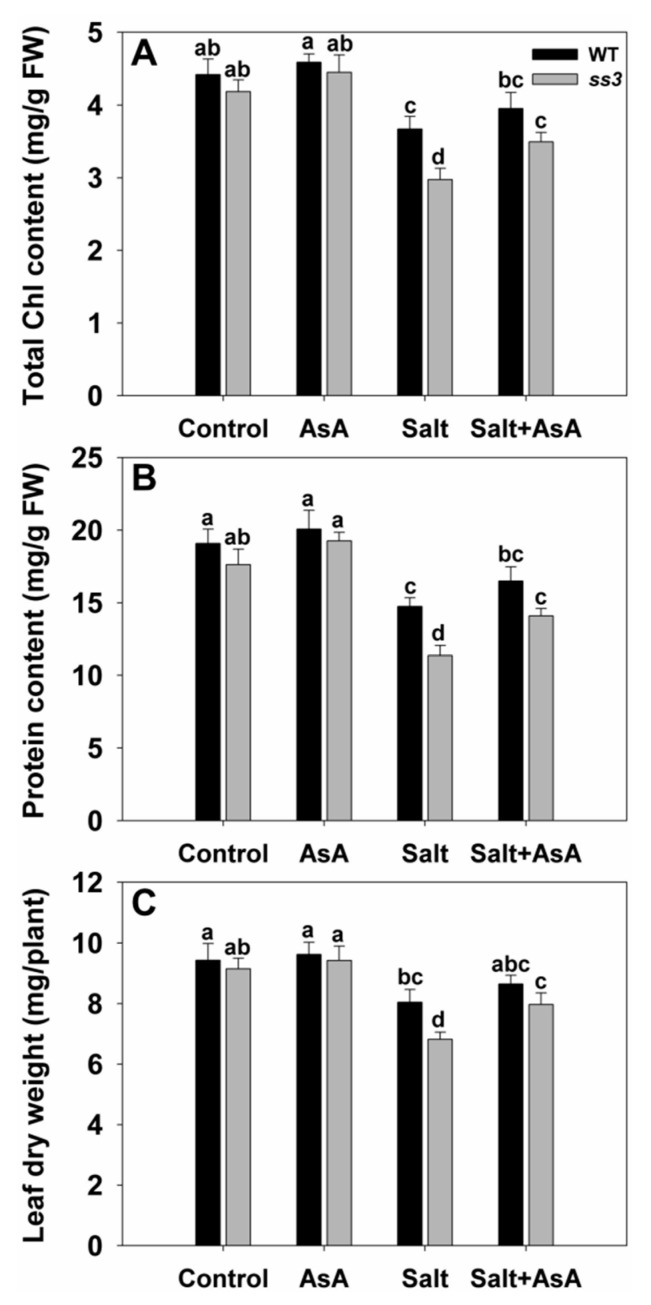
Further, we found that adding exogenous AsA restored the tolerance of ss3 seedlings to salinity. Physiological indicators such as chlorophyll content, protein content, and leaf dry weight of ss3 were then similar to that of WT (Figure 4). Thus, SS3 mediates the response of rice to salt stress through the regulation of AsA biosynthesis.
Figure 4.
Exogenous AsA mitigated the salt hypersensitivity of ss3 mutants. Ten-day-old seedlings were transferred for four days to a nutrient solution containing 0 mM or 100 mM NaCl supplied with or without 1 mM AsA. The chlorophyll (Chl) content (A), protein content (B), and leaf dry weight (C) were assayed. The values are means ± SE of five replicates. Significant differences at p < 0.05 are indicated with different letters. FW—fresh weight.
2.4. Generation of Complementation Lines for SS3
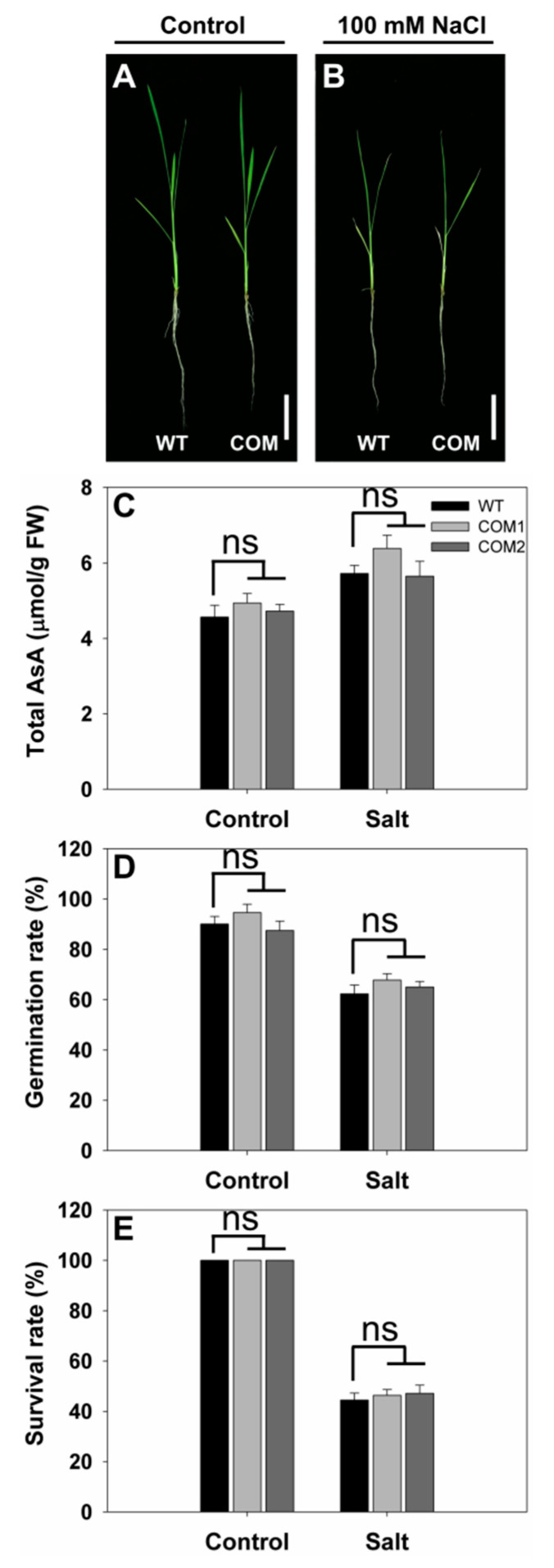
To further confirm that the loss of function of SS3 was responsible for the salt-sensitive phenotype, we generated two complementation lines (COM1 and COM2) for SS3. These lines rescued the salt-hypersensitive phenotype of ss3 mutants (Figure 5A,B). As shown in Figure 5C, the AsA contents of leaves of the complementation lines were restored to WT levels. Under normal and salt stress conditions, there were no obvious differences in the germination rate and survival rate between WT and complementation lines (Figure 5D,E). These results confirm that disruption of SS3 results in hypersensitivity to salinity stress.
Figure 5.
Growth performance in response to salinity stress of complementation lines compared with WT. Growth conditions and treatments were the same as given in Figure 1. Growth performance of WT and the complementation lines under normal (A) or 100 mM NaCl treatment (B). Bars = 5 cm. (C–E), AsA content (C), germination rate (D), and survival rate (E) of the seedlings under normal and salt stress. The values are means ± SE of five replicates. FW—fresh weight; ns indicates non-significant differences.
2.5. SS3 Is Induced by Salinity Stress
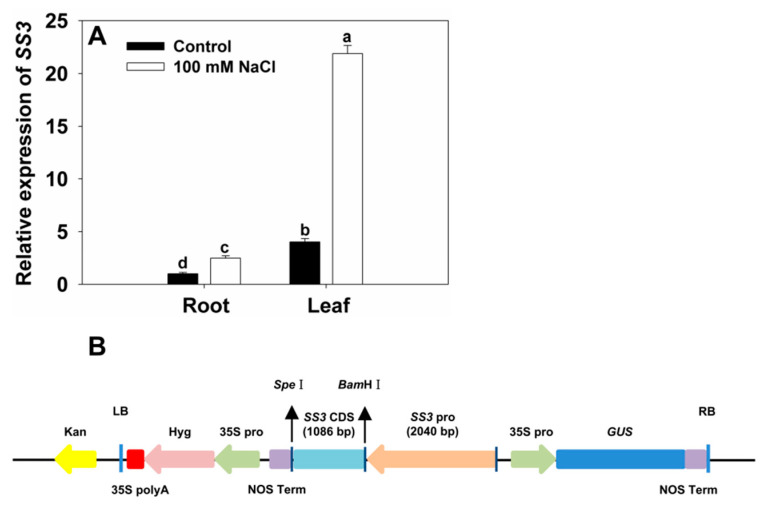
To further assess the role of SS3 in the plant response to salt stress, we tested whether salinity could induce SS3 expression. We grew 10-day-old WT NPB rice plants with the addition of 100 mM NaCl and investigated the expression patterns of SS3 in tissues including roots and leaves using qRT-PCR. SS3 transcript levels increased 1.5- and 4.4-fold in NaCl-treated roots and leaves, respectively, compared with the untreated controls (Figure 6A).
Figure 6.
Effect of salt stress on the expression of SS3, and construction map of SS3p:SS3 vector. (A) Relative expression levels of the SS3 gene in rice roots and leaves under normal and salinity stress. The abundance of SS3 transcript in the non-stressed roots was normalized to 1. The values are means ± SE of three replicates. Significant differences at p < 0.05 are indicated with different letters. (B) Construction map of SS3p:SS3 expression vector.
2.6. Generation of Transgenic Rice Expressing SS3p:SS3
To further determine the relationship between SS3 regulation of AsA synthesis and salt sensitivity, we manipulated the expression of SS3 gene by transforming its native promoter SS3p:SS3 construct into rice (Figure 6B).
We obtained 15 independent SS3p:SS3 transgenic lines in the T0 generation as demonstrated by Southern blot analysis. Four null segregants were detected in the T1 generation by GUS staining, which were used in the phenotype analysis. Since no significant difference in salt sensitivity was observed between WT and the null segregants in the T1 generation, as determined by a comparison of the growth characteristics and chlorosis of leaves, we used WT as the single negative control in hydroponic experiments on the transgenic lines in the T2 generation. In addition, GUS staining was performed on each single-copy transgenic line of the T2 generation, and two homozygous lines (L1 and L2) were selected for further detailed analysis.
2.7. Effects of SS3p:SS3 Expression on Rice Growth and Na+/K+ Homeostasis under Salt Stress
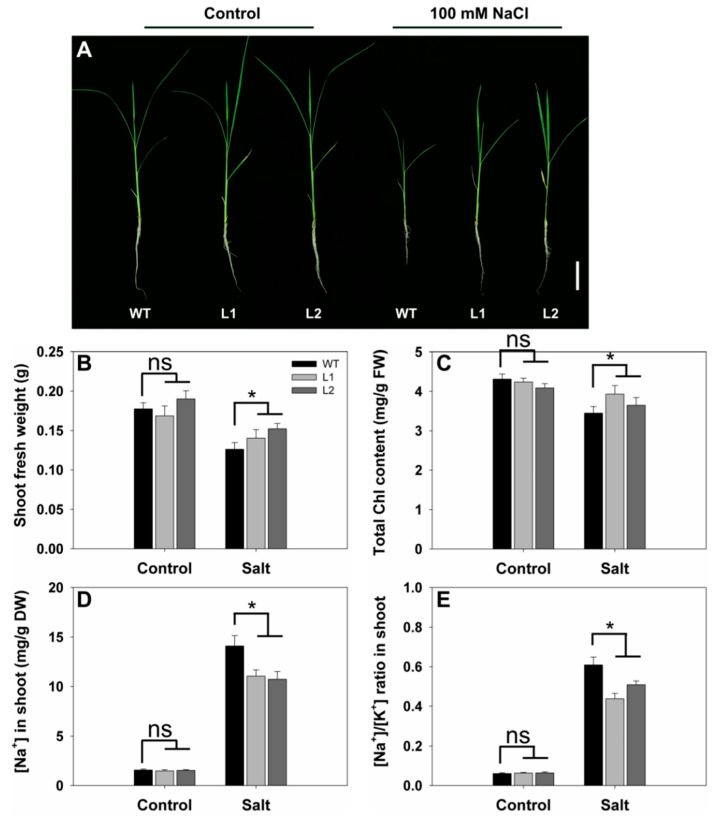
As shown in Figure 7A, no morphological differences were observed between WT and the transgenic lines grown without added salt. Under salt stress, the growth of WT was inhibited to a greater extent than that of the transgenic lines and was accompanied by more severe wilting in the leaves (Figure 7A). To quantify the phenotypes, we compared the shoot fresh weight and chlorophyll contents of the WT and transgenic lines under salt stress. The magnitude of the reduction in shoot growth of SS3p:SS3 transgenic plants was significantly less than that found in WT. When grown in salt solution, the shoot fresh weight of the transgenic lines was approximately 16% higher than that of WT (Figure 7B) and chlorophyll contents were also higher in transgenic lines compared with those in WT plants (Figure 7C). Thus, the SS3p:SS3 transgenic lines were demonstrably more tolerant to salinity stress than WT.
Figure 7.
Growth performance in response to salinity stress of SS3p:SS3 transgenic lines compared with WT. Ten-day-old seedlings were treated for six days with or without addition of 100 mM NaCl. (A) Growth performance of WT and SS3p:SS3 transgenic lines under normal and 100 mM NaCl treatment. Bars = 4 cm. Shoot fresh weight (B), chlorophyll (Chl) content (C), Na+ concentration (D), and Na+/K+ ratio (E) in shoots of the seedlings under normal and salt stress. The values are means ± SE of five replicates. Significant differences between WT and transgenic lines at p < 0.05 are indicated with asterisks; ns denotes non-significant differences. FW—fresh weight. DW—dry weight.
We determined the Na+ and K+ contents in the shoots of the WT and transgenic lines. Under control conditions, we observed no substantial differences in either Na+ concentration or Na+/K+ ratios between the plant materials (Figure 7D,E). Six days after treatment with an added 100 mM NaCl, transgenic plants accumulated less Na+ in shoots than WT plants (Figure 7D). Furthermore, the Na+/K+ ratios in the shoots of the transgenic lines were 17–28% lower than the corresponding values for WT (Figure 7E).
2.8. SS3p:SS3 Transgenic Plants under Salt Stress Present Altered Expression of Senescence- and Autophagy-Related Genes
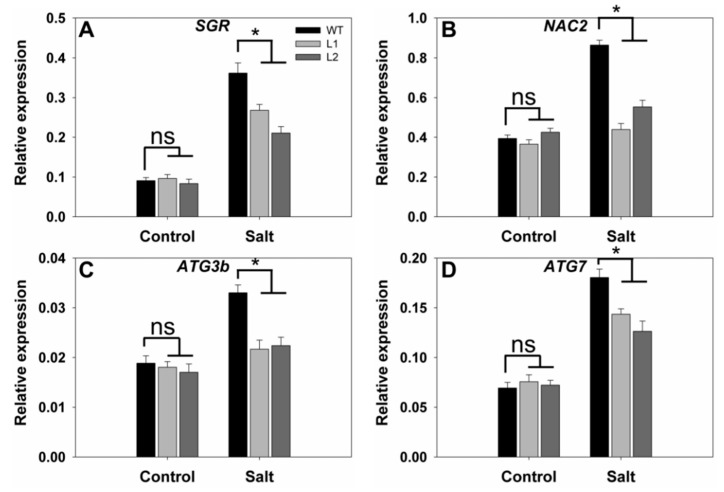
Premature leaf senescence is an important response of plants to various external pressures such as salinity stress [24]. In order to further confirm that SS3 positively regulates the tolerance of rice to salt stress, we performed gene expression profiling of transgenic lines for comparison with WT under normal and salinity conditions. Under normal growth conditions, we observed relatively weak expression and insignificant variations in transcriptional levels for the tested genes in both WT and transgenic plants (Figure 8). The presence of NaCl up-regulated all of the tested genes, although the extent of the induction was less for the transgenic lines than for WT. Expression levels of OsSGR, OsNAC2, OsATG3b, and OsATG7 in the leaves of transgenic lines were 59–74%, 51–64%, 65–68%, and 70–80% of those in WT leaves, respectively (Figure 8). These results indicate that expression of SS3p:SS3 additionally enhances salt tolerance in rice by alleviating leaf senescence induced by salinity stress.
Figure 8.
Expression levels in response to salinity stress of senescence- and autophagy-related genes in SS3p:SS3 transgenic lines compared with WT. Growth conditions and treatments were as described in Figure 7. RNA was extracted from the leaf blades and qRT-PCR was used to detect transcript levels of OsSGR (A), OsNAC2 (B), OsATG3b (C), and OsATG7 (D). The values are means ± SE of three replicates. Significant differences between WT and transgenic lines at p < 0.05 are indicated with asterisks; ns indicates non-significant differences.
3. Discussion
The biosynthetic pathway for ascorbic acid involves a sequence of key enzymes, with MPGase as the first rate-limiting step [25,26]. MPGase has a critical role in the antioxidant response of seedlings to salt stress [23,27]. Many studies have reported that, in plants, changes in the MPGase enzyme can dramatically influence AsA synthesis, modifying tolerance to salt stress [21,28]. In Arabidopsis, transcription factor AtERF98 activates the expression of VTC1, enhancing the production of AsA and improving tolerance [21], while conversely, a point mutation in VTC1 had a severe impact on seedling susceptibility [20]. In contrast, the COP9 complex subunit CSN5B interacts with VTC1 to promote its degradation, but plants bearing a point mutation of CSN5B showed reduced degradation of VTC1, higher levels of AsA, and enhanced tolerance to salt [27].
Other VTC pathway genes play similar roles in the impact of salt stress on seedling growth. Abscisic acid pathway transcription factor ABI4 binds to the promoter of VTC2, reducing the production of the protein and thus the production of AsA during early plant growth, with Arabidopsis seedlings becoming less tolerant to salt [17]. The same effects are found in rice. Transcription of OsVTC1-3 increased under salt stress, while its inhibition resulted in stunting of growth [16]. Again, expression of OsMPG1 increases when rice is grown in saline conditions, while its overexpression in tobacco was found to reduce salt stress [23].
Our results demonstrate that SS3 is up-regulated in seedlings challenged by an increase in salinity (Figure 6). In addition, plasmid SS3p:SS3 expression resulted in improved rice shoot growth and assisted in the maintenance of Na+/K+ ratios in challenged plants. In contrast, ss3 mutants grew less successfully than WT rice under similar salinity conditions (Figure 1 and Figure 7). MPGase SS3 is essential for the normal growth of rice seedlings cultivated under salt stress. The reduction in AsA levels in the ss3 mutant explains its greater susceptibility to salt. Our complementation line experiments show (Figure 3A and Figure 5C) that returning AsA levels in the mutant to those of WT rice restored growth to that found in WT, as did the addition of exogenous AsA (Figure 4).
Damage to plants caused by salt is partly due to the induction of ROS [29]. Increased ROS levels could be a significant factor in the greater severity of leaf senescence in ss3 seedlings compared to WT under identical salt stress conditions. Excessive ROS levels disrupt cell membranes and degrade cell components, leading to premature leaf senescence [30,31,32]. One major consequence of senescence is the loss of chlorophyll [24,33], as was observed for stressed ss3 seedlings relative to WT (Figure 4A). In contrast, salt-tolerant transgenic SS3p:SS3 plants had raised chlorophyll levels (Figure 7C). Loss of protein is another major indicator of senescence [34], and ss3 seedlings under stress contained significantly less protein (Figure 4B). Membrane disruption during senescence may arise via lipid peroxidation [31], which gives rise to MDA as a significant product [24], and increased levels were found in leaves from stressed ss3 seedlings (Figure 3C). In addition, elevated levels of the ROS component H2O2 were found in the leaves of stressed mutants relative to WT (Figure 3B).
Senescence is a dynamic process associated with large-scale transformations in overall gene expression and characteristic increases in the products of a set of senescence-associated genes including OsSGR [31,34]. Similarly, the senescence-related transcription factors OsNAC2, OsWRKY23, and OsWRKY72 were all up-regulated [24,35]. Following salt treatment, we found that both transcription of OsSGR (Figure 8A) and expression of OsNAC2 (Figure 8B) were lower in SS3p:SS3 lines than in WT.
Autophagy is another significant process during leaf senescence [24] and three autophagy-related genes (OsATG4b, OsATG8a, and OsATG18b) were found to show increased expression in rice [34]. In our study, the mRNAs of two other autophagy-related genes (OsATG3b and OsATG7) were less abundant in SS3p:SS3 lines than WT when grown under salt challenge (Figure 8C,D). Expression of these genes (Figure 7A) was also in agreement with the superior performance of SS3p:SS3 lines relative to WT under salt stress.
Neutralization of ROS is critical for plants to adapt to difficult conditions [7,15], and MPGase has been identified as important for ROS scavenging in tobacco and Arabidopsis, both dicots [20,23]. The ability of Arabidopsis to deactivate ROS was compromised by mutations of VTC1. This reduced its ability to withstand ozone and other oxidants along with its tolerance to salinity [17,20]. Similarly, under saline conditions, inhibition of OsVTC1-3 led to a rapid increase in hydrogen peroxide concentration in rice roots [16]. In plants under salt stress, AsA could function as a primary antioxidant, i.e., plants react directly with AsA, and may also remove ROS via the AsA-GSH cycle [15,36]. Our results clearly show that SS3 increases the resistance of rice to saline conditions by control of AsA synthesis and the concomitant removal of ROS.
4. Materials and Methods
4.1. Plant Materials and Growth Conditions
Rice seeds (Oryza sativa ssp. japonica cv. NPB) from a library of EMS-induced mutants were surface-sterilized and germinated. Plants were cultured hydroponically in a growth chamber under a 14 h photoperiod at 30 °C during the light period and 25 °C during the dark period. The hydroponic solution was replaced every two days. The relative humidity was maintained at ~70%.
To screen for salinity stress-responsive mutants and to investigate the effect of high salinity on early seedling growth of WT and complementation lines, uniformly sized 10-day-old seedlings were treated for four days with a nutrient solution [37] containing a final concentration of 100 mM NaCl. The growth performance and the extent of chlorotic damage were determined [1]. To determine the effect of salt stress on the germination rate, rice seeds were sterilized and grown at 30 °C for seven days on 1/2 strength Murashige and Skoog (MS) medium (3% sucrose, 1% agar, pH 5.8) supplemented with or without 100 mM NaCl under a 12 h light/12 h dark photoperiod (100 µmol m−2 s−1 light intensity). The impact of salt stress on the survival rate of WT and complementation lines was assessed by growing them on normal 1/2 MS solid medium for two weeks, followed by irrigation with or without 150 mM NaCl for seven days. After three days of recovery, the survival rates were recorded; seedlings with green leaves were regarded as having survived. The survival rate was the ratio of surviving seedlings to the total number, as previously described [38].
To assess the effect of exogenous AsA on seedling growth and salt tolerance, 10-day-old seedlings were transferred for four days to a nutrient solution containing 0 mM or 100 mM NaCl supplemented with or without 1 mM AsA, as described by Wang et al. [36]. To investigate the differences between SS3p:SS3 transgenic lines and WT in response to salinity stress, 10-day-old seedlings were treated for six days with or without 100 mM NaCl. Shoots, roots, or leaf blades of the seedlings were collected after treatments, and physiological parameters and gene expression patterns were examined. Experiments were performed in triplicate with 5 individuals per genotype for each treatment.
4.2. Map-Based Cloning
For map-based cloning, the mutant (female parent) in the japonica background was hybridized with the indica cultivar TN1 to construct the segregating population. Localization was initially determined with simple sequence repeat (SSR) markers covering all 12 chromosomes [39]. For further mapping, new markers between the two flanking markers were designed based on the differences between the genomic DNA sequences of japonica variety NPB and indica variety 9311. All of the PCR products were separated on 5% agarose gels for visualization. Primers used for mapping the SS3 gene are listed in Table S1. The methods used for marker design and map-based cloning were as described by Yang et al. [39].
4.3. Generation of Transgenic Lines
For complementation of the ss3 mutation, we cloned a 4807 bp genomic DNA fragment containing the SS3 coding region into the binary vector pCAMBIA1300 along with the upstream and downstream sequences. The recombinant vector was introduced into the calli generated from mature seed embryos of the ss3 mutant by the Agrobacterium-mediated method [40].
To construct the SS3p:SS3 vector, the 1086 bp coding sequence (CDS) of SS3 was amplified with NPB cDNA as a template. After purification, it was connected to the pTCK303 linear vector, which was obtained from pTCK303 digested by BamHI and SpeI, using the GBclonart seamless cloning kit (Genebank Biosciences Inc., Zhangjiagang, China), and the intermediate pTCK303-SS3 vector was obtained. The SS3 promoter (2040 bp upstream of the initial codon) was amplified with NPB DNA as the template, and then purified and ligated to the pTCK303-SS3 linear vector, which was obtained from pTCK303-SS3 digested by BamHI, and the final construct was transformed into NPB as described above.
4.4. Determination of AsA Content
Rice seedling leaves were harvested and stored in liquid nitrogen. An aliquot of 0.2 g (fresh weight) was ground to a fine powder in liquid nitrogen. The extraction and measurement of AsA were performed as described by Wang et al. [27].
4.5. Determination of H2O2 and MDA Content
Seedling leaves harvested after salt treatment were ground and stored at −80 °C. The H2O2 content was assessed according to Chen et al. [41], while MDA was determined following Chen et al. [42].
4.6. Measurement of Chlorophyll and Soluble Protein Content
Chlorophyll content was determined by the method of Chen et al. [42], and soluble protein was quantified as described by Chen et al. [24].
4.7. Determination of Sodium and Potassium Ions
Shoots were excised from intact seedlings and dried in air to a constant weight at 70 °C. After digestion with nitric acid at 100 °C, Na+ and K+ were measured with an Optima 2100DV ICP emission spectrometer (Perkin Elmer Inc., Shelton, CT, USA) using the method of Chen et al. [43].
4.8. Quantitative Real Time PCR (qRT-PCR)
qRT-PCR was carried out according to Chen et al. [44]. RNA was extracted from the roots and leaf blades of seedlings grown under both normal and salt stress conditions. The rice gene UBQ5 (LOC_Os01g22490) was chosen as the reference sequence, and relative transcript abundances were calculated as given by Chen et al. [45]. The sequences of all primers used in the qRT-PCR experiments are listed in Table S2.
4.9. Statistical Analysis
Analyses of variance were carried out using SPSS v10 software (SPSS Inc., Chicago, IL, USA). Statistically significant differences between the performance of the various genotypes and WT, or between different treatments (as determined by Tukey’s test), are denoted by the inclusion on the histograms of a specific letter or an asterisk.
5. Conclusions
A salt-hypersensitive mutant was obtained by screening an EMS mutant library under salinity stress, and gene SS3 was identified. We cloned the SS3 gene and demonstrated that it plays a novel role in response to salinity stress, i.e., rice can specifically activate the transcription of SS3 to promote AsA synthesis, thus scavenging ROS in vivo to improve salt tolerance. Further investigations will focus on the SS3-mediated stress signaling pathway in rice to provide both genetic resources and a theoretical basis for enhancing salinity resistance in crops.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms231810338/s1.
Author Contributions
Conceptualization, G.C. and X.W.; formal analysis, G.C. and H.H.; funding acquisition, G.C., R.D. and X.W.; investigation, G.C., H.H. and X.Y.; methodology, G.C., X.Y. and X.W.; project administration, X.W.; resources, R.D. and X.W.; supervision, X.W.; validation, H.H.; writing—original draft preparation, G.C.; writing—review and editing, R.D. and X.W. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are contained within the article and Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by Guangzhou Science and Technology Planning Project (Nos. 202201010032, 202102080370), Guangdong Basic and Applied Basic Research Foundation (No. 2022A1515012580), the National Natural Science Foundation of China (No. 32072662), Special fund for scientific innovation strategy-construction of high level Academy of Agriculture Science (R2021YJ-QG006), Foundation project of Director of Institute of Quality Standard and Monitoring Technology for Agro-products of Guangdong Academy of Agricultural Sciences (DWJJ-202113).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chen G., Hu J., Dong L., Zeng D., Guo L., Zhang G., Zhu L., Qian Q. The tolerance of salinity in rice requires the presence of a functional copy of FLN2. Biomolecules. 2019;10:17. doi: 10.3390/biom10010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Saini S., Kaur N., Pati P.K. Reactive oxygen species dynamics in roots of salt sensitive and salt tolerant cultivars of rice. Anal. Biochem. 2018;550:99–108. doi: 10.1016/j.ab.2018.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Zeng L., Shannon M.C., Lesch S.M. Timing of salinity stress afects rice growth and yield components. Agric. Water Manag. 2001;48:191–206. doi: 10.1016/S0378-3774(00)00146-3. [DOI] [Google Scholar]
- 4.Chen G., Zheng D., Feng N., Zhou H., Mu D., Zhao L., Shen X., Rao G., Meng F., Huang A. Physiological mechanisms of ABA-induced salinity tolerance in leaves and roots of rice. Sci. Rep. 2022;12:8228. doi: 10.1038/s41598-022-11408-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farooq M.A., Niazi A.K., Akhtar J., Farooq M., Souri Z., Karimi N., Rengel Z. Acquiring control: The evolution of ROS-Induced oxidative stress and redox signaling pathways in plant stress responses. Plant Physiol. Biochem. 2019;141:353–369. doi: 10.1016/j.plaphy.2019.04.039. [DOI] [PubMed] [Google Scholar]
- 6.De Pinto M.C., De Gara L. Changes in the ascorbate metabolism of apoplastic and symplastic spaces are associated with cell diferentiation. J. Exp. Bot. 2004;55:2559–2569. doi: 10.1093/jxb/erh253. [DOI] [PubMed] [Google Scholar]
- 7.Akram N.A., Shafiq F., Ashraf M. Ascorbic acid-a potential oxidant scavenger and its role in plant development and abiotic stress tolerance. Front. Plant Sci. 2017;8:613. doi: 10.3389/fpls.2017.00613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shalata A., Mittova V., Volokita M., Guy M., Tal M. Response of the cultivated tomato and its wild salt-tolerant relative Lycopersicon pennellii to salt-dependent oxidative stress: The root antioxidative system. Physiol. Plant. 2001;112:487–494. doi: 10.1034/j.1399-3054.2001.1120405.x. [DOI] [PubMed] [Google Scholar]
- 9.Hancock R.D., Viola R. Biosynthesis and catabolism of L-ascorbic acid in plants. Crit. Rev. Plant Sci. 2005;24:167–188. doi: 10.1080/07352680591002165. [DOI] [Google Scholar]
- 10.Viviani A., Verma B.C., Giordani T., Fambrini M. L-Ascorbic acid in plants: From biosynthesis to its role in plant development and stress response. Agrochim. Int. J. Plant Chem. Soil Sci. Plant Nutr. Univ. Pisa. 2021;65:151–171. doi: 10.12871/00021857202124. [DOI] [Google Scholar]
- 11.Conklin P., Saracco S., Norris S., Last R. Identification of ascorbic acid-deficient Arabidopsis thaliana mutants. Genetics. 2000;154:847–856. doi: 10.1093/genetics/154.2.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lamanchai K., Salmon D.L., Smirnoff N., Sutthinon P., Roytrakul S., Leetanasaksakul K., Kittisenachai S., Jantasuriyarat C. OsVTC1-1 RNAi mutant with reduction of ascorbic acid synthesis alters cell wall sugar composition and cell wall-associated proteins. Agronomy. 2022;12:1272. doi: 10.3390/agronomy12061272. [DOI] [Google Scholar]
- 13.Conklin P., Norris S., Wheeler G., Williams E., Smirnoff N., Last R. Genetic evidence for the role of GDP-mannose in plant ascorbic acid (vitamin c) biosynthesis. Proc. Natl. Acad. Sci. USA. 1999;96:4198–4203. doi: 10.1073/pnas.96.7.4198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qin H., Deng Z., Zhang C., Wang Y., Wang J., Liu H., Zhang Z., Huang R., Zhang Z. Rice GDP-mannose pyrophosphorylase OsVTC1-1 and OsVTC1-3 play different roles in ascorbic acid synthesis. Plant Mol. Biol. 2016;90:317–327. doi: 10.1007/s11103-015-0420-0. [DOI] [PubMed] [Google Scholar]
- 15.Qin H., Wang Y., Wang J., Liu H., Zhao H., Deng Z., Zhang Z., Huang R., Zhang Z. Knocking down the expression of GMPase gene OsVTC1-1 decreases salt tolerance of rice at seedling and reproductive stages. PLoS ONE. 2016;11:e0168650. doi: 10.1371/journal.pone.0168650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Zhao H., Qin H., Li Z., Liu H., Wang J., Zhang H., Quan R., Huang R., Zhang Z. The synthesis of ascorbic acid in rice roots plays an important role in the salt tolerance of rice by scavenging ROS. Int. J. Mol. Sci. 2018;19:3347. doi: 10.3390/ijms19113347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kakan X., Yu Y., Li S., Li X., Huang R., Wang J. Ascorbic acid modulation by ABI4 transcriptional repression of VTC2 in the salt tolerance of Arabidopsis. BMC Plant Biol. 2021;21:112. doi: 10.1186/s12870-021-02882-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang H.S., Yu C., Zhu Z.J., Yu X.C. Overexpression in tobacco of a tomato GMPase gene improves tolerance to both low and high temperature stress by enhancing antioxidation capacity. Plant Cell Rep. 2011;30:1029–1040. doi: 10.1007/s00299-011-1009-y. [DOI] [PubMed] [Google Scholar]
- 19.Conklin P.L., Williams E.H., Last R.L. Environmental stress sensitivity of an ascorbic acid-deficient Arabidopsis mutant. Proc. Natl. Acad. Sci. USA. 1996;93:9970–9974. doi: 10.1073/pnas.93.18.9970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang C., He W., Guo J., Chang X., Su P., Zhang L. Increased sensitivity to salt stress in an ascorbate deficient Arabidopsis mutant. J. Exp. Bot. 2005;56:3041–3049. doi: 10.1093/jxb/eri301. [DOI] [PubMed] [Google Scholar]
- 21.Zhang Z., Wang J., Zhang R., Huang R. Ethylene response factor AtERF98 enhances tolerance to salt through the transcriptional activation of ascorbic acid synthesis in Arabidopsis. Plant J. 2012;71:273–287. doi: 10.1111/j.1365-313X.2012.04996.x. [DOI] [PubMed] [Google Scholar]
- 22.Ismail A.M., Horie T. Genomics, physiology, and molecular breeding approaches for improving salt tolerance. Annu. Rev. Plant Biol. 2017;68:405–434. doi: 10.1146/annurev-arplant-042916-040936. [DOI] [PubMed] [Google Scholar]
- 23.Kumar R., Mustafiz A., Sahoo K.K., Sharma V., Samanta S., Sopory S.K., Pareek A., Singla-Pareek S.L. Functional screening of cDNA library from a salt tolerant rice genotype Pokkali identifies mannose-1-phosphate guanyl transferase gene (OsMPG1) as a key member of salinity stress response. Plant Mol. Biol. 2012;79:555–568. doi: 10.1007/s11103-012-9928-8. [DOI] [PubMed] [Google Scholar]
- 24.Chen G., Wu C., He L., Qiu Z., Zhang S., Zhang Y., Guo L., Zeng D., Hu J., Ren D., et al. Knocking out the gene RLS1 induces hypersensitivity to oxidative stress and premature leaf senescence in rice. Int. J. Mol. Sci. 2018;19:2853. doi: 10.3390/ijms19102853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Smirnoff N., Wheeler G.L. Ascorbic acid in plants: Biosynthesis and function. Crit. Rev. Biochem. Mol. Biol. 2000;35:291–314. doi: 10.1080/10409230008984166. [DOI] [PubMed] [Google Scholar]
- 26.Song W., Wang F., Chen L., Ma R., Zuo X., Cao A., Xie S., Chen X., Jin X., Li H. GhVTC1, the key gene for ascorbate biosynthesis in Gossypium hirsutum, involves in cell elongation under control of ethylene. Cells. 2019;8:1039. doi: 10.3390/cells8091039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang J., Yu Y., Zhang Z., Quan R., Zhang H., Ma L., Deng X., Huang R. Arabidopsis CSN5B interacts with VTC1 and modulates ascorbic acid synthesis. Plant Cell. 2013;25:625–636. doi: 10.1105/tpc.112.106880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Li Y., Chu Z., Luo J., Zhou Y., Cai Y., Lu Y., Xia J., Kuang H., Ye Z., Ouyang B. The C2H2 zinc-finger protein SlZF3 regulates AsA synthesis and salt tolerance by interacting with CSN5B. Plant Biotechnol. J. 2018;16:1201–1213. doi: 10.1111/pbi.12863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inzé D., Van Montague M. Oxidative stress in plants. Curr. Opin. Biotechnol. 1995;6:153–158. doi: 10.1016/0958-1669(95)80024-7. [DOI] [Google Scholar]
- 30.Chen L., Wuriyanghan H., Zhang Y., Duan K., Chen H., Li Q., Lu X., He S., Ma B., Zhang W. An S-domain receptor-like kinase, OsSIK2, confers abiotic stress tolerance and delays dark-induced leaf senescence in rice. Plant Physiol. 2013;163:1752–1765. doi: 10.1104/pp.113.224881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wang Z., Wang Y., Hong X., Hu D., Liu C., Yang J., Li Y., Huang Y., Feng Y., Gong H. Functional inactivation of UDP-N-acetylglucosamine pyrophosphorylase 1 (UAP1) induces early leaf senescence and defence responses in rice. J. Exp. Bot. 2015;66:973–987. doi: 10.1093/jxb/eru456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong Y., Zhang Y., Sinumporn S., Yu N., Zhan X., Shen X., Chen D., Yu P., Wu W., Liu Q., et al. Premature leaf senescence 3, encoding a methyltransferase, is required for melatonin biosynthesis in rice. Plant J. 2018;95:877–891. doi: 10.1111/tpj.13995. [DOI] [PubMed] [Google Scholar]
- 33.Wang M., Zhang T., Peng H., Luo S., Tan J., Jiang K., Heng Y., Zhang X., Guo X., Zheng J., et al. Rice premature leaf senescence 2, encoding a glycosyltransferase (GT), is involved in leaf senescence. Front. Plant Sci. 2018;9:560. doi: 10.3389/fpls.2018.00560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhou Q., Yu Q., Wang Z., Pan Y., Lv W., Zhu L., Chen R., He G. Knockdown of GDCH gene reveals reactive oxygen species-induced leaf senescence in rice. Plant Cell Environ. 2013;36:1476–1489. doi: 10.1111/pce.12078. [DOI] [PubMed] [Google Scholar]
- 35.Akhter D., Qin R., Nath U.K., Alamin M., Jin X., Shi C. The brown midrib leaf (bml) mutation in rice (Oryza sativa L.) causes premature leaf senescence and the induction of defense responses. Genes. 2018;9:203. doi: 10.3390/genes9040203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M., Guo W., Li J., Pan X., Pan L., Zhao J., Zhang Y., Cai S., Huang X., Wang A., et al. The miR528-AO module confers enhanced salt tolerance in rice by modulating the ascorbic acid and abscisic acid metabolism and ROS scavenging. J. Agric. Food Chem. 2021;69:8634–8648. doi: 10.1021/acs.jafc.1c01096. [DOI] [PubMed] [Google Scholar]
- 37.Chen G., Feng H., Hu Q., Qu H., Chen A., Yu L., Xu G. Improving rice tolerance to potassium deficiency by enhancing OsHAK16p:WOX11-controlled root development. Plant Biotechnol. J. 2015;13:833–848. doi: 10.1111/pbi.12320. [DOI] [PubMed] [Google Scholar]
- 38.Wu J., Yu C., Huang L., Gan Y. A rice transcription factor, OsMADS57, positively regulates high salinity tolerance in transgenic Arabidopsis thaliana and Oryza sativa plants. Physiol. Plant. 2021;173:1120–1135. doi: 10.1111/ppl.13508. [DOI] [PubMed] [Google Scholar]
- 39.Yang Y., Xu J., Huang L., Leng Y., Dai L., Rao Y., Chen L., Wang Y., Tu Z., Hu J., et al. PGL, encoding chlorophyllide a oxygenase 1, impacts leaf senescence and indirectly affects grain yield and quality in rice. J. Exp. Bot. 2016;67:1297–1310. doi: 10.1093/jxb/erv529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen G., Hu J., Lian J., Zhang Y., Zhu L., Zeng D., Guo L., Yu L., Xu G., Qian Q. Functional characterization of OsHAK1 promoter in response to osmotic/drought stress by deletion analysis in transgenic rice. Plant Growth Regul. 2019;88:241–251. doi: 10.1007/s10725-019-00504-3. [DOI] [Google Scholar]
- 41.Chen G., Liu C., Gao Z., Zhang Y., Zhang A., Zhu L., Hu J., Ren D., Yu L., Xu G., et al. Variation in the abundance of OsHAK1 transcript underlies the differential salinity tolerance of an indica and a japonica rice cultivar. Front. Plant Sci. 2018;8:2216. doi: 10.3389/fpls.2017.02216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen G., Liu C., Gao Z., Zhang Y., Jiang H., Zhu L., Ren D., Yu L., Xu G., Qian Q. OsHAK1, a high-affinity potassium transporter, positively regulates responses to drought stress in rice. Front. Plant Sci. 2017;8:1885. doi: 10.3389/fpls.2017.01885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen G., Liu C., Gao Z., Zhang Y., Zhu L., Hu J., Ren D., Xu G., Qian Q. Driving the expression of RAA1 with a drought-responsive promoter enhances root growth in rice, its accumulation of potassium and its tolerance to moisture stress. Environ. Exp. Bot. 2018;147:147–156. doi: 10.1016/j.envexpbot.2017.12.008. [DOI] [Google Scholar]
- 44.Chen G., Zhang Y., Ruan B., Guo L., Zeng D., Gao Z., Zhu L., Hu J., Ren D., Yu L., et al. OsHAK1 controls the vegetative growth and panicle fertility of rice by its effect on potassium-mediated sugar metabolism. Plant Sci. 2018;274:261–270. doi: 10.1016/j.plantsci.2018.05.034. [DOI] [PubMed] [Google Scholar]
- 45.Chen G., Hu Q., Luo L., Yang T., Zhang S., Hu Y., Yu L., Xu G. Rice potassium transporter OsHAK1 is essential for maintaining potassium-mediated growth and functions in salt tolerance over low and high potassium concentration ranges. Plant Cell Environ. 2015;38:2747–2765. doi: 10.1111/pce.12585. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are contained within the article and Supplementary Material.