Abstract
Pseudomonas aeruginosa is a ubiquitous environmental bacterium capable of forming biofilms on surfaces as a survival strategy. It exhibits a large variety of competition/virulence factors, such as three types of motilities: flagellum-mediated swimming, flagellum-mediated swarming, and type IV pilus-mediated twitching. A strategy frequently used by bacteria to survive changing environmental conditions is to create a phenotypically heterogeneous population by a mechanism called phase variation. In this report, we describe the characterization of phenotypic variants forming small, rough colonies that spontaneously emerged when P. aeruginosa 57RP was cultivated as a biofilm or in static liquid cultures. These small-colony (S) variants produced abundant type IV fimbriae, displayed defective swimming, swarming, and twitching motilities, and were impaired in chemotaxis. They also autoaggregated in liquid cultures and rapidly initiated the formation of strongly adherent biofilms. In contrast, the large-colony variant (parent form) was poorly adherent, homogeneously dispersed in liquid cultures, and produced scant polar fimbriae. Further analysis of the S variants demonstrated differences in a variety of other phenotypic traits, including increased production of pyocyanin and pyoverdine and reduced elastase activity. Under appropriate growth conditions, cells of each phenotype switched to the other phenotype at a fairly high frequency. We conclude that these S variants resulted from phase variation and were selectively enriched when P. aeruginosa 57RP was grown as a biofilm or in static liquid cultures. We propose that phase variation ensures the prior presence of phenotypic forms well adapted to initiate the formation of a biofilm as soon as environmental conditions are favorable.
Pseudomonas aeruginosa is a gram-negative bacterium found in almost every ecological niche, including soil, water, and plants. It is also an important opportunistic pathogen of humans, primarily infecting immunocompromised patients (17). Recent reports indicate that environmental and clinical P. aeruginosa strains are functionally equivalent and taxonomically indistinguishable (14). The success of P. aeruginosa in various environments is attributed to its broad metabolic versatility and its elaboration of many cell-associated and secreted virulence/survival factors (47).
Among the cell surface structures of P. aeruginosa, the polar flagellum is responsible for a mode of motility in aqueous environment called swimming. As for most other motile bacteria, direction of movement is biased by chemotactic responses to chemical stimuli (6, 31, 46). Flagella also mediate a mode of social motility known as swarming, recently described for the first time in P. aeruginosa (39). Other cell surface structures acting as virulence/survival factors are type IV pili. These polar fimbriae are presumably the principal adhesins, mediating the adherence to eukaryotic cell surfaces (18) and probably to abiotic surfaces as well (41). They are also responsible for the flagellum-independent mode of surface translocation called twitching motility (9, 41, 48).
Bacteria in natural habitats usually grow as biofilms, organized communities of cells embedded in an extracellular polysaccharide matrix and attached to a surface (5). In recent years, much has been learned about how cells initiate biofilm formation (43, 49). Escherichia coli mutants defective in biofilm formation were found either to lack the ability to produce type 1 pili or to be nonmotile (37). Similarly, flagellar motility and type IV pilus-based twitching motility have been shown to be required for the initial attachment and development of a biofilm by P. aeruginosa (34).
Biofilm bacteria display particular phenotypes that distinguish them from their freely growing counterparts (5, 49). The differential expression of a large number of genes is known to occur in the initial steps of biofilm formation (5, 38), such as the upregulation of exopolysaccharide synthesis following bacterial adhesion to a surface (10, 38). However, a regulatory system controlling the conversion to the biofilm phenotype has not been described.
A strategy that bacteria use to rapidly adapt and survive when environmental conditions change is to create a phenotypically diverse population by a mechanism called phase variation, that is, the high-frequency and reversible switching of phenotypic traits (13). In gram-negative bacteria, the expression of a number of cell surface structures and outer membrane proteins, especially those linked to adhesion, aggregation, and colonial morphology, is known to be regulated by phase-variable mechanisms (reviewed in reference 22).
As a part of a research project aimed at understanding the physiological mechanisms used by P. aeruginosa to access and catabolize hydrophobic, poorly bioavailable substrates such petroleum hydrocarbons (11), we have observed the spontaneous emergence of alternate phenotypic forms growing as small, rough colonies when P. aeruginosa 57RP was cultivated as a biofilm or in static liquid cultures (E. Déziel, Y. Comeau, and R. Villemur, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. K-57, 1999). Since colonial morphology reflects the differential expression of components on cell surfaces within the colony, we hypothesized that the adherence and/or motility behavior of this small colony phenotype might be altered.
In this report, we describe the isolation and characterization of phenotypic variants of P. aeruginosa 57RP. In contrast with the large-colony (L) variant (parent form), the small-colony (S) variants produced abundant polar fimbriae, displayed reduced flagellar (chemotactic) and twitching motilities, and rapidly initiated the formation of strongly adherent biofilms. Under appropriate growth conditions, cells of each phenotype switched to the other phenotype at a fairly high frequency, suggesting that these S variants resulted from a reversible phase variation phenomenon and were selectively enriched when P. aeruginosa was grown as a biofilm or in static liquid cultures.
MATERIALS AND METHODS
Bacterial strains and culture media.
P. aeruginosa 57RP was originally isolated from a hydrocarbon-contaminated soil (11). Bacteria were routinely subcultured on tryptic soy agar (TSA) plates from frozen stocks, and overnight cultures in Luria-Bertani broth (LB) at 37°C and 250 rpm were used to prepare inocula, unless stated otherwise.
Evaluation of cell surface hydrophobicity and cell adherence. (i) MATH.
Estimation of microbial cell surface hydrophobicity was performed with a microbial adhesion to hydrocarbon (MATH) test (40). Cells from overnight cultures were washed twice and resuspended in 25 mM phosphate-buffered saline (PBS) to an optical density at 600 nm of approximately 0.6. Then 1.5 ml of this suspension was mixed with various volumes of hexadecane (ranging from 0 to 700 μl) in 16- by 125-mm test tubes and vortexed for 30 s. After 30 min of equilibration, we measured the loss in absorbance of the aqueous phase relative to that of the initial cell suspension and estimated hydrophobicity by calculating the percentage of cells adhering to hexadecane.
(ii) MATS.
To estimate the adhesion potential of the cells (microbial adhesion to silica sand [MATS]), the MATH test was modified by replacing the hexadecane with different amounts (0 to 900 mg) of fine granular silica sand.
Motility and chemotaxis assays. (i) Swimming.
Tryptone swim plates (1% tryptone, 0.5% NaCl, 0.3% agar) were inoculated with a sterile toothpick and incubated for 16 h at 25°C. Motility was then assessed qualitatively by examining the circular turbid zone formed by the bacterial cells migrating away from the point of inoculation.
(ii) Swarming.
Swarm plates were composed of 0.5% Bacto Agar and 8 g of nutrient broth/liter (both from Difco, Detroit, Mich.), supplemented with 5 g of dextrose/liter, and dried overnight at room temperature (39). Cells were point inoculated with a sterile toothpick, and the plates were incubated at 30°C for 24 h.
(iii) Twitching.
Cells were stab inoculated with a toothpick through a thin (approximately 3-mm) LB agar layer (1% agar) to the bottom of the petri dish. After incubation for 24 to 48 h at 30°C, a hazy zone of growth at the interface between the agar and the polystyrene surface was observed (7). The ability of bacteria to strongly adhere and form a biofilm on the polystyrene surface was then examined by removing the agar, washing unattached cells with a stream of tap water, and staining the attached cells with crystal violet (1% [wt/vol] solution).
(iv) Flagellar chemotaxis.
The chemotactic response was quantified by a slightly modified version of the capillary assay of Mazumder et al. (32). A 1-ml tuberculin syringe with a disposable 25-gauge needle (Terumo Medical Corp., Elkton, Md.) was filled with 100 μl of Bushnell-Haas (BH) mineral salts medium (Difco) containing 0.1% tryptone as a chemoattractant. Cells were grown in LB at 37°C to the logarithmic phase, washed, and resuspended in BH. A 100-μl sample of this bacterial suspension was drawn into a 200-μl pipette tip. The syringe was then inserted and tightly fit into the tip with 3 mm of the needle inserted into the cell suspension. Control capillaries containing only BH were performed with each assay. Duplicate apparatus were incubated at 37°C for 45 min, and the content of the syringe was then diluted in 25 mM PBS and plated onto TSA plates for cell enumeration.
Biofilm formation assay with polystyrene culture tubes.
The biofilm formation protocol was adapted from that of O'Toole and Kolter (35). Polystyrene 12- by 75-mm tubes containing 0.5 ml of BDT medium (BH mineral salts medium supplemented with 0.2% dextrose and 0.5% tryptone) were inoculated from overnight LB cultures and incubated at 32°C without agitation. At regular time intervals, triplicate tubes were rinsed thoroughly with water, and a 1% solution of crystal violet was added to stain the attached cells. After 10 to 15 min of incubation at room temperature, the tubes were rinsed with water, and the biomass of attached cells (biofilm) was quantified by solubilization of the dye in 2 ml of 95% ethanol. The absorbance was measured at 600 nm with a spectrophotometer.
Sensitivity to oxidative stress.
The disk assay of Hassett et al. (21) was used to test the sensitivity of cells to oxidative stress. Briefly, 100-μl aliquots from cultures in mid-log or stationary phases of growth were uniformly spread on TSA and medium A (25) plates containing 2% agar. Sterile Whatman no. 1 filter paper disks (7-mm diameter) impregnated with 10 μl of 30% H2O2 were placed in triplicate on each plate. The diameter of the zone of growth inhibition around each disk was measured after 5 h of incubation at 37°C.
Production of exoproducts. (i) Pyocyanin.
Bacteria were grown for 30 h at 37°C and 250 rpm in 2 ml of medium A, which promotes pyocyanin production, and the relative amount of pyocyanin in culture supernatant was measured spectrophotometrically at 695 nm.
(ii) Pyoverdine.
Cells were cultivated at 37°C and 250 rpm for 16 h in 2 ml of medium B (25). The relative concentration of pyoverdine was quantified in the supernatants by measurement of the fluorescence at 460 nm after excitation at 400 nm with a Spectramax Gemini microplate spectrofluorometer (Molecular Devices Corp., Sunnyvale, Calif.).
(iii) Total proteases and elastase.
Elastase (LasB protease) activity was determined in liquid cultures by the elastin-Congo red (ECR) hydrolysis assay as described by Pearson et al. (36).
(iv) Determination of alginate production.
Cells were cultivated in LB supplemented with 0.2% glycerol for 92 h at 37°C and 250 rpm. The cultures were then centrifuged at 8,000 × g for 5 min, and the alginate contained in the supernatants was precipitated at −70°C for 16 h with 3 volumes of 95% ethanol. The precipitate was recovered by centrifugation at 18,000 × g for 15 min and resuspended in water. Alginate was quantified by assaying uronic acids with the borate-carbazole method (26), with d-mannuronate lactone (Sigma Chemical Co., St. Louis, Mo.) as a standard. Values were normalized to cell growth with total cellular protein concentrations. Proteins were solubilized in 0.1 N NaOH at 70°C for 30 min and analyzed by the method of Bradford (Bio-Rad Laboratories, Hercules, Calif.), with bovine serum albumin as a standard.
(v) Rhamnolipids.
Cultures were conducted at 30°C in agitated glass test tubes containing 1 ml of SW1/10F mineral salts medium with 2% mannitol (11, 12). Total production of all isomers of rhamnolipid biosurfactants was estimated in the supernatant by extraction and hydrolysis followed by quantification of rhamnose with the orcinol assay (4). The concentration of rhamnolipids was determined considering that 1 mg of rhamnose corresponds to 2.25 mg of rhamnolipids (12). Because of the highly clumping behavior of the S variants, the whole culture was evaluated for total protein content and correlated to rhamnolipid production.
Electron microscopy.
A drop of water was deposited on the edge of a colony from an overnight-grown LB agar plate. Cells were allowed to become suspended for about 1 min; then a Formvar-coated copper grid was floated on the drop for about 45 s, rinsed in a drop of water, and stained for 15 s with a 2% aqueous solution of phosphotungstic acid. Samples were examined with a Hitachi H-7100 transmission electron microscope.
RESULTS
Emergence of phenotypic variants of P. aeruginosa 57RP correlates with biofilm formation.
The wild-type P. aeruginosa 57RP parent strain usually produces large (∼16-mm diameter after 2 days at 30°C), flat colonies with an irregular, finely mottled periphery on LB plates (Fig. 1A). We had previously observed that when this strain was cultivated in liquid medium with hexadecane as the substrate, there was a lag phase (approximately 5 to 10 days) before significant growth occurred. Interestingly, at the onset of the exponential growth phase, we noticed the formation of a biofilm on the surface of hexadecane droplets, and this was correlated with the appearance of small, dry-looking colonies on agar plates. A progression from the wild-type form to the small rough colony phenotype with transient appearance of intermediate size and roughness was also noticed during the cultivation period on hexadecane (Déziel et al., Abstr. 99th Gen. Meet. Am. Soc. Microbiol.).
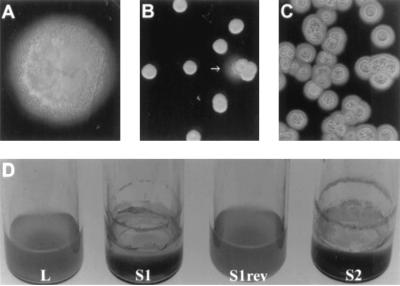
FIG. 1.
Visual differences between growth phenotypes. (A to C) Colonies of L variant (A), S1 variant (B; the arrow indicates the emergence of a L-type revertant sector emerging from the side of a colony), and S2 variant (C) on LB agar plates incubated at 30°C. (D) Overnight growth in broth medium with shaking. The L and S1rev variants grew homogeneously dispersed in the medium, whereas the S1 and S2 variants preferred the interface and the glass surface. S1rev is an L variant resulting from the reversion of an S1 variant.
A variety of colonial forms were recognized and isolated. Two typical small phenotypic variants (called S1 and S2) were selected for a more detailed characterization. When cultivated on LB plates, the S1 variant formed small (∼3-mm diameter after 2 days at 30°C [Fig. 1B]), convex, circular, and opaque colonies, whereas colonies of the S2 variant were larger (∼8-mm diameter after 2 days at 30°C [Fig. 1C]) and flatter, with a granular and irregular surface. This phenomenon was not restricted to strain 57RP; other P. aeruginosa strains demonstrating a lag phase before growth on hexadecane also formed S variants (data not shown). Arbitrarily primed PCRs using four different primers (10 nucleotides each) produced the same DNA patterns with the two variants and the parental strain (designated the L variant), confirming that the S variants were not contaminants (data not shown).
When S variant colonies were cultivated in agitated broth medium, growth appeared along the vessel walls as highly aggregative and adherent cells yielding low-turbidity cultures, whereas the L variant grew as a turbid, homogeneous suspension with no adherent cells (Fig. 1D). The clumping behavior of the S variants prevented representative sampling of liquid culture media. However, no difference in growth kinetics between the L and the S variants was observed when cultures were conducted in glass test tubes and the whole content was evaluated for total proteins. Under static culture conditions, the L variant first grew evenly in suspension in the medium and then slowly formed a surface film. Plating of this pellicle showed that it was essentially composed of S variants. Accordingly, when cultivated in the same conditions, the S1 and S2 variants predominantly grew as a thick pellicle at the surface of the liquid with a clear supernatant.
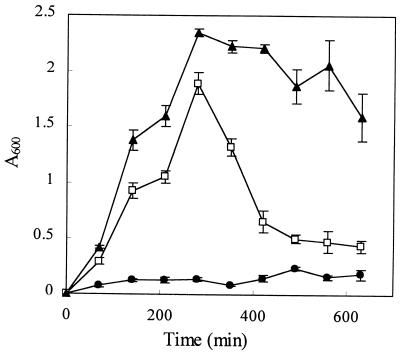
Since the S variants produced adherent growth, we postulated that they could be more efficient than the L variant in initiating the formation of biofilms. In fact, we noticed that biofilms formed in test tubes or in continuous-flow bioreactors after inoculation with the L variant were always predominantly composed of S-type variants. The dynamic of biofilm formation was measured for the three variants by cultivating them in nonagitated polystyrene tubes. As shown in Fig. 2, the L variant did not form a significant biofilm after 10 h of incubation. In contrast, the S variants quickly adhered and formed a dense biofilm within a few hours. The biofilm formed by S1 then rapidly dispersed, probably following exhaustion of the growth substrate, whereas the biofilm developed by S2 was much more stable, eventually coming off as large cell clumps during the washing steps (as shown by the larger error bars at the end of the incubation period).
FIG. 2.
Kinetics of biofilm formation. L (●), S1 (□), and S2 (▴) variants were cultivated in polystyrene tubes at 32°C without agitation. At the indicated time intervals, triplicate tubes were rinsed and stained with crystal violet. The amount of stained cells was then quantified by spectrophotometry (A600) after solubilization of the dye in ethanol.
S variants can revert to the parent L variant phenotype.
Outside a selective environment, the S1 variant reverted to the L phenotype at a relatively high frequency. Transfer plating of an S1 colony on TSA plates consistently resulted in the emergence of L-type revertants forming sectors emerging from the colonies (arrow in Fig. 1B). For example, after 1 week of incubation at room temperature, most colonies on a plate displayed outgrowth of L variant cells. The same phenomenon also occurred with the S2 variant but at a much lower frequency, with only occasional revertants appearing on plates. Because of the differences in growth behavior and environmental niche preferences between the L and S variants, determination of switching rates is not readily possible.
S variants demonstrate increased cell surface hydrophobicity and adhesivity.
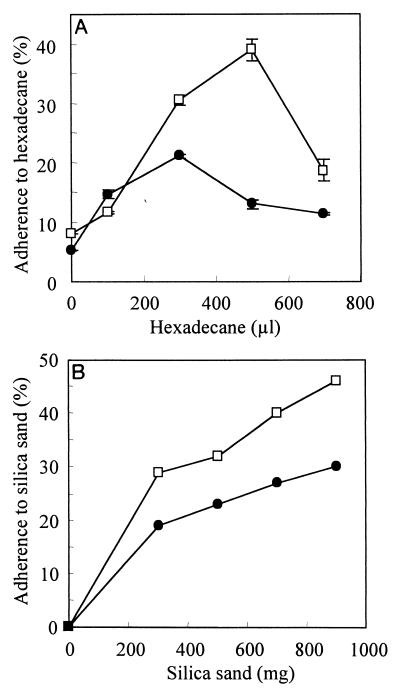
The aggregating and adherent behavior of the S variants suggested that their cell surface hydrophobicity was higher than that of the L variant. According to the standard assay used to quantitatively evaluate cell surface hydrophobicity (MATH test), the cell surface of S1 was much more hydrophobic than the surface of the parent L variant (Fig. 3A). However, this variant was also more adhesive to silica sand, a hydrophilic substratum (Fig. 3B), indicating that the S variants are mainly characterized by their adherence. It was not possible to perform these assays with S2 because of the highly clumping behavior of this variant.
FIG. 3.
Evaluation of cell surface hydrophobicity and adhesion potential by the MATH (A) and MATS (B) tests, respectively, in L (●) and S1 (□) variants. Various amounts of hexadecane (MATH) or silica sand (MATS) were mixed with a washed cell suspension in PBS, and the optical densities at 600 nm before and after were compared. Values for the MATH test are means ± standard deviations of duplicates.
S variants are deficient in swimming, swarming and twitching motilities.
The reduced diameter of S variants colonies suggested that they were impaired in motility and/or chemotaxis. When the S variants were cultivated on soft agar plates, their zones of swimming were smaller than that for the L variant with S2 producing slightly larger zones than S1 (Fig. 4A). There was some dispersion of cells from the point of inoculation but without the formation of concentric chemotactic rings, suggesting that the S variants are not completely defective in motility but may be impaired in chemotaxis. Microscopic examination showed that the S variants were motile, but many cells exhibited a tumbling behavior and random movement and lacked the directional swimming typical of the L phenotype. On swarm agar plates, the S variants remained near the point of inoculation and did not form the expanding and irregular branching pattern which is characteristic of swarming motility in P. aeruginosa, as recently described by Rashid and Kornberg (39) (Fig. 4B).
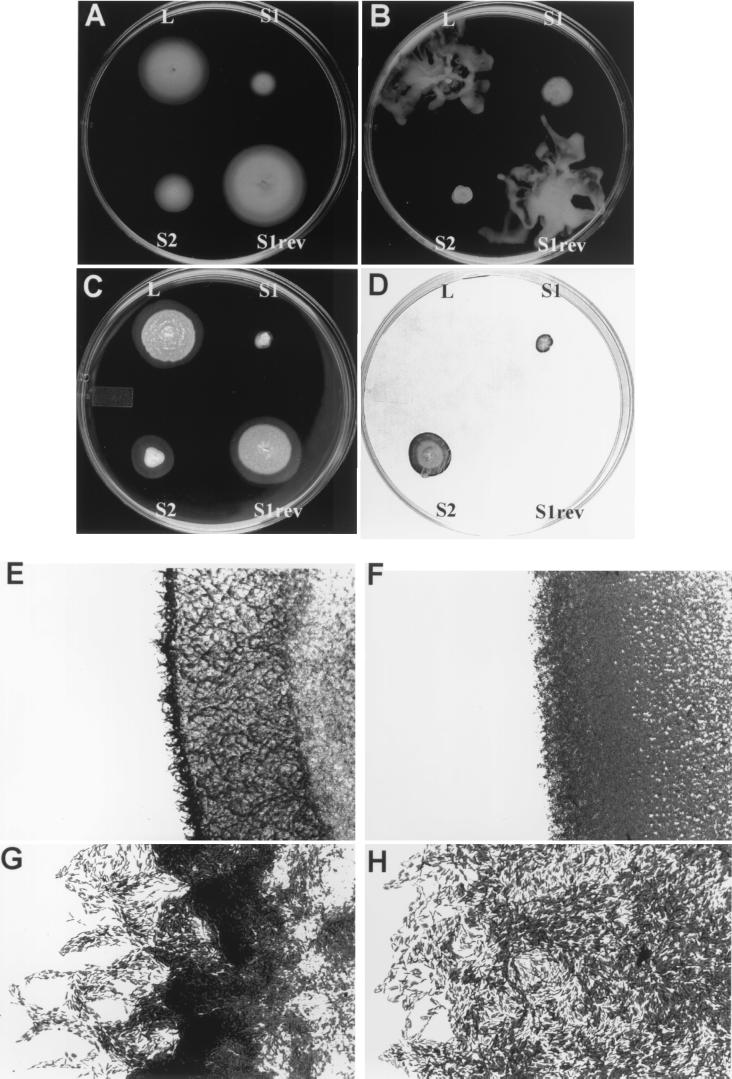
FIG. 4.
Differences in motility phenotypes of L and S variants. (A) Swimming motility on a tryptone swim plate (0.3% agar). (B) Swarming motility on a 0.5% agar plate. (C) Twitching motility on a thin (3-mm) LB plate containing 1% agar. Twitching is observed as a hazy zone of interstitial growth surrounding the surface colony. (D) Staining with crystal violet of cells in twitching zones that remained attached to the polystyrene surface after removing the agar layer and washing with water. (E to H) Light microscopy of the outside of the twitching zone stained with crystal violet. (E and F) S1 and S2 variant cells at a magnification of ×90; (G and H) rafts of S1 and S2 variant cells oriented toward the expending direction of the twitching area at a magnification of ×900.
Finally, when the strains were stabbed through a thin agar layer, the S variants formed a smaller and denser zone of twitching motility at the polystyrene-agar interface than the L variant (Fig. 4C). When the agar was scraped off and the polystyrene surface was rinsed with tap water, the thin layer of L variant growth was readily dispersed by the stream of water, whereas the bacteria in the twitching zone of the S variants remained firmly attached to the polystyrene surface. Staining with crystal violet indicated that the attached cells closely matched the twitching area (Fig. 4D). Furthermore, observation of the stained cells area on the polystyrene plate demonstrated striking differences between the adherence patterns of the S1 and S2 variants. S1 produced an expanding donut-shaped adherent zone, indicating that only the outer side of the twitching area was attached, whereas S2 cells remained adherent to the polystyrene surface, with only few bacteria released from the center of the colony (Fig. 4D). Microscopic analysis revealed that the leading edge of the twitching zone was composed of rafts of cells longitudinally oriented toward the expanding direction of the colony (Fig. 4E to H). These rafts were usually composed of a single layer of cells, but their density varied depending on the culture medium and temperature of incubation. Behind these rafts, a complex lattice-like arrangement of cells was formed, very similar to what was recently reported by others for P. aeruginosa (39, 41). In contrast to what was observed for S1, the rafts of S2 were generally larger, shorter, and often multilayered, and the highly structured fine latticework network behind the rafts of microcolonies was absent (Fig. 4H). Identity of zones of bacterial adherence as a typical biofilm was confirmed by the presence of dispersed microcolonies in an alginate matrix, the latter demonstrated by staining with the exopolysaccharide-specific dye alcian blue (data not shown).
Flagellar chemotactic response is impaired in the S variants.
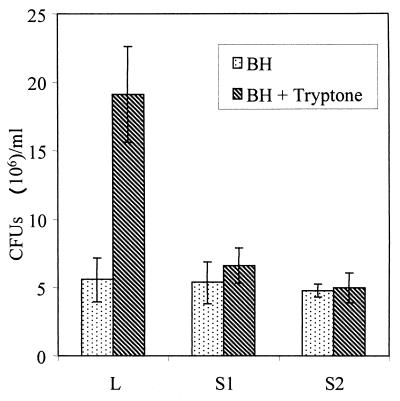
The fact that all three types of motilities were affected, in addition to the absence of clear chemotactic rings on swim plates, suggested a defect in chemotaxis. The capillary chemotaxis assay showed that the S variants were not significantly attracted to tryptone, a strong chemoattractant. The relative chemotaxis response (ratio of bacterial cell number in the capillary with tryptone to that without tryptone) was between 3.5 and 4 for the L variant and between 1 and 1.5 for the S variants (Fig. 5) A ratio of 2 or more is considered significant (32), confirming that the S variants displayed a defective chemotactic response.
FIG. 5.
Comparison of chemotactic responses of L and S variants. Capillary apparatus with or without 0.1% tryptone as a chemoattractant was prepared as described in Materials and Methods and incubated at 37°C for 45 min. The content of the syringe was then plated onto TSA plates for cell enumeration. Error bars represent the standard deviations of duplicates.
Electron microscopy.
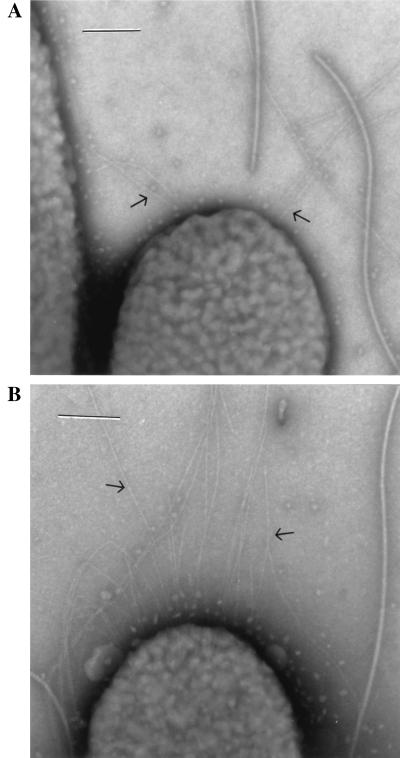
The high adherence and impaired motility of the S variants hinted at abnormal pili or flagella. Transmission electron microscopy of cells directly sampled from the edge of colonies revealed that the S variants are hyperpiliated (Fig. 6), with the environment of the cells surrounded with pili fragments. Pili were especially abundant in cell aggregates. Bundles formed by the entwinement of numerous pili, some larger than flagella, were frequently observed.
FIG. 6.
Transmission electron micrographs of L (A) and S2 (B) variants. Cells were grown overnight on LB agar plates, transferred onto Formvar-coated copper grids, and stained with phosphotungstic acid. Arrows indicate type IV pili. Bars = 0.2 μm.
Expression of various virulence/survival factors is altered in the S variants.
We investigated whether there were any differences in virulence/survival factors other than chemotaxis, motility, and piliation. Pyocyanin, the blue phenazine pigment of P. aeruginosa, is an extracellular secondary metabolite with antibiotic activity. As shown in Table 1, the S variants produced three to fivefold more pyocyanin than the L variant. Furthermore, culture supernatants of the S variants contained about 70% more pyoverdine, the primary siderophore of P. aeruginosa, than the L phenotype. The ECR assay unambiguously demonstrated that the S variants excrete less LasB protease (Table 1). Alginate is an exopolysaccharide that is secreted by P. aeruginosa for the establishment of the biofilm matrix. Considering that the S variants formed a biofilm more readily than the L variants, we examined the production of extracellular alginate. In agitated flask cultures at 37°C, there was no difference in the concentration of uronic acids in the supernatant (Table 1). Rhamnolipids are heat-stable hemolysins, displaying surface-active properties, which are coproduced with other extracellular factors (36, 47). The S variants, especially S2, appeared to produce slightly lower concentrations of this biosurfactant than the parent variant (Table 1). Finally, we examined the ability of the variants to survive stress aggression. As shown in Table 1, there was no clear difference in sensitivity to H2O2 between the L and S variants in stationary-phase cells. However, when cells from the logarithmic phase of growth were plated, the zone of inhibition caused by H2O2 was about 30% smaller for the L variant than for the S variants, indicating hypersensitivity of the latter. Results obtained on TSA plates did not significantly differ from those obtained on medium A agar.
TABLE 1.
Comparison of production of extracellular products and sensitivity to oxidative stress between the L and S variants
| Variant | Pyocyanina | Pyoverdineb | Elastasec | Alginate (mg of uronic acid/mg of protein) | Rhamnolipids (mg/mg proteins) | H2O2 sensitivityd
|
|
|---|---|---|---|---|---|---|---|
| Stationary-phase cells | Log-phase cells | ||||||
| L | 0.27 ± 0.02 | 29,700 ± 2,700 | 2.26 ± 0.27 | 6.53 ± 0.56 | 2.01 ± 0.12 | 17.2 ± 0.7 | 16.4 ± 0.5 |
| S1 | 0.89 ± 0.08 | 50,000 ± 1,100 | 1.43 ± 0.10 | 6.69 ± 0.31 | 1.82 ± 0.08 | 18.3 ± 0.5 | 23.0 ± 0.6 |
| S1reve | 0.23 ± 0.02 | 33,000 ± 1,100 | NDf | ND | ND | 17.0 ± 0.6 | 16.3 ± 0.5 |
| S2 | 1.36 ± 0.04 | 48,100 ± 1,800 | 0.42 ± 0.07 | 7.15 ± 0.27 | 1.58 ± 0.13 | 18.5 ± 0.5 | 24.5 ± 1.0 |
Measured as A695 of the supernatant of a 30-h culture at 37°C in King's A medium.
Measured in the supernatant of a 16-h culture at 37°C in King's B medium. A460 was determined after excitation at 400 nm and reported as relative fluorescence units.
Measured in ECR assays of culture supernatants from 18-h cultures as described in Materials and Methods and reported as A495.
Filter paper disks (7-mm diameter) soaked with 10 μl of 30% H2O2 were placed on top of medium A agar plates covered with P. aeruginosa cells. Bacteria from exponential- or stationary-phase cultures in LB at 37°C were used as the inoculum, and the diameter of the zone of inhibited growth was measured after 5 h of incubation at 37°C. All values are means ± standard errors of triplicates.
S1rev is an L variant resulting from the reversion of an S1 variant.
ND, not determined.
DISCUSSION
Colonial phenotypes and type IV pili.
Our work describes the isolation and characterization of phenotypic variants (S phenotype) selectively enriched when P. aeruginosa 57RP was cultivated as a biofilm, whereas the parent L phenotype predominates in suspended growth. We also observed that the L variant of P. aeruginosa 57RP produced a surface pellicle enriched in S variants when cultivated in static liquid medium. Under these growth conditions, S variants demonstrated a strong preference for aggregative growth at the air-broth interface. The presence of hydrophobic type 1 fimbriae in E. coli, Salmonella, and Shigella is known to mediate the formation of surface film in nonagitated aerobic cultures (20, 33, 42). Hasman et al. (20) reported that the physical presence of type 1 fimbriae on E. coli K-12 is responsible for the formation of small, convex colonies, whereas the absence of fimbriae is correlated with larger, flat colonies. A similar correlation is commonly used as an indicator of pilus expression in Neisseria gonorrhoeae, which display type IV pili (29, 45). Hyperpiliated mutants of P. aeruginosa have been shown to form small colonies very similar to the S1 variant colonies (2). Thus, both surface film formation and small-colony phenotype are in agreement with hyperfimbriation of our S variants, as also confirmed by transmission electron microscopy (Fig. 6). Observations that the cell surface of the S variants is more hydrophobic and their adhesivity is higher than for the parent L variant are also coherent with the hyperpiliated phenotype.
Motility and chemotaxis.
Characterization of P. aeruginosa mutants which lack twitching motility has allowed the identification of about 34 genes involved in type IV pili biogenesis, regulation, and function in twitching (1, 48). Mutation in any of these genes results in nonpiliated cells, with few exceptions such as strains with defects in pilT or pilU, which overexpress surface pili but are incapable of twitching motility (50, 51). The S variants are apparently not directly affected in any of these genes since they actually formed twitching zones at the agar-polystyrene interface, albeit these were smaller than zones formed by the L variants. Another notable exception is pilH, which encodes a homologue of the enteric CheY response regulator. Strains with a defective pilH gene are piliated but form reduced twitching zones, with the presence of donut-shaped swirls at the outer edge of the motile zone (8). We also noticed, especially with S2, many holes and rings of cells reminiscent of the swirls reported by Darzins (8), suggesting that pilH may be affected in the S variants.
Interestingly, cells in the twitching zone of the S variants were highly adherent to the polystyrene surface, suggesting that a biofilm had formed. With the S1 variant, which is the typical S phenotype, only the exterior of the twitching area was adherent, resulting in an expanding donut-shaped biofilm. Accordingly, Semmler et al. (41) have shown by Western blotting with antipilin antisera that type IV fimbriae are expressed only on the outside of active twitching zones. It appears that cells left behind the zone of expansion, where the growth substrate was depleted, were much less adherent and readily detached when the polystyrene surface was rinsed. In this context, the observations that S variants were mostly found attached to surfaces (in biofilms and surface pellicles) and L variant in suspension suggest that the bacteria switched back to the nonadherent, chemotactically swimming L phenotype when growth conditions were no longer favorable. The doughnut-shaped ring of adherent cells therefore appears to extend at the rate of substrate consumption and twitching motility. Twitching motility was recently implicated in P. aeruginosa biofilm movement on abiotic surfaces (41) and in the formation of microcolonies within a differentiating or developing biofilm (34).
Although S variants presented defects in all three known modes of motility in P. aeruginosa, flagellum-mediated swimming, flagellum-mediated swarming, and type IV pilus-mediated twitching, only swarming was completely abrogated. P. aeruginosa is usually strongly attracted to commonly occurring amino acids (6, 46), such as those found in tryptone. However, the lack of chemotactic rings in the swimming assay on soft agar and chemotactic response in the capillary assay indicated that the S variants are deficient in chemotaxis (Fig. 4A and 5). To control the direction of swimming, P. aeruginosa uses a two-component sensor-regulator system with methyl-accepting chemotaxis proteins similar to those found in enteric bacteria (31, 46). Phenotypic differences between the L and the S variants, such as small colony size and defective flagellar and twitching motilities, were observed not only with undefined broth substrates but also with BH mineral salts medium supplemented with succinate or dextrose (data not shown), indicating that the defect is not simply limited to chemotactic transducers. At least two additional signal transduction system regulating pilus biosynthesis and twitching motility have been described in P. aeruginosa. The PilS/PilR network controls fimbrial biogenesis (1), while the pilGHIJKL gene products appear to support both pilus production and twitching motility (1, 9). The latter gene cluster resembles both the chemotaxis (Che) network controlling flagellar rotation in enteric bacteria and the Frz system which controls gliding motility in Myxococcus xanthus (8, 9). Gliding, which was recently shown to be essentially the same as twitching (41), is also mediated by type IV fimbriae (52). Although the environmental signals detected by the twitching motility signal transduction system are still undefined (9), it is suggested that pili might play a role as sensory organs for detecting cells nearby (48). Since twitching motility requires cell-to-cell contacts (41, 48), and our S variants produce denser, less differentiated twitching zones, they may be affected in the ability to sense neighbor cells. In this context, it is pertinent that swarming motility also seems to require cell-cell contacts (15). Swarming was only recently described in P. aeruginosa (39), and any involvement of chemotaxis in this type of motility has yet to be reported. In E. coli, chemotaxis is not required for swarming motility but a functional chemotaxis system is essential (3).
It was recently established that inactivation of the rhlA gene, which is required for rhamnolipid synthesis, abolishes swarming motility in P. aeruginosa (27; our unpublished results). However, the moderate decrease in rhamnolipid production by the S variants (Table 1) does not justify the complete elimination of swarming in these bacteria.
Although a modification of sensory systems is a more likely explanation for the peculiar motility behavior of the S variants, we must consider the possibility that L variant-type motility is simply prevented by the very large number of pili, causing obstruction of the normal flagellar activity and excessive adherence to the solid surface.
In agreement with our results, Pratt and Kolter (37) have shown with E. coli that motility, but not chemotaxis, is essential for normal biofilm formation. Together, our observations (overexpression of surface pili, elevated hydrophobicity and adhesivity, and defective chemosensory response resulting in decreased motilities) imply that the S variants display a modified expression of regulatory genes involved in the rapid initiation of biofilm formation.
In addition to the accelerated initiation of biofilm formation by a more efficient attachment to the surface, we investigated whether the S variants produced higher concentrations of alginate. In agitated liquid cultures, extracellular production of uronic acids polymers did not differ significantly between the S and L variants (Table 1). These results are in agreement with the predominant role of alginate in the consolidation of a biofilm rather than in the initial adhesion process.
Phase variation.
Phase variation is a diversity-generating mechanism ensuring that a portion of a bacterial population will be adapted to survive under new environmental conditions (13). Mechanisms regulated by phase variation are essentially stochastic within a population yet at least partially modulated by environmental signals. We observed that under appropriate environmental and growth conditions, cells of each phenotype could switch to the other phenotype at a fairly high frequency, suggesting that the shift between S and L variant phenotypes is regulated by a phase variation mechanism.
Phenotypic variations of surface structures are common in many pathogenic bacteria. They have been observed in E. coli adhesins, in Salmonella enterica serovar Typhimurium flagellum expression, and in antigenic and phase variation of Neisseria adhesins (22). We have uncovered many activities coordinately regulated by a putative phase variation mechanism, indicating that a major regulator might be the target of the switch (Table 2). Very few examples of phase variation mechanisms regulating simultaneously multiple phenotypic determinants have been reported. Interestingly, in most cases, the phenotypic switch influences the tactic response (23, 24). In the mushroom pathogen P. tolaasii, a spontaneous and reversible duplication within a two-component sensor regulator, causing a frameshift mutation, regulates many phenotypic traits, including attachment, colonial form, and chemotaxis (19). To our knowledge, no typical phenotypic variation switching mechanism has been found in P. aeruginosa.
TABLE 2.
Comparison of phenotypic characteristics between the L and S variantsa
| Phenotype | Descriptionsb
|
|
|---|---|---|
| L variant | S variants | |
| Colony shape | Large, flat, irregular | Small, rounded |
| Adherence to surfaces | + | ++ |
| Hydrophobicity | + | ++ |
| Initiation of biofilm formation | − | ++ |
| Flagella | + | + |
| Polar pili | + | ++ |
| Motility: | ||
| Swimming | ++ | + |
| Swarming | + | − |
| Twitching | ++ | + |
| Chemotaxis | + | − |
| Exoproducts | ||
| Pyoverdine | + | ++ |
| Pyocyanin | + | ++ |
| Alginate | + | + |
| Elastase | ++ | + |
| Rhamnolipids | ++ | +c |
| Sensitivity to H2O2 stress | + | ++ |
The L variant predominates on agar plates or in agitated liquid cultures; S variants predominate in biofilms or in static liquid cultures.
—, absence of phenotype; +, presence of phenotype; ++, expression of phenotype more pronounced than +.
Rhamnolipid production is significantly lower than in the L variant for the S2 variant only.
The motility phenotype that we observed for our S variants, especially S2, is strikingly similar to the one described by Rashid and Kornberg (39) for a polyphosphate kinase knockout mutant of P. aeruginosa PAO1. They proposed that polyphosphate kinase, or its product polyphosphate, might be required for the expression of rpoS, as in E. coli. The alternative sigma factor RpoS was initially identified as a central regulator of stationary-phase-responsive genes and is now associated with the general stress response (28). Our observations of increased pyoverdine and pyocyanin production and decreased swimming and twitching motilities were also reminiscent of a recently described rpoS mutant of P. aeruginosa PAO1 (44). Interestingly, It has been hypothesized that a sigma factor might control the expression of genes responsible for the biofilm phenotype (5). This prompted us to investigate further the possibility that the S variants could be affected in the synthesis of, or response to, RpoS. Like Suh et al. (44), we observed an increased sensitivity to H2O2 (Table 1). However, only our log-phase-grown S cells were more sensitive than the L cells. Also in contrast with the RpoS-negative mutant, we obtained a substantial decrease in elastase production but not in alginate accumulation in liquid medium (Table 1). Moreover, the responses to heat shock (53°C) and osmotic stress (3 M NaCl) did not differ significantly between the S and L variants (data not shown). These results thus invalidate the RpoS hypothesis.
S1 is different from S2.
Although the S1 form is the more abundant phenotypic small variant that we observed, other morphotypes were obtained when the parental L variant was cultivated on hexadecane, in static cultures, or as a biofilm. The S2 variant displayed many differences with the S1 variant, and most of its phenotypic idiosyncrasies were expressed with more amplitude (Table 2). As demonstrated by the retention of adherence in the twitching zone (Fig. 4D) and in the biofilm kinetic assay (Fig. 2), S2 appears to have an impaired detachment phenotype and to form defective biofilms. The fact that this variant did not build the fine lattice-like network of cells typically found behind the rafts in twitching motility expansion zones (41) may also be related to this defect. These features could be explained by the much lower reversion frequency to the L phenotype displayed by S2. It suggests that the S2 variant is blocked in the S phase; a mutation might impede its ability to undergo phase variation.
Biofilm phenotype.
Bacteria in biofilms are phenotypically different from their freely swimming counterpart (5, 38). Our results indicate that the biofilm way of growth selects for a specific phenotypic population that is highly adherent but with reduced motility. Why would chemotactically deficient cells be selected for in biofilms? Chemotaxis is essentially required in environments that are scarce in nutrients. One of the features of biofilms is to provide an environment where nutrients are continuously trapped by the exopolysaccharide matrix and available to the bacteria (5). Obviously, cells inside a biofilm do not require extensive motility until the time they leave to colonize another available surface (49). Mucoid and rough P. aeruginosa strains isolated from cystic fibrosis patients, thus selected for by a biofilm environment (17), lack flagella or are deficient in chemotaxis (16, 30).
S variants demonstrated a preference for growth at interfaces, such as a biofilm (liquid-solid), as a surface pellicle (air-liquid), or on hexadecane (liquid-liquid), suggesting that high surface hydrophobicity is a major characteristic of this phenotype. In contrast, the L variant was predominantly found freely dispersed in liquid medium and was not able to form a biofilm. We propose that the S and L phenotypic forms of P. aeruginosa are adapted to different environmental niches and that growth as a biofilm selects for a phenotypically distinct subpopulation usually found in minority in counterselective environments such as homogeneously agitated liquid cultures or agar plates. Biofilms thus act as an ecological niche colonized by a specific phenotypic population.
Our results suggest that transition between the planktonic and the biofilm phenotype is regulated by phase variation. Therefore, phenotypic diversity determined by phase variation ensures that cells well adapted to initiate the formation of a biofilm are already present as soon as environmental conditions are favorable. This may contribute to explain the major shift in gene expression and physiological properties displayed by bacteria growing as biofilms. Although the molecular mechanisms underlying the regulation of the phase variation control mechanism involved in switching between the L and S phenotypes remain to be elucidated, this work provides useful information that will assist in molecular characterization of the process of biofilm formation in P. aeruginosa.
ACKNOWLEDGMENTS
We are grateful to Réjean Beaudet for photographs, Robert Alain for electron microscopy, and Francine Turcotte-Rivard for technical assistance.
REFERENCES
- 1.Alm R A, Mattick J S. Genes involved in the biogenesis and function of type-4 fimbriae in Pseudomonas aeruginosa. Gene. 1997;192:89–98. doi: 10.1016/s0378-1119(96)00805-0. [DOI] [PubMed] [Google Scholar]
- 2.Bradley D E. The adsorption of Pseudomonas aeruginosa pilus-dependent bacteriophages to a host mutant with nonretractile pili. Virology. 1974;58:149–163. doi: 10.1016/0042-6822(74)90150-0. [DOI] [PubMed] [Google Scholar]
- 3.Burkart M, Toguchi A, Harshey R M. The chemotaxis system, but not chemotaxis, is essential for swarming motility in Escherichia coli. Proc Natl Acad Sci USA. 1998;95:2568–2573. doi: 10.1073/pnas.95.5.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chandrasekaran E V, BeMiller J N. Constituent analysis of glucosaminoglycans. In: Whistler R L, editor. Methods in carbohydrate chemistry. New York, N.Y: Academic Press, Inc.; 1980. pp. 89–96. [Google Scholar]
- 5.Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 6.Craven R, Montie T C. Regulation of Pseudomonas aeruginosa chemotaxis by the nitrogen source. J Bacteriol. 1985;164:544–549. doi: 10.1128/jb.164.2.544-549.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Darzins A. The pilG gene product, required for Pseudomonas aeruginosa pilus production and twitching motility, is homologous to the enteric single-domain response regulator CheY. J Bacteriol. 1993;175:5934–5944. doi: 10.1128/jb.175.18.5934-5944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Darzins A. Characterization of a Pseudomonas aeruginosa gene cluster involved in pilus biosynthesis and twitching motility: sequence similarity to the chemotaxis proteins of enterics and the gliding bacterium Myxococcus xanthus. Mol Microbiol. 1994;11:137–153. doi: 10.1111/j.1365-2958.1994.tb00296.x. [DOI] [PubMed] [Google Scholar]
- 9.Darzins A, Russell M A. Molecular genetic analysis of type-4 pilus biogenesis and twitching motility using Pseudomonas aeruginosa as a model system—a review. Gene. 1997;192:109–115. doi: 10.1016/s0378-1119(97)00037-1. [DOI] [PubMed] [Google Scholar]
- 10.Davies D G, Chakrabarty A M, Geesey G G. Exopolysaccharide production in biofilms: substratum activation of alginate gene expression by Pseudomonas aeruginosa. Appl Environ Microbiol. 1993;59:1181–1186. doi: 10.1128/aem.59.4.1181-1186.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Déziel E, Paquette G, Villemur R, Lépine F, Bisaillon J-G. Biosurfactant production by a soil Pseudomonas strain growing on polycyclic aromatic hydrocarbons. Appl Environ Microbiol. 1996;62:1908–1912. doi: 10.1128/aem.62.6.1908-1912.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Déziel E, Lépine F, Milot S, Villemur R. Mass spectrometry monitoring of rhamnolipids from a growing culture of Pseudomonas aeruginosa 57RP. Biochim Biophys Acta. 2000;1485:145–152. doi: 10.1016/s1388-1981(00)00039-1. [DOI] [PubMed] [Google Scholar]
- 13.Dybvig K. DNA rearrangements and phenotypic switching in prokaryotes. Mol Microbiol. 1993;10:465–471. doi: 10.1111/j.1365-2958.1993.tb00919.x. [DOI] [PubMed] [Google Scholar]
- 14.Foght J M, Westlake D W S, Johnson W M, Ridgway H F. Environmental gasoline-utilizing isolates and clinical isolates of Pseudomonas aeruginosa are taxonomically indistinguishable by chemotaxonomic and molecular techniques. Microbiology. 1996;142:2333–2340. doi: 10.1099/00221287-142-9-2333. [DOI] [PubMed] [Google Scholar]
- 15.Fraser G M, Hughes C. Swarming motility. Curr Opin Microbiol. 1999;2:630–635. doi: 10.1016/s1369-5274(99)00033-8. [DOI] [PubMed] [Google Scholar]
- 16.Garrett E S, Perlegas D, Wozniak D J. Negative control of flagellum synthesis in Pseudomonas aeruginosa is modulated by the alternative sigma factor AlgT (AlgU) J Bacteriol. 1999;181:7401–7404. doi: 10.1128/jb.181.23.7401-7404.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Govan J R, Deretic V. Microbiol pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hahn H P. The type-4 pilus is the major virulence-associated adhesin of Pseudomonas aeruginosa—a review. Gene. 1997;192:99–108. doi: 10.1016/s0378-1119(97)00116-9. [DOI] [PubMed] [Google Scholar]
- 19.Han B, Pain A, Johnstone K. Spontaneous duplication of a 661 bp element within a two-component sensor regulator causes phenotypic switching in colonies of Pseudomonas tolaasii, cause of brown blotch disease of mushrooms. Mol Microbiol. 1997;25:211–218. doi: 10.1046/j.1365-2958.1997.4411811.x. [DOI] [PubMed] [Google Scholar]
- 20.Hasman H, Schembri M A, Klemm P. Antigen 43 and type 1 fimbriae determine colony morphology of Escherichia coli K-12. J Bacteriol. 2000;182:1089–1095. doi: 10.1128/jb.182.4.1089-1095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassett D J, Schweizer H P, Ohman D E. Pseudomonas aeruginosa sodA and sodB mutants defective in manganese- and iron-cofactored superoxide dismutase activity demonstrate the importance of the iron-cofactored form in aerobic metabolism. J Bacteriol. 1995;177:6330–6337. doi: 10.1128/jb.177.22.6330-6337.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henderson I R, Owen P, Nataro J P. Molecular switches—the ON and OFF of bacterial phase variation. Mol Microbiol. 1999;33:919–932. doi: 10.1046/j.1365-2958.1999.01555.x. [DOI] [PubMed] [Google Scholar]
- 23.Kamoun S, Kado C I. Phenotypic switching affecting chemotaxis, xanthan production, and virulence in Xanthomonas campestris. Appl Environ Microbiol. 1990;56:3855–3860. doi: 10.1128/aem.56.12.3855-3860.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kelman A, Hruschka J. The role of motility and aerotaxis in the selective increase of avirulent bacteria in still broth cultures of Pseudomonas solanacearum. J Gen Microbiol. 1973;76:177–188. doi: 10.1099/00221287-76-1-177. [DOI] [PubMed] [Google Scholar]
- 25.King E O, Ward M K, Raney D E. Two simple media for the demonstration of pyocyanin and fluorescin. J Lab Clin Med. 1954;44:301. [PubMed] [Google Scholar]
- 26.Knutson C A, Jeanes A. A new modification of the carbazole analysis: application to heteropolysaccharides. Anal Biochem. 1968;24:470–481. doi: 10.1016/0003-2697(68)90154-1. [DOI] [PubMed] [Google Scholar]
- 27.Köhler T, Kocjancic Curty L, Barja F, Van Delden C, Pechère J-C. Swarming of Pseudomonas aeruginosa is dependent on cell-to-cell signaling and requires flagella and pili. J Bacteriol. 2000;182:5990–5996. doi: 10.1128/jb.182.21.5990-5996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loewen P C, Hengge-Aronis R. The role of sigmaS (KatF) in bacterial global regulation. Annu Rev Microbiol. 1994;48:53–80. doi: 10.1146/annurev.mi.48.100194.000413. [DOI] [PubMed] [Google Scholar]
- 29.Long C D, Madraswala R N, Seifert H S. Comparisons between colony phase variation of Neisseria gonorrhoeae FA1090 and pilus, pilin, and S-pilin expression. Infect Immun. 1998;66:1918–1927. doi: 10.1128/iai.66.5.1918-1927.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luzar M A, Thomassen M J, Montie T C. Flagella and motility alterations in Pseudomonas aeruginosa strains from patients with cystic fibrosis: relationship to patient clinical conditions. Infect Immun. 1985;50:577–582. doi: 10.1128/iai.50.2.577-582.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Masduki A, Nakamura J, Ohga T, Umezaki R, Kato J, Ohtake H. Isolation and characterization of chemotaxis mutants and genes of Pseudomonas aeruginosa. J Bacteriol. 1995;177:948–952. doi: 10.1128/jb.177.4.948-952.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mazumder R, Phelps T J, Krieg N R, Benoit R E. Determining chemotactic responses by two subsurface microaerophiles using a simplified capillary assay method. J Microbiol Methods. 1999;37:255–263. doi: 10.1016/s0167-7012(99)00072-x. [DOI] [PubMed] [Google Scholar]
- 33.Old D C, Duguid J P. Selective outgrowth of fimbriate bacteria in static liquid medium. J Bacteriol. 1970;103:447–456. doi: 10.1128/jb.103.2.447-456.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.O'Toole G A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 35.O'Toole G A, Kolter R. Initiation of biofilm formation in Pseudomonas fluorescens WCS365 proceeds via multiple, convergent signalling pathways: a genetic analysis. Mol Microbiol. 1998;28:449–461. doi: 10.1046/j.1365-2958.1998.00797.x. [DOI] [PubMed] [Google Scholar]
- 36.Pearson J P, Pesci E C, Iglewski B H. Roles of Pseudomonas aeruginosa las and rhl quorum-sensing systems in control of elastase and rhamnolipid biosynthesis genes. J Bacteriol. 1997;179:5756–5767. doi: 10.1128/jb.179.18.5756-5767.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pratt L A, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 38.Prigent-Combaret C, Vidal O, Dorel C, Lejeune P. Abiotic surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli. J Bacteriol. 1999;181:5993–6002. doi: 10.1128/jb.181.19.5993-6002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rashid M H, Kornberg A. Inorganic polyphosphate is needed for swimming, swarming, and twitching motilities of Pseudomonas aeruginosa. Proc Natl Acad Sci USA. 2000;97:4885–4890. doi: 10.1073/pnas.060030097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rosenberg M, Gutnick D, Rosenberg E. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett. 1980;9:29–33. [Google Scholar]
- 41.Semmler A B T, Whitchurch C B, Mattick J S. A re-examination of twitching motility in Pseudomonas aeruginosa. Microbiology. 1999;145:2863–2873. doi: 10.1099/00221287-145-10-2863. [DOI] [PubMed] [Google Scholar]
- 42.Snellings N J, Tall B D, Venkatesan M M. Characterization of Shigella type 1 fimbriae: expression, FimA sequence, and phase variation. Infect Immun. 1997;65:2462–2467. doi: 10.1128/iai.65.6.2462-2467.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stickler D. Biofilms. Curr Opin Microbiol. 1999;2:270–275. doi: 10.1016/S1369-5274(99)80047-2. [DOI] [PubMed] [Google Scholar]
- 44.Suh S-J, Silo-Suh L, Woods D E, Hassett D J, West S E H, Ohman D E. Effect of rpoS mutation on the stress response and expression of virulence factors in Pseudomonas aeruginosa. J Bacteriol. 1999;181:3890–3897. doi: 10.1128/jb.181.13.3890-3897.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Swanson J, Kraus S J, Gotschlich E C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971;134:886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Taguchi K, Fukutomi H, Kuroda A, Kato J, Ohtake H. Genetic identification of chemotactic transducers for amino acids in Pseudomonas aeruginosa. Microbiology. 1997;143:3223–3229. doi: 10.1099/00221287-143-10-3223. [DOI] [PubMed] [Google Scholar]
- 47.Van Delden C, Iglewski B H. Cell-to-cell signaling and Pseudomonas aeruginosa infections. Emerg Infect Dis. 1998;4:551–560. doi: 10.3201/eid0404.980405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wall D, Kaiser D. Type IV pili and cell motility. Mol Microbiol. 1999;32:1–10. doi: 10.1046/j.1365-2958.1999.01339.x. [DOI] [PubMed] [Google Scholar]
- 49.Watnick P, Kolter R. Biofilm, city of microbes. J Bacteriol. 2000;182:2675–2679. doi: 10.1128/jb.182.10.2675-2679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Whitchurch C B, Hobbs M, Livingston S P, Krishnapillai V, Mattick J S. Characterization of a Pseudomonas aeruginosa twitching motility gene and evidence for a specialised protein export system in eubacteria. Gene. 1991;101:33–44. doi: 10.1016/0378-1119(91)90221-v. [DOI] [PubMed] [Google Scholar]
- 51.Whitchurch C B, Mattick J S. Characterization of a gene, pilU, required for twitching motility but not phage sensitivity in Pseudomonas aeruginosa. Mol Microbiol. 1994;13:1079–1091. doi: 10.1111/j.1365-2958.1994.tb00499.x. [DOI] [PubMed] [Google Scholar]
- 52.Wu S S, Kaiser D. Genetic and functional evidence that type IV pili are required for social gliding motility in Myxococcus xanthus. Mol Microbiol. 1995;18:547–558. doi: 10.1111/j.1365-2958.1995.mmi_18030547.x. [DOI] [PubMed] [Google Scholar]