Abstract
Purpose:
The adenoids, or pharyngeal tonsils, consist of a pad of lymphoid tissue, located on the posterior pharyngeal wall of the nasopharynx. During childhood, the adenoid pad serves as a contact site for the soft palate to assist with velopharyngeal closure during oral speech. During adenoidal involution, most children are able to maintain appropriate velopharyngeal closure necessary for normal speech resonance. The purpose of this study is to determine age-related trends of normal adenoid growth and involution from infancy through adulthood.
Method/Description:
Lateral view magnetic resonance imaging was used to analyze velopharyngeal variables among 270 participants, between 3 months and 34 years of age. The velopharyngeal measures of interest included velar length, effective velar length, pharyngeal depth, adenoid height, adenoid thickness, adenoid depth, and adenoid area. Participants were divided into four age groups for statistical comparison.
Results:
There was a statistically significant difference (p < .05) in all linear and area measurements between the four age groups. Adenoid depth reached peak growth at age 4 years, whereas adenoid height and adenoid thickness peaked at 8 years of age. Qualitatively, adenoid growth progresses in an anterior and inferior direction whereas involution occurs in a posterior and superior direction.
Conclusions:
This study contributes to the knowledge of time specific changes across an age span for adenoid growth and involution and presents a visualization of the shape and growth trends of adenoids. A new sequence of involution is reported beginning first with adenoid depth, followed by adenoid height at a slightly faster rate than adenoid thickness.
The adenoid pad is a mass of lymphatic tissue along the posterior-superior wall of the nasopharynx, with a superior connective tissue attachment at the periosteum of the sphenoid bone. Histologically, the adenoid pad is composed of ciliated pseudostriated columnar epithelium, with goblet cells. Unlike the palatine tonsils, the adenoid mass has no crypts. The surface of the adenoids has a layer of respiratory epithelium. Adenoid tissue is present as early as 6 weeks of age (Subtelny & Koepp-Baker, 1956) and forms a convex surface along the posterior nasopharynx by 6 months of age (R. M. Mason, 1973). The adenoids grow rapidly from infancy through the first 2 years of life (C. S. Handelman & Osborne, 1976; Subtelny, 1980), occupying as much as 50% of the nasopharyngeal cavity by 2 years of age (R. M. Mason, 1973; Subtelny, 1980). Growth has been reported to occur in an inferior and anterior direction (R. M. Mason, 1973). Yaseen et al. (2012) reported enlargement of the adenoids to occur from 3 to 5 years of age. Although studies have varied in the reported age of peak adenoid growth, maximal adenoid growth has consistently been reported to occur between 7 and 10 years of age (Gangadhara Somayaji et al., 2012; Haapaniemi, 1995; R. M. Mason, 1973; Papaioannou et al., 2013; Perry, Kollara, et al., 2018)
During childhood, the adenoid pad assists with velopharyngeal (VP) closure by serving as a contact site for the elevated soft palate, resulting in velo-adenoidal closure (Croft et al., 1981; Skolnick et al., 1975; Subtelny & Koepp-Baker, 1956). Perry et al. (2017) reported that individuals with larger adenoids required less levator veli palatini (levator) muscle contraction during phonation and had a smaller change in the levator muscle angle of origin, as compared to individuals with a smaller adenoid pad. This suggests that when the adenoids are larger, the resulting decrease in nasopharyngeal space requires less contraction of the levator muscle to retract and elevate the velum for VP closure. Following peak adenoidal growth, the thickness and overall volume of the adenoids appears to decrease significantly after 9 years of age, causing an increase in the VP depth (Papaioannou et al., 2013; Perry, Kollara, et al., 2018). Nearly all children can maintain appropriate VP closure even as the adenoid gradually involutes (R. M. Mason, 1973). Preservation of normal VP function for speech during growth is largely attributed to the coordinated structural changes to the pharynx during growth and the inherent propensity of the velum to stretch to adjust to growth changes (R. M. Mason, 1973; Siegel-Sadewitz & Shprintzen, 1986). More specifically, with increasing age, the pharynx becomes more vertically oriented.
Growth of the vocal tract and, more specifically, VP structures and muscles demonstrates that VP structures reach peak growth at different rates. For example, among boys, the levator muscle reaches peak growth at 14 years of age, whereas the velum and pharyngeal depth do not peak in growth until 16–17 years of age (Perry et al., 2019). This suggests that although VP structural growth occurs at variable rates through a selected age span, the various and differing growth patterns exhibit an ability to preserve normal VP functions to maintain normal oral-to-nasal balance during speech. These studies also demonstrate that boys and girls have variable growth rates with girls reaching peak growth earlier than boys. Lastly, as sexual dimorphism occurs between the ages of 11 and 21 years, variables such as levator length, distance between muscle origins, angle of origins, velar insertion distance, velar length, and pharyngeal depth demonstrate significant differences between males and females.
Race effects on VP variables also have been reported. Specifically, Black children who are 4–9 years of age have a longer and thicker velum, shorter levator muscle, and a greater effective velar length when compared to White and/or Asian children (Perry, Kollara, et al., 2018). Perry, Kollara, et al. (2018) also reported a race effect in which Black children between 4 and 9 years of age had a larger adenoid mass compared to the other racial groups used in the study. However, it is not known if this racial difference in the configuration of the adenoid pad is apparent through an age span of the adenoid mass or becomes only evident at particular ages, similar to what was observed among VP variables. The sex effect for adenoid tissue is also poorly understood. Such insights are particularly relevant because, as previously mentioned, the adenoids serve as a place of contact during VP closure among children.
Cross-sectional studies and longitudinal studies have identified and reported on adenoid size at specific ages. However, data are lacking regarding the process of adenoid involution (i.e., changes from inferior-to-superior, anterior–posterior depth, or side-to-side) and, more specifically, how these changes occur in coordination and relative to the growth and function of other VP variables important for preserving normal resonance during speech. Individuals with a repaired cleft palate can present VP insufficiency and hypernasal speech. Hypernasal speech occurs in 5%–20% of children following primary palatoplasty (Fisher & Sommerlad, 2011; Lithovius et al., 2014). An understanding of normative growth patterns and the coordination of growth among key VP variables and the adenoids is of clinical importance and can alter surgical intervention for individuals with cleft palate. It is also unclear if growth trends differ based on sex and race, as has been reported among other key VP variables (Perry, Kollara, et al., 2018).
The purpose of this study was to determine age-related trends of normal adenoid development from infancy through adulthood and to examine the coordination of adenoid involution with growth changes among other key VP variables that impact VP functions. The study also aimed to examine if sex and race impact adenoid growth trends. Consistent with prior research (Perry, Kollara, et al., 2018), the hypothesis was made that a race effect would be observed in Black participants who would demonstrate an increase in amount of adenoid tissue as compared to other racial groups. Also, that involution of the adenoid mass would occur at a similar rate to the growth and functional elongation of the velum. It was hypothesized that this would serve to compensate for the increase in pharyngeal depth, as a way to preserve normal VP closure and oral–nasal resonance balance for speech production. As a secondary goal, this study served to provide normative data across an age span from early infancy to adulthood for the key VP variables documented. The VP variables documented in this study will hopefully have value as comparative normative data in studies of individuals with cleft palate.
Method
Participants
Secondary analysis of previously collected data was completed. Specifically, participants were recruited as part of separate studies including an infant magnetic resonance imaging (MRI) study (Schenck et al., 2016), a child MRI study (Perry, Kollara, et al., 2018), and an adult MRI study (Perry et al., 2016), all which were conducted for the purpose of analyzing the VP anatomy among a noncleft control group. In accordance with the local institutional review boards, 242 individuals between 4 months and 34 years of age were recruited to participate in this clinical MRI study. Infant participants (4–23 months of age) were recruited from patients already scheduled for a whole head clinical MRI for reasons unrelated to the study (e.g., seizures). Parental consent for infants was to allow the addition of an MRI sequence (3 min 38 s in length) to the infant's currently scheduled whole-head MRI followed by the release of their child's full MRI study to the researchers. The remainder of the participants were recruited as part of multiple separate studies, all recruiting accomplished via flyers and social media posts within the local community. To be included in the study, participants were required to have normal head and neck anatomy and a report by parents of the participants of a negative history of swallowing, hearing, craniofacial, syndrome, and musculoskeletal disorders. Except for the infants, all other participants were also required to report a negative neurological disorder history. Participants 4 years of age and older completed a speech screening performed by a single speech-language pathologist with over 15 years of experience in resonance assessments using a 5-point rating scale at the sentence and/or conversational level. An oral examination was performed by the lead investigator (speech-language pathologist with over 15 years of experience in performing such evaluations) and/or doctoral students who have been trained by the lead investigator to perform such assessments. The oral examination was used to assess oral abnormalities. Participants with any observed oral abnormality and/or ratings of 2 through 5 on the 5-point scale were excluded from participation.
Additional data were obtained from the National Institute of Mental Health (NIMH) Data Archive to increase enrollments in undersampled age categories. Forty-three participants were selected from the NIMH Data Archive. Specifically, 35 participants were part of the National Database for Autism Research and eight participants were part of the Adolescent Brain Cognitive Development (https://abcdstudy.org) studies. Participants from these archives that were enrolled in this study included 21 children listed as normal control participants and 22 children with a diagnosis of autism spectrum disorder (ASD). Children enrolled as part of this cohort were not screened by a speech-language pathologist as the other participants were. The data achieved do not include speech samples, and therefore an assessment of the child's resonance could not be performed. However, participants were excluded from the database if they have a known anatomic or neurologic disorder.
MRI data from a total of 270 healthy participants were included for analysis in the study (see Table 1). Participants were divided into groups based on age for statistical comparison: Group 1 included 40 infants (ages 4–23 months), Group 2 included 119 children (ages 4–9 years), Group 3 included 30 adolescents (ages 10–19 years), and Group 4 included 81 adults (ages 20–34 years). Groups were further divided into smaller subgroups to document any VP changes and to more discretely collect normative data across smaller age spans more discretely. Division of the groups were as follows: Infants were divided into groups every 3 months (4–7 months, 8–11 months, 12–15 months, 16–19 months, 20–23 months), children were divided into groups every 2 years (4–5 years, 6–7 years, 8–9 years), adolescents were divided into groups every 5 years (10–14 years, 15–19 years), and adults were divided into two groups (20–25 years, 26 years and older; see Table 2).
Table 1.
Participant demographics.
| Group | N | Sex (n) | Caucasian | African American | Other (Asian, Hispanic, interracial) |
|---|---|---|---|---|---|
| Infant | 40 | M (20) | 16 | 4 | 0 |
| (4–23 months) | F (20) | 12 | 6 | 2 | |
| Child | 119 | M (60) | 47 | 11 | 2 |
| (4–9 years) | F (59) | 43 | 12 | 4 | |
| Adolescent | 30 | M (13) | 8 | 0 | 5 |
| (10–19 years) | F (17) | 12 | 3 | 2 | |
| Adult | 81 | M (38) | 14 | 15 | 9 |
| (20 years and older) | F (43) | 15 | 14 | 14 | |
| Total | 270 | M (131) | 167 | 65 | 38 |
| F (139) |
Note. M = male; F = female.
Table 2.
Participant demographics for subgroups.
| Group | N | Sex (n) | Caucasian | African American | Other (Asian, Hispanic, interracial) |
|---|---|---|---|---|---|
| 4–7 months | 9 | M (4); F (5) | 7 | 2 | 0 |
| 8–11 months | 6 | M (6); F (0) | 5 | 1 | 0 |
| 12–15 months | 11 | M (4); F (7) | 6 | 4 | 1 |
| 16–19 months | 8 | M (3); F (5) | 6 | 1 | 1 |
| 20–23 months | 6 | M (3); F (3) | 4 | 2 | 0 |
| 4–5 years | 19 | M (12); F (7) | 13 | 6 | 0 |
| 6–7 years | 17 | M (6); F (11) | 11 | 6 | 0 |
| 8–9 years | 43 | M (19); F (24) | 26 | 11 | 6 |
| 10–14 years | 49 | M (27); F (22) | 45 | 1 | 3 |
| 15–19 years | 21 | M (9); F (12) | 15 | 2 | 4 |
| 20–25 years | 62 | M (26); F (36) | 21 | 24 | 17 |
| 26 years and older | 19 | M (12); F (7) | 8 | 5 | 6 |
| Total | 270 | M (131); F (139) | 167 | 65 | 38 |
Note. M = male; F = female.
MRI and Image Analyses
Infants included in this study were scheduled for a whole-head clinical MRI by a medical provider not associated with the study. Infants were sedated using either chloral hydrate (50–100 mg/kg per rectum with a maximum dose of 2,000 mg), Demerol (1–2 mg/kg intramuscular), Phenegran (0.5–1.0 mg/kg IM), or a combination of the three drugs. These are the standard sedation methods used by the clinical site, and all sedation methods were prescribed as part of the clinical MRI and not associated with this study. The additional MRI sequence that was added to the clinical MRI protocol was a high-resolution, transverse relaxation time–weighted fast-spin echo three-dimensional (3D) anatomical scan (repetition time of 2,250 ms and echo time of 210 ms) using sensitivity encoding for fast MRI with a field of view to capture the oropharyngeal anatomy (150 mm × 127 mm) with an 0.3-mm isotropic resolution.
All other participants were scanned without the use of sedation or contrast medium. Procedures for MRI methods have been described previously (Perry et al., 2014; Perry, Kollara, et al., 2018). Participants were imaged across different MRI sites using 3D MRI sequences with comparable imaging sequence parameters to ensure that image resolution (0.8 in-plane isotropic resolution) was preserved. Each participant was in the supine position, and the velum was in a relaxed and lowered position during the scan. Images that were blurred or for which the velum appeared to be in an elevated position (e.g., such as during a swallow) were excluded from the study.
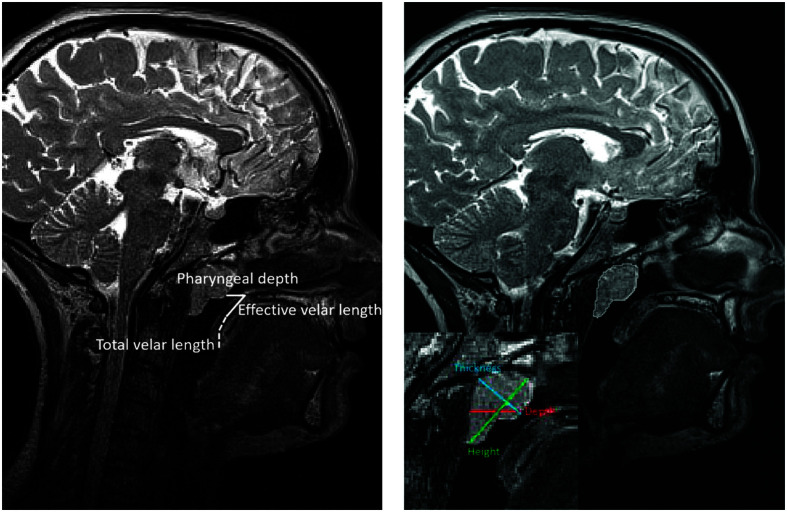
A primary rater, graduate student in engineering, was trained by the primary investigator of this study (who has over 15 years of experience in assessing MRI of this region) to evaluate the MRI data across all participants. The training included the use of data not included in this study followed by an evaluation of the student's reliability with the primary investigator. Once the student was adequately trained, he completed three linear measures of the adenoids (depth, thickness, and height) from a lateral view of the head to examine the direction of adenoidal growth across an age span studied (see Figure 1). A single adenoid area measure was obtained in the lateral view of the midsagittal image plane. Additional lateral-view variables measured included pharyngeal depth, velar length, and effective velar length, and two ratio measures were calculated (VP ratio and effective VP ratio). Complete definitions are provided in Table 3. Magnetic resonance images were measured manually using Amira 6.5 Visualization Volume Modeling software (Visage Imaging GmbH), which has a built-in native Digital Imaging and Communication in Medicine (DICOM) support program. The DICOM support system enables the data to preserve original geometry.
Figure 1.
Visual display of the velopharyngeal variables measured on the midsagittal plane. Adenoid area (right image) visualized following the tracing of the adenoid on the midsagittal images. Adenoid variables (right image) are visualized on a zoomed visual of the adenoid.
Table 3.
Description of measures.
| Measure | Description |
|---|---|
| Pharyngeal depth | Linear distance (millimeters) from PNS to PPW or adenoid pad as seen on the midsagittal image |
| Velar length | Linear distance (millimeters) from the posterior nasal spine to the tip of the uvula as seen on the midsagittal image |
| VP ratio | Calculation obtained from dividing velar length by pharyngeal depth |
| Effective velar length | Linear distance (millimeters) from the posterior nasal spine to the velar knee as seen on the midsagittal image |
| Effective VP ratio | Calculation obtained from dividing effective velar length by the pharyngeal depth |
| Adenoid depth | Linear distance (millimeters) along the palatal plane from the adenoid pad to the posterior end of the adenoid |
| Adenoid thickness | Linear distance (millimeters) from Sy2 (posterior edge of sphenobasioccipital synchondrosis), a common cephalometric landmark, toward the Ad2, the most convex point of the adenoid |
| Adenoid height | Linear distance (millimeters) from the most anterior and superior edge of the adenoid to the most posterior and inferior edge |
| Adenoid cross-sectional area | Segmentation of the adenoid on the midsagittal image (mm2) |
Note. PNS = posterior nasal spine; PPW = posterior pharyngeal wall; VP = velopharyngeal.
Statistical Analysis
A three-way analysis of variance (ANOVA) was performed to investigate the differences across age groups controlling for race and sex effects and using robust standard errors to account for heterogeneous variances (White, 1980). A Bonferroni adjustment was used to account for multiple hypothesis tests. Race and sex were included as fixed factors given the known race and sex effects on the VP variables analyzed in this study. Levene's test of equality of variances was used to verify if the equal variance assumption is true.
Since Levene's test showed variables with unequal variances, Games–Howell post hoc tests were conducted to assess the differences between adjacent age groups. All data analyses were performed using SPSS 22.0 (IBM Corp.). A significance level of 0.05 was adopted for all statistical tests.
Inter- and intrarater reliability were calculated using SPSS 22.0 (IBM Corp.). A secondary rater with 5 years of experience performed measures on 40% of the participants to evaluate the interrater reliability. Measures were obtained using an intraclass correlation coefficient (ICC) performed across 40% of all participants (n = 109). Measurements consisted of adenoid thickness, adenoid depth, and adenoid height. ICC estimates were based on a two-way random model using a 95% confidence interval.
Results
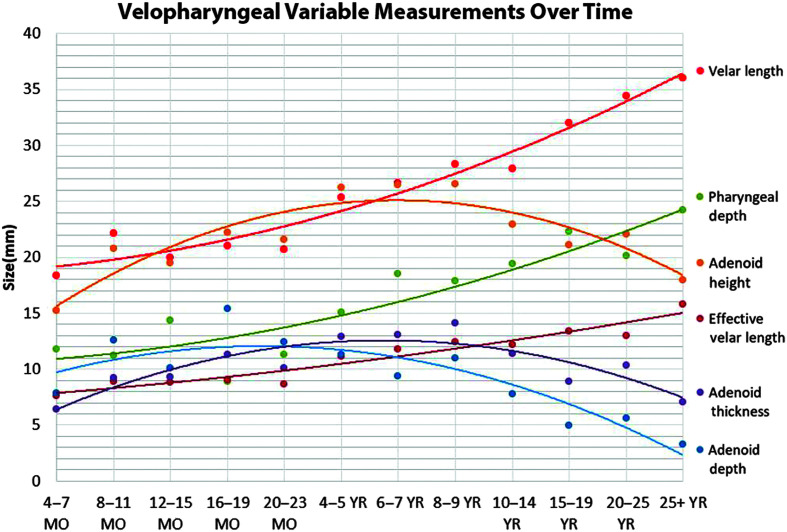
Linear measurements (see Table 3) were visualized to compare growth trends across the age span used in this study (see Figure 2). Means and standard deviations by age groups and smaller subgroups are reported in Tables 4 and 5.
Figure 2.
Graph to display changes in the variables across the selected age span used in this study. MO = month; YR = year.
Table 4.
Means and standard deviations for subgroups.
| Group | Pharyngeal depth | Velar length | VP ratio | Effective velar length | Effective VP ratio | Adenoid depth | Adenoid thickness | Adenoid height | Adenoid area |
|---|---|---|---|---|---|---|---|---|---|
| 4–7 months | 11.79 ± 3.43 | 18.31 ± 2.56 | 1.70 ± .65 | 7.34 ± 1.37 | .67 ± .20 | 7.85 ± 3.28 | 6.40 ± 1.12 | 15.26 ± 2.38 | 488.46 ± 78.51 |
| 8–11 months | 11.23 ± 5.28 | 22.10 ± 1.76 | 2.32 ± .93 | 8.92 ± .72 | .93 ± .39 | 12.56 ± 3.42 | 9.23 ± 2.17 | 20.78 ± 3.35 | 542.96 ± 97.95 |
| 12–15 months | 13.93 ± 5.04 | 19.94 ± 3.80 | 1.68 ± .89 | 8.83 ± 1.54 | .76 ± .45 | 10.05 ± 4.46 | 9.32 ± 1.51 | 19.51 ± 3.42 | 591.00 ± 121.64 |
| 16–19 months | 8.43 ± 2.81 | 20.97 ± 2.12 | 2.82 ± 1.05 | 9.28 ± 1.59 | 1.22 ± .49 | 15.41 ± 2.69 | 11.31 ± 1.28 | 22.21 ± 3.60 | 629.67 ± 119.48 |
| 20–23 months | 10.50 ± 4.11 | 20.66 ± 4.87 | 2.17 ± .82 | 8.68 ± 1.07 | .93 ± .41 | 12.40 ± 3.97 | 10.11 ± 1.57 | 21.56 ± 2.51 | 625.73 ± 155.84 |
| 4–5 years | 14.66 ± 4.36 | 25.60 ± 3.45 | 1.88 ± .55 | 11.47 ± 2.71 | .85 ± .32 | 11.21 ± 3.94 | 12.89 ± 3.58 | 26.22 ± 3.27 | 675.77 ± 320.69 |
| 6–7 years | 18.43 ± 4.26 | 26.62 ± 4.65 | 1.50 ± .54 | 11.74 ± 2.54 | .67 ± .21 | 9.39 ± 5.84 | 13.02 ± 3.02 | 26.43 ± 5.10 | 724.66 ± 228.19 |
| 8–9 years | 17.47 ± 5.13 | 28.66 ± 4.59 | 1.85 ± .93 | 12.50 ± 2.44 | .80 ± .38 | 10.94 ± 5.81 | 14.29 ± 4.39 | 26.89 ± 7.30 | 539.18 ± 219.12 |
| 10–14 years | 19.36 ± 4.11 | 27.91 ± 3.42 | 1.51 ± .38 | 12.26 ± 2.43 | .66 ± .19 | 7.76 ± 5.01 | 11.31 ± 4.47 | 22.82 ± 7.78 | 501.02 ± 233.73 |
| 15–19 years | 22.38 ± 4.56 | 32.33 ± 5.11 | 1.51 ± .45 | 13.04 ± 3.48 | .60 ± .18 | 6.48 ± 5.87 | 9.26 ± 4.47 | 21.83 ± 7.93 | 489.26 ± 261.09 |
| 20–24 years | 19.92 ± 3.72 | 34.49 ± 4.56 | 1.79 ± .42 | 13.07 ± 3.03 | .68 ± .20 | 5.76 ± 4.07 | 10.37 ± 4.63 | 22.08 ± 6.94 | 369.84 ± 200.32 |
| 25 + years | 24.19 ± 3.79 | 36.03 ± 5.44 | 1.53 ± .37 | 15.82 ± 3.98 | .66 ± .13 | 3.28 ± 2.34 | 7.05 ± 3.19 | 17.99 ± 6.42 | 220.75 ± 120.17 |
Note. All distance measurements are in the unit of millimeter. Area measurement is in the unit of millimeter2. VP = velopharyngeal.
Table 5.
Means and standard deviations variables for groups and results of analysis of variance following a Bonferroni adjustment.
| Measure | Group means ± standard deviations |
F(3, 263) | p value | |||
|---|---|---|---|---|---|---|
| Infants (n = 40) | Child (n = 119) | Adolescents (n = 30) | Adults (n = 81) | |||
| Adenoid depth | 11.36 ± 4.38 | 9.66 ± 5.49 | 6.96 ± 5.49 | 5.18 ± 3.88 | 19.328 | .009** |
| Adenoid thickness | 9.16 ± 2.21 | 12.74 ± 4.37 | 10.44 ± 4.54 | 9.59 ± 4.54 | 14.974 | .009** |
| Adenoid height | 19.59 ± 3.91 | 24.84 ± 7.08 | 22.69 ± 7.13 | 21.12 ± 7.01 | 10.486 | .009** |
| Adenoid Area | 573.67 ± 122.19 | 567.70 ± 257.00 | 520.42 ± 252.46 | 334.87 ± 194.65 | 18.748 | .009** |
| Pharyngeal depth | 11.43 ± 4.46 | 17.80 ± 4.79 | 21.45 ± 4.62 | 20.92 ± 4.14 | 42.583 | .009** |
| Velar length | 20.27 ± 3.32 | 27.63 ± 4.17 | 30. 98 ± 5.10 | 34.85 ± 4.79 | 116.776 | .009** |
| Effective velar length | 8.58 ± 1.46 | 12.07 ± 2.43 | 13.12 ± 3.32 | 13.72 ± 3.46 | 33.492 | .009** |
| VP ratio | 2.08 ± .94 | 1.70 ± .69 | 1.51 ± .42 | 1.73 ± .42 | 4.751 | .027* |
| Effective VP ratio | .89 ± .43 | .74 ± .30 | .63 ± .19 | .67 ± .19 | 5.901 | .009** |
Note. All distance measurements are in the unit of millimeter. Area measurement is in the unit of millimeter2. VP = velopharyngeal.
p < .05.
p < .01.
In this study, a primary goal was to examine how adenoid growth and involution changes occur relative to other key VP variables that impact the VP portal. Parallel growth trends are noted for velar length and pharyngeal depth based on visual inspection of the slope of the graphs in Figure 2. Both variables increase in length at a similar rate throughout the age span, as seen in Figure 2. Effective velar length also increases through the life span, similar to velar length. However, starting at approximately 6 years of age, effective velar length increases at a slower rate than velar length. Adenoid depth increases through infancy, but begins to involute at 2 years of age. Adenoid depth and pharyngeal depth present with an inverse growth pattern. Both increase in length through infancy, but, at age 4 years, the rate of which the adenoid depth decreases is approximately the same as the rate the pharyngeal depth increases. Adenoid thickness reaches peak growth at age 8–9 years. Adenoid height also reaches peak growth at age 8–9 years. Adenoid height and thickness present with a similar growth curve. However, adenoid height atrophies at a faster rate than adenoid thickness when examining the slopes after 8 years of age. Whereas adenoid depth peaks at 19 months of age and adenoid thickness and height peak at 8–9 years of age, adenoid area (see Tables 4 and 5) peaks at 6–7 years of age. The VP ratio mean values start at 2.08 (ratio of velar length/pharyngeal depth) at infancy and progress to 1.70 (child), 1.51 (adolescents), and 1.73 (adults). The pharyngeal depth changes most noticeably between infancy and childhood and remains relatively consistent from adolescence to adulthood. The velar length shows a continual increase across the age span. In contrast, the effective VP ratio shows limited change from childhood to adulthood. The most notable change in the effective VP ratio is between infancy (mean of 0.89) and childhood (mean of 0.74). This notable change from infancy to childhood is attributed to both the increase in pharyngeal depth and the increase in effective velar length.
Results from the three-way ANOVA revealed significant age group differences for all variables (see Table 5). Pairwise comparisons focused on growth trends between adjacent groups (see Table 6). Significant decreases in adenoid depth were noted from the child to adolescent group. Adenoid thickness had a significant increase noted between the infant and child group. Adenoid height also had a significant increase noted between the infant and child group. Adenoid area had no significant growth trends between adjacent groups. No other significant growth trends were present between adjacent groups for adenoid measurements. The three-way ANOVA also noted significant gender differences for effective velar length and velar length (see Table 7). Males present with a greater velar length (p = .009) and effective velar length (p = .027) than females. Racial differences were noted for velar length, adenoid thickness, height, and area (see Table 7). Black participants present with a significantly greater velar length (p = .009), adenoid thickness (p = .009), height (p = .009), and area (p = .009) in comparison to White participants and other racial and ethnic groups (Asian, Hispanic, Interracial). The other racial and ethnic groups (Asian, Hispanic, interracial) presented with the smallest measurements for velar length, adenoid thickness, depth, height, and area.
Table 6.
Games–Howell post hoc comparisons among groups.
| Dependent variable | Difference between infant and child (SE) | Difference between child and adolescent (SE) | Difference between adolescent and adult (SE) |
|---|---|---|---|
| Velar length | 7.26* (0.65) | 3.29* (0.99) | 4.04* (1.04) |
| VP Ratio | −.33* (0.12) | −.11 (0.08) | −.22* (0.08) |
| Effective velar length | 3.39* (0.29) | 1.28 (0.56) | .41 (0.64) |
| Effective VP ratio | −.12 (0.05) | −.06 (0.03) | .02 (0.03) |
| Pharyngeal depth | 6.31* (0.75) | 3.30* (0.83) | .30 (0.85) |
| Adenoid depth | −1.67 (0.85) | −3.87* (0.91) | −.77 (0.87) |
| Adenoid area | −5.85 (29.9) | −68.65 (54.10) | −164.30 (53.59) |
| Adenoid thickness | 3.52* (0.52) | −2.50 (0.97) | −.59 (1.03) |
| Adenoid height | 5.48* (0.88) | −2.93 (1.62) | −1.02 (1.69) |
Note. VP = velopharyngeal.
p < .05.
Table 7.
Means and standard deviations of variables and results of analysis of variance for race and gender following a Bonferroni adjustment.
| Gender means ± standard deviation |
F(1, 263) | p value | Race means ± standard deviation |
F(2, 263) | p value | ||||
|---|---|---|---|---|---|---|---|---|---|
| Males (n = 131) | Females (n = 139) | Caucasian (n = 167) |
African American (n = 65) | Other (Asian, Hispanic, interracial; n = 38) | |||||
| Velar length | 30.04 ± 7.13 | 28.17 ± 5.63 | 17.540 | .009** | 28.09 ± 4.62 | 31.00 ± 7.37 | 30.14 ± 4.57 | 7.322 | .009** |
| VP ratio | 1.76 ± .56 | 1.72 ± .74 | 0.228 | 1.000 | 1.71 ± .66 | 1.88 ± .62 | 1.63 ± .69 | 1.638 | 1.000 |
| Effective velar length | 12.64 ± 3.34 | 11.71 ± 3.05 | 9.226 | .027* | 11.87 ± 3.13 | 12.85 ± 3.56 | 12.28 ± 2.88 | 2.884 | .522 |
| Effective VP ratio | .74 ± .23 | 0.72 ± .35 | .134 | 1.000 | 0.72 ± .29 | 0.79 ± .28 | 0.66 ± .31 | 1.606 | 1.00 |
| Pharyngeal depth | 18.26 ± 5.56 | 18.14 ± 5.53 | 0.294 | 1.000 | 17.94 ± 5.56 | 17.66 ± 5.48 | 20.24 ± 5.18 | 1.114 | 2.97 |
| Adenoid depth | 8.56 ± 5.58 | 7.99 ± 5.21 | 0.586 | 1.000 | 8.39 ± 5.47 | 9.34 ± 5.13 | 5.89 ± 4.87 | 4.611 | .099 |
| Adenoid area | 512.52 ± 239.52 | 475.53 ± 250.75 | 1.486 | 1.000 | 505.84 ± 254.09 | 568.90 ± 204.48 | 310.14 ± 177.22 | 13.806 | .009** |
| Adenoid thickness | 11.18 ± 4.26 | 10.85 ± 4.65 | 0.388 | 1.000 | 10.70 ± 4.42 | 12.82 ± 3.81 | 9.25 ± 4.75 | 12.710 | .009** |
| Adenoid height | 23.70 ± 6.7 | 21.78 ± 7.09 | 6.137 | .126 | 22.19 ± 7.44 | 25.34 ± 5.21 | 20.49 ± 6.20 | 10.526 | .009** |
Note. Linear measurements are listed in millimeters. Adenoid area is listed in the unit of millimeter2. VP = velopharyngeal.
p < .05.
p < .01.
Inter- and intrarater reliability were calculated using an ICC performed across 40% of all participants (n = 109). Intrarater agreement for adenoid thickness was .993, indicating excellent reliability. Interrater agreement for adenoid thickness was .940 indicating excellent reliability. Intrarater agreement for adenoid depth was .983, indicating excellent reliability. Interrater agreement for adenoid depth was .855 indicating good reliability. Intrarater agreement for adenoid height was .854, indicating good reliability. Interrater agreement for adenoid height was .898 indicating good reliability.
Discussion
This study contributes new insights into growth trends of adenoids and, more specifically, how these changes across a selected age span relate to the growth changes of other key VP variables. Our results agree with previous literature (Gangadhara Somayaji et al., 2012; Haapaniemi, 1995; R. M. Mason, 1973; Papaioannou et al., 2013; Perry, Kollara, et al., 2018) that states that adenoids continue to increase in size until they peak in growth between 7 and 10 years of age when they begin to involute. This study expands our knowledge on peak in growth by demonstrating that the adenoid thickness and height peak at 8–9 years of age, whereas adenoid area peaks at 6–7 years of age. The variation in the age of peak adenoid growth reported in the literature may be related to the variability in the location in which the measure is taken to define the adenoid size. Our findings also demonstrate that the depth of the adenoids peak quite early, as young as 19 months of age. The adenoid depth represents a measure of the adenoid size along the palatal plane where VP closure typically occurs, particularly in children. In adulthood, the velum is observed to have velar knee flexion that extends superior to the palatal plane particularly among adult males (McKerns & Bzoch, 1970). Because most of childhood and adolescence involves contact of the velum along the palatal plane, adenoid depth may be the most important measure when discussing adenoid size, particularly as it relates to examining the contribution of the adenoid pad in VP closure.
Findings from this study also provide further details on the involution patterns that occur across a selected age span. We observed that adenoid involution first occurs with depth followed by height and thickness. Adenoid depth may involute first due to the constant pressure and contact of the velum to the adenoid. Studies have reported changes to the cellular structure of the adenoid surface with growth and attribute this to the repeated contact and pressure of the velum against the adenoids during early phonation. Specifically, the adenoids, external surface is respiratory epithelium, pseudostratified ciliated columnar epithelium with goblet cells. This is replaced by stratified squamous epithelium over time (C. Handelman et al., 1966). Bunton and Hoit (2018) used longitudinal methods to examine the age at which the velopharynx closes for speech production. The authors observed that VP closure for spontaneous speech was observed to be complete by 19 months of age. It is likely that this repetitive contact of the velum against the adenoid tissue that is visible and consistent in spontaneous speech at 19 months of age contributes to the decrease in the adenoid depth. Our findings demonstrate that, at this time (19 months of age), we saw a continual decrease in the adenoid depth. The decrease in adenoid depth at this time point results in a greater pharyngeal depth, as observed in the child group (compared to the infant group) in our study.
Because participant demographics for race and sex were not equal across each age group within this study, caution should be taken when drawing conclusions on race and sex effects. Findings from this study suggest that race has a significant effect on adenoid area, thickness, and height. Black participants presented with the largest adenoid measurements, followed by White participants and finally other racial and ethnic groups (Asian, Hispanic, interracial), who presented with the smallest adenoid measurements. This finding is consistent with the findings by Perry, Kollara, et al. (2018) who reported that White children have significantly smaller adenoids compared to Black children. In this study, a sex effect was not observed for adenoid size. This is consistent with findings from Perry, Kollara, et al. (2018), who did not observe a sex effect for adenoid size between 43 boys and 42 girls between 4 and 9 years of age. Future research should assess how race and sex impact functional differences that alter VP closure, particularly during involution of the adenoid.
We hypothesized that adenoid depth would demonstrate a coordinated and inverse growth compared to velar length and pharyngeal depth. We anticipated growth patterns to be parallel with growth occurring at the same rate as a means to preserve normal VP function across the selected age span. We expected these three variables to be the most closely coordinated growth variables. Findings from this study support this hypothesis and demonstrate a system that includes variables showing highly similar and consistent growth trends. An early seminal paper by Subtelny and Koepp-Baker (1956) described a “delicate balance” in the growth between the adenoids and nasopharynx to ensure that the airway is maintained. Similar to this early study, we observed that the decrease in adenoid depth was coordinated with the increase in pharyngeal depth and increase in velar length. This preserved a VP ratio between 1.50 and 2.82 across the age span for the subgroups. These values are similar to the normative age ranges of prior reported VP ratios of 1.51–2.08 (Haenssler et al., 2020). Haenssler et al. (2020) suggest the effective VP ratio is strongly correlated to VP function and remains stable throughout the life span even with growth of surrounding VP structures. Therefore, the effective velar length, the portion of the velum used to close the VP port during speech, may be more predictive of VP competence compared to velar length alone.
Understanding the normative growth patterns and coordination of growth among key VP variables and the adenoids is of clinical importance. A short velum and deep pharynx have been attributed to the presence of hypernasal speech (D'Antonio et al., 2000) that is reported to occur in 5%–20% of children with repaired cleft palate (Fisher & Sommerlad, 2011; Lithovius et al., 2014). However, Perry, Kotlarek, et al. (2018) reported abnormal VP measures (shorter and thinner velum, greater pharyngeal depth, shorter hard palate height and length, shorter levator muscle length, shorter intravelar segment, and more acute levator angles of origin) to be common also among adults with repaired cleft palate who have normal speech. This further underscores the complexity of the VP system and the delicate balance of structures for function proposed by Subtelny and Koepp-Baker (1956).
Velar stretch is the ability of the velum to increase in length from rest to elevation during phonation (Pruzansky & Mason, 1969). Velar stretch has been used as a predictor for adequate VP closure necessary for proper resonance. Findings from Mourino and Weinberg (1975) and Simpson and Austin (1972) suggest that the degree of velar stretch may increase with age. This is likely because they require a greater distance (pharyngeal depth) to achieve VP closure due to adenoid involution. The velum can compensate for the gradual increase in size of the nasopharynx during adenoid involution by increasing in functional length as a way to maintain normal VP closure (Pruzansky & Mason, 1969). Velar stretch can be used clinically to predict whether an individual will be able to maintain VP competence and normal resonance following an adenoidectomy.
An abrupt change to the VP portal, such as a surgical removal of the adenoid, can lead to hypernasal speech if the velum cannot overcome the increase in nasopharyngeal depth. This is especially prevalent in individuals with a cleft palate that require VP closure against the adenoid pad for VP competence. R. M. Mason and Warren (1980) assessed nasality following involution of the adenoids and reported one third of the patients developed hypernasality.
Previous literature has reported that the adenoid of individuals with cleft palate grow and involute in a similar pattern to individuals with noncleft anatomy but tend to be larger than individuals without cleft palate (Gohilot et al., 2014; Imamura et al., 2002; K. N. Mason & Perry, 2016; Ren et al., 1993; Sarmadi et al., 2018; Subtelny & Koepp-Baker, 1956). However, a wider range of growth variations that alter the VP system has been reported in individuals with cleft palate, which can put these individuals at a disadvantage to individuals without cleft palate during adenoid involution. Sarmadi et al. (2018) reported individuals with nonsyndromic unilateral cleft lip and palate have a significantly smaller nasopharynx and nasopharyngeal airway. The individuals with cleft palate also presented with significantly larger adenoids and a significantly greater percentage of the nasopharynx that was occupied by the adenoids. Longitudinal studies should be used to evaluate adenoid growth trends for individuals with cleft palate to understand the stability of the VP system at different time points of growth. Data from this study may serve as normative values across an age span to make comparisons to adenoidal growth in disordered anatomy.
This study expands on the growth and involution patterns of the adenoid throughout the life span. Adenoid involution first occurs with depth followed by height and thickness. Adenoid depth peaked at 2 years of age and adenoid thickness and height peak at 8–9 years of age. It is important to know the sequence of involution, as it plays a critical role in VP closure. This knowledge is also important for the timing of surgical intervention for individuals undergoing an adenoidectomy or individuals with VP insufficiency. The adenoid growth trends in relation to VP variables reported in this study are in agreement with previous literature. Adenoid depth was the only variable related to another VP variable. Adenoid depth and pharyngeal depth were inversely related. The pharynx increases in depth as there is a decrease in adenoid depth. It was also found that effective velar length and velar length are parallel to pharyngeal depth, which is crucial to maintain normal VP ratios.
A limitation of this study is the unequal and unbalanced sample sizes across age groups, sex, and race and limited number of participants between the ages of 2 and 4 years. An additional limitation is the inclusion of participants with a diagnosis of ASD. Participants from the archived data were not screened by the primary investigator to evaluate for resonance ratings, and inter- or intrarater reliability of perceptual ratings were not performed on the remaining participants. This is because audio recordings were not a part of the original research studies and therefore data were not able to be rated for reliability. It is unknown if adenoid growth is different in those with ASD.
Conclusions
This study suggests that adenoid growth is represented as a developmental curve. A new sequence of involution is reported beginning first with adenoid depth, followed by adenoid height at a slightly faster rate than adenoid thickness. Future research should analyze the growth trends of the adenoids in those with cleft palate.
Acknowledgments
This study was funded by the National Institute of Deafness and Other Communication Disorders Grant 1R03DC009676-01 awarded to Perry, Sutton, and Kuehn. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH). The study was also funded by the National Science Foundation Research Experience for Undergraduates Biomedical Engineering in Simulations, Imaging and Modeling, Award EEC-1659796. In addition, data and/or research tools used in the preparation of this article were obtained, in part, from the NIH-supported National Database for Autism Research (NDAR). The NDAR is a collaborative informatics system created by the NIH to provide a national resource to support and accelerate research in autism. Data set identifier(s): 10.15154/1524720. This article reflects the views of the authors and may not reflect the opinions or views of the NIH or of the submitters submitting original data to the NDAR. Data used in the preparation of this article were also obtained, in part, from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive. This is a multisite, longitudinal study designed to recruit more than 10,000 children ages 9–10 years and follow them over 10 years into early adulthood. The ABCD Study is supported by the NIH and additional federal partners under Award Numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025. A full list of supporters is available at https://abcdstudy.org/federal-partners.html. A listing of participating sites and a complete listing of the study investigators can be found at https://abcdstudy.org/Consortium_Members.pdf. ABCD consortium investigators designed and implemented the study and/or provided data but did not necessarily participate in analysis or writing of this report. This article reflects the views of the authors and may not reflect the opinions or views of the NIH or ABCD consortium investigators.
Funding Statement
This study was funded by the National Institute of Deafness and Other Communication Disorders Grant 1R03DC009676-01 awarded to Perry, Sutton, and Kuehn. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health (NIH). The study was also funded by the National Science Foundation Research Experience for Undergraduates Biomedical Engineering in Simulations, Imaging and Modeling, Award EEC-1659796. In addition, data and/or research tools used in the preparation of this article were obtained, in part, from the NIH-supported National Database for Autism Research (NDAR). The NDAR is a collaborative informatics system created by the NIH to provide a national resource to support and accelerate research in autism. Data set identifier(s): 10.15154/1524720. This article reflects the views of the authors and may not reflect the opinions or views of the NIH or of the submitters submitting original data to the NDAR. Data used in the preparation of this article were also obtained, in part, from the Adolescent Brain Cognitive Development (ABCD) Study (https://abcdstudy.org), held in the NIMH Data Archive. This is a multisite, longitudinal study designed to recruit more than 10,000 children ages 9–10 years and follow them over 10 years into early adulthood. The ABCD Study is supported by the NIH and additional federal partners under Award Numbers U01DA041022, U01DA041028, U01DA041048, U01DA041089, U01DA041106, U01DA041117, U01DA041120, U01DA041134, U01DA041148, U01DA041156, U01DA041174, U24DA041123, U24DA041147, U01DA041093, and U01DA041025.
References
- Bunton, K. , & Hoit, J. D. (2018). Development of velopharyngeal closure for vocalization during the first 2 years of life. Journal of Speech, Language, and Hearing Research, 61(3), 549–560. https://doi.org/10.1044/2017_JSLHR-S-17-0208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Croft, C. B. , Shprintzen, R. J. , & Ruben, R. J. (1981). Hypernasal speech following adenotonsillectomy. Otolaryngology—Head & Neck Surgery, 89(2), 179–188. https://doi.org/10.1177/019459988108900208 [DOI] [PubMed] [Google Scholar]
- D'Antonio, L. L. , Eichenberg, B. J. , Zimmerman, G. J. , Patel, S. , Riski, J. E. , Herber, S. C. , & Hardesty, R. A. (2000). Radiographic and aerodynamic measures of velopharyngeal anatomy and function following Furlow Z-plasty. Plastic and Reconstructive Surgery, 106(3), 539–549. https://doi.org/10.1097/00006534-200009010-00002 [PubMed] [Google Scholar]
- Fisher, D. M. , & Sommerlad, B. C. (2011). Cleft lip, cleft palate, and velopharyngeal insufficiency. Plastic and Reconstructive Surgery, 128(4), 342e–360e. https://doi.org/10.1097/PRS.0b013e3182268e1b [DOI] [PubMed] [Google Scholar]
- Gangadhara Somayaji, K. S. , Rajeshwari, A. , & Mahaveera, J. (2012). Significance of adenoid nasopharyngeal ratio in the assessment of adenoid hypertrophy in children. Research in Otolaryngology, 1(1), 1–5. [Google Scholar]
- Gohilot, A. , Pradhan, T. , & Keluskar, K. M. (2014). Cephalometric evaluation of adenoids, upper airway, maxilla, velum length, need ratio for determining velopharyngeal incompetency in subjects with unilateral cleft lip and palate. Journal of the Indian Society of Pedodontics and Preventive Dentistry, 32(4), 297–303. https://doi.org/10.4103/0970-4388.140950 [DOI] [PubMed] [Google Scholar]
- Haapaniemi, J. J. (1995). Adenoids in school-aged children. The Journal of Laryngology and Otology, 109(3), 196–202. https://doi.org/10.1017/S0022215100129688 [DOI] [PubMed] [Google Scholar]
- Haenssler, A. E. , Fang, X. , & Perry, J. L. (2020). Effective velopharyngeal ratio: A more clinically relevant measure of velopharyngeal function. Journal of Speech, Language, and Hearing Research, 63(11), 3586–3593. https://doi.org/10.1044/2020_JSLHR-20-00305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Handelman, C. , Pruzansky, S. , & Mason, R. (1966, April). Hypoplastic adenoids and early involution of the adenoid in cleft palate. Paper presented at the American Cleft Palate Association meeting, Mexico City. [Google Scholar]
- Handelman, C. S. , & Osborne, G. (1976). Growth of the nasopharynx and adenoid development from one to eighteen years. The Angle Orthodontics, 46(3), 243–259. [DOI] [PubMed] [Google Scholar]
- Imamura, N. , Ono, T. , Hiyama, S. , Ishiwata, Y. , & Kuroda, T. (2002). Comparison of the sizes of adenoidal tissues and upper airways of subjects with and without cleft lip and palate. American Journal of Orthodontics and Dentofacial Orthopedics, 122(2), 189–194. https://doi.org/10.1067/mod.2002.125234 [DOI] [PubMed] [Google Scholar]
- Lithovius, R. H. , Ylikontiola, L. P. , & Sándor, G. K. (2014). Frequency of pharyngoplasty after primary repair of cleft palate in northern Finland. Oral Surgery, Oral Medicine, Oral Pathology and Oral Radiology, 117(4), 430–434. https://doi.org/10.1016/j.oooo.2013.12.409 [DOI] [PubMed] [Google Scholar]
- Mason, K. N. , & Perry, J. L. (2016). Relationship between age and diagnosis on volumetric and linear velopharyngeal measures in the cleft and noncleft populations. The Journal of Craniofacial Surgery, 27(5), 1340–1345. https://doi.org/10.1097/SCS.0000000000002744 [DOI] [PubMed] [Google Scholar]
- Mason, R. M. (1973). Preventing speech disorders following adenoidectomy by preoperative examination. Clinical Pediatrics, 12(7), 405–414. https://doi.org/10.1177/000992287301200809 [DOI] [PubMed] [Google Scholar]
- Mason, R. M. , & Warren, D. W. (1980). Adenoid involution and developing hypernasality in cleft palate. Journal of Speech and Hearing Disorders, 45(4), 469–480. https://doi.org/10.1044/jshd.4504.469 [DOI] [PubMed] [Google Scholar]
- McKerns, D. , & Bzoch, K. R. (1970). Variations in velopharyngeal valving: The factor of sex. The Cleft Palate Journal, 7(2), 652–662. [PubMed] [Google Scholar]
- Mourino, A. P. , & Weinberg, B. (1975). A cephalometric study of velar stretch in 8 and 10-year old children. The Cleft Palate Journal, 12, 417–435. [PubMed] [Google Scholar]
- Papaioannou, G. , Kambas, I. , Tsaoussoglou, M. , Panaghiotopoulou-Gartagani, P. , Chrousos, G. , & Kaaitis, A. G. (2013). Age-dependent changes in the size of adenotonsillar tissue in childhood: Implications for sleep-disordered breathing. The Journal of Pediatrics, 162(2), 269–274.e4. https://doi.org/10.1016/j.jpeds.2012.07.041 [DOI] [PubMed] [Google Scholar]
- Perry, J. L. , Kollara, L. , Kuehn, D. P. , Sutton, B. P. , & Fang, X. (2018). Examining age, sex, and race characteristics of velopharyngeal structures in 4- to 9-year-old children using magnetic resonance imaging. The Cleft Palate–Craniofacial Journal, 55(1), 21–34. https://doi.org/10.1177/1055665617718549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, J. L. , Kollara, L. , Sutton, B. P. , Kuehn, D. P. , & Fang, X. (2019). Growth effects on velopharyngeal anatomy from childhood to adulthood. Journal of Speech, Language, and Hearing Research, 62(3), 682–692. https://doi.org/10.1044/2018_JSLHR-S-18-0016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, J. L. , Kotlarek, K. J. , Sutton, B. P. , Kuehn, D. P. , Jaskolka, M. S. , Fang, X. , Point, S. W. , & Rauccio, F. (2018). Variations in velopharyngeal structure in adults with repaired cleft palate. The Cleft Palate–Craniofacial Journal, 55(10), 1409–1418. https://doi.org/10.1177/1055665617752803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, J. L. , Kuehn, D. P. , Sutton, B. P. , & Fang, X. (2017). Velopharyngeal structural and functional assessment of speech in young children using dynamic magnetic resonance imaging. The Cleft Palate–Craniofacial Journal, 54(4), 408–422. https://doi.org/10.1597/15-120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, J. L. , Kuehn, D. P. , Sutton, B. P. , Gamage, J. K. , & Fang, X. (2016). Anthropometric analysis of the velopharynx and related craniometric dimensions in three adult populations using MRI. The Cleft Palate–Craniofacial Journal, 53(1), e1–e13. https://doi.org/10.1597/14-015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry, J. L. , Sutton, B. P. , Kuehn, D. P. , & Gamage, J. K. (2014). Using MRI for assessing velopharyngeal structures and function. The Cleft Palate–Craniofacial Journal, 51(4), 476–486. https://doi.org/10.1597/12-083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruzansky, S. , & Mason, R. M. (1969). The “stretch factor” in soft palate function. Journal of Dental Research, 48(5), 972–972. https://doi.org/10.1177/00220345690480057001 [DOI] [PubMed] [Google Scholar]
- Ren, Y. F. , Isberg, A. , & Henningsson, G. (1993). Interactive influence of a pharyngeal flap and an adenoid on maxillofacial growth in cleft lip and palate patients. The Cleft Palate–Craniofacial Journal, 30(2), 144–149. https://doi.org/10.1597/1545-1569_1993_030_0144_iioapf_2.3.co_2 [DOI] [PubMed] [Google Scholar]
- Sarmadi, S. , Chalipa, J. , Tanbakuchi, B. , Mahd, M. J. , Nasiri, M. , & Mehtari, M. R. (2018). Two-dimensional analysis of the size of nasopharynx and adenoids in non-syndromic unilateral cleft lip and palate patients using lateral cephalograms. Journal of Dentistry (Tehran, Iran), 15(3), 161–168. [PMC free article] [PubMed] [Google Scholar]
- Schenck, G. C. , Perry, J. L. , Fang, X. (2016). Normative velopharyngeal data in infants: Implications for treatment of cleft palate. Journal of Craniofacial Surgery, 27(6), 1430–1439. https://doi.org/10.1097/SCS.0000000000002722. [DOI] [PubMed] [Google Scholar]
- Siegel-Sadewitz, V. L. , & Shprintzen, R. J. (1986). Changes in velopharyngeal valving with age. International Journal of Pediatric Otorhinolaryngology, 11(2), 171–182. https://doi.org/10.1016/s0165-5876(86)80011-8 [DOI] [PubMed] [Google Scholar]
- Simpson, R. K. , & Austin, A. A. (1972). A cephalometric investigation of velar stretch. The Cleft Palate Journal, 9(4), 341–351. [PubMed] [Google Scholar]
- Skolnick, M. L. , Shprintzen, R. J. , McCall, G. N. , & Rakoff, S. (1975). Patterns of velopharyngeal closure in subjects with repaired cleft palate and normal speech: A multi-view videofluoroscopic analysis. The Cleft Palate Journal, 12(4), 369–376. [PubMed] [Google Scholar]
- Subtelny, J. D. (1980). Oral respiration: Facial maldevelopment and corrective dentofacial orthopedics. The Angle Orthodontist, 50(3), 147–164. [DOI] [PubMed] [Google Scholar]
- Subtelny, J. D. , & Koepp-Baker, H. (1956). The significance of adenoid tissue in velopharyngeal function. Plastic Reconstruction Surgery, 17(3), 235–250. https://doi.org/10.1097/00006534-195603000-00008 [DOI] [PubMed] [Google Scholar]
- White, H. (1980). A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica, 48(4), 817–838. https://doi.org/10.2307/1912934 [Google Scholar]
- Yaseen, E. T. , Khammas, A. H. , & Al-Anbaky, F. (2012). Adenoid enlargement assessment by plain X-ray & nasoendoscopy. Iraqi Journal of Community Medicine, 1, 88–91. [Google Scholar]