Abstract
We highlight an unusual case of multifocal glioblastoma in an adolescent patient, manifesting as four discrete brain lesions, each distinct in appearance. Familiarity with the diverse imaging features of glioblastoma can reduce misdiagnosis and avoid treatment delays.
Clinical presentation
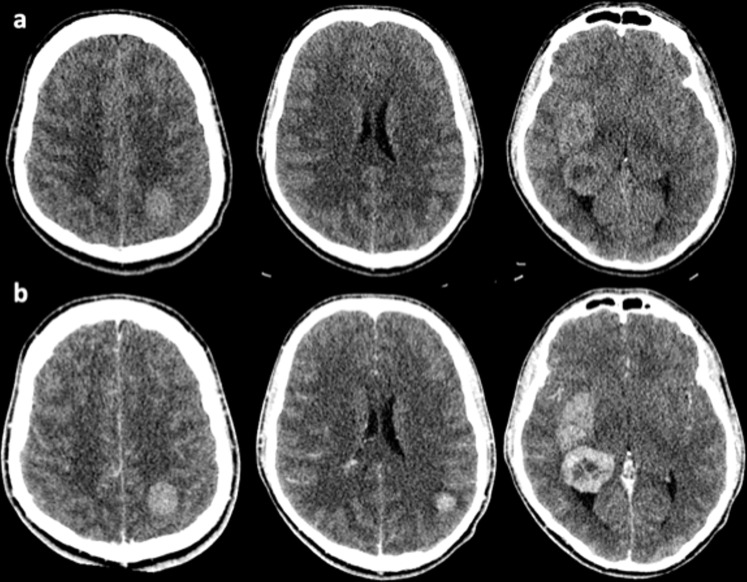
A 17-year-old, previously well male was admitted to the emergency department with a 2-week history of progressive headaches, nausea and vomiting. This was followed by a single seizure with transient loss of consciousness. CT brain imaging performed on admission demonstrated several masses in both cerebral hemispheres (Figure 1). CT imaging of the chest, abdomen and pelvis identified no abnormality, and serum tumour markers (beta-HCG and alpha-fetoprotein) were negative.
Figure 1.
Pre- (a) and post-contrast (b) CT imaging performed on admission demonstrating bilateral cerebral tumours.
MR imaging findings
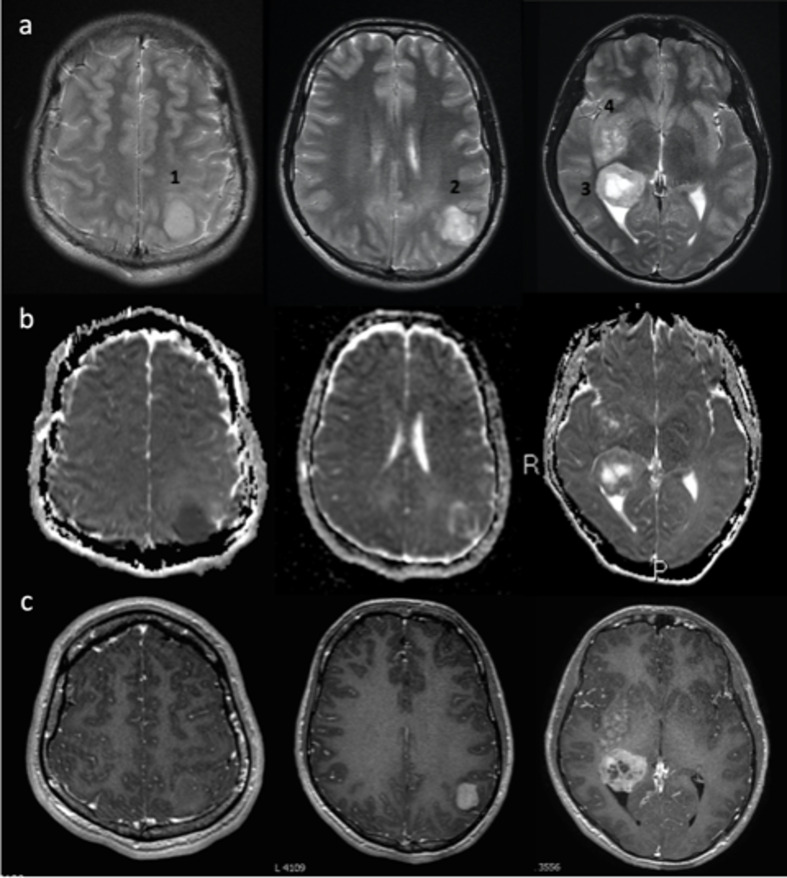
Four tumours varying in size and morphology were present within both cerebral hemispheres (Figure 2): Lesion 1 in the left superior parietal lobule appeared circumscript and markedly T2 hyperintense, showing restricted diffusion without Gadolinium contrast enhancement. Lesion 2 in the left inferior parietal lobule exhibited diffusivity similar to surrounding brain parenchyma and solid contrast enhancement. Lesion 3 was the largest, located at the right dorsal thalamic border. Because this lesion was centred on the lateral ventricular ependymal margin, it appeared partially intraventricular. Lesion 3 demonstrated avid peripheral contrast uptake with central necrosis. Lesion 4 consisted of ill-defined T2 hyperintense expansion of the right lentiform nucleus and surrounding brain parenchyma with patchy contrast uptake. Localised perilesional T2 signal abnormality (oedema ± infiltration) ranged from minimal to diffuse, with areas of normal appearing brain interposed between the lesions.
Figure 2.
T2 (a), ADC (b), T1+Gad (c) images showing multiple masses: Lesion 1 is circumscript with marked diffusion restriction and no significant Gadolinium enhancement. Lesion 2 is heterogenous featuring a rim of oedema/infiltration, ADC values similar to surrounding brain and avid enhancement. Lesion 3 exhibits rim enhancement and central necrosis. Lesion 4 is poorly marginated on all sequences demonstrating mild patchy Gadolinium uptake. ADC, apparent diffusion coefficient.
Therapeutic management
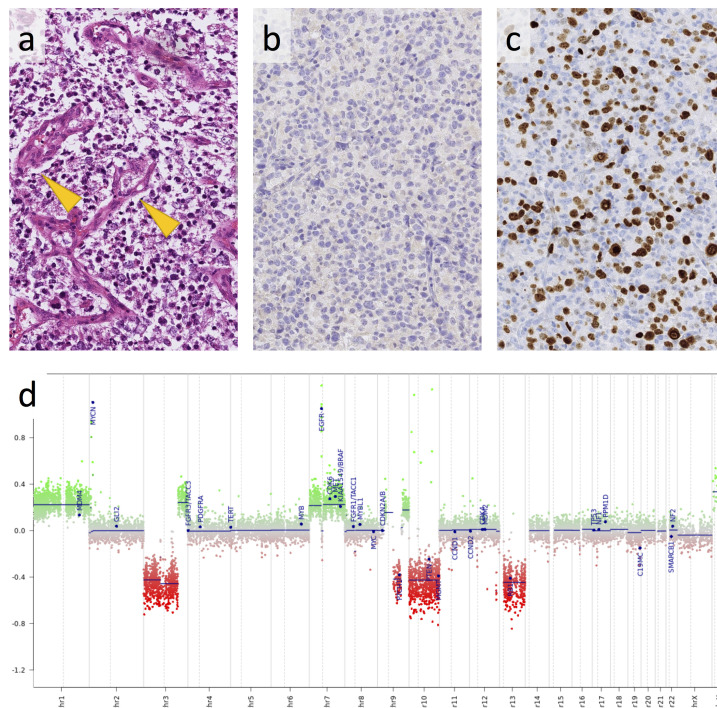
The patient was transferred to the teenage and young adult (TYA) cancer unit, and a biopsy was performed. Histological examination showed morphological evidence of glioblastoma (WHO Grade IV). Post-operatively, the patient suffered a rapid clinical deterioration with increasing headaches, vomiting, blurred vision and new left arm paraesthesia and was commenced on steroids to help alleviate these symptoms. Upon histopathological assessment, immunohistochemical tests showed absence of the most common mutation of isocitrate dehydrogenase (IDH) 1 (R132H) (Figure 3), retained Alpha-thalassaemia X-linked mutant retardation (ATRX) protein expression in tumour cell nuclei, and a very high Ki67 proliferation index (Figure 3). On molecular pathological analysis, 1 the tumour was negative for rare IDH1 and for IDH2 (R172) mutations, BRAF (V600), or Histone 3.3 (K27, G34) mutations. Epidermal growth factor receptor was amplified (18 copies) and the methyl guanine methyl transferase (MGMT) promoter was unmethylated. The unusual presentation of a tumour, which is most commonly found in adults in their fifth-seventh decade, prompted us to perform a methylation array with subsequent algorithmic classification. 1 This investigation confirmed the presence of a classical IDH wild-type glioblastoma with the methylation subclass receptor tyrosine (RTK) kinase II. 2 3 The copy number profile (Figure 3) showed in addition a MYC amplification.
Figure 3.
Histology of the tumour shows a monomorphic population of neoplastic glial glial cells with frequent microvascular proliferations (arrow) (a). Immunoreactivity for mutant IDH1 (R132H) is negative (b) and ki67 immunostain for proliferating tumour cells shows a very high labelling index (c). The copy number profile, derived from the Illumina 450 K methylation array, shows the characteristic chromosome seven gain, 10q loss, and amplification of MYC and EGFR (d). The scale bar corresponds to 200 µm in (a)–(c). EGFR, epidermal growth factor receptor.
As a patient with this histological diagnosis and extent of disease is unlikely to be cured by resection, i.e. when the lesions involve multiple sites and are embedded in deep structures, rapid arc intensity-modulated targeted radiotherapy (54 Gy) was administered alongside adjuvant temozolomide, with palliative intent.
Initially, a partial radiological response to treatment was observed, with complete symptom resolution. However, at 3 months into treatment a new gadolinium enhancing lesion developed in the dorsal medulla, indicating progressive disease. This new deposit and one of the previously treated cerebral lesions continued to grow. Temporary effective symptom control was achieved with further focal radiotherapy to the site of new disease, but disseminated ependymal and parenchymal disease followed, with death at 11 months post diagnosis.
Discussion
Glioblastoma is a relatively uncommon diagnosis in adolescence, 4 and it may be considered low on the differential list - more so if the imaging findings are perceived as atypical. We would like to draw attention to this case, in which the coexistence of several tumour morphologies could create a diagnostic dilemma. A learning point is to consider the possibility of malignant glioma in cases of multiple brain masses with different imaging appearances. 5
The diverse post Gadolinium (non-, solid-, rim-enhancing), T 2 weighted (circumscript vs diffuse) and diffusion-weighted (restricted, intermediate, facilitated) features encountered in this case are all known to exist within the phenotypic spectrum of glioblastoma, 6,7 but may be confused with other entities, even dual pathology, when occurring in combination.
Multifocal cerebral lesions may raise concern for infective-inflammatory aetiologies, especially in a young individual, metastatic disease or lymphoma. 8 Importantly, rapid progression can occur in glioblastoma and should not deter radiologists from suspecting the diagnosis. 9 Physiological MR imaging (perfusion, spectroscopy) can aid the diagnosis of glioblastoma, 10 however, in this case tissue diagnosis was prioritised because of the fulminant clinical decline.
In adult patients, multiple intracranial tumours are present at diagnosis in up to 34% of glioblastomas. 11 It is probable that diverse glioblastoma morphologies represent different stages of the disease. Moreover, evidence exists for significant genetic heterogeneity amongst synchronous tumours 12 as well as within the same glioma through mutational evolution over time 13 ; glioblastomas have been shown to originate up to seven years before diagnosis and acquire most of their driver mutations leading to genetic heterogeneity in this period (i.e. before treatment is initiated). 14
Tumours involving the subventricular zone, as the presumed site of neural stem cell origin of glioblastoma, have been postulated to be more often multifocal and to recur at distant sites 15 ; in our case. the two lesions most closely related to the ventricular surface represented the sites of recurrence and progression.
Multifocal glioblastoma typically have the IDH-wildtype genetic signature 16 associated with poor prognosis and limited response to maximum therapy. Multifocal disease may further shorten the dismal survival of glioblastoma, if gross total resection is difficult to achieve. 17,18 At present, most high-grade gliomas in adolescence undergo standard glioblastoma therapy, consisting of radiation and chemotherapy (Temozolomide). In the future, rapid genetic and epigenetic tumour profiling may improve focused approaches, 19 for which strategies in development include immune modulation and therapies directed against specific signalling pathways. 20
Conclusion
Glioblastoma should be considered as part of the differential diagnosis in young patients presenting with multiple brain masses. The disease may exhibit variable MR imaging features, which can coexist in the same individual.
Learning points
Glioblastoma should be considered in young patients presenting with multiple brain masses.
Glioblastoma manifests with variable MR imaging features, which can coexist in the same individual.
Imaging findings include enhancing and non-enhancing lesions, with or without diffusion restriction.
Footnotes
Acknowledgements: ZJ and SB are supported by the Department of Health’s NIHR Biomedical Research Centre’s funding scheme to UCLH.
Contributor Information
Thomas Campion, Email: campiontc@gmail.com.
Sara Stoneham, Email: sara.stoneham@nhs.net.
Ayisha Al-Busaidi, Email: ayisha.albusaidi@nhs.net.
Atul Kumar, Email: atul.kumar@nhs.net.
Zane Jaunmuktane, Email: zane.jaunmuktane@nhs.net.
Sebastian Brandner, Email: sebastian.brandner@nhs.net.
Neil Kitchen, Email: neilkitchen@nhs.net.
Stefanie Thust, Email: steffi.thust@nhs.net.
REFERENCES
- 1. Jaunmuktane Z, Capper D, Jones DTW, Schrimpf D, Sill M, Dutt M, et al. Methylation array profiling of adult brain tumours: diagnostic outcomes in a large, single centre. Acta Neuropathol Commun 2019; 7: 24. doi: 10.1186/s40478-019-0668-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Capper D, Stichel D, Sahm F, Jones DTW, Schrimpf D, Sill M, et al. Practical implementation of dna methylation and copy-number-based cns tumor diagnostics: the heidelberg experience. Acta Neuropathol 2018; 136: 181–210. doi: 10.1007/s00401-018-1879-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Capper D, Jones DTW, Sill M, Hovestadt V, Schrimpf D, Sturm D, et al. DNA methylation-based classification of central nervous system tumours. Nature 2018; 555: 469–74. doi: 10.1038/nature26000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arora RS, Alston RD, Eden TOB, Estlin EJ, Moran A, Birch JM. Age-incidence patterns of primary cns tumors in children, adolescents, and adults in england. Neuro Oncol 2009; 11: 403–13. doi: 10.1215/15228517-2008-097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Giannopoulos S, Kyritsis AP. Diagnosis and management of multifocal gliomas. Oncology 2010; 79: 306–12. doi: 10.1159/000323492 [DOI] [PubMed] [Google Scholar]
- 6. Rathore S, Akbari H, Rozycki M, Abdullah KG, Nasrallah MP, Binder ZA, et al. Radiomic mri signature reveals three distinct subtypes of glioblastoma with different clinical and molecular characteristics, offering prognostic value beyond idh1. Sci Rep 2018; 8(1): 5087. doi: 10.1038/s41598-018-22739-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hakyemez B, Erdogan C, Yildirim N, Parlak M. Glioblastoma multiforme with atypical diffusion-weighted mr findings. Br J Radiol 2005; 78: 989–92. doi: 10.1259/bjr/12830378 [DOI] [PubMed] [Google Scholar]
- 8. Omuro AM, Leite CC, Mokhtari K, Delattre JY. Pitfalls in the diagnosis of brain tumours. Lancet Neurol 2006; 5: 937–48. doi: 10.1016/S1474-4422(06)70597-X [DOI] [PubMed] [Google Scholar]
- 9. Zhang YY, Ruan LX, Zhang S. Rapid progression of glioblastoma multiforme: a case report. Oncol Lett 2016; 12: 4803–6. doi: 10.3892/ol.2016.5228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Suh CH, Kim HS, Jung SC, Choi CG, Kim SJ. Perfusion mri as a diagnostic biomarker for differentiating glioma from brain metastasis: a systematic review and meta-analysis. Eur Radiol 2018; 28: 3819–31. doi: 10.1007/s00330-018-5335-0 [DOI] [PubMed] [Google Scholar]
- 11. Lasocki A, Gaillard F, Tacey M, Drummond K, Stuckey S. Multifocal and multicentric glioblastoma: improved characterisation with flair imaging and prognostic implications. J Clin Neurosci 2016; 31: 92–98. doi: 10.1016/j.jocn.2016.02.022 [DOI] [PubMed] [Google Scholar]
- 12. Schroeder B, Shah N, Rostad S, McCullough B, Aguedan B, Foltz G, et al. Genetic investigation of multicentric glioblastoma multiforme: case report. J Neurosurg 2016; 124: 1353–58. doi: 10.3171/2015.4.JNS142231 [DOI] [PubMed] [Google Scholar]
- 13. Wang J, Cazzato E, Ladewig E, Frattini V, Rosenbloom DIS, Zairis S, et al. Clonal evolution of glioblastoma under therapy. Nat Genet July 2016; 48: 768–76. doi: 10.1038/ng.3590 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Körber V, Yang J, Barah P, Wu Y, Stichel D, Gu Z, et al. Evolutionary trajectories of idhwt glioblastomas reveal a common path of early tumorigenesis instigated years ahead of initial diagnosis. Cancer Cell 2019; 35: 692–704. doi: 10.1016/j.ccell.2019.02.007 [DOI] [PubMed] [Google Scholar]
- 15. Lim DA, Cha S, Mayo MC, Chen M-H, Keles E, VandenBerg S, et al. Relationship of glioblastoma multiforme to neural stem cell regions predicts invasive and multifocal tumor phenotype. Neuro Oncol 2007; 9: 424–29. doi: 10.1215/15228517-2007-023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Liu Q, Liu Y, Li W, Wang X, Sawaya R, Lang FF, et al. Genetic, epigenetic, and molecular landscapes of multifocal and multicentric glioblastoma. Acta Neuropathol 2015; 130: 587–97. doi: 10.1007/s00401-015-1470-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Thomas RP, Xu LW, Lober RM, Li G, Nagpal S. The incidence and significance of multiple lesions in glioblastoma. J Neurooncol 2013; 112: 91–97. doi: 10.1007/s11060-012-1030-1 [DOI] [PubMed] [Google Scholar]
- 18. Brown TJ, Brennan MC, Li M, Church EW, Brandmeir NJ, Rakszawski KL, et al. Association of the extent of resection with survival in glioblastoma: a systematic review and meta-analysis. JAMA Oncol 2016; 2: 1460–69. doi: 10.1001/jamaoncol.2016.1373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Worst BC, van Tilburg CM, Balasubramanian GP, Fiesel P, Witt R, Freitag A, et al. Next-generation personalised medicine for high-risk paediatric cancer patients - the inform pilot study. Eur J Cancer 2016; 65: 91–101. doi: 10.1016/j.ejca.2016.06.009 [DOI] [PubMed] [Google Scholar]
- 20. Zhang D, Liu X, Fan C, Chen J. Novel drugs in pediatric gliomas. Oncol Lett 2017; 13: 2881–85. doi: 10.3892/ol.2017.5812 [DOI] [PMC free article] [PubMed] [Google Scholar]