Significance
Synchronous neurotransmitter release relies on directed docking of synaptic vesicles at active zones in axon terminals, where calcium influx activates membrane fusion and release. In vitro, the calcium sensor synaptotagmin oligomerizes into rings disassembled by calcium. Here, experiment and modeling suggest the synaptotagmin molecules hosted by an undocked vesicle oligomerize into a tethered, unbound halo in ATP-rich synaptic terminals. The halo directs vesicle docking to phosphatidylinositol 4,5-bisphosphate (PIP2)-rich plasma membrane domains in active zones, where the trans-bound ring conformation is favored, interposed between the membranes to clamp fusion until calcium triggers ring disassembly and neurotransmitter release. The mechanism exploits the extreme sensitivity of synaptotagmin ring binding preferences to solution and membrane composition, with ∼15-fold-enhanced sensitivity for rings of ∼15 molecules.
Keywords: synaptotagmin, synapse, membrane fusion, vesicle docking, neurotransmitter release
Abstract
Synchronous release at neuronal synapses is accomplished by a machinery that senses calcium influx and fuses the synaptic vesicle and plasma membranes to release neurotransmitters. Previous studies suggested the calcium sensor synaptotagmin (Syt) is a facilitator of vesicle docking and both a facilitator and inhibitor of fusion. On phospholipid monolayers, the Syt C2AB domain spontaneously oligomerized into rings that are disassembled by Ca2+, suggesting Syt rings may clamp fusion as membrane-separating “washers” until Ca2+-mediated disassembly triggers fusion and release [J. Wang et al., Proc. Natl. Acad. Sci. U.S.A. 111, 13966–13971 (2014)].). Here, we combined mathematical modeling with experiment to measure the mechanical properties of Syt rings and to test this mechanism. Consistent with experimental results, the model quantitatively recapitulates observed Syt ring-induced dome and volcano shapes on phospholipid monolayers and predicts rings are stabilized by anionic phospholipid bilayers or bulk solution with ATP. The selected ring conformation is highly sensitive to membrane composition and bulk ATP levels, a property that may regulate vesicle docking and fusion in ATP-rich synaptic terminals. We find the Syt molecules hosted by a synaptic vesicle oligomerize into a halo, unbound from the vesicle, but in proximity to sufficiently phosphatidylinositol 4,5-bisphosphate (PIP2)-rich plasma membrane (PM) domains, the PM-bound trans Syt ring conformation is preferred. Thus, the Syt halo serves as landing gear for spatially directed docking at PIP2-rich sites that define the active zones of exocytotic release, positioning the Syt ring to clamp fusion and await calcium. Our results suggest the Syt ring is both a Ca2+-sensitive fusion clamp and a high-fidelity sensor for directed docking.
Neurotransmission is based on the Ca2+-triggered release of neurotransmitters from membrane-enclosed synaptic vesicles, each carrying ∼15 copies of the transmembrane protein synaptotagmin-1 (1–3). During synchronous release, an action potential triggers Ca2+ entry through voltage-gated Ca2+ channels that is sensed by Synaptotagmin (Syt), provoking fusion of the synaptic vesicle with the plasma membrane (PM) and neurotransmitter release. SNARE proteins fuse the vesicle and target membranes, regulated by Syt and other accessory proteins, but the detailed mechanisms remain unclear (4, 5).
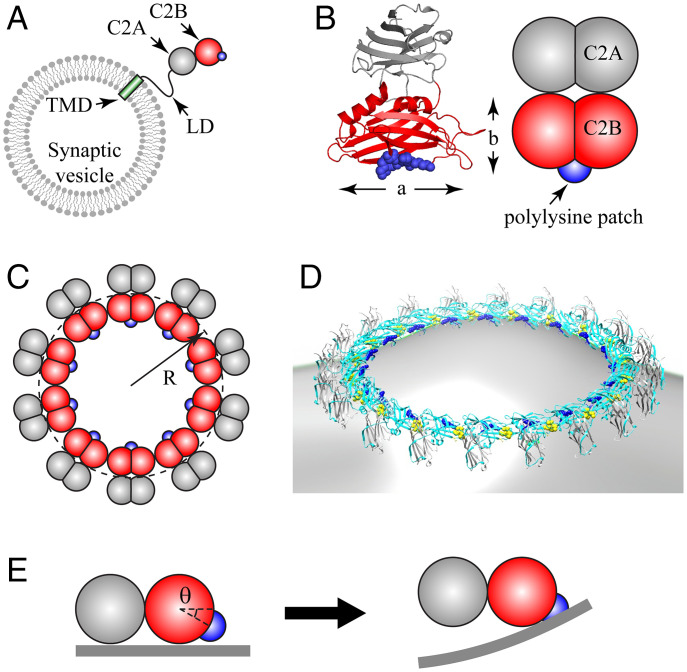
The ability of Syt to bind phospholipid membranes in both a Ca2+-independent and a Ca2+-dependent manner is likely fundamental to its function in synaptic release (6). Syt consists of a transmembrane domain (TMD); a juxtamembrane linker domain (LD); and two cytosolic Ca2+-binding domains, namely, C2A and C2B (Fig. 1A). The polylysine patch of C2B (residues 324 to 327) electrostatically binds anionic phospholipids such as phosphatidylserine (PS), with particularly strong binding to phosphatidylinositol 4,5-bisphosphate (PIP2) (6, 7), in a Ca2+-independent manner. With Ca2+, partial insertion of the calcium-binding loops of the C2 domains into membranes leads to strong Syt-membrane binding (8–10).
Fig. 1.
Coarse-grained model of Syt C2AB domain and oligomeric Syt rings. (A) Synaptic vesicles carry ∼15 copies of Syt, with each comprising a TMD, a flexible juxtamembrane LD, and the C2A and C2B globular domains (schematic, not to scale). The polylysine patch (blue) on C2B mediates Ca2+-independent binding to anionic membranes. (B) Crystal structure of Syt (PDB ID: 2R83) (Left) and our coarse-grained representation (Right). Each C2 domain is represented as two overlapping beads, radius , giving length nm and width nm. The positively charged polylysine patch (blue) on C2B is treated as a point charge. (C) Coarse-grained model representation of a Syt ring of radius R, Top view. (D) Rendition of a Syt ring bound to a membrane, based on EM reconstruction of Syt oligomers assembled on phospholipid monolayer tubes (43) (cyan, C2B; gray, C2A; blue, polylysine patch; yellow, Ca2+ binding loops on C2B). (E) C2AB subunit in a ring interacting with planar membrane (Side view). The polylysine patch lies below the plane of the ring, at an angle suggested by EM reconstruction, D. In the ring, the C2AB unit cannot rotate downward, so electrostatic attraction bends the charged membrane up toward the patch (Right).
Syt facilitates synchronous fusion, as deletion of Syt shifted neurotransmitter release from a synchronous mode to an asynchronous mode in mice (11, 12) and Drosophila (13). Syt plays a role in clamping fusion before Ca2+ entry. Deletion of Syt greatly increased spontaneous release in mice (14, 15) and Drosophila (16–18). The mechanism is unknown, but it was proposed that Syt may clamp fusion in the absence of Ca2+ by imposing a membrane separation too great for SNARE-mediated fusion (19) or by binding SNAREs and locking them in a fusion-incompetent state (20–22). Whatever the mechanism, a critical role is likely played by Syt-SNARE binding, recently characterized by structural studies (23–25).
Syt also plays a role in vesicle docking. Syt promoted docking of liposomes in vitro (26, 27) and of synaptic vesicles in Drosophila (18, 28), in mouse hippocampal neurons (29, 30), and in mouse chromaffin cells (31). Ca2+-dependent trans-binding of Syt to PIP2-containing liposomes from Syt-bearing chromaffin granules was reported, as well as cis-binding to the host granules (32, 33). Importantly, the presence of ATP reduced the cis-binding but not the trans-binding (32). Synaptotagmin mutants lacking the polylysine patch docked vesicles deficiently to the PM before arrival of an action potential (29).
Vesicle fusion occurs at active zones in the presynaptic membrane with high PIP2 content (19, 34). RIM proteins and Munc13 are known to play essential roles in the localization of synaptic vesicles to active zones (35, 36), but the detailed mechanisms that target vesicles to these high PIP2 regions remain uncertain (31, 36, 37). Rapid vesicle fusion requires PIP2 (38, 39). PIP2 regulates fusion machinery components such as Syt to be more effective as facilitators of fast fusion and release (40). The speed at which Syt responds to calcium by penetrating the membrane is enhanced by the presence of PIP2 (41), and PIP2 enhances the sensitivity of Syt to calcium 40-fold (7, 42).
In the absence of Ca2+, Syt oligomerizes into ring-like oligomers. Without ATP, electron microscopy (EM) images showed rings of , the soluble C2AB domain, on carbon-supported anionic phospholipid monolayers (43, 44) that buckled into dome-, volcano-, or tube-like shapes. Ring formation depended on the polylysine patch of the C2B domain, as the KAKA (K326A, K327A) patch mutation abolished rings (43, 44). Higher PS or PIP2 membrane content or lower salt concentration increased the ring density.
These findings suggest that ring formation is mediated by electrostatic interactions between anionic lipids and the polylysine patch of C2B. Rings appear further promoted by the highly charged LD (45) since SytCD, the cytosolic domain including the LD, oligomerized into rings on monolayers even at physiological salt concentrations and in the presence of ATP, while SytC2AB rings required low salt (44). In the presence of ATP or soluble PIP2, rings can also assemble in solution, at physiological and lower salt concentrations (46).
Importantly, membrane-bound Syt rings disassemble at physiological Ca2+ concentrations (43), suggesting an explanation for Syt’s dual role as a fusion clamp and facilitator. In this model, SNAREs are primed for fusion prior to Ca2+ influx, but the Syt ring acts as a “washer” separating the synaptic vesicle and PMs, sterically preventing fusion; injection of Ca2+ rapidly disassembles the ring and fusion proceeds (43). Consistent with a clamping role for Syt oligomers, mutations selectively disrupting Syt oligomerization showed increased spontaneous release frequency in PC12 cells (47) and neocortical synapses (48) and abrogated clamping under in vitro conditions (49, 50). This mutation also abolished a symmetric arrangement of components revealed by electron cryotomography at the synaptic vesicle–PM interface in nerve growth factor–differentiated PC12 cells, suggesting Syt rings template the organization and have a role consistent with this model (51). Ca2+ binding may disassemble rings by driving Ca2+-dependent insertion of the Ca2+-binding loops into membranes, as Ca-triggered disassembly is abolished by the 3xDA (D309A, D363A, D365A) mutation, which disrupts Ca2+ binding to the C2B domain (43, 44).
Here, we assess the feasibility of the hypothesized Syt washer mechanism quantitatively, combining molecularly explicit mathematical modeling and experiment. We measured Syt ring size distributions in bulk solution and used a mathematical model to infer the bending stiffness of rings. We built a model of Syt–Syt and Syt–membrane interactions severely constrained by experimental data, which quantitatively reproduced the Syt-induced monolayer domes and volcanoes. Applying the model to the conditions in synaptic terminals during neurotransmitter release when vesicles hosting ∼15 copies of Syt dock at the PM, we asked if the Syt C2 domains will oligomerize into a ring and, if so, whether the ring will bind the PM as assumed by the washer model (trans-binding), bind the vesicle membrane (cis-binding), or remain in solution (the “halo”). We find the outcome depends with extreme sensitivity on membrane and cytosol composition. These results suggest the Syt halo serves as landing gear for spatially directed vesicle docking to PIP2-rich sites that colocalize with the t-SNARE Syntaxin and other fusion machinery components in active zones, positioning the PM-bound trans-Syt ring to clamp fusion and await calcium. Thus, the Syt ring is both a calcium-sensitive fusion clamp and a high-fidelity sensor for directed docking. The high sensitivity originates in the oligomeric character of the ring, which enhances sensitivities ∼15-fold compared to monomeric Syt.
Results
Model of Syt Oligomerization in Solution to Extract Mechanical Properties of Syt Rings.
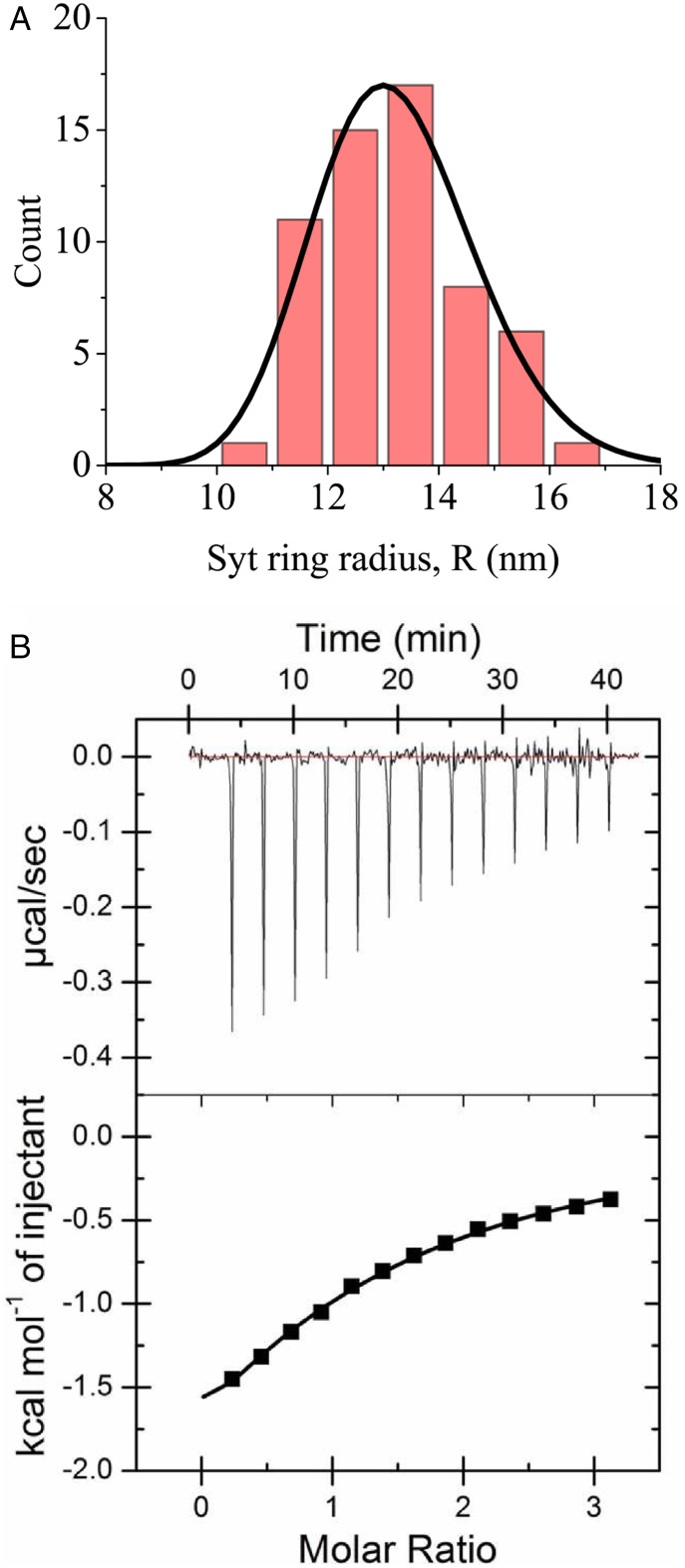
We used EM to image Syt C2AB domains incubated in solution with 15 mM KCl and 1 mM Mg-ATP (see Materials and Methods). To ease notation, hereafter Syt will denote the C2AB domain (referred to as above). Negative stain analysis showed that Syt monomers in solution spontaneously oligomerized into rings (SI Appendix, Fig. S1). Previous observations of Syt rings of similar dimensions using cryo-EM show they are not artifacts of this negative staining procedure (43). We measured the size distribution, revealing a mean radius (mean SD) (Fig. 2A).
Fig. 2.
(A) Experimental Syt ring size distribution in bulk solution with 1 mM Mg-ATP and 15 mM KCl, from electron micrographs (radius is defined in Fig. 1C). Mean radius is 13.1 nm ± 1.4 nm (SD). Solid line represents best fit theoretical distribution (Eq. 1), yielding spontaneous radius of curvature and persistence length (Table 1). (B) Syt-ATP binding measured by ITC. The measured parameters are as follows: ΔH = −4.4 ± 0.7 kcal/mol, dissociation constant .
To extract the stiffness and energetics of Syt rings from this size distribution, which was needed to model Syt ring formation and interaction with membranes (Coarse-Grained Model of Syt Rings Interacting with Membranes), we developed a simple model describing the equilibrium of monomers and rings in solution. Rings were assumed circular, with variable numbers of Syt monomers each of length nm, spontaneous radius of curvature and unknown flexural rigidity , where is the persistence length (52). We find the effective free energy of rings of radius is given by (SI Appendix)
| [1] |
This expression involves neither the Syt–Syt binding energy nor the Syt monomer concentration since in equilibrium the latter self-tunes to a critical value where the free monomer chemical potential equals (SI Appendix). The predicted ring size distribution is the Boltzmann distribution (solid line, Fig. 2A). Fitting to the experimental distribution yielded best fit values nm, nm, or ∼16 Syt subunits per ring, which is in agreement with fluorescence correlation spectroscopy measurements (46) and similar to the number of Syt molecules per synaptic vesicle, namely, ∼15 to 20 (1, 3). This is consistent with the predictions of our model (see below) that the Syt molecules hosted by a synaptic vesicle spontaneously oligomerize into a ring at the synaptic terminal. It was found that heterozygous mice with only one copy of the Syt1 gene are normal (15), suggesting that even when fewer Syt are available in the cell, synaptic vesicles have normal Syt1 copy numbers so Syt1 rings can form normally for normal neurotransmission. Finally, the value we find shows that Syt rings are quite flexible; e.g., a ring of 20 subunits has only 1.7 kT more free energy than a ring with the mean number 16.
Coarse-Grained Model of Syt Rings Interacting with Membranes.
To describe the Ca2+-independent interaction of Syt rings with anionic phospholipid membranes, we developed a highly coarse-grained model, as system size and equilibration timescales are considerable. The main model outputs are as follows: (1) the membrane surface configuration with a bound ring and (2) the free energy of ring-membrane binding. Here, we present the framework, which will be adapted to monolayers and then to physiologically realistic bilayers in later sections (see SI Appendix).
A molecule comprises a C2A and a C2B domain. The crystal structure of human Syt (Protein Data Bank [PDB] identifier [ID]: 2R83) shows the domains are similarly shaped, about nm long and nm wide (Fig. 1B). We represent each C2 domain by two beads of radius nm, overlapping to give length and width (Fig. 1B and SI Appendix).
Syt rings are constructed by interconnecting many subunits. From an EM reconstruction of Syt oligomers on monolayer tubes (43), the C2B domains were proposed to form the inner circle of the ring, with their long axes oriented tangentially (Fig. 1 C and D). The C2B polylysine patches were estimated to lie on the inward face of the ring, tilted an angle out of plane (Fig. 1E). In our model, we assume rings have these features. In addition, we tested a range of possible angles, supporting as proposed in ref. 43 (see below).
Phospholipid membranes were modeled as continuous sheets using a well-established triangulation scheme with a dynamic mesh representing membrane fluidity, bending energy and tension (53–55) (see SI Appendix). The membrane sheet is a network of nodes, with adjacent nodes connected by finitely extensible tethers and interacting via hard-sphere repulsions below a certain separation. Random bond flipping ensures fluidity, continuously redefining node–node connectivity. Bending and tension are discretized versions of the Helfrich energy (56),
| [2] |
Here, is the bending modulus; is the mean of the principal curvatures, discretized using the scheme of ref. 57; is the spontaneous curvature; and the membrane tension.
Syt interacts with the membrane via screened electrostatic and repulsive steric forces. Membranes are assumed uniformly charged, so each node interacts electrostatically with the polylysine patch of each Syt with a strength depending on membrane composition. Taking the reference state as free Syt monomers in solution, the total free energy of formation of a Syt ring bound to a membrane, per Syt monomer, is given by
| [3] |
where denotes the Syt-Syt binding energy, denotes the bending energy, and denotes the electrostatic interaction between the Syt ring and the membrane, all per monomer. The superscript “mb” may denote “pm”, “ves,” or “mono” depending on whether the planar or vesicle membrane or monolayer is involved. In Eq. 3, we neglected contributions from membrane tension, Syt-membrane steric interactions, and Syt ring bending energy (Eq. 1), all of which our simulations showed to be negligible.
To calculate the ring–membrane interaction and membrane bending energies, we placed a Syt ring adjacent to a flat 60-nm diameter monolayer or 50-nm diameter bilayer and equilibrated the system using pseudo Langevin dynamics (58) that decrease free energy with time (SI Appendix) using interaction forces from energy derivatives and fictitious particle drags. In simulations with vesicles, the 40-nm diameter vesicles had pressure-enforced constant volume.
Our overall objective was to determine if Syt monomers spontaneously oligomerize into rings under different circumstances, some with and others without membranes. Consider the simplest case, with Syt monomers and Syt rings of monomers each at densities and , respectively, in a bulk solution. The total free energy density is where is the ring free energy per monomer and are constants. The first two terms describe the entropy of the monomers and rings, respectively. Minimizing this free energy at fixed total monomers yields , where . Since , the solution to this equation is to within small errors. Thus, if is negative with a magnitude much bigger than , all monomers will spontaneously oligomerize except for a small remnant at very low concentration (analogous to the critical micelle concentration of surfactant solutions (59)). Similar conclusions apply in the presence of a membrane.
Using this result, our procedure throughout this study was to calculate for each situation. If is negative and larger than in magnitude, this demonstrates rings will form. When several candidate ring types are possible, a similar calculation shows the ring class with lowest is overwhelmingly selected, provided the free energy difference is well above .
This procedure implicitly assumes enough time is available that the thermodynamically favored state (monomers or rings) is realized. Taking halo ring formation (see below) as a representative case, we demonstrated this assumption is valid using a simple model that predicts ring formation kinetics are very fast (see SI Appendix).
Measurement or Calculation of Synaptotagmin Binding Parameters Needed by the Model.
We first experimentally measured or computed key model parameters (Table 1 and SI Appendix for details).
Table 1.
Model parameters
| Symbol | Description | Value | Source* |
|---|---|---|---|
| Length, width of C2B domain | 5 nm, 3 nm | (43) | |
| Monolayer spontaneous curvature (40% PS, 60% PC) | 0.02 nm−1 | (A) | |
| Hydrophobic interaction length | 2 nm | (60, 61) | |
| Monolayer-carbon support hydrophobic energy density | 0.04 kT/nm2 | (B) | |
| Syt-ATP binding energy | 9.7 kT | (C) | |
| Syt-monolayer binding energy | 18 kT | (D) | |
| Syt-planar-membrane binding energy | 4.9 kT | (D) | |
| Syt-Syt binding energy | 9.5 kT | (E) | |
| Syt-synaptic-vesicle binding energy | 2.2 kT | (D) | |
| Persistence length of Syt oligomer | 170 nm | (F) | |
| Radius of model beads | 1.5 nm | (G) | |
| Vesicle radius | 20 nm | ||
| Spontaneous radius of curvature of Syt oligomers | 13 nm | (F) | |
| Bending modulus of monolayer (bilayer) | 12.5 kT (25 kT) | (H) | |
| Bilayer membrane tension | 0.003 pN/nm | (79, 80) | |
| Viscosity of water | Pa·s | ||
| 2D viscosity of lipid bilayers | Pa·s·m | (81) | |
| Debye length at 140 mM salt | 0.7 nm | (66) |
*(A) Linear interpolation of spontaneous curvatures of DOPS ( (82)) and DOPC ( (83)), based on the molar fractions of the two components. (B) Deduced in the present work (see Fig. 3 C and D). (C) Experimentally measured in the present work. (D) Calculated in the present work, using measurements of ref. 6 as input. (E) Calculated in the present work, using measurements of ref. 46 as input. (F) Calculated in the present work by fitting Eq. 1 to the experimentally measured Syt ring size distribution in bulk solution (Fig. 2A). (G) Estimated from the C2AB crystal structure (Fig. 1B). (H) Bilayer bending modulus from ref. 84, measured in 100 mM NaCl. Monolayer modulus is assumed half the bilayer value.
(1) The Syt-ATP binding energy (Fig. 2B). We used isothermal titration calorimetry (ITC) to measure the dissociation constant at 140 mM KCl. Taking an ATP molecule size gives a local binding energy of . (2) The Syt–Syt binding energy in the presence of 1 mM Mg-ATP. Wang et al. (46) measured with 20 mM KCl and 1 mM Mg-ATP, yielding binding energy , taking a Syt molecule size of 5 nm. We assumed this value is independent of salt concentration and unaltered by membrane binding. (3) The binding energy of Syt to phospholipid bilayers and monolayers in the absence of Ca2+. Using the molar partition coefficient M−1 reported for Syt–liposome binding (25% POPS, 75% POPC) in 100 mM KCl (6), we extrapolated the binding energy to different salt concentrations and membrane charge densities. For physiological conditions, this yielded and for a typical neuronal PM and synaptic vesicle, respectively, and for the monolayers studied here (43) (Table 2 for assumed membrane compositions).
Table 2.
Lipid composition and salt concentration conditions used by model
Syt Oligomerizes into Rings on Phospholipid Monolayers and Generates Domes or Volcanoes.
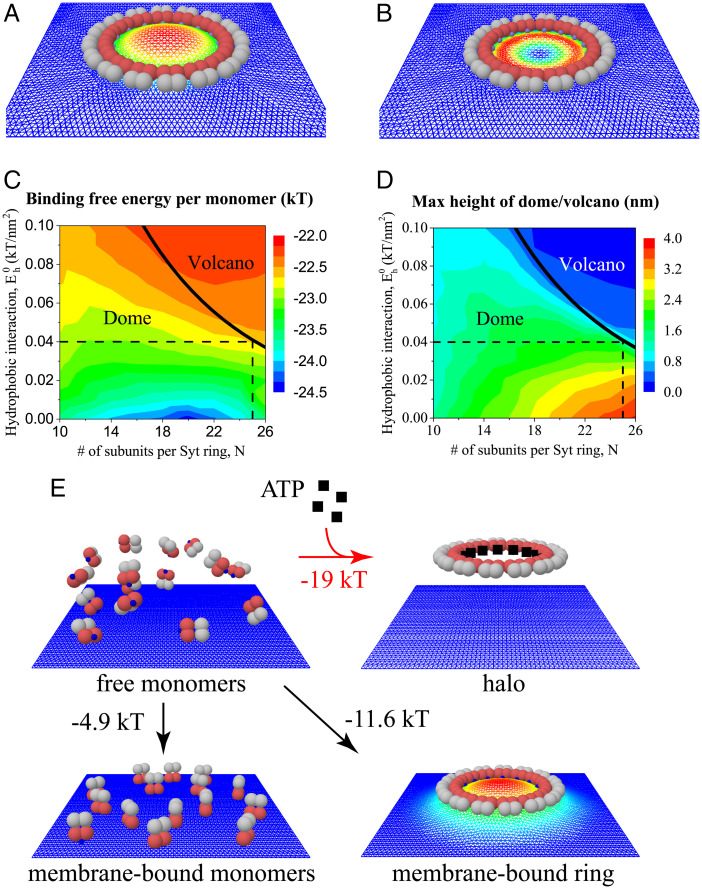
We first asked if our model could reproduce the experiments of ref. 43, in which Syt rings formed on carbon-supported monolayers and the monolayer assumed various shapes at the ring locations. To mimic the experiment, we simulated monolayers at 15 mM KCl (Table 1) and with the uniform charge density corresponding to the lipid composition of 40% phosphatidylserine (PS), 60% phosphatidylcholine (PC) (Table 2). Free energies were calculated from Eq. 2, but with an additional term added to of Eq. 3 representing the hydrophobic attraction (per Syt monomer) between the monolayer and its carbon support (60, 61), . Here, is the hydrophobic energy density, is the separation between the monolayer node and the carbon substrate, is the area of node , and nm is the hydrophobic decay length.
Placing the ring adjacent to the monolayer as an initial condition, independently of the initial condition, the system evolved to an equilibrium with the Syt ring bound to the monolayer that it deformed into a dome or volcano, similarly to the experiments (43) (Fig. 3 A and B). The selected shape depended on the number of Syt molecules per ring N and the hydrophobic interaction (see phase diagrams, Fig. 3 C and D). Volcanoes are favored by large rings and strong hydrophobic attraction.
Fig. 3.
Model results for binding of Syt rings on carbon-supported monolayers and on planar bilayer membranes. (A and B) Simulation snapshots of equilibrium configurations of a Syt ring of subunits binding a monolayer with 40% PS, 60% PC in 15 mM salt solution (conditions as in ref. 43). Warmer colors denote greater monolayer elevations. (A) Monolayer deforms into a dome shape for lower monolayer-carbon hydrophobic interaction energy, . (B) Volcano shape results for larger interaction, . (C and D) Monolayer shape (dome or volcano) and binding free energy per monomer (C) or maximum monolayer height (D) versus N, the number of Syt subunits per ring, and , the monolayer-carbon binding energy density. In the experiments of ref. 43, the dome–volcano transition occurred at , identifying (dashed lines). (E) Possible states for Syt monomers in solution near a planar bilayer membrane with overall composition typical of a plasma membrane, with composition assumed homogenous (Table 2). Energies of formation are per Syt monomer, relative to a reference state with 15 free Syt monomers in solution at physiological salt conditions, 140 mM (Top Left). With ATP, the preferred state is the Syt ring in solution (halo), stabilized by the substantial Syt-ATP binding energy, Without ATP, the Syt ring oligomerizes on the membrane, deforming it into a dome (simulation snapshot, warmer colors denote greater elevation).
To estimate the hydrophobic interaction strength , we used the fact that the dome–volcano transition occurred at in the experiments of ref. 43, corresponding to kT/nm2 on the predicted dome–volcano boundary curve (Fig. 3C). With this value, over a range of N values, the free energy of ring formation is ∼−25 kT/monomer, with dome and volcano heights of ∼1 to 2 nm and <1 nm, respectively. The negative free energy shows that Syt spontaneously forms rings on carbon-supported monolayers from solution.
These results assumed the tilt angle locating the polylysine patch in the ring is (Fig. 1E), following the proposal of ref. 43. To test this further, we ran simulations in which Syt rings bound bilayer membranes, and this angle was varied (SI Appendix, Fig. S2). Domes were only generated when this angle was close to and were absent when the patch was located on the “underside” of the ring () or near the “top” (). Given the experimental observation of domes on monolayers, our results support the location proposed in ref. 43.
The origin of these shapes is clear from the model. When Syt assembles into a ring, the polylysine patches are raised ∼1 nm above the membrane (Fig. 1 C and E), so that binding requires the membrane to deform upward everywhere around the ring into a dome shape (Fig. 1 D and E). This shape incurs a membrane bending energy penalty proportional to ring length, ∼N, being dominated by the severe curvature at the ring boundary. Another candidate shape, the volcano, has a higher bending energy of ∼N, as the high curvature occurs along both the inside and outside of the volcano rim. However, a dome pulls the monolayer away from the carbon substrate, incurring a hydrophobic penalty of ∼N2 proportional to ring area, whereas the hydrophobic penalty is only ∼N for the volcano. Thus, for large enough , the net dome energy exceeds that of the volcano, and volcanoes are selected. The greater , the smaller the value of at the transition. These features are apparent in Fig. 3C.
Syt Forms Rings on Homogenous Planar Bilayers But Rings Prefer an ATP-Rich Solution.
Our model predicts that Syt C2AB molecules in solution spontaneously oligomerize into monolayer-bound rings, as seen experimentally. We next asked if the same is true for physiologically relevant bilayers, which was untested experimentally. This is a priori unclear, as the hydrophobic substrate interaction is absent (favoring ring binding), but a bilayer has twice a monolayer’s bending modulus (disfavoring ring binding). Furthermore, the ∼1 mM ATP in the cytosol (62) may favor rings in solution, an effect seen for carbon-supported monolayers with physiological ATP concentrations (44, 46). All energies below are per Syt monomer, unless otherwise stated.
We simulated Syt rings interacting at physiological salt concentration with planar bilayers of typical PM composition (Table 1), including 25% PS and 2% PIP2 (63, 64) (Table 2). The composition was assumed homogenous. Rings of 10 to 20 subunits bound to the bilayer, producing domes (Fig. 3E). In the final state, the attractive electrostatic energy ∼2.5 kT was offset by a membrane bending energy penalty of ∼0.4 kT. Adding the Syt–Syt binding energy ∼9.5 kT, we conclude that Syt monomers in ATP-free solution bind bilayers as rings, with net binding free energy . This has greater magnitude than the binding energy of free monomers from solution, kT. Thus, membrane-bound rings are stable to fragmentation into membrane-bound monomers (Fig. 3E).
Consider now the effects of ATP in the bulk solution. Thus far, we excluded Syt rings in solution (halos) as candidate structures. With ATP, we find halos are preferred since Syt molecules can release by remaining in solution and binding ATP. Thus, the energy of halo formation is kT, which is less than that of a membrane-bound ring, (Fig. 3E). This argument assumes Syt in solution must unbind ATP in order to bind the membrane (32, 44).
Membrane Curvature and Charge Density Effects: Syt Forms Rings That Bind Anionic Vesicles in the Absence of ATP.
The previous section treated Syt interactions with a model PM. At synaptic terminals, Syt can presumably also interact with its high curvature host synaptic vesicle, lacking PIP2 (1) (Table 2). Thus, we analyzed curvature and charge density effects.
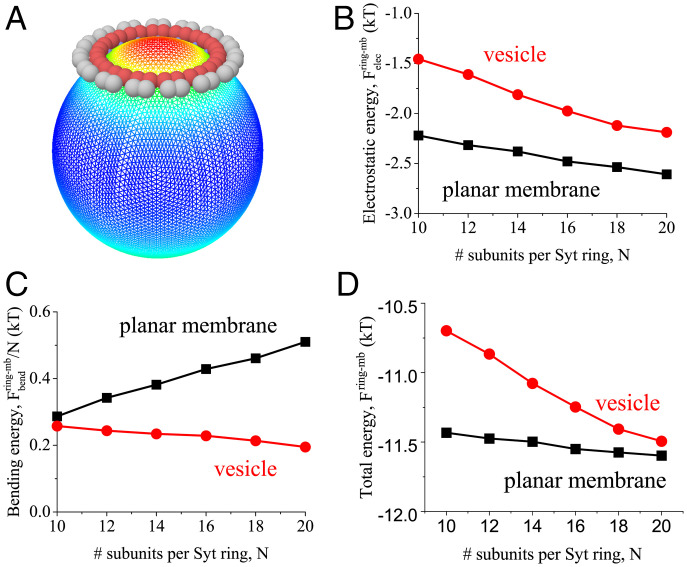
Simulations showed that Syt monomers oligomerize into rings and bind anionic vesicles when ATP is absent. At physiological salt, Syt rings () bound and dimpled 20-nm-radius vesicles with synaptic vesicle lipid composition, a milder version of the dome on planar membranes (Fig. 4A). Typical energies were ∼2 kT (electrostatic advantage) and ∼0.2 kT (membrane bending penalty), giving a net binding energy kT, after adding the Syt–Syt binding advantage (Fig. 4 B–D).
Fig. 4.
Membrane curvature and charge density promote binding of Syt rings. Model results for binding of Syt rings at physiological salt, 140 mM, to a 20-nm-radius lipid vesicle with composition representative of synaptic vesicles (15% PS, Table 2). Vesicle binding energies are compared with binding energies to planar membranes with lipid composition representative of a plasma membrane (Table 2). Energies are per Syt subunit. (A) Simulation snapshot of a Syt ring of subunits binding and dimpling the vesicle, with a mild version of the dome seen on planar bilayers. Warmer colors denote greater distance from vesicle center. (B–D) Model-predicted free energies when a Syt ring binds a vesicle or a planar membrane versus number of Syt monomers per ring. (B) The attractive electrostatic energy is greater for the plasma membrane due to its higher anionic lipid charge density. (C) Vesicle binding incurs less bending energy, due to vesicle curvature. (D) The net binding energy favors the plasma membrane since the electrostatic advantage exceeds the bending disadvantage.
The competition between a planar PM and a vesicle for binding to a Syt ring is quantified in Fig. 4 B–D. Vesicle curvature favors ring binding because the membrane needs to bend less to curve upward and bind the polylysine patch (Fig. 1E). However, electrostatic interactions favor Syt-PM binding due to the higher PM negative charge density. Overall, binding to planar PMs is preferred (Fig. 4D). We stress, however, that the planar membrane energies of Fig. 4 neglect composition inhomogeneities, which are considered next.
PIP2 Clustering Promotes Trans-Binding of Syt Rings to the PM.
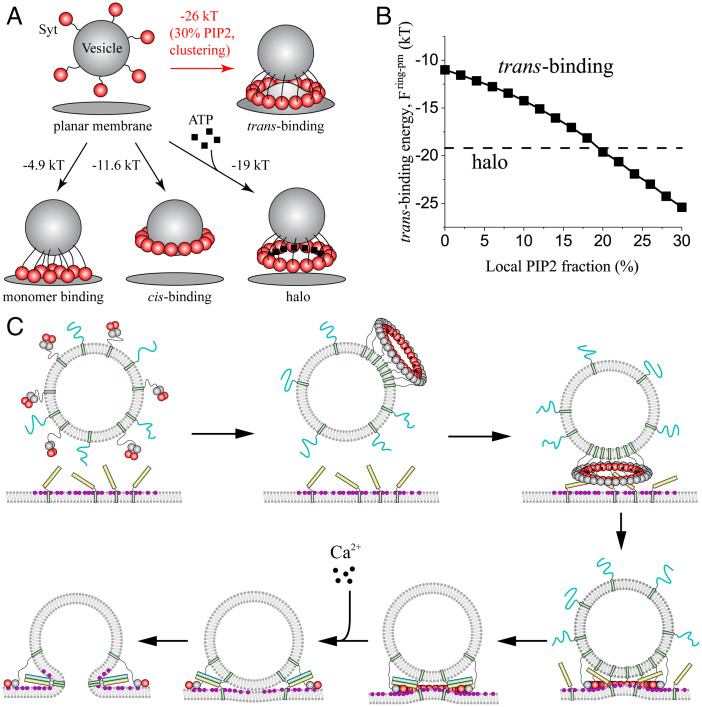
In synaptic terminals, monomers are anchored to a synaptic vesicle by their TMDs and juxtamembrane LDs (1), and mM ATP (62) is present. When a vesicle approaches the PM in an active zone, if the monomers oligomerize into a ring, the ring could bind the PM (trans-binding) as predicted by the washer hypothesis, bind the vesicle membrane (cis-binding), or exist unbound in solution (halo) (Fig. 5A).
Fig. 5.
(A) When a synaptic vesicle bearing 15 Syt molecules approaches the plasma membrane, 4 final states are possible. The model-predicted free energies per molecule are shown. Vesicle size, lipid composition, and salt conditions are as for Fig. 4. At PIP2 clustering sites with sufficiently high local PIP2 concentration, trans-binding of a Syt ring is preferred (e.g., 30% PIP2, as shown) to the ATP-stabilized halo, the cis-bound ring, or unoligomerized membrane-bound monomers. (B) Model calculations of trans-binding energy of a Syt ring to the plasma membrane versus local PIP2 concentration. For [PIP2], trans-binding is favored over the halo, while halos are preferred for [PIP2] < . (C) Model of Syt-mediated regulation of neurotransmitter release, with the Syt ring serving both as a discriminating sensor and as a spacer clamping fusion. In the ATP-rich synaptic terminal, the monomers carried by a synaptic vesicle form a halo (unbound ring) in preference to a ring bound to the PIP2-free vesicle (cis-binding). The oligomeric nature of the Syt ring endows it with high sensitivity to PIP2 levels in the plasma membrane, and trans-binding to the PM is preferred to the halo only for PIP2 content above a threshold. Thus, the Syt halo is spatially directed to dock the vesicle at PIP2-rich sites that colocalize with the t-SNARE Syntaxin (yellow). Docking positions the Syt ring to clamp fusion by spacing the vesicle and PMs and by restraining the SNARE proteins to lie partially within the Syt ring, consistent with measured crystal structures of the Syt-SNARE complex (23, 24). Ca2+ influx disassembles the ring, releasing SNAREs to fuse the membranes rapidly and release neurotransmitters through the fusion pore.
Our results so far show that Syt rings bind the PM without ATP but form halos with ATP, suggesting Syt halos are favored in ATP-rich synaptic terminals. This would seem to argue against the washer hypothesis. However, all calculations thus far assumed uniformly distributed membrane charge, neglecting the established clustering of the highly charged lipid PIP2 (a charge −4e is reported at pH 7 (63, 65)). In the PM, PIP2 clusters into -nm-diameter microdomains (66) with local PIP2 concentrations of 20 to 100% (64). Furthermore, the t-SNARE Syntaxin is thought to colocalize with PIP2 in active zones (67), suggesting that vesicle docking and synaptic release occur within these microdomains.
To account for PIP2 inhomogeneities, we next ran simulations with the PM carrying a charge density based on the local (rather than global) PIP2 concentration, modeling a vesicle near a PIP2 “hot spot” in the active zone. In our model, this is achieved by accordingly increasing the Syt-membrane binding energy parameter in the electrostatic energy [PIP2] (SI Appendix).
Since the precise value of the local [PIP2] is unknown, we ran simulations for a range of values. The ring–PM binding energy increased with increasing local [PIP2] (Fig. 5B). For , the trans-binding energy of a ring became comparable to the halo energy at [PIP2]. Given the reported local PIP2 fractions of ≥20% (64), we conclude that, consistent with the washer hypothesis, trans-binding of Syt rings is favored when vesicles approach PIP2-rich PM domains in synaptic terminals. By contrast, we predict that the halo is favored outside PIP2-rich domains. These conclusions are robust with respect to the presence of cholesterol, which considerably increases membrane bending modulus (68) since simulated trans-binding energies were increased only ∼0.3 kT by a fourfold increase of bending modulus to 100 kT (SI Appendix, Fig. S3).
The principal conclusions of this study are summarized in Fig. 5A. In ATP-rich synaptic terminals away from PIP2 microdomains, halos are favored, having free energy of ∼−19 kT. However, near a cluster with even a relatively low [PIP2] concentration of 30%, trans-binding is favored as the trans-binding energy is ∼7 kT lower than the free energy of halo formation.
Finally, our approach throughout implicitly assumed ring formation kinetics are rapid enough that the thermodynamically preferred state has time to be selected. Taking halo formation as a representative example, we developed a simple model that showed that ring assembly is fast because the polymerizing monomers are confined to the vicinity of the vesicle, yielding an estimated time for halo oligomerization of order 1 msec (SI Appendix). Given a ∼1-s timescale to replenish the readily releasable vesicle pool (69), this suggests more than sufficient time is available for Syt ring assembly during the vesicle docking process.
Discussion
Due to Their Oligomeric Nature, Syt Rings Bind Membranes with High Sensitivity to Lipid Composition and Membrane Curvature.
Here, we predict that Syt rings prefer to bind membranes with higher anionic lipid density or curvature (Fig. 4), and the preference is characterized by high sensitivity. Thus, rings bind vesicles in preference to planar membranes of the same lipid composition, but planar membranes of sufficiently high charge density are preferred, as at neuronal synapses. The transition in preference is predicted to be very sharp, i.e., Syt rings bind specific targets with high sensitivity, an effect well known from the surface adsorption energetics of high-molecular-weight synthetic polymers (70). The origin is the -fold greater binding energy of an oligomer of units, compared to one unit. Since the binding energy is compared to a fixed scale, , this translates to -fold higher sensitivity. It would be interesting to test these and other specific predictions experimentally. In vitro, these predictions might be tested by measurements of interactions of Syt-reconstituted vesicles with suspended bilayers or other vesicles, as a function of vesicle sizes and compositions.
Ca2+-Independent Binding of Syt Rings to Membranes Is Coupled to Membrane Bending.
Dome or volcano shapes were observed in electron micrographs at sites of Syt ring binding on phospholipid monolayers (43). The model developed here explained these as due to electrostatic attraction of the monolayer to the charged polylysine patches of the C2B domains lining the inner edge of the ring that overcomes the cost of membrane bending energy and bulges the monolayer upward. Thus, binding is coupled to monolayer bending. We find the same qualitative effect occurs on bilayers (Figs. 3 and 4). This mechanism is distinct from a previously proposed Ca2+-dependent Syt-mediated membrane bending mechanism, which holds that the insertion of the C2B loop leads to local bending (71).
Syt has been proposed to mechanically reshape membranes, enlarging membrane fusion pores cooperatively with SNARE complexes during Ca2+-evoked neurotransmitter release (72). In this study, the significance of the domes or volcano shapes was as a quantitative test of the model. Whether they have functional significance is unknown. A dome could bring the membrane into closer proximity to the Syt calcium binding loops to accelerate Syt ring disassembly on injection of Ca2+. However, membrane buckling was not resolved beneath docked vesicles in cryo-electron tomographic analysis of PC12 cells and cultured neurons (51, 73) or on supported monolayers when the full cytosolic domain of Syt was included (44).
Syt Halo Formation May Prepare Syt for Its Role as a Fusion Clamp and Direct Synaptic Vesicle Docking.
ATP promotes Syt ring formation in bulk solution (44). The model presented here predicts that, due to the presence of ATP at synaptic terminals, the ∼15 Syt monomers carried by a synaptic vesicle form a tethered halo in preference to a vesicle-bound ring (cis-binding). The halo ring is assembled away from the vesicle surface so that halo formation may prepare the ring for its subsequent role as a trans-binding fusion clamp. In this picture, the Syt ring is the landing gear for docking, ensuring that once the vesicle is docked, the Syt ring is ready formed and correctly positioned at the fusion site to serve as a spacer and fusion clamp.
Furthermore, we predict that the halo fails to bind phospholipid membranes whose PIP2 content is low because the halo is then energetically favored compared to a Syt ring bound in trans to the PM (Fig. 5). By contrast, in the vicinity of PIP2 clusters with local PIP2 concentrations exceeding a threshold of ∼20%, trans-binding is preferred (this threshold is likely underestimated, as divalent cations such as Mg2+ (65) or highly basic protein residues (64, 67) may partially neutralize clustered PIP2; however, local PIP2 concentrations of up to ∼80% have been measured in clusters (64)). Thus, the halo landing gear specifically directs docking to PIP2-rich sites.
These results suggest Syt ring formation may underlie a mechanism to direct the docking of vesicles to PIP2-rich active zones, the sites of vesicle fusion where functionalities of Syt and other fusion machinery components are enhanced by PIP2 content (19, 34, 40). Driven by interactions between PIP2 and polybasic residues in the Syntaxin juxtamembrane LD, Syntaxin and PIP2 colocalize in ∼70-nm clusters that may define sites of synaptic vesicle docking (66, 67). Our model predicts that vesicle docking mediated by trans-binding of the Syt ring occurs specifically at these PIP2- and Syntaxin-rich hot spots. Consistent with this picture, Syt promotes synaptic vesicle docking in Drosophila (18, 28) and chromaffin cells (31), and mutation of the Syt polylysine patch in mouse hippocampal neurons reportedly reduced the number of docked vesicles without affecting the total number of vesicles in the axon terminal (29).
Interestingly, a recent electron tomography study in mouse hippocampal neurons showed that Syt1 interacts with PIP2 to dock synaptic vesicles at a range of ∼12 nm from the active zone membrane, independently of SNAREs but dependent on the C2B polylysine patch and the active zone PIP2 level (74). These findings are consistent with the first docking event being mediated by the long reach of the Syt halo, which binds in trans at active zones of sufficiently high PIP2 density.
A Model of Syt-Mediated Regulation of Synchronous Synaptic Release.
The present results are consistent with the proposed washer mechanism (43) and suggest that Syt rings may serve not only as membrane spacers but also as sensors that help direct synaptic vesicle docking to the active zone with high PIP2 content. In this picture, the ATP-rich solution and negatively charged membranes compete for the Syt ring, a competition whose outcome depends on the PIP2 content of the membranes (Fig. 5C) (1). Prior to vesicle docking, the ATP-rich solution provides a more favorable environment than does the PIP2-free vesicle membrane so that Syt C2AB domains unbind from the vesicle and organize into the halo that will later serve as landing gear and washer (2). Once the vesicle approaches the PM, the PIP2-rich membrane provides a stronger pull for the Syt ring than does the ATP-rich solution but only when the PIP2 content exceeds a threshold. Thus, the Syt halo is spatially directed to dock at PIP2-rich sites that colocalize with other fusion components in the active zone (3). Docking positions the Syt ring to clamp fusion as a spacer between vesicle and PMs and likely by restraining the SNARE proteins via binding the primary interface identified in the Syt-SNARE complex crystal structure (23). The primary interface is positioned roughly opposite the polylysine patch, so it could bind the SNARE complex while simultaneously docking the vesicle to the PM (75). (4) When action potential–evoked Ca2+ influx disassembles the ring, the unrestrained SNAREs are free to fuse the membranes rapidly, facilitating neurotransmitter release through a fusion pore (85).
In this model, the Syt ring functions as a sensor that discriminates with high sensitivity between different levels of PIP2 and ATP. As a high-sensitivity sensor, the Syt ring has a big advantage compared to individual Syt molecules, as rings have much greater binding energies. For example, compared to the ATP-rich solution, Syt rings prefer to bind PM with 20% local PIP2 content but by a margin of only ∼ kT per Syt molecule (i.e., trans-binding is preferred to the halo; Fig. 5B). Were unoligomerized Syt molecules the sensors, this advantage would be marginal, leaving a significant fraction of molecules unbound. By contrast, a 15-monomer Syt ring enjoys a substantial 15-kT binding advantage, so rings overwhelmingly prefer to bind a domain with 20% PIP2 content.
Thus, Syt rings allow regulation to be fine-tuned; the balance between halos and target membrane-bound rings is reversed by only a small change in conditions, such as local PIP2 content or ATP concentration. This translates to superior spatial regulation, ensuring that vesicles are directed to sufficiently PIP2-rich membrane domains with high fidelity.
Materials and Methods
Protein Constructs, Expression, and Purification.
The Syt1 C2AB (residues 143 to 421) was expressed and purified using a pGEX6 vector with an N-terminal GST tag. Similar to previous studies, Escherichia coli BL21 gold (DE3) competent cells expressing Syt constructs were grown to an OD600 of ∼0.7 to 0.8, induced with 0.5 mM isopropyl β-D-1-thiogalactopyranoside (46, 76). The cells were harvested after a 3.5-h incubation at 37 °C and suspended in lysis buffer containing 25 mM Hepes (pH 7.4), 400 mM KCl, 1 mM MgCl2, 0.5 mM TCEP, 4% Triton X-100, and protease inhibitors. The samples were lysed, and the lysate was supplemented with 0.1% polyethylamine before being clarified by ultracentrifugation.
The supernatant was incubated with glutathione-Sepharose beads overnight at 4 °C. The beads were then washed with 20 mL of lysis buffer, followed by 20 mL of 25 mM Hepes and 400 mM KCl buffer containing 2 mM ATP, 10 mM MgSO4, and 1 mM DTT. Subsequently, the beads were resuspended in 5 mL of lysis buffer supplemented with 10 µg/mL DNaseI, 10 µg/mL RNaseA, and 10 µL of benzonase (2,000 units) and incubated at room temperature for 1 h, followed by a quick rinse with 10 mL of high salt buffer (25 mM Hepes, 1.1 M KCl, 1 mM DTT) to remove the nucleotide contamination. The beads were then washed with 20 mL of Hepes and 400 mM KCl buffer containing 0.5 mM EGTA to remove any trace calcium ions.
The N-terminal GST tag was cleaved by incubating with PreScission protease overnight at 4 °C (46, 76). After eluting from the affinity beads, the protein was further purified by Mono-S anionic exchange (GE Healthcare) chromatography.
ITC Measurements.
ITC experiments were performed similarly to those previously described (77). Before the experiments, the protein Syt C2AB (residues 143 to 421) was purified by gel filtration using a Superdex 75 column (GE Healthcare Life Sciences), as before (77). Peak fractions were pooled and concentrated. Syt was then dialyzed overnight at 4 °C. The concentration of the dialyzed protein was determined using the Bradford assay with bovine serum albumin (BSA) as the standard. ATP was first dissolved in the dialysis buffer to a 100 mM stock concentration, its pH was adjusted to 7.4, and it was filtered. Then ATP was diluted to ∼800 µM for ITC experiments by adding the dialysis buffer.
ITC experiments were performed with a Microcal ITC200 instrument similarly to that described before (78). Typically, about 200 µL of dialysis buffer or Syt solution was loaded into the sample cell, and about 60-µL ATP or PIP2 solution was loaded into the syringe. As described before (77, 78), ATP or PIP2 was first titrated in the dialysis buffer to make sure that no heat signal was generated, followed by titrations of ATP into Syt solution. The heat change from each injection was integrated and then normalized by the moles of protein in the injection. The Microcal Origin ITC200 software package was used to analyze the titration calorimetric data and obtain the stoichiometric number (N), the molar binding enthalpy (ΔH), and the association constant (Ka). A nonlinear least-squares fit assuming a simple one site chemical reaction was used. The equilibrium dissociation constant (KD), the binding free energy (ΔG), and the binding entropy (ΔS) were calculated using the thermodynamic equations:
Imaging of Soluble Syt1 Ring Oligomers.
Protein stock (50 µM) was diluted 10-fold in MBS (20 mM MOPS [pH 7.5], 15 mM KCl, 1 mM EGTA, 1 mM Mg(AC)2, 1 mM Mg.ATP, 1 mM DTT, 4% trehalose) at room temperature for 10 min. The diluted protein solution was further centrifuged at 10,000 × g for 10 min at 4 °C to remove large aggregates, and the supernatant (∼8 µL) was applied to a continuous carbon-coated EM grid, which was glow discharged for 10 s prior to application. After 1-min incubation, the grid was blotted dry with Whatman #1 filter paper, stained with 1% uranyl acetate, and air-dried.
Supplementary Material
Acknowledgments
We thank Shuyuan Wang and Rui Ma for valuable discussions. This work was supported by NIH Grants GM071458 (J.E.R.) and R01GM117046 (B.O.); the High Performance Computing (HPC) facilities operated by, and the staff of, the Yale Center for Research Computing; and Yale’s W. M. Keck Biotechnology Laboratory; as well as NIH Grants RR19895 and RR029676-01 that helped fund the cluster.
Footnotes
The authors declare no competing interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.2208337119/-/DCSupplemental.
Data, Materials, and Software Availability
Simulation results supporting the findings of this paper and codes to perform the simulations, to analyze the data, and to generate the technical figures are available in the Zenodo repository (https://zenodo.org/record/6547660) (86). The codes are also available in the GitHub repository (https://github.com/OShaughnessyGroup-Columbia-University/syt_ring_vesicle_docking) (87).
References
- 1.Takamori S., et al. , Molecular anatomy of a trafficking organelle. Cell 127, 831–846 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Mutch S. A., et al. , Protein quantification at the single vesicle level reveals that a subset of synaptic vesicle proteins are trafficked with high precision. J. Neurosci. 31, 1461–1470 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wilhelm B. G., et al. , Composition of isolated synaptic boutons reveals the amounts of vesicle trafficking proteins. Science 344, 1023–1028 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Chapman E. R., How does synaptotagmin trigger neurotransmitter release? Annu. Rev. Biochem. 77, 615–641 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Chapman E. R., Synaptotagmin: A Ca(2+) sensor that triggers exocytosis? Nat. Rev. Mol. Cell Biol. 3, 498–508 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Kuo W., Herrick D. Z., Ellena J. F., Cafiso D. S., The calcium-dependent and calcium-independent membrane binding of synaptotagmin 1: Two modes of C2B binding. J. Mol. Biol. 387, 284–294 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radhakrishnan A., Stein A., Jahn R., Fasshauer D., The Ca2+ affinity of synaptotagmin 1 is markedly increased by a specific interaction of its C2B domain with phosphatidylinositol 4,5-bisphosphate. J. Biol. Chem. 284, 25749–25760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paddock B. E., Striegel A. R., Hui E., Chapman E. R., Reist N. E., Ca2+-dependent, phospholipid-binding residues of synaptotagmin are critical for excitation-secretion coupling in vivo. J. Neurosci. 28, 7458–7466 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Paddock B. E., et al. , Membrane penetration by synaptotagmin is required for coupling calcium binding to vesicle fusion in vivo. J. Neurosci. 31, 2248–2257 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackler J. M., Drummond J. A., Loewen C. A., Robinson I. M., Reist N. E., The C(2)B Ca(2+)-binding motif of synaptotagmin is required for synaptic transmission in vivo. Nature 418, 340–344 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Nishiki T., Augustine G. J., Dual roles of the C2B domain of synaptotagmin I in synchronizing Ca2+-dependent neurotransmitter release. J. Neurosci. 24, 8542–8550 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishiki T., Augustine G. J., Synaptotagmin I synchronizes transmitter release in mouse hippocampal neurons. J. Neurosci. 24, 6127–6132 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yoshihara M., Littleton J. T., Synaptotagmin I functions as a calcium sensor to synchronize neurotransmitter release. Neuron 36, 897–908 (2002). [DOI] [PubMed] [Google Scholar]
- 14.Xu J., Pang Z. P., Shin O. H., Südhof T. C., Synaptotagmin-1 functions as a Ca2+ sensor for spontaneous release. Nat. Neurosci. 12, 759–766 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geppert M., et al. , Synaptotagmin I: A major Ca2+ sensor for transmitter release at a central synapse. Cell 79, 717–727 (1994). [DOI] [PubMed] [Google Scholar]
- 16.Broadie K., Bellen H. J., DiAntonio A., Littleton J. T., Schwarz T. L., Absence of synaptotagmin disrupts excitation-secretion coupling during synaptic transmission. Proc. Natl. Acad. Sci. U.S.A. 91, 10727–10731 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Littleton J. T., Stern M., Perin M., Bellen H. J., Calcium dependence of neurotransmitter release and rate of spontaneous vesicle fusions are altered in Drosophila synaptotagmin mutants. Proc. Natl. Acad. Sci. U.S.A. 91, 10888–10892 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee J., Guan Z., Akbergenova Y., Littleton J. T., Genetic analysis of synaptotagmin C2 domain specificity in regulating spontaneous and evoked neurotransmitter release. J. Neurosci. 33, 187–200 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van den Bogaart G., et al. , Synaptotagmin-1 may be a distance regulator acting upstream of SNARE nucleation. Nat. Struct. Mol. Biol. 18, 805–812 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chicka M. C., Hui E., Liu H., Chapman E. R., Synaptotagmin arrests the SNARE complex before triggering fast, efficient membrane fusion in response to Ca2+. Nat. Struct. Mol. Biol. 15, 827–835 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Voleti R., Jaczynska K., Rizo J., Ca2+-dependent release of synaptotagmin-1 from the SNARE complex on phosphatidylinositol 4,5-bisphosphate-containing membranes. eLife 9, e57154 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grushin K., et al. , Structural basis for the clamping and Ca2+ activation of SNARE-mediated fusion by synaptotagmin. Nat. Commun. 10, 2413 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhou Q., et al. , Architecture of the synaptotagmin-SNARE machinery for neuronal exocytosis. Nature 525, 62–67 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhou Q., et al. , The primed SNARE-complexin-synaptotagmin complex for neuronal exocytosis. Nature 548, 420–425 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brewer K. D., et al. , Dynamic binding mode of a Synaptotagmin-1-SNARE complex in solution. Nat. Struct. Mol. Biol. 22, 555–564 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang Z., Liu H., Gu Y., Chapman E. R., Reconstituted synaptotagmin I mediates vesicle docking, priming, and fusion. J. Cell Biol. 195, 1159–1170 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Parisotto D., Malsam J., Scheutzow A., Krause J. M., Söllner T. H., SNAREpin assembly by Munc18-1 requires previous vesicle docking by synaptotagmin 1. J. Biol. Chem. 287, 31041–31049 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reist N. E., et al. , Morphologically docked synaptic vesicles are reduced in synaptotagmin mutants of Drosophila. J. Neurosci. 18, 7662–7673 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chang S., Trimbuch T., Rosenmund C., Synaptotagmin-1 drives synchronous Ca2+-triggered fusion by C2B-domain-mediated synaptic-vesicle-membrane attachment. Nat. Neurosci. 21, 33–40 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H., Dean C., Arthur C. P., Dong M., Chapman E. R., Autapses and networks of hippocampal neurons exhibit distinct synaptic transmission phenotypes in the absence of synaptotagmin I. J. Neurosci. 29, 7395–7403 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Wit H., et al. , Synaptotagmin-1 docks secretory vesicles to syntaxin-1/SNAP-25 acceptor complexes. Cell 138, 935–946 (2009). [DOI] [PubMed] [Google Scholar]
- 32.Park Y., et al. , Controlling synaptotagmin activity by electrostatic screening. Nat. Struct. Mol. Biol. 19, 991–997 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park Y., et al. , Synaptotagmin-1 binds to PIP(2)-containing membrane but not to SNAREs at physiological ionic strength. Nat. Struct. Mol. Biol. 22, 815–823 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lauwers E., Goodchild R., Verstreken P., Membrane lipids in presynaptic function and disease. Neuron 90, 11–25 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Zarebidaki F., et al. , Disentangling the roles of RIM and Munc13 in synaptic vesicle localization and neurotransmission. J. Neurosci. 40, 9372–9385 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han Y., Kaeser P. S., Südhof T. C., Schneggenburger R., RIM determines Ca2+ channel density and vesicle docking at the presynaptic active zone. Neuron 69, 304–316 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mohrmann R., et al. , Synaptotagmin interaction with SNAP-25 governs vesicle docking, priming, and fusion triggering. J. Neurosci. 33, 14417–14430 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milosevic I., et al. , Plasmalemmal phosphatidylinositol-4,5-bisphosphate level regulates the releasable vesicle pool size in chromaffin cells. J. Neurosci. 25, 2557–2565 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Di Paolo G., et al. , Impaired PtdIns(4,5)P2 synthesis in nerve terminals produces defects in synaptic vesicle trafficking. Nature 431, 415–422 (2004). [DOI] [PubMed] [Google Scholar]
- 40.de Jong A. P. H., et al. , RIM C2B domains target presynaptic active zone functions to PIP2-containing membranes. Neuron 98, 335–349.e7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bai J., Tucker W. C., Chapman E. R., PIP2 increases the speed of response of synaptotagmin and steers its membrane-penetration activity toward the plasma membrane. Nat. Struct. Mol. Biol. 11, 36–44 (2004). [DOI] [PubMed] [Google Scholar]
- 42.van den Bogaart G., Meyenberg K., Diederichsen U., Jahn R., Phosphatidylinositol 4,5-bisphosphate increases Ca2+ affinity of synaptotagmin-1 by 40-fold. J. Biol. Chem. 287, 16447–16453 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang J., et al. , Calcium sensitive ring-like oligomers formed by synaptotagmin. Proc. Natl. Acad. Sci. U.S.A. 111, 13966–13971 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zanetti M. N., et al. , Ring-like oligomers of Synaptotagmins and related C2 domain proteins. eLife 5, e17262 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lu B., Kiessling V., Tamm L. K., Cafiso D. S., The juxtamembrane linker of full-length synaptotagmin 1 controls oligomerization and calcium-dependent membrane binding. J. Biol. Chem. 289, 22161–22171 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang J., et al. , Circular oligomerization is an intrinsic property of synaptotagmin. eLife 6, e27441 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bello O. D., et al. , Synaptotagmin oligomerization is essential for calcium control of regulated exocytosis. Proc. Natl. Acad. Sci. U.S.A. 115, E7624–E7631 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tagliatti E., et al. , Synaptotagmin 1 oligomers clamp and regulate different modes of neurotransmitter release. Proc. Natl. Acad. Sci. U.S.A. 117, 3819–3827 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ramakrishnan S., Bera M., Coleman J., Rothman J. E., Krishnakumar S. S., Synergistic roles of Synaptotagmin-1 and complexin in calcium-regulated neuronal exocytosis. eLife 9, e54506 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ramakrishnan S., et al. , Synaptotagmin oligomers are necessary and can be sufficient to form a Ca(2+) -sensitive fusion clamp. FEBS Lett. 593, 154–162 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Li X., et al. , Symmetrical organization of proteins under docked synaptic vesicles. FEBS Lett. 593, 144–153 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacobs C. R., Huang H., Kwon R. Y., Introduction to Cell Mechanics and Mechanobiology (Garland Science, 2013). [Google Scholar]
- 53.Kroll D. M., Gompper G., The conformation of fluid membranes: Monte Carlo simulations. Science 255, 968–971 (1992). [DOI] [PubMed] [Google Scholar]
- 54.Yazdani A., Bagchi P., Three-dimensional numerical simulation of vesicle dynamics using a front-tracking method. Phys. Rev. E Stat. Nonlin. Soft Matter Phys. 85, 056308 (2012). [DOI] [PubMed] [Google Scholar]
- 55.Sreeja K. K., Ipsen J. H., Kumar P. B. S., Monte Carlo simulations of fluid vesicles. J. Phys. Condens. Matter 27, 273104 (2015). [DOI] [PubMed] [Google Scholar]
- 56.Ou-Yang Z., Helfrich W., Bending energy of vesicle membranes: General expressions for the 1st, 2nd, and 3rd variation of the shape energy and applications to spheres and cylinders. Phys. Rev. A 39, 5280–5288 (1989). [DOI] [PubMed] [Google Scholar]
- 57.Watanabe K., Belyaev A. G., Detection of salient curvature features on polygonal surfaces. Computer Graph. Forum 20 (2001). [Google Scholar]
- 58.Doi M., Edwards S. F., The Theory of Polymer Dynamics (Clarendon Press, 1986). [Google Scholar]
- 59.Evans D. F., Wennerström H.,The Colloidal Domain: Where Physics, Chemistry, Biology, and Technology Meet (1999). [Google Scholar]
- 60.Donaldson S. H. Jr., et al. , Developing a general interaction potential for hydrophobic and hydrophilic interactions. Langmuir 31, 2051–2064 (2015). [DOI] [PubMed] [Google Scholar]
- 61.Parker J. L., Claesson P. M., Attard P., Bubbles, cavities, and the long-ranged attraction between hydrophobic surfaces. J Phys Chem-Us 98, 8468–8480 (1994). [Google Scholar]
- 62.Rangaraju V., Calloway N., Ryan T. A., Activity-driven local ATP synthesis is required for synaptic function. Cell 156, 825–835 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.McLaughlin S., Wang J., Gambhir A., Murray D., PIP(2) and proteins: Interactions, organization, and information flow. Annu. Rev. Biophys. Biomol. Struct. 31, 151–175 (2002). [DOI] [PubMed] [Google Scholar]
- 64.Martin T. F., PI(4,5)P2-binding effector proteins for vesicle exocytosis. Biochim. Biophys. Acta 1851, 785–793 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ellenbroek W. G., et al. , Divalent cation-dependent formation of electrostatic PIP2 clusters in lipid monolayers. Biophys. J. 101, 2178–2184 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.van den Bogaart G., et al. , Membrane protein sequestering by ionic protein-lipid interactions. Nature 479, 552–555 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Honigmann A., et al. , Phosphatidylinositol 4,5-bisphosphate clusters act as molecular beacons for vesicle recruitment. Nat. Struct. Mol. Biol. 20, 679–686 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dimova R., Recent developments in the field of bending rigidity measurements on membranes. Adv. Colloid Interface Sci. 208, 225–234 (2014). [DOI] [PubMed] [Google Scholar]
- 69.Rizzoli S. O., Betz W. J., Synaptic vesicle pools. Nat. Rev. Neurosci. 6, 57–69 (2005). [DOI] [PubMed] [Google Scholar]
- 70.De Gennes P.-G., Scaling Concepts in Polymer Physics (Cornell University Press, 1979). [Google Scholar]
- 71.Wu Z., Schulten K., Synaptotagmin’s role in neurotransmitter release likely involves Ca(2+)-induced conformational transition. Biophys. J. 107, 1156–1166 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wu Z., Dharan N., McDargh Z. A., Thiyagarajan S., O'Shaughnessy B., Karatekin E., The neuronal calcium sensor Synaptotagmin-1 and SNARE proteins cooperate to dilate fusion pores. eLife 10:e68215, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Radhakrishnan A., et al. , Symmetrical arrangement of proteins under release-ready vesicles in presynaptic terminals. Proc. Natl. Acad. Sci. U.S.A. 118, e2024029118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Chen Y., et al. , Synaptotagmin-1 interacts with PI(4,5)P2 to initiate synaptic vesicle docking in hippocampal neurons. Cell Rep. 34, 108842 (2021). [DOI] [PubMed] [Google Scholar]
- 75.Rothman J. E., Krishnakumar S. S., Grushin K., Pincet F., Hypothesis—Buttressed rings assemble, clamp, and release SNAREpins for synaptic transmission. FEBS Lett. 591, 3459–3480 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Li F., et al. , A half-zippered SNARE complex represents a functional intermediate in membrane fusion. J. Am. Chem. Soc. 136, 3456–3464 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cho R. W., et al. , Genetic analysis of the complexin trans-clamping model for cross-linking SNARE complexes in vivo. Proc. Natl. Acad. Sci. U.S.A. 111, 10317–10322 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li F., Tiwari N., Rothman J. E., Pincet F., Kinetic barriers to SNAREpin assembly in the regulation of membrane docking/priming and fusion. Proc. Natl. Acad. Sci. U.S.A. 113, 10536–10541 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hochmuth F. M., Shao J. Y., Dai J., Sheetz M. P., Deformation and flow of membrane into tethers extracted from neuronal growth cones. Biophys. J. 70, 358–369 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dai J., Sheetz M. P., Wan X., Morris C. E., Membrane tension in swelling and shrinking molluscan neurons. J. Neurosci. 18, 6681–6692 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Stanich C. A., et al. , Coarsening dynamics of domains in lipid membranes. Biophys. J. 105, 444–454 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Fuller N., Benatti C. R., Rand R. P., Curvature and bending constants for phosphatidylserine-containing membranes. Biophys. J. 85, 1667–1674 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kollmitzer B., Heftberger P., Rappolt M., Pabst G., Monolayer spontaneous curvature of raft-forming membrane lipids. Soft Matter 9, 10877–10884 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Bouvrais H., Duelund L., Ipsen J. H., Buffers affect the bending rigidity of model lipid membranes. Langmuir 30, 13–16 (2014). [DOI] [PubMed] [Google Scholar]
- 85.Mostafavi H., et al. , Entropic forces drive self-organization and membrane fusion by SNARE proteins. Proc. Natl. Acad. Sci. U.S.A 114, 5455–5460 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhu T., OShaughnessyGroup-Columbia-University/syt_ring_vesicle_docking_fusion: syt_ring_vesicle_docking_fusion. Zenodo. https://zenodo.org/record/6547660#.Yv4UehxByUk. Deposited 13 May 2022.
- 87.Zhu T., OShaughnessyGroup-Columbia-University/syt_ring_vesicle_docking. GitHub. https://github.com/OShaughnessyGroup-Columbia-University/syt_ring_vesicle_docking. Deposited 11 May 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Simulation results supporting the findings of this paper and codes to perform the simulations, to analyze the data, and to generate the technical figures are available in the Zenodo repository (https://zenodo.org/record/6547660) (86). The codes are also available in the GitHub repository (https://github.com/OShaughnessyGroup-Columbia-University/syt_ring_vesicle_docking) (87).