Abstract
Purpose
Left atrial thrombus (LAT)/left atrial spontaneous echo contrast (LASEC) still exists in CHA2DS2-VASc score-defined low/borderline risk population. The purpose of this study is to explore the risk factors that associate with LAT/SEC and to create a nomogram to predict LAT/SEC risk in NVAF patients with low/borderline CHA2DS2-VASc scores.
Patients and Methods
A total of 834 NVAF patients with complete data on transesophageal echocardiography (TEE) were included in this study. Univariate and multivariate logistic regression analyses were performed to identify the risk factors that associate with LAT/SEC, and a nomogram was established based on the results. Receiver operating characteristic curve (ROC), calibration curve and decision curve analysis were performed to verify the predictive power of nomogram.
Results
The rates for LAT/SEC for the training and validation cohorts were 84 (14.7%) and 30 (11.4%), respectively. Independent factors including age, left ventricular ejection fraction (LVEF), left atrial diameter (LAD), smoke, non-paroxysmal AF (NPAF), and E/e’ were considered to construct the nomogram for LAT/SEC. The AUC for nomogram was 0.839 and 0.811 in the training and validation cohorts, respectively. The calibration and decision curve analysis showed that the nomogram had a good prediction capacity and would be clinically useful.
Conclusion
Age, LVEF, LAD, smoke, NPAF, and E/e’ are independently associated with LAT/SEC in NVAF patients with low/borderline CHA2DS2-VASc scores. The nomogram that incorporates these six variables effectively predict LAT/SEC risk in NVAF patients with low/borderline CHA2DS2-VASc scores.
Keywords: left atrial thrombus, spontaneous echo contrast, atrial fibrillation, nomogram, CHA2DS2-VASc score
Introduction
Atrial fibrillation (AF), one of the most common arrhythmia, is increasingly reported to associate with lethal cardiovascular conditions such as cardioembolic stroke which is a subtype of ischemic stroke with poor prognosis compared to other ischemic stroke subtypes.1 Besides, AF is also found to be associated with a higher in-hospital mortality both in cardioembolic and atherothrombotic stroke patients.2 Several risk factors have been reported to be associated with AF and AF-related coronary vascular disease (CVD) complications. Of these risk factors, left atrial thrombus (LAT) and left atrial spontaneous echo contrast (LASEC) have been stated as significant factors that associate with cardiogenic embolism in AF.3–5
The CHA2DS2-VASc score is used to determine future stroke risk and guide anticoagulant therapy for non-valvular atrial fibrillation (NVAF) patients. Recently, the CHA2DS2-VASc score has also been reported as a significant independent predictor of prosthetic valve thrombosis (PVT) in patients with prosthetic valve.6 Current guidelines recommend anticoagulation as a class Ia indication for all AF individuals with 1 non-sex-related CHA2DS2-VASc risk factor.7 Interestingly, patients with low/borderline CHA2DS2-VASc scores have been found to experience LAT/SEC.8,9 Therefore, it would be of great clinical significance to identify the high-risk subgroup among the CHA2DS2-VASc score-defined low/borderline stroke risk population. A powerful model can help estimate LAT/SEC presence among AF patients with low/borderline CHA2DS2-VASc scores and aid clinicians in identifying high-risk patients, thereby leading to a more tailored treatment choice.
In the past, efforts on the prediction of LAT/SEC have been made10–12 but, none of these studies focused on AF individuals with low/borderline CHA2DS2-VASc scores. Indeed, transesophageal echocardiography (TEE) has been considered as the gold standard for diagnosing LA/LAA thrombus. Nonetheless, TEE is often intolerable without conscious sedation and sometimes accompanied by a risk of complications which limits its clinical applications. Besides, as an invasive test, it requires special skills and interpretation. Therefore, a potentially non-invasive and effective risk stratifying model that can be used to detect LAT/SEC would be of crucial importance in clinical settings.
The objectives of this study were to (1) identify the risk factors of LAT/SEC and (2) establish a nomogram to estimate LAT/SEC risk in NVAF patients with low/borderline CHA2DS2-VASc scores.
Methods
Study Participants
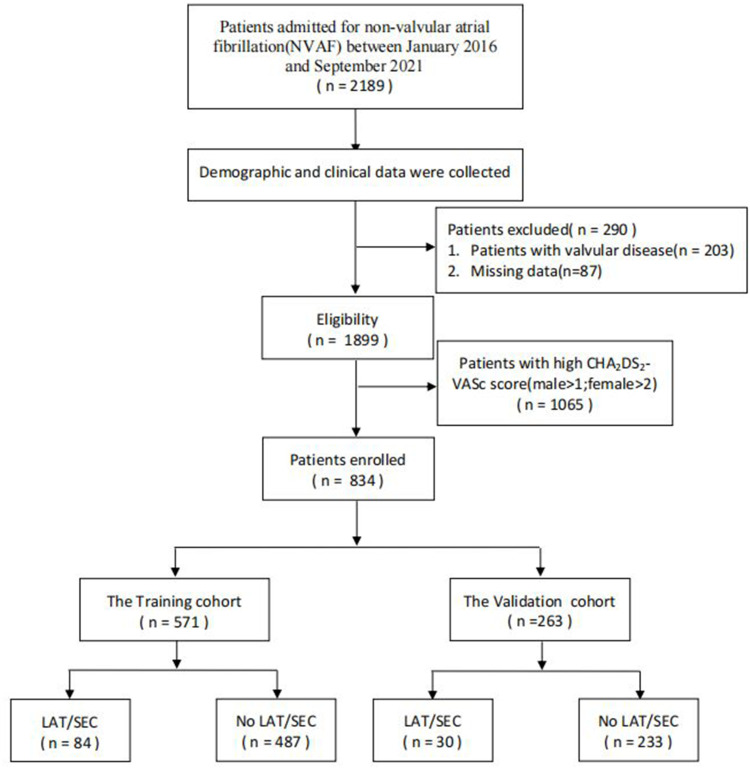
A total of 2189 consecutive NVAF patients were retrospectively enrolled in the First Affiliated Hospital of Dalian Medical University (FAHDMU) from January 2016 to September 2021. Patients who were referred for direct current cardioversion or catheter ablation who underwent TEE were included in the study. Of the recorded patients, those with organic valvular heart diseases and congenital heart diseases were excluded. Likewise, patients with missing/incomplete data were excluded. Finally, 834 patients with low/borderline CHA2DS2-VASc scores (male ≤1; female ≤2) were included in this study. The flow chart of the included patients is summarized in Figure 1. The study was approved by the institutional review board of FAHDMU and the requirement for informed consent was waived. The research was conducted according to the Helsinki declaration guidelines and all procedures were carried out in accordance with the approved guidelines.
Figure 1.
The overview of the selection of study participants.
Abbreviation: LAT/SEC, left atrial thrombus/spontaneous echo contrast.
Definition of the Explanatory Variables
Demographic, clinical, and laboratory data were retrieved from the electronic medical record of FAHDMU. Diabetes mellitus (DM) was defined as serum fasting blood glucose level ≥126 mg/dL, use of antidiabetic medication or previous diagnosis by a physician.13 Coronary artery disease (CAD) was defined if patients have any of the following characteristics: history of physician-diagnosed CAD, use of medications for CAD, the presence of ≥50% luminal stenosis in at least one major coronary artery in coronary angiography.14 Hypertension (HTN) was defined as systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg at two or more visits or a history of hypertension and use of anti-hypertensive drugs.15 Dyslipidemia was defined as having one or more of the following conditions: TC ≥6.22 mmol/L (240 mg/dL), LDL-C ≥4.14 mmol/L (160 mg/dL), HDL-C ≤1.04 mmol/L (40 mg/dL), TG ≥2.26 mmol/L (200 mg/dL), or use of anti-dyslipidemia medication.16 The left ventricular mass index (LVMI) was assessed based on the definition of the American Society of Echocardiography (ASE); briefly, LVMI >115 g/m2 for men and >95 g/m2 for women was considered as left ventricular hypertrophy (LVH).17 AF was defined and classified according to the previously published guideline. Briefly, non-paroxysmal AF (NPAF) was composed of persistent, long-standing persistent, and permanent AF.7
Assessment of CHA2DS2-VASc Score and Risk Classification
The CHA2DS2-VASc score was evaluated according to previous description.18 According to current guidelines, men with a CHA2DS2-VASc score of 2 or greater or women with a CHA2DS2-VASc score of 3 or greater were categorized into high-risk patients. Men with a CHA2DS2-VASc score of 1 or women with a CHA2DS2-VASc score of 2 were considered as borderline risk groups. Men with a CHA2DS2-VASc score of 0 or women with a CHA2DS2-VASc score of 0–1 were classified as low-risk groups.7
Transesophageal Echocardiography Evaluation
TEE was performed before catheter ablation or direct current cardioversion for all patients as described in a previous study.19 Prior to the TEE examination, the details of the procedure were explained for every patient, and all patients provided written informed consent. Thrombus was defined as a circumscribed, uniformly echo dense mass distinct from the underlying left atrial endocardium and pectinate muscles detected in more than 1 imaging plane. Spontaneous echocardiographic contrast (SEC) was defined as dynamic “smoke-like” echoes with a characteristic swirling motion that could not be eliminated despite optimized gain settings.3 All the procedures were performed by well-trained observers who were blind to the study.
Statistical Analysis
All statistical analyses were performed using R software, version 4.0. Continuous data with a normally distributed nature were compared using Student’s t-test and results were expressed as mean and standard deviation (SD). These variables with a skewed distribution were compared using the Mann–Whitney test and expressed as the median and interquartile range (IQR). The categorical data were presented as frequencies and percentages and analyzed by χ2 test or Fisher's exact test.
To identify the independent risk factors associated with LAT/SEC, we run a univariate logistic regression analysis. We further performed stepwise multivariate analysis for all the variables that have shown significant association with LAT/SEC with P-value <0.1 in univariate analyses. Then, a nomogram was constructed by incorporating the independent risk factors in the multivariate logistic regression analysis. The nomogram was established by assigning each variable a risk score. The points of the variables were added up to calculate the total points, which were eventually transformed to LAT/SEC probabilities. The area under curve (AUC) was computed to evaluate the predictive performance of the nomogram. To decrease the overfit bias, calibration with 1000 bootstrap samples was executed. We also conducted the decision curve analysis to evaluate the clinical utility of the nomogram using the R library rmda package.
Receiver operating characteristic curve analysis was performed and the optimal cutoff values were calculated according to the Youden index (sensitivity + specificity-1). We finally evaluated the predictive values, likelihood ratios, sensitivity, and specificity to observe the accuracy of the optimal cutoff value. P-value <0.05 was considered statistically significant.
Results
A total of 834 NVAF patients were enrolled in the study. The patients were divided into the training (571, 68.5%) and validation cohorts (263, 31.5%). There was no significant difference in the baseline data between the two cohorts. LAT/SEC was found in 84 (14.7%) and 30 (11.4%) in the training and validation cohorts, respectively. The baseline characteristics of the patients are described in Table 1.
Table 1.
Baseline Characteristics
| Variable | Cohort | P value | ||
|---|---|---|---|---|
| Overall (n=834) | Training (n=571) | Validation (n=263) | ||
| Age, years | 60(53–64) | 60 (53–63) | 60 (53–64) | 0.488 |
| Male, n (%) | 561(67.3) | 390 (68.3) | 171 (65.0) | 0.390 |
| Smoke, n (%) | 137(16.4) | 93(16.3) | 44(16.7) | 0.952 |
| Drink, n (%) | 97(11.6) | 61(10.7) | 36(13.7) | 0.254 |
| DBP, mmHg | 83(74–90) | 82(75–90) | 84(74–90) | 0.801 |
| SBP, mmHg | 128(116–140) | 128(117–140) | 128(116–140) | 0.874 |
| Medical history | ||||
| HTN, n (%) | 228(27.3) | 157 (27.5) | 71 (27.0) | 0.947 |
| DM, n (%) | 34(4.1) | 23 (4.0) | 11 (4.2) | 1.000 |
| Previous stroke /TIA, n (%) | 0(0) | 0(0) | 0(0) | – |
| Vascular disease, n (%) | 2(0.2) | 2 (0.4) | 0 (0) | 0.842 |
| Dyslipidemia, n (%) | 455(54.6) | 311(54.5) | 144(54.8) | 0.998 |
| NPAF, n (%) | 481(57.7) | 339 (59.4) | 142 (54.0) | 0.166 |
| CAD, n (%) | 93(11.2) | 64 (11.2) | 29 (11.0) | 1.000 |
| CHF, n (%) | 57(6.8) | 47(8.2) | 10(3.8) | 0.027 |
| CHA2DS2-VASc Score | 1(0–1) | 1(0–1) | 1(0–1) | 0.510 |
| Laboratory data | ||||
| Uric Acid, μmol/L | 363(304–430) | 364(305–431) | 359(304–423) | 0.718 |
| eGFR, mL/(min ▪1.73 m2) | 91(81–102) | 91 (80–102) | 91 (82–102) | 0.955 |
| PT-INR | 1.07(1.00–1.24) | 1.07 (1.00–1.24) | 1.06 (1.01–1.25) | 0.667 |
| D-dimer, μg/L | 120(90–230) | 120(90–240) | 130(90–230) | 0.765 |
| Fibrinogen, g/L | 2.56(2.28–2.88) | 2.56(2.28–2.85) | 2.56(2.29–2.92) | 0.889 |
| Echocardiographic parameters | ||||
| LAT/SEC, n (%) | 114(13.7) | 84 (14.7) | 30 (11.4) | 0.237 |
| LAD, mm | 39(37–43) | 39 (37–43) | 39 (37–43) | 0.997 |
| LVEDD, mm | 48(44–50) | 48(44–50) | 48 (45–51) | 0.702 |
| LVEF, % | 58(55–59) | 58 (55–59) | 58(55–59) | 0.650 |
| E/e’ | 7.7(6.0–9.8) | 7.5(6.0–9.6) | 8.0(6.0–10.0) | 0.119 |
| LVMI, g/m2 | 87.7(78.0–101.5) | 87.1(77.5–101.8) | 88.5(78.8–100.6) | 0.419 |
| LVH, n (%) | 138(16.5) | 90(15.8) | 48(18.3) | 0.425 |
| Medication | ||||
| Amiodarone, n (%) | 574(68.8) | 387(67.8) | 187(71.1) | 0.377 |
| Antiplatelet, n (%) | 45(5.4) | 28(4.9) | 17(6.5) | 0.446 |
| Warfarin | 245(29.4) | 163(28.5) | 82(31.2) | 0.488 |
| NOAC | 589(70.6) | 408(71.5) | 181(68.8) | 0.488 |
| Diuretic | 104(12.5) | 75(13.1) | 29(11.0) | 0.457 |
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; DBP, diastolic blood pressure; DM, diabetes mellitus; HTN, hypertension; LAD, left atrium diameter; LAT, left atrial thrombus; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; NOAC, new oral anticoagulants; NPAF, non-paroxysmal AF; SBP, systolic blood pressure; SEC, spontaneous echo contrast; TIA, transient ischemic attack.
Table 2 shows the results of the univariate and multivariate logistic regression analyses. Patients with LAT/SEC were older and had a higher prevalence of congestive heart failure (CHF), non-paroxysmal AF (NPAF), left ventricular hypertrophy (LVH), left ventricular end-diastolic diameter (LVEDD) and larger left atrial diameter (LAD). Likewise, the LAT/SEC group had higher values of E/e’, PT-INR, but a lower left ventricular ejection fraction (LVEF). However, there was no significant difference in the prevalence of hypertension, dyslipidemia, DM, and CAD between the two cohorts. The multivariate analysis showed that age (OR 1.08 95% CI 1.04–1.12, P = 0.001), LAD (OR 1.16 95% CI 1.09–1.24, P = 0.001), LVEF (OR 0.95 95% CI 0.91–0.98, P = 0.005), E/e’ (OR 1.13 95% CI 1.06–1.21, P = 0.001), smoke (OR 2.46 95% CI 1.25–4.78, P = 0.008) and NPAF (OR 5.99 95% CI 2.77–14.67, P = 0.001) were independently associated with LAT/SEC.
Table 2.
Univariate and Multivariate Logistic Regression Analysis of LAT/SEC Presence Based on Peri-Procedural Data in the Training Cohort
| Variable | Univariate Analysis | Multivariate Analysis | ||
|---|---|---|---|---|
| OR(95% CI) | P value | OR(95% CI) | P value | |
| Age, years | 1.04(1.01–1.08) | 0.007 | 1.08(1.04–1.12) | 0.001 |
| Male sex | 0.92(0.56–1.52) | 0.727 | ||
| Smoke | 1.92(1.09–3.30) | 0.021 | 2.46(1.25–4.78) | 0.008 |
| Drink | 2.31(1.21–4.25) | 0.009 | ||
| DBP | 1.02(1.00–1.04) | 0.037 | ||
| SBP | 1.01(0.99–1.02) | 0.393 | ||
| Medical history | ||||
| HTN | 1.06(0.63–1.76) | 0.811 | ||
| DM | 0.25(0.01–1.24) | 0.184 | ||
| Dyslipidemia | 1.35(0.84–2.18) | 0.214 | ||
| NPAF | 6.20(3.27–13.04) | 0.001 | 5.99(2.77–14.67) | 0.001 |
| CAD | 1.39(0.68–2.66) | 0.335 | ||
| CHF | 3.46(1.76–6.59) | 0.001 | ||
| Laboratory data | ||||
| UA | 1.01(0.99–1.02) | 0.053 | ||
| eGFR, mL/(min ▪1.73 m2) | 0.99(0.98–1.01) | 0.447 | ||
| PT-INR | 1.37(1.04–1.81) | 0.024 | ||
| D-D | 1.01(0.98–1.02) | 0.236 | ||
| Fibrinogen | 1.02(0.67–1.51) | 0.924 | ||
| Echocardiographic parameters | ||||
| LAD, mm | 1.19(1.13–1.26) | 0.001 | 1.16(1.09–1.24) | 0.001 |
| LVEDD, mm | 1.08(1.03–1.13) | 0.001 | ||
| LVEF, % | 0.94(0.92–0.97) | 0.001 | 0.95(0.91–0.98) | 0.005 |
| E/e’ | 1.15(1.09–1.22) | 0.001 | 1.13(1.06–1.21) | 0.001 |
| LVMI | 1.02(1.01–1.03) | 0.001 | ||
| LVH | 2.36(1.35–4.04) | 0.002 | ||
| Medication | ||||
| Amiodarone | 1.00(0.62–1.67) | 0.986 | ||
| Antiplatelet | 2.46(1.05–5.78) | 0.039 | ||
| Warfarin | 0.81(0.47–1.36) | 0.436 | ||
| NOAC | 1.23(0.74–2.14) | 0.436 | ||
| Diuretic | 2.06(1.12–3.65) | 0.017 | ||
Abbreviations: CAD, coronary artery disease; CHF, congestive heart failure; DBP, diastolic blood pressure; DM, diabetes mellitus; HTN, hypertension; LAD, left atrium diameter; LAT, left atrial thrombus; LVEDD, left ventricular end diastolic diameter; LVEF, left ventricular ejection fraction; LVH, left ventricular hypertrophy; LVMI, left ventricular mass index; NOAC, new oral anticoagulants; NPAF, non-paroxysmal AF; SBP, systolic blood pressure; SEC, spontaneous echo contrast; TIA, transient ischemic attack.
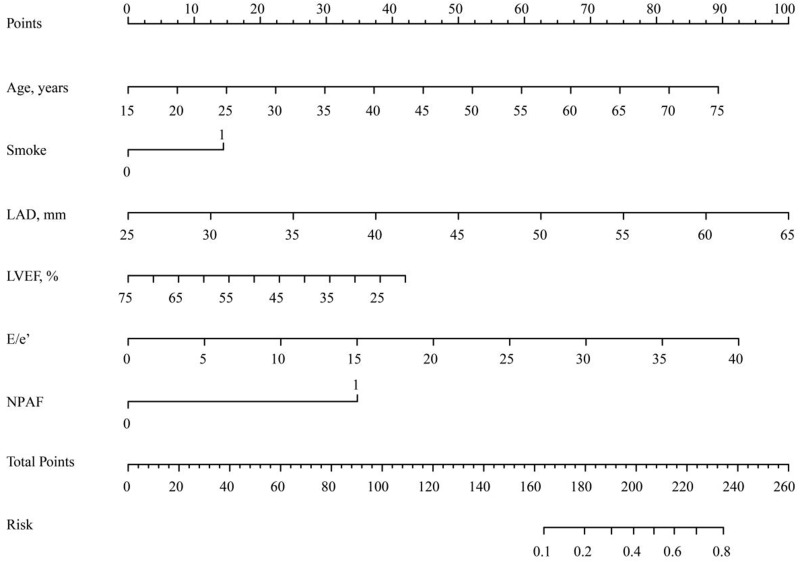
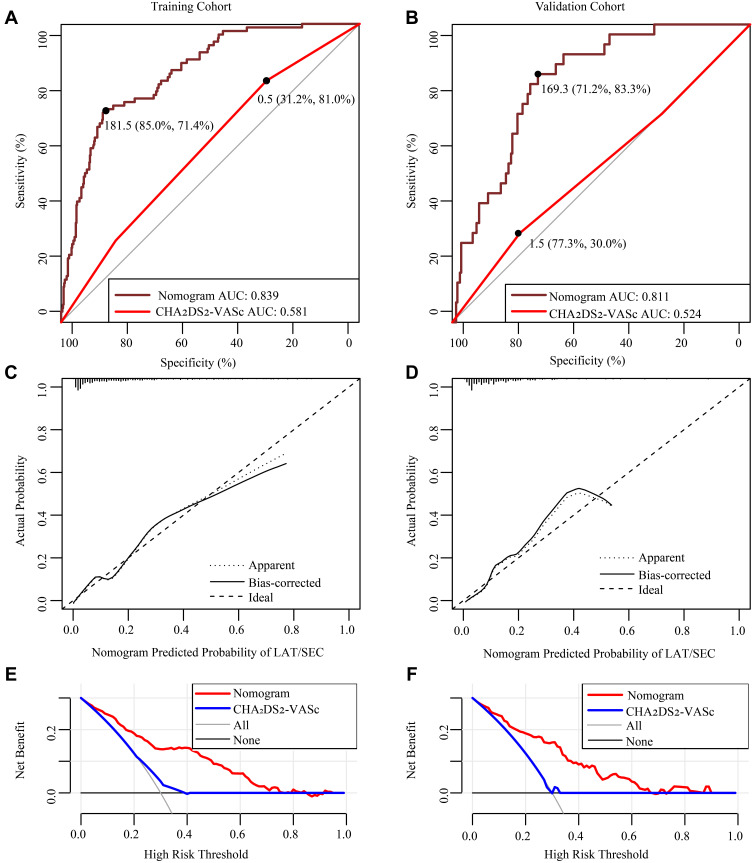
Initially, we evaluated the predictive capacity of the CHA2DS2-VASc score in terms of LAT/SEC using ROC curve analysis. The results demonstrated a AUC of 0.581 for the CHA2DS2-VASc score. Furthermore, we developed a nomogram to predict LAT/SEC according to the results of the multivariate logistic analysis (Figure 2). The nomogram had a good discriminative performance with an AUC of 0.839. Moreover, the AUC of the nomogram for LAT/SEC risk was 0.811 in the validation cohort. Also, the nomogram showed a good calibration curve for the risk estimation in both training and validation cohort (Figure 3A–D). Importantly, the analysis of the final decision curve displayed that the nomogram had a higher net benefit than CHA2DS2-VASc scores at a threshold probability of 0.1–0.8 (Figure 3E and F).
Figure 2.
Nomogram for Estimation of LAT/SEC Risk in NVAF patients with low to borderline CHA2D2VASc score.
Abbreviations: LAD, left atrium diameter; LVEF, left ventricular ejection fraction; NPAF, non-paroxysmal AF.
Figure 3.
Model-comparison results of the nomogram and CHA2DS2-VASc score for estimating the risk of LAT/SEC. (A and B) Receiver operating characteristic curve for models in predicting LAT/SEC in the training cohort and validation cohort. (C and D) Calibration curves for the nomogram in the training cohort and validation cohort. The dotted line represents the entire cohort, the solid line is the result after bias correction by bootstrapping (1000 repetitions), indicating nomogram performance (boot mean absolute error=0.019 and 0.028, respectively). (E and F) Decision curve analysis for the nomogram and CHA2DS2-VASc score in the training cohort and validation cohort. The decision curve of the nomogram is composed of an X-axis which represents continuum of potential thresholds for LAT/SEC risk and a Y-axis which represents the net benefit. The horizontal black line: to assume no patients will experience the event; the light gray line: to assume all patients will experience the event. The nomogram had a higher net benefit than CHA2DS2-VASc scores at a threshold probability of 0.1–0.8.
Abbreviation: AUC, area under ROC curve.
The optimal cutoff value of the sum scores of the constructed nomogram was 181.5. The sensitivity, specificity, positive predictive value, and negative predictive value were 71.4%, 85.0%, 45.1%, and 94.5%, respectively, in the training cohort, and 83.3%, 71.2%, 27.2%, and 97.1%, respectively, in the validation cohort (Table 3).
Table 3.
Comparison of Predictive Accuracy Between the Nomogram and CHA2DS2-VASc Score for Estimating the Risk of LAT/SEC
| Variable | Nomogram | CHA2DS2-VASc Score | ||
|---|---|---|---|---|
| Training Cohort | Validation Cohort | Training Cohort | Validation Cohort | |
| AUC | 0.84 | 0.81 | 0.58 | 0.52 |
| Cutoff score | 181.45 | 169.26 | 0.5 | 1.5 |
| Specificity, % | 85.01 | 71.24 | 31.21 | 77.25 |
| Sensitivity, % | 71.43 | 83.33 | 80.95 | 30.00 |
| NPV, % | 94.52 | 97.08 | 90.48 | 89.55 |
| PPV, % | 45.11 | 27.17 | 16.87 | 14.52 |
Abbreviations: AUC, area under ROC curve; LAT/SEC, left atrial thrombus/spontaneous echo contrast; NPV, negative predictive value; PPV, positive predictive value.
Discussion
In this study, we demonstrated that age, smoke, LAD, LVEF, E/e’, and NPAF were closely associated with LAT/SEC and a nomogram composed of these variables was established to estimate LAT/SEC risk in NVAF patients with low/borderline CHA2DS2-VASc scores. The new model performed better in terms of LAT/SEC prediction compared to CHA2DS2-VASc score, suggesting the model can be used as a noninvasive parameter in predicting the risk of LAT/SEC in NVAF patients with low to borderline CHA2DS2-VASc scores.
LAT/SEC prevalence among AF patients with low/borderline CHA2DS2-VASc scores has been varied across different studies.5,8,20 In the present study, the LAT/SEC prevalence was 13.7%. Furthermore, we noticed that LAD, E/e’, and NPAF were the top three predictors of LAT/SEC. Importantly, these variables are not included in the CHA2DS2-VASc score. Therefore, among NVAF patients with low/borderline CHA2DS2-VASc scores, more intensive follow-up for patients with the enlarged left atrium, higher E/e’, and NPAF type is highly recommended. Significant association between these factors and LAT/SEC was previously reported.21–23 Left atrial (LA) dilatation characterized by the LA structural remodeling has been found to be associated with LAT/SEC, which is a classic stroke risk factor.10,21 The plausible mechanism for the LAT/SEC involves changes in hemodynamics caused by enlarged left atrial, including turbulences, increased blood stasis, decreased flow velocity and endothelial injury.24
In the present study, NPAF remained a significant risk factor of LAT/SEC, a consistent finding with other studies.22,25 However, the current ESC guidelines do not list AF type as a factor of LAA thrombus formation. AF usually starts as paroxysmal AF and transforms into NPAF.26 In the past, persistent AF has been reported to be associated with the existence of an advanced atrial structural, electrical remodeling, and fibroelastosis of the LA/LAA, all of which predispose patients to thrombosis.27,28
E/e’, a parameter to evaluate LV diastolic function,29 can be used as a surrogate marker of the left atrial pressure.30 The possible explanation for the relationship between LV diastolic function and left atrial pressure completes a vicious circle. An elevated left atrial pressure due to left ventricular diastolic dysfunction may amplify left atrial wall stress which could further result in advanced electrical and structural remodeling of the left atrial.31 On the contrary, the dilated left atrium may influence systolic and diastolic function negatively, which could lead to an increased left atrial pressure. Consequently, the decreased LA/LAA hemodynamic function eventually leads to LAT/SEC.23,32
Nomogram is a kind of scoring system, which predicts an individual’s probability of a clinical event using individual factors. Like many other scoring systems,33 the nomogram as a noninvasive technique that provides advantages of simplicity, effectiveness, convenience and a lower cost. In the present study, we found several predictors of LAT/SEC and established a nomogram by combining these predictors. Interestingly, the new model, which is composed of both clinical and echocardiographic parameters, showed a good predictive ability to detect patients with high LAT/SEC risk. From our results, the nomogram had a stronger discriminatory performance compared with the CHA2DS2-VASc score. This suggests that there exists a potential of utilizing the model for clinical use. Based on the analysis of our data, we endorse 181.5 as the cutoff value, and those patients with a score of more than 181.5 should be recognized as a high-risk group for LAT/SEC. According to our results, the nomogram might be used as an alternative to TEE among NVAF patients with low/borderline CHA2DS2-VASc scores. Also, the model may provide an insight into whether to stop anticoagulants after the TEE procedure during the follow-up.
Owing to the lack of a accurate and useful predictive model, developing a new model that incorporates clinical and echocardiographic features associated with LAT/SEC becomes desirable. Therefore, this is the first study to identify the high-risk subgroup among the CHA2DS2-VASc score-defined low stroke risk population. However, limitations can be stated for this study. Our study is limited due to single-centre data, thus it is essential to confirm the findings through multi-center data in the future. Second, due to the retrospective nature of the study, some indicators such as left atrial appendage (LAA) flow velocity, LAA morphology, mean platelet volume (MPV)34 and whole blood viscosity (WBV)35 which might be related to LAT/SEC were not available in the study. Finally, only NVAF with low/borderline CHA2DS2-VASc score patients were included in the present study, therefore our findings may not be representative of all patients with NVAF.
Conclusions
Our study showed that age, smoke, LAD, LVEF, E/e’, and NPAF were associated with LAT/SEC in AF patients with low/borderline CHA2DS2-VASc scores. The nomogram constructed by combining these risk factors effectively predicts LAT/SEC risk in non-valvular atrial fibrillation patients with a low/borderline CHA2DS2-VASc score.
Acknowledgments
We would like to acknowledge Yidu cloud (Beijing) Technology Ltd for their worthy cooperation in data searching, extraction, and processing. Zhitong Lia and Lifei Pan are co-first authors for this study.
Funding Statement
There is no funding to report.
Abbreviations
LAT, left atrial thrombus; LASEC, left atrial spontaneous echo contrast; NPAF, non-paroxysmal AF; NVAF, non-valvular atrial fibrillation; TEE, transesophageal echocardiography.
Data Sharing Statement
The datasets analysed in the current study are available from the corresponding author on reasonable request.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Arboix A, Alio J. Acute cardioembolic stroke: an update. Expert Rev Cardiovasc Ther. 2011;9(3):367–379. doi: 10.1586/erc.10.192 [DOI] [PubMed] [Google Scholar]
- 2.Arboix A, Garcia-Eroles L, Massons JB, Oliveres M, Pujades R, Targa C. Atrial fibrillation and stroke: clinical presentation of cardioembolic versus atherothrombotic infarction. Int J Cardiol. 2000;73(1):33–42. doi: 10.1016/S0167-5273(99)00214-4 [DOI] [PubMed] [Google Scholar]
- 3.Fatkin D, Kelly RP, Feneley MP. Relations between left atrial appendage blood flow velocity, spontaneous echocardiographic contrast and thromboembolic risk in vivo. J Am Coll Cardiol. 1994;23(4):961–969. doi: 10.1016/0735-1097(94)90644-0 [DOI] [PubMed] [Google Scholar]
- 4.Vinereanu D, Lopes RD, Mulder H, et al. Echocardiographic risk factors for stroke and outcomes in patients with atrial fibrillation anticoagulated with apixaban or warfarin. Stroke. 2017;48(12):3266–3273. doi: 10.1161/STROKEAHA.117.017574 [DOI] [PubMed] [Google Scholar]
- 5.Black IW, Hopkins AP, Lee LC, Walsh WF. Left atrial spontaneous echo contrast: a clinical and echocardiographic analysis. J Am Coll Cardiol. 1991;18(2):398–404. doi: 10.1016/0735-1097(91)90592-W [DOI] [PubMed] [Google Scholar]
- 6.Cinar T, Hayiroglu MI, Tanik VO, et al. The predictive value of the CHA2DS2-VASc score in patients with mechanical mitral valve thrombosis. J Thromb Thrombolysis. 2018;45(4):571–577. doi: 10.1007/s11239-018-1640-3 [DOI] [PubMed] [Google Scholar]
- 7.Hindricks G, Potpara T, Dagres N, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European Association for Cardio-Thoracic Surgery (EACTS): the Task Force for the diagnosis and management of atrial fibrillation of the European Society of Cardiology (ESC) Developed with the special contribution of the European Heart Rhythm Association (EHRA) of the ESC. Eur Heart J. 2021;42(5):373–498. doi: 10.1093/eurheartj/ehaa612 [DOI] [PubMed] [Google Scholar]
- 8.Yao Y, Shang MS, Gao LJ, et al. Elevated homocysteine increases the risk of left atrial/left atrial appendage thrombus in non-valvular atrial fibrillation with low CHA2DS2-VASc score. Europace. 2018;20(7):1093–1098. doi: 10.1093/europace/eux189 [DOI] [PubMed] [Google Scholar]
- 9.Chen J, Zhou M, Wang H, et al. Risk factors for left atrial thrombus or spontaneous echo contrast in non-valvular atrial fibrillation patients with low CHA2DS2-VASc score. J Thromb Thrombolysis. 2022;53(2):523–531. doi: 10.1007/s11239-021-02554-9 [DOI] [PubMed] [Google Scholar]
- 10.Huang J, Wu SL, Xue YM, et al. Association of CHADS2 and CHA2DS2-VASc scores with left atrial thrombus with nonvalvular atrial fibrillation: a single center based retrospective study in a cohort of 2695 Chinese subjects. Biomed Res Int. 2017;2017:6839589. doi: 10.1155/2017/6839589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim MN, Kim SA, Choi JI, et al. Improvement of predictive value for thromboembolic risk by incorporating left atrial functional parameters in the CHADS2 and CHA2DS2-VASc scores. Int Heart J. 2015;56(3):286–292. doi: 10.1536/ihj.14-380 [DOI] [PubMed] [Google Scholar]
- 12.Piccini JP, Stevens SR, Chang Y, et al. Renal dysfunction as a predictor of stroke and systemic embolism in patients with nonvalvular atrial fibrillation: validation of the R(2)CHADS(2) index in the ROCKET AF (Rivaroxaban Once-daily, oral, direct factor Xa inhibition Compared with vitamin K antagonism for prevention of stroke and Embolism Trial in Atrial Fibrillation) and ATRIA (AnTicoagulation and Risk factors In Atrial fibrillation) study cohorts. Circulation. 2013;127(2):224–232. [DOI] [PubMed] [Google Scholar]
- 13.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2013;36(Suppl 1):S67–74. doi: 10.2337/dc13-S067 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arbab-Zadeh A, Fuster V. The risk continuum of atherosclerosis and its implications for defining CHD by coronary angiography. J Am Coll Cardiol. 2016;68(22):2467–2478. doi: 10.1016/j.jacc.2016.08.069 [DOI] [PubMed] [Google Scholar]
- 15.Whelton PK, Carey RM, Aronow WS, et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol. 2018;71(19):e127–e248. doi: 10.1016/j.jacc.2017.11.006 [DOI] [PubMed] [Google Scholar]
- 16.Jun-Ren ZH, Run-Lin GA, Shui-Ping ZH, Guo-Ping LU, Dong ZH, Jian-Jun LI. 2016 Chinese guidelines for the management of dyslipidemia in adults. J Geriatr Cardiol. 2018;15(1):1–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2015;28(1):1–39 e14. [DOI] [PubMed] [Google Scholar]
- 18.Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the euro heart survey on atrial fibrillation. Chest. 2010;137(2):263–272. doi: 10.1378/chest.09-1584 [DOI] [PubMed] [Google Scholar]
- 19.Li Z, Liu Q, Liu F, et al. Nomogram to predict left atrial thrombus or spontaneous echo contrast in patients with non-valvular atrial fibrillation. Front Cardiovasc Med. 2021;8:737551. doi: 10.3389/fcvm.2021.737551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen J, Zhou M, Wang H, et al. Risk factors for left atrial thrombus or spontaneous echo contrast in non-valvular atrial fibrillation patients with low CHA2DS2-VASc score. J Thromb Thrombolysis. 2021;53:523–531. [DOI] [PubMed] [Google Scholar]
- 21.Yaghi S, Moon YP, Mora-McLaughlin C, et al. Left atrial enlargement and stroke recurrence: the Northern Manhattan Stroke Study. Stroke. 2015;46(6):1488–1493. doi: 10.1161/STROKEAHA.115.008711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ganesan AN, Chew DP, Hartshorne T, et al. The impact of atrial fibrillation type on the risk of thromboembolism, mortality, and bleeding: a systematic review and meta-analysis. Eur Heart J. 2016;37(20):1591–1602. doi: 10.1093/eurheartj/ehw007 [DOI] [PubMed] [Google Scholar]
- 23.Doukky R, Garcia-Sayan E, Gage H, et al. The value of diastolic function parameters in the prediction of left atrial appendage thrombus in patients with nonvalvular atrial fibrillation. Cardiovasc Ultrasound. 2014;12:10. doi: 10.1186/1476-7120-12-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lau DH, Nattel S, Kalman JM, Sanders P. Modifiable risk factors and atrial fibrillation. Circulation. 2017;136(6):583–596. doi: 10.1161/CIRCULATIONAHA.116.023163 [DOI] [PubMed] [Google Scholar]
- 25.Steinberg BA, Hellkamp AS, Lokhnygina Y, et al. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCKET-AF trial. Eur Heart J. 2015;36(5):288–296. doi: 10.1093/eurheartj/ehu359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kato T, Yamashita T, Sagara K, Iinuma H, Fu LT. Progressive nature of paroxysmal atrial fibrillation. Observations from a 14-year follow-up study. Circ J. 2004;68(6):568–572. doi: 10.1253/circj.68.568 [DOI] [PubMed] [Google Scholar]
- 27.Goette A, Kalman JM, Aguinaga L, et al. EHRA/HRS/APHRS/SOLAECE expert consensus on atrial cardiomyopathies: definition, characterization, and clinical implication. Europace. 2016;18(10):1455–1490. doi: 10.1093/europace/euw161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shirani J, Alaeddini J. Structural remodeling of the left atrial appendage in patients with chronic non-valvular atrial fibrillation: implications for thrombus formation, systemic embolism, and assessment by transesophageal echocardiography. Cardiovasc Pathol. 2000;9(2):95–101. doi: 10.1016/S1054-8807(00)00030-2 [DOI] [PubMed] [Google Scholar]
- 29.Nagueh SF, Smiseth OA, Appleton CP, et al. Recommendations for the evaluation of left ventricular diastolic function by echocardiography: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2016;29(4):277–314. doi: 10.1016/j.echo.2016.01.011 [DOI] [PubMed] [Google Scholar]
- 30.Park J, Joung B, Uhm JS, et al. High left atrial pressures are associated with advanced electroanatomical remodeling of left atrium and independent predictors for clinical recurrence of atrial fibrillation after catheter ablation. Heart Rhythm. 2014;11(6):953–960. doi: 10.1016/j.hrthm.2014.03.009 [DOI] [PubMed] [Google Scholar]
- 31.Hu YF, Hsu TL, Yu WC, et al. The impact of diastolic dysfunction on the atrial substrate properties and outcome of catheter ablation in patients with paroxysmal atrial fibrillation. Circ J. 2010;74(10):2074–2078. doi: 10.1253/circj.CJ-10-0175 [DOI] [PubMed] [Google Scholar]
- 32.Iwakura K, Okamura A, Koyama Y, et al. Effect of elevated left ventricular diastolic filling pressure on the frequency of left atrial appendage thrombus in patients with nonvalvular atrial fibrillation. Am J Cardiol. 2011;107(3):417–422. doi: 10.1016/j.amjcard.2010.09.042 [DOI] [PubMed] [Google Scholar]
- 33.Hayiroglu MI, Cinar T, Cicek V, et al. A simple formula to predict echocardiographic diastolic dysfunction-electrocardiographic diastolic index. Herz. 2021;46(Suppl 2):159–165. doi: 10.1007/s00059-020-04972-6 [DOI] [PubMed] [Google Scholar]
- 34.Cinar T, Hayiroglu MI, Cicek V, et al. Predictors of left atrial thrombus in acute ischemic stroke patients without atrial fibrillation: a single-center cross-sectional study. Rev Assoc Med Bras. 2020;66(10):1437–1443. doi: 10.1590/1806-9282.66.10.1437 [DOI] [PubMed] [Google Scholar]
- 35.Cinar T, Hayiroglu MI, Selcuk M, et al. Association of whole blood viscosity with thrombus presence in patients undergoing transoesophageal echocardiography. Int J Cardiovasc Imaging. 2022;38(3):601–607. doi: 10.1007/s10554-021-02445-3 [DOI] [PubMed] [Google Scholar]