Abstract
Recurrent meningiomas constitute an uncommon but significant problem after standard (surgery and radiation) therapy failure. Current chemotherapies (hydroxyurea, RU-486, and interferon-α) are only of marginal benefit. There is an urgent need for more effective treatments for meningioma patients who have failed surgery and radiation therapy. Limonin, Tangeritin, Zerumbone, 6-Gingerol, Ganoderic Acid A, and Ganoderic Acid DM are some of the plant derivatives that have anti-tumorgenic properties and cause cell death in meningioma cells in vitro. Due to its ease of administration, long-term tolerability, and low incidence of long-term side effects, we explored its potential as a therapeutic agent against meningiomas by examining their efficacy in vitro against meningioma cells. Treatment effects were assessed using MTT assay, Western blot analysis, caspases assay, and DNA fragmentation assay. Results indicated that treatments of IOMM-Lee and CH157MN meningioma cells with Limonin, Tangeritin, Zerumbone, 6-Gingerol, Ganoderic Acid A, and Ganoderic Acid DM induced apoptosis with enhanced phosphorylation of glycogen synthase kinase 3 β (GSK3β) via inhibition of the Wnt5/β-catenin pathway. These drugs did not induce apoptosis in normal human neurons. Other events in apoptosis included downregulation of tetraspanin protein (TSPAN12), survival proteins (Bcl-XL and Mcl-1), and overexpression apoptotic factors (Bax and caspase-3). These results provide preliminary strong evidence that medicinal plants containing Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM can be applied to high-grade meningiomas as a therapeutic agent, and suggests that further in vivo studies are necessary to explore its potential as a therapeutic agent against malignant meningiomas.
Keywords: Meningioma, Apoptosis, Wnt, Catenin, Lemon, Ginger, Mushroom
Introduction
Meningiomas are the second most common central nervous system tumor found in adults [1–4]. Meningiomas arise from arachnoid cells of the meninges, which are the covering layers of the brain. Unlike glioblastomas, meningiomas typically are benign, localized, non-aggressive, and non-invasive (World Health Organization [WHO] Grade I) [4–6]. Only higher-grade meningiomas (WHO Grade II and WHO Grade III) have shown to be aggressive, malignant, and invasive, which can cause multiple neurological and physiological complications [1, 5, 7]. There are a number of factors that can lead to the development of meningiomas: genetic factors (neurofibromatosis, chromosomal deletions, and mutations), ionizing radiation, and other factors such as chemical carcinogens and infections. Though 90 % of meningiomas are histologically classified as benign, more than 20 % cannot be removed completely by surgery due to their inaccessible anatomical location or their extent of invasion; moreover, in about 20 % of the patient’s tumor, recidivism has been observed [5]. Currently used medications (such as hydroxyurea, somatostatin, and interferon-α) and radiation for malignant meningiomas may workfor a while but progression through these treatments is practically universal [8]. Therefore, an additional novel therapy for aggressive and non-resectable meningiomas is desirable. Thus, we propose to evaluate the effect of naturally occurring products: Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM on the growth of meningioma cells in culture.
Recently published results showed that limonoids (citrus products) have a strong potential for the prevention of breast cancer (MCF-7) and colon cancer (SW480) via mitochondria-mediated intrinsic apoptosis (caspase-3/7) pathways [9, 10]. Whereas Tangeretin is a methoxyflavone from citrus fruits that effectively reduced the tumor growth of human breast and promyelocytic leukemia cells by suppressing tumor cell proliferation markers (PCNA, COX-2, and Ki-67), matrix metalloproteinase (MMP-2/9), vascular endothelial growth factor, and extracellular-signal-regulated kinases 1/2 (ERK1/2) phosphorylation and also by arresting the cancer cell division at the G1/S phase via p53/p21 upregulation [11, 12]. [6]-Gingerol and Zerumbone (components of ginger) has been used to treat numerous types of disease of human colon and breast cells [13–16]. Recent reports demonstrated that [6]-gingerol induce apoptosis by suppressing inducible nitric oxide synthase (iNOS), tumor necrosis factor alpha (TNF-α) expression, IkappaB alpha (IκBα) phosphorylation, nuclear factor kappa B (NF-κB) nuclear translocation, and ERK1/2/JNK/AP-1 pathway and also by activating mitochondria pathway [13, 14]. Zerumbone inhibited the NF-kappaB-dependent reporter gene (TNF, TNFR1, TRADD, TRAF2, NIK, and IKK), suppression of antiapoptotic and metastatic gene expression (Akt and FOXO1 activation), upregulation of apoptosis, and downregulation of invasion (MMP9, VEGF, interleukin IL-6/8) in human breast and GBM 8401 cells [15, 16]. It has been demonstrated that ganoderic acids (mushroom products) suppress growth by downregulating the expression of various NF-κB-regulated genes including genes involved in cell proliferation (c-Myc and cyclin D1), anti-apoptosis (Bcl-2), invasion (MMP-9), and angiogenesis [17–20].Taken together, Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM have shown the ability to induce apoptosis in numerous cancer cells, which suggests that they could be a viable option for treatment against these tumors.
Recent investigations suggest that genetic instabilities and Wnt/β-catenin pathway seems to be a central mediator in the pathophysiology of meningioma [21]. While investigating the effect of products from lemons, tangerines, ginger, mushrooms, and garlic in inducing apoptosis in IOMM-Lee and CH157MN cell line, an in vitro model for meningioma, we were intrigued in identifying the influence of Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM on players that respond to Wnt/β-catenin such as Frizzled (Fz), glycogen synthase kinase-3β (GSK-3β), TSPAN12, c-myc, and cyclin D1. The current study aimed to determine the in vitro efficacy of Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM against meningiomas and to elucidate the mechanism of action that may help to explain altered tumor growth and metastasis.
Material and methods
Cell culture and treatments
Human meningioma cell lines (IOMM-Lee, CH157MN) were obtained from Elmar Kirchesa (Institute of Neuropathology, Otto-von-Guericke-University, Germany) and Jerry Jeff Jaboin (Washington University of Medicine, USA) [22–25]. Human neuron culture was purchased from ScienceCell Research Laboratories and maintained under the conditions provided by ScienceCell Research Laboratories. Standard laboratory techniques were used to grow and treat these cells. Drug stock solutions were prepared in dimethyl sulfoxide (DMSO). An equal amount of DMSO (0.01 %) was added to untreated control cells. Dose-response studies were conducted to determine the suitable concentration of the drugs used in the experiments [26, 27]. Cells were treated with 25 μM Limonin (LKT Laboratories), 25 μM Tangeritin (LKT Laboratories), 25 μM 6-Gingerol (Sigma-Aldrich), 25 μM Zerumbone (LKT Laboratories), 25 μM Ganoderic Acid A (ChromaDex), 25 μM Ganoderic Acid DM (ChemFaces), and 25 μM hydroxyurea (Sigma) for 24 h for induction of apoptosis. After treatments, cells were used for determination of the mechanisms of apoptosis.
MTT cell viability assay
IOMM-Lee and CH157MN (5×104 cells per well) were seeded into six-well plate overnight. Cells were treated with various concentrations of compound for 24 h. Next, cells were incubated with 50 μl of 4,5-dimethylthiazol-2-yl-2,5-diphenyltetrazolium-bromide (MTT) (2 mg/ml) at 37 °C for 2 h. Then, the culture supernatant was removed and DMSO was added (400 μl/well). Cells were incubated in a shaker at 37 °C for 10 min until crystals were completely dissolved. Ninety-six-well plates were used to measure the absorbance using Emax Precision Microplate reader at 570 nm with reference wavelength set at 620 nm using SoftMax Pro software (Molecular Devices, Sunnyvale, CA, USA). Optical density was compared setting the control at 100 % and results were analyzed using Microsoft Office Excel©. This experiment was performed in triplicate and repeated for three times. Calculation of cell viability was described previously [28].
DNA fragmentation assay
Genomic DNA fragmentation was analyzed by agarose gel electrophoresis of genomic DNA isolated from cells treated with 25 μM Limonin, 25 μM Tangeritin, 25 μM 6-Gingerol, 25 μM Zerumbone, 25 μM Ganoderic Acid A, and 25 μM Ganoderic Acid DM, as we reported previously [29]. Equal amounts of the DNA samples were loaded onto 1.6 % agarose gels and electrophoresed in a TAE (40 mM Tris-acetate, pH 8.3, 1 mM EDTA) buffer. Gels were stained with ethidium bromide (1 μg/mL), destained the background in water, and photographed on a UV (303 nm) transilluminator using Alpha Innotech (San Leandro, CA, USA)
Western blot analysis
Western blotting was performed as we described previously [26–29]. GAPDH was used to standardize cytosolic protein loading on the sodium dodecyl sulfate-polyacrylamide gel electrophoresis. Mcl-1 antibody (S-19) (sc-819), c-Myc antibody (9E10) (sc-40), cyclin D1 antibody (H-295) (sc-753), VEGF antibody (147) (sc-507), LRP5 antibody (B-9) (sc-390267), frizzled-4 antibody (H-120) (sc-135108), p-GSK-3α/β antibody (6D3) (sc-81496), Wnt-5α/β antibody ( sc-376249), Bcl-xL antibody (H-5) ( sc-8392), TSPAN12 (sc-133810), β-catenin antibody (H-102) (sc-7199), Bax antibody (N-20): sc-493, p-Akt1/2/3 antibody (Ser 473)-R (sc-7985-R), GAPDH antibody (G-9) (sc-365062), and caspase-3 p11 antibody (C-6) (sc-271759) were purchased from Santa Cruz Biotech (Santa Cruz, Calif). The secondary antibodies were horseradish peroxidase-conjugated goat antimouse immunoglobulin (Ig) G (ICN Biomedicals, Aurora, Ohio) and horseradish peroxidase-conjugated goat antirabbit IgG (ICN Biomedicals).
Colorimetric assays for caspase-3 activities
Measurements of caspase activities in cells were performed as we described previously using the commercially available caspase-3 assay kits (Sigma). Experiments were performed in triplicate.
Statistical analysis
All results obtained from different treatments were analyzed using StatView software (Abacus Concepts, Berkeley, CA). Data were expressed as mean+standard error of mean of separate experiments (n≥3) and compared by one-way analysis of variance followed by Fisher post hoc test.
Results
Limonin, Tangeritin, Zerumbone, 6-Gingerol, Ganoderic acid A, and Ganoderic acid DM inhibits survival of high grade meningioma cells but not normal human neurons
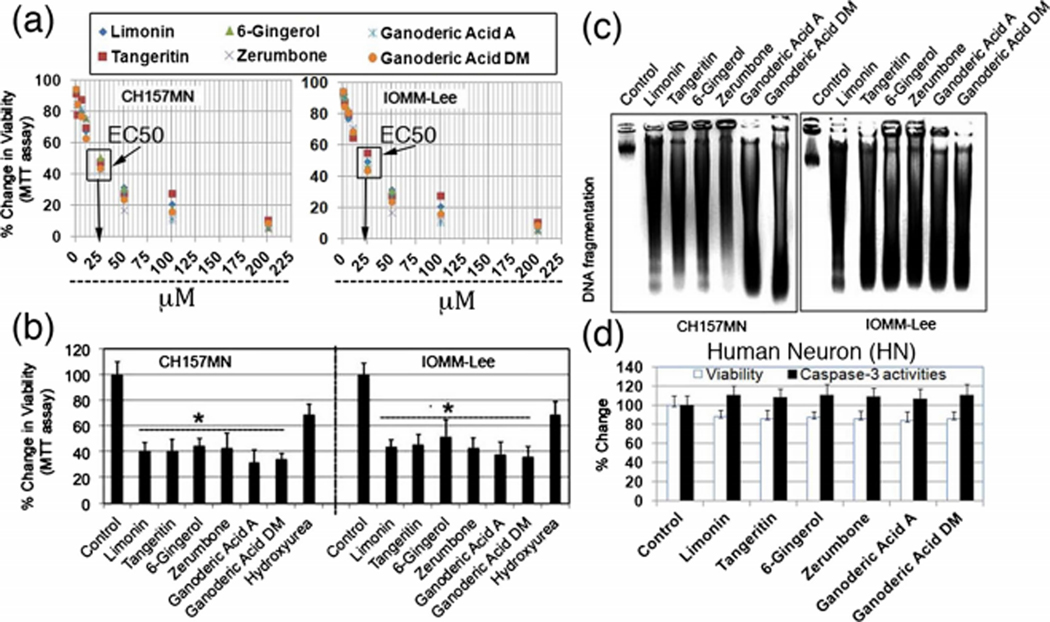
We first conducted dose response experiments to determine median effective concentration (EC50) of Limonin, Tangeritin, Zerumbone, 6-Gingerol, Ganoderic Acid A, and Ganoderic Acid DM on well-characterized human meningioma cell line, IOMM-Lee and CH157MN (Fig. 1a). Our results showed no major differences in EC50 (25 μM) values of these drugs. Thus, we compared the cytotoxic effect of 25 μM Limonin, 25 μM Tangeritin, 25 μM 6-Gingerol, 25 μM Zerumbone, 25 μM Ganoderic Acid A, and 25 μM Ganoderic Acid DM on cell survival using IOMM-Lee and CH157MN. MTT assay was used to determine cell viability. The survival of IOMM Lee and CH157MN decreased significantly (Fig 1b). No significant cell inhibitory effect was observed in DMSO (solvent)-treated samples. As a positive control, we treated IOMM-Lee and CH157MN cells with 25 μM hydroxyurea, a standard meningioma chemotherapy drug, which showed less effect than Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM. We also correlated molecular changes due to apoptosis with internucleosomal DNA fragmentation in untreated or Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM-treated IOMM-Lee, and CH157MN cells. Unlike untreated cells, substantial DNA laddering was found in 25 μM Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM-treated meningioma cells (Fig 1b, c).
Fig. 1.
Determine median effective concentration (EC50) of Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM after exposure to different doses (a) and compared the residual cell viability in IOMM-Lee cells and CH157MN cells using MTT assay (b). c Agarose gel electrophoresis of genomic DNA samples for detection of internucleosomal DNA fragmentation. d Analysis of cytotoxicity effects (MTT assay) and apoptosis (colorimetric assay of caspase-3) of 25 μM Limonin, 25 μM Tangeritin, 25 μM 6-Gingerol, 25 μM Zerumbone, 25 μM Ganoderic Acid A, and 25 μM Ganoderic Acid DM on HN (human normal neuron) cells
We examined the effects of Limonin, Tangeritin, Zerumbone, 6-Gingerol, Ganoderic Acid A, and Ganoderic Acid DM on HN (human normal neurons). Residual cell viability was determined by MTT assay. Compared with control cells, cells treated with Limonin, Tangeritin, Zerumbone, 6-Gingerol, Ganoderic Acid A, and Ganoderic Acid DM did not show any significant change in viability (Fig 1d). These results demonstrated that these drugs were cytotoxic to IOMM-Lee and CH157MN cells but not to HN. Colorimetric assays showed insignificant changes in activities of caspase-3 in HN following treatment with Limonin, Tangeritin, Zerumbone, 6-Gingerol, Ganoderic Acid A, and Ganoderic Acid DM (Fig. 1d). These results indicated that HN did not commit apoptosis due to exposure of these drugs.
Consistent disrupted canonical Wnt signaling and GSK3 inactivation suppresses the expression of β-catenin target genes
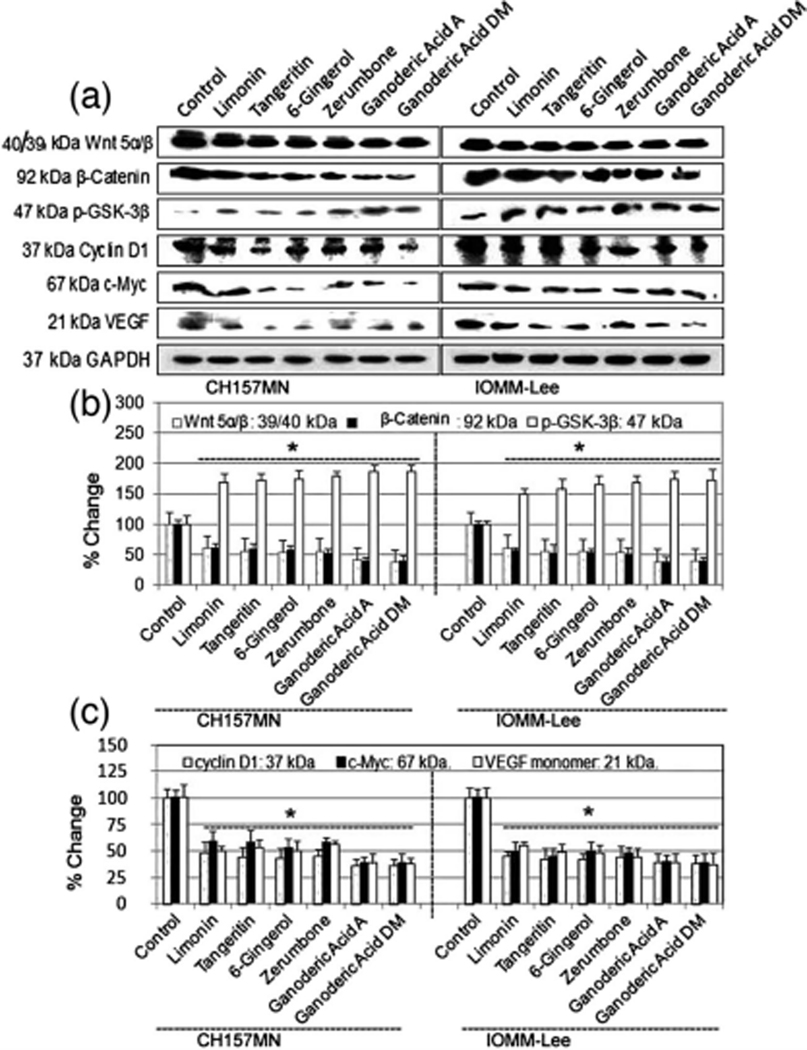
Gsk3β plays a critical role in Wnt/b-catenin signaling pathway [30, 31]. Here, Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM suppressed the expression of Wnt5α/β and β-catenin and enhanced the phosphorylation of GSK3β at Ser 9 in IOMM-Lee and CH157MN cells (Fig. 2a, b). These results imply that Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM-induced apoptosis is controlled through Wnt5/GSK3β/β-catenin signaling. We also identified that these drugs interrupt wnt signaling by decreasing β-catenin activity, which in turn suppresses the expression of β-catenin target genes (c-myc, VEGF, and cyclin D1). This may be one mechanism of apoptosis in meningioma cells.
Fig. 2.
Analysis the expression of GSK3β, Wnt5α/β, β-catenin, c-myc, VEGF and cyclin D1 at protein levels in IOMM-Lee and CH157MN cells. Treatments (24 h): control, 25 μM Limonin, 25 μM Tangeritin, 25 μM 6-Gingerol, 25 μM Zerumbone, 25 μM Ganoderic Acid A, and 25 μM Ganoderic Acid DM. a Western blots and b, c Quantitative analysis of GSK3β, Wnt5α/β, β-catenin, c-myc, VEGF, cyclin D1, and GAPDH. *P<.05; **P<.01, significant difference between control and drug treatments
TSPAN12 elimination from human IOMM-Lee and CH157MN cells also caused diminished association between FZD4 and its co-receptor LRP5
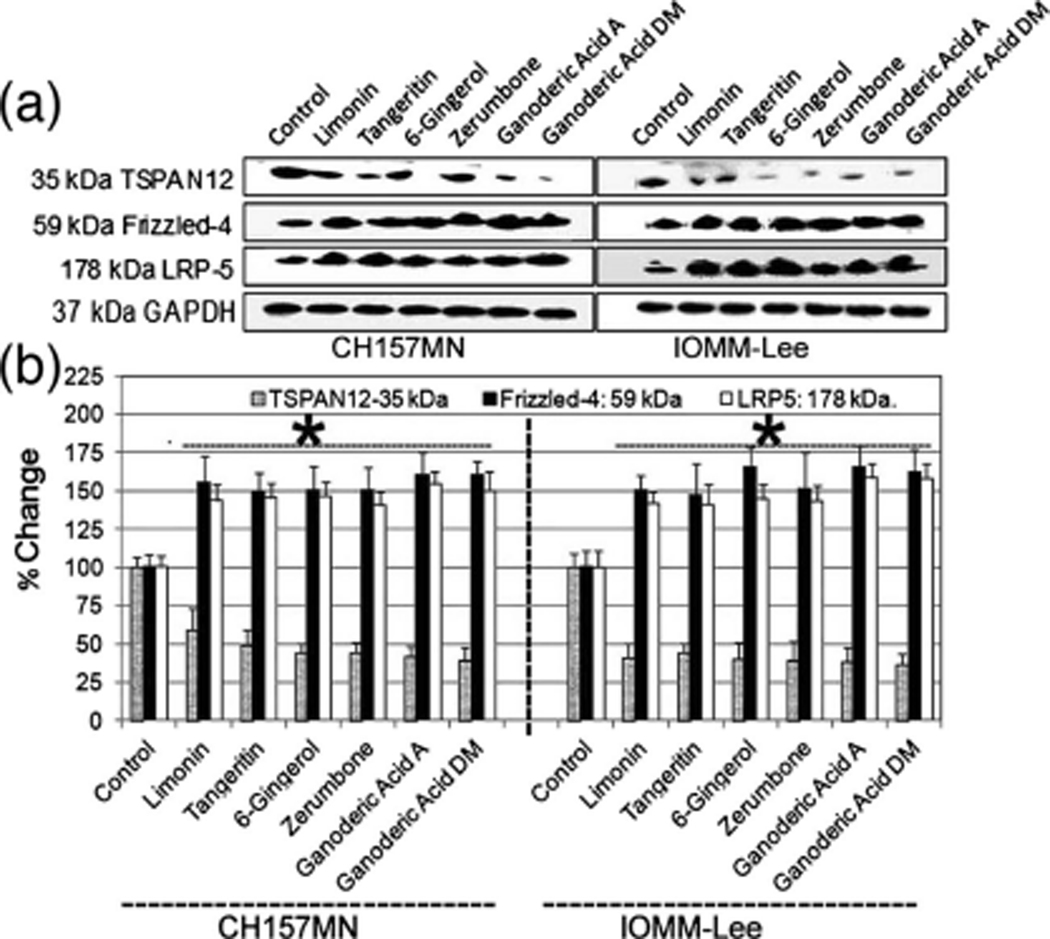
Genetic evidence previously linked TSPAN12 to a FZD4–LRP5 signaling pathway, upstream of β-catenin [32]. The β-catenin protein and associated signaling pathways can play a prominent role in primary tumor growth as well as metastasis [33, 34]. Hence, to find the mechanistic insight into TSPA N12’s role in IOMM-Lee and CH157MN cells treated with Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM, we analyzed the expression of TSPAN12, FZD4, and LRP5. Our results demonstrated TSPA N12 levels were diminished by more than 80 % (Fig. 3a, b), and altered protein levels for LRP5, Dvl1, and Dvl2, (Fig. 3), are suggestive of dysregulated FZD4–LRP5 signaling.
Fig. 3.
Analysis of the expression of TSPAN12, FZD4, and LRP5 at protein levels in IOMM-Lee and CH157MN cells. Treatments (24 h): control, 25 μM Limonin, 25 μM Tangeritin, 25 μM 6-Gingerol, 25 μM Zerumbone, 25 μM Ganoderic Acid A, and 25 μM Ganoderic Acid DM. a Western blots and b quantitative analysis of TSPAN12, FZD4, LRP5, and GAPDH. *P<.05; **P<.01, significant difference between control and drug treatments
Induction of cell death factors in meningioma cells
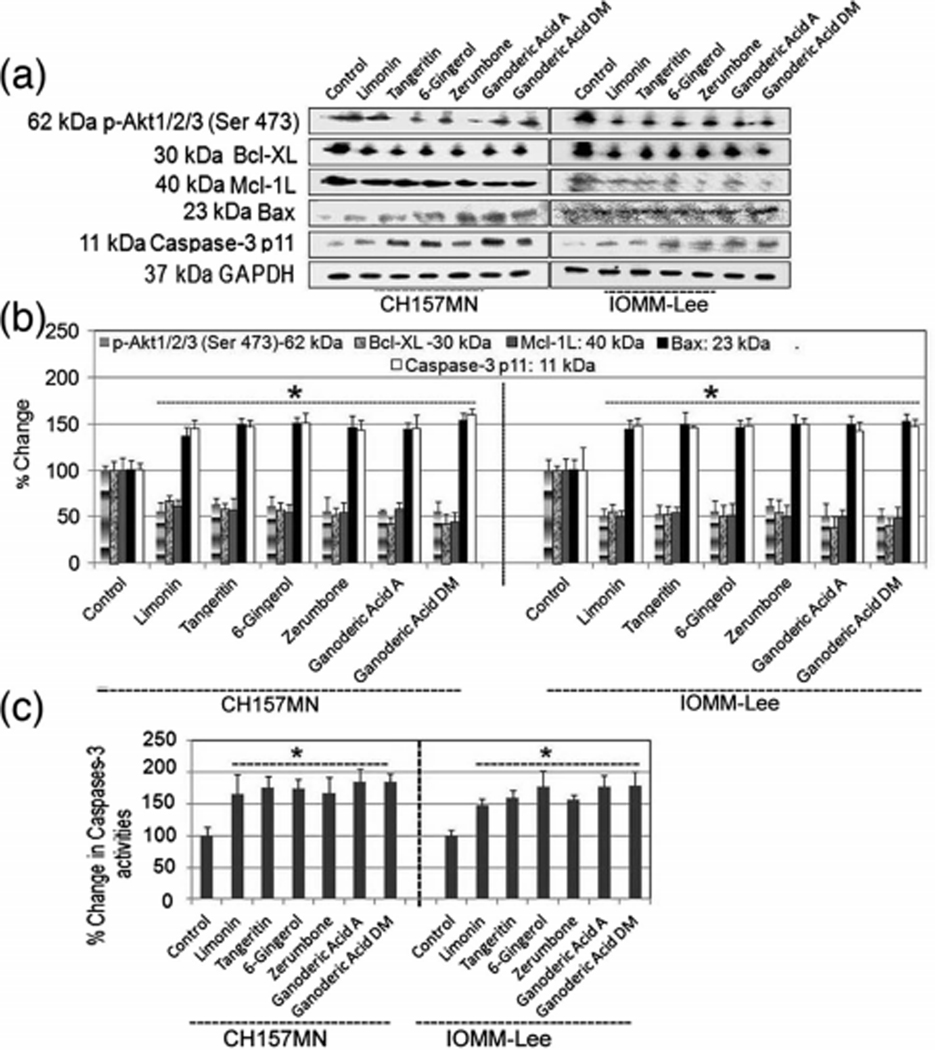
Generally, apoptosis is induced through two distinctive pathways such as cell death extrinsic pathway and mitochondrial-dependent intrinsic pathway [26–29]. To further match up to the apoptotic activity of Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM in meningioma cells, the effects of these drugs on apoptosis-related proteins such as Akt, Bcl-xL, Mcl-1, Bax, and caspase-3 in IOMM-Lee and CH157MN cells were examined by Western blotting and capases activities kit. Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM activated caspase cascades and regulated the Bcl-2 family proteins in IOMM-Lee and CH157MN cells. Western blotting showed that activated caspase-3 as shown in Fig 4a, implying that these drugs induce apoptosis via mitochondrial-dependent pathway. Anti-apoptotic Bcl-2 family proteins such as Akt, Bcl-xL, and Mcl-1 L are frequently overexpressed in cancers [35]. In the present study, Western blotting revealed that Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM suppressed the expression of Akt, Bcl-XL, and Mcl-1 L as antiapoptotic genes and also upregulated the expression of Bax in IOMM-Lee and CH157MN cells as shown in Fig 4a, b. Almost uniform expression of GAPDH served as a loading control for cytosolic proteins. A colorimetric assay indicated very significant increases in total caspase-3 activity in meningioma cells after exposure to Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM (Fig. 4c).
Fig. 4.
Analysis the expression of Akt, Bcl-XL, Mcl-1, Bax, and caspase-3 at protein levels and caspase-3 activities in IOMM-Lee and CH157MN cells. Treatments (24 h): control, 25 μM Limonin, 25 μM Tangeritin, 25 μM 6-Gingerol, 25 μM Zerumbone, 25 μM Ganoderic Acid A, and 25 μM Ganoderic Acid DM. a Western blots and b quantitative analysis of Akt, Bcl-XL, Mcl-1, Bax, caspase-3, and GAPDH. c Colorimetric determination of caspase-3 activity is shown. *P<.05; **P<.01, significant difference between control and drug treatments
Discussion
Clinically, refractory and atypical/anaplastic meningiomas may be difficult to cure surgically and few medical options exist [1–8]. Current guidelines recommend only three drugs that can be used to treat patients with refractory and high-grade meningiomas: hydroxyurea, interferon-α 2B, and Sandostatin long-acting release [1–8]. With the lack of current effective chemotherapy for meningiomas, we proposed Wnt/β-catenin pathway, which involved in the progression from benign to malignant tumors, as a possible candidate for the development of novel therapeutic approaches for the treatment of this aggressive form of meningioma [30, 36]. Several naturally occurring plant derived products have shown tremendous potential in the prevention and treatment of several cancers, especially due to their ability to induce apoptosis. In this study, we demonstrated for the first time the antitumor drugs from natural products: Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM induced cell death in IOMM-Lee and CH157MN cells without affecting HN cells (Fig 1). We explored the signaling pathways activated by these natural products with reference to the Wnt/βcatenin signaling on meningioma cells.
Inappropriate activation of the Wnt/β-catenin signaling pathways plays a critical role in the early stages in a variety of human cancers, including meningioma [30, 36]. However, their respective implication in tumor cell invasion is still hypothetical. Here, we demonstrate that Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM induced cell death (Fig. 1) and also suppressed the expression of Wnt5α/β and β-catenin and induced the phosphorylation of glycogen synthase kinase-3β (GSK-3β), at Ser 9, which is an indicator of GSK3β deactivation in IOMM-Lee and CH157MN cells (Fig. 2). As downstream signaling mediators of the Wnt/β-catenin pathway may play an important role in tumorigenisis in meningioma, it is important to target some of these β-catenin target genes (c-myc, VEGF, and cyclin D1). Current results showed that these drugs not only interrupts wnt signaling but also reduced β-catenin activity, which in turn suppresses the expression of β-catenin target genes (c-myc, VEGF, and cyclin D1). This observation is important as it was well established previously that wnt signaling can restrain the process of apoptosis and increase survival by inhibiting GSK3-β.
The majority of meningioma cases involve the Wnt/β-catenin signaling, Akt pathways, or with components of the MAPK, JAK/STAT, and Notch-1 pathways because these has been linked to the mTORC1/mTORC2 complex and the TSC1/TSC2 protein complex that regulates cell growth. This pathway also regulates the cell cycle through the cyclin D1, cyclin-G2, and cyclin-G1 proteins, which is encoded by the CCND1, CCNG2, and CCNG1 genes. The Wnt/β-catenin signaling is regulated by secreted Frizzled proteins which can bind Wnts, by inhibiting interactions between FZD4 and its co-receptor LRP5. Furthermore, we observed suppression of TSPAN12 expression and overexpression of FZD4 and its co-receptor LRP5 in human IOMM-Lee and CH157MN-treated cells (Fig. 3). The combined result substantially explains disruption of Wnt/β-catenin signaling and dysregulation of FZD4–LRP5 signaling that may help to explain altered meningioma growth.
To date, inhibition of Wnt/β-catenin signaling mechanism(s) has been linked with apoptosis in a number of cancer cells [36]. Our Western blotting and caspases assay consistently revealed that Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM effectively activated caspase-3, suppressed the expression of survival proteins such as Akt, Anti-apoptoitc Bcl-2 family protein (Bcl-XL and Mcl-1), and upregulated the expression of apoptotic Bcl-2 family protein (Bax) in IOMM-Lee and CH157MN cells (Fig 4). These processes subsequently lead to attenuation of MMP and cytochrome c release. Release of cytochrome c activates caspase cascade and PARP cleavage to execute apoptotic program through fragmentation of chromatin DNA, i.e., increase in DNA laddering (the hallmark of apoptosis). We showed increased in caspase-3 activities (Fig. 4) and DNA fragmentation (Fig. 1) in Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM-treated meningioma cells.
The current findings demonstrate that multi-targeting products Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM have potent antitumorigenic actions in meningioma cells, but are not toxic to human normal neuron cells. These might therefore be putative candidates for the pharmacological treatment of meningiomas. The findings in this report indicate potential therapeutic value of Limonin, Tangeritin, 6-Gingerol, Zerumbone, Ganoderic Acid A, and Ganoderic Acid DM. Further research in animal tumor models is necessary to confirm its anti-cancer activity in vivo.
Acknowledgments
This investigation was supported by the Jerry Zucker Fund for Brain Tumor Research at the MUSC Foundation, Department of Neurosurgery (MUSC), American Brain Tumor Association (Medical Student Summer Fellowship in Honor of Paul Fabri), NIH grants (NS-38146, NS-41088), and also, this work in part was supported by a merit award from the Office of Research and Development, Department of Veterans Affairs (101BX001262).
Footnotes
Conflicts of interest None.
Copyright of Tumor Biology is the property of Springer Science & Business Media B.V. and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written permission. However, users may print, download, or email articles for individual use.
References
- 1.Liu L, Stone J, Hoffpauir JT, Xiong Z. Histiocytic meningioma: a distinctive subtype of meningioma? Intractable Rare Dis Res. 2014;3(2):57–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Preusser M, Berghoff AS, Hottinger AF. High-grade meningiomas: new avenues for drug treatment? Curr Opin Neurol. 2013;26(6): 708–15. [DOI] [PubMed] [Google Scholar]
- 3.Norden AD, Drappatz J, Wen PY. Advances in meningioma therapy. Curr Neurol Neurosci Rep. 2009;9(3):231–40. [DOI] [PubMed] [Google Scholar]
- 4.Mawrin C, Perry A. Pathological classification and molecular genetics of meningiomas. J Neurooncol. 2010;99(3):379–91. [DOI] [PubMed] [Google Scholar]
- 5.Modha A, Gutin PH. Diagnosis and treatment of atypical and anaplastic meningiomas: a review. Neurosurgery. 2005;57:538–50. [DOI] [PubMed] [Google Scholar]
- 6.Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, et al. The 2007 WHO classification of tumors of the central nervous system. Acta Neuropathol. 2007;114:97–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pasquier D, Bijmolt S, Veninga T, Rezvoy N, et al. Atypical and malignant meningioma: outcome and prognostic factors in 119 irradiated patients. A multicenter, retrospective study of the Rare Cancer Network. Int J Radiat Oncol Biol Phys. 2008;71:1388–93. [DOI] [PubMed] [Google Scholar]
- 8.Loven D, Hardoff R, Sever ZB, Steinmetz AP, et al. Non-resectable slow-growing meningiomas treated by hydroxyurea. J Neurooncol. 2004;67:221–6. [DOI] [PubMed] [Google Scholar]
- 9.Langeswaran K, Gowthamkumar S, Vijayaprakash S, Revathy R, Balasubramanian MP. Influence of limonin on Wnt signalling molecule in HepG2 cell lines. J Nat Sci Biol Med. 2013;4(1):126–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim J, Jayaprakasha GK, Patil BS. Limonoids and their antiproliferative and anti-aromatase properties in human breast cancer cells. Food Funct. 2013;4(2):258–65. [DOI] [PubMed] [Google Scholar]
- 11.Dong Y, Cao A, Shi J, Yin P, Wang L, et al. Tangeretin, a citrus polymethoxyflavonoid, induces apoptosis of human gastric cancer AGS cells throughextrinsic and intrinsic signalling pathways. Oncol Rep. 2014;31(4):1788–94. [DOI] [PubMed] [Google Scholar]
- 12.Lakshmi A, Subramanian SP. Tangeretin ameliorates oxidative stress in the renal tissues of rats with experimental breast cancer induced by 7,12-dimethylbenz[a]anthracene. Toxicol Lett. 2014;229(2):333–48. [DOI] [PubMed] [Google Scholar]
- 13.Weng HY, Hsu MJ, Wang CC, Chen BC, et al. Zerumbone suppresses IKKα, Akt, and FOXO1 activation, resulting in apoptosis of GBM 8401 cells. J Biomed Sci. 2012;19:86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sehrawat A, Arlotti JA, Murakami A, Singh SV. Zerumbone causes Bax- and Bak-mediated apoptosis in human breast cancer cells and inhibits orthotopic xenograft growth in vivo. Breast Cancer Res Treat. 2012;136(2):429–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kim SO, Kim MR. [6]-Gingerol prevents disassembly of cell junctions and activities of MMPs in invasive human pancreas cancer cells through ERK/NF-κ B/snail signal transduction pathway. Evid Based Complement Alternat Med. 2013;2013:761852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee SH, Cekanova M, Baek SJ. Multiple mechanisms are involved in 6-gingerol-induced cell growth arrest and apoptosis in human colorectal cancer cells. Mol Carcinog. 2008;47(3):197–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao X, Li G, Xu H, Lü C. Inhibition of the JAK-STAT3 signaling pathway by ganoderic acid A enhances chemosensitivity of HepG2 cells to cisplatin. Planta Med. 2012;78(16):1740–8. [DOI] [PubMed] [Google Scholar]
- 18.Jiang J, Grieb B, Thyagarajan A, Sliva D. Ganoderic acids suppress growth and invasive behavior of breast cancer cells by modulating AP-1 and NF-kappaB signaling. Int J Mol Med. 2008;21(5):577–84. [PubMed] [Google Scholar]
- 19.Wu GS, Lu JJ, Guo JJ, Li YB, et al. Ganoderic acid DM, a natural triterpenoid, induces DNA damage, G1 cell cycle arrest and apoptosis in humanbreast cancer cells. Fitoterapia.2012;83(2):408–14. [DOI] [PubMed] [Google Scholar]
- 20.Johnson BM, Doonan BP, Radwan FF, Haque A. Ganoderic Acid DM: an alternative agent for the treatment of advanced prostate cancer. Open Prost Cancer J. 2010;3:78–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park JH, Kwon HY, Sohn EJ, et al. Inhibition of Wnt/β-catenin signaling mediates ursolic acid-induced apoptosis in PC-3 prostate cancer cells. Pharmacol Rep. 2013;65(5):1366–74. [DOI] [PubMed] [Google Scholar]
- 22.Ragel BT, Couldwell WT, Gillespie DL, Wendland MM, et al. A comparison of the cell lines used in meningioma research. Surg Neurol. 2008;70(3):295–307. [DOI] [PubMed] [Google Scholar]
- 23.Tummalapalli P, Gondi CS, Dinh DH, Gujrati M, Rao JS. RNA interference-mediated targeting of urokinase plasminogen activator receptor and matrix metalloproteinase-9 gene expression in the IOMM-lee malignant meningioma cell line inhibits tumor growth, tumor cell invasion and angiogenesis. Int J Oncol. 2007;31(1):05–17. [PMC free article] [PubMed] [Google Scholar]
- 24.Lee WH. Characterization of a newly established malignant meningioma cell line of the human brain: IOMM-Lee. Neurosurgery. 1990;27(3):389–95. [PubMed] [Google Scholar]
- 25.Ragel BT, Elam IL, Gillespie DL, Flynn JR, et al. A novel model of intracranial meningioma in mice using luciferase-expressing meningioma cells. Laboratory investigation. J Neurosurg. 2008;108(2):304–10. [DOI] [PubMed] [Google Scholar]
- 26.Das A, Banik NL, Ray SK. Garlic compounds generate reactive oxygen species leading to activation of stress kinases and cysteine proteases for apoptosis in human glioblastoma T98G and U87MG cells. Cancer. 2007;110(5):1083–95. [DOI] [PubMed] [Google Scholar]
- 27.Das A, Banik NL, Ray SK. Flavonoids activated caspases for apoptosis in human glioblastoma T98G and U87MG cells but not in human normal astrocytes. Cancer. 2010;116(1):164–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sur P, Sribnick EA, Wingrave JM, Nowak MW, et al. Estrogen attenuates oxidative stress-induced apoptosis in C6 glial cells. Brain Res. 2003;971(2):178–88. [DOI] [PubMed] [Google Scholar]
- 29.Wallace GC 4th, Haar CP, Vandergrift WA 3rd, Giglio P, et al. Multi-targeted DATS prevents tumor progression and promotes apoptosis in ectopic glioblastoma xenografts in SCID mice via HDAC inhibition. J Neurooncol. 2013;114(1):43–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wu D, Pan W. GSK3: a multifaceted kinase in Wnt signaling. Trends Biochem Sci. 2010;35:161–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Metcalfe C, Bienz M. Inhibition of GSK3 by Wnt signalling—two contrasting models. J Cell Sci. 2011;124(Pt 21):3537–44. [DOI] [PubMed] [Google Scholar]
- 32.Knoblich K, Wang HX, Sharma C, Fletcher AL, et al. Tetraspanin TSPAN12 regulates tumor growth and metastasis and inhibits β-catenin degradation. Cell Mol Life Sci. 2014;71(7):1305–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Logan CY, Nusse R. The Wnt signaling pathway in development and disease. Annu Rev Cell Dev Biol. 2004;20:781–810. [DOI] [PubMed] [Google Scholar]
- 34.Luo J. Glycogen synthase kinase 3b (GSK3b) in tumorigenesis and cancer chemotherapy. Cancer Lett. 2009;273:194–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang D, Veena MS, Stevenson K, Tang C, et al. Liposome-encapsulated curcumin suppresses growth of head and neck squamous cell carcinoma in vitro and in xenografts through the inhibition of nuclear factor kappaB by an AKT-independent pathway. Clin Cancer Res. 2008;14(19):6228–36. [DOI] [PubMed] [Google Scholar]
- 36.Polakis P. Wnt signaling and cancer. Genes Dev. 2000;14(15): 1837–51. [PubMed] [Google Scholar]