Abstract
The polymeric immunoglobulin receptor (PIGR), an exosome-associated glycoprotein, plays an important role in the occurrence and development of different tumors. This study aimed to investigate whether PIGR is essential for colorectal cancer (CRC). Comprehensive bioinformatics analysis and immunohistochemistry (IHC) revealed that expression of PIGR was significantly decreased in CRC patients. Upregulated PIGR displayed favorable prognostic values in CRC patients. Several algorithms, such as TISIDB and TIMER, were used to evaluate the roles of PIGR expression in the regulation of immune response in CRC. Moreover, GSEA enrichment analysis indicated the underlying role of PIGR in the regulation of fatty acid metabolism in CRC. Taken together, our findings might provide a new potential prognostic and immune-associated biomarker for CRC and supply a new destination for PIGR-related immunotherapy in clinical treatment.
1. Introduction
Colorectal cancer (CRC) is one of the most common cancers worldwide, with a high incidence and mortality rate [1, 2]. In recent years, various types of clinical treatments have been applied to CRC patients, including systemic chemotherapy and radiation. However, the average 5-year survival rate of CRC patients with positive regional lymph nodes is only 40%, while less than 5% of patients with distant metastases survive beyond 5 years [3, 4]. Therefore, it is significantly important to explore a novel biomarker to improve the overall survival rate of CRC patients.
The tumor immune microenvironment (TIME) has an important role in mediating cytotoxic drug response and tumor progression [5]. Exploring the underlying mechanisms of TIME displays an important role in the occurrence and development of CRC [6, 7]. Immune cells combined with signaling biomarkers could play a crucial role in the prognostic prediction of CRC patients [8, 9]. Therefore, it is very important to further study the tumor microenvironment to improve the patients' overall survival. Exosomes, the nano-sized vesicles, have the inherent potential to shuttle diverse biomolecules like proteins, lipids, and nucleic acids to the recipient cells [10, 11]. Employing exosomes as vehicles for the delivery of products to initiate antitumor immune responses shows striking therapeutic effects [12, 13]. Thus, exosomes could be considered as potential therapeutic targets and valuable biomarkers for the treatment of malignancies.
The polymeric immunoglobulin receptor (PIGR), an exosome-associated glycoprotein, picks up its cargo on the basolateral surface and carries it by the process of transcytosis to the apical face. The function and regulation of PIGR may be closely related to the immune defense of organisms [14]. Numerous studies have recently demonstrated the important roles of aberrant PIGR in different tumors' tumorigenesis. Qi et al. considered that the PIGR may be a tumor suppressor in nasopharyngeal carcinoma [15]. Increased expression of PIGR was correlated with hepatic metastasis and poor prognosis in colon carcinoma patients [16]. Whereas, more studies are still required to investigate the relationship between PIGR expression and the prognosis of CRC patients.
In this article, we explored the underlying mechanism of PIGR in CRC. Based on bioinformatics analysis and immunohistochemical technology, it was found that the exosome-related gene PIGR was significantly downregulated in CRC tissues. Survival analysis showed that high expression of PIGR was associated with a good prognosis in CRC patients. Furthermore, we analyzed the relationship between PIGR and tumor-infiltrating immune cells (TIICs) in CRC. These findings indicate that PIGR could be a novel prognostic and immune-related biomarker in CRC patients.
2. Materials and Methods
2.1. Data Acquisition
Three CRC datasets, GSE20842 [17], GSE23878 [18] and GSE25070 [19], were downloaded from gene expression omnibus (GEO) database [20] (Table 1). Then, we explored the codifferently expressed genes (co-DEGs) between CRC tissues and normal colorectal tissues. The screening criteria was shown as follows: |log FC| ≥1.5 and p value <0.05. Next, we used Venn plots to explore the overlapping molecules between the exosome-associated dataset and three GEO datasets. Moreover, we employed the Cancer Genome Atlas (TCGA) database [21] to evaluate the effects of co-DEGs on the clinical characteristics of CRC patients.
Table 1.
The upregulated genes and downregulated genes in the three GEO datasets.
2.2. Bioinformatics Platforms
The profiles of co-DEGs were analyzed by comprehensive bioinformatic technologies (Table 2). The Kaplan–Meier plotter [22] was used to evaluate the prognostic values of the overlapping molecules, including overall survival (OS) and recurrence-free survival (RFS). In addition, several databases, such as TNMplot [23], GEPIA2.0 24 and TCGA-CRC, were used to confirm the downregulated expression level of PIGR. Subsequently, we used the Linked-Omics platform [25] to evaluate the interaction between PIGR and its coexpressed genes. Meanwhile, the Linked-Omics platform was used to analyze the gene enrichment. We employed the TISIDB [26], TIMER [27] and single-sample GSEA (ssGSEA) to evaluate the roles of PIGR expression in the regulation of immune response in CRC. We also evaluated the probable relationships between PIGR expression and several immune checkpoints, such as indoleamine 2,3-dioxygenase 1 (IDO1), CD274, programmed cell death 1 (PDCD1), cytotoxic T-lymphocyte associated protein 4 (CTLA4), and lymphocyte activating 3 (LAG3).
Table 2.
Bioinformatics platforms that are employed to analyze the role of PIGR in colorectal cancer.
| Database | URL | References |
|---|---|---|
| GEO | https://www.ncbi.nlm.nih.gov/gds/?term= | [20] |
| TCGA | https://portal.gdc.cancer.gov/ | [21] |
| Kaplan–Meier plotter | https://kmplot.com/analysis/ | [22] |
| TNMplot | https://www.tnmplot.com | [23] |
| GEPIA2.0 | https://gepia.cancer-pku.cn/ | [24] |
| Linked-Omics | https://www.linkedomics.org/admin.php | [25] |
| TISIDB | https://cis.hku.hk/TISIDB/ | [26] |
| TIMER | https://cistrome.shinyapps.io/timer/ | [27] |
2.3. Immunohistochemistry (IHC)
Tissue sections were deparaffinized in xylene and rehydrated with ethanol, and then preincubated with 10% normal goat serum in pharmaceutical benefits scheme (PBS) (pH 7.5). Then, the tissue slides were incubated with the primary antibody overnight at 4°C and then stained with a biotinylated secondary antibody (SAB4600042, Sigma-Aldrich) for 1 h at room temperature. The peroxidase reaction was visualized with a 3, 3-diaminobenzidine chromogenic kit (ZLI-9019, ORIGENE). After that, the tissues were photographed under a conventional microscope (DMI3000 B, Leica). The formalin-fixed, paraffin-embedded specimens of CRC and adjacent tissues were obtained from the Department of Pathology, Xiangya Hospital, Central South University. The ethics for this study (202205114) was approved by the Ethical Committee of Xiangya Hospital, Central South University.
2.4. Statistical Analysis
In this report, the statistical difference was investigated by t-test assay. And the data were mainly depicted as the mean ± standard deviation (SD). P values <0.05 was considered to demonstrate statistically significant differences.
3. Results
3.1. Identification of the Co-DEGs in Colorectal Cancer
We explored the co-DEGs between CRC tissues and normal colorectal tissues from three GEO-CRC datasets. And we found 1262 upregulated genes and 883 downregulated genes in GSE20842, 258 upregulated genes and 1011 downregulated genes in GSE23878, and 86 upregulated genes and 222 downregulated genes in GSE25070 (Supplementary Table S1). A Venn analysis (http://bioinformatics.psb.ugent.be/webtools/Venn/) was used to explore the potential differently-expressed exosome-related genes in CRC. Accordingly, one downregulated exosome-related gene, PIGR, was identified in CRC tissues (Figure 1(a)). Then, the Kaplan–Meier plotter database was used to analyze the effects of PIGR expression on the prognosis in CRC patients. As shown in Figures 1(b) and 1(c), high expression level of PIGR was related with good OS (HR = 0.39, 95% CI = 0.17–0.88, p=0.018) and RFS (HR = 0, 95% CI = 0-Inf, p=0.013) in CRC patients. These results collectively suggest that PIGR overexpression could be significantly associated with a favorable prognosis in CRC patients.
Figure 1.

Identification of downregulated exosome-related PIGR in CRC. (a) The Venn plot showed one downregulated exosome-correlated gene (PIGR) in CRC progression. (b-c) The prognostic values of PIGR in CRC patients. Abbreviations: OS, overall survival; RFS, recurrence-free survival.
3.2. Downregulated Expression of PIGR in Colorectal Cancer
By comprehensively analyzing the expression levels of PIGR in the three GEO-CRC datasets, we found that PIGR was lowly expressed in CRC tissues (p < 0.0001) (Figures 2(a)–2(c)). Besides, the data results from TCGA-CRC further verified that the expression level of PIGR was significantly different between the normal group and the CRC group (p < 0.0001) (Figure 2(d)). What's more, the TNMplot database indicated that PIGR mRNA expression was both lower in CRC tissues from gene chip data (p=3.06e − 17) and RNA-seq data (p=5.68e − 07) (Figures 2(e) and 2(f)). In addition, the IHC results confirmed that PIGR was downregulated in CRC tissues. Together, these results proved the lower expression of PIGR at mRNA and protein levels in CRC.
Figure 2.

PIGR was downregulated in CRC patients. (a-c) The expression level of PIGR was lower in the three GEO-CRC datasets. (d) The expression level of PIGR was lower in the TCGA-CRC. (e-f) TNMplot database depicting the downregulated PIGR expression in CRC tissues from gene chip data and RNA-seq data. (g) Representative IHC results showing the downregulated PIGR expression in CRC tissues.
3.3. The Coexpression Network of PIGR in Colorectal Cancer
We explored the coexpression network and biological functions of PIGR in the TCGA-Colorectal adenocarcinoma (COADREAD) cohort. In Figure 3(a) and Supplementary Table S2, we presented the PIGR coexpressed genes. Meanwhile, Figures 3(b) and 3(c) and Supplementary Tables S3 and S4 have demonstrated these candidate genes that are positively and negatively correlated with PIGR in CRC patients. Notably, the top 20 positively-related genes were highly likely to be low-risk factors for CRC patients (Figure 3(d)). Furthermore, 1 of top 20 negatively-related genes might be the high-risk factor in CRC (Figure 3(e)). In addition, the Gene Ontology showed that the genes coexpressed with PIGR were mainly involved in multiple biological process categories, such as biological regulation, metabolic processes, and response to stimulus. In the category of cellular component, these genes mainly took part in the membrane, nucleus and membrane-enclosed lumen. Then, in the molecular function categories, these coexpressed genes are involved in the protein binding, ion binding, and nucleic acid binding (Figure 3(f)). Moreover, the KEGG analysis indicated that the most likely enriched pathways were carbon metabolism, fructose and mannose metabolism, hippo signaling pathway, and Notch signaling pathway (Figure 3(g)).
Figure 3.

The coexpression network of PIGR in CRC. (a) The Linked-Omics platform portraying the crucially associated genes with PIGR in CRC patients. (b-c) Heatmaps showing the top genes that were positively and negatively correlated with PIGR in CRC. (d-e) Survival heatmaps downloaded from the GEPIA2.0 database displayed that the top genes that were positively and negatively associated with PIGR in CRC. (f-g) GO and KEGG enrichment of PIGR-coexpressed genes in CRC patients.
Next, we performed GSEA enrichment analysis of PIGR-related genes in CRC. As shown in Figures 4(a)–4(g), aberrantly expressed PIGR might involve in the regulation of fatty acid metabolism-related signaling pathways, such as mitochondrial fatty acid beta oxidation, mitochondrial fatty acid beta oxidation, fatty acid metabolism, nonalcoholic fatty acids. All the statistical significance wasp < 0.05. Overall, our results suggest that PIGR may be involved in the cellular metabolism in CRC.
Figure 4.

The GSEA enrichment analysis of PIGR-related genes in CRC. (a-g) PIGR-related genes were involved in several fatty acid metabolism pathways in CRC, such as mitochondrial fatty acid beta oxidation.
3.4. The Link between PIGR with Immune Regulation
We used ssGSEA to analyze the effects of PIGR on immune regulation in the TCGA-COADREAD cohort. The expression of PIGR was significantly positively correlated with T helper type 17 (Th17) cells, B cells, and so on (Figure 5(a)). The results obtained from TISIDB database also confirmed the similar findings (Figure 5(b)). The scatter plot from TIMER database further demonstrated that the expression level of PIGR was strongly positively correlated with B cell in colon adenocarcinoma (COAD) and rectum adenocarcinoma (READ) patients (Figure 5(c)).
Figure 5.
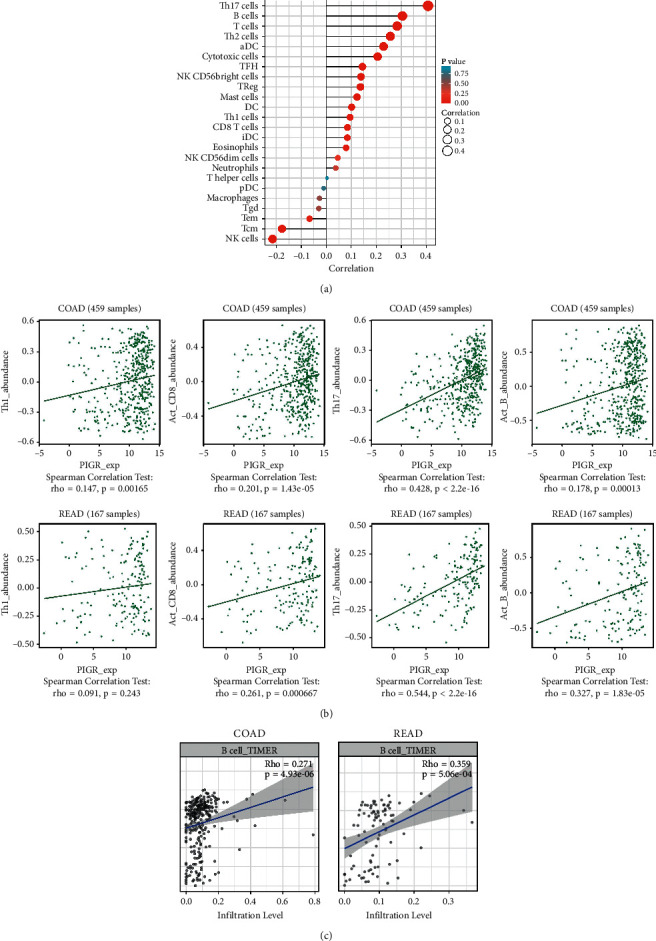
The relationship between the expression level of PIGR and immune cells in CRC patients. (a) The diagraph showing the relation between PIGR expression and 24 types of immune cells. (b) TISIDB database showed the relationship between PIGR and immune infiltration cells, such as T helper type 17 (Th17) cells, B cells, and so on. (c) TIMER database showed the relationship between the expression level of PIGR and immune infiltration cells.
Next, we explored the relationship between PIGR expression and several immune checkpoints and found that the expression level of PIGR was positively correlated with IDO1 (Spearman r = 0.186, p < 0.001), CD274 (Spearman r = 0.147, p < 0.001), PDCD1 (Spearman r = 0.186, p < 0.001), CTLA4 (Spearman r = 0.103, p=0.009), and LAG3 (Spearman r = 0.232, p < 0.001) (Figures 6(a)–6(c)). The patients with high level of PIGR displayed overexpressed IDO1, CD274, PDCD1, CTLA4, and LAG3 (Figure 6(f)). Additionally, the results from the TISIDB platform showed the relationship between PIGR levels and other immune-associated signatures, including immuno inhibitors and cytokine receptors. Figure 7(a) conveyed the correlation between the immuno inhibitors and the expression of PIGR in CRC patients. The top three immuno inhibitors closely related to PIGR were galectin 9 (LGALS9), LAG3, and CD244 (Figure 7(b)). In addition, the correlation between PIGR and cytokine receptors has been displayed in Figure 7(c). And the top three receptors positively associated with PIGR expression were C-X-C motif chemokine ligand 3 (CXCL3), C-X-C motif chemokine ligand 17 (CXCL17), and C–C motif chemokine ligand 28 (CCL28) (Figure 7(d)). Taken together, these findings suggest that aberrantly expressed PIGR is involved in the immune regulation of CRC patients.
Figure 6.

The relationship between PIGR expression and several immune checkpoints. (a-f) The scatterplot and heatmap depicted that PIGR expression was positively correlated to IDO1, CD274, PDCD1, CTLA4, and LAG3.
Figure 7.

The roles of PIGR in the regulation of immunoinhibitors and cytokine receptors in CRC patients. (a) The diagraph showing the correlation between PIGR expression and immunoinhibitors. (b) The scatter plots depicting the top three immunoinhibitors positively-related with PIGR expression. (c) The picture showing the connection between PIGR expression and cytokine receptors. (d) The scatter plots portraying the top three cytokine receptors positively-related with PIGR expression.
4. Discussion
Numerous studies have indicated the important role of exosomes in the occurrence and development of human cancers [28]. In this article, we elucidated the downregulated exosome-associated gene, PIGR, in the prognosis and immune regulation of CRC patients. Using the Kaplan–Meier plotter database, we found that high expression of PIGR was associated with a better prognosis in CRC patients. Gene enrichment analysis indicated that the coexpressed genes of PIGR were involved in the regulation of the immune microenvironment and fatty acid metabolism in CRC.
Exosomes are small extracellular vesicles secreted by almost all types of cells, including tumor cells. As important mediators of intercellular communication, exosomes provide an alternative cargo-handling mechanism to maintain homeostasis and cell survival [29]. Exosomes enhanced or inhibited certain important mediators and changed the tumor microenvironment, thereby altering the occurrence and development of different types of tumors [30–32]. Some differentially expressed RNAs and proteins in exosomes have been identified as potential biomarkers linked to CRC initiation and progression. Wang et al. considered that CRC cell-derived exosomal miR-146a-5p and miR-155-5p could activate the JAK2-STAT3/NF-κB signaling pathways, thereby enhancing the invasive ability of CRC cells [33]. Studies have found that in human CRC cells, exosomal Nrf2 plays a pivotal role in oxaliplatin resistance [34]. Thus, further studies into the underlying mechanisms of exosomes might be beneficial for the treatment management of CRC patients. Accordingly, our study aimed to explore the prognostic values of PIGR in CRC patients, and we concluded that a high expression level of PIGR was associated with a good prognosis.
Nowadays, the roles of aberrant PIGR in tumors remain controversial, which might be due to the tumor heterogeneity or the different underlying mechanisms. In hepatocellular carcinoma, PIGR-loaded extracellular vesicles could activate Akt/GSK3β/β-catenin signaling cascades, driving cancer stemness, tumorigenesis, and metastasis [35]. Ohkuma et al. have assessed the prognostic value of PIGR in pancreatic cancer patients after surgical resection and determined that the overexpression of PIGR was correlated with poor prognosis in pancreatic cancer [36]. Through transcriptomic sequencing analysis, Bao et al. demonstrated that PIGR was downregulated in breast cancer. PIGR overexpression could suppress cell proliferation and adhesion in breast cancer cells [37]. In this paper, the bioinformatics and IHC results revealed the downregulated exosome-related PIGR in CRC tissues.
Immunotherapy is a novel anticancer method in the clinic. The combination of traditional treatment methods with immune checkpoint inhibitors could provide promising treatment strategies for cancer patients, including CRC [38]. Numerous studies have shown that immunotherapy can inhibit the growth of colorectal cancer cells and prolong the survival period of patients [39–41]. In this paper, the correlation between PIGR and immune-associated signatures was explored by comprehensive bioinformatic technologies. PIGR was positive with tumor-infiltrating of B cells, Th17 cells, T cells, and Th2 cells. Meanwhile, PIGR had a negative correlation with tumor-infiltrating NK cells and Tcm cells. Studies have demonstrated that immune checkpoint inhibitors have been regarded as potential strategies for enhancing immune responses in patients with CRC [42]. We found that the expression of PIGR had a positive relationship with several immune checkpoints, including IDO1, CD274, PDCD1, CTLA4, and LAG3. These above results implied that PIGR was strongly associated with immune responses and immune regulation, implying that PIGR could be a novel prognostic and immune-related biomarker of CRC patients.
5. Conclusion
Overall, this paper reported that the downregulated exosome-associated gene PIGR was significantly associated with a good prognosis in CRC patients. Aberrant PIGR expression might participate in the regulation of immune response and fatty acid metabolism. Therefore, we identified PIGR as a novel, valuable prognostic and immune-related biomarker for patients with CRC.
Acknowledgments
This study is supported by the Horizontal Project (2021-021, 1 43010100).
Data Availability
The original contributions presented in the study are included in the article/Supplementary Material and are available upon request to the corresponding authors.
Disclosure
Ying Liu and Yongbin Hu are co-first authors.
Conflicts of Interest
The authors declare that there are no conflicts of interest.
Authors' Contributions
YL and YH performed conception and design and also performed writing, reviewing, and/or revision of the manuscript. Administrative, technical, or material support was provided by YL and LD. All authors approved the final version of the manuscript. Ying Liu and Yongbin Hu contributed equallly to this work.
Supplementary Materials
Supplementary Table 1. The upregulated genes and downregulated genes in the three GEO datasets. Supplementary Table 2. The coexpressed genes possess a positive and negative relationships with PIGR. Supplementary Table 3. The top 20 genes positively correlated with PIGR in colorectal cancer. Supplementary Table 4. The top 20 genes negatively correlated with PIGR in colorectal cancer.
References
- 1.Lei Z. N., Teng Q. X., Wu Z. X., et al. Overcoming multidrug resistance by knockout of ABCB1 gene using CRISPR/Cas9 system in SW620/Ad300 colorectal cancer cells. MedComm . 2021;2(4):765–777. doi: 10.1002/mco2.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li C., Zhang K., Pan G., et al. Dehydrodiisoeugenol inhibits colorectal cancer growth by endoplasmic reticulum stress-induced autophagic pathways. Journal of Experimental & Clinical Cancer Research . 2021;40(1):p. 125. doi: 10.1186/s13046-021-01915-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fonseca A., Ramalhete S. V., Mestre A., et al. Identification of colorectal cancer associated biomarkers: an integrated analysis of miRNA expression. Aging . 2021;13(18) doi: 10.18632/aging.203556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhou R. C., Wang P. Z., Li Y. Y., et al. Quality improvement of sample collection increases the diagnostic accuracy of quantitative fecal immunochemical test in colorectal cancer screening: a pilot study. Frontiers of Medicine . 2021;8 doi: 10.3389/fmed.2021.762560.762560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Shi R., Tang Y. Q., Miao H. Metabolism in tumor microenvironment: implications for cancer immunotherapy. MedComm . 2020;1(1):47–68. doi: 10.1002/mco2.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y. F., Yu Z. L., Lv M. Y., et al. Genome-Wide analysis reveals hypoxic microenvironment is associated with immunosuppression in poor survival of stage II/III colorectal cancer patients. Frontiers of Medicine . 2021;8 doi: 10.3389/fmed.2021.686885.686885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu Y., Cheng L., Li C., Zhang C., Wang L., Zhang J. Identification of tumor microenvironment-related prognostic genes in colorectal cancer based on bioinformatic methods. Scientific Reports . 2021;11(1) doi: 10.1038/s41598-021-94541-6.15040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yin Y., Yao S., Hu Y., et al. The immune-microenvironment confers chemoresistance of colorectal cancer through macrophage-derived IL6. Clinical Cancer Research . 2017;23(23) doi: 10.1158/1078-0432.ccr-17-1283. [DOI] [PubMed] [Google Scholar]
- 9.Schimek V., Strasser K., Beer A., et al. Tumour cell apoptosis modulates the colorectal cancer immune microenvironment via interleukin-8-dependent neutrophil recruitment. Cell Death & Disease . 2022;13(2):p. 113. doi: 10.1038/s41419-022-04585-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim M. Y., Shin H., Moon H. W., Park Y. H., Park J., Lee J. Y. Urinary exosomal microRNA profiling in intermediate-risk prostate cancer. Scientific Reports . 2021;11(1) doi: 10.1038/s41598-021-86785-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koomullil R., Tehrani B., Goliwas K., et al. Computational simulation of exosome transport in tumor microenvironment. Frontiers of Medicine . 2021;8 doi: 10.3389/fmed.2021.643793.643793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Shimada Y., Matsubayashi J., Kudo Y., et al. Serum-derived exosomal PD-L1 expression to predict anti-PD-1 response and in patients with non-small cell lung cancer. Scientific Reports . 2021;11(1) doi: 10.1038/s41598-021-87575-3.7830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thakur A., Ke X., Chen Y. W., et al. The mini player with diverse functions: extracellular vesicles in cell biology, disease, and therapeutics. Protein and cell . 2022;13(9):631–654. doi: 10.1007/s13238-021-00863-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johansen F. E., Kaetzel C. S. Regulation of the polymeric immunoglobulin receptor and IgA transport: new advances in environmental factors that stimulate pIgR expression and its role in mucosal immunity. Mucosal Immunology . 2011;4(6):598–602. doi: 10.1038/mi.2011.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Qi X., Li X., Sun X. Reduced expression of polymeric immunoglobulin receptor (pIgR) in nasopharyngeal carcinoma and its correlation with prognosis. Tumor Biology . 2016;37(8) doi: 10.1007/s13277-016-4791-x. [DOI] [PubMed] [Google Scholar]
- 16.Liu F., Ye P., Bi T., et al. COLORECTAL polymeric immunoglobulin receptor expression is correlated with hepatic metastasis and poor prognosis in colon carcinoma patients with hepatic metastasis. Hepato-Gastroenterology . 2014;61(131):652–659. [PubMed] [Google Scholar]
- 17.Gaedcke J., Grade M., Jung K., et al. Mutated KRAS results in overexpression of DUSP4, a MAP-kinase phosphatase, and SMYD3, a histone methyltransferase, in rectal carcinomas. Genes, Chromosomes and Cancer . 2010;49(11) doi: 10.1002/gcc.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Uddin S., Ahmed M., Hussain A., et al. Genome-wide expression analysis of Middle Eastern colorectal cancer reveals FOXM1 as a novel target for cancer therapy. American Journal Of Pathology . 2011;178(2):537–547. doi: 10.1016/j.ajpath.2010.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hinoue T., Weisenberger D. J., Lange C. P., et al. Genome-scale analysis of aberrant DNA methylation in colorectal cancer. Genome Research . 2012;22(2):271–282. doi: 10.1101/gr.117523.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clough E., Barrett T. The gene expression omnibus database. Methods in Molecular Biology . 2016;1418:93–110. doi: 10.1007/978-1-4939-3578-9_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCain J. The cancer genome atlas: new weapon in old war? Biotechnology Healthcare . 2006;3(2):46–51B. [PMC free article] [PubMed] [Google Scholar]
- 22.Lanczky A., Gyorffy B. Web-Based survival analysis tool tailored for medical research (KMplot): development and implementation. Journal of Medical Internet Research . 2021;23(7) doi: 10.2196/27633.e27633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bartha A., Gyorffy B. TNMplot.com: a web tool for the comparison of gene expression in normal, tumor and metastatic tissues. International Journal of Molecular Sciences . 2021;22(5) doi: 10.3390/ijms22052622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang Z., Kang B., Li C., Chen T., Zhang Z. GEPIA2: an enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Research . 2019;47 doi: 10.1093/nar/gkz430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasaikar S. V., Straub P., Wang J., Zhang B. LinkedOmics: analyzing multi-omics data within and across 32 cancer types. Nucleic Acids Research . 2018;46 doi: 10.1093/nar/gkx1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ru B., Wong C. N., Tong Y., et al. TISIDB: An integrated repository portal for tumor-immune system interactions. Bioinformatics . 2019;35(20) doi: 10.1093/bioinformatics/btz210. [DOI] [PubMed] [Google Scholar]
- 27.Li T., Fan J., Wang B., et al. TIMER: a web server for comprehensive analysis of tumor-infiltrating immune cells. Cancer Research . 2017;77(21):e108–e110. doi: 10.1158/0008-5472.can-17-0307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu W. J., Shen Y. W., Zhang L. J., et al. The multifaceted involvement of exosomes in tumor progression: induction and inhibition. MedComm . 2021;2(3):297–314. doi: 10.1002/mco2.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jiang H., Zhao H., Zhang M., et al. Hypoxia induced changes of exosome cargo and subsequent biological effects. Frontiers in Immunology . 2022;13 doi: 10.3389/fimmu.2022.824188.824188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thakur A., Johnson A., Jacobs E., et al. Energy sources for exosome communication in a cancer microenvironment. Cancers . 2022;14(7) doi: 10.3390/cancers14071698.1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen W. X., Wang D. D., Zhu B., et al. Exosomal miR-222 from adriamycin-resistant MCF-7 breast cancer cells promote macrophages M2 polarization via PTEN/Akt to induce tumor progression. Aging . 2021;13(7) doi: 10.18632/aging.202802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tian W., Yang H., Zhou B. Integrative analysis of exosomal microRNA-149-5p in lung adenocarcinoma. Aging . 2021;13(5) doi: 10.18632/aging.202596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang D., Wang X., Song Y., et al. Exosomal miR-146a-5p and miR-155-5p promote CXCL12/CXCR7-induced metastasis of colorectal cancer by crosstalk with cancer-associated fibroblasts. Cell Death and Disease . 2022;13(4):p. 380. doi: 10.1038/s41419-022-04825-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mostafazadeh M., Kahroba H., Haiaty S., et al. In vitro exosomal transfer of Nrf2 led to the oxaliplatin resistance in human colorectal cancer LS174T cells. Cell Biochemistry and Function . 2022;40(4):391–402. doi: 10.1002/cbf.3703. [DOI] [PubMed] [Google Scholar]
- 35.Tey S. K., Wong S. W. K., Chan J. Y. T., et al. Patient pIgR-enriched extracellular vesicles drive cancer stemness, tumorigenesis and metastasis in hepatocellular carcinoma. Journal of Hepatology . 2022;76(4):883–895. doi: 10.1016/j.jhep.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 36.Ohkuma R., Yada E., Ishikawa S., et al. High expression levels of polymeric immunoglobulin receptor are correlated with chemoresistance and poor prognosis in pancreatic cancer. Oncology Reports . 2020;44(1):252–262. doi: 10.3892/or.2020.7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bao Y., Wang L., Shi L., et al. Transcriptome profiling revealed multiple genes and ECM-receptor interaction pathways that may be associated with breast cancer. Cellular and Molecular Biology Letters . 2019;24(1):p. 38. doi: 10.1186/s11658-019-0162-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shi R., Li Y., Ran L., et al. Screening and identification of HLA-A2-restricted neoepitopes for immunotherapy of non-microsatellite instability-high colorectal cancer. Science China Life Sciences . 2022;65(3):572–587. doi: 10.1007/s11427-021-1944-5. [DOI] [PubMed] [Google Scholar]
- 39.Lv Q., Pan X., Wang D., et al. Discovery of (Z)-1-(3-((1H-Pyrrol-2-yl)methylene)-2-oxoindolin-6-yl)-3-(isoxazol-3-yl)urea derivatives as novel and orally highly effective CSF-1R inhibitors for potential colorectal cancer immunotherapy. Journal of Medicinal Chemistry . 2021;64(23) doi: 10.1021/acs.jmedchem.1c01184. [DOI] [PubMed] [Google Scholar]
- 40.Shi J., Bao M., Wang W., et al. Integrated profiling identifies PLOD3 as a potential prognostic and immunotherapy relevant biomarker in colorectal cancer. Frontiers in Immunology . 2021;12 doi: 10.3389/fimmu.2021.722807.722807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Soh J. E., Abu N., Sagap I., et al. Validation of immunogenic PASD1 peptides against HLA-A∗24:02 colorectal cancer. Immunotherapy . 2019;11(14) doi: 10.2217/imt-2019-0073. [DOI] [PubMed] [Google Scholar]
- 42.Fathi M., Pustokhina I., Kuznetsov S. V., et al. T-cell immunoglobulin and ITIM domain, as a potential immune checkpoint target for immunotherapy of colorectal cancer. IUBMB Life . 2021;73(5):726–738. doi: 10.1002/iub.2461. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1. The upregulated genes and downregulated genes in the three GEO datasets. Supplementary Table 2. The coexpressed genes possess a positive and negative relationships with PIGR. Supplementary Table 3. The top 20 genes positively correlated with PIGR in colorectal cancer. Supplementary Table 4. The top 20 genes negatively correlated with PIGR in colorectal cancer.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material and are available upon request to the corresponding authors.


