Abstract
Background
There is no curative treatment option for patients with unresectable intrahepatic, cholangiocarcinoma (IHC). The aim of this study was to evaluate the efficacy of; radiation segmentectomy/lobectomy using Y90-labeled glass microspheres in patients with; unresectable IHC.
Methods
This IRB-approved, single-center study included, 16 patients (age: 67 ± 7.7 years) with IHC who received radiation segmentectomy or lobectomy, treatment using Y90-labeled glass microspheres between May 2009 and October 2019. Radiation, segmentectomy/lobectomy was defined as at least 190 Gy dose delivered into treated liver; volume.
Results
The median OS from IHC diagnosis was 22.7 months (95% CI: 13.9–66.1) and from, radioembolization it was 7 months (95% CI: 4.33–54.17). Patients who did not receive, chemotherapy before the radioembolization had significantly longer median OS (26.8 vs. 5.9, months, P = 0.03). Four patients had >20 months survival after radioembolization, including 2, patients with survival of 42 and 54 months. There was no 30-day mortality and no severe, complications.
Conclusion
Radiation segmentectomy/lobectomy is safe with minimal side effects. The median, OS of the study group is modest; however, 4 patients (25%) showed excellent survival. These results suggest a need for a larger study to define the IHC patient group who could, most benefit from this procedure.
Keywords: liver, cholangiocarcinoma, radioembolization, radiation segmentectomy, radiation lobectomy
Abbreviations: IHC, Intrahepatic Cholangiocarcinoma; IRB, Institutional Review Board; Y90, Yttrium 90; OS, Overall Survival; Gy, Gray; HCC, Hepatocellular Carcinoma; ECOG, Eastern Cooperative Oncology Group; INR, International Normalized Ratio; TARE, Transarterial Radioembolization
Currently, there is no curative treatment option for patients with unresectable intrahepatic cholangiocarcinoma (IHC). The disease has a dismal prognosis with less than 10% of all IHC patients surviving for more than five years after the diagnosis regardless of the treatment they receive, with palliative treatment offering a median overall survival of three months.1,2 Surgical resection represents the only potentially curative treatment. However, many patients are not surgical candidates due to the advanced stage of the disease at diagnosis or various comorbidities or tumor locations.3 Additionally, an analysis of an international surgical database that included 563 patients demonstrated that 71% of patients had a recurrence following curative intent surgical resection.4
Transarterial radiation segmentectomy and lobectomy with yttrium 90 (Y90)-labeled microspheres is an emerging new technology that could provide curative treatment for patients with liver-only disease who are not surgical candidates, as well as patients looking to avoid major liver surgery with its potential complications and high recurrence rate.5 Radiation segmentectomy is defined as selective delivery of 190 Gy or higher radiation dose to ≤ 2 segments of the liver, while radiation lobectomy involves the delivery of 190 Gy or higher radiation dose to a hepatic lobe.6 Vouche at al. demonstrated complete pathologic necrosis in hepatocellular carcinoma (HCC) treated with 190 Gy or higher radiation dose during radioembolization.6
Currently, there is no literature on survival outcomes of radiation segmentectomy or lobectomy in unresectable IHC, but given the effectiveness of radiation segmentectomy and lobectomy in treating HCC7,8 the aim of this proof of concept retrospective cohort study was to investigate the safety and outcome of radiation segmentectomy and lobectomy in patients with IHC using Y90-labeled glass microspheres.
Materials and methods
Patients
The Institutional Review Board authorized this study. A review of our institution's radioembolization database identified 162 consecutive patients with biopsy proven IHC who underwent radioembolization between May 2009 and October 2019. Based on the report of Vouche et al., radiation segmentectomy/lobectomy was defined as at least 190 Gy dose delivered into the treated liver volume.6 The final study cohort consisted of 16 patients with IHC who received radiation segmentectomy or lobectomy treatment by delivering 190 Gy or higher dose to the treated liver volume. A retrospective review of medical records and imaging studies of these 16 patients was performed. Criteria for receiving radioembolization treatment included Eastern Cooperative Oncology Group (ECOG) performance status score ≤2, total serum bilirubin ≤2 mg/dl, serum creatinine ≤2 mg/dl, international normalized ratio (INR) ≤ 1.5, and platelet count ≥50,000/μl. Patients were not excluded if they had received previous liver-directed therapy or multiple lines of chemotherapy before the radioembolization treatment.
Patient demographics are summarized in Table 1. The study included six men and ten women median age of 66 years (range 48–80 years). Ten patients received radiation segmentectomy and 6 patients received radiation lobectomy. Four patients underwent two radiation segmentectomy treatments. The median interval between the diagnosis of IHC and radiation segmentectomy/lobectomy treatment was 7.4 months (range, 2.0–24.4 months). At the time of the radioembolization treatment, 10 patients had the extrahepatic disease, 10 patients received chemotherapy before the first radioembolization treatment, and 5 patients had prior liver-directed therapy (3 had a surgical resection, 1 had external-beam radiation, and 1 had surgical resection and external-beam radiation). Of the two patients who underwent prior radiation therapy, one underwent intensity modulated radiation therapy and the other underwent stereotactic body radiation therapy. Four patients received radiation segmentectomy/lobectomy as first-line treatment. Nine patients received systemic chemotherapy after the radiation segmentectomy/lobectomy treatment.
Table 1.
Demographic Characteristics of Patients.
| n | % | |
|---|---|---|
| Age (years) | ||
| < 70 | 10 | 60.5 |
| ≥ 70 | 6 | 37.5 |
| Gender | ||
| Male | 6 | 37.5 |
| Female | 10 | 62.5 |
| ECOG | ||
| 0 | 8 | 50.0 |
| 1 | 8 | 50.0 |
| Tumor grade | ||
| Well differentiated | 3 | 18.7 |
| Moderately differentiated | 8 | 50.0 |
| Poorly differentiated | 2 | 12.5 |
| Not reported | 3 | 18.7 |
| Distribution | ||
| Unilobar | 15 | 93.7 |
| Bilobar | 1 | 6.3 |
| Number of tumors | ||
| Solitary | 13 | 81.2 |
| Multiple | 3 | 18.8 |
| Tumor morphology | ||
| Mass forming | 6 | 37.5 |
| Infiltrative | 10 | 62.5 |
| Tumor enhancement | ||
| Hypoenhancing | 9 | 56.2 |
| Hyperenhancing | 7 | 43.8 |
| Extrahepatic metastasis | ||
| No | 6 | 37.5 |
| Yes | 10 | 62.5 |
| Nodal metastasis | 6 | 37.5 |
| Distant metastasis | 4 | 25 |
| Previous chemotherapy | ||
| None | 6 | 37.5 |
| Yes | 10 | 62.5 |
| Previous liver-directed therapy | ||
| None | 11 | 68.7 |
| Resection | 3 | 18.8 |
| Radiation | 1 | 6.2 |
| Radiation + Resection | 1 | 6.2 |
ECOG: Eastern Cooperative Oncology Group.
Radioembolization Procedure
Planning angiogram was performed on all patients before the radioembolization treatment as previously described.9 As part of the planning angiography, tumor-feeding vessels and anatomic variants were identified, and technetium-99-labeled macroaggregated albumin was injected into the hepatic arteries to determine the degree of hepatopulmonary shunting. Radioembolization was performed using Y-90 labeled glass microspheres (TheraSphere; BTG International, London, England). The delivered hepatic dose was determined based on the treated liver volume, the administered activity, and the lung shunt fraction using the medical internal radiation dose equation (MIRD) model. The procedures were performed by 5 interventional radiologists with 6–10 years of experience in radioembolization.
Clinical Outcome Measures
Overall survival (OS) was calculated using the date of the diagnosis, as well as the date of the first treatment to the last encounter or death. Clinical and laboratory assessments were conducted during a follow-up visit three months after radioembolization treatment. Clinical and biochemical toxicities were defined using both subjective reporting by the patient and corresponding measures in the Common Terminology Criteria for Adverse Events scoring system (version 5.0). Response Evaluation Criteria in Solid Tumors (RECIST) was employed to evaluate tumor response three months after treatment.10
Statistical Analysis
The probabilities of OS were estimated using the Kaplan–Meier method to report median OS and 95% confidence intervals (95% CIs). Differences in OS estimates were compared using log–rank tests with Bonferroni's correction. Univariate Cox proportional hazards regression analyses were conducted to investigate the predictors of OS. The pre- and post-radioembolization MELD scores were compared using the Wilcoxson signed rank test. Statistical analyses were performed using MedCalc software (MedCalc Software Ltd, Ostend, Belgium) and the SPSS version 26 software (IBM Corporation, Armonk, New York).
Results
Radiation Segmentectomy/Lobectomy Treatment
Twelve patients received one radioembolization treatment (6 were segmental, and 6 were lobar treatments) and 4 patients received two treatments to 2 different hepatic segments.
The average delivered dose was 309.27 ± 198.94 Gy (median delivered dose was 225.1 Gy). The average lung shunt was 4.8 ± 1.4% (median lung shunt was 4.6%).
Survival Outcomes
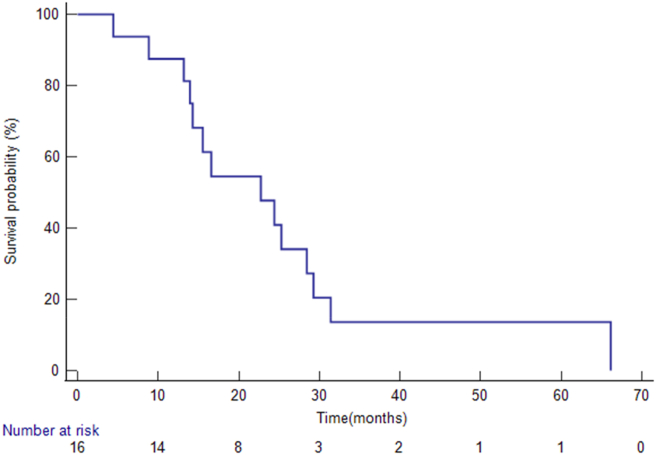
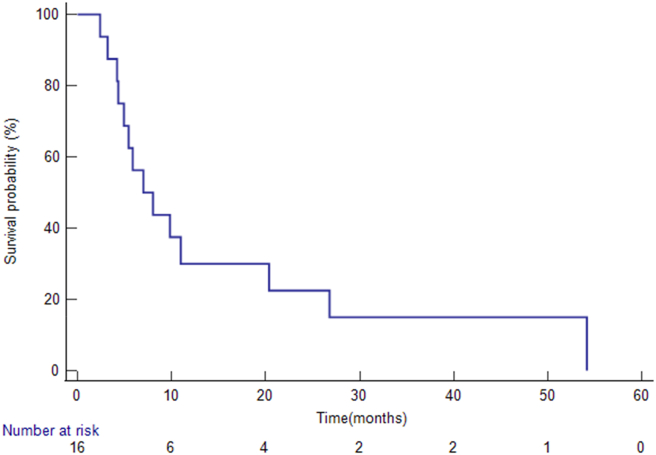
The median follow-up interval from the radiation segmentectomy/lobectomy treatment was 7.5 months (range, 2.4–54.2 months). When the analysis was conducted, 14 patients had died. The median OS from IHC diagnosis was 22.7 months (95% CI: 13.9–66.1) (Figure 1), and the median OS from radiation segmentectomy/lobectomy was 7 months (95% CI: 4.33–54.17) (Figure 2). The six patients who did not undergo systemic chemotherapy before the radioembolization had significantly longer median OS than patients who had pre-embolization systemic chemotherapy (26.8 vs. 5.9 months, P = 0.03). Four patients had survival over 20 months (Table 2).
Figure 1.
Overall survivals from the diagnosis of IHC. The Kaplan–Meier method revealed that the median overall survival from IHC diagnosis was 22.7 months (95% CI: 13.9–66.1).
Figure 2.
Overall survivals from the radiation segmentectomy/lobectomy. The Kaplan–Meier method revealed that the median overall survival from radiation segmentectomy/lobectomy was 7 months (95% CI: 4.33–54.17).
Table 2.
Patients with Long Survival After TARE.
| Patient # | Age at Treatment | Gender | Dose (in Gy) | Treatment Location | Alive | Median OS from Diagnosis (in months) | Median OS from TARE (in months) |
|---|---|---|---|---|---|---|---|
| 2 | 49 | M | 305.9 | Segment 4 | Yes | 44.13 | 41.9 |
| 6 | 66 | M | 202.3 | Left Lobe | No | 25.27 | 20.37 |
| 8 | 77 | F | 350.5 | Segment 4 | No | 29.27 | 26.80 |
| 10 | 71 | F | 198.4 | Segment 7 | No | 66.13 | 54.17 |
TARE: Transarterial Radioembolization; OS: Overall Survival.
Patients who had an albumin toxicity grade of 0 had significantly longer median OS than patients with albumin toxicity grades of 1 or 2 (20.4 vs. 4.9 vs. 3.2 months, P = 0.000). A bilirubin toxicity grade of 0 was associated with a significantly better median OS than a bilirubin toxicity grade of 2 or 4 (11 vs. 5.4 vs. 3.2 months; P = 0.000). Patients with an AST toxicity grade of 0 had a longer median OS than patients with an AST toxicity grade of 1 (20.4 vs. 5.4 months; P = 0.001). An ALT toxicity grade of 0 was associated with a significantly greater median OS than an ALT toxicity grade of 1 (9.8 vs. 3.2 months; P < 0.001).
Clinical and Biochemical Toxicities
No instances of mortality were reported within the 30 days following radioembolization therapy. The reported clinical toxicity after radiation segmentectomy/lobectomy was overall mild, and instances of severe complications were not reported. Two patients did not report side effects from the treatment. In those who did, side effects included fatigue (grades 1 and 2, n = 9), abdominal pain (grades 1 and 2, n = 10), ascites (grade 2, n = 1), and nausea (grade 1, n = 1). Thirty-three events of mild (grades 1 and 2) biochemical toxicities were detected in 14 patients at 3-month follow-up after radiation segmentectomy/lobectomy. The most common biochemical toxicity was mildly elevated alkaline phosphatase (grade 1 and 2 toxicities), which 12 patients experienced. Grade 4 elevated bilirubin was detected in 1 patient at 3-month follow-up. The MELD score was significantly higher at the 3-month follow-up compared with the baseline score before the radioembolization (6.79 ± 1.61 vs. 8.64 ± 3.77, P = 0.01).
Discussion
Limited treatment options exist for patients diagnosed with unresectable cholangiocarcinoma. The current first-line chemotherapeutic regimen, gemcitabine with cisplatin, resulted in a prolonged median OS of 11.7 months, compared to 8.1 months with gemcitabine alone.11 Radioembolization appears to be more effective with a reported median OS of 21.4 months from diagnosis and 12.4 months from the first radioembolization treatment.12
In HCC, radiation segmentectomy has been suggested as a potentially curative treatment for patients with preserved liver function who cannot undergo liver resection. Lewandowski et al. reported a median overall survival of 6.7 years for a cohort of 70 patients with HCC who underwent radiation segmentectomy with Y90-labeled glass microspheres.8 Radiation lobectomy with Y90-labeled glass microspheres has also yielded promising results in HCC, with a high response rate, prolonged survival, and significant contralateral lobar hypertrophy.7
In the present study, radiation segmentectomy and lobectomy of IHC using Y90-labeled glass microspheres resulted in a median OS of 22.7 months from the time of diagnosis. This result compares favorably to treatment with chemotherapy alone, with a reported OS of 11.7 months.11 However, the median OS from the radiation segmentectomy or lobectomy was only 7 months. This result falls short of the 12.4 months median OS reported for radioembolization of IHC patients in a larger scale study.12 This may be due to the low number of patients included in the current study.
The current study hypothesized that radiation segmentectomy/lobectomy of IHC competes with surgical outcomes, similar to the results with HCC where radiation segmentectomy/lobectomy survival outcomes compared favorably to surgical resection.7,8 Data from a recent multi-center database demonstrated 38 months median OS of IHC patients after surgical resection.13 The current study shows that radiation segmentectomy /lobectomy is inferior to surgical resection in patients with IHC based on these findings. On the other hand, very promising results were observed in 4 patients who survived more than 20 months after radioembolization, and 2 of them survived over 3 and 4 years, respectively. One patient is still alive without evidence of disease (Table 2). This may suggest that radiation segmentectomy can be very effective in certain IHC patients. The current study has a small number of patients, and it doesn't allow identifying all the prognostic factors which determine survival after radiation segmentectomy/lobectomy and would help with better patient selection.
In the present study, the radiation segmentectomy and lobectomy treatments were well tolerated by the patients, with only one grade 4 toxicity was observed. This 6.2% major complication rate is much higher than the reported 0–1.2% seen in studies including larger number of IHC patients12,14 and this may be due to the small number of patients in the current analysis. A few factors were identified that were associated with improved survival. Patients who did not undergo systemic chemotherapy before the radiation segmentectomy or lobectomy had excellent survival (26.8 months). This was statistically significant compared to patients who did undergo pre-treatment systemic chemotherapy (5.9 months). As was expected, patients who did not develop post-treatment albumin, bilirubin, AST, and ALT toxicity had significantly longer survival than patients who developed biochemical toxicity after the treatment.
The study has several limitations. This is a retrospective, single-institution study without a control group. Selection bias also may have skewed the results, as the study was performed in a tertiary cancer center, and criteria for radioembolization treatment could vary between our institution and others as based on decisions made by a multidisciplinary tumor board. Most patients received other treatments prior to radioembolization, therefore, lead-time bias may contribute to the overall survival measured from the diagnosis. Additionally, post-radioembolization chemotherapy may contribute to overall survival measured from the radioembolization treatment. Lastly, the relatively small number of patients did not allow identification of all of the prognostic factors that may influence patient outcomes.
In conclusion, radiation segmentectomy and lobectomy are safe with minimal side effects. The median OS of the study group was modest; however, four patients showed excellent survival. These results suggest that radiation segmentectomy and lobectomy are feasible in unresectable IHC and demonstrate the need for a larger-scale study to better define the IHC patient group who could most benefit from these treatments.
Credit authorship contribution statement
Premsai Kumar, BSc: Conceptualization, Methodology, Writing – Original Draft, Investigation, Data Curation, Visualization, Rahul Mhaskar, PhD, MPH: Software, Formal Analysis, Visualization, Richard Kim, MD: Conceptualization, Resources, Daniel Anaya, MD: Conceptualization, Resources, Jessica Frakes, MD: Conceptualization, Resources, Sarah Hoffe, MD: Conceptualization, Resources, Junsung Choi, MD: Conceptualization, Resources, Bela Kis, MD, PhD: Conceptualization, Writing – Review and Editing, Methodology, Resources, Supervision, Project Administration.
Conflicts of interest
The author has none to declare.
References
- 1.Park J., Kim M.H., Kim K.P., et al. Natural history and prognostic factors of advanced cholangiocarcinoma without surgery, chemotherapy, or radiotherapy: a large-scale observational study. Gut Liver. 2009;3:298–305. doi: 10.5009/gnl.2009.3.4.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Simo K.A., Halpin L.E., McBrier N.M., et al. Multimodality treatment of intrahepatic cholangiocarcinoma: a review. J Surg Oncol. 2016;113:62–83. doi: 10.1002/jso.24093. [DOI] [PubMed] [Google Scholar]
- 3.Morise Z., Sugioka A., Tokoro T., et al. Surgery and chemotherapy for intrahepatic cholangiocarcinoma. World J Hepatol. 2010;2:58–64. doi: 10.4254/wjh.v2.i2.58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spolverato G., Kim Y., Alexandrescu S., et al. Management and outcomes of patients with recurrent intrahepatic cholangiocarcinoma following previous curative-intent surgical resection. Ann Surg Oncol. 2016;23:235–243. doi: 10.1245/s10434-015-4642-9. [DOI] [PubMed] [Google Scholar]
- 5.Shaker T.M., Chung C., Varma M.K., et al. Is there a role for Ytrrium-90 in the treatment of unresectable and metastatic intrahepatic cholangiocarcinoma? Am J Surg. 2018;215:467–470. doi: 10.1016/j.amjsurg.2017.11.022. [DOI] [PubMed] [Google Scholar]
- 6.Vouche M., Habib A., Ward T.J., et al. Unresectable solitary hepatocellular carcinoma not amenable to radiofrequency ablation: multicenter radiology-pathology correlation and survival of radiation segmentectomy. Hepatology. 2014;60:192–201. doi: 10.1002/hep.27057. [DOI] [PubMed] [Google Scholar]
- 7.Gaba R.C., Lewandowski R.J., Kulik L.M., et al. Radiation lobectomy: preliminary findings of hepatic volumetric response to lobar yttrium-90 radioembolization. Ann Surg Oncol. 2009;16:1587–1596. doi: 10.1245/s10434-009-0454-0. [DOI] [PubMed] [Google Scholar]
- 8.Lewandowski R.J., Gabr A., Abouchaleh N., et al. Radiation segmentectomy: potential curative therapy for early hepatocellular carcinoma. Radiology. 2018;287:1050–1058. doi: 10.1148/radiol.2018171768. [DOI] [PubMed] [Google Scholar]
- 9.Salem R., Lewandowski R.J., Gates V.L., et al. Research reporting standards for radioembolization of hepatic malignancies. J Vasc Interv Radiol. 2011;22:265–278. doi: 10.1016/j.jvir.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishino M., Jagannathan J.P., Ramaiya N.H., Van den Abbeele A.D. Revised RECIST guideline version 1.1: what oncologists want to know and what radiologists need to know. AJR Am J Roentgenol. 2010;195:281–289. doi: 10.2214/AJR.09.4110. [DOI] [PubMed] [Google Scholar]
- 11.Valle J., Wasan H., Palmer D.H., et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 12.Gangi A., Shah J., Hatfield N., et al. Intrahepatic cholangiocarcinoma treated with transarterial yttrium-90 glass microsphere radioembolization: results of a single institution retrospective study. J Vasc Interv Radiol. 2018;29:1101–1108. doi: 10.1016/j.jvir.2018.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panayotova G., Guerra J., Guarrera J., et al. The role of surgical resection and liver transplantion for the treatment of intrahepatic cholangiocarcinoma. J Clin Med. 2021;10:2428. doi: 10.3390/jcm10112428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paprottka K., Galiè F., Ingrisch M., et al. Outcome and safety after 103 radioembolizations with yttrium-90 resin micropsheres in 73 patients with unresectable intrahepatic cholangiocaricnoma - an evaluation of predictors. Cancers (Basel) 2021;13:5399. doi: 10.3390/cancers13215399. [DOI] [PMC free article] [PubMed] [Google Scholar]