Abstract
Changes in gut microbiota (GM) may be associated with the causation and progression of multiple liver diseases such as metabolic-associated liver disease, alcohol-associated liver disease (ALD), alcohol-associated hepatitis (AH), primary biliary cholangitis, primary sclerosing cholangitis, autoimmune liver disease, and most importantly, complications of cirrhosis and portal hypertension such as hepatic encephalopathy (HE), infection, and hepatocellular carcinoma. ALD includes simple steatosis, steatohepatitis, AH, cirrhosis, and acute-on-chronic liver failure. Alcohol consumption is associated with GM changes even before ALD development, and continued alcohol intake results in progressive dysbiosis and development of clinical events such as AH, infection, and HE. The composition and function of GM, specific changes in bacterial communities, and the functional metabolism of GM are affected in the spectrum of ALD, as revealed using high-throughput sequencing. It was reported in preliminary studies that modulation of disrupted GM improves adverse clinical events and ameliorates disease progression in ALD. In this review, we exhaustively discuss the preclinical and clinical studies on GM in ALD and critically discuss GM modulation and its effects based on various human and animal models of ALD.
Keywords: ALD, ACLF, microbiome, FMT, cirrhosis
Abbreviations: ALD, alcohol-associated liver disease; AH, alcohol-associated hepatitis; ACLF, acute on chronic liver failure; AUD, alcohol use disorder; FMT, fecal microbiota transplantation; GM, gut microbiota; HE, hepatic encephalopathy; IL, interleukin; MAFLD, metabolic-associated fatty liver disease; SCFA, short chain fatty acids
The human gut microbiota (GM) comprises bacteria, viruses (including phages), fungi, and primitive prokaryotic archaea that inhabit the digestive tract, express 100 times more genes than their human host, and play key roles in human health and disease causation and progression. GM facilitates the breakdown and absorption of dietary nutrients and minerals, synthesis of antimicrobial peptides, fermentation of fibers to short-chain fatty acids (SCFAs), neutralization of toxins, and regulation of local and systemic endocrine and immunological functions.1 Compositional and functional profiling revealed the association between human GM and multiple diseases affecting the liver. These diseases include hepatic steatosis, non-alcoholic fatty liver disease, alcohol-associated liver disease (ALD), alcohol-associated hepatitis (AH), chronic cholestatic conditions (e.g., primary biliary cholangitis and primary sclerosing cholangitis), autoimmune liver disease, complications of cirrhosis and portal hypertension such as hepatic encephalopathy (HE), infections such as spontaneous bacterial peritonitis, and hepatocellular carcinoma.2
In microbiome research, the most widely used high-throughput sequencing methods for analyzing bacterial communities include polymerase chain reaction (PCR) amplicon-based sequencing such as 16S ribosomal ribonucleic acid (rRNA) sequencing, deoxyribonucleic acid (DNA)-based shotgun metagenomic sequencing, and ribonucleic acid (RNA)-based meta-transcriptomic sequencing. The 16S rRNA sequencing method utilizes PCR to target and amplify areas of hypervariable regions (V1–V9) of bacterial rRNA subunit genes. In contrast, with the shotgun sequencing method, the entire metagenome of the sample can be randomly sequenced (with difficulty differentiating DNA from viable or dead cells) with no need for a specific primer. Metatranscriptomics is used to identify active microbial members in the biological sample and actively expressed genes in the community under specific conditions.3 Thereafter, the raw sequenced data generated using these methods are entered into bioinformatics software pipelines for taxonomic assignment and microbial community analysis and visualization using operational taxonomic unit-based or amplicon sequence variant-based analysis. As soon as phylogenetic information is collected, functional gene composition of the bacterial community is then predicted using pipelines such as Phylogenetic Investigation of Communities by Reconstruction of Unobserved States. To further identify significant and differentially abundant microbial taxa between communities in specific disease conditions, software tools optimized for statistical analysis of microbiome big-data, such as Linear Discriminant Analysis Effect Size, are used.3,4
ALD comprises simple steatosis, steatohepatitis, AH, cirrhosis, and the syndrome of AH-related acute-on-chronic liver failure (ACLF). Alcohol consumption is associated with GM changes even before ALD development.5 Continued alcohol intake worsens dysbiosis and is associated with disease progression and clinical events such as infection and HE. As reported in bench-to-bedside studies, GM composition and function play a role in the spectrum of ALD, and GM modulation improves adverse clinical events and disease progression.6 In the sections below, we exhaustively discuss relevant preclinical and clinical studies on GM in ALD and critically discuss GM modulation and its effects based on various human and animal models of ALD.
Preclinical studies on the role of GM in ALD
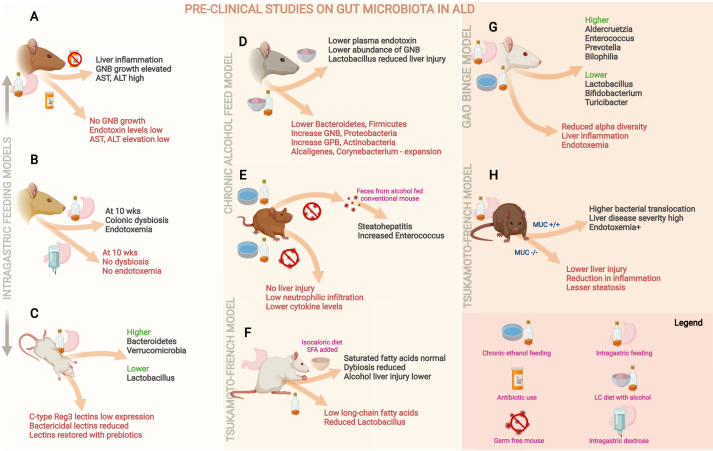
As early as 1995, it was found that fecal cultures of male Wistar rats exposed to alcohol continuously for up to three weeks via intragastric feeding (after undergoing gut sterilization using polymyxin B and neomycin) had virtually no growth of gram-negative bacteria. Antibiotic therapy was reported to significantly reduce endotoxin levels and the average hepatic pathological score and to inhibit aspartate aminotransferase level elevation. The investigators reported that the benefits observed are as a result of reduction in Kupffer cell activation due to antibiotic therapy, without linking antibiotic therapy to the role of GM and GM modulatory effects.7 When intragastric alcohol or dextrose was administered to male Sprague–Dawley rats by gavage twice daily for up to 10 weeks, investigators observed changes in mucosa-associated microbiota composition in the colon, but not at weeks four and six. The authors concluded that daily alcohol consumption may affect colonic microbiome composition and result in dysbiosis that may trigger alcohol-induced endotoxemia. This study offered the first glimpse into alcohol-induced enteric dysbiosis using length-heterogeneity PCR fingerprinting.8 In an intragastric alcohol-fed mouse model, investigators found that bacterial translocation occurred prior to GM changes. Using conventional culture techniques, bacterial overgrowth in the small and large intestine was observed after mice were fed intragastrical alcohol for at least three weeks. Pyrosequencing of 16S rRNA genes revealed the relative abundance of Bacteroidetes and Verrucomicrobia and the low abundance of Lactobacillus (phylum Firmicutes) in alcohol-fed mice associated with down-regulation of gene and protein expression of bactericidal c-type lectins Reg3b and Reg3g in the small intestine. Prebiotics were found to partially restore bactericidal lectins, ameliorate bacterial overgrowth, and reduce steatohepatitis.9 In an experimental ALD model inspired by the Tsukamoto–French model that involved continuous intragastric feeding of wild-type and Muc2-deficient (−/−) mice with an isocaloric diet or alcohol, less alcohol-associated liver injury and steatosis were observed in the Muc2-deficient (−/−) mice than in the wild-type mice. The Muc2-deficient (−/−) mice were protected from alcohol-associated GM changes dependent on intestinal mucins, with lower amounts of bacterial products such as endotoxin found to translocate into the systemic circulation, thereby reducing the severity of liver disease.10 In another study, mice were fed liquid Lieber–DeCarli diet with or without alcohol (5% v/v) for six weeks. The authors reported that chronic ethanol feeding reduces the abundance of Bacteriodetes and Firmicutes and proportionally increases the abundance of gram-negative Proteobacteria and gram-positive Actinobacteria. Furthermore, a significant increase in the abundance of gram-negative alkaline-tolerant Alcaligenes and gram-positive Corynebacterium was observed following alcohol exposure. These GM changes are associated with increased plasma endotoxin, fecal pH, and liver inflammation and injury. Lactobacillus rhamnosus GG supplementation for six to eight weeks after alcohol feeding was reported to reduce GM changes and liver injury. The authors concluded that GM dysbiosis may be an important therapeutic target for the prevention or treatment of chronic alcohol-induced intestinal barrier dysfunction and liver disease.11
In the study by Canesso et al. germ-free and conventional mice were subjected to acute alcohol intake by adding alcohol to their drinking water for seven days, with a higher dose of alcohol added on day seven. Compared to the alcohol-fed conventional mice, the germ-free mice were found to have no liver injury, low neutrophil infiltration, and low pro-inflammatory cytokine levels in the liver. After conventionalization using intestinal contents from the alcohol-fed conventional mice, injury, and inflammation were observed in the liver and intestine of the germ-free mice, suggesting that alcohol intake causes gut dysbiosis and liver injury. In their study, quantitative culturing of stool samples revealed increased abundance of Enterococcus species in the alcohol-fed mice.12 In a Tsukamoto–French mouse model that involved continuous intragastric feeding with an isocaloric diet or alcohol for three weeks, analysis of cecal contents revealed reduced synthesis of saturated long-chain fatty acids (LCFAs) associated with reduced abundance of LCFA-producing Lactobacilli. When intestinal levels of saturated fatty acids were maintained, GM disturbance lessened, resulting in less alcohol-induced liver injury.13 These studies also show that alcohol-treated mice have high intestinal levels of phyla such as Verrucomicrobia, Actinobacteria, and Proteobacteria (and their respective taxa Akkermansia muciniphila, Corynebacterium species, and Alcaligenes species), which support the role of GM dysbiosis associated with alcohol-induced injuries in the liver and intestines due to loss of gut barrier integrity and function and promotion of local and systemic inflammation via portal circulation.
In the Gao-binge mouse model (with chronic plus binge alcohol feeding), significant and reproducible differences in microbiome communities were observed between alcohol-fed mice and control mice on the same diet, excluding alcohol. In addition to reduction in alpha diversity and dispersion in beta diversity in alcohol-fed mice, it was found that the initial effect of alcohol was on gram-positive bacteria (increase in Adlercreutzia and Enterococcus), with increase in gram-negative communities (including Prevotella, Bilophila, Desulfovibrio, and Helicobacter), abundance of Clostridium, and reduction in Bifidobacterium, Lactobacillus (which mitigates alcohol injury), and Turicibacter (which is associated with anxiety-like effects, social avoidance behavior), and fatty liver disease.14 In a study on mice by Kang et al. distinctive GM dysbiosis was observed in chronic alcoholic fatty liver disease (AFLD) and metabolic-associated fatty liver disease (MAFLD). Compared to controls, the AFLD group had significant abundance of Enterococcus and Streptococcus, and in the MAFLD group, Lachnospiraceae was the most abundant bacterial family, Erysipelatoclostridium, Gordonibacter, and Streptococcus were the most abundant bacterial taxa, and there was a reduction of Bifidobacterium.15 Researchers from China showed that GM composition and structure significantly change with ALD progression and that the relative abundance of Streptococcus species significantly increases in alcohol-associated cirrhosis and correlates positively with aspartate aminotransferase level. They concluded that dysbiosis occurs from the early to the end stages of ALD and that Streptococcus may be a microbiological marker for the evaluation of liver injury severity in patients with ALD.16 Thus, preclinical studies have demonstrated the distinct role played by GM in various stages of ALD which are dependent on microbial metabolism, host immune response, gut barrier integrity and systemic inflammation. A summary of pertinent preclinical study models and important outcomes in ALD is shown in Figure 1.
Figure 1.
Pertinent summary of preclinical studies on gut microbiota in alcohol-associated liver disease (ALD). Studies on rodent models of ALD have shown that liver inflammation was ameliorated with “gut sterilization” (A); compared to dextrose feeding, alcohol feeding was associated with colonic dysbiosis and endotoxemia (B); intragastric alcohol feeding resulted in reduced expression of bactericidal lectins leading to increase in relative abundance of pathogenic bacterial communities (C); alcohol feeding led to specific gut microbial changes that led to liver injury (D) which was ameliorated in germ-free mice, but transferable via fecal transplant from conventional mice undergoing alcohol feeding (E); short chain fatty acid diet reduced alcohol liver injury (F); and alcohol feeding was associated with gut barrier dysfunction and dysbiosis (G, H). AST—aspartate transaminase, ALT—alanine transaminase, GNB—Gram-negative bacteria, GPB—Gram-positive bacteria, MUC—mucin expression.
Pre-clinical studies on GM modulation in ALD
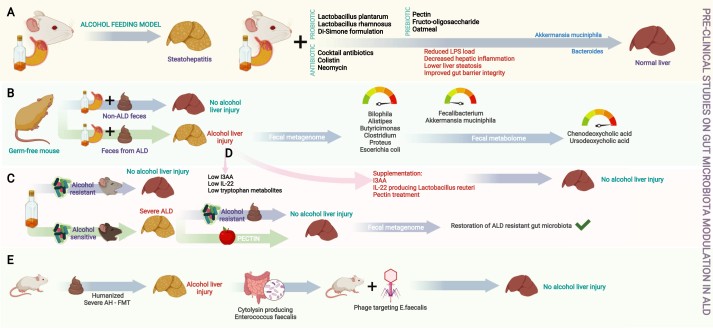
Preliminary alcohol studies using small animal models have shown that probiotic therapy with Lactobacillus plantarum or L. rhamnosus or multi-strain Di-Simone formulation or any of its generics reduces systemic inflammation and gut dysbiosis, ameliorates hepatic steatosis, improves liver tests, and reduces oxidative stress. It was also shown that antibiotic cocktail or colistin therapy decreases liposaccharide and bacterial load, reduces hepatic, neurological, and systemic inflammation, and improves gut barrier integrity by increasing the relative abundance of mucin-secreting A. muciniphila. In animal models of ALD, increased relative abundance of Bacteroides was found to be associated with reduced oxidative stress, improved gut barrier integrity, and reduced liver inflammation due to intake of prebiotic oatmeal, pectin, and fructooligosaccharides.17,18 In a seminal paper, Llopis et al. reported that, compared to germ-free mice transplanted with GM from alcoholic subjects without AH, alcohol-fed germ-free mice developed severe liver inflammation and necrosis and increased intestinal permeability when transplanted with GM isolated from patients with severe AH (SAH). Thus, susceptibility to alcohol-induced liver injury was shown to be transmissible from patients to mice through fecal microbiota transplantation (FMT). Abundance of taxa such as Bilophila wadsworthia, Alistipes, Butyricimonas, Clostridium, Proteus, and Escherichia coli and reduced levels of Faecalibacterium prausnitzii (associated with anti-inflammatory effects and gut barrier health via butyric acid production) and A. muciniphila (associated with the mucin barrier) were found to be associated with SAH. On further assessment of the fecal metabolome, significant differences in bile acid derivative levels were observed between mice with liver injury and mice without liver injury. In mice without liver lesions, it was found that chenodeoxycholic and ursodeoxycholic acid levels are significantly higher in the fecal samples of mice that received FMT from an alcoholic patient without liver disease than in the fecal samples of mice that received intestinal microbiota from a patient with ALD.19
Ferrere et al. fed alcohol and a Lieber–DeCarli diet to mice in two different animal facilities. FMT was performed with fresh feces from alcohol-resistant donor mice to alcohol-sensitive receiver mice three times a week. The control group received pectin during the entire alcohol consumption period. The investigators found that alcohol-induced steatohepatitis is associated with disruption of gut homeostasis in alcohol-sensitive mice, but not in alcohol-resistant mice. It was also found that abundance of Bacteroides is significantly lower in alcohol-sensitive mice than in alcohol-resistant mice and that FMT restores the GM of alcohol-sensitive recipient mice close to that of alcohol-resistant donor mice. Therefore, the investigators concluded that GM modulation may prevent alcohol-induced liver injury.20
In a mouse model of chronic alcohol feeding, researchers found that type 3 innate lymphoid cells of the intestine produce lower-than-normal levels of interleukin (IL)-22 (due to alcohol-induced dysbiosis) and intestinal indole-3-acetic acid (IAA), a microbial metabolite that regulates IL-22 expression. Further, patients with SAH were found to have low fecal levels of IAA. It was reported that IAA supplementation or feeding mice IL-22-producing Lactobacillus reuteri restores IL-22 expression, thereby mitigating alcohol-induced liver injury. The study showed that focused targeting of microbiota and metabolism may ameliorate alcohol-induced liver injury and prevent AH.21 It is evident that chronic alcohol consumption is associated with bacterial dysbiosis. Yang et al. demonstrated that, in mice, chronic alcohol administration increases mycobiota populations and fungal β-glucan translocation into the systemic circulation and that antifungal treatment reduces intestinal fungal overgrowth, decreases β-glucan translocation, and ameliorates alcohol-induced liver disease.22
Duan et al. found that cytolysin, a two-subunit exotoxin secreted by Enterococcus faecalis, causes hepatocyte death and liver injury and that patients with SAH have increased fecal numbers of E. faecalis. It was also found that the abundance of cytolytic E. faecalis is significantly associated with liver disease severity and mortality in patients with SAH. Using humanized mice colonized with bacteria from the feces of patients with AH, the investigators studied bacteriophage targeting of cytolytic E. faecalis. They found that bacteriophage reduces cytolysin in the liver and ameliorates alcohol-induced liver disease in humanized mice, thereby demonstrating that cytolysin-positive E. faecalis is associated with poor clinical outcomes and high mortality rates in patients with AH. The study offered proof of concept that precision therapy using bacteriophages to target cytolytic E. faecalis is a method of intestinal microbiota editing for the improvement of clinical outcomes, pending further prospective clinical trials.23 Wrzosek et al. used the prebiotic pectin to modulate intestinal microbiota in a mouse model of ALD in which the GM of alcoholic patients was transplanted into human-associated mice. They focused on microbiota tryptophan metabolites, which are ligands of the aryl hydrocarbon receptor (AhR), and found that pectin treatment modifies the microbiome and metabolome of human microbiota-associated alcohol-fed mice, resulting in increased production of bacterial tryptophan metabolites that are associated with amelioration of liver injury. The investigators showed that SAH is associated with low levels of bacterial tryptophan derivatives. The use of AhR agonists that target the GM (e.g., moderate amounts of the prebiotic pectin) may positively affect the AhR pathway and ameliorate alcohol-induced liver injury.24 Gut microbiome modulation studies in the preclinical setting have underlined the role of systemic and local inflammation as well as dysregulated host immune functions in alcohol-associated liver injury. The use of prebiotic/probiotic therapy, antimicrobial agents or fecal transplantation in small animal models have demonstrated the role of dysbiosis in driving alcohol-induced liver injury and its amelioration through modification of bacterial communities and beneficial modifications via microbial metabolite generation or systemic immune modulation. Figure 2 summarizes the important preclinical GM modulation studies in ALD.
Figure 2.
Infographic summary of preclinical studies of gut microbial (GM) modulation in ALD. Various studies on probiotics, prebiotics and antimicrobial cocktails have shown that GM modulation leads to downregulation of inflammatory pathways, improvement in gut barrier integrity and thus reduction in liver injury (A); studies on germ-free mice have shown that alcohol-associated hepatitis is transferable and is associated with specific changes in the intestinal bile acid pool driven by modification of bacterial communities (B); restoration of beneficial bacterial communities was possible via prebiotic treatment (C); and supplementation of specific metabolites or beneficial metabolite producing groups of bacteria ameliorated liver injury in ALD models (D), and phage targeting of cytolysin producing Enterococcus fecalis in severe ALD improved liver injury (E). ALD—alcohol associated liver disease, FMT—fecal microbiota transplantation, I3AA—indole-3 acetic acid, IL—interleukin, LPS—lipopolysaccharide.
Clinical studies on GM structure and functional changes across ALD
Alcohol Drinkers and Abstinence
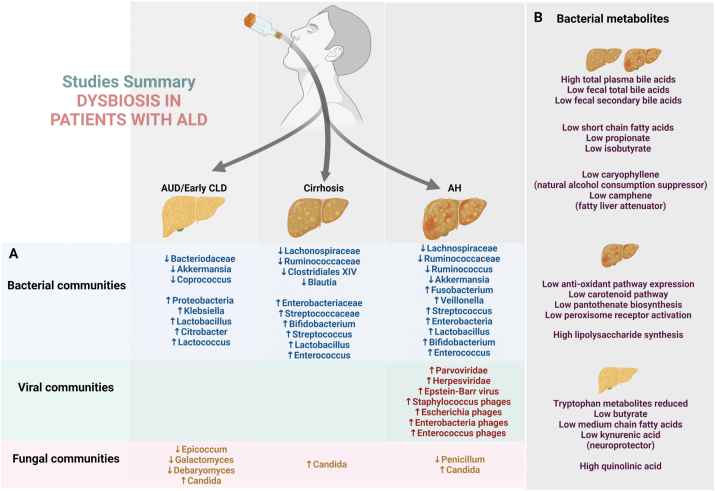
In 1984, Bode et al. reported GM changes associated with chronic alcohol abuse in humans. They analyzed the types and numbers of bacteria in jejunal aspirates from chronic alcoholics and hospitalized control patients. They found that the number of anaerobic and aerobic microbes (gram-negative anaerobic bacteria and endospore-forming rods) is significantly higher and jejunal fluid cultures reveal coliform organisms more frequently in alcoholics than in controls.25 A large body of evidence indicates the role of GM in patients with ALD of different stages. Studies showed that ALD without cirrhosis is associated with reduced Bacteroidetes and increased Proteobacteria that active alcohol use is associated with reduced Ruminococcaceae, which reverses after abstinence, and that dysbiosis is worse in patients with ALD and cirrhosis than in patients with non-ALD cirrhosis of comparable severity.26, 27, 28
Alcohol-associated Cirrhosis and SAH
Depletion of commensal bacteria and increased relative abundance of Lactobacillus and Bifidobacterium were observed in actively drinking patients with alcohol-associated cirrhosis, and oral-predominant bacteria were found to be associated with activation of alcohol metabolism and inflammatory pathways. Researchers also found that alcohol-dependence correlates inversely with the levels of butyrate-producing species from the Clostridiales order. The pro-inflammatory Enterobacteriaceae was significantly elevated in patients with alcohol-dependence, and over-representation of oral microbes in the gut was observed in patients with cirrhosis.29 Bajaj et al. reported that, compared to non-drinkers and controls, actively drinking patients with cirrhosis have significant duodenal, ileal, and colonic mucosal and fecal dysbiosis with low autochthonous bacterial taxa,30 which were linked with alterations in microbial function pertaining to bile acid levels. Studies also showed that reduced abundance of A. muciniphila is associated with increased severity of ALD (lowest in AH) and that secondary bile acid levels are higher in the serum and feces of actively drinking patients with ALD-related cirrhosis than in those of non-drinkers.31,32 Similarly, high plasma levels of total bile acids were observed in patients with advanced ALD-related cirrhosis and patients with SAH, and low fecal levels of total and secondary bile acids were found to be associated with high relative abundance of Actinobacteria and low relative abundance of Bacteroidetes.33 In another study, metabolome analysis of passed stool samples and feces collected directly from the sigmoid lumen revealed significant differences in the metabolomic signatures of volatile organic compounds between alcoholics and healthy controls. The most notable metabolite alterations included tetradecane (associated with oxidative stress), reduced levels of fatty alcohols with antioxidant properties, reduced levels of the SCFAs propionate and isobutyrate (associated with gut barrier integrity), decrease in caryophyllene (a natural alcohol consumption suppressor), decrease in camphene (a natural fatty liver attenuator), and reduced levels of sulfide end products associated with qualitative and quantitative alterations in intestinal microbiota.34 Philips et al. recently reported an association between GM and the clinical complications and treatment response in patients with SAH. Catenibacterium and Lachnobacterium were found to be associated with HE in patients with AH, and fecal metagenomic analysis revealed significantly higher abundance of Pediococcus in corticosteroid non-responders who died over the follow-up period. After growth factor therapy, a significant change from Enterococcus (which promotes AH) to Barnesiella (which inhibits Enterococcus faecium, known to promote SAH) was observed. Mutual interactions between Enterococcus cecorum, Acinetobacter schindleri, and Mitsuokella species correlated with kidney injury at admission. Upregulations of the phenylpropanoid biosynthesis pathway (associated with innate immunity) and the glycerophospholipid metabolism pathway (associated with cellular integrity) were observed in subjects without infections and in deceased subjects, respectively.35
Fungal and Viral Microbiome Changes in ALD
Fungal microbiome analysis using internal transcribed spacer 2 amplicon sequencing of fecal samples revealed significantly higher abundance of the taxa Candida, Debaryomyces, Pichia, Kluyveromyces, and Issatchenkia and of the species Candida albicans and Candida zeylanoides in patients with alcohol use disorder (AUD) than in control subjects. After two weeks of alcohol abstinence, the abundance of these taxa decreased significantly. It was also observed that the serum levels of anti-C. albicans immunoglobulin G and M in patients with AUD decreased with abstinence. Further, the intestinal abundance of the genus Malassezia was reported to be significantly higher in patients with AUD and progressive liver disease than in patients with AUD but without progressive liver disease.36 In addition to bacterial and fungal communities, researchers studied the intestinal virome in patients with AH. They observed increased viral diversity in fecal samples from patients with ALD, with the most significant changes found in patients with AH. Escherichia-, Enterobacteria-, and Enterococcus phages were over-represented, and significant increases in mammalian viruses such as Parvoviridae and Herpesviridae were observed. Antibiotic treatment resulted in increased viral diversity, and specific viral taxa, such as Staphylococcus phages and Herpesviridae, were found to be associated with increased liver disease severity and 90-day mortality.37 An infographic summary of various clinical studies on the role of gut microbiota in human-ALD is shown in Figure 3.
Figure 3.
Summary of various microbial community changes associated with alcohol use and spectrum of alcohol-associated liver disease in humans (A) and the pertinent changes in gut microbial functional metabolism (B). AUD—alcohol use disorder, AH—alcohol-associated hepatitis, and CLD—chronic liver disease.
Clinical studies on GM modulation and outcomes in ALD
Non-FMT-Based Therapeutic Strategies
GM modulation can be performed using prebiotics and probiotics, antimicrobials, or healthy donor FMT. In various studies, the clinical outcomes of probiotic therapy at different stages of ALD were analyzed. In most of the studies, improved liver tests, reduced levels of inflammatory markers (such as C-reactive protein and tumor necrosis factor-alpha) and endotoxin, improved immune function (evaluated using the phagocytic capacity of neutrophils in the short term), and improved Child–Pugh scores (in some studies) were observed. These findings were notable in the presence or absence of qualitative and quantitative GM changes such as increase in Clostridium coccoides and Eubacterium cylindroides and decrease in Enterobacteriaceae and E. coli. In current literature, probiotic therapy in severe stages of ALD is not shown to improve realistic clinical outcomes (such as transplant-free survival and liver disease severity) or reduce the risk of portal hypertension, extrahepatic organ dysfunction, or infection.38 In a recent study by Kim et al. GM composition in patients with SAH was investigated, and microbiome recovery after rifaximin treatment was evaluated. It was found that patients with AH have lower alpha diversity and higher beta diversity than healthy controls and that patients with AH have significantly increased Bacilli, Lactobacillales, and Veillonella but significantly decreased Eubacterium, Oscillibacter, and Clostridiales. It was also found that, after rifaximin treatment, Veillonella decreased and Prevotella increased. The study highlights the role of dysbiosis in SAH and the improvement of dysbiosis with non-absorbable antibiotic-related GM modulation.39
FMT-Based Therapeutic Strategies in Alcohol-use Disorder, Cirrhosis and Infections
The gut-brain communication is mostly driven by the immune, metabolic and neural pathways that are affected by GM structure and functions in health and disease. The metabolites arising from the tryptophan–kynurenine pathway were demonstrated to be at the interface between GM, host immune response and brain functions in a recent study. The authors found that increased concentration of the neurotoxic metabolite quinolinic acid and decreased levels of the neuroprotector metabolite kynurenic acid, both which modulate glutamatergic neurotransmission were observed in AUD patients. Further, tryptophan and kynurenic acid correlated with depression and alcohol craving respectively. On GM analysis, the researchers found that SCFAs producing intestinal bacteria and their metabolites such as butyrate and medium-chain fatty acids were associated with some metabolites of the tryptophan–kynurenine pathway.40 A recent randomized clinical trials of patients with AUD and cirrhosis revealed increased microbial diversity with increased Ruminococcaceae and other SCFA-producing taxa post-FMT, but not with placebo. The investigators reported that FMT is safe and associated with short-term reduction in alcohol craving and consumption and reduction in alcohol use-related events over six months.41 Acute and chronic alcohol use leads to an impaired immune response and dysregulated inflammatory state that is driven by dysbiotic microbiota, which contributes to a markedly increased risk of infection. These effects are most evident in patients with SAH and alcohol-associated cirrhosis. Particularly in SAH patients, the use of corticosteroids increases risk for developing serious infections which was revealed to be dependent on the level of circulating bacterial DNA before treatment. Development of infections in SAH and multi-drug resistance in cirrhosis is associated with very poor prognosis.42, 43, 44 A recent systematic review revealed that FMT could be used as a treatment for eradicating multidrug resistance colonization and possibly prevents recurrent multidrug resistant infections, once more supporting efficacy and safety data were available. Bajaj et al. found that antimicrobial resistance gene expression and abundance was largely reduced after FMT compared to pre-FMT baseline and non-FMT groups in decompensated cirrhosis proving proof of concept of utility of FMT in tackling recurrent infections in patients with advanced liver disease.45,46
Severe Alcohol-associated Hepatitis
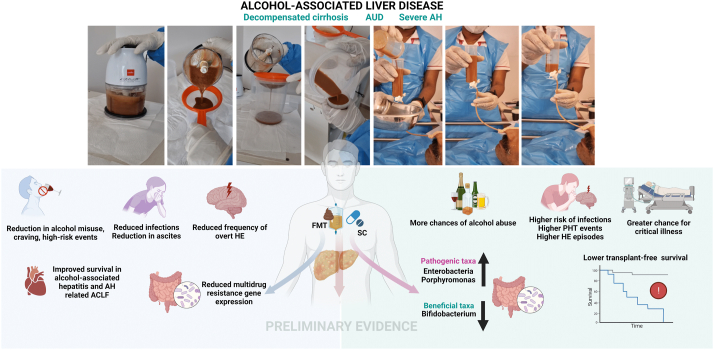
In an open-label study with one-year follow-up period, steroid-ineligible patients with AH underwent daily FMT from several healthy donors for seven days through a nasojejunal tube, and it was found that the patients had higher transplant-free survival than historical controls. This finding is associated with a reduction in potentially pathogenic species and improved liver disease severity scores. Firmicutes phylum dominated in donors and recipients one year after FMT and is associated with reduction in Proteobacteria and increase in Actinobacteria after FMT. In the long term, the relative abundance of Klebsiella pneumonia reduced, and the relative abundance of non-pathogenic species (e.g., Enterococcus villorum and Bifidobacterium longum) and Megasphaera elsdenii increased at six and 12 months after FMT. Methane metabolism and fluorobenzoate acid degradation and bacterial invasion of epithelial cell pathways mediated by Pseudomonas and E. coli, were upregulated at baseline and decreased one year after FMT. It was reported that antioxidant pathways (e.g., the carotenoid and pantothenate biosynthesis pathways) are downregulated in SAH but improve after FMT.47 In another open-label study with a three-month follow-up period, the outcomes of nasojejunal FMT were analyzed and compared to those of pentoxifylline, corticosteroid, and nutritional therapy. Survival was found to be highest with FMT and is associated with beneficial modulation of bacterial communities and functional composition. Beneficial modulation from predominantly pathogenic bacteria such as Bilophila, Citrobacter, Enterobacter, and Klebsiella to Bacteroides, Parabacteroides, and Porphyromonas at the end of one week and to Roseburia and Micrococcus beyond 30 days after FMT was notable. At one month, in subjects that underwent FMT, amino acid biosynthesis, and peroxisome proliferator-activated receptor signaling pathways were significantly active, but lipopolysaccharide signaling pathways were subdued.48
AH-related ACLF is associated with high mortality in the presence of corticosteroid non-response and absence of liver transplantation. The reported 90-day survival rates of steroid non-responders with ACLF grades 0, 1, and (2 + 3) are 68.1%, 45.8%, and 36.7%, respectively. In a retrospective study on FMT in patients with AH-related ACLF ineligible for steroid therapy, an overall survival rate of 66% at 548 days of follow-up and a mean overall survival of 389 days were reported. The survival rates at lower and higher ACLF grades at the end of 548 days of follow-up were 72.7% and 58.3%, respectively, showing proof of concept on the benefits of FMT in a very sick group of patients with ACLF.49
In some recent studies published as abstracts, improved clinical outcomes of FMT in subjects with AH were also reported. A randomized trial reported a survival rate of 83% in patients who underwent healthy donor FMT and compared it to the survival rate of 40% in patients who underwent pentoxifylline therapy. The rates of clinically evident ascites and HE were significantly higher in patients who underwent pentoxifylline therapy than in patients who underwent FMT. Further, it was found that Firmicutes and Bacteroides increase post-FMT (with gradual reduction in Proteobacteria) and that beneficial Bifidobacterium is most abundant three to six months post-FMT.50 In a study by Chandigarh, it was reported that single-session nasojejunal FMT is tolerable and safe for patients with AH. It was also reported that one-month mortality is lower with FMT than with standard-of-care treatment and that FMT is associated a higher rate of ascites resolution than standard-of-care treatment, with a trend toward improved three-month survival and amelioration of HE.51 Another randomized controlled trial compared FMT with corticosteroid therapy and found that patients on FMT have higher survival rates than patients on corticosteroid therapy. Butyricicoccus, Bifidobacterium, Lactobacillus, Collinsella, Roseburia, and Dorea, which were absent pre-FMT, were introduced after therapy. The phylum Firmicutes, family Lachnospiraceae, and genera Veillonella and Prevotella are associated with good FMT outcomes.52 Moreover, a recent study showed that, in patients with SAH, healthy donor FMT is associated with significantly less episodes of HE, critical infections, hospitalizations, and alcohol relapse (with a trend toward higher survival rates) than corticosteroid therapy. High-dimensional biomarker discovery revealed significantly increased relative abundance of Bifidobacterium and a reduction in Acinetobacter in subjects that underwent FMT. It was reported that, beyond one to two years, the relative abundance of Porphyromonas is significantly higher and that of Bifidobacterium is lower in subjects that underwent corticosteroid therapy than in subjects that underwent FMT.53 Figure 4 summarizes preliminary benefits associated with healthy donor FMT in ALD.
Figure 4.
Summary of preliminary evidence for clinical benefits with healthy donor fecal microbiota transplantation (FMT) in patients with severe alcohol-associated liver disease. For detailed discussion, please refer to text section on FMT under “Clinical studies on GM modulation and outcomes in ALD.” AH—alcohol-associated hepatitis, ACLF—acute on chronic liver failure, HE—hepatic encephalopathy, PHT—portal hypertension, and SC—standard care.
Concerns and future directions
Healthy donor FMT is generally considered safe with strict donor screening protocols. Serious adverse events directly related to FMT have not been reported from trials involving liver disease patients. A recent systematic review on the adverse events (AE) associated with FMT in patients with recurrent Clostridioides difficile infections (RCDI) revealed that the total incidence rate of AEs was 28.5% of which only 5 types of events (most common, abdominal discomfort—cramps and bloating) were definitely attributed to FMT. The incidence of serious adverse events (SAEs; death, life-threatening experience, inpatient hospitalization or prolongation of existing hospitalization) was 2% for upper gastrointestinal route.54 Another metanalysis of high-quality studies on FMT revealed that the AE rate of FMT was 39.3%, with most forms of AE mild and self-limiting. SAEs were uncommon at 5.3%, and many were only possibly related to the procedure.55 A prospective survey on long-term safety of FMT demonstrated that new events which included 13% gastrointestinal, 10% weight gain, 11.8% new infections (all deemed unrelated to FMT) were notable.56 Thus, FMT is generally considered safe and is well-tolerated even in high-risk patients in the presence of rigorous donor screening and testing, mandated to minimize risk of multidrug resistant infection spread. Nonetheless, long term impact on human health and disease after FMT beckons further studies.
Currently, the recommended use of FMT is only for RCDI. In patients with liver disease, especially ALD, data on the role of GM is slowly but hopefully emerging and thus, its currently utility remain within clinical research trials. Multiple factors remain vaguely defined and require further studies to improve on our understanding on the role of GM modulation in ALD. These include defining and identifying a “healthy stool donor” including “super donors” the latter, proposed to describe donors whose stool results in significantly more successful FMT outcomes than the stool of other donors. The different (optimal) routes of administration including the use capsule formulations and their associated efficacy as well as risks across the spectrum of ALD needs prospective validation. And finally, efficacy and safety of single FMT compared to repeated sessions and their timings in various forms of ALD also require rigorous studies.
Compositional and functional changes in GM are associated with initiation and progression of severe forms of ALD, especially AH, cirrhosis, and sepsis in cirrhosis. Dysbiosis drives disruption of gut barrier integrity, which leads to pro-inflammatory changes within the liver microenvironment and at the systemic level. Animal model studies have shown that alcohol feeding causes changes in GM composition and function and that interventions targeting the GM (using pre- and probiotics or faeces from healthy donors) improve gut barrier function, reduce endotoxemia, and ameliorate hepatic inflammation. It was also shown that specific bacterial components and metabolites are associated with disease events in ALD models. For example, it was observed that Enterococcus produces cytolysin in subjects with SAH and that the use of phages to target pathogenic bacteria in the gut improves liver inflammation in animal models. The transfer of AH via fecal transplant in animal models confirms a major role of GM in the initiation and progression of severe ALD. Preliminary human studies demonstrated the role of healthy donor FMT in improving transplant-free survival, reducing portal hypertension events and infections, and reducing alcohol craving in patients with AUD. Our understanding of the complexities of GM in ALD is developing, and further bench-to-bedside studies are necessary to improve our understanding of beneficial GM modulation and its long-term effects. Before GM modulation can be recommended for use in specific liver-related events in ALD, it is necessary to conduct large well-controlled studies with comprehensive methodological refinements.
Credit authorship contribution statement
B.S. has been consulting for Ferring Research Institute, Gelesis, HOST Therabiomics, Intercept Pharmaceuticals, Mabwell Therapeutics, Patara Pharmaceuticals and Takeda. B.S. is founder of Nterica Bio. UC San Diego has filed several patents with B.S. as inventor related to this work. JSB has received funding through his institution from Cosmo, Bausch, Grifols, Sequana, and Mallinckrodt Pharmaceuticals.
Conflicts of interest
The authors have none to declare.
Acknowledgements
B.S. is supported by NIH funded San Diego Digestive Diseases Research Center (SDDRC) P30 DK120515. JSB is supported by VA Merit Review 2IOCX001076 and NIH R21TR003095.
References
- 1.Hartmann P., Chu H., Duan Y., Schnabl B. Gut microbiota in liver disease: too much is harmful, nothing at all is not helpful either. Am J Physiol Gastrointest Liver Physiol. 2019;316:G563–G573. doi: 10.1152/ajpgi.00370.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li R., Mao Z., Ye X., Zuo T. Human gut microbiome and liver diseases: from correlation to causation. Microorganisms. 2021;9:1017. doi: 10.3390/microorganisms9051017. Published 2021 May 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Philips C.A., Augustine P., Yerol P.K., et al. Modulating the intestinal microbiota: therapeutic opportunities in liver disease. J Clin Transl Hepatol. 2020;8:87–99. doi: 10.14218/JCTH.2019.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao B., Chi L., Zhu Y., et al. An introduction to next generation sequencing bioinformatic analysis in gut microbiome studies. Biomolecules. 2021 Apr 2;11:530. doi: 10.3390/biom11040530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Albhaisi S.A.M., Bajaj J.S., Sanyal A.J. Role of gut microbiota in liver disease. Am J Physiol Gastrointest Liver Physiol. 2020 Jan 1;318:G84–G98. doi: 10.1152/ajpgi.00118.2019. [DOI] [PubMed] [Google Scholar]
- 6.Schwenger K.J., Clermont-Dejean N., Allard J.P. The role of the gut microbiome in chronic liver disease: the clinical evidence revised. JHEP Rep. 2019;1:214–226. doi: 10.1016/j.jhepr.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Adachi Y., Moore L.E., Bradford B.U., Gao W., Thurman R.G. Antibiotics prevent liver injury in rats following long-term exposure to ethanol. Gastroenterology. 1995 Jan;108:218–224. doi: 10.1016/0016-5085(95)90027-6. [DOI] [PubMed] [Google Scholar]
- 8.Mutlu E., Keshavarzian A., Engen P., Forsyth C.B., Sikaroodi M., Gillevet P. Intestinal dysbiosis: a possible mechanism of alcohol-induced endotoxemia and alcoholic steatohepatitis in rats. Alcohol Clin Exp Res. 2009 Oct;33:1836–1846. doi: 10.1111/j.1530-0277.2009.01022.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yan A.W., Fouts D.E., Brandl J., et al. Enteric dysbiosis associated with a mouse model of alcoholic liver disease. Hepatology. 2011 Jan;53:96–105. doi: 10.1002/hep.24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hartmann P., Chen P., Wang H.J., et al. Deficiency of intestinal mucin-2 ameliorates experimental alcoholic liver disease in mice. Hepatology. 2013 Jul;58:108–119. doi: 10.1002/hep.26321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bull-Otterson L., Feng W., Kirpich I., et al. Metagenomic analyses of alcohol induced pathogenic alterations in the intestinal microbiome and the effect of Lactobacillus rhamnosus GG treatment. PLoS One. 2013;8 doi: 10.1371/journal.pone.0053028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Campos Canesso M., Lacerda Queiroz N., Marcantonio C., et al. Comparing the effects of acute alcohol consumption in germ-free and conventional mice: the role of the gut microbiota. BMC Microbiol. 2014;14:240. doi: 10.1186/s12866-014-0240-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen P., Torralba M., Tan J., et al. Supplementation of saturated long-chain fatty acids maintains intestinal eubiosis and reduces ethanol-induced liver injury in mice. Gastroenterology. 2015 Jan;148:203–214.e16. doi: 10.1053/j.gastro.2014.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LeBrun E.S., Nighot M., Dharmaprakash V., et al. The gut microbiome and alcoholic liver disease: ethanol consumption drives consistent and reproducible alteration in gut microbiota in mice. Life (Basel) 2020 Dec 24;11:7. doi: 10.3390/life11010007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kang K., Sun Y., Pan D., et al. Distinctive gut microbial dysbiosis between chronic alcoholic fatty liver disease and metabolic-associated fatty liver disease in mice. Exp Ther Med. 2021 May;21:418. doi: 10.3892/etm.2021.9862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhong X., Cui P., Jiang J., et al. Streptococcus, the predominant bacterium to predict the severity of liver injury in alcoholic liver disease. Front Cell Infect Microbiol. 2021 Mar 17;11:649060. doi: 10.3389/fcimb.2021.649060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gupta H., Suk K.T., Kim D.J. Gut microbiota at the intersection of alcohol, brain, and the liver. J Clin Med. 2021 Feb 2;10:541. doi: 10.3390/jcm10030541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chi X., Pan C.Q., Liu S., Cheng D., Cao Z., Xing H. Regulating intestinal microbiota in the prevention and treatment of alcohol-related liver disease. Chin J Gastroenterol Hepatol. 2020 Dec 17;2020:6629196. doi: 10.1155/2020/6629196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Llopis M., Cassard A.M., Wrzosek L., et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016 May;65:830–839. doi: 10.1136/gutjnl-2015-310585. [DOI] [PubMed] [Google Scholar]
- 20.Ferrere G., Wrzosek L., Cailleux F., et al. Fecal microbiota manipulation prevents dysbiosis and alcohol-induced liver injury in mice. J Hepatol. 2017 Apr;66:806–815. doi: 10.1016/j.jhep.2016.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Hendrikx T., Duan Y., Wang Y., et al. Bacteria engineered to produce IL-22 in intestine induce expression of REG3G to reduce ethanol-induced liver disease in mice. Gut. 2019 Aug;68:1504–1515. doi: 10.1136/gutjnl-2018-317232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang A.M., Inamine T., Hochrath K., et al. Intestinal fungi contribute to development of alcoholic liver disease. J Clin Invest. 2017 Jun 30;127:2829–2841. doi: 10.1172/JCI90562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Duan Y., Llorente C., Lang S., et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575:505–511. doi: 10.1038/s41586-019-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wrzosek L., Ciocan D., Hugot C., et al. Microbiota tryptophan metabolism induces aryl hydrocarbon receptor activation and improves alcohol-induced liver injury. Gut. 2021 Jul;70:1299–1308. doi: 10.1136/gutjnl-2020-321565. [DOI] [PubMed] [Google Scholar]
- 25.Bode J.C., Bode C., Heidelbach R., Dürr H.K., Martini G.A. Jejunal microflora in patients with chronic alcohol abuse. Hepato-Gastroenterology. 1984 Feb;31:30–34. [PubMed] [Google Scholar]
- 26.Mutlu E.A., Gillevet P.M., Rangwala H., et al. Colonic microbiome is altered in alcoholism. Am J Physiol Gastrointest Liver Physiol. 2012 May 1;302:G966–G978. doi: 10.1152/ajpgi.00380.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Leclercq S., Matamoros S., Cani P.D., et al. Intestinal permeability, gut-bacterial dysbiosis, and behavioral markers of alcohol-dependence severity. Proc Natl Acad Sci U S A. 2014 Oct 21;111:E4485–E4493. doi: 10.1073/pnas.1415174111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bajaj J.S., Heuman D.M., Hylemon P.B., et al. Altered profile of human gut microbiome is associated with cirrhosis and its complications. J Hepatol. 2014 May;60:940–947. doi: 10.1016/j.jhep.2013.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dubinkina V.B., Tyakht A.V., Odintsova V.Y., et al. Links of gut microbiota composition with alcohol dependence syndrome and alcoholic liver disease. Microbiome. 2017 Oct 17;5:141. doi: 10.1186/s40168-017-0359-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bajaj J.S., Kakiyama G., Zhao D., et al. Continued alcohol misuse in human cirrhosis is associated with an impaired gut-liver Axis. Alcohol Clin Exp Res. 2017 Nov;41:1857–1865. doi: 10.1111/acer.13498. [DOI] [PubMed] [Google Scholar]
- 31.Grander C., Adolph T.E., Wieser V., et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut. 2018 May;67:891–901. doi: 10.1136/gutjnl-2016-313432. [DOI] [PubMed] [Google Scholar]
- 32.Kakiyama G., Hylemon P.B., Zhou H., et al. Colonic inflammation and secondary bile acids in alcoholic cirrhosis. Am J Physiol Gastrointest Liver Physiol. 2014 Jun 1;306:G929–G937. doi: 10.1152/ajpgi.00315.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ciocan D., Voican C.S., Wrzosek L., et al. Bile acid homeostasis and intestinal dysbiosis in alcoholic hepatitis. Aliment Pharmacol Ther. 2018 Nov;48:961–974. doi: 10.1111/apt.14949. [DOI] [PubMed] [Google Scholar]
- 34.Couch R.D., Dailey A., Zaidi F., et al. Alcohol induced alterations to the human fecal VOC metabolome. PLoS One. 2015 Mar 9;10 doi: 10.1371/journal.pone.0119362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Philips C.A., Augustine P., Ganesan K., et al. The role of gut microbiota in clinical complications, disease severity, and treatment response in severe alcoholic hepatitis. Indian J Gastroenterol. 2022 January doi: 10.1007/s12664-021-01157-9. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 36.Hartmann P., Lang S., Zeng S., et al. Dynamic changes of the fungal microbiome in alcohol use disorder. Front Physiol. 2021 Jul 19;12:699253. doi: 10.3389/fphys.2021.699253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jiang L., Lang S., Duan Y., et al. Intestinal virome in patients with alcoholic hepatitis. Hepatology. 2020 Dec;72:2182–2196. doi: 10.1002/hep.31459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Leclercq S., de Timary P., Stärkel P. Targeting the gut microbiota to treat alcoholic liver diseases: evidence and promises. Acta Gastroenterol Belg. 2020 Oct-Dec;83:616–621. [PubMed] [Google Scholar]
- 39.Kim S.S., Eun J.W., Cho H.J., et al. Microbiome as a potential diagnostic and predictive biomarker in severe alcoholic hepatitis. Aliment Pharmacol Ther. 2021 Feb;53:540–551. doi: 10.1111/apt.16200. [DOI] [PubMed] [Google Scholar]
- 40.Leclercq S., Schwarz M., Delzenne N.M., Stärkel P., de Timary P. Alterations of kynurenine pathway in alcohol use disorder and abstinence: a link with gut microbiota, peripheral inflammation and psychological symptoms. Transl Psychiatry. 2021;11:503. doi: 10.1038/s41398-021-01610-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bajaj J.S., Gavis E.A., Fagan A., et al. A randomized clinical trial of fecal microbiota transplant for alcohol use disorder. Hepatology. 2020 doi: 10.1002/hep.31496. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 42.Karakike E., Moreno C., Gustot T. Infections in severe alcoholic hepatitis. Ann Gastroenterol. 2017;30:152–160. doi: 10.20524/aog.2016.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vergis N., Atkinson S.R., Knapp S., et al. In patients with severe alcoholic hepatitis, prednisolone increases susceptibility to infection and infection-related mortality, and is associated with high circulating levels of bacterial DNA. Gastroenterology. 2017 Apr;152:1068–1077.e4. doi: 10.1053/j.gastro.2016.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel V.C., Williams R. Antimicrobial resistance in chronic liver disease. Hepatol Int. 2020 Jan;14:24–34. doi: 10.1007/s12072-019-10004-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Saha S., Tariq R., Tosh P.K., Pardi D.S., Khanna S. Faecal microbiota transplantation for eradicating carriage of multidrug-resistant organisms: a systematic review. Clin Microbiol Infect. 2019 Aug;25:958–963. doi: 10.1016/j.cmi.2019.04.006. [DOI] [PubMed] [Google Scholar]
- 46.Bajaj J.S., Shamsaddini A., Fagan A., et al. Fecal microbiota transplant in cirrhosis reduces gut microbial antibiotic resistance genes: analysis of two trials. Hepatol Commun. 2020 Nov 21;5:258–271. doi: 10.1002/hep4.1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Philips C.A., Pande A., Shasthry S.M., et al. Healthy donor fecal microbiota transplantation in steroid-ineligible severe alcoholic hepatitis: a pilot study. Clin Gastroenterol Hepatol. 2017 Apr;15:600–602. doi: 10.1016/j.cgh.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 48.Philips C.A., Phadke N., Ganesan K., Ranade S., Augustine P. Corticosteroids, nutrition, pentoxifylline, or fecal microbiota transplantation for severe alcoholic hepatitis. Indian J Gastroenterol. 2018 May;37:215–225. doi: 10.1007/s12664-018-0859-4. [DOI] [PubMed] [Google Scholar]
- 49.Philips C.A., Augustine P., Padsalgi G., Ahamed R., Jose A., Rajesh S. Only in the darkness can you see the stars: severe alcoholic hepatitis and higher grades of acute-on-chronic liver failure. J Hepatol. 2019 Mar;70:550–551. doi: 10.1016/j.jhep.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 50.Philips C.A., Shasthry S.M., Pande A., et al. Outcomes of fecal microbiota transplantation in steroid ineligible severe alcoholic hepatitis-A randomized control trial ( NCT02458079) Indian J Gastroenterol. 2016;35:1–111. doi: 10.1007/s12664-016-0715-3. [DOI] [Google Scholar]
- 51.Dhiman R.K., Sharma A., Roy A., et al. Role of fecal microbiota transplantation in severe alcoholic hepatitis: assessment of impact on prognosis and short-term outcomes. J Hepatol. 2020;73:S179. [Google Scholar]
- 52.Sharma S., Pande A., Khillan V., et al. Post-fecal microbiota transplant taxa correlate with 3-month survival in severe alcoholic hepatitis patients. J Hepatol. 2020;73:S137. [Google Scholar]
- 53.Philips C.A., Abduljaleel J.K.A., Zulfikar R.A., Augustine P. Three-year follow-up of alcohol-related hepatitis patients undergoing healthy donor fecal transplant: analysis of clinical outcomes, relapse, gut microbiota and comparisons with standard care. Oral Abstracts. Hepatology. 2021;74:1–156. doi: 10.1002/hep.32187. [DOI] [Google Scholar]
- 54.Wang S., Xu M., Wang W., et al. Systematic review: adverse events of fecal microbiota transplantation. PLoS One. 2016 Aug 16;11 doi: 10.1371/journal.pone.0161174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Michailidis L., Currier A.C., Le M., Flomenhoft D.R. Adverse events of fecal microbiota transplantation: a meta-analysis of high-quality studies. Ann Gastroenterol. 2021 Nov-Dec;34:802–814. doi: 10.20524/aog.2021.0655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saha S., Mara K., Pardi D.S., Khanna S. Long-term safety of fecal microbiota transplantation for recurrent Clostridioides difficile infection. Gastroenterology. 2021 May;160:1961–1969.e3. doi: 10.1053/j.gastro.2021.01.010. [DOI] [PubMed] [Google Scholar]