Abstract
Alcohol-associated liver disease is one of the main causes of chronic liver disease. It comprises a clinical-histologic spectrum of presentations, from steatosis, steatohepatitis, to different degrees of fibrosis, including cirrhosis and severe necroinflammatory disease, called alcohol-associated hepatitis. In this focused update, we aim to present specific therapeutic interventions and strategies for the management of alcohol-associated liver disease. Current evidence for management in all spectra of manifestations is derived from general chronic liver disease recommendations, but with a higher emphasis on abstinence and nutritional support. Abstinence should comprise the treatment of alcohol use disorder as well as withdrawal syndrome. Nutritional assessment should also consider the presence of sarcopenia and its clinical manifestation, frailty. The degree of compensation of the disease should be evaluated, and complications, actively sought. The most severe acute form of this disease is alcohol-associated hepatitis, which has high mortality and morbidity. Current treatment is based on corticosteroids that act by reducing immune activation and blocking cytotoxicity and inflammation pathways. Other aspects of treatment include preventing and treating hepatorenal syndrome as well as preventing infections although there is no clear evidence as to the benefit of probiotics and antibiotics in prophylaxis. Novel therapies for alcohol-associated hepatitis include metadoxine, interleukin-22 analogs, and interleukin-1-beta antagonists. Finally, granulocyte colony–stimulating factor, microbiota transplantation, and gut–liver axis modulation have shown promising results. We also discuss palliative care in advanced alcohol-associated liver disease.
Keywords: cirrhosis, alcohol, alcohol-associated hepatitis, ALD, alcohol use disorders, steatosis, fatty liver disease
Abbreviations: AC, Amoxicillin/clavulanate; ADLs, Activities of Daily Living; AKI-HRS, Acute Kidney Injury - Hepatorenal Syndrome; ACLF, Acute-on-Chronic Liver Failure; ALD, Alcohol-Associated Liver Disease; AUD, Alcohol Use Disorder; AH, Alcohol-Associated Hepatitis; ASH, Alcoholic Steatohepatitis; AWS, Alcohol Withdrawal Syndrome; BCAAs, Branched-Chain Amino Acids; CDC, Center for Disease Control; COVID-19, Coronavirus Disease 2019; CI, Confidence Interval; CT, Computerized Tomography; GABA, gamma-aminobutyric acid agonist; HBV, Hepatitis B Virus; HCV, Hepatitis C Virus; HCC, Hepatocellular Carcinoma; HE, Hepatic Encephalopathy; HIV, Human Immunodeficiency Virus; HR, Hazard Ratio; IBW, Ideal Body Weight; ICA, International Club of Ascites; IL-1β, Interleukin-1β; IL-22, Interleukin-22; KPS, Karnofsky Performance Status; LPS, Lipopolysaccharide; LB, Liver Biopsy; LSM, Liver Stiffness Measurement; LT, Liver Transplantation; MDF, Maddrey’s Discriminant Function; MRI, Magnetic Resonance Imaging; MELD, Model of End-Stage Liver Disease; MUST, Malnutrition Universal Screening Tool; NIAAA, National Institute on Alcohol Abuse and Alcoholism; NRS-2002, Nutritional Risk Screening-2002; OR, Odds Ratio; PAMPs, Pathogen-Activated Molecular Patterns; PTX, Pentoxifylline; PMI, Psoas Muscle Index; RAI, Relative Adrenal Insufficiency; RCT, Randomized Clinical Trials; ROS, Reactive Oxygen Species; RR, Relative Risk; RFH-NPT, Royal Free Hospital-Nutritional Prioritizing Tool; SIRS, Systemic Inflammatory Response Syndrome; TNF, Tumor Necrosis Factor; WKS, Wernicke-Korsakoff Syndrome
Alcohol-associated liver disease (ALD) is one of the main causes of chronic liver disease.1 It comprises a clinical-histologic spectrum of presentations, from steatosis, alcohol-associated steatohepatitis (ASH), to different degrees of fibrosis, including cirrhosis. It also can manifest in a severe and acute necroinflammatory disease, called alcohol-associated hepatitis (AH).2 Hazardous alcohol consumption is an essential determinant of disease burden; therefore, the proper management of ALD should be a priority for all clinicians. In general, evidence for ALD management in all spectra of manifestations is derived from general chronic liver disease recommendations. In this focused update, we aim to present specific therapeutic interventions and strategies for the management of ALD.
General management of ALD
Management of ALD begins with adequate screening. Although excessive alcohol consumption is frequent, ALD is usually underdiagnosed. The quantification of alcohol consumption is a challenge in clinical practice. Although total grams of alcohol per day or week is more precise, it may be difficult to obtain accurately. Also, there are discrepancies among experts in certain definitions, such as “standard drink” and “heavy drinker.”3,4,5 Despite this, the latest guidelines suggest standardizing a drink of “pure” alcohol to 10–14 g, heavy episodic drinking as the consumption of more than 60 g of pure alcohol in one occasion,6 and binge drinking as the consumption within two hours of four or more drinks for women and five or more for men.7 Of note, the current coronavirus disease 2019 (COVID-19) pandemic has had an important impact on the incidence of alcohol use disorder (AUD) and ALD. In one study, the one-year increase in alcohol consumption during the COVID-19 pandemic was estimated to result in 8000 (95% confidence interval [CI]: 7500–8600) additional ALD-related deaths, 18,700 (95% CI: 17,600–19,900) cases of decompensated cirrhosis, and 1000 (95% CI: 1000–1100) cases of hepatocellular carcinoma (HCC) and 8.9 million disability-adjusted life-years between 2020 and 2040.8 Another cross-sectional study of ALD at a liver transplantation (LT) center found specific COVID-related stressors that led to early LT.8,9 These findings help confirm the predictions of rising AUD and ALD as an immediate consequence of the COVID-19 pandemic.10 However, a positive impact that the pandemic might have had is the use of new technologies, which were greatly advanced, especially the use of telemedicine to offer reasonable care in patients during social distancing and quarantine. The effectiveness of these strategies has been studied showing a high degree of revolutivity. In fact, 73% (95% CI: 66%–79%) were resolved through telemedicine.11
Compared with other causes of chronic liver disease (i.e., metabolic dysfunction–associated fatty liver disease, viral hepatitis, autoimmune hepatitis), few pharmacological advances have been made for the management of patients with ALD in the past decades. Current guidelines for the management of patients with any form of ALD, including those with nonsevere forms of the AH, mainly advocate interventions directed at abstinence, nutritional support, and the early referral of patients to gastroenterologists or hepatologists in selected cases.12 However, the spectrum of presentation of ALD requires an individualized approach to treatment. It is essential to keep in mind that patients with ALD suffer from two different disorders: AUD, which is defined as “a problematic pattern of alcohol use leading to clinically significant impairment or distress,”13 and the liver disease itself. Both of these should be integrated into the overall management.14
In regards to abstinence, although it does not guarantee complete recovery from the disease (27% of patients achieve histological normalization, while 18% progress to cirrhosis15), it is currently the mainstay of the treatment of ALD.16 Importantly, patients with nonalcoholic steatohepatitis or other liver diseases, such as chronic hepatitis C virus (HCV) infection, should avoid alcohol consumption since it may act synergistically to accelerate the progression to cirrhosis.14 Likewise, it is important for patients with chronic alcohol consumption to prevent and screen for alcohol withdrawal syndrome (AWS), as it may require behavioral and/or pharmacotherapy (discussed in another specific chapter in this focused update in further detail). Broadly speaking, medications for abstinence are effective and safe and should be considered for patients with AUS that present with AWS.17 Screening of advanced fibrosis or cirrhosis and surveillance for HCC should be initiated with liver imaging, with or without alpha-fetoprotein every six months.3,16 Additionally, patients with AH must be quickly identified and the severity stratified to treat optimally.
Nutritional Assessment and Management
ALD, especially in advanced stages, is a major risk factor for malnutrition, sarcopenia, and frailty. It affects as many as 25%–56% of all cirrhotic patients18,19 and up to 90% in those with advanced liver cirrhosis.20 Malnutrition is defined as an imbalance (deficiency or excess) of nutrients that causes measurable adverse effects on the body composition or function and/or clinical outcome.21 It is a multifactorial spectrum of nutritional disorders across a range of patients from underweight to obese.22 It is also a prognostic factor, as patients with cirrhosis have a worse prognosis if they present with sarcopenia (hazard ratio [HR]: 0.95, 95% confidence interval [CI]: 0.93–0.98).23,24 Sarcopenia (defined as the loss of muscle mass and/or function) has also been associated with an increased risk of septic complications, hyperammonemia, overt hepatic encephalopathy, and worse outcomes after LT.25,26
There are many variables that influence malnutrition, for example, reduced oral intake of macronutrients, which in turn is dependent on other factors such as early satiety, anorexia, nausea and vomiting, dysgeusia, unpalatability of the diet (from low sodium or low potassium), impaired level of consciousness, free water restriction, and frequent fasting due to procedures and hospitalizations.27 Also, these patients have an impaired uptake of micronutrients (which is also multifactorial due to malabsorption, maldigestion, and altered macronutrient metabolism).28,29 Furthermore, some factors are related to the cirrhosis itself, such as reduced muscle formation, and increased muscle breakdown.28, 29, 30 These alterations of micronutrients have been broadly reported to be a common characteristic in patients with ALD. This is thought to be caused by the role micronutrients have in the inflammatory response, oxidative stress, and cell proliferation, either as part of enzymes or acting as coenzymes.31 Likewise, ALD-induced lipid peroxidation produces free radicals, which are eliminated by micronutrients that act as antioxidants, helping protect against hepatocyte necrosis and maintain mitochondrial integrity.32 However, it must be noted that obesity is also a manifestation of malnutrition in these patients. In fact, harmful alcohol ingestion and a high body mass index (BMI) are major risk factors for the incidence of cirrhosis with a supra-additive effect.33,34 This may be due to the proinflammatory effect that obesity has, which can prime the liver for alcohol-induced damage.35 Genetic factors such as PNPLA3 polymorphisms add to the proinflammatory state, which can impact the development of ALD.36 Furthermore, obesity is a prevalent, complex, progressive, and relapsing chronic disease37 that it should be managed as an important comorbidity in patients with ALD.
Indeed, some patients develop sarcopenic obesity as a simultaneous loss of skeletal muscle and gain of adipose tissue.36,38 This may lead to myosteatosis (fat deposition in skeletal muscle), which can occur in both sarcopenic and nonsarcopenic patients, with or without obesity.39 Although there is no formal definition,25 nor a consensus, one widely accepted method is the combination of obesity (BMI ≳ 25 kg/m2 corrected for ascites) associated with computerized tomography (CT)–based height corrected definition of sarcopenia.36
Screening and Evaluation of Malnutrition, Sarcopenia, and Frailty
Current guidelines40 recommend that patients with liver disease should be screened for malnutrition using a validated tool,41 such as the Nutritional Risk Screening 2002 (NRS-2002), Malnutrition Universal Screening Tool (MUST), or the Royal Free Hospital-Nutritional Prioritizing Tool (RFH-NPT).42, 43, 44 The RFH-NPT has higher sensibility than the NRS-2002 to identify patients with liver disease at risk for malnutrition45 and is the best currently available option.46
For the assessment of sarcopenia in cirrhosis, CT imaging is the gold standard. However, there are no formal recommendations for the routine use of CT for this purpose due to the increased cost and exposure to ionizing radiation.47 If abdominal CT imaging is performed for other clinical reasons, the psoas muscle index (PMI), which is the cross-sectional measurement of the psoas muscle at the lower level of L3/L4 vertebra, correlates well with the total body muscle mass.25 It is the most consistent and reproducible method to quantify muscle mass in patients with cirrhosis and can be measured using readily available quantitative morphomics software that does not require specialized training.48 Alternatively, muscle mass can be reported as the skeletal mass index (SMI), calculated as the total muscle area at L3 normalized to height.49 It is quantified using tissue-specific Hounsfield unit thresholds.50
Frailty can also be screened and evaluated via specific tools, and guidelines suggest that at least one frailty tool be incorporated during the initial evaluation and longitudinal follow-up.36 One tool is the activities of daily living (ADLs) which measures the ability to conduct basic tasks to function within one's home (e.g., basic hygiene, eating, ambulation)51 and the Karnofsky Performance Status (KPS).52 Other scales are validated to grade the severity of frailty. For example, the KPS thresholds of 80–100, 50–70, or 10–40 establish high, moderate, or low performance status, respectively.52,53 Likewise, poor performance according to ADLs suggests a greater burden of functional deficits than others. Furthermore, the KPS has shown associations between longitudinal follow-up and outcomes in patients with cirrhosis.30,52,53
As mentioned earlier in the article, patients with cirrhosis, in particular ALD, are in a catabolic state characterized by an increase in protein breakdown but a reduction of protein synthesis to fuel gluconeogenesis.36 This in turn means that patients must have a higher intake of energy and proteins, given that, even with a sedentary lifestyle, a patient with cirrhosis has 1.3 times the estimated resting metabolic rate.23 Therefore, current guidelines recommend that the total energy expenditure (TEE) should be calculated based on dry weight.54 This can be estimated by the following36: (1) use of preascites weight, (2) calculation of ideal body weight (IBW) based on height, (3) postparacentesis weight, or (4) empirically corrected body weight by subtracting a percentage of weight based on the severity of ascites (i.e., for mild ascites, 5%; moderate, 10%; severe, 15%).
Evidence for Nutritional Treatment in ALD
Benefit in mortality of nutritional treatment has been proven difficult to prove.55,56 However, evidence from subgroup analyses of nutritional supplementation in hospitalized patients with cirrhosis demonstrated a benefit in mortality in hospitalized patients with cirrhosis (relative risk [RR]: 0.40; 95% CI: 0.18–0.90).56 Furthermore, the benefit was also shown in AH in which adequate oral intake was assured (defined as ≥ 22 kcal/kg/day)—regardless of the mode of administration. This reduced the mortality by 67% (0.19–0.57) compared with patients who consumed <22 kcal/kg/day.56,57 On the other hand, intensive enteral nutrition (i.e., enteral nutrition was given via feeding tube) in patients with AH is difficult to implement and does not increase survival (Table 1).57
Table 1.
Evidence for Nutritional Support.
| Nutritional Support | Outcome | Type of Study | Reference |
|---|---|---|---|
| Nocturnal feeding | Significant improvement in total body protein and fat-free mass in the patients who received nocturnal supplementation. | RCT | Plank LD, et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48:557–566. |
| Nocturnal feeding | Late-evening snack reverses the aberrant substrate utilization pattern, improved substrate utilization and nitrogen retention than daytime calorie supplementation alone, may improve health-related quality of life and survival, and also may reduce the frequency and severity of HE | RCT and SR | Swart, G et al. Effect of a late evening meal on nitrogen balance in patients with cirrhosis of the liver. BMJ 299, 1202–1203 (1989). Zillikens, M. C., et al. Nocturnal oral glucose supplementation. The effects on protein metabolism in cirrhotic patients and in healthy controls. J. Hepatol. 17, 377–383 (1993). Tsien, C. D., et al Late evening snack: Exploiting a period of anabolic opportunity in cirrhosis. Journal of Gastroenterology and Hepatology vol. 27,430–441 (2012). Maharshi S, et al. Efficacy of Nutritional Therapy for Patients With Cirrhosis and Minimal Hepatic Encephalopathy in a Randomized Trial. Clin Gastroenterol Hepatol. 2016 Mar;14(3):454–460.e3. |
| Lifestyle changes | Overweight or obese patients may benefit from progressive weight normalization (reduced BW and portal pressure). | RCT | Berzigotti A, et al Effects of an intensive lifestyle intervention program on portal hypertension in patients with cirrhosis and obesity: The SportDiet study. Hepatology. 2017 Apr;65(4):1293-130 |
| Supplementation in hospitalized patients | Hospitalized patients with cirrhosis have a benefit in mortality with oral supplementation with adequate oral intake. | SR | Ney, M. et al. Meta-analysis: oral or enteral nutritional supplementation in cirrhosis. Aliment. Pharmacol. Ther. 37, 672–679 (2013). |
| Supplementation in hospitalized patients | Hospitalized patients with AH have a benefit in mortality with oral supplementation with adequate oral intake. | RCT | Moreno, C. et al. Intensive enteral nutrition is ineffective for patients with severe alcoholic hepatitis treated with corticosteroids. Gastroenterology 150, 903–10.e8 (2016). |
| BCAA | BCAA supplementation had a beneficial effect on encephalopathy (RR of 0.73). | SR | Gluud, L. L. et al. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst. Rev. 5, CD001939 (2017). Matsuoka S, et al Improvement in the nutritional status and clinical conditions of patients with liver failure using a liver diet combined with a branched-chain amino acids-enriched elemental diet. Hepato-Gastroenterology. 2014;61:1308–1312. |
| Salt restriction | SUD can increase the level of blood sodium and RBF and be beneficial to diuresis and ascite reduction and disappearance. | RCT | Gu XB, et al Effect of a diet with unrestricted sodium on ascites in patients with hepatic cirrhosis. Gut Liver. 2012 Jul;6(3):355–61. |
AH: alcohol-associated hepatitis; BCAA: branched-chain amino acids; HE: hepatic encephalopathy; RBF: renal blood flow; RCT: randomized controlled trial; RR: relative risk; SR: systematic review; SUD: salt-unrestricted diet.
Studies have also shown benefit in other outcomes. For example, in a randomized controlled trial (RCT), patients with cirrhosis and hepatic encephalopathy (HE) were assigned to receive nutritional therapy for a 6-month study period. It showed a higher reversal of HE (71.1% vs 22.8%; P = 0.001), less development of overt HE (10 vs 21.7% P = 0.04), and better quality of life score.58 Late-evening snacks have also shown to have good evidence that shows a positive effect on important outcomes. Studies have shown an improvement in nitrogen balance, with an increase in muscle mass and quality of life, a reduction of the severity and frequency of HE, and an increase in survival.59, 60, 61, 62, 63
Branched-chain amino acids (BCAAs) have also shown to reduce the progression of liver failure, as well as improve quality of life, reduce hepatic encephalopathy, and increase survival.64, 65, 66, 67 Nonetheless, the primary management strategy to improve nitrogen balance and prevent further sarcopenia is a positive protein intake, which in patients with cirrhosis can be difficult due to their increased protein requirements. This supported by several studies has shown that a positive nitrogen balance with recommended levels of protein and energy intake can improve sarcopenia.68, 69, 70, 71, 72 Furthermore, diets with an adequate content of protein can be administered safely to patients with cirrhosis with HE.72
Recommendations on Nutrition
Based on the current evidence, recommendations include the following: (1) calorie intake of at least 35 kcal/kg in nonobese patients,73 (2) protein intake of 1.2–1.5 g per kilogram daily; in case of protein intolerance, BCAAs may be used to achieve the desired target of protein,74 (3) micronutrient repletion of vitamins and trace elements (as patients with ALD and AUD may suffer from deficiency of water- and fat-soluble vitamins, mainly group B vitamins75, 76, 77, 78), (4) minimize fasting (i.e., frequent small meals and late-evening snack), (5) consider ease of sodium restriction to increase oral intake,28,48 (6) adults living with obesity should receive individualized and multidisciplinary medical nutrition therapy, so as to improve BMI that a target of 5–10% weight loss could be achieved by reducing the estimated TEE by 500–800 Kcal/day.36,37 Also, treating comorbid conditions such as metabolic syndrome must be encouraged together with interventions targeting nutritional support.3 Of note, although micronutrient measurement is recommended, its high cost and the low risk of supplementation usually favor empirical treatment.
Guidelines recommend that patients with cirrhosis should have their caloric and protein intake split into multiple, small, frequent meals (4–6 hourly). It is recommended that the highest protein content be at breakfast, with a late-evening snack composed of complex polysaccharide (50 g).36
Finally, resting energy expenditure is usually increased in ALD, AH, and cirrhosis. The European Society for Clinical Nutrition and Metabolism (ESPEN) guidelines recommend that nutritional supplements should be used when patients cannot meet their caloric requirements through normal food to improve survival. Likewise, enteral tube feeding should be considered in patients with poor oral intake; however, it is not recommended as first-line therapy.23,57
Sarcopenia and Frailty
In the context of patients with cirrhosis, including ALD cirrhosis, sarcopenia is defined as “the phenotypic manifestation of the loss of muscle mass.” Its clinical manifestation in hepatology is usually referred to as frailty; however, this is a narrower definition than the commonly used one in geriatric medicine and is limited to physical frailty. Nonetheless, it can lead to decreased physical function, performance, disability, and worse outcomes.28,79 It is especially relevant in patients with ALD as it is more prevalent in this population than in cirrhosis of other etiologies.24 Up to 30–70% of patients with end-stage liver disease suffer from sarcopenia, with higher prevalence among males than among females.24
Similar to malnutrition, frailty and sarcopenia are multifactorial and can be attributed to the altered catabolic state in ALD itself. This leads to an imbalance between energy expenditure, caloric intake, and altered protein metabolism, particularly BCAAs. A Cochrane review of 16 RCTs including 827 participants with HE showed that BCAA supplementation had a beneficial effect on encephalopathy (relative risk [RR: 0.73). However, no benefit was observed in nutritional parameters.80 Other specific recommendations include (1) the use of a diverse range of protein sources, including vegetable and dairy products, (2) a tailored regimen of aerobic and resistance exercises, and (3) testosterone therapy (although not yet ready for prime time since risk/benefit should be considered in case of history of HCC, other malignancy, or thrombosis).28 It is of note that this last point is relevant as mortality is increased in cirrhotic men with low testosterone levels (with a threshold value of 8.3 nmol/L), independently of the Model of End-Stage Liver Disease (MELD) score (hazard ratio [HR]: 2.36; 95% CI: 1.16–4.81; P = 0.02).81 In fact, one RCT showed that testosterone therapy in men with cirrhosis and low serum testosterone safely increases muscle mass, bone mass, and hemoglobin and reduces fat mass and hemoglobin A1c.82 Therefore, in patients with low testosterone and without HCC, other malignancies, or a history of thrombosis, hormonal replacement therapy may be recommended.
Use of Thiamine in Patients with AUD and ALD
Wernicke-Korsakoff syndrome (WKS) is one of the most severe neuropsychiatric disorders associated with AUD. It is caused by thiamine deficiency. The full triad of symptoms (ophthalmoplegia, ataxia, and mental confusion) is present in just 0.05% of all patients with WKS, whereas the whole population prevalence, based on autopsy studies, has been estimated at 1%–2%.83,84 Although WKS is difficult to diagnose, intravenous administration of thiamine should be considered as it is safe, simple, and inexpensive. However, it is important to note that evidence of its efficacy is low.85,86,87 Recommended prophylaxis doses are 100–200 mg once daily for 3–5 days intravenous (which is preferred), intramuscular, or oral.88 For Wernicke encephalopathy treatment, it is recommended with a dose of 200–500 mg 3 times daily for 2–7 days, followed by 250 mg once daily for an additional 3–5 days, then decrease to at least 100 mg daily until no longer at risk.89
Immunizations
For patients with ALD, the same general recommendations for immunizations exist as with other etiologies. For further details on the recommendation by the Centers for Disease Control and Prevention (CDC) for patients with ALD, please refer to Table 2.
Table 2.
The Centers for Disease Control and Prevention (CDC) Recommendations for Vaccinations for patients With ALD.197,198, 199, 200,201,202
| Vaccine | Frequency | Other Recommendations |
|---|---|---|
| Influenza | Yearly | Best before flu season begins in the community Those who have had a severe allergic reaction to egg (any symptom other than hives) should be vaccinated in a medical setting under supervision |
| Tdap Td (protects against tetanus and diphtheria but not pertussis) |
Tdap or Td booster dose every 10 years, or after 5 years in the case of a severe or dirty wound or burn. | May be given at the same time as other vaccines. |
| Pneumococcal polysaccharide vaccine PPSV23 or PCV13 |
Recommended: PPSV23 for 19 through 64 years with chronic liver disease. Recommended: PPSV23 for >65 years if ≥ 5 years after any PPSV23 at < 65 years Not recommended: PCV13 for 19 through 64 years for chronic liver disease. Recommended: PCV13 for ≥65 years based on shared clinical decision-making. Recommended: PPSV23 ≥ 1 year after PCV13 ∗This does not include solid organ transplant recipients. |
For case-specific situation, please refer to CDC guidelines |
| Hepatitis B | Once for all patients not vaccinated | Hepatitis B vaccine may be given as a stand-alone vaccine or as part of a combination vaccine |
| Hepatitis A | Once for all patients not vaccinated | Patients not previously vaccinated and that have direct contact with someone with hepatitis A should get vaccinated within 2 weeks of exposure. |
| Zoster | Two doses separated by 2–6 months for adults aged 50 years and older | |
| HPV | Once in adults under 26 years old. | HPV vaccination in patients over 27 years old provides less benefit, as more people have already been exposed to HPV. |
| MMR | Once if the patient is born in 1957 or after. | |
| Varicella vaccine | Once if the patient is born in 1980 or after. | |
| SARS- CoV2 | For patients aged 18 years or older: Recommended: BNT162b2 (Pfizer-BioNTech COVID-19 vaccine) Recommended: mRNA-1273 (Moderna COVID-19 vaccine) Recommended: Ad26.COV2.S (Janssen COVID-19 vaccine) Recommended: booster shot of Pfizer-BioNTech's COVID-19 vaccine at least 6 months after their Pfizer-BioNTech primary series for patients with chronic liver disease. |
Currently there are insufficient data to inform the efficacy and safety of mixing mRNA vaccines for the primary series. |
Abbreviations: ALD: alcohol-associated liver disease; Tdap: Tetanus diphtheria and pertussis; Td: tetanus and diphtheria; PPSV23: pneumococcal polysaccharide vaccine 23; PCV13: pneumococcal conjugate vaccine 13; HPV: human papillomavirus; MMR: measles, mumps, and rubella; SARS-CoV2: severe acute respiratory syndrome - coronavirus 2; mRNA: messenger RNA.
Management from mild fibrosis to cirrhosis
Screening
To improve outcomes, it is fundamental to diagnose ALD-induced fibrosis in early stages.90 Methods such as enhanced liver fibrosis test and FibroTest have good performance for the diagnosis of advanced fibrosis in ALD, with studies suggesting that they are better than other biological tests (i.e., FIB-4 and AST to Platelet Ratio Index).91 However, emphasis on simple and widely accessible methods such as FIB-4 have shown to improve screening for advanced fibrosis.92 On the other hand, cost-effectiveness studies show that both populations with low and high prevalence of ALD benefit from screening strategies that include liver stiffness measurement (LSM).93 LSM is also advised for screening in patients with AUD.91 Other strategies such as magnetic resonance spectroscopy and magnetic resonance elastography, although highly accurate and reproducible, with the highest sensitivities and specificities for detecting histological steatosis, have high cost and low accessibility, limiting their potential for use broadly in clinical practice.94
General Management
The management of fibrosis and/or cirrhosis from ALD does not differ from other causes. The degree of compensation of the disease should be evaluated, and complications, actively sought. Alcohol and infection with hepatitis virus infection are compounding factors for the progression of liver fibrosis. Therefore, chronic infection with hepatitis B virus (HBV), HCV, or human immunodeficiency virus (HIV) must be examined.3 Routine practices are advised the same as with other etiologies. This includes management of (1) ascites (diagnostic paracentesis, low-sodium diet, diuretics after ruling out spontaneous bacterial peritonitis, and evacuating paracentesis if necessary), (2) esophageal varices (perform upper gastrointestinal endoscopy and evaluate the need for prophylaxis with beta-blockers/banding according to the results), (3) hepatic encephalopathy (lactulose and/or rifaximin), and (4) HCC screening (ultrasound or MRI). Likewise, usual recommendations for primary and secondary prophylaxis of cirrhosis complications (ascites, spontaneous bacterial peritonitis, esophageal varices, encephalopathy, hepatorenal syndrome) are also recommended as standard of care.3
On the other hand, LT should be considered in patients with advanced cirrhosis due to alcohol that have an MELD score >15 points, multiple decompensations of the disease, especially on those who have discontinued consumption and have a favorable psychosocial profile. Indeed, the decision should not be based on solely if a minimum of six months of alcohol abstinence has been achieved; rather, other criteria should be taken into consideration.3,4,95
Management of AH
General Management
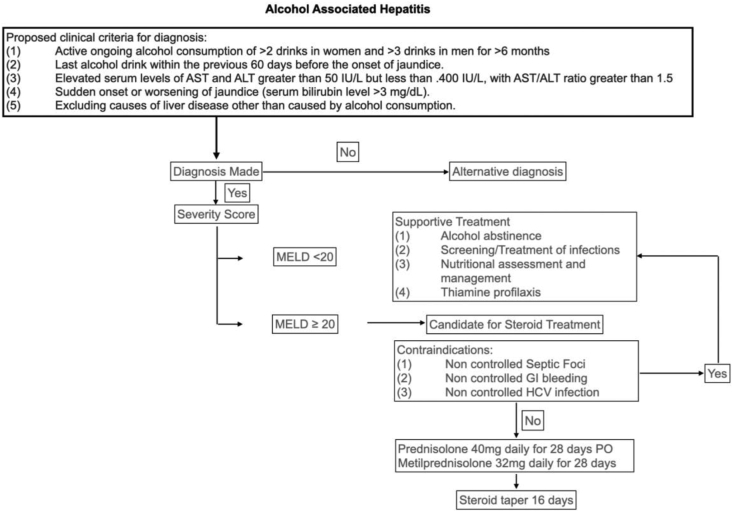
As stated earlier in the article, the most severe acute form of ALD is AH. It is associated with high mortality (20–50%) at 30 days96 and should be promptly suspected in patients with AUD. The National Institute on Alcohol Abuse and Alcoholism (NIAAA) proposed clinical criteria for diagnosis including (1) active ongoing alcohol consumption of >2 drinks in women and >3 drinks in men for >6 months, (2) last alcohol drink within the previous 60 days before the onset of jaundice, (3) elevated serum levels of AST and ALT greater than 50 IU/L but less than 400 IU/L, with AST/ALT ratio greater than 1.5, (4) sudden onset or worsening of jaundice (serum bilirubin level >3 mg/dL), (5) excluding causes of liver disease other than caused by alcohol consumption97 (Algorithm 1). Patients with suspected AH, who do not fulfill these criteria, should be considered for liver biopsy (LB) (discussed later in this review).
Algorithm 1.
General management of alcohol-associated hepatitis. AST: aspartate aminotransferase;ALT: alanine aminotransferase; MELD: Model of End-Stage Liver Disease; GI: gastrointestinal; HCV: hepatitis C virus.
Initially, the severity of the acute event and the risk of complications should be evaluated. In terms of scoring, different systems have been developed to assess patients with AH.98 The MELD score has proven to be a better predictive score in AH than the Maddrey’s discriminant function (MDF).99,100,101,102,103,104 In fact, we have recently demonstrated that the MELD score showed the best performance in predicting short-term mortality compared to previously used scores.105 AH is categorized as severe when associated with encephalopathy or a MELD score of ≥20 and should be considered for steroid therapy (discussed further on). Additionally, a new maker of severity, serum cytokeratin (CK)-18, strongly correlated with histological severity, 90-day mortality and response to prednisolone therapy.106 Another important marker of prognosis and disease severity is the presence of relative adrenal insufficiency (RAI). In one study of 120 patients with AH, 54% of patients with severe AH showed RAI (P ≤ 0.001),107 whereas none of the patients with mild/moderate AH showed RAI. RAI also showed negative correlation with MDF.
Low-risk patients (defined by a MELD score <20) should be managed with abstinence, nutritional support, and general measures. In contrast, patients with severe disease will have a worse prognosis and should be evaluated for additional treatment measures.14 This includes moderate hydration, with emphasis on avoiding overhydration, since it can increase the risk of ascites and increased portal pressure14 and nutritional therapy as per recommendations already stated in this article. Other general measures include treatment of HE (lactulose, rifaximin) and treatment of ascites (salt restriction, paracentesis, or diuretics).3,23
Acute Kidney Injury-hepatorenal Syndrome Prevention and Treatment
In patients with AH, systemic inflammatory response syndrome (SIRS) predisposes them to a reduction in renal blood flow and glomerular filtration rate, leading to acute kidney injury-hepatorenal syndrome (AKI-HRS).108,109 This is heavily associated with poor outcomes.14 As per the International Club of Ascites (ICA) consensus recommendations in 2015, the new definition of AKI-HRS is an increase in serum creatinine ≥0.3 mg/dL (26.5 μmol/L) or increase in serum creatinine ≥1.5-fold to twofold from baseline.110 Up to 90% of patients with stage 1A AKI-HRS (serum creatinine <1.5 g/dL) may resolve with intravenous albumin at 1 g/kg/day for two days.109,111 It is also recommended that diuretics and beta-blockers (given their negative inotropic effect) should be withdrawn.109,111 In patients with AKI-HRS stage 1B (serum creatinine ≥1.5 g/dL) or greater, vasoconstrictors should be used.3,112 These include terlipressin, noradrenaline, and the combination of midodrine plus octreotide.113 All vasoconstrictors should be combined with albumin.114 These drugs augment systolic blood pressure and arterial volume, increasing renal perfusion and producing systemic and splanchnic vasoconstriction.115
Antibiotics in AH
Patients with AH have peripheral neutrophil dysfunction with impaired phagocytic activity, oxidative stress, and complete paralysis of the innate immunity that predispose them to infections.116 Prophylactic use of antibiotics has been considered to reduce the risk of infection, aiming to achieve intestinal decontamination and control the gut–liver axis. In mice models, exotoxin that is secreted by Enterococcus faecalis promotes ethanol-induced liver disease.117,118 In fact, studies with bacteriophages that target cytolytic E. faecalis found that ALD fibrosis could be reversed in tested subjects.119
In human studies, prophylactic antibiotics have been associated with a lower risk of death120; however, results are conflicting. Rifaximin was tested in a small RCT of 30 patients with AH.121 The authors found improved levels of endotoxin, cytokine, cell activation markers, and metabolites during rifaximin administration with 90 days of follow-up (EudraCT 2014-002264-33). Similarly, an RCT subgroup analysis of 33 patients with acute-on-chronic liver failure (ACLF) evaluated the use of norfloxacin as prophylaxis in AH, comparing norfloxacin and steroid vs placebo and steroid. They found that the incidence of infection was lower in patients that used norfloxacin (10% vs. 38.5% P = 0.066) at day 30 and also at day 90 (30% vs. 69.2%, P = 0.027). In both groups, the adverse effects of therapy were similar.122 Other combined therapies with vancomycin, gentamicin, and meropenem were used in an open-label trial in Denmark,123 but this study found no benefit. The authors suggested that bacterial translocation may have less impact once inflammation has been established or that the composition and not reduction of the microbiome is more important. Likewise, amoxicillin/clavulanate (AC) has also been examined in an RCT that included 284 patients with biopsy-proven AH, comparing prednisolone and AC or prednisolone plus placebo. In an intention-to-treat analysis, they found that 2-month survival was not significantly different in the two groups (HR of 0.77, 95% CI: 0.45–1.31). Nevertheless, the incidence of infection at 2 months was lower in the antibiotics group (HR: 0.62; 95% CI: 0.42–0.91). The Lille model at day 7 was not significantly different when using antibiotics vs. placebo (0.37 vs. 0.39, P = 0.80).124 Though these results are interesting, further validation studies are necessary to define the usefulness and safety of antibiotics as prophylaxis of infections in patients with AH.
Steroids
Patients with ALD have increased intestinal permeability due to prolonged or binge alcohol consumption,125 which worsens endotoxemia and stimulates Kupffer cells leading to increased production of proinflammatory cytokines.126 This may lead to hepatocellular damage and failure which causes AH.127 Thus, treatment based on corticosteroids may act by reducing immune activation blocking cytotoxicity and inflammation pathways.128,129 Histological evidence shows that certain findings in AH have a prognostic value: the presence of severe fibrosis, megamitochondria, the level of neutrophil infiltration, and cholestasis.130 All of these factors may be reversible by corticosteroids, even in advanced fibrosis.95 Therefore, corticosteroid treatment is recommended in patients with AH with a MELD score higher than 20131 and an MDF higher than 32.132 In addition, an increase in the MELD score of ≥2 points in the first week of hospitalization may independently predict in-hospital mortality,102 and a score greater than 20 is associated with the development of ACLF and multiorgan failure.133
However, there has been controversy over the effectiveness of pharmacological treatments. Indeed, there have been 13 RCTs and four meta-analyses investigating the role of corticosteroid therapy for AH.134 Ten of these have compared corticosteroids with placebo, with the rest comparing to other anti-inflammatory therapeutic strategies.135 Results across these studies have shown mixed efficacy data due to heterogeneity between studies on patient population and study inclusion/exclusion criteria.136, 137, 138, 139, 140, 141, 142, 143, 144, 145, 146, 147 For example, a retrospective review of patients with AH showed that there was no statistically significant difference between the treatment group vs. nontreatment group in terms of 28-day mortality in patients with an MDF ≥32 (31% vs. 11%, respectively; P = 0.18) and 6-month mortality (45% treatment vs. 38% nontreatment; P = 0.75). Likewise, in patients with an MDF <32, there was no statistically significant difference in 28-day and 6-month mortality between the treatment group vs. nontreatment group (P > 0.99).148 However, a systematic review of individual patient data from the five largest randomized placebo controlled trials142,143,145,149 showed approximately 50% mortality benefit at 28 days with corticosteroids (20 vs. 34%, P < 0.0001).103 This lead to the STOPAH trial, one of the largest RCTs tasked with determining the effectiveness of pharmacological treatment.135 Its 2-by-2 factorial design included 1103 patients and showed mortality at 28 days was lowest in the prednisolone–placebo group in comparison to the pentoxifylline (PTX)–placebo and the placebo–placebo control group (14% vs 19% vs 17%, respectively) with an odds ratio (OR) of 0.72 in the prednisone group (95% CI: 0.52 to 1.01; P = 0.06).150 It suggested a narrow therapeutic window, as at 90 days and at 1 year, there were no significant between-group differences. Furthermore, a recent retrospective, international multicenter cohort study including 3380 adults demonstrated a short-term benefit of corticosteroids even with higher MELD scores, where the highest effect was observed in patients with MELD scores between 25 and 39.101 This benefit was lost only in patients with the most severe liver disease (MELD score higher than 51). Again, benefit seems to be lost in patients past 30 days.
Treatment regimens usually recommended by guidelines, prednisolone or prednisone 40 mg/d, is given orally for 28 days. Methylprednisolone is used in a dose of 32 mg given intravenously daily for patients unable to take medications orally.131,151,152,129 Additionally, the response to corticosteroid treatment can be predicted using the Lille model, where a value ≥ 0.45 predicts failure to corticosteroid treatment. Patients with corticosteroids should be assessed at day 4 or 7 and could be considered for discontinuing therapy if the cutoff is reached. For patients who respond to treatment, prednisolone should be maintained for 28 days and finish therapy with a 16-day prednisolone taper.153,104
Contraindications for Corticosteroid Therapy
As mentioned earlier in the article, patients with AH have several immune system dysfunctions that predispose severe patients with AH to the development of infections. This topic is discussed elsewhere in this review; however, possible infection foci must be adequately controlled before starting corticosteroid therapy.131 Other contraindications are uncontrolled or active gastrointestinal bleeding, which should also warrant a delay in corticosteroid treatment as concurrent steroid is associated with twofold risk of further GI bleeding and perforation.154
Concomitant HCV infection is also a concern in patients with AH as they have worse outcomes than patients with AH without HCV infection.155 This is mostly based on studies where the use of high-dose corticosteroids increases the HCV replication in transplant patients.156,157 Whether this translates in AH, which uses a medium dose for a limited period and worsens outcomes, is still unknown. Current recommendations are that until more data are available, patients who are concomitantly infected with HCV may benefit more from withholding steroids for AH.135,158
Infection Prevention
Several studies have examined clinical predictors in patients with AH who are at risk of developing a nosocomial infection. Factors such as age, baseline liver function, improvement in cholestasis within 7 days, renal function, and the presence of SIRS correlate with the likelihood of developing infections after the start of therapy.120,159,160 Liver function in particular is a key predictor of the likelihood of developing infection in patients with AH with SIRS criteria who did have infection versus those who did not.160 Evidence shows that in ALD, bacterial overgrowth161 and changes in gut permeability result in bacterial translocation and endotoxemia from bacterial products, such as lipopolysaccharide (LPS), which are proinflammatory.162 As a functional group, these bacterial products are known as pathogen-activated molecular patterns (PAMPs). Also, strong evidence suggests that the microbiome (both bacteria and fungi) are altered in patients with AH.163,164,165 This may explain the increased risk of infection in patients with AH and increased mortality (up to 30% at 2 months). In fact, infected patients have a similar survival rate to that of nonresponders to corticosteroids (the mainstay of severe AH treatment).159 Furthermore, the use of corticosteroids can increase this risk and are contraindicated in patients with uncontrolled sites of sepsis.3 Patients with evidence of possible infection (e.g., fever, worsening mental status, hemodynamic instability) should have blood and urine cultures obtained. However, no clinical and biological parameters are able to distinguish between infected and noninfected patients. Procalcitonin, with a cutoff value of 0.45 ng/mL, has shown the best performance (positive predictive value of 83.3% and negative predictive value of 71%).166,160 Another biomarker is high levels of circulating 16S bacterial DNA, which have been shown to have an increased risk of developing infection if patients are treated with prednisolone, independent of liver function.120
Recommendations for the management of patients with AH include limiting the use of indwelling catheters given their high susceptibility to infection.167 Therefore, in patients who are candidates for intravenous nutrition, a cost–benefit analysis between improving nitrogen balance should be weighed against the risk of higher rates of infection.120,168 Finally, as stated earlier, some evidence has shown that the use of prophylactic antibiotics is helpful,169,170 and further validation studies are necessary.
Pentoxifylline
The phosphodiesterase inhibitor, PTX, has been evaluated in patients with AH for its ability to inhibit the production of tumor necrosis factor (TNF).171 High levels of TNF induce the production of reactive oxygen species (ROS) by the hepatocyte mitochondria, leading to cell death.172 As mentioned, initially studies observed some benefit;173 however, survival was not improved with the use of PTX in follow-up studies.150,174,175 Similar results have been found when combining PTX with corticosteroids.176,177 Therefore, evidence for survival benefit with PTX therapy in patients with severe AH is very weak, and the drug can no longer be recommended.3
N-Acetylcysteine
As mentioned, there is an increase in ROS during AH. This is accompanied by severe mitochondrial depletion of glutathione, the primary antioxidant in cells. N-acetylcysteine could act as an antioxidant in the treatment of AH because the thiol group in N-acetylcysteine is able to reduce levels of free radicals.178 This has been demonstrated in acetaminophen-induced hepatitis.179 An RCT evaluating the efficacy of glucocorticoids plus N-acetylcysteine, as compared with glucocorticoids alone in 174 patients with severe AH, demonstrated an increased 1-month survival. However, the 6-month survival was not improved.172 Other similar studies did not show benefit in survival.178,180 Further studies are needed before a recommendation can be made.
As for the risk of infection, a meta-analysis found one study that N-acetylcysteine and corticosteroids (versus N-acetylcysteine alone) had a positive effect on reducing the rate of infection (RR: 0.45, 95% CI: [0.27, 0.75] P = 0.002).181 This was also supported in studies where N-acetylcysteine was added to corticosteroid therapy.172 In this same study, death due to HRS was less frequent in the prednisolone-N-acetylcysteine group than in the prednisolone-only group at 6 months (9% vs. 22%, P = 0.02). In the case of HRS, some evidence exists that N-acetylcysteine can prevent the adverse effects of terlipressin and that its addition could be synergetic in improving response to therapy and survival rate.182, 183, 184, 185 However, more controlled trials are required to be able to adequately recommend it.
When To Biopsy
Clinical criteria proposed by the NIAAA are useful for the diagnosis; however, they may be inaccurate in up to 46% of cases.186 The main confounders in patients with diagnostic uncertainty of AH are the presence of factors such as ischemic hepatitis, possible drug-induced liver injury, serology positive for another liver disease etiology, or uncertain alcohol use.14 Therefore, LB may be required when the diagnosis is uncertain, especially if confounding factors are in place.187 In one study, the success rate for obtaining adequate samples for interpretation was 96%–98% with a median of 2.7 passes, with an excellent safety profile for transjugular procedures.188, 189, 190 Furthermore, the transjugular approach allows measurement of portal pressure that may also be helpful to estimate short-term prognosis because the degree of portal hypertension and hyperdynamic circulation relates to AH severity. LB may also predict the disease severity and prognosis based on bilirubin stasis, fibrosis, neutrophilic infiltration, and presence of megamitochondria.191
Palliative Care
Unfortunately some patients with decompensated cirrhosis who may be nonresponsive to therapy may benefit from palliative care (PC) and hospice interventions. PC should be considered for patients who are not candidates for LT; who have ACLF that is unresponsive to treatment, chronic multiorgan failure, or complications of cirrhosis (i.e., HRS, HE, variceal bleeding or HCC) that are advanced; and who have not responded to or are unlikely to respond to treatment.192 Therefore, it must be considered in cases where treatment is considered futile (i.e., when clinical reasoning or experience suggests that a life-sustaining treatment is highly unlikely to achieve its purpose).193 PC must focus on the quality of life for patients and families through the prevention and relief of suffering. The goal is to neither hasten nor postpone death.193 Patients with advanced stage ALD have a high degree of burden from symptoms. Studies show that patients who receive PC reduce hospital resource utilization (total cost of hospitalization, the number of emergency department visits, hospital and critical care admissions).194 Other studies have examined factors associated with PC in patients with decompensated cirrhosis.195 In multivariable logistic regression, higher comorbidity burden, ascites, and increased MELD-Na score were unlisted for LT or unmarried status were most associated with referral to PC. Worryingly PC referrals were late in 68.5% of cases, and hospice referrals were late in 62.7%. This was higher in younger or married patients or in those with recent alcohol use. Also of concern is the lack of referral in patients that were removed from LT lists. One study found that only 11% were referred for PC.196 Therefore, it is important that patients should be constantly re-evaluated for their symptom control and prognosis, to better the chance of receiving timely PC.
ALD is one of the main causes of cirrhosis. Treatment follows the same principles as with other etiologies but with a higher emphasis on abstinence and nutritional support. Emerging therapies have had some success in treating alcohol-induced fibrosis, but more studies are needed. Likewise, novel therapeutic options in AH that address oxidative stress, inflammation, liver regeneration, and gut–liver axis dysfunction have been examined. Although some have not had positive results, others, such as metadoxine, IL-22 analogs, and fecal microbiota transplantation, have shown promising results. They may offer new avenues for treatment, potentially improving outcomes, and should be explored with RCTs to bring further advances in this area.
Credit authorship contribution statement
Authors confirm contribution to the article as follows: Gustavo Ayares: Investigation, Writing - Original Draft, Writing - Review and Editing Francisco Idalsoaga: Conceptualization, Writing - Review and Editing Luis Antonio Díaz: Writing - Review and Editing Jorge Arnold: Writing - Review and Editing Juan Pablo Arab: Conceptualization, Writing - Review and Editing, Supervision.
Conflicts of interest
The authors declare that there is no conflict of interest.
Funding
This article was partially supported by the Chilean government through the Fondo Nacional de Desarrollo Científico y Tecnológico (FONDECYT 1200227 to JPA) and the Agencia Nacional de Investigación y desarrollo, ANID through ANID ACE 210009 grant.
References
- 1.Website. https://pubs.niaaa.nih.gov/publications/surveillance105/Cirr13.htm Accessed November 9, 2021.
- 2.Gao B., Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.European Association for the Study of the Liver Electronic address: easloffice@easloffice.eu, European association for the study of the liver. EASL clinical practice guidelines: management of alcohol-related liver disease. J Hepatol. 2018;69:154–181. doi: 10.1016/j.jhep.2018.03.018. [DOI] [PubMed] [Google Scholar]
- 4.Crabb D.W., Im G.Y., Szabo G., Mellinger J.L., Lucey M.R. Diagnosis and treatment of alcohol-associated liver diseases: 2019 practice guidance from the American association for the study of liver diseases. Hepatology. 2020;71:306–333. doi: 10.1002/hep.30866. [DOI] [PubMed] [Google Scholar]
- 5.Arab J.P., Roblero J.P., Altamirano J., et al. Alcohol-related liver disease: clinical practice guidelines by the Latin American association for the study of the liver (ALEH) Ann Hepatol. 2019;18:518–535. doi: 10.1016/j.aohep.2019.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Rehm J., Gmel G.E., Sr., Gmel G., et al. The relationship between different dimensions of alcohol use and the burden of disease-an update. Addiction. 2017;112:968–1001. doi: 10.1111/add.13757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Phillips J.A. Dietary guidelines for Americans, 2020-2025. Workplace Health & Saf. 2021;69:395. doi: 10.1177/21650799211026980. [DOI] [PubMed] [Google Scholar]
- 8.Julien J., Ayer T., Tapper E.B., Barbosa C., Dowd W., Chhatwal J. Effect of increased alcohol consumption during COVID-19 pandemic on alcohol-related liver disease: a modeling study. Hepatology. 2021 doi: 10.1002/hep.32272. Published online December 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rutledge S.M., Schiano T.D., Florman S., Im G.Y. COVID-19 aftershocks on alcohol-associated liver disease: an early cross-sectional report from the U.S. Epicenter. Hepatol Commun. 2021;5:1151–1155. doi: 10.1002/hep4.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moon A.M., Curtis B., Mandrekar P., Singal A.K., Verna E.C., Fix O.K. Alcohol-associated liver disease before and after COVID-19-an overview and call for ongoing investigation. Hepatol Commun. 2021;5:1616–1621. doi: 10.1002/hep4.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mauro E., Marciano S., Torres M.C., Roca J.D., Novillo A.L., Gadano A. Telemedicine improves access to Hepatology consultation with high patient satisfaction. J Clin Exp Hepatol. 2020;10:555–562. doi: 10.1016/j.jceh.2020.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Simonetto D.A., Shah V.H., Kamath P.S. Outpatient management of alcohol-related liver disease. Lancet Gastroenterol Hepatol. 2020;5:485–493. doi: 10.1016/S2468-1253(19)30415-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.American Psychiatric Association . American Psychiatric Pub; 2013. Diagnostic and Statistical Manual of Mental Disorders (DSM-5®) [Google Scholar]
- 14.Singal A.K., Bataller R., Ahn J., Kamath P.S., Shah V.H. ACG clinical guideline: alcoholic liver disease. Am J Gastroenterol. 2018;113:175–194. doi: 10.1038/ajg.2017.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lackner C., Spindelboeck W., Haybaeck J., et al. Histological parameters and alcohol abstinence determine long-term prognosis in patients with alcoholic liver disease. J Hepatol. 2017;66:610–618. doi: 10.1016/j.jhep.2016.11.011. [DOI] [PubMed] [Google Scholar]
- 16.Singal A.G., Lampertico P., Nahon P. Epidemiology and surveillance for hepatocellular carcinoma: new trends. J Hepatol. 2020;72:250–261. doi: 10.1016/j.jhep.2019.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Singal A.K., Shah V.H. Zakim and Boyer's Hepatology. 2018. Prevention and management of alcoholic liver disease; pp. 351–368. e7. [DOI] [Google Scholar]
- 18.Huynh D.K., Selvanderan S.P., Harley H.A.J., Holloway R.H., Nguyen N.Q. Nutritional care in hospitalized patients with chronic liver disease. World J Gastroenterol. 2015;21:12835–12842. doi: 10.3748/wjg.v21.i45.12835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao C.K., Fung J., Chu N.H.S., Tan V.P.Y. Dietary interventions in liver cirrhosis. J Clin Gastroenterol. 2018;52:663–673. doi: 10.1097/MCG.0000000000001071. [DOI] [PubMed] [Google Scholar]
- 20.Henkel A.S., Buchman A.L. Nutritional support in patients with chronic liver disease. Nat Clin Pract Gastroenterol Hepatol. 2006;3:202–209. doi: 10.1038/ncpgasthep0443. [DOI] [PubMed] [Google Scholar]
- 21.Merli M., Riggio O., Dally L. Does malnutrition affect survival in cirrhosis? PINC (Policentrica Italiana Nutrizione Cirrosi) Hepatology. 1996;23:1041–1046. doi: 10.1002/hep.510230516. [DOI] [PubMed] [Google Scholar]
- 22.Lochs H., Allison S.P., Meier R., et al. Introductory to the ESPEN guidelines on enteral nutrition: terminology, definitions and general topics. Clin Nutr. 2006;25:180–186. doi: 10.1016/j.clnu.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Plauth M., Bernal W., Dasarathy S., et al. ESPEN guideline on clinical nutrition in liver disease. Clin Nutr. 2019;38:485–521. doi: 10.1016/j.clnu.2018.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tantai X., Liu Y., Yeo Y.H., et al. Effect of sarcopenia on survival in patients with cirrhosis: a systematic review and meta-analysis. J Hepatol. 2021 doi: 10.1016/j.jhep.2021.11.006. Published online November 13. [DOI] [PubMed] [Google Scholar]
- 25.Anand A.C. Nutrition and muscle in cirrhosis. J Clin Exp Hepatol. 2017;7:340–357. doi: 10.1016/j.jceh.2017.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singal A.K., Charlton M.R. Nutrition in alcoholic liver disease. Clin Liver Dis. 2012;16:805–826. doi: 10.1016/j.cld.2012.08.009. [DOI] [PubMed] [Google Scholar]
- 27.Bunchorntavakul C., Reddy K.R. Review article: malnutrition/sarcopenia and frailty in patients with cirrhosis. Aliment Pharmacol Ther. 2020;51:64–77. doi: 10.1111/apt.15571. [DOI] [PubMed] [Google Scholar]
- 28.Tandon P., Montano-Loza A.J., Lai J.C., Dasarathy S., Merli M. Sarcopenia and frailty in decompensated cirrhosis. J Hepatol. 2021;75(Suppl 1):S147–S162. doi: 10.1016/j.jhep.2021.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cruz-Jentoft A.J., Bahat G., Bauer J., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing. 2019;48:16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lai J.C., Tandon P., Bernal W., et al. Malnutrition, frailty, and sarcopenia in patients with cirrhosis: 2021 practice guidance by the American association for the study of liver diseases. Hepatology. 2021;74:1611–1644. doi: 10.1002/hep.32049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wu J., Meng Q.H. Current understanding of the metabolism of micronutrients in chronic alcoholic liver disease. World J Gastroenterol. 2020;26:4567–4578. doi: 10.3748/wjg.v26.i31.4567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Masalkar P.D., Abhang S.A. Oxidative stress and antioxidant status in patients with alcoholic liver disease. Clin Chim Acta. 2005;355:61–65. doi: 10.1016/j.cccn.2004.12.012. [DOI] [PubMed] [Google Scholar]
- 33.Hart C.L., Morrison D.S., Batty G.D., Mitchell R.J., Davey Smith G. Effect of body mass index and alcohol consumption on liver disease: analysis of data from two prospective cohort studies. BMJ. 2010;340:c1240. doi: 10.1136/bmj.c1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Åberg F., Helenius-Hietala J., Puukka P., Färkkilä M., Jula A. Interaction between alcohol consumption and metabolic syndrome in predicting severe liver disease in the general population. Hepatology. 2018;67:2141–2149. doi: 10.1002/hep.29631. [DOI] [PubMed] [Google Scholar]
- 35.Boyle M., Masson S., Anstee Q.M. The bidirectional impacts of alcohol consumption and the metabolic syndrome: cofactors for progressive fatty liver disease. J Hepatol. 2018;68:251–267. doi: 10.1016/j.jhep.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 36.Puri P., Dhiman R.K., Taneja S., et al. Nutrition in chronic liver disease: consensus statement of the Indian national association for study of the liver. J Clin Exp Hepatol. 2021;11:97–143. doi: 10.1016/j.jceh.2020.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wharton S., Lau D.C.W., Vallis M., et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192:E875–E891. doi: 10.1503/cmaj.191707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Carias S., Castellanos A.L., Vilchez V., et al. Nonalcoholic steatohepatitis is strongly associated with sarcopenic obesity in patients with cirrhosis undergoing liver transplant evaluation. J Gastroenterol Hepatol. 2016;31 doi: 10.1111/jgh.13166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gallagher D., Kuznia P., Heshka S., et al. Adipose tissue in muscle: a novel depot similar in size to visceral adipose tissue. Am J Clin Nutr. 2005;81:903–910. doi: 10.1093/ajcn/81.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bischoff S.C., Bernal W., Dasarathy S., et al. ESPEN practical guideline: clinical nutrition in liver disease. Clin Nutr. 2020;39:3533–3562. doi: 10.1016/j.clnu.2020.09.001. [DOI] [PubMed] [Google Scholar]
- 41.Website. https://www.espen.org/files/ESPEN-Guidelines/ESPEN_practical_guideline_Clinical_nutrition_in_liver_disease.pdf
- 42.Kondrup J., Rasmussen H.H., Hamberg O., Stanga Z., Ad Hoc ESPEN Working Group Nutritional risk screening (NRS 2002): a new method based on an analysis of controlled clinical trials. Clin Nutr. 2003;22:321–336. doi: 10.1016/s0261-5614(02)00214-5. [DOI] [PubMed] [Google Scholar]
- 43.Sorensen J., Kondrup J., Prokopowicz J., et al. EuroOOPS: an international, multicentre study to implement nutritional risk screening and evaluate clinical outcome. Clin Nutr. 2008;27:340–349. doi: 10.1016/j.clnu.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 44.Wu Y., Zhu Y., Feng Y., et al. Royal Free Hospital-Nutritional Prioritizing Tool improves the prediction of malnutrition risk outcomes in liver cirrhosis patients compared with Nutritional Risk Screening 2002. Br J Nutr. 2020;124:1293–1302. doi: 10.1017/S0007114520002366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Borhofen S.M., Gerner C., Lehmann J., et al. The royal free hospital-nutritional prioritizing tool is an independent predictor of deterioration of liver function and survival in cirrhosis. Dig Dis Sci. 2016;61:1735–1743. doi: 10.1007/s10620-015-4015-z. [DOI] [PubMed] [Google Scholar]
- 46.Tandon P., Raman M., Mourtzakis M., Merli M. A practical approach to nutritional screening and assessment in cirrhosis. Hepatology. 2017;65:1044–1057. doi: 10.1002/hep.29003. [DOI] [PubMed] [Google Scholar]
- 47.Carey E.J., Lai J.C., Sonnenday C., et al. A north American expert opinion statement on sarcopenia in liver transplantation. Hepatology. 2019;70:1816–1829. doi: 10.1002/hep.30828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Paris M.T., Tandon P., Heyland D.K., et al. Automated body composition analysis of clinically acquired computed tomography scans using neural networks. Clin Nutr. 2020;39:3049–3055. doi: 10.1016/j.clnu.2020.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carey E.J., Lai J.C., Wang C.W., et al. A multicenter study to define sarcopenia in patients with end-stage liver disease. Liver Transplant. 2017;23:625–633. doi: 10.1002/lt.24750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mitsiopoulos N., Baumgartner R.N., Heymsfield S.B., Lyons W., Gallagher D., Ross R. Cadaver validation of skeletal muscle measurement by magnetic resonance imaging and computerized tomography. J Appl Physiol. 1998;85:115–122. doi: 10.1152/jappl.1998.85.1.115. [DOI] [PubMed] [Google Scholar]
- 51.Tapper E.B., Finkelstein D., Mittleman M.A., Piatkowski G., Lai M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology. 2015;62:584–590. doi: 10.1002/hep.27830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Thuluvath P.J., Thuluvath A.J., Savva Y. Karnofsky performance status before and after liver transplantation predicts graft and patient survival. J Hepatol. 2018;69:818–825. doi: 10.1016/j.jhep.2018.05.025. [DOI] [PubMed] [Google Scholar]
- 53.Tandon P., Reddy K.R., O'Leary J.G., et al. A Karnofsky performance status-based score predicts death after hospital discharge in patients with cirrhosis. Hepatology. 2017;65:217–224. doi: 10.1002/hep.28900. [DOI] [PubMed] [Google Scholar]
- 54.Kalafateli M., Mantzoukis K., Choi Yau Y., et al. Malnutrition and sarcopenia predict post-liver transplantation outcomes independently of the Model for End-stage Liver Disease score. J Cachexia Sarcopenia Muscle. 2017;8:113–121. doi: 10.1002/jcsm.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Koretz R.L., Avenell A., Lipman T.O. Nutritional support for liver disease. Cochrane Database Syst Rev. 2012;5:CD008344. doi: 10.1002/14651858.CD008344.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ney M., Vandermeer B., van Zanten S.J.V., Ma M.M., Gramlich L., Tandon P. Meta-analysis: oral or enteral nutritional supplementation in cirrhosis. Aliment Pharmacol Ther. 2013;37:672–679. doi: 10.1111/apt.12252. [DOI] [PubMed] [Google Scholar]
- 57.Moreno C., Deltenre P., Senterre C., et al. Intensive enteral nutrition is ineffective for patients with severe alcoholic hepatitis treated with corticosteroids. Gastroenterology. 2016;150:903–910. doi: 10.1053/j.gastro.2015.12.038. e8. [DOI] [PubMed] [Google Scholar]
- 58.Maharshi S., Sharma B.C., Sachdeva S., Srivastava S., Sharma P. Efficacy of nutritional therapy for patients with cirrhosis and minimal hepatic encephalopathy in a randomized trial. Clin Gastroenterol Hepatol. 2016;14:454–460. doi: 10.1016/j.cgh.2015.09.028. e3; quiz e33. [DOI] [PubMed] [Google Scholar]
- 59.Rivera Irigoin R., Abilés J. [Nutritional support in patients with liver cirrhosis] Gastroenterol Hepatol. 2012;35:594–601. doi: 10.1016/j.gastrohep.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Plank L.D., Gane E.J., Peng S., et al. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: a randomized 12-month trial. Hepatology. 2008;48:557–566. doi: 10.1002/hep.22367. [DOI] [PubMed] [Google Scholar]
- 61.Swart G.R., Zillikens M.C., van Vuure J.K., van den Berg J.W. Effect of a late evening meal on nitrogen balance in patients with cirrhosis of the liver. BMJ. 1989;299:1202–1203. doi: 10.1136/bmj.299.6709.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zillikens M.C., van den Berg J.W., Wattimena J.L., Rietveld T., Swart G.R. Nocturnal oral glucose supplementation. The effects on protein metabolism in cirrhotic patients and in healthy controls. J Hepatol. 1993;17:377–383. doi: 10.1016/s0168-8278(05)80221-1. [DOI] [PubMed] [Google Scholar]
- 63.Tsien C.D., McCullough A.J., Dasarathy S. Late evening snack: exploiting a period of anabolic opportunity in cirrhosis. J Gastroenterol Hepatol. 2012;27:430–441. doi: 10.1111/j.1440-1746.2011.06951.x. [DOI] [PubMed] [Google Scholar]
- 64.Yoshida T., Muto Y., Moriwaki H., Yamato M. Effect of long-term oral supplementation with branched-chain amino acid granules on the prognosis of liver cirrhosis. Gastroenterol Jpn. 1989;24:692–698. doi: 10.1007/BF02774169. [DOI] [PubMed] [Google Scholar]
- 65.Muto Y., Sato S., Watanabe A., et al. Effects of oral branched-chain amino acid granules on event-free survival in patients with liver cirrhosis. Clin Gastroenterol Hepatol. 2005;3:705–713. doi: 10.1016/s1542-3565(05)00017-0. [DOI] [PubMed] [Google Scholar]
- 66.Kawaguchi T., Izumi N., Charlton M.R., Sata M. Branched-chain amino acids as pharmacological nutrients in chronic liver disease. Hepatology. 2011;54:1063–1070. doi: 10.1002/hep.24412. [DOI] [PubMed] [Google Scholar]
- 67.Marchesini G., Bianchi G., Merli M., et al. Nutritional supplementation with branched-chain amino acids in advanced cirrhosis: a double-blind, randomized trial. Gastroenterology. 2003;124:1792–1801. doi: 10.1016/s0016-5085(03)00323-8. [DOI] [PubMed] [Google Scholar]
- 68.Manguso F., D'Ambra G., Menchise A., Sollazzo R., D'Agostino L. Effects of an appropriate oral diet on the nutritional status of patients with HCV-related liver cirrhosis: a prospective study. Clin Nutr. 2005;24:751–759. doi: 10.1016/j.clnu.2005.02.010. [DOI] [PubMed] [Google Scholar]
- 69.Nielsen K., Kondrup J., Martinsen L., et al. Long-term oral refeeding of patients with cirrhosis of the liver. Br J Nutr. 1995;74:557–567. doi: 10.1079/bjn19950158. [DOI] [PubMed] [Google Scholar]
- 70.Ruiz-Margáin A., Macías-Rodríguez R.U., Ríos-Torres S.L., et al. Effect of a high-protein, high-fiber diet plus supplementation with branched-chain amino acids on the nutritional status of patients with cirrhosis. Rev Gastroenterol México. 2018;83:9–15. doi: 10.1016/j.rgmx.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 71.Horst D., Grace N.D., Conn H.O., et al. Comparison of dietary protein with an oral, branched chain-enriched amino acid supplement in chronic portal-systemic encephalopathy: a randomized controlled trial. Hepatology. 1984;4:279–287. doi: 10.1002/hep.1840040218. [DOI] [PubMed] [Google Scholar]
- 72.Córdoba J., López-Hellín J., Planas M., et al. Normal protein diet for episodic hepatic encephalopathy: results of a randomized study. J Hepatol. 2004;41:38–43. doi: 10.1016/j.jhep.2004.03.023. [DOI] [PubMed] [Google Scholar]
- 73.Bémeur C., Butterworth R.F. Reprint of: nutrition in the management of cirrhosis and its neurological complications. J Clin Exp Hepatol. 2015;5(Suppl 1):S131–S140. doi: 10.1016/j.jceh.2015.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Matsuoka S., Tamura A., Nakagawara H., Moriyama M. Improvement in the nutritional status and clinical conditions of patients with liver failure using a liver diet combined with a branched chain amino acids-enriched elemental diet. Hepato-Gastroenterology. 2014;61:1308–1312. [PubMed] [Google Scholar]
- 75.Mills P.R., Shenkin A., Anthony R.S., et al. Assessment of nutritional status and in vivo immune responses in alcoholic liver disease. Am J Clin Nutr. 1983;38:849–859. doi: 10.1093/ajcn/38.6.849. [DOI] [PubMed] [Google Scholar]
- 76.Schenker S., Halff G.A. Nutritional therapy in alcoholic liver disease. Semin Liver Dis. 1993;13:196–209. doi: 10.1055/s-2007-1007349. [DOI] [PubMed] [Google Scholar]
- 77.Lieber C.S. Alcohol, liver, and nutrition. J Am Coll Nutr. 1991;10:602–632. doi: 10.1080/07315724.1991.10718182. [DOI] [PubMed] [Google Scholar]
- 78.Lindor K.D. Management of osteopenia of liver disease with special emphasis on primary biliary cirrhosis. Semin Liver Dis. 1993;13:367–373. doi: 10.1055/s-2007-1007365. [DOI] [PubMed] [Google Scholar]
- 79.Lucidi C., Lattanzi B., Di Gregorio V., et al. A low muscle mass increases mortality in compensated cirrhotic patients with sepsis. Liver Int. 2018;38:851–857. doi: 10.1111/liv.13691. [DOI] [PubMed] [Google Scholar]
- 80.Gluud L.L., Dam G., Les I., et al. Branched-chain amino acids for people with hepatic encephalopathy. Cochrane Database Syst Rev. 2017;5:CD001939. doi: 10.1002/14651858.CD001939.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sinclair M., Gow P.J., Grossmann M., Shannon A., Hoermann R., Angus P.W. Low serum testosterone is associated with adverse outcome in men with cirrhosis independent of the model for end-stage liver disease score. Liver Transplant. 2016;22:1482–1490. doi: 10.1002/lt.24607. [DOI] [PubMed] [Google Scholar]
- 82.Sinclair M., Grossmann M., Hoermann R., Angus P.W., Gow P.J. Testosterone therapy increases muscle mass in men with cirrhosis and low testosterone: a randomised controlled trial. J Hepatol. 2016;65:906–913. doi: 10.1016/j.jhep.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 83.Harper C., Fornes P., Duyckaerts C., Lecomte D., Hauw J.J. An international perspective on the prevalence of the Wernicke-Korsakoff syndrome. Metab Brain Dis. 1995;10:17–24. doi: 10.1007/BF01991779. [DOI] [PubMed] [Google Scholar]
- 84.Torvik A. Wernicke's encephalopathy--prevalence and clinical spectrum. Alcohol Alcohol Suppl. 1991;1:381–384. [PubMed] [Google Scholar]
- 85.Galvin R., Bråthen G., Ivashynka A., et al. EFNS guidelines for diagnosis, therapy and prevention of Wernicke encephalopathy. Eur J Neurol. 2010;17:1408–1418. doi: 10.1111/j.1468-1331.2010.03153.x. [DOI] [PubMed] [Google Scholar]
- 86.Day E., Bentham P.W., Callaghan R., Kuruvilla T., George S. Thiamine for prevention and treatment of Wernicke-Korsakoff Syndrome in people who abuse alcohol. Cochrane Database Syst Rev. 2013;7:CD004033. doi: 10.1002/14651858.CD004033.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Praharaj S.K., Munoli R.N., Shenoy S., Udupa S.T., Thomas L.S. High-dose thiamine strategy in Wernicke-Korsakoff syndrome and related thiamine deficiency conditions associated with alcohol use disorder. Indian J Psychiatr. 2021;63:121–126. doi: 10.4103/psychiatry.IndianJPsychiatry_440_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ganatra R.B., Breu A.C., Ronan M.V. Clinical guideline highlights for the hospitalist: 2020 American society of addiction medicine clinical practice guideline on alcohol withdrawal management. J Hosp Med. 2021 doi: 10.12788/jhm.3729. Published online December 15. [DOI] [PubMed] [Google Scholar]
- 89.Latt N., Dore G. Thiamine in the treatment of Wernicke encephalopathy in patients with alcohol use disorders. Intern Med J. 2014;44:911–915. doi: 10.1111/imj.12522. [DOI] [PubMed] [Google Scholar]
- 90.Shah N.D., Ventura-Cots M., Abraldes J.G., et al. Alcohol-related liver disease is rarely detected at early stages compared with liver diseases of other etiologies worldwide. Clin Gastroenterol Hepatol. 2019;17:2320–2329. doi: 10.1016/j.cgh.2019.01.026. e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Argemi J., Ventura-Cots M., Rachakonda V., Bataller R. Alcoholic-related liver disease: pathogenesis, management and future therapeutic developments. Rev Esp Enferm Dig. 2020;112:869–878. doi: 10.17235/reed.2020.7242/2020. [DOI] [PubMed] [Google Scholar]
- 92.Xu X.L., Jiang L.S., Wu C.S., et al. The role of fibrosis index FIB-4 in predicting liver fibrosis stage and clinical prognosis: a diagnostic or screening tool? J Formos Med Assoc. 2021 doi: 10.1016/j.jfma.2021.07.013. Published online July 26. [DOI] [PubMed] [Google Scholar]
- 93.Asphaug L., Thiele M., Krag A., Melberg H.O. Cost-effectiveness of noninvasive screening for alcohol-related liver fibrosis. Hepatology. 2020;71:2093–2104. doi: 10.1002/hep.30979. [DOI] [PubMed] [Google Scholar]
- 94.Moreno C., Mueller S., Szabo G. Non-invasive diagnosis and biomarkers in alcohol-related liver disease. J Hepatol. 2019;70:273–283. doi: 10.1016/j.jhep.2018.11.025. [DOI] [PubMed] [Google Scholar]
- 95.Stickel F., Datz C., Hampe J., Bataller R. Pathophysiology and management of alcoholic liver disease: update 2016. Gut Liver. 2017;11:173–188. doi: 10.5009/gnl16477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lucey P., Cohn J., Lucey S., Matthews I., Sridharan S., Prkachin K.M. Automatically detecting pain using facial actions. Int Conf Affect Comput Intell Interact Workshops. 2009;2009:1–8. doi: 10.1109/ACII.2009.5349321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Crabb D.W., Bataller R., Chalasani N.P., et al. Standard definitions and common data elements for clinical trials in patients with alcoholic hepatitis: recommendation from the NIAAA alcoholic hepatitis consortia. Gastroenterology. 2016;150:785–790. doi: 10.1053/j.gastro.2016.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Singal A.K., Kamath P.S. Model for end-stage liver disease. J Clin Exp Hepatol. 2013;3:50–60. doi: 10.1016/j.jceh.2012.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kamath P., Wiesner R., Malinchoc M., et al. A model to predict survival in patients with end-stage liver disease. Gastroenterology. 2001;120:A76–A77. doi: 10.1016/s0016-5085(01)80377-2. [DOI] [PubMed] [Google Scholar]
- 100.Farnsworth N., Fagan S.P., Berger D.H., Awad S.S. Child-Turcotte-Pugh versus MELD score as a predictor of outcome after elective and emergent surgery in cirrhotic patients. Am J Surg. 2004;188:580–583. doi: 10.1016/j.amjsurg.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 101.Arab J.P., Díaz L.A., Baeza N., et al. Identification of optimal therapeutic window for steroid use in severe alcohol-associated hepatitis: a worldwide study. J Hepatol. 2021;75:1026–1033. doi: 10.1016/j.jhep.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Srikureja W., Kyulo N.L., Runyon B.A., Hu K.Q. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol. 2005;42:700–706. doi: 10.1016/j.jhep.2004.12.022. [DOI] [PubMed] [Google Scholar]
- 103.Mathurin P., O'Grady J., Carithers R.L., et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis: meta-analysis of individual patient data. Gut. 2011;60:255–260. doi: 10.1136/gut.2010.224097. [DOI] [PubMed] [Google Scholar]
- 104.Forrest E.H., Atkinson S.R., Richardson P., et al. Application of prognostic scores in the STOPAH trial: Discriminant function is no longer the optimal scoring system in alcoholic hepatitis. J Hepatol. 2018;68:511–518. doi: 10.1016/j.jhep.2017.11.017. [DOI] [PubMed] [Google Scholar]
- 105.Arraez D.M., Cots M.V., Altamirano J., et al. A large worldwide study SHOWS that MELD IS the best scoring system to predict mortality IN alcohol-associated hepatitis. In: The Liver Meeting Digital ExperienceTM. AASLD. https://aasld.confex.com/aasld/2020/meetingapp.cgi/Paper/23453
- 106.Atkinson S.R., Grove J.I., Liebig S., et al. In severe alcoholic hepatitis, serum keratin-18 fragments are diagnostic, prognostic, and theragnostic biomarkers. Am J Gastroenterol. 2020;115:1857–1868. doi: 10.14309/ajg.0000000000000912. [DOI] [PubMed] [Google Scholar]
- 107.Kumar M., Gupta G.K., Wanjari S.J., Tak V., Ameta M., Nijhawan S. Relative adrenal insufficiency in patients with alcoholic hepatitis. J Clin Exp Hepatol. 2019;9:215–220. doi: 10.1016/j.jceh.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Altamirano J., Fagundes C., Dominguez M., et al. Acute kidney injury is an early predictor of mortality for patients with alcoholic hepatitis. Clin Gastroenterol Hepatol. 2012;10:65–71. doi: 10.1016/j.cgh.2011.09.011. e3. [DOI] [PubMed] [Google Scholar]
- 109.Simonetto D.A., Gines P., Kamath P.S. Hepatorenal syndrome: pathophysiology, diagnosis, and management. BMJ. 2020;370:m2687. doi: 10.1136/bmj.m2687. [DOI] [PubMed] [Google Scholar]
- 110.Angeli P., Gines P., Wong F., et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64:531–537. doi: 10.1136/gutjnl-2014-308874. [DOI] [PubMed] [Google Scholar]
- 111.Piano S., Rosi S., Maresio G., et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59:482–489. doi: 10.1016/j.jhep.2013.03.039. [DOI] [PubMed] [Google Scholar]
- 112.Martín-Llahí M., Pépin M.N., Guevara M., et al. Terlipressin and albumin vs albumin in patients with cirrhosis and hepatorenal syndrome: a randomized study. Gastroenterology. 2008;134:1352–1359. doi: 10.1053/j.gastro.2008.02.024. [DOI] [PubMed] [Google Scholar]
- 113.Facciorusso A., Chandar A.K., Murad M.H., et al. Comparative efficacy of pharmacological strategies for management of type 1 hepatorenal syndrome: a systematic review and network meta-analysis. Lancet Gastroenterol Hepatol. 2017;2:94–102. doi: 10.1016/S2468-1253(16)30157-1. [DOI] [PubMed] [Google Scholar]
- 114.Solé C., Pose E., Solà E., Ginès P. Hepatorenal syndrome in the era of acute kidney injury. Liver Int. 2018;38:1891–1901. doi: 10.1111/liv.13893. [DOI] [PubMed] [Google Scholar]
- 115.Ojeda-Yuren A.S., Cerda-Reyes E., Herrero-Maceda M.R., Castro-Narro G., Piano S. An integrated review of the hepatorenal syndrome. Ann Hepatol. 2021;22:100236. doi: 10.1016/j.aohep.2020.07.008. [DOI] [PubMed] [Google Scholar]
- 116.Mookerjee R.P., Stadlbauer V., Lidder S., et al. Neutrophil dysfunction in alcoholic hepatitis superimposed on cirrhosis is reversible and predicts the outcome. Hepatology. 2007;46:831–840. doi: 10.1002/hep.21737. [DOI] [PubMed] [Google Scholar]
- 117.Llopis M., Cassard A.M., Wrzosek L., et al. Intestinal microbiota contributes to individual susceptibility to alcoholic liver disease. Gut. 2016;65:830–839. doi: 10.1136/gutjnl-2015-310585. [DOI] [PubMed] [Google Scholar]
- 118.Ike Y., Clewell D.B., Segarra R.A., Gilmore M.S. Genetic analysis of the pAD1 hemolysin/bacteriocin determinant in Enterococcus faecalis: tn917 insertional mutagenesis and cloning. J Bacteriol. 1990;172:155–163. doi: 10.1128/jb.172.1.155-163.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Duan Y., Llorente C., Lang S., et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. 2019;575:505–511. doi: 10.1038/s41586-019-1742-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Vergis N., Atkinson S.R., Knapp S., et al. In patients with severe alcoholic hepatitis, prednisolone increases susceptibility to infection and infection-related mortality, and is associated with high circulating levels of bacterial DNA. Gastroenterology. 2017;152:1068–1077. doi: 10.1053/j.gastro.2016.12.019. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Flamm S.L. Rifaximin treatment for reduction of risk of overt hepatic encephalopathy recurrence. Therap Adv Gastroenterol. 2011;4:199–206. doi: 10.1177/1756283X11401774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kulkarni A., Iyengar S., Fatima S., Sharma M., Rao P.N., Nageshwar Reddy D. Clinical Hepatology. BMJ Publishing Group Ltd and British Society of Gastroenterology; 2021. IDDF2021-ABS-0089 Primary norfloxacin prophylaxis reduces the incidence of infections in severe alcoholic hepatitis-A double-blind, randomized controlled study. [DOI] [Google Scholar]
- 123.Støy S., Laursen T.L., Eriksen L.L., Grønbæk H., Vilstrup H., Sandahl T.D. No effect in alcoholic hepatitis of gut-selective, broad-spectrum antibiotics on bacterial translocation or hepatic and systemic inflammation. Clin Transl Gastroenterol. 2021;12 doi: 10.14309/ctg.0000000000000306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Website. https://easl.eu/wp-content/uploads/2021/06/EASL_2021_Version-5-new.pdf
- 125.Mathurin P. Exacerbation of alcoholic liver injury by enteral endotoxin in rats. Hepatology. 2000;32:1008–1017. doi: 10.1053/jhep.2000.19621. [DOI] [PubMed] [Google Scholar]
- 126.Enomoto N., Ikejima K., Bradford B., et al. Alcohol causes both tolerance and sensitization of rat Kupffer cells via mechanisms dependent on endotoxin. Gastroenterology. 1998;115:443–451. doi: 10.1016/s0016-5085(98)70211-2. [DOI] [PubMed] [Google Scholar]
- 127.Morgan M.Y. The treatment of alcoholic hepatitis. Alcohol Alcohol. 1996;31:117–134. doi: 10.1093/oxfordjournals.alcalc.a008123. [DOI] [PubMed] [Google Scholar]
- 128.Szabo G., Petrasek J., Bala S. Innate immunity and alcoholic liver disease. Dig Dis. 2012;30(Suppl 1):55–60. doi: 10.1159/000341126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Louvet A., Thursz M.R., Kim D.J., et al. Corticosteroids reduce risk of death within 28 Days for patients with severe alcoholic hepatitis, compared with pentoxifylline or placebo-a meta-analysis of individual data from controlled trials. Gastroenterology. 2018;155:458–468. doi: 10.1053/j.gastro.2018.05.011. e8. [DOI] [PubMed] [Google Scholar]
- 130.Altamirano J., Miquel R., Katoonizadeh A., et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. 2014;146:1231–1239. doi: 10.1053/j.gastro.2014.01.018. e6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.O'Shea R.S., Dasarathy S., McCullough A.J. Practice guideline committee of the American association for the study of liver diseases and the practice parameters committee of the American college of gastroenterology. Alcoholic liver disease. Hepatology. 2010;51:307–328. doi: 10.1002/hep.23258. [DOI] [PubMed] [Google Scholar]
- 132.Pavlov C.S., Varganova D.L., Casazza G., Tsochatzis E., Nikolova D., Gluud C. Glucocorticosteroids for people with alcoholic hepatitis. Cochrane Database Syst Rev. 2017;11:CD001511. doi: 10.1002/14651858.CD001511.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Singal A.K., Mathurin P. Diagnosis and treatment of alcohol-associated liver disease: a review. JAMA. 2021;326:165–176. doi: 10.1001/jama.2021.7683. [DOI] [PubMed] [Google Scholar]
- 134.Forrest E., Mellor J., Stanton L., et al. Steroids or pentoxifylline for alcoholic hepatitis (STOPAH): study protocol for a randomised controlled trial. Trials. 2013;14:262. doi: 10.1186/1745-6215-14-262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Singal A.K., Shah V.H. Therapeutic strategies for the treatment of alcoholic hepatitis. Semin Liver Dis. 2016;36:56–68. doi: 10.1055/s-0036-1571297. [DOI] [PubMed] [Google Scholar]
- 136.Maddrey W.C., Boitnott J.K., Bedine M.S., Weber F.L., Mezey E., White R.I. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. 1978;75:193–199. doi: 10.1016/0016-5085(78)90401-8. [DOI] [PubMed] [Google Scholar]
- 137.Blitzer B.L., Mutchnick M.G., Joshi P.H., Phillips M.M., Fessel J.M., Conn H.O. Adrenocorticosteroid therapy in alcoholic hepatitis. A prospective, double-blind randomized study. Am J Dig Dis. 1977;22:477–484. doi: 10.1007/BF01072499. [DOI] [PubMed] [Google Scholar]
- 138.Lesesne H.R., Bozymski E.M., Fallon H.J. Treatment of alcoholic hepatitis with encephalopathy. Comparison of prednisolone with caloric supplements. Gastroenterology. 1978;74(2 Pt 1):169–173. [PubMed] [Google Scholar]
- 139.Shumaker J.B., Resnick R.H., Galambos J.T., Makopour H., Iber F.L. A controlled trial of 6-methylprednisolone in acute alcoholic hepatitis. With a note on published results in encephalopathic patients. Am J Gastroenterol. 1978;69:443–449. [PubMed] [Google Scholar]
- 140.Depew W., Boyer T., Omata M., Redeker A., Reynolds T. Double-blind controlled trial of prednisolone therapy in patients with severe acute alcoholic hepatitis and spontaneous encephalopathy. Gastroenterology. 1980;78:524–529. [PubMed] [Google Scholar]
- 141.Theodossi A., Eddleston A.L., Williams R. Controlled trial of methylprednisolone therapy in severe acute alcoholic hepatitis. Gut. 1982;23:75–79. doi: 10.1136/gut.23.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carithers R.L., Jr., Herlong H.F., Diehl A.M., et al. Methylprednisolone therapy in patients with severe alcoholic hepatitis. A randomized multicenter trial. Ann Intern Med. 1989;110:685–690. doi: 10.7326/0003-4819-110-9-685. [DOI] [PubMed] [Google Scholar]
- 143.Ramond M.J., Poynard T., Rueff B., et al. A randomized trial of prednisolone in patients with severe alcoholic hepatitis. N Engl J Med. 1992;326:507–512. doi: 10.1056/NEJM199202203260802. [DOI] [PubMed] [Google Scholar]
- 144.Cabré E., Rodríguez-Iglesias P., Caballería J., et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomized trial. Hepatology. 2000;32:36–42. doi: 10.1053/jhep.2000.8627. [DOI] [PubMed] [Google Scholar]
- 145.Phillips M., Curtis H., Portmann B., Donaldson N., Bomford A., O'Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis--a randomised clinical trial. J Hepatol. 2006;44:784–790. doi: 10.1016/j.jhep.2005.11.039. [DOI] [PubMed] [Google Scholar]
- 146.Campra J.L., Hamlin E.M., Jr., Kirshbaum R.J., Olivier M., Redeker A.G., Reynolds T.B. Prednisone therapy of acute alcoholic hepatitis. Report of a controlled trial. Ann Intern Med. 1973;79:625–631. doi: 10.7326/0003-4819-79-5-625. [DOI] [PubMed] [Google Scholar]
- 147.Helman R.A., Temko M.H., Nye S.W., Fallon H.J. Alcoholic hepatitis. Natural history and evaluation of prednisolone therapy. Ann Intern Med. 1971;74:311–321. doi: 10.7326/0003-4819-74-3-311. [DOI] [PubMed] [Google Scholar]
- 148.Owens R.E., Snyder H.S., Twilla J.D., Satapathy S.K. Pharmacologic treatment of alcoholic hepatitis: examining outcomes based on disease severity stratification. J Clin Exp Hepatol. 2016;6:275–281. doi: 10.1016/j.jceh.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Mendenhall C.L., Anderson S., Garcia-Pont P., et al. Short-term and long-term survival in patients with alcoholic hepatitis treated with oxandrolone and prednisolone. N Engl J Med. 1984;311:1464–1470. doi: 10.1056/NEJM198412063112302. [DOI] [PubMed] [Google Scholar]
- 150.Thursz M.R., Richardson P., Allison M., et al. Prednisolone or pentoxifylline for alcoholic hepatitis. N Engl J Med. 2015;372:1619–1628. doi: 10.1056/NEJMoa1412278. [DOI] [PubMed] [Google Scholar]
- 151.Singal A.K., Kamath P.S., Gores G.J., Shah V.H. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. 2014;12:555–564. doi: 10.1016/j.cgh.2013.06.013. quiz e31-e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Singal A.K., Shah V.H. Alcoholic hepatitis: prognostic models and treatment. Gastroenterol Clin N Am. 2011;40:611–639. doi: 10.1016/j.gtc.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 153.Louvet A., Naveau S., Abdelnour M., et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. 2007;45:1348–1354. doi: 10.1002/hep.21607. [DOI] [PubMed] [Google Scholar]
- 154.Garcia Rodríguez L.A., Hernández-Díaz S. The risk of upper gastrointestinal complications associated with nonsteroidal anti-inflammatory drugs, glucocorticoids, acetaminophen, and combinations of these agents. Arthritis Res. 2001;3:98–101. doi: 10.1186/ar146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Singal A.K., Kuo Y.F., Anand B.S. Hepatitis C virus infection in alcoholic hepatitis: prevalence patterns and impact on in-hospital mortality. Eur J Gastroenterol Hepatol. 2012;24:1178–1184. doi: 10.1097/MEG.0b013e328355cce0. [DOI] [PubMed] [Google Scholar]
- 156.Segev D.L., Sozio S.M., Shin E.J., et al. Steroid avoidance in liver transplantation: meta-analysis and meta-regression of randomized trials. Liver Transplant. 2008;14:512–525. doi: 10.1002/lt.21396. [DOI] [PubMed] [Google Scholar]
- 157.Shoreibah M., Anand B.S., Singal A.K. Alcoholic hepatitis and concomitant hepatitis C virus infection. World J Gastroenterol. 2014;20:11929–11934. doi: 10.3748/wjg.v20.i34.11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Mathurin P., Bataller R. Trends in the management and burden of alcoholic liver disease. J Hepatol. 2015;62(1 suppl l):S38–S46. doi: 10.1016/j.jhep.2015.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Louvet A., Wartel F., Castel H., et al. Infection in patients with severe alcoholic hepatitis treated with steroids: early response to therapy is the key factor. Gastroenterology. 2009;137:541–548. doi: 10.1053/j.gastro.2009.04.062. [DOI] [PubMed] [Google Scholar]
- 160.Michelena J., Altamirano J., Abraldes J.G., et al. Systemic inflammatory response and serum lipopolysaccharide levels predict multiple organ failure and death in alcoholic hepatitis. Hepatology. 2015;62:762–772. doi: 10.1002/hep.27779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kwong E.K., Puri P. Gut microbiome changes in Nonalcoholic fatty liver disease & alcoholic liver disease. Transl Gastroenterol Hepatol. 2021;6:3. doi: 10.21037/tgh.2020.02.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Tornai D., Szabo G. Emerging medical therapies for severe alcoholic hepatitis. Clin Mol Hepatol. 2020;26:686–696. doi: 10.3350/cmh.2020.0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Smirnova E., Puri P., Muthiah M.D., et al. Fecal microbiome distinguishes alcohol consumption from alcoholic hepatitis but does not discriminate disease severity. Hepatology. 2020;72:271–286. doi: 10.1002/hep.31178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Dong T.S., Katzka W., Lagishetty V., et al. A microbial signature identifies advanced fibrosis in patients with chronic liver disease mainly due to NAFLD. Sci Rep. 2020;10:2771. doi: 10.1038/s41598-020-59535-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Lang S., Duan Y., Liu J., et al. Intestinal fungal dysbiosis and systemic immune response to fungi in patients with alcoholic hepatitis. Hepatology. 2020;71:522–538. doi: 10.1002/hep.30832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Karakike E., Moreno C., Gustot T. Infections in severe alcoholic hepatitis. Ann Gastroenterol Hepatol. 2017;30:152–160. doi: 10.20524/aog.2016.0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Templeton A., Schlegel M., Fleisch F., et al. Multilumen central venous catheters increase risk for catheter-related bloodstream infection: prospective surveillance study. Infection. 2008;36:322–327. doi: 10.1007/s15010-008-7314-x. [DOI] [PubMed] [Google Scholar]
- 168.Simon D., Galambos J.T. A randomized controlled study of peripheral parenteral nutrition in moderate and severe alcoholic hepatitis. J Hepatol. 1988;7:200–207. doi: 10.1016/s0168-8278(88)80483-5. [DOI] [PubMed] [Google Scholar]
- 169.Kulkarni A.V., Tirumalle S., Premkumar M., et al. Primary norfloxacin prophylaxis for APASL-defined acute-on-chronic liver failure: a placebo-controlled double-blind randomized trial. Am J Gastroenterol. 2022 doi: 10.14309/ajg.0000000000001611. Published online January 17. [DOI] [PubMed] [Google Scholar]
- 170.Website. https://easl.eu/wp-content/uploads/2021/06/EASL_2021_Version-5-new.pdf
- 171.Subramaniyan V., Chakravarthi S., Jegasothy R., et al. Alcohol-associated liver disease: a review on its pathophysiology, diagnosis and drug therapy. Toxicol Rep. 2021;8:376–385. doi: 10.1016/j.toxrep.2021.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Nguyen-Khac E., Thevenot T., Piquet M.A., et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. 2011;365:1781–1789. doi: 10.1056/NEJMoa1101214. [DOI] [PubMed] [Google Scholar]
- 173.Akriviadis E., Botla R., Briggs W., Han S., Reynolds T., Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. 2000;119:1637–1648. doi: 10.1053/gast.2000.20189. [DOI] [PubMed] [Google Scholar]
- 174.Lebrec D., Thabut D., Oberti F., et al. Pentoxifylline does not decrease short-term mortality but does reduce complications in patients with advanced cirrhosis. Gastroenterology. 2010;138:1755–1762. doi: 10.1053/j.gastro.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 175.Role Shalimar. Of pentoxifylline and steroids for alcoholic hepatitis - has the last word been said? J Clin Exp Hepatol. 2015;5:170–172. doi: 10.1016/j.jceh.2015.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mathurin P., Louvet A., Duhamel A., et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis. JAMA. 2013;310:1033. doi: 10.1001/jama.2013.276300. [DOI] [PubMed] [Google Scholar]
- 177.Louvet A., Diaz E., Dharancy S., et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol. 2008;48:465–470. doi: 10.1016/j.jhep.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 178.Amjad W., Alukal J., Doycheva I., et al. A combination of N-acetylcysteine and prednisone has No benefit over prednisone alone in severe alcoholic hepatitis: a retrospective analysis. Dig Dis Sci. 2020;65:3726–3733. doi: 10.1007/s10620-020-06142-4. [DOI] [PubMed] [Google Scholar]
- 179.Smilkstein M.J., Knapp G.L., Kulig K.W., Rumack B.H. Efficacy of oral N-acetylcysteine in the treatment of acetaminophen overdose. Analysis of the national multicenter study (1976 to 1985) N Engl J Med. 1988;319:1557–1562. doi: 10.1056/NEJM198812153192401. [DOI] [PubMed] [Google Scholar]
- 180.Moreno C., Langlet P., Hittelet A., et al. Enteral nutrition with or without N-acetylcysteine in the treatment of severe acute alcoholic hepatitis: a randomized multicenter controlled trial. J Hepatol. 2010;53:1117–1122. doi: 10.1016/j.jhep.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 181.Gadour E., Mohamed T., Hassan Z., Hassan A. Meta-analysis and systematic review of primary renal tubular acidosis in patients with autoimmune hepatitis and alcoholic hepatitis. Cureus. 2021;13 doi: 10.7759/cureus.15287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Hiremath S.B., Srinivas L.D. Survival benefits of terlipressin and non-responder state in hepatorenal syndrome: a meta-analysis. Indian J Pharmacol. 2013;45:54–60. doi: 10.4103/0253-7613.106436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Holt S., Goodier D., Marley R., et al. Improvement in renal function in hepatorenal syndrome with N-acetylcysteine. Lancet. 1999;353:294–295. doi: 10.1016/s0140-6736(05)74933-3. [DOI] [PubMed] [Google Scholar]
- 184.Izzedine H., Kheder-Elfekih R., Deray G., Thabut D. Endothelin-receptor antagonist/N-acetylcysteine combination in type 1 hepatorenal syndrome. J Hepatol. 2009;50:1055–1056. doi: 10.1016/j.jhep.2009.02.002. [DOI] [PubMed] [Google Scholar]
- 185.Sen S., Mookerjee R.P., Jalan R. Terlipressin-induced vasoconstriction reversed with N-acetylcysteine: a case for combined use in hepatorenal syndrome? Gastroenterology. 2002;123:2160–2161. doi: 10.1053/gast.2002.37303. [DOI] [PubMed] [Google Scholar]
- 186.Hamid R., Forrest E.H. Is histology required for the diagnosis of alcoholic hepatitis? a review of published randomised controlled trials. Gut. 2011;60(Suppl 1):A233. doi: 10.1136/gut.2011.239301.492. A233. [DOI] [Google Scholar]
- 187.Forrest E., Goldin R. Letter: the diagnostic and prognostic significance of liver histology in alcoholic hepatitis—authors’ reply. Aliment Pharmacol Ther. 2021;54:864. doi: 10.1111/apt.16559. 864. [DOI] [PubMed] [Google Scholar]
- 188.Kalambokis G., Manousou P., Vibhakorn S., et al. Transjugular liver biopsy--indications, adequacy, quality of specimens, and complications--a systematic review. J Hepatol. 2007;47:284–294. doi: 10.1016/j.jhep.2007.05.001. [DOI] [PubMed] [Google Scholar]
- 189.Soyer P., Fargeaudou Y., Boudiaf M., Rymer R. Transjugular liver biopsy using ultrasonographic guidance for jugular vein puncture and an automated device for hepatic tissue sampling: a retrospective analysis of 200 consecutive cases. Abdom Imag. 2008;33:627–632. doi: 10.1007/s00261-007-9357-3. [DOI] [PubMed] [Google Scholar]
- 190.Esposito A.A., Nicolini A., Meregaglia D., Sangiovanni A., Biondetti P. Role of transjugular liver biopsy in the diagnostic and therapeutic management of patients with severe liver disease. Radiol Med. 2008;113:1008–1017. doi: 10.1007/s11547-008-0311-4. [DOI] [PubMed] [Google Scholar]
- 191.Arab J.P., Arrese M., Singal A.K. Diagnosis of alcohol-associated hepatitis: when is liver biopsy required? Clin Liver Dis. 2021;25:571–584. doi: 10.1016/j.cld.2021.03.003. [DOI] [PubMed] [Google Scholar]
- 192.Lucey M.R. Alcohol-associated cirrhosis. Clin Liver Dis. 2019;23:115–126. doi: 10.1016/j.cld.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 193.Kim D.J., Choi M.S. Life-sustaining treatment and palliative care in patients with liver cirrhosis - legal, ethical, and practical issues. Clin Mol Hepatol. 2017;23:115–122. doi: 10.3350/cmh.2017.0018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Mudumbi S.K., Bourgeois C.E., Hoppman N.A., et al. Palliative care and hospice interventions in decompensated cirrhosis and hepatocellular carcinoma: a rapid review of literature. J Palliat Med. 2018;21:1177–1184. doi: 10.1089/jpm.2017.0656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Holden J.H., Shamseddeen H., Johnson A.W., et al. Palliative care and hospice referrals in patients with decompensated cirrhosis: what factors are important? J Palliat Med. 2020;23:1066–1075. doi: 10.1089/jpm.2019.0501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Poonja Z., Brisebois A., van Zanten S.V., Tandon P., Meeberg G., Karvellas C.J. Patients with cirrhosis and denied liver transplants rarely receive adequate palliative care or appropriate management. Clin Gastroenterol Hepatol. 2014;12:692–698. doi: 10.1016/j.cgh.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 197.Vaccination of adults with liver disease. 2021. https://www.cdc.gov/vaccines/adults/rec-vac/health-conditions/liver-disease.html Published May 3. [Google Scholar]
- 198.Website. https://www.fda.gov/media/144413/download
- 199.Website. https://www.fda.gov/media/144637/download?utm_medium=email&utm_source=govdelivery
- 200.Website. https://www.fda.gov/media/146304/download
- 201.Website. https://www.cdc.gov/vaccines/covid-19/clinical-considerations/covid-19-vaccines-us.html
- 202.CDC statement on ACIP booster recommendations. 2021. https://www.cdc.gov/media/releases/2021/p0924-booster-recommendations-.html Published September 27. Accessed October 23, 2021. [Google Scholar]