Abstract
Background
Combination conversion therapies afforded curative surgery chance for initially unresectable hepatocellular carcinoma (uHCC). This study aimed to evaluate the conversion rate and clinical outcomes of a first-line conversion regimen of lenvatinib combined with transarterial chemoembolization (TACE) plus immunotherapy for initial uHCC by interpreting real-world data.
Methods
Conversion therapy data of patients with uHCC from November 2018 to January 2021 were analysed. The regimens included triple combination therapy (t-CT: lenvatinib, TACE, plus toripalimab) and dual combination therapy (d-CT: lenvatinib plus TACE). Another study population diagnosed with hepatocellular carcinoma of macrovascular invasion disease were included as the upfront surgery cohort. Treatment responses and conversion rate were primary outcomes. Survival and adverse events were analysed.
Results
Fifty-one patients receiving t-CT (n = 30) and d-CT (n = 21) were enrolled. Higher overall response rates (76.7 per cent versus 47.6 per cent, P = 0.042) and disease control rates (90.0 per cent versus 57.1 per cent, P = 0.042) were observed via t-CT than d-CT. Both median overall survival and event-free survival were not reached in the t-CT cohort. A higher rate of curative conversion resection was achieved through t-CT than d-CT (50.0 per cent versus 19.0 per cent, P = 0.039). The disease-free survival of patients undergoing conversion resection in the t-CT cohort (n = 15) was higher than that in the upfront surgery cohort (n = 68, P = 0.039). Both t-CT and d-CT regimens were tolerable.
Conclusions
Better treatment responses and conversion rate for patients with uHCC were obtained with first-line t-CT. Neoadjuvant t-CT before surgery should be recommended for patients with macrovascular invasion.
The first-line triple combination therapy (t-CT) of transarterial chemoembolization plus anti-PD-1 antibodies plus lenvatinib increase the conversion rate and treatment responses for patients with unresectable hepatocellular carcinoma. Neoadjuvant t-CT regimen is recommended for China Liver Cancer Staging stage IIIa patients.
Introduction
Hepatocellular carcinoma (HCC) is the most common type of primary liver cancer, ranking the fourth leading cause of cancer-related death worldwide1. Although in early and intermediate stages, patient survival could be improved by curative liver resection, yet for more than half of the patients with HCC, diagnosis is made when locally advanced HCC or distant metastasis is already present2. The prognosis of unresectable HCC (uHCC) remains unfavourable, and effective systemic therapy is urgently needed3. In the past decade, great progress has been made in therapeutic strategies for uHCC, ranging from local therapy to systematic therapy, including external beam radiation, transarterial chemoembolization (TACE), chemotherapy, targeted therapy, and immune therapy4.
The emergence of immunological therapy marks a milestone in cancer treatment. Immune checkpoint inhibitors (ICIs), exemplified by programmed cell death-1 (PD-1) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) antibodies are being incorporated into the HCC treatment schedule. Both mono-immunotherapy5,6 and combination immunotherapy7 showed promising tolerance and efficacy profiles in patients with advanced HCC. In the era of precision treatment, targeted drugs are another attractive approach. Lenvatinib, a multi-kinase inhibitor of vascular endothelial growth factor receptor, fibroblast growth factor receptor, platelet-derived growth factor receptor-a, RET, and KIT, was approved as a first-line treatment in 2018 based on the REFLECT trial, in which lenvatinib exhibited non-inferior efficacy in comparison with sorafenib (median overall survival (OS), 13.6 months versus 12.3 months respectively)8. The utilization of anti-PD-1 antibody plus lenvatinib reached an overall response rate (ORR) of 46.0 per cent and a median OS of 22 months, reflecting promising prospects for the combination regimen9. The synergistic effect between these two drugs was consistent with the results of basic research10.
Conversion therapy offers the chance of radical resection by downstaging tumour burden, leading to better survival and reduced recurrence11–14. Several studies have reported the feasibility of combination conversion therapies. Huang et al. evaluated the response rates of first-line lenvatinib plus anti-PD-1 antibody regimen. The ORR was 33.3 per cent, and six out of 18 patients with macrovascular tumour thrombus underwent liver resection after treatment15. A massive retrospective analysis reported that pembrolizumab–lenvatinib–TACE sequential therapy had an acceptable conversion rate (25.7 per cent) for patients with initial PD-L1-positive uHCC16. Nevertheless, its information on conversion surgery was fragmentary, and the study design was not stringent enough. In addition, conversion therapy with a combination of tyrosine kinase inhibitors and anti-PD-1 antibodies was also proven feasible17. Due to the nearly absent real-world data on conversion therapy in uHCC by the combination of anti-PD-1 antibody, lenvatinib, plus TACE, assessment of the feasibility and efficacy of this therapeutic strategy is in urgent need.
In the present study, the treatment responses and survival of patients with uHCC receiving toripalimab plus lenvatinib and TACE were prospectively assessed. The clinical outcomes and significance of conversion surgery in comparison with upfront surgery were further investigated.
Methods
Patient cohorts
From December 2018 to January 2021, patients with uHCC who received conversion treatment of triple combination therapy (t-CT) using lenvatinib, TACE, plus toripalimab or dual combination therapy (d-CT) using lenvatinib plus TACE at Zhongshan Hospital were prospectively enrolled. The inclusion criteria were as follows: diagnosed as HCC through imaging or biopsy according to the American Association for the Study of Liver Diseases criteria, supported by imaging or biopsy evidence18; no previous anti-tumoural therapies except t-CT and d-CT; Child–Pugh score of 7 or lower; and initially ineligible for R0 resection based on an evaluation of patient’s poor general condition or liver function intolerance, insufficient remaining liver volume or insufficient resection margins. The exclusion criteria included: presence of other types of malignancy; previous anti-tumour treatment within 1 year; missing clinical information; and discontinuity of therapy, irrespective of treatment-related adverse events (AEs). Collection of clinical data is detailed in the Supplementary material.
To evaluate the prognosis of successful conversion cases receiving t-CT regimen, an upfront surgery cohort from January 2019 to December 2020 was enrolled under the same tumour stage.
Treatment regimens and follow-up
The first-line combination therapy was administered following the study protocol. Seven days after the first TACE treatment (details in Supplementary material), lenvatinib was orally administered at 8 mg/day, regardless of bodyweight (Eisai, Tokyo, Japan). Toripalimab (Junshi Bioscience, Shanghai, China)19, an anti-PD-1 antibody, was intravenously administered within 3 days after the initiation of TACE at 240 mg, and then every 3 weeks. A blood sample was taken before and every other day after each treatment until discharge. The patient was followed up 3 weeks after the last treatment. Surgical resection could be considered after a full cycle of treatment if the imaging, including enhanced MRI and/or CT, suggests a good tumour response.
Conversion surgery was defined as surgical treatment with the goal of R0 resection for initially uHCC (originally unresectable or marginally resectable tumours) after a good response to the combination therapy. The same criteria of resectable lesions as in the previous study from our institution were adopted17. Surgical evaluation for each patient was conducted by a single surgery group.
Endpoints and evaluation of adverse events
Tumour responses were graded as complete response (CR), partial response (PR), stable disease (SD), or progressive disease (PD), in accordance with the modified Response Evaluation Criteria in Solid Tumours (mRECIST)20. All evaluation on treatment responses was performed by the investigator and independent imaging review group. The ORR was calculated as the sum of CR and PR. The disease control rate (DCR) was calculated as the sum of CR, PR, and SD. OS was defined as the time interval from treatment initiation to death or last follow-up. Event-free survival (EFS) was defined as the time interval from treatment initiation to tumour progression, tumour recurrence or death, whichever occurred first. HCC recurrence was defined as the appearance of a newly detected HCC tumour confirmed on two radiological images, with or without elevation of serum tumour markers. Disease-free survival (DFS) was defined as the time interval between the date of diagnosis and the date of first recurrence, last follow-up, or death.
Treatment-related AEs were evaluated by the National Cancer Institute Common Terminology Criteria for Adverse Events version 4.0.
Statistical analysis
Continuous variables were expressed as the median (interquartile range (i.q.r.)) and were compared with the Mann–Whitney U test. Categorical variables were expressed as numbers and percentages and compared with the chi-squared test or Fisher’s exact test. Kaplan–Meier curves with log rank tests were used to compare survival. Univariable Cox regression method described by HRs and 95 per cent confidence intervals (c.i.) were used to assess the therapeutic effect of t-CT in subgroups. A two-tailed P value < 0.05 was considered statistically significant. Statistical analysis was performed using R software version 4.1.1 (R Foundation, Vienna, Austria) and SPSS® 22.0 (IBM, Armonk, NY, USA).
Results
Patients’ demographic information
The study enrolled 30 patients in the t-CT cohort and 21 patients in the d-CT cohort. Table 1 demonstrated the baseline characteristics. More than two-thirds of the patients in the t-CT and d-CT cohorts were diagnosed with hepatitis B virus infection and liver cirrhosis. The median largest tumour diameters were 9.2 and 9.7 cm in the t-CT and d-CT cohorts respectively. Vascular invasion detected by preoperative imaging was observed in 27 (90 per cent) and 17 (81.0 per cent) patients in the t-CT and d-CT cohorts respectively. In the t-CT conversion cohort, 27 (90 per cent) patients were diagnosed as China Liver Cancer Staging (CNLC) stage IIIa, whereas a higher proportion of intermediate-stage patients were included in the d-CT cohort. Similarly, most patients were diagnosed as Barcelona Clinic Liver Cancer (BCLC) stage C (96.7 per cent in the t-CT cohort). No significant differences were observed in demographic characteristics between the two cohorts.
Table 1.
Demographic, clinical, and tumour characteristics of patients with unresectable hepatocellular carcinoma
| Patient demographics | t-CT cohort (n = 30) | d-CT cohort (n = 21) | P |
|---|---|---|---|
| Age (years) | 55.5 (47.8, 64.3) | 50.0 (45.0, 61.0) | 0.541 |
| Sex (male) | 26 (86.7) | 20 (95.2) | 0.391 |
| HBsAg (positive) | 23 (76.7) | 18 (85.7) | 0.495 |
| Anti-HCV (positive) | 1 (3.3) | 0 (0.0) | 1.000 |
| Liver cirrhosis (yes) | 20 (66.7) | 16 (76.2) | 0.543 |
| ALT (U/l) | 56.0 (39.3, 74.3) | 42.0 (33.0, 71.0) | 0.309 |
| AFP (ng/ml) | 0.718 | ||
| ≤20 | 5 (16.7) | 3 (14.3) | |
| >20 and ≤400 | 5 (16.7) | 2 (9.5) | |
| >400 | 20 (66.7) | 16 (76.2) | |
| Tumour diameter (cm) | 9.2 (7.2, 12.6) | 9.7 (8.0, 10.3) | 0.755 |
| Tumour number (median) | 2.0 (1.0, 3.3) | 4.0 (1.0, 5.5) | 0.428 |
| Child–Pugh score | 0.506 | ||
| A | 28 (93.3) | 21 (100.0) | |
| B | 2 (6.7) | 0 (0.0) | |
| Vascular invasion (yes) | 27 (90.0) | 17 (81.0) | 0.427 |
| Portal vein | 26 (86.7) | 15 (71.4) | 0.283 |
| Hepatic vein and/or vena cava | 15 (50.0) | 8 (38.1) | 0.568 |
| PVTT, Japanese Vp classification | 0.275 | ||
| I | 0 (0.0) | 0 (0.0) | |
| II | 6 (20.0) | 3 (14.3) | |
| III | 15 (50.0) | 6 (28.6) | |
| IV | 5 (16.7) | 6 (28.6) | |
| Extrahepatic metastasis (yes) | 2 (6.7) | 2 (9.5) | 1.000 |
| CNLC | 0.450 | ||
| IIa | 0 (0.0) | 1 (4.8) | |
| IIb | 1 (3.3) | 2 (9.5) | |
| IIIa | 27 (90.0) | 16 (76.2) | |
| IIIb | 2 (6.7) | 2 (9.5) | |
| BCLC | 0.293 | ||
| B | 1 (3.3) | 3 (14.3) | |
| C | 29 (96.7) | 18 (85.7) | |
| AJCC 8th | 0.317 | ||
| IIIa | 1 (3.3) | 3 (14.3) | |
| IIIb | 27 (90.0) | 16 (76.2) | |
| IVb | 2 (6.7) | 2 (9.5) |
Values are n (%) unless otherwise indicated. Age, ALT, tumour diameter and tumour number are presented in median (i.q.r.). HCC, hepatocellular carcinoma; t-CT, triple combination therapy (TACE + lenvatinib + toripalimab); d-CT, dual combination therapy (TACE + lenvatinib); TACE, transarterial chemoembolization; HBsAg, hepatitis B virus surface antigen; HCV, hepatitis C virus; AFP, α-fetoprotein; ALT, alanine aminotransferase; AJCC, American Joint Committee on Cancer; PVTT, portal vein tumour thrombus; CNLC, China Liver Cancer Staging; BCLC, Barcelona Clinic Liver Cancer.
Treatment responses to combination conversion therapies
Treatment responses in the two conversion cohorts were summarized in Table 2. In contrast to the d-CT cohort, fewer cases (10.0 per cent versus 42.9 per cent, P = 0.016) were classified as PD in the t-CT cohort. Higher ORR (76.7 per cent versus 47.6 per cent, P = 0.042), DCR (90.0 per cent versus 57.1 per cent, P = 0.042), and successful conversion rate (50.0 per cent versus 19.0 per cent, P = 0.039) were observed in the t-CT cohort than in the d-CT cohort.
Table 2.
Treatment responses in triple combination therapy and dual combination therapy cohorts
| Overall response | t-CT (n = 30) | d-CT (n = 21) | P |
|---|---|---|---|
| Complete response | 2 (6.7) | 1 (4.8) | 1.000 |
| Partial response | 21 (70.0) | 9 (42.9) | 0.083 |
| Stable response | 4 (13.3) | 2 (9.5) | 1.000 |
| Progressive disease | 3 (10.0) | 9 (42.9) | 0.016 |
| Overall response rate | 23 (76.7) | 10 (47.6) | 0.042 |
| Disease control rate | 27 (90.0) | 12 (57.1) | 0.016 |
| Successful conversion | 15 (50.0) | 4 (19.0) | 0.039 |
Values are n (%). t-CT, triple combination therapy (TACE + lenvatinib + toripalimab); d-CT, dual combination therapy (TACE + lenvatinib); TACE, transarterial chemoembolization.
Relationship between treatment responses and tumour burden
Previous studies reported that patients with low tumour burden were prone to having better treatment responses from TACE when diagnosed with intermediate HCC21. Thus different stages of tumour burden were evaluated (Fig. S1). Based on 7–11 criteria, patients with a low tumour burden had a higher proportion of CR and PR after t-CT treatment, compared with those with an intermediate and high tumour burden (Fig. S1b, P = 0.025). In the t-CT cohort, worse treatment responses were observed in those who were out of 5–7 criteria (Fig. S1c, P = 0.041).
Survival of combination conversion therapies
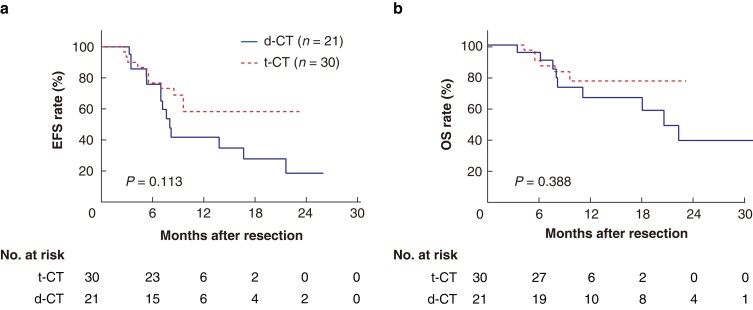
The survival between the two conversion cohorts was compared (Fig. 1). In the d-CT cohort, the median EFS was 8.0 months (95 per cent c.i. 6.8 to 9.3 months), and the median OS was 20.6 months (95 per cent c.i. 14.7 to 26.5 months). In contrast, both median EFS and OS were not reached in the t-CT cohort at the end of follow-up. The 1-year EFS and OS rates in the t-CT cohort were 58.3 per cent and 76.9 per cent respectively. The 1-year EFS and OS rates in the d-CT cohort were 41.7 per cent and 66.3 per cent respectively. The survival was not statistically different between two cohorts. The multivariable Cox regression analysis of EFS is shown in Table S1. Tumour number was an independent predictor for EFS.
Fig. 1.
Kaplan–Meier survival curves in the t-CT and d-CT cohorts
a EFS of cohort. b OS of cohort. EFS, event-free survival; OS, overall survival; t-CT, triple combination therapy (TACE + lenvatinib + toripalimab); d-CT, dual combination therapy (TACE + lenvatinib); TACE, transarterial chemoembolization.
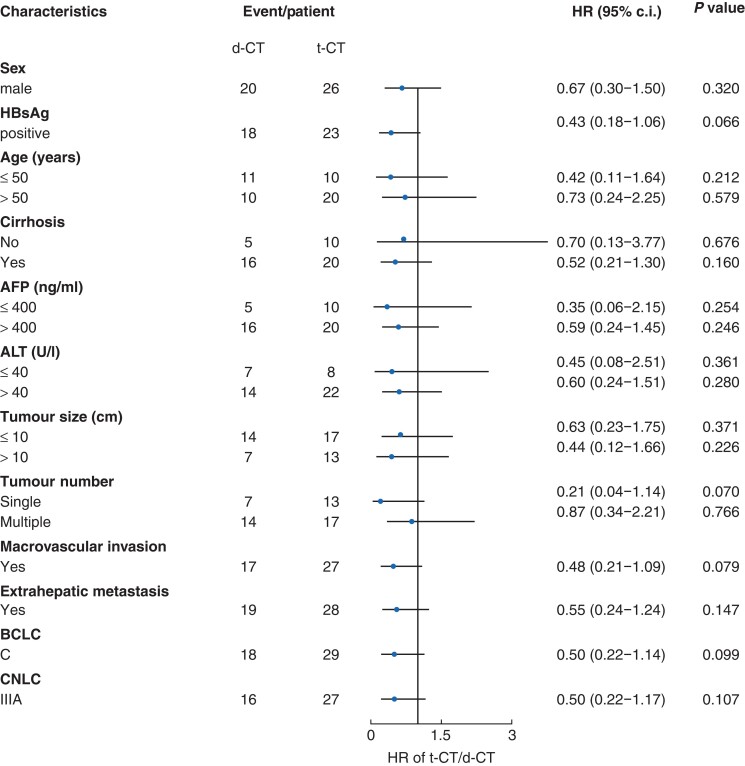
To further elucidate the influences of different regimens on EFS, a forest plot was produced to depict the survival risk in different subgroups (Fig. 2), showing that all HRs were less than one when comparing t-CT with d-CT, though not statistically significant.
Fig. 2.
Forest plot showing the effects of t-CT versus d-CT on event-free survival in different subgroups
t-CT, triple combination therapy (TACE + lenvatinib + toripalimab); d-CT, dual combination therapy (TACE + lenvatinib); HBsAg, hepatitis B virus surface antigen; AFP, α-fetoprotein; ALT, alanine aminotransferase; CNLC, China Liver Cancer Staging; BCLC, Barcelona Clinic Liver Cancer; TACE, transarterial chemoembolization.
Outcomes of the conversion surgery cohort treated by t-CT
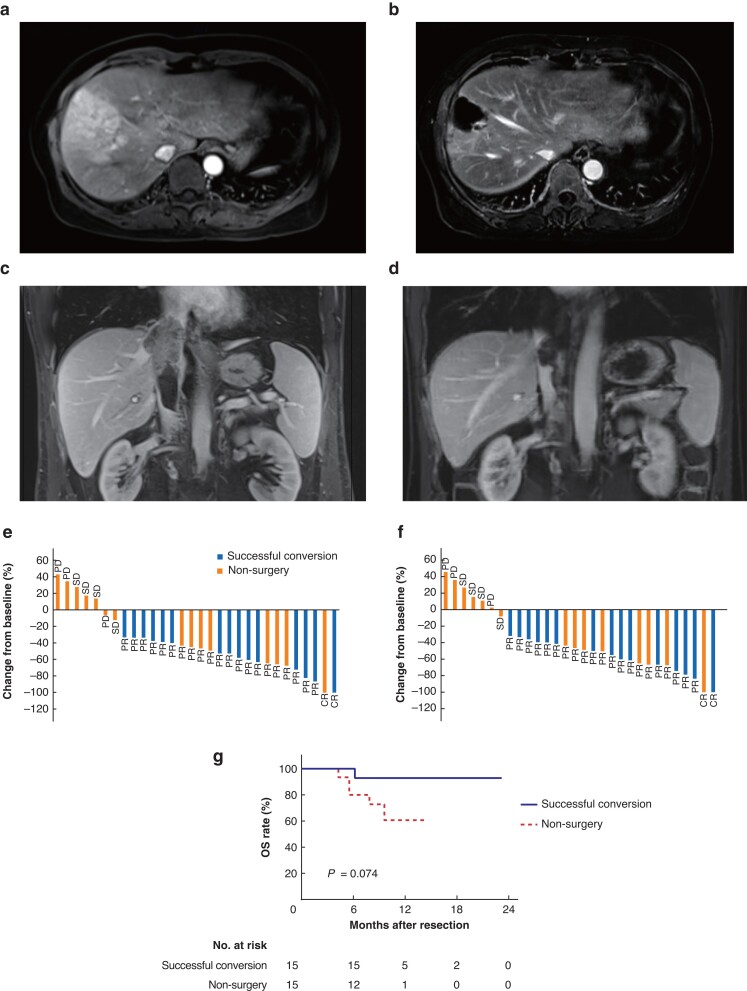
The analysis was focused on the t-CT cohorts to assess the survival outcomes of the successful conversion cases. The median duration between the initiation of t-CT therapy and conversion surgery was 2.1 months (i.q.r. 1.7–3.0 months). The representative images of necrosis and regression of tumour lesion and inferior vena cava tumour thrombus are shown in Fig. 3a–d. Changes from baseline after treatment are summarized in Fig. 3e,f, demonstrating that all patients who underwent liver resections achieved a CR or PR. Survival analysis revealed that successfully converted patients possessed a better trend of OS than the non-surgery population (Fig. 3g). The 1-year OS rates were 92.9 per cent and 60.6 per cent in the surgery group and non-surgery group respectively.
Fig. 3.
The efficacy of conversion therapy by t-CT
a MRI images of necrosis and regression of the tumour lesion before t-CT treatment. b Tumour lesion after t-CT treatment. c IVCTT before t-CT treatment. d IVCTT after t-CT treatment. e Tumour responses in the t-CT cohort: investigator. f Tumour responses in the t-CT cohort: independent review. g Kaplan–Meier curve for OS rate in the t-CT cohort. t-CT, triple combination therapy (TACE + lenvatinib + toripalimab); OS, overall survival; IVCTT, inferior vena cava tumour thrombus; TACE, transarterial chemoembolization; PD, progressive disease; SD, stable disease; CR, complete response; PR, partial response.
To better assess for the surgical effects of conversion therapy, a treatment-naive cohort of CNLC stage IIIa HCC who received surgery without previous anti-tumour therapy was retrospectively collected as the upfront surgery cohort (n = 68). This cohort was also characterized by a predominance of patients with hepatitis B virus (HBV)-related liver cirrhosis (Table S2). Patients in the two cohorts had the same tumour stage.
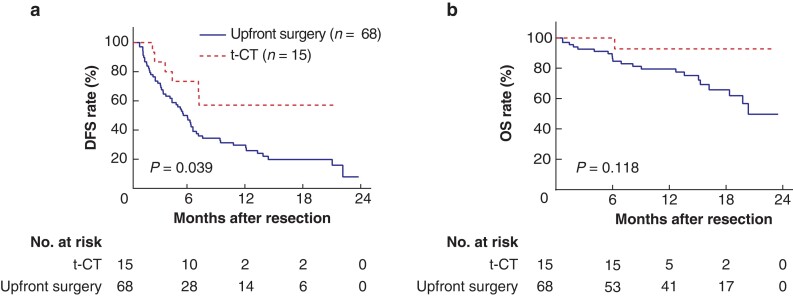
The DFS in the t-CT cohort was significantly higher than that of upfront surgery cohort (P = 0.039, Fig. 4a). The median DFS was 5.4 months (95 per cent c.i. 3.9 to 6.5 months) in the upfront surgery cohort, whereas it was not reached in the t-CT cohort. The OS of the t-CT cohort was also longer in number than that of the upfront surgery cohort, though statistically not significant (P = 0.118, Fig. 4b). These results support the improved efficacy and critical importance of t-CT treatment in CNLC stage IIIa patients before hepatectomy.
Fig. 4.
Differences in survival between the conversion surgery cohort via t-CT regimen and the upfront surgery cohort
a DFS for surgery cohorts. b OS for surgery cohorts. t-CT, triple combination therapy (TACE + lenvatinib + toripalimab); DFS, disease-free survival; OS, overall survival; TACE, transarterial chemoembolization.
Safety
The details of the AEs are summarized in Table S3. The most common grade 1–2 AEs were alanine aminotransferase (ALT)/aspartate transferase (AST) elevation, decreased platelet count, and abdominal pain. Decreased appetite, rash, and nausea were common clinical complaints. There were also cases of severe ALT/AST elevation, categorized as grade 3 AEs (6.7 per cent in the t-CT group versus 19.0 per cent in the d-CT group, P = 0.214). The TACE-related side effects are separately shown in Table S4.
As the study cohorts were composed of Japanese Vp classification III patients predominantly, the ALT level of the initial and last blood sample in both t-CT and d-CT cohorts was compared (Fig. S2, P = 0.631). No significant ALT flare was observed in the study population.
Discussion
In the past decade, great progress has been made in the systemic treatment of uHCC with targeted agents and ICIs. The present study evaluated the efficacy of conversion therapy with lenvatinib combined with TACE and PD-1 antibodies by interpreting real-world data. This combination therapy achieved a satisfactory tumour response and decent conversion rate. Survival benefits from conversion therapy may provide evidence to develop the optimal management for patients with advanced HCC.
Dual combination therapies have shown promising prognostic benefits for uHCC. In a retrospective analysis, lenvatinib and TACE significantly improved clinical outcomes over TACE monotherapy, reaching a DCR of 93.3 per cent, and an ORR of 68.3 per cent22. Similarly, the lenvatinib plus ICI regimen was reported to extend the median progression-free survival (PFS) of patients with uHCC to 9.3 months9. In May 2020, atezolizumab plus bevacizumab was approved by the US Food and Drug Administration as the first-line therapy for uHCC23,24. All these studies hinted at the synergistic effect among local treatment, immunotherapy, and targeted therapy. Herein, we compared the treatment outcomes between triple combination therapy and TACE plus lenvatinib therapy. Significantly higher response rates were found in the t-CT cohort than in the d-CT cohort. Moreover, patients treated with t-CT achieved a longer EFS and OS, although the log rank test did not endorse a significant difference. The forest plot revealed that receiving the t-CT regimen instead of d-CT might provide a positive impact on prolonged EFS in different subgroups. These results all highlight the promising anti-tumour effect of the t-CT combination therapy.
ORR is pivotal for the conversion surgery. Recently, He et al. reported a superior prognosis with the combination of lenvatinib, toripalimab, and the hepatic arterial infusion chemotherapy (HAIC), compared with lenvatinib monotherapy, presenting with an ORR of 67.6 per cent according to mRECIST criteria and a median PFS of 11.1 months25. In the present study, the patients who received t-CT treatment achieved an ORR of 76.7 per cent, which seemed higher than in previous studies investigating combination therapies for uHCC22,25,26. The speculated reason for this difference was individual variations between different studies, as well as a short interval between treatment initiation to conversion hepatectomy.
Whether tumour burden could predict tumour responses in patients with uHCC was further explored. Patients who received TACE plus lenvatinib had similar treatment responses in whatever stage based on up-to-11 or 7–11 or 5–7 criteria. For t-CT, patients with low tumour burden were prone to achieve PR or CR according to the 7–11 and 5–7 criteria. This provides indicative guidance to select beneficial therapeutic strategies.
For patients who are initially diagnosed with uHCC, how to maximally convert the tumour stage to a status appropriate for liver resection is of top priority. Most patients in our study cohort had macrovascular invasion disease, which is not considered a surgical indication by the CNLC or BCLC guidelines27–29. Nonetheless, surgical treatment was made possible by conversion therapy, and survival rates in the conversion surgery cohort and the upfront surgery cohort were compared. The median DFS in the upfront surgery cohort was 5.4 months, approximately at the same level as reported in a previous study11. The DFS rates in the neoadjuvant t-CT cohort at 6 and 12 months were 73.3 per cent and 57.0 per cent respectively, while the 1-year OS rate in the conversion surgery cohort was 92.9 per cent. The great benefits in survival observed after conversion surgery suggest that downstaging of HCC by conversion therapy should be undertaken as a routine for patients at CNLC stage IIIa.
Studies on neoadjuvant therapies for locally advanced HCC demonstrated good feasibility and efficacy of liver resection following downstaging or conversion treatment30. Previously reported DFS rates in patients with portal vein tumour thrombus was 56.9 per cent at 6 months and 33.0 per cent at 12 months after neoadjuvant radiotherapy followed by curative resection11, which was inferior to the present triple combination regimen. In the 2021 American Society of Clinical Oncology conference, a single-arm, phase II ongoing randomized clinical trial reported that eight out of 32 patients with uHCC receiving toripalimab combined with lenvatinib plus HAIC met the criteria for downstaging resection. The ORR achieved 66.7 per cent in the whole cohort. Recently, a retrospective analysis based on the pembrolizumab–lenvatinib–TACE combination regimen revealed a conversion rate of 25.7 per cent and an ORR of 47.1 per cent among 70 patients, despite the limited reliability brought on by the retrospective design and long-time span. It was reported in another study on tyrosine kinase inhibitors plus anti-PD-1 antibodies reported that 15.9 per cent of patients with uHCC underwent R0 resection 3.2 months (range 2.4–8.3 months) after the initiation of conversion therapy17. Notably, 50 per cent of patients (15 of 30) received R0 resection after t-CT treatment in the present study. To our knowledge, this conversion rate is the highest among the exploratory studies on combination therapy, which may be attributed to the active surgery-oriented treatment intention. Furthermore, the preferable DFS and short conversion process in this study suggest that once patient response to the t-CT regimen is sufficient to perform radical surgery safely, and drug-induced AEs are well tolerated, conversion liver resection should be considered. No significant difference was observed between the two treatment cohorts in terms of AEs, indicating satisfactory safety profiles of triple combination therapy. Of note, one specific case of haemangioma was detected in the t-CT population. ALT/AST elevation was the most common grade 3 AE, which was probably caused by liver injury from TACE. TACE is generally contraindicated when there is a tumour thrombosis in main trunk or first branch of portal vein. Recent studies increasingly focused on the TACE and combination therapy in unresectable patients with Vp III and Vp IV31. Even for those with tumour thrombosis in main trunk or first branch of portal vein, the treatment was well tolerated. In the present study, tolerable ALT/AST flare was observed during the treatment. Moreover, patients who underwent conversion surgery shared good liver function of Child–Pugh grade A. These results indicated the feasibility of both t-CT and d-CT in patients with portal vein tumour thrombus.
The present study had several limitations. First, it was an observational analysis which inevitably suffered from selection bias. A more reliable and rigorous randomized clinical trial is required to confirm this effect. Second, the data came from a single institution. Independent external validation cohorts should be included in the future. Third, expansions in sample size and the follow-up duration are needed. Fourth, further analysis of the biomarkers to predict treatment responses and/or to determine whether a patient is suitable for conversion surgery is necessary. Finally, the patients in the data sets were predominantly HBV infected, impairing the representativeness for an extensive uHCC population.
In conclusion, first-line conversion therapy with toripalimab combined with lenvatinib plus TACE resulted in promising treatment responses and conversion rate for patients who were initially uHCC. As an effective strategy for tumour downstaging, the t-CT regimen is recommended for patients with macrovascular invasion disease to achieve a curative opportunity.
Supplementary Material
Acknowledgements
This study protocol was reviewed and approved by the Institutional Review Board of Zhongshan Hospital (approval no. SK2020-054). Written informed consent was obtained from participants to participate in the study. W.-F.Q., Z.-B.D., X.-D.Q. and Z.T. contributed equally to this work. Conception and design were the responsibility of Y.-H.S., W.-R.L., Z.-B.D., J.Z. and J.F. Administrative support was provided by Y.-H.S., W.-R.L. and X.-D.Q. Y.-H.S, Z.-B.D., X.-D.Q., W.-R.L., X.-T.F., Z.-H.Z., X.Z., A.H., J.Z. and J.F. provided study materials or patients. Collection and assembly of data was provided by W.-F.Q., Z.T., G.-Q.Z., M.-X.T., Z.-H. Z., M.T., X.-F.J., R.H., C.-Y.T., Y.F., J.G. and X.-L.W. Data analysis and interpretation was conducted by W.-F.Q., Z.T. and G.-Q.Z. W.-F.Q. and Z.-B.D. wrote the manuscript. Final approval of the manuscript was the responsibility of all authors.
Contributor Information
Wei-Feng Qu, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China.
Zhen-Bin Ding, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China.
Xu-Dong Qu, Department of Interventional Radiology, Zhongshan Hospital, Fudan University, Shanghai, China.
Zheng Tang, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China.
Gui-Qi Zhu, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China.
Xiu-Tao Fu, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China.
Zi-Han Zhang, Department of Interventional Radiology, Zhongshan Hospital, Fudan University, Shanghai, China.
Xin Zhang, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China.
Ao Huang, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China.
Min Tang, Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, China.
Meng-Xin Tian, Department of General Surgery, Zhongshan Hospital, Fudan University, Shanghai, China.
Xi-Fei Jiang, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China.
Run Huang, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China.
Chen-Yang Tao, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China.
Yuan Fang, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China.
Jun Gao, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China.
Xiao-Ling Wu, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China.
Jian Zhou, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China; Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, China.
Jia Fan, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China; Department of Radiology, Zhongshan Hospital, Fudan University, Shanghai, China.
Wei-Ren Liu, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China; State Key Laboratory of Genetic Engineering and Collaborative Innovation Center for Genetics and Development, School of Life Sciences, Fudan University, Shanghai, China.
Ying-Hong Shi, Department of Liver Surgery and Transplantation, Liver Cancer Institute, Zhongshan Hospital, Fudan University, Key Laboratory of Carcinogenesis and Cancer Invasion of Ministry of Education, and Research Unit of Bench and Clinic Research for Liver Cancer Recurrence and Metastasis, Chinese Academy of Medical Sciences, Shanghai, China; Shanghai Key Laboratory of Organ Transplantation, Shanghai, China; State Key Laboratory of Genetic Engineering and Collaborative Innovation Center for Genetics and Development, School of Life Sciences, Fudan University, Shanghai, China.
Funding
This work was supported by the grants from National Natural Science Foundation of China (nos. 81773067, 82073217, 82073218, and 82003084); Shanghai Municipal Science and Technology Major Project (no. 2018SHZDZX05); Shanghai Municipal Key Clinical Specialty; CAMS Innovation Fund for Medical Sciences (CIFMS) (2019-I2M-5-058); National Key R&D Program of China (2018YFC1312100); and Beijing iGandan Foundation (GDXZ-08-15).
Disclosure
The authors declare no conflict of interest.
Supplementary material
Supplementary material is available at BJS Open online.
Data Availability
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.
References
- 1. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424 [DOI] [PubMed] [Google Scholar]
- 2. Park JW, Chen M, Colombo M, Roberts LR, Schwartz M, Chen PJ et al. Global patterns of hepatocellular carcinoma management from diagnosis to death: the BRIDGE study. Liver Int 2015;35:2155–2166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Llovet JM, Montal R, Sia D, Finn RS. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat Rev Clin Oncol 2018;15:599–616 [DOI] [PubMed] [Google Scholar]
- 4. Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP et al. Systemic therapy for advanced hepatocellular carcinoma: ASCO guideline. J Clin Oncol 2020;38:4317–4345 [DOI] [PubMed] [Google Scholar]
- 5. El-Khoueiry AB, Sangro B, Yau T, Crocenzi TS, Kudo M, Hsu C et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 2017;389:2492–2502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu AX, Finn RS, Edeline J, Cattan S, Ogasawara S, Palmer D et al. Pembrolizumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib (KEYNOTE-224): a non-randomised, open-label phase 2 trial. Lancet Oncol 2018;19:940–952 [DOI] [PubMed] [Google Scholar]
- 7. Yau T, Kang Y-K, Kim T-Y, El-Khoueiry AB, Santoro A, Sangro B et al. Efficacy and safety of nivolumab plus ipilimumab in patients with advanced hepatocellular carcinoma previously treated with sorafenib: the CheckMate 040 randomized clinical trial. JAMA Oncol 2020;6:e204564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F et al. Lenvatinib versus sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet 2018;391:1163–1173 [DOI] [PubMed] [Google Scholar]
- 9. Finn RS, Ikeda M, Zhu AX, Sung MW, Baron AD, Kudo M et al. Phase Ib study of lenvatinib plus pembrolizumab in patients with unresectable hepatocellular carcinoma. J Clin Oncol 2020;38:2960–2970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Yi C, Chen L, Lin Z, Liu L, Shao W, Zhang R et al. Lenvatinib targets FGF receptor 4 to enhance antitumor immune response of anti-programmed cell death-1 in HCC. Hepatology 2021;74:2544–2560 [DOI] [PubMed] [Google Scholar]
- 11. Wei X, Jiang Y, Zhang X, Feng S, Zhou B, Ye X et al. Neoadjuvant three-dimensional conformal radiotherapy for resectable hepatocellular carcinoma with portal vein tumor thrombus: a randomized, open-label, multicenter controlled study. J Clin Oncol 2019;37:2141–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Fan J, Tang Z-Y, Yu Y-Q, Wu Z-Q, Ma Z-C, Zhou X-D et al. Improved survival with resection after transcatheter arterial chemoembolization (TACE) for unresectable hepatocellular carcinoma. Dig Surg 1998;15:674–678 [DOI] [PubMed] [Google Scholar]
- 13. Tustumi F, Ernani L, Coelho FF, Bernardo WM, Junior SS, Kruger JAP et al. Preoperative strategies to improve resectability for hepatocellular carcinoma: a systematic review and meta-analysis. HPB (Oxford) 2018;20:1109–1118 [DOI] [PubMed] [Google Scholar]
- 14. Shindoh J, Kawamura Y, Kobayashi Y, Kobayashi M, Akuta N, Okubo S et al. Prognostic impact of surgical intervention after lenvatinib treatment for advanced hepatocellular carcinoma. Ann Surg Oncol 2021;28:7663–7672 [DOI] [PubMed] [Google Scholar]
- 15. Huang C, Zhu X-D, Shen Y-H, Wu D, Ji Y, Ge N-L et al. Organ specific responses to first-line lenvatinib plus anti-PD-1 antibodies in patients with unresectable hepatocellular carcinoma: a retrospective analysis. Biomark Res 2021;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Chen S, Wu Z, Shi F, Mai Q, Wang L, Wang F et al. Lenvatinib plus TACE with or without pembrolizumab for the treatment of initially unresectable hepatocellular carcinoma harbouring PD-L1 expression: a retrospective study. J Cancer Res Clin Oncol 2021;148:2115–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhu X-D, Huang C, Shen Y-H, Ji Y, Ge N-L, Qu XD et al. Downstaging and resection of initially unresectable hepatocellular carcinoma with tyrosine kinase inhibitor and anti-PD-1 antibody combinations. Liver Cancer 2021;10:320–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bruix J, Sherman M, American Association for the Study of Liver Diseases . Management of hepatocellular carcinoma: an update. Hepatology 2011;53:1020–1022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Keam SJ. Toripalimab: first global approval. Drugs 2019;79:573–578 [DOI] [PubMed] [Google Scholar]
- 20. Llovet JM, Lencioni R. mRECIST for HCC: performance and novel refinements. J Hepatol 2020;72:288–306 [DOI] [PubMed] [Google Scholar]
- 21. Hung Y-W, Lee I-C, Chi C-T, Lee R-C, Liu C-A, Chiu N-C et al. Redefining tumor burden in patients with intermediate-stage hepatocellular carcinoma: the seven-eleven criteria. Liver Cancer 2021;10:629–640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N et al. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int 2021;15:663–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Qin S, Ren Z, Feng Y-H, Yau T, Wang B, Zhao H et al. Atezolizumab plus bevacizumab versus sorafenib in the Chinese subpopulation with unresectable hepatocellular carcinoma: phase 3 randomized, open-label IMbrave150 study. Liver Cancer 2021;10:296–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Casak SJ, Donoghue M, Fashoyin-Aje L, Jiang X, Rodriguez L, Shen Y-L et al. FDA approval summary: atezolizumab plus bevacizumab for the treatment of patients with advanced unresectable or metastatic hepatocellular carcinoma. Clin Cancer Res 2021;27:1836–1841 [DOI] [PubMed] [Google Scholar]
- 25. He M-K, Liang R-B, Zhao Y, Xu Y-J, Chen H-W, Zhou Y-M et al. Lenvatinib, toripalimab, plus hepatic arterial infusion chemotherapy versus lenvatinib alone for advanced hepatocellular carcinoma. Ther Adv Med Oncol 2021;13:17588359211002720 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mei J, Tang Y-H, Wei W, Shi M, Zheng L, Li S-H et al. Hepatic arterial infusion chemotherapy combined with PD-1 inhibitors plus lenvatinib versus PD-1 inhibitors plus lenvatinib for advanced hepatocellular carcinoma. Front Oncol 2021;11:618206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Zhou J, Sun H-C, Wang Z, Cong W-M, Wang J-H, Zeng M-S et al. Guidelines for diagnosis and treatment of primary liver cancer in China (2017 edition). Liver Cancer 2018;7:235–260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Department of Medical Administration, National Health and Health Commission of the People's Republic of China . [Guidelines for diagnosis and treatment of primary liver cancer in China (2019 edition)]. Zhonghua Gan Zang Bing Za Zhi 2020;28:112–128 [DOI] [PubMed] [Google Scholar]
- 29. Forner A, Reig ME, de Lope CR, Bruix J. Current strategy for staging and treatment: the BCLC update and future prospects. Semin Liver Dis 2010;30:61–74 [DOI] [PubMed] [Google Scholar]
- 30. Yamamura K, Beppu T, Miyata T, Okabe H, Nitta H, Imai K et al. Conversion surgery for hepatocellular carcinoma following molecular therapy. Anticancer Res 2022;42:35–44 [DOI] [PubMed] [Google Scholar]
- 31. Yang B, Jie L, Yang T, Chen M, Gao Y, Zhang T et al. TACE plus lenvatinib versus TACE plus sorafenib for unresectable hepatocellular carcinoma with portal vein tumor thrombus: a prospective cohort study. Front Oncol 2021;11:821599. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analysed during this study are included in this article. Further enquiries can be directed to the corresponding author.