Abstract
Background
High-quality evidence from trials directly comparing single antiplatelet therapies in symptomatic peripheral arterial disease (PAD) to dual antiplatelet therapies or acetylsalicylic acid (ASA) plus low-dose rivaroxaban is lacking. Therefore, we conducted a network meta-analysis on the effectiveness of all antithrombotic regimens studied in PAD.
Methods
A systematic search was conducted to identify randomized controlled trials. The primary endpoints were major adverse cardiovascular events (MACE) and major bleedings. Secondary endpoints were major adverse limb events (MALE) and acute limb ischaemia (ALI). For each outcome, a frequentist network meta-analysis was used to compare relative risks (RRs) between medication and ASA. ASA was the universal comparator since a majority of studies used ASA as in the reference group.
Results
Twenty-four randomized controlled trials were identified including 48,759 patients. With regard to reducing MACE, clopidogrel [RR 0.78, 95% confidence interval (CI) 0.66–0.93], ticagrelor (RR 0.79, 95% CI 0.65–0.97), ASA plus ticagrelor (RR 0.79, 95% CI 0.64–0.97), and ASA plus low-dose rivaroxaban (RR 0.84, 95% CI 0.76–0.93) were more effective than ASA, and equally effective to one another. As compared to ASA, major bleedings occurred more frequently with vitamin K antagonists, rivaroxaban, ASA plus vitamin K antagonists, and ASA plus low-dose rivaroxaban. All regimens were similar to ASA concerning MALE, while ASA plus low-dose rivaroxaban was more effective in preventing ALI (RR 0.67, 95% CI 0.55–0.80). Subgroup analysis in patients undergoing peripheral revascularization revealed that ≥ 3 months after intervention, evidence of benefit regarding clopidogrel, ticagrelor, and ASA plus ticagrelor was lacking, while ASA plus low-dose rivaroxaban was more effective in preventing MACE (RR 0.87, 95% CI 0.78–0.97) and MALE (RR 0.89, 95% CI 0.81–0.97) compared to ASA. ASA plus clopidogrel was not superior to ASA in preventing MACE ≥ 3 months after revascularization. Evidence regarding antithrombotic treatment strategies within 3 months after a peripheral intervention was lacking.
Conclusion
Clopidogrel, ticagrelor, ASA plus ticagrelor, and ASA plus low-dose rivaroxaban are superior to ASA monotherapy and equally effective to one another in preventing MACE in PAD. Of these four therapies, only ASA plus low-dose rivaroxaban provides a higher risk of major bleedings. More than 3 months after peripheral vascular intervention, ASA plus low-dose rivaroxaban is superior in preventing MACE and MALE compared to ASA but again at the cost of a higher risk of bleeding, while other treatment regimens show non-superiority. Based on the current evidence, clopidogrel may be considered the antithrombotic therapy of choice for most PAD patients, while in patients who underwent a peripheral vascular intervention, ASA plus low-dose rivaroxaban could be considered for the long-term (> 3 months) prevention of MACE and MALE.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40265-022-01756-6.
Key Points
| Clopidogrel should be considered as the first-choice antithrombotic therapy in patients with symptomatic peripheral arterial disease. |
| After peripheral revascularization procedures, acetylsalicylic acid plus low-dose rivaroxaban could be considered for the long-term (> 3 months) secondary prevention, but a higher bleeding risk should be taken into account. |
Introduction
Peripheral arterial disease (PAD) is a manifestation of atherosclerosis in the major arteries of the lower extremities, leading to various clinical symptoms such as intermittent claudication, ischaemic rest pain, and gangrene [1, 2]. Globally, approximately 202 million patients suffer from PAD, of whom 141 live in low-income or middle-income countries and 61 million live in high-income countries [3]. PAD is associated with a significant risk of arterial thrombotic events, such as myocardial infarction, ischaemic stroke, lower extremity amputation, and cardiovascular death [2, 4–8]. The relative risk (RR) of cardiovascular death over a period of 10 years in patients with PAD is six times increased compared to non-PAD individuals [8]. Furthermore, during a person’s lifetime, PAD is associated with impaired physical function, reduced quality of life, and increased healthcare costs [9, 10]. Within the framework of secondary prevention, antithrombotic therapy is recommended. According to the current guidelines, the first choice of antithrombotic therapy in symptomatic PAD is single antiplatelet therapy (SAPT) [1, 11]. The benefit of acetylsalicylic acid (ASA) monotherapy in symptomatic PAD has been extensively studied [12]. Clopidogrel, a P2Y12 inhibitor, was more effective in reducing arterial thrombotic events with a similar safety profile compared to ASA in the CAPRIE study [13]. Therefore, clopidogrel may be preferred over ASA as antithrombotic therapy in patients with symptomatic PAD [1]. The use of alternative P2Y12 inhibitors, such as ticagrelor and prasugrel, is not approved by international authorities (i.e. the European Medicines Agency and the United States Food and Drug Administration) and has therefore no place in the secondary prevention of PAD patients. After peripheral revascularization by endovascular stenting or infra-inguinal prosthetic bypass grafting, dual antiplatelet therapy (DAPT) with ASA plus clopidogrel for at least 1 month is recommended [1, 11]. However, this recommendation is not supported by high-quality trial evidence and the optimal duration of DAPT after an intervention is unknown [14]. After venous bypass surgery, fewer graft occlusions were demonstrated with oral anticoagulation in the Dutch BOA trial, unfortunately, at the expense of a twofold increased risk of major bleeding [15]. Recently, two large randomized controlled trials (RCTs), respectively, including patients with symptomatic PAD or patients undergoing a peripheral vascular intervention (endovascular or surgical), demonstrated superiority of ASA plus low-dose rivaroxaban over ASA monotherapy [16, 17]. Whether the benefits of ASA plus low-dose rivaroxaban also apply when compared to clopidogrel monotherapy is unclear. Choosing the best antithrombotic therapy for PAD patients entails significant uncertainties, since only a few RCTs directly compared the latest treatment options. By use of network meta-analysis, treatment options can be indirectly compared using a universal comparator [18]. This study aimed to evaluate the effectiveness and safety of antithrombotic regimens for secondary prevention in PAD patients.
Methods
Data Sources and Searches
This systematic review and network meta-analysis was performed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-analyses of Health Care Interventions (PRISMA-NMA). The review protocol is registered with Open Science Framework, number 4nz9t. A systematic search was performed using the electronic databases PubMed, MEDLINE, and EMBASE for English language RCTs published from January 1, 1995 up to December 31, 2021. Earlier performed RCTs were excluded to minimize the risk of suboptimal secondary prevention biasing the protective effect of antithrombotic treatment [19]. The search combined terms for PAD with terms for antithrombotic treatment. The electronic database search was supplemented with a manual search for RCTs in the reference list of the selected articles. ClinicalTrials.gov and the Cochrane Central Register of Controlled Trials were searched to identify further studies and unpublished RCTs with results. Details of the search strategies are described in Appendix A (see the Electronic Supplementary Material).
Study Selection
Two authors (LW and DM) independently screened the titles and abstracts of the articles collected through the searches. Of all eligible articles, the full text was independently assessed by two authors (LW and DM). Disagreement was resolved by discussion. In the case where no agreement was obtained, arbitration of a third author was requested (MW). In the case where different articles contained duplicate data, the article with the largest sample size or the most complete information was selected.
Studies on patients with symptomatic lower extremity PAD, based on a clinical presentation of intermittent claudication or chronic limb-threatening ischaemia that either was related to an ankle brachial index below 0.9 and/or resulted in the need for peripheral revascularization, were considered eligible if (1) two or more antithrombotic treatment strategies were compared and (2) patients were followed for clinical outcome measurements. Clinical outcome measurements included death, myocardial infarction, stroke, acute limb ischaemia (ALI), need for revascularization, major amputation, and bleeding events. Study reports meeting one or more of the following criteria were excluded: (1) studies on patients using anticoagulant therapy for venous thromboembolic disease, (2) studies on patients using anticoagulant therapy for the prevention of systemic embolism in atrial fibrillation, (3) studies on patients with known congenital bleeding or thrombotic disorders, (4) studies with intravenous or intra-arterial antithrombotic treatment as intervention, (5) studies in which the clinical outcomes were described for a follow-up period that was twice or more the duration of the intervention period, (6) animal studies, and (7) in vitro studies.
Data Extraction
Data were extracted from the selected articles by two independent reviewers (LW and DM) using a standardized form. Discrepancies were resolved by discussion. Extracted data included the following: study acronym, last name of the first author, full title, publication year, study setting (primary, secondary, tertiary care), population, inclusion criteria, exclusion criteria, clinical severity of PAD, intervention (drug and dose), comparison (drug and dose), sample size, baseline characteristics of the participants, compliance, outcome measurements, and duration of follow-up.
The primary cardiovascular effectiveness endpoint was the occurrence of major adverse cardiovascular events (MACE). The primary safety endpoint was major bleeding. Secondary endpoints were major adverse limb events (MALE) and ALI. Variations in definitions were allowed as long as they assessed cardiovascular complications of atherosclerotic cardiovascular disease, but not endpoints related to other pathophysiological mechanisms such as venous thromboembolism. Endpoints were assessed at the longest follow-up available.
Quality Assessment
Risk of bias was assessed by two independent reviewers (LW and DM) using Cochrane Collaboration’s risk of bias tool for RCTs. In brief, a quality judgment was performed on the randomization process, allocation concealment, missing outcome data, measurement of the outcome, and selection of the reported results [20]. Studies with a low risk of bias in all domains or studies that raised concerns in a maximum of one domain are considered to have a low risk of bias. Studies that raised concerns in two domains are considered to have a medium risk of bias. Studies with a high risk of bias in one or more domains and studies that raised concerns in more than two domains are considered to have a high risk of bias. Discrepancies between reviewers were resolved through discussion.
Certainty of Evidence
We assessed the certainty of evidence contributing to network estimates of the main outcomes with the Grading of Recommendations Assessment, Development and Evaluation (GRADE) framework [21]. Indirect estimates were rated, starting with the lower of the ratings from the direct estimates forming the dominant first order loop. If intransitivity was present, the rating was further downgraded. The estimate, direct or indirect, that contributed most was the basis for the certainty of evidence. In the case of similar amounts of contribution, the higher of certainty judgments was chosen. If there was evidence of imprecision between direct and indirect estimates, the certainty was downgraded. If the RR estimate was ≥ 1 and the lower limit of the confidence interval (CI) was below 0.75, or if the RR estimate was ≤ 1 and the upper limit of the CI was above 1.25, the certainty was downgraded [22].
Statistical Methods
We used a frequentist network meta-analysis with random-effects models to estimate the aggregate effects in MACE, major bleeding, MALE, and ALI for each type of antithrombotic medication compared with ASA and with each other. Pooled estimates were expressed as RRs with their corresponding 95% CIs.
Network plots were produced for each outcome to visualize network geometry and node connectivity. We estimated the ranking probabilities of the different antithrombotic treatments based on their p scores. The p score is measured on a scale from 0 (worst) to 1 (best), with a higher score indicating better overall performance of the competing treatment. The numerical p score values are nearly identical to the surface under the cumulative ranking curve [23]. It is also important to consider the RR and corresponding 95% CI for each comparison when interpreting the ranking results [24].
Network heterogeneity across treatment contrasts was assessed using τ2 and I2 statistics. We applied the Q statistic to test for global inconsistency using a design-by-treatment interaction random effects model [25]. Local inconsistency was evaluated through a node split method by splitting the network estimates into direct and indirect evidence using a back-calculation method [26–28]. P values were two-sided, and p values less than 0.05 were considered statistically significant.
Results were graphically displayed using forest plots.
We used the number needed to treat (NNT) as an absolute measure of effect used to communicate the effectiveness or safety of an intervention. The NNT provides insight into the clinical relevance of an effect size because it is defined as the average number of patients who need to be treated to prevent one extra person from having a bad outcome compared with another treatment. For positive outcomes, the NNT can be equivalently defined as the number of people that need to be treated to have one person with a good outcome. Similarly, the number needed to harm (NNH) indicates how many people need to be treated in order for one patient to have a particular adverse effect. To avoid the unfavourable NNH term [29], we used the terms ‘number needed to treat for an additional beneficial outcome’ (NNTB) and ‘number needed to treat for an additional harmful outcome’ (NNTH) for positive and negative outcomes, respectively.
The NNTB and NNTH with their 95% CIs were calculated by taking the inverse of the risk difference (RD) as estimated from the network meta-analysis [30]. In NNT, values between −1 and 1 are impossible, and the domain of NNT uses two regions: (1) the NNTB region, including the union of 1 (where it is the largest possible beneficial treatment effect) to ∞ (no treatment effect), and (2) the NNTH region, ∞ (no treatment effect) to 1 (where it is the largest possible harmful treatment effect). For example, a non-statistically significant NNT 5 with a CI of −40 to 2 is a combination of the two regions (∞,40) and (2,∞). The suggested presentation of such a non-statistically significant NNT is NNTB 5 (NNTH 40; ∞; NNTB 2) [29, 30]. We used this presentation for the 95% CIs of the NNT. Furthermore, the direct evidence proportion, mean path length, and aggregated minimal parallelism were quantified. The direct evidence proportion is the proportion of direct evidence contained in each network estimate. Minimal parallelism reflects the minimum number of independent paths contributing to the effect estimate on an aggregated level. Large values of parallelism can be interpreted as supporting the robustness of the estimate. Mean path length characterizes the degree of indirectness of an estimate. Higher mean path lengths indicate less reliable estimates, given that more similarity assumptions have to be made when serially combining direct comparisons. Comparisons with mean path lengths greater than two should be interpreted with caution [31].
Population-based subgroup analysis was performed for studies with patients that were selected for undergoing a peripheral vascular intervention.
Sensitivity analyses were performed to assess the robustness of the model by excluding trials with a high risk of bias. We explored the potential for publication bias by visual inspection of the comparison-adjusted funnel plots [32, 33].
Analyses were performed using R (version 4.1.2; R Foundation for Statistical Computing, Vienna, Austria) with package ‘netmeta’ [34].
Results
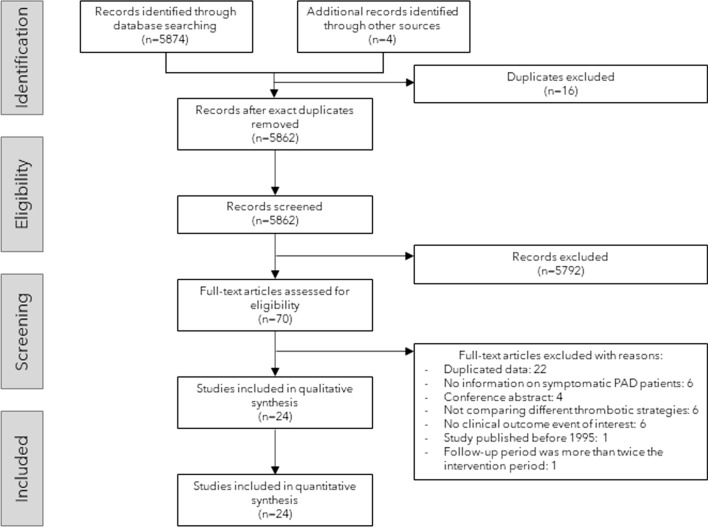
The study flowchart is presented in Fig. 1. In total, 5862 records were identified by electronic database and additional records searching, of which 24 RCTs with 48,759 patients (range 20–13,885) were included in this network meta-analysis [13, 15–17, 35–55]. One of the included studies was retrieved by the search for unpublished literature in the trial registers. A network diagram of all the research scenarios of the primary outcomes is shown in Figure 2. Study characteristics are shown in Table 1. Five studies had broader inclusion criteria than symptomatic lower extremity PAD but were included since a great majority (> 75%) of participants fulfilled the inclusion criteria of our network meta-analysis: (1) the CREDO study [43], which included 61 patients (22.4% of study population) with cerebrovascular disease, (2) the WAVE study [55], which included 394 patients (18.2% of study population) with PAD of the subclavian or carotid arteries, and (3) the CHARISMA [39], (4) CLIPS [40], and (5) PEGASUS TIMI 54 [50] trials, which included, respectively, 258 (8.3%), 82 (22.4%), and 217 patients (19.0%) with asymptomatic PAD, diagnosed by an ankle brachial index below 0.9. Two studies compared three antithrombotic regimens [16, 50]. Drug dose variations were collectively analysed, including ASA 75–325 mg daily, ticagrelor 60–90 mg twice daily, and ticlopidine 200–250 mg twice daily. Target international normalized ratios (INRs) in studies combining SAPT with a vitamin K antagonist (VKA) ranged from INR 1.4 to 3.0. The one study [16] that investigated VKA monotherapy strived for a target INR of 3.0–4.5. Other drug doses were consistent among the included studies. Definitions of MACE, major bleeding and MALE differed between the studies. MACE was mostly defined as the composite of (cardiovascular) death, myocardial infarction, and stroke. Major bleeding was defined according to Thrombolysis In Myocardial Infarction (TIMI), International Society on Thrombosis and Haemostasis (ISTH), or Global Use of Strategies to Open Occluded Arteries (GUSTO) criteria [56], or defined according to the self-defined criteria of the individual articles. MALE was commonly defined as the composite of any peripheral vascular intervention for chronic limb ischaemia or ALI and major amputation, but occasionally included elective peripheral revascularization for non-ischaemic reasons, vascular occlusion without intervention, or death. Table 2 summarizes the variable definitions used in the individual RCTs.
Fig. 1.
Flow chart of study screening and selection. PAD peripheral arterial disease
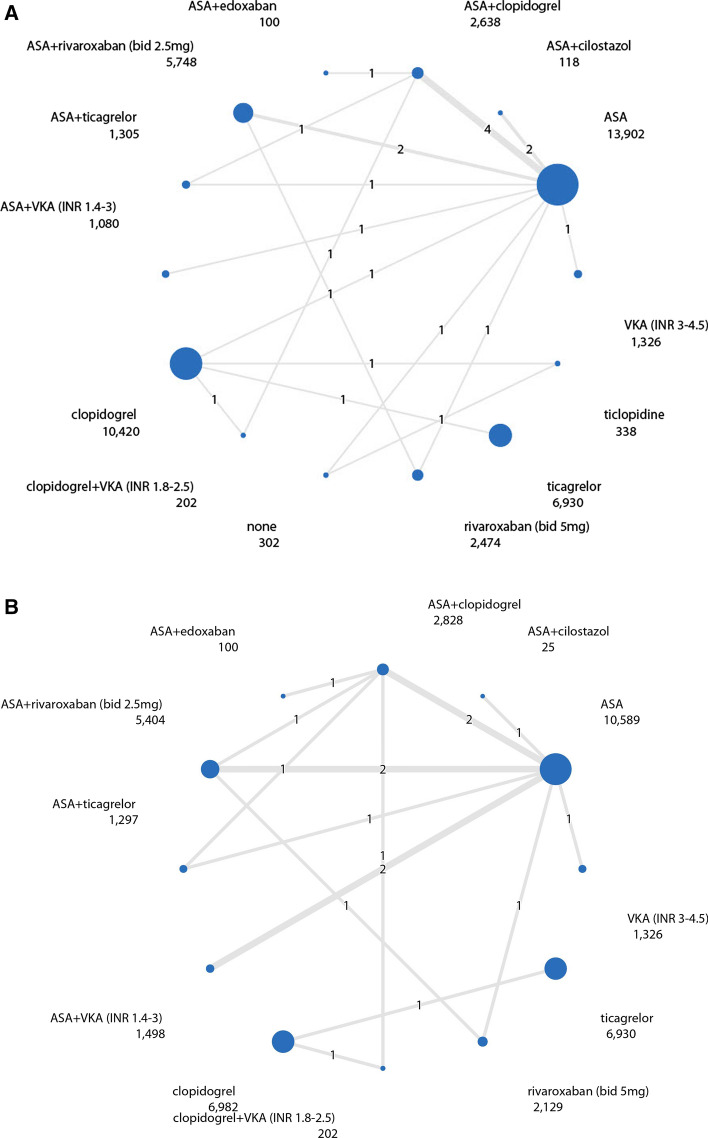
Fig. 2.
Network diagram of antithrombotic regimens. The line width is proportional to the sample size of each direct comparison. The number in the middle of the line represents the number of direct comparisons. The number below the antithrombotic regimen corresponds with the total number of participants on that specific antithrombotic therapy. A Network diagram of MACE. B Network diagram of major bleedings. ASA acetylsalicylic acid, bid bi-daily, INR international normalized ratio, MACE major adverse cardiovascular events, VKA vitamin K antagonist. *Patients who used no antithrombotic treatment, did receive placebo tablets
Table 1.
Study characteristics
| References | Study | Year | Sample size | Antithrombotic regimena | Populationb | Average follow-up (months) | Age (mean) | Male (%) | HT (%) | HL (%) | CSM (%) | HSM (%) | CAD (%) | CVD (%) | DM (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| [36] | Becquemin | 1997 | 243 | P vs TP2 | PVI | 24 | 67 | 77 | 51 | 25 | 22 | 23 | 24 | ||
| [15] | BOA | 2000 | 2690 | A vs VKA2 | PVI | 21 | 69 | 64 | |||||||
| [37] | CABBAGE | 2017 | 50 | A vs A+CI | PVI | 3 | 73 | 74 | 88 | 50 | 14 | 40 | 54 | 26 | 74 |
| [13] | CAPRIE | 1996 | 6452 | A vs C | PAD | 23 | 64 | 72 | 51 | 45 | 38 | 90 | 54 | 14 | 21 |
| [38] | CASPAR | 2010 | 851 | A vs A+C | PVI | 12 | 66 | 76 | 70 | 50 | 38 | 35 | 37 | ||
| [39] | CHARISMA | 2009 | 3096 | A vs A+C | PAD | 26 | 60 | 70 | 72 | 70 | 32 | 85 | 25 | 16 | 36 |
| [40] | CLIPS | 2007 | 366 | A vs P | PAD | 21 | 66 | 77 | 62 | 26 | 80 | 76 | |||
| [16] + [41] | COMPASS | 2018 | 7470 | A vs A+R1 vs R2 | PAD | 21 | 68 | 72 | 79 | 28 | 75 | 65 | 7 | 45 | |
| [42] | COOPER | 2012 | 431 | C vs TP1 | PAD | 3 | 71 | 88 | 74 | 56 | 25 | 88 | 13 | 18 | 33 |
| [33] | CREDO | 2006 | 272 | A vs A+C | CAD | 12 | 67 | 66 | 76 | 74 | 30 | 100 | 32 | ||
| [44] | ePAD | 2018 | 203 | A+C vs A+E | PVI | 2.7 | 67 | 29 | 83 | 35 | 86 | 40 | |||
| [35] | EUCLID | 2017 | 13,885 | C vs TG2 | PAD | 30 | 66 | 72 | 78 | 76 | 31 | 78 | 29 | 12 | 39 |
| [45] | Gresele | 2000 | 159 | A vs A+CC | PAD | 6 | 66 | 86 | 45 | 64 | 84 | 25 | |||
| [46] | Johnson | 2002 | 831 | A vs A+VKA1 | PVI | 38 | 64 | 88 | 25 | 17 | 36 | ||||
| [47] | Li | 2013 | 50 | C vs C+VKA1 | PVI | 12 | 74 | 66 | 70 | 24 | 51 | 59 | 20 | 42 | |
| [48] | MIRROR | 2011 | 80 | A vs A+C | PVI | 6 | 70 | 53 | 78 | 63 | 40 | 33 | 19 | 38 | |
| [49] | Monaco | 2012 | 318 | A+C vs C+VKA1 | PVI | 77 | 67 | 70 | 81 | 24 | 61 | 48 | |||
| [50] | PEGASUS TIMI 54 | 2016 | 1143 | A vs A+TG1 vs A+TG2 | CAD | 36 | 66 | 78 | 85 | 81 | 30 | 100 | 3 | 42 | |
| [51] | PLATO | 2015 | 1144 | A+C vs A+TG2 | CAD | 9 | 66 | 75 | 79 | 66 | 38 | 74 | 100 | 15 | 38 |
| [52] | RIVAL-PAD | 2020 | 20 | A+C vs A+R1 | PVI | 3 | 67 | 60 | 0 | 0 | |||||
| [53] | Soga | 2009 | 80 | A+TP1 vs A+TP1+CI | PVI | 24 | 71 | 83 | 49 | 33 | 39 | 54 | 9 | [36 | |
| [54] | STOP-IC | 2013 | 200 | A vs A+CI | PVI | 12 | 73 | 59 | 81 | 47 | 461 | 39 | 56 | ||
| [17] | VOYAGER-PAD | 2020 | 6564 | A vs A+R1 | PVI | 28 | 67 | 74 | 81 | 60 | 35 | 32 | 40 | ||
| [55] | WAVE | 2007 | 2161 | A vs A+ VKA1 | PAD | 35 | 64 | 74 | 58 | 29 | 39 | 47 | 16 | 27 |
CAD coronary artery disease, CSM current smoker, CVD cerebrovascular disease, DM diabetes mellitus, HL hyperlipidaemia, HSM history of smoking, HT hypertension, INR international normalized ratio
aAntithrombotic regimen: A acetylsalicylic acid 75–325 mg daily, C clopidogrel 75 mg once daily, CC cloricromene 100 mg twice daily, CI cilostazol 200 mg once daily, E edoxaban 60 mg once daily, P placebo only, R1 rivaroxaban 2.5 mg twice daily, R2 rivaroxaban 5 mg twice daily, TG1 ticagrelor 60 mg twice daily, TG2 ticagrelor 90 mg twice daily, TP1 ticlopidine 200 mg twice daily, TP2 ticlopidine 250 mg twice daily, VKA1 vitamin K antagonist with target INR between 1.4 and 3, VKA2 vitamin K antagonist with target INR between 3 and 4.5
bPopulation: CAD studies on patients with coronary artery disease who coincide with peripheral arterial disease, PAD studies on patients who are solely selected for peripheral arterial disease, PVI studies on patients who underwent a peripheral vascular intervention for peripheral arterial disease
Table 2.
Definitions of outcome measurements
| References | Study | MACEa | MBb | MALEc |
|---|---|---|---|---|
| [36] | Becquemin | 1, 3, 4 | – | – |
| [15] | BOA | 2, 3, 4, 6 | 1, 2, 3 | – |
| [37] | CABBAGE | 1, 3, 4 | 1, 2, 3, 4 | 1, 2, 3 |
| [13] | CAPRIE | 2, 3, 4 | – | – |
| [38] | CASPAR | – | GUSTO | 1, 3, 4 |
| [39] | CHARISMA | 1, 3, 4 | GUSTO | – |
| [40] | CLIPS | 1, 3, 4 | – | – |
| [16, 41] | COMPASS | 2, 3, 4 | Modified ISTH | 1, 3, 5 |
| [42] | COOPER | 2, 3, 4 | – | – |
| [43] | CREDO | 1, 3, 4 | – | – |
| [44] | ePAD | 2, 3, 4 | TIMI | – |
| [35] | EUCLID | 2, 3, 5 | TIMI | 1, 5 |
| [45] | Gresele | 2, 3, 4 | – | – |
| [46] | Johnson | – | 1, 2, 3, 5, 6 | – |
| [47] | Li | 1, 3, 4 | 1, 2, 5, 6, 7, 8 | – |
| [48] | MIRROR | NS | – | – |
| [49] | Monaco | 2, 3, 4, 7 | 1, 2, 3, 6 | – |
| [50] | PEGASUS TIMI 54 | 2, 3, 4 | TIMI | 1, 5 |
| [51] | PLATO | 2, 3, 4 | TIMI | – |
| [52] | RIVAL-PAD | – | TIMI | – |
| [53] | Soga | 1, 3, 4 | 5, 6, 9 | – |
| [54] | STOP-IC | 1, 3, 4 | – | 3, 4, 5, 6 |
| [17] | VOYAGER-PAD | 1, 3, 4, 6, 8 | ISTH | 1, 3, 5 |
| [55] | WAVE | 2, 3, 4 | 1, 2, 5, 6, 10 | – |
GUSTO Global Use of Strategies to Open Occluded Arteries,ISTH International Society on Thrombosis and Haemostasis, MACE major adverse cardiovascular events, MALE major adverse limb events, MB major bleeding, NS not specified, TIMI Thrombolysis In Myocardial Infarction,
aMACE: 1 = death; 2 = cardiovascular death; 3 = myocardial infarction; 4 = stroke; 5 = ischaemic stroke; 6 = amputation; 7 = urgent revascularization; 8 = acute limb event
bMB: 1 = fatal bleeding; 2 = intracranial haemorrhage; 3 = bleeding requiring hospitalization; 4 = gastro-intestinal haemorrhage; 5 = bleeding requiring intervention; 6 = bleeding requiring blood product transfusion; 7 = hematoma with diameter > 5 cm; 8 = haemoglobin reduction of > 4 g/dL; 9 = hypotension requiring inotropic support; 10 = intraocular haemorrhage. GUSTO = major bleeding defined as intracranial haemorrhage and/or haemodynamic compromise. ISTH = major bleeding including (1) fatal bleeding, (2) symptomatic bleeding into a critical organ, or (3) bleeding causing a fall in haemoglobin level of 2 g/dL (1.24 mmol/L) or more, or leading to transfusion of two or more units of whole blood or red cells. Modified ISTH = major bleeding including (1) symptomatic bleeding into a critical organ, (2) surgical site bleeding requiring reoperation, or (3) any bleeding requiring hospitalization (including presentation to an acute care facility without an overnight stay). TIMI = major bleeding including (1) any intracranial bleeding, (2) clinically overt signs of haemorrhage associated with a drop in haemoglobin of ≥ 5 g/dL or a ≥ 15% absolute decrease in haematocrit, and (3) fatal bleeding
cMALE: 1 = peripheral revascularization; 2 = any revascularization; 3 = major amputation; 4 =re-occlusion/revascularization of target lesion after intervention; 5 = acute limb event; 6 = death
Risk of Bias Assessment
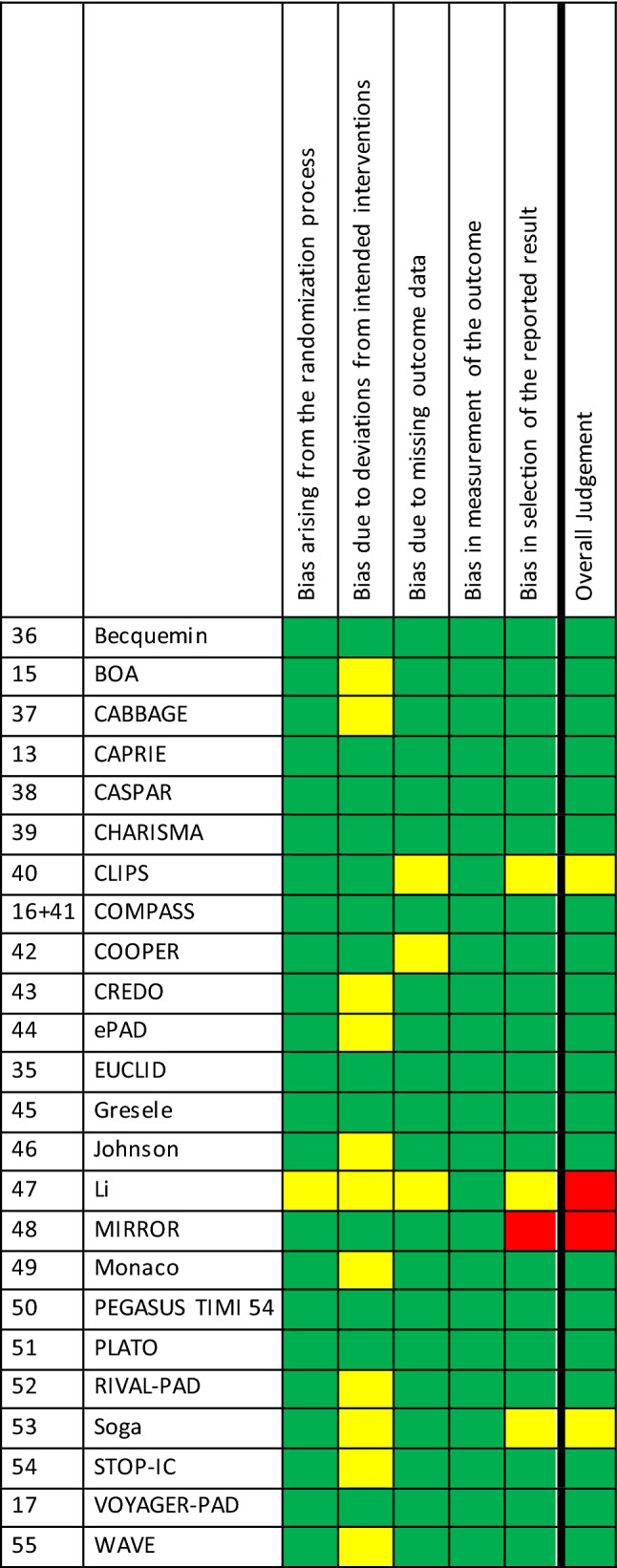
Some concerns about the risk of bias were noted for multiple studies in the following domains: randomization process (n = 1), deviation from intended interventions (n = 11), missing outcome data (n = 3), and selection of the reported result (n = 3). A high risk of bias was found once, in the domain ‘selection of the reported results’. Eventually, of the 24 included studies, most were classified as low risk for bias (n = 20, 83.3%), two raised some concerns (8.3%), and two were classified as high risk of bias (8.3%). The risk of bias is presented in Table 3.
Table 3.
Quality assessment
Visual inspection of the comparison-adjusted funnel plots indicated no clear indication of publication bias; however, to draw definite conclusions, the number of studies appears relatively low.
Certainty of Evidence Assessment
Certainty of evidence contributing to network estimates as assessed by the GRADE framework was rated for each separate comparison of the four outcome measurements. Rates varied between high quality of evidence, moderate quality of evidence, low quality of evidence, and very low quality of evidence. The results of all certainty of evidence assessments are displayed in Supplementary tables 1–4 (see the Electronic Supplementary Material). The certainty of evidence of the main comparisons are discussed per outcome.
Clinical Outcome
The number of events of the primary cardiovascular effectiveness outcome, the primary safety outcome, and secondary outcomes per study are presented in Table 4. Figure 3A shows the results of the network meta-analysis.
Table 4.
Number of events per study, sorted by study population
| References | Study | Antithrombotic regimena | Sample size | MACE | MB | MALE | ALI | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| T1 | T2 | T3 | N1 | N2 | N3 | N1 | N2 | N3 | N1 | N2 | N3 | N1 | N2 | N3 | N1 | N2 | N3 | ||
| Studies on patients who are solely selected for PAD | |||||||||||||||||||
| [13] | CAPRIE | A | C | 3229 | 3223 | 277 | 215 | ||||||||||||
| [39] | CHARISMA | A | A+C | 1551 | 1545 | 138 | 117 | 27 | 26 | ||||||||||
| [40] | CLIPS | A | P | 185 | 181 | 9 | 19 | ||||||||||||
| [16, 41] | COMPASS | A | A+R1 | R2 | 2504 | 2492 | 2474 | 174 | 126 | 149 | 42 | 68 | 66 | 56 | 32 | 40 | 34 | 19 | 19 |
| [42] | COOPER | C | TP1 | 215 | 216 | 0 | 2 | ||||||||||||
| [35] | EUCLID | C | TG2 | 6955 | 6930 | 740 | 751 | 109 | 113 | 115 | 117 | ||||||||
| [45] | Gresele | A | A+CC | 73 | 74 | 0 | 0 | ||||||||||||
| [55] | WAVE | A | A+ VKA1 | 1081 | 1080 | 144 | 132 | 24 | 74 | 44 | 42 | ||||||||
| Cumulative incidence of events with universal comparator A, n (%) | 742/8623 (8.6%) | 93/5136 (1.8%) | 56/2504 (1.6%) | 78/3583 (2.2%) | |||||||||||||||
| Studies on patients who underwent a peripheral vascular intervention for PAD | |||||||||||||||||||
| [36] | Becquemin | P | TP2 | 121 | 122 | 31 | 28 | 17 | 16 | ||||||||||
| [15] | BOA | A | VKA2 | 1324 | 1326 | 275 | 248 | 56 | 108 | ||||||||||
| [37] | CABBAGE | A | A+CI | 25 | 25 | 2 | 1 | 0 | 1 | 2 | 3 | ||||||||
| [38] | CASPAR | A | A+C | 426 | 425 | 5 | 9 | 151 | 149 | ||||||||||
| [44] | ePAD | A+C | A+E | 101 | 100 | 1 | 3 | 2 | 0 | ||||||||||
| [46] | Johnson | A | A+VKA1 | 413 | 418 | 15 | 35 | ||||||||||||
| [47] | Li | C | C+VKA1 | 25 | 25 | 2 | 1 | 0 | 1 | ||||||||||
| [48] | MIRROR | A | A+C | 40 | 40 | 15 | 12 | ||||||||||||
| [49] | Monaco | A+C | C+VKA1 | 157 | 161 | 5 | 7 | 13 | 15 | ||||||||||
| [52] | RIVAL-PAD | A+C | A+R1 | 11 | 9 | 0 | 0 | ||||||||||||
| [53] | Soga | A+TP1 | A+TP1+CI | 39 | 39 | 3 | 1 | 0 | 0 | ||||||||||
| [54] | STOP-IC | A | A+CI | 98 | 93 | 9 | 11 | 28 | 16 | ||||||||||
| [17] | VOYAGER-PAD | A | A+R1 | 3278 | 3286 | 588 | 514 | 100 | 140 | 770 | 687 | 227 | 155 | ||||||
| Cumulative incidence of events with universal comparator A, n (%) | 889/4765 (18.7%) | 676/5466 (12.4%) | 951/3827 (24.8%) | 277/3287 (8.4%) | |||||||||||||||
| Studies on patients with coronary artery disease who coincide with PAD | |||||||||||||||||||
| [43] | CREDO | A | A+C | 140 | 132 | 24 | 12 | ||||||||||||
| [50] | PEGASUS TIMI 54 | A | A+TG1 | A+TG2 | 404 | 368 | 371 | 71 | 47 | 54 | 4 | 4 | 5 | 26 | 23 | 17 | 4 | 2 | 3 |
| [51] | PLATO | A+C | A+TG2 | 578 | 566 | 112 | 93 | 46 | 58 | ||||||||||
| Cumulative incidence of events with universal comparator A, n (%) | 95/544 (17.5%) | 4/404 (0.9%) | 26/404 (6.4%) | 4/404 (0.9%) | |||||||||||||||
ALI acute limb event, INR international normalized ratio, MACE major adverse cardiovascular events, MALE major adverse limb events, MB major bleeding, Nx number of patients in group x PAD peripheral arterial disease, Tx treatment in group x
aAntithrombotic regimen: A acetylsalicylic acid 75–325 mg daily, C clopidogrel 75 mg once daily, CC cloricromene 100 mg twice daily, CI cilostazol 200 mg once daily, E edoxaban 60 mg once daily, P placebo only, R1 rivaroxaban 2.5 mg twice daily, R2 rivaroxaban 5 mg twice daily, TG1 ticagrelor 60 mg twice daily, TG2 ticagrelor 90 mg twice daily, TP1 ticlopidine 200 mg twice daily, TP2 ticlopidine 250 mg twice daily, VKA1 vitamin K antagonist with target INR between 1.4 and 3, VKA2 vitamin K antagonist with target INR between 3 and 4.5
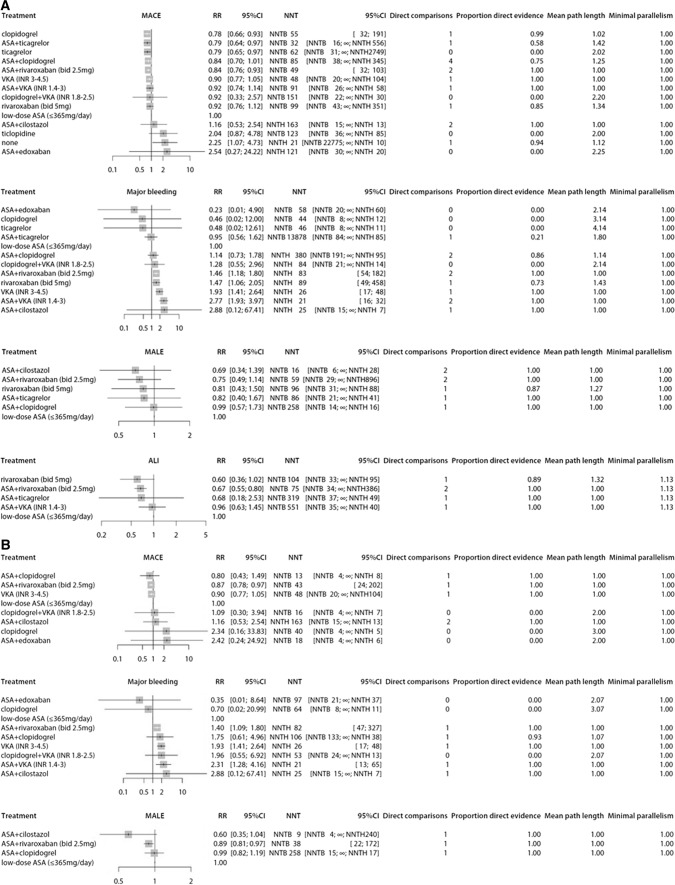
Fig. 3.
Forest plots presenting estimated relative risks (RRs) and corresponding 95% confidence intervals (CIs). Results are presented for different antithrombotic strategies compared to low-dose acetylsalicylic acid (ASA). Direct comparisons are the number of studies that directly compared the treatment option to the universal comparator. Hereafter, the proportion of direct evidence is shown. The mean pathlength characterizes the degree of indirectness of an estimate. Minimal parallelism presents the minimum number of independent paths contributing to the effect estimate [22]. A All patients. B Patients who underwent a peripheral vascular intervention for peripheral arterial disease. For the patients who underwent a peripheral vascular intervention, no network meta-analysis could be performed for acute limb events, since studies had no overlapping antithrombotic regimens. ALI acute limb event, bid bi-daily, INR international normalized ratio, MACE major adverse cardiovascular events, MALE major adverse limb events, NNT number needed to treat, NNTB number needed to treat for an additional beneficial outcome, NNTH number needed to treat for an additional harmful outcome, VKA vitamin K antagonist, ∞ need to treat an infinite number of people to cause or avoid an event (i.e. no effect)
Major Adverse Cardiovascular Events
Twenty-one studies with 46,961 patients reported MACE. One study [53] with 78 patients is not part of the network graph, since its treatment regimens did not connect to other studies. The certainty of evidence was incorporated in Supplementary table 1 (see the Electronic Supplementary Material). Compared to ASA, clopidogrel (RR 0.78, 95% CI 0.66–0.93; p score 0.82), ticagrelor (RR 0.79, 95% CI 0.65–0.97; p score 0.77), ASA plus ticagrelor (RR 0.79, 95% CI 0.64–0.97; p score 0.79), and ASA plus low-dose rivaroxaban (RR 0.84, 95% CI 0.76–0.93; p score 0.67) were more effective in reducing MACE, according to GRADE, with moderate certainty of evidence for the comparison with ticagrelor monotherapy and high certainty of evidence for the other three regimens. None of these four antithrombotic regimens were superior to one another (Supplementary table 1). Only placebo significantly increased the risk of developing MACE (RR 2.25, 95% CI 1.07–4.73; p score 0.09) (Fig. 3A).
In the network meta-analysis, no evidence of heterogeneity was found (τ2 = 0 and I2 = 0%; 95% CI 0–64.8). There was no measurable global inconsistency based on a random effects design-by-treatment model (χ24 = 1.43; p = 0.84) or local inconsistency within the network.
Major Bleeding
Sixteen studies with 39,388 patients reported major bleeding. One study [53] with 78 patients was not part of the network graph. The certainty of evidence was incorporated in Supplementary table 2 (see the Electronic Supplementary Material). High-intensity VKA (RR 1.93, 95% CI 1.41–2.64; p score 0.22), rivaroxaban 5 mg twice daily (RR 1.47, 95% CI 1.06–2.05; p score 0.39), ASA plus low-intensity VKA (RR 2.77, 95% CI 1.93–3.97; p score 0.08), and ASA plus low-dose rivaroxaban (RR 1.46, 95% CI 1.18–1.80; p score 0.40) all significantly increased the risk of major bleeding compared to ASA monotherapy, with high certainty of evidence according to GRADE. No antithrombotic regimen or placebo reduced the risk of major bleeding (Fig. 3A).
No heterogeneity was observed (τ2 = 0 and I2 = 0%; 95% CI 0–74.6), and there was no measurable global inconsistency based on a random effects design-by-treatment model (χ23 = 0.60; p = 0.90) or local inconsistency within the network.
Major Adverse Limb Events
Seven studies with 29,015 patients reported MALE. A network graph could be built of six studies [16, 17, 37, 38, 50, 54] with 15,130 patients. The EUCLID trial [35] was left out since its treatment options did not connect to other studies. The certainty of evidence was incorporated in Supplementary table 3 (see the Electronic Supplementary Material). ASA plus clopidogrel (RR 0.99, 95% CI 0.57–1.73, moderate certainty of evidence), ASA plus ticagrelor (RR 0.82, 95% CI 0.40–1.67, low certainty of evidence), rivaroxaban 5 mg twice daily (RR 0.81, 95% CI 0.43–1.50, moderate certainty of evidence), ASA plus low-dose rivaroxaban (RR 0.75, 95% CI 0.49–1.14, high certainty of evidence) and ASA plus cilostazol (RR 0.69, 95% CI 0.34–1.39, moderate certainty of evidence) were compared to ASA, but none was superior in preventing MALE (Fig. 3A).
Some heterogeneity was observed (τ2 = 0.07 and I2 = 60%; 95% CI 0–88.7). There was measurable inconsistency based on a random effects design-by-treatment model (χ21 = 4.04; p = 0.04).
Acute Limb Ischaemia
Six studies with 31,406 patients reported ALI. A network graph could be built of four studies [16, 17, 50, 55] with 17,278 patients. The certainty of evidence was incorporated in Supplementary table 4 (see the Electronic Supplementary Material). ASA plus low-dose rivaroxaban significantly reduced the occurrence of ALI, compared to ASA monotherapy (RR 0.67, 95% CI 0.55–0.80), with high certainty of evidence according to GRADE. No benefit was established for ASA plus ticagrelor, rivaroxaban 5 mg twice daily, or ASA plus low-intensity VKA (Fig. 3A).
No heterogeneity was observed (τ2 = 0 and I2 = 0%; 95% CI not estimable). There was no measurable global inconsistency based on a random effects design-by-treatment model (χ21 = 0.41; p = 0.52) or local inconsistency within the network.
Sensitivity Analysis
Exclusion of the high risk of bias studies [47, 48] showed our results to be robust, with comparable RRs and CIs for MACE, MALE, and ALI. For major bleeding, besides a high risk of bias study, an additional study [35] was excluded from the network graph because of loss of connection to other studies. Therefore, clopidogrel monotherapy and ticagrelor monotherapy could no longer be compared to ASA for major bleeding. However, all other antithrombotic regimens demonstrated comparable RRs and CIs as in the primary analysis.
Subgroup Analysis
There were 13 RCTs in which patients were selected for undergoing a peripheral vascular intervention (endovascular or surgical). Table 4 provides an overview of the number of events, sorted per population, including the cumulative incidence of events of all patients taking the universal comparator ASA. MACE, major bleeding, MALE, and ALI were all more common in patients who underwent a peripheral intervention for PAD, compared to patients who were solely selected for PAD (18.7% vs 8.6%, 12.4% vs 1.8%, 24.8% vs 1.6%, and 8.4% vs 2.2%, respectively). Figure 3B demonstrates the results of the network meta-analysis of patients who underwent a peripheral vascular intervention for PAD. The duration of antithrombotic treatment and follow-up of all studies was at least 3 months, starting at the day of intervention. Evidence regarding antithrombotic treatment strategies within 3 months after peripheral intervention was lacking.
In patients, ≥ 3 months after a peripheral vascular intervention, MACE was reported for ASA, clopidogrel, ASA plus clopidogrel, high-intensity VKA, ASA plus low-dose rivaroxaban, ASA plus edoxaban, ASA plus cilostazol, and clopidogrel plus low-intensity VKA in eight studies with 10,073 patients. Only ASA plus low-dose rivaroxaban significantly reduced the risk of MACE (RR 0.87, 95% CI 0.78–0.97) compared to ASA. Major bleeding was reported for ASA, clopidogrel, ASA plus clopidogrel, high-intensity VKA, ASA plus low-intensity VKA, ASA plus low-dose rivaroxaban, ASA plus edoxaban, ASA plus cilostazol, and clopidogrel plus low-intensity VKA in nine studies with 11,503 patients. High-intensity VKA (RR 1.93, 95% CI 1.41–2.64), ASA plus low-intensity VKA (RR 2.31, 95% CI 1.28–4.16), and ASA plus low-dose rivaroxaban (RR 1.4, 95% CI 1.09–1.80) increased the risk of major bleeding. MALE was reported for ASA, ASA plus clopidogrel, ASA plus low-dose rivaroxaban, and ASA plus cilostazol in four studies with 7596 patients. ASA plus low-dose rivaroxaban significantly reduced MALE (RR 0.89, 95% CI 0.81–0.97). No network meta-analysis could be performed for ALI, since studies describing ASA had no overlapping antithrombotic regimens.
Discussion
In this systematic review and network meta-analysis, we evaluated the effectiveness and safety of different antithrombotic regimens compared to ASA in symptomatic PAD patients. In the overall network meta-analysis, clopidogrel, ticagrelor, ASA plus ticagrelor, and ASA plus low-dose rivaroxaban, were all more effective than ASA monotherapy, and equally effective to one another in preventing MACE. ASA plus low-dose rivaroxaban also reduced the risk of ALI, but increased the risk of major bleeding. Regarding the efficacy of clopidogrel in reducing MALE and ALI, evidence is lacking, while limited evidence indicates similar safety regarding bleeding complications of clopidogrel compared to ASA.
According to international guidelines, clopidogrel monotherapy is advised in symptomatic PAD, and ASA is an alternative [1, 11]. The slight preference of clopidogrel over ASA, as mentioned by the European guidelines [1], is based on the results from the CAPRIE study [13]. CAPRIE was an RCT assessing the relative efficacy and safety of clopidogrel versus ASA in (stratified) subgroups of patients with atherosclerotic vascular disease (i.e. ischaemic stroke, recent myocardial infarction, or symptomatic PAD). The primary outcome (i.e. a composite of ischaemic stroke, myocardial infarction, and cardiovascular death) was significantly reduced with clopidogrel compared to ASA. The CAPRIE study reported no major differences in bleeding risk; however, safety profiles were not separately analysed for the different subgroups, while we could not retrieve bleeding data of PAD patients from the investigators. In our network meta-analysis, the comparison between ASA and clopidogrel regarding bleedings is based on indirect evidence with a relatively high uncertainty, but demonstrated no increased bleeding risk. Altogether, it is plausible to assume that clopidogrel does not increase the risk of bleeding as compared to ASA.
The use of alternative P2Y12 inhibitors, such as ticagrelor, in PAD patients is not approved by international authorities (i.e. the European Medicines Agency and the United States Food and Drug Administration) and has therefore currently no place in the treatment of PAD. The network meta-analysis demonstrated superiority of ticagrelor over ASA, with similar effectiveness as compared to clopidogrel. This is in line with results from the EUCLID trial [33]. However, since clopidogrel is a prodrug that needs to be converted by the CYP2C19 enzyme, its effectiveness is related to CYP2C19 polymorphisms. The EUCLID trial did not compensate for the presence of CYP2C19 loss-of-function alleles. At least in theory, the effectiveness of clopidogrel could be further improved by CYP2C19 polymorphism-guided prescription, suggesting that clopidogrel might be the more potent antithrombotic regimen. Results of the ongoing GENPAD study (https://clinicaltrials.gov/ct2/show/NCT04619927) will clarify the role of CYP2C19 polymorphism in symptomatic PAD patients treated with clopidogrel monotherapy.
The DAPT ASA plus clopidogrel and ASA plus ticagrelor were also studied in this network meta-analysis. The combination of ASA plus clopidogrel has been studied in several RCTs concerning PAD patients, of which the CHARISMA trial [39] was the largest. In line with the CHARISMA trial, our network meta-analysis found no significant improvement in MACE and no increased rates of major bleeding compared to ASA. In contrast, ASA plus ticagrelor did result in fewer MACE compared to ASA monotherapy. However, this combination has only been studied in populations of PAD patients with manifest concomitant coronary artery disease [50, 51], which might influence the extrapolation of these results to the overall PAD population.
The use of VKA monotherapy has only been studied in patients that underwent infra-inguinal bypass grafting [15], but not in the overall population of symptomatic PAD. Antiplatelet therapy plus VKA, however, was studied in the overall population of symptomatic PAD. Similar to our results of the network meta-analysis, the WAVE trial [55] reported no reduction in MACE, while the bleeding risk increased with ASA plus VKA compared to ASA monotherapy.
The recent COMPASS [16] and VOYAGER-PAD [17] studies demonstrated that the use of ASA plus low-dose rivaroxaban was associated with a reduction of MACE and MALE, but an increased risk of major bleeding, compared to ASA monotherapy. Altogether, a net clinical benefit of ASA plus low-dose rivaroxaban was established over ASA monotherapy [16, 57]. Trials directly comparing ASA plus low-dose rivaroxaban to clopidogrel monotherapy have not been performed. By use of network meta-analysis, we could indirectly compare clopidogrel to ASA plus low-dose rivaroxaban in their effectiveness to reduce MACE (RR 0.93, 95% CI 0.76–1.13) (Supplementary table 1; see the Electronic Supplementary Material), and found no significant difference. This is in line with a recent concise network meta-analysis [58].
In the subgroup analysis of patients who underwent a peripheral vascular intervention, only ASA plus low-dose rivaroxaban was superior to ASA monotherapy for the reduction of MACE and MALE. This benefit, however, coincided with an increased risk of major bleeding. Quality trial evidence on clopidogrel or ticagrelor monotherapy was lacking. Patients undergoing a peripheral vascular intervention display a remarkably high risk of MACE and MALE, compared to the overall group of symptomatic PAD. In this subgroup of patients, a higher bleeding risk would be justified to reduce arterial thrombotic events, resulting in a net clinical benefit. Based on the current evidence and the strongly increased thrombotic risk, the use of ASA plus low-dose rivaroxaban could be considered in patients who underwent a peripheral vascular intervention. This is supported by a commentary of Mukherjee, who argues that adding rivaroxaban to ASA could be considered in PAD patients who have had lower extremity revascularization, with reassessment of the patient-specific risk–benefit ratio beyond 1 year [59]. Furthermore, this is in line with the European Society for Vascular Surgery (ESVS) Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia, which advise consideration of low-dose rivaroxaban to reduce adverse cardiovascular events and lower extremity ischaemic events in patients with chronic limb-threatening ischaemia [60].
Remarkably, no benefits were found for DAPT after peripheral revascularization. The use of DAPT after revascularization procedures has been widely studied in the field of cardiology. The combination of ASA with a P2Y12 inhibitor is recommended for 6–12 months after myocardial revascularization to reduce the risk of stent thrombosis [61, 62]. In PAD, DAPT with ASA plus clopidogrel for at least 1 month after peripheral revascularization procedures (i.e. endovascular stent implantation, below-the-knee bypass with a prosthetic graft) is currently recommended in guidelines [1, 11]. In our network meta-analysis, evidence regarding antithrombotic treatment strategies within 3 months after peripheral intervention was lacking. Therefore, we have no information on whether DAPT might improve outcomes in the first months after peripheral revascularization. For long-term secondary prevention, we identified two RCTs comparing the combination of ASA and clopidogrel to ASA monotherapy. The CASPAR trial [38], which solely studied below-the-knee bypass grafting, indicated a benefit of ASA plus clopidogrel over ASA monotherapy in patients receiving prosthetic grafts. The MIRROR trial demonstrated a reduction in target lesion revascularization with ASA plus clopidogrel compared to ASA, in patients following endovascular femoropopliteal revascularization. These trials were not powered for MACE. A high-quality RCT comparing DAPT to clopidogrel monotherapy for secondary prevention in patients undergoing peripheral vascular interventions is needed to address these important gaps in evidence.
The strengths of our network meta-analysis are mainly related to its comprehensive approach, including all RCTs since 1995 comparing different antithrombotic treatments in symptomatic PAD patients. Also, no evidence of statistical heterogeneity and no measurable global or local inconsistency were found for the primary outcomes (MACE and major bleedings). However, this network meta-analysis also has some limitations that should be addressed. First, the definitions of MACE, MALE, and major bleeding differed between the studies. MACE was generally defined as the composite of cardiovascular death, myocardial infarction, and stroke, and MALE as the composite of chronic limb ischaemia or ALI and major amputation. Broader definitions were accepted if they concerned outcomes of interest. For example, MACE including amputation was acceptable, but MACE including pulmonary embolism was not. In some studies, in which multiple composite outcomes were reported, we selected the composite outcome that was closest to the most common definitions of MACE and MALE. We expect that the differences in definitions of MACE, MALE, and major bleeding did not have much impact on the RRs calculated in our network meta-analyses, as the definitions were similar between the control and intervention arms of the respective studies. Second, the follow-up time varied between the studies. Since re-occlusion is more common in the first year after revascularization [63], this could have resulted in a relatively high risk of events in studies on patients who underwent a peripheral vascular intervention. The increased risk, however, applied to both the control group and intervention group, and therefore did not necessarily affect RRs. Third, ASA was the universal comparator in the network meta-analyses, while current guidelines state that clopidogrel may be preferred over ASA as antithrombotic treatment in symptomatic PAD patients. Since the majority of studies used ASA as the comparator, choosing another antithrombotic regimen as the universal comparator would have increased the indirectness of the evidence, resulting in higher uncertainties. Fourth, there is a lack of individual patient data. Individual patient data network meta-analysis would generate more precise estimates; however, individual patient data were not available for most important trials. Fifth, the subgroup analysis of patients after a peripheral vascular intervention included both endovascular and surgical procedures. With the outcomes of interest mainly focusing on secondary prevention, we chose to select a broad group with relatively severe PAD. However, it is possible that the optimal long-term antithrombotic therapy for a patient after endovascular revascularization is not similar to the optimal therapy for a surgically treated patient.
In conclusion, clopidogrel, ticagrelor, ASA plus ticagrelor, and ASA plus low-dose rivaroxaban are superior to ASA monotherapy and equally effective to one another in preventing MACE in PAD patients. Of these four therapies, ticagrelor is not approved by international authorities, and ASA plus low-dose rivaroxaban provides a higher risk of major bleedings. Therefore, clopidogrel may be considered the first choice in symptomatic PAD patients. In PAD patients undergoing a vascular intervention, ASA plus low-dose rivaroxaban could be considered for the long-term (> 3 months) prevention of MACE and MALE, but a higher bleeding risk should be taken into account.
Supplementary Information
Below is the link to the electronic supplementary material.
Declarations
Funding
No external funds were used to support this work.
Competing interests
Loes H. Willems, Dominique P.M.S.M. Maas, Kees Kramers, Michel M.P.J. Reijnen, Niels P. Riksen, Hugo Ten Cate, Rozemarijn J. van der Vijver-Coppen, Gert J. de Borst, Barend M. E. Mees, Clark J. Zeebregts, Gerjon Hannink, and Michiel C. Warlé declare no relevant financial or non-financial interests.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
The datasets generated during and/or analysed during the current study are available from the corresponding author upon reasonable request.
Code availability
Not applicable.
Authors' Contributions
LHW, DPMSMM, KK, MMPJR, GH, and MCW contributed to the concept of the study and the study design. LHW, DPMSMM, GH, and MCW contributed to the data search. LHW, DPMSMM, and MCW contributed to the data selection and data extraction. LHW, DPMSMM, and GH contributed to the quality assessment. GH performed the statistical analysis. All authors contributed to the interpretation of the data. LHW and MCW wrote the first draft of the manuscript. All authors contributed by editing the manuscript. All authors reviewed and approved the final manuscript.
Footnotes
The original article has been updated: Due to author name update.
Change history
9/7/2022
A Correction to this paper has been published: 10.1007/s40265-022-01776-2
References
- 1.Aboyans V, Ricco JB, Bartelink MEL et al. 2017 ESC Guidelines on the Diagnosis and Treatment of Peripheral Arterial Diseases, in collaboration with the European Society for Vascular Surgery (ESVS): Document covering atherosclerotic disease of extracranial carotid and vertebral, mesenteric, renal, upper and lower extremity arteries. Endorsed by: the European Stroke Organization (ESO)The Task Force for the Diagnosis and Treatment of Peripheral Arterial Diseases of the European Society of Cardiology (ESC) and of the European Society for Vascular Surgery (ESVS). Eur Heart J. 2018;39(9):763–816. [DOI] [PubMed]
- 2.Weitz JI, Byrne J, Clagett GP, Farkouh ME, Porter JM, Sackett DL, Strandness DE, Jr, Taylor LM. Diagnosis and treatment of chronic arterial insufficiency of the lower extremities: a critical review. Circulation. 1996;94(11):3026–3049. doi: 10.1161/01.CIR.94.11.3026. [DOI] [PubMed] [Google Scholar]
- 3.Fowkes FGR, Rudan D, Rudan I, et al. Comparison of global estimates of prevalence and risk factors for peripheral artery disease in 2000 and 2010: a systematic review and analysis. Lancet. 2013;382(9901):1329–1340. doi: 10.1016/S0140-6736(13)61249-0. [DOI] [PubMed] [Google Scholar]
- 4.Steg PG, Bhatt DL, Wilson PWF, et al. One-year cardiovascular event rates in outpatients with atherothrombosis. JAMA. 2007;297(11):1197–1206. doi: 10.1001/jama.297.11.1197. [DOI] [PubMed] [Google Scholar]
- 5.Peacock JM, Keo HH, Duval S, et al. The incidence and health economic burden of ischemic amputation in Minnesota, 2005–2008. Prev Chronic Dis. 2011;8(6):A141. [PMC free article] [PubMed] [Google Scholar]
- 6.Versteeg HH, Heemskerk JW, Levi M, Reitsma PH. New fundamentals in hemostasis. Physiol Rev. 2013;93(1):327–358. doi: 10.1152/physrev.00016.2011. [DOI] [PubMed] [Google Scholar]
- 7.Fowkes FG, Murray GD, Butcher I, et al. Ankle brachial index combined with Framingham Risk Score to predict cardiovascular events and mortality: a meta-analysis. JAMA. 2008;300(2):197–208. doi: 10.1001/jama.300.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Criqui MH, Langer RD, Fronek A, et al. Mortality over a period of 10 years in patients with peripheral arterial disease. N Engl J Med. 1992;326(6):381–386. doi: 10.1056/NEJM199202063260605. [DOI] [PubMed] [Google Scholar]
- 9.Mangiafico RA, Mangiafico M. Medical treatment of critical limb ischemia: current state and future directions. Curr Vasc Pharmacol. 2011;9(6):658–676. doi: 10.2174/157016111797484107. [DOI] [PubMed] [Google Scholar]
- 10.Abola MTB, Bhatt DL, Duval S, et al. Fate of individuals with ischemic amputations in the REACH Registry: three-year cardiovascular and limb-related outcomes. Atherosclerosis. 2012;221(2):527–535. doi: 10.1016/j.atherosclerosis.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 11.Gerhard-Herman MD, Gornik HL, Barrett C, et al. 2016 AHA/ACC Guideline on the management of patients with lower extremity peripheral artery disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2017;135(12):e686–e725. doi: 10.1161/CIR.0000000000000470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Antithrombotic Trialists’ Collaboration Collaborative meta-analysis of randomized trials of antiplatelet therapy for prevention of death, myocardial infarction, and stroke in high risk patients. BMJ. 2002;324(7329):71–86. doi: 10.1136/bmj.324.7329.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.CAPRIE Steering Committee A randomised, blinded, trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE) Lancet. 1996;348(9038):1329–1339. doi: 10.1016/S0140-6736(96)09457-3. [DOI] [PubMed] [Google Scholar]
- 14.Saha SP, Whayne TF, Jr, Mukherjee D. Current evidence for antithrombotic therapy after peripheral vascular interventions. Curr Vasc Pharmacol. 2013;11(4):507–513. doi: 10.2174/1570161111311040014. [DOI] [PubMed] [Google Scholar]
- 15.Dutch Bypass Oral anticoagulants or Aspirin (BOA) Study Group. Efficacy of oral anticoagulants compared with aspirin after infrainguinal bypass surgery (The Dutch Bypass Oral anticoagulants or Aspirin study): a randomised trial. Lancet. 2000;355(9201):346–351. [PubMed]
- 16.Anand SS; COMPASS Investigators Rivaroxaban with or without aspirin in patients with stable peripheral or carotid artery disease: an international, randomized, double-blind, placebo-controlled trial. Lancet. 2018;391(10117):219–229. doi: 10.1016/S0140-6736(17)32409-1. [DOI] [PubMed] [Google Scholar]
- 17.Bonaca MP, Bauersachs RM, Anand SS, et al. Rivaroxaban in peripheral artery disease after revascularization. N Engl J Med. 2020;382(21):1994–2004. doi: 10.1056/NEJMoa2000052. [DOI] [PubMed] [Google Scholar]
- 18.Li T, Puhan MA, Vedula SS, Singh S, Dickersin K. Network meta-analysis-highly attractive but more methodological research is needed. BMC Med. 2011;27(9):79. doi: 10.1186/1741-7015-9-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.EUROASPIRE Study Group. A European Society of Cardiology survey of secondary prevention of coronary heart disease: principal results. EUROASPIRE Study Group. European Action on Secondary Prevention through Intervention To Reduce Events. Eur Heart J 1997;18(10):1569–1582. [DOI] [PubMed]
- 20.Higgins JPT, Altman DG, Gøtzsche PC, et al. The Cochrane Collaboration's tool for assessing risk of bias in randomised trials. BMJ. 2011;18(343):d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brignardello-Petersen R, Bonner A, Alexander PE, et al, GRADE Working Group. Advances in the GRADE approach to rate the certainty in estimates from a network meta-analysis. J Clin Epidemiol. 2018;93:36–44 [DOI] [PubMed]
- 22.Cipriani A, Furukawa TA, Salanti G, et al. Comparative efficacy and acceptability of 21 antidepressant drugs for the acute treatment of adults with major depressive disorder: a systematic review and network meta-analysis. Lancet. 2018;391(10128):1357–1366. doi: 10.1016/S0140-6736(17)32802-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rücker G, Schwarzer G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med Res Methodol. 2015;31(15):58. doi: 10.1186/s12874-015-0060-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eusebi LH, Black CJ, Howden CW, Ford AC. Effectiveness of management strategies for uninvestigated dyspepsia: systematic review and network meta-analysis. BMJ. 2019;11(367):l6483. doi: 10.1136/bmj.l6483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jackson D, Barrett JK, Rice S, White IR, Higgins JPT. A design-by-treatment interaction model for network meta-analysis with random inconsistency effects. Stat Med. 2014;33(21):3639–3654. doi: 10.1002/sim.6188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Higgins JP, Jackson D, Barrett JK, Lu G, Ades AE, White IR. Consistency and inconsistency in network meta- analysis: concepts and models for multi-arm studies. Res Synth Methods. 2012;3(2):98–110. doi: 10.1002/jrsm.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dias S, Welton NJ, Caldwell DM, Ades AE. Checking consistency in mixed treatment comparison meta- analysis. Stat Med. 2010;29(7–8):932–944. doi: 10.1002/sim.3767. [DOI] [PubMed] [Google Scholar]
- 28.Lu G, Ades A. Assessing evidence inconsistency in mixed treatment comparisons. J Am Stat Assoc. 2006;101(474):447–459. doi: 10.1198/016214505000001302. [DOI] [Google Scholar]
- 29.Altman DG. Confidence intervals for the number needed to treat. BMJ. 1998;317(7168):1309–1312. doi: 10.1136/bmj.317.7168.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Veroniki AA, Bender R, Glasziou P, Straus SE, Tricco AC. The number needed to treat in pairwise and network meta-analysis and its graphical representation. J Clin Epidemiol. 2019;111:11–22. doi: 10.1016/j.jclinepi.2019.03.007. [DOI] [PubMed] [Google Scholar]
- 31.König J, Krahn U, Binder H. Visualizing the flow of evidence in network meta-analysis and characterizing mixed treatment comparisons. Stat Med. 2013;32(30):5414–5429. doi: 10.1002/sim.6001. [DOI] [PubMed] [Google Scholar]
- 32.Salanti G, DelGiovane C, Chaimani A, Caldwell DM, HigginsJP. Evaluating the quality of evidence from a network meta-analysis. PLoS One. 2014;9(7):e99682. [DOI] [PMC free article] [PubMed]
- 33.Sterne JA, Sutton AJ, Ioannidis JP, Terrin N, Jones DR, Lau J, Carpenter J, Rücker G, Harbord RM, Schmid CH, Tetzlaff J, Deeks JJ, Peters J, Macaskill P, Schwarzer G, Duval S, Altman DG, Moher D, Higgins JP. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;22(343):d4002. doi: 10.1136/bmj.d4002. [DOI] [PubMed] [Google Scholar]
- 34.Rücker G, Krahn U, König J, Efthimiou O, Papakonstantinou T, Schwarzer G (2021) netmeta: network meta-analysis using frequentist methods. R package version 1.5-0. Accessed 30 Oct 2021. https://CRAN.R-project.org/package=netmeta.
- 35.Hiatt WR, Fowkes FGR, Heizer G, et al. Ticagrelor versus clopidogrel in symptomatic peripheral artery disease. N Engl J Med. 2017;376(1):32–40. doi: 10.1056/NEJMoa1611688. [DOI] [PubMed] [Google Scholar]
- 36.Becquemin J, Castaigne A, Fiessinger J, et al. Effect of ticlopidine on the long-term patency of saphenous-vein bypass grafts in the legs. N Engl J Med. 1997;337(24):1726–1731. doi: 10.1056/NEJM199712113372404. [DOI] [PubMed] [Google Scholar]
- 37.Soga Y, Takahara M, Iida O, et al. Efficacy of CilostAzol for below-the-knee artery disease after balloon AnGioplasty in PatiEnts with Severe Limb Ischemia (CABBAGE Trial) Ann Vasc Surg. 2017;45:22–28. doi: 10.1016/j.avsg.2017.05.029. [DOI] [PubMed] [Google Scholar]
- 38.Belch JJF, Dormandy J, CASPAR Writing Committee et al. Results of the randomized, placebo-controlled clopidogrel and acetylsalicylic acid in bypass surgery for peripheral arterial disease (CASPAR) trial. J Vasc Surg. 2010;52(4):825–833. [DOI] [PubMed]
- 39.Cacoub PP, Bhatt DL, Steg PG, et al. Patients with peripheral arterial disease in the CHARISMA trial. Eur Heart J. 2009;30(2):192–201. doi: 10.1093/eurheartj/ehn534. [DOI] [PubMed] [Google Scholar]
- 40.Critical Leg Ischaemia Prevention Study (CLIPS) Group; M Catalano, G Born, R Peto. Prevention of serious vascular events by aspirin amongst patients with peripheral arterial disease: randomized, double-blind trial. J Intern Med. 2007;261(3):276–284. [DOI] [PubMed]
- 41.Anand SS, Caron F, Eikelboom JW, et al. Major adverse limb events and mortality in patients with peripheral artery disease. J Am Coll Cardiol. 2018;71(20):2306–2315. doi: 10.1016/j.jacc.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 42.Shigematsu H, Komori K, Tanemoto K, et al. Clopidogrel for atherothrombotic event management in patients with peripheral arterial disease (COOPER) study: safety and efficacy of clopidogrel versus ticlopidine inmJapanese patients. Ann Vasc Dis. 2012;5(3):364–375. doi: 10.3400/avd.oa.12.00039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mukherjee D, Topol EJ, Moliterno DJ, et al. Extracardiac vascular disease and effectiveness of sustained clopidogrel treatment. Heart. 2006;92(1):49–51. doi: 10.1136/hrt.2005.064501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Moll F, Baumgartner I, Jaff M, et al. Edoxaban plus aspirin vs dual antiplatelet therapy in endovascular treatment of patients with peripheral artery disease: results of the ePAD trial. J Endovasc Ther. 2018;25(2):158–168. doi: 10.1177/1526602818760488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gresele P, Migliacci R, Di Sante G et al. Effect of cloricromene on intermittent claudication. A randomized, double-blind, placebo-controlled trial in patients treated with aspirin: effect on claudication distance and quality of life. CRAMPS Investigator Group. Cloricromene Randomized Arteriopathy Multicenter Prospective Study. Vasc Med. 2000;5(2):83–89. [DOI] [PubMed]
- 46.Johnson WC, Williford WO, Department of Veterans Affairs Cooperative Study #362. Benefits, morbidity, and mortality associated with long-term administration of oral anticoagulant therapy to patients with peripheral arterial bypass procedures: a prospective randomized study. J Vasc Surg. 2002;35(3):413–421. [DOI] [PubMed]
- 47.Li H, Zhang F, Liang G, et al. A prospective randomized controlled clinical trial on clopidogrel combined with warfarin versus clopidogrel alone in the prevention of restenosis after endovascular treatment of the femoropopliteal artery. Ann Vasc Surg. 2013;27(5):627–633. doi: 10.1016/j.avsg.2012.07.011. [DOI] [PubMed] [Google Scholar]
- 48.Tepe G, Bantleon R, Brechtel K, et al. Management of peripheral arterial interventions with mono or dual antiplatelet therapy—the MIRROR study: a randomised and double-blinded clinical trial. Eur Radiol. 2012;22(9):1998–2006. doi: 10.1007/s00330-012-2441-2. [DOI] [PubMed] [Google Scholar]
- 49.Monaco M, Di Tommaso L, Pinna GB, et al. Combination therapy with warfarin plus clopidogrel improves outcomes in femoropopliteal bypass surgery patients. J Vasc Surg. 2012;56(1):96–105. doi: 10.1016/j.jvs.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 50.Bonaca MP, Bhatt DL, Storey RF, et al. Ticagrelor for prevention of ischemic events after myocardial infarction in patients with peripheral artery disease. J Am Coll Cardiol. 2016;67(23):2719–2728. doi: 10.1016/j.jacc.2016.03.524. [DOI] [PubMed] [Google Scholar]
- 51.Patel MR, Becker RC, Wojdyla DM. Cardiovascular events in acute coronary syndrome patients with peripheral arterial disease treated with ticagrelor compared with clopidogrel: data from the PLATO Trial. Eur J Prev Cardiol. 2015;22(6):734–742. doi: 10.1177/2047487314533215. [DOI] [PubMed] [Google Scholar]
- 52.ClinicalTrials.gov [Internet]. Bethesda (MD): National Library of Medicine (US). 2000 Feb 29 - . Identifier NCT02260622, Pilot Study to Examine the Use of Rivaroxaban After Angioplasty for Critical Limb Ischemia (RIVAL-PAD); 2019 Nov 22 [cited 2021 June 29]. https://clinicaltrials.gov/ct2/show/NCT02260622
- 53.Soga Y, Yokoi H, Kawasaki T, et al. Efficacy of cilostazol after endovascular therapy for femoropopliteal artery disease in patients with intermittent claudication. J Am Coll Cardiol. 2009;53(1):48–53. doi: 10.1016/j.jacc.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 54.Iida O, Yokoi H, Soga Y, et al. Cilostazol reduces angiographic restenosis after endovascular therapy for femoropopliteal lesions in the sufficient treatment of peripheral intervention by cilostazol study. Circulation. 2013;127(23):2307–2315. doi: 10.1161/CIRCULATIONAHA.112.000711. [DOI] [PubMed] [Google Scholar]
- 55.Warfarin Antiplatelet Vascular Evaluation Trial Investigators; Anand S, Yusuf S et al. Oral anticoagulant and antiplatelet therapy and peripheral arterial disease. N Engl J Med. 2007;357(3):217–227. [DOI] [PubMed]
- 56.Bonaca MP, Bhatt DL, Braunwald E, et al. Design and rationale for the prevention of cardiovascular events in patients with prior heart attack using ticagrelor compared to placebo on a background of aspirin-thrombolysis in myocardial infarction 54 (PEGASUS-TIMI 54) trial. Am Heart J. 2014;167(4):437–444.e5. doi: 10.1016/j.ahj.2013.12.020. [DOI] [PubMed] [Google Scholar]
- 57.Petersohn S, Pouwels X, Ramaekers B, Ten Cate-Hoek A, Joore M. Rivaroxaban plus aspirin for the prevention of ischaemic events in patients with cardiovascular disease: a cost-effectiveness study. Eur J Prev Cardiol. 2020;27(13):1354–1365. doi: 10.1177/2047487320913380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ambler GK, Nordanstig J, Behrendt CA, Twine CP. Network meta-analysis of the benefit of aspirin with rivaroxaban vs. clopidogrel for patients with stable symptomatic lower extremity arterial disease. Eur J Vasc Endovasc Surg. 2021;62(4):654–655. [DOI] [PubMed]
- 59.Mukherjee D. After revascularization for PAD, rivaroxaban reduced vascular events with a small increase in major bleeding. Ann Intern Med. 2020;173(4):JC22. [DOI] [PubMed]
- 60.Conte MS, Bradbury AW, Kolh P, et al. Global vascular guidelines on the management of chronic limb-threatening ischemia. Eur J Vasc Endovasc Surg. 2019;58(1S):S1–S109.e33. [DOI] [PMC free article] [PubMed]
- 61.Neumann FJ, Sousa-Uva M, Ahlsson A, et al. 2018 ESC/EACTS Guidelines on myocardial revascularization. Eur Heart J. 2019;40(2):87–165. doi: 10.1093/eurheartj/ehy394. [DOI] [PubMed] [Google Scholar]
- 62.Valgimigli M, Bueno H, Byrne RA, et al. 2017 ESC focused update on dual antiplatelet therapy in coronary artery disease developed in collaboration with EACTS: The Task Force for dual antiplatelet therapy in coronary artery disease of the European Society of Cardiology (ESC) and of the European Association for Cardio-Thoracic Surgery (EACTS) Eur Heart J. 2018;39(3):213–260. doi: 10.1093/eurheartj/ehx419. [DOI] [PubMed] [Google Scholar]
- 63.Laird JR, Schneider PA, Tepe G, Brodmann M, Zeller T, Metzger C, Krishnan P, Scheinert D, Micari A, Cohen DJ, Wang H, Hasenbank MS, Jaff MR. Durability of treatment effect using a drug-coated balloon for femoropopliteal lesions: 24- month results of IN.PACT SFA. J Am Coll Cardiol. 2015;66(21):2329–2338. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.