Abstract
RNA modifications have become hot topics recently. By influencing RNA processes, including generation, transportation, function, and metabolization, they act as critical regulators of cell biology. The immune cell abnormality in human diseases is also a research focus and progressing rapidly these years. Studies have demonstrated that RNA modifications participate in the multiple biological processes of immune cells, including development, differentiation, activation, migration, and polarization, thereby modulating the immune responses and are involved in some immune related diseases. In this review, we present existing knowledge of the biological functions and underlying mechanisms of RNA modifications, including N6-methyladenosine (m6A), 5-methylcytosine (m5C), N1-methyladenosine (m1A), N7-methylguanosine (m7G), N4-acetylcytosine (ac4C), pseudouridine (Ψ), uridylation, and adenosine-to-inosine (A-to-I) RNA editing, and summarize their critical roles in immune cell biology. Via regulating the biological processes of immune cells, RNA modifications can participate in the pathogenesis of immune related diseases, such as cancers, infection, inflammatory and autoimmune diseases. We further highlight the challenges and future directions based on the existing knowledge. All in all, this review will provide helpful knowledge as well as novel ideas for the researchers in this area.
Subject terms: Biochemistry, Immunology
Introduction
Chemical modification occurs on many types of biological macromolecules, such as nucleic acids, sugars, lipids, and proteins, and is specific and efficient for regulating their functions.1–4 RNA modifications, such as N6-methyladenosine (m6A), 5-methylcytosine (m5C), N1-methyladenosine (m1A), N7-methylguanosine (m7G), N4-acetylcytosine (ac4C), pseudouridine (Ψ), uridylation, and adenosine-to-inosine (A-to-I) RNA editing, are RNA features that alter the canonical AUGC bases and function as emerging and critical post-transcriptional regulators.1–3,5 The RNA modifications catalyzed by “writer” enzymes can be removed by “eraser” enzymes.6,7 However, some modifications are further modified by enzymes, which we term for the first time as “modifiers”. The modifications are identified by RNA-binding proteins (RBP) known as “readers” to participate in various physiological and pathological processes.1–3,6,7
The immune cell abnormality in human diseases is also a research focus and progressing rapidly these years. In 2005, a study demonstrated that RNA modifications, such as m6A, m5C, m5U, s2U, or Ψ, may influence the activations of dendritic cells (DCs) and toll-like receptor (TLR)-expressing cells.8 Although this research was a preliminary exploration, it gave us knowledge that RNA modification could affect the biology of immune cells. In recent years, advances in new technologies and ideas have led to an increasing number of researchers focusing on the influence of RNA modifications on immune cell biology, and their roles in immune related diseases. m6A is the modification most frequently studied and there already have some reviews summarized its important roles in immune processes.9,10 Although not thoroughly and comprehensively, other RNA modifications have also been confirmed to participate in the immune cell biology and immune related diseases. However, due to the complexity of RNA modification and the diversity of immune cells, the interaction network between RNA modification and immune cells remains largely unclear, which needs to be further consummated. Hence, based on the research status, we write this review, which we hope to be helpful for researchers and promote progress in this area.
In this review, we clarify the current understanding of eight RNA modifications and focus on their critical roles in regulating immune cell biology and immune related diseases. We also highlight questions that remain to be addressed in this area and provide perspectives for further studies.
RNA modifications
N6-methyladenosine
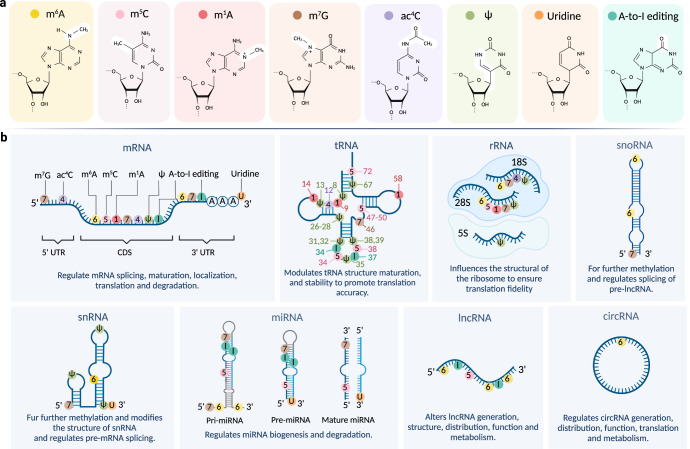
The methylation of adenosine at position N6, m6A modification has emerged as the most prevalent and abundant mRNA modification in eukaryotes (m6A/A = 0.1–0.6%).11,12 It appears in the full-length sequence but is enriched in the vicinity of the stop codon and the 3ʹ untranslated region (3ʹUTR) of mRNAs, within the consensus motif RRACH (R = G or A; H = A, C, or U).13,14 It also occurs in most non-coding RNAs, including ribosomal RNAs (rRNAs), small nuclear RNAs (snRNAs), small nucleolar RNAs (snoRNAs), microRNAs (miRNAs), long non-coding RNAs (lncRNAs), and circular RNAs (circRNAs) (Fig. 1).15–17
Fig. 1.
RNA modifications and their distributions on different RNA subtypes. a Chemical structures of eight RNA modifications. b Distribution of RNA modifications on different RNA subtypes. Indicated modifications are labeled at the corresponding modification sites. m6A N6-methyladenosine, m5C 5-methylcytosine, m1A N1-methyladenosine, m7G 7-methylguanosine, ac4C N4-acetylcytidine, ψ pseudouridine, A-to-I editing adenosine-to-inosine RNA editing, CDS coding sequence, UTR untranslated regions, pri-miRNA primary microRNA, pre-miRNA precursor microRNA
m6A deposition in mRNA is mostly mediated by the m6A methyltransferase complex (MTC) (Fig. 2 and Table 1).18–20 The key MTC components are methyltransferase-like 3 (METTL3), METTL14, Wilms’ tumor 1-associating protein (WTAP), vir-like m6A methyltransferase-associated (VIRMA, also known as KIAA1429), Cbl proto-oncogene-like 1 (HAKAI), zinc finger CCCH-type containing 13 (ZC3H13), and RNA-binding motif protein 15/15B (RBM15/15B).18,21,22 Among them, METTL3 is considered the only putative S-adenosylmethionine (SAM)-dependent methyltransferase with its own catalytic ability and can form a tight heterodimer with METTL14 to perform catalysis.23–25 The other aforementioned writers act as regulatory factors.15,20 Zinc finger CCCH-type containing 4 (ZCCHC4) and METTL5 mediate m6A formation on 28S and 18S rRNA, respectively, to accelerate the global translation rate (Fig. 3 and Table 1).26–30 In U6 snRNA, m6A is executed by METTL16 to participate in RNA splicing regulation (Fig. 4 and Table 1).31–33
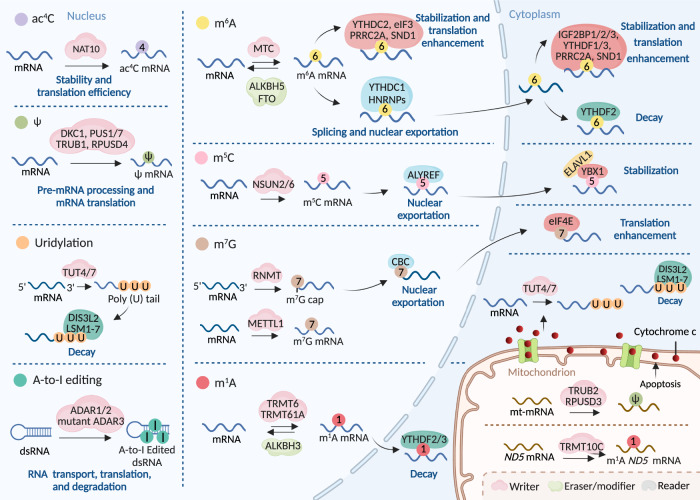
Fig. 2.
The machinery of RNA modifications and their molecular functions in mRNA. All RNA modifications included in this review can be installed on mRNA through their writers, and m6A as well as m1A modifications can be removed by indicated erasers, therefore making these RNA modifications dynamically reversible processes. Some of the RNA modifications can be recognized by their respective reader proteins, which changes the fates of target RNAs via altering generation, transportation, function and metabolization. m6A N6-methyladenosine, m5C 5-methylcytosine, m1A N1-methyladenosine, m7G 7-methylguanosine, ac4C N4-acetylcytidine, ψ pseudouridine, U Uridine, A-to-I editing adenosine-to-inosine RNA editing, dsRNA double-stranded RNA
Table 1.
Characteristics of reviewed RNA modifications
| Modifications | Target RNA | Writer | Eraser/Modifier | Reader | Biological function | Ref. |
|---|---|---|---|---|---|---|
| m6A | mRNA | METTL3/14, WTAP, VIRMA, HAIKAI, ZC3H13, METTL16, RBM15/15B | FTO, ALKBH5 | YTHDF1-3, YTHDC1-2, IGF2BP1-3, HNRNPC/G/A2B1, eIF3, PRRC2A, SND1, FMR1, LRPPRC | Regulates transcription, maturation, localization, translation and degradation. | 68 |
| rRNA | ZCCHC4, METTL5 | N.A. | N.A. | Promotes global translation. | 26,27 | |
| snRNA | METTL16 | FTO | N.A. | Regulates snRNA pre-mRNA splicing. | 31 | |
| snoRNA | METTL3/14 | N.A. | N.A. | Regulates pre-lncRNA splicing. | 69 | |
| miRNA | METTL3/14 | FTO | HNRNPA2B1 | Regulates pri-miRNA processing. | 70 | |
| lncRNA | METTL3/14, WTAP | FTO ALKBH5 | YTHDF1, YTHDF2, IGF2BP1, IGF2BP2 | Regulates generation, structure, distribution, function, metabolism. | 72 | |
| circRNA | METTL3/14 | ALKBH5, FTO | YTHDF2, YTHDF3 | Regulates generation, distribution, function, translation, metabolism. | 66 | |
| eRNA | N.A. | N.A. | YTHDC1 | Activates enhancer. | 452 | |
| m5C | mRNA | NSUN2/6 | N.A. | ALYREF, YBX1, FMRP | Modulates stability, export, translation and promotes mRNA-dependent repair. | 90,106,107 |
| tRNA | NSUN2/3/6, DNMT2 | ALKBH1, TETs | N.A. | Regulates tRNA structure and stability to ensure translation accuracy. | 78 | |
| rRNA | NSUN1/3/4/5 | N.A. | YTHDF2 | Stabilizes ribosome structural conformation to ensure translation fidelity. | 86,104 | |
| vtRNA | NSUN2 | N.A. | N.A. | Promotes small-vault RNAs generation. | 106 | |
| eRNA | NSUN7 | N.A. | N.A. | Protects target RNAs from degradation. | 88 | |
| miRNA | NSUN2 | N.A. | N.A. | Affects miRNA maturation. | 453 | |
| lncRNA | NSUN2 | N.A. | N.A. | Increases stability. | 454 | |
| m1A | mRNA | TRMT6/61A/10C | ALKBH3 | YTHDF1-3, YTHDC1 | Regulates translation. | 115,125,126 |
| tRNA | TRMT6/61A/61B/10B/10C | FTO, ALKBH1/3/7 | N.A. | Stabilizes tRNA structure and promotes translational initiation. | 113,116 | |
| rRNA | NML, TRMT61B | N.A. | N.A. | Maintain ribosomal structure and function. | 118 | |
| m7G | mRNA | METTL1, RNMT | N.A. | eIF4E, CBC | Regulates mRNA transcription elongation, slicing, export, translation and degradation. | 127,147 |
| tRNA | METTL1, WDR4 | N.A. | N.A. | Regulates tRNA structural integrity to promotes stability, translation ability and reduce ribosome pausing. | 133,148 | |
| rRNA | WBSCR22, TRM112 | N.A. | N.A. | Promotes ribosome biogenesis. | 455 | |
| snRNA | N.A. | TGS1 | N.A. | For further methylation. | 456 | |
| snoRNA | N.A. | TGS1, H29K | N.A. | For further methylation. | 456,457 | |
| miRNA | METTL1 | N.A. | N.A. | Enhances miRNA processing via affecting pri-miRNA structure. | 135 | |
| ac4C | mRNA | NAT10 | N.A. | N.A. | Promotes mRNA stability and promote protein translation. | 158,168,169 |
| tRNA | NAT10 | N.A. | N.A. | Enhances its stability and indicates eukaryotic tRNA maturation. | 166,167 | |
| rRNA | NAT10 | N.A. | N.A. | Boosts ribosome synthesis, and influences mRNA translation ability. | 160 | |
| Ψ | mRNA | DKC1, PUS1/7, TRUB1/2, RPUSD3/4 | N.A. | N.A. | Affects multiple steps in translation that could impact fidelity. | 176,178 |
| tRNA | PUS1/3/7/10, TRUB1/2, RPUSD4 | N.A. | N.A. | Maintains stable tRNA structure and mediate tRNA codon-anticodon base pairing to regulate translation. | 175,176,182 | |
| rRNA | DKC1, PUS7, TRUB2, RPUSD3/4 | N.A. | N.A. | Critical for rRNA folding and controls translational fidelity. | 186 | |
| snRNA | PUS1/3/7, TRUB1, H/ACA snoRNPs | N.A. | N.A. | Influence structure, RNA-RNA or RNA-RBP interaction to function in pre-mRNA splicing. | 172 | |
| Uridylation | mRNAs | TUT4, TUT7 | N.A. | LSM1-7, DIS3L2, La | Promotes mRNA decay. | 202,227 |
| miRNA | TUT4, TUT7 | N.A. | DIS3L2 | Regulates miRNA biogenesis and degradation, affects miRNAs recognizing or interacting with target sites. | 220–224,226 | |
| gRNAs | RET1/2 | N.A. | N.A. | Initiates and promotes gRNA maturation. | 217,458 | |
| snRNA | TUT1 | N.A. | N.A. | Promotes stabilization and maturation | 206 | |
| Viral RNA | TUT4, TUT7 | N.A. | N.A. | Facilitates target genes degradation and involves in antiviral defense. | 230 | |
| A-to-I editing | mRNA | ADAR1-3 | N.A. | N.A. | Regulates mRNA transport, translation, and degradation and pre-mRNA splicing. | 259,268–271,275,459 |
| tRNA | ADAR1-3 | N.A. | N.A. | Preserves translational accuracy | 239,243–247 | |
| miRNA | ADAR1/2 | N.A. | N.A. | Influence the biogenesis and function of miRNAs. | 241,459,460 | |
| lncRNA | ADAR1/2 | N.A. | N.A. | Disrupts its interaction with genomic DNA or RNA. | 461,462 | |
| Viral RNA | ADAR1-3 | N.A. | N.A. | Alters dsRNA structure, thereby suppressing innate immune responses. | 459,463 |
m6A N6-methyladenosine, m5C 5-methylcytosine, m1A N1-methyladenosine, m7G 7-methylguanosine, ac4C N4-acetylcytidine, ψ pseudouridine, A-to-I editing adenosine-to-inosine RNA editing
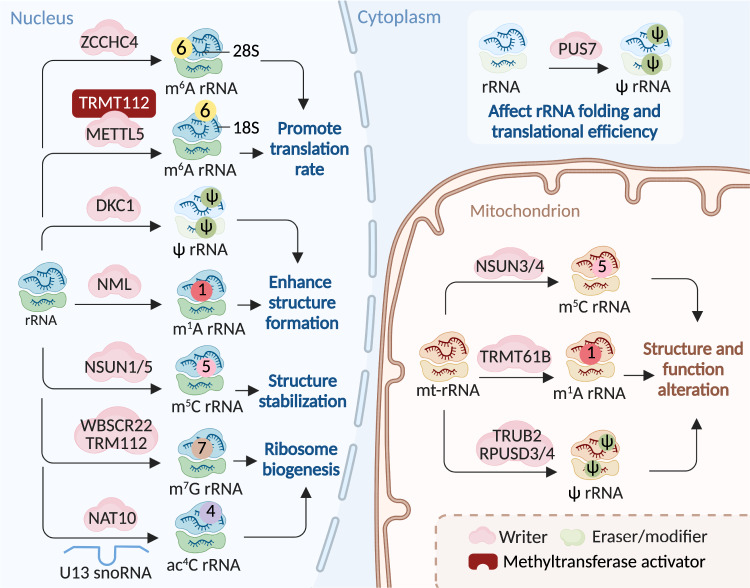
Fig. 3.
The machinery of RNA modifications and their molecular functions in rRNA. The indicated RNA modifications are installed on rRNA via their writers. These modifications occurred on rRNA alter the RNA structure, thereby regulating the function of ribosomes, which in turn affects the translation rate. The same modification can be installed by different writers in different parts of the cell. Besides, m6A modifications on different subunits of the ribosome can be catalyzed by different writers. Some writers also need to form a heterodimeric complex with methyltransferase activators to gain metabolic stability in cells, such as METTL5-TRMT112. m6A, N6-methyladenosine; m5C, 5-methylcytosine; m1A, N1-methyladenosine; m7G, 7-methylguanosine; ac4C, N4-acetylcytidine; ψ, pseudouridine
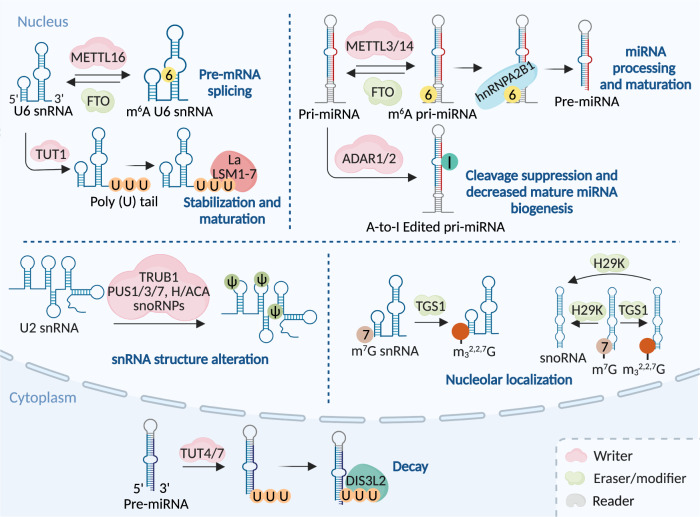
Fig. 4.
The machinery of RNA modifications and their molecular functions in snRNA, snoRNA and miRNA. The indicated RNA modifications are installed on snRNA, snoRNA and miRNA through respective writers. m6A modifications on snRNA and miRNA can be removed by FTO, while m7G modifications on snRNA and snoRNA can be removed by H29K, making RNA modifications on snRNAs, snoRNA or miRNAs dynamically reversible process. Besides, m7G installed snRNA and snoRNA can be further modified as m2,2,7G by modifier TGS1. RNA modifications affect the function of these non-coding RNAs via altering their structures, facilitating fine-tuning in various physiological processes. m6A N6-methyladenosine; m7G 7-methylguanosine, ψ pseudouridine, U Uridine, A-to-I adenosine-to-inosine, m32,2,7G 2,2,7-trimethyl guanosine, pri-miRNA primary microRNA, pre-miRNA precursor microRNA
To date, two m6A erasers have been identified, both belonging to the AlkB family of the Fe (II)/α-ketoglutarate-dependent dioxygenase superfamily.34–37 The first eraser is fat mass and obesity-associated protein (FTO) that mediates mRNA and snRNA demethylation in m6A and m6Am residues, and tRNA in m1A residue (Figs. 2, 4, 5 and Table 1).35,36,38 The other m6A eraser, AlkB homolog 5 (ALKBH5), only oxidatively reverses m6A in mRNA (Fig. 2 and Table 1).39,40
Fig. 5.
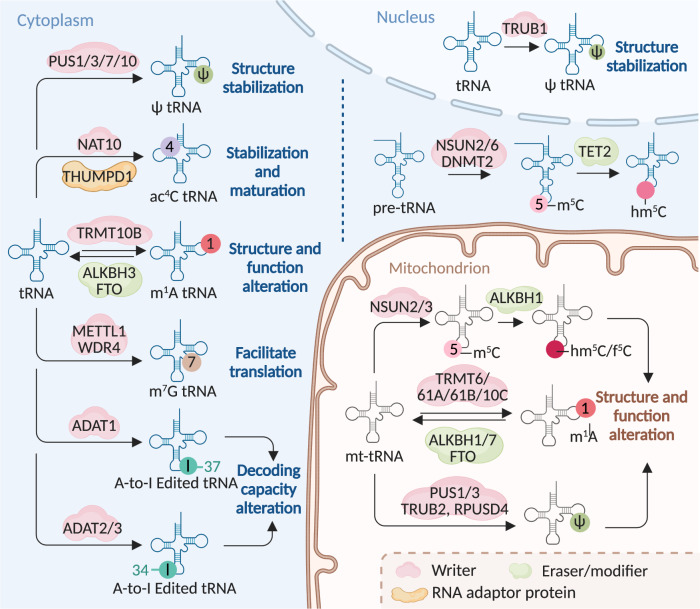
The machinery of RNA modifications and their molecular functions in tRNA. The indicated RNA modifications are installed on tRNA through indicated writers, and m1A modifications can be removed by ALKBH3 and FTO, while m5C modification on pre-tRNA can be converted into hm5C or f5C by TET2. These modifications on tRNA can alter the structure of tRNA, thereby regulating its functions to affect the translation efficiency. The same modification can be installed by different writers on tRNAs in different parts of the cell. A-to-I editing on different tRNA positions can be added by different writers. ac4C writer NAT10 modifies tRNAs assisted by the adaptor Tan1/THUMPD1. m5C 5-methylcytosine, m1A N1-methyladenosine, m7G 7-methylguanosine, ac4C N4-acetylcytidine, ψ pseudouridine, A-to-I adenosine-to-inosine, hm5C 5-hydroxymethylcytidine, f5C 5-formylcytidine
Many reader proteins influence the fate of m6A RNAs in various ways, which is largely determined by their subcellular localization. The most studied readers are the YT521-B homology (YTH) domain family members, which share the m6A-recognizing YTH domain but exert different effects on RNA fate.41,42 The YTH domain family includes YTHDF1–3 and YTHDC1–2.41,42 YTHDF1 and YTHDF3 actively promote protein synthesis by interacting with translation machinery, whereas YTHDF2 recruits RNA-degrading enzymes or adaptor proteins to trigger the rapid degradation of its target mRNA.43–47 YTHDC1 not only facilitates the decay of m6A-modified chromosome-associated regulatory RNAs to influence the open chromatin state and downstream transcription but also mediates mRNA splicing by recruiting and regulating pre-mRNA splicing factors.48–50 YTHDC2 may participate in mRNA stability and translation in an m6A-dependent or -independent manner.51–53 Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs, which include IGF2BP1–3), identify m6A through K homology domains to enhance mRNA stability and translation.54,55 The heterogeneous nuclear ribonucleoprotein (HNRNP) family members, which include HNRNPC, HNRNPG, and HNRNPA2B1, can identify m6A on precursor (pre)-mRNAs and/or primary (pri)-miRNAs to mediate splicing and/or nucleocytoplasmic trafficking.56–60 Eukaryotic initiation factor 3 (eIF3) promotes cap-independent translation upon the induction of cellular stress by recruiting the 43S complex to initiate translation.61,62 Both proline rich coiled-coil 2A (PRRC2A) Staphylococcal nuclease and tudor domain-containing 1 (SND1) function as m6A readers, facilitating the stabilization of modified RNA.63,64 In particular, m6A participates in transcription termination by promoting co-transcriptional R-loop formation to prevent the readthrough activity of Pol II, while it is unclear whether other readers are involved in this process.65 The specific roles of m6A in various RNA molecules are presented in Figs. 2 to 4 and Table 1.
Collectively, m6A modification plays a regulatory role in various cellular processes by affecting transcription, maturation, localization, function, and metabolism in different RNA classes.14,22,66,67 (Table 1) In mRNAs, m6A can affect transcription, maturation, localization, translation and degradation, eventually influencing the proteins encoded (Fig. 2).7,68 In rRNAs, the m6A1832 modification in 18s rRNA as well as the m6A4220 modification in 28S rRNA are required for global translation (Fig. 3).26,27 In snRNAs and snoRNAs, m6A modification may regulate snRNA pre-mRNA or pre-lncRNA splicing processes (Fig. 4).31,69 In miRNAs, m6A facilitates pri-miRNA processing by recruiting the miRNA microprocessor complex protein DGCR8 depending on HNRNPA2B1 (Fig. 4).56,70,71 In lncRNAs and circRNAs, m6A has been verified to modulate generation, structure, distribution, function and metabolism (Fig. 1), and m6A is also a critical translation initiator in circRNAs with coding potential.70,72
Although m6A has been widely investigated, there are still some important questions remain to be solved. For example, m6A is a widespread modification that can affect a variety kind of RNA, existing studies mainly focus on the effect of m6A on mRNA, and its effect on non-coding RNA may be of good research interest. The current study shows that the effect of m6A on RNA stability is bidirectional, i.e., increasing stability or promoting degradation. For this issue, it is necessary to fully consider the modification sites of m6A in RNAs as well as the imbalance of different readers, such as YTHDF2 and IGF2BPs. m6A may affect circRNA generation and circRNA-mRNA imbalance by mediating pre-mRNA splicing, which is also a potential study focus due to the recent research upsurge on circRNAs. Although existing inhibitors can interfere m6A levels by inhibiting writers,25,73,74 they are often nonspecific and affects overall m6A levels. It is more significant to explore gene specific m6A interference.
5-methylcytosine
m5C methylation occurs at position 5 of the cytidine residues of both DNA and RNA. Identified in 1958, m5C is described as a widespread mark in the epitranscriptome on tRNA, rRNA, mRNA, enhancer RNA (eRNA), and miRNA and is most abundant in eukaryotic tRNAs and rRNAs (Fig. 1).75–78
In eukaryotes, m5C methylation is introduced by the NOL1/NOP2/SUN domain (NSUN) family members, NSUN1–7, and DNA methyltransferase-like 2 (DNMT2) as presented in Table 1.76,78,79 Specific m5C writers catalyze different RNA subsets. According to current knowledge, cytoplasmic tRNAs are methylated by NSUN2, NSUN6, and DNMT2, while mitochondrial tRNAs are catalyzed by NSUN2 and NSUN3 (Fig. 5 and Table 1).80–84 rRNAs are methylated by NSUN1 and NSUN5 in the nucleus and by NSUN4 in the mitochondria (Fig. 3 and Table 1).85–87 mRNAs are methylated by NSUN2 and NSUN6, whereas ncRNA and eRNAs can be modified by NSUN2 and NSUN7, respectively (Fig. 2 and Table 1).76,78,79,84,88
Recently, some m5C erasers or modifiers have been concerned in RNA molecules. The known erasers/modifiers include ten-eleven translocation (TET) proteins (TET1–3) and α-ketoglutarate-dependent dioxygenase ABH1 (ALKBH1), which possess the activity of oxidizing m5C to 5-hydroxymethylcytidine (hm5C) (Fig. 5 and Table 1).89–91 In DNA, TETs successively convert m5C to hm5C, 5-formylcytosine (f5C), and 5-carboxylcytosine, the latter two of which are identified and removed by thymine DNA glycosylase;92–95 whereas in RNA, TETs has been only reported to convert m5C to hm5C (Fig. 5).96–98 ALKBH1 can successively convert m5C into hm5C and f5C at position 34 of cytoplasmic and mitochondrial tRNA, and the process in mitochondria is required for mitochondrial functions (Fig. 5).91,99 Under all circumstances, hm5C formation from m5C reduces the m5C modification, which is why previous studies considered TETs and ALKBH1 erasers.
Like m6A modification, m5C also involves binding proteins to change the fate of the modified RNA. The first identified mRNA m5C reader was RNA and export factor-binding protein 2 (ALYREF), a well-known protein complex that facilitates the nuclear export of mRNAs (Fig. 1 and Table 1).100,101 Y-box-binding protein 1 (YBX1) is a reader located in the cytoplasm that enhances the stability of m5C-modified mRNA by recruiting ELAV like RNA binding protein 1 (ELAVL1), an mRNA stability maintainer (Fig. 2 and Table 1).102,103 Besides, YTHDF2, also an m6A reader protein, could directly bind to m5C in RNA to modulate the distribution of m5C in both coding and noncoding RNA and influence rRNA maturation by regulating m5C levels (Table 1).104 Recently, Lan and colleagues presented a novel m5C reader, fragile X messenger ribonucleoprotein 1 (FMRP), which could be recruited to DNA damage sites by DNMT2 and promote TET1-mediated RNA m5C demethylation in DNA:RNA hybrids (Table 1).90
Generally, m5C plays a critical role in stabilizing both non-coding and coding RNAs. In tRNAs, m5C regulates RNA structure and stability and is required for translation accuracy (Fig. 5 and Table 1).78,80,105 m5C methylation at C2278 within a conserved region of 25S rRNA stabilizes the structural conformation of the ribosome, ensures translation fidelity, and recruits oxidative stress-responsive mRNA subsets to polysomes (Fig. 3 and Table 1).86 m5C methylation on vault RNAs affects their processing into derived small RNAs, while m5C in eRNAs protects them from degradation (Table 1).106 In mRNAs, m5C is vital for modulating stability, nuclear export, and translation (Table 1).101,102,107–109 For example, a subset of mRNAs with hypermethylated m5C sites was stabilized in an NSUN2- or YBX1-dependent manner, which influenced bladder carcinogenesis or embryonic development in zebrafish.107 NSUN2 enhanced the recognition of cyclin-dependent kinase inhibitor 1A (CDKN1A) mRNA by ALYREF, which functionally promoted the nuclear export capacity and translation of CDKN1A mRNA in 3T3-L1 preadipocytes.101
As we described above, m5C is abundant and required for maintaining RNA structure and stability in eukaryotic tRNAs and rRNAs, which are vital molecules in maintaining the normal physiology of almost all types of eukaryotic cells. Thus, targeting m5C as a therapeutic approach may have a long way to go. Fortunately, different RNAs possess different writers, and targeting specific writers can affect the function of specific RNAs. For instance, a recent study revealed that targeting NSUN3 to regulate site-specific mitochondrial RNA m5C modification shows therapeutic effects in combating cancer metastasis.105
N1-methyladenosine
Identified in the 1960s, m1A was reported as the methylation of adenosine at position N1 and has been observed in tRNAs, rRNAs, mRNAs, and lncRNAs.110,111 m1A is inextricably linked with m6A modification, as not only does m1A rearrange to m6A under alkaline conditions (Dimroth rearrangement), they also share some regulators (Fig. 1).36,112
The current reported human m1A writers include nucleomethylin (NML, also known as RRP8) (for rRNA), the tRNA methyltransferase 6 non-catalytic subunit (TRMT6)–RNA methyltransferase 61A (TRMT61A) complex (for mRNA and mitochondrial tRNA), TRMT61B (for mitochondrial tRNA and rRNA), TRMT10B (for tRNA), and TRMT10C (for mitochondrial tRNA and mRNA).113–117 m1A erasers, including FTO (for tRNA) and the ALKBH family members ALKBH1 (for mitochondrial tRNA), ALKBH3 (for tRNA and mRNA), and ALKBH7 (for mitochondrial tRNA), overlap or are closely related to some m6A erasers. Accordingly, it has been verified that some m6A readers, i.e., YTH domain family proteins including YTHDF1–3 and YTHDC1, identify m1A modifications (Figs. 2, 3, 5 and Table 1).36,99,118–123
Generally, m1A affects RNA base pairing and subsequently influences the target RNA molecule structure and function.115,119,124 Human rRNAs and tRNAs contain many different m1A modification sites. For example, m1A at position 1322 of 28S rRNA promotes 60S ribosomal subunit formation and m1A at position 947 is essential for mitoribosomal structure and function.113,116 m1A at position 58 of tRNA is essential for tRNA structure, stability, and translational initiation; in this position, absent m1A may promote the generation of tRNA-derived small RNAs (tDRs), enhancing ribosome assembly and causing malignant phenotypes.118,121 In mRNA, m1A is distributed in every mRNA segment, which includes the coding sequence (CDS), 5′UTR, and 3′UTR, and its roles appear region- or subcellular location-dependent.119,125 Near the start codon, m1A might regulate translation initiation by altering the secondary/tertiary structure or reader recognition of translation initiation sites (TISs), thereby promoting translation.125 In mitochondria, m1A in the 5′UTR or CDS repressed translation, probably by affecting ribosome scanning or translation (Figs. 2, 3, 5 and Table 1).115,126
Because m1A shares some regulators, such as YTHDF1–3 and YTHDC1, with m6A modification, the research ideas of m6A can provide reference for m1A. Since m1A modification can affect RNA base pairing, we expect that it might affect the binding of miRNAs with other RNA structures, such as mRNA 3ʹUTR, lncRNA and circRNA. Competing endogenous RNAs (ceRNA) regulatory network is attracting much attention these years, and m1A modification may add novel conceptions to this theory.
N7-methylguanosine
m7G refers to the RNA methylation of guanine at position N7 and is present in approximately 0.4% of all guanosine, a level similar to that of m1A modification.127–129 m7G is well known for the formation of the 5ʹ cap (m7GPPPN) structure of mature mRNA, snRNA, and snoRNA; moreover, it is enriched in all three transcript segments of mRNA 5ʹUTR, CDS, and 3ʹUTR and in pre-mRNAs.127,130–132 m7G is also present in noncoding RNAs, such as position 46 of tRNA, G1575/G1639 of 18S rRNA, and even mature and pre-miRNAs (Fig. 1).133–135
RNA guanine-7 methyltransferase (RNMT), METTL1–WD repeat domain 4 (WDR4) complex, and Williams–Beuren syndrome chromosomal region 22 protein (WBSCR22, also known as BUD23) are considered m7G writers. Activated by RNMT-activating mini-protein (RAM), RNMT is required for efficient cap methylation.136–138 By forming a complex with WDR4 or other partners, METTL1 has m7G methyltransferase activity for tRNA, internal mRNA, and pri-miRNA/miRNA.127,135,139,140 Requiring the methyltransferase adapter protein TRM112, WBSCR22 specifically methylates m7G in 18S rRNA.141 In most non-coding RNAs, the m7G cap can be lost during maturation by cleavage or further modification to m2,2,7G trimethylguanosine.142 For example, trimethylguanosine synthase 1 (TGS1) might function as a modifier, which hypermethylated the m7G caps of snRNAs and snoRNAs to a m2,2,7G cap structure, leading to their concentration in nuclear foci.143 The m7G cap can be recognized by eIF4E and the cap-binding complex (CBC) composed of CBP80 and CBP20, thereby affecting RNA maturation, nuclear export, and translation (Figs. 2 to 5 and Table 1).144–146
On mRNA, the m7G cap regulates multiple stages of mRNA processes, including pre-mRNA slicing, nuclear export, transcription elongation, translation, and degradation and indirectly augments ribosome synthesis and translation rates.127,142,147 On internal mRNA, m7G methylation might influence mRNA translation.127 m7G in tRNAs remodels the mRNA translatome by maintaining tRNA structural integrity to promote its stability, translation ability, and reduce ribosome pausing.133,140,148,149 However, the effects of m7G on rRNA have not been studied in-depth. In miRNA, m7G promoted miRNA processing by antagonizing G-quadruplex structures in pri-miRNA (Figs. 2 to 5 and Table 1).135,150,151
As we know, m7G is widely present in mRNAs and is a critical regulator in the translation process, therefore, it may not be a good therapic target in human diseases. The roles of m7G regulators may vary in different RNAs and diseases. For example, m7G modification on tRNA promoted the progression of lung cancer,139 while m7G modification on let-7 miRNA showed the opposite effect.135 m7G modification promoted the progression of hepatocellular carcinoma and bladder cancer,152,153 while it exerted an opposite opposite effect in teratoma.154
N4-acetylcytosine
Aside from m5C and hm5C, ac4C (acetylation of the N4 position of cytosine) is another conserved modification in cytidine and is currently the only acetylation event described in eukaryotic RNA.155–157 As with many RNA modifications, ac4C was detected initially in tRNA and rRNA, followed by mRNA.158,159 In rRNA, ac4C is distributed in helix 34 and helix 45 near the decoding site of mammalian 18S rRNA; in tRNA, it is detected at the D-stem of tRNASer/Leu in eukaryotes.160–163 In mRNA, the deposition of ac4C sites is detected mainly in the CDS region, and also in the 5ʹUTR (Fig. 1).158
N-acetyltransferase 10 (NAT10), an essential ATP-dependent RNA acetyltransferase, is currently considered the only writer of ac4C.164 It catalyzes ac4C modification in 18S rRNA, tRNA, and a broad range of mRNA.158,160,164,165 Two additional proteins are required in ac4C formation in human rRNA or tRNA, respectively. The first is the box C/D snoRNA U13, which is essential and specific for 18S rRNA acetylation by timely pre-rRNA folding.160 The other is THUMP domain-containing 1 (THUMPD1), a specific RNA adaptor protein harboring an RNA-binding motif that can interact with NAT10 to cooperate in tRNA acetylation (Figs. 2, 3, 5 and Table 1).160,162
In 18S rRNAs, ac4C is critical for pre-rRNA processing and ribosome synthesis and influences translation ability possibly by turning the 18S rRNA 3′ end into an environment rich in base modifications to interact with mRNA or tRNA.160 The function of ac4C formation in tRNA is not fully understood, but ac4C of tRNA can promote its stability and is considered a monitoring indicator of eukaryotic tRNA maturation due to the rapid tRNA degradation pathway.166,167 Furthermore, ac4C can influence mRNA translation. The presence of ac4C on mRNA CDS region robustly boosts mRNA stability and promotes protein translation, probably by affecting its interaction with cognate tRNAs during translation.158,168,169 However, ac4C modification on 5ʹUTR mainly affects translation initiation by directly and indirectly mediating exquisite locational specificity: ac4C modification immediately adjacent to a strong AUG start codon can repress translation, while ac4C modification downstream of a weak translation initiation site can facilitate translation (Figs. 2, 3, 5 and Table 1).170
As a newly identified RNA modification, ac4C remains largely unknown, particularly its regulators and molecular functions. Only one writer and no erasers or readers have been identified. The functions of ac4C in rRNA, tRNA, and the CDS as well as UTR regions of mRNAs have been reported, however, relevant studies are rare. More investigations are required.
Pseudouridine
Identified nearly 70 years ago, Ψ is the C5-glycoside isomer of uridine, of which the C5 atom (instead of N1) of the heterocyclic ring is bonded to the C1′ atom of the pentose.171–173 Ψ is present in almost all kinds of RNAs, including coding and non-coding RNAs, and is highly conserved among species (Fig. 1).79,171,174
Thirteen writers for Ψ have been identified in humans, one of which is Dyskerin pseudouridine synthase 1 (DKC1), a catalytic subunit of the H/ACA snoRNP complex that catalyzes rRNA pseudouridylation, which requires an RNA guide for its catalytic activity.175–177 The remaining 12 writers are RNA-independent single pseudouridine synthases (PUSs): PUS1, PUSL1, PUS3, TRUB1, TRUB2, PUS7, PUS7L, RPUSD1–4, and PUS10; these enzymes have specific cellular localizations and RNA targets.178–181 To date, there are no known Ψ erasers and readers. The absence of erasers may be due to the relatively inert C–C bond formed by the ribose sugar and base, leading to the pseudouridylation process being irreversible (Figs. 2 to 5 and Table 1).175
Previous studies have shown that Ψ plays functional roles in RNA biogenesis, structure, stability, and function to participate in regulating gene expression.79 tRNAs contain many pseudouridylation sites, which are critical for maintaining stable tRNA structure and mediating tRNA codon–anticodon base pairing and are thereby involved in translation processes.175,176,182–184 Ψ also represses aberrant protein synthesis by altering the properties of tRNA-derived fragments.185 Similar to that in tRNA, Ψ is also abundant and present in various rRNA regions, aiding the formation of stable structures.186–188 Moreover, Ψ contributes to ribosome processing and function to ensure translational fidelity in protein synthesis.178,189 In snRNAs, Ψ was predicted to influence structure and RNA–RNA or RNA–RBP interactions to function in pre-mRNA splicing.172,190–192 Ψ is also involved in regulating pre-mRNA processing, mRNA structure, stability, translational fidelity, and termination, which is another mechanism of translation control apart from tRNA and rRNA modification (Figs. 2–5 and Table 1).176,178,193–196
Despite being identified several decades ago, the contributions of Ψ to multiple cellular processes are just starting to be revealed. Similar to ac4C, new things always take time to be understood. The elucidation of erasers and readers of Ψ will be one of the key directions in the future. Specially, Ψ have already been applied to generating highly effective COVID-19 mRNA vaccines,197 which is the clinical application of this modification and has potential value for further research.
Uridylation
In addition to the most widespread homomeric poly (A) tails, uridylation, which consists of the untemplated addition, appears to be the second most prevalent modification at the 3ʹ RNA termini.198–201 Virtually, uridylation can occur on all classes of eukaryotic RNAs including mRNAs, and noncoding RNAs including U6 spliceosomal RNA, guide RNA (gRNA), small interfering RNA (siRNA), miRNA, Piwi-interacting RNA (piRNA), rRNA, and tRNA. Uridylation also targets viral RNA tagging (Fig. 1).198,199,202
In different substrates, uridylation is catalyzed by different terminal uridylyltransferases (TUTases), which belong to the noncanonical terminal nucleotidyltransferases (TENTs).203,204 In nuclear U6 snRNA, the U6 TUTase (TUT1) specifically added or restored at least four uridines at the 3′ end.205,206 TUT4 and/or TUT7 belonging to the TENT3 subfamily are the predominant writers of other cellular uridylation.205,207–210 Uridylation erasers or modifiers have not been reported and the uridylation readers include the LSM1-7 complex (for oligouridylation), DIS3L2 (for oligouridylation and polyuridylation), and La protein (Fig. 2 and Table 1).211–216
Uridylation alters RNA fate from diverse aspects, including RNA maturation, function, stability, and decay. Uridylation is essential for U6 snRNA maturation and 3′ stabilization to perform splicing function and initiating gRNA maturation.206,217–219 The functions of uridylation in miRNAs are diverse. For example, TUTs-mediated pre-miRNA uridylation is a critical step in miRNA biogenesis, which involves repairing or removing defective pre-miRNAs, arm switching, and Dicer processing.220–222 Uridylation on the miRNA 3′ end can recognize noncanonical target sites; on the other hand, it may abrogate target gene repression by directly affecting miRNA 3ʹUTR interactions.223,224 Moreover, the 3ʹ addition of uridine promotes miRNA degradation, which also applies to other small RNAs, such as siRNAs and piRNAs.225,226 Many studies have demonstrated that uridylation facilitates 5ʹ-to-3ʹ or 3ʹ-to-5ʹ mRNA decay, which is mediated by the recruitment of deadenylases, decapping enzymes, and exonucleases.202,227 Uridylation also regulates translation efficiency via various mechanisms, for example, mRNA destabilization, and rRNA and tRNA turnover.202,209,228,229 Moreover, viral RNA uridylation is involved in antiviral defense.230–232 Uridylated ncRNAs appear overrepresented in exosomes, indicating that uridylation directs RNA sorting into exosomes (Figs. 2, 4 and Table 1).233
Uridylation can act on almost all classes of RNAs in eukaryotic cells, further identification of writers and their auxiliary factors in recognizing specific RNA substrates, as well as of erasers and readers that regulate the deuridylation and decide the fate of uridylated transcripts, will no doubt be key to further understanding the regulatory network. The cell-type and disease-specific patterns of uridylation are also crucial in unraveling the roles of uridylation in cellular biology. Viral RNAs are also targets of uridylation, and the contributions of uridylation in fighting viruses and controlling transposons might be interesting topics of future research considering the current epidemic situation of COVID-19.
Adenosine-to-inosine editing
A-to-I editing, which converts adenosines to inosines by deamination in RNA molecules, is a widespread co-transcriptional and post-transcriptional modification in mammals.234,235 A-to-I editing occurs widely in pre-mRNAs, mRNAs, noncoding RNAs such as miRNAs, lncRNAs as well as tRNAs, and even in virus RNAs (Fig. 1).236–240 The most common targets of A-to-I RNA editing are dsRNA hairpin structures forming from inverted Alu repetitive elements, which are located mainly within introns and untranslated regions and fewer in coding exons.241–243
A-to-I editing is the direct conversion of adenosine residues to inosine residues, which is not a conventional “writing” process, so the writers of A-to-I editing are also editors. Adenosine Deaminase TRNA Specific 1 (ADAT1) is responsible for the deamination of adenosine 37 to inosine in eukaryotic tRNA,239,243,244 while A-to-I conversion at position 34 of certain tRNAs is catalyzed by ADAT2 and ADAT3 (Fig. 5 and Table 1).245–247 Other A-to-I editing events are catalyzed by adenosine deaminases acting on RNAs (ADAR) family members, which are conserved in mammals.248–252 ADARs share similar functional domain structures of dsRNA-binding domains (dsRBDs) and a larger catalytic adenosine deaminase domain.253,254 There are three ADAR members: ADAR1 and ADAR2 deaminate double-stranded (ds)RNA, whereas ADAR3 binds to dsRNA as well as single-stranded (ss)RNA .236,237,255 ADAR3 lacks editing activity, and it may competitively bind to dsRNAs with other ADARs to decrease the efficiency of these enzymes.256–258 Different from other additive chemical modifications, A-to-I editing may not be further regulated by erasers/modifiers and readers.
Generally, this specific adenosine editing can cause transcriptomic diversity and influence the functions of the target RNAs.259–262 Although the probability of A-to-I editing occurrence in the coding regions is relatively low,241,263 studies have revealed its role in impacting the protein translation and function by altering the protein codon.264–267 For example, in colorectal cancer, the A-to-I editing of ras homolog family member Q (RHOQ) transcripts results in the substitution of asparagine with serine at residue 136 of RHOQ protein, leading to increased RHOQ activity and cancer invasion potential.264 In UTRs, A-to-I RNA editing can regulate RNA processes including transport, translation, and degradation.259,268–271 For example, ADAR1 directly edits 3ʹ UTR of XIAP and MDM2 mRNAs to promote nuclear retention of these mRNAs.272 A-to-I editing facilitates the recruitment of the stabilizing RNA-binding protein human antigen R (HuR) to the 3ʹ UTR of the CTSS mRNA, thereby enhancing the stability and translation CTSS mRNA.273 In addition, the A-to-I RNA editing in 3ʹUTR has a potential to block the interaction between miRNAs and target genes to hinder the post-transcriptional repression activity.274 The A-to-I RNA editing in introns usually regulates alternative splicing processes.268,275 For example, ADAR1 deficiency may cause alternative splicing in intron 27 of the ABCB1 gene to produce transcripts with retained intron, resulting in nonsense-mediated mRNA decay and decreased ABCB1 mRNA stability.276 The A-to-I RNA editing in miRNAs may influence the biogenesis and function of miRNAs.277,278 A-to-I RNA editing also represses Alu elements in introns to form dsRNA structures, leading to altered linear mRNA and circRNAs generation (Figs. 2, 4 and Table 1).279–281
Some reports indicate that A-to-I RNA editing in pri- or pre-miRNA may induce local structural conformation changes, leading to cleavage suppression and decreased mature miRNA biogenesis.282–285 Conversely, some A-to-I RNA editing may not interfere or promote miRNA biogenesis.282,286 Specially, ADARs may also bind directly to miRNA precursors to promote miRNA processing by acting as an RNA-binding protein, independent of adjacent A-to-I editing events.287–289 The A-to-I editing in mature miRNAs or siRNAs may impact their target mRNA selection and silencing efficiency.290–297 In lncRNAs, A-to-I editing can affect their secondary structures, stability and interactions with other molecules.238,298,299 For example, the A-to-I RNA editing in lncRNAs may impact lncRNA-miRNA interactions and, consequently, change their miRNA sponge function.300 In tRNAs, A-to-I editing is closely associated with their decoding capacity (Fig. 5 and Table 1).245 In virus RNAs, A-to-I RNA editing can directly target the genome or transcriptome of RNA viruses to regulate viral pathogenicity as well as host innate immune response, which we will discuss in detail below.301–303
Nonetheless, there are many questions that remain to be answered in this field. How the target sites of A-to-I editing are chosen by editors? Although previous studies have identified lots of A-to-I editing sites in human RNA molecules, the significance of such editing for the vast majority of RNA sites remains unclear. Due to A-to-I editing can regulate gene expression through multiple mechanisms, it may be a potential approach to assist or replace RNA interference. Except for influencing miRNA-3ʹUTR and miRNA-lncRNA interaction, A-to-I editing may also affect miRNA-circRNA interaction, which has not been validated yet. Further investigations of RNA editing may provide lessons for precise gene editing.
Roles of RNA modification in immune cell biology
RNA modifications and T lymphocytes
T lymphocytes originate from bone marrow progenitors, mature in the thymus, and are transported to the periphery to fulfill immune functions after activation, proliferation, and differentiation.304,305 The m5C methyltransferase NSUN2 mediates hyperhomocysteinemia-induced interleukin-17A (IL-17A) upregulation by methylating IL17A mRNA and enhancing its translation in T lymphocytes.306 A recent study discovered that the m7G cap methyltransferase RNMT plays critical roles in T cell activation by specifically regulating ribosome synthesis.142 Enzymes modulating miRNA uridylation and uridylated miRNAs are regulated during T cell activation; TUT4 is critical for maintaining miRNA uridylation in the steady state of T lymphocytes and is downregulated during T cell activation, leading to the degradation of uridylated miRNAs.226 A-to-I RNA editing induced by ADAT1 prevents the sensing of endogenous dsRNAs by MDA5 to participate in thymic T cell maturation, which includes negative selection.307,308 Specifically, m5C and Ψ mRNA modification may be promising in the systemic delivery of nanoparticle formulations for regulating T cell immunity and inflammation.309 Many studies have uncovered the key functions of RNA modifications in the biology of multiple T lymphocyte subsets, which are presented below.
CD4+ T cells
Naive CD4+ T cells exit the thymus as Th0 cells and differentiate into various cell subsets following different activation signals.310,311 The best understood effector cell subsets include T helper (Th) cells (Th1, Th2, Th9, Th17, Th22, et al.), T follicular helper (Tfh) cells, and T regulatory (Treg) cells.312–314 Up to now, there have been some studies revealed that m6A participates in the biology of CD4+ T cells, as well as several subsets.
First, m6A can affect the functions of CD4+ T cells. For example, ALKBH5 decreases m6A levels in CXCL2 and IFNG mRNA to enhance mRNA stability and translation, thereby promoting CD4+ T cell responses.315 m6A can also influence CD4+ T cell differentiation and subset functions, which are discussed in detail below. In particular, as CD4+ T cells are the target cells of HIV infection, HIV infection leads to an extensive increase in m6A levels in both host and viral mRNAs, thereby influencing HIV replication and viral RNA nuclear export.316 During the latent phase of HIV-1 infection, NSUN1 binds with HIV-1 TAR RNA at the 5′ long terminal repeat and generates its m5C methylation, and NSUN1 binding with TAR competes with Tat–TAR interaction, leading to hampered HIV-1 transcriptional elongation and viral latency in CD4+ T cells.317
m5C levels and NSUN2 expression are decreased in the CD4+ T cells of systemic lupus erythematosus (SLE) patients, and hypermethylated m5C in SLE is closely associated with the immune- and inflammation-related pathways, including the immune system, cytokine signaling, and interferon (IFN) signaling.318 In the CD4+ T cells of SLE patients, ac4C modification in mRNAs is highly conserved and enriched in mRNA CDS regions and participates in critical immune and inflammatory signaling in SLE pathogenesis.319
Th1/Th2 cells Th1 cells are characterized by the expression of the transcription factor T-bet and IFN-γ secretion, and participate in immune responses against intracellular pathogens.320–322 Th2 cells are characterized by the expression of the transcription factor GAΤA3 and IL-4/5/13 and participate in immune responses against larger extracellular pathogens.320–322 A preliminary study using the clustering method demonstrated that m6A may be involved in the Th1/Th2 imbalance and the occurrence of allergic asthma.323
Th17 cells Defined by expression of the master transcription factor RORγt and the production of the lineage cytokines IL-17/IL-22, Th17 cells participate in the elimination of bacteria and fungi and in the pathogenesis of autoimmune diseases.324–326 In enterotoxigenic Bacteroides fragilis-induced intestinal inflammation and tumorigenesis, METTL14-dependent m6A modification promoted the splicing and generation of miR-149-3p to regulate Th17 differentiation.327
Tfh cells With Bcl6 as the lineage-defining transcription factor, Tfh cells are a specialized CD4+ T cell subset essential for germinal centers and B cell responses.328–330 METTL3/METTL14-catalyzed m6A modification of ICOS mRNA suppressed ICOS expression, resulting in impaired Tfh cell differentiation.331 METTL3-catalyzed m6A modification on the Tcf7 mRNA 3ʹUTR enhanced the stability of Tcf7 mRNA, ensuring TCF-1 expression in maintaining Tfh differentiation.332
Treg cells Specifically expressing FoxP3 in the nucleus, Treg cells play immune regulatory roles in maintaining immune cell homeostasis and preventing immunopathology.333–335 Mettl14 deficiency led to the inability to maintain the differentiation of naïve T cells into induced Treg cells and the Mettl14-deficient Treg cells exhibited impaired function in suppressing naïve T cell–induced inflammation.336 Mettl3/m6A deficiency in Treg cells increased Socs mRNA levels, leading to deactivation of the IL-2–STAT5 signaling which is integral in maintaining the functions and stability of Treg cells.337
CD8+ T cells
Naive CD8+ T cells proliferate and differentiate into various effector and memory cell types following different activation signals. CD8+ T cells can persist for years and are involved in protective immunity against intracellular pathogens and tumors.338–341 Many studies have demonstrated that m6A methylation regulators are closely associated with CD8+ T cell infiltration in various cancers.342–345 Furthermore, m6A methylation regulators are involved in regulating CD8+ T cell functions. For example, Ythdf1-deficient mice exhibited an elevated antigen-specific CD8+ T cell antitumor response.346 In tumor-associated macrophages, METTL14 deficiency led to anomalous CD8+ T cell differentiation, driving CD8+ T cell dysfunction and repressing CD8+ T effector cell activation.347 Tumor-intrinsic FTO restricted the activation and effector states of CD8+ T cells; knockdown of FTO impaired tumor cell glycolytic activity, which restored CD8+ T cell function.347 m1A levels were negatively related to CD8+ T effector cell proliferation in colon cancer.348
RNA modifications and B lymphocytes
Generally, B lymphocytes are well known for their function of producing antibodies in the adaptive immune response; they are also key modulators of the innate immune response.349–351 Under antigen stimulation, mature B cells are activated and differentiate into memory B cells or plasma cells, which secrete antibodies.352–354 Some studies have reported that m6A modification also participates in B cell biology. For example, METTL14 deficiency inhibits mRNA m6A methylation in developing B cells and blocks IL-7-induced pro-B cell proliferation and the large-pre-B to small-pre-B transition, resulting in severe B cell development stagnation in mice.355 The RNA exosome cofactor MPP6, m6A modification, and m6A readers play vital roles in modulating lncRNA processing, DNA recombination, and development in B cells.356 m6A methylation was significantly decreased in the plasma cells of patients with multiple myeloma, which was due to the upregulation of FTO; FTO facilitated multiple myeloma cell proliferation, migration, and invasion by targeting HSF1–HSPs in a YTHDF2-dependent manner.357 In addition, ADAR1 is essential for normal B lymphopoiesis in the bone marrow and peripheral maintenance.358
RNA modifications and DCs
DCs are key regulators of the innate and adaptive immune responses. They integrate signals from pathogens or other damage and present processed antigens to naïve T cells to control T cell differentiation.359–362 Similar to other immune cell types, the expression of the m6A methylation regulators in diseases is also associated with DC infiltration or depletion.363,364 Specially, chemokine receptor 7 (CCR7) increases lnc-Dpf3 expression by reducing m6A modification to prevent its degradation, and lnc-Dpf3 functions in the feedback control of DC migration and inflammatory responses by coupling the epigenetic and metabolic pathways.365 YTHDF1 identifies m6A-modified mRNAs encoding lysosomal proteases and promotes the translation of these transcripts in DCs, thereby suppressing the cross-presentation of wild-type DCs.346 DCs exposed to m5C-, m6A-, m5U-, s2U-, or Ψ-modified RNAs express decreased cytokines and activation markers, suggesting that nucleoside modifications repress the latent capacity of RNAs to activate DCs.8 Mettl3-mediated m6A modification maintained DC maturation and activation by promoting the translation of key factors, including CD40, CD80, and the TLR signaling adaptor TIRAP.365 Recognition of mRNA m6A methylation by YTHDF1 promoted the translation of lysosomal proteases in DCs and suppressed cross-priming of CD8+ T cells, resulting in defective immune recognition and tumor immune evasion.346 ADAR1 is required for the differentiation, functionality, and survival of DCs and alveolar macrophages, which involves the A-to-I editing of several coding genes and lncRNAs.366
RNA modifications and natural killer cells
Natural killer (NK) cells are cytotoxic lymphocytes of the innate immune system characterized by target cell killing and cytokine production, functioning in controlling viral and intracellular bacterial infections and tumors, as well as regulating other immune cells.367–370 m6A also influences the functions of NK cells. METTL3-mediated m6A methylation guaranteed the sufficient response of AKT and MAPK signaling to IL-15 by raising SHP-2 expression, thus exerting critical roles in maintaining NK cell homeostasis and anti-tumor immunity.371 YTHDF2 is increased in NK cells activated by cytokines, tumors, and cytomegalovirus infection, and is essential for maintaining NK cell homeostasis and maturation; YTHDF2 is also required for IL-15–mediated NK cell survival, proliferation, and effector functions by forming a STAT5–YTHDF2 positive feedback loop.372 In addition, YTHDF2 modulates NK cell proliferation and division partially via reducing Tardbp mRNA stability.372
RNA modifications and monocytes or macrophages
Monocytes and macrophages play an essential role in the innate immune system and present phagocytic activity to exhibit antimicrobial, homeostatic, and immunoregulatory functions.373–376 Due to the wide application of monocyte/macrophage cell lines such as THP-1 and RAW264.7,377,378 there have been many studies investigating the roles of RNA modifications in monocytes or macrophages.
Some recent studies demonstrate that m6A modification plays critical roles in the antiviral immunity of monocytes and macrophages. For example, m6A-modified HIV-1 RNA escaped RIG-I-mediated RNA sensing and IFN-I-mediated innate antiviral immune responses in differentiated human monocytic cells and primary monocyte-derived macrophages.374 After vesicular stomatitis virus infection, METTL3 in monocytes/macrophages translocated to the cytoplasm to promote m6A modification of viral RNAs. Then, the m6A-modified viral RNAs were reshaped with decreased double-stranded RNA loads to restrain innate sensing efficacy by MDA5 or RIG-I, resulting in inactivation of the global innate immune signaling pathways.379 In response to viral infection, macrophages impaired ALKBH5 enzymatic activity and induced m6A modification-mediated inactivation of the OGDH–itaconate pathway to inhibit viral replication.380 In response to DNA viruses, HNRNPA2B1 promoted m6A modification and nucleocytoplasmic trafficking of CGAS, IFI16, and STING mRNAs, thereby triggering the downstream cytoplasmic TBK1–IRF3 signaling in macrophages.381 DDX46 recruited ALKBH5 via its DEAD helicase domain to demethylate m6A-modified antiviral transcripts, impeding their nuclear exportation and translation and resulting in impaired IFN production and antiviral innate responses.382 Under homeostatic conditions, YTHDF3 cooperated with PABP1 and eIF4G2 to enhance FOXO3 translation by binding to the translation initiation region of FOXO3 mRNA and functioning as a negative regulator of antiviral immunity.381 BCG vaccine exposure can cause increased ADAR1 expression and subsequent enhanced A-to-I editing events in human macrophages to participate in trained immunity.383
m6A modification also regulates monocyte inflammation and immune activity. METTL3-mediated m6A modification and YTHDF2-mediated recognition promoted PGC1A mRNA degradation, leading to insufficient ATP production and excessive reactive oxygen species accumulation in monocyte inflammation.384 In the peripheral blood immune cells from patients with colorectal cancer, m6A modification was the most abundant in monocytes, and the m6A levels in the monocytes were negatively related to the monocyte immune response.385
m6A modification is involved in various aspects of macrophage biology, including polarization, differentiation, activation, inflammation, and pyroptosis.386–389 For example, IGF2BP2 reads the m6A modification on TSC1 and PPARG mRNA to regulate TSC1 and PPAR-γ expression, thereby skewing M1 macrophages to M2 activation through the TSC1–mTORC1 pathway and PPAR-γ-mediated fatty acid uptake.386 METTL3-mediated m6A modification of Irakm mRNA accelerated its degradation, resulting in TLR signaling-mediated macrophage activation.386 METTL3 increased MALAT1 levels through m6A methylation to downregulate USP8; the reduced USP8 decreased TAK1 ubiquitination and degradation, which promoted macrophage pyroptosis and inflammation.389 TUT7 functioned as a regulator in TLR4-mediated inflammation in macrophages by uridylating and thereby destabilizing the mRNAs of inflammatory mediators, including Zc3h12a.227 Through targeting the miR-21 precursor, ADAR1 reduces the generation of mature miR-21, then facilitating the polarization of macrophages toward the M2 phenotype via regulating the Foxo1-IL-10 axis.390
RNA modifications and granulocytes
It is well known that granulocytes are divided into three types—neutrophils, eosinophils and basophils.391,392 There are relatively few and superficial studies on m6A regulation of granulocytes. m6A modification on c-Rel and Rela mRNA inactivated the NF-κB pathway to suppress IL-8 secretion, thereby inhibiting neutrophil infiltration in papillary thyroid cancer progression in a METTL3- and YTHDF2-dependent manner.393 Other studies only found that the expression of m6A methylation regulators in tumors was associated with the infiltration of granulocytes, especially neutrophils.393–395
To summarize, for functions, RNA modifications regulate various biological processes of immune cells, including development, differentiation, activation, migration and polarization, thus modulating the immune responses. For molecular mechanism, RNA modifications target immune cell RNAs that are responsible for those biological processes and influence RNA processes including generation, transportation, function and metabolization, leading to alterant immune cell biology. However, there are many kinds of RNA modifications and their functions are complex; immune cells are also diverse, and each cell type has its own unique cellular processes. Therefore, although the mechanism presented above is a common one explicating the interaction between RNA modifications and immune cells, the roles of a certain RNA modification in a specific immune cell need to be concretely investigated. Since the relevant research is still in its infancy, more work is needed to further improve the interaction network between RNA modification and immune cells.
Roles of RNA modifications in immune related diseases
The immune system, consists of innate and adaptive immune, functions in the host defense against harmful antigens and immune homeostasis.396–398 Immune cells are important constituents of the immune system, the dysregulation of which can result in immune related diseases, such as cancers, infection, inflammatory disorders, and autoimmune diseases.399–402 Therefore, via regulating the biological processes of immune cells, RNA modifications can participate in the pathogenesis of immune related diseases.
Cancers
Although previous research on cancers mainly focused on the malignant phenotypes of the cancer cell itself, in recent years, there are more and more studies on anti-tumor immunity, such as immune checkpoint, immune cell infiltration, cancer immune escape, and cancer immunotherapy.403–407 RNA modifications have been widely investigated in cancers, and they play vital roles in various cellular biology aspects of cancer cells, such as proliferation, metastasis, metabolism, apoptosis, and treatment resistance.1,15,18,79,175 Relatively, the roles of RNA modifications mediating immune cell biology in tumor immunization are not extensively and profoundly considered.
The most reported RNA modification mediating immune cells in cancers is their influence on immune cell infiltration of tumors.408–410 m6A modification and multiple m6A regulators have been verified to be closely associated with the infiltration of various immune cells in plenty of human cancers.408,409,411 Relatively less, m5C, m1A, m7G, ac4C, and Ψ are also found to be related to immune cell infiltration in cancers (Fig. 6).412–417 However, most of these studies investigated the RNA modifications and regulators in cancer tissues and cells, but not in immune cells, and they did not elaborately explain how RNA modification disorders affect immune cell infiltration. Here, we propose an idea that chemokines secreted by tumors may be an intermediate medium regulated by RNA modification in this process.
Fig. 6.
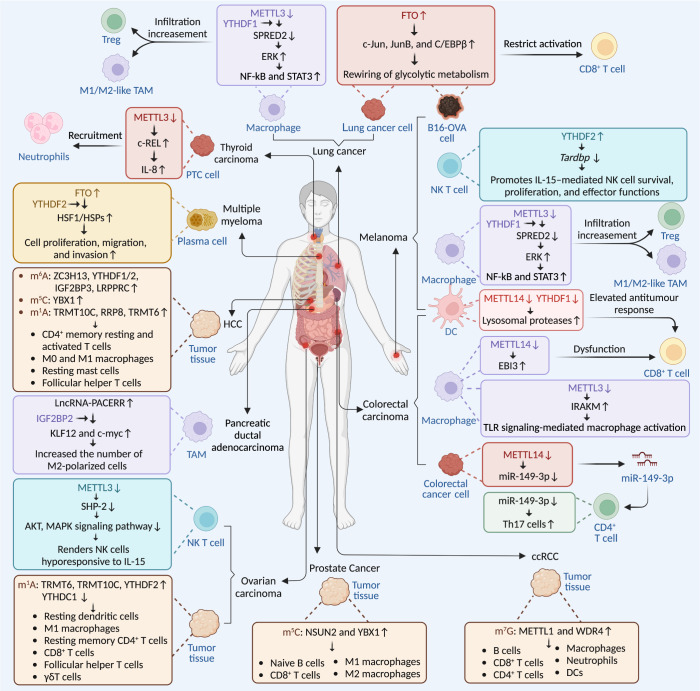
RNA modifications and immune cells in diverse cancers. RNA modifications, especially m6A modification, mainly play a positive role in regulating immune function in various cancers as illustrated in the figure. In melanoma and lung cancer, FTO-mediated m6A demethylation in tumor cells elevates the transcription factors c-Jun, JunB, and C/EBPβ, allowing the rewiring of glycolytic metabolism, thereby restricting the function of CD8+ T cells and inhibiting tumor growth. Besides, ablation of Mettl3 in myeloid cells promotes tumor growth and metastasis via impairing the YTHDF1-mediated translation of SPRED2, which enhances the activation of NF-kB and STAT3 through the ERK pathway, thereby increasing M1/M2-like tumor-associated macrophage and regulatory T cell infiltration into tumors. In melanoma, upregulation of YTHDF2 in NK cells promotes NK cell effector function and is required for IL-15–mediated NK cell survival and proliferation by targeting Tardbp. In melanoma and colorectal carcinoma, loss of YTHDF1 in classical DCs enhanced the cross-presentation of tumor antigens and the cross-priming of CD8+ T cells via increasing the m6A sites on transcripts encoding lysosomal proteases recognized by YTHDF1, which could be written by METTL14. In colorectal carcinoma, Mettl3- or Mettl14-deficient macrophages showed faster tumor growth via slowing down the degradation of Irakm, encoding a negative regulator of TLR4 signaling, or driving CD8+ T cells to dysfunctional ones by directly targeting Ebi3, respectively. Moreover, elevation of METTL14 in colorectal cancer cells promotes the differentiation of CD4+ T cells into Th17 cells via exosomes included miR-149-3p. In Thyroid carcinoma, METTL3 reduction in PTC cells recruits tumor-associated neutrophils into tumor tissue through IL-8, thereby further promoting tumor development, while in ovarian carcinoma, depletion of METTL3 in NK cells inhibits cell infiltration ability and function, leading to accelerated tumor development via reducing SHP-2 expression as well as the activation of AKT and MAPK signaling pathway. In multiple myeloma, upregulation of the demethylase FTO in plasma cells plays a tumor-promoting and pro-metastatic role in MM by targeting HSF1 which could be recognized by YTHDF2. In pancreatic ductal adenocarcinoma, LncRNA-PACERR increased the number of M2-polarized cells and facilized cell proliferation, invasion and migration via binding to IGF2BP2 to enhance the stability of KLF12 and c-myc, thereby activating KLF12/p-AKT/c-myc pathway through binding to miR-671-3p. Extensive bioinformatics analysis revealed the potential key roles of RNA modifications other than m6A modification in immune cell infiltration in diverse types of tumors. DC dendric cell, NK T cell natural killer T cell, PTC papillary thyroid carcinoma, HCC hepatocellular carcinoma, ccRCC clear cell renal cell carcinoma, TAM tumor-associated macrophage, HSF1 heat shock factor 1
There have been some studies exploring the RNA modification dysregulations in immune cells in the tumor immune microenvironment (TIME) and their roles in cancer progression.13,418–420 As expected, m6A is the most extensive and in-depth modification inquired. METTL3 is downregulated in tumor-infiltrating NK cells, which affects Ptpn11 m6A modification and downstream IL-15-induced signaling, leading to homeostasis disruption, impaired infiltration, and function of NK cells in TIME, resulting in cancer development.371 Through targeting and inhibiting the stability of Tardbp mRNA, YTHDF2 is involved in maintaining NK cell homeostasis, maturation, IL-15–mediated survival, and antitumor activity.372 m6A modification can also influence macrophage reprogramming by mediating SPRED2 translation, and, thereby regulating the activation of NF-κB and STAT3 signaling; METTL3 deficiency impairs the YTHDF1-mediated translation of SPRED2, orchestrating growth, metastasis, and anti-PD-1 therapeutic efficacy of cancer.421 Another study confirmed this biological effect of m6A in macrophages from another aspect that loss of Mettl3 impairs the TLR4 signaling in macrophage activation by reducing Irakm mRNA degradation.386 Additionally, lncRNA-PACERR induces the polarization of pro-tumor macrophages in an IGF2BP2 and m6A-dependent manner.422 YTHDF1 negatively regulates the anti-tumor immune responses of DCs by promoting the translation of m6A-modified mRNAs encoding lysosomal proteases to impair immune recognition, leading to tumor immune evasion.346 In T cells, m6A modification targets specific genes to control T cell differentiation and maintains the suppressive effects of Tregs, functioning as a negative regulator in the anti-tumor immune responses.337 CD8+ T cells are direct effector cells of anti-tumor immunity, but there are few studies revealing the m6A disorders in this cell type in cancers. Nevertheless, m6A can influence the anti-tumor response of CD8+ T cells via controlling the biological processes of other related cells in the TIME, such as tumor cells, macrophages, and DCs.346,347,423 Especially, the progression of multiple myeloma, a B-cell lymphoma, is mediated by m6A in an FTO and YTHDF2 dependent manner.357 Relatively, there are no comprehensive studies on the biological functions and molecular mechanisms of other modifications regulating immune cells in cancers till now. (Fig. 6 and Table 2).
Table 2.
Main functions of RNA modifications on various immune cells
| Type of Immune cell | RNA modifications | Regulatory enzymes | Target RNA | Main functions | Ref. |
|---|---|---|---|---|---|
| T cells | m5C | NSUN2 | IL17A | Enhances IL-17A mRNA translation. | 306 |
| (uncategorized) | m7G | RNMT | TOP | Modulates ribosome synthesis and activate T cells. | 142 |
| CD4+ T cells | m6A | ALKBH5 | CXCL2, IFNG | Enhances the pathogenicity of CD4+ T cells. | 315 |
| (uncategorized) | METTL3 | Cd40, Cd80, Tirap | Promotes DC function in CD4+ T-cell activation. | 365 | |
| m5C | NSUN1 | HIV TAR RNA | Hamper HIV-1 transcriptional elongation and viral latency in CD4+ T cells. | 317 | |
| N.A. | N.A. | Associated with immune system, cytokine signaling and interferon signaling in SLE. | 318 | ||
| ac4C | N.A. | USP18, GPX1, RGL1 | Regulates mRNA catabolic processes and translational initiation in SLE. | 319 | |
| Uridylation | TUTases | N.A. | Reduces the stability of miRNAs and promotes CD4+ T cell activation. | 226 | |
| A-to-I editing | ADAT1 | dsRNA | Participates in thymic T cell maturation | 307,308 | |
| Th1/Th2 cells | m6A | N.A. | N.A. | Influences the Th1/Th2 imbalance in allergic asthma. | 323 |
| Th17 cells | m6A | METTL14 | miR-149 | Regulates Th17 differentiation in intestinal inflammation and malignancy. | 327 |
| Tfh cells | m6A | METTL3, METTL14 | ICOS | Attenuates Tfh cell differentiation. | 331 |
| METTL3 | Tcf7 | Activates Tfh transcriptional program to maintain Tfh differentiation. | 332 | ||
| Treg cells | m6A | METTL14 | N.A. | Facilitates the differentiation of Treg and suppress the inflammatory response in IBD. | 336 |
| N.A. | Socs | Maintains the functions and stability of Treg cells. | 337 | ||
| CD8+ T cells | m6A | N.A. | N.A. | Regulates CD8+ T cells infiltration in cancers. | 342–344 |
| YTHDF1 | mRNAs encoding lysosomal proteases | m6A modification in DCs suppresses the cross-priming of CD8+ T cells. | 346 | ||
| METTL14, YTHDF2 | Ebi3 | m6A in macrophages maintains CD8+ T cell differentiation and activation. | 423 | ||
| FTO | c-Jun, JunB, C/EBPβ | Restricts glycolytic metabolism of cancer cells to activate CD8+ T cells. | 347 | ||
| m1A | N.A. | N.A. | Negatively related to CD8+ proliferation ability of T effector cells in colon cancer. | 348 | |
| m5C, Ψ | N.A. | N.A. | Influences immune responses of CD8+ T cells. | 309 | |
| B cells | m6A | METTL14 | N.A. | Mediates IL-7-induced cell proliferation of pro-B cell and large-pre-B-to-small-pre-B transition. | 355 |
| METTL3 | lncRNAs | Promotes DNA recombination and development in B cells. | 356 | ||
| FTO, YTHDF2 | HSF1 | Suppresses proliferation, migration, and invasion in plasma cells of multiple myeloma. | 357 | ||
| A-to-I editing | ADAR1 | N.A. | Critical for normal B lymphopoiesis in the bone marrow and peripheral maintenance. | 358 | |
| DCs | m6A | N.A. | N.A. | Associated with the infiltration or depletion of DCs cancers and IBD. | 363,364 |
| N.A. | lnc-Dpf3 | Facilitates DC migration and inflammatory responses functions in a feedback manner. | 447 | ||
| METTL3 | Tirap, Cd40, Cd80 | Activates DCs through TLR4/NF-κB signaling pathway and T-cell activation. | 365 | ||
| YTHDF1 | mRNAs encoding lysosomal proteases | Restricts cross-priming of CD8+ T cells mediated by DCs. | 346 | ||
| m6A/Ψ | N.A. | N.A. | May influence the activations of DCs. | 8 | |
| A-to-I editing | ADAR1 | N.A. | Essential for the differentiation, functionality, and survival of DCs. | 366 | |
| NK cells | m6A | METTL3 | Ptpn11 | Maintains homeostasis and anti-tumor immunity of NK cells. | 371 |
| YTHDF2 | Tardb | Inhibits IL-15–mediated NK cell survival, proliferation, and effector functions. | 372 | ||
| Macrophages and/or monocytes | m6A | METTL14, YTHDF1 | Socs1 | Declines macrophage responses to acute bacterial infection. | 387 |
| YTHDF2 | MAP2K4, MAP4K4 | Promotes LPS-induced inflammatory response in macrophages. | 388 | ||
| METTL14, YTHDF2 | Ebi3 | Regulates macrophages-mediated CD8+ T cell differentiation and activation to inhibit tumor growth. | 423 | ||
| N.A. | HIV-1 RNA | Facilitates HIV-1 escaping from innate antiviral immune responses of macrophages. | 374 | ||
| METTL3 | viral RNAs | Limits the innate sensing efficacy of macrophages for viral RNA. | 379 | ||
| ALKBH5 | Inhibits viral replication in macrophage. | 380 | |||
| hnRNPA2B1 | CGAS, IFI16, STING | Facilitates immune response to DNA viruses in macrophages. | 434 | ||
| ALKBH5 | antiviral transcripts | Increases interferon production and antiviral innate responses in macrophages. | 382 | ||
| YTHDF3 | FOXO3 | Inhibits antiviral immunity under homeostatic conditions in macrophages. | 381 | ||
| METTL3, YTHDF2 | PGC-1α | Increases ROS accumulation and proinflammatory cytokines level in inflammatory monocytes. | 384 | ||
| N.A. | N.A. | Negatively related to the immune response of monocytes in colorectal cancer. | 385 | ||
| IGF2BP2 | TSC1, PPAR-γ | Promotes M2 macrophages differentiation. | 443 | ||
| METTL3 | Irakm | Activate macrophages via TLR signaling. | 386 | ||
| METTL3 | MALAT | Promotes pyroptosis and inflammation of macrophages. | 389 | ||
| N.A. | N.A. | Possibly promotes infiltration of macrophages in colorectal cancer. | 395 | ||
| Uridylation | TUT7 | Zc3h12a | Stabilize IL6 mRNA expression in TLR4-mediated inflammation in macrophages. | 227 | |
| A-to-I editing | ADAR1 | N.A. | Promotes the differentiation, functionality, and survival of and alveolar macrophages. | 366 | |
| ADAR1 | N.A. | Participates in trained immunity | 383 | ||
| ADAR1 | miR-21 precursor | Reduces the generation of mature miR-21, therefore facilitating the polarization of macrophages toward the M2 phenotype via Foxo1-IL-10 axis. | 390 | ||
| Granulocytes | m6A | METTL3 | c-Rel, RelA | Inhibit neutrophil infiltration in papillary thyroid cancer progression. | 464 |
| N.A. | N.A. | Related to the infiltration of neutrophils in breast cancer and colorectal cancer. | 393–395 |
m6A N6-methyladenosine, m5C 5-methylcytosine, m1A N1-methyladenosine, m7G 7-methylguanosine, ac4C N4-acetylcytidine, ψ pseudouridine, A-to-I editing adenosine-to-inosine RNA editing, DC dendritic cell, SLE systemic lupus erythematosus, IBD inflammatory bowel disease, ROS reactive oxygen species, TLR toll-like receptors, LPS Lipopolysaccharide, HIV human immunodeficiency virus, dsRNA double-stranded RNA
Despite all this, the roles of RNA modifications mediating immune cell biology in cancer immunization remains largely unclear. There seem to be some paradoxes as well as enlightenments. For instance, as we discussed above, m6A deficiency will lead to the disability of some anti-tumor immune cells, whereas YTHDF1 deficiency enhances anti-tumor immune responses. In this regard, we think researchers should comprehensively consider the other roles of writers, erasers, and readers, not just focusing on their regulation of RNA modifications. Moreover, as we know, RNA modifications are vital modulators of normal cell biology, and it is easy to understand that their delicacy may cause the disability of immune cells; but we don’t know what will happen if these modifications are excessive, and whether there is a balance. Besides, the level and functions of some modifications, such as m6A, are diverse between cancer cells and infiltrated immune cells.1,15 This indicates researchers to separate cancer cells and infiltrated immune cells when analyzing human or animal tumor samples.
Infectious diseases
Similar to cancers, the pathogenesis and development of infectious diseases are closely related to the immune defense deficiency that involves the deficiency of the immune system itself and immune escape from pathogens.424–427 According to existing literature, RNA modifications are critical participators in the progression of infectious diseases by affecting the biology of immune cells.
In recent years, many studies have focused attention on the roles of RNA modifications in viral infection.53,302,428–430 On one aspect, RNA modifications, such as m6A, m5C, ac4C, Ψ, and RNA editing, directly act on viral RNAs, thus influencing RNA structure, RNA nuclear export, translation, stability, and replication.53,430–433 On the other aspect, RNA modifications can regulate host responses to viral infection by mediating viral RNA sensing and signaling, cytokine responses, as well as immune cell functions, which are emphasis discussion of this text. The roles of RNA modifications in regulating immune cell functions in antiviral infection can also be explicated from two perspectives. The first one is that RNA modifications on viral RNAs repress innate immune signaling pathways. For example, m6A-modified HIV-1 and vesicular stomatitis virus RNAs restrain the innate sensing efficacy of MDA5 or RIG-I and thereby impaired IFN-I-mediated innate antiviral immune responses in monocytes and macrophages.374,379 The other one is that RNA modifications affect the key factors of antiviral immunity in immune cells, especially in innate immune cells. For example, in monocytes and macrophages, m6A modification affects antiviral transcripts including CGAS, IFI16, STING, Mavs, Traf3, Traf6, and FOXO3, as well as signaling pathways including OGDH–itaconate, TBK1–IRF3, and IFN signaling to function in inhibiting viral replication and antiviral innate immunity.380–382,434 In NK cells, except for the antitumor activity, YTHDF2 is also essential for the antiviral activity of NK cells by targeting Tardbp.372 Especially, as CD4+ T cells are the target of HIV infection, RNA modification participates in the viral processes including replication, nuclear export, transcriptional elongation, and viral latency in CD4+ T cells via modulating biological processes inside CD4+ T cells as described above. After COVID-19 infection, A-to-I editing of endogenous Alu RNAs is decreased in normal human lung cells and in lung biopsies, which may represent the responses of the hosts.435 ADAR1 mediated RNA editing on extensive duplex RNA structures can lead to repressed innate immune responses and is profitable for viral replication, which indicates that A-to-I editing prevents autoimmunity while also favoring pathogens (Table 3).436
Table 3.
RNA modifications and immune cells in infectious, inflammatory and autoimmune diseases
| Type of immune disease | Involved disease | RNA modifications | Main functions | Ref. |
|---|---|---|---|---|
| Infectious diseases | HIV-1 and VSV infection | m6A | Modified HIV-1 and VSV RNAs restrain the innate sensing efficacy of MDA5 or RIG-I and thereby impaired IFN-I-mediated innate antiviral immune responses in monocytes and macrophages. | 3,14 |
| VSV and HSV-1 infection | m6A | Inhibits viral replication and antiviral innate immunity via affecting various antiviral transcripts in in monocytes and macrophages. | 380–382,434 | |
| CMV-1 infection | m6A | Essential for the antiviral activity of NK cells by targeting Tardbp. | 372 | |
| COVID-19 infection | A-to-I editing | Edited endogenous Alu RNAs is decreased in normal human lung cells and in lung biopsies, possibly representing the responses of the hosts. | 435 | |
| Measles virus infection | A-to-I editing | Extensive duplex RNA structure edited by ADAR1 can lead to repressed innate immune responses and is profitable for viral replication. | 436 | |
| DNA and RNA virus infection | m6A, m5C, ac4C, Ψ, A-to-I editing | Affects RNA structure, RNA nuclear export, translation, stability, and replication. | 53,430–433 | |
| Inflammatory and autoimmune diseases | Hyperhomocysteinemia | m5C | NSUN2 upregulates IL-17A expression in an m5C-dependent manner in T lymphocytes. | 306 |
| Allergic asthma | m6A | Participates in the Th1/Th2 imbalance. | 323 | |
| IBD | m6A | Affects immune infiltration and therapeutic response. | 153 | |
| IBD | m6A | Mettl14 deficiency causes impaired induction of naïve T cells into iTreg cells by decreasing RORγt expression, contributing to spontaneous colitis. | 336 | |
| IBD | A-to-I editing | Impaired A-to-I editing due to Adar1 in CD4+ T cell leads to abnormal thymic T cell maturation and impaired negative selection, resulting in spontaneous colitis. | 307,308 | |
| Colon and lung Inflammation | m6A | IGF2BP2 switches M1 macrophages to M2 activation by stabilizing TSC1 and PPARγ in an m6A-dependent manner, leading to inflammatory diseases. | 443 | |
| Acute lung injury and respiratory distress syndrome | m6A | Ablation of METTL14 in myeloid cells exacerbates macrophage responses to acute bacterial infection. | 387 | |
| Liver fibrosis | m6A | Through essentially stimulating pyroptosis and inflammation of macrophages, the signaling cascade METTL3/MALAT1/PTBP1/USP8/TAK1 aggravates liver fibrosis. | 389 | |
| SLE | m5C | m5C level and NSUN2 expression are decreased in CD4+ T cells, and hypermethylated m5C is significantly involved in the immune- and inflammation-related pathways. | 318 | |
| SLE | ac4C | ac4C modification in mRNAs of SLE CD4+ T cells is highly enriched in CDS regions and involved in the immune and inflammatory signaling of SLE pathogenesis. | 319 | |
| SLE | A-to-I editing | Up-regulated ADAR1 in SLE T cells is a potential mechanism accounting for the mutations in the RI alpha subunit of type 1 protein kinase A. | 444 | |
| SLE | A-to-I editing | Involved in generating or elevating the autoantigen load. | 445 | |
| Autoimmune encephalomyelitis | m6A | Ablation of ALKBH5 resulted in increased m6A modification on IFNG and CXCL2 mRNA and impaired responses of CD4+ T cells, leading to repress autoimmunity. | 315 | |
| Systemic sclerosis | A-to-I editing | A-to-I editing mediated by ADAR1p150 in PBMCs are closely related to type I IFN responses. | 270 | |
| Allogeneic transplant | A-to-I editing | Edited RNA can suppress the host antigraft response and promote graft survival through the ADAR1-miR-21-Foxo1-IL-10 axis. | 390,446 |
m6A N6-methyladenosine, m5C 5-methylcytosine, ac4C N4-acetylcytidine, ψ pseudouridine, A-to-I editing adenosine-to-inosine RNA editing, HIV human immunodeficiency virus, VSV vesicular stomatitis virus, CMV cytomegalovirus, COVID-19 Corona Virus Disease 2019, IBD inflammatory bowel disease, SLE systemic lupus erythematosus
Unfortunately, there is no report on the roles of other RNA modifications other than m6A or A-to-I editing in regulating immune cells in infectious diseases. Also, there is few study on the roles of RNA modifications regulating immune cells in infectious diseases induced by other pathogens such as bacteria and fungi. In addition, as we reviewed above, the roles of RNA modifications in antiviral processes are reported only in innate immunity, while its functions in adaptive immunity are ignored and may be of good research interest. Due to the pathogenesis similarity in immune defense deficiency, the research ideas on cancer may provide lessons for infectious diseases in this field.
Inflammatory and autoimmune diseases
Inflammation and immune responses are critical in opposing harmful stimuli and injury, while their overreaction or being out of control will lead to inflammatory and autoimmune diseases, causing tissue damage and organ dysfunction.437–439 Generally speaking, inflammatory diseases and autoimmune diseases are different, but they share some similar pathogenesis,440–442 so we discuss them together here. To date, there have some studies verified that RNA modifications may exert biological functions during inflammation and autoimmunity by regulating immune cells.
In hyperhomocysteinemia, NSUN2 upregulates IL-17A expression by inducing IL-17A mRNA m5C modification in T lymphocytes to mediate chronic inflammation.306 In allergic asthma, m6A may participate in the Th1/Th2 imbalance.323 In inflammatory bowel disease (IBD), m6A modification may affect immune infiltration and therapeutic response,153 and Mettl14 deficiency can cause impaired induction of naïve T cells into iTreg cells by decreasing RORγt expression, thereby leading to spontaneous colitis.336 Deficiency of Adar1 mediated impaired A-to-I editing in CD4+ T cell will lead to abnormal thymic T cell maturation and impaired negative selection, thereby resulting in autoimmunity disease such as spontaneous colitis.307,308 By regulating various aspects of macrophage biology, including polarization, differentiation, activation, inflammation, and pyroptosis, m6A has been found to serve as a mediator in inflammatory diseases including ulcerative colitis, cytokine storm after bacterial infection and liver fibrosis (Table 3).387,389,443
In SLE, m5C level and NSUN2 expression are decreased in CD4+ T cells, and hypermethylated m5C is related to the immune- and inflammation-related pathways.318 ac4C modification in mRNAs of SLE CD4+ T cells is highly conserved and enriched in CDS regions and involved in the immune and inflammatory signaling of SLE pathogenesis.319 ADAR1 mRNA was significantly up-regulated in SLE T cells, which may be a potential mechanism accounting for the mutations in the RI alpha subunit of type 1 protein kinase A.444 In SLE and some other autoimmune diseases, increased A-to-I RNA editing is involved in generating or elevating the autoantigen load to facilitate autoimmunity progression.445 In autoimmune encephalomyelitis, ablation of ALKBH5 resulted in increased m6A modification on IFNG and CXCL2 mRNA, as well as impaired responses of CD4+ T cells to repress autoimmunity.315 In systemic autoimmunity characterized by chronic or acute type I IFN pathway such as systemic sclerosis and other disease contexts, ADAR1p150 isoform mediated A-to-I editing in PBMCs are closely related to type I IFN responses.270 ADAR1 can edit putatively immunogenic dsRNA substrates to evade MDA5-mediated dsRNA sensing to suppress inflammatory and autoimmune diseases.446 In the allogeneic transplant model, ADAR1 mediated RNA editing can suppress the host antigraft response and promote graft survival through the ADAR1-miR-21-Foxo1-IL-10 axis (Table 3).390
Although there are few studies on RNA modification regulating immune cells in inflammatory and autoimmune diseases, as we described above, many studies have confirmed the modulation of RNA modifications on the biology of immune cell closely related to these diseases, which build potential connections between cell biology and human diseases. For example, RNA modifications can influence the biology of DCs, monocytes and macrophages, and mediate inflammatory cytokine secretion,8,142,365,384,447 they also probably function in many inflammatory diseases. RNA modifications can affect the activation of T cells and differentiation of Th17 cells, Tfh cells and Treg cells,142,327,332,337 which play critical roles in the immune dysregulation of some autoimmune diseases such as SLE and multiple sclerosis,448,449 they are likely to participate in the pathogenesis of these diseases. In addition, the research on cancers and infectious diseases listed above may also provide feasible ideas. Due to the contrary roles of immune cell activation and infiltration in cancers and infectious diseases with inflammatory and autoimmune diseases, it is possible that the roles of RNA modifications regulating immune cells are opposite in these diseases.
Conclusions and perspectives
In this review, we introduced eight RNA modifications including m6A, m5C, m1A, m7G, ac4C, Ψ, uridylation, and A-to-I editing, and summarized their influence on the biology of immune cells, as well as their roles in immune related diseases by regulating immune cells. The modifications involve various kinds of RNAs, such as mRNAs, noncoding RNAs, tRNAs, rRNAs, or even exogenous RNAs (eg., viral RNAs and synthetic RNAs). These modifications are executed by RNA-modifying enzymes such as writers, erasers and readers, and influence RNA processes including generation, transportation, function, and metabolization. Based on these molecular functions, RNA modifications participate in various biological processes of immune cells, including development, differentiation, activation, migration, and polarization, thus modulating the immune response and participating in the pathogenesis of immune related diseases (Fig. 6 and Table 3).
Currently and a long time in the future, m6A will continue to be a research hotspot in this field, especially in the field of anti-tumor immunity, with relatively adequate background knowledge and mature research technology available. In addition, RNA modifications are mostly investigated in T lymphocytes and monocytes/macrophages, which are relatively easy to obtain, and in T cells or monocytes/macrophages related immune processes and diseases. Moreover, due to the global epidemic of COVID-19, RNA modifications that are involved in antiviral immunity may also be of promising research interest.
There remain many questions that should be addressed to fully understand the impact of RNA modifications on immune cell biology. Nevertheless, there is continuous progress in this field and there are presently many meaningful points to study. First, to what extent do RNA modifications regulate immune cell biological processes? Are they the main mediators or auxiliary participants? In other words, how helpful would it to rectify RNA modification abnormalities in the treatment of immune related diseases? This is important for designing therapeutic targets and a critical issue to be solved. Second, some RNA modifications have been proven feasible in the systemic delivery of nanoparticle formulations for regulating both immune cell immunity and inflammation in the laboratory.309,450,451 Clinical practice is still the grandest challenge, which will be a valuable research direction. Third, essentially, RNA modifications and their functions are regulated by writers, erasers and readers. However, many studies have failed to elucidate the mechanisms causing the anomalies of these regulators. As RNA modifications can target many downstream RNAs and there are no effective interfering drugs, research on the upstream factors is particularly important. Fourth, further exploration of the role of RNA modifications in various immune cells needs to be done, since the current research is mainly focused on a few modifications (e.g., m6A) and immune cells (e.g., T lymphocytes) while overlooking other modifications and immune cells. Immune cells are interconnected and RNA modifications regulate various immune and inflammatory-related factors and signaling pathways. This is a complicated regulatory network that requires us to consummate. Most important, targeting RNA modifications as a treatment in immune related diseases remains in the theoretical stage, and there are no clinical application examples at present. RNA modifications may influence almost all types of RNA, and interfering RNA modifications may cause a wide range of effects. Therefore, gene-specific RNA modification interference is a vital research bottleneck in this area.
Acknowledgements
This work was supported by grants from National Natural Science Foundation of China (No. 81872522, 82073429), Innovation Program of Shanghai Municipal Education Commission (No. 2019-01-07-00-07-E00046), Clinical Research Plan of SHDC (No. SHDC2020CR1014B, SHDC12018X06) and Program of Shanghai Academic Research Leader (No. 20XD1403300). We thank all of the authors who contributed to the knowledge reviewed in this article. Figures were created with BioRender.com.
Author contributions
L.C., R.M. and J.C. wrote the manuscript; C.G., Z.C., L.Y., Y.W. and R.F. retrieved literature; X.W. and Y.S. critically revised the manuscript. All authors have read and approved the final manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
These authors contributed equally: Lian Cui, Rui Ma, Jiangluyi Cai
Contributor Information
Xin Wang, Email: wx739976260@foxmail.com.
Yuling Shi, Email: shiyuling1973@tongji.edu.cn.
References
- 1.Barbieri I, Kouzarides T. Role of RNA modifications in cancer. Nat. Rev. Cancer. 2020;20:303–322. doi: 10.1038/s41568-020-0253-2. [DOI] [PubMed] [Google Scholar]
- 2.Delaunay S, Frye M. RNA modifications regulating cell fate in cancer. Nat. Cell Biol. 2019;21:552–559. doi: 10.1038/s41556-019-0319-0. [DOI] [PubMed] [Google Scholar]
- 3.Frye M, Harada BT, Behm M, He C. RNA modifications modulate gene expression during development. Science. 2018;361:1346–1349. doi: 10.1126/science.aau1646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao LY, Song J, Liu Y, Song CX, Yi C. Mapping the epigenetic modifications of DNA and RNA. Protein Cell. 2020;11:792–808. doi: 10.1007/s13238-020-00733-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA modifications in gene expression regulation. Cell. 2017;169:1187–1200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shi H, Wei J, He C. Where, when, and how: context-dependent functions of RNA methylation writers, readers, and erasers. Mol. Cell. 2019;74:640–650. doi: 10.1016/j.molcel.2019.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zaccara S, Ries RJ, Jaffrey SR. Reading, writing and erasing mRNA methylation. Nat. Rev. Mol. Cell Biol. 2019;20:608–624. doi: 10.1038/s41580-019-0168-5. [DOI] [PubMed] [Google Scholar]
- 8.Kariko K, Buckstein M, Ni H, Weissman D. Suppression of RNA recognition by toll-like receptors: the impact of nucleoside modification and the evolutionary origin of RNA. Immunity. 2005;23:165–175. doi: 10.1016/j.immuni.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 9.Wang Y, et al. The emerging role of m6A modification in regulating the immune system and autoimmune diseases. Front Cell Dev. Biol. 2021;9:755691. doi: 10.3389/fcell.2021.755691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dong L, Cao Y, Hou Y, Liu G. N(6) -methyladenosine RNA methylation: A novel regulator of the development and function of immune cells. J. Cell Physiol. 2022;237:329–345. doi: 10.1002/jcp.30576. [DOI] [PubMed] [Google Scholar]
- 11.Xiong X, Yi C, Peng J. Epitranscriptomics: toward a better understanding of RNA modifications. Genomics Proteom. Bioinforma. 2017;15:147–153. doi: 10.1016/j.gpb.2017.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jiang X, et al. The role of m6A modification in the biological functions and diseases. Signal Transduct. Target Ther. 2021;6:74. doi: 10.1038/s41392-020-00450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.An Y, Duan H. The role of m6A RNA methylation in cancer metabolism. Mol. Cancer. 2022;21:14. doi: 10.1186/s12943-022-01500-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deng LJ, et al. m6A modification: recent advances, anticancer targeted drug discovery and beyond. Mol. Cancer. 2022;21:52. doi: 10.1186/s12943-022-01510-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang H, Weng H, Chen J. m(6)A modification in coding and non-coding RNAs: roles and therapeutic implications in cancer. Cancer Cell. 2020;37:270–288. doi: 10.1016/j.ccell.2020.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xu T, et al. Novel insights into the interaction between N6-methyladenosine modification and circular RNA. Mol. Ther. Nucleic Acids. 2022;27:824–837. doi: 10.1016/j.omtn.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhu ZM, Huo FC, Pei DS. Function and evolution of RNA N6-methyladenosine modification. Int. J. Biol. Sci. 2020;16:1929–1940. doi: 10.7150/ijbs.45231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu L, et al. Insights into N6-methyladenosine and programmed cell death in cancer. Mol. Cancer. 2022;21:32. doi: 10.1186/s12943-022-01508-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Deng X, et al. RNA N(6)-methyladenosine modification in cancers: current status and perspectives. Cell Res. 2018;28:507–517. doi: 10.1038/s41422-018-0034-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kan RL, Chen J, Sallam T. Crosstalk between epitranscriptomic and epigenetic mechanisms in gene regulation. Trends Genet. 2022;38:182–193. doi: 10.1016/j.tig.2021.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Garcias Morales D, Reyes JL. A birds’-eye view of the activity and specificity of the mRNA m(6) A methyltransferase complex. Wiley Interdiscip. Rev. RNA. 2021;12:e1618. doi: 10.1002/wrna.1618. [DOI] [PubMed] [Google Scholar]
- 22.Huang W, et al. N6-methyladenosine methyltransferases: functions, regulation, and clinical potential. J. Hematol. Oncol. 2021;14:117. doi: 10.1186/s13045-021-01129-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, et al. Structural basis of N(6)-adenosine methylation by the METTL3-METTL14 complex. Nature. 2016;534:575–578. doi: 10.1038/nature18298. [DOI] [PubMed] [Google Scholar]
- 24.Liu J, et al. A METTL3-METTL14 complex mediates mammalian nuclear RNA N6-adenosine methylation. Nat. Chem. Biol. 2014;10:93–95. doi: 10.1038/nchembio.1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang P, Doxtader KA, Nam Y. Structural Basis for Cooperative Function of Mettl3 and Mettl14 Methyltransferases. Mol. Cell. 2016;63:306–317. doi: 10.1016/j.molcel.2016.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma H, et al. N(6-)Methyladenosine methyltransferase ZCCHC4 mediates ribosomal RNA methylation. Nat. Chem. Biol. 2019;15:88–94. doi: 10.1038/s41589-018-0184-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ignatova VV, et al. The rRNA m(6)A methyltransferase METTL5 is involved in pluripotency and developmental programs. Genes Dev. 2020;34:715–729. doi: 10.1101/gad.333369.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ren W, et al. Structure and regulation of ZCCHC4 in m(6)A-methylation of 28S rRNA. Nat. Commun. 2019;10:5042. doi: 10.1038/s41467-019-12923-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.van Tran N, et al. The human 18S rRNA m6A methyltransferase METTL5 is stabilized by TRMT112. Nucleic Acids Res. 2019;47:7719–7733. doi: 10.1093/nar/gkz619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pinto R, et al. The human methyltransferase ZCCHC4 catalyses N6-methyladenosine modification of 28S ribosomal RNA. Nucleic Acids Res. 2020;48:830–846. doi: 10.1093/nar/gkz1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pendleton KE, et al. The U6 snRNA m(6)A methyltransferase METTL16 regulates SAM synthetase intron retention. Cell. 2017;169:824–835.e814. doi: 10.1016/j.cell.2017.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aoyama T, Yamashita S, Tomita K. Mechanistic insights into m6A modification of U6 snRNA by human METTL16. Nucleic Acids Res. 2020;48:5157–5168. doi: 10.1093/nar/gkaa227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Su R, et al. METTL16 exerts an m(6)A-independent function to facilitate translation and tumorigenesis. Nat. Cell Biol. 2022;24:205–216. doi: 10.1038/s41556-021-00835-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Jia G, et al. N6-methyladenosine in nuclear RNA is a major substrate of the obesity-associated FTO. Nat. Chem. Biol. 2011;7:885–887. doi: 10.1038/nchembio.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mauer J, et al. Reversible methylation of m(6)A(m) in the 5’ cap controls mRNA stability. Nature. 2017;541:371–375. doi: 10.1038/nature21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wei J, et al. Differential m(6)A, m(6)A(m), and m(1)A Demethylation Mediated by FTO in the Cell Nucleus and Cytoplasm. Mol. Cell. 2018;71:973–985.e975. doi: 10.1016/j.molcel.2018.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zheng G, et al. ALKBH5 is a mammalian RNA demethylase that impacts RNA metabolism and mouse fertility. Mol. Cell. 2013;49:18–29. doi: 10.1016/j.molcel.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wei J, et al. FTO mediates LINE1 m(6)A demethylation and chromatin regulation in mESCs and mouse development. Science. 2022;376:968–973. doi: 10.1126/science.abe9582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Qu J, et al. RNA demethylase ALKBH5 in cancer: from mechanisms to therapeutic potential. J. Hematol. Oncol. 2022;15:8. doi: 10.1186/s13045-022-01224-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yue B, et al. Essential role of ALKBH5-mediated RNA demethylation modification in bile acid-induced gastric intestinal metaplasia. Mol. Ther. Nucleic Acids. 2021;26:458–472. doi: 10.1016/j.omtn.2021.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shi R, et al. Linking the YTH domain to cancer: the importance of YTH family proteins in epigenetics. Cell Death Dis. 2021;12:346. doi: 10.1038/s41419-021-03625-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lasman L, et al. Context-dependent functional compensation between Ythdf m(6)A reader proteins. Genes Dev. 2020;34:1373–1391. doi: 10.1101/gad.340695.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang X, et al. N(6)-methyladenosine modulates messenger RNA translation efficiency. Cell. 2015;161:1388–1399. doi: 10.1016/j.cell.2015.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Li A, et al. Cytoplasmic m(6)A reader YTHDF3 promotes mRNA translation. Cell Res. 2017;27:444–447. doi: 10.1038/cr.2017.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee Y, Choe J, Park OH, Kim YK. Molecular mechanisms driving mRNA degradation by m(6)A modification. Trends Genet. 2020;36:177–188. doi: 10.1016/j.tig.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Ries RJ, et al. m(6)A enhances the phase separation potential of mRNA. Nature. 2019;571:424–428. doi: 10.1038/s41586-019-1374-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zaccara S, Jaffrey SR. A unified model for the function of YTHDF proteins in regulating m(6)A-modified mRNA. Cell. 2020;181:1582–1595.e1518. doi: 10.1016/j.cell.2020.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J, et al. N (6)-methyladenosine of chromosome-associated regulatory RNA regulates chromatin state and transcription. Science. 2020;367:580–586. doi: 10.1126/science.aay6018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xiao W, et al. Nuclear m(6)A Reader YTHDC1 regulates mRNA splicing. Mol. Cell. 2016;61:507–519. doi: 10.1016/j.molcel.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 50.Widagdo J, Anggono V, Wong JJ. The multifaceted effects of YTHDC1-mediated nuclear m(6)A recognition. Trends Genet. 2022;38:325–332. doi: 10.1016/j.tig.2021.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Mao Y, et al. m(6)A in mRNA coding regions promotes translation via the RNA helicase-containing YTHDC2. Nat. Commun. 2019;10:5332. doi: 10.1038/s41467-019-13317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhou B, et al. N(6) -methyladenosine reader protein YT521-B homology domain-containing 2 suppresses liver steatosis by regulation of mRNA stability of lipogenic genes. Hepatology. 2021;73:91–103. doi: 10.1002/hep.31220. [DOI] [PubMed] [Google Scholar]
- 53.Li, N. & Rana, T. M. Regulation of antiviral innate immunity by chemical modification of viral RNA. Wiley Interdiscip Rev RNA. e1720 (2022). [DOI] [PMC free article] [PubMed]
- 54.Huang H, et al. Recognition of RNA N(6)-methyladenosine by IGF2BP proteins enhances mRNA stability and translation. Nat. Cell Biol. 2018;20:285–295. doi: 10.1038/s41556-018-0045-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sun CY, Cao D, Du BB, Chen CW, Liu D. The role of Insulin-like growth factor 2 mRNA-binding proteins (IGF2BPs) as m(6)A readers in cancer. Int J. Biol. Sci. 2022;18:2744–2758. doi: 10.7150/ijbs.70458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Alarcon CR, et al. HNRNPA2B1 is a mediator of m(6)A-dependent nuclear RNA processing events. Cell. 2015;162:1299–1308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Liu N, et al. N(6)-methyladenosine-dependent RNA structural switches regulate RNA-protein interactions. Nature. 2015;518:560–564. doi: 10.1038/nature14234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Liu N, et al. N6-methyladenosine alters RNA structure to regulate binding of a low-complexity protein. Nucleic Acids Res. 2017;45:6051–6063. doi: 10.1093/nar/gkx141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhou KI, et al. Regulation of co-transcriptional pre-mRNA splicing by m(6)A through the low-complexity protein hnRNPG. Mol. Cell. 2019;76:70–81.e79. doi: 10.1016/j.molcel.2019.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jiang L, et al. Interaction of tau with HNRNPA2B1 and N(6)-methyladenosine RNA mediates the progression of tauopathy. Mol. Cell. 2021;81:4209–4227.e4212. doi: 10.1016/j.molcel.2021.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Meyer KD, et al. 5’ UTR m(6)a promotes cap-independent translation. Cell. 2015;163:999–1010. doi: 10.1016/j.cell.2015.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Choe J, et al. mRNA circularization by METTL3-eIF3h enhances translation and promotes oncogenesis. Nature. 2018;561:556–560. doi: 10.1038/s41586-018-0538-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wu R, et al. A novel m(6)A reader Prrc2a controls oligodendroglial specification and myelination. Cell Res. 2019;29:23–41. doi: 10.1038/s41422-018-0113-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baquero-Perez B, et al. The Tudor SND1 protein is an m(6)A RNA reader essential for replication of Kaposi’s sarcoma-associated herpesvirus. Elife. 2019;8:e47261. doi: 10.7554/eLife.47261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang X, et al. m(6)A promotes R-loop formation to facilitate transcription termination. Cell Res. 2019;29:1035–1038. doi: 10.1038/s41422-019-0235-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wang X, et al. Crosstalk between N6-methyladenosine modification and circular RNAs: current understanding and future directions. Mol. Cancer. 2021;20:121. doi: 10.1186/s12943-021-01415-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.He PC, He C. m(6) A RNA methylation: from mechanisms to therapeutic potential. EMBO J. 2021;40:e105977. doi: 10.15252/embj.2020105977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wiener D, Schwartz S. The epitranscriptome beyond m(6)A. Nat. Rev. Genet. 2021;22:119–131. doi: 10.1038/s41576-020-00295-8. [DOI] [PubMed] [Google Scholar]
- 69.Filippova JA, et al. Are Small Nucleolar RNAs “CRISPRable”? A Report on Box C/D Small Nucleolar RNA Editing in Human Cells. Front Pharm. 2019;10:1246. doi: 10.3389/fphar.2019.01246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Yi YC, Chen XY, Zhang J, Zhu JS. Novel insights into the interplay between m(6)A modification and noncoding RNAs in cancer. Mol. Cancer. 2020;19:121. doi: 10.1186/s12943-020-01233-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Alarcon CR, Lee H, Goodarzi H, Halberg N, Tavazoie SF. N6-methyladenosine marks primary microRNAs for processing. Nature. 2015;519:482–485. doi: 10.1038/nature14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen Y, Lin Y, Shu Y, He J, Gao W. Interaction between N(6)-methyladenosine (m(6)A) modification and noncoding RNAs in cancer. Mol. Cancer. 2020;19:94. doi: 10.1186/s12943-020-01207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yankova E, et al. Small-molecule inhibition of METTL3 as a strategy against myeloid leukaemia. Nature. 2021;593:597–601. doi: 10.1038/s41586-021-03536-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dolbois A, et al. 1,4,9-triazaspiro[5.5]undecan-2-one derivatives as potent and selective METTL3 inhibitors. J. Med. Chem. 2021;64:12738–12760. doi: 10.1021/acs.jmedchem.1c00773. [DOI] [PubMed] [Google Scholar]
- 75.Amos H, Korn M. 5-Methyl cytosine in the RNA of Escherichia coli. Biochim Biophys. Acta. 1958;29:444–445. doi: 10.1016/0006-3002(58)90214-2. [DOI] [PubMed] [Google Scholar]
- 76.Bohnsack KE, Hobartner C, Bohnsack MT. Eukaryotic 5-methylcytosine (m(5)C) RNA methyltransferases: mechanisms, cellular functions, and links to disease. Genes (Basel) 2019;10:102. doi: 10.3390/genes10020102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Garcia-Vilchez R, Sevilla A, Blanco S. Post-transcriptional regulation by cytosine-5 methylation of RNA. Biochim Biophys. Acta Gene Regul. Mech. 2019;1862:240–252. doi: 10.1016/j.bbagrm.2018.12.003. [DOI] [PubMed] [Google Scholar]
- 78.Trixl L, Lusser A. The dynamic RNA modification 5-methylcytosine and its emerging role as an epitranscriptomic mark. Wiley Interdiscip. Rev. RNA. 2019;10:e1510. doi: 10.1002/wrna.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Nombela P, Miguel-Lopez B, Blanco S. The role of m(6)A, m(5)C and Psi RNA modifications in cancer: Novel therapeutic opportunities. Mol. Cancer. 2021;20:18. doi: 10.1186/s12943-020-01263-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Blaze J, et al. Neuronal Nsun2 deficiency produces tRNA epitranscriptomic alterations and proteomic shifts impacting synaptic signaling and behavior. Nat. Commun. 2021;12:4913. doi: 10.1038/s41467-021-24969-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shinoda S, et al. Mammalian NSUN2 introduces 5-methylcytidines into mitochondrial tRNAs. Nucleic Acids Res. 2019;47:8734–8745. doi: 10.1093/nar/gkz575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Van Haute L, et al. NSUN2 introduces 5-methylcytosines in mammalian mitochondrial tRNAs. Nucleic Acids Res. 2019;47:8720–8733. doi: 10.1093/nar/gkz559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li J, et al. Archaeal NSUN6 catalyzes m5C72 modification on a wide-range of specific tRNAs. Nucleic Acids Res. 2019;47:2041–2055. doi: 10.1093/nar/gky1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Squires JE, et al. Widespread occurrence of 5-methylcytosine in human coding and non-coding RNA. Nucleic Acids Res. 2012;40:5023–5033. doi: 10.1093/nar/gks144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heissenberger C, et al. The ribosomal RNA m(5)C methyltransferase NSUN-1 modulates healthspan and oogenesis in Caenorhabditis elegans. Elife. 2020;9:e56205. doi: 10.7554/eLife.56205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schosserer M, et al. Methylation of ribosomal RNA by NSUN5 is a conserved mechanism modulating organismal lifespan. Nat. Commun. 2015;6:6158. doi: 10.1038/ncomms7158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Navarro IC, et al. Translational adaptation to heat stress is mediated by RNA 5-methylcytosine in Caenorhabditis elegans. EMBO J. 2021;40:e105496. doi: 10.15252/embj.2020105496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Aguilo F, et al. Deposition of 5-methylcytosine on enhancer RNAs enables the coactivator function of PGC-1alpha. Cell Rep. 2016;14:479–492. doi: 10.1016/j.celrep.2015.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shen H, et al. TET-mediated 5-methylcytosine oxidation in tRNA promotes translation. J. Biol. Chem. 2021;296:100087. doi: 10.1074/jbc.RA120.014226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Yang H, et al. FMRP promotes transcription-coupled homologous recombination via facilitating TET1-mediated m5C RNA modification demethylation. Proc. Natl. Acad. Sci. USA. 2022;119:e2116251119. doi: 10.1073/pnas.2116251119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Arguello AE, et al. Reactivity-dependent profiling of RNA 5-methylcytidine dioxygenases. Nat. Commun. 2022;13:4176. doi: 10.1038/s41467-022-31876-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Meng H, et al. DNA methylation, its mediators and genome integrity. Int. J. Biol. Sci. 2015;11:604–617. doi: 10.7150/ijbs.11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ito S, et al. Tet proteins can convert 5-methylcytosine to 5-formylcytosine and 5-carboxylcytosine. Science. 2011;333:1300–1303. doi: 10.1126/science.1210597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hu L, et al. Structural insight into substrate preference for TET-mediated oxidation. Nature. 2015;527:118–122. doi: 10.1038/nature15713. [DOI] [PubMed] [Google Scholar]
- 95.Pfaffeneder T, et al. Tet oxidizes thymine to 5-hydroxymethyluracil in mouse embryonic stem cell DNA. Nat. Chem. Biol. 2014;10:574–581. doi: 10.1038/nchembio.1532. [DOI] [PubMed] [Google Scholar]
- 96.Wang L, et al. Molecular basis for 5-carboxycytosine recognition by RNA polymerase II elongation complex. Nature. 2015;523:621–625. doi: 10.1038/nature14482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Delatte B, et al. RNA biochemistry. Transcriptome-wide distribution and function of RNA hydroxymethylcytosine. Science. 2016;351:282–285. doi: 10.1126/science.aac5253. [DOI] [PubMed] [Google Scholar]
- 98.Fu L, et al. Tet-mediated formation of 5-hydroxymethylcytosine in RNA. J. Am. Chem. Soc. 2014;136:11582–11585. doi: 10.1021/ja505305z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kawarada L, et al. ALKBH1 is an RNA dioxygenase responsible for cytoplasmic and mitochondrial tRNA modifications. Nucleic Acids Res. 2017;45:7401–7415. doi: 10.1093/nar/gkx354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Dominissini D, Rechavi G. 5-methylcytosine mediates nuclear export of mRNA. Cell Res. 2017;27:717–719. doi: 10.1038/cr.2017.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu Y, et al. mRNA m5C controls adipogenesis by promoting CDKN1A mRNA export and translation. RNA Biol. 2021;18:711–721. doi: 10.1080/15476286.2021.1980694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yang Y, et al. RNA 5-methylcytosine facilitates the maternal-to-zygotic transition by preventing maternal mRNA decay. Mol. Cell. 2019;75:1188–1202.e1111. doi: 10.1016/j.molcel.2019.06.033. [DOI] [PubMed] [Google Scholar]
- 103.Chen X, et al. 5-methylcytosine promotes pathogenesis of bladder cancer through stabilizing mRNAs. Nat. Cell Biol. 2019;21:978–990. doi: 10.1038/s41556-019-0361-y. [DOI] [PubMed] [Google Scholar]
- 104.Dai X, et al. YTHDF2 binds to 5-methylcytosine in RNA and modulates the maturation of ribosomal RNA. Anal. Chem. 2020;92:1346–1354. doi: 10.1021/acs.analchem.9b04505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Delaunay S, et al. Mitochondrial RNA modifications shape metabolic plasticity in metastasis. Nature. 2022;607:593–603. doi: 10.1038/s41586-022-04898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hussain S, et al. NSun2-mediated cytosine-5 methylation of vault noncoding RNA determines its processing into regulatory small RNAs. Cell Rep. 2013;4:255–261. doi: 10.1016/j.celrep.2013.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhang X, et al. The tRNA methyltransferase NSun2 stabilizes p16INK(4) mRNA by methylating the 3’-untranslated region of p16. Nat. Commun. 2012;3:712. doi: 10.1038/ncomms1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Yang X, et al. 5-methylcytosine promotes mRNA export - NSUN2 as the methyltransferase and ALYREF as an m(5)C reader. Cell Res. 2017;27:606–625. doi: 10.1038/cr.2017.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Selmi T, et al. Sequence- and structure-specific cytosine-5 mRNA methylation by NSUN6. Nucleic Acids Res. 2021;49:1006–1022. doi: 10.1093/nar/gkaa1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Dunn DB. The occurrence of 1-methyladenine in ribonucleic acid. Biochim Biophys. Acta. 1961;46:198–200. doi: 10.1016/0006-3002(61)90668-0. [DOI] [PubMed] [Google Scholar]
- 111.Zhou H, et al. Evolution of a reverse transcriptase to map N(1)-methyladenosine in human messenger RNA. Nat. Methods. 2019;16:1281–1288. doi: 10.1038/s41592-019-0550-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Macon JB, Wolfenden R. 1-Methyladenosine. Dimroth rearrangement and reversible reduction. Biochemistry. 1968;7:3453–3458. doi: 10.1021/bi00850a021. [DOI] [PubMed] [Google Scholar]
- 113.Waku T, et al. NML-mediated rRNA base methylation links ribosomal subunit formation to cell proliferation in a p53-dependent manner. J. Cell Sci. 2016;129:2382–2393. doi: 10.1242/jcs.183723. [DOI] [PubMed] [Google Scholar]
- 114.Saikia M, Fu Y, Pavon-Eternod M, He C, Pan T. Genome-wide analysis of N1-methyl-adenosine modification in human tRNAs. RNA. 2010;16:1317–1327. doi: 10.1261/rna.2057810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Safra M, et al. The m1A landscape on cytosolic and mitochondrial mRNA at single-base resolution. Nature. 2017;551:251–255. doi: 10.1038/nature24456. [DOI] [PubMed] [Google Scholar]
- 116.Bar-Yaacov D, et al. Mitochondrial 16S rRNA is methylated by tRNA methyltransferase TRMT61B in all vertebrates. PLoS Biol. 2016;14:e1002557. doi: 10.1371/journal.pbio.1002557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Howell NW, Jora M, Jepson BF, Limbach PA, Jackman JE. Distinct substrate specificities of the human tRNA methyltransferases TRMT10A and TRMT10B. RNA. 2019;25:1366–1376. doi: 10.1261/rna.072090.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Liu F, et al. ALKBH1-mediated tRNA demethylation regulates translation. Cell. 2016;167:816–828.e816. doi: 10.1016/j.cell.2016.09.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li X, et al. Transcriptome-wide mapping reveals reversible and dynamic N(1)-methyladenosine methylome. Nat. Chem. Biol. 2016;12:311–316. doi: 10.1038/nchembio.2040. [DOI] [PubMed] [Google Scholar]
- 120.Woo HH, Chambers SK. Human ALKBH3-induced m(1)A demethylation increases the CSF-1 mRNA stability in breast and ovarian cancer cells. Biochim Biophys. Acta Gene Regul. Mech. 2019;1862:35–46. doi: 10.1016/j.bbagrm.2018.10.008. [DOI] [PubMed] [Google Scholar]
- 121.Chen Z, et al. Transfer RNA demethylase ALKBH3 promotes cancer progression via induction of tRNA-derived small RNAs. Nucleic Acids Res. 2019;47:2533–2545. doi: 10.1093/nar/gky1250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhang LS, et al. ALKBH7-mediated demethylation regulates mitochondrial polycistronic RNA processing. Nat. Cell Biol. 2021;23:684–691. doi: 10.1038/s41556-021-00709-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dai X, Wang T, Gonzalez G, Wang Y. Identification of YTH Domain-Containing Proteins as the Readers for N1-Methyladenosine in RNA. Anal. Chem. 2018;90:6380–6384. doi: 10.1021/acs.analchem.8b01703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhou H, et al. m(1)A and m(1)G disrupt A-RNA structure through the intrinsic instability of Hoogsteen base pairs. Nat. Struct. Mol. Biol. 2016;23:803–810. doi: 10.1038/nsmb.3270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Dominissini D, et al. The dynamic N(1)-methyladenosine methylome in eukaryotic messenger RNA. Nature. 2016;530:441–446. doi: 10.1038/nature16998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Li X, et al. Base-resolution mapping reveals distinct m(1)A methylome in nuclear- and mitochondrial-encoded transcripts. Mol. Cell. 2017;68:993–1005.e1009. doi: 10.1016/j.molcel.2017.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang LS, et al. Transcriptome-wide mapping of internal N(7)-methylguanosine methylome in Mammalian mRNA. Mol. Cell. 2019;74:1304–1316.e1308. doi: 10.1016/j.molcel.2019.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Enroth C, et al. Detection of internal N7-methylguanosine (m7G) RNA modifications by mutational profiling sequencing. Nucleic Acids Res. 2019;47:e126. doi: 10.1093/nar/gkz736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Chen, Y., Lin, H., Miao, L. & He, J. Role of N7-methylguanosine (m(7)G) in cancer. Trends Cell Biol. 32, 819–824 (2022). [DOI] [PubMed]
- 130.Ramanathan A, Robb GB, Chan SH. mRNA capping: biological functions and applications. Nucleic Acids Res. 2016;44:7511–7526. doi: 10.1093/nar/gkw551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Luo Y, et al. The potential role of N(7)-methylguanosine (m7G) in cancer. J. Hematol. Oncol. 2022;15:63. doi: 10.1186/s13045-022-01285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shatsky IN, Terenin IM, Smirnova VV, Andreev DE. Cap-Independent Translation: What’s in a Name? Trends Biochem Sci. 2018;43:882–895. doi: 10.1016/j.tibs.2018.04.011. [DOI] [PubMed] [Google Scholar]
- 133.Katsara O, Schneider RJ. m(7)G tRNA modification reveals new secrets in the translational regulation of cancer development. Mol. Cell. 2021;81:3243–3245. doi: 10.1016/j.molcel.2021.07.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sloan KE, et al. Tuning the ribosome: The influence of rRNA modification on eukaryotic ribosome biogenesis and function. RNA Biol. 2017;14:1138–1152. doi: 10.1080/15476286.2016.1259781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Pandolfini L, et al. METTL1 promotes let-7 MicroRNA processing via m7G methylation. Mol. Cell. 2019;74:1278–1290.e1279. doi: 10.1016/j.molcel.2019.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Varshney D, et al. Molecular basis of RNA guanine-7 methyltransferase (RNMT) activation by RAM. Nucleic Acids Res. 2016;44:10423–10436. doi: 10.1093/nar/gkw637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Varshney D, et al. mRNA cap methyltransferase, RNMT-RAM, promotes RNA pol II-dependent transcription. Cell Rep. 2018;23:1530–1542. doi: 10.1016/j.celrep.2018.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Aregger M, et al. CDK1-cyclin B1 activates RNMT, coordinating mRNA cap methylation with G1 phase transcription. Mol. Cell. 2016;61:734–746. doi: 10.1016/j.molcel.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Ma J, et al. METTL1/WDR4-mediated m(7)G tRNA modifications and m(7)G codon usage promote mRNA translation and lung cancer progression. Mol. Ther. 2021;29:3422–3435. doi: 10.1016/j.ymthe.2021.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lin S, et al. Mettl1/Wdr4-mediated m(7)G tRNA methylome is required for normal mRNA translation and embryonic stem cell self-renewal and differentiation. Mol. Cell. 2018;71:244–255.e245. doi: 10.1016/j.molcel.2018.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Malbec L, et al. Dynamic methylome of internal mRNA N(7)-methylguanosine and its regulatory role in translation. Cell Res. 2019;29:927–941. doi: 10.1038/s41422-019-0230-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Galloway A, et al. Upregulation of RNA cap methyltransferase RNMT drives ribosome biogenesis during T cell activation. Nucleic Acids Res. 2021;49:6722–6738. doi: 10.1093/nar/gkab465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Monecke T, Dickmanns A, Ficner R. Structural basis for m7G-cap hypermethylation of small nuclear, small nucleolar and telomerase RNA by the dimethyltransferase TGS1. Nucleic Acids Res. 2009;37:3865–3877. doi: 10.1093/nar/gkp249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mars JC, Ghram M, Culjkovic-Kraljacic B, Borden KLB. The cap-binding complex CBC and the eukaryotic translation factor eIF4E: co-conspirators in cap-dependent RNA maturation and translation. Cancers (Basel) 2021;13:6185. doi: 10.3390/cancers13246185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Dou Y, et al. Affinity proteomic dissection of the human nuclear cap-binding complex interactome. Nucleic Acids Res. 2020;48:10456–10469. doi: 10.1093/nar/gkaa743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jensen KB, et al. capCLIP: a new tool to probe translational control in human cells through capture and identification of the eIF4E-mRNA interactome. Nucleic Acids Res. 2021;49:e105. doi: 10.1093/nar/gkab604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Galloway A, Cowling VH. mRNA cap regulation in mammalian cell function and fate. Biochim Biophys. Acta Gene Regul. Mech. 2019;1862:270–279. doi: 10.1016/j.bbagrm.2018.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Orellana EA, et al. METTL1-mediated m(7)G modification of Arg-TCT tRNA drives oncogenic transformation. Mol. Cell. 2021;81:3323–3338.e3314. doi: 10.1016/j.molcel.2021.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Thongdee N, et al. TrmB, a tRNA m7G46 methyltransferase, plays a role in hydrogen peroxide resistance and positively modulates the translation of katA and katB mRNAs in Pseudomonas aeruginosa. Nucleic Acids Res. 2019;47:9271–9281. doi: 10.1093/nar/gkz702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kouzarides T, Pandolfini L, Barbieri I, Bannister AJ, Andrews B. Further evidence supporting N7-methylation of guanosine (m(7)G) in human MicroRNAs. Mol. Cell. 2020;79:201–202. doi: 10.1016/j.molcel.2020.05.023. [DOI] [PubMed] [Google Scholar]
- 151.Boulias K, Greer EL. Put the pedal to the METTL1: adding internal m(7)G increases mRNA translation efficiency and augments miRNA processing. Mol. Cell. 2019;74:1105–1107. doi: 10.1016/j.molcel.2019.06.004. [DOI] [PubMed] [Google Scholar]
- 152.Xia P, et al. MYC-targeted WDR4 promotes proliferation, metastasis, and sorafenib resistance by inducing CCNB1 translation in hepatocellular carcinoma. Cell Death Dis. 2021;12:691. doi: 10.1038/s41419-021-03973-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Ying X, et al. METTL1-m(7) G-EGFR/EFEMP1 axis promotes the bladder cancer development. Clin. Transl. Med. 2021;11:e675. doi: 10.1002/ctm2.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Deng Y, Zhou Z, Ji W, Lin S, Wang M. METTL1-mediated m(7)G methylation maintains pluripotency in human stem cells and limits mesoderm differentiation and vascular development. Stem Cell Res Ther. 2020;11:306. doi: 10.1186/s13287-020-01814-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Bartee D, Nance KD, Meier JL. Site-specific synthesis of N(4)-acetylcytidine in RNA reveals physiological duplex stabilization. J. Am. Chem. Soc. 2022;144:3487–3496. doi: 10.1021/jacs.1c11985. [DOI] [PubMed] [Google Scholar]
- 156.Thalalla Gamage S, Sas-Chen A, Schwartz S, Meier JL. Quantitative nucleotide resolution profiling of RNA cytidine acetylation by ac4C-seq. Nat. Protoc. 2021;16:2286–2307. doi: 10.1038/s41596-021-00501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Thomas JM, et al. A chemical signature for cytidine acetylation in RNA. J. Am. Chem. Soc. 2018;140:12667–12670. doi: 10.1021/jacs.8b06636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Arango D, et al. Acetylation of cytidine in mRNA promotes translation efficiency. Cell. 2018;175:1872–1886.e1824. doi: 10.1016/j.cell.2018.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Jin G, Xu M, Zou M, Duan S. The processing, gene regulation, biological functions, and clinical relevance of N4-acetylcytidine on RNA: a systematic review. Mol. Ther. Nucleic Acids. 2020;20:13–24. doi: 10.1016/j.omtn.2020.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sharma S, et al. Yeast Kre33 and human NAT10 are conserved 18S rRNA cytosine acetyltransferases that modify tRNAs assisted by the adaptor Tan1/THUMPD1. Nucleic Acids Res. 2015;43:2242–2258. doi: 10.1093/nar/gkv075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Sas-Chen A, et al. Dynamic RNA acetylation revealed by quantitative cross-evolutionary mapping. Nature. 2020;583:638–643. doi: 10.1038/s41586-020-2418-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Broly M, et al. THUMPD1 bi-allelic variants cause loss of tRNA acetylation and a syndromic neurodevelopmental disorder. Am. J. Hum. Genet. 2022;109:587–600. doi: 10.1016/j.ajhg.2022.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Bortolin-Cavaille ML, et al. Probing small ribosomal subunit RNA helix 45 acetylation across eukaryotic evolution. Nucleic Acids Res. 2022;50:6284–6299. doi: 10.1093/nar/gkac404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ito S, et al. Human NAT10 is an ATP-dependent RNA acetyltransferase responsible for N4-acetylcytidine formation in 18 S ribosomal RNA (rRNA) J. Biol. Chem. 2014;289:35724–35730. doi: 10.1074/jbc.C114.602698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Wang K, et al. PIWI-interacting RNA HAAPIR regulates cardiomyocyte death after myocardial infarction by promoting NAT10-mediated ac(4) C acetylation of Tfec mRNA. Adv. Sci. (Weinh.) 2022;9:e2106058. doi: 10.1002/advs.202106058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Dewe JM, Whipple JM, Chernyakov I, Jaramillo LN, Phizicky EM. The yeast rapid tRNA decay pathway competes with elongation factor 1A for substrate tRNAs and acts on tRNAs lacking one or more of several modifications. RNA. 2012;18:1886–1896. doi: 10.1261/rna.033654.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Whipple JM, Lane EA, Chernyakov I, D’Silva S, Phizicky EM. The yeast rapid tRNA decay pathway primarily monitors the structural integrity of the acceptor and T-stems of mature tRNA. Genes Dev. 2011;25:1173–1184. doi: 10.1101/gad.2050711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Dominissini D, Rechavi G. N(4)-acetylation of cytidine in mRNA by NAT10 regulates stability and translation. Cell. 2018;175:1725–1727. doi: 10.1016/j.cell.2018.11.037. [DOI] [PubMed] [Google Scholar]
- 169.Tsai K, et al. Acetylation of cytidine residues boosts HIV-1 gene expression by increasing viral RNA stability. Cell Host Microbe. 2020;28:306–312.e306. doi: 10.1016/j.chom.2020.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Arango D, et al. Direct epitranscriptomic regulation of mammalian translation initiation through N4-acetylcytidine. Mol. Cell. 2022;82:2797–2814.e2711. doi: 10.1016/j.molcel.2022.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Zhao BS, He C. Pseudouridine in a new era of RNA modifications. Cell Res. 2015;25:153–154. doi: 10.1038/cr.2014.143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Borchardt EK, Martinez NM, Gilbert WV. Regulation and function of RNA pseudouridylation in human cells. Annu Rev. Genet. 2020;54:309–336. doi: 10.1146/annurev-genet-112618-043830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Ge J, Yu YT. RNA pseudouridylation: new insights into an old modification. Trends Biochem Sci. 2013;38:210–218. doi: 10.1016/j.tibs.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Karijolich J, Yi C, Yu YT. Transcriptome-wide dynamics of RNA pseudouridylation. Nat. Rev. Mol. Cell Biol. 2015;16:581–585. doi: 10.1038/nrm4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Xue C, et al. Role of main RNA modifications in cancer: N(6)-methyladenosine, 5-methylcytosine, and pseudouridine. Signal Transduct. Target Ther. 2022;7:142. doi: 10.1038/s41392-022-01003-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Cerneckis J, Cui Q, He C, Yi C, Shi Y. Decoding pseudouridine: an emerging target for therapeutic development. Trends Pharm. Sci. 2022;43:522–535. doi: 10.1016/j.tips.2022.03.008. [DOI] [PubMed] [Google Scholar]
- 177.Jobert L, et al. The human base excision repair enzyme SMUG1 directly interacts with DKC1 and contributes to RNA quality control. Mol. Cell. 2013;49:339–345. doi: 10.1016/j.molcel.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 178.Penzo M, Guerrieri AN, Zacchini F, Trere D, Montanaro L. RNA pseudouridylation in physiology and medicine: for better and for worse. Genes (Basel) 2017;8:301. doi: 10.3390/genes8110301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Song J, et al. Differential roles of human PUS10 in miRNA processing and tRNA pseudouridylation. Nat. Chem. Biol. 2020;16:160–169. doi: 10.1038/s41589-019-0420-5. [DOI] [PubMed] [Google Scholar]
- 180.De Zoysa MD, Yu YT. Posttranscriptional RNA pseudouridylation. Enzymes. 2017;41:151–167. doi: 10.1016/bs.enz.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Purchal MK, et al. Pseudouridine synthase 7 is an opportunistic enzyme that binds and modifies substrates with diverse sequences and structures. Proc. Natl. Acad. Sci. USA. 2022;119:e2109708119. doi: 10.1073/pnas.2109708119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Lorenz C, Lunse CE, Morl M. tRNA modifications: impact on structure and thermal adaptation. Biomolecules. 2017;7:35. doi: 10.3390/biom7020035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Guzzi N, et al. Pseudouridylation of tRNA-derived fragments steers translational control in stem cells. Cell. 2018;173:1204–1216.e1226. doi: 10.1016/j.cell.2018.03.008. [DOI] [PubMed] [Google Scholar]
- 184.Guegueniat J, et al. The human pseudouridine synthase PUS7 recognizes RNA with an extended multi-domain binding surface. Nucleic Acids Res. 2021;49:11810–11822. doi: 10.1093/nar/gkab934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Guzzi N, et al. Pseudouridine-modified tRNA fragments repress aberrant protein synthesis and predict leukaemic progression in myelodysplastic syndrome. Nat. Cell Biol. 2022;24:299–306. doi: 10.1038/s41556-022-00852-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.McCown PJ, et al. Naturally occurring modified ribonucleosides. Wiley Interdiscip. Rev. RNA. 2020;11:e1595. doi: 10.1002/wrna.1595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Leppik M, Liiv A, Remme J. Random pseuoduridylation in vivo reveals critical region of Escherichia coli 23S rRNA for ribosome assembly. Nucleic Acids Res. 2017;45:6098–6108. doi: 10.1093/nar/gkx160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Antonicka H, et al. A pseudouridine synthase module is essential for mitochondrial protein synthesis and cell viability. EMBO Rep. 2017;18:28–38. doi: 10.15252/embr.201643391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Penzo M, Montanaro L. Turning uridines around: Role of rRNA pseudouridylation in ribosome biogenesis and ribosomal function. Biomolecules. 2018;8:38. doi: 10.3390/biom8020038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Rajan KS, et al. Pseudouridines on Trypanosoma brucei spliceosomal small nuclear RNAs and their implication for RNA and protein interactions. Nucleic Acids Res. 2019;47:7633–7647. doi: 10.1093/nar/gkz477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wu G, et al. Pseudouridines in U2 snRNA stimulate the ATPase activity of Prp5 during spliceosome assembly. EMBO J. 2016;35:654–667. doi: 10.15252/embj.201593113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang Q, et al. The PSI-U1 snRNP interaction regulates male mating behavior in Drosophila. Proc. Natl. Acad. Sci. USA. 2016;113:5269–5274. doi: 10.1073/pnas.1600936113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kariko K, et al. Incorporation of pseudouridine into mRNA yields superior nonimmunogenic vector with increased translational capacity and biological stability. Mol. Ther. 2008;16:1833–1840. doi: 10.1038/mt.2008.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Carlile TM, et al. Pseudouridine profiling reveals regulated mRNA pseudouridylation in yeast and human cells. Nature. 2014;515:143–146. doi: 10.1038/nature13802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Martinez NM, et al. Pseudouridine synthases modify human pre-mRNA co-transcriptionally and affect pre-mRNA processing. Mol. Cell. 2022;82:645–659.e649. doi: 10.1016/j.molcel.2021.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Kohama Y, et al. Studies on thermophile products. V. Immunosuppressive profile in vitro of Bacillus stearothermophilus component, Fr.5-B. Chem. Pharm. Bull. (Tokyo) 1992;40:3017–3020. doi: 10.1248/cpb.40.3017. [DOI] [PubMed] [Google Scholar]
- 197.Morais P, Adachi H, Yu YT. The critical contribution of pseudouridine to mRNA COVID-19 vaccines. Front Cell Dev. Biol. 2021;9:789427. doi: 10.3389/fcell.2021.789427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lee M, Kim B, Kim VN. Emerging roles of RNA modification: m(6)A and U-tail. Cell. 2014;158:980–987. doi: 10.1016/j.cell.2014.08.005. [DOI] [PubMed] [Google Scholar]
- 199.Song J, Song J, Mo B, Chen X. Uridylation and adenylation of RNAs. Sci. China Life Sci. 2015;58:1057–1066. doi: 10.1007/s11427-015-4954-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Clamer M, Hofler L, Mikhailova E, Viero G, Bayley H. Detection of 3’-end RNA uridylation with a protein nanopore. ACS Nano. 2014;8:1364–1374. doi: 10.1021/nn4050479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Wang J, Yang YN, Piao WL, Jin H. Uridylation: a vital way for cellular RNA surveillance. Yi Chuan. 2022;44:449–465. doi: 10.16288/j.yczz.22-094. [DOI] [PubMed] [Google Scholar]
- 202.Zigackova D, Vanacova S. The role of 3’ end uridylation in RNA metabolism and cellular physiology. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;373:20180171. doi: 10.1098/rstb.2018.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Warkocki Z, Liudkovska V, Gewartowska O, Mroczek S, Dziembowski A. Terminal nucleotidyl transferases (TENTs) in mammalian RNA metabolism. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2018;373:20180162. doi: 10.1098/rstb.2018.0162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Aphasizhev R, Suematsu T, Zhang L, Aphasizheva I. Constructive edge of uridylation-induced RNA degradation. RNA Biol. 2016;13:1078–1083. doi: 10.1080/15476286.2016.1229736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Yu S, Kim VN. A tale of non-canonical tails: gene regulation by post-transcriptional RNA tailing. Nat. Rev. Mol. Cell Biol. 2020;21:542–556. doi: 10.1038/s41580-020-0246-8. [DOI] [PubMed] [Google Scholar]
- 206.Yamashita S, Takagi Y, Nagaike T, Tomita K. Crystal structures of U6 snRNA-specific terminal uridylyltransferase. Nat. Commun. 2017;8:15788. doi: 10.1038/ncomms15788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lim J, et al. Uridylation by TUT4 and TUT7 marks mRNA for degradation. Cell. 2014;159:1365–1376. doi: 10.1016/j.cell.2014.10.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Morgan M, et al. mRNA 3’ uridylation and poly(A) tail length sculpt the mammalian maternal transcriptome. Nature. 2017;548:347–351. doi: 10.1038/nature23318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Warkocki Z, et al. Uridylation by TUT4/7 Restricts Retrotransposition of Human LINE-1s. Cell. 2018;174:1537–1548.e1529. doi: 10.1016/j.cell.2018.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Thornton JE, et al. Selective microRNA uridylation by Zcchc6 (TUT7) and Zcchc11 (TUT4) Nucleic Acids Res. 2014;42:11777–11791. doi: 10.1093/nar/gku805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Hoefig KP, Heissmeyer V. Degradation of oligouridylated histone mRNAs: see UUUUU and goodbye. Wiley Interdiscip. Rev. RNA. 2014;5:577–589. doi: 10.1002/wrna.1232. [DOI] [PubMed] [Google Scholar]
- 212.Chung CZ, et al. RNA surveillance by uridylation-dependent RNA decay in Schizosaccharomyces pombe. Nucleic Acids Res. 2019;47:3045–3057. doi: 10.1093/nar/gkz043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Wolin SL, Cedervall T. The La protein. Annu Rev. Biochem. 2002;71:375–403. doi: 10.1146/annurev.biochem.71.090501.150003. [DOI] [PubMed] [Google Scholar]
- 214.Rissland OS, Norbury CJ. Decapping is preceded by 3’ uridylation in a novel pathway of bulk mRNA turnover. Nat. Struct. Mol. Biol. 2009;16:616–623. doi: 10.1038/nsmb.1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Faehnle CR, Walleshauser J, Joshua-Tor L. Mechanism of Dis3l2 substrate recognition in the Lin28-let-7 pathway. Nature. 2014;514:252–256. doi: 10.1038/nature13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ustianenko D, et al. TUT-DIS3L2 is a mammalian surveillance pathway for aberrant structured non-coding RNAs. EMBO J. 2016;35:2179–2191. doi: 10.15252/embj.201694857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.De Almeida, C., Scheer, H., Zuber, H. & Gagliardi, D. RNA uridylation: a key posttranscriptional modification shaping the coding and noncoding transcriptome. Wiley Interdiscip Rev RNA. 9, (2018). [DOI] [PubMed]
- 218.Yashiro Y, Tomita K. Function and regulation of human terminal uridylyltransferases. Front Genet. 2018;9:538. doi: 10.3389/fgene.2018.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Menezes MR, Balzeau J, Hagan JP. 3’ RNA uridylation in epitranscriptomics, gene regulation, and disease. Front Mol. Biosci. 2018;5:61. doi: 10.3389/fmolb.2018.00061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Kim B, et al. TUT7 controls the fate of precursor microRNAs by using three different uridylation mechanisms. EMBO J. 2015;34:1801–1815. doi: 10.15252/embj.201590931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Kim H, et al. A mechanism for microRNA arm switching regulated by uridylation. Mol. Cell. 2020;78:1224–1236.e1225. doi: 10.1016/j.molcel.2020.04.030. [DOI] [PubMed] [Google Scholar]
- 222.Heo I, et al. Mono-uridylation of pre-microRNA as a key step in the biogenesis of group II let-7 microRNAs. Cell. 2012;151:521–532. doi: 10.1016/j.cell.2012.09.022. [DOI] [PubMed] [Google Scholar]
- 223.Yang A, et al. 3’ Uridylation Confers miRNAs with non-canonical target repertoires. Mol. Cell. 2019;75:511–522.e514. doi: 10.1016/j.molcel.2019.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Jones MR, et al. Zcchc11-dependent uridylation of microRNA directs cytokine expression. Nat. Cell Biol. 2009;11:1157–1163. doi: 10.1038/ncb1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Ji L, Chen X. Regulation of small RNA stability: methylation and beyond. Cell Res. 2012;22:624–636. doi: 10.1038/cr.2012.36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Gutierrez-Vazquez C, et al. 3’ Uridylation controls mature microRNA turnover during CD4 T-cell activation. RNA. 2017;23:882–891. doi: 10.1261/rna.060095.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Lin CC, et al. Terminal uridyltransferase 7 regulates TLR4-triggered inflammation by controlling Regnase-1 mRNA uridylation and degradation. Nat. Commun. 2021;12:3878. doi: 10.1038/s41467-021-24177-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Scheer H, et al. The TUTase URT1 connects decapping activators and prevents the accumulation of excessively deadenylated mRNAs to avoid siRNA biogenesis. Nat. Commun. 2021;12:1298. doi: 10.1038/s41467-021-21382-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Eberhardt W, Doller A, Akool el S, Pfeilschifter J. Modulation of mRNA stability as a novel therapeutic approach. Pharm. Ther. 2007;114:56–73. doi: 10.1016/j.pharmthera.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 230.Le Pen J, et al. Terminal uridylyltransferases target RNA viruses as part of the innate immune system. Nat. Struct. Mol. Biol. 2018;25:778–786. doi: 10.1038/s41594-018-0106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Felix MA, Wang D. Natural viruses of caenorhabditis nematodes. Annu Rev. Genet. 2019;53:313–326. doi: 10.1146/annurev-genet-112618-043756. [DOI] [PubMed] [Google Scholar]
- 232.Frazier MN, et al. Characterization of SARS2 Nsp15 nuclease activity reveals it’s mad about U. Nucleic Acids Res. 2021;49:10136–10149. doi: 10.1093/nar/gkab719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Koppers-Lalic D, et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8:1649–1658. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 234.Wulff BE, Sakurai M, Nishikura K. Elucidating the inosinome: global approaches to adenosine-to-inosine RNA editing. Nat. Rev. Genet. 2011;12:81–85. doi: 10.1038/nrg2915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Li JB, Church GM. Deciphering the functions and regulation of brain-enriched A-to-I RNA editing. Nat. Neurosci. 2013;16:1518–1522. doi: 10.1038/nn.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Quin J, et al. ADAR RNA Modifications, the Epitranscriptome and Innate Immunity. Trends Biochem Sci. 2021;46:758–771. doi: 10.1016/j.tibs.2021.02.002. [DOI] [PubMed] [Google Scholar]
- 237.Ganem NS, Ben-Asher N, Lamm AT. In cancer, A-to-I RNA editing can be the driver, the passenger, or the mechanic. Drug Resist Updat. 2017;32:16–22. doi: 10.1016/j.drup.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 238.Liao Y, Jung SH, Kim T. A-to-I RNA editing as a tuner of noncoding RNAs in cancer. Cancer Lett. 2020;494:88–93. doi: 10.1016/j.canlet.2020.08.004. [DOI] [PubMed] [Google Scholar]
- 239.Macbeth MR, et al. Inositol hexakisphosphate is bound in the ADAR2 core and required for RNA editing. Science. 2005;309:1534–1539. doi: 10.1126/science.1113150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Vlachogiannis NI, Verrou KM, Stellos K, Sfikakis PP, Paraskevis D. The role of A-to-I RNA editing in infections by RNA viruses: Possible implications for SARS-CoV-2 infection. Clin. Immunol. 2021;226:108699. doi: 10.1016/j.clim.2021.108699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Nishikura K. A-to-I editing of coding and non-coding RNAs by ADARs. Nat. Rev. Mol. Cell Biol. 2016;17:83–96. doi: 10.1038/nrm.2015.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Baker AR, Slack FJ. ADAR1 and its implications in cancer development and treatment. Trends Genet. 2022;38:821–830. doi: 10.1016/j.tig.2022.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Walkley CR, Li JB. Rewriting the transcriptome: adenosine-to-inosine RNA editing by ADARs. Genome Biol. 2017;18:205. doi: 10.1186/s13059-017-1347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Maas S, Kim YG, Rich A. Sequence, genomic organization and functional expression of the murine tRNA-specific adenosine deaminase ADAT1. Gene. 2000;243:59–66. doi: 10.1016/S0378-1119(99)00562-4. [DOI] [PubMed] [Google Scholar]
- 245.Bertotti S, et al. Characterization of ADAT2/3 molecules in Trypanosoma cruzi and regulation of mucin gene expression by tRNA editing. Biochem J. 2022;479:561–580. doi: 10.1042/BCJ20210850. [DOI] [PubMed] [Google Scholar]
- 246.Liu X, et al. Crystal structure of the yeast heterodimeric ADAT2/3 deaminase. BMC Biol. 2020;18:189. doi: 10.1186/s12915-020-00920-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Liu H, et al. Genome-wide A-to-I RNA editing in fungi independent of ADAR enzymes. Genome Res. 2016;26:499–509. doi: 10.1101/gr.199877.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Keegan LP, et al. The properties of a tRNA-specific adenosine deaminase from Drosophila melanogaster support an evolutionary link between pre-mRNA editing and tRNA modification. Mol. Cell Biol. 2000;20:825–833. doi: 10.1128/MCB.20.3.825-833.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Elbarbary RA, Lucas BA, Maquat LE. Retrotransposons as regulators of gene expression. Science. 2016;351:aac7247. doi: 10.1126/science.aac7247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Werry TD, Loiacono R, Sexton PM, Christopoulos A. RNA editing of the serotonin 5HT2C receptor and its effects on cell signalling, pharmacology and brain function. Pharm. Ther. 2008;119:7–23. doi: 10.1016/j.pharmthera.2008.03.012. [DOI] [PubMed] [Google Scholar]
- 251.Jain M, Jantsch MF, Licht K. The Editor’s I on disease development. Trends Genet. 2019;35:903–913. doi: 10.1016/j.tig.2019.09.004. [DOI] [PubMed] [Google Scholar]
- 252.Gagnidze K, Rayon-Estrada V, Harroch S, Bulloch K, Papavasiliou FN. A new chapter in genetic medicine: RNA editing and its role in disease pathogenesis. Trends Mol. Med. 2018;24:294–303. doi: 10.1016/j.molmed.2018.01.002. [DOI] [PubMed] [Google Scholar]
- 253.Zipeto MA, Jiang Q, Melese E, Jamieson CH. RNA rewriting, recoding, and rewiring in human disease. Trends Mol. Med. 2015;21:549–559. doi: 10.1016/j.molmed.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 254.Farajollahi S, Maas S. Molecular diversity through RNA editing: a balancing act. Trends Genet. 2010;26:221–230. doi: 10.1016/j.tig.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Savva YA, Rieder LE, Reenan RA. The ADAR protein family. Genome Biol. 2012;13:252. doi: 10.1186/gb-2012-13-12-252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Tan MH, et al. Dynamic landscape and regulation of RNA editing in mammals. Nature. 2017;550:249–254. doi: 10.1038/nature24041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Oakes E, Anderson A, Cohen-Gadol A, Hundley HA. Adenosine deaminase that acts on RNA 3 (ADAR3) binding to glutamate receptor subunit B Pre-mRNA inhibits RNA editing in glioblastoma. J. Biol. Chem. 2017;292:4326–4335. doi: 10.1074/jbc.M117.779868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 258.Raghava Kurup R, et al. RNA binding by ADAR3 inhibits adenosine-to-inosine editing and promotes expression of immune response protein MAVS. J. Biol. Chem. 2022;298:102267. doi: 10.1016/j.jbc.2022.102267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Teoh PJ, Koh MY, Chng WJ. ADARs, RNA editing and more in hematological malignancies. Leukemia. 2021;35:346–359. doi: 10.1038/s41375-020-01076-2. [DOI] [PubMed] [Google Scholar]
- 260.Aquino-Jarquin G. Novel engineered programmable systems for ADAR-mediated RNA editing. Mol. Ther. Nucleic Acids. 2020;19:1065–1072. doi: 10.1016/j.omtn.2019.12.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Yablonovitch AL, Deng P, Jacobson D, Li JB. The evolution and adaptation of A-to-I RNA editing. PLoS Genet. 2017;13:e1007064. doi: 10.1371/journal.pgen.1007064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Xu X, Wang Y, Liang H. The role of A-to-I RNA editing in cancer development. Curr. Opin. Genet Dev. 2018;48:51–56. doi: 10.1016/j.gde.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Ganem NS, Lamm AT. A-to-I RNA editing - thinking beyond the single nucleotide. RNA Biol. 2017;14:1690–1694. doi: 10.1080/15476286.2017.1364830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Han SW, et al. RNA editing in RHOQ promotes invasion potential in colorectal cancer. J. Exp. Med. 2014;211:613–621. doi: 10.1084/jem.20132209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Daniel C, Wahlstedt H, Ohlson J, Bjork P, Ohman M. Adenosine-to-inosine RNA editing affects trafficking of the gamma-aminobutyric acid type A (GABA(A)) receptor. J. Biol. Chem. 2011;286:2031–2040. doi: 10.1074/jbc.M110.130096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Garrett S, Rosenthal JJ. RNA editing underlies temperature adaptation in K+ channels from polar octopuses. Science. 2012;335:848–851. doi: 10.1126/science.1212795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Chen L, et al. Recoding RNA editing of AZIN1 predisposes to hepatocellular carcinoma. Nat. Med. 2013;19:209–216. doi: 10.1038/nm.3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Nakano M, Nakajima M. Significance of A-to-I RNA editing of transcripts modulating pharmacokinetics and pharmacodynamics. Pharm. Ther. 2018;181:13–21. doi: 10.1016/j.pharmthera.2017.07.003. [DOI] [PubMed] [Google Scholar]
- 269.Slotkin W, Nishikura K. Adenosine-to-inosine RNA editing and human disease. Genome Med. 2013;5:105. doi: 10.1186/gm508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Vlachogiannis NI, et al. Adenosine-to-inosine RNA editing contributes to type I interferon responses in systemic sclerosis. J. Autoimmun. 2021;125:102755. doi: 10.1016/j.jaut.2021.102755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Jiang L, et al. ADAR1-mediated RNA editing links ganglioside catabolism to glioblastoma stem cell maintenance. J. Clin. Invest. 2022;132:e143397. doi: 10.1172/JCI143397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Yang CC, et al. ADAR1-mediated 3’ UTR editing and expression control of antiapoptosis genes fine-tunes cellular apoptosis response. Cell Death Dis. 2017;8:e2833. doi: 10.1038/cddis.2017.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Stellos K, et al. Adenosine-to-inosine RNA editing controls cathepsin S expression in atherosclerosis by enabling HuR-mediated post-transcriptional regulation. Nat. Med. 2016;22:1140–1150. doi: 10.1038/nm.4172. [DOI] [PubMed] [Google Scholar]
- 274.Liang H, Landweber LF. Hypothesis: RNA editing of microRNA target sites in humans? RNA. 2007;13:463–467. doi: 10.1261/rna.296407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Banerjee A, Vest KE, Pavlath GK, Corbett AH. Nuclear poly(A) binding protein 1 (PABPN1) and Matrin3 interact in muscle cells and regulate RNA processing. Nucleic Acids Res. 2017;45:10706–10725. doi: 10.1093/nar/gkx786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Omata Y, et al. RNA editing enzyme ADAR1 governs the circadian expression of P-glycoprotein in human renal cells by regulating alternative splicing of the ABCB1 gene. J. Biol. Chem. 2021;296:100601. doi: 10.1016/j.jbc.2021.100601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Cho CJ, Myung SJ, Chang S. ADAR1 and MicroRNA; a hidden crosstalk in cancer. Int. J. Mol. Sci. 2017;18:799. doi: 10.3390/ijms18040799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Marceca GP, et al. Detecting and characterizing A-To-I microRNA editing in cancer. Cancers (Basel) 2021;13:1699. doi: 10.3390/cancers13071699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.Kokot KE, et al. Reduction of A-to-I RNA editing in the failing human heart regulates formation of circular RNAs. Basic Res. Cardiol. 2022;117:32. doi: 10.1007/s00395-022-00940-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Omata Y, et al. RNA editing enzyme ADAR1 controls miR-381-3p-mediated expression of multidrug resistance protein MRP4 via regulation of circRNA in human renal cells. J. Biol. Chem. 2022;298:102184. doi: 10.1016/j.jbc.2022.102184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Shen H, et al. ADARs act as potent regulators of circular transcriptome in cancer. Nat. Commun. 2022;13:1508. doi: 10.1038/s41467-022-29138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Yang W, et al. Modulation of microRNA processing and expression through RNA editing by ADAR deaminases. Nat. Struct. Mol. Biol. 2006;13:13–21. doi: 10.1038/nsmb1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Kawahara Y, et al. Frequency and fate of microRNA editing in human brain. Nucleic Acids Res. 2008;36:5270–5280. doi: 10.1093/nar/gkn479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Zipeto MA, et al. ADAR1 activation drives leukemia stem cell self-renewal by impairing let-7 biogenesis. Cell Stem Cell. 2016;19:177–191. doi: 10.1016/j.stem.2016.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Kawahara Y, Zinshteyn B, Chendrimada TP, Shiekhattar R, Nishikura K. RNA editing of the microRNA-151 precursor blocks cleavage by the Dicer-TRBP complex. EMBO Rep. 2007;8:763–769. doi: 10.1038/sj.embor.7401011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Kawahara Y, et al. Redirection of silencing targets by adenosine-to-inosine editing of miRNAs. Science. 2007;315:1137–1140. doi: 10.1126/science.1138050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Vesely C, et al. ADAR2 induces reproducible changes in sequence and abundance of mature microRNAs in the mouse brain. Nucleic Acids Res. 2014;42:12155–12168. doi: 10.1093/nar/gku844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Chen T, et al. ADAR1 is required for differentiation and neural induction by regulating microRNA processing in a catalytically independent manner. Cell Res. 2015;25:459–476. doi: 10.1038/cr.2015.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Ota H, et al. ADAR1 forms a complex with Dicer to promote microRNA processing and RNA-induced gene silencing. Cell. 2013;153:575–589. doi: 10.1016/j.cell.2013.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Shoshan E, et al. Reduced adenosine-to-inosine miR-455-5p editing promotes melanoma growth and metastasis. Nat. Cell Biol. 2015;17:311–321. doi: 10.1038/ncb3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Magnaye KM, et al. A-to-I editing of miR-200b-3p in airway cells is associated with moderate-to-severe asthma. Eur. Respir. J. 2021;58:2003862. doi: 10.1183/13993003.03862-2020. [DOI] [PubMed] [Google Scholar]
- 292.van der Kwast R, et al. Adenosine-to-inosine editing of MicroRNA-487b alters target gene selection after ischemia and promotes neovascularization. Circ. Res. 2018;122:444–456. doi: 10.1161/CIRCRESAHA.117.312345. [DOI] [PubMed] [Google Scholar]
- 293.Li L, et al. The landscape of miRNA editing in animals and its impact on miRNA biogenesis and targeting. Genome Res. 2018;28:132–143. doi: 10.1101/gr.224386.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Kume H, Hino K, Galipon J, Ui-Tei K. A-to-I editing in the miRNA seed region regulates target mRNA selection and silencing efficiency. Nucleic Acids Res. 2014;42:10050–10060. doi: 10.1093/nar/gku662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Mallela A, Nishikura K. A-to-I editing of protein coding and noncoding RNAs. Crit. Rev. Biochem Mol. Biol. 2012;47:493–501. doi: 10.3109/10409238.2012.714350. [DOI] [PubMed] [Google Scholar]
- 296.Pasquinelli AE. A rADAR defense against RNAi. Genes Dev. 2018;32:199–201. doi: 10.1101/gad.313049.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Reich DP, Tyc KM, Bass BL. C. elegans ADARs antagonize silencing of cellular dsRNAs by the antiviral RNAi pathway. Genes Dev. 2018;32:271–282. doi: 10.1101/gad.310672.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Vlachogiannis NI, et al. Adenosine-to-inosine Alu RNA editing controls the stability of the pro-inflammatory long noncoding RNA NEAT1 in atherosclerotic cardiovascular disease. J. Mol. Cell Cardiol. 2021;160:111–120. doi: 10.1016/j.yjmcc.2021.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Silvestris DA, Scopa C, Hanchi S, Locatelli F, Gallo A. De novo A-to-I RNA editing discovery in lncRNA. Cancers (Basel) 2020;12:2959. doi: 10.3390/cancers12102959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 300.Gong J, et al. LNCediting: a database for functional effects of RNA editing in lncRNAs. Nucleic Acids Res. 2017;45:D79–D84. doi: 10.1093/nar/gkw835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Di Giorgio S, Martignano F, Torcia MG, Mattiuz G, Conticello SG. Evidence for host-dependent RNA editing in the transcriptome of SARS-CoV-2. Sci. Adv. 2020;6:eabb5813. doi: 10.1126/sciadv.abb5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Gonzales-van Horn SR, Sarnow P. Making the mark: the role of adenosine modifications in the life cycle of RNA viruses. Cell Host Microbe. 2017;21:661–669. doi: 10.1016/j.chom.2017.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Wulff BE, Nishikura K. Substitutional A-to-I RNA editing. Wiley Interdiscip. Rev. RNA. 2010;1:90–101. doi: 10.1002/wrna.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Kumar BV, Connors TJ, Farber DL. Human T cell development, localization, and function throughout Life. Immunity. 2018;48:202–213. doi: 10.1016/j.immuni.2018.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Gaber T, Chen Y, Krauss PL, Buttgereit F. Metabolism of T lymphocytes in health and disease. Int Rev. Cell Mol. Biol. 2019;342:95–148. doi: 10.1016/bs.ircmb.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 306.Wang N, Tang H, Wang X, Wang W, Feng J. Homocysteine upregulates interleukin-17A expression via NSun2-mediated RNA methylation in T lymphocytes. Biochem Biophys. Res Commun. 2017;493:94–99. doi: 10.1016/j.bbrc.2017.09.069. [DOI] [PubMed] [Google Scholar]
- 307.Nakahama T, et al. ADAR1-mediated RNA editing is required for thymic self-tolerance and inhibition of autoimmunity. EMBO Rep. 2018;19:e46303. doi: 10.15252/embr.201846303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 308.Chung H, Rice CM. T time for ADAR: ADAR1 is required for T cell self-tolerance. EMBO Rep. 2018;19:e47237. doi: 10.15252/embr.201847237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Udhayakumar, V. K. et al. Arginine-rich peptide-based mRNA nanocomplexes efficiently instigate cytotoxic T cell immunity dependent on the amphipathic organization of the peptide. Adv Healthc Mater. 6, (2017). [DOI] [PubMed]
- 310.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30:646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 311.Zhu J, Paul WE. CD4 T cells: fates, functions, and faults. Blood. 2008;112:1557–1569. doi: 10.1182/blood-2008-05-078154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 312.Taniuchi I. CD4 helper and CD8 cytotoxic T cell differentiation. Annu Rev. Immunol. 2018;36:579–601. doi: 10.1146/annurev-immunol-042617-053411. [DOI] [PubMed] [Google Scholar]
- 313.Zhu, J., Yamane, H. & Paul, W. E. Differentiation of effector CD4 T cellpopulations (*). Annu Rev. Immunol. 28, 445–489 (2010). [DOI] [PMC free article] [PubMed]
- 314.Saravia J, Chapman NM, Chi H. Helper T cell differentiation. Cell Mol. Immunol. 2019;16:634–643. doi: 10.1038/s41423-019-0220-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 315.Zhou J, et al. m(6)A demethylase ALKBH5 controls CD4(+) T cell pathogenicity and promotes autoimmunity. Sci. Adv. 2021;7:eabg0470. doi: 10.1126/sciadv.abg0470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Lichinchi G, et al. Dynamics of the human and viral m(6)A RNA methylomes during HIV-1 infection of T cells. Nat. Microbiol. 2016;1:16011. doi: 10.1038/nmicrobiol.2016.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Kong W, et al. Nucleolar protein NOP2/NSUN1 suppresses HIV-1 transcription and promotes viral latency by competing with Tat for TAR binding and methylation. PLoS Pathog. 2020;16:e1008430. doi: 10.1371/journal.ppat.1008430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 318.Guo G, et al. Disease activity-associated alteration of mRNA m(5) C methylation in CD4(+) T cells of systemic lupus erythematosus. Front Cell Dev. Biol. 2020;8:430. doi: 10.3389/fcell.2020.00430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Guo G, et al. Epitranscriptomic N4-acetylcytidine profiling in CD4(+) T cells of systemic lupus erythematosus. Front Cell Dev. Biol. 2020;8:842. doi: 10.3389/fcell.2020.00842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 320.Ruterbusch M, Pruner KB, Shehata L, Pepper M. In vivo CD4(+) T cell differentiation and function: revisiting the Th1/Th2 paradigm. Annu Rev. Immunol. 2020;38:705–725. doi: 10.1146/annurev-immunol-103019-085803. [DOI] [PubMed] [Google Scholar]
- 321.Romagnani S. Th1/Th2 cells. Inflamm. Bowel Dis. 1999;5:285–294. doi: 10.1097/00054725-199911000-00009. [DOI] [PubMed] [Google Scholar]
- 322.Romagnani S. T-cell subsets (Th1 versus Th2) Ann. Allergy Asthma Immunol. 2000;85:9–18. doi: 10.1016/S1081-1206(10)62426-X. [DOI] [PubMed] [Google Scholar]
- 323.Dai B, et al. Significance of RNA N6-Methyladenosine Regulators in the Diagnosis and Subtype Classification of Childhood Asthma Using the Gene Expression Omnibus Database. Front Genet. 2021;12:634162. doi: 10.3389/fgene.2021.634162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Yasuda K, Takeuchi Y, Hirota K. The pathogenicity of Th17 cells in autoimmune diseases. Semin Immunopathol. 2019;41:283–297. doi: 10.1007/s00281-019-00733-8. [DOI] [PubMed] [Google Scholar]
- 325.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 Cells. Annu Rev. Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 326.Knochelmann HM, et al. When worlds collide: Th17 and Treg cells in cancer and autoimmunity. Cell Mol. Immunol. 2018;15:458–469. doi: 10.1038/s41423-018-0004-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Cao Y, et al. Enterotoxigenic bacteroidesfragilis promotes intestinal inflammation and malignancy by inhibiting exosome-packaged miR-149-3p. Gastroenterology. 2021;161:1552–1566.e1512. doi: 10.1053/j.gastro.2021.08.003. [DOI] [PubMed] [Google Scholar]
- 328.Crotty ST. Follicular helper cell biology: a decade of discovery and diseases. Immunity. 2019;50:1132–1148. doi: 10.1016/j.immuni.2019.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 329.Varricchi G, et al. T follicular helper (Tfh) cells in normal immune responses and in allergic disorders. Allergy. 2016;71:1086–1094. doi: 10.1111/all.12878. [DOI] [PubMed] [Google Scholar]
- 330.Crotty S. T follicular helper cell differentiation, function, and roles in disease. Immunity. 2014;41:529–542. doi: 10.1016/j.immuni.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 331.Zhu Y, et al. The E3 ligase VHL promotes follicular helper T cell differentiation via glycolytic-epigenetic control. J. Exp. Med. 2019;216:1664–1681. doi: 10.1084/jem.20190337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 332.Yao Y, et al. METTL3-dependent m(6)A modification programs T follicular helper cell differentiation. Nat. Commun. 2021;12:1333. doi: 10.1038/s41467-021-21594-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 333.Ohkura N, Sakaguchi S. Transcriptional and epigenetic basis of Treg cell development and function: its genetic anomalies or variations in autoimmune diseases. Cell Res. 2020;30:465–474. doi: 10.1038/s41422-020-0324-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 334.Goschl L, Scheinecker C, Bonelli M. Treg cells in autoimmunity: from identification to Treg-based therapies. Semin Immunopathol. 2019;41:301–314. doi: 10.1007/s00281-019-00741-8. [DOI] [PubMed] [Google Scholar]
- 335.Scheinecker C, Goschl L, Bonelli M. Treg cells in health and autoimmune diseases: New insights from single cell analysis. J. Autoimmun. 2020;110:102376. doi: 10.1016/j.jaut.2019.102376. [DOI] [PubMed] [Google Scholar]
- 336.Lu TX, et al. A new model of spontaneous colitis in mice induced by deletion of an RNA m(6)A methyltransferase component METTL14 in T cells. Cell Mol. Gastroenterol. Hepatol. 2020;10:747–761. doi: 10.1016/j.jcmgh.2020.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 337.Tong J, et al. m(6)A mRNA methylation sustains Treg suppressive functions. Cell Res. 2018;28:253–256. doi: 10.1038/cr.2018.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 338.Henning AN, Roychoudhuri R, Restifo NP. Epigenetic control of CD8(+) T cell differentiation. Nat. Rev. Immunol. 2018;18:340–356. doi: 10.1038/nri.2017.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Raskov H, Orhan A, Christensen JP, Gogenur I. Cytotoxic CD8(+) T cells in cancer and cancer immunotherapy. Br. J. Cancer. 2021;124:359–367. doi: 10.1038/s41416-020-01048-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Reading JL, et al. The function and dysfunction of memory CD8(+) T cells in tumor immunity. Immunol. Rev. 2018;283:194–212. doi: 10.1111/imr.12657. [DOI] [PubMed] [Google Scholar]
- 341.Zhang N, Bevan MJ. CD8(+) T cells: foot soldiers of the immune system. Immunity. 2011;35:161–168. doi: 10.1016/j.immuni.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Tsuchiya K, et al. YTHDF1 and YTHDF2 are associated with better patient survival and an inflamed tumor-immune microenvironment in non-small-cell lung cancer. Oncoimmunology. 2021;10:1962656. doi: 10.1080/2162402X.2021.1962656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 343.He X, Tan L, Ni J, Shen G. Expression pattern of m(6)A regulators is significantly correlated with malignancy and antitumor immune response of breast cancer. Cancer Gene Ther. 2021;28:188–196. doi: 10.1038/s41417-020-00208-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Wang L, et al. m(6) A RNA methyltransferases METTL3/14 regulate immune responses to anti-PD-1 therapy. EMBO J. 2020;39:e104514. doi: 10.15252/embj.2020104514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Liu Z, et al. N(6)-methyladenosine-modified circIGF2BP3 inhibits CD8(+) T-cell responses to facilitate tumor immune evasion by promoting the deubiquitination of PD-L1 in non-small cell lung cancer. Mol. Cancer. 2021;20:105. doi: 10.1186/s12943-021-01398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 346.Han D, et al. Anti-tumour immunity controlled through mRNA m(6)A methylation and YTHDF1 in dendritic cells. Nature. 2019;566:270–274. doi: 10.1038/s41586-019-0916-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Liu Y, et al. Tumors exploit FTO-mediated regulation of glycolytic metabolism to evade immune surveillance. Cell Metab. 2021;33:1221–1233.e1211. doi: 10.1016/j.cmet.2021.04.001. [DOI] [PubMed] [Google Scholar]
- 348.Gao Y, et al. Integrated analyses of m(1)A regulator-mediated modification patterns in tumor microenvironment-infiltrating immune cells in colon cancer. Oncoimmunology. 2021;10:1936758. doi: 10.1080/2162402X.2021.1936758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 349.Adamo L, Rocha-Resende C, Mann DL. The emerging role of B lymphocytes in cardiovascular disease. Annu Rev. Immunol. 2020;38:99–121. doi: 10.1146/annurev-immunol-042617-053104. [DOI] [PubMed] [Google Scholar]
- 350.LeBien TW, Tedder TF. B lymphocytes: how they develop and function. Blood. 2008;112:1570–1580. doi: 10.1182/blood-2008-02-078071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Viau M, Zouali M. B-lymphocytes, innate immunity, and autoimmunity. Clin. Immunol. 2005;114:17–26. doi: 10.1016/j.clim.2004.08.019. [DOI] [PubMed] [Google Scholar]
- 352.Lee DSW, Rojas OL, Gommerman JL. B cell depletion therapies in autoimmune disease: advances and mechanistic insights. Nat. Rev. Drug Disco. 2021;20:179–199. doi: 10.1038/s41573-020-00092-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 353.Cancro MP, Tomayko MM. Memory B cells and plasma cells: The differentiative continuum of humoral immunity. Immunol. Rev. 2021;303:72–82. doi: 10.1111/imr.13016. [DOI] [PubMed] [Google Scholar]
- 354.Ripperger TJ, Bhattacharya D. Transcriptional and metabolic control of memory b cells and plasma cells. Annu Rev. Immunol. 2021;39:345–368. doi: 10.1146/annurev-immunol-093019-125603. [DOI] [PubMed] [Google Scholar]
- 355.Zheng Z, et al. Control of early B cell development by the RNA N(6)-methyladenosine methylation. Cell Rep. 2020;31:107819. doi: 10.1016/j.celrep.2020.107819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 356.Nair L, et al. Mechanism of noncoding RNA-associated N(6)-methyladenosine recognition by an RNA processing complex during IgH DNA recombination. Mol. Cell. 2021;81:3949–3964.e3947. doi: 10.1016/j.molcel.2021.07.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 357.Xu A, et al. FTO promotes multiple myeloma progression by posttranscriptional activation of HSF1 in an m(6)A-YTHDF2-dependent manner. Mol. Ther. 2022;30:1104–1118. doi: 10.1016/j.ymthe.2021.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 358.Marcu-Malina V, et al. ADAR1 is vital for B cell lineage development in the mouse bone marrow. Oncotarget. 2016;7:54370–54379. doi: 10.18632/oncotarget.11029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Nutt SL, Chopin M. Transcriptional Networks Driving Dendritic Cell Differentiation and Function. Immunity. 2020;52:942–956. doi: 10.1016/j.immuni.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 360.Eisenbarth SC. Dendritic cell subsets in T cell programming: location dictates function. Nat. Rev. Immunol. 2019;19:89–103. doi: 10.1038/s41577-018-0088-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 361.Gardner A, de Mingo Pulido A, Ruffell B. Dendritic cells and their role in immunotherapy. Front Immunol. 2020;11:924. doi: 10.3389/fimmu.2020.00924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 362.Gardner A, Ruffell B. Dendritic cells and cancer immunity. Trends Immunol. 2016;37:855–865. doi: 10.1016/j.it.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 363.Shen S, et al. N6-methyladenosine (m6A)-mediated messenger RNA signatures and the tumor immune microenvironment can predict the prognosis of hepatocellular carcinoma. Ann. Transl. Med. 2021;9:59. doi: 10.21037/atm-20-7396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 364.Chen Y, Lei J, He S. m(6)A modification mediates mucosal immune microenvironment and therapeutic response in inflammatory bowel disease. Front Cell Dev. Biol. 2021;9:692160. doi: 10.3389/fcell.2021.692160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Wang H, et al. Mettl3-mediated mRNA m(6)A methylation promotes dendritic cell activation. Nat. Commun. 2019;10:1898. doi: 10.1038/s41467-019-09903-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Baal N, et al. ADAR1 is required for dendritic cell subset homeostasis and alveolar macrophage function. J. Immunol. 2019;202:1099–1111. doi: 10.4049/jimmunol.1800269. [DOI] [PubMed] [Google Scholar]
- 367.Myers JA, Miller JS. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021;18:85–100. doi: 10.1038/s41571-020-0426-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 368.Bjorkstrom NK, Strunz B, Ljunggren HG. Natural killer cells in antiviral immunity. Nat. Rev. Immunol. 2022;22:112–123. doi: 10.1038/s41577-021-00558-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Wu SY, Fu T, Jiang YZ, Shao ZM. Natural killer cells in cancer biology and therapy. Mol. Cancer. 2020;19:120. doi: 10.1186/s12943-020-01238-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Caligiuri MA. Human natural killer cells. Blood. 2008;112:461–469. doi: 10.1182/blood-2007-09-077438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 371.Song H, et al. METTL3-mediated m(6)A RNA methylation promotes the anti-tumour immunity of natural killer cells. Nat. Commun. 2021;12:5522. doi: 10.1038/s41467-021-25803-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 372.Ma S, et al. The RNA m6A reader YTHDF2 controls NK cell antitumor and antiviral immunity. J. Exp. Med. 2021;218:e20210279. doi: 10.1084/jem.20210279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Germic N, Frangez Z, Yousefi S, Simon HU. Regulation of the innate immune system by autophagy: monocytes, macrophages, dendritic cells and antigen presentation. Cell Death Differ. 2019;26:715–727. doi: 10.1038/s41418-019-0297-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Chen S, et al. N6-methyladenosine modification of HIV-1 RNA suppresses type-I interferon induction in differentiated monocytic cells and primary macrophages. PLoS Pathog. 2021;17:e1009421. doi: 10.1371/journal.ppat.1009421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Hume DA, Irvine KM, Pridans C. The mononuclear phagocyte system: the relationship between monocytes and macrophages. Trends Immunol. 2019;40:98–112. doi: 10.1016/j.it.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 376.Merad M, Martin JC. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat. Rev. Immunol. 2020;20:355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Chanput W, Mes JJ, Wichers HJ. THP-1 cell line: an in vitro cell model for immune modulation approach. Int Immunopharmacol. 2014;23:37–45. doi: 10.1016/j.intimp.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 378.Khatua S, Simal-Gandara J, Acharya K. Understanding immune-modulatory efficacy in vitro. Chem. Biol. Interact. 2022;352:109776. doi: 10.1016/j.cbi.2021.109776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 379.Qiu W, et al. N(6)-methyladenosine RNA modification suppresses antiviral innate sensing pathways via reshaping double-stranded RNA. Nat. Commun. 2021;12:1582. doi: 10.1038/s41467-021-21904-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 380.Liu Y, et al. N (6)-methyladenosine RNA modification-mediated cellular metabolism rewiring inhibits viral replication. Science. 2019;365:1171–1176. doi: 10.1126/science.aax4468. [DOI] [PubMed] [Google Scholar]
- 381.Zhang Y, et al. RNA-binding protein YTHDF3 suppresses interferon-dependent antiviral responses by promoting FOXO3 translation. Proc. Natl. Acad. Sci. USA. 2019;116:976–981. doi: 10.1073/pnas.1812536116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Zheng Q, Hou J, Zhou Y, Li Z, Cao X. The RNA helicase DDX46 inhibits innate immunity by entrapping m(6)A-demethylated antiviral transcripts in the nucleus. Nat. Immunol. 2017;18:1094–1103. doi: 10.1038/ni.3830. [DOI] [PubMed] [Google Scholar]
- 383.Bannister S, et al. Neonatal BCG vaccination is associated with a long-term DNA methylation signature in circulating monocytes. Sci. Adv. 2022;8:eabn4002. doi: 10.1126/sciadv.abn4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Zhang X, Li X, Jia H, An G, Ni J. The m(6)A methyltransferase METTL3 modifies PGC-1alpha mRNA promoting mitochondrial dysfunction and oxLDL-induced inflammation in monocytes. J. Biol. Chem. 2021;297:101058. doi: 10.1016/j.jbc.2021.101058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 385.Xie J, et al. Elevated N6-methyladenosine RNA levels in peripheral blood immune cells: a novel predictive biomarker and therapeutic target for colorectal cancer. Front Immunol. 2021;12:760747. doi: 10.3389/fimmu.2021.760747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 386.Tong J, et al. Pooled CRISPR screening identifies m(6)A as a positive regulator of macrophage activation. Sci. Adv. 2021;7:eabd4742. doi: 10.1126/sciadv.abd4742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 387.Du J, et al. N(6)-adenosine methylation of Socs1 mRNA is required to sustain the negative feedback control of macrophage activation. Dev. Cell. 2020;55:737–753. doi: 10.1016/j.devcel.2020.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 388.Yu R, Li Q, Feng Z, Cai L, Xu Q. m6A reader YTHDF2 regulates LPS-induced inflammatory response. Int J. Mol. Sci. 2019;20:1323. doi: 10.3390/ijms20061323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 389.Shu B, et al. The METTL3/MALAT1/PTBP1/USP8/TAK1 axis promotes pyroptosis and M1 polarization of macrophages and contributes to liver fibrosis. Cell Death Disco. 2021;7:368. doi: 10.1038/s41420-021-00756-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 390.Li J, et al. ADAR1 attenuates allogeneic graft rejection by suppressing miR-21 biogenesis in macrophages and promoting M2 polarization. FASEB J. 2018;32:5162–5173. doi: 10.1096/fj.201701449R. [DOI] [PubMed] [Google Scholar]
- 391.Geering B, Stoeckle C, Conus S, Simon HU. Living and dying for inflammation: neutrophils, eosinophils, basophils. Trends Immunol. 2013;34:398–409. doi: 10.1016/j.it.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 392.Duffin R, Leitch AE, Fox S, Haslett C, Rossi AG. Targeting granulocyte apoptosis: mechanisms, models, and therapies. Immunol. Rev. 2010;236:28–40. doi: 10.1111/j.1600-065X.2010.00922.x. [DOI] [PubMed] [Google Scholar]
- 393.Zeng H, et al. Construction and analysis of a colorectal cancer prognostic model based on N6-methyladenosine-related lncRNAs. Front Cell Dev. Biol. 2021;9:698388. doi: 10.3389/fcell.2021.698388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 394.Gong PJ, et al. Analysis of N6-methyladenosine methyltransferase reveals METTL14 and ZC3H13 as tumor suppressor genes in breast cancer. Front Oncol. 2020;10:578963. doi: 10.3389/fonc.2020.578963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 395.He Z, et al. PSMC5 promotes proliferation and metastasis of colorectal cancer by activating epithelial-mesenchymal transition signaling and modulating immune infiltrating cells. Front Cell Dev. Biol. 2021;9:657917. doi: 10.3389/fcell.2021.657917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 396.Zhernakova A, van Diemen CC, Wijmenga C. Detecting shared pathogenesis from the shared genetics of immune-related diseases. Nat. Rev. Genet. 2009;10:43–55. doi: 10.1038/nrg2489. [DOI] [PubMed] [Google Scholar]
- 397.Shilts J, et al. A physical wiring diagram for the human immune system. Nature. 2022;608:397–404. doi: 10.1038/s41586-022-05028-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 398.Suo C, et al. Mapping the developing human immune system across organs. Science. 2022;376:eabo0510. doi: 10.1126/science.abo0510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 399.Roy P, Orecchioni M, Ley K. How the immune system shapes atherosclerosis: roles of innate and adaptive immunity. Nat. Rev. Immunol. 2022;22:251–265. doi: 10.1038/s41577-021-00584-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 400.Jacquelot N, Seillet C, Vivier E, Belz GT. Innate lymphoid cells and cancer. Nat. Immunol. 2022;23:371–379. doi: 10.1038/s41590-022-01127-z. [DOI] [PubMed] [Google Scholar]
- 401.Blander JM, Longman RS, Iliev ID, Sonnenberg GF, Artis D. Regulation of inflammation by microbiota interactions with the host. Nat. Immunol. 2017;18:851–860. doi: 10.1038/ni.3780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 402.Sogkas G, et al. Cellular and molecular mechanisms breaking immune tolerance in inborn errors of immunity. Cell Mol. Immunol. 2021;18:1122–1140. doi: 10.1038/s41423-020-00626-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 403.Kalaora S, Nagler A, Wargo JA, Samuels Y. Mechanisms of immune activation and regulation: lessons from melanoma. Nat. Rev. Cancer. 2022;22:195–207. doi: 10.1038/s41568-022-00442-9. [DOI] [PubMed] [Google Scholar]
- 404.Johnson DB, Nebhan CA, Moslehi JJ, Balko JM. Immune-checkpoint inhibitors: long-term implications of toxicity. Nat. Rev. Clin. Oncol. 2022;19:254–267. doi: 10.1038/s41571-022-00600-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 405.Sullivan RJ, Weber JS. Immune-related toxicities of checkpoint inhibitors: mechanisms and mitigation strategies. Nat. Rev. Drug Disco. 2022;21:495–508. doi: 10.1038/s41573-021-00259-5. [DOI] [PubMed] [Google Scholar]
- 406.Tie Y, Tang F, Wei YQ, Wei XW. Immunosuppressive cells in cancer: mechanisms and potential therapeutic targets. J. Hematol. Oncol. 2022;15:61. doi: 10.1186/s13045-022-01282-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 407.Liu J, Peng Y, Wei W. Cell cycle on the crossroad of tumorigenesis and cancer therapy. Trends Cell Biol. 2022;32:30–44. doi: 10.1016/j.tcb.2021.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 408.Gu Y, et al. The evolving landscape of N(6)-methyladenosine modification in the tumor microenvironment. Mol. Ther. 2021;29:1703–1715. doi: 10.1016/j.ymthe.2021.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 409.Li X, Ma S, Deng Y, Yi P, Yu J. Targeting the RNA m(6)A modification for cancer immunotherapy. Mol. Cancer. 2022;21:76. doi: 10.1186/s12943-022-01558-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 410.Natua S, Dhamdhere SG, Mutnuru SA, Shukla S. Interplay within tumor microenvironment orchestrates neoplastic RNA metabolism and transcriptome diversity. Wiley Interdiscip. Rev. RNA. 2022;13:e1676. doi: 10.1002/wrna.1676. [DOI] [PubMed] [Google Scholar]
- 411.Li M, Zha X, Wang S. The role of N6-methyladenosine mRNA in the tumor microenvironment. Biochim Biophys. Acta Rev. Cancer. 2021;1875:188522. doi: 10.1016/j.bbcan.2021.188522. [DOI] [PubMed] [Google Scholar]
- 412.Li D, et al. The m6A/m5C/m1A regulated gene signature predicts the prognosis and correlates with the immune status of hepatocellular carcinoma. Front Immunol. 2022;13:918140. doi: 10.3389/fimmu.2022.918140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 413.Yu G, et al. Comprehensive analysis of m5C methylation regulatory genes and tumor microenvironment in prostate cancer. Front Immunol. 2022;13:914577. doi: 10.3389/fimmu.2022.914577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 414.Liu J, et al. Comprehensive of N1-methyladenosine modifications patterns and immunological characteristics in ovarian cancer. Front Immunol. 2021;12:746647. doi: 10.3389/fimmu.2021.746647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 415.Dong K, et al. Identification and verification of m(7)G modification patterns and characterization of tumor microenvironment infiltration via multi-omics analysis in clear cell renal cell carcinoma. Front Immunol. 2022;13:874792. doi: 10.3389/fimmu.2022.874792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 416.Yang C, et al. Prognostic and immunological role of mRNA ac4C regulator NAT10 in pan-cancer: new territory for cancer research? Front Oncol. 2021;11:630417. doi: 10.3389/fonc.2021.630417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 417.Zhang M, et al. H/ACA snoRNP gene family as diagnostic and prognostic biomarkers for hepatocellular carcinoma. Pharmgenomics Pers. Med. 2021;14:1331–1345. doi: 10.2147/PGPM.S333838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 418.Li B, et al. Surmounting cancer drug resistance: New insights from the perspective of N(6)-methyladenosine RNA modification. Drug Resist Updat. 2020;53:100720. doi: 10.1016/j.drup.2020.100720. [DOI] [PubMed] [Google Scholar]
- 419.Zhang F, et al. Crosstalk among m(6)A RNA methylation, hypoxia and metabolic reprogramming in TME: from immunosuppressive microenvironment to clinical application. J. Hematol. Oncol. 2022;15:84. doi: 10.1186/s13045-022-01304-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 420.Jia J, et al. Novel insights into m(6)A modification of coding and non-coding RNAs in tumor biology: From molecular mechanisms to therapeutic significance. Int J. Biol. Sci. 2022;18:4432–4451. doi: 10.7150/ijbs.73093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 421.Yin H, et al. RNA m6A methylation orchestrates cancer growth and metastasis via macrophage reprogramming. Nat. Commun. 2021;12:1394. doi: 10.1038/s41467-021-21514-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 422.Liu Y, et al. LncRNA-PACERR induces pro-tumour macrophages via interacting with miR-671-3p and m6A-reader IGF2BP2 in pancreatic ductal adenocarcinoma. J. Hematol. Oncol. 2022;15:52. doi: 10.1186/s13045-022-01272-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 423.Dong L, et al. The loss of RNA N(6)-adenosine methyltransferase Mettl14 in tumor-associated macrophages promotes CD8(+) T cell dysfunction and tumor growth. Cancer Cell. 2021;39:945–957.e910. doi: 10.1016/j.ccell.2021.04.016. [DOI] [PubMed] [Google Scholar]
- 424.Wykes MN, Lewin SR. Immune checkpoint blockade in infectious diseases. Nat. Rev. Immunol. 2018;18:91–104. doi: 10.1038/nri.2017.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 425.Galluzzi L, Buque A, Kepp O, Zitvogel L, Kroemer G. Immunogenic cell death in cancer and infectious disease. Nat. Rev. Immunol. 2017;17:97–111. doi: 10.1038/nri.2016.107. [DOI] [PubMed] [Google Scholar]
- 426.McBride AA. Human papillomaviruses: diversity, infection and host interactions. Nat. Rev. Microbiol. 2022;20:95–108. doi: 10.1038/s41579-021-00617-5. [DOI] [PubMed] [Google Scholar]
- 427.Mohammadi M, Akhoundi M, Malih S, Mohammadi A, Sheykhhasan M. Therapeutic roles of CAR T cells in infectious diseases: Clinical lessons learnt from cancer. Rev. Med Virol. 2022;32:e2325. doi: 10.1002/rmv.2325. [DOI] [PubMed] [Google Scholar]
- 428.Tan, B. & Gao, S. J. RNA epitranscriptomics: Regulation of infectionof RNA and DNA viruses by N(6) -methyladenosine (m(6) A. Rev. Med. Virol28, e1983 (2018). [DOI] [PMC free article] [PubMed]
- 429.Yu PL, Cao SJ, Wu R, Zhao Q, Yan QG. Regulatory effect of m(6) A modification on different viruses. J. Med. Virol. 2021;93:6100–6115. doi: 10.1002/jmv.27246. [DOI] [PubMed] [Google Scholar]
- 430.McFadden MJ, Horner SM. N(6)-methyladenosine regulates host responses to viral infection. Trends Biochem Sci. 2021;46:366–377. doi: 10.1016/j.tibs.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 431.Thompson MG, Sacco MT, Horner SM. How RNA modifications regulate the antiviral response. Immunol. Rev. 2021;304:169–180. doi: 10.1111/imr.13020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 432.Williams GD, Gokhale NS, Horner SM. Regulation of viral infection by the RNA modification N6-methyladenosine. Annu Rev. Virol. 2019;6:235–253. doi: 10.1146/annurev-virology-092818-015559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 433.Kostyusheva A, Brezgin S, Glebe D, Kostyushev D, Chulanov V. Host-cell interactions in HBV infection and pathogenesis: the emerging role of m6A modification. Emerg. Microbes Infect. 2021;10:2264–2275. doi: 10.1080/22221751.2021.2006580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 434.Wang L, Wen M, Cao X. Nuclear hnRNPA2B1 initiates and amplifies the innate immune response to DNA viruses. Science. 2019;365:eaav0758. doi: 10.1126/science.aav0758. [DOI] [PubMed] [Google Scholar]
- 435.Crooke PS, 3rd, Tossberg JT, Porter KP, Aune TM. Reduced A-to-I editing of endogenous Alu RNAs in lung after SARS-CoV-2 infection. Curr. Res Immunol. 2021;2:52–59. doi: 10.1016/j.crimmu.2021.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 436.Pfaller CK, Donohue RC, Nersisyan S, Brodsky L, Cattaneo R. Extensive editing of cellular and viral double-stranded RNA structures accounts for innate immunity suppression and the proviral activity of ADAR1p150. PLoS Biol. 2018;16:e2006577. doi: 10.1371/journal.pbio.2006577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 437.Yuan Y, et al. Therapeutic potential of interleukin-2 in autoimmune diseases. Trends Mol. Med. 2022;28:596–612. doi: 10.1016/j.molmed.2022.04.010. [DOI] [PubMed] [Google Scholar]
- 438.Krovi SH, Kuchroo VK. Activation pathways that drive CD4(+) T cells to break tolerance in autoimmune diseases() Immunol. Rev. 2022;307:161–190. doi: 10.1111/imr.13071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 439.Brannon, E. R. et al. Polymeric particle-based therapies for acute inflammatory diseases. Nat Rev Mater. 1–18 (2022). [DOI] [PMC free article] [PubMed]
- 440.Efferth T, Oesch F. The immunosuppressive activity of artemisinin-type drugs towards inflammatory and autoimmune diseases. Med. Res. Rev. 2021;41:3023–3061. doi: 10.1002/med.21842. [DOI] [PubMed] [Google Scholar]
- 441.Damsky W, et al. The emerging role of Janus kinase inhibitors in the treatment of autoimmune and inflammatory diseases. J. Allergy Clin. Immunol. 2021;147:814–826. doi: 10.1016/j.jaci.2020.10.022. [DOI] [PubMed] [Google Scholar]
- 442.Papotto PH, Reinhardt A, Prinz I, Silva-Santos B. Innately versatile: gammadelta17 T cells in inflammatory and autoimmune diseases. J. Autoimmun. 2018;87:26–37. doi: 10.1016/j.jaut.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 443.Wang X, et al. The m6A reader IGF2BP2 regulates macrophage phenotypic activation and inflammatory diseases by stabilizing TSC1 and PPARgamma. Adv. Sci. (Weinh.) 2021;8:2100209. doi: 10.1002/advs.202100209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 444.Laxminarayana D, Khan IU, Kammer G. Transcript mutations of the alpha regulatory subunit of protein kinase A and up-regulation of the RNA-editing gene transcript in lupus T lymphocytes. Lancet. 2002;360:842–849. doi: 10.1016/S0140-6736(02)09966-X. [DOI] [PubMed] [Google Scholar]
- 445.Roth SH, et al. Increased RNA editing may provide a source for autoantigens in systemic lupus erythematosus. Cell Rep. 2018;23:50–57. doi: 10.1016/j.celrep.2018.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 446.Li Q, et al. RNA editing underlies genetic risk of common inflammatory diseases. Nature. 2022;608:569–577. doi: 10.1038/s41586-022-05052-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 447.Liu J, et al. CCR7 chemokine receptor-inducible lnc-Dpf3 restrains dendritic cell migration by inhibiting HIF-1alpha-mediated glycolysis. Immunity. 2019;50:600–615.e615. doi: 10.1016/j.immuni.2019.01.021. [DOI] [PubMed] [Google Scholar]
- 448.Kaul A, et al. Systemic lupus erythematosus. Nat. Rev. Dis. Prim. 2016;2:16039. doi: 10.1038/nrdp.2016.39. [DOI] [PubMed] [Google Scholar]
- 449.Attfield, K. E., Jensen, L. T., Kaufmann, M., Friese, M. A. & Fugger, L. The immunology of multiple sclerosis. Nat Rev Immunol. (2022). [DOI] [PubMed]
- 450.Van der Jeught K, et al. Dendritic cell targeting mRNA lipopolyplexes combine strong antitumor T-cell immunity with improved inflammatory safety. ACS Nano. 2018;12:9815–9829. doi: 10.1021/acsnano.8b00966. [DOI] [PubMed] [Google Scholar]
- 451.Krienke C, et al. A noninflammatory mRNA vaccine for treatment of experimental autoimmune encephalomyelitis. Science. 2021;371:145–153. doi: 10.1126/science.aay3638. [DOI] [PubMed] [Google Scholar]
- 452.Lee JH, et al. Enhancer RNA m6A methylation facilitates transcriptional condensate formation and gene activation. Mol. Cell. 2021;81:3368–3385.e3369. doi: 10.1016/j.molcel.2021.07.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 453.Chellamuthu A, Gray SG. The RNA methyltransferase NSUN2 and its potential roles in cancer. Cells. 2020;9:1758. doi: 10.3390/cells9081758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 454.Sun Z, et al. Aberrant NSUN2-mediated m(5)C modification of H19 lncRNA is associated with poor differentiation of hepatocellular carcinoma. Oncogene. 2020;39:6906–6919. doi: 10.1038/s41388-020-01475-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 455.Letoquart J, et al. Structural and functional studies of Bud23-Trm112 reveal 18S rRNA N7-G1575 methylation occurs on late 40S precursor ribosomes. Proc. Natl. Acad. Sci. USA. 2014;111:E5518–E5526. doi: 10.1073/pnas.1413089111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 456.Boon KL, Pearson MD, Kos M. Self-association of Trimethylguanosine Synthase Tgs1 is required for efficient snRNA/snoRNA trimethylation and pre-rRNA processing. Sci. Rep. 2015;5:11282. doi: 10.1038/srep11282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 457.Peculis BA, Reynolds K, Cleland M. Metal determines efficiency and substrate specificity of the nuclear NUDIX decapping proteins X29 and H29K (Nudt16) J. Biol. Chem. 2007;282:24792–24805. doi: 10.1074/jbc.M704179200. [DOI] [PubMed] [Google Scholar]
- 458.Aphasizhev R, Aphasizheva I, Simpson L. A tale of two TUTases. Proc. Natl. Acad. Sci. USA. 2003;100:10617–10622. doi: 10.1073/pnas.1833120100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 459.Pfaller CK, George CX, Samuel CE. Adenosine deaminases acting on RNA (ADARs) and viral infections. Annu Rev. Virol. 2021;8:239–264. doi: 10.1146/annurev-virology-091919-065320. [DOI] [PubMed] [Google Scholar]
- 460.Cesarini V, et al. ADAR2/miR-589-3p axis controls glioblastoma cell migration/invasion. Nucleic Acids Res. 2018;46:2045–2059. doi: 10.1093/nar/gkx1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 461.Figueroa T, Boumart I, Coupeau D, Rasschaert D. Hyperediting by ADAR1 of a new herpesvirus lncRNA during the lytic phase of the oncogenic Marek’s disease virus. J. Gen. Virol. 2016;97:2973–2988. doi: 10.1099/jgv.0.000606. [DOI] [PubMed] [Google Scholar]
- 462.Song C, Sakurai M, Shiromoto Y, Nishikura K. Functions of the RNA editing enzyme ADAR1 and their relevance to human diseases. Genes (Basel) 2016;7:129. doi: 10.3390/genes7120129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 463.Ringlander J, et al. Impact of ADAR-induced editing of minor viral RNA populations on replication and transmission of SARS-CoV-2. Proc. Natl. Acad. Sci. USA. 2022;119:e2112663119. doi: 10.1073/pnas.2112663119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 464.He J, et al. METTL3 restrains papillary thyroid cancer progression via m(6)A/c-Rel/IL-8-mediated neutrophil infiltration. Mol. Ther. 2021;29:1821–1837. doi: 10.1016/j.ymthe.2021.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]