Abstract
Infection by the gram-negative bacterium Shigella flexneri results in dysentery, an acute inflammatory disease of the colon. Essential events in the pathogenesis of Shigella infections include bacterial invasion of epithelial cells, escape from the phagosome, and induction of apoptosis in macrophages. The Shigella virulence factor invasion plasmid antigen B (IpaB) is required for all of these processes. Induction of apoptosis is dependent on IpaB binding to the cysteine protease caspase-1 (Casp-1). The activation of this enzyme triggers both apoptosis and release of the proinflammatory cytokine interleukin-1β. Several IpaB mutants were generated to correlate function with protein subdomains. We determined that the N-terminal portion of IpaB is necessary for stable expression of IpaB. A putative amphipathic α-helical domain preserves the structure of IpaB. We found 10 consecutive residues within the amino terminus of the hydrophobic region that play a critical role in invasion, phagosomal escape, and cytotoxicity. An IpaB mutant carrying a mutation in this region binds to Casp-1 yet is not cytotoxic, even following direct delivery to the macrophage cytoplasm. These results indicate that the association between IpaB and Casp-1 is only a step in the activation of macrophage apoptosis.
Infections with enterobacteria of the genus Shigella cause dysentery, a severe bloody diarrhea. Dysentery is characterized by an acute inflammation of the colon with mucosal erosion (11, 23). Development of shigellosis requires bacterial penetration across the intestinal epithelial barrier via M cells. Upon reaching the underlying lymphoid follicles, the bacteria are engulfed by resident macrophages (30, 38). Once inside a macrophage, Shigella escapes from the phagosome into the cytoplasm and kills this cell by inducing apoptosis (42). The dying macrophage releases mature interleukin-1β (IL-1β) (40) and IL-18 (32), two cytokines important in the initiation of inflammation (34). Shigella also invades epithelial cells through pathogen-directed endocytosis (23). Invasion of enterocytes and bacterial cell-to-cell spread enhance tissue damage (20, 33).
The Shigella invasion plasmid antigens B (IpaB), IpaC, and IpaD are required for epithelial cell entry and phagosome escape (14, 23). However, IpaB alone is sufficient to activate macrophage apoptosis (5). The Ipa proteins interact with host cells upon being secreted by a type III secretion apparatus (26). In the macrophage cytoplasm, IpaB binds to caspase-1 (Casp-1; also called IL-1β converting enzyme [ICE]), a proapoptotic and proinflammatory cysteine protease that cleaves IL-1β and IL-18 to their biologically active forms (6, 37). The activation of Casp-1 leads to macrophage apoptosis by an as yet ill-defined pathway (15, 40). Casp-1-deficient macrophages' resistance to Shigella-induced cell death demonstrates that Casp-1 is essential for this process (16).
IpaB contains a hydrophobic region (amino acids [aa] 310 to 430) that contains two putative membrane-spanning domains (aa 313 to 346 and 400 to 423) (3). IpaB is homologous to the Salmonella invasion protein B (SipB) and to the Yersinia outer protein B (YopB). The similarity of IpaB to these proteins is particularly high in the hydrophobic region (65 and 30% identity to SipB and YopB, respectively) (9, 18). Interestingly, SipB is required for Salmonella invasion of epithelial cells (12, 17, 18) and interacts with Casp-1 to trigger macrophage apoptosis (13). Unlike IpaB and SipB, YopB is not the effector molecule of Yersinia-induced apoptosis (27, 28), and its function remains controversial (10, 21).
In this study, we investigate the regions of IpaB that are necessary for invasion, phagosome escape, Casp-1 binding, and cytotoxicity. We generated ipaB mutants which were analyzed by functional complementation of a nonpolar ipaB deletion mutant strain (SF620) (25) or by testing purified recombinant IpaB mutant proteins in functional and binding assays. We found a region at the amino terminus of the hydrophobic domain that is required for invasion of epithelial cells, escape from the phagosome, and induction of cell death. Unexpectedly, this region is dispensable for Casp-1 binding.
MATERIALS AND METHODS
Bacterial strains and cell culture.
The Shigella flexneri wild-type strain M90T (serotype 5A) was described previously (35). The ipaB deletion mutant strain SF620 (25) is an avirulent derivative of M90T that contains a nonpolar deletion of the ipaB gene. SF620 containing pGEX-KG-ipaB (gst-ipaB) or pGEX-KG (gst) was described before (5). SF620 was transformed with plasmid pUC19 (39) or with plasmids carrying either wild-type or mutant ipaB. Bacteria were grown at 37°C in tryptic soy broth supplemented with ampicillin (100 μg/ml) or kanamycin (10 μg/ml) when necessary.
J774 and HeLa cells were grown at 37°C with 5% CO2 in RPMI 1640 medium supplemented with 10% decomplemented fetal calf serum (Gibco-BRL), 2 mM glutamine, and 50 μg each of penicillin and streptomycin per ml.
Cloning and mutagenesis of ipaB.
Full-length ipaB was amplified by PCR from p179 (22) using primers ipa-F (forward) and ipa-R (reverse) (Table 1) and cloned into pUC19 (pipaB) (39) by ligation to the HindIII and PstI sites of the polylinker. Similarly, we constructed pipaBN75 and pipaBN146 by cloning a fragment of ipaB (starting at positions corresponding to aa 75 and 146, respectively) into pUC19, using primers N75-F or N146-F, respectively, and ipa-R. Site-directed mutations and internal deletions in ipaB were created using pipaB or pGEX-KG-ipaB as templates. Mutations were produced using the QuickChange site-directed mutagenesis kit (Stratagene) according to the manufacturer's protocol. This method uses complementary oligonucleotides encoding the desired mutation. The sense strand oligonucleotides used in the mutagenesis reactions are listed in Table 1. All mutations were confirmed by restriction analysis and/or automated DNA sequencing.
TABLE 1.
Oligonucleotides used in this studya
Underlined letters represent non-ipaB sequences, such as restriction endonuclease sites generated to facilitate either cloning or screening of mutants and nucleotides introduced by site-directed mutagenesis.
Mutagenic primers. Complementary oligonucleotides were also used but are not listed.
Protein analysis.
Cultures of exponentially growing bacteria were standardized by measuring the optical density at 600 nm and harvested by centrifugation at 10,000 × g for 10 min. Crude bacterial extracts were obtained from the pellets, and proteins of filtered (0.2-μm pore size) culture supernatants were precipitated with 10% trichloroacetic acid. Protein secretion was analyzed in basal conditions from supernatants of cultures grown without specific inducers (24). Protein samples were analyzed by sodium dodecyl sulfate–10% polyacrylamide gel electrophoresis (SDS-PAGE). Immunoblotting procedures were carried out with the mouse anti-IpaB monoclonal antibody (MAb) H16 and anti-IpaC MAb J22, kindly provided by Armelle Phalipon, Institut Pasteur (2, 31). Horseradish peroxidase-labeled sheep anti-mouse immunoglobulin antibodies were used as secondary antibodies and visualized by enhanced chemiluminescence.
For limited proteolysis of IpaB and IpaB mutants, supernatants from exponentially growing cultures were harvested and concentrated 50-fold by filtration through a membrane with a cutoff value of 50 kDa. Samples were diluted fourfold in 50 mM NaHCO3 and digested with 0.6 mg of trypsin (Sigma) per ml at 37°C. At different time intervals, aliquots (20 μl) were sampled and snap-frozen to stop the proteolysis. The protein samples were analyzed by immunoblotting as described above.
Virulence assays.
Infections of J774 and HeLa cells were performed as previously described (35, 41) using a multiplicity of infection of 100. For macrophage cytotoxicity assays, J774 cells were grown in 96-well plates and infected in serum-free medium for 5 h. Cytotoxicity was quantified by measuring the release of lactate dehydrogenase enzyme from infected cells using the CytoTox 96 kit (Promega) following the manufacturer's instructions.
Phagosomal escape was evaluated with a chloroquine resistance assay (7). Briefly, J774 cells infected for 1 h were incubated in the presence of gentamicin (50 μg/ml) with or without chloroquine (100 μg/ml) for an additional 1 h. The cells were subsequently lysed and plated to determine the number of intracellular bacteria surviving the treatment. The percentage of bacteria that escaped from the phagosome was calculated as [(CFU from cells treated with gentamicin and chloroquine, corresponding to bacteria in the cytoplasm)/(CFU from cells treated with gentamicin alone, corresponding to total intracellular bacteria)] × 100. SF620 complemented with wild-type ipaB but not with ipaBC401 caused significant macrophage cytotoxicity in the time course of this experiment. Since dying cells become permeable to gentamicin, the percentage of mutant bacteria escaping the phagosome was evaluated relative to SF620 carrying vector alone.
To test for epithelial cell invasion, the number of intracellular bacteria in infected HeLa cells was determined using a gentamicin protection assay as reported before (29). Briefly, HeLa cells infected for 1 h were incubated in the presence of gentamicin (50 μg/ml) for an additional 3 h. Intracellular bacteria were determined after lysing the infected cells, plating dilutions of the lysates, and counting the CFU. In the assays described above, the standard error was calculated based on at least three independent determinations.
Purification of GST fusion proteins.
Glutathione-S-transferase (GST), GST-IpaB, and GST-IpaB mutant proteins were produced as described before (5) from SF620 harboring plasmids encoding the corresponding genes. Bacterial cultures were induced with IPTG (isopropyl thiogalactopyronoside) for 3 h and pellets were subsequently lysed by French press. The lysates were incubated with glutathione-Sepharose beads (Pharmacia) for 4 h at 4°C, followed by three washes of the beads with phosphate-buffered saline (PBS). The GST and GST fusion proteins were used either coupled to the beads or after elution with glutathione, following the manufacturer's protocol.
Microinjection.
Microinjection experiments and the isolation of peritoneal macrophages were performed as previously described (5). Briefly, a monolayer of cells was microinjected (0.3 × 10−11 to 0.7 × 10−11 ml/cell) with coded samples (750 μg of protein per ml, 2.25 to 5.25 fg of protein per cell) using an Eppendorf microinjection system. After microinjection, cells were incubated for 4 to 6 h at 37°C and then stained with 1 μM propidium iodide in PBS. Injected cells were identified and scored for propidium iodide uptake into the nucleus, which allows visualization of dead cells. The results are the averages of at least four experiments with a minimum of 600 cells microinjected per sample.
Casp-1 binding assay.
J774 cells were radiolabeled with [35S]methionine, lysed, and centrifuged to obtain a nucleus-free supernatant. GST or GST fusion proteins coupled to beads were incubated with the cell lysate for 3 h at 4°C and then washed five times with RIPA buffer (1% Triton X-100, 0.5% deoxycholic acid, 0.1% SDS, 50 mM Tris-HCl [pH 7.5], 0.15 M NaCl). The proteins bound to the beads were resolved in a 5 to 15% gradient SDS-PAGE gel and exposed to a PhosphorImager. As described before (5), the only radioactive protein binding to IpaB was identified as Casp-1 by immunoblotting. Since the Casp-1 antibodies cross-react with IpaB mutant degradation products, the detection of bound radiolabeled Casp-1 is more sensitive and reproducible than the immunoblot in these cases.
RESULTS AND DISCUSSION
ipaB truncation mutants.
The S. flexneri nonpolar ipaB deletion mutant strain SF620 is unable to invade epithelial cells or cause macrophage apoptosis. Introduction of wild-type ipaB in trans (SF620/pipaB) fully complements SF620 for these two phenotypes (see Fig. 4) (25, 36, 41). The levels of cytoplasmic and secreted IpaB in SF620/pipaB were slightly higher but not significantly different from those in wild-type Shigella by immunoblot analysis (see Fig. 2A), even though ipaB is a high-copy-number plasmid (pUC19) and under a heterologous promoter. Ménard et al. (25) originally described comparable results obtained using the same ipaB mutant strain (SF620) complemented with a similar plasmid.
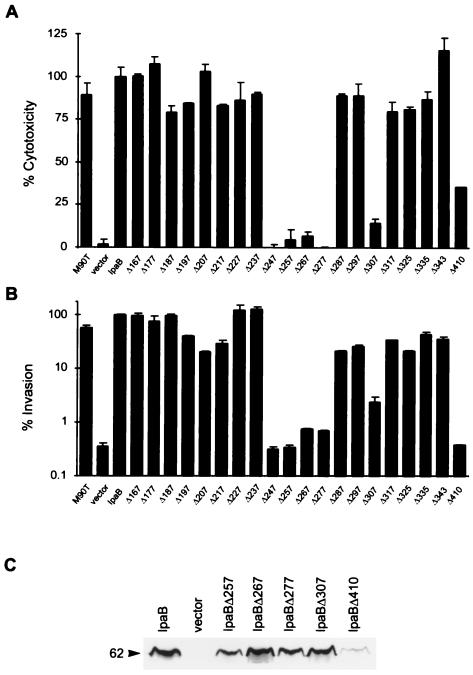
FIG. 4.
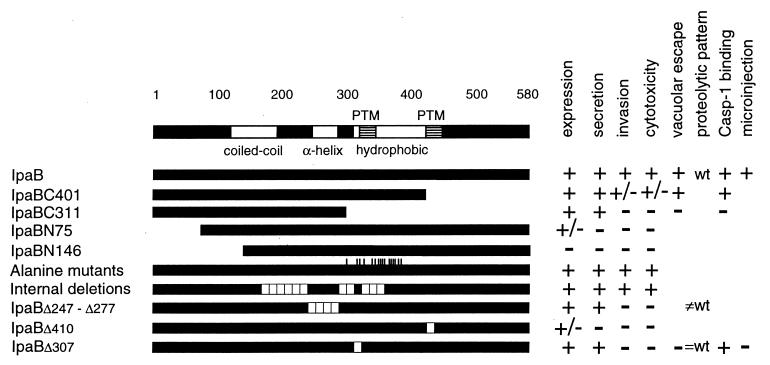
Cytotoxicity, invasiveness, and expression of ipaB internal deletion mutants. Macrophage cytotoxicity (A) and epithelial cell invasion (B) of SF620 complemented with plasmids encoding ipaB internal deletion mutants. Cells were infected with SF620 carrying vector alone or SF620 expressing IpaB or the indicated IpaB deletion mutants. Mutants are labeled with the corresponding starting position. The wild-type strain M90T was also included as a control. Cytotoxicity and invasiveness were assayed as described in Materials and Methods. (C) Whole-cell extracts of SF620 carrying vector alone or SF620 expressing IpaB or the indicated IpaB deletion mutants were analyzed by immunoblotting. IpaBΔ257–266, IpaBΔ267–276, IpaBΔ277–286, and IpaBΔ307–316 are expressed at levels comparable to wild-type IpaB. In contrast, IpaBΔ410–417 is expressed at significantly lower levels.
FIG. 2.
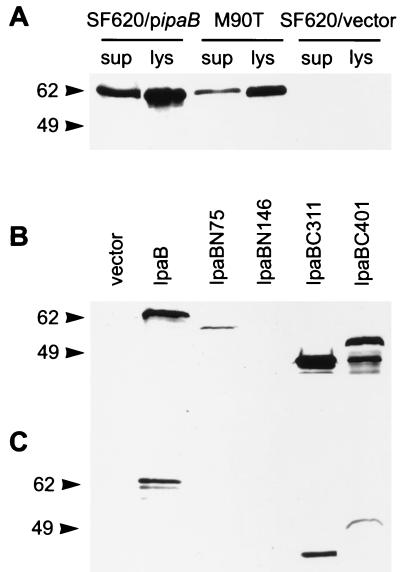
Expression and secretion of IpaB truncates. (A) Whole-cell lysates (lys) and culture supernatants (sup) of SF620 carrying pipaB, wild-type strain M90T, and SF620 carrying vector alone were analyzed by immunoblotting with an anti-IpaB MAb. The expression of IpaB in SF620/pipaB is slightly higher but not significantly different from that in the wild-type Shigella strain. Whole-cell extracts (B) and culture supernatants (C) of SF620 carrying vector alone or SF620 expressing IpaB or the indicated truncates were analyzed by immunoblotting with an anti-IpaB MAb. IpaBC311 and IpaBC401 are expressed and secreted at detectable levels. In contrast, IpaBN75 is expressed but not secreted, and IpaB146 is neither expressed nor secreted. The molecular sizes are indicated (in kilodaltons).
To determine the regions necessary for the different functions of IpaB, we designed truncations of either the amino-terminal (IpaBN75 and IpaBN146) or the carboxy-terminal (IpaBC311 and IpaBC401) segments of the protein (Fig. 1A). We tested constructs bearing these deletions for their ability to complement SF620 in assays for expression, secretion, macrophage cytotoxicity, phagosome escape, and epithelial cell invasion.
FIG. 1.
Schematic representation of mutations generated in IpaB. (A) We generated two C-terminal (IpaBC401 and IpaBC311) and two N-terminal (IpaBN75 and IpaBN146) constructs. The positions of the corresponding first and last aa in each truncate are indicated. (B) Alanine-scanning. The indicated amino acids, either charged or polar, were substituted by alanine. (C) Internal, nonoverlapping deletions. Twenty deletions of either 8 or 10 residues were generated. Only the starting positions of the deletions are shown.
To examine whether these IpaB truncations were expressed and secreted, we analyzed the bacterial extracts and culture supernatants by immunoblotting. IpaB lacking the N terminus (IpaBN75) was detected, albeit in low amounts, in the bacteria, but was not secreted (Fig. 2B and C). We could not detect secreted IpaBN75 even after overexposure of the film (data not shown). Further truncation of the N terminus (IpaBN146) appears to make expression of the protein even lower, since it was not detected in bacterial extracts.
We could detect the translated product of ipaBN146 in highly concentrated (at least 10-fold) samples of bacterial extracts, confirming that this truncated product was synthesized (data not shown). In contrast, loss of the C-terminal region in IpaB (IpaBC311 and IpaBC401) did not abrogate the expression or the secretion of the protein compared to wild-type IpaB levels (Fig. 2B and C). In the immunoblot analysis, we detected several IpaBC401 degradation products that are not present in the other truncates or in wild-type IpaB. The significance of this degradation remains to be determined.
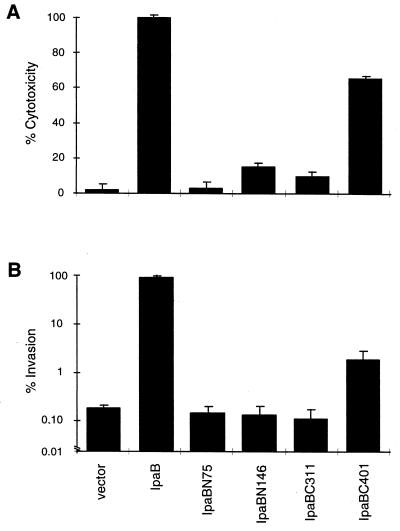
We measured the capacity of the ipaB truncates to complement SF620 for killing of the macrophage-like cell line J774. The level of cytotoxicity observed for SF620 with wild-type ipaB expressed in trans was used as a reference (100%) to calculate the killing potential of SF620 carrying the truncates or vector alone. Strains encoding ipaBN75 and ipaBN146 were severely attenuated for macrophage cytotoxicity (Fig. 3A). Among the C-terminal truncation mutants, IpaBC311 was not cytotoxic, while IpaBC401 retained an intermediate ability to kill macrophages.
FIG. 3.
Cytotoxicity and invasiveness of ipaB truncation mutants. Macrophage cytotoxicity (A) and epithelial cell invasion (B) of SF620 complemented with plasmids encoding ipaB truncates. Cells were infected with SF620 carrying vector alone or SF620 expressing IpaB or the indicated truncates. Cytotoxicity and invasiveness were assayed as described in Materials and Methods.
Using a gentamicin protection assay, we evaluated the capacity of the ipaB truncates to complement SF620 for invasion of HeLa cells, an epithelial cell line. The number of intracellular bacteria recovered after infection with SF620 complemented with wild-type ipaB was used as a reference (100%). Similar to the results obtained for cytotoxicity, ipaBN75 and ipaBN146 did not complement SF620 for HeLa cell invasion (Fig. 3B). ipaBC311 was also unable to restore invasion, while SF620 containing ipaBC401 was 10-fold more invasive than SF620 with vector alone.
Escape from the phagosome is a prerequisite for Shigella to induce macrophage apoptosis (41). We tested whether SF620 complemented with either ipaBC311 or ipaBC401 escapes from the phagosome using a chloroquine resistance assay (7). This assay was done in J774 macrophages, which are phagocytic and therefore internalize both invasive and noninvasive bacteria. The percentage of cytoplasmic bacteria was calculated relative to the total number of intracellular bacteria as described in Materials and Methods. Both the control strain carrying the vector alone and the one with ipaBC311 were unable to lyse the phagosomal vacuole, displaying 7% ± 1% and 6% ± 1% escape, respectively. Conversely, a high percentage of bacteria expressing IpaBC401 (62% ± 6%) reached the cytoplasm of the macrophage.
The N-terminal region of IpaB appears to be relevant for maintaining normal levels of cytoplasmic protein. Not surprisingly, mutants IpaBN75 and IpaBN146 were impaired in cytotoxicity and invasion. The region truncated in IpaBN146 maps, in part, to a coiled-coil structure between aa 120 and 180, as predicted by the algorithm of Berger et al. (4). The low protein levels detected for the amino-terminal IpaB truncation mutants may be due to lower transcription or translation. Both of these mutants use a different translation initiation site, which could modify the translational level. In the bacterial cytoplasm, the chaperone IpgC prevents IpaB degradation prior to its secretion (26). This interaction could also be altered in mutants IpaBN75 and IpaBN146 and cause protein instability. Further tests are necessary to determine why the N-terminal region of IpaB affects protein levels.
We determined that the C-terminal 179 aa in IpaB are, to some degree, dispensable for function, since a mutant with a deletion in this region (ipaBC401) partially complements SF620. In contrast, truncation of the entire hydrophobic region (ipaBC311) causes a complete loss of activity. These data imply that the hydrophobic domain is either necessary for function or important to maintain the structure of another critical domain. HeLa cell invasion was complemented to a lesser extent than cytotoxicity by ipaBC401. This discrepancy may reflect differences in the sensitivity of each method in measuring IpaB activity. Alternatively, the C terminus of IpaB may play a more important role in epithelial cell invasion than in macrophage killing.
Alanine-scanning mutagenesis of ipaB.
The results described above indicate that the hydrophobic core of IpaB is essential for activity. Since charged or polar residues in hydrophobic domains may play a role in protein function (19), we mutated each of these residues. Our mutations resulted in alanine substitution of 3 polar and 13 charged aa between residues 306 and 400 in IpaB (Fig. 1B). Each mutant was tested for its capacity to complement SF620 for macrophage cytotoxicity and epithelial cell invasion. All of these ipaB mutants complemented SF620 for killing and invasion similar to wild-type levels (data not shown). These data suggest that the charged and polar residues in the hydrophobic domain do not play a crucial role. Alternatively, single substitutions of these aa may be insufficient to cause a significant alteration in protein function.
Internal-deletion ipaB mutants.
We constructed independent and consecutive internal deletions of 8 or 10 aa in the region between aa 167 and 352 and a deletion between aa 410 and 417 (Fig. 1C). These deletions covered part of the coiled-coil region and the two putative transmembrane domains in the hydrophobic region. The phenotypes of these deletions were assayed as described above. Six of these deletion mutants, encoding ipaBΔ247–256, ipaBΔ257–266, ipaBΔ267–276, ipaBΔ277–286, ipaBΔ307–316, and ipaBΔ410–417, killed macrophages very inefficiently (Fig. 4A). Furthermore, these mutants could not invade HeLa cells (Fig. 4B). The rest of the deletion mutants fully complemented SF620 for both cytotoxicity and HeLa cell invasion.
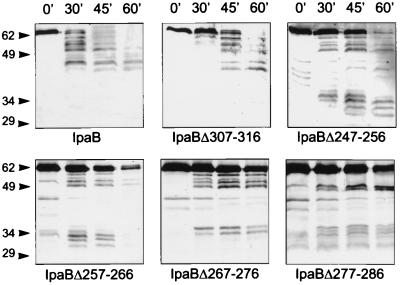
Of the mutants impaired in function, only IpaBΔ410–417 was expressed at very low levels, while IpaBΔ247–256 (data not shown), IpaBΔ257–266, IpaBΔ267–276, IpaBΔ277–286, and IpaBΔ307–316 were expressed (Fig. 4C) and secreted (Fig. 5, lane 0′) at levels similar to wild-type IpaB. To investigate whether these IpaB mutants had conformational alterations, we tested their susceptibility to limited proteolysis by trypsin (1) and analyzed them by immunoblotting. IpaBΔ307–316 (Fig. 5) and the fully functional deletion mutant IpaBΔ237–246 (data not shown) had a proteolytic pattern similar to that of wild-type IpaB, suggesting that IpaBΔ307–316 folds correctly. In contrast, the proteolysis of IpaBΔ247– 256, IpaBΔ257–266, IpaBΔ267–276, and IpaBΔ277–286 yielded products of 29 to 34 kDa which are not observed in wild-type IpaB. Also, the full-length products of these mutants seem to be more resistant to trypsin digestion. Taken together, these results suggest that these IpaB mutants do not fold correctly. The loss of function observed in strains expressing IpaBΔ247–256, IpaBΔ257–266, and IpaBΔ277–286 is probably due to their structural alterations. According to our secondary-structure analysis, these mutations map to an amphipathic α-helix between aa 240 and 280 that is predicted by the algorithms of Frishman and Argas (Fig. 6) (8). Our data suggest that this structure may be necessary to maintain IpaB in its native conformation.
FIG. 5.
Proteolytic profile of IpaB internal deletion mutants. Culture supernatants from SF620 expressing IpaB or the indicated IpaB deletion mutants were subjected to limited proteolysis by trypsin and analyzed by immunoblot. The time course of trypsin digestion is indicated (in minutes), and molecular sizes are shown in kilodaltons. The pattern observed for IpaBΔ307–316 but not for IpaBΔ247–256, IpaBΔ257–266, IpaBΔ267–276 or IpaBΔ277–286 is similar to that for wild-type IpaB.
FIG. 6.
Summary of IpaB mutant results. IpaB and IpaB mutants are schematically represented by solid bars with the corresponding results of the assays performed. The predicted domains in IpaB, coiled-coil, amphipathic α-helix, hydrophobic, and putative transmembrane (PTM), are indicated. Wild-type IpaB, truncation mutants, alanine mutants, and internal deletion mutants are shown. Internal deletion mutants with a phenotype similar to the wild type are grouped together and independently from those with a different phenotype. The results described in this work are summarized as positive (+), intermediate (+/−), and negative (−); refer to the text for quantitative details. For the proteolytic pattern, wt indicates a wild-type pattern, =wt indicates similar to wild-type pattern, and ≠wt indicates different from wild-type pattern.
There are two putative transmembrane regions in IpaB (Fig. 6) (3). The mutations in IpaBΔ307–316, IpaBΔ317–324, IpaBΔ325–334, and IpaBΔ335–342 all map within the first of these regions (aa 313 to 346). Interestingly, among these mutants only IpaBΔ307–316, in which only 3 aa of the putative transmembrane region are deleted, was impaired in function, as tested by complementation of SF620. The second putative membrane-spanning region in IpaB (aa 400 to 423) maps beyond the IpaBC401 truncate, which conserves function. A deletion mutation in the second putative membrane-spanning region, IpaBΔ410– 417, was poorly expressed and therefore was inactive in our functional assays. It remains to be determined whether the inefficiency of this mutant in complementing for invasion and cytotoxicity is intrinsic to the mutant's function or a reflection of the low level of expression.
Analysis of IpaBΔ307 and Casp-1 binding domain in IpaB.
The partial tryptic digestion of IpaBΔ307–316 suggests that this mutant has a normal structure. Nevertheless, IpaBΔ307–316 is unable to mediate macrophage cytotoxicity and HeLa cell invasion. To further investigate ipaBΔ307–316, we tested its ability to complement SF620 for phagosome escape. The percentage of SF620 complemented with ipaBΔ307–316 that escapes to the cytoplasm (10% ± 1%) is similar to that of SF620 carrying vector alone (7% ± 1%). This result suggests that aa 307 to 316 are also critical for lysing the phagosome.
Since escape from the phagosomal vacuole is a prerequisite for cytotoxicity and IpaBΔ307–316 is impaired in this function, we microinjected purified protein into the macrophage cytoplasm to test the cytotoxicity of this mutant. Microinjection of GST-IpaB fusion protein but not of GST into macrophages causes cell death (5). After microinjection of purified GST fusion proteins, macrophage cytotoxicity was evaluated by propidium iodide exclusion (5). The number of dead macrophages was scored by fluorescence microscopy. Our positive control, GST-IpaB, and our negative control (GST) caused 85% ± 5% and 24% ± 4% cytotoxicity, respectively. GST-IpaBΔ307–316 was poorly cytotoxic (30% ± 5%). Thus, residues 307 to 316 in IpaB are crucial for the induction of macrophage death.
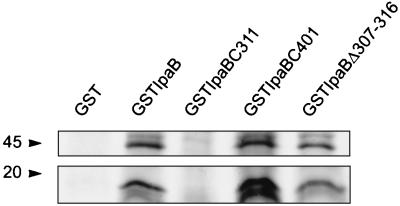
The interaction of IpaB with Casp-1 triggers the cell death program in Shigella-infected macrophages (5, 16). To map the region of IpaB involved in this interaction, we purified the GST fusion proteins GST-IpaB, GST-IpaBC311, GST-IpaBΔ307–316, and GST-IpaBC401 and tested their ability to associate with Casp-1. The recombinant proteins were incubated with radiolabeled J774 lysates as previously described (5). Casp-1 precursor and mature forms bound to GST-IpaB but not to the negative control GST (Fig. 7). Casp-1 interacted with GST-IpaBC401 and GST-IpaBΔ307–316 but did not associate with GST-IpaBC311. These results indicate that the Casp-1 binding domain is located in the hydrophobic region of IpaB that is amino terminal to aa 401. In addition, since GST-IpaBΔ307–316 binds to Casp-1 but is not cytotoxic, it is possible that residues between 307 and 316 in IpaB are involved in regulating the activation of Casp-1. The precise mechanism of this process is still under study.
FIG. 7.
Binding of IpaB mutants to Casp-1. GST-IpaB mutants coupled to beads were incubated with lysates from metabolically labeled J774 macrophages. Proteins eluted from the beads were resolved in a 5 to 15% gradient SDS-PAGE. GST-IpaB and GST are the positive and negative controls, respectively. The molecular sizes are indicated (in kilodaltons). The bands observed in the top and bottom panels correspond to the Casp-1 precursor and mature forms, respectively. GST-IpaBC401 and GST-IpaBΔ307–316 but not GST-IpaBC311 bind to Casp-1.
Between residues 307 and 316 there is only one polar (C309) and one charged (K312) aa. As described in the section on alanine-scanning mutagenesis, the replacement of these residues with alanine did not affect IpaB function, suggesting that the activity resides in the hydrophobic residues of this sequence. These residues are necessary to maintain a functional IpaB either by having intrinsic activity or by indirectly affecting a different functional region. Interestingly, the sequence of IpaB between aa 307 and 316 is identical, except for a single conservative substitution, to the corresponding segment in the IpaB homologue SipB. In contrast, YopB, which is not involved in the induction of apoptosis (27, 28), does not contain this aa sequence. Since the hydrophobic region in IpaB is homologous to SipB, and SipB can complement SF620 for HeLa cell invasion (12) and macrophage cytotoxicity (our unpublished observation), our results provide a structure-function link between these two proteins. We speculate that the hydrophobic domain of SipB also constitutes the functional core. Future research on the interaction between IpaB and Casp-1 as well as on the role of IpaB in invasion will allow us to better understand how IpaB and its homologues work.
ACKNOWLEDGMENTS
This work was supported by grants from the NIH (AI 42780–01) and the WHO (V27/181/108).
We thank A. B. Hittelman for his help with the pipaBN75 construct. We acknowledge Rashmi Hegde and Tim Cardozo for their valuable advice. We thank A. Aliprantis, H. Hilbi, R. Menard, J. Moss, W. Navare, R. Puro, and Y. Weinrauch for careful revision of the manuscript.
REFERENCES
- 1.Barzu S, Arondel J, Guillot S, Sansonetti P J, Phalipon A. Immunogenicity of IpaC hybrid proteins expressed in the Shigella flexneri 2a vaccine candidate SC602. Infect Immun. 1998;66:77–82. doi: 10.1128/iai.66.1.77-82.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barzu S, Nato F, Rouyre S, Mazie J-C, Sansonetti P J, Phalipon A. Characterization of B-cell epitopes on IpaB, an invasion-associated antigen of Shigella flexneri: identification of an immunodominant domain recognized during natural infection. Infect Immun. 1993;61:3825–3831. doi: 10.1128/iai.61.9.3825-3831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Baudry B, Kaczorek M, Sansonetti P J. Nucleotide sequence of the invasion plasmid antigen B and C genes (ipaB and ipaC) of Shigella flexneri. Microb Pathog. 1988;4:345–357. doi: 10.1016/0882-4010(88)90062-9. [DOI] [PubMed] [Google Scholar]
- 4.Berger B, Wilson D B, Wolf E, Tonchev M M, Kim P S. Predicting coiled coils by use of pairwise residue correlations. Proc Natl Acad Sci USA. 1995;92:8359–8263. doi: 10.1073/pnas.92.18.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y, Smith M R, Thirumalai K, Zychlinsky A. A bacterial invasin induces macrophage apoptosis by directly binding ICE. EMBO J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 6.Dinarello C. Interleukin-1β, interleukin-18, and the interleukin-1 beta converting enzyme. Ann NY Acad Sci. 1998;856:1–11. doi: 10.1111/j.1749-6632.1998.tb08307.x. [DOI] [PubMed] [Google Scholar]
- 7.Finlay B B, Falkow S. Comparison of the invasion strategies used by Salmonella cholera-suis, Shigella flexneri and Yersinia enterocolitica to enter cultured animal cells: endosome acidification is not required for bacterial invasion or intracellular replication. Biochimie. 1988;70:1089–1099. doi: 10.1016/0300-9084(88)90271-4. [DOI] [PubMed] [Google Scholar]
- 8.Frishman D, Argos P. Incorporation of non-local interactions in protein secondary structure prediction from the amino acid sequence. Protein Eng. 1996;9:133–142. doi: 10.1093/protein/9.2.133. [DOI] [PubMed] [Google Scholar]
- 9.Hakansson S, Bergman T, Vanooteghem J C, Cornelis G, Wolf-Watz H. YopB and YopD constitute a novel class of Yersinia Yop proteins. Infect Immun. 1993;61:71–80. doi: 10.1128/iai.61.1.71-80.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hakansson S, Schesser K, Persson C, Galyov E E, Rosqvist R, Homble F, Wolf-Watz H. The YopB protein of Yersinia pseudotuberculosis is essential for the translocation of Yop effector proteins across the target cell plasma membrane and displays a contact-dependent membrane disrupting activity. EMBO J. 1996;15:5812–5823. [PMC free article] [PubMed] [Google Scholar]
- 11.Hale T L. Genetic basis of virulence in Shigella species. Microbiol Rev. 1991;55:206–224. doi: 10.1128/mr.55.2.206-224.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hermant D, Ménard R, Arricau N, Parsot C, Popoff M Y. Functional conservation of the Salmonella and Shigella effectors of entry into epithelial cells. Mol Microbiol. 1995;17:781–789. doi: 10.1111/j.1365-2958.1995.mmi_17040781.x. [DOI] [PubMed] [Google Scholar]
- 13.Hersh D, Monack D, Smith M, Ghori N, Falkow S, Zychlinsky A. The Salmonella invasin SipB induces macrophage apoptosis by binding to caspase-1. Proc Natl Acad Sci USA. 1999;96:2396–2401. doi: 10.1073/pnas.96.5.2396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.High N, Mounier J, Prevost M C, Sansonetti P J. IpaB of Shigella flexneri causes entry into epithelial cells and escape from the phagocytic vacuole. EMBO J. 1992;11:1991–1999. doi: 10.1002/j.1460-2075.1992.tb05253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hilbi H, Chen Y, Thirumalai K, Zychlinsky A. The interleukin 1β-converting enzyme, caspase 1, is activated during Shigella flexneri-induced apoptosis in human monocyte-derived macrophages. Infect Immun. 1997;65:5165–5170. doi: 10.1128/iai.65.12.5165-5170.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilbi H, Moss J E, Hersh D, Chen Y, Arondel J, Banerjee S, Flavell R A, Yuan J, Sansonetti P J, Zychlinsky A. Shigella-induced apoptosis is dependent on caspase-1 which binds to IpaB. J Biol Chem. 1998;273:32895–32900. doi: 10.1074/jbc.273.49.32895. [DOI] [PubMed] [Google Scholar]
- 17.Hueck C, Hantman M, Bajaj V, Johnston C, Lee C, Miller S. Salmonella typhimurium secreted invasion determinants are homologous to Shigella Ipa proteins. Mol Microbiol. 1995;18:479–490. doi: 10.1111/j.1365-2958.1995.mmi_18030479.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaniga K, Tucker S, Trollinger D, Galan J E. Homologs of the Shigella IpaB and IpaC invasins are required for Salmonella typhimurium entry into cultured epithelial cells. J Bacteriol. 1995;177:3965–3971. doi: 10.1128/jb.177.14.3965-3971.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klann A G, Hull R A, Palzkill T, Hull S I. Alanine-scanning mutagenesis reveals residues involved in binding of pap-3-encoded pili. J Bacteriol. 1994;176:2312–2317. doi: 10.1128/jb.176.8.2312-2317.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaBrec E H, Schneider H, Magnani T J, Formal S B. Epithelial cell pentetration as an essential step in the pathogenesis of bacillary dysentery. J Bacteriol. 1964;88:1503–1518. doi: 10.1128/jb.88.5.1503-1518.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee V T, Schneewind O. Type III machines of pathogenic yersiniae secrete virulence factors into the extracellular milieu. Mol Microbiol. 1999;31:1619–1629. doi: 10.1046/j.1365-2958.1999.01270.x. [DOI] [PubMed] [Google Scholar]
- 22.Maurelli A T, Baudry B, d'Hauteville H, Hale T L, Sansonetti P J. Cloning of plasmid DNA sequences involved in invasion of HeLa cells by Shigella flexneri. Infect Immun. 1985;49:164–171. doi: 10.1128/iai.49.1.164-171.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ménard R, Dehio C, Sansonetti P J. Bacterial entry into epithelial cells: the paradigm of Shigella. Trends Microbiol. 1996;4:220–226. doi: 10.1016/0966-842X(96)10039-1. [DOI] [PubMed] [Google Scholar]
- 24.Ménard R, Sansonetti P, Parsot C. The secretion of the Shigella flexneri Ipa invasins is activated by epithelial cells and controlled by IpaB and IpaD. EMBO J. 1994;13:5293–5302. doi: 10.1002/j.1460-2075.1994.tb06863.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ménard R, Sansonetti P J, Parsot C. Nonpolar mutagenesis of the ipa genes defines IpaB, IpaC, and IpaD as effectors of Shigella flexneri entry into epithelial cells. J Bacteriol. 1993;175:5899–5906. doi: 10.1128/jb.175.18.5899-5906.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ménard R, Sansonetti P J, Parsot C, Vasselon T. Extracellular association and cytoplasmic partitioning of the IpaB and IpaC invasins of S. flexneri. Cell. 1994;79:515–525. doi: 10.1016/0092-8674(94)90260-7. [DOI] [PubMed] [Google Scholar]
- 27.Mills S D, Boland A, Sory M-P, van der Smissen P, Kerbourch C, Finlay B B, Cornelis G R. Yersinia enterocolitica induces apoptosis in macrophages by a process requiring functional type III secretion and translocation mechanisms and involving YopP, presumably acting as an effector protein. Proc Natl Acad Sci USA. 1997;94:12638–12643. doi: 10.1073/pnas.94.23.12638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Monack D M, Mecsas J, Ghori N, Falkow S. Yersinia signals macrophages to undergo apoptosis and YopJ is necessary for this cell death. Proc Natl Acad Sci USA. 1997;94:10385–10390. doi: 10.1073/pnas.94.19.10385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mounier J, Vasselon T, Hellio R, Lesourd M, Sansonetti P J. Shigella flexneri enters human colonic Caco-2 epithelial cells through the basolateral pole. Infect Immun. 1992;60:237–248. doi: 10.1128/iai.60.1.237-248.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perdomo O J, Cavaillon J M, Huerre M, Ohayon H, Gounon P, Sansonetti P J. Acute inflammation causes epithelial invasion and mucosal destruction in experimental shigellosis. J Exp Med. 1994;180:1307–1319. doi: 10.1084/jem.180.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Phalipon A, Arondel J, Nato F, Rouyre S, Mazie J C, Sansonetti P J. Identification and characterization of B-cell epitopes of IpaC, an invasion-associated protein of Shigella flexneri. Infect Immun. 1992;60:1919–1926. doi: 10.1128/iai.60.5.1919-1926.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sansonetti P, Phalipon A, Arondel J, Thirumalai K, Banerjee S, Akira S, Takeda K, Zychlinsky A. Caspase-1 activation of IL-1b and IL-18 are essential for Shigella flexneri induced inflammation. Immunity. 2000;12:581–590. doi: 10.1016/s1074-7613(00)80209-5. [DOI] [PubMed] [Google Scholar]
- 33.Sansonetti P J, Arondel J. Construction and evaluation of a double mutant of Shigella flexneri as a candidate for oral vaccination against shigellosis. Vaccine. 1989;7:443–450. doi: 10.1016/0264-410x(89)90160-6. [DOI] [PubMed] [Google Scholar]
- 34.Sansonetti P J, Arondel J, Cavaillon J-M, Huerre M. Role of IL-1 in the pathogenesis of experimental shigellosis. J Clin Investig. 1995;96:884–892. doi: 10.1172/JCI118135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sansonetti P J, Kopecko D J, Formal S B. Involvement of a plasmid in the invasive ability of Shigella flexneri. Infect Immun. 1982;35:852–860. doi: 10.1128/iai.35.3.852-860.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thirumalai K, Kim K, Zychlinsky A. IpaB, a Shigella flexneri invasin, colocalizes with interleukin-1β converting enzyme in the cytoplasm of macrophages. Infect Immun. 1997;65:787–793. doi: 10.1128/iai.65.2.787-793.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thornberry N A. Interleukin-1β converting enzyme. Methods Enzymol. 1994;244:615–631. doi: 10.1016/0076-6879(94)44045-x. [DOI] [PubMed] [Google Scholar]
- 38.Wassef J S, Keren D F, Mailloux J L. Role of M cells in initial antigen uptake and in ulcer formation in rabbit intestinal loop model of shigellosis. Infect Immun. 1989;57:858–863. doi: 10.1128/iai.57.3.858-863.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]
- 40.Zychlinsky A, Fitting C, Cavaillon J M, Sansonetti P J. Interleukin-1 is released by murine macrophages during apoptosis induced by Shigella flexneri. J Clin Investig. 1994;94:1328–1332. doi: 10.1172/JCI117452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zychlinsky A, Kenny B, Ménard R, Prévost M C, Holland I B, Sansonetti P J. IpaB mediates macrophage apoptosis induced by Shigella flexneri. Mol Microbiol. 1994;11:619–627. doi: 10.1111/j.1365-2958.1994.tb00341.x. [DOI] [PubMed] [Google Scholar]
- 42.Zychlinsky A, Prévost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–168. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]