Abstract
Background & Aims.
Many of the reported adverse events in clinical trials of IBS are extra-intestinal symptoms, which are typically assessed by open-ended questions during the trial and not at baseline. This may lead to misattribution of some pre-existing symptoms as side effects to the treatment.
Methods.
The current study analyzed data from a 6-week clinical trial of irritable bowel syndrome (IBS). Participants were randomized to receive double-blind peppermint oil (DBM), double-blind placebo (DBP), or treatment as usual (TAU). Extra-intestinal symptoms were assessed at baseline and end-of-study.
Results.
This analysis included 173 participants (30 in DBM, 72 in TAU, and 71 in DBP). At baseline, each group reported approximately five extra-intestinal symptoms per participant. The number of symptoms per participant decreased to an average of three by the end-of-study visit, and this change was statistically significant in all groups (p<0.001 for each group). When evaluating individual extra-intestinal symptoms, the majority of participants did not report new/worse symptoms. In fact, between the baseline assessment and the final assessment, the average symptom severity decreased significantly in all three groups (p<0.001,).
Conclusions.
Our study suggests that participants with IBS often experience extra-intestinal symptoms at baseline and that these symptoms generally improve in severity over the course of a clinical trial, regardless of the treatment arm. Systematic assessment of extra-intestinal symptoms at the beginning of a clinical trial is necessary to determine more definitively whether these symptoms may be considered an adverse event attributable to a study medication.
Keywords: Clinical trials, Irritable Bowel Syndrome, Extra-intestinal symptoms, Adverse events
INTRODUCTION
Adverse events in clinical trials are typically assessed by open-ended questions, as opposed to detailed and standardized symptom checklists. Recently, this methodology has come into question for several reasons. First, open-ended assessment of adverse events in clinical trials is not a validated methodology and is prone to participant and investigator bias1. Second, this methodology has been shown to underestimate the prevalence of adverse events, with reported rates of common symptoms (e.g., headache or fatigue) in some studies being much lower than the rates in the general population2,3. And third, this methodology does not distinguish between symptoms present at baseline and those that develop during a trial, which may lead, in some instances, to misattribution of symptoms present at baseline as side effects to the treatment.
Several authors have suggested that systematically assessing symptoms using standardized questionnaires at baseline and at the end of the trial can decrease bias and increase detection of true drug side effects4–7, while simultaneously reducing the likelihood of false positive side effects. Indeed, previous studies have shown that so-called “non-specific” symptoms (e.g. headache, fatigue, pain) are very common in the general population. For example, a large survey of medical students and hospital staff (n=414) who self-reported being healthy and not taking any medications during the previous three days found that 81% had experienced at least one of 25 common symptoms in the previous 72 hours8. The median number of symptoms experienced per person was two, and 30 participants reported six or more, suggesting that many such symptoms reported in clinical trials are common in the general, healthy population and may have been present prior to entering the trial. Other surveys have found similar results9. Furthermore, a recent RCT tested the effects of a systematic assessment of adverse events at baseline and end of study in a 4-day trial of amitriptyline in healthy controls. That study demonstrated that only 4 of the 32 symptoms had a clear causal relationship with amitriptyline, while the rest were attributable to symptoms present at baseline4. Similarly, an elegant study of desipramine in IBS found that most reported side effects to the drug were present at baseline and that most of the reported symptoms did not get worse between baseline and follow up visits, suggesting that many of the commonly reported side effects to desipramine may be falsely attributed to the drug10.
Systematically assessing common symptoms at baseline as well as during a clinical trial would be especially important in conditions associated with high rates of comorbidities, such as disorders of gut-brain interaction (DGBIs, previously known as functional gastrointestinal disorder). IBS is a common DGBI, affecting approximately 4.1% of the population globally11. IBS patients often have comorbidities such as psychiatric disorders (e.g., anxiety, depression and somatoform disorders), chronic pain, chronic fatigue, and headaches12,13. Given the large number of gastrointestinal and extra-intestinal symptoms present in IBS, assessment of adverse events in IBS clinical trials is particularly challenging. In the current study, we analyzed data from a randomized controlled trial of irritable bowel syndrome (IBS) that included a standardized measure of extra-intestinal symptoms at baseline and end-of-study, which allowed us to evaluate how such symptoms change over time, in both the treatment and the control groups.
METHODS
This study was based on the data collected in a 6-week, randomized controlled trial evaluating placebo effects in IBS (Clinicaltrials.gov identifier: NCT02802241). Participants were randomized in a 2:2:2:1 ratio to one of four groups: (1) treatment as usual (TAU); (2) open-label placebo (OLP); (3) double-blind placebo (DBP); and (4) double-blind peppermint oil (DBM, enteric-coated, 180mg). Fewer patients were allocated to the peppermint oil group because the primary aims of the larger study focused on placebo effects. Detailed methods and results from the larger study, as well as the results of the nested peppermint oil RCT, are reported elsewhere14–16. Because we were interested in applying our study results to double blind, randomized controlled trials, we did not include the open label placebo group in our analyses; and instead, we only analyzed data from the double-blind RCT of peppermint out (DBM vs. DBP), as well as the TAU control group.
Participants
Participants were eligible if they fulfilled Rome IV criteria for IBS17, and had moderate to severe IBS symptoms based on the IBS Severity Scoring System18. Exclusion criteria included pregnancy, established or suspected diagnosis of concomitant bowel disturbance, and/or a history of bowel surgery. Participants were allowed to continue IBS medications as long as the regimen was stable (>30 days). In addition, they were asked to avoid any changes to diet, exercise, or medication regimens for the duration of the study. Detailed inclusion/exclusion criteria have been published elsewhere14.
Measures
Extra-intestinal Symptom questionnaire.
Extra-intestinal symptoms were measured at baseline and at the end of the study using a checklist consisting of 15 common symptoms that are often reported as adverse events in drug trials9. The severity of each symptom was rated by participants on a 6-point scale ranging from 0=not present to 5=very severe (Full scale shown in Supplementary Table 1).
A composite score for each participant was calculated by summing reported severity (05) across each of the 15 extra-intestinal symptoms, which yielded a symptom burden score (range 0–75).
Irritable Bowel Syndrome Severity Scoring System (IBS-SSS).
The IBS-SSS scale is a validated questionnaire consisting of 5 questions about severity of abdominal pain, severity of abdominal distension, number days with abdominal pain, dissatisfaction with bowel habits, and interference with quality of life. The total score of the IBS-SSS ranges from 0–500, with higher scores indicating worse IBS severity, and scores of ≥175 indicating at least moderate severity18.
Changes in the severity of each symptom were calculated by subtracting the symptom severity at baseline from symptom severity at end of study. Thus, a negative number indicates a decrease in symptom severity and a positive number indicates an increase in symptom severity.
Patient Health Questionnaire-8 (PHQ-8).
The PHQ-8 is a validated, 8-item scale that is used as a diagnostic and severity measure for depressive disorders in clinical studies19.
Generalized Anxiety Disorder-7 (GAD-7).
The GAD-7 is a validated, 7-item scale used as a diagnostic and severity measure for anxiety disorders in clinical practice and research20.
Statistics
SPSS (IBM SPSS Statistics version 26, IBM Corp., Armonk, NY, U.S.A.) was used for analyses. Descriptive statistics are reported as mean (standard deviation [SD]) for continuous variables and percentages for frequencies. Analysis of variance (ANOVA) was used to compare the differences across the treatment groups (DBM, DBP and TAU) at each baseline, while analysis of covariance (ANCOVA), controlling for baseline symptom burden, was used to compare differences across treatment groups at follow-up. Effect sizes were calculated using Cohen’s d. Paired t-tests were used to compare symptom burdens at baseline and end-of-study within each group. Correlations were calculated between change in extra-intestinal symptom burden and IBS-SSS scores (at baseline as well as IBS-SSS change). All tests were two-tailed with alpha set at 5%.
RESULTS
Demographics
Between June 2016 and December 2019, 219 participants were randomized to either double-blind peppermint oil (n=46, DBM), double-blind placebo (n=87, DBP), or treatment as usual (n=86, TAU). Mean age of the participants was 41.8 years (SD=17.9), the majority were women (73.1%), and 82% reported their race as white (Table 1). A total of 46 participants (21%) withdrew or were lost to follow-up (16 in DBM, 14 in TAU, and 16 in DBP). Thus, our analysis included 173 participants (30 in DBM, 72 in TAU, and 71 in DBP). Detailed demographics of the larger study are published elsewhere15.
Table 1:
Baseline demographics
| Demographics and Baseline Characteristics | DBP N=87 | DBM N=46 | TAU N = 86 |
|---|---|---|---|
| Mean Age (SD) | 43.8 (19.2) | 41.0(17.4) | 40.0 (17.0) |
| Female N (%) | 64 (73.6%) | 34 (73.9%) | 63 (73.3%) |
| Caucasian N (%) | 75 (86.2%) | 35 (76.1%) | 69 (80.2%) |
| African American N (%) | 3 (3.4%) | 3 (6.5%) | 3 (3.5%) |
| Asian N (%) | 4 (4.6%) | 2 (4.3%) | 9 (10.5%) |
| IBS-Constipation N (%) | 18 (20.7%) | 9 (19.6%) | 24 (27.9%) |
| IBS-Mixed N (%) | 27 (31.0%) | 20 (43.5%) | 26 (30.2%) |
| IBS-Diarrhea N (%) | 39 (44.8%) | 15 (32.6%) | 34 (39.5%) |
| IBS-Undefined | 3 (3.4%) | 2 (4.3%) | 2 (2.3%) |
DBP = Double Blind Placebo; DPM = Double Blind Mint; TAU=Treatment as Usual; IBS = irritable bowel syndrome
Number of nonspecific extra-intestinal symptoms at baseline and end of study
At baseline, each group reported approximately five extra-intestinal symptoms per participant (Table 2). The five most commonly reported symptoms at baseline were fatigue, excessive sleepiness, nasal congestion, inability to concentrate, and irritability (Table 3). Between baseline and the final 6-week assessment, the number of extra-intestinal symptoms per participant decreased to an average of three symptoms, and this change was statistically significant in all three groups (p<0.001 for each group, Table 2). When evaluating individual symptoms, the majority of participants did not report new/worse symptoms (supplementary table 2).
Table 2:
Average (SD) number of extra-intestinal Symptoms per participant at baseline and end of study
| Baseline Mean (SD) | End of Study Mean (SD) | p value | |
|---|---|---|---|
| DBM (N=30) | 5.03 (3.30) | 2.93 (3.06) | <0.001 |
| DBP (N=71) | 4.93 (3.19) | 3.38 (2.66) | <0.001 |
| TAU (N=72) | 5.35 (3.29) | 4.04 (3.11) | <0.001 |
DBP = Double Blind Placebo; DPM = Double Blind Mint; TAU=Treatment as Usual
Table 3.
Percentage of participants reporting extra-intestinal symptoms at Baseline and End-of-Study
| Symptoms at Baseline | Symptoms at End of Study | ||||||
|---|---|---|---|---|---|---|---|
| DBM (N=30) | DBP (N=71) | TAU (N=72) | DBM (N=30) | DBP (N=71) | TAU (N=72) | ||
| SLEEP | Bad dreams (%) | 40.0 | 40.8 | 45.8 | 26.7* | 25.4 | 29.2 |
| Excessive sleepiness (%) | 43.3 | 38.0 | 47.2 | 30.0* | 25.4 | 26.4 | |
| Insomnia (%) | 36.7 | 33.8 | 40.3 | 20.0 | 26.8 | 31.9 | |
| Fatigue (%) | 63.3 | 52.1 | 70.8 | 30.0 | 52.1 | 61.1 | |
| NEURO | Inability to concentrate (%) | 43.3 | 43.7 | 38.9 | 23.3 | 35.2 | 26.4 |
| Irritability (%) | 33.3 | 43.7 | 44.4 | 10.0 | 21.1 | 36.1 | |
| Dry mouth (%) | 33.3 | 23.9 | 25.0 | 20.0* | 15.5 | 19.4 | |
| Headache (%) | 36.7 | 36.6 | 47.2 | 30.0* | 23.9 | 38.9 | |
| Weakness (%) | 13.3 | 14.1 | 23.6 | 13.3* | 5.6 | 11.1 | |
| Dizziness (%) | 26.7 | 22.5 | 25.0 | 26.7* | 11.3 | 18.1 | |
| MSK | Joint pain (%) | 33.3 | 35.2 | 33.3 | 13.3 | 21.1 | 27.8 |
| Muscle pain (%) | 30.0 | 32.4 | 27.8 | 6.7 | 15.5 | 20.8 | |
| RESP | Nasal congestion (%) | 46.7 | 42.3 | 38.9 | 26.7 | 38.0 | 37.5 |
| DERM | Skin rash (%) | 10.0 | 18.3 | 16.7 | 13.3* | 9.9 | 11.1 |
| Bruising (%) | 13.3 | 15.5 | 9.7 | 3.3 | 11.3 | 8.3 | |
Denotes symptoms that would be attributed to the study drug using the following, common definition of side effects: present in at least 2% of the drug group and at a higher incidence than placebo group at study end point.
Seven of the 15 extra-intestinal symptoms measured in this study could have been identified as side effects using a common standard for reporting adverse events in drug trials (that the symptom should occur in at least 2% of the drug group, and at a higher incidence as compared to the placebo group (table 3)). However, 6 out of these 7 symptoms were equally or more prevalent at baseline compared to the final assessment at 6 weeks, and therefore they cannot be side effects to the drug treatment.
Severity of nonspecific extra-intestinal symptoms at baseline and end of study
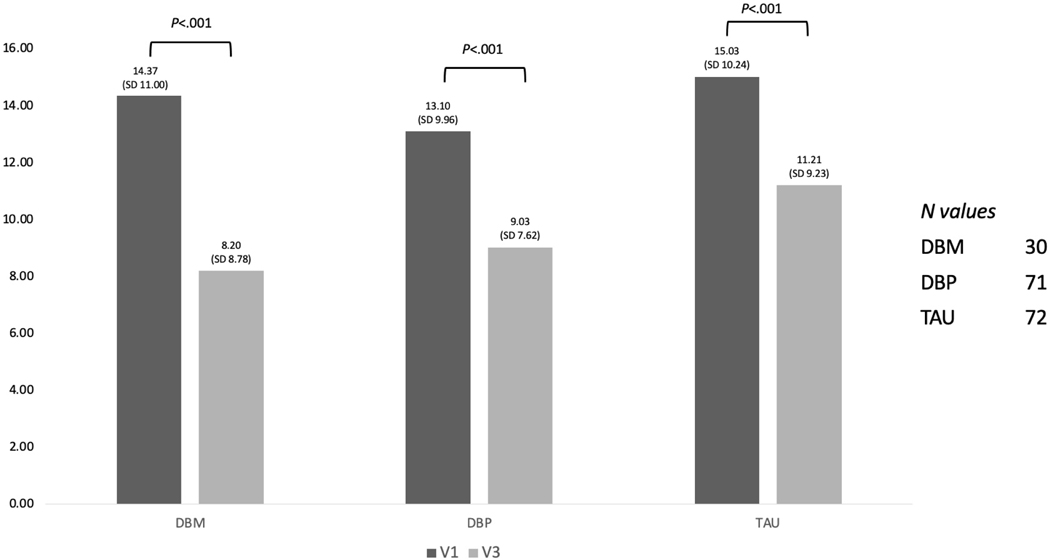
Between the baseline assessment and the final 6-week assessment, the average symptom burden decreased significantly from an average of 14.1 (SD=10.2) to an average of 9.8 (SD=8.6) and this decrease was statistically significant in all three groups (p<0.001, see Figure 1). There was a 43% decrease in symptom burden in DBM (d=0.94, large effect size), a 31% decrease in DBP (d=0.48, medium effect size), and a 25% decrease in TAU (d=0.50, medium effect size). ANOVA revealed that there were no statistically significant differences in symptom burden at baseline between the groups, F(2,170)=0.62, p=.53. Similarly, using an analysis of covariance (ANCOVA) that controlled for baseline symptom burden, the three groups did not differ significantly on overall symptom burden at the end of the study, F(2,169)=1.88, p=.16.
Figure 1:
Symptom burden of extra-intestinal symptoms at baseline and end of study
**There was no statistical differences between the Tx groups at each time point – ANOVA
**The overall symptom burden decreased significantly P<0.01 (T-Test) from Baseline (V1) to the end point (V3) for all treatment arms
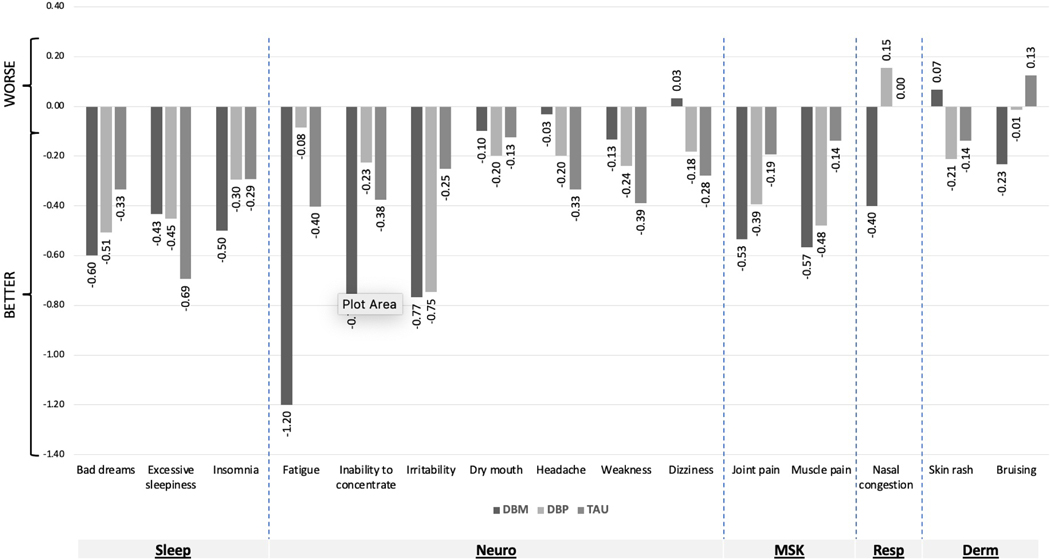
In addition, as shown in Figure 2, for all three groups the symptom burden decreased for nearly all of the individual extra-intestinal symptoms. Indeed, 91% (41 of 45) of the symptom burden change scores shown in Figure 2 showed a reduction in symptoms, whereas only 9% (4 of 45) showed an increase in symptoms.
Figure 2:
Change in individual symptom burden
IBS symptom severity at baseline was weakly, but significantly correlated with extra-intestinal symptom burden at baseline (r=0.265, p=0.001). In addition, change in symptom burden between baseline and the end of the study at 6 weeks was also weakly, but significantly correlated with change in IBS severity (r=.257, p=0.001). In other words, to the degree that patients experienced an improvement in their IBS symptoms, they were also likely to experience an improvement in their extra-intestinal symptoms. Baseline depression and anxiety scores were also each significantly and positively correlated with higher extraintestinal symptom burden at baseline (r=0.627 and r=0.519, respectively, p<0.001 for both) and at followup (r=0.458 and r=0.362, respectively, p<0.001 for both). Interestingly, baseline depression and anxiety scores were significantly and positively correlated with change (improvement) in extraintestinal symptom severity (r=0.319 and r=0.323, respectively p<0.001 for both). When controlling for baseline anxiety and depression, the relationship between change in IBS symptom severity and change in extraintestinal symptom burden remained significant (r=.233, p=0.002). However, the association between baseline IBS symptom severity and baseline extra-intestinal symptom burden was no longer significant after controlling for baseline anxiety and depression (r=.053, p=.48)
DISCUSSION
In this six-week, double-blind, placebo-controlled trial of peppermint oil in IBS, we found that many of the extra-intestinal symptoms that are often reported as adverse events in IBS clinical trials were present, and often more severe, at baseline. This is consistent with findings previously reported by Thiwan et al10, which found that many reported side effects to the drug desipramine were present at baseline and at least as severe as they were at followup visits in a sample of patients with IBS. Therefore, our findings lend support to previous arguments that nonspecific symptoms ought to be assessed systematically at baseline and throughout a clinical trial to improve accuracy in assessing whether any adverse events are attributable to a drug under investigation2.
A common standard for reporting adverse events of a medication based on clinical trial data is that (1) the symptom should occur in at least 2% of the drug group, and (2) the symptom should occur at a higher incidence in the drug group as compared to the placebo group, although it should be noted that there is no universally accepted or standardized cutoff for reporting adverse events in clinical trials. Using this definition, 7 of the 15 extra-intestinal symptoms measured in this study would have been identified as side effects to the study drug. However, 6 out of these 7 symptoms were equally or more prevalent at baseline compared to the final assessment at 6 weeks, and therefore they cannot be side effects to the drug. Thus, assessment of symptoms at baseline decreased the number of potential side effects from 7 to just 1, a decrease of 86%.
The current study expands upon the findings of Thiwan et al10 by including a general measure of common extra-intestinal symptoms (vs. anticipated side effects specific to a specific drug) and by including a Treatment as Usual group. The inclusion of a TAU group is uncommon in clinical trials, but offers valuable information about the natural history of IBS as well as which extra-intestinal symptoms might be related to worry, hypervigilance, or anticipation of adverse events related to a particular treatment (i.e., “nocebo effects” associated with both placebo and drug treatments). Indeed, the frequency and severity of symptoms reported by Thiwan et al did not correlate with drug blood levels, further highlighting the role of nocebo effects in clinical trials of IBS.
In our study, the nonspecific symptoms reported in the TAU group were similar, and in some case more frequent, than the symptoms reported in the placebo and drug arms at study end point. For example, at the end-of-study visit, 13% of participants in the DBM group reported weakness, compared to 6% in the placebo group. In a typical trial, without a TAU control arm, this might seem like a noteworthy difference indicating that the study drug causes, or contributes to, weakness. However, in the current trial we can see that 11% of the TAU arm reported weakness, which suggests that weakness may not be strongly associated with the study treatment.
Notably, almost all extra-intestinal symptoms improved during our trial irrespective of the study group; and the degree of improvement in each symptom did not vary significantly between groups. This improvement in extra-intestinal symptoms during the trial could be due to: (1) Hawthorne effects21 (e.g., improvement attributable to the attention associated with being enrolled in a study); (2) participants’ expectations of improvement22; (3) natural history (e.g., many common symptoms such as headache tend to wax and wane over time); and (4) regression to the mean23.
Our finding that patients with higher baseline levels of anxiety and depression also had higher extra-intestinal symptoms at baseline is consistent with Thiwan et al as well as with the literature on neuroticism and somatic symptoms24. This finding may also hint at another possible explanation for the general improvement in extra-intestinal symptoms over the course of the trial. In particular, patients with higher baseline anxiety and depression also showed greater improvements in extra-intestinal symptoms. Perhaps participation in the trial reduced patients’ anxiety and depression, which in turn, reduced their experience of, and/or attention to extra-intestinal symptoms. Unfortunately, anxiety and depression were not measured at followup in this study, so we were not able to test this specific hypothesis. Relatedly, improvement in IBS symptom severity was correlated with improvement in extra-intestinal symptom burden, even after controlling for baseline anxiety and depression, which could reflect a tendency for those with more severe baseline symptoms in general (including anxiety, depression, IBS, and extra-intestinal symptoms) to improve more over time.
Our study has several limitations. First, the data was collected from a trial that was mainly focused on evaluating the placebo effect and was not specifically designed to address adverse events. Nonetheless, the nested DBM versus DBP was itself a carefully designed drug vs. placebo RCT14, which increases the generalizability of our study to more typical RCTs of pharmaceuticals. Second, our overall drop-out rate was 21%, and it was 35% in the DBM group, which may have led to an underestimation of the symptom burden for that group. Third, participants in this study were allowed to remain on IBS medications throughout the study and, therefore, it is possible that some patients were taking medications whose side effects may have contributed to the relatively high percentage of patients reporting extraintestinal symptoms at baseline. However, participants were only enrolled in the study if they had been on a stable dose of their medication for at least 30 days prior to enrollment and they agreed not to make any medication changes for the duration of the study. Any participants who reported major medication changes were withdrawn from study participation. Unfortunately, data regarding medications at baseline was not collected and it is possible that some of the extraintestinal symptoms reported at baseline could have reflected side effects to medications started 30 days or more before enrollment. In that case, reduction in extraintestinal symptoms during the RCT could be due to the patient’s increased tolerance to medication with time. Randomization should have addressed any potential baseline group differences in medication and their potential side effects. Finally, and related to the previous limitation, because participants were allowed to continue on their IBS medications, it is possible that our sample includes a more severe group of patients (i.e. those who met criteria for moderate symptom severity despite being on medications), which may limit generalizability of our findings to the typical participant of a double-blind, placebo controlled, IBS treatment trial.
In summary, our study suggests that participants with IBS in clinical trials often experience extra-intestinal symptoms at baseline and that these symptoms generally improve in severity over the course of the study, regardless of the treatment arm. Our findings suggest that systematic assessment of symptoms at the beginning and end of a clinical trial would be a valuable addition to the current methodology of using open-ended questions assessing adverse events throughout a trial in order to determine more definitively whether adverse events are attributable to a study medication. Additionally, these findings could be applied to clinical practice by systematically assessing symptoms before and after prescribing a medication in order to promote a data-driven dialogue with patients about possible side effects to medications.
Supplementary Material
Supplementary Table 1: Extra-intestinal Symptom Questionnaire
Supplementary Table 2: Percentage of participants reporting new/worse and better/unchanged/resolved symptoms DBP = Double Blind Placebo; DPM = Double Blind Mint; TAU=Treatment as Usual
What You Need to Know.
Background:
Many reported adverse events in IBS clinical trials are extra-intestinal symptoms, which are not assessed at baseline. This may lead to misattribution of some pre-existing symptoms as side effects.
Findings:
Our study suggests that participants in IBS clinical trials often experience extra-intestinal symptoms at baseline and these symptoms generally improve in severity over the course of the study.
Implications for patient care:
Baseline assessment of symptoms could help determine whether adverse events are attributable to the study medication in a clinical trial.
Financial Disclosures:
This study was supported by NIH grant R01AT008573. TJK is partially supported by a grant from the Sciences of the Therapeutic Encounter.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Rief W, Nestoriuc Y, von Lilienfeld-Toal A, et al. Differences in adverse effect reporting in placebo groups in SSRI and tricyclic antidepressant trials: a systematic review and meta-analysis. Drug Saf 2009;32:1041–1056. [DOI] [PubMed] [Google Scholar]
- 2.Rief W, Avorn J, Barsky AJ. Medication-Attributed Adverse Effects in Placebo Groups: Implications for Assessment of Adverse Effects. Arch Intern Med 2006;166:155–160. [DOI] [PubMed] [Google Scholar]
- 3.Ioannidis JPA. Adverse events in randomized trials: neglected, restricted, distorted, and silenced. Arch Intern Med 2009;169:1737–1739. [DOI] [PubMed] [Google Scholar]
- 4.Rheker J, Rief W, Doering BK, et al. Assessment of adverse events in clinical drug trials: Identifying amitriptyline’s placebo- and baseline-controlled side effects. Exp Clin Psychopharmacol 2018;26:320–326. [DOI] [PubMed] [Google Scholar]
- 5.Avery CW, Ibelle BP, Allison B, et al. Systematic errors in the evaluation of side effects. Am J Psychiatry 1967;123:875–878. [DOI] [PubMed] [Google Scholar]
- 6.Barsky AJ, Saintfort R, Rogers MP, et al. Nonspecific medication side effects and the nocebo phenomenon. JAMA 2002;287:622–627. [DOI] [PubMed] [Google Scholar]
- 7.Rief W, Glombiewski JA, Barsky AJ. Generic Assessment of Side Effects. 2009. www.GASEscale.com.
- 8.Reidenberg MM, Lowenthal DT. Adverse nondrug reactions. N Engl J Med 1968;279:678–679. [DOI] [PubMed] [Google Scholar]
- 9.Green DM. Pre-existing Conditions, Placebo Reactions, and “Side Effects.” Ann Intern Med 1964;60:255. [DOI] [PubMed] [Google Scholar]
- 10.Thiwan S, Drossman DA, Morris CB, et al. Not All Side Effects Associated With Tricyclic Antidepressant Therapy Are True Side Effects. Clinical Gastroenterology and Hepatology 2009;7:446–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sperber AD, Bangdiwala SI, Drossman DA, et al. Worldwide Prevalence and Burden of Functional Gastrointestinal Disorders, Results of Rome Foundation Global Study. Gastroenterology April 2020. [DOI] [PubMed] [Google Scholar]
- 12.Mikocka-Walus A, Turnbull D, Moulding N, et al. Psychological comorbidity and complexity of gastrointestinal symptoms in clinically diagnosed irritable bowel syndrome patients. J Gastroenterol Hepatol 2008;23:1137–1143. [DOI] [PubMed] [Google Scholar]
- 13.Riedl A, Schmidtmann M, Stengel A, et al. Somatic comorbidities of irritable bowel syndrome: a systematic analysis. J Psychosom Res 2008;64:573–582. [DOI] [PubMed] [Google Scholar]
- 14.Ballou S, Kaptchuk TJ, Hirsch W, et al. Open-label versus double-blind placebo treatment in irritable bowel syndrome: study protocol for a randomized controlled trial. Trials 2017;18:234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lembo A, Kelley JM, Nee J, et al. Open-label placebo vs double-blind placebo for irritable bowel syndrome: a randomized clinical trial. Pain February 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nee J, Ballou S, Kelley JM, et al. Peppermint Oil Treatment for Irritable Bowel Syndrome: A Randomized Placebo-Controlled Trial. Am J Gastroenterol 2021;116:2279–2285. [DOI] [PubMed] [Google Scholar]
- 17.Mearin F, Lacy BE, Chang L, et al. Bowel Disorders. Gastroenterology February 2016. [DOI] [PubMed] [Google Scholar]
- 18.Francis CY, Morris J, Whorwell PJ. The irritable bowel severity scoring system: a simple method of monitoring irritable bowel syndrome and its progress. Aliment Pharmacol Ther 1997;11:395–402. [DOI] [PubMed] [Google Scholar]
- 19.Kroenke K, Strine TW, Spitzer RL, et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–173. [DOI] [PubMed] [Google Scholar]
- 20.Spitzer RL, Kroenke K, Williams JBW, et al. A brief measure for assessing generalized anxiety disorder: the GAD-7. Arch Intern Med 2006;166:1092–1097. [DOI] [PubMed] [Google Scholar]
- 21.McCambridge J, Witton J, Elbourne DR. Systematic review of the Hawthorne effect: New concepts are needed to study research participation effects. Journal of Clinical Epidemiology 2014;67:267–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kirsch I. Response expectancy as a determinant of experience and behavior. American Psychologist 1985;40:1189–1202. [Google Scholar]
- 23.Barnett AG, van der Pols JC, Dobson AJ. Regression to the mean: what it is and how to deal with it. Int J Epidemiol 2005;34:215–220. [DOI] [PubMed] [Google Scholar]
- 24.Denovan A, Dagnall N, Lofthouse G. Neuroticism and Somatic Complaints: Concomitant Effects of Rumination and Worry. Behav Cogn Psychother 2019;47:431–445. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1: Extra-intestinal Symptom Questionnaire
Supplementary Table 2: Percentage of participants reporting new/worse and better/unchanged/resolved symptoms DBP = Double Blind Placebo; DPM = Double Blind Mint; TAU=Treatment as Usual