Abstract
Objectives:
Persistent functional impairment is common in bipolar disorder (BD) and is influenced by a number of demographic, clinical, and cognitive features. The goal of this project was to estimate and compare the influence of key factors on community function in multiple cohorts of well-characterized samples of individuals with BD.
Methods:
Thirteen cohorts from 7 countries included n=5,882 individuals with BD across multiple sites. The statistical approach consisted of a systematic uniform application of analyses across sites. Each site performed a logistic regression analysis with empirically derived “higher versus lower function” as the dependent variable and selected clinical and demographic variables as predictors.
Results:
We found high rates of functional impairment, ranging from 41–75%. Lower community functioning was associated with depressive symptoms in 10 of 12 of the cohorts that included this variable in the analysis. Lower levels of education, a greater number of prior mood episodes, presence of a comorbid substance use disorder, and a greater total number of psychotropic medications were also associated with low functioning.
Conclusions:
The bipolar clinical research community is poised to work together to characterize the multi-dimensional contributors to impairment and address the barriers that impede patients’ complete recovery. We must also identify the core features which enable many to thrive and live successfully with BD. A large-scale, worldwide, prospective longitudinal study focused squarely on BD and its heterogeneous presentations will serve as a platform for discovery and promote major advances toward optimizing outcomes for every individual with this illness.
Introduction
Bipolar disorder (BD) is one of the most impairing mental health conditions worldwide1. A meta-analysis by Léda-Rêgo (2020) focused on community function in BD, as measured by the Functional Assessment Short Test (FAST) indicated that 65.6% of people with euthymic BD experience work-related impairment (e.g., unable to maintain a job and reduced efficiency performing tasks). The same study reported that 49.2% of patients had cognitive impairments on the FAST (e.g., difficulty in concentrating, performing mental calculations and solving problems), 42.6% had impairments on autonomy, and 42.1% reported impairment in maintaining interpersonal relationships.
The BD-related factors that contribute to functional impairment are not fully understood but include: primary affective symptoms, both syndromal and subsyndromal2, high rates of comorbid psychiatric3 and medical disorders4–6, substance misuse7, sleep quality deficits8, and cognitive impairment (deficits in attention, memory and executive functioning)9, among other factors. An enhanced understanding of the key predictors of functional impairment in individuals with BD would chart a clearer path to interventions that promote full recovery for every individual.
The field has embraced a mandate to improve the lives of individuals suffering from this disease; many challenges and hurdles impede progress, yet offer opportunities to deepen the knowledge base behind this complex disorder: 1) BD is heterogeneous. This contributes to a high rate of partial treatment response, and in many individuals, treatment resistance. This heterogeneity has also confounded our ability to identify pathophysiological mechanisms that drive disease, and limited the development of novel, more effective treatments. Heterogeneity in BD exists at the level of diagnosis, stage, clinical profile and course, cognitive capacity10–12 and everyday functioning13; 2) BD is a highly comorbid disease. As noted above, common mental health conditions (e.g. substance use disorders, personality disorder, anxiety disorders) and environmental influences (e.g. lifestyle risks, early life exposure to trauma) add to the complexity of illness presentation and comorbid chronic cardiometabolic conditions14 lead to a shortened life span15. While there may be risk factors that are shared among all individuals diagnosed with BD, each patient has a unique combination that complicates the illness trajectory and related outcomes; 3) BD is a dynamic illness. After diagnosis, the average BD patient is symptomatic 50% of the time with changes in symptom status approximately 6 times/year, and switches in polarity about 3 times/year16–19. Few longitudinal studies of representative first-episode cohorts have been conducted and thus that our knowledge about the course of BD is still limited. Alongside the episodic mood state changes that define the illness are impairments in sleep, cognitive capacity, substance use, motivation, and energy. There are intermittent stressors in social support systems and socio-economic health. There is evidence of cumulative burden of illness recurrence, with effects on the brain (e.g., grey matter abnormalities, cognitive decline)20–22 that contribute to the high rates of functional impairment in individuals with BD. There are also indications of improvement in cognitive function after the first manic episode in those who do not relapse23; 4) Finally, beyond challenges inherent to the illness and its care, resources for BD research remain insufficient. Research focused on individuals with BD has consistently been, and remains, underfunded relative to other serious psychiatric disorders24, despite the exceptionally high rates of disability and societal costs. Splitting of existing funds across smaller and shorter cohort studies25 that are generally underpowered to examine the clinical variability inhibits our ability to truly parse the dynamics of illness course. This has wide ranging implications that include the support for ongoing research and the challenges of attracting early career researchers to the field, all of which contribute to the slow pace at which new discoveries are made.
The recent progress in the field has been largely due to collaborative efforts of prescient researchers with a shared vision. A better, albeit very much incomplete, understanding of the underlying genetic architecture of BD has been mustered through massive data sharing efforts. Genotype data from tens of thousands of individuals with BD have been merged in a common platform for genome-wide association studies through the Psychiatric Genomics Consortium, and replicable risk loci have successfully been identified26. The collective energy of the ENIGMA neuroimaging consortium further exemplifies a highly collaborative international network which has made major contributions to the knowledge of the neurobiology of the disorder. Cognitive impairment, previously considered more relevant to schizophrenia, is now known to be a core component of BD22, and a key contributor to the noted functional disability1,27. This has led to efforts in BD to target cognition with specific treatment28. Finally, more recent collaborative studies have begun to elucidate biological mechanisms that drive the disease, including evidence from neuroimaging paradigms, immune biomarkers, and epigenetics. Inducible pluripotent stem cells and other cutting-edge methodologies are now being applied in BD. It is an exciting time in the field, but more coordinated efforts are needed if we are to gain a full understanding of this highly complex and disabling condition.
The primary goal of the current study was to pool resources from multiple international groups in a capacity building exercise to define the current state of collaborative research opportunities and identify the barriers that need to be lifted to realize a common vision that includes optimal outcomes for every single person living with BD. With a common mission, an international group of investigators leveraged the added value from combining existing BD data to investigate core predictors of functional outcome, independent of treatment organization or societal differences. The present work, as well as our continued collaboration, will allow for us to disentangle core bipolar disorder features from secondary phenomena which is not possible in small, one-site studies. Ultimately, we plan to launch a worldwide prospective longitudinal study of BD to more rapidly advance progress via large-scale collaborations.
Methods
Participants:
Initially, 11 groups were identified via ongoing collaborative relationships. These groups do not represent all ongoing BD research worldwide, rather a starting point and an outline of an iterative process. There are many investigators worldwide conducting high quality BD research in cohorts of variable sizes. As is illustrated in Table 1, thirteen independent BD cohorts are included, with two sites contributing 2 cohorts (Mass General Hospital, MGH; and King’s College London, KCL) and two cohorts representing consortium-based samples that were collected at multiple different sites (FondaMental Advanced Centers of Expertise for Bipolar Disorder, (FACE-BD, Fondation FondaMental, France29) and Global Aging and Geriatric Experiments in Bipolar Disorder, GAGE-BD30). Each of the other 7 sites contributed data from a single cohort.
Table 1.
Defining “lower” functioning at each site
| Cohort | Country | Lower function defined |
|---|---|---|
| FACE-BD (Fondation FondaMental) | France | FAST≥21 |
| University of Michigan | USA | Best Estimate Illness Impact ≥2 |
| King’s College London PROMPT | England | WSAS ≥20 |
| King’s College London CRIB | England | FAST ≥21 |
| University of British Colombia | Canada | MSIF ≥4 |
| University of Barcelona | Spain | FAST≥21 |
| Deakin University | Australia | GAF≤60 |
| Mass General Hospital LITMUS | USA | LIFTRIFT ≥14 |
| Mass General Hospital CHOICE | USA | LIFTRIFT ≥14 |
| Mayo Clinic | USA | Not working full time |
| Oslo University Hospital | Norway | GAF <60 |
| GAGE-BD (consortium) | USA | GAF≤60 |
| Brigham and Women’s Hospital/Icahn School of Medicine at Mount Sinai | USA | WHODAS >5 |
Functional Assessment Short Test (FAST)33; Work and Social Adjustment Scale (WSAS)32; Multidimensional Scale of Independent Functioning (MSIF)38; Global Assessment of Function (GAF)39; Longitudinal Interval Follow Up Evaluation-Range of Impaired Functioning Tool (LIFE-RIFT)40; WHO Disability Adjustment Schedule (WHODAS)31; Best Estiamte of Illness Impact from Diagnostic Interview for Genetic Studies (DIGS)41.
FAST is a 24 item assessment of 6 domains of function (autonomy, occupational functioning, cognitive functioning, financial, interpersonal, and leisure time); MSIF assesses occupational, education and residential domains of function; WSAS is a brief, 5-item questionnaire that assesses one’s ability to work, home management, social and private leisure activities and close relationships; WHODAS assesses 6 domains of function (cognition, mobility, self-care, getting along, life activities, and participation); LIFE-RIFT assesses 4 domains of function (work, interpersonal, satisfaction, and recreation); GAF is a continuous scale of function based on the DSM-IV, rated from 0–100, with higher scores indicating higher functional capacity; Best Estimate of Illness Impact is derived from a diagnostic interview that classifies the impact of the patient’s illness on their life.
Measures:
The approach was planned as purposefully simplistic with a focus on one question via systematic uniform application of analyses across sites. Because the existing data at each site are highly variable, we allowed for substantial flexibility in the measures that were included from each site. First, each site was asked to empirically identify how they defined “high versus low functioning” in their BD cohort. This was kept purposefully coarse in its definition, as some sites use detailed questionnaires (e.g., WHO Disability Adjustment Schedule (DAS)31, Work and Social Adjustment Scale32, Functional Assessment Short Test (FAST)33 etc.), while others may only have documented employment status, or some other high-level indicator of everyday functionality. Sites were required to dichotomize whatever measure was chosen for defining function in their study, such that individuals with BD were described as having either higher or lower functional status. The way in which the variable was dichotomized was also up to the investigator, based upon the measure used at each site (e.g., some scales have recognized cutoffs, some used median splits, etc.), and are defined in Table 1.
Each site was then asked to provide detailed characteristics on their cohort(s), including demographics, diagnostic, clinical, cognitive, and functional features in the format of Table 2. Each site provided results from a Shapiro-Wilk statistical test for normality (for relevant variables), where a p<0.05 indicated non-normal distribution.
Table 2.
An informational table completed by each site
| Mean (SD) | Range | Distribution** Shapiro-Wilk (p) | Descriptor | |
|---|---|---|---|---|
| LEVEL ONE MEASURES | --- | --- | --- | --- |
| Age | In years | e.g., first episode | ||
| Sex | %Female | |||
| Race | %White | |||
| Education level | In years | Primary/secondary | ||
| LEVEL TWO MEASURES | --- | --- | --- | --- |
| BD subtype | %BDI/BDII/NOS | |||
| Psychosis Hx | %yes/no | Lifetime presence | ||
| Current depressive sx | HDRS/MDRS score | mild/mod/severe | ||
| Current manic sx | YMRS/other score | mild/mod/severe | ||
| Age at onset dep | In years | |||
| Age at onset mania | In years | |||
| # Prior manias | ||||
| #Prior depressions | ||||
| # total episodes | Full mania/depression | |||
| #Hospitalizations | ||||
| #Suicide attempts | ||||
| Comorbid substance dx | %yes/no | Lifetime presence | ||
| Comorbid anxiety dx | %yes/no | Lifetime presence | ||
| LEVEL THREE MEASURES | --- | --- | --- | --- |
| Global cognition | Calculated g using PCA | |||
| Premorbid IQ | e.g., proxy WRAT/NART | |||
| Medications | ||||
| Lithium | % Yes | |||
| Anticonvulsants | % Yes | |||
| Antipsychotics | % Yes | |||
| Antidepressants | % Yes | |||
| Benzodiazepines | % Yes | |||
| Total number of psychotropic meds | Mean (SD) | |||
| --- | --- | --- | --- | --- |
| OUTCOME MEASURES | ||||
| Global functioning | Provide name of scale | |||
| Global functioning impaired | Provide name of scale | |||
| Social functioning | Provide name of scale | |||
| Occupational fx | Provide name of scale | |||
| Independent living | Provide name of scale | |||
| Employed | Yes/no |
Shapiro-Wilk test for normality should be reported for each measure
Participating sites were not required to have all of the variables described in Table 2. Only “level one” measures were required. For those sites with cognitive data, we included a composite of general cognitive ability (g) (see below for derivation of g) and a premorbid estimate of IQ (typically a reading-based task).
The listing of the variables available at each site were reviewed by the first and second authors (KEB; CEM) and recommendations were made as to which variables should be included in the regression model at each site. This decision was based upon considerations of statistical power (e.g., the sample size at each site) and the validity of the data collected for each variable. After a consensus was achieved, sites were instructed to conduct a logistic regression with functional outcome as the dependent variable (dichotomized as higher versus lower global functioning) using the entry method while model-level and predictor-level results were collated. Example analysis scripts and instructions were provided to each participating site for consistency and checked upon completion. These are provided in the supplemental materials.
Analyses:
In preparation for collating results, each site coded dichotomous variables as 0 or 1 (yes) to allow for uniform interpretation of results and to label variables in English. Any site that had cognitive data from at least 3 different cognitive domains was instructed to derive a general cognitive ability index “g” using an unrotated principal components analysis (PCA) with up to two representative measures per cognitive domain. Estimates of g can be reliably derived with only 3 measures provided that they assay different domains and the measure is stable across different datasets regardless of the measures included in analyses34. Sites were instructed to select one or more tests within a purported ‘domain’ as most representative of that domain, allowing for more than one test per domain (e.g. ‘attention’) if available, provided they did not violate the 10:1 ratio rule (10 participants per one variable). Next, they were asked to select the “best” or most representative item-level variable from each test to be included in the analysis (only one per test). These variables were standardized on a z-score scale using the mean and standard deviation from their own BD sample (within-sample normalization). Finally, an unrotated PCA was conducted to include factors with eigenvalues > 1.0 and component 1 was defined as g. Individual scores for each g factor for each patient were analyzed by logistic regression.
The results of each site’s logistic regression were then compared descriptively side-by-side, i.e., the results were not directly combined as each site had different predictors/definitions of outcome. The goal herein was to find consistencies across samples and to identify where differences exist by individual site. Thus, meta-analyses were not conducted, rather a multiple cohort replication and expansion approach was used.
Results
In total, analyses included 5,882 individuals with a diagnosis of BD. Each site was different with regard to sample characteristics and measures included. These individual cohort descriptives are presented by site in Supplemental Table 1.
The prevalence of a priori defined “lower” functioning across sites ranged from 41–75% (Figure 1). Although different measures were used at nearly every site, each of which had had different ascertainment criteria and target populations ranging from population-based through to tertiary referral centers, these estimates are consistent with prior literature reporting high rates of lower functioning or impairment in BD.
Figure 1.
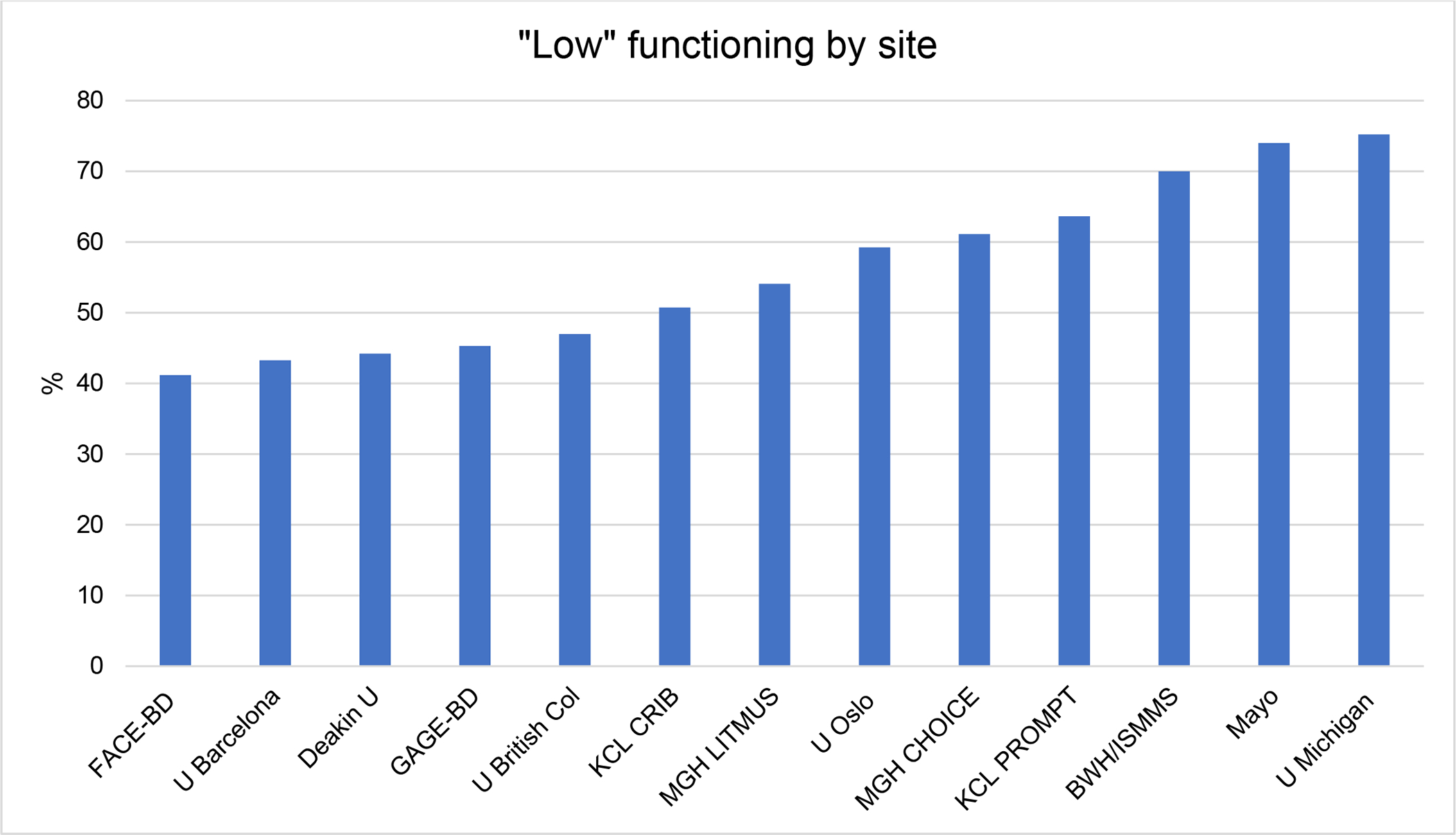
The rate of low functioning at each site.
Regression results by site are presented in detail in Supplemental Table 2. The differences in the regression results at each site likely reflect differences in the sample characteristics, sample size, and the measures used at each site.
Table 3 illustrates the frequency at which each of the measures contributed at a statistically-significant or trend level to the regression models predicting outcome (e.g., how many sites reported any given factor as significant in their regression model). There are several findings that are consistent across sites, and some that are relatively site-specific. The most commonly reported finding was that depressive symptoms (even subthreshold symptoms) were the strongest predictor of lower community functioning (10/12 sites reported). Severel additional factors that were noted as significant or trend level predictors of lower function across at least 25% of the sites reporting on that factor included: 1) Lower levels of education; 2) Greater number of prior manic episodes; 3) Greater number of prior depressive episodes; 4) Comorbid substance use; 5) Comorbid anxiety disorders; and 6) Greater of total number of psychotropic medications. We include a detailed discussion below on any factor that was found to be significant or trend-level significant (p≤ 0.10) in at least 25% of the sites reporting on that measure.
Table 3.
Summary of results from logistic regression analyses
| Characteristic | Proportion of sites reporting significance | Direction of results |
|---|---|---|
| Age | 1/13 (8%) | Older age → more impairment |
| Sex | 2/13 (16%) | Female → more impairment |
| Race | 0/8 (0%) | --- |
| Education level | 3/12 (25%) | Lower education → more impairment |
| BD subtype | 3/12 (25%) | BD I and SZA/BD → more impairment |
| Psychosis history | 3/11 (27%) | Mixed direction: 2 sites Psychosis hx → more impairment; 1 site Psychosis hx → less impairment |
| Current depressive symptoms | 10/12 (83%) | More severe depression → more impairment |
| Current manic symptoms | 2/11 (18%) | More severe mania → more impairment |
| Age at onset depression | 0/6 (0%) | --- |
| Age at onset mania | 0/6 (0%) | --- |
| # Prior manias | 2/7 (29%) | More prior episodes → more impairment |
| #Prior depressions | 2/8 (25%) | More prior episodes → more impairment |
| # Total episodes | 0/2 (0%) | --- |
| Comorbid substance diagnosis | 2/8 (25%) | Comorbid substance use d/o → more impairment |
| Comorbid anxiety diagnosis | 3/6 (50%) | Comorbid anxiety d/o → more impairment |
| Global cognition (g) | 1/6 (17%) | Lower g → more impairment |
| Premorbid intelligence quotient | 0/5 (0%) | --- |
| Lithium | 1/6 (17%) | Lithium use → less impairment |
| Anticonvulsants | 2/6 (33%) | Anticonvulsant use → more impairment |
| Antipsychotics | 3/7 (43%) | Antipsychotic use → more impairment |
| Antidepressants | 0/7 (0%) | --- |
| Benzodiazepines | 0/6 (0%) | --- |
| Total number of psychotropic meds | 2/7 (29%) | More psychotropic meds → more impairment |
Discussion
The current study set out to measure the influence of key demographic, clinical, and cognitive factors on community function in multiple international cohorts of well-characterized populations with BD (n=5,882) and to collate and compare results. The study design was intentionally inclusive, allowing sites to contribute results regardless of the specific measures used to assess mood, disability, and other illness features. As such, and for other practical reasons, we did not combine data across cohorts, but used a distributed data analyses framework. This approach has inherent limitations, but served as an opportunity to pool resources from multiple international groups, as a necessary first step in building the field-wide capacity for a scalable global initiative and to define the current state of the field, recognizing that more cohorts are available that were not included in this project.
First, our results are consistent with prior reports of high rates of functional impairment in individuals diagnosed with BD. This is consistent with a multitude of single-site studies as well as a recent meta-analysis of 13 studies that reported prevalence rates of functional impairment in several domains using the FAST (global, 58.6%; occupational, 65.6%; cognitive, 49.2%; autonomy, 42.6%; interpersonal relationships, 42.1%; leisure, 29.2%; and financial issues, 28.8%)27. The range of functional impairment that we report here is broad; being dependent upon site-specific sample characteristics as well as the granularity of the measure used to assess function. Still, these data capture the pressing need to assess everyday functioning in BD and to consider interventions that aggressively target those illness features that contribute to disability in hopes of promoting full recovery.
Second, across the majority of our cohorts, our results were consistent with prior reports showing the critical role that depressive symptoms play in functional status. Of the 12 cohorts where depression was examined (one site, Mayo Clinic, did not include current mood symptoms in the regression due to a loss of sample size), 10 report that the severity of depression symptoms present at the time of assessment significantly contributed to low functional status. Consistent with prior work1,27 this points to the consistent and deleterious effects of depressive symptoms on how people with BD function in their daily lives, and further emphasizes the need for treatment beyond the acute phase of the illness to include the low-grade persistent affective symptoms that are common even in “remitted” individuals.
Third, additional factors that were significant predictors of lower function across at least 25% of the sites reporting on that feature included: 1) Lower levels of education; 2) Greater number of prior manic episodes; 3) Greater number of prior depressive episodes; 4) Comorbid substance use; 5) Comorbid anxiety disorders; and 6) Greater of total number of psychotropic medications. Each of these premorbid and illness-related factors has been previously reported as contributing to poor outcomes1,27.
With regard to medication, several sites were able to evaluate the effects of the use of a specific medication classese in their cohort (including lithium, anticonvulsants, antidepressants, antipsychotics, and benzodiazepines); however, we did not examine polypharmacy, which is highly prevalent in BD. Further, we did not explicitly ask for timing and length of treatment with each medication class. One site reported that lithium use was associated with better community functioning, consistent with a recent report of benefits to cognition35; while other medication classes showed negative effects (anticonvulsants and antipsychotics) on broad community functioning. These effects may be related to the medications themselves or the patient characteristics that are associated with the use of certain medications but are concordant with historical and emergent data on the primacy of lithium for bipolar disorder36. Of note, we cannot rule out the possibility that those taking anticonvulsants and antipsychotics were initial lithium non-responders (i.e., these individuals would have a poor outcome regardless)—more granular, time-course data is necessary to tease apart the subtleties in medication usage and outcomes.
Measures of cognitive function were of great interest to us, as many studies have reported a relationship between cognitive impairment and psychosocial impairment in BD9,37; however, only 6 of the sites had cognitive data available for inclusion in the prediction models. Somewhat surprisingly, only 1 site found cognitive impairment was a significant predictor of lower function. This may be due to cohort characteristic differences by site, differences in the cognitive tasks included at each site, relatively small sample sizes of some cohorts or different functional outcome measures. Indeed, since the cognitive batteries did not overlap completely, we opted to calculate a general cognitive ability (g) index using factor analyses to capture a single measure of global cognition that has been shown to be stable and valid regardless of the tasks included in the factor analysis34. As a result, we sacrificed potentially relevant granularity in our measurement, as we were unable to look at deficits in specific cognitive domains (e.g., attention, memory, executive function) as potential predictors of functioning. Often used as a proxy of intellectual capacity, educational attainment was found to be a significant predictor of lower functioning in 25% of the sites who collected that information, notwithstanding that education is also a proxy of other variables such as socio-economic advantage or disadvantage.
There are several limitations to this study. Whilst we were able to convene a large team of collaborators, we did not directly combine data; therefore, the limitations that apply to single-site data (e.g. limited sample sizes) still apply here. Moreover, there are a number of additional factors that contribute to community functioning in BD beyond what we were able to capture, including financial and social support, among others. We acknowledge that the planned analyses across cohorts using the identicial analytic methods inherently reduced the granularity that could be achieved if any single site attempted to address the same question independently (e.g., by dichotomizing the functional measure). This indeed was part of the exercise and illustrates the need for a unified protocol to be adopted for future prospective studies.
Our findings are consistent with a convergent body of literature on functional impairment in BD. However, the primary purpose of this study was to demonstrate the value of large-scale collaborative networks in order to provide a snapshot of the BD global research landscape, and to highlight the current barriers to move beyond typical within-cohort analytic frameworks in order to advance progress in BD research and care. While we provide data and results from parallel analyses across cohorts, this manuscript is, in many ways, a perspective piece that serves as a call to arms. Specifically, the information gathered emphasizes several necessary next steps: 1) We need large samples, that reach beyond single sites and encompass the full spectrum of diversity within this heterogeneous condition, taking geographic, cultural and societal aspects into account. It is critical to include participants from non-European ancestries, to address the global impact of BD, which were not included in these analyses. Existing consortia have been fruitful and highly successful (e.g., Psychiatric Genomics Consortium, ENIGMA) but are inherently limited for fine-grained analysis, typically only including relatively superficial clinical phenotype data, allowing for limited to no follow-up over time. 2) We need comprehensive and integrated phenotyping with standardized measures which allow for data sharing and harmonization across studies. Many individual investigators are successful at collecting well-characterized cohorts but sample size is inherently limited at any single site and merging of data across sites is difficult because measures are not uniform, preventing more optimal meta- or mega-analyses. 3) Long-term, repeated assessment of clinical, cognitive, and functional measures alongside key biomarkers (e.g., genetic, immune, imaging, digital) is necessary if we are to unravel the complexity of this illness. There are several longitudinal studies that are ongoing around the world, but the duration and frequency of follow-up is both limited and variable, usually due to funding constraints, and protocols are not uniform across sites. 4) A centralized coordinating center to direct the complex infrastructure of a global research effort (e.g., compliance/human subjects oversight, data use and sharing agreements, and database management). We propose the imminent and urgent need for a carefully designed, well-resourced, prospective global longitudinal study of BD that will allow the necessary methods to be applied in order to make game-changing discoveries and substantive improvements to the lives of individuals living with BD.
Data sharing: Deidentified data that underlie the results reported in this Article will be made available upon reasonable request to the PI at the relevant organizing institution.
Supplementary Material
Acknowledgements:
The authors are grateful to all people with BD who participated and research staff at each site.
Funding sources:
Supported in part by 1) National Alliance for Mental Illness (NAMI) gift to KEB, AN, and MGM; 2) The Baszucki Brain Research Foundation. Individual investigator funding sources include: KEB is supported by R01MH124381; CEM is supported by the Stuart T. Hauser Research Training Program in Biological and Social Psychiatry Federal Postdoctoral Training Grant NIMH T32 016259-40. OAA received support from Research Council of Norway (223273), the KG Jebsen Stiftelsen (SKGJ-MED-021), EU’s H2020 RIA grant # 847776 CoMorMent, South-East Norway Health Authority (2019-108). HPB is supported by R01MH113230. MB is supported by a NHMRC Senior Principal Research Fellowship (1156072). The FACE-BD cohort was supported by the Foundation FondaMental, Institut National de la Santé et de la Recherche Médicale (INSERM), AP-HP, and by the Investissements d’Avenir program managed by the ANR under reference ANR-11-IDEX-0004-02 and ANR-10-COHO-10-01. LTE is supported by the International Society for Bipolar Disorder Bowden-Massey Strategic Research Initiative and the Desert-Pacific VA Mental Illness Research Education and Clinical Center. MAF, JMB and SLM are supported by the Marriott Foundation and the Mayo Foundation. JMF acknowledges the Janette Mary O’Neil Research Fellowship. TVL received support from the Research Council of Norway (288542). FK is supported by Early Postdoc Mobility Fellowship of the Swiss National Science Foundation (SNSF) and Fellowship by the Novartis Foundation for medical-biological Research. BPF has recently received funding from NIH, Biogen, Lilly, Eisai, Rogers Family Foundation, Spier Family Foundation. MGM is supported by the HC Prechter Bipolar Research Program, the Richard Tam Foundation, NIMH (MH114835 & UL1TR002240). PBM is supported by an Australian NHMRC Investigator Grant (1177991). AAN Supported, in part, by the Dauten Family Center for Bipolar Treatment Innovation and the Thomas P. Hackett, MD Chair in Psychiatry at Massachusetts General Hospital. AP is supported by NIMH 1K23MH122676. MS has received research grants within past 3 years: Nuromate, Otsuka, Alkermes, International Society for Bipolar Disorders (ISBD), National Institutes of Health (NIH), Centers for Disease Control and Prevention (CDC), Patient-Centered Outcomes Research Institute (PCORI). EV thanks the support of the Spanish Ministry of Science and Innovation (PI15/00283, PI18/00805) integrated into the Plan Nacional de I+D+I and co-financed by the ISCIII-Subdirección General de Evaluación and the Fondo Europeo de Desarrollo Regional (FEDER); the Instituto de Salud Carlos III; the CIBER of Mental Health (CIBERSAM); the Secretaria d’Universitats i Recerca del Departament d’Economia i Coneixement (2017 SGR 1365), the CERCA Programme, and the Departament de Salut de la Generalitat de Catalunya for the PERIS grant SLT006/17/00357. LJW is supported by a NHMRC Emerging Leadership Fellowship (1174060). AHY has received funding from NIMH (USA); CIHR (Canada); NARSAD (USA); Stanley Medical Research Institute (USA); MRC (UK); Wellcome Trust (UK); Royal College of Physicians (Edin); BMA (UK); UBC-VGH Foundation (Canada); WEDC (Canada); CCS Depression Research Fund (Canada); MSFHR (Canada); NIHR (UK). Janssen (UK). AS, LW and MB (Deakin) are supported by a competitive project grant from the National Health and Medical Research Council (NHMRC; ID 1104438).
Disclosures:
KEB has served as a consultant or advisor to Sunovion and Dainippon Sumitomo Pharma; OAA has received speaker’s honorarium from Lundbeck and Sunovion, and is a consultant to HealthLytix. MB has received Grant/Research Support from the NIH, Cooperative Research Centre, Simons Autism Foundation, Cancer Council of Victoria, Stanley Medical Research Foundation, Medical Benefits Fund, National Health and Medical Research Council, Medical Research Futures Fund, Beyond Blue, Rotary Health, A2 milk company, Meat and Livestock Board, Woolworths, Avant and the Harry Windsor Foundation, has been a speaker for Abbot, Astra Zeneca, Janssen and Janssen, Lundbeck and Merck and served as a consultant to Allergan, Astra Zeneca, Bioadvantex, Bionomics, Collaborative Medicinal Development, Janssen and Janssen, Lundbeck Merck, Pfizer and Servier – all unrelated to this work. AJC has received honoraria for lectures or consulting from Lundbeck, Livanova, Janssen & Allergan, and a research grant from Protexin Probiotics International Ltd. MAF has had grant seed support from Assurex Health, Mayo Foundation, Medibio. Mayo Clinic has a financial interest in AssureRX and OneOme. BPF has served on advisory boards for Biogen and Acadia Pharmaceuticals, as an Advisor to Patina Health, and has served on the Pharmacy and Therapeutics Committee for CVS Health. SLM has been a consultant to or member of the scientific advisory boards of Avanir, F. Hoffmann-La Roche Ltd., Idorsia, Intra-Cellular Therapies, Janssen, Mitsubishi Tanabe Pharma America, Myriad, Novo Nordisk, Otsuka, Signant Health (formerly Brackett), Sunovion, and Takeda (formerly Shire), has been a principal or co-investigator on studies sponsored by Allergan, Brainsway, Janssen, Jazz, Marriott Foundation, Myriad, National Institute of Mental Health, Novo Nordisk, Otsuka, Sunovion, and Takeda (formerly Shire), is also an inventor on United States Patent No. 6,323,236 B2, Use of Sulfamate Derivatives for Treating Impulse Control Disorders, and along with the patent’s assignee, University of Cincinnati, Cincinnati, Ohio, has received payments from Johnson & Johnson, which has exclusive rights under the patent. MGM has received consulting fees and research support from Janssen Pharmaceuticals. In the past 12 months, AAN received consulting fees, grants, or honoraria from Alkermes, Belvior Publishing, Ginger Inc., Merck, Myriad, Neuronetics, Patient Centered Outcomes Research Institute, Physician’s Postgraduate Press, Protagenics, Slack Publishing, Sunovion, UpToDate Wolters Kluwer, and Wiley Publishing. PBM has received honoraria from Sanofi (Hangzhou) and Janssen Australia. MS has served as a consultant for Alkermes, Otsuka, Janssen, Myriad, Health Analytics, Frontline Medical Communications, has received royalties from Springer Press, Johns Hopkins University Press, Oxford Press, UpToDate, and received compensation for preparation of CME activities: American Physician’s Institute, MCM Education, CMEology, Potomac Center for Medical Education, Global Medical Education, Creative Educational Concepts, Psychopharmacology Institute. IJT has served as consultant for Community Living British Columbia. RS has received honoraria from Lundbeck. EV has received grants and served as consultant, advisor or CME speaker for the following entities: AB-Biotics, Abbott, AbbVie, Angelini, Boehringer-Ingelheim, Dainippon Sumitomo Pharma, Ferrer, Gedeon Richter, GH Research, Janssen, Lundbeck, Novartis, Otsuka, Sage, Sanofi-Aventis, Sunovion, and Takeda, outside the submitted work. LJW has received Grant/Research support from Eli Lilly, Pfizer, The University of Melbourne, Deakin University and the NHMRC. LNY has been on speaker/advisory boards for, or has received research grants from Abbvie, Alkermes, Allergan, Canadian Network for Mood and Anxiety Treatments (CANMAT), Canadian Institutes of Health Research (CIHR), DSP, Gideon Richter, Intracellular Therapies, Merck, Sanofi and Sunovion. AHY has received honoraria for lectures and advisory boards for all major pharmaceutical companies with drugs used in affective and related disorders including: Astrazenaca, Eli Lilly, Lundbeck, Sunovion, Servier, Livanova, Janssen, Allegan, Bionomics, Sumitomo Dainippon Pharma, COMPASS, as well as investigator-initiated studies from AZ, Eli Lilly and Lundbeck. CMA, JMB, HPB, CDB, FK, AMA, CEM, PO, DLP, AP, JP, MS, BS, AY have nothing to disclose.
References
- 1.Sanchez-Moreno J, Martinez-Aran A, Tabares-Seisdedos R, Torrent C, Vieta E, Ayuso-Mateos JL. Functioning and disability in bipolar disorder: an extensive review. Psychother Psychosom. 2009;78(5):285–297. [DOI] [PubMed] [Google Scholar]
- 2.Altshuler LL, Post MR, Black OD, et al. Subsyndromal depressive symptoms are associated with functional impairment in patients with bipolar disorder: results of a large, multisite study. The Journal of clinical psychiatry. 2006;67(10):1551–1560. [DOI] [PubMed] [Google Scholar]
- 3.Nabavi B, Mitchell AJ, Nutt D. A Lifetime Prevalence of Comorbidity Between Bipolar Affective Disorder and Anxiety Disorders: A Meta-analysis of 52 Interview-based Studies of Psychiatric Population. EBioMedicine. 2015;2(10):1405–1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Crump C, Sundquist K, Winkleby MA, Sundquist J. Comorbidities and mortality in bipolar disorder: A Swedish national cohort study. JAMA Psychiatry. 2013;70(9):931–939. [DOI] [PubMed] [Google Scholar]
- 5.Carney CP, Jones LE. Medical comorbidity in women and men with bipolar disorders: A population-based controlled study. Psychosomatic Medicine. 2006;68(5):684–691. [DOI] [PubMed] [Google Scholar]
- 6.Fornaro M, Solmi M, Veronese N, et al. The burden of mood-disorder/cerebrovascular disease comorbidity: essential neurobiology, psychopharmacology, and physical activity interventions. In. Vol 29: Taylor and Francis Ltd; 2017:425–435. [DOI] [PubMed] [Google Scholar]
- 7.Cerullo MA, Strakowski SM. The prevalence and significance of substance use disorders in bipolar type I and II disorder. In. Vol 2: BioMed Central; 2007:29–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ng TH, Chung KF, Ho FYY, Yeung WF, Yung KP, Lam TH. Sleep-wake disturbance in interepisode bipolar disorder and high-risk individuals: A systematic review and meta-analysis. In. Vol 20: W.B. Saunders Ltd; 2015:46–58. [DOI] [PubMed] [Google Scholar]
- 9.Depp CA, Mausbach BT, Harmell AL, et al. Meta-analysis of the association between cognitive abilities and everyday functioning in bipolar disorder. Bipolar disorders. 2012;14(3):217–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burdick KE, Russo M, Frangou S, et al. Empirical evidence for discrete neurocognitive subgroups in bipolar disorder: clinical implications. Psychol Med. 2014;44(14):3083–3096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Martino DJ, Strejilevich SA, Scápola M, et al. Heterogeneity in cognitive functioning among patients with bipolar disorder. Journal of Affective Disorders. 2008;109(1–2):149–156. [DOI] [PubMed] [Google Scholar]
- 12.Green MJ, Girshkin L, Kremerskothen K, Watkeys O, Quidé Y. A Systematic Review of Studies Reporting Data-Driven Cognitive Subtypes across the Psychosis Spectrum. In. Vol 30: Springer; 2020:446–460. [DOI] [PubMed] [Google Scholar]
- 13.Solé B, Bonnin CM, Jiménez E, et al. Heterogeneity of functional outcomes in patients with bipolar disorder: a cluster-analytic approach. Acta Psychiatr Scand. 2018;137(6):516–527. [DOI] [PubMed] [Google Scholar]
- 14.Momen NC, Plana-Ripoll O, Agerbo E, et al. Association between Mental Disorders and Subsequent Medical Conditions. New England Journal of Medicine. 2020;382(18):1721–1731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wahlbeck K, Westman J, Nordentoft M, Gissler M, Laursen TM. Outcomes of Nordic mental health systems: Life expectancy of patients with mental disorders. British Journal of Psychiatry. 2011;199(6):453–458. [DOI] [PubMed] [Google Scholar]
- 16.Judd LL, Akiskal HS, Schettler PJ, et al. A prospective investigation of the natural history of the long-term weekly symptomatic status of bipolar II disorder. Arch Gen Psychiatry. 2003;60(3):261–269. [DOI] [PubMed] [Google Scholar]
- 17.Judd LL, Akiskal HS, Schettler PJ, et al. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry. 2002;59(6):530–537. [DOI] [PubMed] [Google Scholar]
- 18.Altshuler LL, Kupka RW, Hellemann G, et al. Gender and depressive symptoms in 711 patients with bipolar disorder evaluated prospectively in the Stanley Foundation bipolar treatment outcome network. Am J Psychiatry. 2010;167(6):708–715. [DOI] [PubMed] [Google Scholar]
- 19.Gignac A, McGirr A, Lam R, Yatham L. Recovery and recurrence following a first episode of mania: a systematic review and meta-analysis of prospectively characterized cohorts. The Journal of clinical psychiatry. 2015;76(9):1241–1248. [DOI] [PubMed] [Google Scholar]
- 20.Kessing LV, Andersen PK. Evidence for clinical progression of unipolar and bipolar disorders. Acta psychiatrica Scandinavica. 2017;135(1):51–64. [DOI] [PubMed] [Google Scholar]
- 21.Kapczinski F, Magalhães PVS, Balanzá-Martinez V, et al. Staging systems in bipolar disorder: an International Society for Bipolar Disorders Task Force Report. Acta Psychiatrica Scandinavica. 2014;130(5):354–363. [DOI] [PubMed] [Google Scholar]
- 22.Vieta E, Berk M, Schulze TG, et al. Bipolar disorders. Nature reviews Disease primers. 2018;4. [DOI] [PubMed] [Google Scholar]
- 23.Demmo C, Lagerberg T, Kvitland L, et al. Neurocognitive functioning, clinical course and functional outcome in first-treatment bipolar I disorder patients with and without clinical relapse: A 1-year follow-up study. Bipolar disorders. 2018;20(3):228–237. [DOI] [PubMed] [Google Scholar]
- 24.Post RM. A shocking deficit in bipolar disorder treatment research funding. Bipolar disorders. 2020;22(8):864–865. [DOI] [PubMed] [Google Scholar]
- 25.Vieta E, Angst J. Bipolar disorder cohort studies: Crucial, but underfunded. European neuropsychopharmacology : the journal of the European College of Neuropsychopharmacology. 2021;47:31–33. [DOI] [PubMed] [Google Scholar]
- 26.Mullins N, Forstner AJ, O’Connell KS, et al. Genome-wide association study of more than 40,000 bipolar disorder cases provides new insights into the underlying biology. Nature Genetics. 2021;53(6):817–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Léda-Rêgo G, Bezerra-Filho S, Miranda-Scippa Â. Functioning in euthymic patients with bipolar disorder: A systematic review and meta-analysis using the Functioning Assessment Short Test. Bipolar Disorders. 2020;22(6):569–581. [DOI] [PubMed] [Google Scholar]
- 28.Miskowiak KW, Rush AJ, Gerds TA, Vinberg M, Kessing LV. Targeting Treatments to Improve Cognitive Function in Mood Disorder: Suggestions From Trials Using Erythropoietin. The Journal of clinical psychiatry. 2016;77(12):e1639–e1646. [DOI] [PubMed] [Google Scholar]
- 29.Henry C, Godin O, Courtet P, et al. Outcomes for bipolar patients assessed in the French expert center network: A 2-year follow-up observational study (FondaMental Advanced Centers of Expertise for Bipolar Disorder [FACE-BD]). Bipolar Disorders. 2017;19(8):651–660. [DOI] [PubMed] [Google Scholar]
- 30.Sajatovic M, Eyler LT, Rej S, et al. The Global Aging & Geriatric Experiments in Bipolar Disorder Database (GAGE-BD) project: Understanding older-age bipolar disorder by combining multiple datasets. Bipolar Disorders. 2019;21(7):642–649. [DOI] [PubMed] [Google Scholar]
- 31.Üstün TB, Chatterji S, Kostanjsek N, et al. Developing the world health organization disability assessment schedule 2.0. Bulletin of the World Health Organization. 2010;88(11):815–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mundt JC, Marks IM, Shear MK, Greist JH. The Work and Social Adjustment Scale: A simple measure of impairment in functioning. British Journal of Psychiatry. 2002;180(MAY):461–464. [DOI] [PubMed] [Google Scholar]
- 33.Rosa AR, Sánchez-Moreno J, Martínez-Aran A, et al. Validity and reliability of the Functioning Assessment Short Test (FAST) in bipolar disorder. Clinical Practice and Epidemiology in Mental Health. 2007;3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lyall DM, Cullen B, Allerhand M, et al. Cognitive Test Scores in UK Biobank: Data Reduction in 480,416 Participants and Longitudinal Stability in 20,346 Participants. PLOS ONE. 2016;11(4):e0154222–e0154222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Burdick KE, Millett CE, Russo M, et al. The association between lithium use and neurocognitive performance in patients with bipolar disorder. Neuropsychopharmacology 2020 45:10. 2020;45(10):1743–1749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Del Matto L, Muscas M, Murru A, et al. Lithium and suicide prevention in mood disorders and in the general population: A systematic review. Neuroscience and biobehavioral reviews. 2020;116:142–153. [DOI] [PubMed] [Google Scholar]
- 37.Burdick KE, Millett CE. Cognitive heterogeneity is a key predictor of differential functional outcome in patients with bipolar disorder. European Neuropsychopharmacology. 2021;53:4–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jaeger J, Berns SM, Czobor P. The Multidimensional scale of independent functioning: A new instrument for measuring functional disability in psychiatric populations. In. Vol 29: DHHS Public Health Service; 2003:153–167. [DOI] [PubMed] [Google Scholar]
- 39.Spitzer R, Gibbon MJ, Williams J, Endicott J. Global Assessment of Functioning (GAF) Scale. undefined. 1996. [Google Scholar]
- 40.Leon AC, Solomon DA, Mueller TI, Turvey CL, Endicott J, Keller MB. The Range of Impaired Functioning Tool (LIFE-RIFT): A brief measure of functional impairment. Psychological Medicine. 1999;29(4):869–878. [DOI] [PubMed] [Google Scholar]
- 41.Nurnberger JI, Blehar MC, Kaufmann CA, et al. Diagnostic interview for genetic studies: Rationale, unique features, and training. In. Vol 51: American Medical Association; 1994. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


