Abstract
Introduction
Once-weekly (OW) glucagon-like peptide 1 receptor agonist (GLP-1RA) semaglutide has been shown to have a more potent glycated hemoglobin (HbA1c)-lowering effect than other oral hypoglycemic agents and existing GLP-1RAs in global randomized controlled trials. The study aim was to evaluate the safety and effectiveness of OW semaglutide in Japanese patients with type 2 diabetes mellitus (T2DM) in a real-world clinical setting and identify pre- and post-treatment predictors of good response.
Methods
We investigated the change in HbA1c, percentage of patients achieving < 7% HbA1c, and factors contributing to the effect 6 months after OW semaglutide use in Japanese patients with T2DM. We also examined differences in effectiveness between patients with different backgrounds.
Results
At baseline, the 77 patients had a mean baseline HbA1c of 8.1% ± 1.23%, 74% of the patients were injecting another GLP-1RA, and 42.9% of the patients were being treated with insulin. HbA1c decreased by 0.89% and by 0.66% in the other GLP-1RA users. The rate of achievement of < 7% HbA1c increased from 21% to 43%. There were no differences in effect by age, sex, or body mass index. Higher baseline HbA1c and shorter duration of diabetes were associated with greater HbA1c reduction. OW semaglutide was tolerable for the majority of our study population.
Conclusion
This study provided real-world evidence showing that OW semaglutide significantly reduced HbA1c in Japanese patients with T2DM who had inadequate HbA1c control.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13300-022-01313-0.
Keywords: Glucagon-like peptide 1 receptor agonist, Once-weekly semaglutide, Type 2 diabetes mellitus, Real-world evidence
Key Summary Points
| Why carry out this study? |
| Once-weekly (OW) semaglutide has been reported to lower glycated hemoglobin (HbA1c) more potently than other glucagon-like peptide 1 receptor agonists (GLP-1RAs) and dipeptidyl peptidase 4 inhibitors in global randomized controlled trials that included Japanese people. |
| Few studies have been conducted using real-world evidence (RWE) in Japanese patients with type 2 diabetes mellitus (T2DM). |
| This retrospective study provides RWE for enhanced treatment using OW semaglutide in Japanese patients with T2DM. |
| What was learned from this study? |
| Add-on or switching from another GLP-1RA to OW semaglutide significantly reduced HbA1c at 6 months relative to those supported by RCT. The rate of achievement of HbA1c < 7% also increased. |
| Patients with higher baseline HbA1c and shorter duration of diabetes were associated with greater reduction of HbA1c. |
Introduction
The pathophysiology of type 2 diabetes mellitus (T2DM) involves decreased insulin secretion from pancreatic β-cells and decreased activity in target organs [1]. Insulin secretion capacity and resistance are lower in East Asians, including Japanese people, than in Caucasians [2, 3]. In particular, acute insulin secretion in response to higher glucose levels is decreased and causes postprandial hyperglycemia in early-stage T2DM [4]. Glucagon-like peptide 1 (GLP-1), a gut-derived hormone, maintains glucose homeostasis through glucose-stimulated insulin secretion (GSIS) from pancreatic β-cells (pancreatic action) and other direct or indirect actions on the central nervous system, liver, gut, and adipose tissue (extrapancreatic action) [5, 6]. GLP-1 stimulates GSIS in pancreatic β-cells by activating the ATP-sensitive K+ channel pathway via GLP-1/cyclic adenosine 3′,5′-monophosphate (cAMP)/exchange protein directly activated by the cAMP 2 (EPAC2) pathway or another mechanism [7–9] and improves glucose intolerance. Administration of GLP-1, not incretin enhancers such as dipeptidyl peptidase 4 inhibitors, also reduces appetite and food intake [10]. GLP-1 receptor agonists (GLP-1RAs) are an established glucose-lowering therapeutic option for treatment of T2DM proven in numerous clinical trials [11]. Presently, once-weekly (OW) administered GLP-1RA semaglutide is available (in Japan since June 2020), and patients can choose the most appropriate formulation (oral or injectable) depending on their individual factors [12]. On the other hand, other GLP-1RAs and/or new sodium-glucose transporter 2 (SGLT2) inhibitors have become widely used in Japan, but some patients still have difficulty reducing glycated hemoglobin (HbA1c) [13–15]. OW semaglutide has been reported to have a more potent effect on HbA1c than placebo or existing drugs or another GLP-1 receptor agonist in the Semaglutide Unabated Sustainability in Treatment of Type 2 Diabetes (SUSTAIN) clinical trial program, which includes clinical trials on Japanese patients [16, 17]. The SUSTAIN-6 trial, a cardiovascular outcome trial, showed significant reduction in major adverse cardiovascular events by treatment with semaglutide relative to those in the placebo group [18]. Currently published clinical trials suggest that semaglutide may be useful for therapeutic intensification of diabetes treatment and provide a cardiovascular benefit, but few studies have been conducted in real-world clinical settings in Japanese patients with T2DM. A recent meta-analysis of six GLP-1RA clinical trials, including the SUSTAIN-6 trial, reported that the benefit of GLP-1 on major adverse cardiovascular events was greater in Asians than in White patients [19]. We planned a study of the efficacy and safety of semaglutide in Japanese patients with T2DM. To reinforce the previous randomized clinical trial (RCT) findings, the present study aim was to evaluate the safety and effectiveness of OW semaglutide and identify pre- and post-treatment predictors of good response in Japanese patients with T2DM in a real-world clinical setting.
Methods
Study Design and Participants
This was a retrospective cohort study conducted at a single center. We enrolled patients with T2DM who had been treated with OW semaglutide (Ozempic® subcutaneous injection SD) in the Jichi Medical University Saitama Medical Center between June 2021 and December 2021. Semaglutide was used for better glycemic control at the discretion of the attending physician according to the Japanese package insert, with a starting dose of 0.25 mg. We evaluated clinical parameters 6 months after OW semaglutide initiation. The inclusion criteria were as follows: Japanese patients with T2DM who initiated OW semaglutide in an outpatient setting. The exclusion criteria were patients with acute metabolic disorders, such as diabetic ketoacidosis and hyperosmolar hyperglycemic syndrome; who were taking steroids, had acute infectious disease, or had any newly diagnosed cancer; who required hospitalization, self-discontinued, or had poor adherence to injections; who exhibited a change in an oral antidiabetic drug (OAD) or new insulin induction during the observation period; and whose adverse events led to discontinuation within 6 months.
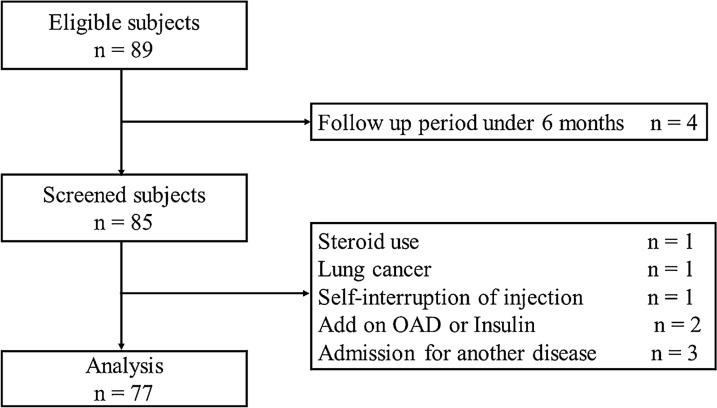
Figure 1 shows the flowchart of patient enrollment. After application of the inclusion and exclusion criteria, we analyzed the data from 77 patients. No patient discontinued the treatment because of side effects. The primary outcome was the change in HbA1c at 6 months after initiation of OW semaglutide. The secondary outcome was the proportion of participants achieving HbA1c < 7.0%. Additionally, we investigated clinical factors that potentially caused a change in HbA1c, and the information in the medical records on adverse events. We obtained baseline demographic data of the patients, such as age, sex, body mass index (BMI), duration of diabetes, presence of macroangiopathy and cardiovascular disease, concomitant medications, adverse events, and OW semaglutide dose at 6 months, from the medical records. Additionally, we collected the following clinical laboratory data: HbA1c, total cholesterol, high-density lipoprotein cholesterol, triglyceride, and estimated glomerular filtration rate (eGFR). This study was conducted in accordance with the Declaration of Helsinki of 1964, as revised in 2013, and was approved by the Ethics Committee at Jichi Medical University Saitama Medical Center (No. S19-005). This study used non-identifiable data obtained by the treating physicians and therefore, on the basis of the decision from our local Ethics Committee of Jichi Medical University Saitama Medical Center (No. S19-005), informed consent was not required. Patients had the opportunity to object to the use of their data for retrospective scientific research; however, none of the patients objected.
Fig. 1.
Flowchart of patient enrollment
Statistical Analysis
We expressed continuous variable data as means (± standard deviations) and categorical variable data as numbers and percentages. We compared each parameter before and after initiating OW semaglutide by using paired t tests or the t test. One-way analysis of variance (ANOVA) or the Kruskal–Wallis test was used to compare the clinical characteristics among three or more groups. Categorical variables were compared by performing Fisher’s exact test. We defined patients who showed a change in HbA1c greater than the median (≥ 0.7%) as the good-response group and those who showed a change in HbA1c less than the median (< 0.7%) as the poor-response group.
To explore the effects of various clinical factors on the deterioration of OW semaglutide in the good-response group, we performed logistic regression analysis. We divided the patients into the younger and elderly groups (age < 65, ≥ 65 years), non-obese and obese groups (BMI < 25, ≥ 25 kg/m2), and the chronic kidney disease (CKD) and non-CKD groups (eGFR < 60, ≥ 60 mL/min/1.73 m2). We defined baseline HbA1c < 8.1% or ≥ 8.1% and duration of diabetes as < 14 or ≥ 14 years according to these medians. We performed all statistical analyses using EZR (Jichi Medical University, Saitama Medical Center), a graphical user interface for R (The R Foundation for Statistical Computing), and a modified version of R commander designed to add statistical functions frequently used in biostatistics. We accepted all P values less than 0.05 as indicating statistical significance.
Results
Baseline Characteristics
The baseline characteristics of the 77 patients (aged 64.6 ± 11.6 years, 50 men) are shown in Table 1. The average baseline HbA1c and BMI were 8.1% ± 1.23% and 27.3 ± 5.03 kg/m2, respectively. The elderly group (age ≥ 65 years) comprised 41 patients, and the obese group (BMI ≥ 25 kg/m2) comprised 49 patients. The most used OADs were the SGLT2 inhibitor (66.2%) and biguanides (52%). The percentage of patients treated only by an OAD was 14.3%. We found that 74% of the patients were injecting GLP-1RAs and 42.9% of the patients were being treated with insulin. All patients were initially administered semaglutide 0.25 mg, and at the time of data evaluation, the semaglutide dosages were 0.25 mg in 13 patients, 0.5 mg in 59 patients, and 1.0 mg in 5 patients.
Table 1.
Clinical baseline characteristics of the patients
| Variables | |
| Number of patients, n | 77 |
| Men/women, n | 50/27 |
| Age, years | 64.6 (11.6) |
| BMI, kg/m2 | 27.3 (5.03) |
| BW, kg | 70.4 [65–82] |
| Duration of diabetes, years | 14 [9–22] |
| HbA1c, % | 8.1 (1.23) |
| eGFR, mL/min/1.73 m2 | 65.5 (26.9) |
| TC, mg/dL | 178 (37.3) |
| TG, mg/dL | 146 [103–205] |
| HDL-C, mg/dL | 49 [20–56] |
| Dyslipidemia, yes, n (%) | 51 (66.2) |
| Hypertension, yes, n (%) | 48 (62.3) |
| Ischemic heart disease, yes, n (%) | 17 (22.1) |
| Diabetic retinopathy yes, n (%) | 11 (14.3) |
| Diabetic nephropathy yes, n (%) | 27 (35.1) |
| Medications | |
| Sulfonylurea, n (%) | 14 (18.2) |
| Biguanide, n (%) | 40 (52) |
| Glinide, n (%) | 23 (29.8) |
| α-Glucosidase inhibitor, n (%) | 21 (27.3) |
| Thiazolidinedione, n (%) | 7 (9.1) |
| SGLT2 inhibitor, n (%) | 51 (66.2) |
| DPP4 inhibitor n (%) | 14 (18.2) |
| OAD only, n (%) | 11 (14.3) |
| Insulin therapy, n (%) | 33 (42.9) |
| Basal insulin only, n (%) | 21 (27.3) |
| Bolus insulin only, n (%) | 0 (0) |
| Basal–bolus therapy, n (%) | 12 (15.6) |
| GLP-1 receptor agonist, n (%) | 57 (74) |
| Liraglutide, n (%) | 10 (13) |
| Lixisenatide, n (%) | 10 (13) |
| Dulaglutide, n (%) | 37 (48.1) |
Data are presented as n (%) for categorical variables and as the mean (standard deviation) or median [interquartile range] for continuous variables
BMI body mass index, BW body weight, HbA1c glycated hemoglobin, eGFR estimated glomerular filtration rate, TC total cholesterol, HDL-C high-density lipoprotein cholesterol, TG triglyceride, SGLT2 sodium-glucose cotransporter 2, DPP4 dipeptidyl peptidase 4, OAD oral antidiabetic drug, GLP-1 glucagon-like peptide 1
Change in HbA1c and Response Predictors
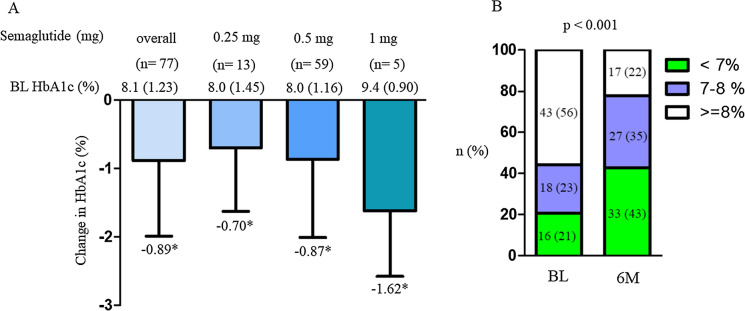
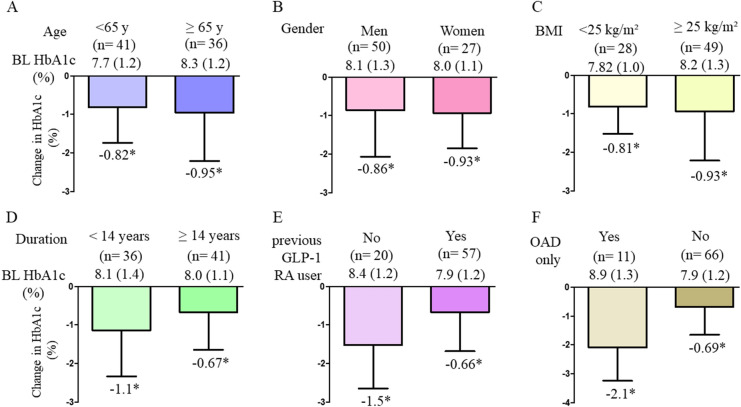
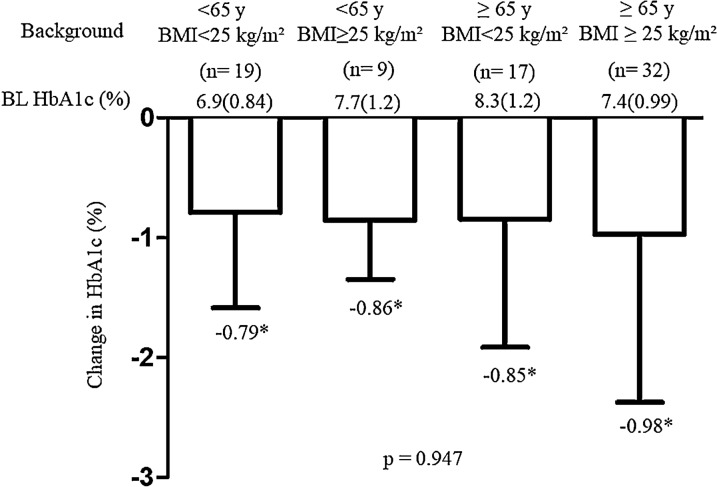
Overall, reduction of HbA1c was − 0.89% from baseline HbA1c at 6 months. The percentage of patients who achieved HbA1c < 7% was significantly changed from 21% at baseline to 43% (Fig. 2). The changes in HbA1c in the two groups were significantly improved from their baseline HbA1c values (Fig. 2). The degree of change in HbA1c between the two groups did not differ by age, sex, BMI, and duration (Fig. 3a–d), but significant changes were observed between previous GLP-1RA users and OAD-only users (Fig. 3e, f). Among the following four categories, patients who were < 65 years with BMI < 25 kg/m2, < 65 years with BMI ≥ 25 kg/m2, ≥ 65 years with BMI < 25 kg/m2, and ≥ 65 years with BMI ≥ 25 kg/m2, the changes in HbA1c decreased relative to their respective baseline HbA1c values. However, the degree of change in HbA1c by category was not significant (Fig. 4). Weight data were available for 64 patients. The overall change in body weight was a decrease of 1.25 kg, with a decrease of 4.2 kg for semaglutide 0.25 mg (n = 4), 0.63 kg for 0.5 mg (n = 55), and 5.7 kg for 1.0 mg (n = 5), and significant differences in weight changes were observed. (p = 0.016). Significant reductions from baseline weight were also observed overall and in the group using OW semaglutide 0.5 mg (Supplementary Material 1).
Fig. 2.
Change in HbA1c from baseline overall and in the semaglutide dose subgroup (a) and proportion of patients achieving HbA1c < 7% (b). *P < 0.05 in the paired t test (vs. BL). BL baseline, HbA1c glycated hemoglobin
Fig. 3.
Change in HbA1c by subgroup a age, b sex, c BMI, d duration of diabetes, e previous GLP-1RA user or not, and f OAD user or not. *P < 0.05 in paired t test (vs. BL HbA1c). BL baseline, HbA1c glycated hemoglobin, BMI body mass index, GLP-1RA glucagon-like peptide 1 receptor agonist, OAD oral antidiabetic drug
Fig. 4.
Change in baseline HbA1c by age and BMI. *P < 0.05 in the paired t test (vs. BL HbA1c). BL baseline, HbA1c glycated hemoglobin, BMI body mass index
Multivariate analysis of the potential predictors of a good response to OW semaglutide (> 0.7% HbA1c improvement as defined in the “Methods” section) revealed that shorter duration of diabetes and higher baseline HbA1c level were more likely than the other predictors to achieve a good response (Table 2).
Table 2.
Independent clinical factors associated with good response
| Parameters | OR | 95% CI | P value |
|---|---|---|---|
| Age ≥ 65 years | 1.29 | 0.376–4.44 | 0.685 |
| Sex, women | 0.948 | 0.282–3.18 | 0.931 |
| BMI ≥ 25 kg/m2 | 0.680 | 0.211–2.20 | 0.520 |
| Duration of diabetes ≥ 14 years | 0.312 | 0.101–0.962 | 0.043 |
| eGFR ≥ 60 mL/min/1.73 m2 | 0.367 | 0.103–1.30 | 0.121 |
| Baseline HbA1c ≥ 8.1% | 8.87 | 2.83–27.8 | < 0.001 |
BMI body mass index, eGFR estimated glomerular filtration rate, HbA1c glycated hemoglobin, OR odds ratio, CI confidence interval
Safety
In this study, no patients were discontinued because of side effects. Nausea occurred in eight (10.4%) patients, out of which six whose previous therapy was not GLP-1RA and two who had switched from GLP-1RA. By OW semaglutide dose, seven patients received 0.5 mg, and one patient received 1.0 mg. Seven (9.1%) patients complained of injection pain, but all were able to continue treatment. No hypoglycemic episodes were reported.
Discussion
We showed that the addition of semaglutide or switching from other GLP-1RAs was effective in Japanese patients with T2DM and inadequate HbA1c control using existing therapy. OW semaglutide was particularly effective in patients with high baseline HbA1c, but less effective in those with longer disease duration of diabetes.
In the safety and effectiveness of monotherapy with OW semaglutide versus one additional OAD in Japanese people with T2DM (SUSTAIN OAD combination trial in Japan), OW semaglutide 0.5 mg and 1 mg reduced HbA1c by 1.7% and 2.0% from baseline HbA1c (8.1%, overall), respectively, compared with 0.7% for additional OAD [16]. In the present study, treatment with OW semaglutide reduced HbA1c by 0.89%; however, in previously untreated GLP-1 or treated by OAD-only patients, the addition of OW semaglutide reduced HbA1c by 1.5% to 2.1%, which was similar to the results for the SUSTAIN Japan OAD combination [16]. Otherwise, people who switched from another GLP-1RA to OW semaglutide showed reduction of 0.66% (baseline HbA1c 7.9%) of HbA1c in the present study. In a retrospective study conducted in Canada, switching to OW semaglutide from liraglutide or dulaglutide decreased HbA1c by 0.65% (baseline HbA1c, 7.9%) in 164 patients after 6 months (REALISE-DM study) [20]. Most prior GLP-1RA usage (liraglutide and dulaglutide) was of high doses [20]. In our study, there were few previous users of high-dose GLP-1RAs. Additionally, the final dose of OW semaglutide was 1.0 mg in the REALISE-DM study [20], whereas the average dose in our study was 0.49 mg, and only five (6.5%) patients received 1.0 mg. A prospective, observational study conducted in Switzerland (SURE Switzerland) showed a 0.8% reduction in HbA1c (baseline HbA1c 7.8%) with the introduction of OW semaglutide (mean dose 0.78 mg) at approximately 30 weeks [21]. Patients treated with insulin or other GLP-1RAs were included in SURE Switzerland, and, similar to the results of our study, semaglutide may contribute to HbA1c improvement regardless of prior therapy and without using up to 1.0 mg.
Introduction of OW semaglutide significantly reduced HbA1c levels from the baseline level; however, there were no significant differences among subgroups divided by age and BMI (Fig. 4). The degree of improvement in HbA1c was greater in the higher HbA1c group (Supplementary Material 2). A post hoc analysis of two SUSTAIN Japan trials also showed that OW semaglutide was effective regardless of age and BMI subgroups (age < 65 and ≥ 65 years and/or BMI < 25 and ≥ 25 kg/m2) [22]. A large US database used to examine the effect of switching other GLP-1RAs to OW semaglutide showed that semaglutide was effective regardless of prior GLP-1RAs, age, or gender [23]. Our real-world evidence (RWE) is consistent with the US RWE, and semaglutide may be effective for further improvement of HbA1c in poorly controlled T2DM regardless of patient background, as well as in Japanese patients.
In the present study, nausea occurred in eight (10.4%) patients, and compared with the SUSTAIN Japan post hoc analysis [22], no new adverse effect concerns were identified. Injection site pain was thought to be due to the use of the SD pen. We concluded that OW semaglutide was tolerable for the majority of our study population.
The current study data showed that baseline HbA1c and disease duration of diabetes were predictors of effectiveness. The results of several real-world studies have shown that higher baseline HbA1c was associated with a greater effect of OW semaglutide [20, 24, 25], consistent with our results (Table 2, Supplemental Fig. 1). Marzullo et al. reported that the shorter duration of diabetes was a significant predictor of a good response after 6 months OW semaglutide induction [25]. Since the retention of GSIS capacity in patients with T2DM is inversely correlated with the duration of diabetes [26], it is speculated that longer duration is responsible for the attenuated effect of insulin secretagogues. Ohbatake et al. reported that the best predictor of GLP-1RA efficacy was residual β-cell function as assessed by the glucagon stimulation test [27]. Consequently, it would be useful to examine the relationship between β-cell function and semaglutide efficacy in the future.
A study showed that weekly dulaglutide was superior to liraglutide with respect to treatment satisfaction in Japanese patients with T2DM [28]. OW semaglutide may also affect treatment satisfaction, but treatment quality of life (QOL) could not be assessed in this study. Future studies on treatment QOL are also needed.
This study had several limitations. First, this was a single-center retrospective observational study without a control group; therefore, there may have been confounding factors that were not eliminated, and the results may not be applicable to the general Japanese diabetes population. Side effects, such as nausea or hypoglycemic episodes, were not documented in the medical records and may have been underestimated. Second, OW semaglutide usage was uneven, with most patients using 0.5 mg (76.6%), and the follow-up period was short (6 months), which may have resulted in inadequate semaglutide dose titration. Third, there was variability in the drug and dose of pre-treatment GLP-1RAs. There was no statistical power to analyze each drug subgroup; consequently, analysis of a large number of cases is desired in the future.
Conclusion
Our study results showed that the introduction of semaglutide in Japanese patients with T2DM who had not achieved the target reduction in HbA1c level with existing therapy, including another GLP-1RA, was useful for lowering HbA1c levels. In addition, patients with shorter disease duration and higher baseline HbA1c were found to be more likely to benefit from treatment. Semaglutide was useful for earlier treatment intensification.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We thank all of the study participants. We also appreciate all members of the medical staff of Jichi Medical University Saitama Medical Center.
Funding
This work was supported by a Grant‐in‐Aid for Young Scientists (JSPS KAKENHI Grant Number 19K18012), Scientific Research (C) (JSPS KAKENHI Grant Number 18K08525). The journal’s Rapid Service Fee was funded by the authors.
Medical Writing, Editorial and Other Assistance
The authors thank Enago (www.enago.jp) for the English language review. This was funded by the authors.
Authorship
All named authors meet the International Committee of Medical Journal Editors criteria for authorship of this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Conceptualization and methodology: Hodaka Yamada, Masashi Yoshida, and Daisuke Suzuki; investigation: Hodaka Yamada, Shunsuke Funazaki, Shuichi Nagashima, Masahiko Kimura, Otsuka Kiyoshi, and Kazuo Hara; formal analysis: Hodaka Yamada, Masashi Yoshida, and Daisuke Suzuki. All authors contributed to the data interpretation, drafting, critical review, and revision of the manuscript and read and approved the final draft for submission.
Disclosures
Hodaka Yamada, Masashi Yoshida, Daisuke Suzuki, Shunsuke Funazaki, Shuichi Nagashima, Masahiko Kimura, Otsuka Kiyoshi, and Kazuo Hara declare that they have no conflict of interest.
Compliance with Ethics Guidelines
This study was conducted in accordance with the Declaration of Helsinki of 1964, as revised in 2013. The study was approved by the Ethics Committee of Jichi Medical University Saitama Medical Center (No. S19-005). This study used non-identifiable data obtained by the treating physicians and therefore, on the basis of the decision from our local Ethics Committee of Jichi Medical University Saitama Medical Center (No. S19-005), informed consent was not required. Patients had the opportunity to object to the use of their data for retrospective scientific research; however, none of the patients objected.
Data Availability
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Footnotes
The original online version of this article was revised due to update in figure 3.
Change history
1/11/2023
A Correction to this paper has been published: 10.1007/s13300-022-01365-2
References
- 1.Kendall DM, Cuddihy RM, Bergenstal RM. Clinical application of incretin-based therapy: therapeutic potential, patient selection and clinical use. Am J Med. 2009;122(6 Suppl):S37–50. doi: 10.1016/j.amjmed.2009.03.015. [DOI] [PubMed] [Google Scholar]
- 2.Yabe D, Seino Y, Fukushima M, Seino S. β cell dysfunction versus insulin resistance in the pathogenesis of type 2 diabetes in East Asians. Curr Diabetes Rep. 2015;15(6):602. doi: 10.1007/s11892-015-0602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Seino Y, Kuwata H, Yabe D. Incretin-based drugs for type 2 diabetes: focus on East Asian perspectives. J Diabetes Investig. 2016;7(Suppl 1):102–109. doi: 10.1111/jdi.12490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukushima M, Suzuki H, Seino Y. Insulin secretion capacity in the development from normal glucose tolerance to type 2 diabetes. Diabetes Res Clin Pract. 2004;66(Suppl 1):S37–43. doi: 10.1016/j.diabres.2003.11.024. [DOI] [PubMed] [Google Scholar]
- 5.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 6.Drucker DJ. The biology of incretin hormones. Cell Metab. 2006;3(3):153–165. doi: 10.1016/j.cmet.2006.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Kakei M, Yoshida M, Dezaki K, et al. Glucose and GTP-binding protein-coupled receptor cooperatively regulate transient receptor potential-channels to stimulate insulin secretion. Endocr J. 2016;63(10):867–876. doi: 10.1507/endocrj.EJ16-0262. [DOI] [PubMed] [Google Scholar]
- 8.Seino S, Shibasaki T, Minami K. Dynamics of insulin secretion and the clinical implications for obesity and diabetes. J Clin Invest. 2011;121(6):2118–2125. doi: 10.1172/JCI45680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rorsman P, Ashcroft FM. Pancreatic β-cell electrical activity and insulin secretion: of mice and men. Physiol Rev. 2018;98(1):117–214. doi: 10.1152/physrev.00008.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Holst JJ, Deacon CF, Vilsbøll T, Krarup T, Madsbad S. Glucagon-like peptide-1, glucose homeostasis and diabetes. Trends Mol Med. 2008;14(4):161–168. doi: 10.1016/j.molmed.2008.01.003. [DOI] [PubMed] [Google Scholar]
- 11.Nauck MA, Quast DR, Wefers J, Meier JJ. GLP-1 receptor agonists in the treatment of type 2 diabetes - state-of-the-art. Mol Metab. 2021;46:101102. doi: 10.1016/j.molmet.2020.101102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Meier JJ. Efficacy of semaglutide in a subcutaneous and an oral formulation. Front Endocrinol. 2021;12:645617. doi: 10.3389/fendo.2021.645617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ohsugi M, Eiki JI, Iglay K, Tetsuka J, Tokita S, Ueki K. Comorbidities and complications in Japanese patients with type 2 diabetes mellitus: retrospective analyses of J-DREAMS, an advanced electronic medical records database. Diabetes Res Clin Pract. 2021;178:108845. doi: 10.1016/j.diabres.2021.108845. [DOI] [PubMed] [Google Scholar]
- 14.Bouchi R, Sugiyama T, Goto A, et al. Retrospective nationwide study on the trends in first-line antidiabetic medication for patients with type 2 diabetes in Japan. J Diabetes Investig. 2022;13(2):280–291. doi: 10.1111/jdi.13636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yagi N, Komiya I, Arai K, et al. Current status of oral antidiabetic drug prescribing patterns based on the body mass index for Japanese type 2 diabetes mellitus patients and yearly changes in diabetologists' prescribing patterns from 2002 to 2019 (JDDM61) J Diabetes Investig. 2022;13(1):65–73. doi: 10.1111/jdi.13621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kaku K, Yamada Y, Watada H, et al. Safety and efficacy of once-weekly semaglutide vs additional oral antidiabetic drugs in Japanese people with inadequately controlled type 2 diabetes: a randomized trial. Diabetes Obes Metab. 2018;20(5):1202–1212. doi: 10.1111/dom.13218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Seino Y, Terauchi Y, Osonoi T, et al. Safety and efficacy of semaglutide once weekly vs sitagliptin once daily, both as monotherapy in Japanese people with type 2 diabetes. Diabetes Obes Metab. 2018;20(2):378–388. doi: 10.1111/dom.13082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375(19):1834–1844. doi: 10.1056/NEJMoa1607141. [DOI] [PubMed] [Google Scholar]
- 19.Lee MMY, Ghouri N, McGuire DK, Rutter MK, Sattar N. Meta-analyses of results from randomized outcome trials comparing cardiovascular effects of SGLT2is and GLP-1RAs in Asian versus white patients with and without type 2 diabetes. Diabetes Care. 2021;44(5):1236–1241. doi: 10.2337/dc20-3007. [DOI] [PubMed] [Google Scholar]
- 20.Jain AB, Kanters S, Khurana R, Kissock J, Severin N, Stafford SG. Real-world effectiveness analysis of switching from liraglutide or dulaglutide to semaglutide in patients with type 2 diabetes mellitus: the retrospective REALISE-DM study. Diabetes Ther. 2021;12(2):527–536. doi: 10.1007/s13300-020-00984-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rudofsky G, Catarig AM, Favre L, et al. Real-world use of once-weekly semaglutide in patients with type 2 diabetes: results from the SURE Switzerland multicentre, prospective, observational study. Diabetes Res Clin Pract. 2021;178:108931. doi: 10.1016/j.diabres.2021.108931. [DOI] [PubMed] [Google Scholar]
- 22.Yabe D, Yamada Y, Kaku K, Nishida T, Sato T, Seino Y. Efficacy and safety of once-weekly semaglutide in Japanese individuals with type 2 diabetes by baseline age and body mass index. J Diabetes Investig. 2022;13(7):1161–1174. doi: 10.1111/jdi.13773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lingvay I, Kirk AR, Lophaven S, Wolden ML, Shubrook JH. Outcomes in GLP-1 RA-experienced patients switching to once-weekly semaglutide in a real-world setting: the retrospective, observational EXPERT study. Diabetes Ther. 2021;12(3):879–896. doi: 10.1007/s13300-021-01010-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Williams DM, Ruslan AM, Khan R, et al. Real-world clinical experience of semaglutide in secondary care diabetes: a retrospective observational study. Diabetes Ther. 2021;12(3):801–811. doi: 10.1007/s13300-021-01015-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marzullo P, Daffara T, Mele C, et al. Real-world evaluation of weekly subcutaneous treatment with semaglutide in a cohort of Italian diabetic patients. J Endocrinol Invest. 2022 doi: 10.1007/s40618-022-01799-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaku K, Kawasaki F, Kanda Y, Matsuda M. Retained capacity of glucose-mediated insulin secretion in patients with type 2 diabetes mellitus inversely correlates with the duration of diabetes. Diabetes Res Clin Pract. 2004;64(3):221–223. doi: 10.1016/j.diabres.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 27.Ohbatake A, Yagi K, Karashima S, et al. C-peptide area under the curve at glucagon stimulation test predicts glucose improvements by GLP-1 receptor analogue: a retrospective observational study. Diabetes Ther. 2019;10(2):673–681. doi: 10.1007/s13300-019-0586-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takase T, Nakamura A, Yamamoto C, et al. Improvement in treatment satisfaction after switching from liraglutide to dulaglutide in patients with type 2 diabetes: a randomized controlled trial. J Diabetes Investig. 2019;10(3):699–705. doi: 10.1111/jdi.12906. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data sets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.