Abstract
Objective
The present manuscript aims to describe an international, electronic-based, user-friendly and interoperable patient registry for monogenic autoinflammatory diseases (mAIDs), developed in the contest of the Autoinflammatory Diseases Alliance (AIDA) Network.
Methods
This is an electronic platform, based on the Research Electronic Data Capture (REDCap) tool, used for real-world data collection of demographics, clinical, laboratory, instrumental and socioeconomic data of mAIDs patients. The instrument has flexibility, may change over time based on new scientific acquisitions, and communicate potentially with other similar registries; security, data quality and data governance are corner stones of the platform.
Results
AIDA project will share knowledge and expertise on mAIDs. Since its start, 118 centers from 24 countries and 4 continents have joined the AIDA project. Fifty-nine centers have already obtained the approval from their local Ethics Committees. Currently, the platform counts 337 users (122 Principal Investigators, 210 Site Investigators, 2 Lead Investigators, and 3 data managers). The Registry collects baseline and follow-up data using 3,748 fields organized into 21 instruments, which include demographics, patient history, symptoms, trigger/risk factors, therapies, and healthcare information for mAIDs patients.
Conclusions
The AIDA mAIDs Registry, acts both as a research tool for future collaborative real-life studies on mAIDs and as a service to connect all the figures called to participate. On this basis, the registry is expected to play a pivotal role in generating new scientific evidence on this group of rare diseases, substantially improving the management of patients, and optimizing the impact on the healthcare system. NCT 05200715 available at https://clinicaltrials.gov.
Keywords: autoinflammatory diseases, international registry, personalized medicine, precision medicine, rare diseases
Introduction
Monogenic autoinflammatory diseases (mAIDs) are a group of rare inborn errors of immunity caused by mutations in genes linked to the innate immune pathways. Constitutive overactivation of these pathways leads to increased release of monocyte- and neutrophil-derived cytokines, such as interleukin-1β, tumor necrosis factor α and type 1 interferon, which results clinically in periodic fevers and a variety of unprovoked inflammatory symptoms affecting any organ or system (1). Since the candidate gene for familial Mediterranean fever (FMF) was identified in 1997, the spectrum of mAIDs has rapidly broadened especially after the remarkable advances in molecular techniques and the extensive use of next-generation sequencing (NGS): this has led to the description of more than 50 new rare diseases in the last 10 years (2, 3). Given the heterogeneity and low prevalence of mAIDs, transnational collaboration is critical in order to collect an adequate volume of data and perform groundbreaking high-impact research. To this end, the Autoinflammatory Diseases Alliance (AIDA) Network was established in 2019 to provide an international collaborative framework for scientific research and education on rare autoinflammatory diseases (https://aidanetwork.org/en/).
In the field of rare diseases, patient registries are recognized at the international level as invaluable tools that support clinical research and orientate clinical trial design, improving both the management of patients and the overall healthcare system. According to the definition provided by the Agency for Healthcare Research and Quality, a registry is “an organized system that uses observational study methods to collect uniform data (clinical and other) to evaluate specified outcomes for a population defined by a particular disease, condition, or exposure, and that serves one or more predetermined scientific, clinical, or policy purposes” (4). In this respect, it is now acknowledged that a circular flow of quality actions—governance, data source identification, standardization of data, FAIRness (findable, accessible, interoperable, and reusable) of information technology (IT) infrastructures, data/information quality check, training, and audits—are seminal to create tools of significant clinical impact (5). Furthermore, clinical registries are increasingly perceived as a service facilitating learning networks and establishing virtuous collaborations among the scientific community, industry, regulative agencies, patients, and their support networks (6). Indeed, one of the primary aims of the European Reference Networks is “to reinforce research and epidemiological surveillance like registries” (7). Also, the development of the European Platform on Rare Disease Registration (EU RD), providing common services and tools to rare disease registries operating across Europe, accounts for a strategic objective of the European Commission (EC). Through the EPIRARE project (“Building Consensus and Synergies for the EU Registration of Rare Disease Patients”, www.epirare.eu) the EC seeks to harmonize registry data to ensure interoperability, standardization, and data comparability (8). In the field of rare diseases, the AIDA Network has already worked to develop different registries dedicated to specific autoinflammatory diseases and ocular inflammatory disorders (9–11).
The present manuscript aims to describe an international, electronic-based, user-friendly and interoperable patient registry for mAIDs, the Autoinflammatory Diseases Alliance Registry of Monogenic Autoinflammatory Diseases (AIDA mAIDs), developed in the context of the AIDA Network program (ClinicalTrials.gov, Identifier: NCT05200715).
Methods
To be as comprehensive as possible, the authors adopted the registry description template questionnaire developed by Kodra et al. (12).
Registry organization
The AIDA mAIDs registry was launched on June 25, 2020 in the context of the AIDA Network program. English is the official language of the platform.
The University of Siena is the promoter of the AIDA Project. The promoter center is responsible for the registry governance and the coordination of the AIDA Network program. The role of registry/data manager is held by the personnel of the biomedical engineering department of the University of Siena, also in charge of the IT personnel of the AIDA project. The medical staff involved in the conceptual design and development of the registry and clinical advice to enrolling centers, includes physicians with expertise in rheumatology, pediatric rheumatology, ophthalmology, immunology, gastroenterology, and medical genetics. Methodologic, statistical, ethical, legal, and social issues (ELSI) and administrative support are provided by the University of Siena.
Type of registry
The AIDA mAIDs is an electronic-based, non-population based, physician-driven, clinical/genetic research registry (5).
Objectives
The overarching scope of the AIDA registry for mAIDs is declined into the following general aims: (i) to share knowledge and expertise on mAIDs by linking key referral centers for these rare diseases at an international level; (ii) to raise awareness among physicians and the general population about mAIDs, improving early diagnosis; (iii) to promote future multicenter studies based on a critical volume of data from patients with mAIDs.
The AIDA mAIDs-based studies will pursue the following specific objectives:
to depict the clinical phenotype of newly identified mAIDs and broaden the phenotypic spectrum of well-established nosological entities;
to describe genotype-phenotype correlations;
to describe clinical manifestations based on patients' ethnicity;
to identify age- and gender-related factors that may affect disease onset and progression;
to outline the long-term prognosis for these diseases and identify potential prognostic factors for negative outcomes;
to evaluate response to different therapeutic strategies and safety of drugs;
to recognize the possible impact of mAIDs on fertility and course of pregnancy;
to estimate the socio-economic burden of mAIDs.
Further objectives may be added over time thanks to the flexible modular infrastructure of the registry.
List of diseases under registration
All hereditary autoinflammatory diseases (monogenic forms) will be included in the registry. As new diseases are identified in this group, the registry shall be updated accordingly given its flexible framework. The list of genes/diseases currently included in the registry is given in Table 1. However, it is also possible to enroll patients carrying mutations in genes associated with mAIDs not yet included in the list.
Table 1.
List of genes/diseases covered by the Autoinflammatory Diseases Alliance Registry of monogenic autoinflammatory diseases.
| Gene | Inheritance | Disease | ORPHA number |
|---|---|---|---|
| ADA2 (HGNC:1839) | AR | ADA2 deficiency vasculitis | ORPHA:404553 |
| AP1S3 (HGNC:18971) | AD | Generalized pustular psoriasis | ORPHA:247353 |
| CARD14 (HGNC:16446) | AD | Pityriasis rubra pilaris | ORPHA:2897 |
| IL1RN (HGNC:6000) | AR | DIRA | ORPHA:210115 |
| IL36RN (HGNC:15561) | AR | DITRA | ORPHA:404546 |
| LACC1 (HGNC:26789) | AR | LACC1 deficiency | – |
| LPIN2 (HGNC:14450) | AR | Majeed syndrome | ORPHA:77297 |
| MEFV (HGNC:6998) | AR | FMF | ORPHA:342 |
| AD | PAAND | – | |
| MVK (HGNC:7530) | AR | MKD | ORPHA:343 |
| NLRC4 (HGNC:16412) | AD | AIFEC | ORPHA:436166 |
| NLRP3 (HGNC:16400) | AD | CAPS-FCAS 1 | ORPHA: 47045 |
| NLRP3 (HGNC:16400) | AD | CAPS-MWS | ORPHA:575 |
| NLRP3 (HGNC:16400) | AD | CAPS-NOMID | ORPHA:1451 |
| NLRP12 (HGNC:22938) | AD | FCAS 2 | ORPHA:247868 |
| NOD2 (HGNC:5331) | AD | Blau syndrome | ORPHA:90340 |
| OTULIN (HGNC:25118) | AR | ORAS | ORPHA:500062 |
| PLCG2 (HGNC:9066) | AD | APLAID | ORPHA:324530 |
| PLCG2 (HGNC:9066) | AD | PLAID | ORPHA:300359 |
| POMP (HGNC:20330) PSMA3 (HGNC:9532) PSMB4 (HGNC:9541) PSMB8 (HGNC:9545) PSMB9 (HGNC:9546) PSMG2 (HGNC:24929) |
AR | PRAAS | ORPHA:324977 |
| PSTPIP1 (HGNC:9580) | AD | PAID | ORPHA:69126 |
| RBCK1/ HOIL1 (HGNC:15864) | AR | HOIL1 deficiency | ORPHA:329173 |
| SH3BP2 (HGNC:10825) | AD/AR | Cherubism | ORPHA:184 |
| SLC29A3 (HGNC:23096) | AR | H syndrome | ORPHA:168569 |
| TMEM173 (HGNC:27962) | AD | SAVI | ORPHA:425120 |
| TNFAIP3 (HGNC:11896) | AD | Hereditary pediatric Behçet-like disease | ORPHA:476102 |
| TNFRSF1A (HGNC:11916) | AD | TRAPS | ORPHA: 32960 |
| Others | – | Hereditary periodic fever syndrome | ORPHA:324924 |
AD, autosomal dominant; ADA, adenosine deaminase; AIFEC, periodic fever infantile enterocolitis autoinflammatory syndrome; APLAID, autoinflammation, PLCG2-associated antibody deficiency and immune dysregulation; AR, autosomal recessive; CAPS, cryopyrin-associated periodic syndromes; DIRA, sterile multifocal osteomyelitis with periostitis and pustulosis; DITRA, deficiency of interleukin-36 receptor antagonist; FCAS, familial cold autoinflammatory syndrome; FMF, familial Mediterranean fever; IL, interleukin; LACC1, laccase domain containing 1; MKD, mevalonate kinase deficiency; MWS, Muckle Wells syndrome; NOMID, neonatal-onset multisystem inflammatory disease; ORAS, OTULIN-related autoinflammatory syndrome; PAAND, pyrin-associated autoinflammation with neutrophilic dermatosis; PAID, PSTPIP1-associated inflammatory diseases; PLAID, PLCG2-associated antibody deficiency and immune dysregulation; PRAAS, proteasome-associated autoinflammatory syndrome; SAVI, STING-associated vasculopathy with onset in infancy; TRAPS, tumor necrosis factor receptor-associated periodic syndrome.
Inclusion and exclusion criteria
Patients will be enrolled on the AIDA registry if they meet all the following criteria:
Subjects diagnosed and/or treated at the AIDA network partner centers (the full list is available at https://aidanetwork.org/en/clinical-sites).
-
Diagnosis of mAIDs based on one of the following scenarios:
a) the presence of a confirmatory1 genotype AND at least 1 among the clinical items included in the Eurofever classification criteria for cryopyrin-associated periodic syndromes (CAPS), FMF, tumor necrosis factor receptor-associated periodic syndrome (TRAPS) or mevalonate kinase deficiency (MKD) (14);
b) the presence of a not confirmatory (see text footnote 1) genotype AND at least 2 among the clinical items included in the Eurofever classification criteria for CAPS, FMF or TRAPS;
c) fulfillment of the Tel Hashomer or the Yalcinkaya criteria for FMF, irrespectively of the genotype (13, 15);
d) fulfillment of the Kuemmerle-Deschner criteria for CAPS, irrespectively of the genotype (16);
e) the presence of a clinical picture consistent with MKD or adenosine deaminase (ADA)2 deficiency AND a positive biomarker test for DADA2 (ADA2 enzyme activity) or MKD (MVK enzyme activity or mevalonic aciduria) AND an inconclusive genotype2 (17);
f) the presence of a clinical picture consistent with Blau syndrome, pyogenic arthritis-pyoderma gangrenosum-acne (PAPA) syndrome, A20 haploinsufficiency, ADA2 deficiency or pyrin-associated autoinflammation with neutrophilic dermatosis (PAAND) syndrome AND the detection of a confirmatory or consistent genotype (see text footnote 2) (17);
g) the presence of a clinical picture consistent with any monogenic autoinflammatory disease covered by the registry other than FMF, MKD, TRAPS, CAPS, Blau syndrome, PAPA syndrome, A20 haploinsufficiency, DADA2 and PAAND syndrome (Table 1) AND a confirmative genotype according to the expert clinician and genetic counseling of the reference center.
3. Willing of the subject (and/or his/her parents or legal guardian where applicable according to the national regulatory frameworks) to join the project.
Subjects carrying benign variants, likely benign variants (based on the INFEVERS classification) or no variants in genes known to be responsible for mAIDs, except for the scenarios described in 2c and 2d, are excluded from the registry (18). Specific scenarios other than those described above should be discussed with the AIDA medical staff to be considered as eligible.
Data sources and data flow
The data sources for the registry are extracted from (i) hospital clinical charts, (ii) laboratory reports, (iii) genetic laboratory reports, (iv) instrumental exams reports, (v) patient reported outcomes. The registry system is designed to capture both retrospective and longitudinal data.
To minimize recall bias for self- or proxy-reported information in the retrospective section, participants are notified before sensitive information is asked and they are allowed to provide secondary sources of information to update or validate previously collected data. As for the prospective section of the registry, a streamlined approach through real-time ad-hoc updates to the records during routine follow-up visits is suggested. Recruiting centers are advised to enter at least one follow-up record per year or when therapeutic changes are made.
The system includes mandatory and non-mandatory fields. However, it allows editing and completing any previously unanswered fields at the user's convenience. User-friendly data collecting tools are in place, such as automatic calculation fields, calendar fields, branching logics, electronic joint count homunculus and integrated Online Mendelian Inheritance in Man (OMIM), HUGO Gene Nomenclature Committee (HGNC) and International Classification of Diseases (ICD) 10 databases. Direct explanations or Internet addresses referring to external resources useful to the interpretation of specific fields are provided when required [i.e., INFEVERS classification of gene variants (18), diagnostic/classification criteria, clinimetric scores, laboratory reference values]. Complex branching logics allow the registry system to unfold following the patient's clinical history, making data collection straightforward. Each field includes a free text area for comments and queries.
Population under surveillance of the registry
The target population includes subjects affected by mAIDs. No specific demographic, geographic, clinical, or genetic determinants are foreseen.
Geographic coverage
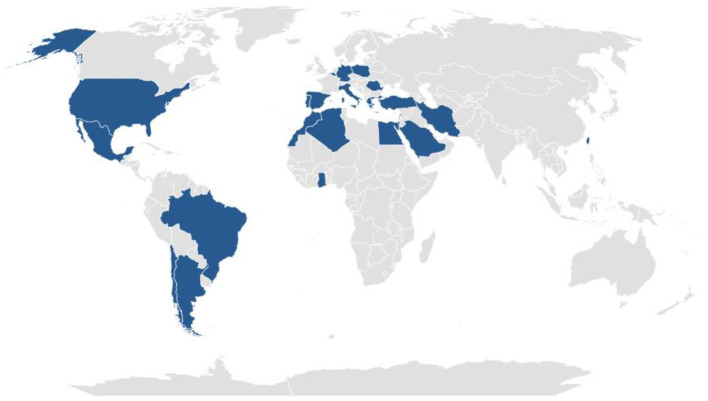
The AIDA mAIDs registry is a non-population-based registry. The geographic coverage is the catchment area of the clinical centers affiliated to the AIDA Network. The countries with at least one AIDA partner center are shown in Figure 1 (updated to June 20th, 2022). Moreover, the updated geographic coverage of the registry can be found at https://aidanetwork.org/en/clinical-sites.
Figure 1.
Current geographical coverage of the Autoinflammatory Diseases Alliance (AIDA) Registry of Monogenic Autoinflammatory Diseases. Countries highlighted in violet are those with at least one AIDA partner center (updated to June 20th, 2022).
Specification of the information to report
The instruments constituting the registry investigate the following sets of variables:
demographics
consents
diagnostic data and family history
-
general genetic information:
- gene mutations
-
features of inflammatory attacks:
- at disease onset
- between disease onset and diagnosis
- between diagnosis and the time of enrolment
clinical diagnostic and classification criteria
laboratory data
cardiovascular risk
-
past and current treatments:
- treatment with corticosteroids as main therapy
- treatment with colchicine
- treatment with conventional disease-modifying anti-rheumatic drugs
- treatment with small molecules
- treatment with biotechnological agents
-
fertility and pregnancy
- disease course and treatment during pregnancies
follow-up visits: clinical manifestations and treatment
Death of the patient (to open ONLY in case of patient's death)
The following common data elements (CDE) are included in the registry, according to the EPIRARE set of CDE for the European RDR platform (19): patient consent, patient sex, patient date of birth, patient country of birth, diagnosis (standardized according to the OMIM classification), patient country of residence, ID treatment center, other cases in the family (if Yes: degree of kinship), case parents are consanguineous (Yes/No), genetic features of the patient (gene-HGNC Gene Symbol, variant description in HGVS format, variant description in other formats), date of symptom onset, date of final diagnosis, current drug treatment, hospitalizations, patient vital status (and date of death), comorbidity (standardized according to the ICD10 format).
Registry regulatory status
The ELSI and privacy expertise are provided by the University of Siena. The AIDA project has been firstly approved by the Tuscany Region Ethics Committee - South-East (C.E.A.V.S.E.) area on 24/06/2019 (Ref. N. 14951). The last amendment to the protocol was approved on 02/05/2022. The approval of the protocol should be provided by local Ethics Committee for each of the Centers joining AIDA, whenever required by local regulations. The approval is essential for data collection, but does not affect the participation to the other branches of the AIDA project, such as AIDA Academy or AIDA for patients.
The registry has been developed in accordance with the World Medical Association (WMA) Helsinki declaration 2013 (20) and with ELSI principles and rules, including international and local data protection regulations. The registry conforms to the General Data Protection Regulation (GDPR) (21) ensuring compliance with legal requirements regarding the processing of personal data.
To be eligible for inclusion in the AIDA registry for mAIDs, patients (or their parents/legal guardians) have to provide written opt-in consent. Patients receive from the investigator appropriate information about registry objectives, the type of information collected, how data will be used, the governance and data access rules for third parties and how to withdraw consent at any time.
Collaborative framework status
The AIDA registry for mAIDs was developed as a specific action of the AIDA Network program (https://aidanetwork.org/en/). Established in 2019, the program aims to move beyond the isolation of reference centers for rare autoinflammatory diseases and autoimmune ocular diseases, facilitating the collection and exchange of clinical data, conduction of multicenter studies and dissemination of scientific knowledge at an international level.
The registry management function and high-level decision making are responsibility of the AIDA Network program governance, chaired by the principal investigator of the AIDA promoter center.
The AIDA registry stakeholders include clinicians, patients, family, and patient organizations (to date, the Italian associations AIFP-Italian Association of Periodic Fevers, ANMAR-National Association of Patients with Rheumatic Diseases, and APMARR-Association of People with Rare Rheumatic Diseases), researchers, the European Reference Network (ERN) RITA.
Inspired by the FAIR guiding principles for scientific data management and stewardship, the AIDA mAIDs registry is included in the European Rare Disease Registry Infrastructure directory (ERDRI.dor, available at https://eu-rd-platform.jrc.ec.europa.eu/erdridor/home) and is committed to promote the interoperability with the other ERN RITA registries and potentially with the other ERNs, within the context of the MeRITA (Metadata registry for the ERN RITA) project (22, 23).
Informatics infrastructure
The AIDA registry for mAIDs is hosted by a virtual server in the platform of the Laboratory of Bioengineering of the University of Siena, which is located in the Data Center of the Azienda Ospedaliero-Universitaria Senese at the Santa Maria alle Scotte hospital in Siena (Italy).
The registry service is based on REDCap (Research Electronic Data Capture, https://projectredcap.org) a secure web application designed to support data capture for research studies. REDCap provides (i) an intuitive interface for validated data capture; (ii) audit trails for tracking data manipulation and export procedures; (iii) automated export procedures for seamless data downloads to common statistical packages; and (iv) procedures for data integration and interoperability with external sources (24, 25). REDCap requires a typical web infrastructure including one or more secure web servers running a standard software stack (LAMP) that can be deployed “on-premise” (i.e., on the local institution's hardware servers or virtualized servers) or on various cloud-based infrastructures. REDCap can integrate new software modules to extend functionality, such as data query workflow, support direct data capture from patients via user-facing surveys and patient pseudonymization. Even though it is not open source, REDCap software is licensed at no-cost for academic, non-profit, and government institutions.
The AIDA Network program developed a standard scheme to build a federated infrastructure of interoperable systems for registry services between partner sites to securely share data and collaborate on research goals. This scheme takes advantage of the portability of project metadata across REDCap installations and on a set of standard operating procedures with rapid turnaround times to: (i) assess, deploy and manage multiple aligned REDCap framework instances and project registries; (ii) efficiently share and deploy standard resources, such as medical data models and ontologies, that already exist or that are newly released by international framework initiatives (such as ERDRI-JRC and ERNs), integrating them into the network of project registries and data collection instruments; (iii) ensure compliance with privacy and security requirements throughout the project partners; and, (iv) guarantee global sharing and FAIR access to data.
Data management procedure/quality control
The principal investigators of each site are responsible for the validation of data of the corresponding records. They will be also responsible for the accuracy of the information accrued, with the Principal Investigator required to supervise the goodness of overall data. Each Principal Investigator may analyze data gathered in his/her center. Site opening visits are scheduled virtually for each new partner center in order to train the investigators in the correct use of the registry. Further assistance from the AIDA team may be asked by the investigators by email (http://support@aidanetwork.org). The REDCap system allows quality data control at the moment of the data entry by users. In addition, internal data quality audits are periodically performed by the registry staff at the time of sample data extraction or when significant sources of error are identified. The quality control at the time of data extraction is aimed at the control of duplicate records from different investigator sites or logical inconsistencies and range errors on individual data batches sent by each site. When range errors or logical inconsistencies are found, a query is sent to the correspondent principal investigator in order to check the data source. When duplicate records are identified, the record management is dependent on the type of duplication and objective of analysis.
Security standards and procedures
Data are accessed through secure channels and there are procedures ensuring traceability of their processing, including login authorization procedures and history logs. Confidentiality is ensured on an operational level by pseudonymization and double codification if data are shared, in accordance with the GDPR rules. Full on-site and off-site encrypted data and system back-ups are programmed periodically.
Results
The AIDA mAIDs registry is running since June 2020.
The registry is composed of 21 instruments and 3,748 fields. By data lock in June 2022, the network counts 118 partner centers and 24 countries in 4 continents and is open to new membership applications. The protocol has been already approved by the local Ethics Committees of 59 partner centers; the remaining EC submissions performed in the last few weeks are in progress or, in a few cases, are not required by local regulations.
At last evaluation (June 20th, 2022), 418 subjects (M:F = 195:212, transsexual n = 1, missing n = 10) from 35 centers in 11 countries have been enrolled in the registry. Enrolling countries listed in alphabetical order are the following: Algeria, Belgium, Brazil, Egypt, Greece, Iran, Italy, Poland, Romania, Spain, Turkey. Patients' country of birth are Algeria (n = 23), Armenia (n = 1), Belgium (n = 4), Brazil (n = 1), China (n = 1), Egypt (n = 58), Greece (n = 6), Iran (n = 3), Italy (n = 195), Lebanon (n = 1), Morocco (n = 2), Palestine (n = 1), Poland (n = 27), Romania (n = 4), Spain (n = 2), Turkey (n = 78), Ukraine (n = 1), missing (n = 10). Mean age at the time of enrolment is 33.9 ± 16.7 years (range 0–75.9). A positive family history for the same mAID is recorded for 162 subjects (40.9%). Proband's parents are consanguineous in 40 cases (10.2%).
Discussion
The AIDA mAIDs registry is a powerful infrastructure embedded in the solid collaborative framework of the AIDA program. The registry enables the collection, sharing and valorization of a critical volume of data on mAIDs. Collecting data on autoinflammatory diseases is hampered by the well-known obstacles that rare disease research has to deal with, including the limited number of patients, isolation of research centers, and difficulty in obtaining the correct diagnosis in non-specialized clinical settings. Launched in June 2020, this AIDA registry received favorable attention on the European stage, showing increasing attractiveness, including 118 partner centers from 24 countries in a relatively short time.
According to the results of a survey conducted among the ERN RITA members in 2018, there are twenty-two registries collecting data on monogenic and/or multifactorial autoinflammatory diseases in Europe, with different scopes and geographic coverage: five of them (Eurofever, Infevers, Blau Cohort Study, Pediatric Behçet's Disease Registry, and ImmunAID) are transnational and devoted exclusively to AIDs (in two cases to a single specific disorder); four other registries (ESID, JIR-cohort, Brainworks Study, European Society for Blood and Marrow Transplantation Registry) are transnational and include both autoinflammatory diseases and primary immunodeficiencies and/or autoimmune diseases. The remaining registries collect regional or national data about rare immune diseases, including autoinflammatory diseases, with variable specificity (23). Therefore, it is of the greatest relevance to adopt a standardized scheme and provide a detailed description of the instrument when developing a new registry, in order to ensure adequate quality, efficient interoperability, and long-term sustainability. The AIDA Registry has been developed in such a way to potentially communicate with the existing registries in order to analyze the current clinical and scientific issues from different perspectives. In this regard, the AIDA registry for patients with monogenic autoinflammatory disease is already included within the context of the MeRITA.
In the scene of European registries for mAIDs, the AIDA registry stands out for its disease-specificity, richness in details, flexibility, complex branching logics allowing smart and time-saving data collection, and wide geographic and demographic coverage. With specific regard to the latter, the choice of including subjects of any age naturally follows from the well-established evidence that mAIDs may start from the very first hours of life to late adulthood, and also that adults with pediatric-onset disease may obtain the correct diagnosis with a delay of several decades. The inclusion of adult patients along with children is an asset to this registry, allowing the design of comparative and longitudinal studies, research focused on childhood-to-adult transition and adult-specific issues not yet explored, such as pregnancy, fertility, adult vaccination, comorbidities, long-term disease-related damage, adult-specific outcome measures and PROMs, workability and further socio-economic issues. With this respect, the AIDA actions will be aligned to the research agenda set by the EULAR/ACR points to consider for diagnosis, management and monitoring of the IL-1 mediated AIDs and autoinflammatory type I interferonopathies (26, 27), also in the context of possible future collaborations at the international level.
The registry has been conceived as a flexible and modular tool able to capture the evolving landscape of this field of research. Whensoever new theoretical or practical knowledge is generated, the system enables agile updating of data collection tools. As an example, new modules may be added to include newly identified genes, new treatments that become available or to address to future unmet needs with new research objectives. Moreover, direct queries to the investigators can address specific gaps in the data collection. The AIDA mAIDs registry is inspired by the principles of FAIRness and is committed to adopt the instruments that the EC suggests for the development of registry platforms. Data are standardized by the use of shared libraries such as the ICD-10 and the OMIM classification; the EPIRARE set of CDE for the European RDR platform has been employed when possible (19). The registry has been already registered on the ERDRI directory (https://eu-rd-platform.jrc.ec.europa.eu/erdridor/) and will adopt the new SPIDER tool in the future, with the aim of facilitating the pseudonymization, linkage and transfer of encrypted pseudonymized data among European rare disease registries.
On the other hand, the registry platform supports data capture via user-facing surveys and the MyCap application (https://projectmycap.org/), which leverages REDCap, ResearchKit, and ResearchStack tools to capture patient reported outcomes via mobile devices. Later, the tool synchronizes the results back to the registry project. The integration of data from the “AIDA for patients” action into the AIDA mAIDs registry highlights the huge potentiality of this instrument. Actually, data obtained through the surveys proposed by “AIDA for patients” will complement the registry with patient-reported data about quality of life, fatigue, the socio-economic burden of the disease, psychological health, experience of the healthcare pathway and beliefs about medicines. This will allow four-hands studies with the active participation of both patients and their physicians. With this regard, a pilot project co-designed with the patient's association S.I.M.B.A. (Associazione Italiana Sindrome e Malattia di Behçet) has been already launched by the “AIDA for patients” action for Italian patients with Behçet's disease. A similar experience may be reproduced also in the context of mAIDs and other multifactorial AIDs, with the collaboration of local patient associations (https://aidanetwork.org/en/magazine/aida-for-patients-is-ready-for-launch).
As for the long-term sustainability of the registry, it seems relevant to highlight the lively interest raising from a growing number of international partners both in and outside European borders. Moreover, the AIDA Network program strategic communication and dissemination activities (website, magazine, web events) are equally important to the registry promotion. The program also includes the provision of high-level specialized education in the field of AID, through web-based seminars, face-to-face meetings, and a permanent education archive in collaboration with a renowned international faculty (AIDA Academy action). Of note, the program is endorsed by a growing number of patient associations, whose active involvement enhances a multiplier effect, by giving resonance to both AIDA Network program and AIDA mAIDs registry.
Furthermore, the AIDA mAIDs registry has been designed for the implementation of top-down and bottom-up research initiatives. Each Principal Investigator and Site Investigator may provide their study proposals during dedicated meetings. In particular, each Principal Investigator may analyze data collected in their own center for clinical and administrative use. On the contrary, the whole data will be managed by statistics and physicians involved in the network, selected by the Promoter on a case-by-case basis according to their field of expertise. Aggregated CDE are also used for periodic AIDA progress reports and are ready to be shared at the national and European level in the context of the ERNs as publicly indexed metadata and for clinical benchmarking.
In conclusion, we provide a new powerful instrument, the AIDA mAIDs registry, acting both as a research tool for future collaborative real-life studies on mAIDs and as a service to connect all the figures called to participate. These include international researchers, non-specialized clinicians, patients and their representatives, regulatory agencies as well as institutions at the national and supranational level. On this basis, the registry is expected to play a pivotal role in generating new scientific evidence on this group of rare diseases, substantially improving the management of patients and optimizing their impact on the healthcare system.
Ethics statement
The studies involving human participants were reviewed and approved by Tuscany Region Ethics Committee - South-East (C.E.A.V.S.E.) area on 24/06/2019 (Ref. N. 14951). Written informed consent to participate in this study was provided by the participants' legal guardian/next of kin.
Author contributions
CG wrote the first draft of the manuscript. AV conceived and designed the study and revised the draft of the manuscript. DR revised the draft of the manuscript. LC conceived and designed the study and accounts for AIDA Registries Coordinator. AB is the bioengineer involved in the technical management of the platform and registries. AT, GR, EA, EW-S, DA-I, GC, LI, MCM, MC, FLT, GL, EV, AP, AS, AI, PPS, AM, MF, BO, DO-B, PP, GE, FS, FCa, MP, JH-R, RP, MA, RNu, AO, ED, PS, PR, FLG, HK, JS, MAH, GM, KJ-R, RS-H, MR, FR, FCi, FI, FD, MF, KL, TG, FF, VSa, DY, VC, MTH, RD, AK, and GK were involved in data recruitment in the Registry dedicated to patients with mAIDs. MT, IA, AL, VM, LD, LB, SGe, FCr, VP, AA-MAM, RNa, MD, CBC, SGr, MM, ID, VSp, HAMG, IV, AR, AF, MAM, BF, GT, and CF were included in the authorship as investigators from the top contributor centers for any of the other seven AIDA Registries. ML was included as leading AIDA expert in the field of mAIDs. All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Footnotes
1According to Gattorno et al., the genotype is considered confirmatory if 1 pathogenic/likely pathogenic mutation (for CAPS and TRAPS) or 2 pathogenic/likely pathogenic mutations (for FMF and MKD) in the causative genes are detected; the genotype is considered not confirmatory if (1) trans compound heterozygous for 1 pathogenic MEFV variant and 1 variant of uncertain significance (VUS), or biallelic VUS, or heterozygous for 1 pathogenic MEFV variant is detected, or if (2) 1 VUS in MVK or TNFRSF1A genes are detected. The pathogenicity of gene variants shall be based on the Infevers classification (10, 13).
2According to Shinar et al., the genotype is considered confirmatory when 1 (likely) pathogenic variant in NOD2, PSTPIP1, TNFAIP3 genes or 1 pathogenic dominant variant in MEFV gene or 2 (likely) pathogenic variants in ADA2 gene are detected; the genotype is considered consistent when 1 novel likely pathogenic variant in NOD2, PSTPIP1, TNFAIP3 genes or 2 (likely) pathogenic not phased variants in ADA2 gene or 1 (likely) pathogenic and 1 rare/novel VUS variants in ADA2 gene are detected; the genotype is considered inconclusive when 1 (likely) pathogenic or 2 rare VUS in ADA2 or MVK are detected (15).
References
- 1.Manthiram K, Zhou Q, Aksentijevich I, Kastner DL. The monogenic autoinflammatory diseases define new pathways in human innate immunity and inflammation. Nat Immunol. (2017) 18:832–42. 10.1038/ni.3777 [DOI] [PubMed] [Google Scholar]
- 2.Rigante D, Frediani B, Galeazzi M, Cantarini L. From the Mediterranean to the sea of Japan: the transcontinental odyssey of autoinflammatory diseases. Biomed Res Int. (2013) 2013:485103. 10.1155/2013/485103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Georgin-Lavialle S, Ducharme-Benard S, Sarrabay G, Savey L, Grateau G, Hentgen V. Systemic autoinflammatory diseases: clinical state of the art. Best Pract Res Clin Rheumatol. (2020) 34:101529. 10.1016/j.berh.2020.101529 [DOI] [PubMed] [Google Scholar]
- 4.Gliklich RE, Dreyer NA, Leavy MB. Registries for Evaluating Patient Outcomes: A User's Guide. 3rd ed. Rockville, MD: Agency for Healthcare Research and Quality (US); 2014 Apr. Report No.: 13-EHC111. [PubMed] [Google Scholar]
- 5.Kodra Y, Weinbach J, Posada-de-la-Paz M, Coi A, Lemonnier SL, van Enckevort D, et al. Recommendations for improving the quality of rare disease registries. Int J Environ Res Public Health. (2018) 15:1644. 10.3390/ijerph15081644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boulanger V, Schlemmer M, Rossov S, Seebald A, Gavin P. Establishing patient registries for rare diseases: rationale and challenges. Pharmaceut Med. (2020) 34:185–90. 10.1007/s40290-020-00332-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Directive 2011/24/EU of the European Parliament and of the Council of 9 March 2011 on the application of patients' rights in cross-border healthcare . Off J Eur Union. (2011) L88:45–65. [Google Scholar]
- 8.Vittozzi L, Gainotti S, Mollo E, Donati C, Taruscio D. A model for the European platform for rare disease registries. Public Health Genomics. (2013) 16:299–304. 10.1159/000355935 [DOI] [PubMed] [Google Scholar]
- 9.Della Casa F, Vitale A, Pereira RM, Guerriero S, Ragab G, Lopalco G, et al. Development and implementation of the AIDA international registry for patients with non-infectious scleritis. Ophthalmol Ther. (2022) 11:887–97. 10.1007/s40123-022-00466-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casa FD, Vitale A, Guerriero S, Sota J, Cimaz R, Ragab G, et al. Development and implementation of the AIDA international registry for patients with non-infectious uveitis. Ophthalmol Ther. (2022) 11:899–911. 10.1007/s40123-022-00459-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vitale A, Della Casa F, Lopalco G, Pereira RM, Ruscitti P, Giacomelli R, et al. Development and implementation of the AIDA international registry for patients with still's disease. Front Med. (2022) 9:878797. 10.3389/fmed.2022.878797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kodra Y, Posada de la Paz M, Coi A, Santoro M, Bianchi F, Ahmed F, et al. Data quality in rare diseases registries. Adv Exp Med Biol. (2017) 1031:149–64. 10.1007/978-3-319-67144-4_8 [DOI] [PubMed] [Google Scholar]
- 13.Yalçinkaya F, Ozen S, Ozçakar ZB, Aktay N, Cakar N, Düzova A, et al. A new set of criteria for the diagnosis of familial Mediterranean fever in childhood. Rheumatology. (2009) 48:395–8. 10.1093/rheumatology/ken509 [DOI] [PubMed] [Google Scholar]
- 14.Gattorno M, Hofer M, Federici S, Vanoni F, Bovis F, Aksentijevich I, et al. Classification criteria for autoinflammatory recurrent fevers. Ann Rheum Dis. (2019) 78:1025–32. 10.1136/annrheumdis-2019-215048 [DOI] [PubMed] [Google Scholar]
- 15.Livneh A, Langevitz P, Zemer D, Zaks N, Kees S, Lidar T, et al. Criteria for the diagnosis of familial Mediterranean fever. Arthritis Rheum. (1997) 40:1879–85. 10.1002/art.1780401023 [DOI] [PubMed] [Google Scholar]
- 16.Kuemmerle-Deschner JB, Ozen S, Tyrrell PN, Kone-Paut I, Goldbach-Mansky R, Lachmann H, et al. Diagnostic criteria for cryopyrin-associated periodic syndrome (CAPS). Ann Rheum Dis. (2017) 76:942–47. 10.1136/annrheumdis-2016-209686 [DOI] [PubMed] [Google Scholar]
- 17.Shinar Y, Ceccherini I, Rowczenio D, Aksentijevich I, Arostegui J, Ben-Chétrit E, et al. ISSAID/EMQN best practice guidelines for the genetic diagnosis of monogenic autoinflammatory diseases in the next-generation sequencing era. Clin Chem. (2020) 66:525–36. 10.1093/clinchem/hvaa024 [DOI] [PubMed] [Google Scholar]
- 18.Van Gijn ME, Ceccherini I, Shinar Y, Carbo EC, Slofstra M, Arostegui JI, et al. New workflow for classification of genetic variants' pathogenicity applied to hereditary recurrent fevers by the International Study Group for Systemic Autoinflammatory Diseases (INSAID). J Med Genet. (2018) 55:530–7. 10.1136/jmedgenet-2017-105216 [DOI] [PubMed] [Google Scholar]
- 19.Taruscio D, Mollo E, Gainotti S, Posada de la Paz M, Bianchi F, Vittozzi L. The EPIRARE proposal of a set of indicators and common data elements for the European platform for rare disease registration. Arch Public Health. (2014) 72:35. 10.1186/2049-3258-72-35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.World Medical Association . World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. (2013) 310:2191–4. 10.1001/jama.2013.281053 [DOI] [PubMed] [Google Scholar]
- 21.Regulation Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the Protection of Natural Persons with Regard to the Processing of Personal Data and on the Free Movement of Such Data and Repealing Directive 95/46/EC (General Data Protection Regulation) (Text with EEA Relevance) . Available online at: http://eur-lex.europa.eu/eli/reg/2016/679/oj (accessed June 1, 2022).
- 22.Wilkinson MD, Dumontier M, Aalbersberg IJ, Appleton G, Axton M, Baak A, et al. The FAIR guiding principles for scientific data management and stewardship. Sci Data. (2016) 3:160018. 10.1038/sdata.2016.18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Papa R, Cant A, Klein C, Little MA, Wulffraat NM, Gattorno M, et al. Towards European harmonisation of healthcare for patients with rare immune disorders: outcome from the ERN RITA registries survey. Orphanet J Rare Dis. (2020) 15:33. 10.1186/s13023-020-1308-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)–a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. (2009) 42:377–81. 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O'Neal L, et al. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. (2019) 95:103208. 10.1016/j.jbi.2019.103208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Romano M, Arici ZS, Piskin D, Alehashemi S, Aletaha D, Barron K, et al. The 2021 EULAR/American College of Rheumatology Points to Consider for Diagnosis, Management and Monitoring of the Interleukin-1 Mediated Autoinflammatory Diseases: cryopyrin-associated periodic syndromes, tumour necrosis factor receptor-associated periodic syndrome, mevalonate kinase deficiency, and deficiency of the interleukin-1 receptor antagonist. Arthritis Rheumatol. (2022) 74:1102–21. 10.1002/art.42139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cetin Gedik K, Lamot L, Romano M, Demirkaya E, Piskin D, Torreggiani S, et al. The 2021 European Alliance of Associations for Rheumatology/American College of Rheumatology points to consider for diagnosis and management of autoinflammatory type I interferonopathies: CANDLE/PRAAS, SAVI and AGS. Ann Rheum Dis. (2022) 81:601–13. 10.1136/annrheumdis-2021-221814 [DOI] [PMC free article] [PubMed] [Google Scholar]