Abstract
Introduction:
Molecular profiling of tumor tissue is the gold standard for treatment decision-making in advanced non-small cell lung cancer (NSCLC). Results may be delayed or unavailable due to insufficient tissue, prolonged wait times for biopsy, pathology assessment and testing. We piloted the use of plasma testing in the initial diagnostic workup for patients with suspected advanced lung cancer.
Methods:
Patients with ⩽15 pack-year smoking history and suspected advanced lung cancer referred to the lung cancer rapid diagnostic program underwent plasma circulating-tumor DNA testing using a DNA-based mutation panel. Tissue testing was performed per standard of care, including comprehensive next-generation sequencing (NGS). The primary endpoint was time from diagnostic program referral to cancer treatment in stage IV NSCLC patients (Cohort A) compared to a contemporary cohort not enrolled in the study (Cohort B) and an historical pre-COVID cohort referred to the program between 2018 and 2019 (Cohort C).
Results:
From January to June 2021, 20 patients were enrolled in Cohort A; median age was 70.5 years (range 33–87), 70% were female, 55% Caucasian, 85% never smokers, and 75% were diagnosed with NSCLC. Seven had actionable alterations detected in plasma or tissue (4/7 concordant). Fusions, not tested in plasma, were identified by immunohistochemistry for three patients. Mean result turnaround time was 17.8 days for plasma NGS and 23.6 days for tissue (p = 0.10). Mean time from referral to treatment initiation was significantly shorter in cohort A at 32.6 days (SD 13.1) versus 62.2 days (SD 31.2) in cohort B and 61.5 days (SD 29.1) in cohort C, both p < 0.0001.
Conclusion:
Liquid biopsy in the initial diagnostic workup of patients with suspected advanced NSCLC can lead to faster molecular results and shorten time to treatment even with smaller DNA panels. An expansion study using comprehensive NGS plasma testing with faster turnaround time is ongoing (NCT04862924).
Keywords: liquid biopsy, lung cancer, plasma-first, time to treatment
Background
In the current era of precision medicine, clinical management of newly diagnosed non-small cell lung cancer (NSCLC) patients requires knowledge of molecular alterations to guide treatment decisions.1,2 Targeted therapies have changed the management of molecularly-driven NSCLC and new treatments targeting actionable alterations are being continuously developed. Molecular testing of tumor tissue is the gold standard for diagnosis and genotyping. However, 15–40% of lung cancer patients do not have enough tissue for successful molecular testing.3–6 As such a significant proportion of lung cancer patients do not have test results available at the time of oncology consultation.7–9
The wait time for starting treatment is a period of uncertainty for patients that causes fear and anxiety. 10 Prolonged wait times may have a detrimental impact on patient outcomes, with fewer patients able to access effective therapy contributing to reduced survival and quality of life.11–13 Delayed tumor biopsy and molecular profiling results are associated with significant patient attrition, with up to 17% of lung cancer patients dying or becoming unfit for therapy before results are available. 13 In a previous report, nearly 20% of patients eligible for targeted therapy started chemotherapy instead because molecular results were not available at the time of treatment decision-making. 11
Multidisciplinary centralized referral programs can help shorten wait times for diagnosis and treatment of lung cancer. 14 However, many programs in managed care or publicly funded systems are impacted by limited resources for biopsy, pathology, and molecular testing. Wait times for patients to access diagnostic tests, including imaging and biopsies, have also increased significantly during the COVID-19 pandemic. 15
Liquid biopsies are minimally invasive tests that can detect circulating-tumor DNA (ctDNA) and identify targetable alterations in a proportion of lung cancer patients, with a sensitivity of 80% in plasma.16,17 Although the gold standard for molecular testing is tumor tissue genotyping, plasma ctDNA analysis has demonstrated clinical utility as an alternative or complementary tool in NSCLC, especially in clinical scenarios where tissue or time for genotyping is limited. 17 Liquid biopsies using plasma are more convenient and safer for patients than repeat tissue biopsies and their use for molecular profiling may lead to cost savings in some patient populations.16,18–21 Although considered a valid tool for genotyping in newly diagnosed patients with advanced pathologically proven NSCLC, 17 the clinical utility of liquid biopsies as an initial approach for biomarker evaluation prior to cancer diagnosis remains to be demonstrated in prospective studies. 22 Preliminary studies suggest that turnaround times (TATs) and time to treatment (TTT) may be shortened with a complementary approach.23,24 However, the role of liquid biopsy in the pre-diagnostic phase, a plasma-first approach, has not yet been established.
The ACCELERATE study (NCT04863924) aims to prospectively assess the utility of liquid biopsy to accelerate TTT for patients with radiographic evidence of advanced lung cancer. Herein we report the results from a pilot cohort using liquid biopsy in the pre-diagnostic phase in patients with suspected advanced lung cancer.
Methods
The pilot study was a prospective, single arm, nontherapeutic, minimally invasive study conducted at the University Health Network (UHN), Toronto, Canada. The study was registered on ClinicalTrials.gov on 14 January 2021 (ACCELERATE, NCT04863924). Conduct of this study was reviewed and approved by the institutional research ethics board (UHN Board C). All participants provided written informed consent before study enrolment. Between 14 January and 30 June 2021, eligible patients referred to the Lung Rapid Access Management Program (Lung RAMP) with suspected advanced lung cancer (stage IVA or B) based on imaging who were nonsmokers or with ⩽15 pack-year smoking history, were enrolled. Patients were enrolled after eligibility confirmation by the Lung RAMP Multidisciplinary Case Conference (MCC), including representation from Thoracic Surgery, Interventional Respirology, Radiology, Radiation, and Medical Oncology. Imaging tests to document stage IV disease included computed tomography (CT) of chest and abdomen and also positron emission tomography and total body bone scan as needed. Patients deemed eligible were approached to participate in the study, including liquid biopsy as part of their ongoing diagnostic workup. Other eligibility criteria included: (a) measurable disease with >1 cm of disease on CT in patients with solid lesions; (b) diagnostic tissue biopsy ordered or planned. Patients with pleural effusions and no measurable disease were eligible if the MCC favored a malignant diagnosis based on imaging. Patients were excluded if they had concurrent cancer, a cancer diagnosis other than lung cancer in the past 2 years, or if they were pregnant due to concerns of potentially confounding ctDNA results.
Patients underwent plasma ctDNA testing using a DNA-based mutation panel of single nucleotide variants (SNVs) and indels in 38 cancer-associated genes (Follow It®, Imagia Canexia Health, Vancouver, BC, Canada) through the Project Access to Cancer Testing and Treatment (supported by the Canadian Technology Digital Supercluster). The Follow It® assay uses a multiplexed polymerase chain reaction (PCR) DNA-based panel targeting hotspots in clinically actionable genes in lung cancer, allowing for the detection of single-base substitutions (SNVs), small deletions, and insertions (up to 24 bp). It evaluates the mutation status of tumor DNA at 337 hotspots and 26 exons in 38 known cancer-associated genes simultaneously, including clinically relevant variants in Epidermal Growth Factor Receptor gene (EGFR), Erb-B2 Receptor Tyrosine Kinase 2 gene (ERBB2), B-Raf Proto-Oncogene (BRAF), Kirsten rat sarcoma virus gene (KRAS), and MET proto-oncogene (MET). Ret Proto-Oncogene (RET) mutations can also be detected. Reflex tissue testing was performed per institutional standard of care, including comprehensive next-generation sequencing (NGS, Oncomine Comprehensive Assay v3, Thermo Fisher Scientific, Waltham, MA, USA) and immunohistochemistry (IHC) for tumor programmed death-ligand 1.25,26 For samples with insufficient tissue for NGS or expedited requests, single gene testing for EGFR mutations (RT-52, EntroGen Inc., Woodland Hills, CA, USA), ALK (5A4 antibody), and ROS-1 (D4D6 antibody, fluorescence in situ hybridization) was performed.27,28
Time from referral to treatment initiation was analyzed and compared between patients in the pilot cohort that underwent liquid biopsy (cohort A, n = 20) and two control cohorts (contemporaneous and historic). The contemporaneous cohort included patients with stage IV NSCLC referred to Lung RAMP during the same time period in which the pilot study was conducted (cohort B, n = 26). The third cohort was derived from an historical cohort of patients with advanced NSCLC harboring actionable genomic alterations that were light or never smokers referred to Lung RAMP in 2018, prior to the COVID-19 pandemic (cohort C, n = 41). In 2018 (cohort C), institutional standard tissue profiling included single gene testing for ALK and ROS1 gene fusions, described above, NGS assay targeting 15 genes (Trusight Tumor 15 Panel, Illumina™). 29
The primary endpoint was TTT, defined as the time from diagnostic program referral to treatment initiation for cohort A using plasma ctDNA, compared to cohorts B and C. Secondary endpoints included the identification of actionable targets, time to sample collection, and result TAT of plasma-based versus standard of care tissue profiling.
Statistical methods
Patient characteristics were summarized using descriptive statistics. Two-sample t-test was used to compare TTT between the pilot and the historical comparison cohorts, and the paired t-test was used to compare TAT between plasma molecular testing and tissue NGS testing within the pilot cohort.
Results
From 14 January to 31 June 2021, 20 eligible patients were enrolled in cohort A (Supplemental Figure 1). The median age was 70.5 years (range 33–87). 70% were female, 55% Caucasian, 85% never smokers (see Table 1). All patients underwent testing of diagnostic tumor tissue as per standard of care. Twelve (60%) had lung adenocarcinoma upon final pathology reporting, five (25%) had lung non-adenocarcinoma histology (one each of large cell neuroendocrine, large cell, sarcomatoid, lymphoepithelioma-like carcinoma, and atypical carcinoid). Three (15%) had non-lung cancer diagnoses (one carcinoma of unknown primary, one gastric adenocarcinoma, one diffuse B-cell lymphoma).
Table 1.
Patient characteristics.
| Cohort A, N (%) | Cohort B, N (%) | Cohort C, N (%) | |
|---|---|---|---|
| Sex | |||
| Female | 14 (70) | 10 (38) | 30 (73) |
| Male | 6 (30) | 16 (62) | 11 (27) |
| Mean age at diagnosis in years | 70.5 | 72 | 72.1 |
| Smoking history | |||
| Never smoker | 17 (85) | 7 (27) | 30 (73) |
| Light ex-smoker (<15 pack-year) | 3 (15) | 0 | 6 (15) |
| Former | 0 | 9 (35) | 3 (7) |
| Current | 0 | 10 (38) | 2 (5) |
| Final histological diagnosis | |||
| Adenocarcinoma | 12 (60) | 22 (85) | 39 (95) |
| Squamous cell | 0 | 2 (8) | 0 |
| NSCLC NOS | 0 | 1 (4) | 2 (5) |
| Large cell carcinoma | 1 (5) | 1 (4) | 0 |
| LCNEC | 1 (5) | 0 | 0 |
| Lymphoepithelioma-like | 1 (5) | 0 | 0 |
| Carcinoid | 1 (5) | 0 | 0 |
| Sarcomatoid | 1 (5) | 0 | 0 |
| Not lung primary a | 2 (10) | 0 | 0 |
| Unknown b | 1 (5) | 0 | 0 |
One patient was diagnosed with lung metastases from gastric adenocarcinoma, one patient with diffuse large-B lymphoma (JAK2, IDH1 gene alterations).
One patient underwent ctDNA plasma testing and CT-guided liver biopsy both negative for malignancy.
CT, computed tomography; ctDNA, circulating-tumor DNA; LCNEC, large cell neuroendocrine carcinoma; NSCLC, non-small cell lung cancer: NOS, not otherwise specified.
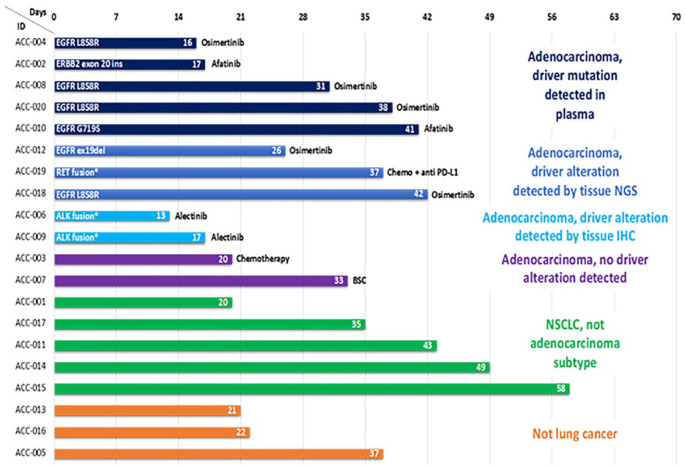
Of the 12 patients with tissue biopsy-proven adenocarcinoma of the lung, 10 (83%) had actionable oncogenic driver alterations detected in plasma or tissue (Figure 1). Using the plasma DNA mutation hotspot panel, five patients had actionable alterations detected in EGFR and went on to receive targeted therapy (EGFR kinase inhibitors). These were later confirmed by tissue testing in four of five cases. Three patients had alterations detected by tissue NGS only (2 EGFR, 1 RET fusion not included in plasma panel); both patients with negative ctDNA results had stage IVB NSCLC metastatic to the bone (M1c), but no other distant metastases. Two received targeted therapy while the third received standard chemo-immunotherapy as targeted therapy could not be accessed at that time (RET). Two patients had ALK fusion proteins identified through reflex single gene testing (IHC) in tissue, which was not included in the plasma panel, and received targeted therapy. Of the other five patients with NSCLC other than adenocarcinoma, three patients underwent tissue NGS as per institutional standard of care (large cell neuroendocrine, large cell, sarcomatoid carcinoma) with no actionable driver alterations identified.
Figure 1.
Cohort A swimmer plot of time from referral to the program to treatment initiation.
Overall concordance was 71% between plasma and tissue for gene alterations that were assayed using both methods, and sensitivity ranged between 60 and 100% (Supplemental Table 2).
Comparison cohort characteristics
Cohort B included all patients referred to Lung RAMP that were not enrolled in the study (N = 26) during the same time period as study conduct (1 January 2021 to 1 July 2021) that were diagnosed with advanced NSCLC. These patients were either ineligible due to smoking history or were potentially eligible but not recruited as they were not seen in person due to COVID-19 restrictions. The median age in cohort B was 71.5 years (range 38–85), 38% were female, 27% were never smokers, 81% had lung adenocarcinoma. Nine patients (35%) had actionable alterations detected in tissue, for which eight patients received targeted therapy (Table 2).
Table 2.
Molecular testing method and results.
| Cohort A, Plasma + tissue, N (%) | Cohort B, Tissue (Contemporaneous), N (%) | Cohort C, Tissue (Historical), N (%) | |
|---|---|---|---|
| Tissue sampling method | |||
| EBUS/TBNA | 10 (50) | 14 (54) | 26 (63) |
| CT-guided biopsy | 5 (25) | 7 (27) | 9 (22) |
| Thoracentesis | 3 (15) | 3 (12) | 4 (10) |
| Other | 2 (10) | 2 (7) | 2 (5) |
| Tissue molecular profiling method | |||
| Oncomine | 7 (35) | 19 (73) | 0 |
| 15-gene panel (TST15) | 0 | 1 (4) | 29 (71) |
| Single gene PCR + IHC | 2 (10) | 4 (15) | 12 (29) |
| N/A | 11 (55) | 2 (8) | 0 |
| Oncogenic alterations | |||
| EGFRex19 del/L858R | 5 (25) | 6 (23) | 33 (80) |
| EGFR (uncommon) | 1 (5) | 1 (4) | 3 (7) |
| ALK fusion | 2 (10) | 1 (4) | 3 (7) |
| ERBB2 ex20 insertion | 1 (5) | 0 | 2 (5) |
| MET ex14 skip | 0 | 1 (4) | 0 |
| RET fusion | 1 (5) | 0 | 0 |
| KRASG12C | 0 | 4 (15) | 0 |
| BRAFV600E | 0 | 1 (4) | 0 |
| No alteration detected | 10 (50) | 12 (46) | 0 |
CT, computed tomography; EBUS, endobronchial ultrasound; IHC, immunohistochemistry; TBNA, transbronchial needle aspiration.
Cohort C included patients with advanced NSCLC harboring an actionable alteration that were light or never smokers referred to Lung RAMP during 2018, prior to the COVID-19 pandemic (n = 41). Median age was 72.1 years (range 39–92), 73% were female, 88% were never smokers or had ⩽15 pack-year smoking history, 95% had adenocarcinoma. All patients had an actionable alteration per selection criteria (Table 2), and 83% received targeted therapy (Figure 1)
Turnaround times
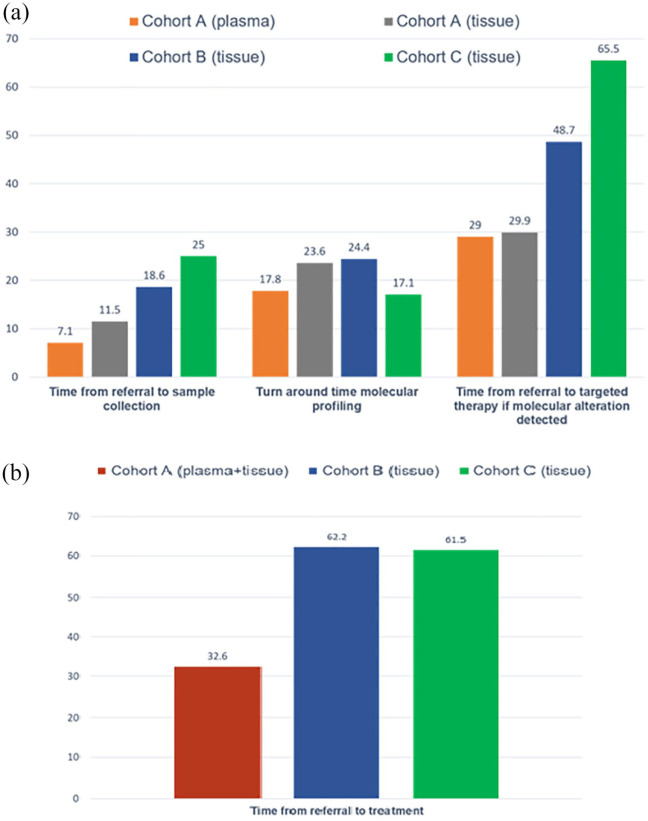
TATs are displayed in Figure 2(a). Mean time from program referral to plasma testing was 7.1 days (SD 6.9) in cohort A. Tissue biopsy for study participants was performed a mean of 11.5 days (SD 9.8) after referral. The mean TAT for plasma molecular results was 17.8 days (SD 5.6) and 23.6 days (SD 9.0) for tissue results using a larger comprehensive NGS panel (OCAv3, p = 0.10).
Figure 2.
Mean wait times (days) in cohort A NSCLC (n = 17), plasma versus tissue and compared to cohort B (n = 26) and C (n = 41). (a) Turnaround times. (b) Time from referral to treatment initiation.
In the contemporaneous cohort B, the mean time from referral to biopsy was 18.6 days (SD 10.5) and the mean TAT for tissue NGS results was 24.4 days (SD 7.2). In the pre-COVID-19 cohort C, the mean time from referral to biopsy was 25 days (SD 16.7) and the mean TAT for tissue results using a 15-gene NGS panel was 17.1 days (SD 5.5).
Time to systemic treatment initiation
Time to treatment initiation is showed in Figure 2(b), with individual treatment data detailed in Figure 1. In the cohort A, using liquid biopsy, mean time to systemic treatment initiation (TTT), was 32.6 days (n = 17, SD 13.1) for the NSCLC cohort and 27.8 days (n = 12, SD 10.8) for the adenocarcinoma cohort; compared to 62.2 days (n = 26, SD 31.2) in the Cohort B and compared to 61.5 days (n = 40, SD 29.1) in the Cohort C, both p < 0.0001.
In the cohort A, patients with alterations identified in plasma (n = 5) had a mean TTT of 29.0 days (SD 11.8). Those with alterations identified by tissue NGS only (n = 5) had a mean TTT of 29.9 days (SD 10.4); those with fusions detected by IHC (n = 2) had a mean TTT of 15 days (SD not applicable) and those with no actionable alterations identified in plasma or tissue (n = 2) had a mean TTT of 36.9 days (SD 14.3).
In the contemporaneous cohort B, mean time from referral to targeted therapy treatment in the subgroup of patients with actionable alterations detected by tissue NGS was 48.7 days (n = 8, SD 14.7). Only one patient had a fusion detected in tissue (ALK rearrangement), and TTT was 20 days.
In the pre-COVID cohort C, three patients had an ALK fusion detected by IHC and time from referral to treatment was 30, 36, and 63 days. Nine patients had sensitizing EGFR mutations detected by PCR (Entrogen); time from referral to treatment was 55.2 days (SD 15.8). For the remaining 29 patients with alterations detected by tissue NGS, TTT was 65.5 days (SD 32.6).
Discussion
In this pilot study of never or light smokers (⩽15 pack-years) with suspected advanced lung cancer, we explored the feasibility of liquid biopsy for molecular genotyping as part of the pre-diagnostic workup for lung cancer. In our study, this approach was easily integrated and accepted by patients and providers. Time to treatment initiation in patients with lung cancer was significantly improved with a ‘plasma first’ approach compared to both contemporary and historical cohorts that did not undergo plasma testing as part of their diagnostic workup (mean 32.6 days versus 62.2 days versus 61.5 days, respectively, p < 0.0001). Time to treatment was shortened for all patients participating in the plasma-first approach, even if targetable alterations were not identified.
Our pilot study is limited by small numbers. Also, despite the selection of never or light smokers with radiological evidence of advanced lung cancer confirmed by a multidisciplinary committee, only 12 (60%) had lung adenocarcinoma, while 3 had a non-lung cancer diagnosis. These results highlight the need for tissue biopsy for lung cancer diagnosis and pathologic subtyping: ‘plasma first’ does not mean ‘plasma only’. For this pilot study, patients underwent plasma ctDNA testing using a DNA-based mutation panel which detected SNVs, and indels in 38 cancer-associated genes, but not fusions, with a mean TAT for molecular results of 17.8 days. The concordance between plasma and tissue for identification of actionable mutations (excluding fusions) was 71%. Plasma NGS before tissue NGS can increase detection of therapeutically targetable mutations, especially when tissue DNA is insufficient or unavailable. However, despite ctDNA sensitivity continues to improve, it is not perfect. Some tumors may not shed ctDNA, likely explaining why two patients with NSCLC harboring an EGFR mutation in tissue had negative plasma results. Finally, larger comprehensive NGS assays using DNA and RNA-based panels may have greater ability to detect fusions and more mutations with faster TAT, and may in turn lead to better results.10,12
In our study, we also found that time from referral to targeted therapy initiation was shorter in cohort B versus cohort C (48.7 days versus 65.5 days), suggesting a potential impact of access to care during the COVID-19 pandemic. A lower volume of referrals was seen during the pandemic and more patients had suspected advanced disease. Patients with suspected metastatic disease were prioritized for diagnostic tests, which likely contributed to shorter waiting times for diagnostic biopsies and faster access to treatment in cohort B compared to in the pre-pandemic era.
Aggarwal et al. 30 conducted a retrospective study showing that concurrent tissue and plasma genotyping significantly increased the proportion of patients undergoing guideline concordant comprehensive molecular testing versus tissue alone, with increased delivery of targeted therapy leading to an improvement in overall survival. Preliminary studies suggest that TATs and TTT may be shortened by incorporating a ‘plasma-first’ approach. Thompson et al. 23 explored a plasma-first approach in a prospective study of 55 patients with suspected advanced lung cancer in the United States. Plasma-based molecular profiling was performed at the time of diagnostic biopsy. Results from plasma were highly concordant with tissue sequencing and associated with shorter TTT initiation. In a similar study by Cui et al. 24 in the United Kingdom, 51 patients with suspected advanced lung cancer had plasma testing as part of their diagnostic workup. Of these, 22% commenced targeted therapy based on plasma results without awaiting tissue profiling.
Our study showed similar results in a more selected population of never or light smokers, and also highlighted the impact of multidisciplinary diagnostic assessment programs. However, our pilot study also highlights potential limitations of a plasma-first approach, including the need for tissue to confirm histological diagnosis and the importance of using large panel comprehensive NGS assays with rapid TAT to maximize benefit.
In summary, a ‘plasma-first’ approach appears to accelerate molecular genotyping and TTT in selected patients with advanced NSCLC. However, the impact on clinically meaningful outcomes for patients such as quality of life, survival, and cost still needs to be demonstrated in prospective trials. An expansion study (NCT04863924) is ongoing, with a target accrual of 150 patients regardless of smoking history. A comprehensive DNA-based assay that also detects fusions with a 7-day TAT will be used (SNV, indels, fusions, CNV; InVisionFirst™, Inivata). Clinical outcomes including quality of life and cost effectiveness of a plasma-first approach will be collected.
Conclusion
A plasma-first approach in the diagnostic algorithm for patients with suspected advanced lung cancer may increase TTT, access to precision medicine, and potentially even improve patient outcomes.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221126151 for Plasma-first: accelerating lung cancer diagnosis and molecular profiling through liquid biopsy by Miguel Garcia-Pardo, Kasia Czarnecka, Jennifer H. Law, Alexandra Salvarrey, Roxanne Fernandes, Jason Fan, Lucy Corke, Thomas K. Waddell, Kazuhiro Yasufuku, Laura L. Donahoe, Andrew Pierre, Lisa W. Le, Noor Ghumman, Geoffrey Liu, Frances A. Shepherd, Penelope Bradbury, Adrian Sacher, Tracy Stockley, Prodipto Pal, Patrik Rogalla, Ming Sound Tsao and Natasha B. Leighl in Therapeutic Advances in Medical Oncology
Acknowledgments
I would like to thank Dr. Laura Mezquita, my thesis supervisor and my mentor, for all her support and help during my research career and my thesis project.
Footnotes
ORCID iD: Miguel Garcia-Pardo  https://orcid.org/0000-0001-6339-8501
https://orcid.org/0000-0001-6339-8501
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Miguel Garcia-Pardo, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada.
Kasia Czarnecka, Division of Respirology, Toronto General Hospital, University Health Network, Toronto, ON, Canada.
Jennifer H. Law, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada
Alexandra Salvarrey, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, CanadaDivision of Thoracic Surgery, Toronto General Hospital, University Health Network, Toronto, ON, Canada.
Roxanne Fernandes, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada.
Jason Fan, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada.
Lucy Corke, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada.
Thomas K. Waddell, Division of Thoracic Surgery, Toronto General Hospital, University Health Network, Toronto, ON, Canada
Kazuhiro Yasufuku, Division of Thoracic Surgery, Toronto General Hospital, University Health Network, Toronto, ON, Canada.
Laura L. Donahoe, Division of Thoracic Surgery, Toronto General Hospital, University Health Network, Toronto, ON, Canada
Andrew Pierre, Division of Thoracic Surgery, Toronto General Hospital, University Health Network, Toronto, ON, Canada.
Lisa W. Le, Department of Biostatistics, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada
Noor Ghumman, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada.
Geoffrey Liu, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada.
Frances A. Shepherd, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada
Penelope Bradbury, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada.
Adrian Sacher, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, Toronto, ON, Canada.
Tracy Stockley, Department of Laboratory Medicine and Pathobiology, Toronto General Hospital, University Health Network, Toronto, ON, Canada.
Prodipto Pal, Department of Laboratory Medicine and Pathobiology, Toronto General Hospital, University Health Network, Toronto, ON, Canada.
Patrik Rogalla, Joint Department of Medical Imaging, Toronto General Hospital, University Health Network, Toronto, ON, Canada.
Ming Sound Tsao, Department of Laboratory Medicine and Pathobiology, Toronto General Hospital, University Health Network, Toronto, ON, Canada.
Natasha B. Leighl, Division of Medical Oncology and Hematology, Princess Margaret Cancer Centre, University Health Network, 7-913 700 University Avenue, Toronto, ON M5G 1Z5, Canada.
Declarations
Ethics approval and consent to participate: Conduct of this study was reviewed and approved by the institutional research ethics board (UHN Board C, 20-591). All participants provided written informed consent before study enrolment.
Consent for publication: The authors consent to publish this work.
Author contribution(s): Miguel Garcia-Pardo: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Validation; Visualization; Writing – original draft; Writing – review & editing.
Kasia Czarnecka: Investigation; Writing – review & editing.
Jennifer H. Law: Data curation; Project administration.
Alexandra Salvarrey: Resources.
Roxanne Fernandes: Data curation; Resources.
Jason Fan: Data curation; Writing – review & editing.
Lucy Corke: Investigation; Writing – review & editing.
Thomas K. Waddell: Investigation; Writing – review and editing.
Kazuhiro Yasufuku: Investigation; Writing – review & editing.
Laura L. Donahoe: Investigation; Writing – review & editing.
Andrew Pierre: Investigation; Writing – review & editing.
Lisa W. Le: Formal analysis.
Noor Ghumman: Data curation; Investigation.
Geoffrey Liu: Investigation; Writing – review & editing.
Frances A. Shepherd: Investigation; Writing – review & editing.
Penelope Bradbury: Investigation; Writing – review & editing.
Adrian Sacher: Investigation; Writing – review & editing.
Tracy Stockley: Investigation; Writing – review & editing.
Prodipto Pal: Writing – review & editing.
Patrik Rogalla: Writing – review & editing.
Ming Sound Tsao: Investigation; Writing – review & editing.
Natasha B. Leighl: Conceptualization; Formal analysis; Funding acquisition; Investigation; Methodology; Writing – review & editing.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Funding support is gratefully acknowledged from the Lung Health Foundation Breathe Better Grant Program and from the Princess Margaret Cancer Foundation (Invest in Research Grant), the Division of Medical Oncology/Hematology Fellowship Award (MG) and Merck LCIC. Liquid biopsy testing was obtained free-of-charge through Project ACTT (Imagia-Canexia Health, supported by the Canadian Technology Digital Supercluster).
Competing interests: The authors declare that there is no conflict of interest.
Availability of data and materials: Data and materials are available upon request to corresponding authors.
References
- 1. Ettinger DS, Wood DE, Aisner DL, et al. NCCN guidelines insights: non-small cell lung cancer, version 2.2021. J Natl Compr Canc Netw 2021;19: 254–266. [DOI] [PubMed] [Google Scholar]
- 2. Planchard D, Popat S, Kerr K, et al. Metastatic non-small cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2018; 29: iv192–iv237. [DOI] [PubMed] [Google Scholar]
- 3. Lim C, Sekhon HS, Cutz JC, et al. Improving molecular testing and personalized medicine in non-small-cell lung cancer in Ontario. Curr Oncol 2017; 24: 103–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gutierrez ME, Choi K, Lanman RB, et al. Genomic profiling of advanced non-small cell lung cancer in community settings: gaps and opportunities. Clin Lung Cancer 2017; 18: 651–659. [DOI] [PubMed] [Google Scholar]
- 5. Ferry-Galow KV, Datta V, Makhlouf HR, et al. What can be done to improve research biopsy quality in oncology clinical trials? J Oncol Pract 2018; 14: e722–e728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Griesinger F, Eberhardt W, Nusch A, et al. Biomarker testing in non-small cell lung cancer in routine care: analysis of the first 3,717 patients in the German prospective, observational, nation-wide CRISP Registry (AIO-TRK-0315). Lung Cancer 2021; 152: 174–184. [DOI] [PubMed] [Google Scholar]
- 7. Gordan LN, Diaz M, Patel AJ, et al. Effective biomarker testing rates in a large U.S. community practice. J Clin Oncol 2022; 40: e21093. [Google Scholar]
- 8. Nadler E, Vasudevan A, Wang Y, et al. Real-world patterns of biomarker testing and targeted therapy in de novo metastatic non-small cell lung cancer patients in the US oncology network. Cancer Treat Res Commun 2022; 31: 100522. [DOI] [PubMed] [Google Scholar]
- 9. Robert NJ, Espirito JL, Chen L, et al. Biomarker testing and tissue journey among patients with metastatic non-small cell lung cancer receiving first-line therapy in The US Oncology Network. Lung Cancer 2022; 166: 197–204. [DOI] [PubMed] [Google Scholar]
- 10. Labbé C, Anderson M, Simard S, et al. Wait times for diagnosis and treatment of lung cancer: a single-centre experience. Curr Oncol 2017; 24: 367–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lim C, Tsao MS, Le LW, et al. Biomarker testing and time to treatment decision in patients with advanced nonsmall-cell lung cancer. Ann Oncol 2015; 26: 1415–1421. [DOI] [PubMed] [Google Scholar]
- 12. Kasymjanova G, Small D, Cohen V, et al. Lung cancer care trajectory at a Canadian centre: an evaluation of how wait times affect clinical outcomes. Curr Oncol 2017; 24: 302–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lim C, Sung M, Shepherd FA, et al. Patients with advanced non-small cell lung cancer: are research biopsies a barrier to participation in clinical trials? Journal of Thoracic Oncology 2016; 11: 79–84. [DOI] [PubMed] [Google Scholar]
- 14. Common JL, Mariathas HH, Parsons K, et al. Reducing wait time for lung cancer diagnosis and treatment: impact of a multidisciplinary, centralized referral program. Can Assoc Radiol J 2018; 69: 322–327. [DOI] [PubMed] [Google Scholar]
- 15. Maringe C, Spicer J, Morris M, et al. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: a national, population-based, modelling study. The Lancet Oncology 2020; 21: 1023–1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Leighl NB, Page RD, Raymond VM, et al. Clinical utility of comprehensive cell-free DNA analysis to identify genomic biomarkers in patients with newly diagnosed metastatic non-small cell lung cancer. Clin Cancer Res 2019; 25: 4691–4700. [DOI] [PubMed] [Google Scholar]
- 17. Rolfo C, Mack P, Scagliotti GV, et al. Liquid biopsy for advanced NSCLC: A consensus statement from the International Association for the Study of Lung Cancer. J Thorac Oncol 2021; 16: 1647–1662. [DOI] [PubMed] [Google Scholar]
- 18. Aggarwal C, Thompson JC, Black TA, et al. Clinical implications of plasma-based genotyping with the delivery of personalized therapy in metastatic non-small cell lung cancer. JAMA Oncol 2019; 5: 173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sabari JK, Santini F, Bergagnini I, et al. Changing the therapeutic landscape in non-small cell lung cancers: the evolution of comprehensive molecular profiling improves access to therapy. Curr Oncol Rep 2017; 19: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Juergens RA, Ezeife DA, Laskin JJ, et al. Demonstrating the value of liquid biopsy for lung cancer in a public health care system. J Clin Oncol 2020; 38: 3546. [Google Scholar]
- 21. Ezeife D, Spackman E, Juergens R, et al. OA16.02 the economic value of liquid biopsy for genomic profiling in advanced non-small cell lung cancer. J Thorac Oncol 2021; 16: S876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Makarem M, Leighl NB. Molecular testing for lung adenocarcinoma: is it time to adopt a ‘plasma-first’ approach? Cancer 2020; 126: 3176–3180. [DOI] [PubMed] [Google Scholar]
- 23. Thompson JC, Aggarwal C, Wong J, et al. BRIEF REPORT: plasma genotyping at the time of diagnostic tissue biopsy decreases time to treatment in patients with advanced NSCLC – results from a prospective pilot study. JTO Clin Res Rep 2022; 3: 100301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Cui W, Milner-Watts C, McVeigh TP, et al. A pilot of blood-first diagnostic cell free DNA (cfDNA) next generation sequencing (NGS) in patients with suspected advanced lung cancer. Lung Cancer 2022; 165: 34–42. [DOI] [PubMed] [Google Scholar]
- 25. Perdrizet K, Stockley T, Law JH, et al. Non-small cell lung cancer (NSCLC) next generation sequencing (NGS) using the Oncomine Comprehensive Assay (OCA) v3: integrating expanded genomic sequencing into the Canadian publicly funded health care model. J Clin Oncol 2019; 37: 2620.31408415 [Google Scholar]
- 26. Hwang DM, Albaqer T, Santiago RC, et al. Prevalence and heterogeneity of PD-L1 expression by 22C3 assay in routine population-based and reflexive clinical testing in lung cancer. J Thorac Oncol 2021; 16: 1490–1500. [DOI] [PubMed] [Google Scholar]
- 27. Fiset PO, Labbé C, Young K, et al. Anaplastic lymphoma kinase 5A4 immunohistochemistry as a diagnostic assay in lung cancer: a Canadian reference testing center’s results in population-based reflex testing. Cancer 2019; 125: 4043–4051. [DOI] [PubMed] [Google Scholar]
- 28. Cheung CC, Smith AC, Albadine R, et al. Canadian ROS proto-oncogene 1 study (CROS) for multi-institutional implementation of ROS1 testing in non-small cell lung cancer. Lung Cancer 2021; 160: 127–135. [DOI] [PubMed] [Google Scholar]
- 29. Perdrizet K, Stockley T, Tsao MS, et al. Upfront next generation sequencing in NSCLC: a publicly funded perspective. J Clin Oncol.2018; 36: 12062. [Google Scholar]
- 30. Aggarwal C, Marmarelis ME, Hwang WT, et al. Association of comprehensive molecular genotyping and overall survival in patients with advanced non-squamous non-small cell lung cancer. J Clin Oncol 2022; 40: 9022. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221126151 for Plasma-first: accelerating lung cancer diagnosis and molecular profiling through liquid biopsy by Miguel Garcia-Pardo, Kasia Czarnecka, Jennifer H. Law, Alexandra Salvarrey, Roxanne Fernandes, Jason Fan, Lucy Corke, Thomas K. Waddell, Kazuhiro Yasufuku, Laura L. Donahoe, Andrew Pierre, Lisa W. Le, Noor Ghumman, Geoffrey Liu, Frances A. Shepherd, Penelope Bradbury, Adrian Sacher, Tracy Stockley, Prodipto Pal, Patrik Rogalla, Ming Sound Tsao and Natasha B. Leighl in Therapeutic Advances in Medical Oncology