Abstract
Chaperone-mediated autophagy (CMA) plays an important role in regulating a variety of cellular functions by selectively degrading damaged or functional proteins in the cytoplasm. One of the cellular processes in which CMA participates is the oxidative stress response. Oxidative stress regulates CMA activity, while CMA protects cells from oxidative damage by degrading oxidized proteins and preventing the accumulation of excessive reactive oxygen species (ROS). Changes in CMA activity have been found in many human diseases, and oxidative stress is also involved. Therefore, understanding the interaction mechanism of ROS and CMA will provide new targets for disease treatment. In this review, we discuss the role of CMA in combatting oxidative stress during the development of different conditions, such as aging, neurodegeneration, liver diseases, infections, pulmonary disorders, and cancers.
Keywords: reactive oxygen species, autophagy, neurodegeneration, metabolism, immunity, pulmonary disease, tumor
Introduction
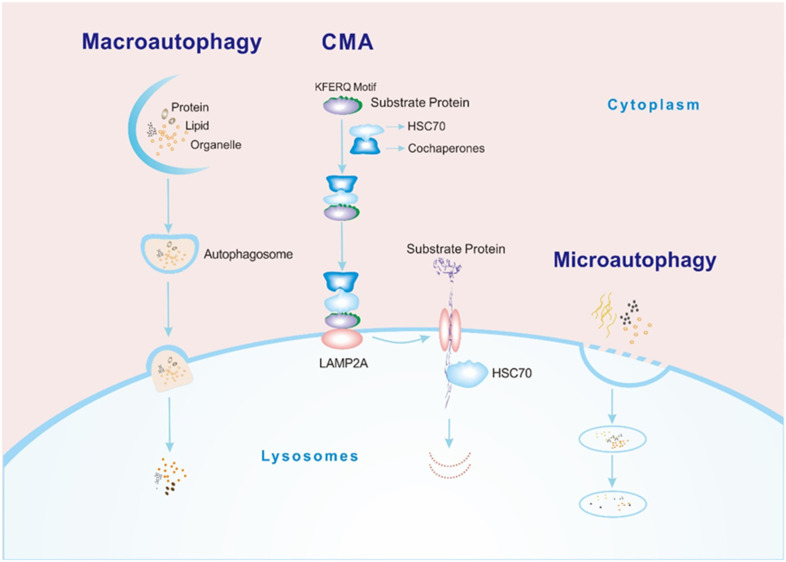
Autophagy is a cellular self-digestion process in which macromolecules, aggregated proteins, damaged organelles, and pathogens are digested by lysosomal hydrolases. This digestion produces nucleotides, amino acids, fatty acids, glucose, and ATP for use by the cell. 1 Based on the method by which degraded cargos enter the lysosome, there are 3 types of autophagy, macroautophagy, microautophagy, and chaperone-mediated autophagy (CMA). During macroautophagy, cytosolic components bind to vesicles to form autophagosomes, which then fuse with the lysosomal membrane. In microautophagy, the lysosomal membrane collapses directly to form vesicles, and the cargos are enveloped in the lysosome. The third protein degradation pathway, called CMA, involves direct crossing of the lysosomal membrane without reliance on vesicles 2 (Figure 1).
Figure 1.
Processes of 3 types of autophagy. Macroautophagy: cargos (such as proteins, lipids, and organelles) combine with vesicles in the cytoplasm to form an autophagosome. The autophagosome fuses with the lysosomal membrane, and then the cargos enter the lysosomal cavity and are degraded by hydrolase. CMA: (1) The HSC70-cochaperone complex recognizes the KFERQ sequence of the substrate protein. (2) The substrate binds to the cytoplasmic segment of LAMP2A on the lysosomal membrane. (3) The LAMP2A monomer polymerizes to form a transport complex, and the substrate adopts an extended conformation and passes through the lysosomal membrane into the lysosomal cavity with the assistance of HSC70 in the lysosomal cavity. (4) The substrate is degraded. Microautophagy: the lysosomal membrane directly invaginates to form vesicles, and the cargos are enveloped within the lysosomal cavity for degradation.
Abbreviations: CMA, chaperone-mediated autophagy; HSC70, heat shock cognate 71 kDa protein; LAMP2A, lysosome-associated membrane protein type 2A.
One of the most prominent features of CMA is that the degraded cargos are limited to proteins, a significant difference from macroautophagy and microautophagy, which transport any type of macromolecule, including glycogen, lipids, proteins, organelles, and even pathogens. Because CMA specifically degrades damaged and nonfunctional proteins, it is necessary for protein quality control and intracellular homeostasis. 3 Another very important feature of CMA is its high selectivity. CMA regulates many physiological processes by selectively degrading key proteins. 4 Therefore, CMA dysfunction may disrupt normal cell function and subsequently lead to the occurrence of human diseases.
One of the cellular processes in which CMA participates is the oxidative stress response. Oxidative stress is a state of imbalance between intracellular oxidation and antioxidation in which the levels of intracellular reactive oxygen species (ROS) increase. ROS are molecules with dual effects: they can regulate normal cell proliferation, differentiation, and apoptosis, 5 but the high concentrations of ROS produced by excessive oxidative stress are toxic and therefore lead to cell death. 6 Studies have shown that CMA and ROS are involved in aging and in many disease processes, including neurodegenerative disease, liver disease, infection, pulmonary disease, and tumor processes, but their interactions are unclear. Therefore, elucidating the molecular mechanisms by which CMA combats oxidative stress will provide new insights into a disease treatment. Here, we summarize the process and physiological functions of CMA and discuss the relationship between CMA and oxidative stress during the occurrence and development of human diseases. We also discuss the potential therapeutic value of CMA.
Mechanisms of CMA
The process of substrate degradation through CMA, which includes recognition, binding, transport, and degradation, is shown in Figure 1. 7 The hallmark of a CMA substrate protein is the presence of the pentapeptide motif KFERQ. 8 The motif is specifically recognized by the molecular chaperone heat shock cognate 71 kDa protein (HSC70), and then, the cargo is transported into lysosomes for degradation with the help of the lysosomal membrane receptor lysosome-associated membrane protein type 2A (LAMP2A).9,10 Pentapeptide motifs are necessary for substrate recognition in CMA, but not all proteins containing pentapeptide motifs are substrates of CMA. The pentapeptide motif is (Q)-(K/R)-(F/V/L/I)-(E/D)-(K/R/F/V/L/I), in which Q can also be at the end, and the order of the other four amino acids can be changed. 11 The identification of CMA substrates based on pentapeptide motifs alone is unreliable, and studies have shown that this type of motif is also a target for microautophagy. 12 Therefore, the gold standard for verifying CMA substrates is a lysosome binding and uptake assay. 13
HSC70 and LAMP2A are two key molecules in the CMA pathway (Figure 1). Molecular chaperones are involved in many processes of CMA, especially HSC70, which is the only chaperone that directly binds to the KFERQ-like motif. Other molecular chaperones form complexes to regulate CMA in an HSC70-dependent manner. 14 In addition to HSC70, LAMP2A is a molecule that transports substrates into the lysosomal lumen during CMA. LAMP2A, as a receptor protein on the lysosomal membrane, usually exists as a monomer and forms a multiprotein translocation complex with other proteins when transporting substrates. 15 After the substrate binds to LAMP2A, it transitions from a folded to an extended formation with the help of HSC70 and other chaperones on the membrane in preparation for subsequent passage through the lysosomal membrane 16 (Figure 1). After the LAMP2A polymer transports the substrate to the lysosomal cavity, it is immediately depolymerized into a monomer with the help of HSC70 to continue to function as a receptor. 15 The content of LAMP2A directly determines the rate of CMA. To date, upregulation or inhibition of LAMP2A expression via transgenic techniques has been the most common means to change the activity of CMA. Oxidative stress, long-term starvation, and other stressors all lead to the activation of CMA by upregulating the level of LAMP2A.17,18 CMA activity can be measured according to the expression of the substrate proteins, LAMP2A or HSC70.19–21
Physiological Roles of CMA
The removal of damaged proteins to maintain protein quality balance is the basic function of CM. In recent years, it has been found that CMA can regulate cellular pathways by selectively degrading key proteins, broadening the physiological processes in which CMA is known to participate.
Amino Acid Recycling, Protein Quality Control, and Response to Stress
CMA shows a certain basal activity in most cells, but it is activated to the maximum extent under conditions of long-term starvation, 22 oxidative stress, 18 hypoxia, 23 and DNA damage. 24 The first identified physiological function of CMA was the promotion of the recycling of amino acids. Under nutrient deficiency, CMA degrades nonessential proteins to supplement the levels of free amino acids in cells and uses them as raw materials to synthesize new proteins for cell use.7,22 In the early stage of starvation, macroautophagy degrades proteins; at the eighth hour of starvation, the level of macroautophagy peaks, and lipid degradation begins. Interestingly, CMA is activated only after 8 h of starvation, and its level is maintained for 3 days. Therefore, the amino acid pool needed for protein synthesis induced by nutritional deficiency lasting more than 8 h may be supplemented mainly by CMA.25–27 In addition, enhancing CMA activity combats oxidative stress and aging to promote cell survival.18,28,29
Regulation of Glucose and Lipid Metabolism
CMA selectively degrades important enzymes in lipid and glucose metabolism to terminate their functions and reduce the activity of the metabolic pathways in which they are involved. 21 One study on a mouse model with liver-specific conditional knockout of LAMP2A has shown that blocking CMA results in hepatic glycogen depletion, increased energy consumption, and changes in glucose homeostasis. During starvation, CMA degrades glycolytic enzymes in the liver at several times the basal rate, which blocks the glycolysis of liver glycogen and prevents subsequent energy shortages in peripheral organs. Lysosomal proteomic analysis has further confirmed that CMA degrades most of the enzymes involved in glycolysis and the tricarboxylic acid cycle, such as fructose-bisphosphate aldolase A (ALDOA), pyruvate kinase PKM (PKM2), malate dehydrogenase 1 (Mdh1), and isocitrate dehydrogenase 1 (Idh1), thus maintaining metabolism under starvation. 21 Similar to the mechanism by which CMA regulates glucose metabolism, proteins involved in lipid metabolism have been identified as CMA substrates. The levels of lipid metabolic enzymes are altered in the livers of LAMP2A-knockout mice, and 30% of these lipid metabolic enzymes are substrates of CMA. Loss of CMA results in reduced degradation of lipogenic enzymes such as glycerol-3-phosphate dehydrogenase 2 (GPD2), long-chain specific acyl-CoA dehydrogenase (ACADL), cytochrome P450 family 27 subfamily A member 1 (CYP27A1), and long-chain-fatty-acid-CoA ligase 1 (ACSL1), as well as reduced lipolysis. 21 In addition, CMA initiates lipolysis by selectively degrading perilipin-2 (PLIN2), perilipin-3 (PLIN3), and perilipin-5 (PLIN5), allowing lipases to access the lipids stored in the center of lipid droplets.30–32 CMA reduces fat production and promotes fat consumption and utilization to maintain cellular lipid homeostasis.
Control of the Cell Cycle
In response to genotoxicity, CMA degrades checkpoint kinase 1 (Chk1) in time to ensure cell cycle progression after DNA repair. In the absence of CMA, Chk1 remains at a high level in the nucleus, resulting in a decrease in the stability of the MRN complex (responsible for signal transduction before DNA repair) and thus destroying the DNA repair mechanism. 24 In addition, CMA controls the cell cycle by indirectly regulating the level of myc proto-oncogene protein (MYC). 33 MYC is a transcription factor that promotes or inhibits the expression of cell cycle regulatory factors through several mechanisms and ultimately stimulates the cell cycle. 34 In fibroblasts, CMA promotes MYC degradation and thus inhibits malignant transformation. 33
Immune Response
The ubiquitin ligase Itch and the calcineurin inhibitor RCAN1 are considered to inhibit T-cell receptor (TCR) signaling. High levels of ITCH and RCAN1 inhibit T-cell proliferation and cytokine secretion. CMA activates T cells by degrading ITCH and RCAN1 to promote the immune response. 35 A study also revealed that CMA regulates the function of antigen-presenting cells (APCs). In that study, the activity of CMA was regulated by changing the expression of LAMP2A or HSC70 in APCs, and analyses of the functions of these cells revealed that overexpression of LAMP2A or HSC70 increased antigen presentation via cytoplasmic MHCII molecules.36,37
Transcriptional Regulation
CMA directly degrades transcription factors to regulate transcriptional programs. The paired box protein Pax-2 (Pax2) is a very important transcription factor for cell proliferation and differentiation. CMA degrades Pax2 to maintain kidney size and growth. 38 In neurons, CMA degrades the transcription factors MEF2A and MEF2D to promote neuronal survival.39,40 CMA also regulates transcription via the degradation of activators or inhibitors of transcription factors. For example, CMA indirectly regulates the transcriptional processes mediated by nuclear factor-κB (NF-κB) through the degradation of NF-κB inhibitor-α (IκBα) during starvation. 41
Regulation of Pluripotency in Stem Cells
Stem cells with self-renewal and unlimited reproductive abilities have great application value in organ regeneration, repair, and disease treatment. Recent studies have shown that CMA regulates mammalian stem cells, including embryonic stem cells (ESCs), hematopoietic stem cells (HSCs), and mesenchymal stem cells (MSCs). ESCs generally maintain low CMA activity. However, CMA is upregulated during differentiation and reaches its highest level in differentiated cells. Activated CMA promotes the differentiation of ESCs and inhibits ESC self-renewal. 42 In contrast, HSC proliferation in LAMP2A-knockout mice is decreased, and HSC self-renewal is impaired, but the inhibition of CMA does not significantly affect HSC differentiation. Therefore, CMA is essential for the activation and self-renewal of HSCs because it controls the quality of metabolic enzymes to maintain normal glycolysis and lipid metabolism. 43 Additionally, the differentiation of rat bone marrow MSCs into osteoblasts has been shown to be mediated by inhibition of CMA. 44 Therefore, the function of CMA in stem cells may be closely related to cell type, and more studies are needed to explore the exact roles of CMA in various stem cells.
Interaction Between ROS and CMA
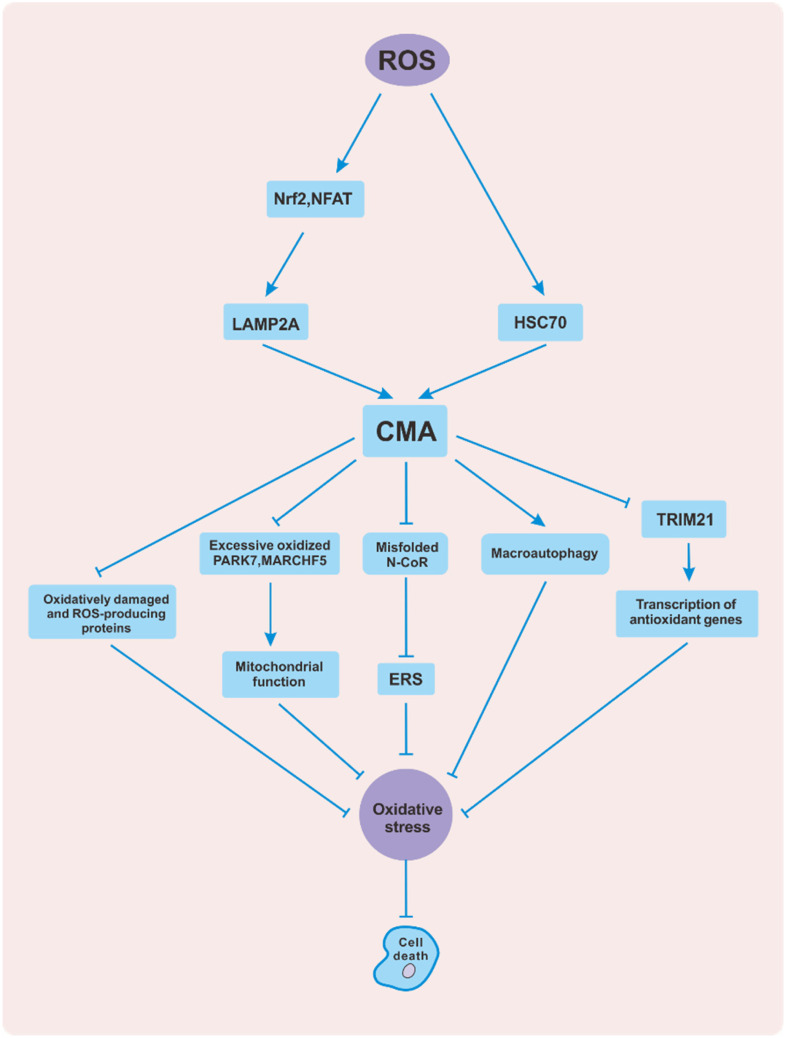
ROS, including peroxides, superoxides, and hydroxyl radicals, are oxygen-containing chemical species with reactive properties. 45 Excessive ROS can lead to the peroxidation of intracellular components such as DNA,46,47 proteins, 48 and lipids, 49 thus destroying cellular homeostasis. CMA is activated during oxidative stress and acts as a damage response. Inhibition of CMA leads to increased ROS levels and cell apoptosis 18 (Figure 2). The mechanisms by which ROS activate CMA mainly include increases in the susceptibility of proteins to degradation by lysosomes and enhancement of the ability of lysosomes to take up substrates. In one study, lysosomes isolated from rat livers were incubated with the CMA substrate protein GAPDH. Lysosome binding and uptake assays showed that the number of proteins bound to the lysosomal membrane and transported into the lysosomal cavity increased with increasing GAPDH oxidation time. These observations reveal that oxidative stress leads to oxidative modification of substrate proteins, which makes the proteins more easily degraded by the CMA pathway. 18 Additionally, oxidative stress stimulates CMA by upregulating the CMA-related proteins LAMP2A and HSC70, whose content can reflect CMA activity and the efficiency of substrate degradation.19,20 Compared with those in control rats, HSC70 in the lysosomal cavity and LAMP2A on the lysosomal membrane in the livers of rats treated with paraquat (an inducer of oxidative stress) are significantly upregulated. 18 In addition, oxidative stress upregulates LAMP2A in the hippocampus of epileptic rats. 50 Further studies have confirmed that ROS activates the T-cell transcription factor nuclear factor of activated T cells (NFAT) to promote LAMP2 gene transcription, consequently increasing both the mRNA and protein levels of LAMP2A. 35 ROS also activate nuclear factor erythroid 2-related factor 2 (Nrf2) to promote the expression of LAMP2A in bronchial epithelial cells. 51 In contrast, the LAMP2A level is reduced in the presence of the antioxidant vitamin E. 50
Figure 2.
Interaction between ROS and CMA. Reactive oxygen species (ROS) activate CMA by promoting the expression of LAMP2A and HSC70. In contrast, CMA defends against oxidative stress in several ways to protect cells from apoptosis. For example, CMA directly scavenges oxidatively damaged and ROS-producing proteins. In addition, CMA indirectly regulates mitochondrial function, ERS, the macroautophagy pathway, and antioxidant gene transcription. Abbreviations: ROS, reactive oxygen species; CMA, chaperone-mediated autophagy; HSC70, heat shock cognate 71 kDa protein; LAMP2A, lysosome-associated membrane protein type 2A; ERS, endoplasmic reticulum stress; NFAT, nuclear factor of activated T cells; Nrf2, nuclear factor erythroid 2-related factor 2; PARK7, parkinsonism associated deglycase; MARCHF5, membrane-associated ring-CH-type finger 5; N-CoR, nuclear receptor corepressor; TRIM21, tripartite motif containing 21.
CMA protects cells from oxidative stress in several ways (Figure 2), the most typical of which is selective scavenging of oxidative and prooxidative proteins. For example, MEF2D and MEF2A are transcription factors related to neuronal survival, and impairment of their function can lead to neuronal death. 52 It has been found that 6-hydroxy dopamine (6-OHDA) or hydrogen peroxide (H2O2) can stimulate neuronal cells to induce oxidative modification of the MEF2A and MEF2D proteins, which causes them to lose their transcriptional activity and play a neurotoxic role. However, oxidative stress upregulates CMA, which can degrade oxidatively damaged and nonfunctional MEF2D and MEF2A to protect neurons from apoptosis.39,53 Both mutant alpha-synuclein (SNCA) and aggregated amyloid β (Aβ) peptides can promote oxidation and induce ROS.54,55 SNCA has been proven to be a substrate of CMA. 56 In addition, studies have shown that overexpression of LAMP2A in neurons protects Drosophila from oxidative stress by upregulating CMA to clear abnormally aggregated SNCA. 54 In addition, in a mouse model of Alzheimer’s disease (AD), CMA has been found to downregulate Aβ plaques and reduce neurotoxicity. 57 Furthermore, LAMP2A overexpression can reduce protein carbonyl content (PCC; used as a measure of total protein oxidation) and inhibit apoptosis in breast cancer cells, but the mechanism is not clear. 58 Another way in which CMA defends against oxidative stress is by regulating the function of mitochondria. Mitochondria are the main sites for the respiratory production of ROS. Mitochondrial impairment leads to an imbalance between oxidation and antioxidation, resulting in oxidative stress. The antioxidant protein Parkinson’s disease (PD) 7 (parkinsonism associated deglycase [PARK7], also known as DJ-1) is essential for mitochondria to maintain their structure and function. 59 Evidence suggests that overoxidation of PARK7 in mouse dopaminergic neurons is harmful and increases mitochondrial fragmentation. CMA promotes the degradation of PARK7 under oxidative stress and protects the function of mitochondria. 60 In vivo and in vitro studies have revealed that CMA inhibits mitochondrial dynamin-1-like protein (DNM1L) through the degradation of membrane-associated ring-CH-type finger 5 (MARCHF5), thus reducing mitochondrial breakage induced by oxidative stress. 61 In addition, CMA reduces the production of ROS induced by endoplasmic reticulum stress (ERS) by degrading misfolded nuclear receptor corepressor (N-CoR).62,63 Overexpression of LAMP2A in Drosophila neurons reduces the accumulation of ROS by increasing macroautophagy. 54 Finally, CMA enhances the antioxidant capacity of cells. Tripartite motif containing 21 (TRIM21) is a ubiquitin E3 ligase that inhibits p62-mediated antioxidant gene transcription and increases cell sensitivity to oxidative stress. 64 The degradation of TRIM21 by CMA during oxidative stress enhances the antioxidant capacity of mouse macrophages and reduces cell death induced by Salmonella Typhimurium. 65
In summary, ROS activates intracellular CMA. Subsequently, as an antioxidant defense mechanism, CMA protects cells from harmful oxidative stress by reducing the levels of oxidative and prooxidative proteins and regulating mitochondrial function, ERS, the macroautophagy pathway, and the antioxidant pathway.
CMA and Oxidative Stress in Human Diseases
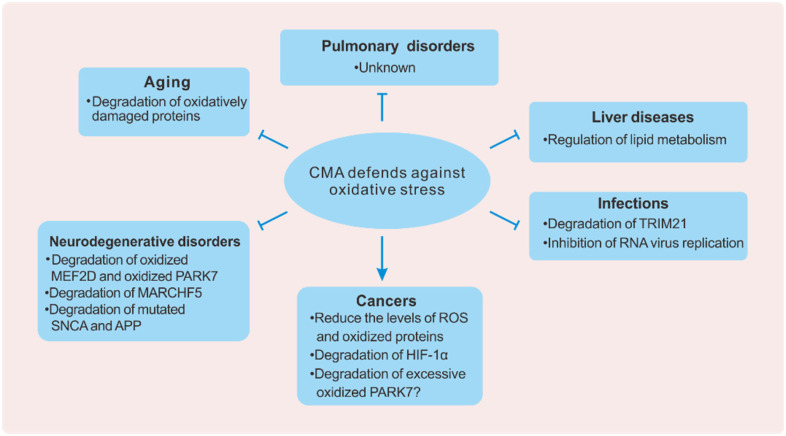
CMA participates in regulating the physiological function of cells, and its dysfunction is closely related to human diseases. A decrease in CMA activity can lead to aging, neurodegenerative diseases, liver diseases, infections, and pulmonary disorders, while upregulation of CMA activity can promote the development of tumors. Oxidative stress is also involved in these diseases and processes. Here, we mainly discuss how CMA defends against oxidative stress under different conditions (Figure 3).
Figure 3.
CMA and oxidative stress in diseases. CMA plays a protective or pathogenic role in different diseases by counteracting oxidative stress. CMA protects organisms against aging, neurodegeneration, liver disease, and infection. However, CMA also promotes the development of cancers. The picture above shows the mechanisms of CMA in specific diseases.
Abbreviations: CMA, chaperone-mediated autophagy; MEF2D, myocyte-specific enhancer factor 2D; PARK7, parkinsonism associated deglycase; MARCHF5, membrane-associated ring-CH-type finger 5; SNCA, alpha-synuclein; APP, amyloid-beta precursor protein; TRIM21, tripartite motif containing 21; HIF-1α, hypoxia-inducible factor 1-alpha.
Aging
One of the characteristics of aging is the increased sensitivity of cells to oxidative stress, which leads to the accumulation of oxidative damage. 66 Decreased CMA activity is observed in the cells and tissues of aged animals.67,68 Age-dependent changes in the properties of the lysosomal membrane disrupt the dynamic balance of LAMP2A in the membrane and cavity, resulting in increased degradation of LAMP2A and reduced LAMP2A content on the membrane, thus reducing CMA efficiency. 68 Therefore, oxidative damage caused by impaired CMA may be one of the pathogenic mechanisms of aging (Figure 3). In general, the activation of the macroautophagy and proteasome pathways can compensate for the impairment of CMA function, but this compensation is blocked by stressors (such as oxidative stress and lipotoxicity). 29 In one study, oxidative stress was induced in paraquat-treated mice. Compared with the control mice, liver-specific LAMP2A-knockout mice showed increased fibrosis and inclusion bodies, but there was no significant difference in young mice. Interestingly, a fluorescent probe injected into the tail vein showed that ROS levels were increased in both old and young LAMP2A-knockout mice, but old LAMP2A-knockout mice were more sensitive to ROS-induced liver damage than young LAMP2A-knockout mice. The inhibition of CMA in young LAMP2A-knockout mice was believed to reduce the degradation of antioxidant enzymes (found to be CMA substrates), promoting ROS clearance, while old LAMP2A-knockout mice had impaired antioxidant enzymes due to disrupted protein homeostasis and were thus more susceptible to oxidative stress. 29 Studies have suggested that oxidized proteins and lipofuscin (mainly from mitochondrial damage, which is considered to be a marker of aging) 69 are decreased after the restoration of CMA activity in the livers of old mice and that the structure of mitochondria becomes closer to that in young mice, indicating that CMA can improve the life span of old animals. 28 These data show the antiaging role of CMA. However, the interaction between CMA and oxidative stress in aging needs to be further explored.
Neurodegenerative Diseases
Various neurodegenerative diseases are related to the failure of the protein degradation system to effectively remove harmful proteins. The decrease in the ability to deal with abnormal proteins changes protein homeostasis and causes the accumulation of neurotoxic proteins, which promotes neuronal death. Oxidative stress destroys the structure and function of neurons and leads to several typical neurodegenerative diseases, such as PD and AD, which are related to oxidative damage to intracellular proteins.53,70 Normally, CMA removes pathogenic proteins to prevent the pathological process of neurodegeneration (Figure 3). Studies have shown that oxidized MEF2D levels are dramatically increased in the brains of PD animals and PD patients. 53 However, high MEF2D activity protects PD animal neurons from toxicity. Further studies have suggested that MEF2D is modified before it is degraded by CMA and that the modified MEF2D is inactive and even harmful to neurons. For example, 6-OHDA is a neurotoxin that induces PD in animals. After stimulating mouse neurons, 6-OHDA promotes the oxidation of MEF2D and decreases MEF2D DNA binding activity. The oxidative modification of MEF2D mediates neuron death induced by oxidative stress. CMA degrades oxidatively damaged MEF2D, thereby hindering the progression of PD. 53 PARK7 is a PD gene that plays a role in combatting oxidative stress. 59 Hyperoxidized and damaged forms of PARK7 have been detected in the brains of PD patients. 71 Downregulation of LAMP2A results in the accumulation of oxidized PARK7, increases in ROS levels, and mitochondrial rupture in mouse dopaminergic neurons. The restoration of CMA reverses these pathological changes. However, PARK7 gene silencing eliminates the protective effect of CMA on neurons, indicating that CMA reduces the toxicity of ROS to cells through PARK7 degradation, thus inhibiting neuronal death in the process of PD. 60 Moreover, PARK7 overexpression not only inhibits rotenone-induced pathological changes in PD neurons but also promotes the expression of LAMP2A. PARK7 may accelerate the degradation of harmful proteins by upregulating CMA. 72 Additionally, abnormal aggregation of mutated SNCA is the most important cause of PD. It has been suggested that the neurotoxicity of SNCA may be mediated in part by oxidative stress. 54 CMA degrades mutant SNCA, reduces ROS levels, and prolongs mouse lifespan. 73
In addition to its role in PD, accumulating evidence shows that the ability of CMA to resist oxidative stress is related to AD. The aggregation of mutated TAU protein and Aβ in the brain is a pathological process of AD. Amyloid precursor protein (APP) is cleaved to form Aβ plaques and the decrease in APP-scavenging ability in AD results in the accumulation of Aβ and APP. Aggregated Aβ induces oxidative stress, which destroys cellular homeostasis and leads to neuronal damage. Meanwhile, the generated ROS promotes the expression and aggregation of Aβ. 74 TAU is a microtubule-associated protein that maintains the stability of the neuronal skeleton. Mutated TAU cannot bind normally to microtubules and is neurotoxic. 75 Activation of the p38 pathway by oxidative stress leads to tau hyperphosphorylation and promotes the expression of RCAN1 to prevent TAU dephosphorylation. 76 Evidence has been reported that both APP and TAU are substrate proteins of CMA.57,77 CMA directly scavenges APP and phosphorylated TAU to protect neurons from oxidative stress and protein toxicity. Additionally, CMA activity in the brains of AD mice and AD patients has been found to be significantly decreased. 70 Further analysis of proteins in the brains of LAMP2A-deficient mice revealed increased levels of ubiquitinated and oxidized proteins, as well as accumulation of TAU and many other KFERQ-like proteins. However, macroautophagy activity is not upregulated in the brains of CMA-deficient mice. The restoration of CMA activity decreases mutant TAU and Aβ plaques and improves learning and memory in AD mice. 70
Defects in CMA have been found in several neurodegenerative diseases.70,78 Most pathological proteins associated with neurodegeneration bind tightly to the LAMP2A receptor on the lysosomal membrane and cannot be transported to the lysosomal cavity, thus destroying the stability of the CMA transport complex.56,79,80 In addition, the increased expression of microRNAs that negatively regulate LAMP2A and HSC70 is also the reason for the decrease in CMA activity. 81 In summary, CMA scavenges pathogenic proteins related to PD and AD to protect neurons from oxidative stress. Considering the pathological function of oxidative stress in many neurodegenerative diseases, 82 restoring CMA activity may be an effective treatment.
Liver Diseases
Nonalcoholic fatty liver disease (NAFLD) is a clinicopathological syndrome characterized by excessive intracellular fat deposition in the liver. NAFLD includes simple fatty liver (SFL), nonalcoholic steatohepatitis (NASH), and liver cirrhosis, which can eventually develop into hepatocellular carcinoma. Lipotoxicity, oxidative stress, and inflammation are involved in these pathological processes and promote disease progression. 83 Most of the proteins involved in lipid metabolism have been identified as substrates of CMA.21,30–32 Activating CMA in the liver reduces the damage to hepatocytes caused by lipotoxicity and oxidative stress (Figure 3). Decreases in LAMP2A and positive regulators of CMA are found in the livers of both NAFLD patients and high-fat diet (HFD)-fed mice, while the negative regulator of CMA is continuously activated during hepatic steatosis. 31 Additionally, dietary fat alters lysosomal membrane components, reducing the stability of LAMP2A at the membrane and leading to increased LAMP2A degradation. 84 LAMP2A-knockout mice fed an HFD have enlarged, pale livers; increased accumulation of low-density lipoprotein, triglycerides, and cholesterol in hepatocytes; and elevated levels of triglycerides and alanine aminotransferase (ALT) in serum. 21 Excessive lipid accumulation leads to increased ROS and oxidative stress. 85 Lipid and protein peroxidation occurs in the liver tissue of NASH mice, which enhances macroautophagy and CMA. These results suggest that metabolic oxidative stress aggravates disease progression and also reduces lipid accumulation by activating CMA. 86 In addition, the incidence of liver tumors is increased in aged LAMP2A-knockout mice, which may be attributed to the fact that the increase in oxidative damage with age promotes malignant transformation in livers with CMA deficiency. 29
In summary, oxidative stress produced by self-metabolism drives NAFLD/NASH progression. CMA protects against oxidative stress by maintaining the balance of lipid metabolism. Therefore, CMA may be a promising therapeutic target for liver diseases.
Infection and Immunity
There is growing evidence that CMA is involved in immune response processes, including the responses to viral 87 and bacterial 65 infections, T-cell function regulation, 35 antigen presentation, 36 and tumor immunity. 88 CMA interacts with oxidative stress and plays an important role in innate immunity and adaptive immunity (Figure 3). Salmonella Typhimurium activates mTORC2/Akt signaling to inhibit CMA activity, resulting in reduced TRIM21 degradation. The increase in TRIM21 damages the antioxidative resistance of cells and promotes the death of macrophages induced by Salmonella Typhimurium. 65 Studies have also revealed that hypericin-based photodynamic therapy (Hyp-PDT) can induce ERS and consequently induce ROS production and activate CMA during tumor therapy. CMA promotes the exposure of calreticulin on the surfaces of tumor cells and leads to immunogenic cell death, which is helpful for stimulating antitumor immunity. 89 In contrast to the function of CMA in tumor cells, CMA in the tumor microenvironment can inhibit the immune response and promote tumor survival. For example, glioblastoma (GB) activates CMA in perivascular cells (PCs) by inducing ROS. CMA inhibits antitumor protein secretion and T-cell activation, resulting in low immune function or immunosuppression. In a GB mouse model, LAMP2A knockout in PCs has been found to promote T-cell infiltration around the tumor and tumor clearance. This evidence suggests that abnormal CMA upregulation in PCs is necessary for the maintenance of GB cell survival and the induction of immune tolerance. 88 Additionally, CMA degrades the negative TCR regulatory factors Rcan1 and Itch to activate CD4+ T cells. Activated CD4+ T cells produce ROS and induce LAMP2A transcription to further enhance CMA activity. In LAMP2A-deficient mice, the spleen and thymus are reduced in size, the percentages of CD4+ T and CD8+ T cells in peripheral blood are decreased, the proliferation of CD4+ T cells and the secretion of cytokines are significantly inhibited, and the serum antibody levels in mice infected with Listeria monocytogenes are decreased. Moreover, CMA activity in mouse and human T cells decreases with age. 35 Therefore, the restoration of CMA activity in T cells in elderly individuals may ameliorate the age-related decline in immunity.
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was found in Wuhan, China, in 2019 and is spreading around the world, causing coronavirus disease 2019 (COVID-19) and posing a serious threat to human health. Because of the protective role of CMA in the antiinfection immune system, activating CMA may effectively prevent SARS-CoV-2 infection. Recent studies have revealed LAMP2A, a key CMA receptor, to be involved in SARS-CoV-2 infection. The host LAMP2A protein acts on SARS-CoV-2 RNA, affecting virus replication. A lack of LAMP2A increases the level of viral RNA, while overexpression of LAMP2A has the opposite result. This finding indicates that LAMP2A has antiviral potential. 90 Furthermore, oxidative stress promotes SARS-CoV-2 replication, 91 and virus-induced oxidative stress may aggravate COVID-19. 92 Considering the antioxidant effect of CMA, we speculate that CMA may be targetable for the treatment of COVID-19.
In summary, CMA functions in the prevention of microbial infection and tumor immunity by regulating immune cell function and resisting oxidative stress.
Pulmonary Disorders
Research on CMA in pulmonary disorders other than lung cancers is very limited. Recently, CMA has been found to be involved in the pathological process of chronic obstructive pulmonary disease (COPD), 51 and oxidative stress also plays an important role in it. 93 COPD is a respiratory disease characterized by persistent airflow limitation, which is related to an abnormal inflammatory response to harmful gases and particles. Cigarette smoke (CS) is the main risk factor for COPD. 94 One study showed that injection of CS extract (CSE) into the trachea of mice leads to emphysema and increased apoptosis of bronchial epithelial cells. However, it also upregulates the expression of CMA-related proteins and activated CMA reduces apoptosis, 95 indicating that CMA plays a protective role in COPD. The mechanism of CMA in COPD was further analyzed. The results showed that CSE induces the expression of LAMP2A in bronchial epithelial cells in an Nrf2-dependent manner, thus enhancing CMA activity. Nrf2 is activated under oxidative stress, and CS is rich in oxidants. LAMP2A overexpression significantly inhibits the unfolded protein response (UPR) and reduces cell death. 51 In addition, the levels of Nrf2 and LAMP2A decrease significantly in COPD lungs compared with nonsmokers and non-COPD smokers, whereas the LAMP2A level is positively correlated with pulmonary function tests. 51 Therefore, restoring CMA activity may be effective in patients with COPD.
Cancers
It is well-accepted that CMA inhibits malignant transformation under physiological conditions but has a pro-oncogenic function in cancer cells. The expression of LAMP2A, a key molecule reflecting the level of CMA, is significantly increased in most human tumors, 96 indicating that the basal activity of CMA in tumors is enhanced. The characteristics of the tumor microenvironment, such as oxidative stress, hypoxia, and nutritional deficiency, are CMA activators.18,22,23 Tumor cells upregulate CMA activity by reducing lysosomal LAMP2A degradation. 97 Crosstalk between macroautophagy and CMA is preserved, and blockade of macroautophagy also activates CMA. 98 Furthermore, the TORC2/AKT1 signaling pathway no longer inhibits CMA in many tumors. 99 Oxidative stress plays a dual role in tumors. ROS regulates signaling pathways that promote tumor formation, but high ROS levels are also harmful to cells. In cancer cells, CMA combats oxidative stress to promote cancer survival (Figure 3). However, the interaction mechanism of CMA and ROS in cancers remains to be explored.
Our previous study on gastric cancer revealed that the antioxidant protein PARK7 may be a substrate of CMA. 96 PARK7 is a protein that regulates cellular oxidative stress. In tumor cells, moderate oxidation of PARK7 maintains cell activity by activating autophagy, while excessive oxidation of PARK7 promotes apoptosis by activating p38. 100 Considering the pro-oncogenic effect of CMA in tumors, excessive oxidized PARK7 is believed to be a CMA substrate. CMA may promote cell survival by scavenging excessive oxidized PARK7 in response to oxidative stress. This possibility is supported by a recent study showing that CMA degrades oxidatively damaged PARK7 in neurons and protects mitochondrial function. 60 Additionally, LAMP2A levels are upregulated in the tumor tissue of patients with breast cancer, which is beneficial for tumor survival under oxidative stress. In vitro experiments have shown that the overexpression of LAMP2A under stimulation with H2O2 decreases the content of oxidized protein and protects breast cancer cells from oxidative damage, while CMA inhibition results in increased ROS production and apoptosis. 58 In nonsmall-cell lung cancer (NSCLC), CMA reduces ERS-induced apoptosis by degrading misfolded N-CoR. 62 ERS releases calcium ions and triggers the production of ROS, which further activates the apoptosis signaling pathway and causes cell death. 63 Furthermore, hypoxia damages the electron transport chain of mitochondria and induces mitochondrial ROS production, which leads to the accumulation of hypoxia-inducible factor-1α (HIF-1α). 101 HIF-1 is a transcription factor that mediates the adaptive response to hypoxia. As a negative regulator of DNA replication, the HIF-1α subunit plays a nontranscriptional role by activating helicases. HIF-1α is a CMA substrate in many tumors. CMA degrades HIF-1α to maintain the cell cycle and proliferation under hypoxia.23,102
The function of CMA in untransformed cells is opposite to its function in tumor cells: CMA has an antitumor effect under physiological conditions. CMA prevents malignant transformation through many mechanisms, such as maintaining genomic stability, 24 reducing the levels of cancer-promoting proteins, inhibiting tumor-promoting signals,33,103 promoting immunogenic apoptosis of tumor cells, 104 and combating metabolic oxidative stress.31,84,86 As previously described, CMA attenuates the damage to hepatocytes caused by oxidative stress, thus preventing the occurrence of hepatocellular carcinoma.
Therefore, most of the evidence supports the ability of CMA to resist oxidative stress to promote tumor development or inhibit tumor occurrence in nontransformed cells. Modulating CMA activity according to the different functions of CMA in various cell types will be beneficial for the treatment of cancers.
Targeting CMA and ROS in the Treatment of Diseases
CMA activity is abnormal (downregulated or upregulated) in many human diseases. The common result is that CMA dysfunction destroys the physiological function of cells and leads to the occurrence of disease. The restoration of normal CMA activity can be an effective method to prevent or treat diseases. Oxidative stress is involved in the pathogenesis of diseases in complex roles. Therefore, targeting CMA and ROS is a very promising treatment strategy (Figure 3).
Aging
The efficiency of CMA decreases with age.67,68 Gene intervention to restore CMA function in the livers of aged mice can significantly improve age-related changes in this organ. Overexpression of LAMP2A enhances the ability to combat oxidative stress in the aging liver and helps to maintain protein homeostasis. 28 Since neurodegeneration, liver metabolic disorders, immune decline, and tumors all occur with age, interventions to improve the function of CMA are important for the prevention of aging-related diseases.
Neurodegenerative Diseases
As mentioned above, LAMP2A overexpression to enhance CMA activity can protect neuronal function and improve related symptoms in AD and PD animal models. Activating CMA may have a beneficial effect on neurodegenerative diseases. A CMA-specific small molecule activator (retinoic acid derivative) has been developed to effectively protect cells from oxidative stress and protein toxicity. 105 Recent studies have reported that the retinoic acid receptor alpha antagonist AR7 inhibits the accumulation of SNCA oligomers in the brains of AD mice and increases the clearance rate of defective proteins, thus ameliorating the pathology of AD. 106 In addition, therapeutic measures related to antioxidative stress have also made progress in neurodegenerative disease treatment. Tacrine is a cholinesterase inhibitor, and its therapeutic effect in AD is partly achieved through the inhibition of Aβ aggregation to attenuate oxidative stress. A series of multifunctional hybrids, including tacrine and antioxidants, have been used in AD. Clioquinol and its derivatives and flavonoids also exert ameliorative effects in AD and/or PD by antagonizing oxidative stress. 107 In addition, overexpression of the antioxidant protein DJ-1 significantly reduces neuronal damage in PD. 72 However, the effects of antioxidants in several epidemiological experiments have not been ideal. 108 Therefore, targeting CMA may be a more beneficial treatment than the administration of antioxidants alone because the latter can inhibit the function of CMA.
Liver Diseases
CMA is important for maintaining lipid balance in hepatocytes. 31 As described earlier, mice with CMA blockage are more likely to develop steatosis in their livers. 21 It has been reported that interfering with the expression of negative regulatory factors of CMA upregulates the level of LAMP2A, leading to the activation of CMA, which reduces liver inflammation and fibrosis in NASH mice. 86 The CMA activity in the liver of patients with NAFLD is decreased. 31 Accordingly, the restoration of normal CMA activity may be a potential target for NAFLD and NASH therapy.
Infection and Immunity
Several studies have shown that CMA in host cells can effectively inhibit virus replication and enhance the body’s resistance to viral infection.90,109,110 In the process of bacterial infection, LAMP2A-deficient macrophages and T cells are more vulnerable to pathogen damage, and the cellular immune response is impaired.35,65 LAMP2A overexpression promotes defense against infection in mice by activating CMA. 35 In conclusion, CMA has the dual functions of interfering with pathogens and improving immunization levels and activating CMA may have potential as a new therapeutic approach.
Pulmonary Disorders
CMA plays a protective role in COPD, 51 but the relationship between CMA and other pulmonary disorders remains to be further studied. CMA activation significantly reduces the death of bronchial epithelial cells in COPD mice. 95 In addition, antioxidants show great potential in the treatment of COPD. 111 Considering the role of CMA in combatting oxidative stress, targeting CMA in combination with antioxidants may be more effective.
Cancers
The blockade of CMA in various cancers via transgenic techniques has been found to inhibit tumor cell proliferation and growth and to increase sensitivity to radiotherapy and chemotherapy,96,112,113 supporting the idea that targeting CMA is a potential therapeutic strategy for cancer. Several clinical trials have shown that hydroxychloroquine (HCQ) exerts a therapeutic effect by inhibiting the autophagic flux of tumor cells. In addition to affecting autophagic activity, HCQ increases the pH of the lysosomal cavity and destroys the stability of HSC70, thus impairing CMA. 114 However, the extent to which CMA participates in the antitumor effect of HCQ remains unclear. Considering the complex function of oxidative stress in tumors, both ROS scavengers and activators may interfere with normal intracellular ROS levels and lead to tumor cell death. Several antioxidant nutrients, such as vitamin D, 115 epigallocatechin gallate, 116 and genistein, 117 have been shown to inhibit tumor stem cells. Similarly, various chemotherapeutic drugs (such as 5-FU, cisplatin, and doxorubicin)118–120 and radiotherapy 121 can kill tumor cells by inducing oxidative stress. Therefore, combination therapy targeting oxidative stress and CMA may have advantages.
Conclusions and Perspectives
Due to the ability of CMA to degrade oxidatively damaged proteins and functional proteins, it has been gradually revealed that CMA regulates a variety of physiological functions in mammalian cells. Maintaining normal CMA activity is very important for health maintenance. We found that there is a complex interaction between CMA and ROS (Figure 2). These 2 factors are involved in aging and the pathogeneses of neurodegenerative diseases, liver diseases, infections, pulmonary disorders, and cancers (Figure 3). Therefore, elucidating the molecular mechanism by which CMA counteracts oxidative stress will provide new targets for treatment.
There is a lack of specific inhibitors of CMA, and the existing chemical regulator HCQ, which inhibits CMA activity, also affects other types of autophagy. Furthermore, CMA has been studied only in vitro thus far. The recent development of a transgenic reporting mouse makes it possible to monitor CMA activity dynamically in vivo with image-based procedures. This will enable better evaluation of the in vivo effects of CMA modulators and the development of more drugs for clinical use.
CMA is activated during oxidative stress as an antiinjury response. Excessive ROS damage lipids, proteins, and nucleic acids, which can disrupt cellular function, so measures related to antioxidative stress are effective in preventing disease progression. Although many antioxidants have been used for the treatment of AD, their effects have been proven to be unsatisfactory in several epidemiological studies. Considering the negative regulation of CMA by antioxidants, inhibition of CMA activity may contribute to the failure of antioxidant therapy. Due to the different functions of CMA and ROS in different disease types, combination therapies that simultaneously regulate CMA and ROS to address specific diseases will likely be more effective than monotherapies.
Acknowledgments
The authors thank Dr. Renlong Li and Dr. Tianyu Cao for their suggestions for this article.
Abbreviations
- Aβ
amyloid β
- ACADL
long-chain specific acyl-CoA dehydrogenase
- ACSL1
long-chain-fatty-acid-CoA ligase 1
- AD
Alzheimer’s disease
- ALDOA
fructose-bisphosphate aldolase A
- ALT
alanine aminotransferase
- APC
antigen-presenting cell
- Chk1
checkpoint kinase 1
- CMA
chaperone-mediated autophagy
- COPD
chronic obstructive pulmonary disease
- COVID-19
coronavirus disease 2019
- CS
cigarette smoke
- CSE
CS extract
- CYP27A1
cytochrome P450 family 27 subfamily A member 1
- DNM1L
dynamin-1-like protein
- ERS
endoplasmic reticulum stress
- ESC
embryonic stem cell
- GB
glioblastoma
- GPD2
glycerol-3-phosphate dehydrogenase 2
- HCQ
hydroxychloroquine
- HFD
high-fat diet
- H2O2
hydrogen peroxide
- HSC70
heat shock cognate 71 kDa protein
- HSC
hematopoietic stem cell
- Hyp-PDT
hypericin-based photodynamic therapy
- Idh1
isocitrate dehydrogenase 1
- IκBα
NF-κB inhibitor-α
- LAMP2A
lysosome-associated membrane protein type 2A
- MARCHF5
membrane-associated ring-CH-type finger 5
- Mdh1
malate dehydrogenase 1
- MEF2D
myocyte-specific enhancer factor 2D
- MSC
mesenchymal stem cell
- MYC
myc proto-oncogene protein
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- N-CoR
nuclear receptor corepressor
- NFAT
nuclear factor of activated T cells
- NF-κB
nuclear factor-κB
- Nrf2
nuclear factor erythroid 2-related factor 2
- NSCLC
nonsmall-cell lung cancer
- 6-OHDA
6-hydroxy dopamine
- PARK7
parkinsonism associated deglycase
- Pax2
paired box protein Pax-2
- PCC
protein carbonyl content
- PC
perivascular cell
- PD
Parkinson’s disease
- PKM2
pyruvate kinase PKM
- PLIN2
perilipin-2
- PLIN3
perilipin-3
- PLIN5
perilipin-5
- ROS
reactive oxygen species
- SARS-CoV-2
severe acute respiratory syndrome coronavirus 2
- SFL
simple fatty liver
- SNCA
alpha-synuclein
- TCR
T-cell receptor
- TRIM21
tripartite motif containing 21
Footnotes
Authors’ Note: Our study did not require ethical board approval because it did not involve human or animal trials.
Ethics Statement: Our study did not require an ethical board approval because it did not contain human or animal trials.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Guangxi Key Research and Development Project (No. AB20117001), the State Project for Essential Drug Research and Development (No. 2019ZX09301132), the Scientific and Technological Innovation Major Base of Guangxi (No. 2018-15-Z04), and the National Natural Science Foundation of China (31601123).
ORCID iD: Shuangshuang Le https://orcid.org/0000-0003-3431-8458
References
- 1.Kitada M, Koya D. Autophagy in metabolic disease and ageing. Nat Rev Endocrinol. 2021;17(11):647‐661. doi: 10.1038/s41574-021-00551-9. [DOI] [PubMed] [Google Scholar]
- 2.Kaushik S, Cuervo AM. Chaperone-mediated autophagy: a unique way to enter the lysosome world. Trends Cell Biol. 2012;22(8):407‐417. doi: 10.1016/j.tcb.2012.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arias E, Cuervo AM. Chaperone-mediated autophagy in protein quality control. Curr Opin Cell Biol. 2011;23(2):184‐189. doi: 10.1016/j.ceb.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaushik S, Cuervo AM. The coming of age of chaperone-mediated autophagy. Nat Rev Mol Cell Biol. 2018;19(6):365‐381. doi: 10.1038/s41580-018-0001-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sies H, Jones DP. Reactive oxygen species (ROS) as pleiotropic physiological signalling agents. Nat Rev Mol Cell Biol. 2020;21(7):363‐383. doi: 10.1038/s41580-020-0230-3. [DOI] [PubMed] [Google Scholar]
- 6.Vaccaro A, Kaplan Dor Y, Nambara K, et al. Sleep loss can cause death through accumulation of reactive oxygen species in the gut. Cell. 2020;181(6):1307‐1328. e15. doi: 10.1016/j.cell.2020.04.049. [DOI] [PubMed] [Google Scholar]
- 7.Cuervo AM, Wong E. Chaperone-mediated autophagy: roles in disease and aging. Cell Res. 2014;24(1):92‐104. doi: 10.1038/cr.2013.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cuervo AM, Gomes AV, Barnes JA, Dice JF. Selective degradation of annexins by chaperone-mediated autophagy. J Biol Chem. 2000;275(43):33329‐33335. doi: 10.1074/jbc.M005655200. [DOI] [PubMed] [Google Scholar]
- 9.Franch HA. Chaperone-mediated autophagy in the kidney: the road more traveled. Semin Nephrol. 2014;34(1):72‐83. doi: 10.1016/j.semnephrol.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hayes SA, Dice JF. Roles of molecular chaperones in protein degradation. J Cell Biol. 1996;132(3):255‐258. doi: 10.1083/jcb.132.3.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dice JF. Peptide sequences that target cytosolic proteins for lysosomal proteolysis. Trends Biochem Sci. 1990;15(8):305‐309. doi: 10.1016/0968-0004(90)90019-8. [DOI] [PubMed] [Google Scholar]
- 12.Sahu R, Kaushik S, Clement CC, et al. Microautophagy of cytosolic proteins by late endosomes. Dev Cell. 2011;20(1):131‐139. doi: 10.1016/j.devcel.2010.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaushik S, Cuervo AM. Chapter 19 methods to monitor chaperone-mediated autophagy. 2009;452:297‐324. doi: 10.1016/s0076-6879(08)03619-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Agarraberes FA, Dice JF. A molecular chaperone complex at the lysosomal membrane is required for protein translocation. J Cell Sci. 2001;114(Pt 13):2491‐2499. doi: 10.1242/jcs.114.13.2491. [DOI] [PubMed] [Google Scholar]
- 15.Bandyopadhyay U, Kaushik S, Varticovski L, Cuervo AM. The chaperone-mediated autophagy receptor organizes in dynamic protein complexes at the lysosomal membrane. Mol Cell Biol. 2008;28(18):5747‐5763. doi: 10.1128/MCB.02070-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salvador N, Aguado C, Horst M, Knecht E. Import of a cytosolic protein into lysosomes by chaperone-mediated autophagy depends on its folding state. J Biol Chem. 2000;275(35):27447‐27456. doi: 10.1074/jbc.M001394200. [DOI] [PubMed] [Google Scholar]
- 17.Li CL, Wei HL, Chen J, et al. Ebb-and-flow of macroautophagy and chaperone-mediated autophagy in Raji cells induced by starvation and arsenic trioxide. Asian Pac J Cancer Prev. 2014;15(14):5715‐5719. doi: 10.7314/apjcp.2014.15.14.5715. [DOI] [PubMed] [Google Scholar]
- 18.Kiffin R, Christian C, Knecht E, Cuervo AM. Activation of chaperone-mediated autophagy during oxidative stress. Mol Biol Cell. 2004;15(11):4829‐4840. doi: 10.1091/mbc.e04-06-0477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cuervo AM, Dice JF. A receptor for the selective uptake and degradation of proteins by lysosomes. Science. 1996;273(5274):501‐503. doi: 10.1126/science.273.5274.501. [DOI] [PubMed] [Google Scholar]
- 20.Agarraberes FA, Terlecky SR, Dice JF. An intralysosomal hsp70 is required for a selective pathway of lysosomal protein degradation. J Cell Biol. 1997;137(4):825‐834. doi: 10.1083/jcb.137.4.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schneider JL, Suh Y, Cuervo AM. Deficient chaperone-mediated autophagy in liver leads to metabolic dysregulation. Cell Metab. 2014;20(3):417‐432. doi: 10.1016/j.cmet.2014.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cuervo AM, Knecht E, Terlecky SR, Dice JF. Activation of a selective pathway of lysosomal proteolysis in rat liver by prolonged starvation. Am J Physiol. 1995;269(5 Pt 1):C1200‐8. doi: 10.1152/ajpcell.1995.269.5.C1200. [DOI] [PubMed] [Google Scholar]
- 23.Hubbi ME, Hu H, Kshitiz AI, Levchenko A, Semenza GL. Chaperone-mediated autophagy targets hypoxia-inducible factor-1alpha (HIF-1alpha) for lysosomal degradation. J Biol Chem. 2013;288(15):10703‐10714. doi: 10.1074/jbc.M112.414771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Park C, Suh Y, Cuervo AM. Regulated degradation of Chk1 by chaperone-mediated autophagy in response to DNA damage. Nat Commun. 2015;6:6823. doi: 10.1038/ncomms7823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Singh R, Kaushik S, Wang Y, et al. Autophagy regulates lipid metabolism. Nature. 2009;458(7242):1131‐1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mizushima N, Yamamoto A, Matsui M, Yoshimori T, Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Mol Biol Cell. 2004;15(3):1101‐1111. doi: 10.1091/mbc.e03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Massey AC, Kaushik S, Sovak G, Kiffin R, Cuervo AM. Consequences of the selective blockage of chaperone-mediated autophagy. Proc Natl Acad Sci U S A. 2006;103(15):5805‐5810. doi: 10.1073/pnas.0507436103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C, Cuervo AM. Restoration of chaperone-mediated autophagy in aging liver improves cellular maintenance and hepatic function. Nat Med. 2008;14(9):959‐965. doi: 10.1038/nm.1851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schneider JL, Villarroya J, Diaz-Carretero A, et al. Loss of hepatic chaperone-mediated autophagy accelerates proteostasis failure in aging. Aging Cell. 2015;14(2):249‐264. doi: 10.1111/acel.12310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kaushik S, Cuervo AM. Degradation of lipid droplet-associated proteins by chaperone-mediated autophagy facilitates lipolysis. Nat Cell Biol. 2015;17(6):759‐770. doi: 10.1038/ncb3166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma SY, Sun KS, Zhang M, et al. Disruption of Plin5 degradation by CMA causes lipid homeostasis imbalance in NAFLD. Liver Int. 2020;40(10):2427‐2438. doi: 10.1111/liv.14492. [DOI] [PubMed] [Google Scholar]
- 32.Zhang T, Liu J, Tong Q, Lin L. SIRT3 acts as a positive autophagy regulator to promote lipid mobilization in adipocytes via activating AMPK. Int J Mol Sci. 2020;21(2). doi: 10.3390/ijms21020372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomes LR, Menck CFM, Cuervo AM. Chaperone-mediated autophagy prevents cellular transformation by regulating MYC proteasomal degradation. Autophagy. 2017;13(5):928‐940. doi: 10.1080/15548627.2017.1293767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia-Gutierrez L, Delgado MD, Leon J. MYC oncogene contributions to release of cell cycle brakes. Genes (Basel). 2019;10(3). doi: 10.3390/genes10030244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Valdor R, Mocholi E, Botbol Y, et al. Chaperone-mediated autophagy regulates T cell responses through targeted degradation of negative regulators of T cell activation. Nat Immunol. 2014;15(11):1046‐1054. doi: 10.1038/ni.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhou D, Li P, Lin Y, et al. Lamp-2a facilitates MHC class II presentation of cytoplasmic antigens. Immunity. 2005;22(5):571‐581. doi: 10.1016/j.immuni.2005.03.009. [DOI] [PubMed] [Google Scholar]
- 37.Bonam SR, Ruff M, Muller S. HSPA8/HSC70 in immune disorders: a molecular rheostat that adjusts chaperone-mediated autophagy substrates. Cells. 2019;8(8). doi: 10.3390/cells8080849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franch HA, Sooparb S, Du J, Brown NS. A mechanism regulating proteolysis of specific proteins during renal tubular cell growth. J Biol Chem. 2001;276(22):19126‐19131. doi: 10.1074/jbc.M101777200. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Sun Y, Fei M, et al. Disruption of chaperone-mediated autophagy-dependent degradation of MEF2A by oxidative stress-induced lysosome destabilization. Autophagy. 2014;10(6):1015‐1035. doi: 10.4161/auto.28477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang Q, She H, Gearing M, et al. Regulation of neuronal survival factor MEF2D by chaperone-mediated autophagy. Science. 2009;323(5910):124‐127. doi: 10.1126/science.1166088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cuervo AM, Hu W, Lim B, Dice JF. Ikappab is a substrate for a selective pathway of lysosomal proteolysis. Mol Biol Cell. 1998;9(8):1995‐2010. doi: 10.1091/mbc.9.8.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu Y, Zhang Y, Garcia-Canaveras JC, et al. Chaperone-mediated autophagy regulates the pluripotency of embryonic stem cells. Science. 2020;369(6502):397‐403. doi: 10.1126/science.abb4467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dong S, Wang Q, Kao YR, et al. Chaperone-mediated autophagy sustains haematopoietic stem-cell function. Nature. 2021;591(7848):117‐123. doi: 10.1038/s41586-020-03129-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.He Q, Qin R, Glowacki J, et al. Synergistic stimulation of osteoblast differentiation of rat mesenchymal stem cells by leptin and 25(OH)D3 is mediated by inhibition of chaperone-mediated autophagy. Stem Cell Res Ther. 2021;12(1):557. doi: 10.1186/s13287-021-02623-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gorrini C, Harris IS, Mak TW. Modulation of oxidative stress as an anticancer strategy. Nat Rev Drug Discov. 2013;12(12):931‐947. doi: 10.1038/nrd4002. [DOI] [PubMed] [Google Scholar]
- 46.Yuan LQ, Wang C, Lu DF, Zhao XD, Tan LH, Chen X. Induction of apoptosis and ferroptosis by a tumor suppressing magnetic field through ROS-mediated DNA damage. Aging (Albany NY). 2020;12(4):3662‐3681. doi: 10.18632/aging.102836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ahmad MI, Zafeer MF, Javed M, Ahmad M. Pendimethalin-induced oxidative stress, DNA damage and activation of anti-inflammatory and apoptotic markers in male rats. Sci Rep. 2018;8(1):17139. doi: 10.1038/s41598-018-35484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cafe SL, Nixon B, Dun MD, Roman SD, Bernstein IR, Bromfield EG. Oxidative stress dysregulates protein homeostasis within the male germ line. Antioxid Redox Signal. 2020;32(8):487‐503. doi: 10.1089/ars.2019.7832. [DOI] [PubMed] [Google Scholar]
- 49.Choromanska B, Mysliwiec P, Kozlowski T, et al. Antioxidant barrier and oxidative damage to proteins, lipids, and DNA/RNA in adrenal tumor patients. Oxid Med Cell Longev. 2021;2021:5543531. doi: 10.1155/2021/5543531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cao L, Chen R, Xu J, Lin Y, Wang R, Chi Z. Vitamin E inhibits activated chaperone-mediated autophagy in rats with status epilepticus. Neuroscience. 2009;161(1):73‐77. doi: 10.1016/j.neuroscience.2009.02.059. [DOI] [PubMed] [Google Scholar]
- 51.Hosaka Y, Araya J, Fujita Y, et al. Chaperone-mediated autophagy suppresses apoptosis via regulation of the unfolded protein response during chronic obstructive pulmonary disease pathogenesis. J Immunol. 2020;205(5):1256‐1267. doi: 10.4049/jimmunol.2000132. [DOI] [PubMed] [Google Scholar]
- 52.Li M, Linseman DA, Allen MP, et al. Myocyte enhancer factor 2A and 2D undergo phosphorylation and caspase-mediated degradation during apoptosis of rat cerebellar granule neurons. J Neurosci. 2001;21(17):6544‐6552. doi: 10.1523/JNEUROSCI.21-17-06544.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gao L, She H, Li W, et al. Oxidation of survival factor MEF2D in neuronal death and Parkinson's disease. Antioxid Redox Signal. 2014;20(18):2936‐2948. doi: 10.1089/ars.2013.5399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Issa AR, Sun J, Petitgas C, et al. The lysosomal membrane protein LAMP2A promotes autophagic flux and prevents SNCA-induced Parkinson disease-like symptoms in the Drosophila brain. Autophagy. 2018;14(11):1898‐1910. doi: 10.1080/15548627.2018.1491489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zhou WW, Lu S, Su YJ, et al. Decreasing oxidative stress and neuroinflammation with a multifunctional peptide rescues memory deficits in mice with Alzheimer disease. Free Radic Biol Med. 2014;74:50‐63. doi: 10.1016/j.freeradbiomed.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 56.Cuervo AM, Stefanis L, Fredenburg R, Lansbury PT, Sulzer D. Impaired degradation of mutant alpha-synuclein by chaperone-mediated autophagy. Science. 2004;305(5688):1292‐1295. doi: 10.1126/science.1101738. [DOI] [PubMed] [Google Scholar]
- 57.Xu X, Sun Y, Cen X, et al. Metformin activates chaperone-mediated autophagy and improves disease pathologies in an Alzheimer disease mouse model. Protein Cell. 2021. doi: 10.1007/s13238-021-00858-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Saha T. LAMP2A Overexpression in breast tumors promotes cancer cell survival via chaperone-mediated autophagy. Autophagy. 2012;8(11):1643‐1656. doi: 10.4161/auto.21654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McCoy MK, Cookson MR. DJ-1 regulation of mitochondrial function and autophagy through oxidative stress. Autophagy. 2011;7(5):531‐532. doi: 10.4161/auto.7.5.14684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang B, Cai Z, Tao K, et al. Essential control of mitochondrial morphology and function by chaperone-mediated autophagy through degradation of PARK7. Autophagy. 2016;12(8):1215‐1228. doi: 10.1080/15548627.2016.1179401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nie T, Tao K, Zhu L, et al. Chaperone-mediated autophagy controls the turnover of E3 ubiquitin ligase MARCHF5 and regulates mitochondrial dynamics. Autophagy. 2021;17(10):2923‐2938. doi: 10.1080/15548627.2020.1848128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ali AB, Nin DS, Tam J, Khan M. Role of chaperone mediated autophagy (CMA) in the degradation of misfolded N-CoR protein in non-small cell lung cancer (NSCLC) cells. PLoS One. 2011;6(9):e25268. doi: 10.1371/journal.pone.0025268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hung JY, Hsu YL, Ni WC, et al. Oxidative and endoplasmic reticulum stress signaling are involved in dehydrocostuslactone-mediated apoptosis in human non-small cell lung cancer cells. Lung Cancer. 2010;68(3):355‐365. doi: 10.1016/j.lungcan.2009.07.017. [DOI] [PubMed] [Google Scholar]
- 64.Pan JA, Sun Y, Jiang YP, et al. TRIM21 ubiquitylates SQSTM1/p62 and suppresses protein sequestration to regulate redox homeostasis. Mol Cell. 2016;61(5):720‐733. doi: 10.1016/j.molcel.2016.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hos NJ, Fischer J, Hos D, et al. TRIM21 is targeted for chaperone-mediated autophagy during Salmonella Typhimurium infection. J Immunol. 2020;205(9):2456‐2467. doi: 10.4049/jimmunol.2000048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Harman D. Aging: a theory based on free radical and radiation chemistry. J Gerontol. 1956;11(3):298‐300. doi: 10.1093/geronj/11.3.298. [DOI] [PubMed] [Google Scholar]
- 67.Cuervo AM, Dice JF. Age-related decline in chaperone-mediated autophagy. J Biol Chem. 2000;275(40):31505‐31513. doi: 10.1074/jbc.M002102200. [DOI] [PubMed] [Google Scholar]
- 68.Kiffin R, Kaushik S, Zeng M, et al. Altered dynamics of the lysosomal receptor for chaperone-mediated autophagy with age. J Cell Sci. 2007;120(Pt 5):782‐791. doi: 10.1242/jcs.001073. [DOI] [PubMed] [Google Scholar]
- 69.Brunk UT, Terman A. Lipofuscin: mechanisms of age-related accumulation and influence on cell function. Free Radic Biol Med. 2002;33(5):611‐619. doi: 10.1016/s0891-5849(02)00959-0. [DOI] [PubMed] [Google Scholar]
- 70.Bourdenx M, Martin-Segura A, Scrivo A, et al. Chaperone-mediated autophagy prevents collapse of the neuronal metastable proteome. Cell. 2021;184(10):2696‐2714. e25. doi: 10.1016/j.cell.2021.03.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choi J, Sullards MC, Olzmann JA, et al. Oxidative damage of DJ-1 is linked to sporadic Parkinson and Alzheimer diseases. J Biol Chem. 2006;281(16):10816‐10824. doi: 10.1074/jbc.M509079200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.De Miranda BR, Rocha EM, Bai Q, et al. Astrocyte-specific DJ-1 overexpression protects against rotenone-induced neurotoxicity in a rat model of Parkinson's disease. Neurobiol Dis. 2018;115:101‐114. doi: 10.1016/j.nbd.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gan L, Vargas MR, Johnson DA, Johnson JA. Astrocyte-specific overexpression of Nrf2 delays motor pathology and synuclein aggregation throughout the CNS in the alpha-synuclein mutant (A53T) mouse model. J Neurosci. 2012;32(49):17775‐17787. doi: 10.1523/JNEUROSCI.3049-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jiang T, Sun Q, Chen S. Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson's disease and Alzheimer's disease. Prog Neurobiol. 2016;147:1‐19. doi: 10.1016/j.pneurobio.2016.07.005. [DOI] [PubMed] [Google Scholar]
- 75.Rane JS, Kumari A, Panda D. An acetylation mimicking mutation, K274Q, in tau imparts neurotoxicity by enhancing tau aggregation and inhibiting tubulin polymerization. Biochem J. 2019;476(10):1401‐1417. doi: 10.1042/BCJ20190042. [DOI] [PubMed] [Google Scholar]
- 76.Lloret A, Badia MC, Giraldo E, et al. Amyloid-beta toxicity and tau hyperphosphorylation are linked via RCAN1 in Alzheimer's disease. J Alzheimers Dis. 2011;27(4):701‐709. doi: 10.3233/JAD-2011-110890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Caballero B, Bourdenx M, Luengo E, et al. Acetylated tau inhibits chaperone-mediated autophagy and promotes tau pathology propagation in mice. Nat Commun. 2021;12(1):2238. doi: 10.1038/s41467-021-22501-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Xilouri M, Brekk OR, Polissidis A, Chrysanthou-Piterou M, Kloukina I, Stefanis L. Impairment of chaperone-mediated autophagy induces dopaminergic neurodegeneration in rats. Autophagy. 2016;12(11):2230‐2247. doi: 10.1080/15548627.2016.1214777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Orenstein SJ, Kuo SH, Tasset I, et al. Interplay of LRRK2 with chaperone-mediated autophagy. Nat Neurosci. 2013;16(4):394‐406. doi: 10.1038/nn.3350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Caballero B, Wang Y, Diaz A, et al. Interplay of pathogenic forms of human tau with different autophagic pathways. Aging Cell. 2018;17(1). doi: 10.1111/acel.12692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Alvarez-Erviti L, Seow Y, Schapira AH, Rodriguez-Oroz MC, Obeso JA, Cooper JM. Influence of microRNA deregulation on chaperone-mediated autophagy and alpha-synuclein pathology in Parkinson's disease. Cell Death Dis. 2013;4:e545. doi: 10.1038/cddis.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Singh A, Kukreti R, Saso L, Kukreti S. Oxidative stress: a key modulator in neurodegenerative diseases. Molecules. 2019;24(8). doi: 10.3390/molecules24081583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kumar S, Duan Q, Wu R, Harris EN, Su Q. Pathophysiological communication between hepatocytes and non-parenchymal cells in liver injury from NAFLD to liver fibrosis. Adv Drug Deliv Rev. 2021;176:113869. doi: 10.1016/j.addr.2021.113869. [DOI] [PubMed] [Google Scholar]
- 84.Rodriguez-Navarro JA, Kaushik S, Koga H, et al. Inhibitory effect of dietary lipids on chaperone-mediated autophagy. Proc Natl Acad Sci USA. 2012;109(12):E705‐E714. doi: 10.1073/pnas.1113036109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Chen Z, Tian R, She Z, Cai J, Li H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic Biol Med. 2020;152:116‐141. doi: 10.1016/j.freeradbiomed.2020.02.025. [DOI] [PubMed] [Google Scholar]
- 86.Das S, Seth RK, Kumar A, et al. Purinergic receptor X7 is a key modulator of metabolic oxidative stress-mediated autophagy and inflammation in experimental nonalcoholic steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2013;305(12):G950‐G963. doi: 10.1152/ajpgi.00235.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zhao X, Di Q, Yu J, et al. USP19 (ubiquitin specific peptidase 19) promotes TBK1 (TANK-binding kinase 1) degradation via chaperone-mediated autophagy. Autophagy. 2021:1‐18. doi: 10.1080/15548627.2021.1963155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Valdor R, Garcia-Bernal D, Riquelme D, et al. Glioblastoma ablates pericytes antitumor immune function through aberrant up-regulation of chaperone-mediated autophagy. Proc Natl Acad Sci USA. 2019;116(41):20655‐20665. doi: 10.1073/pnas.1903542116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Garg AD, Dudek AM, Agostinis P. Calreticulin surface exposure is abrogated in cells lacking, chaperone-mediated autophagy-essential gene, LAMP2A. Cell Death Dis. 2013;4:e826. doi: 10.1038/cddis.2013.372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Verma R, Saha S, Kumar S, Mani S, Maiti TK, Surjit M. RNA-protein interaction analysis of SARS-CoV-2 5' and 3' untranslated regions reveals a role of lysosome-associated membrane protein-2a during viral infection. mSystems. 2021;6(4):e0064321. doi: 10.1128/mSystems.00643-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Du L, Xie Y, Zheng K, et al. Oxidative stress transforms 3CLpro into an insoluble and more active form to promote SARS-CoV-2 replication. Redox Biol. 2021;48:102199. doi: 10.1016/j.redox.2021.102199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laforge M, Elbim C, Frere C, et al. Tissue damage from neutrophil-induced oxidative stress in COVID-19. Nat Rev Immunol. 2020;20(9):515‐516. doi: 10.1038/s41577-020-0407-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yamada K, Asai K, Nagayasu F, et al. Impaired nuclear factor erythroid 2-related factor 2 expression increases apoptosis of airway epithelial cells in patients with chronic obstructive pulmonary disease due to cigarette smoking. BMC Pulm Med. 2016;16:27. doi: 10.1186/s12890-016-0189-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Labaki WW, Rosenberg SR. Chronic obstructive pulmonary disease. Ann Intern Med. 2020;173(3):ITC17‐ITC32. doi: 10.7326/AITC202008040. [DOI] [PubMed] [Google Scholar]
- 95.Lee CH, Lee KH, Jang AH, Yoo CG. The impact of autophagy on the cigarette smoke extract-induced apoptosis of bronchial epithelial cells. Tuberc Respir Dis (Seoul). 2017;80(1):83‐89. doi: 10.4046/trd.2017.80.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Zhou J, Yang J, Fan X, et al. Chaperone-mediated autophagy regulates proliferation by targeting RND3 in gastric cancer. Autophagy. 2016;12(3):515‐528. doi: 10.1080/15548627.2015.1136770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang S, Hu B, You Y, et al. Sorting nexin 10 acts as a tumor suppressor in tumorigenesis and progression of colorectal cancer through regulating chaperone mediated autophagy degradation of p21(Cip1/WAF1). Cancer Lett. 2018;419:116‐127. doi: 10.1016/j.canlet.2018.01.045. [DOI] [PubMed] [Google Scholar]
- 98.Xia HG, Najafov A, Geng J, et al. Degradation of HK2 by chaperone-mediated autophagy promotes metabolic catastrophe and cell death. J Cell Biol. 2015;210(5):705‐716. doi: 10.1083/jcb.201503044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Arias E, Koga H, Diaz A, Mocholi E, Patel B, Cuervo AM. Lysosomal mTORC2/PHLPP1/Akt regulate chaperone-mediated autophagy. Mol Cell. 2015;59(2):270‐284. doi: 10.1016/j.molcel.2015.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cao J, Ying M, Xie N, et al. The oxidation states of DJ-1 dictate the cell fate in response to oxidative stress triggered by 4-hpr: autophagy or apoptosis? Antioxid Redox Signaling. 2014;21(10):1443‐1459. doi: 10.1089/ars.2013.5446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Chandel NS, McClintock DS, Feliciano CE, et al. Reactive oxygen species generated at mitochondrial complex III stabilize hypoxia-inducible factor-1alpha during hypoxia: a mechanism of O2 sensing. J Biol Chem. 2000;275(33):25130‐8. doi: 10.1074/jbc.M001914200. [DOI] [PubMed] [Google Scholar]
- 102.Hubbi ME, Gilkes DM, Hu H, Kshitiz AI, Semenza GL. Cyclin-dependent kinases regulate lysosomal degradation of hypoxia-inducible factor 1alpha to promote cell-cycle progression. Proc Natl Acad Sci USA. 2014;111(32):E3325‐E3334. doi: 10.1073/pnas.1412840111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Tang J, Zhan MN, Yin QQ, et al. Impaired p65 degradation by decreased chaperone-mediated autophagy activity facilitates epithelial-to-mesenchymal transition. Oncogenesis. 2017;6(10):e387. doi: 10.1038/oncsis.2017.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Spisek R, Dhodapkar MV. Towards a better way to die with chemotherapy: role of heat shock protein exposure on dying tumor cells. Cell Cycle. 2007;6(16):1962‐1965. doi: 10.4161/cc.6.16.4601. [DOI] [PubMed] [Google Scholar]
- 105.Anguiano J, Garner TP, Mahalingam M, Das BC, Gavathiotis E, Cuervo AM. Chemical modulation of chaperone-mediated autophagy by retinoic acid derivatives. Nat Chem Biol. 2013;9(6):374‐382. doi: 10.1038/nchembio.1230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ho PW, Leung CT, Liu H, et al. Age-dependent accumulation of oligomeric SNCA/alpha-synuclein from impaired degradation in mutant LRRK2 knockin mouse model of Parkinson disease: role for therapeutic activation of chaperone-mediated autophagy (CMA). Autophagy. 2020;16(2):347‐370. doi: 10.1080/15548627.2019.1603545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci. 2017;38(7):592‐607. doi: 10.1016/j.tips.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 108.Persson T, Popescu BO, Cedazo-Minguez A. Oxidative stress in Alzheimer's disease: why did antioxidant therapy fail? Oxid Med Cell Longev. 2014;2014:427318. doi: 10.1155/2014/427318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhao X, Di Q, Yu J, et al. USP19 (ubiquitin specific peptidase 19) promotes TBK1 (TANK-binding kinase 1) degradation via chaperone-mediated autophagy. Autophagy. 2022;18(4):891‐908. doi: 10.1080/15548627.2021.1963155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Liang J, Sagum CA, Bedford MT, et al. Chaperone-mediated autophagy protein BAG3 negatively regulates Ebola and Marburg VP40-mediated egress. PLoS Pathog. 2017;13(1):e1006132. doi: 10.1371/journal.ppat.1006132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bernardo I, Bozinovski S, Vlahos R. Targeting oxidant-dependent mechanisms for the treatment of COPD and its comorbidities. Pharmacol Ther. 2015;155:60‐79. doi: 10.1016/j.pharmthera.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 112.Wu JH, Guo JP, Shi J, et al. CMA down-regulates p53 expression through degradation of HMGB1 protein to inhibit irradiation-triggered apoptosis in hepatocellular carcinoma. World J Gastroenterol. 2017;23(13):2308‐2317. doi: 10.3748/wjg.v23.i13.2308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cao D, Shan D, Yan W, et al. Chaperone-mediated autophagy affects tumor cell proliferation and cisplatin resistance in esophageal squamous cell carcinoma. Thorac Cancer. 2021;12(7):1048‐1057. doi: 10.1111/1759-7714.13849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Verbaanderd C, Maes H, Schaaf MB, et al. Repurposing drugs in oncology (ReDO)-chloroquine and hydroxychloroquine as anti-cancer agents. Ecancermedicalscience. 2017;11:781. doi: 10.3332/ecancer.2017.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Jenab M, Bueno-de-Mesquita HB, Ferrari P, et al. Association between pre-diagnostic circulating vitamin D concentration and risk of colorectal cancer in European populations: a nested case-control study. Br Med J. 2010;340:b5500. doi: 10.1136/bmj.b5500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Asano Y, Okamura S, Ogo T, Eto T, Otsuka T, Niho Y. Effect of (-)-epigallocatechin gallate on leukemic blast cells from patients with acute myeloblastic leukemia. Life Sci. 1997;60(2):135‐142. doi: 10.1016/s0024-3205(96)00603-0. [DOI] [PubMed] [Google Scholar]
- 117.Carlo-Stella C, Dotti G, Mangoni L, et al. Selection of myeloid progenitors lacking BCR/ABL mRNA in chronic myelogenous leukemia patients after in vitro treatment with the tyrosine kinase inhibitor genistein. Blood. 1996;88(8):3091‐3100. doi: 10.1182/blood.V88.8.3091.bloodjournal8883091. [DOI] [PubMed] [Google Scholar]
- 118.Dey DK, Chang SN, Vadlamudi Y, Park JG, Kang SC. Synergistic therapy with tangeretin and 5-fluorouracil accelerates the ROS/JNK mediated apoptotic pathway in human colorectal cancer cell. Food Chem Toxicol. 2020;143:111529. doi: 10.1016/j.fct.2020.111529. [DOI] [PubMed] [Google Scholar]
- 119.Kleih M, Bopple K, Dong M, et al. Direct impact of cisplatin on mitochondria induces ROS production that dictates cell fate of ovarian cancer cells. Cell Death Dis. 2019;10(11):851. doi: 10.1038/s41419-019-2081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zhang S, Liu X, Bawa-Khalfe T, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639‐1642. doi: 10.1038/nm.2919. [DOI] [PubMed] [Google Scholar]
- 121.Yoshida T, Goto S, Kawakatsu M, Urata Y, Li TS. Mitochondrial dysfunction, a probable cause of persistent oxidative stress after exposure to ionizing radiation. Free Radic Res. 2012;46(2):147‐153. doi: 10.3109/10715762.2011.645207. [DOI] [PubMed] [Google Scholar]