Abstract
Osteosarcoma, one of the common malignant tumors in the skeletal system, originates in mesenchymal tissue, and the most susceptible area of occurrence is the metaphysis with its abundant blood supply. Tumors are characterized by highly malignant spindle stromal cells that can produce bone-like tissue. Most of the osteosarcoma are primary, and a few are secondary. Osteosarcoma occurs primarily in children and adolescents undergoing vigorous bone growth and development. Most cases involve rapid tumor development and early blood metastasis. In recent years, research has grown in the areas of molecular biology, imaging medicine, biological materials, applied anatomy, surgical techniques, biomechanics, and comprehensive treatment of tumors. With developments in molecular biology and tissue bioengineering, treatment methods have also made great progress, especially in comprehensive limb salvage treatment, which significantly enhances the quality of life after surgery and improves the 5-year survival rate of patients with malignant tumors. This article provides a review of limb salvage, immunotherapy, gene therapy, and targeted therapy from traditional amputation to neoadjuvant chemotherapy, providing a reference for current clinical treatments for osteosarcoma.
Keywords: osteosarcoma, treatment, chemotherapy, immunotherapy, prognosis
Introduction
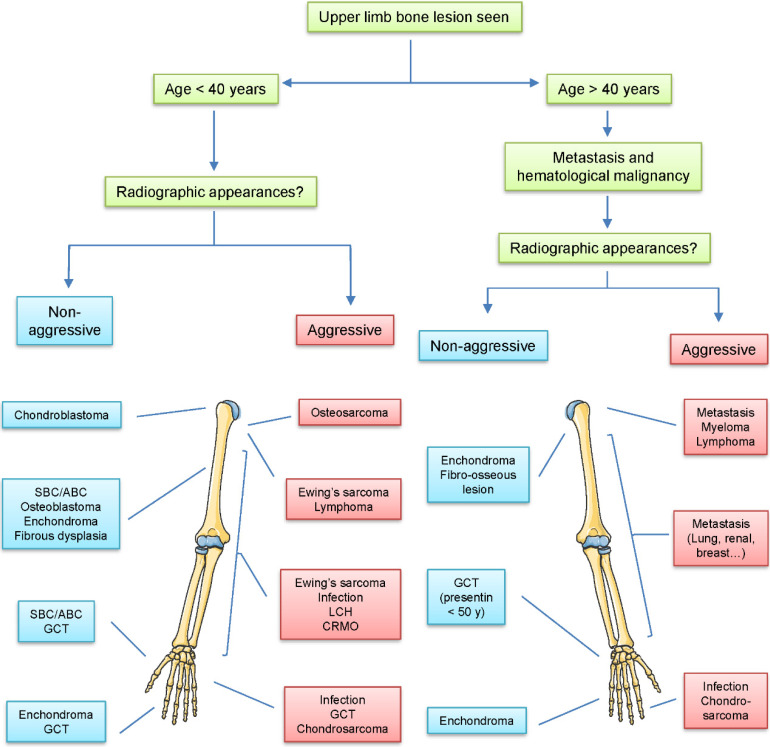
The diagnosis and treatment of tumors in bone is a difficult problem in orthopedics. The incidence of different bone lesions significantly varies at various sites within the limb, and age is one of the important factors in tumorigenesis (Figure 1). The 5-year survival rate of patients with osteosarcoma has been about 20% due to limitations in treatment. Despite recent advances in molecular biology, biomaterials, imaging medicine, applied anatomy, biomechanics, surgical techniques, and comprehensive treatment of tumors, progress has been slow.1–3 Neoadjuvant chemotherapy has been recognized as an especially effective treatment for osteosarcoma since the initiation of its use in the 1980s. 4 The survival rate of osteosarcoma has improved considerably, and the rate of limb salvage has reached more than 65%.5, 6 However, since 80% of patients with osteosarcoma have distant metastasis at the time of initial diagnosis, the prognosis is extremely poor regardless of the treatment taken by 20% to 30% of patients. 7 Therefore, improving the treatment efficacy and survival rate of patients with osteosarcoma remains an urgent issue to be solved in clinical practice. In this review, we provide limb salvage, immunotherapy, gene therapy, and targeted therapy from traditional amputation to neoadjuvant chemotherapy for current clinical treatments for osteosarcoma.
Figure 1.
Common bone lesions according to age and location in the upper limb. The incidence of different bone lesions varies at different sites within the upper limb and age.
Abbreviations: CRMO, chronic recurrent multifocal osteomyelitis; GCT, giant cell tumor; LCH, Langerhans cell histiocytosis; SBC/ABC, simple bone cysts/aneurysmal bone cysts.
Chemotherapy
Clinical treatment of osteosarcoma has made great progress in recent years largely due to improvements in chemotherapy. With the development of limb salvage surgery, neoadjuvant chemotherapy, and immunotherapy, the 5-year survival rate has significantly improved.8–11 However, malignant bone tumors still result in extremely high mortality and disability so a treatment multidisciplinary treatment strategy is important. Surgery and neoadjuvant chemotherapy have become widely recognized as the standard mode for the treatment of osteosarcoma. 12
Traditional chemotherapy treatments used in clinical practice are based on several regimens proposed by Rosen et al in the 1980s, and there is currently no breakthrough in curative effect. 13 Because osteosarcoma tumors are relatively resistant, the efficacy of single-agent chemotherapy has been less than ideal and only a few drugs can produce more than 15% efficiency. 14 The main drugs currently used for osteosarcoma chemotherapy include methotrexate (MTX), adriamycin (ADM), cisplatin (DDP), ifosfamide, vincristine, epirubicin, cyclophosphamide, and etoposide.15–17 MTX, ADM, DDP, and ifosfamide are the most commonly used and are combined in different ways. At present, the map scheme composed of high-dose MTX, ADM, and DDP is the standard scheme for most treatment centers in Europe and America.
Importantly, the sensitivity of chemotherapy for osteosarcoma cells directly affects the survival rate of patients. Chemotherapy must be systematic and regularized to reduce drug resistance.18–20 To eliminate the characteristics of drug resistance caused by single-agent chemotherapy, combined chemotherapy has been developed. The main objective is to synergize the effects of combined antineoplastic drugs, reduce the toxicity of a single drug, and reduce drug resistance. Besides, the use of natural compounds in the treatment of cancers has received much attention in occurring years. 21
The emergence of neoadjuvant chemotherapy in recent years has improved survival and reduced recurrence and metastasis; in addition, it has increased limb salvage rate. Surgery supplemented with chemotherapy is not simply a matter of “preoperative chemotherapy + surgery + postoperative chemotherapy.” The new approach, based on the pathological grade of postoperative histopathology, is to adjust the postoperative chemotherapy regimen according to the tumor necrosis rate after preoperative chemotherapy. 21 Neoadjuvant chemotherapy has become the standard protocol for osteosarcoma, and treating early micrometastases and primary lesions improves the postoperative success rate of limb salvage surgery.
Attention should be paid to the basic principle of neoadjuvant chemotherapy—that is, the evaluation of preoperative chemotherapy efficacy and assessment of whether to adjust the postoperative chemotherapy regimen.9, 22 Because the evaluation of tumor necrosis rate needs to happen after surgical removal of pathological specimens, a judgment is not possible before surgery. A noninvasive means to evaluate the degree of preoperative tumor necrosis before surgery would have even greater value in judging prognosis and guiding treatment. 23 Some valuable factors for judging the effect of chemotherapy include clinical observations, such as the alleviation of pain or symptoms, whether the tumor mass is reduced, and whether isocitrate dehydrogenase (IDH) and alkaline phosphatase (AKP) are decreased on testing. 24 Also important are the presence and extent of metastasis and characteristics such as tumor size, soft tissue mass, reaction area, degree of ossification, and appearance of blood vessels on computed tomography, x-ray, and magnetic resonance imaging. 25
Molecular Targeted Therapy
After decades of development, the conventional treatment for osteosarcoma has been standardized. In various bone tumor centers in China, the comprehensive treatment of surgery, neoadjuvant chemotherapy, and postoperative adjuvant chemotherapy have obtained a 5-year survival rate of 60% to 70%. 26 However, for patients with poor chemotherapy efficacy and difficulty tolerating chemotherapy, the current second-line treatment is still challenging. Besides, due to the high heterogeneity and low incidence of osteosarcoma, it is difficult to target specific driving genes.
Receptor tyrosine kinase (RTK) is a key upstream molecule, which participated in a variety of signal pathways related to cell growth, apoptosis, and survival.27–29 Abnormal amplification or activating mutation of RTK leads to continuous activation of downstream signals, resulting in uncontrolled growth of tumor cells. 30 The multi-target tyrosine kinase inhibitor (TKI) designed for RTK is the drug category that has made the fastest progress in the targeted therapy of osteosarcoma. The drugs regorafenib and pazopanib have clearly established their value in many clinical practices for the treatment of osteosarcoma. 31 It is worth mentioning that the use of a TKI in combination with chemotherapy in the preoperative neoadjuvant treatment stage has been explored to reduce the toxicity of chemotherapy and shorten the treatment course. As such, relevant clinical trials are in progress.
Mutations of cell cycle-related molecules such as CCNE1 amplification, retinoblastoma gene 1 inactivation, and cyclin-dependent kinase (CDK)4/6 amplification can often be detected in osteosarcoma. 32 CCNE1 amplification is thought to be related to chemotherapy resistance in many tumor species.33–36 The frequency of CCNE1 amplification in osteosarcoma can be as high as 30%. Although there is no drug directly targeting CCNE1 at present, it is theoretically feasible to treat it by acting on its downstream cyclin CDK1/2/5/9 and using multi-target CDK inhibitors such as dinaciclib.37–39 Some teams have also used patient-derived xenograft animal models to obtain clear therapeutic effects in osteosarcoma.40–42
CDK4/6 inhibitors such as piperacillin and abemaciclib are among the most mature drugs in the arena of cyclins, and have occupied a high position in the treatment of breast cancer and liposarcoma.43–45 However, because CDK4/6 amplification alone is rare in high-grade osteosarcoma, it would be difficult to design clinical trials for this mutation. For osteosarcoma with multiple suspected carcinogenic mutations, it is also difficult to determine the indication by defining the cutoff of CDK4/6 amplified copy number. 46 In addition, the treatment cost of CDK4/6 inhibitors, and the risk of blood toxicity and other risks associated with off-label use are great obstacles to their individualized use. 47
PTEN-PI3K-AKT-TSC1/2-MTOR is an important signaling pathway related to protein synthesis and cell growth.48–50 Many molecules associated with this pathway have been detected in osteosarcoma.51–53 Mutations of the genes PTEN and TSC1/2 occur with relatively high frequency, with PTEN, a tumor suppressor gene, found in more than 50% of high-grade osteosarcoma cases. 54 The PTEN-P13K pathway involves many molecules, providing opportunities for targeted therapy. For example, everolimus, a second-line drug for osteosarcoma, is a typical mTOR inhibitor and has been listed in the drug guidelines of the National Comprehensive Cancer Network. 55 However, everolimus inhibits only mTORC1 and has very little effect on mTORC2, which may also be an important reason for its limited effect on osteosarcoma. 56
BRCA is an important tumor suppressor gene, and its mutation frequency in osteosarcoma is also very high, making it a possible initiator of tumorigenesis.57–60 Some studies have found that more than 80% of osteosarcomas show a BRCA-like phenotype, with characteristics including large single-nucleotide mutations, loss of heterozygosity, and telomere allele imbalance.61–63 Homologous DNA repair-deficient cancers caused by BRCA mutation provide opportunities for the use of poly ADP ribose polymerase (PARP) inhibitors such as olaparib to produce a “synthetic lethal” effect, resulting in the death of large numbers of tumor cells. With impressive clinical results, it has become a first-line drug in the treatment of some ovarian and breast cancers. 64 Considering the widespread presence of BRCA mutations in osteosarcoma, it is desirable to try PARP inhibitors in osteosarcoma cases with mutation-positive/homologous DNA repair-deficient cancer. 65
Radiotherapy
Ionizing radiation in radiotherapy acts on the target molecule DNA, and tumor cells produce a huge number of reactive oxygen molecules, causing DNA oxidative damage, which ultimately leads to tumor cell death. 66 Radiotherapy is a critical method for the treatment of many malignant bone tumors. In the treatment of spinal vertebral tumors, radiotherapy can directly kill tumor cells while reducing the tumor size for surgical resection. 67 The significance of radiotherapy is greater for spinal tumors that are resected in the lesion and for palliative resection. 68 Moreover, post-loading radiotherapy is radiation therapy at close range and high dose rate. 69 This technique is suitable for locally recurrent tumors or tumors that have been inoperable. 70
Recent research has aimed at the use of radiotherapy sensitizers in cancer treatment. Their application avoids damage to normal tissues while improving the sensitivity of tumor cells to radiotherapy. 71 Although osteosarcoma has poor sensitivity to radiotherapy, radiosensitizers assist in treatment by increasing the sensitivity of tumor cells to radiotherapy and improving the lethality of radiation on tumor cells.72–76
Limb Salvage Treatment
Improvements in chemotherapy, imaging technology, and limb reconstruction technology have promoted the development of limb salvage surgery. There is no statistically significant difference in postoperative survival rates between patients undergoing limb salvage surgery and radical surgery. 77 During the surgical treatment of malignant bone tumors, patients and their families are more likely to request limb salvage surgery; thus, the use of the technique in that setting has developed rapidly. 78 At the same time, advances in related technical fields of surgery including materials science, prosthetic manufacturing processes, radiographic diagnostics, physical therapy, interventional techniques, and surgical techniques, have contributed to the wider use of limb salvage surgery when treat malignant bone tumors.79–82
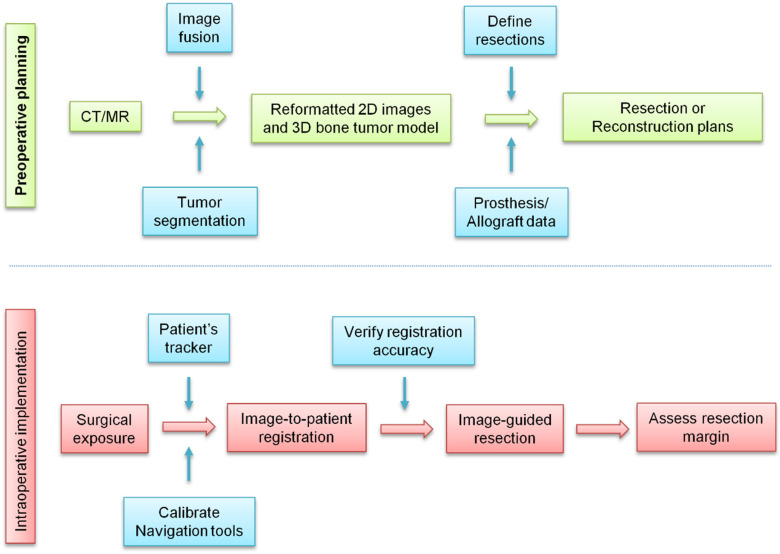
After resection, the bone must be reconstructed to restore limb function. In the past, orthopedic tumor surgeons analyzed two-dimensional imaging information and mentally integrated the data to make three-dimensional operation plans. 83 In complex cases, it is difficult to convert the operation plan into physical practice in an operating room. In the past decade, the development of computer-aided tumor surgery has enabled surgeons to plan three-dimensional operations and perform an image-guided osteotomy. 84 This technique results in safer tumor resection and can improve the accuracy of the surgery by copying the preoperative plan, resulting in clinical benefits (Figure 2).
Figure 2.
Clinical workflow of computer-assisted tumor surgery. In the surgical treatment of osteosarcoma, multimode image data are obtained using advanced imaging equipment. The computer processes the medical image data and aids in displaying and positioning the anatomical structure of the bones, planning the surgical path, formulating a reasonable and quantitative surgical scheme, conducting the preoperative surgical simulation, and using robot-assisted accurate guidance during surgery. With monitoring and improved accuracy of positioning, this technique can reduce surgical injury and improve the success rate of complex surgical operations.
Before deciding whether to perform limb-salvage surgery, it should be fully evaluated whether the function of the limb after salvage surgery would be better than prosthetic function after amputation. The scope of resection of the tumor should be fully estimated before surgery. The resection range should include the tumor body, capsule, reaction zone, and surrounding normal tissues. 85 In fact, such resection is difficult to achieve in most malignant bone tumors, and in clinical practice, most resections are performed closer to 1 cm outside the reaction zone.
If the vascular and nerve is involved, surgery is more difficult. Generally, for patients with sacral nerve invasion or anterior tibial artery damage, limb salvage surgery can still be attempted. If other vascular or nerves are involved, limb salvage surgery should not be performed. However, for tumors around the elbow joint and the knee joint, the affected vascular or nerve can be resected and reconstructed as long as the general condition is good and there is no metastasis. 86 If metastasis has already occurred, surgical resection can be performed under adjuvant chemotherapy and before limb salvage surgery, with an approximate success rate of 30% according to one study. 87 If a pathological fracture has occurred, limb salvage surgery may also be attempted as appropriate.
Artificial prosthesis replacement is currently the most widely used and best-performing method of limb salvage reconstruction. In recent years, the variety and suitability of adjustable and individualized prostheses have improved greatly. Recent surveys have shown that the clinical 10-year satisfaction rate of a good quality prosthesis can reach 90%.88–91 Allogeneic bone transplantation has relatively narrow indications of infection, fracture, absorption, rejection, and infectious diseases. However, its advantages in biological reconstruction have led many institutions to study it. 92
It is worth noting that tumor prosthesis replacement is different from ordinary joint replacement. Most cancer patients are young and desire a very active life. Meantime, a large defect of the bone tissue requires extensive soft tissue resection, resulting in the need for a stable prosthesis to compensate for poor balance. Patients need early recovery activities after surgery to upgrade their quality of life and minimize movement limitations after surgery. 93
Although limb salvage surgery has become the treatment of choice for malignant bone tumors, tumor residuals are still the primary cause of recurrence. However, removing too much tissue affects the reconstruction of bone and soft tissue. Intraoperative radiotherapy can help locally control the tumor and improve postoperative functional recovery. 94
Interventional Therapy
Interventional treatment of osteosarcoma is both a palliative treatment for tumors and an adjuvant treatment for limb salvage surgery. The 2 main types of therapy are intravascular and nonvascular intervention.95, 96 Nonvascular intervention refers to tumor ablation with a percutaneous puncture, while intravascular intervention includes intra-arterial infusion chemotherapy and arterial embolization. 97 Arterial infusion chemotherapy can effectively control tumor growth, reduce the probability of tumor border implantation, and improve the resection rate and limb salvage rate in tumor surgery. Moreover, arterial infusion chemotherapy can improve clinical symptoms, reduce tumor size, and decrease vascular endothelial cell necrosis, vascular occlusion, and blood supply to the tumor. 98
Arterial embolization refers to inject an embolic agent into the main artery supplying blood to embolize the blood vessels and cause tumor necrosis due to ischemia. In the treatment of malignant bone tumors, simple embolization can decrease intraoperative bleeding and improve the resection rate and limb salvage rate. 99
Selective arterial embolization and transcatheter arterial chemoembolization (TACE) are the most common methods used in the clinic. Selective arterial embolization uses either spring coils or small gelatin microspheres to cut off the blood supply of the tumor, thus resulting in ischemia and tumor cell necrosis. 100 Mavrogenis et al 101 employed this method to treat 19 patients with advanced osteosarcoma. The pain of all patients was relieved 3 days after embolization, and 5 patients achieved continuous relief after repeated embolization. Because it reduces pain rapidly and can be repeated multiple times, arterial embolization has advantages over radiotherapy and chemotherapy for patients with advanced cancer.
The TACE procedure involves inserting the catheter selectively and injecting chemotherapy drugs into the target artery supplying blood to the tumor. TACE is mainly used in limb salvage surgery or adjuvant therapy before radical surgery, to promote tumor necrosis, reduce complications, and improve the success rate of surgery.102, 103
Immunotherapy
Immunotherapy modulates the body's own immune function to kill tumor cells. Techniques include specific and nonspecific immunotherapy, immuno-directed therapy, and adoptive immunotherapy.104–107 T cell-mediated cellular immunity makes a valuable contribution toward the fight against tumor cells, and natural killer (NK) cell-mediated natural immunity is the first line of defense against tumors. 108 Due to the development of new tumor molecular biology techniques and the establishment of immunoassay indicators, doctors can accurately assess quantitative and qualitative changes in tumor molecules and cellular immune functions.109–111 Moreover, immunotherapy can play an important role along with traditional surgery, radiotherapy, and chemotherapy.
At present, research achievements in osteosarcoma immunotherapy primarily involve cytokine therapy and dendritic cell (DC) therapy. 8 Chimeric antigen receptor T (CAR-T) cell immunotherapy and immune checkpoint blocking drugs are also becoming hotspots for tumor research.
DCs have a strong antigen-presenting ability. They can be incubated with tumor neoantigen and were injected into patients as a vaccine to produce a specific antitumor immune response. In one experiment using a rat osteosarcoma model, DCs were combined by electrofusion with UMR106 cell line and injected into rats. The results showed that the fusion vaccine could effectively activate cytotoxic T cells and stimulate T lymphocyte proliferation, leading to atrophy and even the disappearance of the tumor. 112
Cytokines take part in the regulation of multiple cellular physiological and immune response. The interleukin-2 (IL-2) cytokine is an essential element of immunotherapy whose role includes activating effector T cells and enhancing the function of NK cells. 113 Meazza et al 114 used chemotherapy and IL-2 to treat patients with primary pulmonary metastatic osteosarcoma. The results indicated that IL-2 involved in immunotherapy can potentially increase the survival rate of osteosarcoma patients. Interferon has also been used in the cancer treatment and studies have confirmed that it can enhance the sensitivity of chemotherapy drugs during the treatment of osteosarcoma. 12
CAR-T is a new immunotherapy method that has been successfully applied to treat blood tumors. In the process of identifying tumors, CAR-T after genetic engineering transformation is no longer limited by proteins that evade the major histocompatibility complex.115–117 CAR-T recognizes tumor antigens in a way independent of human leukocyte antigen (HLA) molecules, recognizes and kills tumor cells across the down-regulation mechanism of HLA expression, and has the advantages of diverse recognition of tumor antigen targets, lasting effects in vivo, and reducing the incidence of rejection reactions.118–121
The key to the application of CAR-T cell immunotherapy in osteosarcoma treatment is to find suitable target molecules. At present, HER2, the IL-11 receptor chain, and ganglioside GD2 have been confirmed to be overexpressed in multiple osteosarcoma cells.122, 123 These receptors may serve as effective targets for CAR-T cell immunotherapy and inhibition of tumor metastasis in osteosarcoma. 124 The antitumor effect and persistence of HER2-CAR-T cells in a certain time range show promise for clinical application. 125
PD-L1 and PD-1 are immune checkpoint proteins involved in modulating the body's immune response.126–128 Although checkpoint proteins have a role in preventing autoimmune disease, they can also suppress the immune system's ability to attack tumor cells. Research is deepening on the drug's use to inhibit the action of PD-1/PD-L1 and thereby increase the body's ability to fight tumor cells. 129 Shimizu et al 130 employed a mouse osteosarcoma model to evaluate the efficacy of immune checkpoint inhibitors on lung metastasis. They found that the proliferation and invasion of osteosarcoma cells were inhibited in the PD-1/PD-L1 antibody treatment group. This suggests that PD-1/PD-L1 antibody therapy may be valuable in the treatment of osteosarcoma.131, 132
Ablation Treatment
Surgery, radiotherapy, and chemotherapy have traditionally been the main treatments for osteosarcoma. However, functional reconstruction has a high incidence of complications, and radiotherapy and chemotherapy can damage normal tissues and reduce the immune function of the host in addition to killing tumor cells. Because radiofrequency ablation (RFA) is noninvasive or minimally invasive, it has some unique advantages in the treatment of osteosarcoma. 133 Techniques that have been applied in the clinic with good curative effects include RFA, high-intensity focused ultrasound (HIFU) ablation, cryoablation, and microwave ablation.133–135
The main mechanisms of HIFU ablation include cavitation effect, thermal effect, and mechanical effect. Among them, thermal effect plays a vital role. Thermal effect refers to the heating of the tissue through the propagation of focused ultrasonic waves. 136 The particles of tissue vibrate at high speed, and part of the acoustic vibration energy converts into molecular thermal motion energy, resulting in increased temperature. The target area is heated to above 60 °C instantaneously, resulting in protein degeneration, coagulation necrosis, and structural and functional changes of cells in the target tissue. 137
Cavitation effect involves the formation, expansion, and explosion or implosion of small air bubbles caused by the ultrasound wave vibration. 138 Cavitation can produce locally high temperature, high pressure, free radicals, and strong shock waves, which can denature enzymes and proteins in cells. Cavitation effect can also enhance the thermal effect. 139 Mechanical effect refers to the vibration of tissue caused by ultrasound. Vibration that exceeds the elastic limit of the tissue causes damage. However, some studies have shown that mechanical effects only cause damage to the diseased bone tissue, does not damage the mechanical function of bone. 140
At present, HIFU treatment is guided by MRI or ultrasound. Ultrasound-guided treatment has the advantages of economy and convenience, but its imaging contrast is poor, quantitative temperature measurement is impossible, and the accuracy of postoperative evaluation is not high. 141 MRI-guided ultrasound has good imaging quality and high resolution, but the imaging speed is relatively slow and can be easily affected by patients’ breathing movements. 142 Patients with cardiac pacemakers and metal parts are prohibited, and the cost is high.
HIFU has unique advantages when treating malignant bone tumors. Because it is noninvasive or minimally invasive, it reduces complications such as bone nonunion and infection that may result from invasive surgery. Further, it does not require functional reconstruction and it can enhance the immune function of the body. The treatment is rapid and has high accuracy. As one of the comprehensive treatment methods for tumors, HIFU can effectively relieve pain and improve patients’ quality of life. 143 However, there is no unified standard for the treatment dose of HIFU for tumors of different pathological types at different sites. Practices need to be adjusted based on intraoperative real-time feedback. Newer HIFU technologies such as ultrasound microbubble angiography still need to be evaluated for their usefulness in treating osteosarcoma. 144
Furthermore, RFA and cryoablation are other ablation techniques recommended for the treatment of osteosarcoma metastasis. However, there are differences between the two because of their different mechanisms of action. 145 RFA is not strong enough to easily penetrate bone, so it is often used to treat lung metastasis, whereas a cold probe can penetrate deep bone tissue, so it can be used in bone metastases. Saumet et al 133 employed RFA to treat 10 patients with lung metastases of osteosarcoma under the age of 25 years. The results showed that 7 patients were completely relieved and no metastasis occurred at the treatment site.
Stem Cell Therapy
Osteosarcoma occurs when genetic or other factors interfere with the normal process by which mesenchymal stem cells differentiate into bone cells. Because mesenchymal stem cells can transform into tumor stem cells (TSCs), there is an opportunity to study the relationship between TSCs and osteosarcoma. 146 TSCs are closely related to tumorigenesis, proliferation, recurrence, and chemotherapy resistance. 147 Therefore, the study of TSCs can help solve the problem of recurrence and metastasis of osteosarcoma at the source.
Based on isolating TSCs and identifying different specific surface antigens expressed by osteosarcoma stem cells, researchers can create a targeted neutralizing antibody to inactivate TSCs and block the development of osteosarcoma. 148 It has been found that in a hypoxic environment, TSCs can activate lysine oxidase by upregulating the expression of hypoxia-inducible factors and promoting the formation of metastatic tumors. 149 This suggests that changing the microenvironment in which TSC survives may be another way to treat osteosarcoma. In summary, the introduction of TSC research has elevated the diversification of osteosarcoma treatment to a new height.
Outlook
The clinical treatment of osteosarcoma has gone through a long period of exploration and development. So far, great progress has been made and treatment techniques have matured. The application of high-dose chemotherapy increases the intensity of action on tumor tissues so that the patient's prognosis is greatly improved. Neoadjuvant chemotherapy combined with extensive resection of tumors not only improves the survival rate of patients but also preserves better limb function. The popularity of large-scale medical testing instruments, such as MRI and radionuclide scanners, has made early detection, early diagnosis, and early treatment of osteosarcoma possible.
Great progress has been made in the reconstruction of bone materials suitable for bone tumor salvage surgery. Extensive resection of the tumor has become possible, resulting in a significantly prolonged local tumor-free period, improved quality of life, and prolonged life expectancy for the patient. Finally, the study and application of new technologies such as radiotherapy, molecular targeted therapy, immunotherapy, ablation, and stem cell therapy have brought new hope in controlling local recurrence and distant metastasis.
Conclusion
Osteosarcoma, one of the most common malignant tumors in the skeletal system, occurs primarily in children and adolescents and most cases involve rapid tumor development and early blood metastasis. Since 80% of patients have metastasis at the time of initial diagnosis, the prognosis is extremely poor regardless of the treatment taken by 20% to 30% of patients. In recent years, research has grown in the areas of molecular biology, biological materials, imaging medicine, applied anatomy, biomechanics, surgical techniques, and comprehensive treatment of tumors. With developments in molecular biology and tissue bioengineering, treatment methods have also made great progress, especially in comprehensive limb salvage treatment, which significantly enhances the quality of life after surgery and improves the 5-year survival rate of patients with malignant tumors. Improving the treatment efficacy and survival rate of patients with osteosarcoma remains an urgent issue to be solved in clinical practice.
Abbreviations
- ADH
alkaline phosphatase
- ADM
adriamycin
- CAR-T
chimeric antigen receptor T
- CDK
cyclin-dependent kinase
- DC
dendritic cell
- DDP
cisplatin
- HIFU
high-intensity focused ultrasound
- HLA
human leukocyte antigen
- IDH
isocitrate dehydrogenase
- IL-2
interleukin-2
- PARP
poly ADP ribose polymerase
- RFA
radiofrequency ablation
- RTX
receptor tyrosine kinase
- MTX
methotrexate
- NK
natural killer
- TSCs
tumor stem cells
- TACE
transcatheter arterial chemoembolization
- TKI
tyrosine kinase inhibitor
Footnotes
Author’s Contribution: YSM, ZYJ, and DF designed and supervised research. All authors interpreted the data and contributed to the final version of the manuscript.
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Wu Jieping Medical Foundation (grant number 320.6750.14326).
Availability of Supporting Data: The datasets supporting the conclusions of this article are included within the article.
ORCID iD: Da Fu https://orcid.org/0000-0002-0878-2575
References
- 1.Zhu S, Liu Y, Wang X, et al. lncRNA SNHG10 promotes the proliferation and invasion of osteosarcoma via Wnt/β-Catenin signaling. Mol Ther Nucleic Acids. 2020;22:957-970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zheng D, Liu W, Xie W, et al. AHA1 upregulates IDH1 and metabolic activity to promote growth and metastasis and predicts prognosis in osteosarcoma. Signal Transduct Target Ther. 2021;6(1):25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liang X, Wang X, He Y, et al. Acetylation dependent functions of Rab22a-NeoF1 fusion protein in osteosarcoma. Theranostics . 2020;10(17):7747-7757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Du M, Gu J, Liu C, et al. Genome-wide CRISPR screen identified Rad18 as a determinant of doxorubicin sensitivity in osteosarcoma. J Exp Clin Cancer Res. 2022;41(1):154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zalacain M, Bunuales M, Marrodan L, et al. Local administration of IL-12 with an HC vector results in local and metastatic tumor control in pediatric osteosarcoma. Mol Ther Oncolytics. 2020;20:23-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shen S, Yao T, Xu Y, et al. CircECE1 activates energy metabolism in osteosarcoma by stabilizing c-Myc. Mol Cancer. 2020;19(1):151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bielack SS, Kempf-Bielack B, Delling G, et al. Prognostic factors in high-grade osteosarcoma of the extremities or trunk: An analysis of 1,702 patients treated on neoadjuvant cooperative osteosarcoma study group protocols. J Clin Oncol. 2002;20(3):776-790. [DOI] [PubMed] [Google Scholar]
- 8.Chen C, Xie L, Ren T, et al. Immunotherapy for osteosarcoma: Fundamental mechanism, rationale, and recent breakthroughs. Cancer Lett. 2021;500:1-10. [DOI] [PubMed] [Google Scholar]
- 9.Corre I, Verrecchia F, Crenn V, et al. The osteosarcoma microenvironment: A complex but targetable ecosystem. Cells . 2020;9(4):976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Benjamin RS, Patel SR. Pediatric and adult osteosarcoma: Comparisons and contrasts in presentation and therapy. Cancer Treat Res. 2009;152:355-363. [DOI] [PubMed] [Google Scholar]
- 11.Christie JD, Appel N, Canter H, et al. Systemic delivery of TNF-armed myxoma virus plus immune checkpoint inhibitor eliminates lung metastatic mouse osteosarcoma. Mol Ther Oncolytics. 2021;22:539-554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smrke A, Anderson PM, Gulia A, et al. Future directions in the treatment of osteosarcoma. Cells. 2021;10(1):172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benjamin RS. Adjuvant and neoadjuvant chemotherapy for osteosarcoma: A historical perspective. Adv Exp Med Biol. 2020;1257:1-10. [DOI] [PubMed] [Google Scholar]
- 14.Zheng C, Tang F, Min L, et al. PTEN In osteosarcoma: Recent advances and the therapeutic potential. Biochim Biophys Acta Rev Cancer. 2020;1874(2):188405. [DOI] [PubMed] [Google Scholar]
- 15.Wippel B, Gundle KR, Dang T, et al. Safety and efficacy of high-dose methotrexate for osteosarcoma in adolescents compared with young adults. Cancer Med. 2019;8(1):111-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gu Z, Zhou Y, Cao C, et al. TFAP2C-mediated LINC00922 signaling underpins doxorubicin-resistant osteosarcoma. Biomed Pharmacother. 2020;129:110363. [DOI] [PubMed] [Google Scholar]
- 17.Yang D, Xu T, Fan L, et al. microRNA-216b enhances cisplatin-induced apoptosis in osteosarcoma MG63 and SaOS-2 cells by binding to JMJD2C and regulating the HIF1α/HES1 signaling axis. J Exp Clin Cancer Res. 2020;39(1):201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He J, Liu L, Tang F, et al. Paradoxical effects of DNA tumor virus oncogenes on epithelium-derived tumor cell fate during tumor progression and chemotherapy response. Signal Transduct Target Ther. 2021;6(1):408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Soghli N, Ferns GA, Sadeghsoltani F, et al. MicroRNAs and osteosarcoma: Potential targets for inhibiting metastasis and increasing chemosensitivity. Biochem Pharmacol. 2022;201:115094. [DOI] [PubMed] [Google Scholar]
- 20.Hattinger CM, Patrizio MP, Fantoni L, et al. Drug resistance in osteosarcoma: Emerging biomarkers, therapeutic targets and treatment strategies. Cancers (Basel) . 2021;13(12):2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rabelo ACS, Borghesi J, Noratto GD. The role of dietary polyphenols in osteosarcoma: A possible clue about the molecular mechanisms involved in a process that is just in its infancy. J Food Biochem. 2022;46(1):e14026. [DOI] [PubMed] [Google Scholar]
- 22.Li CJ, Liu XZ, Zhang L, et al. Advances in bone-targeted drug delivery systems for neoadjuvant chemotherapy for osteosarcoma. Orthop Surg. 2016;8(2):105-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang H, Gao Y, Vafaei S, et al. A chemoresistance lncRNA signature for recurrence risk stratification of colon cancer patients with chemotherapy. Mol Ther Nucleic Acids. 2021;27:427-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu XK, Rao SS, Tan YJ, et al. Fructose-coated angstrom silver inhibits osteosarcoma growth and metastasis via promoting ROS-dependent apoptosis through the alteration of glucose metabolism by inhibiting PDK. Theranostics. 2020;10(17):7710-7729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaffe N. Adjuvant chemotherapy in osteosarcoma: An odyssey of rejection and vindication. Cancer Treat Res. 2009;152:219-237. [DOI] [PubMed] [Google Scholar]
- 26.Meltzer PS, Helman LJ. New horizons in the treatment of osteosarcoma. N Engl J Med. 2021;385(22):2066-2076. [DOI] [PubMed] [Google Scholar]
- 27.Chabot T, Cheraud Y, Fleury F. Relationships between DNA repair and RTK- mediated signaling pathways. Biochim Biophys Acta Rev Cancer. 2021;1875(1):188495. [DOI] [PubMed] [Google Scholar]
- 28.Du Z, Lovly CM. Mechanisms of receptor tyrosine kinase activation in cancer . Mol Cancer . 2018;17(1):58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Huang H. Proteolytic cleavage of receptor tyrosine kinases . Biomolecules. 2021;11(5):660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Correll PH, Paulson RF, Wei X. Molecular regulation of receptor tyrosine kinases in hematopoietic malignancies. Gene. 2006;374:26-38. [DOI] [PubMed] [Google Scholar]
- 31.Cren PY, Lebellec L, Ryckewaert T, et al. Anti-angiogenic agents in management of sarcoma patients: Overview of published trials. Front Oncol. 2020;10:594445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fan Q, Zuo J, Tian H, et al. Nanoengineering a metal-organic framework for osteosarcoma chemo-immunotherapy by modulating indoleamine-2,3-dioxygenase and myeloid-derived suppressor cells. J Exp Clin Cancer Res. 2022;41(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang R, Xing L, Zheng X, et al. The circRNA circAGFG1 acts as a sponge of miR-195-5p to promote triple-negative breast cancer progression through regulating CCNE1 expression. Mol Cancer. 2019;18(1):4. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 34.Wu Y, Zheng Q, Li Y, et al. Metformin targets a YAP1-TEAD4 complex via AMPKα to regulate CCNE1/2 in bladder cancer cells. J Exp Clin Cancer Res. 2019;38(1):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gallo D, Young JTF, Fourtounis J, et al. CCNE1 Amplification is synthetic lethal with PKMYT1 kinase inhibition. Nature. 2022;604(7907):749-756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Li X, Zhang J, et al. E6 hijacks KDM5C/lnc_000231/miR-497-5p/ CCNE1 axis to promote cervical cancer progression. J Cell Mol Med . 2020;24(19):11422-11433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Han W, Liu M, Han D, et al. Exploiting the tumor- suppressive activity of the androgen receptor by CDK4/6 inhibition in castration-resistant prostate cancer. Mol Ther. 2022;30(4):1628-1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lou W, Ding B, Zhong G, et al. RP11-480I12.5-004 promotes growth and tumorigenesis of breast cancer by relieving miR-29c-3p-mediated AKT3 and CDK6 degradation. Mol Ther Nucleic Acids. 2020;21:916-931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang QF, Li J, Jiang K, et al. CDK4/6 Inhibition promotes immune infiltration in ovarian cancer and synergizes with PD-1 blockade in a B cell-dependent manner. Theranostics. 2020;10(23):10619-10633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Oshiro H, Tome Y, Miyake K, et al. Combination of CDK4/6 and mTOR inhibitors suppressed doxorubicin-resistant osteosarcoma in a patient-derived orthotopic xenograft mouse model: A translatable strategy for recalcitrant disease. Anticancer Res. 2021;41(7):3287-3292. [DOI] [PubMed] [Google Scholar]
- 41.Higuchi T, Igarashi K, Yamamoto N, et al. Osteosarcoma patient-derived orthotopic Xenograft (PDOX) models used to identify novel and effective therapeutics: A review. Anticancer Res. 2021;41(12):5865-5871. [DOI] [PubMed] [Google Scholar]
- 42.Landuzzi L, Manara MC, Lollini PL, et al. Patient derived xenografts for genome-driven therapy of osteosarcoma. Cells. 2021;10(2):416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Xin X, Lu Y, Xie S, et al. miR-155 accelerates the growth of human liver cancer cells by activating CDK2 via targeting H3F3A. Mol Ther Oncolytics. 2020;17:471-483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang L, Li Y, Hu C, et al. CDK6-PI3K signaling axis is an efficient target for attenuating ABCB1/P-gp mediated multi- drug resistance (MDR) in cancer cells. Mol Cancer. 2022;21(1):103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chaudhary S, Pothuraju R, Rachagani S, et al. Dual blockade of EGFR and CDK4/6 delays head and neck squamous cell carcinoma progression by inducing metabolic rewiring. Cancer Lett. 2021;510:79-92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang R, Xu K, Gao F, et al. Clinical considerations of CDK4/6 inhibitors in triple-negative breast cancer. Biochim Biophys Acta Rev Cancer. 2021;1876(2):188590. [DOI] [PubMed] [Google Scholar]
- 47.Guenther LM, Dharia NV, Ross L, et al. A combination CDK4/6 and IGF1R inhibitor strategy for ewing sarcoma. Clin Cancer Res . 2019;25(4):1343-1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Li C, Li X. circPTEN suppresses colorectal cancer progression through regulating PTEN/AKT pathway. Mol Ther Nucleic Acids. 2021;26:1418-1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhao S, Li W, Yu W, et al. Exosomal miR-21 from tubular cells contributes to renal fibrosis by activating fibroblasts via targeting PTEN in obstructed kidneys. Theranostics. 2021;11(18):8660-8673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu Y, Xu C, Xiao X, et al. Overcoming resistance to oncolytic virus M1 by targeting PI3K-γ in tumor-associated myeloid cells. Mol Ther. 2022;.S1525-0016(22):00306-00309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chen C, Guo Y, Huang Q, et al. PI3K Inhibitor impairs tumor progression and enhances sensitivity to anlotinib in anlotinib-resistant osteosarcoma. Cancer Lett. 2022;536:215660. [DOI] [PubMed] [Google Scholar]
- 52.Zhu D, Chen C, Liu X, et al. Osteosarcoma cell proliferation suppression via SHP-2-mediated inactivation of the JAK/STAT3 pathway by tubocapsenolide A. J Adv Res. 2021;34:79-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wei QT, Liu BY, Ji HY, et al. Exosome-mediated transfer of MIF confers temozolomide resistance by regulating TIMP3/PI3K/AKT axis in gliomas. Mol Ther Oncolytics. 2021;22:114-128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu W, Jing D, Meng Z, et al. FGD1 Promotes tumor progression and regulates tumor immune response in osteosarcoma via inhibiting PTEN activity. Theranostics. 2020;10(6):2859-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Grignani G, Palmerini E, Ferraresi V, et al. Sorafenib and everolimus for patients with unresectable high-grade osteosarcoma progressing after standard treatment: A non-randomised phase 2 clinical trial. Lancet Oncol. 2015;16(1):98-107. [DOI] [PubMed] [Google Scholar]
- 56.Pignochino Y, Dell’Aglio C, Basiricò M, et al. The combination of sorafenib and everolimus abrogates mTORC1 and mTORC2 upregulation in osteosarcoma preclinical models. Clin Cancer Res. 2013;19(8):2117-2131. [DOI] [PubMed] [Google Scholar]
- 57.Yong H, Wu G, Chen J, et al. lncRNA MALAT1 accelerates skeletal muscle cell apoptosis and inflammatory response in sepsis by decreasing BRCA1 expression by recruiting EZH2. Mol Ther Nucleic Acids. 2020;21:1120-1121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun H, Zeng J, Miao Z, et al. Dissecting the heterogeneity and tumorigenesis of BRCA1 deficient mammary tumors via single cell RNA sequencing. Theranostics. 2021;11(20):9967-9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Buttarelli M, Ciucci A, Palluzzi F, et al. Identification of a novel gene signature predicting response to first-line chemotherapy in BRCA wild-type high-grade serous ovarian cancer patients. J Exp Clin Cancer Res. 2022;41(1):50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Chen D, Gervai JZ, Póti Á, et al. BRCA1 deficiency specific base substitution mutagenesis is dependent on translesion synthesis and regulated by 53BP1. Nat Commun. 2022;13(1):226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Trusler O, Goodwin J, Laslett AL. BRCA1 and BRCA2 associated breast cancer and the roles of current modelling systems in drug discovery. Biochim Biophys Acta Rev Cancer. 2021;1875(1):188459. [DOI] [PubMed] [Google Scholar]
- 62.Lee O, Bosland MC, Wang M, et al. Selective progesterone receptor blockade prevents BRCA1-associated mouse mammary tumors through modulation of epithelial and stromal genes. Cancer Lett . 2021;520:255-266. [DOI] [PubMed] [Google Scholar]
- 63.Zoumpoulidou G, Alvarez-Mendoza C, Mancusi C, et al. Therapeutic vulnerability to PARP1,2 inhibition in RB1-mutant osteosarcoma. Nat Commun. 2021;12(1):7064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kovac M, Blattmann C, Ribi S, et al. Exome sequencing of osteosarcoma reveals mutation signatures reminiscent of BRCA deficiency. Nat Commun. 2015;6:8940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Feng Y, Sassi S, Shen JKet al. Targeting CDK11 in osteosarcoma cells using the CRISPR-Cas9 system. J Orthop Res. 2015;33(2):199-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rühle A, Ping D, Lopez Perez R, et al. Human mesenchymal stromal cells maintain their stem cell traits after high-LET particle irradiation – potential implications for particle radiotherapy and manned space missions. Cancer Lett . 2022;524:172-181. [DOI] [PubMed] [Google Scholar]
- 67.Koukourakis MI, Giatromanolaki A. Tumor draining lymph nodes, immune response, and radiotherapy: Towards a revisal of therapeutic principles. Biochim Biophys Acta Rev Cancer . 2022;1877(3):188704. [DOI] [PubMed] [Google Scholar]
- 68.Ma YS, Shi BW, Lu HM, et al. MicroRNA-499 serves as a sensitizer for lung cancer cells to radiotherapy by inhibition of CK2α-mediated phosphorylation of p65. Mol Ther Oncolytics. 2021;21:171-182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang D, Zhong D, Ouyang J, et al. Microalgae-based oral microcarriers for gut microbiota homeostasis and intestinal protection in cancer radiotherapy. Nat Commun. 2022;13(1):1413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Telarovic I, Wenger RH, Pruschy M. Interfering with tumor hypoxia for radiotherapy optimization. J Exp Clin Cancer Res. 2021;40(1):197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Jagodinsky JC, Jin WJ, Bates AM, et al. Temporal analysis of type 1 interferon activation in tumor cells following external beam radiotherapy or targeted radionuclide therapy. Theranostics. 2021;11(13):6120-6137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Eke I, Bylicky MA, Sandfort V, et al. The lncRNAs LINC00261 and LINC00665 are upregulated in long-term prostate cancer adaptation after radiotherapy. Mol Ther Nucleic Acids. 2021;24:175-187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chen EL, Yoo CH, Gutkin PM, et al. Outcomes for pediatric patients with osteosarcoma treated with palliative radiotherapy. Pediatr Blood Cancer. 2020;67(1):e27967. [DOI] [PubMed] [Google Scholar]
- 74.Matsunobu A, Imai R, Kamada T, et al. Impact of carbon ion radiotherapy for unresectable osteosarcoma of the trunk. Cancer. 2012;118(18):4555-4563. [DOI] [PubMed] [Google Scholar]
- 75.Du C, Zhou M, Jia F, et al. D-arginine-loaded metal-organic frameworks nanoparticles sensitize osteosarcoma to radiotherapy. Biomaterials. 2021;269:120642. [DOI] [PubMed] [Google Scholar]
- 76.PosthumaDeBoer J, Würdinger T, Graat HC, et al. WEE1 Inhibition sensitizes osteosarcoma to radiotherapy. BMC Cancer. 2011;11:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Xu M, Wang Z, Yu XC, et al. Guideline for limb-salvage treatment of osteosarcoma. Orthop Surg. 2020;12(4):1021-1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Han G, Bi WZ, Xu M, et al. Amputation versus limb-salvage surgery in patients with osteosarcoma: A meta-analysis. World J Surg. 2016;40(8):2016-2027. [DOI] [PubMed] [Google Scholar]
- 79.Evans DR, Lazarides AL, Visgauss JD, et al. Limb salvage versus amputation in patients with osteosarcoma of the extremities: An update in the modern era using the national cancer database. BMC Cancer. 2020;20(1):995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Harrison DJ, Geller DS, Gill JD, et al. Current and future therapeutic approaches for osteosarcoma. Expert Rev Anticancer Ther. 2018;18(1):39-50. [DOI] [PubMed] [Google Scholar]
- 81.Mangat KS, Jeys LM, Carter SR. Latest developments in limb-salvage surgery in osteosarcoma. Expert Rev Anticancer Ther. 2011;11(2):205-215. [DOI] [PubMed] [Google Scholar]
- 82.Qi L, Ren X, Liu Z, et al. Predictors and survival of patients with osteosarcoma after limb salvage versus amputation: A population-based analysis with propensity score matching. World J Surg. 2020;44(7):2201-2210. [DOI] [PubMed] [Google Scholar]
- 83.Traven SA, Brinton DL, Walton ZJ, et al. A propensity-score matched analysis of limb salvage vs amputation for osteosarcoma. J Surg Oncol. 2019;120(7):1252-1258. [DOI] [PubMed] [Google Scholar]
- 84.Agrawal N, DeFazio MV, Bird JE, et al. Computer-aided design and computer-aided manufacturing for pelvic tumor resection and free fibula flap reconstruction. Plast Reconstr Surg. 2020;145(4):889e-890e. [DOI] [PubMed] [Google Scholar]
- 85.Wong KC, Kumta SM. Computer-assisted tumor surgery in malignant bone tumors. Clin Orthop Relat Res. 2013;471(3):750-761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Suzuki K, Yasuda T, Suzawa S, et al. Fibroma of tendon sheath around large joints: Clinical characteristics and literature review. BMC Musculoskelet Disord. 2017;18(1):376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Haynes KK, Rosenthal HG. The ever-changing world of limb salvage surgery for malignant bone tumors. Nurs Clin North Am. 2020;55(2):251-266. [DOI] [PubMed] [Google Scholar]
- 88.Miao J, Shen Y, Li C, et al. Cervical artificial disc replacement with discover prosthesis does not reduce the midterm risk of heterotopic ossification: Results of a cohort study. Clin Spine Surg. 2018;31(3):E204-E208. [DOI] [PubMed] [Google Scholar]
- 89.Ferraro D, Siegler S, Belvedere C, et al. Effect of artificial surface shapes and their malpositioning on the mechanics of the replaced ankle joint for possible better prosthesis designs . Clin Biomech (Bristol, Avon) . 2021;90:105489. [DOI] [PubMed] [Google Scholar]
- 90.Walker MJ. On replacement body parts. J Bioeth Inq. 2019;16(1):61-73. [DOI] [PubMed] [Google Scholar]
- 91.Iorio ML. Hand, wrist, forearm, and arm replantation. Hand Clin. 2019;35(2):143-154. [DOI] [PubMed] [Google Scholar]
- 92.Shegarfi H, Reikeras O. Review article: Bone transplantation and immune response. J Orthop Surg (Hong Kong) . 2009;17(2):206-211. [DOI] [PubMed] [Google Scholar]
- 93.Meikle MC. On the transplantation, regeneration and induction of bone: The path to bone morphogenetic proteins and other skeletal growth factors. Surgeon. 2007;5(4):232-243. [DOI] [PubMed] [Google Scholar]
- 94.Chen K, Huang L, Cai Z, et al. Micro-invasive surgery combined with intraoperative radiotherapy for the treatment of spinal metastasis. Eur Spine J. 2017;26(7):1893-1901. [DOI] [PubMed] [Google Scholar]
- 95.Kager L, Tamamyan G, Bielack S. Novel insights and therapeutic interventions for pediatric osteosarcoma. Future Oncol. 2017;13(4):357-368. [DOI] [PubMed] [Google Scholar]
- 96.Chen H, Yao Y. Progress of biomaterials for bone tumor therapy . J Biomater Appl . 2022;36(6):945-955. [DOI] [PubMed] [Google Scholar]
- 97.Dupin CM, Estaquio C, Nabi H. Theoretical conceptions of intervention research addressing cancer control issues. Health Promot Int. 2021;36(1):206-215. [DOI] [PubMed] [Google Scholar]
- 98.Zhang S, Liu Y, Sun S, et al. Catalytic patch with redox Cr/CeO2 nanozyme of noninvasive intervention for brain trauma. Theranostics. 2021;11(6):2806-2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Dalili D, Isaac A, Cazzato RL, et al. Interventional techniques for bone and musculoskeletal soft tissue tumors: Current practices and future directions - part II. Stabilization. Semin Musculoskelet Radiol. 2020;24(6):710-725. [DOI] [PubMed] [Google Scholar]
- 100.Liapi E, Geschwind JF. Transcatheter and ablative therapeutic approaches for solid malignancies. J Clin Oncol. 2007;25(8):978-986. [DOI] [PubMed] [Google Scholar]
- 101.Mavrogenis AF, Rossi G, Palmerini E, et al. Palliative treatments for advanced osteosarcoma. J BUON . 2012;17(3):436-445. [PubMed] [Google Scholar]
- 102.Jiang C, Wang J, Wang Y, et al. Treatment outcome following transarterial chemoembolization in advanced bone and soft tissue sarcomas. Cardiovasc Intervent Radiol. 2016;39(10):1420-1428. [DOI] [PubMed] [Google Scholar]
- 103.Chu JP, Chen W, Li JP, et al. Clinicopathologic features and results of transcatheter arterial chemoembolization for osteosarcoma. Cardiovasc Intervent Radiol. 2007;30(2):201-206. [DOI] [PubMed] [Google Scholar]
- 104.Kawai Y, Kawana-Tachikawa A, Kitayama S, et al. Generation of highly proliferative, rejuvenated cytotoxic T cell clones through pluripotency reprogramming for adoptive immunotherapy. Mol Ther. 2021;29(10):3027-3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Ji P, Yang Z, Li H, et al. Smart exosomes with lymph node homing and immune-amplifying capacities for enhanced immunotherapy of metastatic breast cancer. Mol Ther Nucleic Acids. 2021;26:987-996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jiang M, Zhao L, Cui X, et al. Cooperating minimalist nanovaccine with PD-1 blockade for effective and feasible cancer immunotherapy. J Adv Res. 2021;35:49-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chaurasiya S, Kim SI, O’Leary M, et al. Toward comprehensive imaging of oncolytic viroimmunotherapy. Mol Ther Oncolytics. 2021;23:303-310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Ogiwara Y, Nakagawa M, Nakatani F, et al. Blocking FSTL1 boosts NK immunity in treatment of osteosarcoma. Cancer Lett. 2022;537:215690. [DOI] [PubMed] [Google Scholar]
- 109.Qian H, Fu Y, Guo M, et al. Dual-aptamer-engineered M1 macrophage with enhanced specific targeting and checkpoint blocking for solid-tumor immunotherapy. Mol Ther. 2022;.30(8):2817–2827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Li H, Dai H, Li J. Immunomodulatory properties of mesenchymal stromal/stem cells: the link with metabolism. J Adv Res. 2022;S2090-1232(22):00125-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kandel S, Adhikary P, Li G, et al. The TIM3/Gal9 signaling pathway: An emerging target for cancer immunotherapy. Cancer Lett. 2021;510:67-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yu Z, Ma B, Zhou Y, et al. Allogeneic tumor vaccine produced by electrofusion between osteosarcoma cell line and dendritic cells in the induction of antitumor immunity. Cancer Invest. 2007;25(7):535-541. [DOI] [PubMed] [Google Scholar]
- 113.Dhupkar P, Gordon N. Interleukin-2: Old and new approaches to enhance immune-therapeutic efficacy. Adv Exp Med Biol. 2017;995:33-51. [DOI] [PubMed] [Google Scholar]
- 114.Meazza C, Cefalo G, Massimino M, et al. Primary metastatic osteosarcoma: Results of a prospective study in children given chemotherapy and interleukin-2. Med Oncol . 2017;34(12):191. [DOI] [PubMed] [Google Scholar]
- 115.Tang Y, Yin H, Zhao X, et al. High efficacy and safety of CD38 and BCMA bispecific CAR-T in relapsed or refractory multiple myeloma. J Exp Clin Cancer Res. 2022;41(1):2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Jain MD. Preliminary outcomes reported from three randomized controlled trials of CD19 CAR-T cell therapies in large B cell lymphoma. Mol Ther. 2022;30(1):14-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Jia Q, Qin D, He F, et al. Peripheral eosinophil counts predict efficacy of anti-CD19 CAR-T cell therapy against B-lineage non-Hodgkin lymphoma. Theranostics . 2021;11(10):4699-4709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang Q, Liu G, Liu J, et al. The antitumor capacity of mesothelin-CAR-T cells in targeting solid tumors in mice. Mol Ther Oncolytics. 2021;20:556-568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang C, Palashati H, Rong Z, et al. Pre-depletion of TRBC1 + T cells promotes the therapeutic efficacy of anti- TRBC1 CAR-T for T-cell malignancies . Mol Cancer. 2020;19(1):162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Huang R, Wang X, Zhang X. Unity brings strength: Combination of CAR-T cell therapy and HSCT. Cancer Lett. 2022;1(1):215721. [DOI] [PubMed] [Google Scholar]
- 121.Li H, Harrison EB, Li H, et al. Targeting brain lesions of non-small cell lung cancer by enhancing CCL2-mediated CAR-T cell migration. Nat Commun. 2022;13(1):2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhu J, Simayi N, Wan R, et al. CAR T targets and microenvironmental barriers of osteosarcoma. Cytotherapy. 2022;24(6):567-576. [DOI] [PubMed] [Google Scholar]
- 123.Lin Z, Wu Z, Luo W. Chimeric antigen receptor T-cell therapy: The light of day for osteosarcoma. Cancers (Basel). 2021;13(17):4469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wang Y, Yu W, Zhu J, et al. Anti-CD166/4-1BB chimeric antigen receptor T cell therapy for the treatment of osteosarcoma. J Exp Clin Cancer Res. 2019;38(1):168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Ahmed N, Brawley VS, Hegde M, et al. Human epidermal growth factor receptor 2 (HER2) -specific chimeric antigen receptor-modified T cells for the immunotherapy of HER2-positive sarcoma. J Clin Oncol. 2015;33(15):1688-1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Safi M, Ahmed H, Al-Azab M, et al. PD-1/PDL-1 inhibitors and cardiotoxicity; molecular, etiological and management outlines. J Adv Res. 2020;29:45-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Li P, Rozich N, Wang J, et al. Anti- IL-8 antibody activates myeloid cells and potentiates the anti-tumor activity of anti-PD-1 antibody in the humanized pancreatic cancer murine model. Cancer Lett. 2022;539:215722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sitnik S, Masemann D, Leite Dantas R, et al. PD-1 IC inhibition synergistically improves influenza A virus-mediated oncolysis of metastatic pulmonary melanoma. Mol Ther Oncolytics. 2020;17:190-204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Wu CC, Wang YA, Livingston JA, et al. Prediction of biomarkers and therapeutic combinations for anti-PD-1 immunotherapy using the global gene network association. Nat Commun. 2022;13(1):42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Shimizu T, Fuchimoto Y, Okita H, et al. A curative treatment strategy using tumor debulking surgery combined with immune checkpoint inhibitors for advanced pediatric solid tumors: An in vivo study using a murine model of osteosarcoma. J Pediatr Surg. 2018;53(12):2460-2464. [DOI] [PubMed] [Google Scholar]
- 131.Kaneda M.M., Messer K.S., Ralainirina N., et al. PI3Kγ is a molecular switch that controls immune suppression. Nature. 2016;539:437-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cersosimo F, Lonardi S, Bernardini Get al. et al. Tumor-associated macrophages in osteosarcoma: From mechanisms to therapy. Int J Mol Sci. 2020;21(15):5207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Saumet L, Deschamps F, Marec-Berard P, et al. Radiofrequency ablation of metastases from osteosarcoma in patients under 25 years: The SCFE experience. Pediatr Hematol Oncol. 2015;32(1):41-49. [DOI] [PubMed] [Google Scholar]
- 134.Yu W, Tang L, Lin F, et al. High-intensity focused ultrasound: Noninvasive treatment for local unresectable recurrence of osteosarcoma. Surg Oncol. 2015;24(1):9-15. [DOI] [PubMed] [Google Scholar]
- 135.Li J, Lu Y, Chen G, et al. Cryoablation-aided joint retention surgery for epiphysis involvement in osteosarcoma compared with endoprosthetic replacement. Bone Joint J. 2021;103-B(8):1421-1427. [DOI] [PubMed] [Google Scholar]
- 136.Li C, Wu P, Zhang L, et al. Osteosarcoma: Limb salvaging treatment by ultrasonographically guided high-intensity focused ultrasound. Cancer Biol Ther. 2009;8(12):1102-1108. [DOI] [PubMed] [Google Scholar]
- 137.Wang C. Therapeutic effects of adriamycin combined with high-intensity focused ultrasound on osteosarcoma. J BUON. 2019;24(2):826-831. [PubMed] [Google Scholar]
- 138.Agnese V, Costa V, Scoarughi GL, et al. Focused ultrasound effects on osteosarcoma cell lines. Biomed Res Int. 2019;2019:6082304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Zhang L, Wang ZB. High-intensity focused ultrasound tumor ablation: Review of ten years of clinical experience . Front Med China. 2010;4(3):294-302. [DOI] [PubMed] [Google Scholar]
- 140.Bielack SS, Marina N, Bernstein M. High-intensity focused ultrasound (HIFU) is not indicated for treatment of primary bone sarcomas. Cancer. 2011;117(12):2822. [DOI] [PubMed] [Google Scholar]
- 141.Orgera G, Monfardini L, Della Vigna P, et al. High-intensity focused ultrasound (HIFU) in patients with solid malignancies: Evaluation of feasibility, local tumour response and clinical results. Radiol Med. 2011;116(5):734-748. [DOI] [PubMed] [Google Scholar]
- 142.Chen W, Zhu H, Zhang L, et al. Primary bone malignancy: Effective treatment with high-intensity focused ultrasound ablation. Radiology. 2010;255(3):967-978. [DOI] [PubMed] [Google Scholar]
- 143.Wu F, Wang ZB, Chen WZ, et al. Extracorporeal high intensity focused ultrasound ablation in the treatment of 1038 patients with solid carcinomas in China: An overview. Ultrason Sonochem. 2004;11(3–4):149-154. [DOI] [PubMed] [Google Scholar]
- 144.Yevich S, Gaspar N, Tselikas L, et al. Percutaneous computed tomography-guided thermal ablation of pulmonary osteosarcoma metastases in children. Ann Surg Oncol. 2016;23(4):1380-1386. [DOI] [PubMed] [Google Scholar]
- 145.Koo JS, Chung SH. The efficacy of radiofrequency ablation for bone tumors unsuitable for radical excision. Clin Orthop Surg. 2021;13(2):278-285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tang QL, Zhao ZQ, Li JC, et al. Salinomycin inhibits osteosarcoma by targeting its tumor stem cells. Cancer Lett. 2011;311(1):113-121. [DOI] [PubMed] [Google Scholar]
- 147.Wang L, Mei Q, Xie Q, et al. A comparative study of mesenchymal stem cells transplantation approach to antagonize age-associated ovarian hypofunction with consideration of safety and efficiency. J Adv Res. 2021;38:245-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Brown HK, Tellez-Gabriel M, Heymann D. Cancer stem cells in osteosarcoma. Cancer Lett. 2017;386:189-195. [DOI] [PubMed] [Google Scholar]
- 149.Hammad M, Cornejo YR, Batalla-Covello J, et al. Neural stem cells improve the delivery of oncolytic chimeric orthopoxvirus in a metastatic ovarian cancer model. Mol Ther Oncolytics. 2020;18:326-334. [DOI] [PMC free article] [PubMed] [Google Scholar]