Abstract
Objective
Rapid eye movement (REM) obstructive sleep apnea (OSA) is associated with the risk of cardiovascular events. Arterial stiffness and carotid artery intima-media thickness (IMT) predict these events, but few relevant studies have been conducted. We compared long-term changes in arterial stiffness and IMT between patients with REM OSA and non-REM (NREM) OSA receiving continuous positive airway pressure (CPAP) or oral appliance (OA) therapy.
Methods
Newly diagnosed female patients with OSA received CPAP (n = 6) or OA (n = 7). Pulse wave velocity (PWV) and carotid artery ultrasound were performed before and 60 months after treatment.
Results
There were no differences in baseline characteristics (mean age: 56.0 vs. 61.3 years; mean body mass index: 22.6 vs. 21.7 kg/m2) between the REM OSA and non-REM OSA groups. The median apnea-hypopnea index was lower in the REM OSA group than in the non-REM OSA group. Increased PWV (12.92 ± 1.64 to 14.56 ± 2.73 m/s) and deteriorated glucose metabolism were observed in the REM OSA group after treatment. PWV, IMT, and cardiovascular risk factors were unaffected in the non-REM OSA group.
Conclusion
Arterial stiffness and glucose metabolism are deteriorated in patients with REM OSA compared with these parameters in patients with non-REM OSA after CPAP or OA treatment.
Keywords: Rapid eye movement, obstructive sleep apnea, cardiovascular event, arterial stiffness, intima-media thickness, glucose metabolism
Introduction
Obstructive sleep apnea syndrome (OSA) is a common sleep-associated breathing disorder characterized by repeated narrowing or closure of the upper airway, intermittent hypoxia or apnea, and negative pressure changes in the chest cavity during sleep.1,2 OSA is associated with cardiovascular events and adverse metabolic outcomes as shown in epidemiological research. 3 Rapid eye movement (REM) OSA, which is observed in 12% to 36% of patients with OSA, is defined by a disease presentation of apnea and hypopnea that frequently occur during REM sleep.4,5 REM OSA generally presents with mild to moderate severity and occurs more commonly in women. 6 Moreover, REM OSA is independently associated with cardiovascular events and metabolic outcomes.7,8
Carotid-femoral pulse wave velocity (PWV) and carotid arterial wall intima-media thickness (IMT) are commonly measured and easily obtainable parameters that are useful markers of early atherosclerosis. 9 PWV is also a measure of arterial stiffness. PWV and IMT in the carotid artery are associated with a range of chronic conditions and health behaviors, such as metabolic syndrome, hypertension, diabetes, hyperlipidemia, chronic obstructive pulmonary disease, 10 and smoking. PWV and IMT have a significantly higher prevalence in patients with severe OSA than in patients with mild to moderate OSA or healthy controls.11–14 Furthermore, a high risk of OSA was associated with greater PWV and IMT in a cohort study. 15
Continuous positive airway pressure (CPAP) is considered the gold standard therapy for moderate to severe OSA, but is not effective in preventing cardiovascular events in moderate to severe OSA in the long term.16–18 Better outcomes were reported in patients with good adherence to CPAP treatment protocols (≥4 hours/night) than in patients who did not use CPAP or had poor adherence to their prescribed CPAP protocol. 19 Moreover, therapy with an adjustable oral appliance (OA) is associated with a higher adherence compared with CPAP. This intervention is frequently prescribed for patients with mild to moderate OSA or for those with severe OSA who cannot use CPAP.20,21 OA is effective in patients with moderate to severe OSA.20,21 However, the long-term efficacy of OA in preventing cardiovascular events remains to be determined.21,22
In a short-term observational study, CPAP therapy significantly improved PWV. 23 However, to date, literature regarding the long-term efficacy of CPAP or OA for improving PWV and IMT outcomes is limited.24,25 To the best of our knowledge, no data on either modality in patients with REM OSA have been reported. Therefore, in this study, we aimed to prospectively compare long-term changes in PWV, carotid artery IMT, and cardiovascular risk factors between female patients with REM OSA and those with non-REM OSA treated with CPAP or OA.
Patients and methods
Patients
This study enrolled patients aged ≥45 years who were newly diagnosed with OSA and who were evaluated by full laboratory polysomnography (PSG) at the Division of Comprehensive Sleep Medicine at a Tokyo Women’s Medical University Hospital between August 2011 and May 2013. We excluded men and those with cardiovascular diseases and other sleep disorder complications from the current analysis. Patients using antihypertensive, antihyperlipidemic, or hypoglycemic medications were included in this study. The women enrolled in this study agreed to undergo carotid artery ultrasonography and a PWV examination before and 60 months after receiving OSA treatment. This study was conducted in accordance with the tenets of the amended Declaration of Helsinki. The study was approved by the ethics committee of Tokyo Women’s Medical University (approval number: 2235), and written informed consent was obtained from all participants. To de-identify all patients’ details, each patient was assigned a random number, and their name was omitted. A table correlating numbers and participants’ information was maintained by a person who was not directly involved in the current study.
Twenty-three women were included in the study after men were excluded. Eleven patients were treated with OA and 12 were treated with CPAP. Two patients treated with OA and six patients treated with CPAP did not attend the follow-up study visit. Two patients treated with OA were excluded from the final analysis because their medications were changed within the 60-month follow-up. Among the 23 patients originally enrolled, the data of 13 (6 treated with CPAP and 7 treated with OA) patients were included in the final analysis.
REM OSA was defined according to an apnea-hypopnea index (AHI) of ≥5 and an AHI-REM/AHI-NREM (AHI during REM divided by the AHI during non-REM sleep) value of ≥2. The patients were divided into the two groups of REM OSA and non-REM OSA.
Study design
We conducted a prospective, observational study. We evaluated carotid ultrasonography, PWV, blood pressure, laboratory findings (blood samples), Epworth Sleepiness Scale (ESS) scores, Athene Insomnia Scale (AIS) scores, and adherence to treatment before and after the follow-up. Adherence (%) to CPAP was obtained from the card reader in the CPAP device and was defined as daily CPAP use for ≥4 hours/night divided by the total days of CPAP use. We obtained information regarding the adherence to OA from patients’ interviews evaluating the ratio of daily use of OA >4 hours. Among CPAP users, CPAP titrations were examined through full laboratory PSG readings at the start of treatment. AHI values were obtained from the card reader of the CPAP device at the follow-up. Among OA users, sleep parameters (including AHI) were derived from full laboratory PSG that was applied 6 months after treatment. The reporting of this study conforms to the STROBE guidelines. 26
Cardiovascular risk factors
Blood pressure was measured at the hospital with the patient in a sitting position after a rest period of 15 minutes. Hypertension was diagnosed if blood pressure values exceeded 140/90 mmHg or if the patient was currently using antihypertensive medications. A fasting blood screening examination was performed to determine the presence of diabetes mellitus and hyperlipidemia. Patients were diagnosed with diabetes mellitus if they had a glycated hemoglobin value of ≥ 47.5 mmol/L (in accordance with the guidelines set forth by the National Glycohemoglobin Standardization Program), a history of diabetes, or if they were receiving antidiabetic therapy. Hyperlipidemia was defined as a low-density lipoprotein concentration of ≥3.6 mmol/L, triglyceride concentration of ≥1.7 mmol/L, or the use of lipid-lowering medication.
Laboratory PSG
A full laboratory PSG examination was performed using a Neurofax EEG-9200 reader (Nihon Kohden, Tokyo, Japan). Sleep parameters were recorded using electroencephalography, electrocardiography, electromyography, and electro-oculography. Additional evaluated parameters were a nasal thermistor and prong pressure, respiratory inductance bands, peripheral arterial oxygen saturation, body position, and snoring sounds. All sleep parameters were scored manually by a well-trained technician in accordance with the recommendations of the American Academy of Sleep Medicine. Mild, moderate, and severe OSA were defined as AHI levels of 5 to 14.9/hour, 15 to 29.9/hour, and ≥30/hour, respectively.
Carotid IMT
Carotid artery IMT was determined by measuring the distance between the leading edge of the luminal echo and the media/adventitia echo by high-resolution B-mode ultrasonography and a 7.5-MHz linear array probe (LOGIQ-S8; GE Healthcare Japan, Tokyo, Japan). We implemented the ultrasonographic protocol reported in a prior Japanese cohort study, 27 including a single longitudinal lateral view of the distal 10 mm of the right and left common carotid arteries (CCAs) and three longitudinal views of the internal carotid artery (ICA). The severity of carotid atherosclerosis was assessed using the maximal carotid wall IMT of the ICA (ICA-MaxIMT) and CCA (CCA-MaxIMT) to determine the single thickest wall of the right and left ICA/CCA. 27
Carotid-femoral PWV
PWV was recorded in the morning. This value was calculated from the obtained pulse transit time and the distance traveled between the two recording sites using a noninvasive automatic device (BP-203 RPE; Fukuda-Colin, Tokyo, Japan) after 15 minutes of bed rest in fasting patients.
Statistical analysis
We applied the two-sample t-test to compare age and body mass index between the REM OSA and non-REM OSA groups. The Mann–Whitney test was used to compare other (i.e., categorical/non-continuous) variables. Wilcoxon analysis was applied to compare ICA-MaxIMT values, CCA-MaxIMT values, high-sensitivity C-reactive protein concentrations, and adherence to the prescribed CPAP or OA protocols before and after follow-up. Wilcoxon analysis was used to evaluate the AHI, the ratio of stages 1 to 3 sleep to the total sleep time (TST) (%), and REM sleep to TST (%) in the REM OSA group before and after follow-up. The paired t-test was used to compare other relevant variables before and after follow-up. All calculations were performed using SPSS statistical software (version 17; SPSS Inc., Chicago, IL, USA). Statistical significance was set to a P value of <0.05.
Results
Patients’ characteristics
Table 1 shows comparison of the patients’ characteristics between the REM OSA and non-REM OSAOSA groups. There were no significant differences in baseline characteristics (e.g., mean age, 56.0 ± 5.6 vs. 61.3 ± 10.3 years; mean body mass index, 22.6 ± 2.8 vs. 21.7 ± 2.6 kg/m2), including current treatments and overall medical history, between the REM OSA and non-REM OSA groups. The median AHI was significantly lower in the REM OSA group than in the non-REM-OSA group (P < 0.01). One mild and six moderate OSA cases, as well as three moderate and three severe OSA cases, were observed in the REM OSA and non-REM OSA groups, respectively.
Table 1.
Participants’ baseline medical and demographic characteristics.
| Variables | REM OSA group | Non-REM OSA group | P |
|---|---|---|---|
| Subjects | 7 | 6 | |
| Age (years) | 56.0 ± 5.6 | 61.3 ± 10.3 | 0.45 |
| BMI (kg/m2) | 22.6 ± 2.8 | 21.7 ± 2.6 | 0.56 |
| AHI (/hour) | 20.2 (18.9–21.6) | 31.0 (22.2–39.8) | <0.01 |
| CPAP (n): OA (n) | 2:5 | 4:2 | 0.30 |
| Medical history (%: no./total no.) | |||
| Hypertension | 2 (29) | 3 (50) | 0.53 |
| Diabetes mellitus | 0 | 0 | |
| Dyslipidemia | 4 (57) | 4 (67) | 0.84 |
| Past smoking | 1 (14) | 1 (17) | 0.95 |
| Current smoking | 0 | 0 | |
| Medications (%: no./total no.) | |||
| Antihypertensive medications | 2 (29) | 1 (17) | 0.73 |
| Ca blockers | 1 (14) | 1 (17) | 1.00 |
| Ca blockers + ARB | 1 (14) | 0 | |
| Statins | 0 | 2 (33) | 0.37 |
| Hypnotics | 1 (14) | 0 | |
| EPA | 0 | 1 (17) | 0.63 |
REM, rapid eye movement; OSA, obstructive sleep apnea; BMI, body mass index; AHI, apnea-hypopnea index; CPAP, continuous positive air pressure; OA, oral appliance; Ca, calcium; ARB, angiotensin receptor blocker; EPA, eicosapentaenoic acid.
Follow-up
Table 2 shows comparison of PSG parameters before and during treatment between the two groups. Before treatment, the mean AHI score and arousal index were significantly higher in the non-REM OSA group than in the REM OSA group (both P < 0.05). The arousal index and stage 1 sleep/TST ratio were significantly lower, whereas the stage 3 sleep/TST ratio and the minimum arterial oxygen saturation (SpO2) level were significantly higher during treatment than before treatment in the non-REM-OSA group (all P < 0.05). The stage 3 sleep/TST ratio and the REM sleep/TST ratio were significantly higher during treatment than before treatment in the REM-OSA group (both P < 0.05). However, the arousal index, stage 1 sleep/TST ratio, and minimum SpO2 level did not change during treatment within this group. The number of patients meeting the criteria for REM OSA decreased after treatment in the REM OSA group.
Table 2.
Changes in polysomnographic variables and symptoms between the REM OSA and non-REM OSA groups.
| Variables | REM OSA group (n = 7) |
Non-REM OSA group (n = 6) |
||||
|---|---|---|---|---|---|---|
| 0 months | CPAP or OA | P | 0 months | CPAP or OA | P | |
| Apnea-hypopnea index (/hour) | 20.2 (18.9–21.6)* | 3.2 (0.0–8.2) | 0.03 | 30.9 ± 9.1* | 5.6 ± 6.1 | <0.01 |
| Min SpO2 (%) | 82.5 ± 6.2 | 86.7 ± 8.1 | 0.27 | 83.2 ± 5.7 | 92.3 ± 4.3 | <0.01 |
| SpO2 <90% | 12.8 ± 13.5 | 0.5 ± 0.8 | 0.08 | 5.8 ± 5.6 | 0.1 ± 0.2 | 0.06 |
| Arousal index (/h) | 23.4 ± 4.6* | 18.1 ± 6.2 | 0.22 | 39.9 ± 10.3* | 19.0 ± 10.5 | 0.02 |
| N1 sleep/TST (%) | 48.5 (40.9–56.1) | 14.6 (11.2–18.1) | 0.11 | 45.2 ± 26.4 | 13.1 ± 8.1 | 0.04 |
| N2 sleep/TST (%) | 38.5 (28.8–57.9) | 55.1 (49.2–61.0) | 0.60 | 27.8 ± 19.4 | 52.4 ± 7.3 | 0.01 |
| N3 sleep/TST (%) | 0.3 (0.0–2.3) | 12.6 (6.5–18.7) | 0.03 | 9.0 ± 8.5 | 19.4 ± 2.1 | <0.05 |
| REM sleep/TST (%) | 10.1 (7.3–13.0) | 19.0 (13.6–24.4) | 0.03 | 18.1 ± 9.9 | 15.2 ± 4.2 | 0.59 |
| REM OSA (n) | 7 | 2 | <0.05 | 0 | 1 | 0.32 |
REM, rapid eye movement; OSA, obstructive sleep apnea; CPAP, continuous positive air pressure; OA, oral appliance; Min SpO2, minimum arterial oxygen saturation; N1, stage 1; N2, stage 2; N3, stage 3; TST, total sleep time.
*P < 0.05, compared between the REM and non-REM OSA groups before treatment.
Table 3 shows the comparative variables, such as PWV, CCA-maxIMT, ICAmaxIMT, cardiovascular risk factors, patient characteristics, ESS scores, and AIS scores before and after follow-up between the two groups. Before treatment, none of the variables were statistically significantly different between the REM OSA and nonREM OSA groups. The mean PWV was significantly increased after treatment compared with before treatment in the REM OSA group (12.92 ± 1.64 m/s to 14.56 ± 2.73 m/s, P = 0.04). Glycated hemoglobin and fasting blood sugar concentrations were significantly increased after treatment compared with before treatment in the REM OSA group (both P = 0.01), but body weight did not change. Adherence to treatment was satisfactory in all patients. The CCA-maxIMT, ICA-maxIMT, and blood pressure values did not significantly change in the REM OSA group or in the non-REM OSA group.
Table 3.
Changes in PWV, CCA-MaxIMT, ICA-MaxIMT, cardiovascular risk factors, and other data over the course of 60 months in female patients with OSA who received treatment with continuous positive air pressure or oral appliance.
| Variables | REM OSA group (n = 7) |
Non-REM OSA group (n = 6) |
||||
|---|---|---|---|---|---|---|
| 0 months | 60 months | P | 0 months | 60 months | P | |
| PWV (m/s) | 12.92 ± 1.64 | 14.56 ± 2.73 | 0.04 | 14.51 ± 2.64 | 15.38 ± 2.96 | 0.47 |
| CCA-MaxIMT (mm) | 0.8 (0.6–1.0) | 0.8 (0.5–1.1) | 0.19 | 0.9 (0.8–1.0) | 0.9 (0.7–1.1) | 0.89 |
| ICA-MaxIMT (mm) | 1.3 (1.1–1.5) | 1.3 (1.1–1.6) | 0.59 | 1.1 (0.6–1.6) | 1.2 (0.6–1.9) | 0.79 |
| Systolic blood pressure (mmHg) | 124.6 ± 14.7 | 117.7 ± 13.6 | 0.19 | 125.2 ± 18.0 | 122.0 ± 6.7 | 0.62 |
| Diastolic blood pressure (mmHg) | 77.6 ± 6.9 | 73.3 ± 9.4 | 0.28 | 78.0 ± 8.6 | 80.3 ± 4.1 | 0.58 |
| Triglycerides (mmol/L) | 1.5 ± 0.8 | 0.9 ± 0.5 | 0.02 | 1.9 ± 1.0 | 1.3 ± 0.9 | 0.24 |
| LDL cholesterol (mmol/L) | 3.4 ± 1.1 | 3.4 ± 0.6 | 0.96 | 3.3 ± 0.6 | 3.1 ± 0.7 | 0.48 |
| HbA1c (mmol/L) | 37.4 ± 3.7 | 39.1 ± 2.7 | 0.01 | 37.3 ± 3.3 | 40.4 ± 2.3 | 0.10 |
| FBS (mmol/L) | 4.7 ± 0.3 | 5.3 ± 0.4 | 0.01 | 5.4 ± 0.6 | 5.2 ± 0.2 | 0.47 |
| Adiponectin (mg/L) | 1.4 ± 0.7 | 1.7 ± 1.0 | 0.15 | 1.1 ± 0.4 | 1.2 ± 0.3 | 0.38 |
| CRP (mg/L) | 0.290 (0.000–0.675) | 0.506 (0.154–0.859) | 0.12 | 0.925 (0.000–2.087) | 0.168 (0.000–0.488) | 0.35 |
| Adherence (%) | 100.0 (75.0–100.0)* | 85.5 (46.4–100.0) | 0.46 | 87.5 (65.0–100.0)* | 75.0 (63.0–97.5) | 0.79 |
| Body weight (kg) | 55.7 ± 6.1 | 56.7 ± 7.1 | 0.64 | 51.5 ± 6.4 | 50.7 ± 6.2 | 0.58 |
| Waist circumference (cm) | 82.3 ± 10.0 | 83.1 ± 6.2 | 0.75 | 81.8 ± 7.6 | 80.9 ± 8.0 | 0.43 |
| ESS (points) | 10.3 ± 5.7 | 7.7 ± 2.8 | 0.44 | 5.5 ± 4.5 | 5.7 ± 4.3 | 0.87 |
| AIS (points) | 6.4 ± 5.8 | 6.0 ± 3.2 | 0.33 | 5.2 ± 3.1 | 4.2 ± 2.7 | 0.14 |
REM, rapid eye movement; OSA, obstructive sleep apnea; PWV, pulse wave velocity; CCA-Max IMT, maximal intima-media thickness in the common carotid artery; ICA-MaxIMT, maximal intima-media thickness in the internal carotid artery; LDL, low-density lipoprotein; HbA1c, glycated hemoglobin; FBS, fasting blood sugar; CRP, high-sensitivity C-reactive protein; ESS, Epworth Sleepiness Scale; AIS, Athens Insomnia Scale.
*Six months after treatment.
Discussion
In the current study, increased PWV values and a deterioration in glucose metabolism were observed in non-obese female patients with REM OSA compared with these parameters in patients with non-REM OSA who were treated with CPAP or OA for 60 months. The severity of OSA, as designated by the AHI score, was milder in patients with REM OSA than in those with non-REM OSA before treatment. We found that the arousal index, sleep structure, and minimum SpO2 improved with CPAP or OA in the non-REM OSA group. These changes were not observed in the REM OSA group.
A previous report showed that age and the minimum SpO2 level were independent factors correlated with PWV in patients with OSA. 28 In the current study, the minimum SpO2 level did not improve with CPAP or OA in the REM OSA group, which might have been related to the increased PWV values after treatment.
Prior reports have suggested that sympathetic tone does not decrease in patients with REM OSA. 29 Whereas, the current study showed a deterioration in glucose metabolism after treatment. These findings might affect the progression of arterial stiffness in patients with REM OSA. Moreover, Hoshino et al. reported a low respiratory arousal threshold in patients with REM OSA than in those with non-REM OSA. 30 In the current study, although the arousal index in the enrolled patients with REM OSA was lower than that in patients with non-REM OSA before treatment, sympathetic tone might have been elevated in patients with REM-OSA. This in turn might have affected the progression of arterial stiffness.
A previous report showed that PWV values were significantly increased following CPAP treatment in accordance with age and hypertension, regardless of the severity of OSA at diagnosis or adherence to CPAP. 24 In the present study, an increased PWV was observed only in the REM OSA group, while blood pressure did not change in the REM OSA or non-REM OSA group. Therefore, we conclude that a range of factors (e.g., minimum SpO2 level, sympathetic tone, and glucose metabolism) might affect arterial stiffness in patients with REM OSA. Additional studies with larger sample sizes are necessary to determine the effects of CPAP or OA on arterial stiffness in non-obese female patients with REM OSA.
The current study showed no differences in carotid artery IMT during treatment with CPAP or OA in the REM OSA and non-REM OSA groups. We previously reported that the arousal index, diabetes mellitus status, and age were independent predictors of an ICA-MaxIMT of ≥1.5 mm in patients with OSA. 31 In the current study, carotid artery atherosclerosis was mild before treatment, the deterioration in glucose metabolism was limited, and the IMT was not different between the REM OSA and non-REM OSA groups.
There were some limitations to this study. First, this was an observational study with a small sample size. This study excluded men because REM OSA is more frequently observed in women. However, patients with OSA have been reported to be mostly men (66.7%–77.4%) in cohorts aged 50 to 75 years. 15 All eligible patients were enrolled between August 2011 and May 2013 as a total sample of 23 women, after excluding men, and prospectively followed up for 60 months. Because of the small sample size, future studies with a larger number of patients are warranted. Second, most patients had one or more cardiovascular risk factors, which might have affected inflammation and worsened arterial stiffness and carotid artery atherosclerosis. However, we did not examine the disease severity or management with specific medications in detail. Another potential limitation of this study is that, although past smokers were included in our study, we could not investigate the effects of smoking owing to lack of statistical power. Future highly-powered studies should be conducted with the aim of comprehensively determining the long-term effects of CPAP or OA on PWV and IMT in patients with OSA and minimal risk factors.
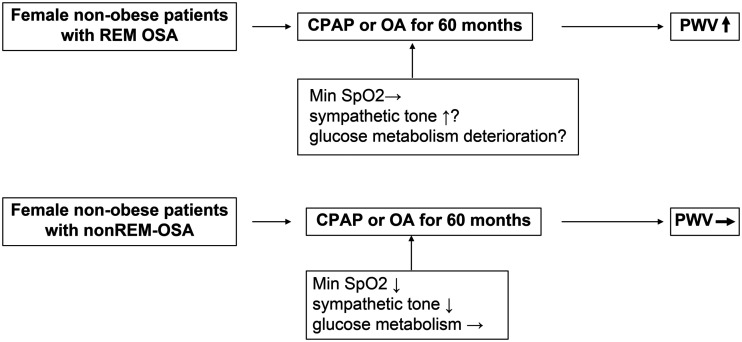
In conclusion, arterial stiffness and glucose metabolism worsened in non-obese female patients with REM OSA compared with these parameters in patients with non-REM-OSA who were treated with CPAP or OA over the course of 60 months (Figure 1).
Figure 1.
Main results of the study.
REM, rapid eye movement; OSA, obstructive sleep apnea; CPAP, continuous positive air pressure; OA, oral appliance; PWV, pulse wave velocity; Min SpO2, minimum arterial oxygen saturation.
Supplemental Material
Supplemental material, sj-pdf-1-imr-10.1177_03000605221121941 for Long-term outcomes regarding arterial stiffness and carotid artery atherosclerosis in female patients with rapid eye movement obstructive sleep apnea by Mayumi Suzuki, Ken Shimamoto, Fujio Tatsumi, Takao Tsuji, Natsumi Satoya, Yuji Inoue, Tetsuro Hoshino, Toshiaki Shiomi and Nobuhisa Hagiwara in Journal of International Medical Research
Acknowledgements
The authors wish to thank the technicians who recorded the PWV values and performed the carotid artery ultrasound examinations, including Takamitsu Harada, Akiko Sato, and Yuko Oikawa.
Author contributions: Mayumi Suzuki contributed to the conception and design of the study, acquisition, analysis and interpretation of data, original draft preparation, and reviewing and editing the manuscript. Ken Shimamoto contributed to the conception and design of the study. Fujio Tatsumi, Takao Tsuji, Natsumi Satoya, and Yuji Inoue contributed to the acquisition of data. Tetsuro Hoshino contributed to reviewing and editing the manuscript. Toshiaki Shiomi contributed to the conception and design of the study and reviewing and editing the manuscript. Nobuhisa Hagiwara contributed to reviewing the manuscript and supervising the study.
Declaration of conflicting interest: The authors declare that there is no conflict of interest.
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Data availability statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
ORCID iD
Mayumi Suzuki https://orcid.org/0000-0003-0655-4760
References
- 1.Mone P, Kansakar U, Varzideh F, et al. Epidemiology of obstructive sleep apnea: What is the contribution of hypertension and arterial stiffness? J Clin Hypertens 2022; 24: 395–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Young T, Palta M, Dempsey J, et al. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med 1993; 328: 1230–1235. [DOI] [PubMed] [Google Scholar]
- 3.Yaggi HK, Concertos J, Kernan WN, et al. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med 2005; 353: 2034–2041. [DOI] [PubMed] [Google Scholar]
- 4.Mano M, Hoshino T, Sasanabe R, et al. Impact of gender and age on rapid eye movement-related obstructive sleep apnea: a clinical study of 3234 Japanese OSA patients. Int J Environ Res Public Health 2019; 16: E1068. 10.3390/ijerph16061068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Conwell W, Patel B, Doeing D, et al. Prevalence, clinical features, and CPAP adherence in rem-related sleep-disordered breathing: A cross-sectional analysis of a large clinical population. Sleep Breath 2012; 16: 519–526. [DOI] [PubMed] [Google Scholar]
- 6.Koo BB, Dostal J, Ioachimescu O, et al. The effects of gender and age on REM-related sleep-disordered breathing. Sleep Breath 2008; 12: 259–264. DOI 10.1007/s11325-007-0161-7. [DOI] [PubMed] [Google Scholar]
- 7.Acosta-Castro P, Hirotsu C, Marti-Soler H, et al. REM-associated sleep apnoea: prevalence and clinical significance in the HypnoLaus cohort. Eur Respir J 2018; 52: 1702484. [DOI] [PubMed] [Google Scholar]
- 8.Varga AW, Mokhlesi B. REM obstructive sleep apnea: risk for adverse health outcomes and novel treatments. Sleep Breath 2019; 23: 413–423. 10.1007/s11325-018-1727-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Polak JF, Pencina MJ, Pencina KM, et al. Carotid-wall intima-media thickness and cardiovascular events. N Engl J Med 2011; 365: 213–221. 10.1056/NEJMoa1012592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clímaco DCS, Lustosa T, de FP MV, et al. Is obstructive sleep apnea associated with increased arterial stiffness in patients with COPD? Sleep Breath 2022. doi: 10.1007/s11325-022-02635-w. [DOI] [PubMed] [Google Scholar]
- 11.Chung S, Yoon IY, Lee CH, et al. The association of nocturnal hypoxemia with arterial stiffness and endothelial dysfunction in male patients with obstructive sleep apnea syndrome. Respiration 2010; 79: 363–369. [DOI] [PubMed] [Google Scholar]
- 12.Minoguchi K, Yokoe T, Tazaki T, et al. Increased carotid intima-media thickness and serum inflammatory markers in obstructive sleep apnea. Am J Respir Crit Care Med 2005; 172: 625–630. 10.1164/rccm.200412-1652OC. [DOI] [PubMed] [Google Scholar]
- 13.Suzuki T, Nakano H, Maekawa J, et al. Obstructive sleep apnea and carotid-artery intima-media thickness. Sleep 2004; 27: 129–133. 10.1093/sleep/27.1.129. [DOI] [PubMed] [Google Scholar]
- 14.Kaynak D, Göksan B, Kaynak H, et al. Is there a link between the severity of sleep-disordered breathing and atherosclerotic disease of the carotid arteries? Eur J Neurol 2003; 10: 487–493. 10.1046/j.1468-1331.2003.00658.x. [DOI] [PubMed] [Google Scholar]
- 15.Lisan Q, Sloten T, Boutouyrie P, et al. Sleep apnea is associated with accelerated vascular aging: Results from 2 European community-based cohort studies. J Am Heart Assoc 2021; 10: e021318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McEvoy RD, Antic NA, Heeley E, et al. CPAP for prevention of cardiovascular events in obstructive sleep apnea. N Engl J Med 2016; 375: 919–931. doi: 10.1056/NEJMoa1606599. (Epub 2016 August 28). PMID: 27571048 Clinical Trial. [DOI] [PubMed] [Google Scholar]
- 17.Barbé F, Durán-Cantolla J, Sánchez-de-la-Torre M, et al. Effect of continuous positive airway pressure on the incidence of hypertension and cardiovascular events in nonsleepy patients with obstructive sleep apnea: a randomized controlled trial. JAMA 2012; 307: 2161–2168. [DOI] [PubMed] [Google Scholar]
- 18.Parra O, Sánchez-Armengol A, Bonnin M, et al. Early treatment of obstructive apnoea and stroke outcome: a randomised controlled trial. Eur Respir J 2011; 37: 1128–1136. [DOI] [PubMed] [Google Scholar]
- 19.Peker Y, Glantz H, Eulenburg C, et al. Effect of positive airway pressure on cardiovascular outcomes in coronary artery disease patients with non-sleepy obstructive sleep apnea: the RICCADSA randomized controlled trial. Am J Respir Crit Care Med 2016; 194: 613–620. [DOI] [PubMed] [Google Scholar]
- 20.Lettieri CJ, Paolino N, Eliasson AH, et al. Comparison of adjustable and fixed oral appliances for the treatment of obstructive sleep apnea. J Clin Sleep Med 2011; 7: 439–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vanderveken OM. Perspectives on the reduction in cardiovascular mortality with oral appliance therapy for patients with severe obstructive sleep apnoea intolerant to continuous positive airway pressure. Respirology 2013; 18: 1161–1162. doi: 10.1111/resp.12157. [DOI] [PubMed] [Google Scholar]
- 22.Gleadhill IC, Schwartz AR, Schubert N, et al. Upper airway collapsibility in snorers and in patients with obstructive hypopnea and apnea. Am Rev Respir Dis 1991; 143: 1300–1303. [DOI] [PubMed] [Google Scholar]
- 23.Zota I.M, Stătescu C, RaduSascău R.A, et al. Arterial stiffness assessment using the arteriograph in patients with moderate-severe OSA and metabolic syndrome-A pilot study. J Clin Med 2021; 10: 4238. doi.org/10.3390/jcm10184238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Galerneau LM, Bailly S, Borel JC, et al. Long-term variations of arterial stiffness in patients with obesity and obstructive sleep apnea treated with continuous positive airway pressure. PLOS One 2020; 15: e0236667. doi: 10.1371/journal.pone.0236667. eCollection 2020.PMID: 32756570 Free PMC article. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kasiakogias A, Tsioufis C, Thomopoulos C, et al. Effects of continuous positive airway pressure on blood pressure in hypertensive patients with obstructive sleep apnea: a 3-year follow-up. J Hypertens 2013; 31: 352–360. doi: 10.1097/HJH.0b013e32835bdcda, 23235356. [DOI] [PubMed] [Google Scholar]
- 26.Von Elm E, Altman DG, Egger M, et al. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 2007; 147: 573–577. [DOI] [PubMed] [Google Scholar]
- 27.Kitamura A, Iso H, Imano H, et al. Carotid intima-media thickness and plaque characteristics as a risk factor for stroke in Japanese elderly men. Stroke 2004; 35: 2788–2794. 10.1161/01.STR.0000147723.52033.9e. [DOI] [PubMed] [Google Scholar]
- 28.Song F, Zou J, Song Z, et al. Association of adipocytokines with carotid intima media thickness and arterial stiffness in obstructive sleep apnea patients. Front Endocrinol 2020; 11: 177. doi: 10.3389/fendo.2020.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mokhlesi B, Hagen EW, Finn LA, et al. Obstructive sleep apnoea during REM sleep and incident non-dipping of nocturnal blood pressure: a longitudinal analysis of the Wisconsin Sleep Cohort. Thorax 2015; 70: 1062–1069. doi:10.1136/thoraxjnl-2015-207231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoshino T, Sasanabe R, Murotani K, et al. Estimated respiratory arousal threshold in patients with rapid eye movement obstructive sleep apnea. Sleep Breath 2021; 26: 347–353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Suzuki M, Shimamoto K, Sekiguchi H, et al. Arousal index as a marker of carotid artery atherosclerosis in patients with obstructive sleep apnea syndrome. Sleep Breath 2019; 23: 87–94. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-imr-10.1177_03000605221121941 for Long-term outcomes regarding arterial stiffness and carotid artery atherosclerosis in female patients with rapid eye movement obstructive sleep apnea by Mayumi Suzuki, Ken Shimamoto, Fujio Tatsumi, Takao Tsuji, Natsumi Satoya, Yuji Inoue, Tetsuro Hoshino, Toshiaki Shiomi and Nobuhisa Hagiwara in Journal of International Medical Research
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.