Abstract
Backgroundand objective
Studies have highlighted the significant role of staged percutaneous coronary intervention (PCI) for a multivessel disease (MVD) among patients with ST-elevation myocardial infarction (STEMI). However, the relative benefit of staged vs. culprit-only PCI for MVD in elderly patients with STEMI remains undetermined. Thus, the present study compared the clinical outcomes of staged and culprit-only PCI in this cohort.
Methods
From January 2014 to September 2019, 617 patients aged ≥65 years with STEMI and MVD who underwent primary PCI of the culprit vessels within 12 h of symptom onset were enrolled. They were then categorized into the staged and culprit-only PCI groups according to intervention strategy. Propensity score matching (PSM) was conducted to adjust for confounding factors between groups. The primary end point was major adverse cardiac and cerebrovascular events (MACCE), a composite of all-cause death, cardiac death, recurrent myocardial infarction (MI), stroke, and ischemia-driven revascularization.
Results
During a mean follow-up of 56 months, 209 patients experienced MACCE and 119 died. Staged revascularization was associated with a lower risk of MACCE, all-cause death, and cardiac death than culprit-only PCI in both overall patients and the PSM cohorts. In contrast, there was no significant difference in stroke or ischemia-driven revascularization. Moreover, on multivariate Cox regression analysis, staged PCI was a significant predictor of a lower incidence of MACCE and all-cause death.
Conclusion
In elderly patients with STEMI and MVD, staged PCI is superior to culprit-only PCI.
Keywords: elderly, ST-segment elevation myocardial infarction, multivessel disease, percutaneous coronary intervention, staged revascularization
Introduction
An ST-segment elevation myocardial infarction (STEMI) is the most threatening type of coronary heart disease. Primary percutaneous coronary intervention (PCI) within 12 h of symptom onset is the best revascularization strategy for patients with STEMI (1). Approximately 40–65% of patients with STEMI have multivessel disease (MVD) (2–5). A lower incidence of reperfusion success, a lower left ventricular ejection fraction (LVEF), and a higher mortality rate were reported for STEMI patients with MVD vs. single-vessel disease. There are three intervention strategies for MVD in patients with STEMI: (1) culprit-only vessel PCI during the index procedure; (2) culprit vessel revascularization during primary PCI with non-culprit vessels staged revascularized after primary PCI; and (3) multivessel revascularization during primary PCI. Previous studies (6–9) confirmed that culprit-only PCI is superior to multivessel revascularization during primary PCI. Recent studies (10) elucidated that for MVD in patients with STEMI, staged PCI could reduce mortality more than culprit-only PCI. Moreover, recent meta-analyses (11) demonstrated that staged PCI has a better effect than multivessel primary PCI. Therefore, the latest guidelines (1) upgraded the recommendation of staged PCI for MVD over culprit-only PCI. Moreover, elderly patients were classified as a special cohort in the guidelines and featured in some specific sections considering their extremely high risk.
Recent studies have shown that the populations of elderly patients are increasing with the increasing human life expectancy. Elderly patients always have more complex clinical conditions and worse prognoses than younger patients (1). Therefore, the effect of staged PCI on MVD in such patients is uncertain. However, the most randomized controlled trials (RCTs) exclude elderly patients. No study has aimed to identify the preferred strategy for these patients. Therefore, this study conducted a comprehensive analysis to compare the effects of staged PCI vs. culprit-only PCI on MVD in elderly patients with STEMI to provide ideas for future treatment strategies.
Materials and methods
Study design and population
This single-center retrospective observational study received no sponsorship from enterprises. A total of 1,592 patients aged ≥65 years with STEMI who had undergone primary PCI of the culprit vessels within 12 h of symptom onset from January 2014 to September 2019 at Tianjin Chest Hospital were included. MVD was defined as angiographic stenosis ≥70% in ≥2 major coronary arteries (diameter ≥ 2.5 mm). Patients were excluded if they met the following criteria: (1) single-vessel disease (n = 622); (2) planned coronary artery bypass graft (CABG) surgery after primary PCI (n = 209); (3) immediate complete revascularization and staged PCI performed >90 days after primary PCI (n = 36); (4) stenosis of the left main coronary artery ≥50% or concomitant chronic occlusion disease (n = 56); (5) cardiac shock or death before hospital discharge (n = 7); (6) previous CABG (n = 3); and (7) lack of complete clinical data (n = 20). A total of 639 patients were remained; of them, 617 patients for whom full clinical data with follow-up information were available were included in the final analyses. The research protocol was authorized by the Tianjin Chest Hospital Ethical Committee and conducted in accordance with the Declaration of Helsinki. This was a retrospective cohort study from real-world situations; therefore, written informed consent from the enrolled patients was not required. The authors vouch for the completeness and accuracy of the data used in this study.
Study procedures
All primary PCI procedures were performed according to current guidelines. The culprit vessel was identified based on electrocardiographic changes and echocardiographic and angiographic findings. According to the treatment strategies, the patients were further categorized into the culprit-only PCI group (only culprit vessels were revascularized by primary PCI) and staged PCI groups (non-culprit vessels were staged revascularized after primary PCI). Non-culprit vessels of patients in the staged PCI group were revascularized within 90 days (12) after the primary PCI. All baseline data were obtained from the patients’ medical records by two independent investigators who were blinded to the study parameters. Baseline information included demographics, clinical characteristics, laboratory data, angiographic and procedural details, and medication at discharge.
Follow-up and endpoints
All patients were followed up from December 2021 to January 2022 by telephone or outpatient visits. The primary endpoint was major adverse cardiac and cerebrovascular events (MACCE), defined as a composite of all-cause death, recurrent myocardial infarction (MI), stroke, and ischemia-driven revascularization. The secondary endpoints included all-cause death, cardiac death, recurrent MI, stroke, and ischemia-driven revascularization. All-cause death was defined as death of any cause. Cardiac death was defined as death caused by cardiovascular disease, such as acute MI, heart failure, and cardiac arrhythmia.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) if normally distributed or as median and interquartile range if non-normally distributed. These were compared using the t-test or the Mann-Whitney U-test. Categorical variables were presented as frequency or percentage and were contrasted using the chi-squared test or Fisher’s exact test. Survival analyses were conducted using Kaplan-Meier curves and contrasted using the log-rank test. Multivariate Cox proportional hazard regression analysis with an entry/exit criterion of 0.1/0.1 was used to acquire the hazard ratios (HRs) and 95% confidence intervals (CIs) to identify independent predictors of MACCE and all-cause death. All variables in Tables 1, 2, except total stent length, are input into the univariate model and then input into a multivariate model with p < 0.10. Before propensity score matching (PSM), possible factors of MACCE included staged PCI, diabetes, previous stroke, estimated glomerular filtration rate (eGFR), heart rate, LVEF, Killip class, use of beta-blocker, intra-aortic balloon pump (IABP) use, number of narrowed coronary arteries, non-culprit lesion diameter stenosis, and use of drug-eluting stents. Possible factors of all-cause death included staged PCI, age, hypertension, diabetes, atrial fibrillation, previous stroke, previous smoking, heart rate, LVEF, eGFR, peak troponin level, Killip class, use of beta-blockers, IABP, culprit vessel, number of narrowed coronary arteries, non-culprit lesion diameter stenosis, and use of drug-eluting stents. After PSM, the possible factors of MACCE included staged PCI, previous stroke, peak creatine kinase myocardial band (CK-MB) level, culprit vessel, and the number of narrowed coronary arteries. Possible factors for all-cause death included staged PCI, previous stroke, LVEF, and eGFR. To adjust for confounding factors, differences in clinical outcomes between the two groups were compared using PSM with a matching ratio of 1:1 and caliper value of 0.02. The matching variables included all baseline, angiographic, and procedural characteristics, as well as medication at discharge. All analyses were performed using SPSS version 25.0 (IBM Corp., Armonk, NY, United States). Two-tailed tests were considered statistically significant at a level of 0.05.
TABLE 1.
Baseline characteristics of included patients before and after propensity score matching (PSM).
| Characteristic | Before PSM analysis |
After PSM analysis |
||||
| Staged PCI (n = 154) | Culprit-only PCI (n = 463) | P-value | Staged PCI (n = 80) | Culprit-only PCI (n = 80) | P-value | |
| Age, years | 70.6 ± 4.7 | 73.8 ± 6.5 | <0.001 | 68.7 ± 2.8 | 68.7 ± 2.8 | 0.772 |
| Male, no. (%) | 105 (68.2) | 267 (57.7) | 0.021 | 51 (63.8) | 54 (67.5) | 0.736 |
| Hypertension, no. (%) | 107 (69.5) | 318 (68.7) | 0.853 | 53 (66.3) | 52 (65.0) | 1.000 |
| Diabetes, no. (%) | 51 (33.1) | 149 (32.2) | 0.830 | 23 (28.8) | 24 (30.0) | 1.000 |
| Dyslipidemia, no. (%) | 93 (60.4) | 285 (61.6) | 0.311 | 49 (61.3) | 49 (61.3) | 1.000 |
| Atrial fibrillation, no. (%) | 5 (3.2) | 22 (4.8) | 0.429 | 4 (5.0) | 3 (3.8) | 1.000 |
| Previous stroke, no. (%) | 29 (18.8) | 128 (27.6) | 0.030 | 20 (25.0) | 23 (28.8) | 0.711 |
| Previous MI, no. (%) | 8 (5.2) | 27 (5.8) | 0.767 | 4 (5.0) | 8 (10.0) | 0.388 |
| Previous PCI, no. (%) | 12 (7.8) | 55 (11.9) | 0.158 | 6 (7.5) | 11 (13.8) | 0.332 |
| Previous smoker, no. (%) | 26 (16.9) | 59 (12.7) | 0.197 | 11 (13.8) | 13 (16.3) | 0.804 |
| Current smoker, no. (%) | 59 (38.3) | 168 (36.3) | 0.651 | 31 (38.8) | 29 (36.3) | 0.878 |
| Systolic blood pressure, mmHg | 128.7 ± 24.5 | 131.1 ± 26.1 | 0.298 | 128.9 ± 23.7 | 124.2 ± 26.4 | 0.265 |
| Heart rate, beats/min | 68.0 ± 13.0 | 70.8 ± 16.8 | 0.035 | 68.8 ± 13.0 | 67.0 ± 13.8 | 0.406 |
| LVEF,% | 52 (46−57) | 49 (43−55) | <0.001 | 51.5 (46.0−56.0) | 50.0 (44.0−55.0) | 0.252 |
| Triglycerides, mmol/L | 1.4 (1.0−1.9) | 1.2 (0.9−1.7) | 0.044 | 1.4 (1.0−1.8) | 1.3 (1.0−1.8) | 0.685 |
| Total cholesterol, mmol/L | 4.6 ± 1.0 | 4.5 ± 1.0 | 0.663 | 4.6 ± 1.1 | 4.7 ± 1.0 | 0.371 |
| HDL-C, mmol/L | 1.1 ± 0.2 | 1.1 ± 0.4 | 0.002 | 1.1 ± 0.2 | 1.1 ± 0.3 | 0.699 |
| LDL-C, mmol/L | 3.1 ± 0.9 | 3.1 ± 0.9 | 0.299 | 3.1 ± 1.0 | 3.2 ± 0.8 | 0.782 |
| eGFR, mL/min/1.73 m2 | 79.0 ± 19.7 | 74.2 ± 23.3 | 0.012 | 80.5 ± 19.9 | 81.5 ± 20.4 | 0.716 |
| Peak troponin, ng/mL | 3.7 (1.4−7.7) | 4.2 (1.4−9.0) | 0.354 | 3.7 (1.1−8.4) | 4.3 (1.4−8.2) | 0.705 |
| Peak CK-MB, U/L | 114 (53.8−201.3) | 142 (65.8−255.0) | 0.053 | 112.5 (58.3−211.0) | 132.0 (66.3−246.3) | 0.379 |
| Killip class, no. (%) | 0.059 | 0.919 | ||||
| 1 | 145 (94.2) | 399 (86.2) | 75 (93.8) | 73 (91.3) | ||
| 2 | 6 (3.9) | 46 (9.9) | 3 (3.8) | 5 (6.3) | ||
| 3 | 1 (0.6) | 8 (1.7) | 1 (1.3) | 1 (1.3) | ||
| 4 | 2 (1.3) | 10 (2.2) | 1 (1.3) | 1 (1.3) | ||
| Time from symptom onset to primary PCI, h | 4 (2−6) | 4 (2−6) | 0.991 | 3.5 (2.0−5.8) | 3.3 (2.0−6.0) | 0.826 |
| Medications at discharge, no. (%) | ||||||
| Aspirin | 152 (98.7) | 453 (97.8) | 0.503 | 79 (98.8) | 80 (100.0) | 1.000 |
| Clopidogrel | 123 (79.9) | 384 (82.9) | 0.389 | 64 (80.0) | 64 (80.0) | 1.000 |
| Ticagrelor | 31 (20.1) | 74 (16.0) | 0.235 | 16 (20.0) | 16 (20.0) | 1.000 |
| Beta-blocker | 121 (78.6) | 365 (78.8) | 0.945 | 63 (78.8) | 64 (80.0) | 1.000 |
| ACEI/ARB | 121 (78.6) | 345 (74.5) | 0.310 | 63 (78.8) | 59 (73.8) | 0.597 |
| Statins | 148 (96.1) | 441 (95.2) | 0.659 | 77 (96.3) | 76 (95.0) | 1.000 |
Data are expressed as percentages, mean ± standard deviation (SD) or median and interquartile range. The p-Values represent differences in baseline characteristics between the two groups. PSM, propensity score matching; PCI, percutaneous coronary intervention; MI, myocardial infarction; LVEF, left ventricular ejection fractions; HDL-C, high-density lipoprotein-cholesterol; LDL-C, low-density lipoprotein-cholesterol; eGFR, estimated glomerular filtration rate; CK-MB, creatine kinase myocardial band; ACEI, angiotensin-converting enzyme inhibitor; ARB, angiotensin receptor blocker.
TABLE 2.
Angiographic characteristics and procedural characteristics of included patients before and after PSM.
| Characteristic | Before PSM analysis |
After PSM analysis |
||||
| Staged PCI (n = 154) | Culprit-only PCI (n = 463) | P-value | Staged PCI (n = 80) | Culprit-only PCI (n = 80) | P-value | |
| Thrombus aspiration, no. (%) | 56 (36.4) | 180 (38.9) | 0.578 | 21 (26.3) | 33 (41.3) | 0.081 |
| IABP, no. (%) | 2 (1.3) | 47 (10.2) | <0.001 | 1 (1.3) | 3 (3.8) | 0.625 |
| Culprit vessel, no./total culprit vessels (%) | 0.007 | 0.489 | ||||
| Left anterior descending | 39/156 (25.0) | 179/470 (38.1) | 22 (27.5) | 24 (30.0) | ||
| Left circumflex | 24/156 (15.4) | 48/470 (10.2) | 11 (13.8) | 14 (17.5) | ||
| Right | 93/156 (59.6) | 243/470 (51.7) | 47 (58.8) | 42 (52.5) | ||
| No. of narrowed coronary arteries | 0.582 | 0.871 | ||||
| 2 | 96 (62.3) | 300 (64.8) | 48 (60.0) | 50 (62.5) | ||
| 3 | 58 (37.7) | 163 (35.2) | 32 (40.0) | 30 (37.5) | ||
| Non-culprit vessel, no./total non-culprit vessels (%) | 0.019 | 0.657 | ||||
| Left anterior descending | 94/212 (44.3) | 210/625 (33.6) | 42/112 (36.8) | 47/110 (42.7) | ||
| Left circumflex | 79/212 (37.3) | 281/625 (45.0) | 46/112 (41.1) | 39/110 (35.5) | ||
| Right coronary artery | 39/212 (18.4) | 134/625 (21.4) | 24/112 (21.4) | 24/110 (21.8) | ||
| Non-culprit vessel diameter stenosis, no./total non-culprit vessels (%) | <0.001 | 0.012 | ||||
| 70−79% | 3/212 (1.4) | 43/625 (6.9) | 1/112 (0.9) | 5/110 (4.5) | ||
| 80−89% | 54/212 (25.5) | 202/625 (32.3) | 34/112 (30.4) | 36/110 (32.7) | ||
| 90−99% | 136/212 (64.2) | 275/625 (44.0) | 68/112 (60.7) | 48/110 (43.6) | ||
| 100% | 19/212 (9.0) | 105/625 (16.8) | 9/112 (8.0) | 21/110 (19.1) | ||
| Type of PCI, no. (%) | 0.002 | 1.000 | ||||
| Drug-eluting stent | 152 (98.7) | 424 (91.6) | 78 (97.5) | 79 (98.8) | ||
| PTCA | 2 (1.3) | 39 (8.4) | 2 (2.5) | 1 (1.3) | ||
| PCI access, no. (%) | 0.348 | 0.359 | ||||
| Femoral artery | 124 (80.5) | 388 (83.8) | 67 (83.8) | 72 (90.0) | ||
| Radial artery | 30 (19.5) | 75 (16.2) | 13 (16.3) | 8 (10.0) | ||
| Minimum stent diameter, mm | 2.8 (2.5−3.0) | 3.0 (2.8−3.5) | <0.001 | 2.8 (2.5−3.0) | 2.8 (2.5−3.0) | 0.124 |
| Total stent length, mm | 74.4 ± 27.0 | 37.7 ± 19.4 | <0.001 | 72.4 ± 28.0 | 40.2 ± 21.8 | <0.001 |
PSM, propensity score matching; PCI, percutaneous coronary intervention; IABP, intra-aortic balloon pump; PTCA, percutaneous transluminal coronary angioplasty.
Results
Baseline, angiographic, and procedural characteristics
Among the 617 elderly patients with STEMI and MVD who underwent primary PCI of the culprit vessels, 463 (75%) underwent culprit-only PCI and 154 (25%) underwent staged PCI.
After PSM, 160 patients were matched. The mean follow-up period was 56 months (interquartile range, 40–89 months). The baseline information and laboratory, angiographic, and procedural characteristics of the overall patients and the PSM cohort are listed in Tables 1, 2.
Before PSM, patients from the staged PCI group were more likely to be male (p = 0.021) and younger (p < 0.001); have a history of previous stroke (p = 0.030); have a higher LVEF (p < 0.001), triglyceride level (p = 0.044), and eGFR (p = 0.012); and have a lower heart rate (p = 0.035) and high-density lipoprotein cholesterol (HDL-C) level (p = 0.002) than those in the culprit-only PCI group. Moreover, patients who underwent staged PCI were less likely to receive IABP (p < 0.001), more likely to receive drug-eluting stents (p = 0.002), more likely to have non-culprit vessels including the left circumflex artery and right coronary artery (p = 0.019), and less likely to have culprit vessels including the left anterior descending coronary artery (p = 0.007). What is more, they had a shorter minimum stent diameter and total stent length (both p < 0.001). Non-culprit vessel diameter stenosis in the staged PCI group was prone to be 90–99%, while that in the culprit-only PCI group was prone to be 70–79%, 80–89%, and 100% (p < 0.001). The other characteristics did not differ significantly between groups.
After PSM, baseline information and laboratory, angiographic, and procedural characteristics were fully consistent between groups except for non-culprit lesion diameter stenosis (p = 0.012; Tables 1, 2).
Clinical outcomes
During the follow-up period of 56 months, 209 patients had developed MACCE and 119 died.
Before PSM, staged PCI had a lower risk of MACCE (HR, 0.487; 95% CI, 0.332–0.713; p < 0.001), all-cause death (HR, 0.264; 95% CI, 0.138–0.504; p < 0.001), cardiac death (HR, 0.247; 95% CI, 0.114–0.534; p < 0.001), and recurrent MI (HR, 0.318; 95% CI, 0.126–0.799; p = 0.015) than culprit-only PCI (Figure 1 and Table 3). Other clinical outcomes, such as stroke and ischemia-driven revascularization, did not differ significantly between groups. Cox proportional hazard regression revealed that the staged PCI group had a reduced risk of MACCE (HR, 0.579; 95% CI, 0.390–0.860; p = 0.007) and all-cause death (HR, 0.420; 95% CI, 0.212–0.832; p = 0.013) in contrast with culprit-only PCI (Tables 4, 5). Staged PCI played a robust role in predicting the risk of a lower incidence of MACCE and all-cause death. Furthermore, previous stroke (p = 0.006) and Killip class (p = 0.015) were independent predictors of MACCE (Table 4). Age (p = 0.006), previous smoking status (p = 0.044), eGFR (p = 0.003), and Killip class (p = 0.014) were also independent predictors of all-cause mortality (Table 5).
FIGURE 1.

Kaplan-Meier curves of clinical outcomes in the two groups before the propensity score matching analysis. (A) MACCE; (B) all-cause death; and (C) cardiac death. MACCE, major adverse cardiac and cerebrovascular event; PCI, percutaneous coronary intervention.
TABLE 3.
Clinical outcomes between the two groups before and after the PSM analysis.
| Outcome | Before PSM analysis |
After PSM analysis |
||||||
| Staged PCI (n = 154) | Culprit-only PCI (n = 463) | HR (95% CI) | P-value | Staged PCI (n = 80) | Culprit-only PCI (n = 80) | HR (95% CI) | P-value | |
| MACCE | 31 (20.1) | 178 (38.4) | 0.487 (0.332−0.713) | <0.001 | 15 (18.8) | 31 (38.8) | 0.468 (0.252−0.867) | 0.016 |
| All-cause death | 10 (6.5) | 109 (23.5) | 0.264 (0.138−0.504) | <0.001 | 3 (3.8) | 15 (18.8) | 0.185 (0.054−0.636) | 0.007 |
| Cardiac death | 7 (4.5) | 82 (17.7) | 0.247 (0.114−0.534) | <0.001 | 1 (1.3) | 13 (16.3) | 0.075 (0.010−0.577) | 0.013 |
| Recurrent MI | 5 (3.2) | 47 (10.2) | 0.318 (0.126−0.799) | 0.015 | 3 (3.8) | 9 (11.3) | 0.347 (0.094−1.281) | 0.112 |
| Stroke | 6 (3.9) | 20 (4.3) | 0.953 (0.382−2.379) | 0.918 | 4 (5.0) | 2 (2.5) | 2.192 (0.400−12.021) | 0.366 |
| Ischemia-driven revascularization | 16 (10.4) | 57 (12.3) | 0.868 (0.498−1.513) | 0.619 | 9 (11.3) | 13 (16.3) | 0.735 (0.314−1.720) | 0.478 |
PSM, propensity score matching; PCI, percutaneous coronary intervention; HR, hazard ratio; CI, confidence interval; MACCE, major adverse cardiac and cerebrovascular events; MI, myocardial infarction.
TABLE 4.
Cox proportion hazards analysis for predictors of major adverse cardiac and cerebrovascular events (MACCE) before PSM analysis.
| Variable | Univariate analysis |
Multivariate analysis |
||
| HR (95% Cl) | P-value | HR (95% Cl) | P-value | |
| Staged PCI (vs. culprit-only PCI) | 0.487 (0.332−0.713) | <0.001 | 0.579 (0.390−0.860) | 0.007 |
| Diabetes | 1.487 (1.127−1.961) | 0.005 | 1.243 (0.928−1.664) | 0.145 |
| Previous stroke | 1.822 (1.370−2.422) | <0.001 | 1.531 (1.133−2.068) | 0.006 |
| eGFR, mL/min/1.73 m2 | 0.994 (0.987−1.000) | 0.045 | 0.997 (0.991−1.004) | 0.397 |
| Heart rate, beats/min | 1.015 (1.006−1.023) | <0.001 | 1.007 (0.999−1.016) | 0.102 |
| LVEF, % | 0.981 (0.964−0.998) | 0.026 | 0.998 (0.979−1.017) | 0.833 |
| Killip class | 1.411 (1.171−1.701) | <0.001 | 1.285 (1.049−1.574) | 0.015 |
| Use of beta-blocker | 1.391 (0.967−2.000) | 0.075 | 1.210 (0.830−1.764) | 0.323 |
| IABP | 1.929 (1.279−2.910) | 0.002 | 1.1094 (0.755−1.889) | 0.449 |
| No. of narrowed coronary arteries | 1.259 (0.982−1.709) | 0.067 | 1.163 (0.856−1.581) | 0.333 |
| Non-culprit lesion diameter stenosis | 1.197 (0.999−1.433) | 0.051 | 1.126 (0.927−1.368) | 0.231 |
| Use of drug-eluting stent | 0.657 (0.410−1.053) | 0.081 | 0.820 (0.502−1.341) | 0.429 |
HR, hazard ratio; CI, confidence interval; PCI, percutaneous coronary intervention; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; IABP, intra-aortic balloon pump.
TABLE 5.
Cox proportion hazards analysis for predictors of all-cause death before PSM analysis.
| Variable | Univariate analysis |
Multivariate analysis |
||
| HR (95% Cl) | P-value | HR (95% Cl) | P-value | |
| Staged PCI (vs. culprit-only PCI) | 0.264 (0.138−0.504) | <0.001 | 0.420 (0.212−0.832) | 0.013 |
| Age | 1.069 (1.041−1.097) | <0.001 | 1.043 (1.012−1.075) | 0.006 |
| Hypertension | 1.422 (0.940−2.151) | 0.096 | 1.104 (0.713−1.710) | 0.656 |
| Diabetes | 1.653 (1.149−2.376) | 0.007 | 1.352 (0.900−2.028) | 0.146 |
| Atrial fibrillation | 2.119 (1.018−4.051) | 0.023 | 1.182 (0.586−2.386) | 0.641 |
| Previous stroke | 1.928 (1.329−2.799) | <0.001 | 1.460 (0.978−2.179) | 0.064 |
| Previous smoker | 1.609 (1.028−2.518) | 0.037 | 1.605 (1.012−2.547) | 0.044 |
| Heart rate, beats/min | 1.027 (1.017−1.037) | <0.001 | 1.010 (0.999−1.021) | 0.069 |
| LVEF, % | 0.949 (0.929−0.970) | <0.001 | 0.978 (0.952−1.006) | 0.117 |
| eGFR, mL/min/1.73 m2 | 0.976 (0.968−0.984) | <0.001 | 0.987 (0.978−0.996) | 0.003 |
| Peak troponin, μmol/L | 1.052 (1.001−1.106) | 0.047 | 0.990 (0.939−1.044) | 0.715 |
| Killip class | 1.703 (1.388−2.088) | <0.001 | 1.357 (1.063−1.733) | 0.014 |
| Use of beta-blocker | 1.552 (0.940−2.562) | 0.086 | 1.127 (0.664−1.912) | 0.658 |
| IABP | 3.780 (2.431−5.878) | <0.001 | 1.502 (0.886−2.547) | 0.110 |
| Culprit vessel | 0.719 (0.538−0.960) | 0.025 | 1.048 (0.739−1.485) | 0.792 |
| No. of narrowed coronary arteries | 1.393 (0.968−2.005) | 0.074 | 1.087 (0.719−1.642) | 0.694 |
| Non-culprit lesion diameter stenosis | 1.358 (1.067−1.728) | 0.013 | 1.202 (0.932−1.550) | 0.156 |
| Use of drug-eluting stent | 0.530 (0.298−0.944) | 0.031 | 0.842 (0.449−1.576) | 0.590 |
HR, hazard ratio; CI, confidence interval; PCI, percutaneous coronary intervention; LVEF, left ventricular ejection fractions; eGFR, estimated glomerular filtration rate; IABP, intra-aortic balloon pump.
After PSM, the staged PCI group still had a lower risk of MACCE (HR, 0.468; 95% CI, 0.252–0.867; p = 0.016), all-cause death (HR, 0.185; 95% CI, 0.054–0.636; p = 0.007), and cardiac death (HR, 0.075; 95% CI, 0.010–0.577; p = 0.013) than the culprit-only group (Figure 2 and Table 3). Other clinical outcomes rarely showed significant differences, such as recurrent MI, stroke, and ischemia-driven revascularization. In addition, Cox proportional hazard regression analysis indicated that staged PCI reduced the risk of MACCE (HR, 0.499; 95% CI, 0.267–0.932; p = 0.029) and all-cause death (HR, 0.192; 95% CI, 0.056–0.660; p = 0.009; Tables 6, 7). After PSM, the role of staged PCI in predicting the risk of MACCE and all-cause mortality remained robust. Moreover, the previous stroke was an independent predictor of MACCE (p = 0.019) and all-cause death (p = 0.009; Tables 6, 7).
FIGURE 2.
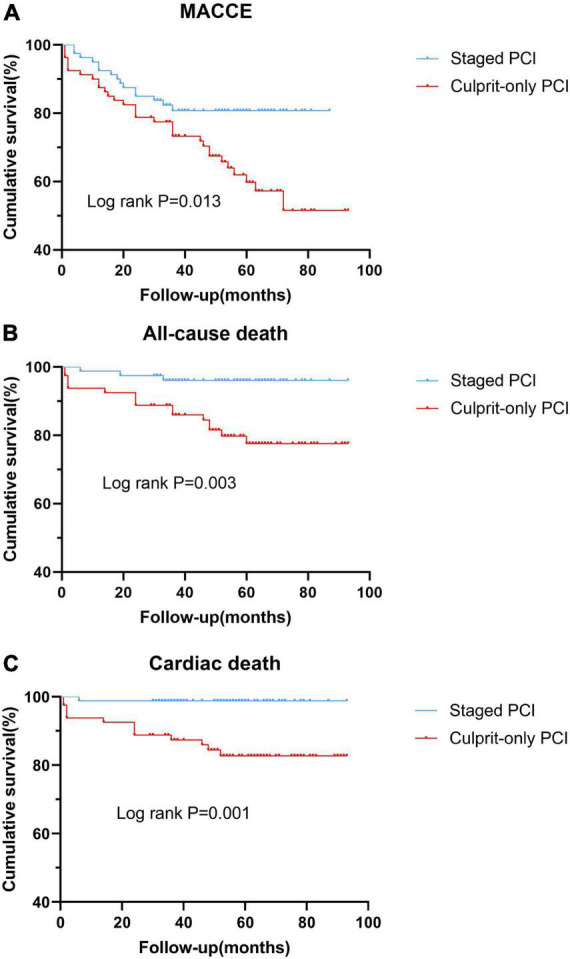
Kaplan-Meier curves of clinical outcomes in the two groups after the propensity score matching analysis. (A) MACCE; (B) all-cause death; and (C) cardiac death. MACCE, major adverse cardiac and cerebrovascular event; PCI, percutaneous coronary intervention.
TABLE 6.
Cox proportion hazards analysis for predictors of the MACCE after PSM analysis.
| Variable | Univariate analysis |
Multivariate analysis |
||
| HR (95% Cl) | P-value | HR (95% Cl) | P-value | |
| Staged PCI (vs. culprit-only PCI) | 0.468 (0.252−0.867) | 0.016 | 0.499 (0.267−0.932) | 0.029 |
| Previous stroke | 2.477 (1.380−4.448) | 0.002 | 2.076 (1.126−3.829) | 0.019 |
| Peak CK-MB, U/L | 1.001 (1.000−1.002) | 0.082 | 1.001 (1.000−1.002) | 0.168 |
| Culprit vessel | 1.491 (0.955−2.327) | 0.079 | 1.388 (0.887−2.173) | 0.151 |
| No. of narrowed coronary arteries | 2.099 (1.175−3.752) | 0.012 | 1.729 (0.935−3.195) | 0.081 |
HR, hazard ratio; CI, confidence interval; PCI, percutaneous coronary intervention; CK-MB, creatine kinase myocardial band.
TABLE 7.
Cox proportion hazards analysis for predictors of all-cause death after PSM analysis.
| Variable | Univariate analysis |
Multivariate analysis |
||
| HR (95% Cl) | P-value | HR (95% Cl) | P-value | |
| Staged PCI (vs. culprit-only PCI) | 0.185 (0.054−0.636) | 0.007 | 0.192 (0.056−0.660) | 0.009 |
| Previous stroke | 3.461 (1.405−8.526) | 0.007 | 3.383 (1.354−8.450) | 0.009 |
| LVEF,% | 0.942 (0.895−0.991) | 0.021 | 0.948 (0.897−1.001) | 0.056 |
| eGFR, mL/min/1.73 m2 | 0.977 (0.956−0.999) | 0.041 | 0.979 (0.957−1.002) | 0.071 |
HR, hazard ratio; CI, confidence interval; PCI, percutaneous coronary intervention; LVEF, left ventricular ejection fractions; eGFR, estimated glomerular filtration rate.
Discussion
In this study, among patients with STEMI and MVD aged ≥ 65 years, staged PCI showed a lower incidence of MACCE, all-cause death, and cardiac death than culprit-only PCI in the overall patients and PSM cohorts. This confirmed that, even in elderly adults, staged PCI was more beneficial for patients with STEMI and MVD.
ST-segment elevation myocardial infarction and MVD are associated with worse clinical prognoses. The optimal revascularization strategy for these patients was determined several years ago. Culprit-only PCI was recommended based on previous RCTs and observational studies (6–9). Early practice guidelines (13, 14) also unequivocally supported culprit-only PCI as long as patient hemodynamics remained stable. However, over time, several RCTs, such as the Third Danish Study of Optimal Acute Treatment of Patients with ST-segment Elevation Myocardial Infarction (DANAMI3-PRIMULTI) trial (15) and the Complete vs. Lesion-only Primary PCI Trail (CvLPRIT) (16), elucidated the promising effects of complete revascularization (at primary or staged PCI). The 2015 American College of Cardiology (ACC)/American Heart Association (AHA) updated the guidelines for complete revascularization during primary PCI from III to IIb for MVD in patients with STEMI and stable hemodynamics (17). Subsequently, the Complete vs. Culprit-Only Revascularization Strategies to Treat Multivessel Disease after Early PCI for STEMI (COMPLETE) trial (10) showed that staged PCI had better efficacy when compared with culprit-only PCI. Bates et al. (11) confirmed that when significant non-culprit vessels were suitable candidates for PCI, staged procedures had better efficacy than multivessel primary PCI. After that, the latest 2017 European Society of Cardiology (ESC) guidelines upgraded staged PCI to a class IIaA recommendation (1). Staged PCI has become the best treatment strategy for patients with STEMI and MVD. However, its relative benefit in elderly patients with STEMI remains unclear.
As a higher-risk cohort, elderly patients always have lower cardiac reserve, poorer heart function, more comorbidities, higher risk of bleeding, and worse prognosis (1, 18–20), especially those with STEMI and MVD. Coronary arteries in elderly patients tend to complicate endothelial dysfunction, inflammatory reaction, increased vascular fragility, and severe diffused calcification, meaning that unstable plaques may not reside in the culprit vessels only (21, 22). Because of the increased burden of coronary artery disease, they develop the greater potential to benefit from aggressive revascularization treatment (23–26). However, in clinical practice, the greater the risk, the more conservative the strategy, especially in elderly patients (23). Considering the advanced age and complex clinical condition, many elderly patients, their relatives, and doctors often hesitate to choose staged PCI. Their higher tendency to choose culprit-only PCI led to delayed or absent revascularization for non-culprit vessels and may cause high mortality and recurrent MI rates. However, elderly patients were excluded from most RCTs of MVD in patients with STEMI (27). The efficacy of staged revascularization in these patients is uncertain.
The present study indicated that staged PCI significantly reduced the risk of MACCE (HR, 0.487; 95% CI, 0.332–0.713; p < 0.001) as compared to culprit-only PCI for MVD in elderly patients with STEMI, primarily due to the reduction in all-cause death (HR, 0.264; 95% CI, 0.138–0.504; p < 0.001) and cardiac death (HR, 0.247; 95% CI, 0.114–0.534; p < 0.001). This was obviously due to the ability of staged PCI to reduce the ischemic burden of non-culprit vessels and stabilize vulnerable plaques. In addition, staged PCI can increase blood flow to the watershed areas of infarction to improve myocardial salvage and resolve early myocardial stunning/hibernation. It can also improve collateral circulation, reduce MI areas, and slow the deterioration of heart function, especially in elderly patients (16). This may be the reason why staged PCI reduced the mortality of elderly patients with STEMI and MVD. These outcomes were consistent with subgroup analyses of the CvLPRIT, which suggested the benefit of complete revascularization during index admission for patients older than 65 years (16).
A meta-analysis also revealed that complete revascularization decreased ischemic events in patients with MVD at 63 ± 7 years of age (28). Moreover, the present study found that staged PCI decreased the risk of recurrent MI (before PSM: HR, 0.318; 95% CI, 0.126–0.799; p = 0.015; after PSM: HR, 0.347; 95% CI, 0.094–1.281; p = 0.112). The high rate of recurrence of MI in elderly patients is the result of plaque rupture of non-culprit vessels and may be associated with their more extensive atherosclerosis (29). Delayed or absent complete revascularization may cause patients to miss timely reperfusion of non-culprit vessels and lead to high recurrent MI rates. This may also be the reason that, compared with culprit-only PCI, staged revascularization could reduce the prevalence rates of cardiac death. There were also some outcomes that rarely differed between the two groups, including stroke and ischemia-driven revascularization. This is slightly different from the results of previous studies. Iqbal et al. (8) reported that staged PCI was less prone to developing repeat ischemia-driven revascularization (HR, 0.64; 95% CI, 0.46–0.90; p = 0.012) than culprit-only PCI for all ages. This difference is possibly due to the fact that vascular and hemodynamic compromise with age is responsible for adverse outcomes in elderly patients, even given the optimal revascularization treatment (24). It was also influenced by the negative attitude of elderly patients toward repeated interventions.
The differences between this and other studies are as follows. Firstly, the subject design and study population differ from the others. To our knowledge, this is the first study to explore the efficacy of staged vs. culprit-only PCI for MVD in elderly patients with STEMI. Secondly, we had a long mean follow-up period of approximately 56 months (interquartile range, 40–89 months). Thirdly, PSM was conducted to eliminate the influence of confounding factors between groups to increase the study’s reliability and feasibility. Our study supports the view that aggressive staged PCI for MVD in elderly patients with STEMI could improve their prognosis and that staged PCI should not be withheld only due to age. These findings will encourage more elderly patients with STEMI and MVD to receive timely staged revascularization treatment. We believe that with the progressive availability of newer-generation stent devices, angiography techniques, and intra-vascular molecular imaging, PCI presents fewer intra- and postoperative risks. This may enable non-culprit vessels to receive more adequate and standardized revascularization to reduce unnecessary mortality. Further large-scale prospective cohort studies are also needed to compare the strategies of drug therapy, multivessel primary PCI, and staged PCI and clarify the optimal timing for staged PCI.
This study had some limitations. Firstly, for many reasons, such as inconvenient transportation or a low tendency to seek medical treatment, the number of patients in this field is low in most hospitals. Although this study included all patients treated in our hospital in recent years, the sample size was still small. Therefore, we did not discuss the optimal timing for staged PCI. In the future, we will conduct this study and examine this topic in greater depth. Secondly, after PSM, there was still a difference in non-culprit lesion diameter stenosis (p = 0.012), but it was rarely a significant factor in the multivariate analysis. Thirdly, in this study, the coronary arteries were evaluated based on ≥70% stenosis of two or more major coronary branches (diameter ≥ 2.5 mm). Future trials will address the value of endovascular imaging and fractional flow reserve and so on.
Conclusion
For MVD in elderly patients with STEMI, staged PCI showed a reduced long-term risk of MACCE, all-cause death, and cardiac death as compared to culprit-only PCI. Moreover, staged PCI was a significant independent predictor of MACCE and all-cause mortality. This study confirmed the benefits of staged PCI for MVD in elderly patients with STEMI, providing a reference for future revascularization strategies for such patients.
Data availability statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.
Ethics statement
The studies involving human participants were reviewed and approved by the Tianjin Chest Hospital. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
JL, CW, LW, HC, and YL took part in design of this study. JL, JZ, and YH participated in the clinical data collection. JL, LW, and HS performed the statistical analyses. JL drafted this article. All authors made contributions to the present study and approved the submitted manuscript.
Acknowledgments
We sincerely thank all the patients and investigators who participated in this study. We thank Tianjin Key Medical Discipline (Specialty) Construction Project.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- 1.Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. (2018) 39:119–77. [DOI] [PubMed] [Google Scholar]
- 2.Goldstein JA, Demetriou D, Grines CL, Pica M, Shoukfeh M, O’Neill WW. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. (2000) 343:915–22. 10.1056/NEJM200009283431303 [DOI] [PubMed] [Google Scholar]
- 3.Kotani J, Mintz GS, Castagna MT, Pinnow E, Berzingi CO, Bui AB, et al. Intravascular ultrasound analysis of infarct-related and non-infarct-related arteries in patients who presented with an acute myocardial infarction. Circulation. (2003) 107:2889–93. 10.1161/01.CIR.0000072768.80031.74 [DOI] [PubMed] [Google Scholar]
- 4.Kim DH, Burton JR, Fu Y, Lindholm L, Van de Werf F, Armstrong PW. What is the frequency and functional and clinical significance of complex lesions in non-infarct-related arteries after fibrinolysis for acute ST-elevation myocardial infarction? Am Heart J. (2006) 151:668–73. 10.1016/j.ahj.2005.05.006 [DOI] [PubMed] [Google Scholar]
- 5.Cavender MA, Milford-Beland S, Roe MT, Peterson ED, Weintraub WS, Rao SV. Prevalence, predictors, and in-hospital outcomes of non-infarct artery intervention during primary percutaneous coronary intervention for ST-segment elevation myocardial infarction (from the National Cardiovascular Data Registry). Am J Cardiol. (2009) 104:507–13. 10.1016/j.amjcard.2009.04.016 [DOI] [PubMed] [Google Scholar]
- 6.Hannan EL, Samadashvili Z, Walford G, Holmes DJ, Jacobs AK, Stamato NJ, et al. Culprit vessel percutaneous coronary intervention versus multivessel and staged percutaneous coronary intervention for ST-segment elevation myocardial infarction patients with multivessel disease. JACC Cardiovasc Interv. (2010) 3:22–31. [DOI] [PubMed] [Google Scholar]
- 7.Iqbal MB, Ilsley C, Kabir T, Smith R, Lane R, Mason M, et al. Culprit vessel versus multivessel intervention at the time of primary percutaneous coronary intervention in patients with ST-segment-elevation myocardial infarction and multivessel disease: real-world analysis of 3984 patients in London. Circ Cardiovasc Qual Outcomes. (2014) 7:936–43. 10.1161/CIRCOUTCOMES.114.001194 [DOI] [PubMed] [Google Scholar]
- 8.Iqbal MB, Nadra IJ, Ding L, Fung A, Aymong E, Chan AW, et al. Culprit vessel versus multivessel versus in-hospital staged intervention for patients with ST-segment elevation myocardial infarction and multivessel disease: stratified analyses in high-risk patient groups and anatomic subsets of nonculprit disease. JACC Cardiovasc Interv. (2017) 10:11–23. 10.1016/j.jcin.2016.10.024 [DOI] [PubMed] [Google Scholar]
- 9.Vlaar PJ, Mahmoud KD, Holmes DJ, van Valkenhoef G, Hillege HL, van der Horst IC, et al. Culprit vessel only versus multivessel and staged percutaneous coronary intervention for multivessel disease in patients presenting with ST-segment elevation myocardial infarction: a pairwise and network meta-analysis. J Am Coll Cardiol. (2011) 58:692–703. 10.1016/j.jacc.2011.03.046 [DOI] [PubMed] [Google Scholar]
- 10.Mehta SR, Wood DA, Storey RF, Mehran R, Bainey KR, Nguyen H, et al. Complete revascularization with multivessel PCI for myocardial infarction. N Engl J Med. (2019) 381:1411–21. 10.1056/NEJMoa1907775 [DOI] [PubMed] [Google Scholar]
- 11.Bates ER, Tamis-Holland JE, Bittl JA, O’Gara PT, Levine GN. PCI strategies in patients with st-segment elevation myocardial infarction and multivessel coronary artery disease. J Am Coll Cardiol. (2016) 68:1066–81. 10.1016/j.jacc.2016.05.086 [DOI] [PubMed] [Google Scholar]
- 12.Toyota T, Shiomi H, Taniguchi T, Morimoto T, Furukawa Y, Nakagawa Y, et al. Culprit vessel-only vs. Staged multivessel percutaneous coronary intervention strategies in patients with multivessel coronary artery disease undergoing primary percutaneous coronary intervention for st-segment elevation myocardial infarction. Circ J. (2016) 80:371–8. 10.1253/circj.CJ-15-0493 [DOI] [PubMed] [Google Scholar]
- 13.Pollack A, Mohanty BD, Handa R, Looser PM, Fuster V, King SR, et al. Preventive stenting in acute myocardial infarction. JACC Cardiovasc Interv. (2015) 8:131–8. 10.1016/j.jcin.2014.09.006 [DOI] [PubMed] [Google Scholar]
- 14.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the american college of cardiology foundation/American heart association task force on practice guidelines and the society for cardiovascular angiography and interventions. Circulation. (2011) 124:e574–651. 10.1161/CIR.0b013e31823a5596 [DOI] [PubMed] [Google Scholar]
- 15.Engstrom T, Kelbaek H, Helqvist S, Hofsten DE, Klovgaard L, Holmvang L, et al. Complete revascularisation versus treatment of the culprit lesion only in patients with ST-segment elevation myocardial infarction and multivessel disease (DANAMI-3-PRIMULTI): an open-label, randomised controlled trial. Lancet. (2015) 386:665–71. 10.1016/s0140-6736(15)60648-1 [DOI] [PubMed] [Google Scholar]
- 16.Gershlick AH, Khan JN, Kelly DJ, Greenwood JP, Sasikaran T, Curzen N, et al. Randomized trial of complete versus lesion-only revascularization in patients undergoing primary percutaneous coronary intervention for STEMI and multivessel disease: the CvLPRIT trial. J Am Coll Cardiol. (2015) 65:963–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with st-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention and the 2013 ACCF/AHA guideline for the management of st-elevation myocardial infarction. J Am Coll Cardiol. (2016) 67:1235–50. [DOI] [PubMed] [Google Scholar]
- 18.Amsterdam EA, Wenger NK, Brindis RG, Casey DJ, Ganiats TG, Holmes DJ, et al. 2014 AHA/ACC guideline for the management of patients with non-st-elevation acute coronary syndromes: a report of the American college of cardiology/American heart association task force on practice guidelines. J Am Coll Cardiol. (2014) 64:e139–228. 10.1161/CIR.0000000000000133 [DOI] [PubMed] [Google Scholar]
- 19.Newman AB, Naydeck BL, Sutton-Tyrrell K, Feldman A, Edmundowicz D, Kuller LH. Coronary artery calcification in older adults to age 99: prevalence and risk factors. Circulation. (2001) 104:2679–84. 10.1161/hc4601.099464 [DOI] [PubMed] [Google Scholar]
- 20.Chang SS, Lu CR, Chen KW, Kuo ZW, Yu SH, Lin SY, et al. Prognosis between st-elevation and non-st-elevation myocardial infarction in older adult patients. Front Cardiovasc Med. (2021) 8:749072. 10.3389/fcvm.2021.749072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Allison MA, Criqui MH, Wright CM. Patterns and risk factors for systemic calcified atherosclerosis. Arterioscler Thromb Vasc Biol. (2004) 24:331–6. 10.1161/01.ATV.0000110786.02097.0c [DOI] [PubMed] [Google Scholar]
- 22.Saw J, Bhatt DL, Moliterno DJ, Brener SJ, Steinhubl SR, Lincoff AM, et al. The influence of peripheral arterial disease on outcomes: a pooled analysis of mortality in eight large randomized percutaneous coronary intervention trials. J Am Coll Cardiol. (2006) 48:1567–72. 10.1016/j.jacc.2006.03.067 [DOI] [PubMed] [Google Scholar]
- 23.Wang TY, Gutierrez A, Peterson ED. Percutaneous coronary intervention in the elderly. Nat Rev Cardiol. (2011) 8:79–90. 10.1038/nrcardio.2010.184 [DOI] [PubMed] [Google Scholar]
- 24.Khera S, Kolte D, Palaniswamy C, Mujib M, Aronow WS, Singh T, et al. ST-elevation myocardial infarction in the elderly–temporal trends in incidence, utilization of percutaneous coronary intervention and outcomes in the United States. Int J Cardiol. (2013) 168:3683–90. 10.1016/j.ijcard.2013.06.021 [DOI] [PubMed] [Google Scholar]
- 25.Franken M, Nussbacher A, Liberman A, Wajngarten MST. Elevation myocardial infarction in the elderly. J Geriatr Cardiol. (2012) 9:108–14. 10.3724/SP.J.1263.2011.12297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.O’Gara PT, Kushner FG, Ascheim DD, Casey DJ, Chung MK, de Lemos JA, et al. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American heart association task force on practice guidelines. Circulation. (2013) 127:e362–425. [DOI] [PubMed] [Google Scholar]
- 27.Navarese EP, Rao SV, Krucoff MW. Age, STEMI, and cardiogenic Shock: never Too Old for PCI? J Am Coll Cardiol. (2019) 73:1901–4. 10.1016/j.jacc.2018.12.088 [DOI] [PubMed] [Google Scholar]
- 28.Garcia S, Sandoval Y, Roukoz H, Adabag S, Canoniero M, Yannopoulos D, et al. Outcomes after complete versus incomplete revascularization of patients with multivessel coronary artery disease: a meta-analysis of 89,883 patients enrolled in randomized clinical trials and observational studies. J Am Coll Cardiol. (2013) 62:1421–31. 10.1016/j.jacc.2013.05.033 [DOI] [PubMed] [Google Scholar]
- 29.Claessen B, Kikkert WJ, Hoebers LP, Bahadurzada H, Vis MM, Baan J, et al. Long-term ischaemic and bleeding outcomes after primary percutaneous coronary intervention for ST-elevation myocardial infarction in the elderly. Neth Heart J. (2015) 23:477–82. 10.1007/s12471-015-0733-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in this study are included in the article/supplementary material, further inquiries can be directed to the corresponding author/s.


