Abstract
The maintenance of intestinal homeostasis is fundamentally important to health. Intestinal barrier function and immune regulation are key determinants of intestinal homeostasis and are therefore tightly regulated by a variety of signaling mechanisms. The endocannabinoid system is a lipid mediator signaling system widely expressed in the gastrointestinal tract. Accumulating evidence suggests the endocannabinoid system is a critical nexus involved in the physiological processes that underlie the control of intestinal homeostasis. In this review we will illustrate how the endocannabinoid system is involved in regulation of intestinal permeability, fluid secretion, and immune regulation. We will also demonstrate a reciprocal regulation between the endocannabinoid system and the gut microbiome. The role of the endocannabinoid system is complex and multifaceted, responding to both internal and external factors while also serving as an effector system for the maintenance of intestinal homeostasis.
Keywords: Anandamide, 2-Arachidonoylglycerol, Cannabinoid, CB1 Receptor, CB2 Receptor, Intestinal Epithelium, Barrier Function
Abbreviations used in this paper: 2-AG, 2-arachidonoylglycerol; 2-OG, 2-oleoylglycerol; 2-PG, 2-palmitoylglycerol; ABHD, α/β-hydrolase domain-containing; AEA, anandamide; cAMP, cyclic adenosine monophosphate; CB, cannabinoid; CBD, cannabidiol; CNS, central nervous system; DAGL, diacylglycerol lipase; DIO, diet-induced obesity; DSS, dextran sodium sulfate; ECS, endocannabinoid system; ENS, enteric nervous system; FAAH, fatty acid amide hydrolase; GI, gastrointestinal; GPCR, G protein-coupled receptor; IBD, inflammatory bowel disease; MAGL, monoacylglycerol lipase; NAAA, N-acylethanolamine-hydrolyzing acid amidase; NAE, N-acylethanolamide; NAPE, N-arachidonoyl phosphatidylethanolamine; OEA, N-oleoylethanolamide; PEA, N-palmitoylethanolamide; PLC, phospholipase C; PLD, phospholipase D; PPAR, peroxisome proliferator-activated receptor; TEER, transepithelial electrical resistance; THC, Δ9-tetrahydrocannabinol; TNBS, trinitrobenzene sulfonic acid; TRPV1, transient receptor potential vanilloid type 1; ZO, zonula occludens
Summary.
The endocannabinoid system is a lipid mediator signaling system widely distributed throughout the gastrointestinal tract. The endocannabinoid system plays a pivotal role in the maintenance of intestinal homeostasis and gut barrier integrity, responding to internal and external environmental factors while also serving as a homeostatic effector system.
The maintenance of intestinal homeostasis is of fundamental importance to health. Intestinal homeostasis requires the integration of digestive and defensive functions of the gut to protect against the insults that arise from digestion, harmful pathogens and toxins, and the commensal microbiota that live in the gut, while simultaneously promoting the efficient utilization of food. A central determinant of intestinal homeostasis is intestinal barrier function.1, 2, 3 The intestinal barrier is a dynamic arrangement composed of a physical barrier (tight junctions) between the epithelial cells and a variety of secretory processes that together preserve the integrity of the epithelium, while being sufficiently permeable to allow for antigen sampling and the passage of nutrients, electrolytes, and water. The gut is poised to trigger effective mechanisms to rapidly rid itself of unwanted luminal contents through enteric neuroimmune mechanisms and has enormous capacity to mount immune responses to microbial pathogens and potentially harmful food antigens.4, 5, 6 Dysregulation of intestinal homeostasis has serious consequences, driving a variety of pathologic conditions, including inflammatory bowel disease (IBD), celiac disease, and diseases of gut-brain interaction such as irritable bowel syndrome.2,7, 8, 9
Because of the importance of preserving the integrity of the gastrointestinal (GI) tract, it is not surprising that there are numerous, highly sophisticated systems controlling various aspects of intestinal homeostasis. These include intrinsic cellular mechanisms, autocrine and paracrine factors, immune and microbial mediators, and intercellular signaling molecules, as well as intrinsic and extrinsic nervous mechanisms.2, 3, 4,10, 11, 12 Accumulating evidence indicates that the endocannabinoid system (ECS) is a vital nexus in the brain-gut-microbiota axis, serving as a critical regulator of intestinal homeostasis.13,14 The ECS is a widely distributed lipid mediator signaling system affecting a multitude of physiological and pathophysiological processes throughout the body.15,16 In this review we will highlight the complex role the ECS plays in the maintenance of intestinal homeostasis and gut barrier integrity. We will develop the idea that it is pivotal because it responds to internal and external environmental factors, while also serving as a homeostatic effector system. We have not addressed the effects of the ECS on gut motility, because this topic has been comprehensively reviewed.14,17,18
Endocannabinoid System
The ECS or endocannabinoidome has a multitude of functions in physiological and pathophysiological processes in the brain and body.19 It is perhaps best known for playing important roles in food intake and energy metabolism, regulation of the hypothalamic-pituitary-adrenal axis, pain transmission, and a variety of emotional and behavioral conditions.14,19, 20, 21, 22, 23, 24, 25
The ECS was discovered after the isolation of Δ9-tetrahydrocannabinol (THC) as the major psychoactive constituent of cannabis.26 This finding led to the development of ligands that were used to identify the receptors for cannabis. There are now 3 major classes of cannabinoids that are recognized: phytocannabinoids that are derived from the cannabis plant, such as THC and cannabidiol (CBD); synthetic cannabinoids, such as the chemical agonists HU210, CP55,940, and WIN55,212-2 and antagonists rimonabant, AM251, and AM630; and endocannabinoids, the lipid mediators, anandamide (AEA) and 2-arachidonoylglycerol (2-AG), that we will discuss further in this review.27 Interested readers are directed to excellent reviews of cannabinoids.15,27, 28, 29, 30
In its original description, the ECS consists of two G protein-coupled receptors (GPCRs), cannabinoid (CB) receptor 1 (CB1) and 2 (CB2), their endogenous ligands endocannabinoids, and the biosynthetic and degradative enzymes that control the availability of these ligands.15,31 Since this original description, many other receptors and ligands are now considered part of the ECS,19 as we will outline below.
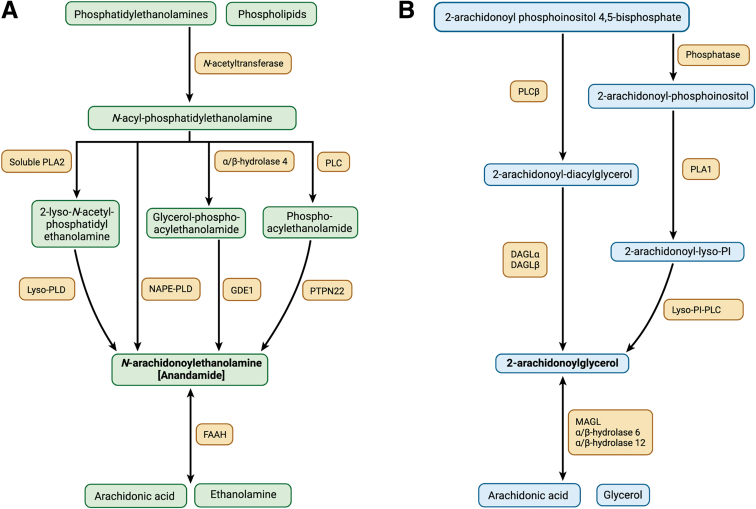
Endocannabinoids are membrane-derived lipid signaling molecules. The 2 primary endocannabinoids are N-arachidonoyl ethanolamine (AEA) and 2-AG. Unlike classical neurotransmitters that are stored in vesicles, endocannabinoids are synthesized “on demand” in response to a stimulus.32 Primary stimuli for their synthesis are an elevation of intracellular calcium and activation of a number of GPCRs.32 The synthesis and degradation pathways of the 2 main endocannabinoids are shown in Figure 1.
Figure 1.
Endocannabinoid synthesis and degradation pathways. Synthesis and degradation of the 2 major endocannabinoids, anandamide (A) and 2-AG (B). Further details are provided in the text. 2-AG, 2-arachidonoylglycerol; DAGL, diacylglycerol lipase; FAAH, fatty acid amide hydrolase; GDE1, glycerophosphodiesterase E1; MAGL, monoacylglycerol lipase; NAPE-PLD, N-acylphosphatidylethanolamine-hydrolyzing phospholipase D; PI, phosphatidylinositol; PLA, phospholipase A; PLC, phospholipase C; PLD, phospholipase D; PTPN22, protein tyrosine phosphatase non-receptor type 22. Figure created with BioRender.com.
Anandamide synthesis begins with the conversion of phosphatidylethanolamine into N-arachidonoyl phosphatidylethanolamine (NAPE) by the calcium-dependent enzyme N-acyltransferase. Anandamide is then produced from the hydrolysis of NAPE by N-acylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD). Three additional pathways for AEA synthesis have also been described.33, 34, 35 (1) Phospholipase C (PLC) converts NAPE into phosphoanandamide, which is then dephosphorylated to form AEA. (2) α/β-hydrolase 4 produces glycerophosphoanandamide from NAPE, which is then converted into AEA by glycerophosphodiesterase-1, and (3) soluble phospholipase A2 acts on NAPE to form 2-lyso-NAPE, which is then converted to AEA by lyso-PLD. The primary enzyme responsible for the degradation of AEA is fatty acid amide hydrolase (FAAH). FAAH hydrolyzes AEA into arachidonic acid and ethanolamine.36,37
The synthesis of 2-AG is dependent on the calcium-dependent enzyme PLC and the activity of diacylglycerol lipase (DAGL). PLC hydrolyzes arachidonic acid-containing membrane lipids to produce diacylglycerol, which is then converted into 2-AG by DAGL.38 In addition, 2-AG may be made from the conversion of 2-arachidonoyl-phosphoinositol to 2-arachidonoyl-lyso-phosphoinositol by phospholipase A1, which is subsequently converted to 2-AG by lyso-phosphoinositol-PLC.38,39 2-AG is primarily metabolized by monoacylglycerol lipase (MAGL) into glycerol and arachidonic acid.39,40 However, studies in mice have revealed 2 additional degradation pathways via the enzymes α/β-hydrolase domain-containing (ABHD)-6 and ABHD-12.41, 42, 43
In the GI tract, the primary cellular sources of endocannabinoids are identified by the expression of the genes for the relevant synthesizing enzymes and include enteric neurons and glia and the extrinsic projections from neurons in the dorsal root ganglia.44, 45, 46 Recent single cell RNA expression studies have suggested other interesting, albeit minor, cellular sources including Paneth and goblet cells.47 However, although we are gaining an understanding of expression of the relevant ECS-related genes, the functional cellular sources of endocannabinoids in the gut are not entirely clear, and we do not have a complete understanding of how sources of endocannabinoids change during pathophysiological conditions.
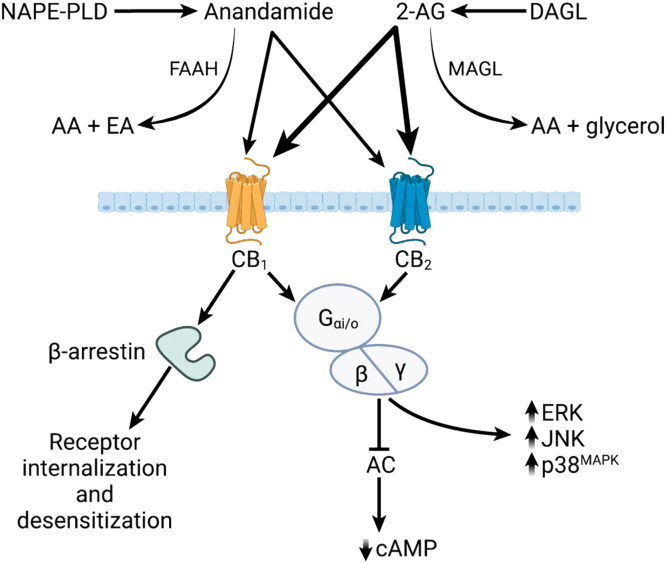
The CB receptors are GPCRs coupled to Gi/o proteins leading to an inhibition of adenylyl cyclase and decreased production of cyclic adenosine monophosphate (cAMP).48 The release of βγ dimers after CB receptor activation also causes inhibition of N- and P/Q-type Ca2+ channels and activation of inwardly rectifying and A-type potassium channels.32,48 The βγ dimers initiate Src-mediated signaling cascades leading to activation of mitogen-activated protein kinases and focal adhesion kinases. Activation of CB receptors is generally inhibitory, serving as a “molecular brake”, to limit excitation across the synapse, reduce secretory processes by epithelial cells, and reduce mediator release from immune cells. Interaction of ligand-bound CB receptor with β-arrestin is required for internalization and termination of extracellular signaling, but β-arrestins are themselves signal transducers for intracellular signaling pathways, including extracellular signal-regulated kinase and c-Jun terminal kinase, which mediate some of the actions of cannabinoids.32,48 CB1 receptor signaling is also regulated by the cannabinoid receptor-interacting protein 1a, which interacts with GPCR and β-arrestin.49 Some cannabinoid ligands are biased toward β-arrestin over cAMP signaling, leading to cell-specific actions, which can potentially be exploited therapeutically.50,51 Cannabinoid receptor signaling is illustrated in Figure 2.
Figure 2.
Signaling pathways induced by endocannabinoid receptor activation. Anandamide is produced from N-arachidonoyl phosphatidylethanolamine (NAPE) by the action of N-acylphosphatidylethanolamine-hydrolyzing phospholipase D (NAPE-PLD). It is degraded by fatty acid amide hydrolase (FAAH). FAAH hydrolyzes AEA into arachidonic acid (AA) and ethanolamine (EA). 2-Arachidonoylglycerol (2-AG) is primarily produced through the action of diacylglycerol lipase (DAGL). It is degraded by monoacylglycerol lipase into AA and glycerol. Inhibition of these degradative enzymes increases levels of endocannabinoid to enhance signaling. Both anandamide and 2-AG signal through the CB1 and CB2 receptors where anandamide is a partial agonist (light arrows) and 2-AG is a full agonist (thick arrows). Upon ligand binding, both receptors activate Gαi/o, which then interacts with β and γ subunits to initiate downstream signaling. The primary response is inhibition of adenylate cyclase (AC) and therefore reduction in the cytosolic levels of cyclic adenosine monophosphate (cAMP). There is evidence suggesting that these receptors can also activate MAP kinase (MAPK) pathways including ERK, JNK, and p38MAPK. Activation of the CB1 receptor also triggers β-arrestin, which is involved in receptor internalization, desensitization, and degradation, and which may also be involved in intracellular signaling pathways. Figure created with BioRender.com.
Cannabinoid receptors may exist in multiple forms as heterodimers or homodimers, are very widely distributed in essentially every organ and tissue in the body, and are subject to dysregulation in pathophysiological conditions.15,48,52 Of particular relevance, CB1 is expressed in the enteric nervous system (ENS) and the autonomic and central nervous systems (CNS) and is one of the most abundant GPCRs in the brain.53 In the GI tract, CB1 is expressed presynaptically on all classes of enteric neurons, except nitric oxide synthase-expressing inhibitory motor neurons.52,53 It is also found on the terminals of primary afferent nerves innervating the gut and on glucose-dependent insulinotropic polypeptide (K cells) and cholecystokinin (I cells) enteroendocrine cells in the upper small intestine.54,55 Activation of the CB1 receptor on enteric nerves leads to a decrease in neurotransmitter release into the synapse and therefore a depression of excitatory neurotransmission in the ENS. CB2 is predominantly expressed by immune cells including B cells, neutrophils, mast cells, and macrophages; however, it is also found in limited regions of the CNS, in the ENS, and on the terminals of primary afferent nerves.56, 57, 58, 59, 60, 61 Like CB1, activation of CB2 inhibits the release of immune mediators or neurotransmitters.
To rapidly activate their receptors, endocannabinoids, as lipids, require a carrier protein to overcome the hydrophilic environment of the extracellular space or synapse. Fatty acid-binding proteins, albumin, and heat shock protein 70 have been described as endocannabinoid carrier proteins.62, 63, 64 Fatty acid-binding proteins were discovered as mediators of the intracellular transport of AEA from the plasma membrane for degradation by FAAH.63 Whether they are used by AEA extracellularly remains uncertain. Fatty acid-binding protein 5 was found to regulate 2-AG signaling at excitatory glutamatergic synapses in the brain.65 In vitro evidence was that the source of the fatty acid-binding protein 5 was astrocytes,65 which suggests that fatty acid-binding proteins are extracellular carrier proteins. These data suggest that glial cells serve to limit the extent of synaptic endocannabinoid signaling. Whether this mechanism exists in vivo remains to be determined.
Recent single cell RNA sequencing of neurons and glia in the ENS reveal fatty acid-binding protein 5 gene is highly expressed by enteric neurons, enteroendocrine cells, and also in enteric glia.45,46 What role it plays in the ENS remains to be determined. It also remains to be determined how endocannabinoid signaling between other cell types in the gut, eg, immune cells, occurs in vivo.
The ECS is not restricted to the actions of its 2 primary ligands. Several related bioactive lipids have been identified that structurally resemble AEA and 2-AG. These include the N-acylethanolamides (NAEs), N-palmitoylethanolamide (PEA), N-oleoylethanolamide (OEA), N-stearoylethanolamide, and N-linoleoylethanolamide, as well as lipid mediators from the acylglycerol family, 2-palmitoylglycerol (2-PG) and 2-oleoylglycerol (2-OG). These bioactive lipids are thought to modulate endocannabinoid signaling through interactions with synthetic and degradative enzymes and other non-canonical endocannabinoid receptors.19,66,67
In addition, endocannabinoids do not interact exclusively with the canonical CB receptors, but also with several other classes of receptors.27 One of the best examples of endocannabinoid action at a non-cannabinoid receptor is the affinity of AEA for the transient receptor potential vanilloid type-1 (TRPV1) channel.27 Endocannabinoids and NAEs also have affinity for peroxisome proliferator-activated receptor (PPAR)-α, PPAR-γ, G protein-coupled receptor 55 (GPR55), GPR119, and GPR18.13,68, 69, 70, 71
Thus, the ECS consists of lipid mediators, their associated biosynthetic and degradative enzymes, receptors, and intracellular signaling systems capable of exquisite local regulatory control in physiological and pathophysiological conditions. There remains much we do not know about this system in the gut, notably the exact sites and specific conditions in which endocannabinoids are released. We know little about the genetic and epigenetic regulation of CB receptor expression in the GI tract, or that of other components of the ECS in the gut. Moreover, we know relatively little about how the ECS is regulated or dysregulated in pathophysiological conditions, although we have evidence for alterations in the expression of the components of the ECS in various GI diseases.
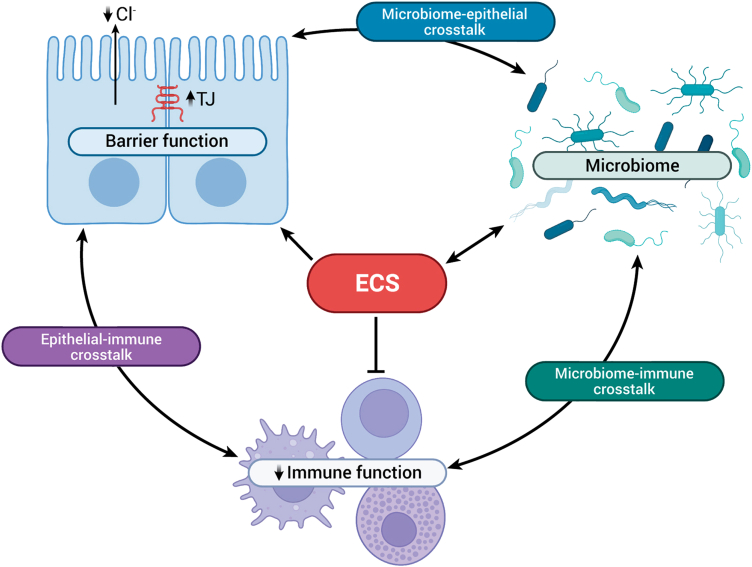
In the following sections of this review, we will discuss how the ECS is involved in the control of intestinal homeostasis by considering the elements that make up the physical, secretory, and immunologic components of the intestinal barrier (Figure 3). Finally, we will consider the reciprocal regulation of the ECS and the gut microbiome.
Figure 3.
Endocannabinoid system (ECS) interactions with barrier, immune, and microbiome systems. The ECS can alter the barrier function of the epithelium by increasing insertion of tight junction (TJ) proteins to enhance the physical barrier of the epithelium. Endocannabinoids also decrease chloride secretion to control the secretory barrier. The ECS, primarily through activation of CB2 receptors, regulate immune cell function by decreasing cytokine release. There are reciprocal interactions between the ECS and the microbiome. By affecting each of these systems, the ECS therefore also regulates the complex crosstalk that exists between the epithelium and the immune system, the immune system and the microbiome, and the microbiome and the epithelium. Figure created with BioRender.com.
Regulation of Epithelial Tight Junctions by the Endocannabinoid System
A single layer of epithelial cells lines the GI tract and is all that separates the contents of the gut from the rest of the body.72 The intestinal epithelium is a selectively permeable barrier that facilitates the transcellular and paracellular transport of nutrients, water, and electrolytes, enables antigen sampling for oral tolerance, while inhibiting the passage of possibly harmful substances such as toxins, foreign antigens, pathogens, and commensal bacteria.73,74
Paracellular transport across the epithelial barrier is regulated by tight junctions. Tight junctions are protein complexes located between adjacent epithelial cells that form a selectively permeable barrier.75 There are 4 groups of transmembrane proteins: occludin,76 claudins,77 junctional adhesion molecule,78 and tricellulin.79 Scaffold proteins, such as zonula occludens (ZO) proteins, anchor the intracellular domains of the transmembrane proteins to the actin cytoskeleton.73 The regulation of tight junctions is dynamic and controls the paracellular permeability of ions, nutrients, and water.73,75 When the integrity of the tight junction barrier is compromised, paracellular permeability increases and allows the passage of large molecules and microbes that reside in the gut lumen. This can lead to inflammation and tissue damage from the infiltration of bacteria and their metabolites into the mucosa.73
The intestinal epithelium expresses CB receptors, although this varies according to species, location along the GI tract, and pathophysiological state. The human colon expresses CB1 receptors with what appears to be primarily apical expression based on immunohistochemical studies.58 Colonic epithelia also express CB2 receptors in Crohn’s disease. The epithelium of mouse small intestine and colon do not display a substantial degree of CB1 expression under normal conditions.80,81 Caco-2 cells, a cancer cell line often used to study epithelial cell biology, express CB1 receptors.82
The ECS has been shown to play a role in the regulation of tight junction proteins essential for the maintenance of intestinal barrier function.82, 83, 84 In an in vitro model of the intestinal epithelium using Caco-2 cell monolayers, Muccioli et al83 demonstrated that simultaneous application of the CB1 agonist HU210 and lipopolysaccharide reduced transepithelial electrical resistance (TEER), and this was associated with a reduction in the expression of tight junction proteins occludin and ZO-1. The effect on TEER and tight junction protein expression was blocked by the CB1 antagonist rimonabant.83 Similarly, Alhamoruni et al84 showed that apical application of the endocannabinoids AEA and 2-AG reduced TEER, increased mRNA expression of ZO-1, and reduced mRNA expression of claudin-1. This effect was also blocked by the CB1 antagonist AM251, suggesting that ligands of the ECS modulate intestinal permeability under baseline conditions by altering the expression of tight junction proteins.
The ECS has also been shown to play a role in the regulation of intestinal permeability in conditions where baseline intestinal permeability is perturbed.82,84, 85, 86 In an in vitro model of ethylenediamine tetraacetic acid–induced increased permeability, basolateral application of AEA and 2-AG decreased permeability in Caco-2 cell monolayers.84 Although the effect of AEA and 2-AG is mediated by CB1, AEA relied on the additional recruitment of TRPV1.84 In a model of inflammation-induced increase in permeability where Caco-2 cells are exposed to tumor necrosis factor and interferon gamma, basolateral application of AEA and 2-AG had no effect on permeability.86 However, in both models, apical application of AEA and 2-AG increased permeability through a CB1-dependent mechanism.84,86 Apical application of the phytocannabinoids THC and CBD were protective and reduced permeability through a CB1-dependent mechanism.84,86 Interestingly, co-application of PEA to the basolateral compartment and cannabidiol to the apical compartment decreased permeability, and this was dependent on PPARα and CB1, respectively.82 Altogether, these data demonstrate that the ECS is involved in the regulation of gut barrier function largely through a CB1-dependent mechanism but may also rely on the recruitment of TRPV1 and PPARα.
Additional studies in vivo have further supported the role of the ECS in the regulation of intestinal permeability. Chronic activation of the CB1 receptor with the potent full agonist HU210 in wild-type mice led to an increase in permeability to 4 kDa FITC-dextran.83 In contrast, Zoppi et al87 demonstrated that CB1 knockout mice experience a greater degree of intestinal barrier dysfunction after exposure to immobilization and acoustic stress compared with wild-type mice, suggesting that the CB1 receptor exerts a protective role in the colon in the regulation of paracellular permeability. Consistent with these findings, Chen et al88 showed that the beneficial effect of resveratrol on intestinal permeability in a high-fat diet–induced nonalcoholic steatohepatitis model in rats was blocked by the synthetic CB1 receptor agonist arachidonyl-2´-chloroethylamide. Interestingly, recent work demonstrated that CB1 receptor agonists and antagonists reduce intestinal permeability acutely in mice exposed to a 2-week high-fat diet. Although changes in the expression of claudin-2 may underlie the effect of the CB1 agonist, further studies are required to understand the mechanism by which the CB1 antagonist reduces intestinal permeability.89
Obesity is a metabolic disorder associated with an altered gut microbiota, defects in barrier function, and increased endocannabinoid tone. In genetically obese (Ob/Ob) mice and dietary-induced obese mice, treatment with the CB1 antagonist rimonabant reduced plasma lipopolysaccharide levels and led to a change in the distribution and localization of tight junction proteins ZO-1 and occludin, suggesting a decrease in intestinal permeability.83,90 Together, these data suggest that the ECS is indeed involved in the chronic regulation of intestinal permeability in vivo through a CB1-dependent mechanism. CB1 agonists disrupt the gut barrier and, as suggested by Cani et al,13 act as a gate opener (increasing intestinal permeability), whereas CB1 antagonists protect the gut barrier and are considered gate keepers. This CB1-mediated disruption of intestinal permeability could be explained partially by changes in the distribution and localization of tight junction proteins after CB1 receptor activation. CB1 receptor blockade restores the tight junction barrier and the integrity of the gut barrier. However, in recent studies, we found that CB1 agonists could also reduce intestinal permeability in vitro under baseline conditions,89 suggesting that the pharmacologic regulation of CB1 receptors may depend on the level of endocannabinoid tone or other factors.
The literature has suggested that AEA and 2-AG, two endogenous CB1 agonists, exert differential effects on gut barrier integrity, acting themselves as a gate opener and gate keeper, respectively. Although the literature consistently establishes the role of AEA as a gate opener, the evidence to support 2-AG as a gate keeper is somewhat limited. In vitro data have suggested that AEA and 2-AG exert the same effects on barrier function such that they increase permeability when applied to the apical compartment and decrease permeability when applied to the basolateral compartment of Caco-2 cell monolayers. However, in vivo data have indirectly suggested a role for 2-AG as a gate keeper. Administration of Akkermansia muciniphila in mice with diet-induced obesity (DIO) is associated with an increase in 2-AG and an improvement of gut barrier function. From this study, the authors suggested that 2-AG is indeed a gate keeper, although the data to support this are quite limited. The role of 2-AG as a gate keeper is somewhat challenging to understand because 2-AG is a full CB1 agonist and typically produces effects associated with CB1 agonism.91 If 2-AG is indeed a gate keeper, then this CB1 agonist is behaving more like an antagonist, because CB1 antagonists have been shown to decrease permeability. On the other hand, AEA is a partial CB1 agonist with lower efficacy than 2-AG92 but is considered a gate opener and is therefore behaving like a CB1 agonist. On the basis of this information, one might predict the opposite of what has been shown, such that 2-AG would be the gate opener and AEA would be the gate keeper. Whether 2-AG is indeed a gate keeper remains unclear and warrants further investigation. Further studies will need to address the concept of biased agonism in CB1-mediated effects on epithelial permeability; AEA and 2-AG, while both acting at epithelially expressed CB1 receptor, could activate different signaling pathways resulting in different cellular responses. In addition, it is not known whether differences in the polarization of distribution of CB1 receptor, apical vs basolateral, could be associated with coupling to different signaling pathways and, hence, different effects on epithelial cell function.
Regulation of Secretory Function by the Endocannabinoid System
The secretion of fluid, mucus, antimicrobial peptides, and secretory immunoglobulin A are key elements of intestinal barrier function.12,93, 94, 95 The role of the ECS in the control of intestinal mucus, antimicrobial peptide, and secretory immunoglobulin A secretion is not well-understood. However, the ECS is involved in the regulation of neurogenic intestinal fluid secretion under baseline conditions96, 97, 98 and when fluid secretion is enhanced to respond to luminal toxin. Oral administration of cholera toxin to mice results in a large increase in fluid accumulation in the small intestine.99 This is associated with increased levels of AEA and increased expression of the CB1 receptor mRNA. These responses are involved in homeostatic control because endogenous CB1 receptor agonists reduce secretion to control levels, and a CB1 receptor antagonist further exacerbates secretion.99 In in vitro preparations of guinea pig ileum, CB1 agonism seems to reduce chloride ion transport (the main driver of fluid secretion) through a reduction of neurotransmitter release from primary sensory afferents, not through direct effects on the epithelium.96 There is limited evidence supporting a role for CB2 receptors in the regulation of intestinal secretion.98
Regulation of Local Gastrointestinal Immune Function by Endocannabinoids
The ECS is an important regulator of the immune system.100, 101, 102 Of note, cells of the innate and adaptive immune systems express CB2 receptors that control their activity. Within the GI tract, neutrophils, macrophages, and T and B cells express CB2 receptor as well as other receptors of the endocannabinoidome (eg, PPARα and GPR55).81,103, 104, 105, 106, 107 The ECS acts as a regulator of immune homeostasis in the gut. For example, CB2 receptor regulates the numbers of CX3CR1Hi macrophages in the intestinal lamina propria and their tolerogenic potential.104 Similarly, activation of CB2 enhances the expansion of regulatory T cells in the gut with concomitant anti-inflammatory actions.104, 105, 106, 107 In the section below we will expand on these homeostatic actions by focusing on the role of the ECS in regulating intestinal homeostasis in response to bacterial pathogens and intestinal inflammation.
Role of the Endocannabinoid System in Regulating Intestinal Homeostasis in Response to Bacterial Pathogens and Intestinal Inflammation
One of the first lines of defense against invading pathogens is the recruitment of neutrophils to the site of infection. The rapid transepithelial migration of neutrophils is a critical response to combat enteric infection. However, the products of neutrophil degranulation and the oxidative burst that are used to kill bacteria are associated with significant damage to surrounding tissues that if unchecked can lead to tissue damage and dysfunction.108 There are various lipid mediators involved in the resolution of inflammation and tissue restitution including the resolvins and lipoxin A4,109,110 but recently, endocannabinoids were discovered as endogenous regulators of the proinflammatory actions of the eicosanoid hepoxilin A3. Szabady et al103 investigated the role of the epithelial P-glycoprotein efflux pump in countering the hepoxilin A3 proinflammatory pathway. They discovered that N-acylethanolamines (AEA, OEA, and α-linolenoylethanolamide), effluxed via P-glycoprotein, suppressed neutrophil migration mediated by hepoxilin A3. This effect was sensitive to FAAH, but not MAGL, and was shown to be mediated by the CB2 receptor localized on neutrophils. Interestingly, Szabady et al did not find evidence for a role for 2-AG or its congeners in inhibiting neutrophil migration, despite the fact it is a full CB2 receptor agonist.103 Thus, although AEA is a (relatively weak) CB2 agonist, exactly how other NAEs regulate CB2 receptor function and the specific receptor mechanisms responsible for these effects remain to be explained.
Consistent with these data, CB2 receptor knockout mice treated with either dextran sodium sulfate (DSS) or trinitrobenzene sulfonic acid (TNBS) to induce colitis have more severe disease and dramatic increases in neutrophil accumulation in the intestinal lumen.103,111 Similarly, activation of CB2 receptors directly with specific agonists or indirectly by inhibiting FAAH and elevating endogenous ligands, protects against experimental colitis in mice.105,112, 113, 114, 115 In accordance with these findings, the CB2-Q63R variant contributes to the risk for pediatric IBD, particularly Crohn’s disease,116 illustrating the clinical significance of endocannabinoid receptor mechanisms to intestinal homeostasis. In fact, the endocannabinoidome is markedly dysregulated in IBD,117 as are the expression and distribution of the ECS receptors and biosynthetic and degradative enzymes.118 Although it is tempting to speculate that these changes contribute to the breakdown in homeostasis in these diseases, this remains to be directly demonstrated.
Szabady et al103 did not find a role for the NAE, PEA, in inhibiting neutrophil migration; however, PEA has been shown by others to be a potent anti-inflammatory mediator acting via CB2 receptors and, in addition, GPR55 and PPARα, as well as through the modulation of TRPV1.119 In elegant work, Esposito et al120 demonstrated that PEA dose-dependently reduced colonic damage, inflammatory mediator expression and release, and immune cell infiltration in DSS colitis and inflammatory mediator expression and release in biopsy samples from patients with ulcerative colitis via PPARα activation. They demonstrated that the effects of PEA were mediated through an action on enteric glia by reducing the expression of toll-like receptor 4 and S100B.120 In an interesting extension of this work, this group recently developed a probiotic-based delivery system for PEA. Using genetic engineering, they developed a strain of Lactobacillus paracasei with the human NAPE-PLD gene inserted into it to produce an in situ delivery system for the release of PEA in the GI tract. They demonstrated that this approach was effective in a mouse model of Clostridium difficile colitis where colonic damage, inflammatory mediator release, and tight junction protein expression were all improved.121 It was similarly effective in DSS colitis.121 The effects of the probiotic bacterium were abolished in PPARα knockout mice, suggesting they were mediated by PEA. However, because NAPE-PLD can synthesize a variety of NAEs and these were not assessed, it remains to be determined whether the effects observed are solely mediated by PEA. These studies placed enteric glia as critically important cellular intermediaries in the regulation of intestinal inflammation and homeostasis, a role that is gaining increasing recognition and clinical relevance.122, 123, 124 For example, it was recently shown that toll-like receptor 4 on enteric glia is critical for the development of necrotizing enterocolitis.125 Little is known about how the ECS regulates enteric glial function. Sharkey and colleagues showed that CB2 receptors could attenuate activation of enteric glia, which is consistent with other actions of cannabinoids, but beyond that this remains an area for further investigation.57
The anti-inflammatory actions of PEA in the gut have been extended to intestinal inflammation in mouse models of Alzheimer’s disease, radiation injury, and ischemia-reperfusion injury.126, 127, 128 Although the actions of PEA are terminated by hydrolysis by FAAH, they are also regulated by N-acylethanolamine-hydrolyzing acid amidase (NAAA).129 Inhibiting NAAA elevates levels of PEA, while not altering those of AEA. In TNBS colitis in mice, a NAAA inhibitor significantly reduced the degree of colitis and the release of inflammatory mediators,129 indicating that endogenously produced PEA can regulate inflammation and mucosal integrity. Interestingly, the related NAE, OEA, is also anti-inflammatory in DSS colitis,130 although the receptor mechanisms remain to be determined.
Activation of CB1 receptors in the gut is also anti-inflammatory in models of experimental colitis,112,114,131 and consistent with those observations, CB1 receptor knockout mice have exacerbated disease.111,131 Although CB1 receptors are expressed throughout the GI tract,14,52 a peripherally restricted CB1 receptor agonist does not protect against colitis,132,133 whereas a centrally administered CB1 agonist was protective.132 This puzzling finding requires further study.
Endogenous 2-AG levels can be selectively elevated by inhibiting the primary enzyme responsible for its metabolism, MAGL. Elevating levels of 2-AG using a pharmacologic MAGL inhibitor attenuated murine TNBS colitis and significantly improved intestinal barrier function.134 Interestingly, the effects of the MAGL inhibitor were blocked by both CB1 and CB2 receptor antagonists, as they are when FAAH inhibitors are used to elevate endogenous endocannabinoids.114 Although it seems likely that some of the effects of inhibiting MAGL are localized to the gut, it may be that the CB1 effects are also (or only) centrally mediated. This remains to be determined and is an exciting area for future study.
Mice with a genetic deletion of MAGL (Mgll knockout mice) have chronic elevations of 2-AG, leading to the desensitization, accumulation and functional inhibition of CB1 receptors in the gut and brain.135,136 Using this model, Ellerman et al137 recently showed that these mice were protected from the effects of enteric bacterial infection with the attaching and effacing enteric pathogen Citrobacter rodentium, which causes a breakdown of barrier function and marked intestinal inflammation. They made the remarkable discovery that the effect was mediated via an action of 2-AG on the virulence programs essential for successful bacterial infection.137 2-AG works by antagonizing QseC, the bacterial histidine kinase that promotes the activation of the type III secretion system, used to infect host cells. QseC is a quorum sensing receptor that was also discovered to be a bacterial adrenergic receptor, responding to epinephrine and norepinephrine, to enhance bacterial virulence.138,139 It therefore appears that 2-AG counteracts the effects of adrenergic stimuli, reducing virulence of C rodentium, thereby also providing an interesting example of interkingdom signaling in which host signaling molecules have the capacity to regulate the degree of bacterial virulence resulting in the maintenance of intestinal homeostasis.
Taken together, these data strongly suggest that the local production and release of endocannabinoids in the GI tract maintain an anti-inflammatory environment in the face of enteric infection or mucosal aggravation and damage. Future studies aimed at discovering the cellular sources of endocannabinoids and the mechanisms that govern their production and release in the intestines are critical for a full understanding of the role of the ECS in regulating intestinal homeostasis in response to bacterial pathogens and intestinal inflammation.
Reciprocal Regulation of the Endocannabinoid System and the Gut Microbiome
The gut microbiota is a critical environmental determinant of host physiology. Studies using germ-free mice, or mice treated with antibiotics to deplete the gut microbiota, have revealed that the microbiota shapes gut function and enteric neural control mechanisms.140, 141, 142, 143, 144, 145 Microbial dysbiosis is associated with a breakdown in epithelial barrier function and is associated with both local GI diseases, eg, IBD, and various systemic conditions, eg, obesity and diabetes. Investigations into the regulation of the ECS by the gut microbiome and vice versa are at an early stage, but some intriguing results have emerged that support the general hypothesis that the ECS regulates intestinal homeostasis through interactions with the microbiota.
In the absence of a gut microbiota, significant changes in the expression of the genes for receptors, biosynthetic and degradative enzymes of the ECS were observed throughout the gut.146 For example, cnr1 (CB1 receptor) is markedly elevated in the colon of germ-free mice. Overall, more striking changes were seen in the small intestine compared with the colon, and when mice were younger (4 weeks compared with 13 weeks of age). In concert with these changes, there were also alterations in the levels of endocannabinoids, NAEs, and other related lipid mediators, to a variable degree along the length of the gut.146 Most of the changes observed were reversible when a normal gut flora was introduced using fecal microbial transplant.146 However, it should be noted that the germ-free animals were only recolonized in these studies for 1 week, which may not have been sufficient for stable microbial recolonization. Nevertheless, these data demonstrate that the gut microbiota is directly impacting all aspects of the ECS.
The ECS has also been examined after antibiotic administration was used to deplete the gut microbiota.147, 148, 149 Modest changes to AEA levels were noted along the length of small intestine with no changes to 2-AG147; however, there were marked changes to NAEs and other endocannabinoidome ligands. Interestingly, increased antibiotic treatment resulted in increased CB2 receptor expression.148,149 CB2 receptor expression was further increased in animals exposed to antibiotics who were also exposed to a period of water-avoidance stress,148 which is illustrative of how the ECS responds to perturbations that lead to elevated visceral sensitivity.
Taken together, these studies reveal how the ECS is relatively altered by marked changes in the microbial environment, although the mechanism underlying these alterations remains to be determined. Importantly, microbial changes to endocannabinoid signaling result in biologically relevant functional effects at the level of the gut and the brain; it should be noted that commensal bacteria make endocannabinoid ligands,150 although their role in GI physiology remains obscure.
Administration of prebiotics or probiotics alters the composition of the gut microbiota (transiently) and can be used therapeutically. The benefits of probiotics may be mediated, at least in part, by the ECS. For example, treatment with the probiotic Lactobacillus acidophilus up-regulated CB2 receptors on intestinal epithelial cells. When animals with visceral hypersensitivity were treated with this probiotic, it gave rise to a pronounced visceral analgesia to colorectal distention, sensitive to CB2 receptor antagonism.151 The exact mechanisms behind these interesting effects remain to be determined because the source and nature of the ligand activating the CB2 receptors were not determined. However, the inhibitory actions of CB2 receptor activation are consistent with recent studies that show that optogenetic inhibition of the colonic epithelium reduces visceral hypersensitivity in mice with DSS colitis.152 In another example, treatment with the probiotic Lactobacillus plantarumWJL reduced despair behavior and increased hippocampal neurogenesis associated with a chronic stress paradigm by altering endocannabinoid levels in the hippocampus.153 The gut-brain pathways involved in these effects remain to be determined.
Just as probiotics can alter the ECS in ways that are beneficial, altering the microbiota can also give rise to effects that are detrimental. Recently, Markey et al154 colonized healthy mice with the commensal fungus Candida albicans for 48 hours. Candida colonization caused no changes to the cecal bacterial populations in the gut and no intestinal inflammation. However, there were marked increases in anxiety-like behavior, accompanied by elevations in plasma corticosterone that were inversely correlated with forebrain AEA levels. Colonization with Candida disrupted the metabolism of endocannabinoids in the gut, notably the NAEs. Consistent with these observations, when mice were treated with the FAAH inhibitor URB597, corticosterone levels were reduced to control levels, as was anxiety-like behavior. This finding is illustrative of the importance of the microbiota-gut-brain axis in regulating behavior and how the ECS is an important component of this signaling system.
In an interesting study in human subjects, Vijay et al155 studied the relationships between the gut microbiome, ECS, and inflammatory cytokines after 6-week exercise intervention. They demonstrated that under baseline conditions, the NAEs were positively associated with bacterial alpha diversity and with short-chain fatty acid producing bacterial species including Bifidobacterium and Faecalibacterium and negatively associated with the pathogenic bacterium Escherichia Shigella. The NAEs increased significantly after the exercise intervention. Changes in AEA correlated with elevated butyrate levels, increases in AEA and PEA correlated with reductions in the inflammatory cytokines, tumor necrosis factor and interleukin 6, whereas 2-AG and OEA levels were correlated with anti-inflammatory cytokine interleukin 10.155 This study again highlights the interactions between the ECS and the gut microbiota and reveals that the ECS is involved in mediating homeostatic anti-inflammatory actions in humans.
The connection between gut microbiota and the ECS has been studied in the context of the regulation of energy homeostasis. Diet-induced obesity in mice is associated with an altered gut microbiota. Chronic administration of the CB1/CB2 partial agonist, THC, prevented the change in gut microbiota in the DIO mice.156 Chronic THC administration in DIO mice increases the abundance of Akkermansia muciniphila.156 Interestingly, THC had no effect on gut microbiota in lean mice.156 Administration of A. muciniphila in DIO mice also increases levels of 2-AG, 2-OG, and 2-PG in the gut.157 Furthermore, blockade of the CB1 receptor with the CB1 antagonist rimonabant increased the abundance of A. muciniphila in the gut but also led to a decreased relative abundance of Lachnospiraceae and Erysipelotrichaceae.90 Insights into how the ECS might regulate the gut microbiota in the context of a high-fat diet were obtained by Everard et al158 using molecular genetics. They selectively knocked out nape-pld (the gene for NAPE-PLD) in intestinal epithelial cells in mice fed a high-fat diet. This resulted in marked changes to the gut microbiota composition that were shown not to be due to the diet per se. These data suggest that epithelially derived NAEs are the mediators of signaling that ultimately alter microbial composition. It remains to be shown how this occurs, but one possibility is through the regulation of local innate immune mechanisms.
Specific changes in the gut microbiota through several models (eg, prebiotic treatment, high-fat diet, antibiotic treatment, and germ-free mice) selectively alter CB1, FAAH, and MAGL mRNA expression in the colon.159 The gut microbiota appears to modulate intestinal endocannabinoid tone in the colon but has no effect in the small intestine, which is likely due to the greater bacterial load in the colon. Although evidence suggests that changes in the composition of the gut microbiota affect colonic endocannabinoid tone, the exact mechanism involved in this regulation remains unknown.
Conclusions and Perspective
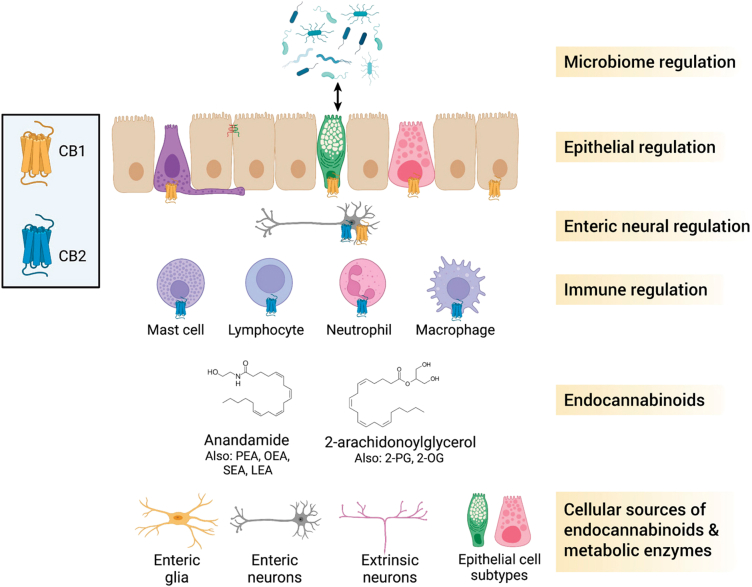
A summary of the material we have presented in this review is presented in Figure 4. Although there are numerous unanswered questions, the wealth of evidence accumulated to date suggests that the ECS is intimately involved in the physiological processes that underlie the control of intestinal homeostasis. The pivotal role the ECS plays in the maintenance of gut barrier integrity is complex and multifaceted because it responds to internal (microbial) and external (diet, stress, etc) environmental factors, while also serving as a homeostatic effector system. A lot of the evidence for the role of the ECS is based on the use of pharmacologic tools or a global knockout of the CB receptors or degradative enzymes. The application of cell-specific gene deletion technologies is required to causally determine the (patho)physiological roles of the ECS. Where this has been used, for example to understand the role of epithelial NAPE-PLD in DIO,158 very novel findings have emerged that greatly advanced our understanding of the physiology of the ECS.
Figure 4.
Overview of the endocannabinoid system in the gut. Endocannabinoids in the intestine are produced primarily by enteric neurons and by subpopulations of extrinsic neurons. Some epithelial subtypes, including goblet and Paneth cells, may also produce endocannabinoids, based on the presence of synthetic enzymes identified in single cell expression studies. The main endocannabinoids are anandamide (AEA) and 2-arachidonoylglycerol (2-AG). Other N-acylethanolamides and acylglycerols act to regulate gut functions. Endocannabinoids act at CB1 and CB2 receptors to regulate various functions in the gut. Various immune cells express CB2 receptors, which function primarily to dampen immune responses. Enteric neurons express CB1 receptors, which modulate enteric neural control of various gastrointestinal functions. Various epithelial subtypes express CB1 receptor under physiological conditions, which acts to suppress secretory responses. CB1 activation also decreases epithelial permeability by altering tight junction protein expression and localization. Endocannabinoids have a reciprocal relationship with the gut microbiota; endocannabinoids can affect the composition of the microbiota, and different commensals (bacteria, fungi) can alter the endocannabinoid system. 2-OG, 2-oleoylglycerol; 2-PG, 2-palmitoylglycerol; LEA, N-linoleoylethanolamide; OEA, N-oleoylethanolamide; PEA, N-palmitoylenthanolamide; SEA, N-stearoylethanolamide. Figure created with BioRender.com.
An important consideration of the ECS that needs to be borne in mind is the context dependent nature of CB receptor activation by endocannabinoids. By this we refer to the fact that the 2 major endocannabinoids AEA and 2-AG are partial and full agonists of the CB1 receptor, respectively, and as we have discussed, they can have opposite effects in the GI tract or individual effects rather than identical actions. Because of the complexity of lipid signaling in inflammatory states in particular, where there may be a diversity of precursors according to the expression of the rate limiting enzymes for the synthesis of the lipid moieties, ECS signaling may be altered in ways that are not easy to predict. Furthermore, many experimental, synthetic cannabinoids have biased signaling effects at CB receptors that do not precisely reflect signaling effects observed with endocannabinoids. Hence, future research that takes an integrative lipidomic approach to studies of the GI tract in health and disease are much needed to reconcile some of the disparate observations in the literature.
The ECS has been proposed as a good therapeutic target for the treatment of GI inflammatory diseases and conditions that involve a breakdown in intestinal homeostasis.160, 161, 162, 163 However, because of the ubiquitous distribution of the ECS in the GI tract and its complex physiology, careful consideration needs to be given to the best molecular target. This is currently challenging because the precise sites of endocannabinoid production are not well-understood, and a detailed description of the distribution of the biosynthetic and degradative enzymes for the expanded ECS is also lacking. Future studies addressing these limitations will greatly advance our understanding of the potential of the ECS to be selectively targeted for the treatment of disease as well as shedding new light on the physiology of the ECS in the GI tract.
Acknowledgments
CRediT Authorship Contributions
Hailey Cuddihey (Conceptualization: Supporting; Writing – original draft: Equal; Writing – review & editing: Equal)
Wallace K. MacNaughton (Conceptualization: Supporting; Resources: Equal; Writing – original draft: Equal; Writing – review & editing: Equal)
Keith A. Sharkey, PhD (Conceptualization: Lead; Resources: Lead; Writing – original draft: Equal; Writing – review & editing: Equal)
Footnotes
Conflicts of interest: This author reports the following: KAS has provided scientific advice and assistance to Arena Pharmaceuticals and GW Pharmaceuticals; has served on a speaker bureau for AbbVie; and has received research support from Abalone Inc. The remaining authors disclose no conflicts.
Funding: Supported by grants from the Canadian Institutes of Health Research (FDN148380 to KAS, PJT153290 to WKM) and the Natural Sciences and Engineering Research Council of Canada (RGPIN/04321-2018 to WKM). HC was a recipient of a Cumming School of Medicine Graduate Scholarship.
Supplementary Material
References
- 1.Zuo L., Kuo W.T., Turner J.R. Tight junctions as targets and effectors of mucosal immune homeostasis. Cell Mol Gastroenterol Hepatol. 2020;10:327–340. doi: 10.1016/j.jcmgh.2020.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Odenwald M.A., Turner J.R. The intestinal epithelial barrier: a therapeutic target? Nat Rev Gastroenterol Hepatol. 2017;14:9. doi: 10.1038/nrgastro.2016.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Allaire J.M., Crowley S.M., Law H.T., Chang S.Y., Ko H.J., Vallance B.A. The intestinal epithelium: central coordinator of mucosal immunity. Trends Immunol. 2018;39:677–696. doi: 10.1016/j.it.2018.04.002. [DOI] [PubMed] [Google Scholar]
- 4.Sharkey K.A., Beck P.L., McKay D.M. Neuroimmunophysiology of the gut: advances and emerging concepts focusing on the epithelium. Nat Rev Gastroenterol Hepatol. 2018;15:765–784. doi: 10.1038/s41575-018-0051-4. [DOI] [PubMed] [Google Scholar]
- 5.Chu C., Artis D., Chiu I.M. Neuro-immune interactions in the tissues. Immunity. 2020;52:464–474. doi: 10.1016/j.immuni.2020.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veiga-Fernandes H., Mucida D. Neuro-immune interactions at barrier surfaces. Cell. 2016;165:801–811. doi: 10.1016/j.cell.2016.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farré R., Vicario M. Abnormal barrier function in gastrointestinal disorders. Handb Exp Pharmacol. 2017;239:193–217. doi: 10.1007/164_2016_107. [DOI] [PubMed] [Google Scholar]
- 8.Barbara G., Barbaro M.R., Fuschi D., Palombo M., Falangone F., Cremon C., Marasco G., Stanghellini V. Inflammatory and microbiota-related regulation of the intestinal epithelial barrier. Front Nutr. 2021;8 doi: 10.3389/fnut.2021.718356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Camilleri M., Madsen K., Spiller R., Van Meerveld B.G., Verne G.N. Intestinal barrier function in health and gastrointestinal disease. Neurogastroenterol Motil. 2012;24:503–512. doi: 10.1111/j.1365-2982.2012.01921.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Peterson L.W., Artis D. Intestinal epithelial cells: regulators of barrier function and immune homeostasis. Nat Rev Immunol. 2014;14:141–153. doi: 10.1038/nri3608. [DOI] [PubMed] [Google Scholar]
- 11.Honda K., Littman D.R. The microbiota in adaptive immune homeostasis and disease. Nature. 2016;535:75–84. doi: 10.1038/nature18848. [DOI] [PubMed] [Google Scholar]
- 12.Wells J.M., Brummer R.J., Derrien M., MacDonald T.T., Troost F., Cani P.D., Theodorou V., Dekker J., Méheust A., De Vos W.M., Mercenier A., Nauta A., Garcia-Rodenas C.L. Homeostasis of the gut barrier and potential biomarkers. Am J Physiol Gastrointest Liver Physiol. 2017;312:G171–G193. doi: 10.1152/ajpgi.00048.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cani P.D., Plovier H., Van Hul M., Geurts L., Delzenne N.M., Druart C., Everard A. Endocannabinoids: at the crossroads between the gut microbiota and host metabolism. Nat Rev Endocrinol. 2016;12:133–143. doi: 10.1038/nrendo.2015.211. [DOI] [PubMed] [Google Scholar]
- 14.Sharkey K.A., Wiley J.W. The role of the endocannabinoid system in the brain–gut axis. Gastroenterology. 2016;151:252–266. doi: 10.1053/j.gastro.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lu H.C., Mackie K. Review of the endocannabinoid system. Biol Psychiatry Cogn Neurosci Neuroimaging. 2021;6:607–615. doi: 10.1016/j.bpsc.2020.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pertwee R.G. Endocannabinoids and their pharmacological actions. Handb Exp Pharmacol. 2015;231:1–37. doi: 10.1007/978-3-319-20825-1_1. [DOI] [PubMed] [Google Scholar]
- 17.Taschler U., Hasenoehrl C., Storr M., Schicho R. Cannabinoid receptors in regulating the GI tract: experimental evidence and therapeutic relevance. Handb Exp Pharmacol. 2017;239:343–362. doi: 10.1007/164_2016_105. [DOI] [PubMed] [Google Scholar]
- 18.Izzo A.A., Sharkey K.A. Cannabinoids and the gut: new developments and emerging concepts. Pharmacol Ther. 2010;126:21–38. doi: 10.1016/j.pharmthera.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 19.Cristino L., Bisogno T., Di Marzo V. Cannabinoids and the expanded endocannabinoid system in neurological disorders. Nature Reviews Neurology. 2020;16:9–29. doi: 10.1038/s41582-019-0284-z. [DOI] [PubMed] [Google Scholar]
- 20.Di Marzo V., Silvestri C. Lifestyle and metabolic syndrome: contribution of the endocannabinoidome. Nutrients. 2019;11:1956. doi: 10.3390/nu11081956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iannotti F.A., Di Marzo V. The gut microbiome, endocannabinoids and metabolic disorders. J Endocrinol. 2021;248:R83–R97. doi: 10.1530/JOE-20-0444. [DOI] [PubMed] [Google Scholar]
- 22.Maldonado R., Cabañero D., Martín-García E. The endocannabinoid system in modulating fear, anxiety, and stress. Dialogues Clin Neurosci. 2020;22:229–239. doi: 10.31887/DCNS.2020.22.3/rmaldonado. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Micale V., Drago F. Endocannabinoid system, stress and HPA axis. Eur J Pharmacol. 2018;834:230–239. doi: 10.1016/j.ejphar.2018.07.039. [DOI] [PubMed] [Google Scholar]
- 24.Gallego-Landin I., García-Baos A., Castro-Zavala A., Valverde O. Reviewing the role of the endocannabinoid system in the pathophysiology of depression. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.762738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van den Hoogen N.J., Harding E.K., Davidson C.E.D., Trang T. Cannabinoids in chronic pain: therapeutic potential through microglia modulation. Front Neural Circuits. 2022;15 doi: 10.3389/fncir.2021.816747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mechoulam R., Hanuš L.O., Pertwee R., Howlett A.C. Early phytocannabinoid chemistry to endocannabinoids and beyond. Nat Rev Neurosci. 2014;15:757–764. doi: 10.1038/nrn3811. [DOI] [PubMed] [Google Scholar]
- 27.Pertwee R.G., Howlett A.C., Abood M.E., Alexander S.P.H., Di Marzo V., Elphick M.R., Greasley P.J., Hansen H.S., Kunos G., Mackie K., Mechoulam R., Ross R.A. International union of basic and clinical pharmacology: LXXIX—cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pacher P., Kogan N.M., Mechoulam R. Beyond THC and endocannabinoids. Annu Rev Pharmacol Toxicol. 2020;60:637–659. doi: 10.1146/annurev-pharmtox-010818-021441. [DOI] [PubMed] [Google Scholar]
- 29.Di Marzo V., Piscitelli F. The endocannabinoid system and its modulation by phytocannabinoids. Neurotherapeutics. 2015;12:692–698. doi: 10.1007/s13311-015-0374-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ligresti A., De Petrocellis L., Di Marzo V. From phytocannabinoids to cannabinoid receptors and endocannabinoids: pleiotropic physiological and pathological roles through complex pharmacology. Physiol Rev. 2016;96:1593–1659. doi: 10.1152/physrev.00002.2016. [DOI] [PubMed] [Google Scholar]
- 31.Piomelli D. The molecular logic of endocannabinoid signalling. Nat Rev Neurosci. 2003;4:873–884. doi: 10.1038/nrn1247. [DOI] [PubMed] [Google Scholar]
- 32.Lutz B. Neurobiology of cannabinoid receptor signaling. Dialogues Clin Neurosci. 2020;22:207–222. doi: 10.31887/DCNS.2020.22.3/blutz. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu J., Wang L., Harvey-White J., Osei-Hyiaman D., Razdan R., Gong Q., Chan A.C., Zhou Z., Huang B.X., Kim H.-Y., Kunos G. A biosynthetic pathway for anandamide. Proc Natl Acad Sci U S A. 2006;103:13345–13350. doi: 10.1073/pnas.0601832103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hussain Z., Uyama T., Tsuboi K., Ueda N. Mammalian enzymes responsible for the biosynthesis of N-acylethanolamines. Biochim Biophys Acta - Mol Cell Biol Lipids. 2017;1862:1546–1561. doi: 10.1016/j.bbalip.2017.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Simon G.M., Cravatt B.F. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for α/β-hydrolase 4 in this pathway. J Biol Chem. 2006;281:26465–26472. doi: 10.1074/jbc.M604660200. [DOI] [PubMed] [Google Scholar]
- 36.Fowler C.J., Jonsson K.O., Tiger G. Fatty acid amide hydrolase: biochemistry, pharmacology, and therapeutic possibilities for an enzyme hydrolyzing anandamide, 2-arachidonoylglycerol, palmitoylethanolamide, and oleamide. Biochem Pharmacol. 2001;62:517–526. doi: 10.1016/s0006-2952(01)00712-2. [DOI] [PubMed] [Google Scholar]
- 37.Van Egmond N., Straub V.M., Van Der Stelt M. Targeting endocannabinoid signaling: FAAH and MAG lipase inhibitors. Annu Rev Pharmacol Toxicol. 2021;61:441–463. doi: 10.1146/annurev-pharmtox-030220-112741. [DOI] [PubMed] [Google Scholar]
- 38.Fowler C.J., Doherty P., Alexander S.P.H. Endocannabinoid turnover. Adv Pharmacol. 2017;80:31–66. doi: 10.1016/bs.apha.2017.03.006. [DOI] [PubMed] [Google Scholar]
- 39.Labar G., Wouters J., Lambert D.M. A review on the monoacylglycerol lipase: at the interface between fat and endocannabinoid signalling. Curr Med Chem. 2010;17:2588–2607. doi: 10.2174/092986710791859414. [DOI] [PubMed] [Google Scholar]
- 40.Fowler C.J. Monoacylglycerol lipase: a target for drug development? Br J Pharmacol. 2012;166:1568–1585. doi: 10.1111/j.1476-5381.2012.01950.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cao J.K., Kaplan J., Stella N. ABHD6: its place in endocannabinoid signaling and beyond. Trends Pharmacol Sci. 2019;40:267–277. doi: 10.1016/j.tips.2019.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Navia-Paldanius D., Savinainen J.R., Laitinen J.T. Biochemical and pharmacological characterization of human α/β-hydrolase domain containing 6 (ABHD6) and 12 (ABHD12) J Lipid Res. 2012;53:2413–2424. doi: 10.1194/jlr.M030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ogasawara D., Ichu T.A., Jing H., Hulce J.J., Reed A., Ulanovskaya O.A., Cravatt B.F. Discovery and optimization of selective and in vivo active inhibitors of the lysophosphatidylserine lipase α/β-hydrolase domain-containing 12 (ABHD12) J Med Chem. 2019;62:1643–1656. doi: 10.1021/acs.jmedchem.8b01958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hockley J.R.F., Taylor T.S., Callejo G., Wilbrey A.L., Gutteridge A., Bach K., Winchester W.J., Bulmer D.C., McMurray G., Smith E.S.J. Single-cell RNAseq reveals seven classes of colonic sensory neuron. Gut. 2019;68:633–644. doi: 10.1136/gutjnl-2017-315631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zeisel A., Hochgerner H., Lönnerberg P., Johnsson A., Memic F., van der Zwan J., Häring M., Braun E., Borm L.E., La Manno G., Codeluppi S., Furlan A., Lee K., Skene N., Harris K.D., Hjerling-Leffler J., Arenas E., Ernfors P., Marklund U., Linnarsson S. Molecular architecture of the mouse nervous system. Cell. 2018;174:999–1014.e22. doi: 10.1016/j.cell.2018.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Drokhlyansky E., Smillie C.S., Van Wittenberghe N., Ericsson M., Griffin G.K., Eraslan G., Dionne D., Cuoco M.S., Goder-Reiser M.N., Sharova T., Kuksenko O., Aguirre A.J., Boland G.M., Graham D., Rozenblatt-Rosen O., Xavier R.J., Regev A. The human and mouse enteric nervous system at single-cell resolution. Cell. 2020;182:1606–1622.e23. doi: 10.1016/j.cell.2020.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Haber A.L., Biton M., Rogel N., Herbst R.H., Shekhar K., Smillie C., Burgin G., Delorey T.M., Howitt M.R., Katz Y., Tirosh I., Beyaz S., Dionne D., Zhang M., Raychowdhury R., Garrett W.S., Rozenblatt-Rosen O., Shi H.N., Yilmaz O., Xavier R.J., Regev A. A single-cell survey of the small intestinal epithelium. Nature. 2017;551:333–339. doi: 10.1038/nature24489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Howlett A.C., Abood M.E. CB1 and CB2 receptor pharmacology. Adv Pharmacol. 2017;80:169–206. doi: 10.1016/bs.apha.2017.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Oliver E.E., Hughes E.K., Puckett M.K., Chen R., Lowther W.T., Howlett A.C. Cannabinoid receptor interacting protein 1a (CRIP1a) in health and disease. Biomolecules. 2020;10:1–22. doi: 10.3390/biom10121609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Al-Zoubi R., Morales P., Reggio P.H. Structural insights into CB1 receptor biased signaling. Int J Mol Sci. 2019;20:1837. doi: 10.3390/ijms20081837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ibsen M.S., Connor M., Glass M. Cannabinoid CB1 and CB2 receptor signaling and bias. Cannabis Cannabinoid Res. 2017;2:48–60. doi: 10.1089/can.2016.0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Trautmann S.M., Sharkey K.A. The endocannabinoid system and its role in regulating the intrinsic neural circuitry of the gastrointestinal tract. Int Rev Neurobiol. 2015;125:85–126. doi: 10.1016/bs.irn.2015.10.002. [DOI] [PubMed] [Google Scholar]
- 53.Duncan M., Davison J.S., Sharkey K.A. Review article: endocannabinoids and their receptors in the enteric nervous system. Aliment Pharmacol Ther. 2005;22:667–683. doi: 10.1111/j.1365-2036.2005.02648.x. [DOI] [PubMed] [Google Scholar]
- 54.Glass L.L., Calero-Nieto F.J., Jawaid W., Larraufie P., Kay R.G., Göttgens B., Reimann F., Gribble F.M. Single-cell RNA-sequencing reveals a distinct population of proglucagon-expressing cells specific to the mouse upper small intestine. Mol Metab. 2017;6:1296–1303. doi: 10.1016/j.molmet.2017.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Argueta D.A., Perez P.A., Makriyannis A., Di Patrizio N.V. Cannabinoid CB1 receptors inhibit gut-brain satiation signaling in diet-induced obesity. Front Physiol. 2019;10:704. doi: 10.3389/fphys.2019.00704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Atwood B.K., Mackie K. CB2: a cannabinoid receptor with an identity crisis. Br J Pharmacol. 2010;160:467–479. doi: 10.1111/j.1476-5381.2010.00729.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Duncan M., Mouihate A., Mackie K., et al. Cannabinoid CB2 receptors in the enteric nervous system modulate gastrointestinal contractility in lipopolysaccharide-treated rats. Am J Physiol Liver Physiol. 2008;295:G78–G87. doi: 10.1152/ajpgi.90285.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wright K., Rooney N., Feeney M., Tate J., Robertson D., Welham M., Ward S. Differential expression of cannabinoid receptors in the human colon: cannabinoids promote epithelial wound healing. Gastroenterology. 2005;129:437–453. doi: 10.1016/j.gastro.2005.05.026. [DOI] [PubMed] [Google Scholar]
- 59.Galiègue S., Mary S., Marchand J., Dussossoy D., Carrière D., Carayon P., Bouaboula M., Shire D., Le Fur G., Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte subpopulations. Eur J Biochem. 1995;232:54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- 60.Beltramo M., Bernardini N., Bertorelli R., Campanella M., Nicolussi E., Fredduzzi S., Reggiani A. CB2 receptor-mediated antihyperalgesia: possible direct involvement of neural mechanisms. Eur J Neurosci. 2006;23:1530–1538. doi: 10.1111/j.1460-9568.2006.04684.x. [DOI] [PubMed] [Google Scholar]
- 61.Cabral G.A., Staab A. Effects on the immune system. Handb Exp Pharmacol. 2005;168:385–423. doi: 10.1007/3-540-26573-2_13. [DOI] [PubMed] [Google Scholar]
- 62.Oddi S., Fezza F., Pasquariello N., D’Agostino A., Catanzaro G., De Simone C., Rapino C., Finazzi-Agrò A., Maccarrone M. Molecular identification of albumin and Hsp70 as cytosolic anandamide-binding proteins. Chem Biol. 2009;16:624–632. doi: 10.1016/j.chembiol.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 63.Kaczocha M., Glaser S.T., Deutsch D.G. Identification of intracellular carriers for the endocannabinoid anandamide. Proc Natl Acad Sci U S A. 2009;106:6375–6380. doi: 10.1073/pnas.0901515106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kaczocha M., Vivieca S., Sun J., Glaser S.T., Deutsch D.G. Fatty acid-binding proteins transport N-acylethanolamines to nuclear receptors and are targets of endocannabinoid transport inhibitors. J Biol Chem. 2012;287:3415–3424. doi: 10.1074/jbc.M111.304907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Haj-Dahmane S., Shen R.Y., Elmes M.W., Studholme K., Kanjiya M.P., Bogdan D., Thanos P.K., Miyauchi J.T., Tsirka S.E., Deutsch D.G., Kaczocha M. Fatty-acid-binding protein 5 controls retrograde endocannabinoid signaling at central glutamate synapses. Proc Natl Acad Sci U S A. 2018;115:3482–3487. doi: 10.1073/pnas.1721339115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hansen H.S., Vana V. Non-endocannabinoid N-acylethanolamines and 2-monoacylglycerols in the intestine. Br J Pharmacol. 2019;176:1443–1454. doi: 10.1111/bph.14175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsuboi K., Uyama T., Okamoto Y., Ueda N. Endocannabinoids and related N-acylethanolamines: biological activities and metabolism. Inflamm Regen. 2018;38:28. doi: 10.1186/s41232-018-0086-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ryberg E., Larsson N., Sjögren S., Hjorth S., Hermansson N.-O., Leonova J., Elebring T., Nilsson K., Drmota T., Greasley P.J. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kohno M., Hasegawa H., Inoue A., Muraoka M., Miyazaki T., Oka K., Yasukawa M. Identification of N-arachidonylglycine as the endogenous ligand for orphan G-protein-coupled receptor GPR18. Biochem Biophys Res Commun. 2006;347:827–832. doi: 10.1016/j.bbrc.2006.06.175. [DOI] [PubMed] [Google Scholar]
- 70.De Petrocellis L., Di Marzo V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J Neuroimmune Pharmacol. 2010;5:103–121. doi: 10.1007/s11481-009-9177-z. [DOI] [PubMed] [Google Scholar]
- 71.Syed S.K., Hoang Bui H., Beavers L.S., Farb T.B., Ficorilli J., Chesterfield A.K., Kuo M.-S., Bokvist K., Barrett D.G., Efanov A.M. Regulation of GPR119 receptor activity with endocannabinoid-like lipids. Am J Physiol Endocrinol Metab. 2012;303:1469–1478. doi: 10.1152/ajpendo.00269.2012. [DOI] [PubMed] [Google Scholar]
- 72.Furness J.B., Rivera L.R., Cho H.-J., Bravo D.M., Callaghan B. The gut as a sensory organ. Nat Rev Gastroenterol Hepatol. 2013;10:729–740. doi: 10.1038/nrgastro.2013.180. [DOI] [PubMed] [Google Scholar]
- 73.Lee S.H. Intestinal permeability regulation by tight junction: implication on inflammatory bowel diseases. Intest Res. 2015;13:11–18. doi: 10.5217/ir.2015.13.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Loh G., Blaut M. Role of commensal gut bacteria in inflammatory bowel diseases. Gut Microbes. 2012;3:544–555. doi: 10.4161/gmic.22156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Nusrat A., Turner J.R., Madara J.L. Molecular physiology and pathophysiology of tight junctions: IV—regulation of tight junctions by extracellular stimuli: nutrients, cytokines, and immune cells. Am J Physiol Gastrointest Liver Physiol. 2000;279:G851–G857. doi: 10.1152/ajpgi.2000.279.5.G851. [DOI] [PubMed] [Google Scholar]
- 76.Furuse M., Hirase T., Itoh M., Nagafuchi A., Yonemura S., Tsukita S., Tsukita S. Occludin: a novel integral membrane protein localizing at tight junctions. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Furuse M., Fujita K., Hiiragi T., Fujimoto K., Tsukita S. Claudin-1 and -2: novel integral membrane proteins localizing at tight junctions with no sequence similarity to occludin. J Cell Biol. 1998;141:1539–1550. doi: 10.1083/jcb.141.7.1539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Martìn-Padura I., Lostaglio S., Schneemann M., Williams L., Romano M., Fruscella P., Panzeri C., Stoppacciaro A., Ruco L., Villa A., Simmons D., Dejana E. Junctional adhesion molecule, a novel member of the immunoglobulin superfamily that distributes at intercellular junctions and modulates monocyte transmigration. J Cell Biol. 1998;142:117–127. doi: 10.1083/jcb.142.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ikenouchi J., Furuse M., Furuse K., Sasaki H., Tsukita S., Tsukita S. Tricellulin constitutes a novel barrier at tricellular contacts of epithelial cells. J Cell Biol. 2005;171:939–945. doi: 10.1083/jcb.200510043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Casu M.A., Porcella A., Ruiu S., Saba P., Marchese G., Carai M.A.M., Reali R., Gessa G.L., Pani L. Differential distribution of functional cannabinoid CB1 receptors in the mouse gastroenteric tract. Eur J Pharmacol. 2003;459:97–105. doi: 10.1016/s0014-2999(02)02830-3. [DOI] [PubMed] [Google Scholar]
- 81.Grill M., Hasenoehrl C., Kienzl M., Kargl J., Schicho R. Cellular localization and regulation of receptors and enzymes of the endocannabinoid system in intestinal and systemic inflammation. Histochem Cell Biol. 2019;151:5–20. doi: 10.1007/s00418-018-1719-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Karwad M.A., Couch D.G., Theophilidou E., Sarmad S., Barrett D.A., Larvin M., Wright K.L., Lund J.N., O’Sullivan S.E. The role of CB1 in intestinal permeability and inflammation. FASEB J. 2017;31:3267–3277. doi: 10.1096/fj.201601346R. [DOI] [PubMed] [Google Scholar]
- 83.Muccioli G.G., Naslain D., Bäckhed F., Reigstad C.S., Lambert D.M., Delzenne N.M., Cani P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alhamoruni A., Lee A.C., Wright K.L., Larvin M., O’Sullivan S.E. Pharmacological effects of cannabinoids on the Caco-2 cell culture model of intestinal permeability. J Pharmacol Exp Ther. 2010;335:92–102. doi: 10.1124/jpet.110.168237. [DOI] [PubMed] [Google Scholar]
- 85.Wang J., Zhang X., Yang C., Zhao S. Effect of monoacylglycerol lipase inhibition on intestinal permeability in chronic stress model. Biochem Biophys Res Commun. 2020;525:962–967. doi: 10.1016/j.bbrc.2020.02.173. [DOI] [PubMed] [Google Scholar]
- 86.Alhamoruni A., Wright K.L., Larvin M., O’Sullivan S.E. Cannabinoids mediate opposing effects on inflammation-induced intestinal permeability. Br J Pharmacol. 2012;165:2598–2610. doi: 10.1111/j.1476-5381.2011.01589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zoppi S., Madrigal J.L.M., Pérez-Nievas B.G., Marín-Jiménez I., Caso J.R., Alou L., García-Bueno B., Colón A., Manzanares J., Luisa Gómez-Lus M., Menchén L., Leza J.C. Endogenous cannabinoid system regulates intestinal barrier function in vivo through cannabinoid type 1 receptor activation. Am J Physiol Gastrointest Liver Physiol. 2012;302:G565–G571. doi: 10.1152/ajpgi.00158.2011. [DOI] [PubMed] [Google Scholar]
- 88.Chen M., Hou P., Zhou M., Ren Q., Wang X., Huang L., Hui S., Yi L., Mi M. Resveratrol attenuates high-fat diet-induced non-alcoholic steatohepatitis by maintaining gut barrier integrity and inhibiting gut inflammation through regulation of the endocannabinoid system. Clin Nutr. 2020;39:1264–1275. doi: 10.1016/j.clnu.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 89.Cuddihey H., Cavin J.B., Wallace L.E., et al. Role of CB1 receptors in the acute regulation of small intestinal permeability: effects of high-fat diet. Am J Physiol Gastrointest Liver Physiol. 2022 doi: 10.1152/ajpgi.00341.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Mehrpouya-Bahrami P., Chitrala K.N., Ganewatta M.S., Tang C., Murphy E.A., Enos R.T., Velazquez K.T., McCellan J., Nagarkatti M., Nagarkatti P. Blockade of CB1 cannabinoid receptor alters gut microbiota and attenuates inflammation and diet-induced obesity. Sci Rep. 2017;7 doi: 10.1038/s41598-017-15154-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Reggio P. Endocannabinoid binding to the cannabinoid receptors: what is known and what remains unknown. Curr Med Chem. 2010;17:1468–1486. doi: 10.2174/092986710790980005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Cristino L., Becker T., Di Marzo V. Endocannabinoids and energy homeostasis: an update. BioFactors. 2014;40:389–397. doi: 10.1002/biof.1168. [DOI] [PubMed] [Google Scholar]
- 93.Vancamelbeke M., Vermeire S. The intestinal barrier: a fundamental role in health and disease. Expert Rev Gastroenterol Hepatol. 2017;11:821–834. doi: 10.1080/17474124.2017.1343143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pietrzak B., Tomela K., Olejnik-Schmidt A., Mackiewicz A., Schmidt M. Secretory IgA in intestinal mucosal secretions as an adaptive barrier against microbial cells. Int J Mol Sci. 2020;21:1–15. doi: 10.3390/ijms21239254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Bevins C.L., Salzman N.H. Paneth cells, antimicrobial peptides and maintenance of intestinal homeostasis. Nat Rev Microbiol. 2011;9:356–368. doi: 10.1038/nrmicro2546. [DOI] [PubMed] [Google Scholar]
- 96.MacNaughton W.K., Van Sickle M.D., Keenan C.M., Cushing K., Mackie K., Sharkey K.A. Distribution and function of the cannabinoid-1 receptor in the modulation of ion transport in the guinea pig ileum: relationship to capsaicin-sensitive nerves. Am J Physiol Liver Physiol. 2004;286:G863–G871. doi: 10.1152/ajpgi.00482.2003. [DOI] [PubMed] [Google Scholar]
- 97.Tyler K., Hillard C.J., Greenwood-Van Meerveld B. Inhibition of small intestinal secretion by cannabinoids is CB1 receptor-mediated in rats. Eur J Pharmacol. 2000;409:207–211. doi: 10.1016/s0014-2999(00)00843-8. [DOI] [PubMed] [Google Scholar]
- 98.Wasilewski A., Sacharczuk M., Fichna J. Modulation of the endocannabinoid system by the fatty acid amide hydrolase, monoacylglycerol and diacylglycerol lipase inhibitors as an attractive target for secretory diarrhoea therapy. J Physiol Pharmacol. 2017;68:591–596. [PubMed] [Google Scholar]
- 99.Izzo A.A., Capasso F., Costagliola A., Bisogno T., Marsicano G., Ligresti A., Matias I., Capasso R., Pinto L., Borrelli F., Cecio A., Lutz B., Mascolo N., Di Marzo V. An endogenous cannabinoid tone attenuates cholera toxin-induced fluid accumulation in mice. Gastroenterology. 2003;125:765–774. doi: 10.1016/s0016-5085(03)00892-8. [DOI] [PubMed] [Google Scholar]
- 100.Rahaman O., Ganguly D. Endocannabinoids in immune regulation and immunopathologies. Immunology. 2021;164:242–252. doi: 10.1111/imm.13378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cabral G.A., Ferreira G.A., Jamerson M.J. Endocannabinoids and the immune system in health and disease. Endocannabinoids. 2015;231:185–211. doi: 10.1007/978-3-319-20825-1_6. [DOI] [PubMed] [Google Scholar]
- 102.Pandey R., Mousawy K., Nagarkatti M., Nagarkatti P. Endocannabinoids and immune regulation. Pharmacol Res. 2009;60:85–92. doi: 10.1016/j.phrs.2009.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Szabady R.L., Louissaint C., Lubben A., Xie B., Reeksting S., Tuohy C., Demma Z., Foley S.E., Faherty C.S., Llanos-Chea A., Olive A.J., Mrsny R.J., McCormick B.A. Intestinal P-glycoprotein exports endocannabinoids to prevent inflammation and maintain homeostasis. J Clin Invest. 2018;128:4044–4056. doi: 10.1172/JCI96817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Acharya N., Penukonda S., Shcheglova T., Hagymasi A.T., Basu S., Srivastava P.K. Endocannabinoid system acts as a regulator of immune homeostasis in the gut. Proc Natl Acad Sci U S A. 2017;114:5005–5010. doi: 10.1073/pnas.1612177114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gentili M., Ronchetti S., Ricci E., Di Paola R., Gugliandolo E., Cuzzocrea S., Bereshchenko O., Migliorati G., Riccardi C. Selective CB2 inverse agonist JTE907 drives T cell differentiation towards a Treg cell phenotype and ameliorates inflammation in a mouse model of inflammatory bowel disease. Pharmacol Res. 2019;141:21–31. doi: 10.1016/j.phrs.2018.12.005. [DOI] [PubMed] [Google Scholar]
- 106.Leinwand K.L., Jones A.A., Huang R.H., Jedlicka P., Kao D.J., de Zoeten E.F., Ghosh S., Moaddel R., Wehkamp J., Ostaff M.J., Bader J., Aherne C.M., Collins C.B. Cannabinoid receptor-2 ameliorates inflammation in murine model of Crohn’s disease. J Crohns Colitis. 2017;11:1369–1380. doi: 10.1093/ecco-jcc/jjx096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ziring D., Wei B., Velazquez P., Schrage M., Buckley N.E., Braun J. Formation of B and T cell subsets require the cannabinoid receptor CB2. Immunogenetics. 2006;58:714–725. doi: 10.1007/s00251-006-0138-x. [DOI] [PubMed] [Google Scholar]
- 108.Nusrat A., Parkos C.A., Liang T.W., Carnes D.K., Madara J.L. Neutrophil migration across model intestinal epithelia: monolayer disruption and subsequent events in epithelial repair. Gastroenterology. 1997;113:1489–1500. doi: 10.1053/gast.1997.v113.pm9352851. [DOI] [PubMed] [Google Scholar]
- 109.Gewirtz A.T., Collier-Hyams L.S., Young A.N., Kucharzik T., Guilford W.J., Parkinson J.F., Williams I.R., Neish A.S., Madara J.L. Lipoxin A 4 analogs attenuate induction of intestinal epithelial proinflammatory gene expression and reduce the severity of dextran sodium sulfate-induced colitis. J Immunol. 2002;168:5260–5267. doi: 10.4049/jimmunol.168.10.5260. [DOI] [PubMed] [Google Scholar]
- 110.Serhan C.N., Levy B.D. Resolvins in inflammation: emergence of the pro-resolving superfamily of mediators. J Clin Invest. 2018;128:2657–2669. doi: 10.1172/JCI97943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Engel M.A., Kellerman C.A., Burnat G., Hahn E.G., Rau T., Konturek P.C. Mice lacking cannabinoid CB1-, CB2-receptors or both receptors show increased susceptibility to trinitrobenzene sulfonic acid (TNBS)-induced colitis. J Physiol Pharmacol. 2010;61:89–97. [PubMed] [Google Scholar]
- 112.D’Argenio G., Valenti M., Scaglione G., Cosenza V., Sorrentini I., Di Marzo V. Up-regulation of anandamide levels as an endogenous mechanism and a pharmacological strategy to limit colon inflammation. FASEB J. 2006;20:568–570. doi: 10.1096/fj.05-4943fje. [DOI] [PubMed] [Google Scholar]
- 113.Storr M.A., Keenan C.M., Zhang H., Patel K.D., Makriyannis A., Sharkey K.A. Activation of the cannabinoid 2 receptor (CB2) protects against experimental colitis. Inflamm Bowel Dis. 2009;15:1678–1685. doi: 10.1002/ibd.20960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Storr M.A., Keenan C.M., Emmerdinger D., Zhang H., Yüce B., Sibaev A., Massa F., Buckley N.E., Lutz B., Göke B., Brand S., Patel K.D., Sharkey K.A. Targeting endocannabinoid degradation protects against experimental colitis in mice: involvement of CB1 and CB2 receptors. J Mol Med. 2008;86:925–936. doi: 10.1007/s00109-008-0359-6. [DOI] [PubMed] [Google Scholar]
- 115.Sałaga M., Mokrowiecka A., Zakrzewski P.K., Cygankiewicz A., Leishman E., Sobczak M., Zatorski H., Małecka-Panas E., Kordek R., Storr M., Krajewska W.M., Bradshaw H.B., Fichna J. Experimental colitis in mice is attenuated by changes in the levels of endocannabinoid metabolites induced by selective inhibition of fatty acid amide hydrolase (FAAH) J Crohns Colitis. 2014;8:998–1009. doi: 10.1016/j.crohns.2014.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Strisciuglio C., Bellini G., Miele E., Martinelli M., Cenni S., Tortora C., Tolone C., Miraglia Del Giudice E., Rossi F. Cannabinoid receptor 2 functional variant contributes to the risk for pediatric inflammatory bowel disease. J Clin Gastroenterol. 2018;52:e37–e43. doi: 10.1097/MCG.0000000000000755. [DOI] [PubMed] [Google Scholar]
- 117.Grill M., Högenauer C., Blesl A., Haybaeck J., Golob-Schwarzl N., Ferreirós N., Thomas D., Gurke R., Trötzmüller M., Köfeler H.C., Gallé B., Schicho R. Members of the endocannabinoid system are distinctly regulated in inflammatory bowel disease and colorectal cancer. Sci Rep. 2019;9:2358. doi: 10.1038/s41598-019-38865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Marquéz L., Suárez J., Iglesias M., Bermudez-Silva F.J., de Fonseca F.R., Andreu M. Ulcerative colitis induces changes on the expression of the endocannabinoid system in the human colonic tissue. PLoS One. 2009;4 doi: 10.1371/journal.pone.0006893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Borrelli F., Romano B., Petrosino S., Pagano E., Capasso R., Coppola D., Battista G., Orlando P., Di Marzo V., Izzo A.A. Palmitoylethanolamide, a naturally occurring lipid, is an orally effective intestinal anti-inflammatory agent. Br J Pharmacol. 2015;172:142–158. doi: 10.1111/bph.12907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Esposito G., Capoccia E., Turco F., Palumbo I., Lu J., Steardo A., Cuomo R., Sarnelli G., Steardo L. Palmitoylethanolamide improves colon inflammation through an enteric glia/toll like receptor 4-dependent PPAR-α activation. Gut. 2014;63:1300–1312. doi: 10.1136/gutjnl-2013-305005. [DOI] [PubMed] [Google Scholar]
- 121.Esposito G., Corpetti C., Pesce M., Seguella L., Annunziata G., Del Re A., Vincenzi M., Lattanzi R., Lu J., Sanseverino W., Sarnelli G. A palmitoylethanolamide producing lactobacillus paracasei improves clostridium difficile toxin A-induced colitis. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.639728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Progatzky F., Shapiro M., Chng S.H., Garcia-Cassani B., Classon C.H., Sevgi S., Laddach A., Bon-Frauches A.C., Lasrado R., Rahim M., Amaniti E.M., Boeing S., Shah K., Entwistle L.J., Suárez-Bonnet A., Wilson M.S., Stockinger B., Pachnis V. Regulation of intestinal immunity and tissue repair by enteric glia. Nature. 2021;599:125–130. doi: 10.1038/s41586-021-04006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Benvenuti L., D’antongiovanni V., Pellegrini C., Antonioli L., Bernardini N., Blandizzi C., Fornai M. Enteric glia at the crossroads between intestinal immune system and epithelial barrier: implications for parkinson disease. Int J Mol Sci. 2020;21:1–16. doi: 10.3390/ijms21239199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Seguella L., Gulbransen B.D. Enteric glial biology, intercellular signalling and roles in gastrointestinal disease. Nat Rev Gastroenterol Hepatol. 2021;18:571–587. doi: 10.1038/s41575-021-00423-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kovler M.L., Gonzalez Salazar A.J., Fulton W.B., Lu P., Yamaguchi Y., Zhou Q., Sampah M., Ishiyama A., Prindle T., Wang S., Jia H., Wipf P., Sodhi C.P., Hackam D.J. Toll-like receptor 4–mediated enteric glia loss is critical for the development of necrotizing enterocolitis. Sci Transl Med. 2021;13 doi: 10.1126/scitranslmed.abg3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.D’Antongiovanni V., Pellegrini C., Antonioli L., Benvenuti L., Di Salvo C., Flori L., Piccarducci R., Daniele S., Martelli A., Calderone V., Martini C., Fornai M. Palmitoylethanolamide counteracts enteric inflammation and bowel motor dysfunctions in a mouse model of Alzheimer’s disease. Front Pharmacol. 2021;12 doi: 10.3389/fphar.2021.748021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wang J., Zheng J., Kulkarni A., Wang W., Garg S., Prather P.L., Hauer-Jensen M. Palmitoylethanolamide regulates development of intestinal radiation injury in a mast cell-dependent manner. Dig Dis Sci. 2014;59:2693–2703. doi: 10.1007/s10620-014-3212-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Di Paola R., Impellizzeri D., Torre A., Mazzon E., Cappellani A., Faggio C., Esposito E., Trischitta F., Cuzzocrea S. Effects of palmitoylethanolamide on intestinal injury and inflammation caused by ischemia-reperfusion in mice. J Leukoc Biol. 2012;91:911–920. doi: 10.1189/jlb.0911485. [DOI] [PubMed] [Google Scholar]
- 129.Alhouayek M., Bottemanne P., Subramanian K.V., Lambert D.M., Makriyannis A., Cani P.D., Muccioli G.G. N-acylethanolamine-hydrolyzing acid amidase inhibition increases colon N-palmitoylethanolamine levels and counteracts murine colitis. FASEB J. 2015;29:650–661. doi: 10.1096/fj.14-255208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lama A., Provensi G., Amoriello R., Pirozzi C., Rani B., Mollica M.P., Raso G.M., Ballerini C., Meli R., Passani M.B. The anti-inflammatory and immune-modulatory effects of OEA limit DSS-induced colitis in mice. Biomed Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110368. [DOI] [PubMed] [Google Scholar]
- 131.Massa F., Marsicano G., Hermann H., Cannich A., Monory K., Cravatt B.F., Ferri G.-L., Sibaev A., Storr M., Lutz B. The endogenous cannabinoid system protects against colonic inflammation. J Clin Invest. 2004;113:1202–1209. doi: 10.1172/JCI19465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Fichna J., Bawa M., Thakur G.A., Tichkule R., Makriyannis A., McCafferty D.M., Sharkey K.A., Storr M. Cannabinoids alleviate experimentally induced intestinal inflammation by acting at central and peripheral receptors. PLoS One. 2014;9 doi: 10.1371/journal.pone.0109115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Cluny N.L., Keenan C.M., Duncan M., Fox A., Lutz B., Sharkey K.A. Naphthalen-1-yl-(4-pentyloxynaphthalen-1-yl)methanone (SAB378), a peripherally restricted cannabinoid CB1/CB2 receptor agonist, inhibits gastrointestinal motility but has no effect on experimental colitis in mice. J Pharmacol Exp Ther. 2010;334:973–980. doi: 10.1124/jpet.110.169946. [DOI] [PubMed] [Google Scholar]
- 134.Alhouayek M., Lambert D.M., Delzenne N.M., Cani P.D., Muccioli G.G. Increasing endogenous 2-arachidonoylglycerol levels counteracts colitis and related systemic inflammation. FASEB J. 2011;25:2711–2721. doi: 10.1096/fj.10-176602. [DOI] [PubMed] [Google Scholar]
- 135.Taschler U., Eichmann T.O., Radner F.P.W., Grabner G.F., Wolinski H., Storr M., Lass A., Schicho R., Zimmermann R. Monoglyceride lipase deficiency causes desensitization of intestinal cannabinoid receptor type 1 and increased colonic μ-opioid receptor sensitivity. Br J Pharmacol. 2015;172:4419–4429. doi: 10.1111/bph.13224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Schlosburg J.E., Blankman J.L., Long J.Z., Nomura D.K., Pan B., Kinsey S.G., Nguyen P.T., Ramesh D., Booker L., Burston J.J., Thomas E.A., Selley D.E., Sim-Selley L.J., Liu Q.S., Lichtman A.H., Cravatt B.F. Chronic monoacylglycerol lipase blockade causes functional antagonism of the endocannabinoid system. Nat Neurosci. 2010;13:1113–1119. doi: 10.1038/nn.2616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ellermann M., Pacheco A.R., Jimenez A.G., Russell R.M., Cuesta S., Kumar A., Zhu W., Vale G., Martin S.A., Raj P., McDonald J.G., Winter S.E., Sperandio V. Endocannabinoids inhibit the induction of virulence in enteric pathogens. Cell. 2020;183:650–665.e15. doi: 10.1016/j.cell.2020.09.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Moreira C.G., Russell R., Mishra A.A., Narayanan S., Ritchie J.M., Waldor M.K., Curtis M.M., Winter S.E., Weinshenker D., Sperandio V. Bacterial adrenergic sensors regulate virulence of enteric pathogens in the gut. MBio. 2016;7:e00826–e008216. doi: 10.1128/mBio.00826-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Clarke M.B., Hughes D.T., Zhu C., Boedeker E.C., Sperandio V. The QseC sensor kinase: a bacterial adrenergic receptor. Proc Natl Acad Sci U S A. 2006;103:10420–10425. doi: 10.1073/pnas.0604343103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vicentini F.A., Keenan C.M., Wallace L.E., Woods C., Cavin J.B., Flockton A.R., Macklin W.B., Belkind-Gerson J., Hirota S.A., Sharkey K.A. Intestinal microbiota shapes gut physiology and regulates enteric neurons and glia. Microbiome. 2021;9:210. doi: 10.1186/s40168-021-01165-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Muller P.A., Matheis F., Schneeberger M., Kerner Z., Jové V., Mucida D. Microbiota-modulated CART+ enteric neurons autonomously regulate blood glucose. Science. 2020;370:314–321. doi: 10.1126/science.abd6176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Obata Y., Pachnis V. The effect of microbiota and the immune system on the development and organization of the enteric nervous system. Gastroenterology. 2016;151:836–844. doi: 10.1053/j.gastro.2016.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Obata Y., Castaño Á., Boeing S., Bon-Frauches A.C., Fung C., Fallesen T., de Agüero M.G., Yilmaz B., Lopes R., Huseynova A., Horswell S., Maradana M.R., Boesmans W., Vanden Berghe P., Murray A.J., Stockinger B., Macpherson A.J., Pachnis V. Neuronal programming by microbiota regulates intestinal physiology. Nature. 2020;578:284–289. doi: 10.1038/s41586-020-1975-8. [DOI] [PubMed] [Google Scholar]
- 144.Niesler B., Kuerten S., Demir I.E., Schäfer K.H. Disorders of the enteric nervous system: a holistic view. Nat Rev Gastroenterol Hepatol. 2021;18:393–410. doi: 10.1038/s41575-020-00385-2. [DOI] [PubMed] [Google Scholar]
- 145.De Vadder F., Grasset E., Holm L.M., Karsenty G., Macpherson A.J., Olofsson L.E., Bäckhed F. Gut microbiota regulates maturation of the adult enteric nervous system via enteric serotonin networks. Proc Natl Acad Sci U S A. 2018;115:6458–6463. doi: 10.1073/pnas.1720017115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Manca C., Boubertakh B., Leblanc N., Deschênes T., Lacroix S., Martin C., Houde A., Veilleux A., Flamand N., Muccioli G.G., Raymond F., Cani P.D., Di Marzo V., Silvestri C. Germ-free mice exhibit profound gut microbiota-dependent alterations of intestinal endocannabinoidome signaling. J Lipid Res. 2020;61:70–85. doi: 10.1194/jlr.RA119000424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Guida F., Turco F., Iannotta M., De Gregorio D., Palumbo I., Sarnelli G., Furiano A., Napolitano F., Boccella S., Luongo L., Mazzitelli M., Usiello A., De Filippis F., Iannotti F.A., Piscitelli F., Ercolini D., de Novellis V., Di Marzo V., Cuomo R., Maione S. Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav Immun. 2018;67:230–245. doi: 10.1016/j.bbi.2017.09.001. [DOI] [PubMed] [Google Scholar]
- 148.Aguilera M., Vergara P., Martínez V. Stress and antibiotics alter luminal and wall-adhered microbiota and enhance the local expression of visceral sensory-related systems in mice. Neurogastroenterol Motil. 2013;25:e515–e529. doi: 10.1111/nmo.12154. [DOI] [PubMed] [Google Scholar]
- 149.Aguilera M., Cerdà-Cuéllar M., Martínez V. Antibiotic-induced dysbiosis alters host-bacterial interactions and leads to colonic sensory and motor changes in mice. Gut Microbes. 2015;6:10–23. doi: 10.4161/19490976.2014.990790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cohen L.J., Esterhazy D., Kim S.H., Lemetre C., Aguilar R.R., Gordon E.A., Pickard A.J., Cross J.R., Emiliano A.B., Han S.M., Chu J., Vila-Farres X., Kaplitt J., Rogoz A., Calle P.Y., Hunter C., Bitok J.K., Brady S.F. Commensal bacteria make GPCR ligands that mimic human signalling molecules. Nature. 2017;549:48–53. doi: 10.1038/nature23874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Rousseaux C., Thuru X., Gelot A., Barnich N., Neut C., Dubuquoy L., Dubuquoy C., Merour E., Geboes K., Chamaillard M., Ouwehand A., Leyer G., Carcano D., Colombel J.F., Ardid D., Desreumaux P. Lactobacillus acidophilus modulates intestinal pain and induces opioid and cannabinoid receptors. Nat Med. 2007;13:35–37. doi: 10.1038/nm1521. [DOI] [PubMed] [Google Scholar]
- 152.Najjar S.A., Ejoh L.L., Loeza-Alcocer E., Edwards B.S., Smith-Edwards K.M., Epouhe A.Y., Gold M.S., Davis B.M., Albers K.M. Optogenetic inhibition of the colon epithelium reduces hypersensitivity in a mouse model of inflammatory bowel disease. Pain. 2021;162:1126–1134. doi: 10.1097/j.pain.0000000000002110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Chevalier G., Siopi E., Guenin-Macé L., Pascal M., Laval T., Rifflet A., Boneca I.G., Demangel C., Colsch B., Pruvost A., Chu-Van E., Messager A., Leulier F., Lepousez G., Eberl G., Lledo P.M. Effect of gut microbiota on depressive-like behaviors in mice is mediated by the endocannabinoid system. Nat Commun. 2020;11:6363. doi: 10.1038/s41467-020-19931-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Markey L., Hooper A., Melon L.C., Baglot S., Hill M.N., Maguire J., Kumamoto C.A. Colonization with the commensal fungus Candida albicans perturbs the gut-brain axis through dysregulation of endocannabinoid signaling. Psychoneuroendocrinology. 2020;121 doi: 10.1016/j.psyneuen.2020.104808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Vijay A., Kouraki A., Gohir S., Turnbull J., Kelly A., Chapman V., Barrett D.A., Bulsiewicz W.J., Valdes A.M. The anti-inflammatory effect of bacterial short chain fatty acids is partially mediated by endocannabinoids. Gut Microbes. 2021;13 doi: 10.1080/19490976.2021.1997559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Cluny N.L., Keenan C.M., Reimer R.A., Foll B Le, Sharkey K.A. Prevention of diet-induced obesity effects on body weight and gut microbiota in mice treated chronically with Δ9-tetrahydrocannabinol. PLoS One. 2015;10 doi: 10.1371/journal.pone.0144270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Everard A., Belzer C., Geurts L., Ouwerkerk J.P., Druart C., Bindels L.B., Guiot Y., Derrien M., Muccioli G.G., Delzenne N.M., De Vos W.M., Cani P.D. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc Natl Acad Sci U S A. 2013;110:9066–9071. doi: 10.1073/pnas.1219451110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Everard A., Plovier H., Rastelli M., Van Hul M., de Wouters d’Oplinter A., Geurts L., Druart C., Robine S., Delzenne N.M., Muccioli G.G., de Vos W.M., Luquet S., Flamand N., Di Marzo V., Cani P.D. Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nat Commun. 2019;10:457. doi: 10.1038/s41467-018-08051-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Muccioli G.G., Naslain D., Bäckhed F., Reigstad C.S., Lambert D.M., Delzenne N.M., Cani P.D. The endocannabinoid system links gut microbiota to adipogenesis. Mol Syst Biol. 2010;6:392. doi: 10.1038/msb.2010.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Picardo S., Kaplan G.G., Sharkey K.A., Seow C.H. Insights into the role of cannabis in the management of inflammatory bowel disease. Therap Adv Gastroenterol. 2019;12 doi: 10.1177/1756284819870977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Kienzl M., Storr M., Schicho R. Cannabinoids and opioids in the treatment of inflammatory bowel diseases. Clin Transl Gastroenterol. 2020;11 doi: 10.14309/ctg.0000000000000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Gotfried J., Naftali T., Schey R. Role of cannabis and its derivatives in gastrointestinal and hepatic disease. Gastroenterology. 2020;159:62–80. doi: 10.1053/j.gastro.2020.03.087. [DOI] [PubMed] [Google Scholar]
- 163.Maselli D.B., Camilleri M. Pharmacology, clinical effects, and therapeutic potential of cannabinoids for gastrointestinal and liver diseases. Clin Gastroenterol Hepatol. 2021;19:1748–1758.e2. doi: 10.1016/j.cgh.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.