Abstract
The nasal microbiome of patients with cutaneous T-cell lymphoma (CTCL) remains unexplored despite growing evidence connecting nasal bacteria to skin health and disease. Nasal swabs from 45 patients with CTCL (40 with mycosis fungoides, 5 with Sézary syndrome) and 20 healthy controls from the same geographical region (Chicago Metropolitan Area, Chicago, IL) were analyzed using sequencing of 16S ribosomal RNA and tuf2 gene amplicons. Nasal α-diversity did not differ between mycosis fungoides/Sézary syndrome and healthy controls (Shannon index, genus level, P = 0.201), but distinct microbial communities were identified at the class (R2 = 0.104, P = 0.023) and order (R2 = 0.0904, P = 0.038) levels. Increased relative abundance of the genera Catenococcus, Vibrio, Roseomonas, Acinetobacter, and unclassified Clostridiales was associated with increased skin disease burden (P < 0.005, q < 0.05). Performed to accurately resolve nasal Staphylococcus at the species level, tuf2 gene amplicon sequencing revealed no significant differences between mycosis fungoides/Sézary syndrome and healthy controls. Although S. aureus has been shown to worsen CTCL through its toxins, no increase in the relative abundance of this taxon was observed in nasal samples. Despite the lack of differences in Staphylococcus, the CTCL nasal microbiome was characterized by shifts in numerous other bacterial taxa. These data add to our understanding of the greater CTCL microbiome and provide context for comprehending nasal-skin and host‒tumor‒microbial relationships.
Abbreviations: CTCL, cutaneous T-cell lymphoma; HC, healthy control; MF, mycosis fungoides; mSWAT, modified Severity Weight Assessment Tool; rRNA, ribosomal RNA; SS, Sézary syndrome
Introduction
Cutaneous T-cell lymphoma (CTCL) comprises a heterogeneous group of T-lymphocyte malignancies that infiltrate the skin. Patients with advanced, progressive disease often suffer from profound immune dysregulation and recurrent skin infections. Previous research suggests that the microbiome may influence CTCL pathogenesis, flares, and progression (Harkins et al., 2021; Lindahl et al., 2019; Willerslev-Olsen et al., 2013). Moreover, distinct microbe-precipitated metabolic and immunologic pathways have been linked to the pathobiology of atopic dermatitis (Nørreslet et al., 2020; Paller et al., 2019), psoriasis (Hidalgo-Cantabrana et al., 2019), hidradenitis suppurativa (McCarthy et al., 2022), and various malignancies (Goodman and Gardner, 2018)—conditions similarly known to be associated with immune dysregulation.
The ecosystem encompassing the nares may be a principal reservoir for self-contamination through nose-to-skin bacterial spread or vice versa. The importance of the nasal microbiome is further emphasized by recent literature suggesting that altered nasal bacterial diversity is associated with gut and skin dysbiosis in hidradenitis suppurativa (McCarthy et al., 2022). Although early culture-based studies have suggested that higher rates of Staphylococcus aureus skin and nasal colonization occur in patients with CTCL (Nguyen et al., 2008; Talpur et al., 2008), the complete nasal microbiome in CTCL has yet to be described. Although the CTCL skin microbiota is currently being investigated (Harkins et al., 2021; Salava et al., 2020), its nasal microbial profile is a missing piece of data because CTCL dysbiosis likely extends beyond the skin.
To better understand the CTCL nasal microbiome, we conducted a cross-sectional analysis of the nasal microbiota present in patients with CTCL and healthy controls (HCs) using 16S ribosomal RNA (rRNA) gene sequencing and further determined staphylococcal species relative abundances using tuf2 gene amplicon sequencing.
Results
Patient characteristics
A total of 45 patients comprised the patient group with CTCL, of which 40 had been diagnosed with mycosis fungoides (MF), and 5 had been diagnosed with Sézary syndrome (SS) (Table 1 and Supplementary Table S1). All patients and HCs were from the same geographical region (Chicago Metropolitan Area, Chicago, IL) to control for environmental influences on the microbiome (Rothschild et al., 2018). Four HC‒CTCL pairs sharing a home were selected for even closer matching. To avoid bias in sample collection, manipulation, and analysis, we concurrently enrolled patients and controls rather than rely on publicly available human microbiome data. There was no significant difference in age, sex, race/ethnicity, or phototype between the two groups (Table 1).
Table 1.
Characteristics of Patients (n = 45) and Healthy Controls (n = 20)
| Characteristics | Patients | Controls | P-Value |
|---|---|---|---|
| n | 45 | 20 | |
| Sex1 | 0.27972 | ||
| Male | 29 (64.4) | 10 (50.0) | |
| Female | 16 (35.6) | 10 (50.0) | |
| Age (y)3 | 62.7 (17.5–83.4) | 54.5 (24.4–79.1) | 0.13932 |
| Race/Ethnicity1 | 0.90412 | ||
| Asian | 2 (4.4) | 3 (15.0) | |
| Black | 5 (11.1) | 0 (0.0) | |
| White | 30 (68.9) | 15 (75.0) | |
| White/Hispanic | 6 (13.3) | 1 (5.0) | |
| Other/Hispanic | 1 (2.2) | 1 (5.0) | |
| Phototype1 | 0.23982 | ||
| Light (FST I‒III) | 45 (100.0) | 19 (95.0) | |
| Dark (FST IV‒VI) | 0 (0.0) | 1 (5.0) | |
| Comorbidities1 | |||
| HTN | 20 (44.4) | 6 (30.0) | 0.4114 |
| DLP | 24 (53.3) | 7 (35.0) | 0.1924 |
| GERD | 11 (24.4) | 6 (30.0) | 0.7614 |
| Diagnosis Subtype1 | |||
| MF | 40 (88.9) | — | |
| SS | 5 (11.1) | — | |
| Clinical stage1 | |||
| Early (IA‒IIA) | 26 (57.8) | — | |
| Advanced (IIB‒IVB) | 19 (42.2) | — | |
| Disease duration (y)3 | 3.1 (0.2–30.0) | — | |
| mSWAT3 | 22 (3–100) | — |
Abbreviations: DLP, dyslipidemia; FST, Fitzpatrick skin phototype; GERD, gastroesophageal reflux; HTN, hypertension; MF, mycosis fungoides; mSWAT, modified Severity Weighted Assessment Tool; SS, Sézary syndrome.
Data are presented as n (%).
Data were analyzed with two-tailed t-test.
Data are presented as median (range).
Data were analyzed with Fisher’s exact test.
A total of 26 patients had the early-stage disease (stages IA‒IIA; 57.8%), and 19 had the advanced-stage disease (stages IIB‒IVB; 42.2%); stage IB was the most common overall (n = 18, 40.0%). The median modified Severity-Weight Assessment Tool (mSWAT) score was 22 (range = 3‒100). The most common comorbidities reported were dyslipidemia (MF/SS = 53.3%, HCs = 35.0%), hypertension (MF/SS = 44.4%, HCs = 30.0%), and gastroesophageal reflux (MF/SS = 24.4%, HCs = 30.0%). There were no significant differences in comorbidities between the two groups for these three conditions (Fisher’s exact test: dyslipidemia P = 0.192; hypertension P = 0.411; and gastroesophageal reflux P = 0.761) or for any other comorbidity.
Differences in nasal microbiota between patients and controls
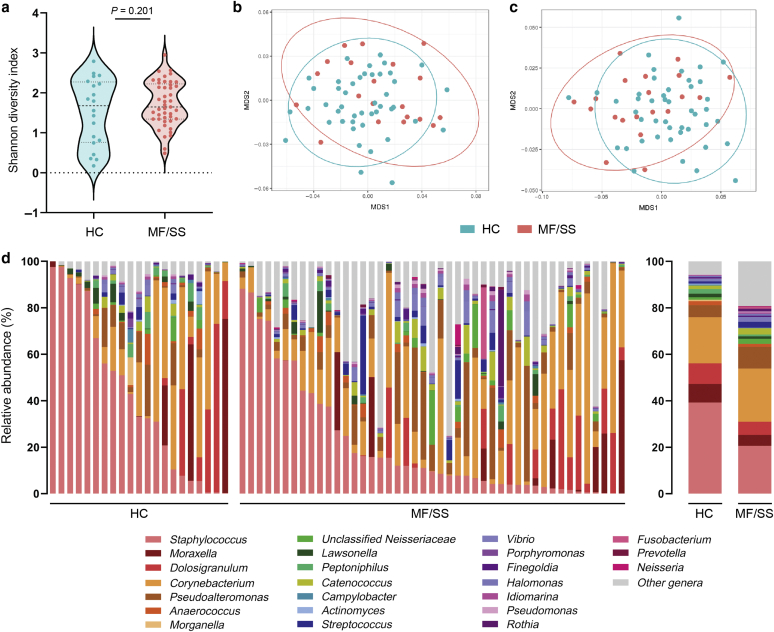
The 16S rRNA gene amplicon sequence data identified a total of 720 genera, 285 families, 139 orders, 67 classes, and 28 phyla. Swab, reagent, and PCR controls were negative for any significant contamination. The most abundant phyla in both groups were the three most frequently encountered in the human nares: Proteobacteria, Actinobacteria, and Firmicutes. At the genus level, there was no significant difference in biodiversity between MF/SS and HC samples as assessed by Shannon diversity index (P = 0.201) (Figure 1a). Notably, β-diversity revealed a small but globally significant difference in the microbial community structure between patients and controls on the basis of Adonis/permutational ANOVA (R2 = 0.104, P = 0.023 for class level; R2 = 0.0904, P = 0.038 for order level) (Figure 1b and c). The most abundant genera in both groups were Corynebacterium and Staphylococcus (Figure 1d).
Figure 1.
Distinct nasal bacterial communities were identified in patients with MF/SS versus in HCs. (a) α-Diversity was not significantly different between HCs and patients with MF/SS at the genus level (Shannon diversity index, P = 0.201). MDS plots using the Bray‒Curtis dissimilarity index of β-diversity analyses show significant differential clustering of HCs and patients with MF/SS at the taxonomic levels of (b) class (Adonis/PERMANOVA R2 = 0.104, P = 0.023) and (c) order (R2 = 0.0904, P = 0.038). (d) Relative abundance (%) of the 20 most abundant genera in nasal samples of HCs and patients with MF/SS (left, individual subjects; right, mean relative abundances per group [HC, MF/SS]). HC, healthy control; MDS, multidimensional scaling; MF, mycosis fungoides; PERMANOVA, permutational ANOVA; SS, Sézary syndrome.
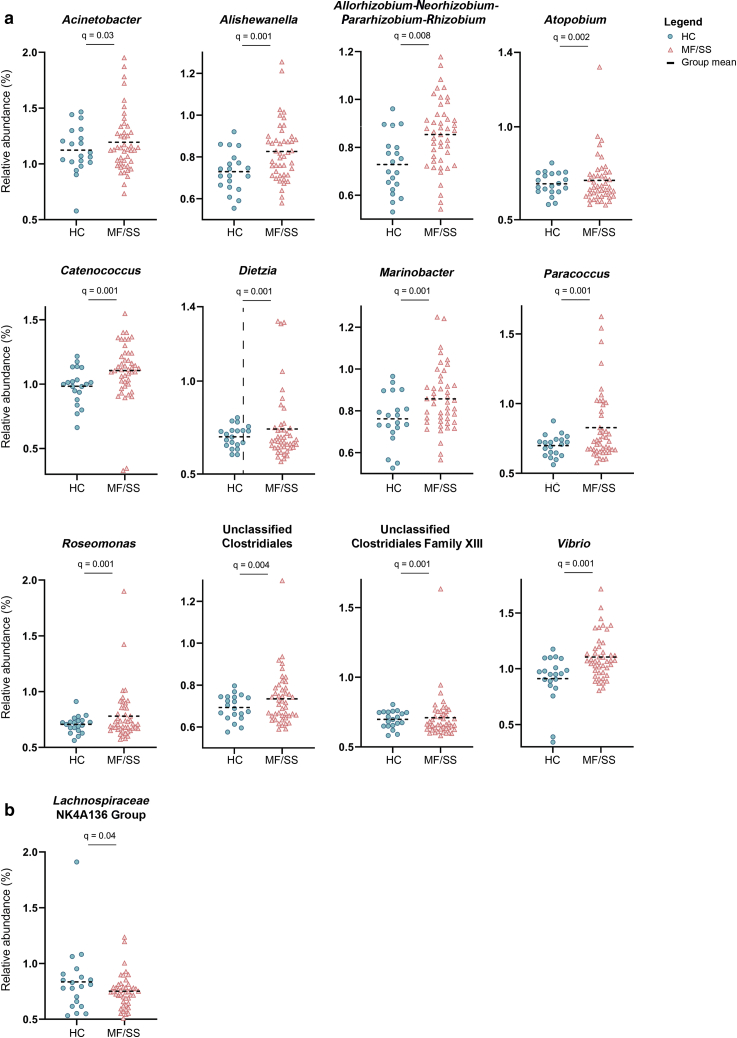
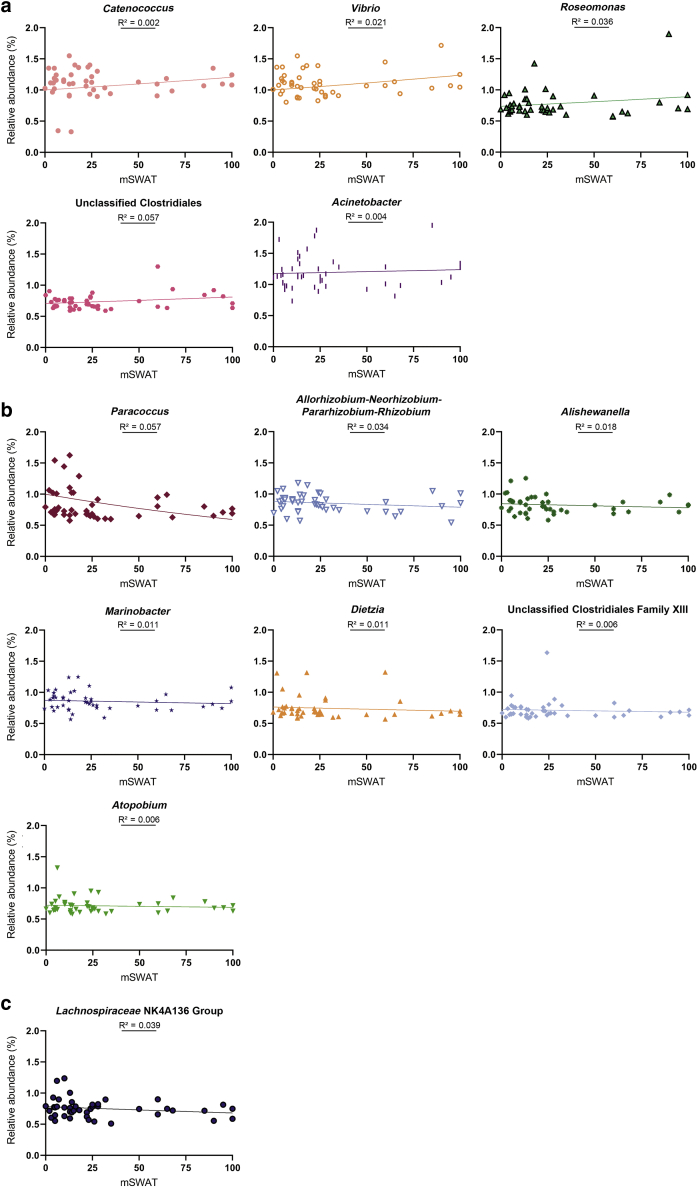
Specific taxa contributing to the distinct nasal microbiota of patients with MF/SS were then investigated. Several genera were significantly higher in patients than in HCs (q < 0.05): Roseomonas, Catenococcus, Vibrio, Marinobacter, Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium, Acinetobacter, Alishewanella, Paracoccus, unclassified Clostridiales and unclassified Clostridiales family XIII, Atopobium, and Dietzia (Table 2 and Figure 2). Meanwhile, Lachnospiraceae NK4A136 group was reduced in patient samples (q < 0.05). Regression analyses revealed a positive association between the relative abundance of Catenococcus, Vibrio, Roseomonas, unclassified Clostridiales, and Acinetobacter genera and increased mSWAT, an indicator of skin disease burden; reduced Lachnospiraceae NK4A136 group relative abundance was associated with higher mSWAT scores (Figure 3). One-way ANOVA revealed significant differences in the mean relative abundance of Alishewanella, Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium, Marinobacter, and Vibrio between HCs and patients as grouped by low versus high mSWAT and early versus advanced disease (Supplementary Table S2, Supplementary Table S3, Supplementary Table S4, Supplementary Table S5). Sidak test for pairwise comparisons showed that the mean relative abundance of Vibrio was significantly different between HCs and patients with low (P = 0.034) and high (P = 0.004) mSWAT and between HCs and patients with early-stage (P = 0.011) and advanced-stage (P = 0.007) disease.
Table 2.
Differential Taxonomic Analysis Shows Unique Microbial Signatures at the Genus Level in Nasal Samples from Patients with MF/SS Versus HCs
| Genus | HC/Patient LogFC | P-Value | q-Value1 | |
|---|---|---|---|---|
| Reduced abundance in MF/SS | Lachnospiraceae NK4A136 group | 0.51 | 0.005 | 0.04 |
| Ruminococcus | 0.12 | 0.02 | 0.14 | |
| Ruminoclostridium | 0.48 | 0.03 | 0.15 | |
| Enriched abundance in MF/SS | Catenococcus | ‒1.41 | <0.001 | 0.001 |
| Alishewanella | ‒1.07 | <0.001 | 0.001 | |
| Vibrio | ‒1.96 | <0.001 | 0.001 | |
| Unclassified Clostridiales family XIII | ‒0.35 | <0.001 | 0.001 | |
| Roseomonas | ‒0.89 | <0.001 | 0.001 | |
| Unclassified Clostridiales | ‒0.62 | <0.001 | 0.001 | |
| Paracoccus | ‒1.36 | <0.001 | 0.001 | |
| Marinobacter | ‒1.07 | <0.001 | 0.001 | |
| Atopobium | ‒0.38 | <0.001 | <0.005 | |
| Dietzia | ‒0.62 | <0.001 | <0.005 | |
| Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium | ‒1.32 | <0.001 | <0.01 | |
| Acinetobacter | ‒0.99 | 0.005 | 0.04 | |
| Unclassified bacteria | ‒0.59 | 0.01 | 0.09 | |
| Christensenellaceae R-7 group | ‒0.04 | 0.01 | 0.09 | |
| Cutibacterium | ‒0.21 | 0.02 | 0.11 | |
| Escherichia/Shigella | ‒1.04 | 0.02 | 0.13 | |
| Neisseria | ‒0.81 | 0.03 | 0.14 | |
| Pseudoalteromonas | ‒1.7 | 0.03 | 0.14 | |
| Subdoligranulum | ‒0.53 | 0.04 | 0.15 | |
| Veillonella | ‒0.93 | 0.04 | 0.17 | |
| Actinomyces | ‒1.18 | 0.04 | 0.17 | |
| Unclassified Gammaproteobacteria | ‒0.64 | 0.05 | 0.17 |
Abbreviations: FC, fold change; HC, healthy control; MF, mycosis fungoides; SS, Sézary syndrome.
The q-value is the FDR-adjusted P-value (Benjamini and Hochberg, 1995).
Figure 2.
Changes in the abundance of specific bacterial genera present in the nares of patients with MF/SS compared with those in the nares of the HCs. Dot plots illustrate the relative sequence abundance (%) of genera that are (a) significantly enriched and (b) significantly reduced in patients with MF/SS versus in HCs. Mean relative abundances are indicated by black horizontal bars. Significance is determined by q ≤ 0.05; the q-value is the FDR-adjusted P-value (Benjamini and Hochberg, 1995). FDR, false discovery rate; HC, healthy control; MF, mycosis fungoides; SS, Sézary syndrome.
Figure 3.
Relationship between the relative abundances of significantly enriched and depleted genera and skin disease burden in patients with MF/SS. The relative abundance (%) of each genus is plotted versus mSWAT score (an indicator of skin disease burden) with line of best fit. (a) Increased mSWAT score was associated with an increased relative abundance of several genera that were enriched in patients with MF/SS: Catenococcus, Vibrio, Roseomonas, unclassified Clostridiales, and Acinetobacter. (b) Lower relative abundances were associated with increased mSWAT scores for the remaining enriched genera: Paracoccus, Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium, Alishewanella, Marinobacter, Dietzia, unclassified Clostridiales family XIII, and Atopobium. (c) Regression analysis of Lachnospiraceae NK4A136 group (reduced in patients with MF/SS compared with that in the HCs) revealed that lower relative abundances were associated with higher mSWAT scores. HC, healthy control; MF, mycosis fungoides; mSWAT, modified Severity-Weight Assessment Tool; SS, Sézary syndrome.
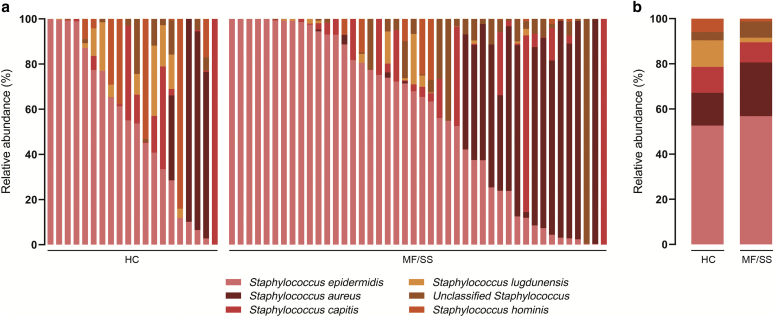
Given the known role of S. aureus in worsening CTCL through its toxins (Fujii, 2022), we next examined whether the relative abundances of Staphylococcus species differed between patients with MF/SS and HCs. We performed targeted sequencing of the bacterial tuf gene, which provides accurate species-level resolution of Staphylococcus communities (Ahle et al., 2021). S. epidermidis and S. aureus were the most abundant staphylococcal species in both groups: these species comprised 56.8% and 23.8% of all staphylococcal species for patients with MF/SS and 52.7% and 14.5% for HCs, respectively (Supplementary Figure S1). There was no statistically significant difference between the relative abundance of any Staphylococcus species (including S. aureus and S. epidermidis) between patients with MF/SS and HCs (Supplementary Table S6).
Supplementary Figure S1.
Relative abundance of staphylococcal species in patients with MF/SS compared with those in HCs. (a) Relative abundance (%) of Staphylococcus species present in the nasal samples of HCs and patients by individual subjects. (b) Mean relative abundances (%) per study group. HC, healthy control; MF, mycosis fungoides; SS, Sézary syndrome.
Discussion
Our results show that the nasal microbiomes of patients with MF/SS and HCs are different. These data add to the existing body of knowledge that supports the importance of the nasal microbiome in skin disease (McCarthy et al., 2022; Olesen et al., 2021; Totté et al., 2019). Nasal microbiota are already known to be important in atopic dermatitis, in which increased relative abundance of nasal Staphylococcus and Moraxella and decreased Dolosigranulum are associated with disease severity (Totté et al., 2019), and increased nasal S. hominis is linked to skin S. hominis abundance and disease improvement (Olesen et al., 2021). The nares of patients with hidradenitis suppurativa are characterized by enriched Proteus communities and reduced Corynebacterium (McCarthy et al., 2022). In addition, loss of nasal Proteobacteria has been associated with skin and soft tissue infections (Johnson et al., 2015), and nasal S. aureus colonization has been implicated in disease activity in various inflammatory skin conditions, including CTCL (Ng et al., 2017; Nørreslet et al., 2020; Talpur et al., 2008). The nasal microbiome could also feasibly serve as a source for bacterial recolonization of the skin after systemic antibiotic treatment (Lindahl et al., 2021), if not also increase the risk of recurrent infections.
Although the exact mechanisms for how the nasal microbiome influences CTCL pathogenesis and vice versa are unclear, the data included in this study provide greater context from which ongoing CTCL skin microbiome research can be understood. We found that enrichment of the genera Vibrio, Roseomonas, and Acinetobacter and depletion of Paracoccus are associated with increased skin severity. From the literature, we know that Vibrio, Roseomonas, and Acinetobacter bacteria are important in causing necrotizing fasciitis, aggravating atopic dermatitis, and instigating skin and soft tissue infections, respectively (Table 3 and Supplementary Discussion) (Cerqueira and Peleg, 2011; Janda et al., 1988; Myles et al., 2018); however, their role as nasal bacteria requires further study because there remains an extreme paucity of knowledge on the biological relationships shared by non‒Staphylococcus species in the nasal microbiome in healthy and disease states.
Table 3.
Summary of Human Disease Associations of Significantly Enriched/Reduced Genera Found in the Anterior Nares of Patients with MF/SS
| Genus | Associations with Human Disease | Associations with Human Cutaneous Disease |
|---|---|---|
| Acinetobacter | Hospital- and community-acquired pneumonia, invasive bloodstream infections, urinary tract infections, hospital-acquired meningitis, osteomyelitis, pericarditis | Skin and soft tissue infections |
| Alishewanella | None available | None available |
| Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium | None available | None available |
| Atopobium | Bacteremia, dental infections, bacterial vaginosis | Bacteremia in the setting of Fournier’s gangrene |
| Catenococcus | None available | None available |
| Dietzia | Bacteremia, prosthetic hip infection, pacemaker infection, pleural fluid isolate | Confluent and reticulated papillomatosis |
| Lachnospiraceae NK4A136 group | Decreased abundance in the gut microbiome after Trichinella spiralis infection and in patients with dementia; biomarker for lean body habitus | None available |
| Marinobacter | None available | None available |
| Paracoccus | P. yeei: myocarditis, peritonitis, bacteremia | None available |
| Roseomonas | Septicemia R. mucosa: catheter-related infections, dialysis and surgical wound infections, bacteremia |
Skin and soft tissue infections, atopic dermatitis |
| Unclassified Clostridiales | Mediates allergic immune activity | Reduced in the gut microbiome of alopecia areata and pediatric atopic dermatitis |
| Unclassified Clostridiales family XIII | Mediates mood disorders | None available |
| Vibrio | Cholera, gastroenteritis, sepsis, less commonly otitis media, meningitis, peritonitis, and pneumonia | Necrotizing fasciitis |
Abbreviations: MF, mycosis fungoides; SS, Sézary syndrome.
Importantly, our data showed that the nasal relative abundances of Staphylococcus species in patients with CTCL did not differ significantly from those of HCs. Although the pathogenic role of S. aureus toxins in CTCL has been well established over the years (Fujii, 2022), it had been unclear whether this translates to a higher relative abundance of S. aureus in the skin and/or nose. Previous culture-based studies have either failed to show a statistically significant difference in nasal S. aureus colonization rates between patients with CTCL and HCs (Nguyen et al., 2008) or did not include a matched comparison group (Talpur et al., 2008). Our nasal data, together with recent CTCL skin microbiome data (Harkins et al., 2021), suggest that the effects of S. aureus in CTCL may not translate to the actual increased relative abundance of S. aureus. Instead, it remains possible that S. aureus toxin production—and not relative abundance—differs between patients with CTCL and HCs. These differences between patients and controls may be mediated by shifts in the abundances of the other bacterial taxa. In fact, in atopic dermatitis, quorum sensing between bacterial species in the skin revealed that coagulase-negative staphylococci species produce autoinducing peptides that inhibit S. aureus phenol-soluble modulin α, a proinflammatory virulence factor capable of mediating epidermal injury (Williams et al., 2019).
Nasal dysbiosis carries intriguing insights for pathophysiology in a disease where advanced-stage patients often suffer from recurrent skin infections (Blaizot et al., 2018). Through the accurate characterization of the nasal microbial profiles associated with worse disease, we can conceivably intervene by altering the nasal microbiome through the decolonization of high-risk bacteria or reconstitution with bacteria associated with healthy individuals. The nasal microbiome may have the potential to serve as an additional and accessible biomarker for the determination of disease progression risk. Eventual matched patient skin and nasal microbiome analyses can further elucidate these relationships.
In this study, we establish that CTCL is characterized by nasal dysbiosis composed of shifts in specific non‒Staphylococcus taxa compared with that of healthy individuals. Because bacterial activity perpetuates CTCL disease progression and because infection is the most common cause of death in this patient population (Tsambiras, 2001; Willerslev-Olsen et al., 2013), attention to the nasal microbiome and its relationship with other microbial reservoirs is crucial to our understanding of the CTCL disease state and pathogenesis.
Materials and Methods
Participants
Ethical approval was obtained from the Northwestern University Institutional Review Board (STU00209226). Written informed consent, nasal samples, and personal data were obtained at the Northwestern University Cutaneous Lymphoma Clinic (Chicago, Illinois) between 2019 and 2021 in compliance with the Declaration of Helsinki. Each patient had clinically and biopsy-proven CTCL, as reviewed by an expert dermatopathologist (JG). At the time of sample collection, patients were receiving standard-of-care therapies, including skin-directed (n = 36, 80.0%) and select systemic (n = 13, 28.9%) treatments or were treatment naive (n = 9, 18.9%) (Supplementary Table S1). Subjects on any antibiotics within the preceding 4 weeks were excluded. Clinical staging and mSWAT were assessed by the study’s principal investigator (XAZ) at sample collection. The HC group (n = 20) was composed of age-matched volunteers without CTCL or other skin diseases from the same geographical region.
Sample collection and DNA extraction
Nasal samples were obtained through sterile swabs (FLOQSwabs, Copan Diagnostics, Murrieta, CA) with hands covered in sterile gloves. All specimens were placed immediately in sterile cryovials and promptly stored at ‒80°C until DNA extraction. Genomic DNA was extracted using a Maxwell 16 LEV Blood DNA Kit (Promega, Madison, WI) implemented on a Maxwell 16 Instrument, following the manufacturer’s instructions with minor modifications: a lysozyme incubation (10 ng/μl lysozyme; Thermo Fisher Scientific, Waltham, MA) for 30 minutes at 37°C and bead beating (40 seconds at 6 min/sec) using a FastPrep-24 System (MP Biomedicals, Irvine, CA). Homogenized samples were transferred to the Maxwell cartridges for final DNA purification.
16S rRNA gene amplicon sequencing
Genomic DNA was prepared for sequencing using a two-stage amplicon sequencing workflow, as described previously (Naqib et al., 2018), using primers targeting the V4 (fourth hypervariable) region of microbial 16S rRNA genes. The 515 forward modified and 806 reverse modified primers contained 5′ linker sequences compatible with access array primers for Illumina sequencers (Fluidigm, South San Francisco, CA) (Walters et al., 2015). PCRs were performed in a total volume of 10 μl using MyTaq HS 2X Mix (Meridian Bioscience, Cincinnati, OH) primers at 500 nM concentration and approximately 1,000 copies per reaction of a synthetic double-stranded DNA template (described below). Extraction blanks and PCR blanks were treated as independent samples and sequenced with unique barcodes. Thermocycling conditions were 95 °C for 5 minutes (initial denaturation), followed by 28 cycles of 95°C for 30 seconds, 55°C for 45 seconds, and 72°C for 30 seconds. Second-stage reactions contained 1 μl of PCR product and a unique primer pair of access array primers; thermocycling conditions consisted of 95°C for 5 minutes (initial denaturation), followed by 8 cycles of 95°C for 30 seconds, 60°C for 30 seconds, and 72°C for 30 seconds. Libraries were pooled and sequenced on an Illumina MiniSeq sequencer (Illumina, San Diego, CA) with 15% phiX spike-in and paired-end 2 × 153 base sequencing reads.
A synthetic double-stranded DNA spike-in was synthesized as a gBLOCK by Integrated DNA Technologies (Coralville, IA). The basis of the design was a 999 base pairs region of the 16S rRNA gene of Rhodanobacter denitrificans strain 2APBS1T (NC_020541) (Prakash et al., 2012). Portions of V1, V2, and V4 variable regions were replaced by eukaryotic mRNA sequences (Apostichopus japonicus Gapdh mRNA, HQ292612; and Strongylocentrotus intermedius Gapdh mRNA, KC775387). Primer sites were preserved, and the overall length in the base pair of the synthetic DNA did not differ from the equivalent R. denitrificans fragment. PCR amplicons generated from this synthetic DNA do not differ in size from bacterial amplicons and can only be identified and removed through postsequencing bioinformatics analysis. The sequence can be accessed through GenBank using the accession number OK324963.
tuf2 amplicon next-generation sequencing
Genomic DNA was PCR amplified with primers ACACTGACGACATGGTTCTACAACAGGCCGTGTTGAACGTG for CS1_tuf2 forward and TACGGTAGCAGAGACTTGGTCTACAGTACGTCCACCTTCACG for CS2_tuf2 reverse (Ahle et al., 2021, 2020) targeting the Staphylococcus tuf gene. Amplicons were generated using a two-stage PCR amplification protocol as previously described (Naqib et al., 2018). First-stage PCR amplifications were performed in 10 μl reactions in 96-well plates using MyTaq HS 2X mastermix (Meridian Bioscience). PCR conditions were 95°C for 5 minutes, followed by 28 cycles of 95°C for 30 seconds, 55°C for 30 seconds, and 72°C for 60 seconds. Second-stage reactions using access array primers were performed as described earlier. Samples were pooled, purified, and sequenced on an Illumina MiSeq with 10% phiX spike-in and paired-end 2 × 300 base sequencing reads (i.e., V3 chemistry). Library preparation, pooling, and sequencing were performed at the Genome Research Core within the Research Resources Center at the University of Illinois Chicago (Chicago, IL).
Sequence data processing (DADA2)
To check for contamination, control swab, PCR, and reagent/kit samples were performed. In total, 22 PCR and 32 extraction controls were analyzed, all of which yielded very low sequence counts (mean ± SD: 122.5 ± 61.4), far below the 5,000 counts per sample threshold needed for inclusion in data analyses.
16S rRNA gene amplicon reads were merged using PEAR, version 0.9.6 (Zhang et al., 2014), and trimmed using cutadapt, version 1.18, to remove ambiguous nucleotides and primer sequences on the basis of a quality threshold of P = 0.01 (Martin, 2011). Reads lacking the primer sequence and/or sequences <225 base pairs after merging and quality trimming were discarded. Chimeric sequences were identified and removed using the USEARCH algorithm with a comparison with Silva (version 132) reference sequence (Edgar, 2010; Glöckner et al., 2017). Amplicon sequence variants were identified using DADA2, version 1.18 (Callahan et al., 2016), and annotated taxonomically using the Naive Bayesian classifier included in DADA2 with the Silva (version 132) training set. Synthetic spike-in sequences were removed before proceeding with downstream bioinformatics analyses. Diversity analyses were performed in R using the vegan library, version 2.5-6 (Okansen et al., 2018). Biodiversity (α-diversity) was calculated using the Shannon index modeled with the sample covariates using a generalized linear model assuming Gaussian distribution. Bray‒Curtis indices were calculated to assess sample dissimilarity (β-diversity).
For the tuf2 next-generation sequencing, merged reads that lacked either primer sequence or were <400 base pairs were discarded. Chimeric sequences were identified and removed in a de novo fashion using USEARCH, version 8.1.1861 (Edgar, 2010). Amplicon sequence variants were identified using the protocol described earlier and taxonomically annotated using alignment from BLAST (blastn) with the RefSeq Prokaryotic Genomes reference, downloaded on 1 December 2021 (NCBI Resource Coordinators, 2017).
Differential analysis of microbial taxa
Differential analyses of taxa as compared with experimental covariates were performed using edgeR (version 3.28.1) on raw sequence counts (McCarthy et al., 2012). The 16S data were filtered to remove sequences of chloroplast, mitochondrial, or eukaryotic origin and taxa present in <30% of all samples and with <500 total sequence counts across all samples. The tuf2 next-generation sequencing data were filtered to retain only species belonging to the genus Staphylococcus and to remove taxa following the same parameters as mentioned earlier. Data were normalized as counts per million and fit using a negative binomial generalized linear model using experimental covariates.
Statistical analyses
Statistical analyses were performed in R and STATA SE. Significance of the α-diversity model (ANOVA) was tested using the F-test. Posthoc, pairwise analyses were performed using the Mann‒Whitney test (Wickham, 2009). The dissimilarity indices were tested for significance using Adonis/permutational ANOVA, and additional comparisons of the individual covariates were performed using analysis of similarities. Statistical tests for the differential analyses were performed using a likelihood ratio test. Adjusted P-values (q-values) were calculated using the Benjamini‒Hochberg false discovery rate correction (Benjamini and Hochberg, 1995). Significant taxa were determined on the basis of a false discovery rate threshold of 5.0% (0.05). Plots were generated using GraphPad Prism, version 9.2, (GraphPad Software, San Diego, CA) and the ggplot2 library in R (Wickham, 2009).
Data availability statement
Datasets related to this article can be found at https://dataview.ncbi.nlm.nih.gov/object/PRJNA768111?reviewer=pd94ec0d6iurp8k0gbs5evtjhj (National Center for Biotechnology Information Short Read Archive, accession number PRJNA768111).
ORCIDs
Madeline J. Hooper: http://orcid.org/0000-0003-1334-5342
Tessa M. LeWitt: http://orcid.org/0000-0001-8935-2165
Francesca L. Veon: http://orcid.org/0000-0002-0232-1671
Yanzhen Pang: http://orcid.org/0000-0003-1825-9603
George E. Chlipala: http://orcid.org/0000-0003-0203-3191
Leo Feferman: http://orcid.org/0000-0002-5821-3434
Stefan J. Green: http://orcid.org/0000-0003-2781-359X
Dagmar Sweeney: http://orcid.org/0000-0001-6320-8931
Katherine T. Bagnowski: http://orcid.org/0000-0002-7482-4454
Michael B. Burns: http://orcid.org/0000-0001-9791-4359
Patrick C. Seed: http://orcid.org/0000-0001-8998-8374
Joan Guitart: http://orcid.org/0000-0001-7635-9237
Xiaolong A. Zhou: http://orcid.org/0000-0002-6177-2472
Author Contributions
Conceptualization: XAZ, TML, FLV, MJH, JG; Data Curation: XAZ, TML, YP, KTB; Formal Analysis: GEC, LF, SJG, XAZ; Funding Acquisition: XAZ; Investigation: XAZ, TML, FLV, MJH, YP, KTB, DS; Methodology: GEC, LF, SJG; Project Administration: XAZ, JG; Resources: XAZ, KTB, JG, DS, SJG; Software: GEC, LF; Supervision: XAZ, PCS, JG; Validation: XAZ, MBB, GEC; Visualization: GEC, LF, XAZ, FLV; Writing - Original Draft Preparation: TML, FLV, MJH, XAZ; Writing - Review and Editing: XAZ, JG, MBB, SJG, GEC, FLV, MJH, TML
Conflict of Interest
The authors state no conflict of interest.
Acknowledgments
The authors would like to thank the patients who contributed to the study. XAZ is supported in part by a career development award from the Dermatology Foundation, a Cutaneous Lymphoma Foundation Catalyst Research Grant, and an institutional grant from Northwestern University Clinical and Translational Sciences Institute and the National Institutes of Health (grant number 5KL2TR001424).
accepted manuscript published online 28 April 2022; corrected proof published online 16 August 2022
Footnotes
Cite this article as: JID Innovations 2022;X:100132
Supplementary material is linked to the online version of the paper at www.jidonline.org, and at https://doi.org/10.1016/j.xjidi.2022.100132.
Supplementary Materials and Discussion
Acinetobacter
The genus Acinetobacter comprises a complex and heterogeneous group of Gram-negative, strictly aerobic coccobacilli (Visca et al., 2011). Acinetobacter is an emergent pathogen that can cause life-threatening infections (Cerqueira and Peleg, 2011). Owing to its extensive virulence and profound resistance to currently available antibiotics, this genus is responsible for substantial morbidity and mortality among critically ill patients in both hospital and community settings (Visca et al., 2011). Notably, the species A. baumannii has been described as the Gram-negative equivalent to methicillin-resistant Staphylococcus aureus (Visca et al., 2011). In addition to bloodstream infections, A. baumannii causes ventilator-associated and community-acquired pneumonia—the most frequent clinical manifestations of Acinetobacter infection (Cerqueira and Peleg, 2011; Munoz-Price and Weinstein, 2008). Of note, higher rates of morbidity are attributed to Acinetobacter-related pneumonia versus to any other species (Wisplinghoff et al., 2012). Recently, a rare case of purulent endocarditis caused by carbapenem-resistant A. baumannii was reported (Liu et al., 2019). Other clinically relevant Acinetobacter species include A. pittii and A. nosocomiali, which commonly cause urinary tract infections, hospital-acquired meningitis, traumatic skin and soft tissue infections, and osteomyelitis (Cerqueira and Peleg, 2011).
Alishewanella
Alishewanella is a genus of Gram-negative, facultatively anaerobic bacteria with four known species, of which only one has been identified within human tissue (Vogel et al., 2000); however, this genus has yet to be linked to human disease.
Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium
Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium is a genus of Gram-negative bacteria found in soil. This genus has yet to be linked to human disease.
Atopobium
The genus Atopobium represents anaerobic, Gram-positive bacteria commensal to the vagina and oral mucosa (Angelakis et al., 2009; Dauby et al., 2019). A. vaginae is a common cause of bacterial vaginosis, but it has also been associated with intrapartum bacteremia (Dauby et al., 2019). Typically found in the gingiva, A. rimae and A. parvulum are known to precipitate chronic periodontitis, and more rarely, cases of A. rimae bacteremia have been reported (Angelakis et al., 2009; Devresse et al., 2016). Two cases of Atopobium-related sepsis have occurred in patients with Fournier’s gangrene, thus indicating that these species may contribute to the pathogenesis of this fulminant form of necrotizing fasciitis (Cools et al., 2014; Oyaert et al., 2014). Furthermore, studies of the lung microbiota in sarcoidosis and cystic fibrosis have suggested that Atopobium is a disease-associated bacterial genus (Surette, 2014; Zimmermann et al., 2017).
Catenococcus
The Catenococcus genus represents a group of Gram-negative, facultatively anaerobic bacteria with only one known species, C. thiocycli. This genus has yet to be linked to human disease.
Dietzia
Members of the Dietzia genus are Gram-positive, aerobic, environmental actinomycetes (Koerner et al., 2009). Three Dietzia species are known to be pathogenic to humans. D. maris has been implicated in bacteremia and infectious aortitis in immunocompromised patients, as well as prosthetic hip and pacemaker infections in immunocompetent individuals (Bemer-Melchior et al., 1999; Perkin et al., 2012; Pidoux et al., 2001; Reyes et al., 2006). One case of pleural D. cinnamea isolated from a patient with stage IV mesothelioma has been published, but the patient was asymptomatic (Cawcutt et al., 2016). Finally, D. pappillomatosis was identified within skin scrapings from a patient with confluent and reticulated papillomatosis, also known as Gougerot-Carteaud syndrome, a benign and rare skin disorder thought to have a bacterial etiology given its response to antibiotics (Jones et al., 2008).
Lachnospiraceae NK4A136 group
A short-chain fatty acid (SCFA) producer, the Lachnospiraceae NK4A136 group has been identified as an anti-inflammatory, probiotic bacterium with potent beneficial effects as a member of the gut microbiome (Stadlbauer et al., 2020). Recent research on the gut microbiota in individuals with obesity versus in lean individuals showed that this bacterium is negatively associated with body fat and is significantly depleted in obese groups, thus suggesting that Lachnospiraceae NK4A136 group may be a biomarker for lean habitus in humans (Companys et al., 2021). Reduced levels of Lachnospiraceae NK4A136 group in the gut have also been identified in patients with dementia and after Trichinella spiralis infection (Chen et al., 2021; Stadlbauer et al., 2020).
Marinobacter
Marinobacter is a genus of Gram-negative bacteria commonly found in seawater. This genus has yet to be linked to human disease.
Paracoccus
A total of 17 soil- and brine-based species are categorized within the genus Paracoccus, but only P. yeei has been associated with human infections. A study of P. yeei revealed that its unique virulence is due to the acquisition of specific, pathoadaptive genomic sequences (Lasek et al., 2018). Still, infections with this species are rare and only known to occur in immunocompromised individuals (Fosso et al., 2021). Case reports involving P. yeei consist of myocarditis in a transplanted heart, peritonitis in peritoneal dialysis, and bacteremia in the setting of cirrhosis (Fosso et al., 2021)
Roseomonas
Roseomonas represents an opportunistic group of pink-pigmented, Gram-negative coccobacilli that is typically associated with septicemia, followed by urogenital and soft tissue infections in patients with underlying immunocompromising conditions (Struthers et al., 1996). The species R. mucosa and R. gilardii have been identified as virulent and highly infectious microbes (Struthers et al., 1996). R. mucosa has been cited in catheter, dialysis, and surgery-related infections (Romano-Bertrand et al., 2016), and a rare case of infective endocarditis bacteremia caused by R. mucosa in a patient with systemic lupus erythematosus has been described (Shao et al., 2019). Moreover, research suggests that opportunistic infections caused by R. mucosa are due to patient skin microbiota rather than the environment (Romano-Bertrand et al., 2016). Roseomonas infections have been associated with various coexisting diseases such as peritonitis, abscess formation, bacteremia, community-acquired secondary bacterial infections, and infectious spondylitis (Shao et al., 2019).
A recent study of the role of Gram-negative skin bacteria in atopic dermatitis (AD) revealed that application of R. mucosa isolates collected from healthy volunteers improved outcomes in mouse and cell culture models of AD, whereas application of AD-sourced R. mucosa had either no impact on or worsened outcomes in the same models (Myles et al., 2018). Treatment of AD with topical R. mucosa obtained from healthy volunteers was associated with significant decreases in measures of AD severity, topical steroid requirements, and S. aureus burden (Myles et al., 2018). These preclinical results suggest that interventions targeting the microbiome could provide therapeutic benefits for patients with AD.
Unclassified Clostridiales and unclassified Clostridiales family XIII
The class Clostridia encompasses a group of SCFA producers that are commensal to the human gut, including unclassified Clostridiales and unclassified Clostridiales family XIII. Largely considered beneficial to human health, Clostridia can modulate immune activity and allergic reactions through interactions with colonic regulatory T cells (Furusawa et al., 2013). SCFAs are also considered anti-inflammatory; depleted levels of the order Clostridiales have been observed in the guts of patients with alopecia areata, and the loss of anti-inflammatory SCFAs is postulated to bridge this microbial alteration and autoimmune skin disease (Moreno-Arrones et al., 2020). In this same study about the gut microbiome in alopecia areata, Clostridiales family XIII was significantly more abundant in controls (Moreno-Arrones et al., 2020). Loss of gut Clostridia has also been associated with increased eosinophilia and earlier age of onset in pediatric AD (Lee et al., 2016). Finally, an investigation of the microbial influence on mood disorders showed that serum Clostridiales family XIII DNA levels are positively correlated with anxiety symptoms, but the gut microbiota of patients with anxiety is characterized by decreased Clostridiales family XIII abundance compared with that of the controls (Rhee et al., 2021). As a growing area of study, it is hypothesized that reduced gut Clostridiales mediates psychiatric diseases through the gut‒brain axis and downstream effects of abnormal intestinal amino acid metabolism and SCFA formation (Li et al., 2020).
Vibrio
Vibriosis represents a general term for a group of clinical conditions of varying severity associated with the genus Vibrio, whose members are facultatively anaerobic, Gram-negative bacilli. The three most common Vibrio species involved in human illness in the United States include V. parahaemolyticus, V. vulnificus, and V. alginolyticus. Infections range from mild gastroenteritis to septicemia or invasive skin and soft tissue infections. Less common, Vibrio is responsible for cases of otitis media, meningitis, peritonitis, and pneumonia. Life-threatening Vibrio infections include cholera and necrotizing fasciitis (Janda et al., 1988). Miscellaneous Vibrio species have been isolated from numerous anatomic sites, including the ear, eye, gallbladder, sinuses, peritoneal fluid, and urine; these account for <5% of all noncholera infections. The chief risk factors for Vibrio infection include the consumption of raw, undercooked seafood or shellfish and trauma associated with a marine environment. Less than 15% of all Vibrio species have been associated with human disease, whereas the remaining taxa are solely environmental (Janda et al., 1988).
Supplementary Table S1.
Detailed Demographic Characteristics of Patients with MF/SS (n = 45) and Healthy Controls (n = 20)
| Gender | Age (y) | Race/Ethnicity | Diagnosis | Stage | mSWAT | Comorbidities | Skin-Directed Therapy | Systemic Therapy |
|---|---|---|---|---|---|---|---|---|
| Healthy controls (n = 20) | ||||||||
| F | 34 | Asian | — | — | — | None | — | — |
| M | 25 | Asian | — | — | — | None | — | — |
| M | 26 | White | — | — | — | None | — | — |
| M | 37 | White | — | — | — | None | — | — |
| M | 24 | Asian | — | — | — | None | — | — |
| F | 27 | White/Hispanic | — | — | — | None | — | — |
| F | 60 | White | — | — | — | GERD, DLP, hypothyroidism, migraines, rheumatoid arthritis | — | — |
| F | 68 | White | — | — | — | DLP | — | — |
| M | 76 | White | — | — | — | GERD, DLP, HTN, T2DM | — | — |
| F | 74 | White | — | — | — | Asthma, CM, CKD (stage III), GERD, DLP, HTN, hypothyroidism, T2DM | — | — |
| F | 54 | White | — | — | — | None | — | — |
| M | 55 | White | — | — | — | None | — | — |
| F | 65 | White | — | — | — | GERD, DLP, T2DM | — | — |
| F | 74 | White | — | — | — | GERD, DLP, HTN | — | — |
| M | 38 | Other/Hispanic | — | — | — | GERD, HTN | — | — |
| M | 79 | White | — | — | — | HTN | — | — |
| F | 54 | White | — | — | — | None | — | — |
| F | 58 | White | — | — | — | DLP, HTN, hypothyroidism, T2DM | — | — |
| M | 66 | White | — | — | — | None | — | — |
| M | 47 | White | — | — | — | None | — | — |
| Patient with MF/SS (n = 45) | ||||||||
| M | 71 | White | FMF | IIIB | 35 | Bladder cancer, GERD, DLP, T2DM |
TCS | Acitretin |
| M | 65 | White | FMF | IIIA | 90 | GERD | TCS | — |
| F | 18 | White/Hispanic | FMF | IB | 26 | Asthma, GERD | TCS, NBUVB | IFN-α-2b |
| M | 56 | White/Hispanic | FMF | IB | 16 | Anemia, CKD, GERD, glaucoma, DLP, HTN, T2DM, vertebral osteomyelitis | TCS, TCI, NBUVB | — |
| F | 72 | White | FMF | IB | 5 | Diverticulitis, GERD, DLP, hypothyroidism, infiltrating ductal carcinoma | TCS | IFN-α-2b, bexarotene |
| M | 36 | White/Hispanic | FMF | IB | 3 | DLP, T2DM | TCS | — |
| F | 74 | White | FMF | IIB | 18 | DLP, HTN | TCS, TCI | Acitretin |
| F | 35 | Other/Hispanic | FMF | IB | 28 | None | — | — |
| F | 63 | Black | FMF | IIB | 13 | DLP, HTN, hypothyroidism, obesity | TCS, NBUVB | — |
| M | 67 | White | FMF/SMF | IB | 13 | DLP, HTN | TCS, NBUVB, XRT | IFN-α-2b, acitretin |
| M | 55 | White | FMF/SMF | IB | 3 | None | TCS, NBUVB | — |
| M | 69 | White | CD4+ MF | IIB | 7 | CAD, insomnia, obesity, overactive bladder | TCS | — |
| M | 49 | Asian | CD4+ MF | IB | 60 | Cataracts, ED, mitral regurgitation, obesity, T2DM | — | — |
| M | 37 | White/Hispanic | CD4+ MF | IIB | 65 | None | TCS, NBUVB | IFN-α-2b |
| M | 47 | White | CD4+ MF | IB | 4 | GERD, obesity | TCS | — |
| M | 58 | White | CD4+ MF | IIA | 22 | Autoimmune hemolytic anemia, anaplastic large cell lymphoma, asthma, HTN, hyperthyroidism, obesity, OSA | TCS | Acitretin |
| M | 65 | White | CD4+ MF | IA | 13 | GERD, HTN, DLP, OSA, T2DM | TCS | — |
| F | 37 | White/Hispanic | CD4+ MF | IB | 25 | HTN, hypothyroidism, OSA | TCS, NBUVB | Bexarotene, acitretin |
| F | 72 | Black | CD4+ MF | IIIA | 100 | DLP, HTN, T2DM | TCS | — |
| M | 69 | White | CD4+ MF | IIIA | 14 | AF, BPH, CAD, DLP, T2DM | TCS | — |
| M | 67 | White | CD4+ MF | IIB | 4.5 | CRC, DLP | TCS, Imiquimod | Acitretin |
| M | 52 | White | CD4+ MF | IB | 13 | Allergic rhinitis | TCS | — |
| M | 81 | White | CD4+ MF | IB | 10 | BPH, DLP, PAF, prostate cancer | TCS | Bexarotene |
| F | 74 | White | CD4+ MF | IA | 5 | Hypothyroidism | TCS | — |
| M | 65 | White | CD4+ MF | IA | 5 | DLP, GERD, HTN, obesity, T2DM | TCS | — |
| M | 61 | White | CD4+ MF | IA | 6 | HTN | TCS | — |
| F | 26 | White | CD4+ MF | IB | 68 | Migraine | — | — |
| M | 63 | White | CD4+ MF | IIB | 6 | Hypothyroidism | TCS | — |
| F | 72 | Black | CD4+ MF | IIIA | 85 | Glaucoma, DLP, HTN | TCS | — |
| F | 61 | White | CD4+ MF | IA | 2 | GERD, endometriosis, osteoporosis, primary hyperparathyroidism | — | — |
| M | 74 | White | CD4+ MF | IA | 22 | Osteoarthritis, BPH, CKD, GERD, glaucoma, HTN, ulnar neuropathy | TCS | Methotrexate, bexarotene |
| M | 46 | Black | CD4+ MF | IIB | 60 | Asthma, bronchitis | TCS | — |
| M | 62 | White | CD4+ MF | IB | 23 | DLP | — | — |
| F | 55 | White | CD4+ MF | IB | 25 | Asthma, COPD, GERD, DLP, HTN, PAD, PFO | — | — |
| M | 68 | White | CD4+ MF | IIB | 50 | DLP, HTN | — | — |
| M | 55 | White | CD4+ MF | IB | 10 | DLP, HTN, obesity | — | — |
| M | 72 | White | CD4+ MF | IIIB | 95 | Atrial flutter, anxiety, cervical stenosis, CKD, ED, DLP, HTN, lumbar disc herniation, OSA, PAF, prostate cancer, tachycardia-induced CM | TCS | Brentuximab vedotin |
| F | 58 | Asian | CD4+ MF | IB | 14 | Breast cancer, DLP, HTN, multinodular goiter, T2DM | TCS | — |
| M | 29 | White/Hispanic | CD8+ MF | IB | 24 | None | TCS | — |
| F | 46 | White | CD8+ MF | IA | 15 | Allergic rhinitis, fibroids, obesity | — | — |
| M | 66 | White | SS | IV | 10 | AF, dysphagia, DLBCL, DLP, HSV keratitis, hypothyroidism | TCS | — |
| F | 56 | White | SS | IV | 24 | DLP, HTN, hypothyroidism | TCS | — |
| M | 83 | White | SS | IV | 100 | CAD, HTN | TCS | Methotrexate |
| F | 66 | White | SS | IV | 28 | DLP, hypothyroidism | TCS | — |
| M | 67 | Black | SS | IV | 32 | Atrial flutter, CAD, ED, HFrEF, DLP, HTN, obesity, T2DM | TCS | — |
Abbreviations: AF, atrial fibrillation; BPH, benign prostatic hyperplasia; CAD, coronary artery disease; CM, cardiomyopathy; CKD, chronic kidney disease; COPD, chronic obstructive pulmonary disease; CRC, colorectal carcinoma; DLBCL, diffuse large B-cell lymphoma; DLP, dyslipidemia; ED, erectile dysfunction; F, female; FMF, folliculotropic mycosis fungoides; GERD, gastroesophageal reflux disease; HFrEF, heart failure with reduced ejection fraction; HSV, herpes simplex virus; HTN, hypertension; LCT, large cell transformation; M, male; MF, mycosis fungoides; mSWAT, modified Severity Weighted Assessment Tool; NBUVB, narrowband UVB; OSA, obstructive sleep apnea; PAD, peripheral artery disease; PAF, paroxysmal atrial fibrillation; PFO, patent foramen ovale; SMF, syringotropic mycosis fungoides; SS, Sézary syndrome; T2DM, type 2 diabetes mellitus; TCI, topical calcineurin inhibitor; TCS, topical corticosteroids; XRT, localized radiotherapy.
Supplementary Table S2.
Mean Relative Abundances (%) of Genera Identified on Differential Analysis Comparing Bacterial Communities in the Samples of HCs Versus in Patients with MF/SS—One-Way ANOVA: HCs Versus MF/SS, Organized by mSWAT Score
| Genus | HCs | Patients with MF/SS |
P-Value | |
|---|---|---|---|---|
| mSWAT < 10 | mSWAT ≥ 10 | |||
| Acinetobacter | 1.123 | 1.132 | 1.213 | 0.386 |
| Alishewanella | 0.729 | 0.884 | 0.807 | 0.006 |
| Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium | 0.729 | 0.869 | 0.850 | 0.005 |
| Atopobium | 0.693 | 0.748 | 0.700 | 0.405 |
| Catenococcus | 0.984 | 1.079 | 1.115 | 0.087 |
| Dietzia | 0.701 | 0.788 | 0.727 | 0.329 |
| Lachnospiraceae NK4A136 group | 0.835 | 0.786 | 0.740 | 0.269 |
| Marinobacter | 0.761 | 0.882 | 0.849 | 0.042 |
| Paracoccus | 0.699 | 0.887 | 0.807 | 0.054 |
| Roseomonas | 0.707 | 0.742 | 0.792 | 0.303 |
| Unclassified Clostridiales | 0.693 | 0.731 | 0.736 | 0.378 |
| Unclassified Clostridiales family XIII | 0.698 | 0.713 | 0.708 | 0.950 |
| Vibrio | 0.912 | 1.111 | 1.104 | 0.003 |
Abbreviations: HC, healthy control; MF, mycosis fungoides; mSWAT, modified Severity Weighted Assessment Tool; SS, Sézary syndrome.
Supplementary Table S3.
Mean Relative Abundances (%) of Genera Identified on Differential Analysis Comparing Bacterial Communities in the Samples of HCs Versus in Patients with MF/SS—Sidak Method for Pairwise Comparisons: HCs Versus MF/SS, Organized by mSWAT Score
| Group 1 | Group 2 | Mean Difference | P-Value |
|---|---|---|---|
| Alishewanella | |||
| HC | MF/SS, mSWAT < 10 | 0.155 | 0.005 |
| HC | MF/SS, mSWAT ≥ 10 | 0.078 | 0.096 |
| MF/SS, mSWAT < 10 | MF/SS, mSWAT ≥ 10 | ‒0.077 | 0.232 |
| Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium | |||
| HC | MF/SS, mSWAT < 10 | 0.140 | 0.028 |
| HC | MF/SS, mSWAT ≥ 10 | 0.121 | 0.009 |
| MF/SS, mSWAT < 10 | MF/SS, mSWAT ≥ 10 | ‒0.019 | 0.971 |
| Marinobacter | |||
| HC | MF/SS, mSWAT < 10 | 0.120 | 0.082 |
| HC | MF/SS, mSWAT ≥ 10 | 0.088 | 0.095 |
| MF/SS, mSWAT < 10 | MF/SS, mSWAT ≥ 10 | ‒0.03 | 0.885 |
| Vibrio | |||
| HC | MF/SS, mSWAT < 10 | 0.200 | 0.034 |
| HC | MF/SS, mSWAT ≥ 10 | 0.192 | 0.004 |
| MF/SS, mSWAT < 10 | MF/SS, mSWAT ≥ 10 | ‒0.008 | 0.999 |
Abbreviations: HC, healthy control; MF, mycosis fungoides; mSWAT, modified Severity Weighted Assessment Tool; SS, Sézary syndrome.
Supplementary Table S4.
Mean Relative Abundances (%) of Genera Identified on Differential Analysis Comparing Bacterial Communities in the Samples of HCs Versus in Patients with MF/SS—One-Way ANOVA: HC Versus MF/SS, Organized by Clinical Stage
| Genus | HC | Pateints with MF/SS |
P-Value | |
|---|---|---|---|---|
| IA‒IIA | IIB‒IVB | |||
| Acinetobacter | 1.123 | 1.181 | 1.210 | 0.551 |
| Alishewanella | 0.729 | 0.843 | 0.803 | 0.016 |
| Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium | 0.729 | 0.881 | 0.818 | 0.002 |
| Atopobium | 0.693 | 0.692 | 0.739 | 0.332 |
| Catenococcus | 0.984 | 1.157 | 1.036 | 0.015 |
| Dietzia | 0.701 | 0.736 | 0.750 | 0.600 |
| Lachnospiraceae NK4A136 group | 0.835 | 0.707 | 0.813 | 0.077 |
| Marinobacter | 0.761 | 0.868 | 0.842 | 0.044 |
| Paracoccus | 0.699 | 0.858 | 0.784 | 0.049 |
| Roseomonas | 0.707 | 0.714 | 0.869 | 0.011 |
| Unclassified Clostridiales | 0.693 | 0.720 | 0.755 | 0.220 |
| Unclassified Clostridiales family XIII | 0.698 | 0.739 | 0.669 | 0.242 |
| Vibrio | 0.912 | 1.096 | 1.119 | 0.003 |
Abbreviations: HC, healthy control; MF, mycosis fungoides; SS, Sézary syndrome.
Supplementary Table S5.
Mean Relative Abundances (%) of Genera Identified on Differential Analysis Comparing Bacterial Communities in the Samples of HCs Versus in Patients with MF/SS—Sidak Method for Pairwise Comparisons: HC Versus MF/SS, Organized by Clinical Stage
| Group 1 | Group 2 | Mean Difference | P-Value |
|---|---|---|---|
| Alishewanella | |||
| HC | MF/SS, IA‒IIA | 0.114 | 0.013 |
| HC | MF/SS, IIB‒IVB | 0.074 | 0.220 |
| MF/SS, IA‒IIB | MF/SS, IIB‒IVB | ‒0.040 | 0.663 |
| Allorhizobium-Neorhizobium-Pararhizobium-Rhizobium | |||
| HC | MF/SS, IA‒IIA | 0.152 | 0.001 |
| HC | MF/SS, IIB‒IVB | 0.089 | 0.130 |
| MF/SS, IA‒IIB | MF/SS, IIB‒IVB | ‒0.063 | 0.350 |
| Catenococcus | |||
| HC | MF/SS, IA‒IIA | 0.173 | 0.016 |
| HC | MF/SS, IIB‒IVB | 0.053 | 0.800 |
| MF/SS, IA‒IIB | MF/SS, IIB‒IVB | ‒0.120 | 0.148 |
| Marinobacter | |||
| HC | MF/SS, IA‒IIA | 0.106 | 0.044 |
| HC | MF/SS, IIB‒IVB | 0.081 | 0.225 |
| MF/SS, IA‒IIB | MF/SS, IIB‒IVB | ‒0.026 | 0.912 |
| Paracoccus | |||
| HC | MF/SS, IA‒IIA | 0.159 | 0.043 |
| HC | MF/SS, IIB‒IVB | 0.085 | 0.512 |
| MF/SS, IA‒IIB | MF/SS, IIB‒IVB | ‒0.744 | 0.581 |
| Roseomonas | |||
| HC | MF/SS, IA‒IIA | 0.159 | 0.043 |
| HC | MF/SS, IIB‒IVB | 0.085 | 0.512 |
| MF/SS, IA‒IIB | MF/SS, IIB‒IVB | ‒0.744 | 0.581 |
| Vibrio | |||
| HC | MF/SS, IA‒IIA | 0.184 | 0.011 |
| HC | MF/SS, IIB‒IVB | 0.207 | 0.007 |
| MF/SS, IA‒IIB | MF/SS, IIB‒IVB | 0.024 | 0.974 |
Abbreviations: HC, healthy control; MF, mycosis fungoides; SS, Sézary syndrome.
Supplementary Table S6.
Differential Analysis for Staphylococcus Species Reveals No Statistically Significant Differences between the Nasal Microbiota of Patients with MF/SS and HCs
| Staphylococcus Species | Patient/HC LogFC | P-Value | q-Value1 | |
|---|---|---|---|---|
| Reduced abundance in MF/SS | S. lugdunensis | ‒2.54 | 0.14 | 0.43 |
| S. hominis | ‒1.92 | 0.16 | 0.43 | |
| S. capitis | ‒0.29 | 0.73 | 0.82 | |
| Enriched abundance in MF/SS | Unclassified Staphylococcus | 1.48 | 0.22 | 0.43 |
| S. aureus | 0.72 | 0.82 | 0.82 | |
| S. epidermidis | 0.19 | 0.74 | 0.82 |
Abbreviations: FC, fold change; FDR, false discovery rate; HC, healthy control; MF, mycosis fungoides; SS, Sézary syndrome.
The q-value is the FDR-adjusted P-value (Benjamini and Hochberg, 1995).
References
- Ahle C.M., Stødkilde K., Afshar M., Poehlein A., Ogilvie L.A., Söderquist B., et al. Staphylococcus saccharolyticus: an overlooked human skin colonizer. Microorganisms. 2020;8:1105. doi: 10.3390/microorganisms8081105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahle C.M., Stødkilde-Jørgensen K., Poehlein A., Streit W.R., Hüpeden J., Brüggemann H. Comparison of three amplicon sequencing approaches to determine staphylococcal populations on human skin. BMC Microbiol. 2021;21:221. doi: 10.1186/s12866-021-02284-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Blaizot R., Ouattara E., Fauconneau A., Beylot-Barry M., Pham-Ledard A. Infectious events and associated risk factors in mycosis fungoides/Sézary syndrome: a retrospective cohort study. Br J Dermatol. 2018;179:1322–1328. doi: 10.1111/bjd.17073. [DOI] [PubMed] [Google Scholar]
- Callahan B.J., McMurdie P.J., Rosen M.J., Han A.W., Johnson A.J.A., Holmes S.P. DADA2: high-resolution sample inference from Illumina amplicon data. Nat Methods. 2016;13:581–583. doi: 10.1038/nmeth.3869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira G.M., Peleg A.Y. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life. 2011;63:1055–1060. doi: 10.1002/iub.533. [DOI] [PubMed] [Google Scholar]
- Edgar R.C. Quality measures for protein alignment benchmarks. Nucleic Acids Res. 2010;38:2145–2153. doi: 10.1093/nar/gkp1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii K. Pathogenesis of cutaneous T cell lymphoma: involvement of Staphylococcus aureus. J Dermatol. 2022;49:202–209. doi: 10.1111/1346-8138.16288. [DOI] [PubMed] [Google Scholar]
- Glöckner F.O., Yilmaz P., Quast C., Gerken J., Beccati A., Ciuprina A., et al. 25 years of serving the community with ribosomal RNA gene reference databases and tools. J Biotechnol. 2017;261:169–176. doi: 10.1016/j.jbiotec.2017.06.1198. [DOI] [PubMed] [Google Scholar]
- Goodman B., Gardner H. The microbiome and cancer. J Pathol. 2018;244:667–676. doi: 10.1002/path.5047. [DOI] [PubMed] [Google Scholar]
- Harkins C.P., MacGibeny M.A., Thompson K., Bubic B., Huang X., Brown I., et al. Cutaneous T-cell lymphoma skin microbiome is characterized by shifts in certain commensal bacteria but not viruses when compared with healthy controls. J Invest Dermatol. 2021;141:1604–1608. doi: 10.1016/j.jid.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hidalgo-Cantabrana C., Gómez J., Delgado S., Requena-López S., Queiro-Silva R., Margolles A., et al. Gut microbiota dysbiosis in a cohort of patients with psoriasis. Br J Dermatol. 2019;181:1287–1295. doi: 10.1111/bjd.17931. [DOI] [PubMed] [Google Scholar]
- Janda J.M., Powers C., Bryant R.G., Abbott S.L. Current perspectives on the epidemiology and pathogenesis of clinically significant Vibrio spp. Clin Microbiol Rev. 1988;1:245–267. doi: 10.1128/cmr.1.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.C., Ellis M.W., Lanier J.B., Schlett C.D., Cui T., Merrell D.S. Correlation between nasal microbiome composition and remote purulent skin and soft tissue infections. Infect Immun. 2015;83:802–811. doi: 10.1128/IAI.02664-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl L.M., Iversen L., Ødum N., Kilian M. Staphylococcus aureus and antibiotics in cutaneous T-cell Lymphoma. Dermatology. 2021:1–3. doi: 10.1159/000517829. [DOI] [PubMed] [Google Scholar]
- Lindahl L.M., Willerslev-Olsen A., Gjerdrum L.M.R., Nielsen P.R., Blümel E., Rittig A.H., et al. Antibiotics inhibit tumor and disease activity in cutaneous T-cell lymphoma. Blood. 2019;134:1072–1083. doi: 10.1182/blood.2018888107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy D.J., Chen Y., Smyth G.K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–4297. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy S., Barrett M., Kirthi S., Vlckova K., Tobin A.M., Murphy M., et al. Altered skin and gut microbiome in hidradenitis suppurativa. J Invest Dermatol. 2022;142:459–468.e15. doi: 10.1016/j.jid.2021.05.036. [DOI] [PubMed] [Google Scholar]
- Martin M. Cutadapt removes adapter sequences from high-throughput sequencing reads. EMBnetjournal. 2011;17:10–12. [Google Scholar]
- Myles I.A., Earland N.J., Anderson E.D., Moore I.N., Kieh M.D., Williams K.W., et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight. 2018;3 doi: 10.1172/jci.insight.120608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naqib A., Poggi S., Wang W., Hyde M., Kunstman K., Green S.J. Making and sequencing heavily multiplexed, high-throughput 16S ribosomal RNA gene amplicon libraries using a flexible, two-stage PCR protocol. Methods Mol Biol. 2018;1783:149–169. doi: 10.1007/978-1-4939-7834-2_7. [DOI] [PubMed] [Google Scholar]
- NCBI Resource Coordinators Database resources of the National Center for Biotechnology Information. Nucleic Acids Res. 2017;45:D12–D17. doi: 10.1093/nar/gkw1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng C.Y., Huang Y.H., Chu C.F., Wu T.C., Liu S.H. Risks for Staphylococcus aureus colonization in patients with psoriasis: a systematic review and meta-analysis. Br J Dermatol. 2017;177:967–977. doi: 10.1111/bjd.15366. [DOI] [PubMed] [Google Scholar]
- Nguyen V., Huggins R.H., Lertsburapa T., Bauer K., Rademaker A., Gerami P., et al. Cutaneous T-cell lymphoma and Staphylococcus aureus colonization. J Am Acad Dermatol. 2008;59:949–952. doi: 10.1016/j.jaad.2008.08.030. [DOI] [PubMed] [Google Scholar]
- Nørreslet L.B., Edslev S.M., Andersen P.S., Plum F., Holt J., Kjerulf A., et al. Colonization with Staphylococcus aureus in patients with hand eczema: prevalence and association with severity, atopic dermatitis, subtype and nasal colonization. Contact Dermatitis. 2020;83:442–449. doi: 10.1111/cod.13679. [DOI] [PubMed] [Google Scholar]
- Okansen J, Blanchet FG, Kindt R, Legendre P, Minchin P, O'Hara R, et al. Vegan: community ecology package. R package version 2018;2:4–6.
- Olesen C.M., Ingham A.C., Thomsen S.F., Clausen M.L., Andersen P.S., Edslev S.M., et al. Changes in skin and nasal microbiome and staphylococcal species following treatment of atopic dermatitis with dupilumab. Microorganisms. 2021;9:1487. doi: 10.3390/microorganisms9071487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paller A.S., Kong H.H., Seed P., Naik S., Scharschmidt T.C., Gallo R.L., et al. The microbiome in patients with atopic dermatitis. J Allergy Clin Immunol. 2019;143:26–35. doi: 10.1016/j.jaci.2018.11.015. [published correction appears in J Allergy Clin Immunol 2019;143:1660] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prakash O., Green S., Jasrotia P., Overholt W., Canion A., Watson D.B., et al. Description of Rhodanobacter denitrificans sp. nov., isolated from nitrate-rich zones of a contaminated aquifer. Int J Syst Evol Microbiol. 2012;62:2457–2462. doi: 10.1099/ijs.0.035840-0. [DOI] [PubMed] [Google Scholar]
- Rothschild D., Weissbrod O., Barkan E., Kurilshikov A., Korem T., Zeevi D., et al. Environment dominates over host genetics in shaping human gut microbiota. Nature. 2018;555:210–215. doi: 10.1038/nature25973. [DOI] [PubMed] [Google Scholar]
- Salava A., Deptula P., Lyyski A., Laine P., Paulin L., Väkevä L., et al. Skin microbiome in cutaneous T-cell lymphoma by 16S and whole-genome shotgun sequencing. J Invest Dermatol. 2020;140:2304–2308.e7. doi: 10.1016/j.jid.2020.03.951. [DOI] [PubMed] [Google Scholar]
- Talpur R., Bassett R., Duvic M. Prevalence and treatment of Staphylococcus aureus colonization in patients with mycosis fungoides and Sezary syndrome. Br J Dermatol. 2008;159:105–112. doi: 10.1111/j.1365-2133.2008.08612.x. [DOI] [PubMed] [Google Scholar]
- Totté J.E.E., Pardo L.M., Fieten K.B., Vos M.C., Broek T.J., Schuren F.H.J., et al. Nasal and skin microbiomes are associated with disease severity in paediatric atopic dermatitis. Br J Dermatol. 2019;181:796–804. doi: 10.1111/bjd.17755. [DOI] [PubMed] [Google Scholar]
- Tsambiras P.E., Patel S., Greene J.N., Sandin R.L., Vincent A.L. Infectious complications of cutaneous T-cell lymphoma. Cancer Control. 2001;8:185–188. doi: 10.1177/107327480100800213. [DOI] [PubMed] [Google Scholar]
- Walters W., Hyde E.R., Berg-Lyons D., Ackermann G., Humphrey G., Parada A., et al. Improved bacterial 16S rRNA gene (V4 and V4-5) and fungal internal transcribed spacer marker gene primers for microbial community surveys. mSystems. 2015;1 doi: 10.1128/mSystems.00009-15. e00009‒15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H., ggplot2 . Springer New York; New York: 2009. Elegant graphics for data analysis. [Google Scholar]
- Willerslev-Olsen A., Krejsgaard T., Lindahl L.M., Bonefeld C.M., Wasik M.A., Koralov S.B., et al. Bacterial toxins fuel disease progression in cutaneous T-cell lymphoma. Toxins (Basel) 2013;5:1402–1421. doi: 10.3390/toxins5081402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams M.R., Costa S.K., Zaramela L.S., Khalil S., Todd D.A., Winter H.L., et al. Quorum sensing between bacterial species on the skin protects against epidermal injury in atopic dermatitis. Sci Transl Med. 2019;11 doi: 10.1126/scitranslmed.aat8329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Kobert K., Flouri T., Stamatakis A. PEAR: a fast and accurate Illumina Paired-End reAd mergeR. Bioinformatics. 2014;30:614–620. doi: 10.1093/bioinformatics/btt593. [DOI] [PMC free article] [PubMed] [Google Scholar]
Supplementary References
- Angelakis E., Roux V., Raoult D., Drancourt M. Human case of Atopobium rimae bacteremia. Emerg Infect Dis. 2009;15:354–355. doi: 10.3201/eid1502.071399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bemer-Melchior P., Haloun A., Riegel P., Drugeon H.B. Bacteremia due to Dietzia maris in an immunocompromised patient. Clin Infect Dis. 1999;29:1338–1340. doi: 10.1086/313490. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J R Stat Soc Series B Stat Methodol. 1995;57:289–300. [Google Scholar]
- Cawcutt K.A., Bhatti M.M., Nelson D.R. Pleural fluid infection caused by Dietzia cinnamea. Diagn Microbiol Infect Dis. 2016;85:496–497. doi: 10.1016/j.diagmicrobio.2016.04.007. [DOI] [PubMed] [Google Scholar]
- Cerqueira G.M., Peleg A.Y. Insights into Acinetobacter baumannii pathogenicity. IUBMB Life. 2011;63:1055–1060. doi: 10.1002/iub.533. [DOI] [PubMed] [Google Scholar]
- Chen H.L., Xing X., Zhang B., Huang H.B., Shi C.W., Yang G.L., et al. Higher mucosal type II immunity is associated with increased gut microbiota diversity in BALB/c mice after Trichinella spiralis infection. Mol Immunol. 2021;138:87–98. doi: 10.1016/j.molimm.2021.07.014. [DOI] [PubMed] [Google Scholar]
- Companys J., Gosalbes M.J., Pla-Pagà L., Calderón-Pérez L., Llauradó E., Pedret A., et al. Gut microbiota profile and its association with clinical variables and dietary intake in overweight/obese and lean subjects: a cross-sectional study. Nutrients. 2021;13:2032. doi: 10.3390/nu13062032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools P., Oyaert M., Vaneechoutte M., De Laere E., Vervaeke S. Atopobium deltae sp. nov., isolated from the blood of a patient with Fournier’s gangrene. Int J Syst Evol Microbiol. 2014;64:3140–3145. doi: 10.1099/ijs.0.065243-0. [DOI] [PubMed] [Google Scholar]
- Dauby N., Martiny D., Busson L., Cogan A., Meghraoui A., Argudín M.A., et al. Atopobium vaginae intrapartum bacteremia: a case report with a literature review. Anaerobe. 2019;59:212–214. doi: 10.1016/j.anaerobe.2018.09.010. [DOI] [PubMed] [Google Scholar]
- Devresse A., Labriola L., Dossin T., Vatlet M., Hantson P. Atopobium rimae bacteremia complicated by an infection-related glomerulonephritis in a cardiac transplanted patient. Transpl Infect Dis. 2016;18:637–638. doi: 10.1111/tid.12544. [DOI] [PubMed] [Google Scholar]
- Fosso C., Maillart E., Beun B., Touzani F., Mahadeb B., Clevenbergh P. Opportunistic peritonitis in peritoneal dialysis: the example of Paracoccus yeei. Clin Case Rep. 2021;9 doi: 10.1002/ccr3.4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furusawa Y., Obata Y., Fukuda S., Endo T.A., Nakato G., Takahashi D., et al. Commensal microbe-derived butyrate induces the differentiation of colonic regulatory T cells. Nature. 2013;504:446–450. doi: 10.1038/nature12721. [published correction appears in Nature 2014;506:254] [DOI] [PubMed] [Google Scholar]
- Janda J.M., Powers C., Bryant R.G., Abbott S.L. Current perspectives on the epidemiology and pathogenesis of clinically significant Vibrio spp. Clin Microbiol Rev. 1988;1:245–267. doi: 10.1128/cmr.1.3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones A.L., Koerner R.J., Natarajan S., Perry J.D., Goodfellow M. Dietzia papillomatosis sp. nov., a novel actinomycete isolated from the skin of an immunocompetent patient with confluent and reticulated papillomatosis. Int J Syst Evol Microbiol. 2008;58:68–72. doi: 10.1099/ijs.0.65178-0. [DOI] [PubMed] [Google Scholar]
- Koerner R.J., Goodfellow M., Jones A.L. The genus Dietzia: a new home for some known and emerging opportunist pathogens. FEMS Immunology & Medical Microbiology. 2009;55(3):296–305. doi: 10.1111/j.1574-695X.2008.00513.x. [DOI] [PubMed] [Google Scholar]
- Lasek R., Szuplewska M., Mitura M., Decewicz P., Chmielowska C., Pawłot A., et al. Genome Structure of the Opportunistic Pathogen Paracoccus yeei (Alphaproteobacteria) and Identification of Putative Virulence Factors. Front Microbiol. 2018;9:2553. doi: 10.3389/fmicb.2018.02553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E.M.D.P., Lee S.-Y.M.D.P., Kang M.-J.P., Kim K.B.S., Won S.P., Kim B.-J.M.D.P., et al. Clostridia in the gut and onset of atopic dermatitis via eosinophilic inflammation. Ann Allergy Asthma Immunol. 2016;117:91–92.e1. doi: 10.1016/j.anai.2016.04.019. [DOI] [PubMed] [Google Scholar]
- Li J., Ma Y., Bao Z., Gui X., Li A.N., Yang Z., et al. Clostridiales are predominant microbes that mediate psychiatric disorders. Journal of Psychiatric Research. 2020;130:48–56. doi: 10.1016/j.jpsychires.2020.07.018. [DOI] [PubMed] [Google Scholar]
- Liu J., Xiao X., Cen C., Yuan H., Yang M. Rare purulent pericarditis caused by carbapenem-resistant Acinetobacter baumannii: a case report. Medicine (Baltimore) 2019;98 doi: 10.1097/MD.0000000000017034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Arrones O.M., Serrano-Villar S., Perez-Brocal V., Saceda-Corralo D., Morales-Raya C., Rodrigues-Barata R., et al. Analysis of the gut microbiota in alopecia areata: identification of bacterial biomarkers. J Eur Acad Dermatol Venereol. 2020;34:400–405. doi: 10.1111/jdv.15885. [DOI] [PubMed] [Google Scholar]
- Munoz-Price L.S., Weinstein R.A. Acinetobacter infection. N Engl J Med. 2008;358:1271–1281. doi: 10.1056/NEJMra070741. [DOI] [PubMed] [Google Scholar]
- Myles I.A., Earland N.J., Anderson E.D., Moore I.N., Kieh M.D., Williams K.W., et al. First-in-human topical microbiome transplantation with Roseomonas mucosa for atopic dermatitis. JCI Insight. 2018;3 doi: 10.1172/jci.insight.120608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyaert M., Cools P., Breyne J., Heyvaert G., Vandewiele A., Vaneechoutte M., et al. Sepsis with an Atopobium-like species in a patient with Fournier's gangrene. J Clin Microbiol. 2014;52:364–366. doi: 10.1128/JCM.02310-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perkin S., Wilson A., Walker D., McWilliams E. Dietzia species pacemaker pocket infection: an unusual organism in human infections. BMJ Case Rep. 2012;2012 doi: 10.1136/bcr.10.2011.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pidoux O., Argenson J.N., Jacomo V., Drancourt M. Molecular identification of a Dietzia maris hip prosthesis infection isolate. J Clin Microbiol. 2001;39:2634–2636. doi: 10.1128/JCM.39.7.2634-2636.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes G., Navarro J.L., Gamallo C., delas Cuevas M.C. Type A aortic dissection associated with Dietzia maris. Interact Cardiovasc Thorac Surg. 2006;5:666–668. doi: 10.1510/icvts.2006.135640. [DOI] [PubMed] [Google Scholar]
- Rhee S.J., Kim H., Lee Y., Lee H.J., Park C.H.K., Yang J., et al. The association between serum microbial DNA composition and symptoms of depression and anxiety in mood disorders. Sci Rep. 2021;11:13987. doi: 10.1038/s41598-021-93112-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romano-Bertrand S., Bourdier A., Aujoulat F., Michon A.L., Masnou A., Parer S., et al. Skin microbiota is the main reservoir of Roseomonas mucosa, an emerging opportunistic pathogen so far assumed to be environmental. Clin Microbiol Infect. 2016;22:737.e1–737.e7. doi: 10.1016/j.cmi.2016.05.024. [DOI] [PubMed] [Google Scholar]
- Shao S., Guo X., Guo P., Cui Y., Chen Y. Roseomonas mucosa infective endocarditis in patient with systemic lupus erythematosus: case report and review of literature. BMC Infect Dis. 2019;19:140. doi: 10.1186/s12879-019-3774-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadlbauer V., Engertsberger L., Komarova I., Feldbacher N., Leber B., Pichler G., et al. Dysbiosis, gut barrier dysfunction and inflammation in dementia: a pilot study. BMC Geriatr. 2020;20:248. doi: 10.1186/s12877-020-01644-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struthers M., Wong J., Janda J.M. An initial appraisal of the clinical significance of Roseomonas species associated with human infections. Clin Infect Dis. 1996;23:729–733. doi: 10.1093/clinids/23.4.729. [DOI] [PubMed] [Google Scholar]
- Surette M.G. The cystic fibrosis lung microbiome. Ann Am Thorac Soc. 2014;11(Suppl 1):S61–S65. doi: 10.1513/AnnalsATS.201306-159MG. [DOI] [PubMed] [Google Scholar]
- Visca P., Seifert H., Towner K.J. Acinetobacter infection – an emerging threat to human health. IUBMB Life. 2011;63:1048–1054. doi: 10.1002/iub.534. [DOI] [PubMed] [Google Scholar]
- Vogel B.F., Venkateswaran K., Christensen H., Falsen E., Christiansen G., Gram L. Polyphasic taxonomic approach in the description of Alishewanella fetalis gen. nov., sp. nov., isolated from a human foetus. Int J Syst Evol Microbiol. 2000;50:1133–1142. doi: 10.1099/00207713-50-3-1133. [DOI] [PubMed] [Google Scholar]
- Wisplinghoff H., Paulus T., Lugenheim M., Stefanik D., Higgins P.G., Edmond M.B., et al. Nosocomial bloodstream infections due to Acinetobacter baumannii, Acinetobacter pittii and Acinetobacter nosocomialis in the United States. J Infect. 2012;64:282–290. doi: 10.1016/j.jinf.2011.12.008. [DOI] [PubMed] [Google Scholar]
- Zimmermann A., Knecht H., Häsler R., Zissel G., Gaede K.I., Hofmann S., et al. Atopobium and Fusobacterium as novel candidates for sarcoidosis-associated microbiota. Eur Respir J. 2017;50 doi: 10.1183/13993003.00746-2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Datasets related to this article can be found at https://dataview.ncbi.nlm.nih.gov/object/PRJNA768111?reviewer=pd94ec0d6iurp8k0gbs5evtjhj (National Center for Biotechnology Information Short Read Archive, accession number PRJNA768111).