Abstract
Objective
Older gastric cancer survivors account for a high proportion of cancer survivors. To improve their quality of life, a cancer survivorship care plan with a consideration of the late effects is required. This study aimed to understand the extent and type of evidence in relation to the late effects in older gastric cancer survivors.
Methods
We conducted a scoping review based on the JBI scoping review framework. We explored articles in the Cumulative Index to Nursing and Allied Health Literature (CINAHL), Medical Literature Analysis and Retrieval System Online (MEDLINE), SCOPUS, Web of science, Excerpta Medica dataBASE (EMBASE), Research InformationSharing Service (RISS), Korean Medical dataBASE(KMBase), and National Digital Science Library (NDSL) databases published from January 1, 2012, to December 31, 2021. The keywords used for search are “gastric cancer”, “aged”, “survivors”, and “late effect or long-term effect or late symptom or time factors”. While 439 records were initially identified, 14 articles were eventually selected based on the inclusion criteria.
Results
Most studies were conducted in 2019 (4 studies, 28.6%), and more than half (8 studies, 57.1%) were conducted in Asia. In total, six definitions of cancer survivors were found in the studies. The most common age range in the studies was 60–64 years (7 studies, 50.0%). The second primary cancer risk was the most common late effects (5 studies, 20.8%). Among 14 studies, there was only one study of intervention study (7.1%).
Conclusions
It is time to shift the focus from survival to care that improve the quality of life after treatment. We suggest future studies to define cancer survivors, set the age criteria and characterize the late effects in older gastric cancer survivors.
Keywords: Gastric cancer, Cancer survivorship, Aged, Late effect, Late symptom, Scoping review, Oncology nursing
Gastric cancer is one of the most prevalent cancer types. In 2020,1 it ranked fifth in incidence and fourth in mortality, worldwide. Furthermore, East Asian countries such as China, Japan, and Korea have high incidence and mortality rates.1, 2, 3 In addition, the older population is more vulnerable as the incidence and mortality rates of gastric cancer increase progressively with age.1,4, 5, 6 Meanwhile, due to advances in early detection, surgical techniques, and targeted therapies in the past decade, mortality in gastric cancer has remained low compared to other cancer types.7 In China, one of the countries with a high incidence of gastric cancer, a significant increase in survival rates for gastric cancer over the last 10 years has been recorded.3 Moreover, in Japan, the 5-year relative survival rates for gastric cancer were slightly higher than for other cancers.1 This trend is even more prominent in Korea; among gastric cancer survivors, the proportion of those over 65 is 49.4%.8
Several definitions of a cancer survivor have been proposed; over time, it has evolved to include the entire cancer trajectory, from diagnosis to completion of active treatment and long-term survival.9 Cancer survivors are defined as patients with cancer who have completed active treatment10 or those who have lived five years after the initial diagnosis and completed active treatment.11 Institute of Medicine defines a cancer survivor as a patient with cancer who does not require hospice or palliative care after the completion of active treatment and before the diagnosis of new cancer.12 Therefore, it is expected that understanding which criteria were mainly applied when defining gastric cancer survivors will help optimize care.
Cancer survivors often report distressing symptoms that appear months or years after treatment. Late effects are chronic disorders resulting from cancer treatment that cause physical and psychological problems, including secondary cancer.11 Late effects have been reported for all cancer types and commonly include the risk of a second primary cancer, anxiety, depression, trauma, cardiovascular disease risk, cognitive dysfunction, employment and return to work, pain, and sexual dysfunction.13 One study on symptom burden of cancer survivors reported that they suffered from late effects even more than 10 years after the termination of primary cancer treatment.14 Wu reported that about a third of cancer survivors experience symptom burden due to late effects, which has a significant impact on their quality of life (QOL) and is related to disability and health care use. Depending on the diagnosis or time since diagnosis, the most prevalent concerns differ among the physical, social, spiritual, emotional domains, and other symptoms also vary.16 Therefore, identification and management of the various late and long-term effects is a crucial aspect of cancer survivors’ care plan.10,17
In particular, gastric cancer survivors have reported late effects such as weight loss, diarrhea, chemotherapy-induced neuropathy, fatigue, bone health, indigestion, vitamin B12 deficiency, iron deficiency, postprandial fullness or eating dysfunction, dumping syndrome, and small intestine bacterial overgrowth.18 Previous studies have also reported other late effects in gastric cancer survivors including secondary new cancer,6 survival rate,19, 20, 21, 22 vitamin D deficiency,23 and osteopathy24 associated with gastrectomy. Although many studies have examined the characteristics and burden of late effects,25, 26, 27 studies on late effects specifically experienced by older gastric cancer survivors, who account for a large proportion of cancer survivors, are sparse.
A scoping review is one of the most recently used methodologies for the preliminary assessment of potential size and scope of available research literature.28 It involves a mapping of existing literature to identify trends in research conducted in a field of interest.29 Such mapping can help us establish where we are and could be used to determine the direction we need to go. A scoping review is used as a standalone project or as a preliminary step for a systematic review.30 In this study, we designed a scoping review to identify how research on the late effects in older gastric cancer survivors has been conducted to provide fundamental and comprehensive understanding of the current research trends of the same.
This study aimed to understand the extent and type of evidence related to the late effects in older gastric cancer survivors over the past decade. The research objectives are as follows: (1) identify the recent research trends; (2) describe the characteristics of the study participants; (3) demonstrate the types of confirmed late effects; (4) explore the interventions used in previous studies, if applicable. With this scoping review, we expect to suggest appropriate interventions and research domains to future researchers for the further study of late effects in older gastric cancer survivors and to consequently improve their health-related QOL.
Methods
Study design and research questions
We conducted this scoping review in accordance with the JBI scoping review framework.31 We aimed to answer the following research questions in order to identify the characteristics of late effects in older gastric cancer survivors:
-
(1)
How have studies on late effects been conducted in older gastric cancer survivors over the past decade?
-
(2)
What are the characteristics of the study participants?
-
(3)
What symptoms and variables have been identified as late effects?
-
(4)
What interventions have been implemented to manage late effects?
Search strategy
We searched for relevant studies using the following four search concepts “gastric cancer”, “aged”, “survivors”, and “late effect or long-term effect or late symptom or time factors”, in conjunction with Boolean operators, proximity locators, and MeSH terms in the following databases: Cumulative Index to Nursing and Allied Health Literature (CINAHL), Medical Literature Analysis and Retrieval System Online (MEDLINE), SCOPUS, Web of Science, Excerpta Medica dataBASE (EMBASE), Research information sharing service (RISS), Koreamed, Korean medical database (KMBase), and National digital science library (NDSL). As subject headings varied in the databases, the modification of terms was repeated in each database. Research papers published included those in English and Korean in the last 10 years between January 1, 2012 and December 31, 2021.
Selection of evidence sources
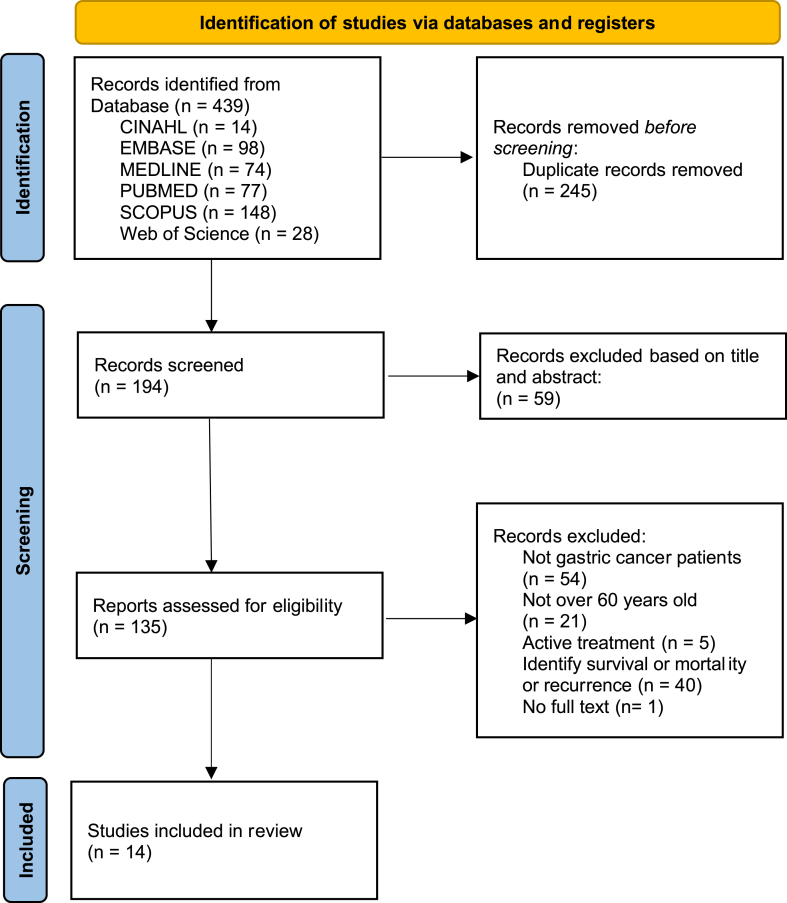
We identified 439 studies from our search of the above-mentioned databases. Duplicates were removed, and 194 research papers were extracted. In the primary screening process, two researchers independently reviewed titles and abstracts with keywords. Researchers excluded 59 papers and selected 135 studies. In the second screening process, two researchers independently reviewed the full text and selected the papers based on the pre-established selection criteria. The inclusion criteria were as follows: (1) studies presenting results related to the elderly or with participants aged 60 years or older; (2) participants primarily diagnosed with gastric cancer; (3) participants’ completion of active cancer treatment and being under observation; (4) description of late effects in general cancer survivors as provided by Survivorship version 1, 2021 NCCN guidelines32 or as stated by the NCCN guidelines-gastric cancer, 202218; (5) study types such as systematic review, descriptive study of late effects, experimental study of interventions for late effects, clinical trial studies of late effects, protocol, review, letter, and gray literature. The exclusion criteria were studies that (1) cover only survival rate, mortality, and recurrence rates without exploring the symptoms experienced by the participants; (2) describe the effects of recurrent gastric cancer after treatment according to the type of treatment; (3) include gastric cancer patients undergoing active treatment; (4) do not present the characteristics of gastric cancer survivors over 60 years of age separately; (5) do not have the primary cancer diagnosis as gastric cancer; (6) examined esophagogastric junction cancer and gastrointestinal stromal tumor; (7) do not include the full text; or (8) do not include gastric cancer survivors or are not able to distinguish gastric cancer survivors from other cancer types.
The differences of opinions were resolved through a consensus process within the research team by a third researcher. Finally, 14 papers were selected (Fig. 1).
Fig. 1.
PRISMA, flow diagram of study selection process.
Data-charting process and analysis
The following data on general information of the selected studies and on the aforementioned four research questions were collated using Microsoft Excel 2020.
-
(1)
General information: Research author, publication year, title, digital object identifier, research purpose, research design, research country, population, number of the study participants, and age of the population.
-
(2)
Information related to the study questions: measurement of late effect, outcome, and summary of study results.
The collected data were encoded and analyzed using descriptive statistics, such as frequency and percentage, on Microsoft Excel 2020.
Results
Research trends on older cancer survivors with gastric cancer in the last decade
Table 1 shows the characteristics of studies targeting older gastric cancer survivors. Since 2018, studies have been published at a steady rate. Most of them were performed in 2019 (4 studies, 28.6%).33, 34, 35, 36 After 2019, four studies (28.6%) investigated late effects related to gastrectomy (risk of dementia,33 osteoporotic fractures,34 nutrition status,37 sarcopenia,37 gastrointestinal symptoms,37 QOL,37 presence of Enterobacteriaceae38) and four studies (28.6%) performed secondary cancer studies.35,36,39,40 More than half studies (8 studies, 57.1%) were conducted in Asian countries, including Korea33,41, 42, 43, 44 and Japan.34,38,39 Prospective studies (8 studies, 57.1%) were mainly performed. Among the prospective studies, more than half studies (7 studies, 50.0%)34,35,37,38,43, 44, 45 were prospective observational studies, while one was interventional study (7.1%).45 Retrospective studies were conducted using big data from hospitals or countries. Most studies focused on the risk of second primary cancer (4 studies, 23.5%),35,36,39,40 followed by psychosocial symptoms such as QOL (2 studies, 11.8%),37,43 and healthy behavior (2 studies, 11.8%).37,43 Studies on specific physical symptoms such as anemia,41 bone metabolism,34,44,45 gastrointestinal symptoms,43 intestinal bacterial,38 vitamin B12 deficiency,33,42 and nutritional status37 in gastric cancer survivors were also performed. Other research aimed at exploring the risk of dementia (1 study, 5.9%)33 and renal function after radiochemotherapy (1 study, 5.9%).46
Table 1.
Characteristics and summary of studies on late effects in older gastric cancer survivors.
| Reference; first author; date published; country | Aim of the study | Methodology: study design, data analysis | Sample size (total or older/total) | Participant characteristics | Mean age ± SD (years old) (total or older/total) | Outcomes and measures | Key findings related to older gastric cancer survivors | Late effects from key findings based on NCCN guidelines |
|---|---|---|---|---|---|---|---|---|
| Hu Y, 2013 42 Republic of Korea |
Identification of risk factors for vitamin B12 deficiency and changes in vitamin B12 over time post gastrectomy | Prospective observational study, the Kaplan–Meier method, Cox proportional hazards model | 184/645 | Gastric cancer patients who underwent STG or TG | 61.0 ± 11.0/58.5 ± 12.2 | Cumulative vitamin B12 deficiency after surgery | Mean time from surgery was 24 months (range: 3–72 months). Risk factors for vitamin B12 deficiency in gastric cancer survivors include age during STGa and preoperative vitamin B12 level. Patients with vitamin B12 deficiency had an average age of 60 years or older. |
Vitamin B12 deficiency |
| Haneder S, 2015 46 Germany |
Evaluating renal function in long-term gastric cancer survivors receiving combined radiochemotherapy | Retrospective study Univariate analysis of variance Linear correlations |
1/18 | Patients after chemoradiotherapy 105 months passed since last treatment. |
63.0/52.1 (range: 24–69, No information of SD) |
3T functional MRIc including DWId,23Na imaging Late toxicity: CTCe questionnaire, creatinine values |
Radiation therapy for gastric cancer causes kidney damage. | Renal dysfunction |
| Lee JE, 2016 44 Republic of Korea |
Describing the health care status of gastric cancer survivors and reporting the experience of using the shared-care model during a one-year experience at the cancer survivorship clinic | Prospective observational study | 250 | Patients with gastric cancer . 3 years post surgery | 62 (range: 36–85 No information of SD) |
Health behaviors, comorbid conditions, secondary cancer screenings, survivorship care status (bone density, vaccinations) | Among the survivors, 7.2% were current smokers, 8.8% were at-risk drinkers, and 32.4% were physically inactive. Among the patients who did not know their bone density status, the majority were in the osteopenic (37.1%) or osteoporotic range (24.1%). Screening among the eligible population within the recommended time intervals were 76.3% for colorectal cancer, but only 13.6% for lung cancer. | Bone health, screening new primary cancer, cardiovascular disease risk, healthy lifestyle, vaccination |
| Jun JH, 2016 41 Republic of Korea |
Exploring the incidence and risk factors of anemia in long-term gastric cancer survivors | Retrospective cohort study Kaplan–Meier survival analysis Cox regression analysis |
106/385 | Gastric cancer patients who survived more than 5 years after gastrectomy | No information /53.21 ± 9.77 |
The cumulative incidence rate of anemia after surgery | The incidence of anemia steadily increased in patients who had underwent gastrectomy. The 5-year cumulative incidence of anemia was almost 40%. The risk of anemia was higher in women and in patients with TGb, diabetes, and low body mass index. There was no significant increased risk of anemia with age. | Iron deficiency |
| Lee SS, 2016 43 Republic of Korea |
Assessment of long-term QOL 5 years after STG and TG by comparing groups matched by a set of patient factors at and beyond postoperative 5 years | Prospective observational study | 5-year survivors after STG and TG: 53 pairs Long-term survivors after STG and TG (> 5 years): 36 pairs |
Gastric cancer survivors of more than 5 years after surgery | 5-year survivors: STG: 61.0 ± 8.3 TG: 61.0 ± 8.3 Long-term survivors (> 5 years) STG: 62.3 ± 10.7 TG: 62.3 ± 10.2 |
QLQf -C30 QLQf -STO22 |
Five-year survivors after TGb showed significantly worse QOLg in social functioning, nausea and vomiting, eating restrictions, and taste. For long-term survivors, QOLg inferiority of the TGb group was observed only in eating restrictions. Among 4 items constituting eating restrictions, the TGb group tended to exhibit worse QOLg in 2 items (enjoyable meals and social meals). | Employment and return symptoms, healthy lifestyle, nausea and vomiting, postprandial fullness or eating dysfunction |
| Climent M, 2018 45 Spain |
Investigating bone health after curative resection of gastric cancer and the consequences of high-dose vitamin D supplementation in patients with low levels of 25-(OH)-vitamin D | Prospective, non-selected, observational, clinical cohort study | 40 | Disease-free patients at least 24 months after gastrectomy | 68.9 ± 11.7 | 25-(OH)-vitamin D iPTH | Mean time from surgery was 48.9 (range: 24–109) months. Vitamin D insufficiency, secondary hyperparathyroidism, osteoporosis, and prevalent fractures were observed at baseline. Vitamin D supplementation led to reaching 25-(OH)-vitamin D values above 30 ng/mL and helped reduce iPTH levels and markers of bone turnover. |
Bone health |
| Morton LM, 2019 36 US |
Quantification of tMDS/AML risk among adults who were diagnosed with a first primary solid cancer and correlation tMDS/AML risk patterns with chemotherapy treatment practices | Retrospective population-based cohort study Poisson-based relative risk estimation |
10,329 /32,239 |
Patients with first primary solid cancer who survived 1 year or more without developing a second cancer | 73 (range: 66–84, No information of SD) /61 |
Second primary tMDS/AMLh Patterns of chemotherapy use |
The risk of tMDS/AMLh was higher at <5 years after diagnosis compared to >5 years after diagnosis of gastric cancer. SIRis were higher among patients who received initial chemoradiotherapy than those with chemotherapy alone for gastric cancers. The EARj of tMDS/AMLh for gastric cancer was significantly higher in the age group 65 years or older [SPCk SIRi 2.0(95% CIl: 1.2–3.3), EARj 5.8] than in the age group 50–64 years [SPCk SIRi4.1(95% CIl: 20.-7.2), EAR5.2]. | Screening new primary cancer |
| Choi YJ, 2019 33 Republic of Korea |
Comparing the risk of dementia, including AD and VaD, between gastric cancer patients who underwent gastrectomy and the general population | Retrospective study Cox regression analysis |
63,998 /267,274 |
Patients with gastric cancer who underwent gastrectomy for more than 2 years and are over 50 years of age | 63.3 ± 8.1 /63.2 ± 8.1 |
Incidence of ADs and VaDt | Gastric cancer patients who underwent gastrectomy had increased risk of ADs [adjusted HRr 1.08, 95% CIl 1.03–1.14]. The risk of ADs was especially marked for those who received a total gastrectomy [adjusted HRr 1.39, 95% CIl 1.25–1.54]. VaDt risk was not high in gastric cancer patients [adjusted HRr 0.85, 95% CIl 0.73–0.98]. | Vit B12 deficiency, cognitive function |
| Iki M, 2019 34 Japan |
Clarification about the association of gastrectomy with aBMD, bone metabolism markers, and fracture risk in community-dwelling elderly Japanese men (aged ≥ 65 years) | Prospective cohort study A 5-year longitudinal study Cox proportional hazards models |
74 /1985 |
Elderly patient with gastric cancer who underwent gastrectomy | 74.2 ± 5.6 /73.0 ± 5.2 |
aBMD at the spine and hip, serum levels of intact PTHm, intact OCn, TRACP5bo, ucOCp, OPFsq |
Mean time from surgery was 9.75(±8.3) years. The risk of osteoporotic fractures in men who underwent gastrectomy for gastric cancer was not significant [HRr: 1.94, 95% CIl: 0.60–6.32]. Patients who survived 20 years or more after surgery were at risk of osteoporotic fractures [HRr: 5.38, 95% CIl: 1.43–9.60]. | Bone health |
| Morais S, 2019 35 Portugal |
Quantification of the association between prediagnosis lifestyles with the risk of SPCs and survival of patients with gastric FPC | Prospective observational study, case–control study. Cox proportional hazards regression analysis |
207 /574 |
Gastric cancer survivors who underwent gastrectomy | No information /No information |
Smoking, drinking, BMI, dietary exposures, second primary cancers, mortality | SPCks occurred more often in males than in females [HRr 3.67, 95% CIl 1.26–10.65], and in older patients [55–69 and ≥ 70: HRr 9.03, 95% CIl 1.16–70.57 vs. <55 16.39, 2.16–124.43]. Significantly higher HRrs (95% CIl) were found for older patients [55–69 and ≥70: 1.53 (1.17–2.00) vs. <55: 2.09 (1.61–2.71)]. No statistically significant estimates were observed between smoking or alcohol intake and the occurrence of an SPCk and between smoking or dietary exposures and mortality. | Screening new primary cancer |
| Gharagozlian S, 2020 37 Norway |
Assessment of nutritional status, GI-symptoms and QOL 2–5 years after gastrectomy for malignancy | Prospective observational study | 21 | No recurrence among patients with gastric cancer who underwent gastrectomy | 60.0 ± 12.6 | Nutritional status: SGAw. Dietary intake: repeated 24-h dietary recalls GSRSx, SF-36 |
Mean time from surgery was 28.7(±8.3) months. A high prevalence of weight loss and pre-sarcopenia was observed. Malnutrition as assessed by SGAw was associated with more GIv-symptoms (abdominal pain syndrome) and reduced QOLg scores (bodily pain and vitality). |
Weight Loss, pain, physical activity |
| Schonfeld SJ, 2020 40 US |
Evaluation of second PTC risk among ≥ 1-year adult survivors of non-thyroid malignancies from US population-based cancer registries | Retrospective study SIRs Multivariable Poisson regression models |
35,039/3,175,216 | Cancer survivors one year after diagnosis | Mean age at diagnosis: 63.2 (range: 20–84) No information of SD/61.5 (No information of SD) |
Incidence of second PTCy | SIRis tended to be higher among patients diagnosed with first primary malignancy at < 50 versus ≥50 years (SIRi 1.9, 95% CIl 1.2–2.7, Phet 0.62). | Screening new primary cancer |
| Amikura K, 2020 39 Japan |
Evaluation of MPC and delayed stomach carcinoma recurrence in long-term survivors over 5 years after gastrectomy | Retrospective cohort study | 325/4883 | Long-term gastric cancer survivors of more than 5 years who underwent gastrectomy | Mean age at diagnosis of MPC: 72.4 (range: 38–95)/No information | Incidence of MPCz, delayed stomach carcinoma recurrence | In the elderly patients, 80 years or older, the mortality and the survival rate after MPCz diagnosis were significantly worse than those in younger patients. The onset of MPCz 5 years after surgery was significantly higher in patients over 80 years of age than within 5 years after surgery. Those diagnosed with MPCz within 5 years of surgery were younger than those diagnosed 5 years after surgery. | Screening new primary cancer |
| Horvath A, 2021 38 Japan |
Investigating whether SGB2 is associated with specific changes in gut microbiome composition and intestinal inflammation. | Prospective cross-sectional proof-of-concept study | 14 /22 |
Patients gastric cancer who underwent gastrectomy and treatment for more than 1 year | 68 (range: 64–74, No information of SD) /No information |
Sequencing from stool sample. Lithuanian versions of EORTC QLQ-C30‡, EORTC QLQ-STO22§ |
The most documented GIv symptoms after SGB2† were abdominal discomfort (n = 9; 69%), diarrhea (n = 7; 54%), and bloating (n = 6; 46%). Fecal calprotectin was increased in SGB2† group, and calprotectin levels positively correlated with the abundance of Streptococcus. GIv symptoms in SGB2† patients were associated with distinct taxonomic changes of the gut microbiome. | Small intestine bacterial overgrowth, GIv symptoms |
STG, subtotal gastrectomy; TG, total gastrectomy; MRI, magnetic resonance imaging; DWI, diffusion-weighted imaging; CTC, Common Toxicity Criteria; QLQ, The European Organization for Research and Treatment of Cancer QOL Questionnaire; QOL, quality of life; tMDS/AML, therapy-related myelodysplastic syndrome/acute myeloid leukemia; SIR, standardized incidence ratio; EAR, excess absolute risk; SPC, second primary cancer; CI, confidence Interval; PTH, parathyroid hormone; OC, osteocalcin; TRACP5b, tartrate-resistant acid phosphatase isoenzyme 5b; ucOC, undercarboxylated OC; OPFs, osteoporotic fractures; HR, hazard ratio; AD, Alzheimer's disease; VaD, vascular dementia; FPC, first primary cancer; GI, gastrointestinal; SGA, subjective global assessment; GSRS, GI-Symptom Rating Scale; PTC, primary papillary thyroid cancer; MPC, multiple primary cancers; SGB2, subtotal gastrectomy with Billroth II reconstruction; EORTC QLQ-C30, the European Organization for Research and Treatment Quality of Life Questionnaire Core 30; EORTC QLQ-STO22, gastric cancer-specific module EORTC QLQ.
Characteristics of older gastric cancer survivors
In the selected studies, six operational definitions of cancer survivors were noted. We presented the characteristics of participants in Table 1. Undergoing surgery, being diagnosed, and completing active treatment were described as the beginning of survivorship. The most commonly used definitions of gastric cancer survivors included (1) patient who lived for five years or more after gastrectomy (4 studies, 28.6%)35,39,41,43 and (2) active treatment completion to cancer recurrence (4 studies, 28.6%).33,37,40,44 The criteria for old age also varied, as shown in the mean age of the participants in Table 1. Seven studies (50%)34, 35, 36,38,39,41,45 were conducted with older gastric cancer survivors over 65 years age. Looking at the mean age of participants, 60–64 years was the most common age range (7 studies, 50.0%),33,37,40,42, 43, 44,46 and in one study, the participants were over 80 years old (7.1%).39
Types of late effects in older gastric cancer survivors
Concerning late effects in older gastric cancer survivors, studies on the risk of second primary cancer were the most common (5 studies, 20.8%),35,36,39,40,44 followed by bone health degradation due to vitamin deficiency (3 studies, 12.5%)34,44,45 and vitamin deficiency resulting from gastrectomy (2 studies, 8.3%).33,42 Moreover, iron deficiency (1 study, 4.2%),41 postprandial fullness or eating dysfunction (1 study, 4.2%),43 bacterial overgrowth in the small intestine (1 study, 4.2%)38 were also evaluated. Three studies (12.5%) related to the late effects experienced by cancer survivors in general as well as gastric cancer survivors (cardiovascular disease risk,43,44 employment and return to work,43 healthy lifestyle,43,44 pain,37 physical activity,37 weight loss37) were also conducted. Three studies (12.5%) examined renal dysfunction,46 vaccination,44 and nausea and vomiting,43 which have not been previously studied as late effects in cancer survivors. The identified late effects shown in Table 1 highlight the detailed results of late effects in older gastric cancer survivors.
Types of interventions for managing late effects
Among the 14 studies, only one study (7.1%)45 included an intervention. This study was a prospective cohort study. To confirm the effect of preventing osteoporosis, patients with 25-(OH)-vitamin D ≤ 30 ng/mL at baseline received 16,000 IU of vitamin D3 oral supplements every 10 days for three months during the 1-year follow-up.45 They reported that the oral administration of high doses of vitamin D was easily performed and restored 25-(OH)-vitamin D and iPTH values, which were often disturbed after gastrectomy.
Discussion
This study demonstrated the research trends on the late effects of older gastric cancer survivors. The varied definitions of gastric cancer survivors have been employed depending on the trajectory of cancer treatment. Furthermore, the baselines for classifying older people based on age were mixed: 60 years old, 65 years old, 70 years old, and 80 years old or more. Most studies focused on the risk of second primary cancer and physical symptoms related to gastrectomy as the late effects. However, contrary to our expectations, interventional studies to manage the late effects were rare.
Although we found many studies on older gastric cancer survivors, most studies were conducted with respect to survival, mortality, or recurrence rates.20,22,26,47 Therefore, more studies are required to understand the late symptoms, experience, and difficulties of older gastric cancer survivors as patients with cancer have low average QOL,48 which affects their symptoms.49 Older cancer survivors are more likely to suffer from comorbid chronic conditions, such as late effects, and poor health status.50 Therefore, we need to prioritize research on QOL with a consideration of the late effects in older gastric cancer survivors, given that the proportion of them among cancer survivors is gradually increasing.
Six late effects in overall cancer survivors, including screening for new primary cancer, healthy lifestyle, cardiovascular disease risk, employment and return to work, pain, and physical activity were identified. We also identified six late effects in gastric cancer survivors such as bone health, vitamin B12 deficiency, iron deficiency, postprandial fullness or eating dysfunction, small intestine bacterial overgrowth, and weight loss. However, there was little research on the symptom burden of late effects such as anxiety, depression, trauma, sexual function, diarrhea, chemotherapy-induced neuropathy, and fatigue.13 Moreover, a study reported that one-third of cancer survivors experienced adverse effects that had not been studied before.15 This implies that researchers should expand and explore research topics related to cancer survivors. In future studies, it is necessary to describe unexamined late effects in older gastric cancer survivors, the differences in symptom burden among late effects, and the impact of late effects on QOL. Beyond exploring late effect experiences in cancer survivors, further studies should be conducted with specific age groups and characteristics that indicate a higher risk. Factors contributing to late effects should be efficiently assessed as well.
We found that studies on late effects in older gastric cancer survivors were mainly conducted in Asia. This is because the incidence of gastric cancer is the highest in East Asia and Eastern Europe, while the incidence in North America and Northern Europe is generally low and equal to that in Africa. The age-standardized incidence rates for both regions are higher than the global age-standardized incidence rate(males: 15.8, females: 7.0) per 100,000 population.2 This may explain why studies of older gastric cancer survivors have been rare in Western countries such as North America, Central and South America, and Oceania. Nevertheless, gastric cancer remains one of the most common and deadly cancers worldwide, especially among older males.2 Therefore, it is necessary to actively research older gastric cancer survivors in countries where gastric cancer is prevalent and share the evidence with the world. These efforts will contribute to the development of effective care plans for gastric cancer survivors.
The stage in the cancer trajectory at which patients with gastric cancer are viewed as gastric cancer survivors varied in the 14 studies included in this review. Defining cancer survivors may improve communication among patients with cancer and organizations that use and work with cancer survivors.51 For patients, self-identifying as a cancer survivor is related to better QOL and mental health.52 Defining cancer survivors also helps us compare the results of many studies and use them to assess how well they adapt to their transitions.
A unified definition of the elderly is also required. Studies included in this review classified the elderly based on various baselines (eg., 60 or 70 or 80 years old). Currently, many studies on cancer survivors using big data are being conducted.53, 54, 55 Without standardization and harmonization processes of rigorous data, leveraging data from electronic health records seems challenging due to the inconsistency in terminology usage.56 Therefore, it is essential to define cancer survivors and classify the elderly.
Among the 14 studies, only one intervention study was included. Given the increasing number of older gastric cancer survivors, studies on managing late effects seem insufficient. Numerous studies have shown that cancer survivorship care plans improve satisfaction in cancer survivors.57,58 Therefore, to develop an appropriate care plan, further research on diverse care models or interventions for older gastric cancer survivors is needed. Moreover, further studies on suitable tools for screening late effects in gastric cancer survivors remain to be conducted.
This study has several limitations. First, in our research, we searched databases using the keywords “late effects”, “long term effects”, and “late symptoms”. Therefore, studies not using these terms or not specifically classifying symptoms as late effects might not have been included. Second, we included studies published only in English and Korean. Therefore, we could not include the results of domestic studies in Japan or Eastern Europe, where the incidence of gastric cancer is fairly high. Despite these limitations, this study is meaningful, as it highlights the research trends in studies on older gastric cancer survivors and identifies the characteristics of late effects in older gastric cancer survivors.
After active treatment completion, most patients with cancer go through transition phases and become cancer survivors. They sometimes have physical and psychological problems during transitions, moving forward to new satisfactory lives. Difficulties during transition may include persisting symptoms of disease and treatment, such as late effects. With the identification of the characteristics and factors related to late effects in older gastric cancer survivors, further studies may help understand the basis for caring and providing appropriate management. Hence, identifying the unmet needs of older gastric cancer survivors who experience late effects and conducting research on interventions will contribute to the successful transition of patients with cancer to cancer survivors and achieving improved health-related QOL in older gastric cancer survivors.
Conclusions
With advances in medical science and technology, the number of older gastric cancer survivors has increased. Accordingly, many older gastric cancer survivors are likely to suffer from the burden of late effects even after the completion of cancer treatment. This review highlights recent research trends on late effects in older gastric cancer survivors. Although many studies on gastric cancer survivors have been conducted, we found that the definition of cancer survivors in terms of the stage of treatment varied amongst the studies. In addition, late effects were mainly explored for secondary cancer and physical symptoms related to gastrectomy. Thus, further studies need to identify the undiscovered issues associated with older gastric cancer survivors. Also, the interaction mechanisms of late effects and symptom burden need to be investigated, focusing on the older population. Intervention studies of late effects were also limited. Experimental studies are needed to validate the effectiveness of interventions on various late effects, personalized care planning, and their sustainability over time. Although this review on gastric cancer clearly correlates with regional specificity in gastric cancer prevalence, we suggest that these findings have global currency given that older gastric cancer survivors are increasing worldwide.
Author contributions
MJ: Conceptualization, Data curation, Project administration, Writing—original draft, review and editing, Formal analysis, Visualization, Final approval of the version to be published. SK: Conceptualization, Data curation, Methodology, Funding acquisition, Project administration, Supervision, Validation, Writing—review and editing, Formal analysis. NY: Data curation, Formal analysis, Writing—review and editing, Formal analysis.
Declaration of competing interest
None declared.
Finding
This work was supported by the Basic Science Research Program through the National Research Foundation (NRF), funded by the Ministry of Education, Korea (Grant No.2020R1A6A1A03041989). Misun Jeon received a scholarship from Brain Korea 21 FOUR Project funded by the NRF of Korea, Yonsei University College of Nursing.
References
- 1.National Cancer Center Japan . 2022. Cancer Statistics in Japan ’22. [Google Scholar]
- 2.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 3.Zeng H., Chen W., Zheng R., et al. Changing cancer survival in China during 2003-15: a pooled analysis of 17 population-based cancer registries. Lancet Global Health. 2018;6:e555–e567. doi: 10.1016/S2214-109X(18)30127-X. [DOI] [PubMed] [Google Scholar]
- 4.Lyons K., Le L.C., Pham Y.T.-H., et al. Gastric cancer: epidemiology, biology, and prevention: a mini review. Eur J Cancer Prev. 2019;28:397–412. doi: 10.1097/CEJ.0000000000000480. [DOI] [PubMed] [Google Scholar]
- 5.National Cancer Institute . Cancer Stat Facts: Stomach Cancer; 2021. Recent Trends in SEER Age-Adjusted Incidence Rates, 2000-2018.https://seer.cancer.gov/explorer/application.html?site=18&data_type=1&graph_type=2&compareBy=age_range&chk_age_range_1=1&chk_age_range_160=160&chk_age_range_166=166&rate_type=2&sex=1&race=1&stage=101&advopt_precision=1&advopt_show_ci=on&advopt_display=2 Available from: [Google Scholar]
- 6.Thrift A.P., El-Serag H.B. Burden of gastric cancer. Clin Gastroenterol Hepatol. 2020;18:534–542. doi: 10.1016/j.cgh.2019.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Siegel R.L., Miller K.D., Fuchs H.E., Jemal A. Cancer statistics. CA Cancer J Clin. 2022;2022 doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 8.Ministry of Health and Welfare, Korea Central Cancer Registry, National Cancer Center . 2021. Annual Report of Cancer Statistics in Korea in 2018.https://ncc.re.kr/cancerStatsView.ncc?bbsnum=558&searchKey=total&searchValue=&pageNum=1 Available from: [Google Scholar]
- 9.American Cancer Society . American Cancer Society; Atlanta: 2019. Cancer Treatment & Survivorship Facts & Figures 2019-2021. [Google Scholar]
- 10.ASCO Long-term side effects of cancer treatment. 2019. https://www.cancer.net/survivorship/long-term-side-effects-cancer-treatment Available from.
- 11.National Cancer Institute Late effects of cancer treatment. 2021. https://www.cancer.gov/about-cancer/coping/survivorship/late-effects Available from.
- 12.Institute of Medicine, National Research Council . The National Academies Press; Washington, DC: 2006. From Cancer Patient to Cancer Survivor: Lost in Transition. [Google Scholar]
- 13.NCCN . 2021. NCCN Guidelines-General Survivorship.https://www.nccn.org/guidelines/guidelines-detail?category=3&id=1466 Available from: [Google Scholar]
- 14.Harrington C.B., Hansen J.A., Moskowitz M., Todd B.L., Feuerstein M. It's not over when it's over: long-term symptoms in cancer survivors—a systematic review. Int J Psychiatr Med. 2010;40:163–181. doi: 10.2190/PM.40.2.c. [DOI] [PubMed] [Google Scholar]
- 15.Wu H.-S., Harden J.K. Symptom burden and quality of life in survivorship: a review of the literature. Cancer Nurs. 2015;38:E29–E54. doi: 10.1097/NCC.0000000000000135. [DOI] [PubMed] [Google Scholar]
- 16.Sheryl Ness R., Janine Kokal R., Fee-Schroeder K., Novotny P., Satele D., Debra Barton R. Oncology Nursing Forum. Oncology Nursing Society; 2013. Concerns across the survivorship trajectory: results from a survey of cancer survivors; p. 35. [DOI] [PubMed] [Google Scholar]
- 17.McCabe M.S., Faithfull S., Makin W., Wengstrom Y. Survivorship programs and care planning. Cancer. 2013;119:2179–2186. doi: 10.1002/cncr.28068. [DOI] [PubMed] [Google Scholar]
- 18.NCCN N.C.C.N. guidelines-gastric cancer. 2022. https://www.nccn.org/guidelines/guidelines-detail?category=1&id=1434 Available from:
- 19.Jin Won L., Ali B., Han Mo Y., et al. Conditional survival analysis in Korean patients with gastric cancer undergoing curative gastrectomy. BMC Cancer. 2015;15:1–7. doi: 10.1186/s12885-015-2022-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kadowaki S., Komori A., Narita Y., et al. Long-term outcomes and prognostic factors of patients with advanced gastric cancer treated with S-1 plus cisplatin combination chemotherapy as a first-line treatment. Int J Clin Oncol. 2014;19:656–661. doi: 10.1007/s10147-013-0610-1. [DOI] [PubMed] [Google Scholar]
- 21.Hochwald S.N., Kim S., Klimstra D.S., Brennan M.F., Karpeb M.S. Analysis of 154 actual five-year survivors of gastric cancer. J Gastrointest Surg. 2000;4:520–525. doi: 10.1016/s1091-255x(00)80095-5. [DOI] [PubMed] [Google Scholar]
- 22.Yuri I., Tomio N., Isao M., et al. Conditional survival for longer-term survivors from 2000-2004 using population-based cancer registry data in Osaka, Japan. BMC Cancer. 2013;13:1–7. doi: 10.1186/1471-2407-13-304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li Z., Shi J., Wang Z., Chen H., Liu Y. Nutrient status of vitamin D among cancer patients. Zhongguo Fei Ai Za Zhi. 2021;24:345–350. doi: 10.3779/j.issn.1009-3419.2021.101.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leach J. Osteopathic support for a survivor of gastric cancer: a case report. Int J Osteopath Med. 2008;11:106–111. [Google Scholar]
- 25.Duijts S.F., Van Egmond M.P., Spelten E., Van Muijen P., Anema J.R., van der Beek A.J. Physical and psychosocial problems in cancer survivors beyond return to work: a systematic review. Psycho Oncol. 2014;23:481–492. doi: 10.1002/pon.3467. [DOI] [PubMed] [Google Scholar]
- 26.Lee J.A., Kim S.Y., Kim Y., et al. Comparison of health-related quality of life between cancer survivors treated in designated cancer centers and the general public in Korea. Jpn J Clin Oncol. 2014;44:141–152. doi: 10.1093/jjco/hyt184. [DOI] [PubMed] [Google Scholar]
- 27.Seiler A., Klaas V., Tröster G., Fagundes C.P. eHealth and mHealth interventions in the treatment of fatigued cancer survivors: a systematic review and meta-analysis. Psycho Oncol. 2017;26:1239–1253. doi: 10.1002/pon.4489. [DOI] [PubMed] [Google Scholar]
- 28.Daudt H.M.L., van Mossel C., Scott S.J. Enhancing the scoping study methodology: a large, inter-professional team's experience with Arksey and O'Malley's framework. BMC Med Res Methodol. 2013;13:48. doi: 10.1186/1471-2288-13-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arksey H., O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. 2005;8:19–32. [Google Scholar]
- 30.Tricco A.C., Lillie E., Zarin W., et al. A scoping review on the conduct and reporting of scoping reviews. BMC Med Res Methodol. 2016;16:15. doi: 10.1186/s12874-016-0116-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peters MDJ G.C., McInerney P., Munn Z., Tricco A.C., Khalil H. JBI Manual for Evidence Synthesis. 2020. [Chapter 11]: scoping reviews (2020 version)https://synthesismanual.jbi.global Available from. [Google Scholar]
- 32.Tevaarwerk A., Denlinger C.S., Sanft T., et al. Survivorship, version 1.2021. J Natl Compr Cancer Netw. 2021;19:676–685. doi: 10.6004/jnccn.2021.0028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choi Y.J., Shin D.W., Jang W., et al. Risk of dementia in gastric cancer survivors who underwent gastrectomy: a nationwide study in Korea. Ann Surg Oncol. 2019;26:4229–4237. doi: 10.1245/s10434-019-07913-8. [DOI] [PubMed] [Google Scholar]
- 34.Iki M., Fujita Y., Kouda K., et al. Increased risk of osteoporotic fracture in community-dwelling elderly men 20 or more years after gastrectomy: the Fujiwara-Kyo Osteoporosis Risk in Men (FORMEN) Cohort Study. Bone. 2019;127:250–259. doi: 10.1016/j.bone.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Morais S., Castro C., Antunes L., Peleteiro B., Bento M.J., Lunet N. Second primary cancers and survival in patients with gastric cancer: association with prediagnosis lifestyles. Eur J Cancer Prev. 2019;28:159–166. doi: 10.1097/CEJ.0000000000000447. [DOI] [PubMed] [Google Scholar]
- 36.Morton L.M., Dores G.M., Schonfeld S.J., et al. Association of chemotherapy for solid tumors with development of therapy-related myelodysplastic syndrome or acute myeloid leukemia in the modern era. JAMA Oncol. 2019;5:318–325. doi: 10.1001/jamaoncol.2018.5625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gharagozlian S., Mala T., Brekke H.K., Kolbjørnsen L.C., Ullerud Å.A., Johnson E. Nutritional status, sarcopenia, gastrointestinal symptoms and quality of life after gastrectomy for cancer – a cross-sectional pilot study. Clin. Nutr. ESPEN. 2020;37:195–201. doi: 10.1016/j.clnesp.2020.03.001. [DOI] [PubMed] [Google Scholar]
- 38.Horvath A., Bausys A., Sabaliauskaite R., et al. Distal gastrectomy with Billroth II reconstruction is associated with oralization of gut microbiome and intestinal inflammation: a proof-of-concept study. Ann Surg Oncol. 2021;28:1198–1208. doi: 10.1245/s10434-020-08678-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Amikura K., Ehara K., Kawashima Y. The risk of developing multiple primary cancers among long-term survivors five years or more after stomach carcinoma resection. Tohoku J Exp Med. 2020;250:31–41. doi: 10.1620/tjem.250.31. [DOI] [PubMed] [Google Scholar]
- 40.Schonfeld S.J., Morton L.M., de González A.B., Curtis R.E., Kitahara C.M. Risk of second primary papillary thyroid cancer among adult cancer survivors in the United States, 2000-2015. Cancer Epidemiol. 2020;64 doi: 10.1016/j.canep.2019.101664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jun J.H., Yoo J.E., Lee J.A., et al. Anemia after gastrectomy in long-term survivors of gastric cancer: a retrospective cohort study. Int J Surg. 2016;28:162–168. doi: 10.1016/j.ijsu.2016.02.084. [DOI] [PubMed] [Google Scholar]
- 42.Hu Y., Kim H.I., Hyung W.J., et al. Vitamin B12 deficiency after gastrectomy for gastric cancer: an analysis of clinical patterns and risk factors. Ann Surg. 2013;258:970–975. doi: 10.1097/SLA.0000000000000214. [DOI] [PubMed] [Google Scholar]
- 43.Lee S.S., Chung H.Y., Kwon O.K., Yu W. Long-term quality of life after distal subtotal and total gastrectomy: symptom- and behavior-oriented consequences. Ann Surg. 2016;263:738–744. doi: 10.1097/SLA.0000000000001481. [DOI] [PubMed] [Google Scholar]
- 44.Lee J.E., Shin D.W., Lee H., et al. One-year experience managing a cancer survivorship clinic using a shared-care model for gastric cancer survivors in Korea. J Kor Med Sci. 2016;31:859–865. doi: 10.3346/jkms.2016.31.6.859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Climent M., Pera M., Aymar I., Ramón J.M., Grande L., Nogués X. Bone health in long-term gastric cancer survivors: a prospective study of high-dose vitamin D supplementation using an easy administration scheme. J Bone Miner Metabol. 2018;36:462–469. doi: 10.1007/s00774-017-0856-1. [DOI] [PubMed] [Google Scholar]
- 46.Haneder S., Budjan J.M., Schoenberg S.O., et al. Dose-dependent changes in renal (1)H-/(23)Na MRI after adjuvant radiochemotherapy for gastric cancer. Strahlenther Onkol. 2015;191:356–364. doi: 10.1007/s00066-014-0787-x. [DOI] [PubMed] [Google Scholar]
- 47.Rutkowski P., Andrzejuk J., Bylina E., et al. What are the current outcomes of advanced gastrointestinal stromal tumors: who are the long-term survivors treated initially with imatinib? Med Oncol. 2013;30 doi: 10.1007/s12032-013-0765-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Firkins J., Hansen L., Driessnack M., Dieckmann N. Quality of life in "chronic" cancer survivors: a meta-analysis. J Cancer Surviv. 2020;14:504–517. doi: 10.1007/s11764-020-00869-9. [DOI] [PubMed] [Google Scholar]
- 49.Nayak M.G., George A., Vidyasagar M.S., et al. Quality of life among cancer patients. Indian J Palliat Care. 2017;23:445–450. doi: 10.4103/IJPC.IJPC_82_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Holmes H.M., Nguyen H.T., Nayak P., Oh J.H., Escalante C.P., Elting L.S. Chronic conditions and health status in older cancer survivors. Eur J Intern Med. 2014;25:374–378. doi: 10.1016/j.ejim.2013.12.003. [DOI] [PubMed] [Google Scholar]
- 51.Marzorati C., Riva S., Pravettoni G. Who is a cancer survivor? A systematic review of published definitions. J Cancer Educ. 2017;32:228–237. doi: 10.1007/s13187-016-0997-2. [DOI] [PubMed] [Google Scholar]
- 52.Cheung S.Y., Delfabbro P. Are you a cancer survivor? A review on cancer identity. J. Cancer Surviv. 2016;10:759–771. doi: 10.1007/s11764-016-0521-z. [DOI] [PubMed] [Google Scholar]
- 53.Major A., Cox S.M., Volchenboum S.L. Using big data in pediatric oncology: current applications and future directions. Semin Oncol. 2020;47:56–64. doi: 10.1053/j.seminoncol.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vromans R.D., van Eenbergen M.C., Geleijnse G., Pauws S., van de Poll-Franse L.V., Krahmer E.J. Exploring cancer survivor needs and preferences for communicating personalized cancer statistics from registry data: qualitative multimethod study. JMIR Cancer. 2021;7 doi: 10.2196/25659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Warrington L., Absolom K., Velikova G. Integrated care pathways for cancer survivors–a role for patient-reported outcome measures and health informatics. Acta Oncol. 2015;54:600–608. doi: 10.3109/0284186X.2014.995778. [DOI] [PubMed] [Google Scholar]
- 56.Meehan R.A., Mon D.T., Kelly K.M., et al. Increasing EHR system usability through standards: conformance criteria in the HL7 EHR-system functional model. J Biomed Inf. 2016;63:169–173. doi: 10.1016/j.jbi.2016.08.015. [DOI] [PubMed] [Google Scholar]
- 57.Brennan M.E., Gormally J.F., Butow P., Boyle F.M., Spillane A.J. Survivorship care plans in cancer: a systematic review of care plan outcomes. Br J Cancer. 2014;111:1899–1908. doi: 10.1038/bjc.2014.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Williams V.A., Brown N.I., Johnson R., et al. A web-based lifestyle intervention for cancer survivors: feasibility and acceptability of survivorSHINE. J Cancer Educ. 2021:1–9. doi: 10.1007/s13187-021-02026-x. [DOI] [PMC free article] [PubMed] [Google Scholar]