Abstract
Objective
To assess the effect of ERAS on clinical prognosis in perioperative patients following lung cancer surgery.
Methods
PubMed, Web of Science, MEDLINE, EMBASE, and other databases were systematically searched from inception to December 2021. Randomized controlled trials and peer-reviewed cohort studies on the use of ERAS in lung cancer surgery patients were included. Primary outcomes comprised visual analog scale scores after treatment and quality of life. Secondary outcomes comprised complication rate, function-related outcomes (chest tube indwelling time and first ambulation), and length of stay. Statistical analysis was performed using RevMan 5.4.1 software.
Results
Finally, 23 studies were included (12 cohort studies and 11 randomized controlled trials) with a total of 8094 patients. Meta-analysis showed that ERAS significantly reduced visual analog scale scores (mean difference [MD] = −1.99, 95% confidence interval [CI] = −2.45, −1.54, P < 0.01), reduced the incidence of complications (odds ratio = 0.48, 95% CI = 0.37, 0.61, P < 0.01), shortened chest tube indwelling time (MD = −2.20, 95% CI = −2.75, −1.64, P < 0.01), accelerated first ambulation (MD = −1.48, 95% CI = −1.77, −1.19, P < 0.01), shortened length of stay (MD = −2.70, 95% CI = −3.05, −2.36, P < 0.01), and improved quality of life (MD = 10.3, 95% CI = 9.59, 11.02, P < 0.01).
Conclusions
ERAS can accelerate postoperative recovery and improve quality of life. These findings support the use of ERAS as a standard of care for lung cancer surgery patients. However, the evidence quality was moderate and there were significant differences among studies. More high-quality studies incorporating relevant outcomes are needed for confirmation.
Keywords: Enhanced recovery after surgery, lung cancer, perioperative care, Meta-analysis, systematic review
Introduction
The morbidity and mortality of lung cancer are high worldwide.1 Surgical resection is the preferred treatment for patients with stage I–IIIA lung cancer.2 To improve the treatment effect, a minimally invasive technique was introduced in the field of lung cancer several years ago.3 Concomitant with economic development, research on minimally invasive surgery continues to progress, the technology continues to mature, and video-assisted thoracoscopic surgery (VATS) is becoming increasingly popular. VATS is a non-rib-spreading thoracic procedure. It enables the real-time observation of the surgical procedure in the chest cavity via TV screen and thoracoscope. The VATS incision is approximately 5–8 cm. It comprises a true anatomic lobectomy with the individual dissection of lobar vessels and bronchus, as well as standard lymph node dissection or sampling.4,5 Despite the acceptance of VATS, it is associated with several serious postoperative complications, such as pleural effusion and pneumothorax.6 Poor lung function before the operation, incorrect intraoperative procedure, and postoperative sputum accumulation are some of the factors that cause complications. Complications can have many negative effects on patients and can increase the risk of cancer recurrence.7 Patients who have had technical surgical complications are more likely to experience dyspnea, fatigue, and vomiting, which can substantially affect their overall quality of life.8 Therefore, perioperative management must be strengthened to reduce adverse clinical outcomes.
Enhanced recovery after surgery (ERAS) is a multidisciplinary perioperative care program that includes strategies such as preoperative education, shortening of fasting time, optimization of anesthesia protocols, and early mobilization.9 By implementing these strategies, it is possible to accelerate recovery and improve quality of life.10,11 ERAS was originally implemented in patients with colorectal cancer and has been widely used in various disciplines in recent years.12 Meta-analyses have shown that ERAS has substantial positive effects in colorectal, liver, and pancreatic surgery.13 In recent years, ERAS has been used in lung cancer surgery; however, its safety and effectiveness remain controversial.14,15
The number of systematic reviews of ERAS is limited. Three systematic reviews of patients undergoing lung cancer surgery concluded that ERAS can substantially accelerate postoperative recovery; however, the overall reliability of the evidence is poor.16, 17, 18 The effect of ERAS on postoperative pain and quality of life had not been examined. Therefore, this meta-analysis aimed to further investigate the effect of ERAS on clinical outcomes, comprising postoperative pain, quality of life, complication rate, function-related outcomes, and length of stay (LOS) in patients who had undergone lung cancer surgery.
Methods
Eligibility criteria
Inclusion criteria
Participants
The review included studies of patients with lung cancer undergoing surgery whose clinical diagnosis complied with the guidelines for the diagnosis and treatment of non-small cell lung cancer.19
Interventions
Studies in which the ERAS measures included at least one strategy before, during, and after the surgery compared with standard care were included.
Outcomes
We assessed the following outcomes: visual analog scale (VAS) score, quality of life (36-item Short-Form, SF-36), complication rate, function-related outcomes (chest tube indwelling time and first ambulation), and LOS. All included studies reported on at least one of the outcome measures.
Study design
We included peer-reviewed cohort studies and randomized controlled trials (RCTs).
Exclusion criteria
Participants
Studies with a sample size of < 30 cases were excluded.20 Smaller sample sizes introduce greater random error coupled with publication bias, which may exaggerate the effectiveness of interventions.21
Studies
The following study types were excluded: studies in languages other than Chinese and English, conference abstracts, reviews, studies for which the full text was not available, and studies lacking sufficient data.
Data sources and search strategy
We searched PubMed, Cochrane Library, Web of Science, MEDLINE, EMBASE, CNKI, WanFang, and VIP from database inception to December 2021. The focus of the review was lung cancer and ERAS. Details of the Web of Science search strategies are shown in Table 1; the other databases were searched using the same strategies. We also manually searched the gray literature to ensure that no relevant sources were omitted.
Table 1.
Details of the Web of Science search strategies.
| Web of Science | Search strategy |
|---|---|
| #1 | (((((((((((((((((TS = (Lung neoplasms)) OR TS = (Pulmonary Neoplasms)) OR TS = (Neoplasms, Lung)) OR TS = (Lung Neoplasm)) OR TS = (Neoplasm, Lung)) OR TS = (Neoplasms, Pulmonary)) OR TS = (Neoplasm, Pulmonary)) OR TS = (Pulmonary Neoplasm)) OR TS = (Lung Cancer)) OR TS = (Cancer, Lung)) OR TS = (Cancers, Lung)) OR TS = (Lung Cancers)) OR TS = (Pulmonary Cancer)) OR TS = (Cancer, Pulmonary)) OR TS = (Cancers, Pulmonary)) OR TS = (Pulmonary Cancers)) OR TS = (Cancer of the Lung)) OR TS = (Cancer of Lung) |
| #2 | (((((((((TS = (enhanced recovery after surgery)) OR TS = (fast-track surgery)) OR TS = (fast-track rehabilitation)) OR TS = (enhanced recovery)) OR TS = (enhanced recovery after surgery program)) OR TS = (ERAS)) OR TS = (FTS)) OR TS = (Early recovery)) OR TS = (clinical pathway)) OR TS = (critical pathways) |
| #3 | (TS = (Randomized controlled trial)) OR TS = (cohort study) |
| #4 | ((#1) AND #2) AND #3 |
Data extraction
Data extraction followed the principles of Hozo et al22 It was important to obtain detailed data for each study to address the purpose of this review. The main data extracted were study characteristics (first author, country, year, and study design), patient characteristics (age, sample size per arm, and percentage of male participants), interventions, and outcome measures. Two evaluators (ZW and ZYT) independently selected studies and extracted data from each study, then jointly compared the collected data. Any disagreements about the results were resolved through consensus or consultation with a third evaluator.
Risk of bias assessment
The Newcastle–Ottawa Quality Assessment Scale (NOS)23 and the Cochrane risk of bias tool24 was used for the quality assessment of cohort studies and RCTs. The NOS assesses three quality parameters: selection, comparability, and outcome. A cohort study with a NOS score of ≥ 7 is regarded as having low risk of bias; low NOS scores indicate high risk of bias. The risk of bias tool assesses the following domains: selection bias, performance bias, detection bias, attrition bias, reporting bias, and other risks of bias. Studies were judged on each domain as showing high, low, or unclear risk of bias. Two evaluators jointly checked all studies and reached a consensus.
Data analysis
Statistical analysis was performed using Review Manager 5.4.1 (The Cochrane Collaboration, London, United Kingdom). The combined effect size was obtained by calculating the mean difference (MD) for continuous variables and the odds ratio (OR) for dichotomous variables. The effect size was calculated using the 95% confidence interval (CI). Moreover, for studies that expressed data using interquartile ranges or medians, the data were transformed using the estimation method proposed by Wan et al25 Heterogeneity was inevitable because the setting of each study was different and was assessed using the Q test and I2. The random-effects model was used if the heterogeneity was significant (I2 > 50% or P < 0.10). Otherwise, the fixed-effects model was used.26,27 Subgroup analysis was used to confirm the robustness of the meta-analysis. Sensitivity analysis was performed by excluding one study at a time. We also re-analyzed the data using a fixed-effects model. P < 0.05 was considered statistically significant. Publication bias was assessed using Egger's test; values of P < 0.05 indicate publication bias.28
Results
Study characteristics
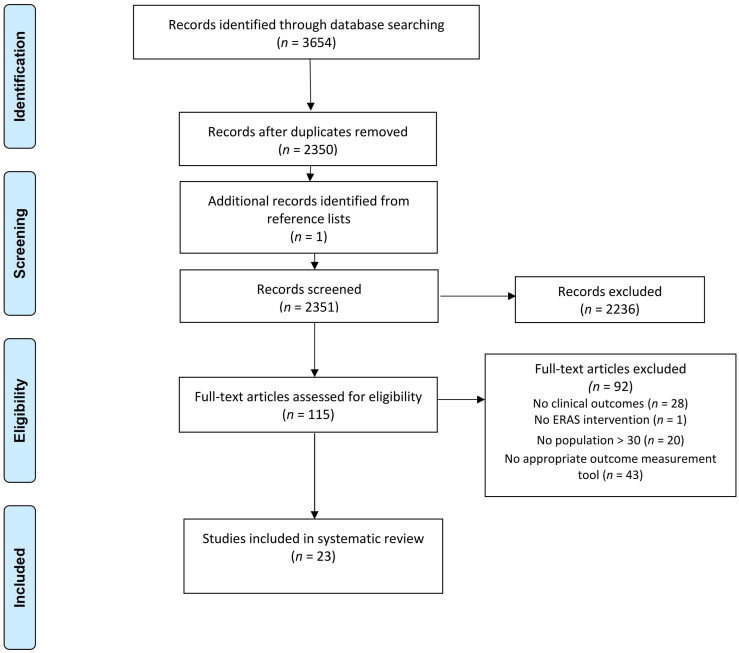
In total, 3654 studies were retrieved. After removing duplicates, we reviewed 2351 titles and abstracts. We read the full text of 115 studies and finally included 23 studies according to the inclusion criteria.29, 30, 31, 32, 33, 34, 35, 36, 37, 38, 39, 40, 41, 42, 43, 44, 45, 46, 47, 48, 49, 50, 51 A flow chart outlining the search strategy is shown in Fig. 1. The 23 studies involved a total of 8094 patients, 3151 in the ERAS group and 4943 in the control group. Of the included studies, 12 were cohort studies and 11 were RCTs. The average age of the study population ranged from 55 to 80 years, and approximately 65% of participants were men. Table 2 summarizes the baseline characteristics of each included study. Each study used different ERAS measures; details of the perioperative measures are shown in Table 2.
Fig. 1.
Study selection flowchart. Transparent reporting outline of the search strategy results from initial search to included studies.
Table 2.
Basic characteristics of included studies.
| Study | Country | Study design | Cases ERAS/control | % Male |
Intervention measures | Outcomes |
|---|---|---|---|---|---|---|
| Alessan 2017 | United Kingdom | RCS | 235/365 | 42.1/40 | A, B, C, E, H, J, I, M | complication |
| Amin 2015 | Canada | RCS | 107/127 | 61/45 | A, F, H, I, J, K | Complication, LOS, chest tube indwelling time |
| Cai 2018 | China | PCS | 62/59 | 66.1/66.1 | A, C, E, F, H, I, J, K | VAS, LOS, first ambulation, complication |
| Che 2018 | China | RCT | 75/75 | 66.7/64 | A, E, F, H, I | VAS, chest tube indwelling time, LOS, first ambulation, complication |
| Fan 2019 | China | RCT | 100/80 | 63/63.8 | A, C, E, F, H, I, J, L | LOS, chest tube indwelling time, first ambulation, complication |
| Forster 2021 | Switzerland | RCS | 140/167 | 47.1/58.7 | A, E, F, H, I, J | Complication, LOS, chest tube indwelling time |
| Greg 2019 | USA | PCS | 126/169 | 31/43.8 | C, E, F, H, I | LOS |
| Huang 2018 | China | RCS | 38/45 | 42.1/55.6 | A, B, C, F, H, I, J | Complication, VAS, chest tube indwelling time, LOS |
| Li 2017 | China | RCT | 80/80 | 66.3/61.3 | A, F, H, J, K | VAS, LOS, complication, chest tube indwelling time, first ambulation |
| Li 2018 | China | RCT | 50/50 | 60/62 | A, B, F, H, I, J, K | VAS, LOS, complication, chest tube indwelling time |
| Li 2020 | China | RCT | 40/40 | 67.5/62.5 | A, C, E, F, H, I, J, K | QoL, complication |
| Michele 2012 | Italy | RCS | 232/232 | NR | A, B, C, D, E, F, H, I, J, K, M | Complication, LOS |
| Robert 2018 | USA | RCS | 342/1615 | 47.4/50 | A, B, E, F, H, I, J, K, L | Complication, LOS, chest tube indwelling time |
| Satoshi 2019 | Japan | RCS | 130/405 | 66.2/57 | A, B, C, D, E, F, G, H, I, J, K, L, M | Complication |
| Tahiri 2020 | Canada | RCS | 98/98 | 36.7/29.6 | A, C, E, F, H, I, J, K | Complication, LOS, chest tube indwelling time, first ambulation |
| Wang 2015 | China | RCT | 54/54 | 68.5/64.8 | A, B, C, E, F, H, I, J, K, L | VAS, chest tube indwelling time, first ambulation, LOS, complication |
| Wang 2019 | China | RCT | 45/45 | 68.9/64.4 | A, E, H, I, J, K, L | VAS, LOS, first ambulation, complication, chest tube indwelling time |
| Wang 2021 | China | RCS | 691/1058 | 50.8/49.8 | A, C, D, E, F, H, J, K | Complication, LOS, chest tube indwelling time |
| Xu 2020 | China | PCS | 60/60 | 46.7/55 | A, B, C, E, F, H, I, J, K | VAS, LOS, complication |
| Zhang 2017 | China | RCT | 50/50 | 52/50 | A, B, C, E, F, H, I, J, K | VAS, chest tube indwelling time, QoL, complication, LOS |
| Zhang 2019 | China | RCT | 106/106 | 65.1/51.9 | A, B, D, E, F, H, I, J, K, L | VAS, chest tube indwelling time, LOS, first ambulation, complication |
| Zhao 2010 | China | RCT | 38/36 | 63.2/69.4 | C, D, E, F, H, I | VAS, LOS, complication |
| Zheng 2019 | China | RCT | 43/43 | 67.4/72.1 | A, E, F, G, H, I, J, K | VAS, LOS, chest tube indwelling time, QoL, complication |
ERAS, enhanced recovery after surgery; RCT, randomized controlled trial; PCS, prospective cohort study; RCS, retrospective cohort study; VAS, visual analog scale; QoL, quality of life; LOS, length of stay.
Intervention measures. Preoperative (A) Patient education, the importance of smoking and alcohol reduction, and nutritional supplements (B) Respiratory function exercise and incentive spirometer instruction (C) Shortened fasting and water period (D) Psychological care, good communication through understanding needs. Intraoperative (E) Intraoperative warming, such as controlling the temperature of the operating room, applying warm water bags and other devices (F) Optimizing the anesthesia method, selecting the appropriate anesthetic drugs (G) Avoidance of fluid overload. Postoperative (H) Multimodal analgesia (I) Restriction of use/early removal of surgical drains (J) Early mobilization, basic activities in bed after awakening, and getting out of bed 1 day after surgery (K) Early feeding (L) Respiratory function exercise (M) Fluid therapy targeting euvolemia.
Risk of bias
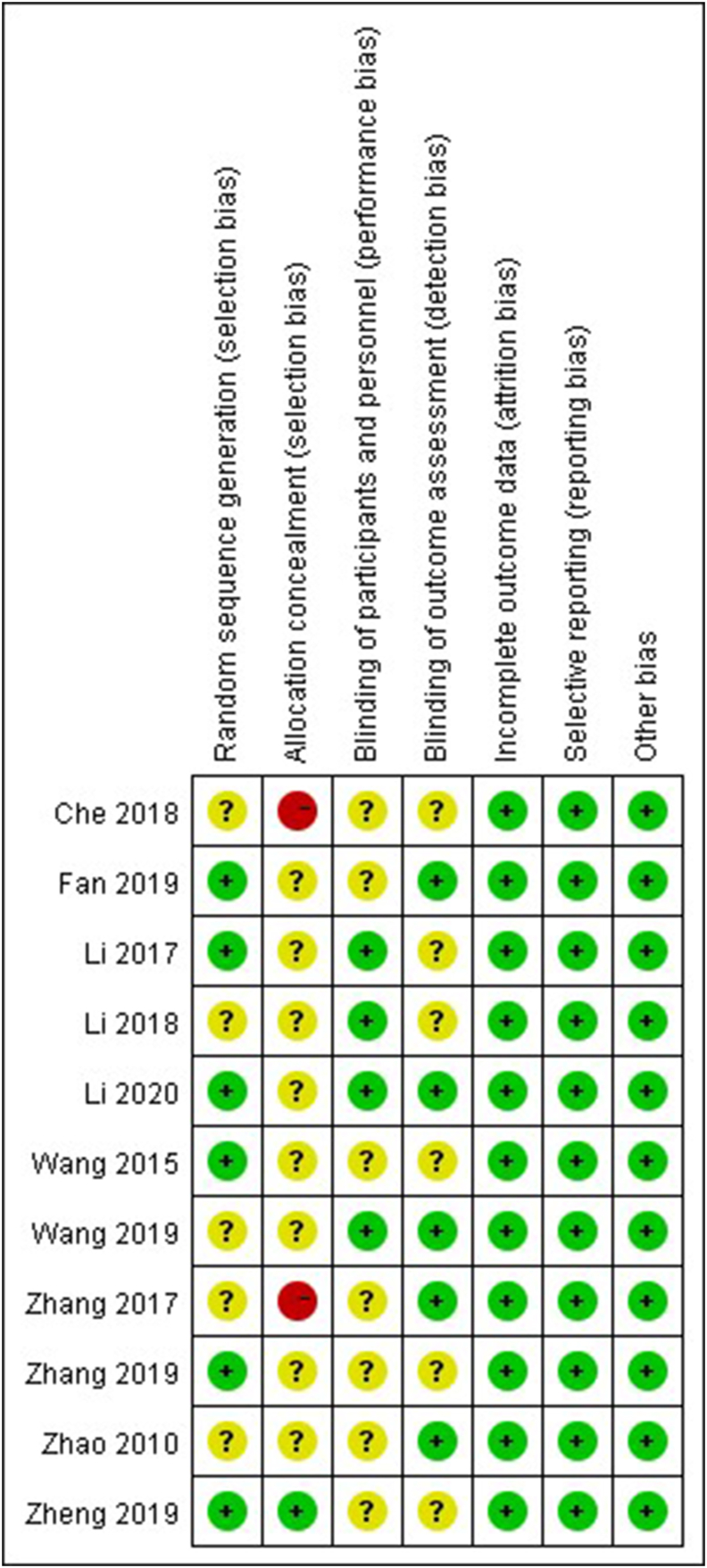
Fig. 2 and Table 3 summarize the risk of bias in the RCTs and cohort studies. The overall quality of the included studies was good. All studies compared the baseline characteristics of the two groups and found that these were consistent. The included studies also showed consistent findings regarding the promotion of patient recovery by the ERAS program. The NOS scores of the included cohort studies were all ≥ 6, and most studies showed a low risk of bias. Studies showed comprehensive selection and comparability parameters, but most studies ignored the adequacy of cohort follow-up in relation to the outcome parameters. Most of the included RCTs had moderate selection bias; no other serious bias was found. However, the risk of bias was increased owing to the lack of allocation concealment.52
Fig. 2.
The risk of bias of randomized controlled trials. Green represents low risk; yellow represents unclear risk; red represents high risk. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 3.
Risk of bias assessment: NOS scores for cohort studies.
| Items of NOS | Studies | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Alessa 2017 |
Amin 2015 | Cai 2018 |
Forster 2021 |
Greg 2019 | Huang 2018 | Michele 2012 |
Robert 2018 | Satoshi 2019 | Tahiri 2020 | Wang 2021 | Xu 2020 |
|
| Selection | ||||||||||||
| Representativeness of the exposed cohort | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | |
| Selection of the non-exposed cohort | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
| Ascertainment of exposure | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
| Demonstration that outcome of interest was not present at start of study | ∗ | |||||||||||
| Comparability | ||||||||||||
| Comparability of cohorts on basis of the design or analysis | ∗∗ | ∗∗ | ∗∗ | ∗∗ | ∗∗ | ∗∗ | ∗∗ | ∗ | ∗∗ | ∗ | ∗∗ | ∗ |
| Outcome | ||||||||||||
| Assessment of outcome | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | |||
| Was follow-up long enough for outcomes to occur | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ | ∗ |
| Adequacy of follow-up of cohorts | ∗ | ∗ | ∗ | |||||||||
| Total | 6 | 7 | 8 | 6 | 8 | 6 | 7 | 6 | 7 | 6 | 7 | 7 |
NOS, Newcastle–Ottawa Quality Assessment Scale.
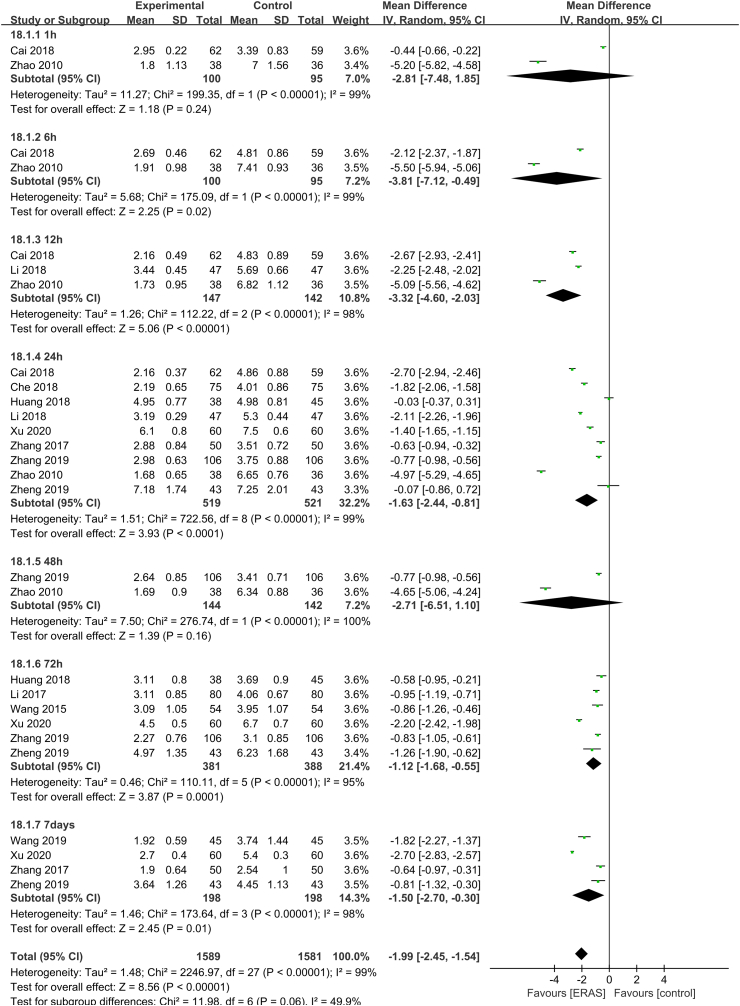
Meta-analysis of VAS scores after treatment
Of the 23 included studies, 12 studies31,32,36, 37, 38,44,45,47, 48, 49, 50, 51 with 3170 patients (1589 ERAS and 1581 control) were included in the meta-analysis of VAS scores after treatment. The heterogeneity test showed significant heterogeneity (P < 0.01, I2 = 99%), so the random-effects model was used. ERAS significantly improved postoperative pain in patients with lung cancer (MD = −1.99, 95% CI [−2.45, −1.54], P < 0.01) (Fig. 3). The subgroup analysis of VAS at 1 h, 6 h, 12 h, 24 h, 48 h, 72 h, and 7 days after surgery showed that the heterogeneity was reduced (P > 0.05 and I2 < 50%) and the results of the meta-analysis were robust. As shown in Fig. 3, compared with the control group, the ERAS group experienced a significant improvement in postoperative pain at 6 h (MD = −3.81, 95% CI [−7.12, −0.49], P < 0.05), 12 h (MD = −3.32, 95% CI [−4.60, −2.03], P < 0.01), 24 h (MD = −1.63, 95% CI [−2.44, −0.81], P < 0.01), 72 h (MD = −1.12, 95% CI [−1.68, −0.55], P < 0.01), 7 days (MD = −1.50, 95% CI [−2.70, −0.30], P < 0.05). However, there was no significant difference in pain at 1 h (MD = −2.81, 95% CI [−7.48, 1.85], P > 0.05) and 48 h (MD = −2.71, 95% CI [−6.51, 1.10], P > 0.05) after surgery. Considering the significant heterogeneity among studies, sensitivity analysis was performed to identify the source of the difference. However, the heterogeneity did not change.
Fig. 3.
Forest plot of VAS scores after treatment. Meta-analysis comparing ERAS versus standard recovery for postoperative pain after lung cancer surgery. ERAS, enhanced recovery after surgery; VAS, visual analog scale.
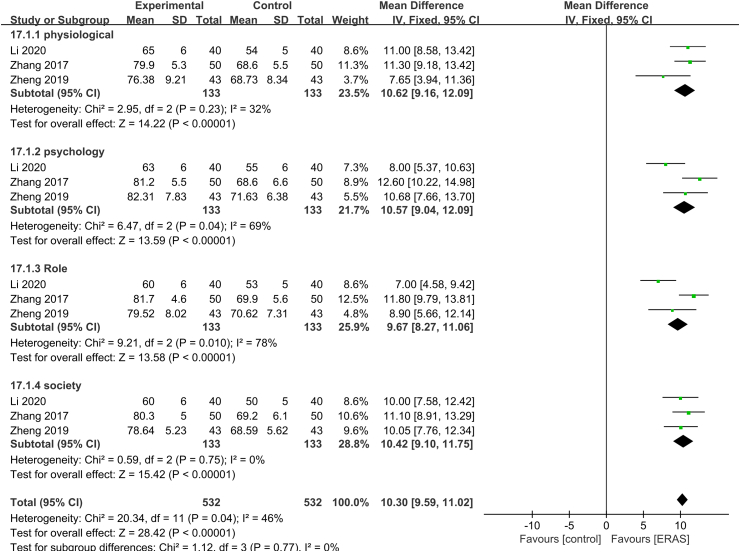
Meta-analysis of quality of life
Three studies39,48,51 with 1064 patients (532 ERAS and 532 control) were included in the meta-analysis of quality of life. The heterogeneity test showed no significant heterogeneity (P = 0.04, I2 = 46%), so the fixed-effects model was used. The results showed that ERAS significantly improved quality of life in patients with lung cancer (MD = 10.3, 95% CI [9.59, 11.02], P < 0.01) (Fig. 4). Subgroup analysis was performed on the four dimensions of quality of life: physiological, psychological, role, and social function. The results were robust (P = 0.77 and I2 = 0%).
Fig. 4.
Forest plot of quality of life. Meta-analysis comparing ERAS versus standard recovery for quality of life after lung cancer surgery. ERAS, enhanced recovery after surgery.
Meta-analysis of complication rate
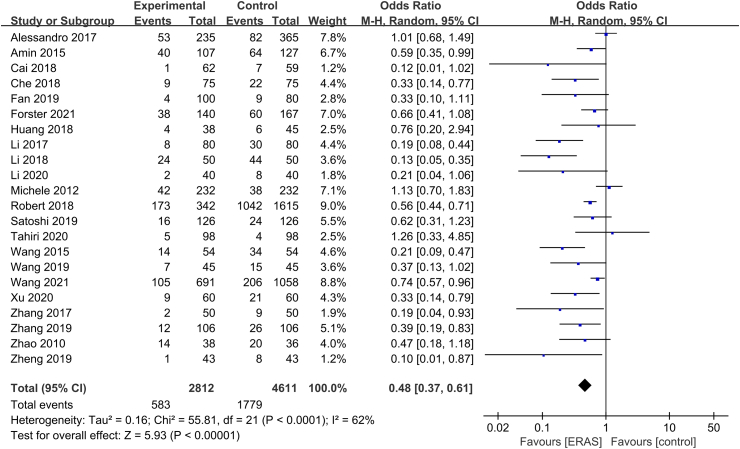
Except for Greg et al,35 22 RCTs with 7423 patients (2812 ERAS and 4611 control) analyzed postoperative complications, and the incidence of complications was described using a binary variable. There was heterogeneity among studies (P < 0.01, I2 = 62%), so the random-effects model was used. As shown in Fig. 5, ERAS significantly reduced the incidence of complications in patients with lung cancer (OR = 0.48, 95% CI [0.37, 0.61], P < 0.01). After excluding the study by Alessandro et al, the heterogeneity was significantly reduced, and the result was stable. However, the incidence of specific complications, such as reoperation, readmission, and mortality, was very low. We performed a subgroup analysis, which showed that there was no significant difference in reoperation rate (OR = 0.87, 95% CI [0.49, 1.55], P > 0.05), readmission rate (OR = 1.03, 95% CI [0.75, 1.40], P > 0.05), and mortality rate (OR = 1.15, 95% CI [0.60, 2.22], P > 0.05).
Fig. 5.
Forest plot of the complication rate. Meta-analysis comparing ERAS versus standard recovery for the complication rate after lung cancer surgery. ERAS, enhanced recovery after surgery.
Meta-analysis of function-related outcomes
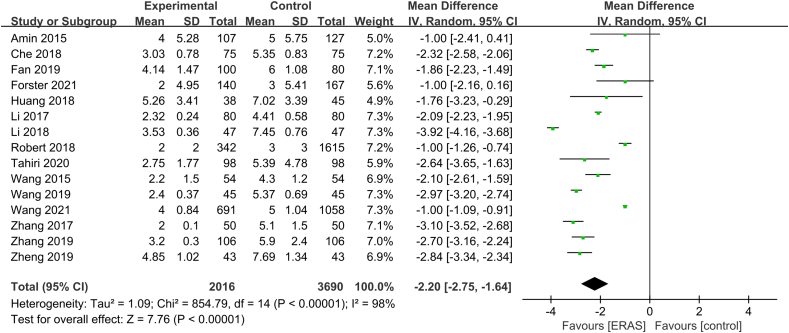
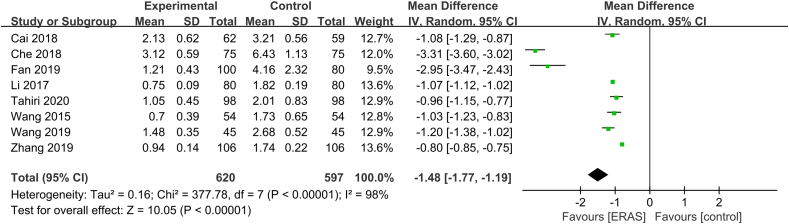
Postoperative recovery mainly includes chest tube indwelling time and first ambulation. Fifteen studies30,32, 33, 34,36, 37, 38,41,43, 44, 45, 46,48,49,51 and eight studies31, 32, 33,37,43, 44, 45,49 analyzed the effect of ERAS on chest tube indwelling time and first ambulation, respectively. The heterogeneity test indicated high heterogeneity among studies regarding chest tube indwelling time (P < 0.01, I2 = 98%). The random-effects model was used, and the combined effect size was statistically significant (MD = −2.20, 95% CI [−2.75, −1.64], P < 0.01) (Fig. 6). There was significant heterogeneity among studies for first ambulation data (P < 0.01, I2 = 98%), so the random-effects model was used. The combined effect size was significant (MD = −1.48, 95% CI [−1.77, −1.19], P < 0.01) (Fig. 7). ERAS significantly accelerated recovery after surgery.
Fig. 6.
Forest plot of chest tube indwelling time. Meta-analysis comparing ERAS versus standard recovery for chest tube indwelling time after lung cancer surgery. ERAS, enhanced recovery after surgery.
Fig. 7.
Forest plot of first ambulation. Meta-analysis comparing ERAS versus standard recovery for first ambulation after lung cancer surgery. ERAS: enhanced recovery after surgery.
Meta-analysis of LOS
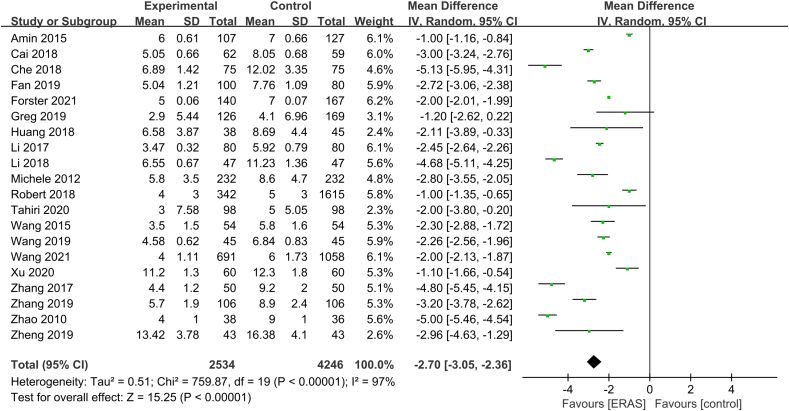
As shown in Fig. 8, 20 studies30, 31, 32, 33, 34, 35, 36, 37, 38,40,41,43, 44, 45, 46, 47, 48, 49, 50, 51 with 6780 patients (2534 ERAS and 4246 control) were included in the meta-analysis for LOS. The heterogeneity test showed high heterogeneity among studies (P < 0.01, I2 = 97%), and the random-effects model was used. LOS reduced after the implementation of ERAS (MD = −2.70, 95% CI [−3.05, −2.36], P < 0.01).
Fig. 8.
Forest plot of length of stay. Meta-analysis comparing ERAS versus standard recovery for length of stay after lung cancer surgery. ERAS, enhanced recovery after surgery.
Discussion
The ERAS research group has published specific perioperative care pathways for thoracic surgery.53 The presentation of a consensus may facilitate an understanding of the priorities for applying ERAS principles in clinical practice. However, implementing an ERAS program in a specific institution remains a daunting task because of the influence of historical practices, resource challenges, and other factors.54
Overall adherence to the ERAS program improved patient outcomes.55 As technology develops, the ERAS program could incorporate more care elements at each stage of the perioperative period. Synergy of these elements may reduce stress response and catabolism.56 Some elements (such as preoperative respiratory function exercise and early postoperative mobilization) are more effective than others.57 Preoperative respiratory function exercise benefits the physiology of surgical patients and may reduce the incidence of pulmonary complications.58 The present review found consistent reports of such effects. Furthermore, postoperative immunosuppression caused by surgery59 may prolong wound healing time and hast cancer cell development. ERAS may reduce postoperative infection in patients and accelerate postoperative recovery by reducing inflammation,60 which is consistent with our pooled estimates. Therefore, nurses should provide timely health education, such as multimedia playback, to increase patient awareness of the importance of measures such as early postoperative mobilization, thereby improving compliance.
This meta-analysis showed that following ERAS, patients reported relief of postoperative pain, and the chest tube indwelling time was shortened, which indirectly reduced the incidence of postoperative complications. Pain is the most common postoperative problem in all types of surgery. Typically, the cause of patient-reported symptoms of pain is investigated and treated with appropriate drugs, such as Celecoxib61 and Dezocine.62 However, some drugs have delayed effects. Because of this, most patients resist engaging in activities because of fear of pain, which leads to problems such as prolonged drainage, followed by an inflammatory response.63 Postoperative inflammatory responses are associated with the occurrence of complications.64 The present study also showed that ERAS was directly related to a reduction in complications and improvement in quality of life. Improved life quality is a goal of humanistic care.65 The reported improvements in outcomes contribute toward ensuring the health and well-being of patients. Therefore, nurses should pay more attention to the ERAS program and implement appropriate ERAS measures for patients with lung cancer.
Egger's test indicated publication bias among studies (P = 0.001), possibly because several included studies did not account for potential confounders. After using the alternative approach described by Zwetsloot et al,66 the risk of publication bias remained. For example, we found that the LOS improvement after the implementation of ERAS was conservative. This may be because LOS is affected by many factors in addition to readiness for discharge; non-medical factors such as surgeon habits and patient expectations67 may explain why some studies reported a lack of effect for ERAS. To some extent, the personal habits of surgeons affect ERAS outcomes. Surgeons in different research institutions use different ERAS measures, such as LOS criteria,68 based on their own experience. Additionally, most measures in the ERAS program require patient cooperation. High patient compliance may be needed to ensure the effectiveness of ERAS implementation.69 Research shows that patient compliance before surgery is high. However, disease progression and psychological pressure lead to reduced compliance.70 Therefore, nurses should pay more attention to the needs of patients and consider providing individualized ERAS measures for specific lung cancer disease sites or surgical interventions based on ERAS guidelines.
Our results are partly consistent with previous studies18; however, we included and analyzed more relevant outcome measures. Research by Huang et al36 showed that compared with traditional perioperative care, ERAS reduced postoperative pain and shortened chest tube indwelling time. Furthermore, previous studies have found a substantial difference in quality of life between the ERAS program and standard care, which indicates that ERAS is beneficial.71,72 There is also evidence that the LOS of patients treated with the ERAS program is shorter.73
This meta-analysis indicated significant heterogeneity among studies. During the study design process, we specified subgroup analyses of potential sources of heterogeneity in advance, including the number of ERAS measures, and risk of bias. However, these factors did not seem to explain the heterogeneity.74 A possible explanation is the differences in case mix among studies. The studies involved different patients in different countries. The diversity of patient types suggests the general applicability of our findings regarding the safety and efficacy of ERAS but inevitably led to heterogeneity. An in-depth analysis of sources of heterogeneity is required in the future.
Teamwork is the basis for the success of the ERAS program. Some research75 has shown that good patient outcomes are inseparable from teamwork and effective communication. Many ERAS measures, such as multimodal analgesia, are not only relevant to nurses but also affect surgeon judgment.53 Therefore, nurses should work collaboratively with surgeons and anesthesiologists to provide care throughout the perioperative period to ensure that patients receive optimal treatment.
The main advantage of this meta-analysis was the inclusion of more studies and patients compared with previous analyses. It increased the focus on the needs of patients and postoperative recovery. However, this meta-analysis had several limitations. First, only Chinese and English articles were finally included. Because articles published in other languages were excluded, the findings do not reflect the status of these populations. Second, some studies did not satisfy the requirements for blinding or allocation concealment, resulting in biased results. Third, a unified ERAS guideline for lung cancer surgery remains to be developed, and indicators such as the inconsistency of chest tube removal criteria and discharge criteria may have affected the results. Moreover, the sample size of the included studies varied greatly, which may have introduced clinical heterogeneity. All these factors may limit the international application and generalizability of findings.
Conclusions
This systematic review indicated that ERAS may lead to significant reductions in pain conditions, postoperative complications, and LOS. Additionally, ERAS may accelerate postoperative recovery and improve quality of life. This analysis provides strong evidence for the efficacy and safety of ERAS for patients with lung cancer. Additional research is needed to investigate the effects of individual elements of the ERAS program. This would help identify important aspects of the program, gradually improve the program, and develop an ERAS application standard for with lung cancer.
Acknowledgments
The authors acknowledge the major medical databases for facilitating our research. We thank Diane Williams, PhD, from Liwen Bianji (Edanz) (www.liwenbianji.cn/), for editing the English text of a draft of this manuscript.
Author contributions
Wenhui Zhang: Study design, literature search, critical appraisal of included papers, extraction of data, data analysis, manuscript writing, and manuscript revision. Yuting Zhang: Critical appraisal of included papers, extraction of data, and data analysis. Yi Qin: Literature review and search, and study supervision. Jiahai Shi: Study design, study supervision, and manuscript revision.
Declaration of competing interest
None declared.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 81770266); the China Postdoctoral Science Foundation (Grant No. 2019M661907); the Clinical Medical Research Center of Cardiothoracic Diseases in Nantong (Grant No. HS2019001); the Innovation Team of Cardiothoracic Disease at the Affiliated Hospital of Nantong University (Grant No. TECT-A04); and the Nantong Key Laboratory of Translational Medicine of Cardiothoracic Diseases.
References
- 1.Sung H., Ferlay J., Siegel R.L., et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. Epub 2021/02/05. PubMed PMID: 33538338. [DOI] [PubMed] [Google Scholar]
- 2.Nasim F., Sabath B.F., Eapen G.A. Lung cancer. Med Clin North Am. 2019;103(3):463–473. doi: 10.1016/j.mcna.2018.12.006. Epub 2019/04/09. PubMed PMID: 30955514. [DOI] [PubMed] [Google Scholar]
- 3.Flores R.M., Alam N. Video-assisted thoracic surgery lobectomy (VATS), open thoracotomy, and the robot for lung cancer. Ann Thorac Surg. 2008;85(2):S710–S715. doi: 10.1016/j.athoracsur.2007.09.055. Epub 2008/01/29. PubMed PMID: 18222202. [DOI] [PubMed] [Google Scholar]
- 4.Swanson S.J., Herndon J.E., 2nd, D'Amico T.A., et al. Video-assisted thoracic surgery lobectomy: report of CALGB 39802--a prospective, multi-institution feasibility study. J Clin Oncol. 2007;25(31):4993–4997. doi: 10.1200/JCO.2007.12.6649. Epub 2007/11/01. PubMed PMID: 17971599. [DOI] [PubMed] [Google Scholar]
- 5.Yan T.D., Cao C., D'Amico T.A., et al. Video-assisted thoracoscopic surgery lobectomy at 20 years: a consensus statement. Eur J Cardio Thorac Surg. 2014;45(4):633–639. doi: 10.1093/ejcts/ezt463. Epub 2013/10/17. PubMed PMID: 24130372. [DOI] [PubMed] [Google Scholar]
- 6.Manerikar A., Querrey M., Cerier E., et al. Comparative effectiveness of surgical approaches for lung cancer. J Surg Res. 2021;263:274–284. doi: 10.1016/j.jss.2020.10.020. Epub 2020/12/15. PubMed PMID: 33309173; PubMed Central PMCID: PMCPMC8169528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lerut T., Moons J., Coosemans W., et al. Postoperative complications after transthoracic esophagectomy for cancer of the esophagus and gastroesophageal junction are correlated with early cancer recurrence: role of systematic grading of complications using the modified Clavien classification. Ann Surg. 2009;250(5):798–807. doi: 10.1097/SLA.0b013e3181bdd5a8. Epub 2009/10/08. PubMed PMID: 19809297. [DOI] [PubMed] [Google Scholar]
- 8.Derogar M., Orsini N., Sadr-Azodi O., Lagergren P. Influence of major postoperative complications on health-related quality of life among long-term survivors of esophageal cancer surgery. J Clin Oncol. 2012;30(14):1615–1619. doi: 10.1200/JCO.2011.40.3568. Epub 2012/04/05. PubMed PMID: 22473157. [DOI] [PubMed] [Google Scholar]
- 9.Ljungqvist O., Scott M., Fearon K.C. Enhanced recovery after surgery: a review. JAMA Surg. 2017;152(3):292–298. doi: 10.1001/jamasurg.2016.4952. Epub 2017/01/18. PubMed PMID: 28097305. [DOI] [PubMed] [Google Scholar]
- 10.Ljungqvist O., Hubner M. Enhanced recovery after surgery-ERAS-principles, practice and feasibility in the elderly. Aging Clin Exp Res. 2018;30(3):249–252. doi: 10.1007/s40520-018-0905-1. Epub 2018/02/18. PubMed PMID: 29453605; PubMed Central PMCID: PMCPMC5856872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Keil D.S., Schiff L.D., Carey E.T., et al. Predictors of admission after the implementation of an enhanced recovery after surgery pathway for minimally invasive gynecologic surgery. Anesth Analg. 2019;129(3):776–783. doi: 10.1213/ANE.0000000000003339. Epub 2019/08/20. PubMed PMID: 31425219. [DOI] [PubMed] [Google Scholar]
- 12.Gustafsson U.O., Scott M.J., Schwenk W., et al. Guidelines for perioperative care in elective colonic surgery: enhanced Recovery after Surgery (ERAS((R))) Society recommendations. World J Surg. 2013;37(2):259–284. doi: 10.1007/s00268-012-1772-0. Epub 2012/10/12. PubMed PMID: 23052794. [DOI] [PubMed] [Google Scholar]
- 13.Visioni A., Shah R., Gabriel E., Attwood K., Kukar M., Nurkin S. Enhanced recovery after surgery for noncolorectal surgery?: a systematic review and meta-analysis of major abdominal surgery. Ann Surg. 2018;267(1):57–65. doi: 10.1097/SLA.0000000000002267. Epub 2017/04/25. PubMed PMID: 28437313. [DOI] [PubMed] [Google Scholar]
- 14.Huang L., Kehlet H., Petersen R.H. Reasons for staying in hospital after video-assisted thoracoscopic surgery lobectomy. BJS Open. 2022;6(3) doi: 10.1093/bjsopen/zrac050. Epub 2022/05/06. PubMed PMID: 35511502; PubMed Central PMCID: PMCPMC9070644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kehlet H. Enhanced postoperative recovery: good from Afar, but far from good? Anaesthesia. 2020;75(1):e54–e61. doi: 10.1111/anae.14860. Epub 2020/01/07. PubMed PMID: 31903577. [DOI] [PubMed] [Google Scholar]
- 16.Fiore J.F., Jr., Bejjani J., Conrad K., et al. Systematic review of the influence of enhanced recovery pathways in elective lung resection. J Thorac Cardiovasc Surg. 2016;151(3):708–715. doi: 10.1016/j.jtcvs.2015.09.112. e6. Epub 2015/11/11. PubMed PMID: 26553460. [DOI] [PubMed] [Google Scholar]
- 17.Li S., Zhou K., Che G., et al. Enhanced recovery programs in lung cancer surgery: systematic review and meta-analysis of randomized controlled trials. Cancer Manag Res. 2017;9:657–670. doi: 10.2147/CMAR.S150500. Epub 2017/11/29. PubMed PMID: 29180901; PubMed Central PMCID: PMCPMC5695257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li R., Wang K., Qu C., et al. The effect of the enhanced recovery after surgery program on lung cancer surgery: a systematic review and meta-analysis. J Thorac Dis. 2021;13(6):3566–3586. doi: 10.21037/jtd-21-433. Epub 2021/07/20. PubMed PMID: 34277051; PubMed Central PMCID: PMCPMC8264698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.NCCN clinical practice guidelines in oncology: non-small cell lung cancer. Available from: https://www.nccn.org/professionals/physician_gls/.
- 20.Triantafyllou T., Olson M.T., Theodorou D., Schizas D., Singhal S. Enhanced recovery pathways vs standard care pathways in esophageal cancer surgery: systematic review and meta-analysis. Esophagus. 2020;17(2):100–112. doi: 10.1007/s10388-020-00718-9. Epub 2020/01/25. PubMed PMID: 31974853. [DOI] [PubMed] [Google Scholar]
- 21.Dechartres A., Trinquart L., Boutron I., Ravaud P. Influence of trial sample size on treatment effect estimates: meta-epidemiological study. BMJ. 2013;346:f2304. doi: 10.1136/bmj.f2304. Epub 2013/04/26. PubMed PMID: 23616031; PubMed Central PMCID: PMCPMC3634626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hozo S.P., Djulbegovic B., Hozo I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med Res Methodol. 2005;5:13. doi: 10.1186/1471-2288-5-13. Epub 2005/04/21. PubMed PMID: 15840177; PubMed Central PMCID: PMCPMC1097734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. Epub 2010/07/24. PubMed PMID: 20652370. [DOI] [PubMed] [Google Scholar]
- 24.Cumpston M., Li T., Page M.J., et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019;10:ED000142. doi: 10.1002/14651858.ED000142. Epub 2019/10/24. PubMed PMID: 31643080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Luo D., Wan X., Liu J., Tong T. Optimally estimating the sample mean from the sample size, median, mid-range, and/or mid-quartile range. Stat Methods Med Res. 2018;27(6):1785–1805. doi: 10.1177/0962280216669183. Epub 2016/09/30. PubMed PMID: 27683581. [DOI] [PubMed] [Google Scholar]
- 26.DerSimonian R., Laird N. Meta-analysis in clinical trials. Contr Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. Epub 1986/09/01. PubMed PMID: 3802833. [DOI] [PubMed] [Google Scholar]
- 27.Mantel N., Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959;22(4):719–748. Epub 1959/04/01. PubMed PMID: 13655060. [PubMed] [Google Scholar]
- 28.Sterne J.A., Sutton A.J., Ioannidis J.P., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. Epub 2011/07/26. PubMed PMID: 21784880. [DOI] [PubMed] [Google Scholar]
- 29.Brunelli A., Thomas C., Dinesh P., Lumb A. Enhanced recovery pathway versus standard care in patients undergoing video-assisted thoracoscopic lobectomy. J Thorac Cardiovasc Surg. 2017;154(6):2084–2090. doi: 10.1016/j.jtcvs.2017.06.037. Epub 2017/07/22. PubMed PMID: 28728783. [DOI] [PubMed] [Google Scholar]
- 30.Madani A., Fiore J.F., Jr., Wang Y., et al. An enhanced recovery pathway reduces duration of stay and complications after open pulmonary lobectomy. Surgery. 2015;158(4):899–908. doi: 10.1016/j.surg.2015.04.046. discussion -10. Epub 2015/07/21. PubMed PMID: 26189953. [DOI] [PubMed] [Google Scholar]
- 31.Cai Q. Multidisciplinary enhanced recovery after surgery in video-assisted thoracoscopic surgery for lung cancer. Journal of Nursing Science. 2018;33(19):26–28. doi: 10.3870/j.issn.1001-4152.2018.19.02. [DOI] [Google Scholar]
- 32.Che Q., Wang H., Li Y., Xia H. Application of rapid rehabilitation in perioperative nursing of early non- small cell lung cancer. Chin J Clin Oncol R ehabil. 2018;25:320–323. doi: 10.13455/j.cnki.cjcor.2018.03.17. 03. [DOI] [Google Scholar]
- 33.Fan X., Jiao J., Zhu W., Yin J. Application of enhanced recovery after surgery in perioperative nursing of patients undergoing radical resection of lung cancer. Chin Nurs Res. 2019;33(15):2724–2726. doi: 10.12102/j.issn.1009-6493.2019.15.043. [DOI] [Google Scholar]
- 34.Forster C., Doucet V., Perentes J.Y., et al. Impact of an enhanced recovery after surgery pathway on thoracoscopic lobectomy outcomes in non-small cell lung cancer patients: a propensity score-matched study. Transl Lung Cancer Res. 2021;10(1):93–103. doi: 10.21037/tlcr-20-891. Epub 2021/02/12. PubMed PMID: 33569296; PubMed Central PMCID: PMCPMC7867780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haro G.J., Sheu B., Marcus S.G., et al. Perioperative lung resection outcomes after implementation of a multidisciplinary, evidence-based thoracic ERAS program. Ann Surg. 2019;274(6):e1008–e1013. doi: 10.1097/SLA.0000000000003719. Epub 2019/12/19. PubMed PMID: 31851005. [DOI] [PubMed] [Google Scholar]
- 36.Huang H., Ma H., Chen S. Enhanced recovery after surgery using uniportal video-assisted thoracic surgery for lung cancer: a preliminary study. Thorac Cancer. 2018;9(1):83–87. doi: 10.1111/1759-7714.12541. Epub 2017/11/01. PubMed PMID: 29087621; PubMed Central PMCID: PMCPMC5754309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Li Y., Yi H., Xiao Y., Chen X., Guo L. Fast rehabilitation surgery in application of radical operation for lung cancer under thoracoscope. Clinical Misdiagnosis & Mistherapy. 2017;30:86–89. doi: 10.3969/j.issn.1002-3429.2017.02.026. 02. [DOI] [Google Scholar]
- 38.Li L., Zhao R., Wang H., Zheng X., Zhao R. Interventional effect of fast track suegery concept on lung cancer patients during perioperative period. Chin J Nosocomio. 2018;28(22):3438–3441. doi: 10.11816/cn.ni.2018-173589. [DOI] [Google Scholar]
- 39.Li J. The effect of rapid rehabilitation surgical nursing on postoperative lung function recovery and quality of life in patients undergoing thoracoscopic radical resection of lung cancer. Chinese Remedies & Clinics,December. 2020;20(24):4191–4193. [Google Scholar]
- 40.Salati M., Brunelli A., Xiume F., Refai M., Pompili C., Sabbatini A. Does fast-tracking increase the readmission rate after pulmonary resection? A case-matched study. Eur J Cardio Thorac Surg. 2012;41(5):1083–1087. doi: 10.1093/ejcts/ezr171. PubMed PMID: WOS:000303161800035. [DOI] [PubMed] [Google Scholar]
- 41.Van Haren R.M., Mehran R.J., Mena G.E., et al. Enhanced recovery decreases pulmonary and cardiac complications after thoracotomy for lung cancer. Ann Thorac Surg. 2018;106(1):272–279. doi: 10.1016/j.athoracsur.2018.01.088. Epub 2018/03/14. PubMed PMID: 29530770. [DOI] [PubMed] [Google Scholar]
- 42.Shiono S., Endo M., Suzuki K., Hayasaka K. Impact of enhanced recovery after surgery on outcomes of elderly patients undergoing open thoracic surgery. Gen Thorac Cardiovas. 2019;67(10):867–875. doi: 10.1007/s11748-019-01099-2. PubMed PMID: WOS:000487282100007. [DOI] [PubMed] [Google Scholar]
- 43.Tahiri M., Goudie E., Jouquan A., Martin J., Ferraro P., Liberman M. Enhanced recovery after video-assisted thoracoscopic surgery lobectomy: a prospective, historically controlled, propensity-matched clinical study. Can J Surg. 2020;63(3):E233–E240. doi: 10.1503/cjs.001919. Epub 2020/05/11. PubMed PMID: 32386474; PubMed Central PMCID: PMCPMC7829001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J., Ni B., Ma H. The application of fast- track surgery in thoracoscopic lung cancer operation for the aged patients. Journal of Laparoscopic Surgery. 2015;20:581–585. doi: 10.13499/jcnki.fqjwkzz.2015.08.581. 08. [DOI] [Google Scholar]
- 45.Wang T. Clinical study of perioperative and serum indexex variation and prognostic relevance in the application of FTS in treatment of stage Ⅰ~Ⅱ non-small cell lung cancer. The Practical Journal of Cancer. 2019;34(3):411–414. doi: 10.3969/j.issn.1001-5930.2019.03.017. [DOI] [Google Scholar]
- 46.Wang C., Lai Y., Li P., Su J., Che G. Influence of enhanced recovery after surgery (ERAS) on patients receiving lung resection: a retrospective study of 1749 cases. BMC Surg. 2021;21(1):115. doi: 10.1186/s12893-020-00960-z. Epub 2021/03/08. PubMed PMID: 33676488; PubMed Central PMCID: PMCPMC7936477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Xu F. Dalian Medical University; 2020. Application of Rapid Rehabilitation (ERAS) in Thoracoscopy for Radical Lung Cancer Surgery. [Google Scholar]
- 48.Zhang X., Xie D., Chen C., Lin J. Investigation on the application of the concept of rapid rehabilitation surgery in the perioperative period of thoracoscopic lung cancer radical surgery. China Modern Doctor. 2017;55(36):105–108. [Google Scholar]
- 49.Zhang Z. Application of the concept of enhanced recovery after surgery in thoracoscopic radical resection of lung cancer. Chin J Rehabil Med. 2019;34(8):950–953. [Google Scholar]
- 50.Zhao G., Huang Y., Chen X., et al. Research on fast track surgery application in lung cancer surgery. Zhongguo Fei Ai Za Zhi. 2010;13(2):102–106. doi: 10.3779/j.issn.1009-3419.2010.02.04. Epub 2010/08/03. PubMed PMID: 20673500; PubMed Central PMCID: PMCPMC6000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zheng C. Effect of enhanced recovery after surgery on postoperative rehabilitation and quality of life of elderly patients undergoing thoracoscopic radical resection of lung cancer. Zhejiang JITCWM. 2019;29(8):639–641. [Google Scholar]
- 52.Guyatt G.H., Oxman A.D., Sultan S., et al. GRADE guidelines: 9. Rating up the quality of evidence. J Clin Epidemiol. 2011;64(12):1311–1316. doi: 10.1016/j.jclinepi.2011.06.004. Epub 2011/08/02. PubMed PMID: 21802902. [DOI] [PubMed] [Google Scholar]
- 53.Batchelor T.J.P., Rasburn N.J., Abdelnour-Berchtold E., et al. Guidelines for enhanced recovery after lung surgery: recommendations of the enhanced recovery after surgery (ERAS(R)) society and the European society of thoracic surgeons (ESTS) Eur J Cardio Thorac Surg. 2019;55(1):91–115. doi: 10.1093/ejcts/ezy301. Epub 2018/10/12. PubMed PMID: 30304509. [DOI] [PubMed] [Google Scholar]
- 54.Batchelor T.J.P. Implementing enhanced recovery after thoracic surgery-no easy task. Eur J Cardio Thorac Surg. 2022 doi: 10.1093/ejcts/ezac011. Epub 2022/01/14. PubMed PMID: 35025984. [DOI] [PubMed] [Google Scholar]
- 55.Group E.C. The impact of enhanced recovery protocol compliance on elective colorectal cancer resection: results from an international registry. Ann Surg. 2015;261(6):1153–1159. doi: 10.1097/SLA.0000000000001029. Epub 2015/02/12. PubMed PMID: 25671587. [DOI] [PubMed] [Google Scholar]
- 56.Schatz C. Enhanced recovery in a minimally invasive thoracic surgery program. AORN J. 2015;102(5):482–492. doi: 10.1016/j.aorn.2015.09.006. Epub 2015/10/31. PubMed PMID: 26514705. [DOI] [PubMed] [Google Scholar]
- 57.Rogers L.J., Bleetman D., Messenger D.E., et al. The impact of enhanced recovery after surgery (ERAS) protocol compliance on morbidity from resection for primary lung cancer. J Thorac Cardiovasc Surg. 2018;155(4):1843–1852. doi: 10.1016/j.jtcvs.2017.10.151. Epub 2018/01/21. PubMed PMID: 29352586. [DOI] [PubMed] [Google Scholar]
- 58.Sebio Garcia R., Yanez Brage M.I., Gimenez Moolhuyzen E., Granger C.L., Denehy L. Functional and postoperative outcomes after preoperative exercise training in patients with lung cancer: a systematic review and meta-analysis. Interact Cardiovasc Thorac Surg. 2016;23(3):486–497. doi: 10.1093/icvts/ivw152. Epub 2016/05/27. PubMed PMID: 27226400. [DOI] [PubMed] [Google Scholar]
- 59.Kadosawa T., Watabe A. The effects of surgery-induced immunosuppression and angiogenesis on tumour growth. Vet J. 2015;205(2):175–179. doi: 10.1016/j.tvjl.2015.04.009. Epub 2015/05/10. PubMed PMID: 25956342. [DOI] [PubMed] [Google Scholar]
- 60.Grant M.C., Yang D., Wu C.L., Makary M.A., Wick E.C. Impact of enhanced recovery after surgery and fast track surgery pathways on healthcare-associated infections: results from a systematic review and meta-analysis. Ann Surg. 2017;265(1):68–79. doi: 10.1097/SLA.0000000000001703. Epub 2016/12/24. PubMed PMID: 28009729. [DOI] [PubMed] [Google Scholar]
- 61.Ueda K., Hayashi M., Murakami J., Tanaka T., Utada K., Hamano K. Intercostal block vs. epidural analgesia in thoracoscopic lung cancer surgery: a randomized trial. Gen Thorac Cardiovasc Surg. 2020;68(3):254–260. doi: 10.1007/s11748-019-01197-1. Epub 2019/09/02. PubMed PMID: 31473913. [DOI] [PubMed] [Google Scholar]
- 62.Huang X., Cui Y., Xiao Y., Zhao X., Xu J., Yang L. Combined programmed intermittent bolus infusion with continuous infusion for the thoracic paravertebral block in patients undergoing thoracoscopic surgery: a prospective, randomized, and double-blinded study. Clin J Pain. 2022 doi: 10.1097/AJP.0000000000001037. Epub 2022/04/21. PubMed PMID: 35442613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Grant M.C., Sommer P.M., He C., et al. Preserved analgesia with reduction in opioids through the use of an acute pain protocol in enhanced recovery after surgery for open hepatectomy. Region Anesth Pain M. 2017;42(4):451–457. doi: 10.1097/Aap.0000000000000615. PubMed PMID: WOS:000404062300006. [DOI] [PubMed] [Google Scholar]
- 64.Alazawi W., Pirmadjid N., Lahiri R., Bhattacharya S. Inflammatory and immune responses to surgery and their clinical impact. Ann Surg. 2016;264(1):73–80. doi: 10.1097/SLA.0000000000001691. Epub 2016/06/09. PubMed PMID: 27275778. [DOI] [PubMed] [Google Scholar]
- 65.Ha D.M., Prochazka A.V., Bekelman D.B., Stevens-Lapsley J.E., Studts J.L., Keith R.L. Modifiable factors associated with health-related quality of life among lung cancer survivors following curative intent therapy. Lung Cancer. 2021;163:42–50. doi: 10.1016/j.lungcan.2021.11.012. Epub 2021/12/14. PubMed PMID: 34896804. [DOI] [PubMed] [Google Scholar]
- 66.Zwetsloot P.P., Van Der Naald M., Sena E.S., et al. Standardized mean differences cause funnel plot distortion in publication bias assessments. Elife. 2017;6 doi: 10.7554/eLife.24260. Epub 2017/09/09. PubMed PMID: 28884685; PubMed Central PMCID: PMCPMC5621838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fiore J.F., Jr., Faragher I.G., Bialocerkowski A., Browning L., Denehy L. Time to readiness for discharge is a valid and reliable measure of short-term recovery after colorectal surgery. World J Surg. 2013;37(12):2927–2934. doi: 10.1007/s00268-013-2208-1. Epub 2013/10/09. PubMed PMID: 24101012. [DOI] [PubMed] [Google Scholar]
- 68.Leeds I.L., Sadiraj V., Cox J.C., Schnier K.E., Sweeney J.F. Assessing clinical discharge data preferences among practicing surgeons. J Surg Res. 2013;184(1):42–48. doi: 10.1016/j.jss.2013.03.064. e3. Epub 2013/05/28. PubMed PMID: 23706559; PubMed Central PMCID: PMCPMC3758431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Seow-En I., Wu J., Yang L.W.Y., et al. Results of a colorectal enhanced recovery after surgery (ERAS) programme and a qualitative analysis of healthcare workers' perspectives. Asian J Surg. 2021;44(1):307–312. doi: 10.1016/j.asjsur.2020.07.020. Epub 2020/08/31. PubMed PMID: 32863145. [DOI] [PubMed] [Google Scholar]
- 70.Rattray M., Roberts S., Desbrow B., et al. A qualitative exploration of factors influencing medical staffs' decision-making around nutrition prescription after colorectal surgery. BMC Health Serv Res. 2019;19(1):178. doi: 10.1186/s12913-019-4011-7. Epub 2019/03/21. PubMed PMID: 30890125; PubMed Central PMCID: PMCPMC6425714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Xu F., Yu P., Li L. Rapid rehabilitation nursing in postoperative patients with colorectal cancer and quality of life. Oncol Lett. 2019;18(1):651–658. doi: 10.3892/ol.2019.10379. Epub 2019/07/11. PubMed PMID: 31289538; PubMed Central PMCID: PMCPMC6546976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zheng Y., Mao M., Ji M., et al. Does a pulmonary rehabilitation based ERAS program (PREP) affect pulmonary complication incidence, pulmonary function and quality of life after lung cancer surgery? Study protocol for a multicenter randomized controlled trial. BMC Pulm Med. 2020;20(1):44. doi: 10.1186/s12890-020-1073-6. Epub 2020/02/20. PubMed PMID: 32070326; PubMed Central PMCID: PMCPMC7029521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Chau J.P.C., Liu X., Lo S.H.S., et al. Perioperative enhanced recovery programmes for women with gynaecological cancers. Cochrane Database Syst Rev. 2022;3:CD008239. doi: 10.1002/14651858.CD008239. Epub 2022/03/16. pub5. PubMed PMID: 35289396; PubMed Central PMCID: PMCPMC8922407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schneider S., Armbrust R., Spies C., du Bois A., Sehouli J. Prehabilitation programs and ERAS protocols in gynecological oncology: a comprehensive review. Arch Gynecol Obstet. 2020;301(2):315–326. doi: 10.1007/s00404-019-05321-7. Epub 2019/10/17. PubMed PMID: 31616986. [DOI] [PubMed] [Google Scholar]
- 75.Wick E.C., Hobson D.B., Bennett J.L., et al. Implementation of a surgical comprehensive unit-based safety program to reduce surgical site infections. J Am Coll Surg. 2012;215(2):193–200. doi: 10.1016/j.jamcollsurg.2012.03.017. Epub 2012/05/29. PubMed PMID: 22632912. [DOI] [PubMed] [Google Scholar]