Abstract
Rubia cordifolia (family: Rubiaceae) L (R. cordifolia) is a perennial botanical drug climbing vine. As the main part of the traditional Chinese medicine, the rhizome has a long history. A great number of literary studies have reported that it can be used for the improvement of blood circulation, hemostasis, activation of collaterals, etc. When it comes to the wide application of R. cordifolia in traditional medicine, we systematically review its traditional uses, phytochemistry and pharmacological effects. Literatures were systematically searched using several scientific databases, including China National Knowledge Infrastructure (CNKI), Baidu Scholar, PubMed, Web of Science, and other professional websites. Kew Botanical Garden and the iPlant were used for obtaining the scientific names and plant images of R. cordifolia. In addition, other information was also gathered from books including traditional Chinese herbal medicine, the Chinese Pharmacopoeia, and Chinese Materia Medica. So far, many prescriptions containing R. cordifolia have been widely used in the clinical treatment of abnormal uterine bleeding, primary dysmenorrhea and other gynecological diseases, allergic purpura, renal hemorrhage and other diseases. The phytochemistry studies have reported that more than 100 compounds are found in R. cordifolia, such as bicyclic peptides, terpenes, polysaccharides, trace elements, flavonoids, and quinones. Among them, quinones and peptides are the types of components with the highest contents in R. cordifolia. The modern pharmacological studies have revealed that R. cordifolia and its derived components have anti-tumor, anti-oxidative, anti-platelet aggregation, and anti-inflammatory effects. However, most studies are preclinical. The pharmacological mechanism of R. cordifolia has not been thoroughly studied. In addition, there are few pharmacokinetic and toxicity studies of R. cordifolia, therefore the clinical safety data for R. cordifolia is lacking. To sum up, this review for the first time summarizes a systemic and integrated traditional uses, chemical compositions, pharmacological actions and clinical applications of R. cordifolia, which provides the novel and full-scale insight for the drug development, medicinal value, and application of R. cordifolia in the future.
Keywords: Rubia cordifolia L., traditional uses, pharmacological activities, phytochemistry, clinical application
Introduction
Plants are invaluable reservoir for the discovery of new drugs, which have been used for medicinal purposes across history and cultures. As the World Health Organization (WHO) stated, approximately 80% of people all over the world relies heavily on botanical medicine for their primary health care (Ekor, 2014). Traditional Chinese medicine (TCM) are a treasure trove of drugs, which have been widely spread and applied in more than 100 countries for the treatment of diverse diseases (Wang et al., 2016). With the Coronavirus disease (COVID-19) pandemic quickly spreads across the whole world, TCM have become a key component in the treatment of COVID-19 and play an irreplaceable role in the treatment of SARS-CoV-2 infection (Wang and Yang, 2021). TCM have the unique advantage of whole course and all-round treatment that could improve symptoms, early prevention, early treatment and reduce mortality, and reduce the occurrence and recurrence of complications, which have broad application prospect (Dai et al., 2020).
The Rubiaceae family consists of about 450 genera and 6,500 species, including trees, shrubs and herbs. Rubia has about 60 species, of which R. cordifolia L. (Rubiaceae) is a perennial herbaceous climbing plant with long, cylindrical and red roots (Adwankar et al., 1980; Dengre et al., 1993). R. cordifolia is widely distributed in Africa, tropical Asia, India, Malaysia, China, Japan, and tropical Australia (Adwankar et al., 1980). In China, the root of R. cordifolia is known as Qiancaogen and Chien-tsao, which is widely distributed in most areas of China, especially shaanxi, Henan, Anhui, Hebei, Shandong, Jiangsu and Zhejiang provinces (Dengre et al., 1993). In Chinese medicine, R. cordifolia is bitter with cold property, which is able to eliminate pathogenic heat from blood, remove blood stasis and dredging collaterals, and promote hemostasis. It is mainly used as a hemostatic agent in folk medicine to treat hematemesis, metrorrhagia, epistaxis, wounds, injuries and strains (Xu et al., 2002; Li et al., 2016). In addition, the aerial part of the R. cordifolia plant, known locally as “Guoshan dragon” in Shaanxi province in China, has been used to treat diarrhea more than a century. The plant water decoction can be taken orally to treat diarrhea, which can also be used externally for foot bath when children are too young to take it. R. cordifolia is also a main ingredient of formula named “Er-Xie-Ting granule”, which is used to treat acute infantile diarrhea in China (Shi et al., 2014). In India, R. cordifolia is often known as Madder or India Madder. Locals in India call it “Manjistha”. Its dried samples are sold in the market under the name “Manjith”. R. cordifolia is very common at high altitudes, Mahabaleswar, Amboli and Maharastra state in India (Adwankar et al., 1980; Siril, 2013). This drug is used to treat Shi Feng Bi (rheumatism), menstrual pain, urinary system diseases, dropsy, paralysis, amenorrhea and jaundice in India (Tripathi et al., 1993; Adams et al., 2009). In Ayurveda, R. cordifolia has been used as a coloring agent for medicinal oils, and applied externally to inflamed areas, ulcers and fractures. R. cordifolia root has been treated various chronic inflammations (Deshkar et al., 2008; Patil et al., 2009). A paste made from honey is applied to the skin to remove brown spots, freckles and other skin discoloration and is used to promote wound healing (Adwankar et al., 1980; Pawar et al., 2009). In Korea, R. cordifolia is widely utilized as a traditional remedy for dysmenorrhea, arthritis, rheumatism, hematorrhea and urinary disorders (Do et al., 2013). In Unani medicine, the dark red root of R. cordifolia is used to invigorate spleen and soothing liver, dysmenorrhea, diuresis, paralysis, jaundice, amenorrhea, skin disorders of many varieties, renal stone and blood detoxification. In Uganda medicine, traditional healers use the drug to treat tuberculosis cases. In the Philippines medicine, root decoction of R. cordifolia is used to treat urinary tract disorders (Patil et al., 2009). In traditional Asian medicine, the roots of R. cordifolia have become an important drug for the treatment of abnormal uterine bleeding (AUB), purpura lupus erythematosus, hemorrhage syndrome, arthritis, kidney stones, hemostasis, hysteresis, and psoriasis (Chang et al., 2000; Basu et al., 2005; Tse et al., 2007; Son et al., 2008; Wang K. et al., 2020). In ancient Europe, R. cordifolia is one of the most commonly used dyes, which is used for dyeing wool, silk, linen, cotton fabrics, as well as basket-making material. The roots of the plant are also one of important ingredients in recipes of red inks. Alizarin is the main component of dyes (Wu and Yang, 2016). On the whole, R. cordifolia is the first batch of plants known to have commercial and medicinal value in the world (Feng et al., 2021). The drug is highly valued for its pharmacological, cardioprotective and industrial approach. Except for its high medicinal value, the drug is also an important source of natural dye used by many flavours and pharmaceutical industries (Pankaj and A., 2013bib_Pankaj_and_Ajay_2013bib_Pankaj_and_Ajay_2013bib_Pankaj_and_Ajay_2013). R. tinctorum L. another member of Rubiaceae family, is also a well-known traditional medicinal plant, which is native to Europe, west of Asia and Africa. Like R. cordifolia, R. tinctorum L. has been used as a dye for over 2,000 years (Kalyoncu et al., 2006). In Morocco, R. tinctorum L. has been usded to treat renal disease, cardiac disease, hypertension and diarrhoea (Jouad et al., 2001; Eddouks et al., 2002; Karim et al., 2010). R. tinctorum L. is recorded in the 14th edition of the modern Russian Pharmacopoeia with a diuretic effect (Shikov et al., 2021). Due to the genotoxic activity and oncogenic potential of R. tinctorum L. Commission of the European Communities has considered R. tinctorum L. as a plant with serious risks (Cpmp, 2004).
Studies have found that R. cordifolia is rich in more than 100 compounds, mainly including anthraquinones, naphthoquinones, anthraquinone glycosides, naphthoquinone glycosides, bicyclic hexapeptides, triterpenoids and polysaccharides (Son et al., 2008; Itokawa et al., 1993; Rao et al., 2006; Gao et al., 2016; Natarajan et al., 2019). R. cordifolia has multiple pharmacological activities, such as neuroprotective, anti-tumor, antibacterial, anti-inflammatory, anti-oxidant, and immunosuppressive effects (Lee et al., 2008a; Son et al., 2008; Shilpa et al., 2012b; Shen et al., 2018; Balachandran et al., 2021).
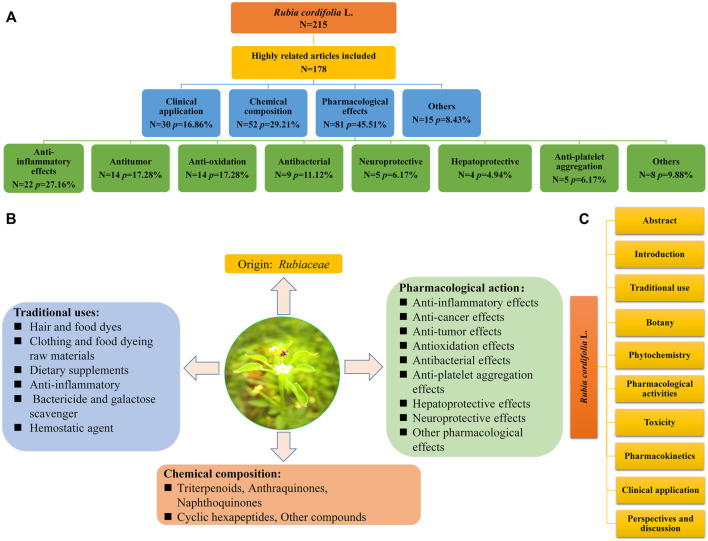
In present review, the keyword Rubia cordifolia L. was used to search the literatures from Google scholar, Web of Science, and PubMed. The latest publication time of these literatures were up to May 2022. We had analyzed these articles and made a classification summary. By document retrieval, we summarized the total number and proportion of articles on R. cordifolia, such as the total number and proportion of clinical application, pharmacological action, or active ingredients (Figure 1A). Meanwhile, we also introduced the origin, traditional application, chemical compounds and pharmacological effects of R. cordifolia (Figure 1B). Then the overall framework of this article was summarized (Figure 1C). Finally, we hope this review can provide an integrated insight for the drug development, medicinal value, and application of R. cordifolia in the future.
FIGURE 1.
Reports on R. cordifolia were collected from PubMed database. Systematic classification of retrieved literatures R. cordifolia (A). Origin, traditional uses, pharmacological effects, and chemical compositions of R. cordifolia (B). Classification and analyzation of the content of the articles (C).
Traditional use
In view of the widespread distribution and application of R. cordifolia in the world, there are differences in application in different countries and regions. According to historical records, R. cordifolia was used as an important natural dye for hair, food and clothing (Ni et al., 2022; Yusuf et al., 2013). It was widely used in ancient China and India with a long history. As early as 2000 Before Christ (B.C.), it had been used as a folk medicine to treat wounds, ulcers, skin diseases, rheumatic, and inflammatory-related diseases. As an important traditional medicine, R. cordifolia was firstly recorded in the Huangdi Neijing (Li et al., 2018), also recorded in some Chinese classical prescriptions, such as Bencaijing Jizhu, 52 Neijing, Shu Bencao, History of Chinese Traditional Medicine, and so on. Nowadays, R. cordifolia is officially listed in the Chinese Pharmacopoeia.
The theory of TCM is the understanding of the general laws of drugs, involving four odors and five flavors, ascending and descending, channel tropism, toxicity and side effects (Zhong et al., 2019). In the field of TCM, the durg R. cordifolia is benefit for cooling blood, hemostasis, removing blood stasis, and relieving pain, with bitter taste, cold property and distribution to liver meridian. This traditional efficacy of R. cordifolia can be translated into modern pharmacological effects such as anti-inflammatory, anti-coagulant and anti-platelet activities as well as spasmolysis (Tripathi et al., 1993; Pawar et al., 2011). R. cordifolia with different processing methods has different curative effects (Li et al., 2018). For example, the raw R. cordifolia product has the effect of promoting blood circulation, removing blood stasis, clearing heat and cooling blood (Chen, 2013). However, after frying with charcoal, the coldness of R. cordifolia will be weakened, property and taste will be astringent, and it is mainly applied to stop bleeding (Gao et al., 2020). Based on the theory of TCM, R. cordifolia belongs to the liver meridian and has anti-liver cancer effect (Li et al., 2016). Furthermore, R. cordifolia is traditionally used as an a ntioxidation, bactericide, and galactose scavenger, and is also widely used in the pharmaceutical industry (Shilpa et al., 2012a). In ancient Chinese classic prescriptions and some minority medical books, R. cordifolia can be combined with other Chinese medicines to treat a variety of symptoms. For instance, combination Cirsium arvense var, Gardenia jasminoides Ellis with R. cordifolia can enhance the hemostatic effect (Wang et al., 2021). Moreover, joint prescription with Sanguisorba officinalis L. is able to treat the symptoms of intestinal dryness and bleeding. When combined with Salvia miltiorrhiza Bunge and Angelica sinensis (Oliv.) Diels, it can effectively improve the symptoms of dysmenorrhea (Li et al., 2009), so as to achieve the effect of multi-drug compatibility in treating various diseases ( Table 1 ).
TABLE 1.
Clinical application of R. cordifolia in traditional prescriptions.
| Classic prescriptions | Composition of medicines | Symptoms of treatment | Type and number of participants | References |
|---|---|---|---|---|
| Puji Benshifang | R. cordifolia, Artemisia argyi Levl.et Vant, Prunus mume (Sieb.) Sieb.et Zuce | Treatment of nosebleeds | 50 patients with nosebleeds. (age range, 14–76 years old) | (Li, 2000; Meng, 2010) |
| Qixiongwan | R. cordifolia, Aconitum kusnezoffii Reichb, Moschus, Terminalia chebula Retz, Ruta graveolens L, Mercury (II) Sulfide, O. chiliophyllaRoyle | Treatment of Mycoplasma pneumoniae pneumonia | 21 patients with positive serum mycoplasma antibody test. (age range, 3–39 years old) | Du, (2002) |
| Shengjie zonglu | R. cordifolia, Glycyrrhiza uralensis Fisch | Treatment of vomiting blood, antipyretic and thirst, detoxification | 60 patients (36 female and 24 male) with fever. (age range, 15–60 years old) | (Gupta et al., 2008; Li and Meng, 2018; Wang et al., 2022) |
| Sanhongtang | R. cordifolia, Eriobotrya japonica Thunb, Laccifer lacca Kerr | Treatment of kidney damage, lung heat, cough, blood in sputum, bladder heat, dysuria and frequent urination | Not available | Larga et al. (2021) |
| Sanwei Chafenliaotang | R. cordifolia, Ophiopogon japonicus (Linn. f.) Ker-Gawl, Polygonum divaricatum L | Treatment of fever in the lungs and tingling in the intestines | Not available | Liu et al. (2021) |
| Sibu Yidian | R. cordifolia, Terminalia chebula Retz. Laccifer lacca Kerr. Symplocos caudata Wall | Treatment of kidney and enteric fever | Not available | Niang et al. (2021) |
| Shisanwei Ximingwan | R. cordifolia, Patrinia scabiosaefolia, Erminalia chebula Retz, Laccifer lacca Kerr, Caesalpinia decapetala (Roth) Alston, Mangifera indica L, Syzygium jambos (L.) Alston, Shanfanshi, Cupressus funebris Endl, Amomum cardamon, Herpetospermum pedunculosum (Ser.) C. B, veronica eriogyne H. Wink | Treatment of chronic prostatitis | 93 patients with chronic prostatitis. (age range, 30–60 years old) | (Zhou, 2017; Liu et al., 2020) |
| Tangyao Jingyanfang | R. cordifolia, Asini Corii Colla, Platycladus orientalis (L.) Franco, Platycladus orientalis (L.) Franco | Pentecostal acts for healing women | 181 patients with blood deficiency. (age range, 18–60 years old) | (Li and Meng, 2018; Zhang et al., 2021) |
| Taiping Shenghuifang | R. cordifolia, Punica granatum L | Treatment of prolapse of the anus and menorrhagia | 48 female patients. (age range, 15–50 years old) | (Shen, 2013; Bryant-Smith et al., 2018) |
| Yiashen Shiqiweiwan | R. cordifolia, Terminalia chebula Retz, Moschus, Aconitum kusnezoffii Reichb, Acorus tatarinowii, Aucklandia lappa Decne, Haliotis discus hannai, Mercury (II) Sulfide, Pulvis billis bovis, Ruta graveolens L. Canavalia gladiata (Jacq.) DC, Carthamus tinctorius L, Eriobotrya japonica Thunb, Xiangmo, Amomum kravanh Pierre ex Gagnep, Laccifer lacca Kerr. Dashua Jihua | Treatment of chronic epididymal and spermatic hydrocele | 324 patients with chronic epididymis. (age range, 7–89 years old) | (Wu, 2008a; Wu, 2008b; Wang, 2013) |
| 32 patients with hydrocele of the spermatic cord. (age range, 3–12 years old) |
Not available: The combination of R. cordifolia and other drugs for the treatment of some diseases has been recorded in traditional Chinese prescriptions, but these clinical data have not been reported in any literatures.
Botany
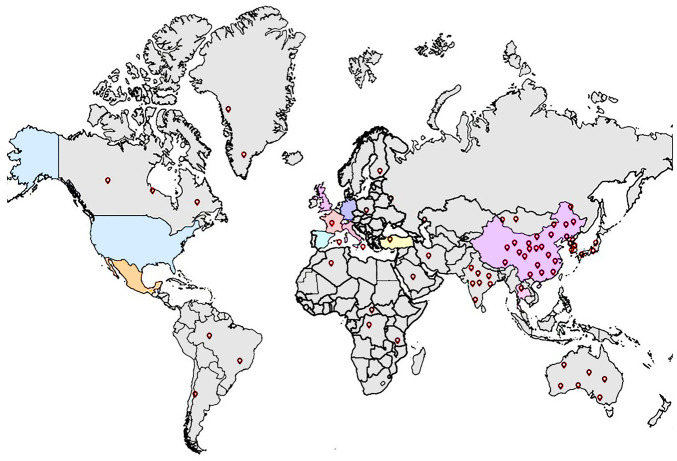
R. cordifolia is distributed all over the world and is extremely widespread in many provinces of China, such as Shanxi, Henan, Anhui, Hebei, Shandong, Hubei, Jiangsu, Zhejiang, etc. (Figure 2). The global distribution data of this plant comes from the websites: iplant (http://ppbc.iplant.cn/) and Kew Botanical Garden (https://powo.science.kew.org/). Among them, Weinan city in Shanxi and Songxian city in Henan have the highest yields and the best quality. R. cordifolia is a perennial herbaceous climbing vine, scrambling, climbing or creeping plant. The root is brown or red. The stem is slender and rough, and the base is lignified. Its branching stems are 0.3–6 m long with brittle stems, strong curved prickles on the four ribs, or fully pubescent, or at least hairy below the nodes. Its roots are usually woody.
FIGURE 2.
Geographical distribution of Rubia cordifolia L (world map from: https://www.onlinedown.net/soft/197029.htm) in the world (The red surface symbol map shows the distribution area of R. cordifolia).
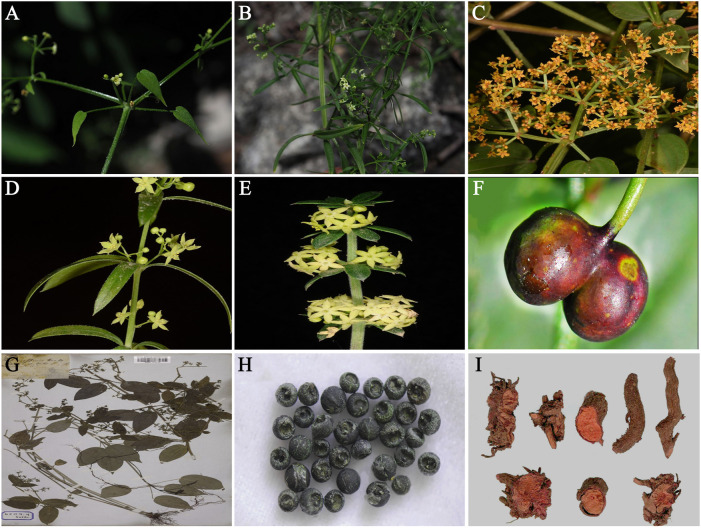
The leaves of R. cordifolia are ovate or ovate-lanceolate, 2–6 cm long and 1–3 cm wide. Leaves in whorls of 4–8 or sometimes paired and blades lanceolate to broadly ovate, 0.7–8.5 cm long, 0.2–4.2 cm wide, acuminate at the apex, and rounded to cordate at the base, margins often with curved prickles, with recurved prickles and often pubescent as well. The plant morphology of R. cordifolia was shown in Figure 3. The flowers of R. cordifolia are characterized by cymes, located in the axilla or at the top, and are usually large pine cones in shape. Flowers glabrous, inflorescences usually numerous, scattered along stem, very loose to fairly dense, 0.5–2.5 cm long, pedicel 1–2.5 cm long, pedicel 0.2–6 mm long, bracts elliptic, 1.2–1.5 mm long, wide 0.3–0.4 mm. The calyx tube is 0.5–0.8 mm long and 0.8–1.4 mm wide. Corolla yellowish, green, with green cream or chartreuse, pink, or with purplish tips in buds, 4–6 mm wide, tube 0.2–0.8 mm long, lobes usually triangular, 1.5–3 mm long, wide 0.6–1.3 mm, apiculate, margin minutely papillary. Fruit glabrous, brownish black, lobes globose, 2.5–5 mm in diameter, pyrenes globose, 3 mm in diameter (Xun, 2014). The harvest season is generally in the third or fourth year of spring or autumn.
FIGURE 3.
Botanical diagram of R. cordifolia (The images (A,B,C,D,G) are obtained from Kew Botanical Garden (http://powo.science.kew.org/):and (F,H,I) the iPlant (ppbc.iplant.cn). The whole plant of R. cordifolia (A,B,C); the flowers of R. cordifolia (D,E) the fruits of R. cordifolia (F); The specimen of R. cordifolia (G); dry fruit of R. cordifolia (H); the dried root of R. cordifolia (I).
Phytochemistry
Due to the extensive use of R. cordifolia in TCM, the chemical components and pharmacological effects of R. cordifolia have largely attracted the attention of scholars inside and outside the country. At present, hundreds of components have been isolated and identified from R. cordifolia. The reported chemical compounds of R. cordifolia were shown in Supplementary Table S1.
Anthraquinones
Anthraquinones are a class of representative compounds in R. cordifolia, including alizarin, munjistin, rubiadin, purpurin, techoquinone and xanthopurpurin (King, 1992). At present, 28 anthraquinones were isolated from R. cordifolia. Dosseh et al. isolated four new anthraquinones from R. cordifolia root, which were 1-hydroxy 2-methoxy anthraquinone, 1,4-dihydroxy 2-methyl 5-methoxy anthraquinone or 1,4-dihydroxy 2-methyl 8-methoxy anthraquinone, 1,3-dimethoxy 2-carboxy anthraquinone and rubiadin (Dosseh et al., 1981a). Wang et al. used ethanol to extract R. cordifolia root and isolated seven anthraquinone compounds (2-methyl-1,3,6-trihydroxy-9,10-anthraquinone, 1-hydroxy-9,10-anthraquinone, 1,2,4-trihydroxy-9,10-anthrequinone, 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone-3-O-β-D-glucoside, 1,2-dijhydroxy-9,10-anthraquinone-2-O-β-D-xylosyl (1→6)-β-D-glucoside and 1,3-dihydroxy-2-hydroxymethyl1-9,10-anthraquinone-3-O-β-D-xylosyl (1→6)-β-D-glucoside) (Wang et al., 1992). Above seventh compounds are a new class of compounds discovered. Their structures were elucidated to be 2-methyl-1,3,6-trihydroxy-9,10-anthraquinone-3-O-β-D-xylosyl (1→2)-β-D-(6′-O-acetyl) glucoside (Wang et al., 1992).
Bicyclic hexapeptides
Bicycle hexapeptides are commonly considered as the most important bioactive compounds in R. cordifolia, which are extremely abundant. So far, 19 bicyclic peptides were found in R. cordifolia. Lots of rubiaakane series peptides (RAs) compounds with anti-tumor bicyclic hexapeptides were isolated from R. cordifolia, such as RA-XVIII, RA-XII (Lee et al., 2008a). Up to now, numerous cyclic hexapeptides have been discovered and isolated from R. cordifolia. Each RA compound contains an 18-membered ring and a 14-membered ring system, which includes amino acids such as n-methyl-O-methyl-l-tyrosine, pyroglutamic acid, l-alanine and d-alanine. Among them, RA-V and RA-VII are the most abundant ingredients with 100 μg/g in R. cordifolia, while the rest are less than 1 μg/g (Hitotsuyanagi et al., 2004). In addition, some RAs analogues or precursors have been isolated from R. cordifolia in recent years, such as neo-RA-V, allo-RA-V, O-seco-RA-V and O-seco-RA-XXIV (Hitotsuyanagi et al., 2012).
Naphthoquinones
Naphthoquinone is a representative classification of components in R. cordifolia. To date, 16 naphthoquinones have been identified in R. cordifolia, such as mollugin (Wang et al., 2017), furomollugin (Ho et al., 1996), 2′-hydroxymollugin, 2′-methyoxymollogin, 1′,2′-dihydroxydihydromollugin, 1-methoxy-2-hydroxydihydromollugin (Hideji et al., 1993), epoxymollugin (Son et al., 2008), dihydromollugin and 2-carbomethoxy-2,3-epoxy-3-prenyl-1,4-naphthoquinone (CMEP-NQ) (Jun et al., 2011).
Pharmacological activities
A number of researches have reported that R. cordifolia has numerous pharmacological activities, including anti-inflammatory, anti-cancer, anti-tumor, antioxidation, antibacterial, anti-platelet aggregation, anti-nephrotoxicity, anti-urolithiasis, hepatoprotective effects, and neuroprotective effects (Rawal et al., 2004; Joy and Nair, 2008; Divakar et al., 2010; Do et al., 2013; Gong et al., 2017; Luo et al., 2022). We summarized the pharmacological effects of R. cordifolia in vitro and in vivo, which were shown in Table 2 and Table 3.
TABLE 2.
In vitro study on the pharmacological effects of R. cordifolia.
| Bioactivities | Cell line | Compound/extract | Tested concentration | Active concentration | Positive control | Negative control | Result/mechanism | References |
|---|---|---|---|---|---|---|---|---|
| Antiadipogenic activity | 3T3-L1 preadipocytes | 2-Carbomethoxy-2,3-epoxy-3-prenyl-1,4-naphthoquinone (CMEP-NQ) | 0, 10, 20, 40 μM | 10, 20, 40 μM | Not available | Dulbecco’s modified eagle’s medium (DMEM) | CMEP-NQ (20, 40 μM) reduced viability of 3T3-L1 preadipocytes and mature adipocytes in a time- and dose-dependent manner. CMEP-NQ (10 μM) had no effect on the viability of these 2 cells, but the accumulation of less differentiation-related intracellular lipids was about 48.5% | (Jun et al., 2011) |
| CMEP-NQ (10 μM) suppressed adipocytic differentiation of 3T3-L1 preadipocytes, and down-regulated the expression of transcription factors, including C/EBPα, PPARγ1 and PPARγ2 | ||||||||
| Anti-cancer | HEp-2 cell | Methanol extract | 5, 10.15, 20, 25, 30 mg/ml | 5, 10.15, 20, 25, 30 mg/ml | Not available | DMEM | The viable cell rates of R. cordifolia methanol extract (5, 10, 15, 20, 25, and 30 mg/ml) for HEp-2 were 62.87, 54.67, 38.99, 26.92, 20.32, and 15%, respectively, and the control group was 79.06%. The methanol extract of R. cordifolia (5, 10, 15, 20, 25 and 30 mg/ml) with LD50 = 10 mg/ml inhibited the proliferation, promoted LDH release, reduced the levels of reduced GSH and GST, increased lipid peroxidation on human laryngeal carcinoma HEp-2 cells in a dose- dependent manner | Shilpa et al. (2012a) |
| HER2-overexpressing SK-BR-3, BT-474 human breast cancer cells, SK-OV-3 human ovarian cancer cells | Mollugin | 1, 5, 25, 50,100 μM | 1, 5, 25, 50,100 μM | Not available | Dimethyl sulfoxide (DMSO) | Mollugin (1, 5, 25, 50 and 100 μM) inhibited HER2-overexpressing cancer cells with IC50 of 50 μM in a dose- and time-dependent manner. Inhibited cell proliferation and promoted apoptosis of breast and ovarian cancer cells by suppressing FAS expression through modulation of a HER2/Akt/SREBP-1c signaling pathway. Inhibited HER2 expression by suppression of NF-kB activation | Do et al. (2013) | |
| Anti-inflammatory | RAW 264.7 cell | Mollugin | 7.5, 15, 30 μM | 7.5, 15, 30 μM | Not available | DMEM | In LPS-induced RAW264.7 inflammatory cells, mollugin (7.5, 15 and 30 μM) inhibited NO release, suppressed the expression of iNOS, IL-1β and IL-6 in a dose-dependent manner, and dose-dependent inhibition of LPS-induced activation of JAK2, STAT1 and STAT3 in RAW264.7 macrophages. Mollugin might be a JAK2 inhibitor that inhibited LPS-induced inflammatory response by blocking the activation of the JAK-STAT pathway | Zhu et al. (2013) |
| LPS/IFN-g stimulated murine peritoneal macrophages | Methanol extract (1-hydrotectoquinone) | 10, 20, and 40 μM | 10, 20, and 40 μM | NG-monomethyl-L-arginine (L-NMMA) | RPMI-1640 | The lowest tested concentration (10 μM) of 1-hydroxytectoquin could significantly inhibit the production of nitric oxide (NO•) compared to the control group treated with LPS/IFN-g. 1-Hydroxytectoquin (10, 20, and 40 μM) dose-dependently inhibited NO• production and iNOS expression in LPS/IFN-g stimulated murine peritoneal macrophages. These results were similar to the positive control group | Ghosh et al. (2010) | |
| Antitumor | A375 cell, Hep2 cell, U937 cell, murine carcinoma | 1-Hydroxytectoquinone (methanol extract) | 10, 20, 40 μM | 10, 20, 40 μM | Doxorubicin, camptothecin | RPMI-1640 | 1-Hydroxytectoquinone inhibited 50% of murine carcinoma (EAC) cell proliferation at less than 10 μM concentration, and inhibited the proliferation of A375 malignant skin melanoma cells with IC50 value of 3.2 μM. But relatively low toxicity against Hep2 cells (IC50 > 50 μM). The inhibitory effect on the U937 cell line was moderately cytotoxic with IC50 values of 19–28 μM | Ghosh et al. (2010) |
| Neuroprotective effects | mouse hippocampal HT22 cell | Mollugin | 2.5, 5, 10 and 20 μM | 2.5, 5, 10 and 20 μM | Trolox (50 μM) | DMEM | Mollugin (2.5, 5, 10 and 20 μM) promoted reactive oxygen species scavenging activity against glutamate-induced reactive oxygen generation in HT22 cells in a dose-dependent manner | Jeong et al. (2011) |
| Mollugin (2.5, 5, 10 and 20 μM) suppressed pro-inflammatory mediators, including pro-inflammatory enzymes (iNOS and COX-2) and cytokines (TNF-α and IL-1β) in BV2 cells stimulated with LPS in a concentration-dependent manner | ||||||||
| The neuroprotective effects of mollugin might be related to inhibition of pro-inflammatory mediators, upregulation of HO-1 expression and HO activity, nuclear accumulation of Nrf2, and activation of MAPK pathway |
TABLE 3.
In vivo study on the pharmacological effects of R. cordifolia.
| Bioactivities | Animal/model | Compound/Extract | Tested concentration | Effective concentration | Positive/Negative control | Result/Mechanism | Reference | |
|---|---|---|---|---|---|---|---|---|
| Anti-inflammatory | Wistar rat/indomethacin-induced enterocolitis | 300 mg/kg, 600 mg/kg body weight | 300 mg/kg, 600 mg/kg body weight | Water | Reduced serum lactate dehydrogenase (LDH) activity levels | Pawar et al. (2011) | ||
| Hydroalcoholic root extract | ||||||||
| #212121; Wistar rat/trinitrobenzenesulfonic acid (TNBS)-induced colonic inflammation | Aqueous extract of the aerial part | 250, 500, 1,000 mg/kg body weight | 500 mg/kg body weight | Dexamethasone (0.3 mg/kg body weight) | Decreased the macroscopic damage area | Gong et al. (2017) | ||
| Improved microstructure and reduced malondialdehyde content in the colon | ||||||||
| Reduced levels of interleukin-1β (IL-1β) and tumor necrosis factor (TNF-α) | ||||||||
| C57BL/6 mice/dextran sulfate sodium (DSS)-induced ulcerative colitis (UC) | Mollugin | 10, 20, 40 mg/kg body weight | 20, 40 mg/kg body weight | Distilled water | Reduced weight loss and the diseased activity index, ameliorated colon injury in ulcerative colitis (UC) mice | Li et al. (2020) | ||
| Mollugin treatment (20 or 40 mg/kg) inhibited the production of pro-inflammatory cytokines IL-1β and TNF-α, and decreased the expression of IFN-γ and TLR4 in the DSS-induced UC mouse model | ||||||||
| Antioxidation | Wistar rat/aspirin plus pyloric ligation -induced ulcer | Chloroform extract/methanol extract | Methanol extract (100, 200, 400 mg/kg body weight) | 400 mg/kg body weight | Reduced the ulcer index, total acidity, protein, and pepsin content of gastric juice, and increased mucoprotein content | Deoda et al. (2011) | ||
| Chloroform extract (50,100,200 mg/kg body weight) | Ranitidine (10 mg/kg body weight) | Reduced lipid peroxidase (LPO) content, increased catalase (CAT), superoxide dismutase (SOD) and glutathione (GSH) content | ||||||
| Swiss albino mice | Ethanol extract | 50,100 mg/kg body weight | 50, 100 mg/kg body weight | Ethanol extract (100 mg/kg body weight) | Enhanced superoxide dismutase (SOD) and catalase (CAT) activities, increased glutathione (GSH) content, inhibited lipid peroxidase (LPO), reduced macrophage yield, macrophage viability, phagocytic index, serum immunoglobulin levels and renal PFC. | Lodi et al. (2011) | ||
| Wistar rat/n-nitrosodiethylamine-induced experimental hepatocellular carcinogenesis | Methanol extract | 250, 500, 750 mg/kg body weight | 750 mg/kg body weight | Methanol extract (500 mg/kg body weight) | Reduced serum marker enzyme levels, including aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP), and lactate dehydrogenase (LDH).Decreased the level of LPO and hydroxyl radicals in liver | Shilpa et al. (2012b) | ||
| Increased activity of several antioxidants in the liver, including SOD, CAT, glutathione peroxidase (GPx), glutathione S-transferase (GST), increased mitochondrial enzymes such as isocitrate dehydrogenase (ICDH), the level of succinate dehydrogenase | ||||||||
| Anti-tumor | Swiss albino mice/C57BL/6 mice | RC-18 | 1.25–5 mg/kg body weight | 5 mg/kg body weight | Saline | Inhibited activity and proliferation of P388 cells and L1210 cells, but failed to show any inhibitory effect on solid tumors, Lewis lung cancer and sarcoma 180 | Adwankar and Chitnis, (1982) | |
| Anti-urolithiasis | Wistar albino rat/ethylene glycol induced urolithiasis | Hydro-alcoholic extract of root | 286, 667 mg/kg body weight | 667 mg/kg body weight | Cistone (750 mg/kg body weight) | Decreased calcium, oxalate levels and number of calcium oxalate crystals deposits in kidney tissue | Divakar et al. (2010) | |
| Anti-nephrotoxicity | Swiss albino mice/cisplatin- induced renal damage | Hydro-alcoholic extract | 250, 500 mg/kg body weight | 500 mg/kg body weight | #212121; Hydro-alcoholic extract (500 mg/kg body weight) | Decreased values of serum urea and creatinine | Joy and Nair, (2008) | |
| Increased GPx, SOD and CAT. | ||||||||
| Hepatoprotective effects | Sprague-dawley Rat/carbon tetrachloride-induced liver injury | Rubiadin | 50, 100, 200 mg/kg body weight | 100 and 200 mg/kg body weight | Silymarin (100 mg/kg body weight) | Prevented the increase in malondialdehyde content and the decrease in reduced glutathione content in the liver of CCl4 poisoned rats in a dose-dependent manner | #080000; Rao et al. (2006) | |
| Histopathological examination confirmed the effective protective effect of rubiadin on carbon tetrachloride-induced liver injury in rats | ||||||||
| Neuroprotective effects | Sprague-dawley Rat/reserpine- induced movement disorders | Methanol extract | 100, 200, 300 mg/kg body weight | Methanol extract (300 mg/kg) combined with Vitamin E (10 mg/kg) | Methanol extract (300 mg/kg) | Inhibited cavitary chewing movements, tongue protrusion, and increased the ability of exercise | Patil and Kasture, (2012) | |
| Increased the levels of SOD, CAT, and GSH in the forebrain region of rats, inhibited LPO and the level of dopamine | ||||||||
Anti-inflammatory effects
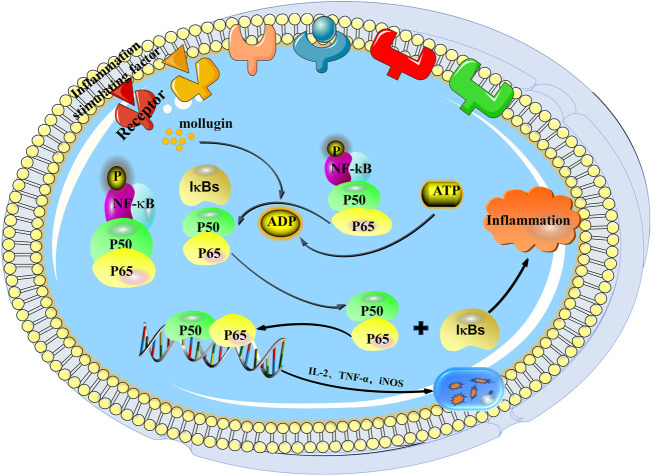
R. cordifolia has been used for the treatment of inflammatory diseases, such as colitis (Pawar et al., 2011), for a long time. In the study of indomethacin-induced enterocolitis in wistar rats, there were some acute intestinal inflammation effects, such as mesenteric haemorrhage, bowel wall thickening, mesenteric adhesion and multiple mucosal ulcerations of the small intestine, increased lactate dehydrogenase (LDH) activity, and so on. After treatment with hydro-alcoholic root extract of R. cordifolia (300 mg/kg and 600 mg/kg body weight) for 11 consecutive days, reduced intensity of lesions and inflammatory reaction in both ileum and colon tissue were observed, and reduced LDH activity was detected in indomethacin-induced enterocolitis rat model. These data suggested that R. cordifolia had a protective effect on indomethacin-induced colitis in rats, which may be used to treat patients with inflammatory bowel diseases (Pawar et al., 2011). Although the therapeutic effects of R. cordifolia on enterocolitis were investigated in rat, but the detection indexes were very simple, the evidences were weak, lacking of positive group, and the mechanism of action had not been confirmed, which should be investigated later. As described earlier, R. cordifolia was used to manage diarrhea in Chinese folk. Gong et al. investigated the anti-diarrheal and anti-inflammatory effects of the aqueous extract of R. cordifolia’s aerial part (AERCAP). They firstly measured the acute toxicity by acute oral toxicity test. Via LD50 study, they found that orally administered with graded doses of AERCAP (1, 2, 4, or 8 g/kg body weight) did not cause any mortality or obvious toxicity in the observation period. Orally administered with a single or maximum dose (13.2 g/kg body weight) of AERCAP also did not cause any mortality or behavioral and physical changes during the observation period, indicating that AERCAP had no acute oral toxicity in Male Swiss albino mice. Then, in a senna leaf-induced diarrhea mice model, administration with AERCAP (500 mg/kg and 1,000 mg/kg body weight) significantly inhibited the onset of semi-solid feces and reduced the evacuation index (EI) when compared with the model mice. But the effects of AERCAP was slightly weaker than the standard anti-diarrheal drug loperamide (4 mg/kg body weight). Finally, the anti-inflammatory activity of AERCAP was evaluated by a trinitrobenzenesulfonic acid (TNBS)-induced colonic inflammation rat model. They found that oral treatment with AERCAP or positive drug dexamethasone (0.3 mg/kg body weight) decreased the macroscopic damage area, improved the microscopic structure, reduced the malondialdehyde (MDA) content and levels of pro-inflammatory cytokines tumor necrosis factor (TNF-α) and interleukin-1β (IL-1β) in colonic tissue when compared with the model rat (Gong et al., 2017). The study provided the scientific evidences of the traditional use of AERCAP for treating diarrhea in folk, but did not elucidate its underlying mechanisms. Besides, the dose of 500 and 1,000 mg/kg of AERCAP exhibited a great therapeutic effect against diarrhea, but the high dose of AERCAP (2,000 mg/kg body weight) had no effects on senna leaf-induced diarrhea in mice. This was a common phenomenon in crude extract preparation. Although the acute toxicity of AERCAP was detected by observation of mortality, behavioral and physical changes, the systemic toxicity should be measured through viscera indexes, H&E staining of primary organs, as well as biochemical indicators of peripheral blood. The systemic toxicity might be an influence factor on the therapeutic effects of AERCAP on diarrhea. Mollugin, a major compound of R. cordifolia, was reported to possess an anti-inflammatory activity (Figure 4). MTT assay was performed to assess the cytotoxicity of mollugin on RAW264.7 cells and showed that the different concentration of mollugin (7.5, 15 and 30 μM) had no cytotoxicity on RAW264.7 cells after treatment for 24 h. In lipopolysaccharide (LPS)-induced RAW264.7 inflammatory cells, mollugin (7.5, 15 and 30 μM) was able to inhibit nitric oxide (NO) release, suppress the expression of nitric oxide synthase (iNOS), IL-1β and IL-6 in a concentration-dependent manner. Mechanically, mollugin (7.5, 15 and 30 μM) dose-dependently inhibited the activation of JAK2, STAT1 and STAT3 induced by LPS in RAW264.7 macrophages. Molecular docking analysis further demonstrated that mollugin exhibited a high binding ability with JAK2, and the binding pattern was similar to AG490, a specific JAK2 inhibitor, indicating that mollugin was a JAK2 inhibitor. These data suggested that mollugin inhibited LPS-induced inflammatory responses in RAW264.7 macrophages via blocking the activation of the JAK-STAT pathway (Zhu et al., 2013). This finding is interesting because they demonstrate the direct target of mollugin against inflammatory response. However, the binding between mollugin with JAK2 should be proved by experiments, such as surface plasmon resonance technology (SPR), cellular thermal shift assay (CETSA) (Martinez et al., 2013) or isothermal dose-response fingerprint (ITDRFCETSA) (Chang et al., 2016), and the in vivo anti-inflammatory activity of mollugin should be investigate. Moreover, the authors confirms the anti-inflammatory activity of mollugin, but the lack of positive control reduces reliability of research which requires further validation. Another study demonstrated that mollugin could also ameliorated dextran sulfate sodium (DSS)-induced ulcerative colitis (UC) in C57BL/6 mice. Intragastric administrated with mollugin (10, 20, and 40 mg/kg body weight) significantly reduced weight loss and the diseased activity index, ameliorated colon injury in UC mice. In addition, mollugin treatment (20 or 40 mg/kg body weight) markedly inhibited the production of pro-inflammatory cytokines IL-1β and TNF-α, and decreased the expression of interferon γ (IFN-γ) and toll-like receptor (TLR4) in the DSS-induced UC mouse model. Therefore, the improvement of DSS-induced ulcerative colitis by mollugin might be by suppressing the production of pro-inflammatory cytokines (Li et al., 2020). 1-hydrotectoquinone (10, 20 and 40 μM) was reported to reduce nitric oxide (NO•) production in LPS and IFN-g stimulated murine peritoneal macrophages, and inhibit iNOS expression in LPS induced cultured murine peritoneal macrophages in a dose dependent manner (Ghosh et al., 2010). The in vivo activity and mechanistic investigation of 1-hydrotectoquinone against inflammatory need for further study. Furthermore, in addition to the drug group and the negative control group, the positive group should be designed to enhance the reliability of the study and the rationality of the experiment. So far, there is a relatively wide selection of anti-inflammatory positive drugs, such as aspirin, celecoxib, diclofenac, diflunisal and ibuprofen etc (Khandia and Munjal, 2020). Taken together, these findings demonstrate that R. cordifolia extract and part of its compounds have anti-inflammatory activity in different models, but the mechanism of regulating inflammatory was still unclear. Further research and clinical trial data are needed to confirm its anti-inflammatory activity and mechanism of action.
FIGURE 4.
Anti-inflammatory mechanism of mollugin.
Anti-cancer effects
Although great progress has been made in various anti-cancer treatments, such as targeted therapy and immunotherapy (Torre et al., 2016), chemotherapy is still the most commonly used treatment (Bukowski et al., 2020). The methanol extract of R. cordifolia (5, 10, 15, 20, 25 and 30 mg/ml) with LD50 = 10 mg/ml could inhibit the proliferation, promote LDH release, reduce the levels of reduced glutathione (GSH) and glutathione transferase (GST), increase lipid peroxidation on human laryngeal carcinoma HEp-2 cells in a dose dependent manner. Moreover, R. cordifolia extract (30 mg/ml) could induce apoptotic cell death of HEp-2 cells, indicating its potential for treating laryngeal squamous cell carcinoma (Shilpa et al., 2012a). The study of R. cordifolia against laryngeal squamous cell carcinoma just using 1 cell line is not sufficient, the other cell lines of laryngeal squamous cell carcinoma and xenograft mouse model should be used to assess the activity of R. cordifolia. Moreover, this study lacks a positive control, and this result needs to be further validated. A cyclic hexapeptide compound (RC-18) (1.25, 2.5 and 5 mg/kg body weight) isolated from R. cordifolia had been demonstrated to had a significant anti-cancer effect against ascites tumors L1210, P338 and L5178Y, as well as solid tumor B16 melanoma, which could prolong median survival time in corresponding tumor mice models. RC-18 had no inhibitory effect on two solids tumors, Lewis lung carcinoma and sarcoma 180, which had no remarkable effect on mean tumor weight (Adwankar and Chitnis, 1982). This study reveals the ability of RC-18 against a spectrum of murine tumor models. A number of natural products have activities against cancer or tumor in mice model, but have no therapeutic actions on humans. P388 and L1210 tumor mice models used in this study have got predictive value in the clinic. Plenty of compounds are able to inhibit P388 and L1210, which are later demonstrated to have activity against cancer or tumor in clinic, indicating RC-18 may be a promising agent against cancer for the clinical use. The toxicity study, mechanism research and clinical research of RC-18 should be carried out in the further. Mollugin had been shown preclinical anti-cancer actions in varieties of cancer models. Mollugin (20, 40, 60 and 80 μM) was able to suppress cell viability of U251MG and U87MG cells (glioblastoma cells), MKN45 cells (gastric cancer cell), MCF-7 cells (breast cancer cell), A549 cells (lung cancer cell) and HT29 cells (colon cancer cell) in a concentration- and time-dependent manner, but had no effect on cell viability of mouse primary neurons. Mollugin (10, 20, and 40 μM) could induce mitochondria apoptosis and autophagy in glioblastoma cells by inhibiting PI3K/AKT/mTOR/p70S6K and ERK signaling pathways (Zhang et al., 2014). The experimental design of this study is rigorous. The multiple cancer cell lines and mouse primary cell were used to evaluate the cell viability of mollugin on cancer cells and normal cells. For mechanism research, the pan-caspase inhibitor Z-VAD, RNA interference with shRNA for knockdown an autophagic regulator Beclin-1, PI3K kinase inhibitor LY294002, and ERK inhibitor U0126 were carried out to clarify the relationship between mollugin with apoptosis and autophagy, as well as the mechanism of action of mollugin. These evidences are solid. However, there are still many questions need to be solve. For instance, what’s the relationship between apoptosis and autophagy induced by mollugin in glioblastoma cells. There may be a switch locates in the upstream of PI3K/AKT/mTOR/p70S6K and ERK signaling pathways, which is regulated by mollugin that should be identified. Moreover, the in vivo study is extremely necessary to carry out to prove the anti-cancer ability of mollugin. Mollugin (1, 5, 25, 50, and 100 μM) was also reported to possess the anti-cancer abilities in HER2-overexpressing cancer cells in a dose- and time-dependent manner with an IC50 value of 50 mM. Further study demonstrated that mollugin inhibited cell proliferation and promoted apoptosis of breast and ovarian cancer cells by suppressing FAS expression through modulation of a HER2/Akt/SREBP-1c signaling pathway. In addition, mollugin could inhibit HER2 expression by suppression of NF-kB activation (Do et al., 2013). In summary, these findings suggest that R. cordifolia and its ingredients may be clinically useful as anti-cancer agent. The in vivo activities against cancers should be explored using cancer animal models. And authors ought to design a positive drug group in the study. For example, paclitaxel is known as one of the most successful natural anticancer drugs (Zhu and Chen, 2019). Vinblastine, podophyllotoxin and camptothecin have also been used as anticancer drugs in clinical practice (Gezici and Sekeroglu, 2019), which is conducive to enhance the credibility, scientificity and rationality of the article.
Anti-tumor effects
Many studies had shown that cyclic peptides were bioactive compounds responsible for anti-tumor activity of R. cordifolia. The majority of the RA series compounds indicated cytotoxicity against a number of tumor cells, including P-388 leukaemia cells, SGC-7901 human gastric adenocarcinoma cells, A-549 human non-small cell lung carcinoma cells, and HeLa (human cervical carcinoma) cells (Chen et al., 2015). For example, RA-XVIII (IC50 = 0.012 μg/ml) (Lee et al., 2008a), RA-XXIII (IC50 = 0.16 μg/ml) and RA-XXIV (IC50 = 0.48 μg/ml) (Lee et al., 2008b) could effectively inhibit the proliferation of P-388 leukaemia cells. The studies of the RA series compounds are pretty simple, which all focus on their cytotoxicity on several tumor cell lines. Whether they have significant cytotoxicity on normal cells should be evaluated. The in vivo activities of RA series compounds against above tumors are the core for further study. Wang et al. found that mollugin isolated from R. cordifolia root had anti-tumor properties. Mollugin (20, 40, and 80 μM) could not only significantly inhibit the expression of NF-κB receptors induced by TNF-α in a dose-dependent manner, but also inhibit p65 phosphorylation and nuclear metastasis induced by TNF-α, phosphorylation and degradation of κB inhibitor (IκBα), and IκB kinase (IKK) phosphorylation. Moreover, mollugin (20, 40, and 80 μM) could inhibit the proliferation of HeLa cells. Ex vivo experimental showed that mollugin (25 or 75 mg/kg body weight) effectively inhibited the growth of heterogeneous transplanted tumors from HeLa cells. The author concludes that mollugin might be a potential drug for treating cancer by targeting NF-κB (Wang et al., 2017). There are some questions need to be solved. For example, what’s the direct target of mollugin against cancer? Both TNFR1 and NF-κB are potential targets of mollugin. The inhibitors of TNFR1 and NF-κB can be used to demonstrate whether mollugin have an anti-tumor effect through regulation of TNFR1 or NF-κB. Besides, the direct interaction relationship between mollugin with TNFR1 or NF-κB should be verified by molecular docking simulation, molecular dynamics simulation, SPR, CETSA or ITDRFCETSA. 1-hydroxytectoquinone was another active ingredient in R. cordifolia, which could inhibit 50% of murine carcinoma (EAC) cell proliferation at less than 10 μM concentration. In addition, it had an inhibitory effect on A375 malignant skin melanoma cells with an IC50 value of 3.2 μM (Ghosh et al., 2010). The study of 1-hydroxytectoquinone only on several tumor cell lines are not enough. Also, this study lacks positive control. Epothilone, a newly developed antitumor drug, can be used as a positive drug in antitumor experiments (Cheng and Huang, 2018). The widely research of 1-hydroxytectoquinone against tumor on xenograft mice model should be initiated. Overall, the underling molecular mechanisms of the ingredients of R. cordifolia against tumors need to be further studied in the future.
Antioxidation effects
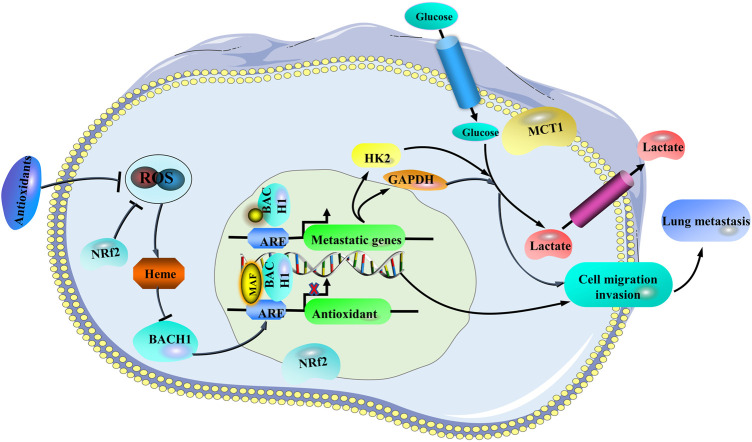
Natural antioxidants are a variety of potential plant drug resources, with a long history of ethnopharmacology (Dkhil et al., 2016). Therefore, it is vital to obtain antioxidant components from natural medicines. Study had demonstrated that R. cordifolia had antioxidant activity, the hydroxyl group on the benzene ring in R. cordifolia played a crucial role in scavenging free radicals, while the hydroxyl structure in hydroxyanthraquinone could effectively enhance the free radical scavenging ability (Cai et al., 2004). The antioxidant mechanism (Lignitto et al., 2019) was shown in Figure 5. Deoda et al. found that methanolic extract of R. cordifolia (100, 200 or 400 mg/kg body weight) and chloroform fraction of R. cordifolia (50, 100 or 200 mg/kg body weight) brought notable decrease of ulcer index, total acidity, protein, pepsin content of the gastric fluid, and increased of the mucin content in an aspirin plus pylorus-ligated ulcer rat model. Several key antioxidant parameters, lipid peroxidase (LPO) content was reduced, catalase (CAT), superoxide dismutase (SOD) and GSH contents were elevated by methanolic extract of R. cordifolia (100, 200 or 400 mg/kg body weight) and chloroform fraction of R. cordifolia (50, 100 or 200 mg/kg body weight) treatment. In above study, the standard antiulcer drug ranitidine (10 mg/kg body weight) was used as positive drug. It was concluded that the gastroprotective effect of R. cordifolia was partly due to its antioxidant activity (Deoda et al., 2011). Lodi et al. assessed the antioxidant activity of the ethanolic extract of the roots of R. cordifolia in vivo. Lead nitrate (40 mg/kg body weight) treatment cause an increase in LPO, CAT and GSH contents, reduction in macrophage yield, viability of macrophage, phagocyte index, serum immunoglobulin level, and PFC in kidney in Swiss albino mice. However, the ethanolic extract of the roots of R. cordifolia (50 and 100 mg/kg body weight) administration significantly reversed lead nitrate-induced toxicity on oxidative stress and immunological parameters, demonstrating that R. cordifolia had an antioxidant property (Lodi et al., 2011). Shilpa et al. reported that the methanol extract of R. cordifolia (750 mg/kg body weight) could reduce levels of serum marker enzymes including aspartate transaminase (AST), alanine aminotransferase (ALT), alkaline phosphatase (ALP) and LDH, decrease the level of LPO and hydroxyl radicals in liver, increase the activities of several antioxidants including SOD, CAT, glutathione peroxidase (GPx), GST in liver, elevate the levels of mitochondrial enzymes like isocitrate dehydrogenase (ICDH), succinate dehydrogenase (SDH), α-ketoglutarate dehydrogenase (α-KGDH) in a N-nitrosodiethylamine-induced experimental hepatocellular carcinogenesis rat model. These data demonstrated that R. cordifolia might be applied as an antioxidant for the treatment of some cancers (Shilpa et al., 2012b). In above studies, the antioxidant activity of R. cordifolia are demonstrated by detection of several antioxidant indexes, however, the reason why R. cordifolia possesses the antioxidant ability should be further study. In addition, it is necessary to include a positive drug such as vitamin E in the experiment to make the results more convincing (Mohd et al., 2020). Besides, what are the antioxidant active components of R. cordifolia need to be confirmed. Overall, these studies demonstrate the antioxidation effects of R. cordifolia or its ingredients in different models, but none of them carry out the mechanism research, which require further study.
FIGURE 5.
Antioxidative mechanisms.
Antibacterial effects
Antibacterial drugs are an important class of therapeutic drugs used to treat bacterial infectious diseases (Sun et al., 2004). Unnecessary and overuse of antibiotics is of particular concern, as this could lead to several adverse drug events, including end-organ toxicity, allergic reactions, subsequent infection with drug-resistant organisms, and Clostridium difficile infection (Tamma et al., 2017). The demand for new antibacterial drugs that are effective against drug-resistant microorganisms has greatly increased (Wright et al., 2017). The plant materials are generally preferred now for use as natural antibacterial agents in the treatment of various infections (Ginovyan et al., 2020). Basu et al. found that the methanol extracts of R. cordifolia (1, 2.5, 5, 7.5, and 10 mg/ml) exhibited activity against 6 g-positive bacteria (Bacillus cereus, Bacillus pumilus, Bacillus subtilis, Micrococcus luteus, Mycobacterium luteum and Mycobacterium luteum) in a concentration-dependent manner. Besides, the methanol extracts of R. cordifolia (1, 2.5, 5, 7.5, and 10 mg/ml) could also inhibit Pseudomonas aeruginosa in a concentration-dependent manner. Mover, the water extracts of R. cordifolia (1, 2.5, 5, 7.5, and 10 mg/ml) could only inhibit Bacillus subtilis and Staphylococcus aureus. All these bacterial were highly susceptible to inhibition by streptomycin (0.1 mg/ml) used as standards, and part of them were significantly inhibited by penicillin G (0.01 mg/ml) used as positive drug (Basu et al., 2005). The study demonstrates the excellent antibacterial ability of R. cordifolia and shows the methanol, methanol and water extracts of R. cordifolia can inhibit different bacteria. The reason why different extracts of R. cordifolia exhibit different antibacterial abilities should be explained. The comprehensive chemical profiling of R. cordifolia is worthy of being explored by using chemical analysis method, such as two-dimensional mixed-mode liquid chromatography × reversed-phase liquid chromatography system (Dai et al., 2022), which is beneficial to antibiotic discovery from R. cordifolia.
Anti-platelet aggregation effects
Thromboembolic disease is one of the most common clinical diseases (Nagalla and Bray, 2016). Platelet aggregation is an important cause of thrombosis. The research and exploration of anti-platelet aggregation drugs is still an important topic of concern (Xie et al., 2019). There are a few reports on anti-platelet aggregation effects of R. cordifolia. Research found that the partially purified fraction of R. cordifolia could inhibit rabbit platelet aggregation induced by platelet activating factor (PAF), and inhibit the binding of 3H-PAF to platelets (Tripathi et al., 1993). Although this study had demonstrated the anti-platelet aggregation effects of R. cordifolia, further pre-clinical research on the effect of R. cordifolia on thrombosis in mice, and clinical research should be performed to confirm their mechanism of action and therapeutic effects in clinic.
Hepatoprotective effects
The liver is an important organ for the regulation of human metabolism. Hepatic damage is associated with disorder of metabolic functions (Limon et al., 2021). Liver ailments are still a worldwide health problem (Venkateswaran et al., 1997; Daniyal et al., 2019). At present, the use of herbal medicine to treat liver ailments has huge potential. Rao et al. evaluated the effect of rubiadin isolated from R. cordifolia on carbon tetrachloride (CCl4)-induced liver injury in rats. They found that rubiadin (50, 100, and 200 mg/kg body weight) significantly prevented the increase in malondialdehyde content and the decrease in reduced glutathione content in the liver of CCl4 poisoned rats in a dose-dependent manner. The hepatoprotective effects of rubiadin were similar to silymarin (100 mg/kg body weight), a known hepatoprotective compound. Histopathological examination of rat liver sections also confirmed that rubiadin had an effective protective effect on rat liver injury induced by carbon tetrachloride (Rao et al., 2006). Although rubiadin has a protective effect on liver damage, its mechanism of action is still unclear, which needs to investigate in further study. Except for rubiadin, other hepatoprotective compounds of R. cordifolia is necessary to identified.
Neuroprotective effects
The neuroprotective effects of R. cordifolia have been studied. Patil et al. found that methanol extract of R. cordifolia (100, 200, and 300 mg/kg body weight) combined with vitamin E (10 mg/kg body weight) treatment significantly inhibited cavitary chewing movements, tongue protrusion, and increased the ability of exercise in a reserpine-induced orofacial dyskinesia model. The authors also found that R. cordifolia treatment could increase the levels of SOD, CAT, and GSH in the forebrain region of rats, inhibit LPO, and increase the level of dopamine. However, the dose alone methanol extract of R. cordifolia (300 mg/kg body weight) did not produce any significant change. These findings suggested that R. cordifolia had a neuroprotective effect (Patil and Kasture, 2012). It is worth noting that R. cordifolia and vitamin E co-treatment play a role in protecting animals against reserpine-induced orofacial dyskinesia. But R. cordifolia treatment alone don’t have any effects on the disease. It is known that vitamin E, a free-radical scavenger, can improve symptoms of antipsychotic-induced tardive dyskinesia (Xu et al., 2022). It is highly possible that R. cordifolia may strengthen the therapeutic effect of vitamin E on neuroleptic induced orofacial dyskinesia. Therefore, R. cordifolia may be an adjuvant therapy when combined with vitamin E for treating antipsychotic-induced tardive dyskinesia. Rawal et al. showed that R. cordifolia (2 mg/ml) exerted neuroprotective properties through elevating the GSH levels by promoting GCLC expression, directly scavenging free radicals, and inhibiting the expression of iNOS gene which was essential for neuronal injury during hypoxia/ischemia in rat hippocampal slices subjected to oxygen glucose deprivation (Rawal et al., 2004). Mollugin (2.5, 5, 10, and 20 μM) could also promote reactive oxygen species scavenging activity against glutamate-induced reactive oxygen generation in HT22 cells in a concentration-dependent manner. In this study, trolox (50 μM) was used as a positive drug, which showed an obvious cytoprotective effect and reactive oxygen species scavenging activity. Besides, mollugin (2.5, 5, 10, and 20 μM) was able to suppress pro-inflammatory mediators, including pro-inflammatory enzymes [inducible nitric oxide synthase (iNOS) and cyclooxygenase-2 (COX-2)] and cytokines (TNF-α and IL-1β) in BV2 cells stimulated with LPS in a concentration-dependent manner. Its neuroprotection might be mediated by the effects on inhibition of pro-inflammatory mediators, up-regulation of heme oxygenase-1 (HO-1) expression and the heme oxygenase (HO) activity, nuclear accumulation of Nrf2 and activation of p38 mitogen-activated protein kinase (MAPK) pathway (Jeong et al., 2011). The study only demonstrates the neuroprotective effect of mollugin in vitro, the more solid evidence should be acquired form animal model of neurological disease.
Toxicity
Although a large number of research have proved that R. cordifolia has numerous pharmacological activities, some compounds isolated from this plant are still toxic. Furthermore, it is extremely necessary to investigate its toxicological effects in order to determine the clinical use of the drug. The toxicity of the crude ethanol extracts of R. cordifolia fruit (100, 500, and 1,000 mg/kg body weight) was evaluated by biochemical parameters and histopathological changes and found that it had harmful effects on liver. The LD50 value was higher than 1,000 mg/kg. In addition, dibutyl phthalate identified from R. cordifolia fruits was showed a holistic toxicity via in silico analysis (Anantharaman et al., 2016). However, this experimental time is short, and further in-depth studies and developmental toxicity studies are needed to determine the effects of R. cordifolia on long-term administration to animals. In addition, its toxic dose can’t be determined by animal toxicity studies, and clinical trials are needed to evaluate its safety in humans. Studies had found that rubiadin, a compound in R. cordifolia, had carcinogenic potential. A rat medium-term multi-organ carcinogenesis model was used and Male F344 ⁄ DuCrj rats were given 0.008% or 0.04% containing diet. The effects of rubiadin on the kidney, liver, and large intestine were explored. The results showed that rubiadin treatment increased renal cell adenomas and carcinomas in the outer medulla of the kidney, preneoplastic lesions, hepatocellular foci, dysplasia, as well as adenomas and adenocarcinomas in the large intestine (Inoue et al., 2009). Therefore, we should avoid exposure to carcinogenic substances containing rubiadin.
Pharmacokinetics
Up to now, there are few reports on the pharmacokinetics of R. cordifolia. Gao et al. used Ultra High- Performance Liquid Chromatography-tandem Mass Spectrometry (UHPL-MS/MS) method to simultaneously determine munjistin, purpurin, and mollugin in rat plasma. The Sprague-Dawley (SD) rats (220–20 g body weight) were oral administrated with R. cordifolia (0.82 g/kg body weight). The 0.4 ml of blood were collected from the orbital venous plexus at the indicated time points (0, 0.33, 0.67, 1, 1.3, 1.67, 2, 2.5, 3, 4, 6, 8, 12, and 24 h) and the plasmas were collected for pharmacokinetic study. The results showed that after treatment with R. cordifolia, the maximum plasma concentrations (C max) for purpurin, munjistin and munjistin were 70.10–11.78 ng/ml, 26.09–6.6 ng/ml, and 52.10–6.71 ng/ml, respectively. The time for maximal concentration (T max) for purpurin, munjistin and mollugin were 1.61–0.24 h, 2.58–0.19 h, and 1.99–0.21 h, respectively (Gao et al., 2016). Except for this study, more pharmacokinetic studies should be performed in order to better understand the absorption, distribution, metabolism and excretion of the active ingredients in R. cordifolia.
Clinical application
Hemorrhage syndrome is a common symptom in clinic, such as acute hemorrhage, cerebral hemorrhage, vascular rupture and bleeding (Dima et al., 2018). As a traditional Chinese medicine, R. cordifolia has been used for many years in the treatment of hemorrhage in clinical (Chen, 2013). R. cordifolia could effectively treat most hemorrhages, such as hematemesis, hematuria, dysmenorrhea, and abnormal uterine bleeding (Li et al., 2009). In a clinical research, 110 patients (25–49 years old) with abnormal uterine bleeding caused by intrauterine device were treated with R. cordifolia decoction (18 g/d) or control drug yunnanbaiyao (1.5 g/d). The blood routine examination and blood coagulation were evaluated of each patient before and after medication. They found that the total effective rate of R. cordifolia-treated group was 94.5%, which was higher than the yunnanbaiyao-treated group (81.8%). The hemostatic ability of R. cordifolia-treated group was much stronger than that of yunnanbaiyao-treated group. The platelet levels of two drug-treated groups were much higher than those before treatment. Yunnanbaiyao is a famous traditional Chinese medicine which is widely used in clinic for treating various bleeding disorders in China. The better performance of R. cordifolia in treating abnormal uterine bleeding compared with Yunnanbaiyao suggests that R. cordifolia has a huge potential for clinical application. The clinical research of R. cordifolia for the treatment of other bleeding disorders is worthy of investigation (Hu, 2019). This study is meaningful. The therapeutic schedule should be further optimized. For instance, the accepted drug for treating abnormal uterine bleeding in the domestic and overseas, such as tranexamic acid should be used as positive drug in patients (Bradley and Gueye, 2016). The treatment groups should be divided into low, medium, and high dose of R. cordifolia, which can demonstrate the optimal treatment dosage. Also, combination therapy of R. cordifolia and other effective drugs is worthy of in-depth study. In another clinical study, the decoction of R. cordifolia combined with other herbs were used to treat dysfunctional uterine bleeding. 184 patients (18–51 years old) participated in the research. Among them, 64 patients were treated with norethisterone, a synthetic hormone for treating a range of menstrual problems in clinic. 120 patients were treated with R. cordifolia decoction. They found that the total effective rate of in R. cordifolia-treated group was 90%, and the norethisterone-treated group was 78.1%. Therefore, according to this clinical data, it can prove that R. cordifolia combined with other drugs are able to effectively treat dysfunctional uterine bleeding (Table 4) (Zhang, 2011). Due to the hemostatic effect of R. cordifolia and the strongly wound healing effect of Bletilla striata Reichb. f, the two drugs were used in combination to treat vaginal bleeding and wound healing after hysterectomy (Li, 2011). However, the detailed data of these clinical studies were not exhibited in any scientific literatures. Although R. cordifolia has been used in clinical for many years, further research is needed on the medicinal value and clinical application of R. cordifolia, in order to discover its medicinal value potential and efficacy and improve the safety and use of medicine.
TABLE 4.
Clinical application of R. cordifolia.
| Function | Symptoms | The number of patients | Age | Dose | Effective rate | References | |
|---|---|---|---|---|---|---|---|
| R. cordifolia | Control drug | ||||||
| Treatment of abnormal uterine bleeding | Uterine bleeding caused by intrauterine device | 110 patients. (50 patients were treated with R. cordifolia and 50 patients were treated with yunnanbaiyao) | 25–49 years old | R. cordifolia decoction. (18 g/d) | Yunnanbaiyao. (1.5 g/d) | R. cordifolia-treated group was 94.5%, and yunnanbaiyao-treated group was 81.8% | Hu, (2019) |
| Treatment of dysfunctional uterine bleeding | Dysfunctional uterine bleeding | 184 patients. (120 patients were treated with R. cordifolia and 64 patients were treated with norethisterone) | 18–51 years old | R. cordifolia decoction. (800 ml/d) | Norethisterone. (2.5–20 mg/d) | R. cordifolia-treated group was 90%, and norethisterone- treated group was 78.1% | Zhang, (2011) |
Perspectives and discussion
As a famous TCM, R. cordifolia has high commercial and medicinal value. R. cordifolia is one of the earliest and most widely used red dyestuff in ancient China. People also use R. cordifolia for treating a wide variety of diseases in folk for thousand years. Due to its excellent therapeutic effects and few side effects in clinic, the plant has received increasing attention in recent years. More than 100 compounds of R. cordifolia are identified. The pharmacological activities of R. cordifolia and its compounds were explored. All these findings are beneficial for drug discovery form R. cordifolia and lay the foundation for its clinical application. However, there are still a number of questions about R. cordifolia research need to be solved. The following aspects should be considered in future study.
Firstly, until now, R. cordifolia don’t has its own genetic identity card. The genomic information of R. cordifolia has not been uncovered. It is necessary to perform genomic sequencing and acquire genome sequence of R. cordifolia, which is essential for identification of key active ingredients, exploration of synthesis pathway of the active compounds, quality improvement, as well as its industrial production. Moreover, genomic sequencing should combine with comparative genomics, metabonomic and transcriptomics, which can give more information of R. cordifolia itself. All these explorations will give R. cordifolia a pair of wings to fly to modernization of TCM.
Secondly, although more than 100 compounds of R. cordifolia have been identified, pharmacologic actions of the most compounds have not been explored. For instance, R. cordifolia is traditionally applied for treating various hemorrhage syndromes, but its active compounds in stopping bleeding are largely unknown. Conversely, several compounds with anti-platelet aggregation effects are found. As is well known, pharmaceutical activity screening form TCM is usually a time-consuming, labor-intensive and cost-intensive process. Fortunately, the artificial intelligence (AI) brings a new dawn for medical field, which has excellently facilitated the modern drug discovery (Gupta et al., 2021). In our previous study, we developed a drug screening model based on machine learning (ML), a primary subfield of AI, to high-throughput screening the active compounds in promoting platelet production from thousands of natural compounds of TCM. As a result, we found indenol had a high activity to accelerate platelet production and exhibited an excellent hemostatic ability in radiation-induced thrombocytopenia mice (Wang L. et al., 2022). We believe that drug screening model based on AI algorithm is a powerful tool which can be applied to identify the active compounds from R. cordifolia in treating hemorrhage syndromes as well as other diseases. Except for AI high throughput virtual screening, other drug screening method can be used to R. cordifolia study, such as reporter gene system, high-throughput microscopy assays.
Thirdly, almost all the research on R. cordifolia is not deep enough. Most studies on pharmacological activities of R. cordifolia or its compounds are in vitro cell levels. Whether they have therapeutic effects on some animal models are still unclear. Besides, nearly all of the mechanism research of R. cordifolia or its compounds are not sufficient. Due to multiple components, targets and effects of R. cordifolia, it is difficult to elucidate its mechanism of action. Network pharmacology is an interdisciplinary science based on system biology, network science, bioinformatics and other related disciplines, which can clarify the relationship between drug, disease and drug targets. Using network pharmacology and experimental verifications, we explored the therapeutic effects of Sanguisorba Offcinalis L. a TCM against leukopenia, identified its active compounds, explored the targets of these active compounds, and illuminated the signal pathways regulated by Sanguisorba Offcinalis L. (Wang et al., 2020b). Network pharmacology are also specialized in illustrating the mode of action of Chinese herbal compound prescription. Our previous study demonstrated the therapeutic mechanisms underlying Beimu-Gualou Formula for the treatment of bronchiectasis using network pharmacology methods. (Shen et al., 2021). Thereby, combination of network pharmacology, high throughput sequencing including transcriptomics, proteomics, metabolomics and intestinal flora sequencing, as well as experimental verifications can deeply elucidate the effects of R. cordifolia or R. cordifolia related prescriptions on gene and protein expression, metabolites and gut microbiota changes in bodies. Another research core is to identify the direct targets of compounds derived from R. cordifolia against diseases. For this purpose, some technologies for identification and confirmation of drug targets should be carried out. For example, our team built a novel high-throughput screening method that combination use of biolayer interferometry (BLI) and ultra-high-performance liquid chromatography coupled with diode-array detector and quadrupole/time-of-flight tandem mass spectrometry (UHPLC−DAD-Q/TOF-MS/MS) to rapidly and effectively discover the small-molecules with amyloid-β (Aβ) binding affinity from natural medicines (Guo et al., 2022). This study strategy can be also applied to explore R. cordifolia.
Fourthly, most pharmacological studies of R. cordifolia and its active compounds lack of positive control, which reduces reliability of the data. Scientific researchs are always performed with controls to obtain reliable results. The experimental results can be critically compared, analyzed and explained with reference to the control treatments. The posive control is an experimental control that shows an experiment is working as intended and gives a positive result at the end of the experiment. If the positive control do not show the expected result, it means the design of the experiment is deficient. Therefore, further study about pharmacological activities of R. cordifolia and its active compounds should designs the posive control to insure the experimental results scientific, credible and solid.
Finally, the pharmacokinetics study and clinical research are extremely insufficient. Via pharmacokinetics study, we can confirm the medication regimen, predict the clinical efficacy and toxicity of the drugs, and provide patients with rational drug use. The shortage of pharmacokinetics study of R. cordifolia largely limits its clinical translation. Although R. cordifolia has long been used in clinic for treating different diseases in China, its clinical research is also seriously inadequate. Clinical research is a key step for clinical application of a new drug from the laboratory. The only two clinical research of R. cordifolia are evaluation of therapeutic effects of R. cordifolia on abnormal uterine bleeding, which are come from two different hospitals and the findings are published in Chinese periodicals. Others clinical data of R. cordifolia are not detailed record and published. We hope this herb with ancient history can be brought to the forefront by more researchers, the clinical research can be promoted and recorded, and the data can be published, which are all conducive to boost development of modernization of TCM.
In summary, this article provides a comprehensive review of the traditional applications, chemical compounds, and pharmacological activities of R. cordifolia. Further studies are still needed to widely identify the active compounds of R. cordifolia, deeply explore the pharmacological mechanisms of R. cordifolia and its compounds against different diseases. Meanwhile, the toxicity, pharmacokinetics and clinical research should be the focus of future investigations. We hope this review can provide some interesting information and useful suggestions for further research on TCM.
Author contributions
JW, MW, AW, FH, QC, and WC contributed to the study conception and design. Conceptualization, Material preparation, data collection and analysis were performed by MW, QC, WC, JY, XZ, JL, SY, and CL. The first draft of the manuscript was written by MW, QC, and WC. CZ, JC, QM, AW, FH, and JW revised the manuscript. All authors commented on previous versions of the manuscript.
Funding
This research was funded by grants from the National Natural Science Foundation of China [Grant Nos. 82074129, 81903829, and 81804221], the Science and Technology Planning Project of Sichuan Province, China [Grant Nos. 2022JDJQ0061, 2019YJ0484, 2019YFSY0014, 2019JDPT0010, and 2019YJ0473], Science and Technology Program of Luzhou, China [Grant Nos. 2019LZXNYD-J11, 2019LZXNYDJ05, 2020LZXNYDZ03 and 2020LZXNYDP01], the School-level Fund of Southwest Medical University, China [Grant Nos. 2021ZKMS044, 2021ZKQN022, 2021ZKMS041].
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2022.965390/full#supplementary-material
References
- Abdullah S. T., Ali A., Hamid H., Ali M., Ansari S. H., Alam M. S. (2003). Two new anthraquinones from the roots of Rubia cordifolia Linn. Pharmazie 58 (3), 216–217. 10.1023/A:1024895515316 [DOI] [PubMed] [Google Scholar]
- Adams M., Berset C., Kessler M., Hamburger M. (2009). Medicinal herbs for the treatment of rheumatic disorders-a survey of European herbals from the 16th and 17th century. J. Ethnopharmacol. 121 (3), 343–359. 10.1016/j.jep.2008.11.010 [DOI] [PubMed] [Google Scholar]
- Adwankar M. K., Chitnis M. P. (1982). In vivo anti-cancer activity of RC-18: A plant isolated from Rubia cordifolia, linn. Against a spectrum of experimental tumour models. Chemotherapy 28 (4), 291–293. 10.1159/000238092 [DOI] [PubMed] [Google Scholar]
- Adwankar M. K., Chitnis M. P., Khandalekar D. D., Bhadsavale C. G. (1980). Anti-cancer activity of the extracts of Rubia cordifolia Linn. (NSC b668893). Indian J. Exp. Biol. 18 (1), 102. [PubMed] [Google Scholar]
- Akhtar M., Ali M., Mir S. R., Singh O. (2006). New anthraquinones from Rubia cordifolia roots. ChemInform 45, 1945–1950. 10.1002/chin.200701211 [DOI] [Google Scholar]
- Anantharaman A., Priya R. R., Hemachandran H., Akella S., Rajasekaran C., Ganesh J., et al. (2016). Toxicity study of dibutyl phthalate of Rubia cordifolia fruits: In vivo and in silico analysis. Environ. Toxicol. 31 (9), 1059–1067. 10.1002/tox.22115 [DOI] [PubMed] [Google Scholar]
- Balachandran P., Ibrahim M. A., Zhang J., Wang M., Pasco D. S., Muhammad I. (2021). Crosstalk of cancer signaling pathways by cyclic hexapeptides and anthraquinones from Rubia cordifolia. Molecules 26 (3), 735. 10.3390/molecules26030735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu S., Ghosh A., Hazra B. (2005). Evaluation of the antibacterial activity of ventilago madraspatana gaertn., Rubia cordifolia linn. And lantana camara linn.: Isolation of emodin and physcion as active antibacterial agents. Phytother. Res. 19 (10), 888–894. 10.1002/ptr.1752 [DOI] [PubMed] [Google Scholar]
- Bradley L. D., Gueye N. A. (2016). The medical management of abnormal uterine bleeding in reproductive-aged women. Am. J. Obstet. Gynecol. 214 (1), 31–44. 10.1016/j.ajog.2015.07.044 [DOI] [PubMed] [Google Scholar]
- Bryant-Smith A. C., Lethaby A., Farquhar C., Hickey M. (2018). Antifibrinolytics for heavy menstrual bleeding. Cochrane Database Syst. Rev. 4, CD000249. 10.1002/14651858.CD000249.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukowski K., Kciuk M., Kontek R. (2020). Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 21 (9), E3233–33. 10.3390/ijms21093233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y., Sun M., Xing J., Corke H. (2004). Antioxidant phenolic constituents in roots of rheum officinale and Rubia cordifolia: Structure-radical scavenging activity relationships. J. Agric. Food Chem. 52 (26), 7884–7890. 10.1021/jf0489116 [DOI] [PubMed] [Google Scholar]
- Chang J., Kim Y., Kwon H. J. (2016). Advances in identification and validation of protein targets of natural products without chemical modification. Nat. Prod. Rep. 33 (5), 719–730. 10.1039/c5np00107b [DOI] [PubMed] [Google Scholar]
- Chang L. C., Chavez D., Gills J. J., Fong H. H. S., Pezzuto J. M., Kinghorn A. D. (2000). Rubiasins A-C, new anthracene derivatives from the roots and stems of Rubia cordifolia . Tetrahedron Lett. 41, 7157–7162. 10.1016/s0040-4039(00)01205-3 [DOI] [Google Scholar]
- Chen X. (2013). Research on the material basis of Rubia cordifolia L. in compound recipes. china: Nanjing Univ. Chin. Med, p135. [Google Scholar]
- Chen X., Zhao S., Wang Z., Zeng G., Huang M., Tan N. (2015). Rubicordins A-C, new cyclopeptides from Rubia cordifolia with cytotoxicity and inhibiting NF-kappa B signaling pathway. Tetrahedron 71 (52), 9673–9678. 10.1016/j.tet.2015.10.044 [DOI] [Google Scholar]
- Chen Y., Chen P. D., Bao B. H., Shan M. Q., Zhang K. C., Cheng F. F., et al. (2018). Anti-thrombotic and pro-angiogenic effects of Rubia cordifolia extract in zebrafish. J. Ethnopharmacol. 219, 152–160. 10.1016/j.jep.2017.11.005 [DOI] [PubMed] [Google Scholar]
- Chen Y., Shan M. Q., Wang H. L., Xue L., Zhang L., Ding A. W. (2017). Changes of chemical constituents in Rubiae Radix et Rhizoma before and after carbonized by UPLC-Q-TOF-MS method. Zhongguo Zhong Yao Za Zhi 42 (5), 923–930. 10.19540/j.cnki.cjcmm.20170121.035 [DOI] [PubMed] [Google Scholar]
- Cheng H., Huang G. (2018). Synthesis & antitumor activity of epothilones B and D and their analogs. Future Med. Chem. 10 (12), 1483–1496. 10.4155/fmc-2017-0320 [DOI] [PubMed] [Google Scholar]
- Cpmp C. F. P. M. (2004). Committee for proprietary medicinal products (CPMP): Points to consider on adjustment for baseline covariates. Stat. Med. 23 (5), 701–709. 10.1002/sim.1647 [DOI] [PubMed] [Google Scholar]
- Dai Y. J., Wan S. Y., Gong S. S., Liu J. C., Li F., Kou J. P. (2020). Recent advances of traditional Chinese medicine on the prevention and treatment of COVID-19. Chin. J. Nat. Med. 18 (12), 881–889. 10.1016/S1875-5364(20)60031-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai Y., Zhang K., Xiong L., Wang L., Guo Z., Yang J., et al. (2022). Comprehensive profiling of Sanguisorba officinalis using offline two-dimensional mixed-mode liquid chromatography × reversed-phase liquid chromatography, tandem high-resolution mass spectrometry, and molecular network. J. Sep. Sci. 45 (10), 1727–1736. 10.1002/jssc.202200013 [DOI] [PubMed] [Google Scholar]
- Daniyal M., Akram M., Zainab R., Munir N., Sharif A., Shah S., et al. (2019). Prevalence and current therapy in chronic liver disorders. Inflammopharmacology 27 (2), 213–231. 10.1007/s10787-019-00562-z [DOI] [PubMed] [Google Scholar]
- Dengre R. G., Patel K. N., Chauhan M. B. (1993). Comparative studies of Rubia cordifolia linn. And Rubia tinctorum linn (Rubiaceae). Anc. Sci. Life 13 (1-2), 165–179. [PMC free article] [PubMed] [Google Scholar]
- Deoda R. S., Kumar D., Bhujbal S. S. (2011). Gastroprotective effect of Rubia cordifolia Linn. on aspirin plus pylorus-ligated ulcer. J. Evidence-Based Complement . Evid. Based. Complement. Altern. Med. 20 (11), 541624–624. 10.1155/2011/541624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshkar N., Tilloo S., Pande V. B. (2008). A comprehensive review of Rubia cordifolia Linn. Pharmacogn. Rev. 2 (3), 124–134. [Google Scholar]
- Dima A., Jurcut C., Manolache A., Balaban D. V., Popp A., Jinga M. (2018). Hemorrhagic events in adult celiac disease patients. Case report and review of the literature. J. Gastrointestin. Liver Dis. 27 (1), 93–99. 10.15403/jgld.2014.1121.271.cld [DOI] [PubMed] [Google Scholar]
- Divakar K., Pawar A. T., Chandrasekhar S. B., Dighe S. B., Divakar G. (2010). Protective effect of the hydro-alcoholic extract of Rubia cordifolia roots against ethylene glycol induced urolithiasis in rats. Food Chem. Toxicol. 48 (4), 1013–1018. 10.1016/j.fct.2010.01.011 [DOI] [PubMed] [Google Scholar]
- Dkhil M. A., Delic D., El E. H., Abdel M. A. (2016). Medicinal plants in therapy: Antioxidant activities. Oxid. Med. Cell. Longev. 1, 7468524. 10.1155/2016/7468524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Do M. T., Hwang Y. P., Kim H. G., Na M., Jeong H. G. (2013). Mollugin inhibits proliferation and induces apoptosis by suppressing fatty acid synthase in HER2-overexpressing cancer cells. J. Cell. Physiol. 228 (5), 1087–1097. 10.1002/jcp.24258 [DOI] [PubMed] [Google Scholar]
- Dosseh C., Tessier A. M., Delaveau P. (1981a). Rubia cordifolia roots. II: New quinones. Planta Med. 43 (2), 141–147. 10.1055/s-2007-971490 [DOI] [PubMed] [Google Scholar]
- Dosseh C., Tessier A. M., Delaveau P. (1981b). Rubia cordifolia roots. II: New quinones]. Planta Med. 43 (2), 141–147. 10.1055/s-2007-971490 [DOI] [PubMed] [Google Scholar]
- Du Aa. (2002). Clinical experience of Mongolian medicine qixiongpill in treating 21 cases of mycoplasma pneumoniae pneumonia. Natl. Med. J. China. 1 (3), 28. 10.16041/j.cnki.cn15-1175.2002.03.027 [DOI] [Google Scholar]
- Eddouks M., Maghrani M., Lemhadri A., Ouahidi M. L., Jouad H. (2002). Ethnopharmacological survey of medicinal plants used for the treatment of diabetes mellitus, hypertension and cardiac diseases in the south-east region of Morocco (Tafilalet). J. Ethnopharmacol. 82 (2-3), 97–103. 10.1016/s0378-8741(02)00164-2 [DOI] [PubMed] [Google Scholar]
- Ekor M. (2014). The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 4, 177. 10.3389/fphar.2013.00177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng L., Zhang A. X., Li L., Zhang X. J., Wang Z., Tan N. H. (2021). Diversity of cultivable endophytic fungi in two Rubia plants and their potential for production of anti-tumour Rubiaceae-type cyclopeptides. Lett. Appl. Microbiol. 73 (6), 759–769. 10.1111/lam.13571 [DOI] [PubMed] [Google Scholar]
- Gao L., Wang K., Jia Y., Rong L. (2020). Effects of different processed products of Rubia cordifolia Linn. on endometrial hemorrhage model rats. J. Chin. Med. 35 (01), 144–148. 10.16368/j.issn.1674-8999.2020.01.033 [DOI] [Google Scholar]
- Gao M., Yang J., Wang Z., Yang B., Kuang H., Liu L., et al. (2016). Simultaneous determination of purpurin, munjistin and mollugin in rat plasma by ultra-high -performance liquid chromatography-tandem mass spectrometry: Application to a pharmacokinetic study after oral administration of Rubia cordifolia L. Extr. Mol. 21 (6), 717. 10.3390/molecules21060717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gezici S., Sekeroglu N. (2019). Current perspectives in the application of medicinal plants against cancer: Novel therapeutic agents. Anticancer. Agents Med. Chem. 19 (1), 101–111. 10.2174/1871520619666181224121004 [DOI] [PubMed] [Google Scholar]
- Ghosh S., Das S. M., Patra A., Hazra B. (2010). Anti-inflammatory and anticancer compounds isolated from ventilago madraspatana gaertn., Rubia cordifolia linn. And lantana camara linn. J. Pharm. Pharmacol. 62 (9), 1158–1166. 10.1111/j.2042-7158.2010.01151.x [DOI] [PubMed] [Google Scholar]
- Ginovyan M., Ayvazyan A., Nikoyan A., Tumanyan L., Trchounian A. (2020). Phytochemical screening and detection of antibacterial components from crude extracts of some Armenian herbs using TLC-bioautographic technique. Curr. Microbiol. 77 (7), 1223–1232. 10.1007/s00284-020-01929-0 [DOI] [PubMed] [Google Scholar]
- Gong X. P., Sun Y. Y., Chen W., Guo X., Guan J. K., Li D. Y., et al. (2017). Anti-diarrheal and anti-inflammatory activities of aqueous extract of the aerial part of Rubia cordifolia . BMC Complement. Altern. Med. 17 (1), 20. 10.1186/s12906-016-1527-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo M., Zhu F., Qiu W., Qiao G., Law B. Y. K., Yu L., et al. (2022). High-throughput screening for amyloid-β binding natural small-molecules based on the combinational use of biolayer interferometry and UHPLC− DAD-Q/TOF-MS/MS. Acta Pharm. Sin. B 12 (4), 1723–1739. 10.1016/j.apsb.2021.08.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gupta M., Shaw B., Mukherjee A. (2008). Evaluation of antipyretic effect of a traditional polyherbal preparation: A double-blind, randomized clinical trial. Int. J. Pharmacol. 4 (3), 190–195. 10.3923/ijp.2008.190.195 [DOI] [Google Scholar]
- Gupta R., Srivastava D., Sahu M., Tiwari S., Ambasta R. K., Kumar P. (2021). Artificial intelligence to deep learning: Machine intelligence approach for drug discovery. Mol. Divers. 25 (3), 1315–1360. 10.1007/s11030-021-10217-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassanean H. A., Ibraheim Z. Z., Takeya K., Itorawa H. (2000). Further quinoidal derivatives from Rubia cordifolia L. Pharmazie 55 (4), 317–319. 10.1023/A:1007549625317 [DOI] [PubMed] [Google Scholar]
- Hideji I., Kazuhiko M., Koichi T. (1983). Studies on a novel anthraquinone and its glycosides isolated from Rubia cordifolia and R. akane . Chem. Pharm. Bull. 31 (7), 2353–2358. 10.1248/cpb.31.2353 [DOI] [Google Scholar]
- Hideji I., Zedan Z. I., Ya-Fang Q., Koichi T. (1993). Anthraquinones, naphthohydroquinones and naphthohydroquinone dimers from Rubia cordifolia and their cytotoxic activity. Chem. Pharm. Bull. 41 (10), 1869–1872. 10.1248/cpb.41.1869 [DOI] [PubMed] [Google Scholar]
- Hitotsuyanagi Y., Hasuda T., Aihara T., Ishikawa H., Yamaguchi K., Itokawa H., et al. (2004). Synthesis of [Gly-1]RA-VII, [Gly-2]RA-VII, and [Gly-4]RA-VII. Glycine-containing analogues of RA-VII, an antitumor bicyclic hexapeptide from Rubia plants. J. Org. Chem. 69 (5), 1481–1486. 10.1021/jo030293p [DOI] [PubMed] [Google Scholar]
- Hitotsuyanagi Y., Hirai M., Odagiri M., Komine M., Hasuda T., Fukaya H., et al. (2019). RA-XXV and RA-XXVI, bicyclic hexapeptides from Rubia cordifolia L.: Structure, synthesis, and conformation. Chem. Asian J. 14 (1), 205–215. 10.1002/asia.201801466 [DOI] [PubMed] [Google Scholar]
- Hitotsuyanagi Y., Odagiri M., Kato S., Kusano J., Hasuda T., Fukaya H., et al. (2012). Isolation, structure determination, and synthesis of allo-RA-V and neo-RA-V, RA-series bicyclic peptides from Rubia cordifolia L. Chemistry 18 (10), 2839–2846. 10.1002/chem.201103185 [DOI] [PubMed] [Google Scholar]
- Hitotsuyanagi Y., Tsuchiya T., Ohata M., Yoshida A., Fukaya H., Park H. S., et al. (2016). RA-dimer B, a new dimeric RA-series cyclopeptide incorporating two different types of cycloisodityrosine units, from Rubia cordifolia L. Chem. Asian J. 11 (23), 3389–3397. 10.1002/asia.201601138 [DOI] [PubMed] [Google Scholar]
- Ho L. K., Don M. J., Chen H. C., Yeh S. F., Chen J. M. (1996). Inhibition of Hepatitis B surface antigen secretion on human hepatoma cells. Components from Rubia cordifolia. J. Nat. Prod. 59 (3), 330–333. 10.1021/np960200h [DOI] [PubMed] [Google Scholar]
- Hu T. (2019). Clinical study in Rubia cordifolia in the treatment of abnormal uterine beeding by intrauterine device placed. china: Hangzhou Normal University, 39. [Google Scholar]
- Hua H. M., Wang S. X., Wu L. J., Li X., Zhu T. R. (1992). Studies on naphthoic acid esters from the roots of Rubia cordifolia L. Yao Xue Xue Bao 27 (4), 279–282. [PubMed] [Google Scholar]
- Ibraheim Z. Z., Gouda Y. G. (2010). Minor constituents from Rubia cordifolia L. root. Bull. Pharm. Sci. Assiut 33 (2), 225–233. 10.21608/bfsa.2010.64774 [DOI] [Google Scholar]
- Inoue K., Yoshida M., Takahashi M., Fujimoto H., Shibutani M., Hirose M., et al. (2009). Carcinogenic potential of alizarin and rubiadin, components of madder color, in a rat medium-term multi-organ bioassay. Cancer Sci. 100 (12), 2261–2267. 10.1111/j.1349-7006.2009.01342.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itokawa H., Ibraheim Z. Z., Qiao Y. F., Takeya K. (1993). Anthraquinones, naphthohydroquinones and naphthohydroquinone dimers from Rubia cordifolia and their cytotoxic activity. Chem. Pharm. Bull. (Tokyo). 41 (10), 1869–1872. 10.1248/cpb.41.1869 [DOI] [PubMed] [Google Scholar]
- Itokawa H., Morita H., Takeya K., Tomioka N., Itai A., Iitaka Y. (1991). New antitumor bicyclic hexapeptides, RA-VI and-VIII from Rubia cordifolia; conformation-activity relationship II. Tetrahedron 47 (34), 7007–7020. 10.1016/s0040-4020(01)96155-1 [DOI] [Google Scholar]
- Itokawa H., Qiao Y. F., Takeya K., Iitaka Y. (1989a). New triterpenoids from Rubia cordifolia var pratensis (Rubiaceae). Chem. Pharm. Bull. 37, 1670–1672. 10.1248/cpb.37.1670 [DOI] [Google Scholar]
- Itokawa H., Qiao Y., Takeya K. (1989b). Anthraquinones and naphthohydroquinones from Rubia cordifolia . Phytochemistry 28 (12), 3465–3468. 10.1016/0031-9422(89)80365-6 [DOI] [Google Scholar]
- Itokawa H., Saitou K., Morita H., Takeya K., Yamada K. (1992). Structures and conformations of metabolites of antitumor cyclic hexapeptides, RA-VII and RA-X. Chem. Pharm. Bull. 40 (11), 2984–2989. 10.1248/cpb.40.2984 [DOI] [PubMed] [Google Scholar]
- Jeong G. S., Lee D. S., Kim D. C., Jahng Y., Son J. K., Lee S. H., et al. (2011). Neuroprotective and anti-inflammatory effects of mollugin via up-regulation of heme oxygenase-1 in mouse hippocampal and microglial cells. Eur. J. Pharmacol. 654 (3), 226–234. 10.1016/j.ejphar.2010.12.027 [DOI] [PubMed] [Google Scholar]
- Jeong S. Y., Zhao B. T., Lee C. S., Son J. K., Min B. S., Woo M. H. (2012). Constituents with DNA topoisomerases I and II inhibitory activity and cytotoxicity from the roots of Rubia cordifolia . Planta Med. 78 (2), 177–181. 10.1055/s-0031-1280265 [DOI] [PubMed] [Google Scholar]
- Jouad H., Haloui M., Rhiouani H., El H. J., Eddouks M. (2001). Ethnobotanical survey of medicinal plants used for the treatment of diabetes, cardiac and renal diseases in the North centre region of Morocco (Fez-Boulemane). J. Ethnopharmacol. 77 (2-3), 175–182. 10.1016/s0378-8741(01)00289-6 [DOI] [PubMed] [Google Scholar]
- Joy J., Nair C. K. (2008). Amelioration of cisplatin induced nephrotoxicity in Swiss albino mice by Rubia cordifolia extract. J. Cancer Res. Ther. 4 (3), 111–115. 10.4103/0973-1482.43139 [DOI] [PubMed] [Google Scholar]
- Jun D. Y., Cr H., Jy L., W P., Ms C., Mh W., et al. (2011). Anti-adipogenic activity of 2-carbomethoxy-2, 3-epoxy-3-prenyl-1, 4-naphthoquinone from Rubia cordifolia L. J. Med. Food 14 (5), 454–461. 10.1089/jmf.2010.1385 [DOI] [PubMed] [Google Scholar]
- Kalyoncu F., Cetin B., Saglam H. (2006). Antimicrobial activity of common madder (Rubia tinctorum L.). Phytother. Res. 20 (6), 490–492. 10.1002/ptr.1884 [DOI] [PubMed] [Google Scholar]
- Karim A., Mekhfi H., Ziyyat A., Legssyer A., Bnouham M., Amrani S., et al. (2010). Anti-diarrhoeal activity of crude aqueous extract of Rubia tinctorum L. roots in rodents. J. Smooth Muscle Res. 46 (2), 119–123. 10.1540/jsmr.46.119 [DOI] [PubMed] [Google Scholar]
- Khandia R., Munjal A. (2020). Interplay between inflammation and cancer. Adv. Protein Chem. Struct. Biol. 119, 199–245. 10.1016/bs.apcsb.2019.09.004 [DOI] [PubMed] [Google Scholar]
- King Suxian H. M. L. W. (1992). Study on anthraquinones in. rubia. Chin. J. Pharm. 10, 743–747. [Google Scholar]
- Larga X. T., Orchid W., le Chau B. (2021). Determination of alizarin in Mongolian yao sanhong decoction. Mod. Chin. Med. 41 (02), 35–39. 10.13424/j.cnki.mtcm.2021.02.007 [DOI] [Google Scholar]
- Lee J. E., Hitotsuyanagi Y., Fukaya H., Kondo K., Takeya K. (2008b). New cytotoxic bicyclic hexapeptides, RA-XXIII and RA-XXIV, from Rubia cordifolia L. . Chem. Pharm. Bull. (Tokyo). 56 (5), 730–733. 10.1248/cpb.56.730 [DOI] [PubMed] [Google Scholar]
- Lee J. E., Hitotsuyanagi Y., Kim I. H., Hasuda T., Takeya K. (2008a). A novel bicyclic hexapeptide, RA-XVIII, from Rubia cordifolia: Structure, semi-synthesis, and cytotoxicity. Bioorg. Med. Chem. Lett. 18 (2), 808–811. 10.1016/j.bmcl.2007.11.030 [DOI] [PubMed] [Google Scholar]
- Li H., Meng X. (2018). Textual research on Chinese herbal medicine Rubia cordifolia L. Chin. Herb. Med. 41 (10), 2462–2466. 10.13863/j.issn1001-4454.2018.10.045 [DOI] [Google Scholar]
- Li H., Xiao L., Zhang J., Wang H., Han W., Huang Z. (2016). Research progress on chemical constituents and pharmacological effects of Rubia cordifolia L. Chin. Tradit. Herb. Drugs. 39 (06), 1433–1436. 10.13863/j.issn1001-4454.2016.06.058 [DOI] [Google Scholar]
- Li J., Zhang J. L., Gong X. P., Xiao M., Song Y. Y., Pi H. F., et al. (2020). Anti-inflammatory activity of mollugin on DSS-induced colitis in mice. Curr. Med. Sci. 40 (5), 910–916. 10.1007/s11596-020-2262-5 [DOI] [PubMed] [Google Scholar]
- Li X. (2011). Bletilla striata Reichb.f Zhixue decoction in the treatment of 40 cases of vaginal stump bleeding. Nei Mongol . J. Trad. Chin. Med. 30 (15), 2. 10.16040/j.cnki.cn15-1101.2011.15.013 [DOI] [Google Scholar]
- Li X., Liu Z., Chen Y., Wang L. J., Zheng Y. N., Sun G. Z., et al. (2009). Rubiacordone A: A new anthraquinone glycoside from the roots of Rubia cordifolia . Molecules 14 (1), 566–572. 10.3390/molecules14010566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X. (2000). On the application of Rubia cordifolia L. to remove blood stasis and stop bleeding. Prac. Med. Technolo. 2 (7), 39–40. [Google Scholar]
- Lignitto L., Leboeuf S. E., Homer H., Jiang S., Askenazi M., Karakousi T. R., et al. (2019). Nrf2 activation promotes lung cancer metastasis by inhibiting the degradation of Bach1. Cell 178 (2), 316–329. e18. 10.1016/j.cell.2019.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limon I. D., Angulo-Cruz I., Sanchez-Abdon L., Patricio-Martinez A. (2021). Disturbance of the glutamate-glutamine cycle, secondary to hepatic damage, compromises memory function. Front. Neurosci. 15, 578922. 10.3389/fnins.2021.578922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Zhang B., Zhang Y., Zheng B. (2020). Research status and prospect of Chinese herbal Helichrysum fructus. J. Agric. Sci. 48 (22), 15–21. 10.15889/j.issn.1002-1302.2020.22.003 [DOI] [Google Scholar]
- Liu N., Bai Y., Ji X. (2021). Determination of the contents of hydroxy alizarin and rubidin in Mongolian medicine Wulan Sanwei Tang powder by HPLC. Chin. J. Ethn. Med. 27 (6), 46–48. 10.16041/j.cnki.cn15-1175.2021.06.027 [DOI] [Google Scholar]
- Lodi S., Sharma V., Kansal L. (2011). The protective effect of Rubia cordifolia against lead nitrate-induced immune response impairment and kidney oxidative damage. Indian J. Pharmacol. 43 (4), 441–444. 10.4103/0253-7613.83118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y., Hu R., Dai Z., Pan Y. (2010). Preparative separation of anti-oxidative constituents from Rubia cordifolia by column-switching counter-current chromatography. J. Sep. Sci. 33 (14), 2200–2205. 10.1002/jssc.201000173 [DOI] [PubMed] [Google Scholar]
- Luo H., Qin W., Zhang H., Ren F. C., Fang W. T., Kong Q. H., et al. (2022). Anthraquinones from the aerial parts of Rubia cordifolia with their NO inhibitory and antibacterial activities. Molecules 27 (5), 1730. 10.3390/molecules27051730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez M. D., Jafari R., Ignatushchenko M., Seki T., Larsson E. A., Dan C., et al. (2013). Monitoring drug target engagement in cells and tissues using the cellular thermal shift assay. Science 341 (6141), 84–87. 10.1126/science.1233606 [DOI] [PubMed] [Google Scholar]
- Meng H. (2010). 50 Cases of epistaxis treated with Liangxuezhixue decoction. Shaanxi J. Trad. Chin. Med. 31 (08), 1010–1011. [Google Scholar]
- Mohd Z. A., Ng S. F., Ng M. H., Hassan H., Alias E. (2020). Pharmacology and pharmacokinetics of vitamin E: Nanoformulations to enhance bioavailability. Int. J. Nanomedicine 15, 9961–9974. 10.2147/IJN.S276355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morita H., Yamamiya T., Takeya K., Itokawa H. (1992). New antitumor bicyclic hexapeptides, RA-XI, -XII, -XIII and -XIV from Rubia cordifolia . Chem. Pharm. Bull. 40 (5), 1352–1354. 10.1248/cpb.40.1352 [DOI] [PubMed] [Google Scholar]
- Nagalla S., Bray P. F. (2016). Personalized medicine in thrombosis: Back to the future. Blood 127 (22), 2665–2671. 10.1182/blood-2015-11-634832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natarajan S., Mishra P., Vadivel M., Basha M. G., Kumar A., Velusamy S. (2019). ISSR characterization and quantification of purpurin and alizarin in Rubia cordifolia L. populations from India. Biochem. Genet. 57 (1), 56–72. 10.1007/s10528-018-9875-4 [DOI] [PubMed] [Google Scholar]
- Ni Y., Xia J., Dong C. (2022). Study on dyeing of silk fabric with Rubia cordifolia Linn. root dye. Dye. Finish. 5 (19), 1–9. [Google Scholar]
- Niang Q., Ren Z., Bao D., Cuo J., Nao H., Li X. (2021). Analysis of the medication rules and medicinal properties of external prescriptions for traumatic injuries in "Four Medical Canons. World Sci. Techn. Mod. Trad. Chi. Med. 23 (05), 1698–1704. [Google Scholar]
- Pankaj B., Ajay S. K. 2013). Rubia cordifolia overview: A new approach to treat cardiac disorders. Inter. J. Drug Dev. Res. 5 (2). [Google Scholar]
- Patil R. A., Kasture S. B. (2012). Protective effect of Rubia cordifolia on reserpine-induced orofacial dyskinesia. Nat. Prod. Res. 26 (22), 2159–2161. 10.1080/14786419.2011.635341 [DOI] [PubMed] [Google Scholar]
- Patil R., Mohan M., Kasture V., Kasture S. (2009). Rubia cordifolia: A review. Orient. Pharm. Exp. Med. 9 (1), 1–13. 10.3742/OPEM.2009.9.1.001 [DOI] [Google Scholar]
- Pawar A. T., Anap R. M., Ghodasara J. V., Kuchekar B. S. (2011). Protective effect of hydroalcoholic root extract of Rubia cordifolia in indomethacin-induced enterocolitis in rats. Indian J. Pharm. Sci. 73 (2), 250–253. 10.4103/0250-474x.91577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar A. T., Kalyani D., Chandrasekar S. B., Divakar G. (2009). Diuretic activity of root extract of Rubia cordifolia Linn. Pharmacologyonline 1, 597–603. [Google Scholar]
- Qiao Y. F., Wang S. X., Wu L. J., Li X., Zhu T. R. (1990). Studies on antibacterial constituents from the roots of Rubia cordifolia L. Acta. Pharm. Sin. 25 (11), 834–839. 10.1152/ajplung.00508.2005 [DOI] [PubMed] [Google Scholar]
- Rao G. M. M., Rao C. V., Pushpangadan P., Shirwaikar A. (2006). Hepatoprotective effects of rubiadin, a major constituent of Rubia cordifolia Linn. Watroly. J. Ethnopharmacol. 103 (3), 484–490. 10.1016/j.jep.2005.08.073 [DOI] [PubMed] [Google Scholar]
- Rawal A. K., Muddeshwar M. G., Biswas S. K. (2004). Rubia cordifolia, fagonia cretica linn and tinospora cordifolia exert neuroprotection by modulating the antioxidant system in rat hippocampal slices subjected to oxygen glucose deprivation. BMC Complement. Altern. Med. 4 (11). 10.1186/1472-6882-4-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen C. H., Liu C. T., Song X. J., Zeng W. Y., Lu X. Y., Zheng Z. L., et al. (2018). Evaluation of analgesic and anti-inflammatory activities of Rubia cordifolia L. by spectrum-effect relationships. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 1090, 73–80. 10.1016/j.jchromb.2018.05.021 [DOI] [PubMed] [Google Scholar]
- Shen R. (2013). Study on the dietary recipe of "taiping shenghui prescription, 63. china, Beijing Univ. Chin. Med. [Google Scholar]
- Shen X., Li H., Zou W. J., Wu J. M., Wang L., Wang W., et al. (2021). Network pharmacology analysis of the therapeutic mechanisms underlying beimu-gualou formula activity against bronchiectasis with in silico molecular docking validation. Evid. Based. Complement. Altern. Med. 2021, 3656272. 10.1155/2021/3656272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Ji H. (2014). Efficacy observation of infantile acute diarrhea treated adjunctively with Er-Xie-Ting . Electron. Lett. 9, 1318–1320. 10.1049/el.2014.1458 [DOI] [Google Scholar]
- Shikov A. N., Narkevich I. A., Flisyuk E. V., Luzhanin V. G., Pozharitskaya O. N. (2021). Medicinal plants from the 14(th) edition of the Russian Pharmacopoeia, recent updates. J. Ethnopharmacol. 268, 113685. 10.1016/j.jep.2020.113685 [DOI] [PubMed] [Google Scholar]
- Shilpa P. N., Sivaramakrishnan V., Niranjali D. S. (2012a). Induction of apoptosis by methanolic extract of Rubia cordifolia Linn in HEp-2 cell line is mediated by reactive oxygen species. Asian pac. J. Cancer Prev. 13 (6), 2753–2758. 10.7314/apjcp.2012.13.6.2753 [DOI] [PubMed] [Google Scholar]
- Shilpa P. N., Venkatabalasubramanian S., Devaraj S. N. (2012b). Ameliorative effect of methanol extract of Rubia cordifolia in N-nitrosodiethylamine-induced hepatocellular carcinoma. Pharm. Biol. 50 (3), 376–383. 10.3109/13880209.2011.608073 [DOI] [PubMed] [Google Scholar]
- Singh J., Hussain Y., Luqman S., Meena A. (2020). Purpurin: A natural anthraquinone with multifaceted pharmacological activities. Phytotherapy Res. 11 (30), 2418–2428. 10.1002/ptr.6965 [DOI] [PubMed] [Google Scholar]
- Singh R., Geetanjali G. (2005). Isolation and synthesis of anthraquinones and related compounds of Rubia cordifolia . J. Serbian. Chem. Soc. 70 (7), 937–942. 10.2298/JSC0507937S [DOI] [Google Scholar]
- Siril E. A. (2013). Pharmacognostic studies on Indian madder (Rubia cordifolia L.). J. Pharm. Phytochem. 1 (5). [Google Scholar]
- Son J. K., Jung J. H., Lee C. S., Moon D. C., Choi S. W., Min B. S., et al. (2006). DNA Topoisomerases I and II inhibition and cytotoxicity of constituents from the roots of Rubia cordifolia . Bull. Korean Chem. Soc. 27 (8), 1231–1234. 10.5012/bkcs.2006.27.8.1231 [DOI] [Google Scholar]
- Son J. K., Jung S. J., Jung J. H., Fang Z., Lee C. S., Seo C. S., et al. (2008). Anticancer constituents from the roots of Rubia cordifolia L. Chem. Pharm. Bull. 56 (2), 213–216. 10.1248/cpb.56.213 [DOI] [PubMed] [Google Scholar]
- Sun Y., Scruggs D. W., Peng Y., Johnson J. R., Shukla A. J. (2004). Issues and challenges in developing long-acting veterinary antibiotic formulations. Adv. Drug Deliv. Rev. 56 (10), 1481–1496. 10.1016/j.addr.2004.02.009 [DOI] [PubMed] [Google Scholar]
- Takeya K., Yamamiya T., Morita H., Itokawa H. (1993). Two antitumour bicyclic hexapeptides from Rubia cordifolia. Phytochemistry 33 (3), 613–615. 10.1016/0031-9422(93)85458-4 [DOI] [PubMed] [Google Scholar]
- Talapatra S. K., Sarkar A. C., Talapatra B. (1981). Two pentacyclic triterpenes from Rubia cordifolia . Phytochemistry 20, 1923–1927. 10.1016/0031-9422(81)84036-8 [DOI] [Google Scholar]
- Tamma P. D., Avdic E., Li D. X., Dzintars K., Cosgrove S. E. (2017). Association of adverse events with antibiotic use in hospitalized patients. JAMA Intern. Med. 177 (9), 1308–1315. 10.1001/jamainternmed.2017.1938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessier A. M., Delaveau P., Champion B. (1981). New anthraquinones in Rubia cordifolia roots. Planta Med. 41 (4), 337–343. 10.1055/s-2007-971724 [DOI] [PubMed] [Google Scholar]
- Torre L. A., Siegel R. L., Ward E. M., Jemal A. (2016). Global cancer incidence and mortality rates and trends-an update. Cancer Epidemiol. Biomarkers Prev. 25 (1), 16–27. 10.1158/1055-9965.EPI-15-0578 [DOI] [PubMed] [Google Scholar]
- Tripathi Y. B., Pandey S., Shukla S. D. (1993). Anti-platelet activating factor property of Rubia cordifolia Linn. Indian J. Exp. Biol. 31 (6), 533–535. [PubMed] [Google Scholar]
- Tse W. P., Cheng C. H., Che C. T., Zhao M., Lin Z. X. (2007). Induction of apoptosis underlies the radix rubiae-mediated anti-proliferative action on human epidermal keratinocytes: Implications for psoriasis treatment. Int. J. Mol. Med. 20 (5), 663–672. 10.3892/ijmm.20.5.663 [DOI] [PubMed] [Google Scholar]
- Venkateswaran S., Pari L., Viswanathan P., Menon V. P. (1997). Protective effect of Livex, a herbal formulation against erythromycin estolate induced hepatotoxicity in rats. J. Ethnopharmacol. 57 (3), 161–167. 10.1016/s0378-8741(97)00062-7 [DOI] [PubMed] [Google Scholar]
- Wang A. K., Geng T., Jiang W., Zhang Q., Zhang Y., Chen P. D., et al. (2020a). Simultaneous determination of twelve quinones from Rubiae radix et Rhizoma before and after carbonization processing by UPLC-MS/MS and their antithrombotic effect on zebrafish. J. Pharm. Biomed. Anal. 191, 113638. 10.1016/j.jpba.2020.113638 [DOI] [PubMed] [Google Scholar]
- Wang J., Guo Y., Li G. L. (2016). Current status of standardization of traditional Chinese medicine in China. Evid. Based. Complement. Altern. Med. 2016, 9123103. 10.1155/2016/9123103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J. (2014). Study on the chemical and biological activity of Rubia cordifolia L. Kaifeng, China: Henan University, 69. [Google Scholar]
- Wang K., Gao L., Zhang Q., Zhang Y., Yao W., Zhang M., et al. (2020c). Revealing the mechanisms and the material basis of Rubia cordifolia L. on abnormal uterine bleeding with uniting simultaneous determination of four components and systematic pharmacology approach-experimental validation. J. Pharm. Biomed. Anal. 189, 113475. 10.1016/j.jpba.2020.113475 [DOI] [PubMed] [Google Scholar]
- Wang L., Li H., Shen X., Zeng J., Yue L., Lin J., et al. (2020b). Elucidation of the molecular mechanism of Sanguisorba Officinalis L. against leukopenia based on network pharmacology. Biomed. Pharmacother. 132, 110934. 10.1016/j.biopha.2020.110934 [DOI] [PubMed] [Google Scholar]
- Wang L., Zhang T., Liu S., Mo Q., Jiang N., Chen Q., et al. (2022b). Discovery of a novel megakaryopoiesis enhancer, ingenol, promoting thrombopoiesis through PI3K-Akt signaling independent of thrombopoietin. Pharmacol. Res. 177, 106096. 10.1016/j.phrs.2022.106096 [DOI] [PubMed] [Google Scholar]
- Wang S. X., Hua H. M., Wu L. J., Li X., Zhu T. R. (1992). Studies on anthraquinones from the roots of Rubia cordifolia L. Acta Pharm. Sin. 27 (10), 743–747. [PubMed] [Google Scholar]
- Wang T., Tian X., Cheng F., Zhang X., Liu S., Wang Q. (2021). Analysis of the efficacy and compatibility of Rubia cordifolia Linn. and safflower. Glob. Tradit. Chin. Med. 14 (05), 873–875. 10.3969/1749.2021.05.018 [DOI] [Google Scholar]
- Wang X. (2013). Observation on the curative effect of Mongolian medicine Yishen Qiwei pill and Western medicine in the treatment of chronic pelvic inflammatory disease. J. Inn. Mong. Univ. Natl. 28 (01), 81–82. 10.14045/j.cnki.15-1220.2013.01.017 [DOI] [Google Scholar]
- Wang Y., Yang R., Zhang R. (2022a). Research progress in study of sheng ji zong Lu in last ten years. J. Basic Chin. Med. 28 (03), 486–490. 10.19945/j.cnki.issn.1006-3250.2022.03.007 [DOI] [Google Scholar]
- Wang Z., Li M. Y., Mi C., Wang K. S., Ma J., Jin X. (2017). Mollugin has an anti-cancer therapeutic effect by inhibiting TNF-α-Induced NF-κB activation. Int. J. Mol. Sci. 18 (8), 1619. 10.3390/ijms18081619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z., Yang L. (2021). Chinese herbal medicine: Fighting SARS-CoV-2 infection on all fronts. J. Ethnopharmacol. 270, 113869. 10.1016/j.jep.2021.113869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watroly M. N., Sekar M., Fuloria S., Gan S. H., Jeyabalan S., Wu Y. S., et al. (2021). Chemistry, biosynthesis, physicochemical and biological properties of rubiadin: A promising natural anthraquinone for new drug discovery and development. Drug Des. devel. Ther. 15, 4527–4549. 10.2147/DDDT.S338548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright G. D. (2017). Opportunities for natural products in 21(st) century antibiotic discovery. Nat. Prod. Rep. 34 (7), 694–701. 10.1039/c7np00019g [DOI] [PubMed] [Google Scholar]
- Wu M., Yang X. (2016). Application of madder dyes in ancient Europe. J. Silk. 53 (10), 28–32. 10.3969.1001/7003.2016.10.006 [DOI] [Google Scholar]
- Wu Y. (2008a). 324 cases of chronic epididymitis treated with Mongolian medicine yishen shiqiwei pill. J. Med Phar. Chin. Minoritie. (4), 29. 10.16041/j.cnki.cn15-1175.2008.04.025 [DOI] [Google Scholar]
- Wu Y. (2008b). Treatment of 32 cases of spermatic cord hydrocele with Mongolian medicine Yishen Qiwei Pill. Joumal Inn. Mong. Univ. Natl. 14 (02), 102. [Google Scholar]
- Xie Z., Tian Y., Ma L., Lv X., Cheng K., Li S., et al. (2019). Developments in inhibiting platelet aggregation based on different design strategies. Future Med. Chem. 11 (14), 1757–1775. 10.4155/fmc-2018-0345 [DOI] [PubMed] [Google Scholar]
- Xu H., Qin H., Chen S., Guan M. (2022). Vitamin E in the treatment of tardive dyskinesia: A meta-analysis. Int. Clin. Psychopharmacol. 37 (2), 60–66. 10.1097/YIC.0000000000000387 [DOI] [PubMed] [Google Scholar]
- Xu L., Hu S., Hu Q., Leng P., Gao E. (2002). Effect of total anthraquinone of Rubia cordifolia L. On adjuvant arthritis of rat and its cytokine. Acta acade. Med. weifang. 1, 11–13. [Google Scholar]
- Xu L., Xing M., Xu X., Saadeldeen F. S., Liu Z., Wei J., et al. (2019). Alizarin increase glucose uptake through PI3K/Akt signaling and improve alloxan-induced diabetic mice. Future Med. Chem. 11 (5), 395–406. 10.4155/fmc-2018-0515 [DOI] [PubMed] [Google Scholar]
- Xun L. (2014). Quality control study of Rubia cordifolia L. And the fakes. Master’s thesis . Beijing, China: Beijing University of Chinese Medicine. [Google Scholar]
- Yusuf M., Shahid M., Khan S. A., Khan M. I., Islam S. U., Mohammad F., et al. (2013). Eco-dyeing of wool using aqueous extract of the roots of Indian madder (Rubia cordifolia) as natural dye. J. Nat. Fibers 10 (1), 14–28. 10.1080/15440478.2012.738026 [DOI] [Google Scholar]
- Zhang C. (2011). 120 cases of dysfunctional uterine bleeding treated by Rubia cordifolia decoction. Mod. J. Int. Trad. Chi. West. Med. 20 (14), 1747–1748. [Google Scholar]
- Zhang L., Wang H., Zhu J., Xu J., Ding K. (2014). Mollugin induces tumor cell apoptosis and autophagy via the PI3K/AKT/mTOR/p70S6K and ERK signaling pathways. Biochem. Biophys. Res. Commun. 450 (1), 247–254. 10.1016/j.bbrc.2014.05.101 [DOI] [PubMed] [Google Scholar]
- Zhang L., Xu Z., Jiang T., Zhang J., Huang P., Tan J., et al. (2021). Efficacy and safety of ejiao (asini corii colla) in women with blood deficient symptoms: A randomized, double-blind, and placebo-controlled clinical trial. Front. Pharmacol. 12, 718154. 10.3389/fphar.2021.718154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong L. Y., Cui M. N., Yang M., Gong Q. F. (2019). Modern researches on effect of processing of Chinese herb medicine on Chinese medical properties. Zhongguo Zhong Yao Za Zhi 44 (23), 5109–5113. 10.19540/j.cnki.cjcmm.20190916.301 [DOI] [PubMed] [Google Scholar]
- Zhou C., Jeon T. H., Jun S. H., Kwon J. J. (2017). Treatment of 93 cases of damp-heat syndrome (chronic prostatitis) with Tibetan medicine shisanwei ximingwan . Maxillofac. Plast. Reconstr. Surg. 23 (08), 30–53. 10.1186/s40902-017-0128-y [DOI] [Google Scholar]
- Zhu L., Chen L. (2019). Progress in research on paclitaxel and tumor immunotherapy. Cell. Mol. Biol. Lett. 24, 40. 10.1186/s11658-019-0164-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z. G., Jin H., Yu P. J., Tian Y. X., Zhang J. J., Wu S. G. (2013). Mollugin inhibits the inflammatory response in lipopolysaccharide-stimulated RAW264.7 macrophages by blocking the janus kinase-signal transducers and activators of transcription signaling pathway. Biol. Pharm. Bull. 36 (3), 399–406. 10.1248/bpb.b12-00804 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.