Abstract
Introduction
The frequencies and functions of T stem cell memory (TSCM) subsets vary in autoimmune diseases. We evaluated the frequencies of CD4+ and CD8+ TSCM subsets as well as their PD‐1 expression levels in patients with T1D.
Methods
Blood samples were collected from new case (NC) (n = 15), and long‐term (LT) (n = 15) groups and healthy controls (n = 15). Five subsets of T cells including TCM(CD4+/CD8+ CCR7+ CD45RO+ CD95+), TCMhi (CD4+/CD8+ CCR7+ CD45ROhi CD95+), TEM(CD4+/CD8+ CCR7− CD45RO+ CD95+), TSCM(CD4+/CD8+ CCR7+ CD45RO− CD95+), and T naive (CD4+/CD8+ CCR7+ CD45RO− CD95−) were detected by flow‐cytometry.
Results
The frequency of CD4+ TSCM was higher in NC patients than LT patients and controls (p < .0001 and p = .0086, respectively). A higher percentage of the CD8+ T naive cells was shown in NC patients as compared with LT and healthy individuals (p = .0003 and p = .0002, respectively). An increased level of PD‐1 expression was observed on the CD4+TCM and TCMhi cells in LT patients as compared with healthy controls (p = .0037 and p = .0145, respectively). Also, the higher PD‐1 expression was observed on the CD8+ TCM and TCMhi in NC and LT patients as compared with controls (p = .0068 and p < .0001; p = .0012 and p = .0012, respectively).
Conclusion
Considering TSCMs' capacities to generate all memory and effector T cells, our results may suggest a potential association between the increased frequencies of TSCMs and T1D progression.
Keywords: autoimmunity, CD4 T cell, CD8 T cell, T memory stem cells, type 1 diabetes
In this study, we have checked the frequency of T stem cell memory (TSCM) in type 1 diabetes (T1D) and our results may suggest a potential association between the increased frequencies of TSCMs and T1D progression.

1. INTRODUCTION
Type 1 diabetes (T1D) is a chronic autoimmune disease resulting from the destruction of insulin‐producing β‐cells with strong genetic background and environmental triggers. 1 , 2 Insulitis, an inflammatory lesion of the islet due to β cell loss, is the pathologic hallmark of T1D. 2 Different immune cells such as CD4+ and CD8+ T cells, B cells, dendritic cells (DC), and macrophages, as well as the production of islet‐specific autoantibodies, are involved in insulitis and T1D progression. 3 , 4 , 5 The recognition of autoantigens like insulin, the 65‐kDa form of glutamic acid decarboxylase (GAD65), insulinoma‐associated protein 2 (IA‐2), and zinc transporter 8 (ZnT8) through the major histocompatibility complex (MHC), leads to T cell activation and contributes to disease development. 6 Previous documents showed that GAD65 and (pro) insulin‐responsive T cells exhibited the properties of naive T cell compartment in healthy individuals, while in patients with T1D, GAD65 and (pro) insulin‐responsive T cells showed the characteristics of the naive and memory T cell compartments. 7 Moreover, autoantigen‐responsive memory T cells in patients with T1D display a higher proliferative capacity to generate memory autoimmune T cell repertoire. 7 , 8 It has been shown that β‐cells‐responsive T cells found in T1D display features of antigen experience, expression of memory markers, decreased telomere length, and activation in the absence of costimulatory molecules. 7 , 9 , 10 Furthermore, IL‐7 signaling plays an important role in the generation and maintenance of autoimmune T cells in T1D, as well as providing crucial signals for the generation of TSCM from naive precursors. 11 , 12 Additionally, the activation of autoimmune memory T cells was found in patients with T1D receiving pancreas and islet allografts 1 year after disease progression, which suggests the autoimmunity recurrence due to long‐lasting memory autoimmunity. 13 The extent of T cell response depends on the activation of costimulatory or co‐inhibitory receptors after the engagement of T cell receptor (TCR) with the specific peptide‐MHC complex. Programmed cell death protein 1 (PD‐1), cytotoxic T‐lymphocyte‐associated protein 4 (CTLA‐4), lymphocyte‐activation gene 3 (LAG‐3), and T cell immunoglobulin and ITIM domain (TIGIT) are known as co‐inhibitory receptors. 14 PD‐1 expression on T cells upon TCR stimulation induces anergy and exhaustion of T cells and is associated with persistent activation of self‐reactive T cells in autoimmune diseases. Certainly, depletion of PD‐1+ T cells has given beneficial effect in autoimmune disease through restriction of inflammation and disease progression. 15 , 16 , 17
T1D pathogenesis involves the three main memory CD4+ and CD8+ T cell subsets, including central memory (CM; CD45RO+ CD45RA− CCR7+ CD28+), transitional memory (TM; CD45RO+CD45RA−CCR7− CD28+), and effector memory (EM; CD45RO+ CD45RA− CCR7−CD28− CD62Llow). 18 In addition to the previously known memory T cell subsets, a distinct subset of peripheral blood CD4+and CD8+ memory T cells, T stem cell memory (TSCM), has recently been discovered. 19 TSCM is described as CD45RA+, CD45RO−, CCR7+, CD28+, CD95+, CD122+ (IL‐2Rβ), CD58+ and CD11a+ cell and comprises 2%–3% of memory T cells in humans. 20 , 21 Although TSCM preserves their naive‐like phenotype, they have self‐renewal capacity and are able to differentiate into CM and EM subsets. 22 , 23 The stem cell‐like functional properties of TSCM empower them to drive different diseases like aplastic anemia (AA), autoimmune uveitis, 24 systemic lupus erythematosus (SLE), 25 juvenile idiopathic arthritis or chronic lymphocytic leukemia (CML), 26 immune thrombocytopenia (ITP), 27 and HIV‐1 infection. 28 , 29 Previous studies have shown that TSCM can generate all memory and effector T cell subsets, which contribute to the progression of autoimmune diseases. 30 , 31 , 32 In this context, these cells exhibit effector functions due to TNF‐α, IFN‐γ, and IL‐2 secretion. 33 Therefore, imbalances in TSCM frequencies and functions can participate in the pathogenesis of different diseases. Collectively, the mentioned characteristics of the autoreactive T cell response in patients with T1D indicate that autoreactive TSCM can be generated during the pathogenesis of the disease. However, little is known about the distribution and function of different TSCM subsets in T1D.
In the present study, we investigated the frequency of CD4+ and CD8+ TSCM subsets as well as their PD‐1 expression levels in patients with T1D.
2. MATERIALS AND METHODS
2.1. Patients
A total of 15 new cases (NCs) with at least 1 year of T1D (5 females and 10 males, mean age ± SEM = 11.11 ± 1.36 years), 15 long‐term (LT) individuals with at least 5 years of T1D (3 females and 12 males, mean age ± SEM = 15.6 ± 1.34 years) and 15 healthy age and sex‐matched controls (six females and nine males, mean age ± SEM = 16.4 ± 1.72 years) were entered in this case‐control study. All subjects with T1D were selected from individuals referred to the Imam Reza clinic affiliated with Shiraz University of Medical Sciences over the past year. Demographic characteristics and laboratory data were collected during admission and are summarized in Table 1. The exclusion criteria included a positive history of alcohol, hepatic infections, HIV, cancers, autoimmune diseases, and pregnancy. The protocols were approved by the ethics committee of the medical school of Shiraz University of Medical Sciences (IR.sums.med.rec.1398.1107), and as the participants of the study were under 18 years old, the consent form was signed by their parents.
Table 1.
The demographic and clinical characteristics of the study participants
| Characteristic (mean ± SEM) | Patients | HC (N = 15) | p‐value | |
|---|---|---|---|---|
| NC (N = 15) | LT (N = 15) | |||
| Age (years) | 11.11 ± 1.36 | 15.6 ± 1.34 | 16.4 ± 1.72 | .16 |
| Gender (M/F) | 10/5 | 12/3 | 9/6 | .9 |
| Diabetes duration (year) | 1.5 ± 0.18 | 8.38 ± 0.88 | _ | <.0001 |
| FBS (mg/dl) | 261.64 ± 21.68 | 225.46 ± 24.48 | _ | .25 |
| 2hpp (mg/dl) | 378.22 ± 61.94 | 221.45 ± 21.9 | _ | .021 |
| HbA1C (%) | 9.71 ± 0.39 | 9.19 ± 0.63 | _ | .44 |
| Total daily insulin (U/kg/day) | 27.13 ± 4.48 | 53 ± 7.81 | _ | .012 |
| BMI (kg/m2) | 16.75 ± 0.86 | 18.41 ± 0.94 | _ | .16 |
| Plasma albumin (mmol/L) | 9.55 ± 1.78 | 25.2 ± 12.6 | _ | .22 |
| TG (mmol/L) | 152.38 ± 48.4 | 101.43 ± 20.18 | _ | .77 |
| HDL cholesterol (mmol/L) | 48.57 ± 4.42 | 48.5 ± 3.99 | _ | .8 |
| LDL cholesterol (mmol/L) | 58.57 ± 11.6 | 104.5 ± 14.72 | _ | .34 |
Abbreviations: LT, long‐term; NC, new case.
2.2. Peripheral blood mononuclear cell (PBMC) isolation
Fresh heparinized blood samples (6 ml) were collected separately from each participant, and PBMCs were isolated by density‐gradient centrifugation at ×800g for 30 min at 25°C using Ficoll‐Paque Plus (inno‐Train Diagnostic GmbH). Then, freshly isolated PBMCs were resuspended to 1 × 106 per ml in RPMI‐1640 culture medium (Shellmax), containing 10% fetal bovine serum and incubated overnight at 37°C.
2.3. Flow cytometry
PBMCs were stained at 4°C for 30 min with fluorochrome‐conjugated antibodies to characterize TSCM subsets. The following human conjugated monoclonal antibodies were used: anti‐CD4‐PerCP‐Cy5.5 (RPA‐T4), anti‐CD8‐APC‐Fire (RPA‐T8), anti‐CD45RO‐APC (UCHL1), anti‐CCR7FITC (G043H7), anti‐CD95‐PE (Dx2), and anti‐PD‐1‐PE‐Dazzle (EH12.2H7) from BioLegend. Mononuclear cells were separated from peripheral blood and live lymphocytes were identified by forward and side angle light scattering characteristics. Each T cell subset was defined as follows based on previous studies 24 , 34 : T central memory (TCM): CD4 + (CD8) + CCR7 + CD45RO + CD95 + T cells; TCMhi: CD4 + (CD8) + CCR7 + CD45ROhi CD95+ T cells; T effector memory (TEM): CD4+ (CD8)+ CCR7− CD45RO+ CD95+ T cells; TSCM: CD4+ (CD8)+ CCR7+ CD45RO− CD95+ T cells and T naive: CD4+ (CD8)+ CCR7+ CD45RO− CD95− T cells. At least 100,000 events per sample were collected using FACS Aria II (BD Sciences); and results were analyzed using FlowJo software (v9.6).
2.4. Statistical analysis
All statistical analyses were carried out using SPSS version 25 and Graphpad Prism version 8 software, and data were expressed as mean ± SEM. Normality was assessed by the Kolmogorov–Smirnov test. The student's t‐test or Mann–Whitney U test was used to evaluate the differences between two groups. The Kruskal–Wallis test was used for comparison of variables between more than two groups. The Spearman's rho method was used to evaluate the potential correlation between variables. p < .05 were considered statistically significant. The following symbols were applied to indicate statistically significant findings: *p < .05, **p < .01, ***p < .001, and ****p < .0001.
3. RESULTS
3.1. Demographic and clinical parameters
The demographic and clinical characteristics of patients with T1D and controls are demonstrated in Table 1. The duration of diabetes (p < .0001) and the total daily dose of insulin (p = .012) were significantly higher in patients with LT T1D compared to NC‐T1D individuals. Conversely, the 2hpp blood glucose level (p = .021) was higher in the NCs of T1D patients as compared with LT‐T1D individuals. Additionally, there were no significant differences in FBS, HbA1C, BMI, plasma albumin, TG, HDL, and LDL cholesterol between NC and LT T1D patients.
3.2. Frequency of CD4+ T cell subsets in T1D
All CD4+T cell subsets (TN, TSCM, TCM, TCMhi, and TEM) in the peripheral blood of all study subjects were identified by sequential surface marker gating as shown in Figure 1A. We found that the frequency of CD4+CCR7+CD45RO−CD95+ TSCM was higher in NC patients than LT patients and controls (2.87 ± 0.69 vs. 1.55 ± 0.38, p < .0001 and 2.87 ± 0.69 vs. 0.88 ± 0.12, p = .0086, respectively, Figure 1B). The percentage of CD4+CCR7+CD45RO+CD95+TCM was decreased in NC patients as compared with healthy controls (22.48 ± 2.4 vs. 31.23 ± 2.18, p = .0023, Figure 1B). As well, the frequency of CD4+CCR7+ CD45ROhi CD95+TCMhi was lower in NC patients than in healthy controls (14.21 ± 2.26 vs. 23.43 ± 1.94, p = .0014, Figure 1B). The percentage of CD4+CCR7−CD45RO+CD95+TEM was lower in NC patients than in healthy controls (5.08 ± 0.40 vs. 10.01 ± 1.57, p = .0033, Figure 1B).
Figure 1.
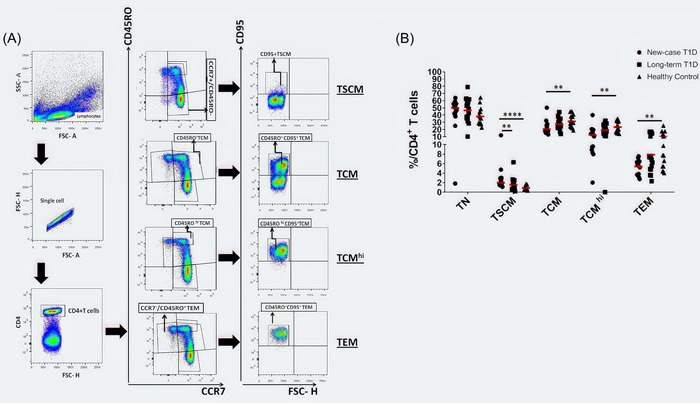
Changes in the percentages of CD4+T cell subsets in T1D patients. (A) The schematic diagram of gating of CD4+ T cell subsets in FCM. (B) The percentages of CD4+T cell subsets (TN, TSCM, TCM, TCMhi, and TEM) in new‐case T1D patients (n = 15), long‐term T1D patients (n = 15), and healthy controls (n = 15) are shown. Each symbol represents an individual. The means are represented by horizontal lines. *p < .05, **p < .01, ***p < .001, and ****p < .0001. Data analysis using the Kruskal–Wallis test followed by Bonferroni. TCM, T central memory.
3.3. Frequency of CD8+ T cell subsets in T1D
The frequencies of CD8+ T cell populations were also analyzed in all study groups. The representative dot plots and the gating strategy used to detect CD8+ T cell subsets are shown in Figure 2A. The frequency of the CD8+CCR7+CD45RO−CD95− T naive cells was higher in NC patients than in LT and healthy individuals (45.26 ± 15.26 vs. 25.37 ± 2.41, p = .0003 and 45.26 ± 15.26 vs. 30.46 ± 1.76, p = .0002, respectively, Figure 2B).
Figure 2.
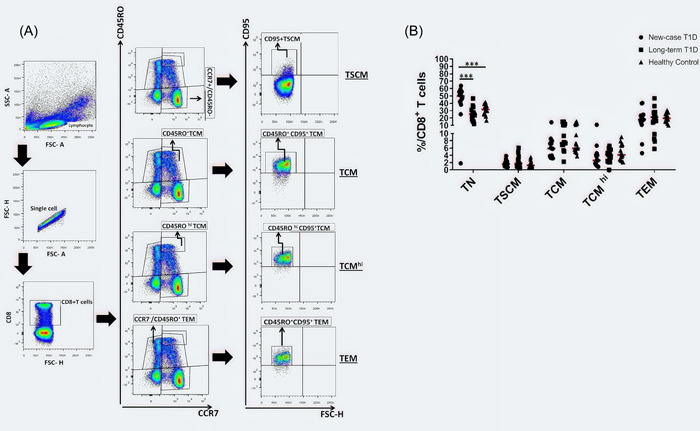
Changes in the percentages of CD8+T cell subsets in T1D patients. (A) The schematic diagram of gating of CD8+ T cell subsets in FCM. (B) The percentages of CD8+T cell subsets (TN, TSCM, TCM, TCMhi, and TEM) in new‐case T1D patients (n = 15), long‐term T1D patients (n = 15), and healthy controls (n = 15) are shown. Each symbol represents an individual. The means are represented by horizontal lines. *p < .05, **p < .01, ***p < .001, and ****p < .0001. Data analysis using the Kruskal–Wallis test followed by Bonferroni. TCM, T central memory.
3.4. Frequency of PD‐1 expressing CD4+and CD8+ T cell subsets
We examined the frequency of PD‐1 expressing CD4+ or CD8+ T subsets in NC, LT and healthy groups, the representative dot plots of CD4+/CD8+ T cells, and the gating strategy used to detect PD‐1+CD4+ or PD‐1+CD8+ T cells are shown in Figures 3 and 4, respectively. In CD4+T cell subsets, the highest percentage of PD‐1 expression was observed on the CCR7+ CD45RO+ CD95+ TCM and CD4+ CCR7+ CD45ROhi CD95+TCMhi cells in LT patients as compared with healthy controls (6.09 ± 1.68 vs. 2.32 ± 0.3, p = .0037 and 5.35 ± 1.05 vs. 2.62 ± 0.35, p = .0145, respectively, Figure 3B). In CD8+ T cell subsets, the highest percentage of PD‐1 expression was observed on the CCR7+ CD45RO+ CD95+ TCM and CCR7+ CD45ROhi CD95+TCMhi in NC and LT patients as compared with controls (10.06 ± 1.84 vs. 4.27 ± 0.43, p = .0068 and 20.11 ± 5.14 vs. 4.27 ± 0.43, p < .0001; 10.59 ± 1.72 vs. 4.35 ± 0.53, p = .0012 and 15.71 ± 3.96 vs. 4.35 ± 0.53, p = .0012, respectively, Figure 4B). Furthermore, LT patients have a higher percentage of PD‐1 in their CD8+ CCR7− CD45RO+ CD95+ TEM subsets as compared with healthy subjects (18.66 ± 3.12 vs. 7.97 ± 1.04, p = .0032, Figure 4B).
Figure 3.
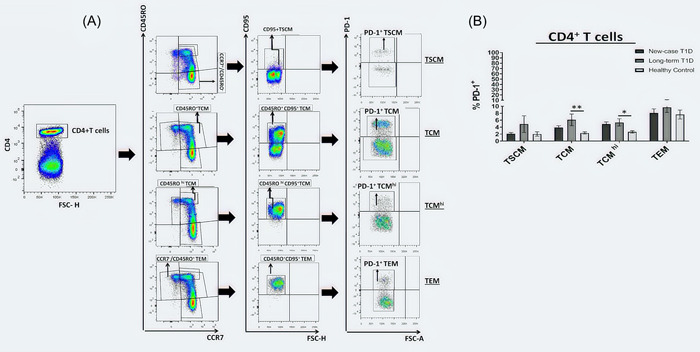
Inhibitory receptor expression on CD4+T cell subsets. (A) Representative dot plot of PD‐1 expression on CD4+ T cell subsets in T1D patients and healthy controls. (B) Surface PD‐1 expression level on CD4+ T cell subsets (TSCM, TCM, TCMhi, and TEM) were analyzed in new‐case T1D patients (n = 15), long‐term T1D patients (n = 15), and healthy controls (n = 15). Data are presented as mean ± SEM and analyzed by Kruskal–Wallis test followed by Bonferroni; *p < .05, **p < .01, ***p < .001, and ****p < .0001. TCM, T central memory.
Figure 4.
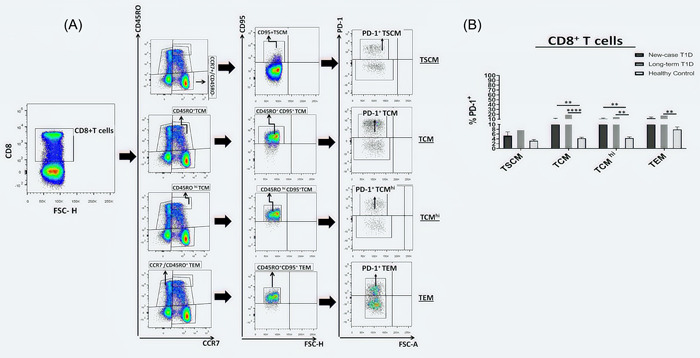
Inhibitory receptor expression on CD8+T cell subsets. (A) Representative dot plot of PD‐1 expression on CD8+ T cell subsets in T1D patients and healthy controls. (B) Surface PD‐1 expression level on CD8+ T cell subsets (TSCM, TCM, TCMhi, and TEM) were analyzed in new‐case T1D patients (n = 15), long‐term T1D patients (n = 15), and healthy controls (n = 15). Data are presented as mean ± SEM and analyzed by Kruskal–Wallis test followed by Bonferroni; *p < .05, **p < .01, ***p < .001, and ****p < .0001. TCM, T central memory.
3.5. The relationship between different CD4+ and CD8+ T subsets
We analyzed the correlation between different CD4+ and CD8+ T subsets in all study groups. As shown in Figure 5A, the frequency of CD4+ naive T cells negatively correlated with frequencies of CD4+ TCM (p < .001; r = −0.804) and CD4+TCMhi (p < .001; r = −0.871) in healthy controls. The percentage of CD4+ TCM positively correlated with CD4+TCMhi cells in the healthy group (p < .001; r = 0.979, Figure 5A). Moreover, the frequency of CD8+ TCM was positively associated with CD8+TCMhi (p < .001; r = 0.796) and CD8+ TSCM (p = .018; r = 0.599) cells in healthy subjects (Figure 5B). As shown in Figure 5C, significant positive correlations were found between the frequency of CD4+ TCM and CD4+TCMhi (p = .002; r = 0.721) as well as CD4+ TEM (p = .035; r = 0.546) in NC individuals. The percentage of CD4+TCMhi was positively correlated with CD4+ TEM (p = .020; r = 0.593). A positive correlation between CD8+ naive T and CD8+ TSCM (p = .025; r = 0.575) as well as CD8+ TCM and CD8+ TCMhi (p < .0001; r = 0.886) were also found in the NC group (Figure 5D). As shown in Figure 5E, IN LT patients the percentage of CD4+ naive T cells was negatively correlated with CD4+ TCM (p = .005; r = −0.682), CD4+TCMhi (p = .001; r = −0.786), CD4+ TEM (p = .016; r = −0.611) and was positively correlated with CD4+ TSCM (p = .005; r = 0.682). There were significant positive correlations between CD4+ TCM and CD4+TCMhi (p < .0001; r = 0.954) and CD4+ TEM (p = .015; r = 0.614) in the LT group. The percentage of CD4+TCMhi was positively correlated with CD4+ TEM (p = .003; r = 0.704) in LT patients. Furthermore, a positive correlation between CD8+ TCM and CD8+TCMhi (p < .0001; r = 0.829) was also found in the LT group (Figure 5F).
Figure 5.
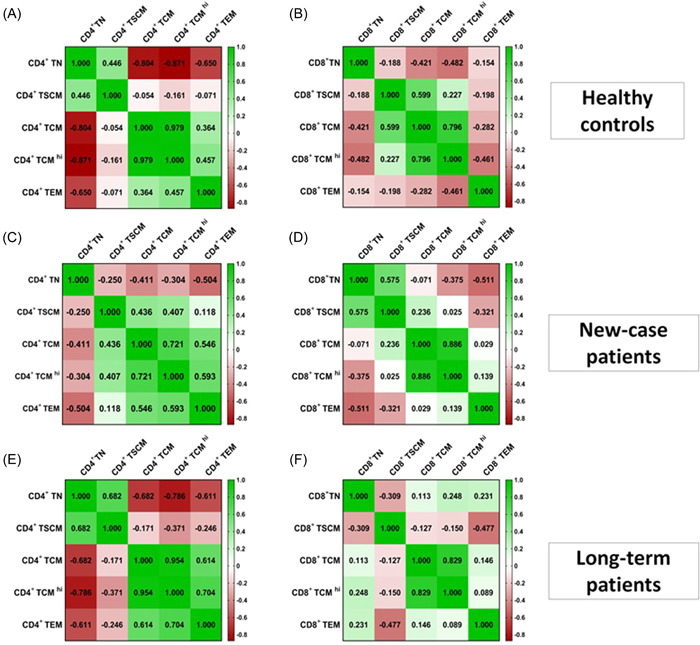
The correlation between CD4+and CD8+T cell subsets with each other in all study groups. (A) Heat map correlation of CD4+ T cell populations with each other in healthy controls. (B) Heat map correlation of CD8+ T cell populations with each other in healthy controls. (C) Heat map correlation of CD4+ T cell populations with each other in new‐case (NC) patients. (D) Heat map correlation of CD8+ T cell populations with each other in NC patients. (E) Heat map correlation of CD4+ T cell populations with each other in long‐term (LT) patients. (F) Heat map correlation of CD8+ T cell populations with each other in LT patients (green: positive correlation, red: negative correlation). The p‐value and R were determined according to Spearman's rank correlation test.
4. DISCUSSION
The relevance of altered frequency and function of TSCMs in the wide spectrum of clinical conditions has received considerable attention recently. While TSCMs exhibit desirable effects in the context of infectious diseases and cancer immunotherapy, these cells may be detrimental in autoimmune diseases due to their stem cell‐like properties. 29 , 35 , 36 , 37 , 38 Certainly, the long‐lived and self‐renewal characteristics of TSCMs may represent a reservoir of autoreactive lymphocytes with undesired and detrimental specificities responsible for autoimmune disease perpetuation.
In the current study, we evaluated the frequencies of CD4+ and CD8+ T subsets in patients with T1D. Our results demonstrated that the frequency of CD4+ TSCM was higher in NC patients than in LT cases and healthy controls. This finding is in line with a previous study which reported an increased percentage of CD4+ TSCM cells in patients with SLE and that isolated TSCM cells secreted IFN‐γ, TNF‐α, and IL‐2 and can differentiate into T follicular helper (Tfh) cells that provide B cell help to produce autoantibodies. 25 Moreover, another study represented higher CD8+ TSCM cells in patients with AA and uveitis as well as higher CD4+ TSCM proportions in SLE patients. 24 Interestingly, profound expansion of CD4+ TSCM is reported during acute HIV infection, which is related to disease progression. 39 A higher frequency of CD4+ TSCM cells is shown to cause disease severity in rheumatoid arthritis (RA) patients. 40 Indeed, these cells displayed the Th17 phenotype and were enriched in putative autoreactive specificities in RA patients compared to healthy donors. 40 In patients with ITP, a higher frequency of CD4+TSCM has been associated with treatment efficacy and disease outcome. 27 A recent study reported higher circulating autoreactive CD8+ TSCM cells specific for GAD65 and insulin in patients with T1D, which are generated in the presence of IL‐7 in vitro. They showed IL‐7 increases glucose uptake by overexpression of GLUT1 and oxidation of pyruvate in the mitochondria as well as upregulation of the glycolytic enzyme hexokinase 2, which are necessary for TSCM cells generation from naive precursors. 41 Previous studies have shown that TSCMs are the least differentiated memory T cells and have introduced them as an intermediate stage between naive and conventional memory T cells. 30 , 31 Accordingly, these populations can differentiate into other memory and effector subsets. 16 , 42 Furthermore, they are antigen experienced and secrete cytokines such as TNF‐α, IFN‐γ, and IL‐2 following activation of T cells. 33 Considering TSCMs' capacities to generate all memory and effector T cells, rapid proliferation and effector molecule generation after TCR stimulation, as well as a potential link between the IL‐7/IL‐7 receptor axis and the development of T‐cell responses toward β‐cells, we hypothesized that the increased frequency of TSCMs is associated with T1D progression.
We also showed the lower percentage of CD4+ TCM, TCMhi, and TEM cells as well as a higher frequency of CD8+ naive T cells in NC patients as compared with LT and healthy individuals. Further analysis of CD4+ and CD8+ T cell subsets in our study also revealed a negative association between the frequency of CD4+ naive T cells and the frequency of CD4+CM, CMhi, and EM T cells in LT patients and healthy controls. Moreover, our results showed positive correlations between the frequency of CD4+ and CD8+ memory T cells with each other in all 3 study groups. A previous study demonstrated that the percentages and absolute numbers of CD4+ CM cells were significantly reduced in recent‐onset diabetic patients compared with control subjects. 43 Also, a lower frequency of CD4+ CM T cells was reported in acute progressive multifocal leukoencephalopathy (PML) that was preserved in patients with a fatal PML outcome. 44 A significant increase in naive CD4+ and CD8+ T cells as well as a significant reduction in the frequency of CD4+ CM and EM T cells was found in patients with thyroid‐associated ophthalmopathy (TAO) as compared with controls. 45 In addition, a higher percentage of CD4+ and CD8+ naive T cells was found in SLE patients. 25 These data may represent dysregulated lymphocyte homeostasis, with favored generation or survival of naive T cells and increased sequestration of central and EM T cells in the inflamed tissues. Furthermore, in autoimmune disease settings, the constant presence of antigen and subsequent chronic nature of the ongoing immune responses may cause memory T cell exhaustion as well as reduced development of traditional memory response and consequently a reduction in their frequency. 46 , 47
Another important finding of our study was the higher expression of PD‐1 on CD4+/CD8+ TCM and TCMhi in the PBMC of NC and LT patients. Previously, the higher expression level of PD‐1 was observed in CD4+ and CD8+ T cells in AA patients. 48 In patients with SLE, higher expression level of PD‐1 was identified in CD4+ T cells, which is associated with IFN‐γ expression on CD3+ T cells. 49 It is also reported that PD‐1 expression on tumor‐infiltrating T‐cells (TILs) correlates with tumor prognosis. 50 , 51 Moreover, increased PD‐1 expression in CD8+ TSCM in AA patients represented this subset as the least exhausted and self‐reactive T cell. 24 Also, it has been reported that PD‐1 expression in healthy individuals correlates with differentiation into an EM phenotype. 24 , 42 , 52 Therefore, it seems that increased expression of PD‐1 in CD4+/CD8+ TCM and TCMhi of T1D patients might be associated with enhanced cytolytic effector activity and persistence of self‐reactive T cells.
In conclusion, we provide evidence for increased circulating CD4+ TSCM in T1D patients, highlighting the importance of this subset in the regulation of immune responses and the pathology of T1D by its ability to differentiate into effector and memory T cells. It would, therefore, be interesting to investigate the significance of TSCMs and their novel mechanistic insight in the progression of T1D and the prediction of disease outcome in a large longitudinal cohort of patients.
AUTHOR CONTRIBUTIONS
Pooriya Fazeli and Atefe Ghamar Talepoor: Performed the experiments, analyzed and interpreted the data, and wrote the draft of the paper. Zahra Faghih, Mohammad Reza Ataollahi, Mohammad Ali‐Hassanzadeh, and Hossein Moravej: Conceived and designed the experiments and corrected the draft of the paper. Nasser Gholijani and Kurosh Kalantar: Conceived and designed the experiments, analyzed and interpreted the data, corrected the draft of the paper, and supervised the research.
CONFLICT OF INTEREST
The authors declare no conflict of interest.
ACKNOWLEDGMENTS
This project was approved by the ethical committee of Shiraz University of Medical Sciences (SUMS, code IR.SUMS.REC.1398.1107) and was financially supported by a grant (97‐18814) from SUMS, Shiraz, Iran. We appreciate Dr. Enayat Nikopoor for helpful discussion and review of this manuscript.
Fazeli P, Talepoor AG, Faghih Z, et al. The frequency of CD4+ and CD8+ circulating T stem cell memory in type 1 diabetes. Immun Inflamm Dis. 2022;10:e715. 10.1002/iid3.715
Contributor Information
Nasser Gholijani, Email: Kalantark@sums.ac.ir, Email: Gholijanin@sums.ac.ir.
Kurosh Kalantar, Email: Kalantark@sums.ac.ir, Email: Gholijanin@sums.ac.ir.
DATA AVAILABILITY STATEMENT
Data sharing is not applicable—no new data is generated, or the article describes entirely theoretical research.
REFERENCES
- 1. Ehlers MR, Rigby MR. Targeting memory T cells in type 1 diabetes. Curr Diab Rep. 2015;15(11):84. [DOI] [PubMed] [Google Scholar]
- 2. Clark M, Kroger CJ, Ke Q, Tisch RM. The role of T cell receptor signaling in the development of type 1 diabetes. Front Immunol. 2020;11:615371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Campbell‐Thompson M, Fu A, Kaddis JS, et al. Insulitis and β‐cell mass in the natural history of type 1 diabetes. Diabetes. 2016;65(3):719‐731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Coppieters KT, Dotta F, Amirian N, et al. Demonstration of islet‐autoreactive CD8 T cells in insulitic lesions from recent onset and long‐term type 1 diabetes patients. J Exp Med. 2012;209(1):51‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Yu L, Rewers M, Gianani R, et al. Antiislet autoantibodies usually develop sequentially rather than simultaneously. J Clin Endocrinol Metab. 1996;81(12):4264‐4267. [DOI] [PubMed] [Google Scholar]
- 6. Arif S, Pujol‐Autonell I, Eichmann M. Assessing effector T cells in type 1 diabetes. Curr Opin Endocrinol Diabetes Obes. 2020;27(4):240‐247. [DOI] [PubMed] [Google Scholar]
- 7. Monti P, Scirpoli M, Rigamonti A, et al. Evidence for in vivo primed and expanded autoreactive T cells as a specific feature of patients with type 1 diabetes. J Immunol. 2007;179(9):5785‐5792. [DOI] [PubMed] [Google Scholar]
- 8. Bingley PJ, Bonifacio E, Mueller PW, Laboratories P. Diabetes Antibody Standardization Program: first assay proficiency evaluation. Diabetes. 2003;52(5):1128‐1136. [DOI] [PubMed] [Google Scholar]
- 9. Danke NA, Yang J, Greenbaum C, Kwok WW. Comparative study of GAD65‐specific CD4+ T cells in healthy and type 1 diabetic subjects. J Autoimmun. 2005;25(4):303‐311. [DOI] [PubMed] [Google Scholar]
- 10. Viglietta V, Kent SC, Orban T, Hafler DA. GAD65‐reactive T cells are activated in patients with autoimmune type 1a diabetes. J Clin Invest. 2002;109(7):895‐903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monti P, Bonifacio E. Interleukin‐7 and type 1 diabetes. Curr Diab Rep. 2014;14(9):1‐7. [DOI] [PubMed] [Google Scholar]
- 12. Cieri N, Camisa B, Cocchiarella F, et al. IL‐7 and IL‐15 instruct the generation of human memory stem T cells from naive precursors. Blood. 2013;121(4):573‐584. [DOI] [PubMed] [Google Scholar]
- 13. Huurman VAL, Hilbrands R, Pinkse GGM, et al. Cellular islet autoimmunity associates with clinical outcome of islet cell transplantation. PLoS One. 2008;3(6):e2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yasuma‐Mitobe K, Matsuoka M. The roles of coinhibitory receptors in pathogenesis of human retroviral infections. Front Immunol. 2018;9:2755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cencioni MT. The immune regulation of PD‐1/PDL‐1 axis, a potential biomarker in multiple sclerosis. Neuroimmunol Neuroinflamm. 2020;7(3):277‐90. [Google Scholar]
- 16. Gianchecchi E, Delfino DV, Fierabracci A. Recent insights into the role of the PD‐1/PD‐L1 pathway in immunological tolerance and autoimmunity. Autoimmun Rev. 2013;12(11):1091‐1100. [DOI] [PubMed] [Google Scholar]
- 17. Jiao Q, Liu C, Yang Z, et al. Upregulated PD‐1 expression is associated with the development of systemic lupus erythematosus, but not the PD‐1.1 allele of the PDCD1 gene. Int J Genomics. 2014;2014:950903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rahimi RA, Luster AD. Redefining memory T cell subsets. Trends Immunol. 2020;41(8):645‐648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Caccamo N, Joosten SA, Ottenhoff THM, Dieli F. Atypical human effector/memory CD4(+) T cells with a naive‐like phenotype. Front Immunol. 2018;9:2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Sarkar I, Pati S, Dutta A, Basak U, Sa G. T‐memory cells against cancer: remembering the enemy. Cell Immunol. 2019;338:27‐31. [DOI] [PubMed] [Google Scholar]
- 21. Flynn JK, Gorry PR. T cell therapies‐are T memory stem cells the answer? Ann Transl Med. 2015;3(17):251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. van der Waart AB, van de Weem NM, Maas F, et al. Inhibition of Akt signaling promotes the generation of superior tumor‐reactive T cells for adoptive immunotherapy. Blood. 2014;124(23):3490‐3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Flynn JK, Gorry PR. Stem memory T cells (TSCM)—their role in cancer and HIV immunotherapies. Clin Transl Immunol. 2014;3(7):e20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Hosokawa K, Muranski P, Feng X, et al. Memory stem T cells in autoimmune disease: high frequency of circulating CD8+ memory stem cells in acquired aplastic anemia. J Immunol. 2016;196(4):1568‐1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lee YJ, Park JA, Kwon H, et al. Role of stem cell‐like memory T cells in systemic lupus erythematosus. Arthritis Rheumatol. 2018;70(9):1459‐1469. [DOI] [PubMed] [Google Scholar]
- 26. Roederer M, Quaye L, Mangino M, et al. The genetic architecture of the human immune system: a bioresource for autoimmunity and disease pathogenesis. Cell. 2015;161(2):387‐403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cao J, Zhang C, Han X, et al. Emerging role of stem cell memory‐like T cell in immune thrombocytopenia. Scand J Immunol. 2019;89(3):e12739. [DOI] [PubMed] [Google Scholar]
- 28. Buzon MJ, Sun H, Li C, et al. HIV‐1 persistence in CD4+ T cells with stem cell‐like properties. Nature Med. 2014;20(2):139‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jaafoura S, de Goër de Herve MG, Hernandez‐Vargas EA, et al. Progressive contraction of the latent HIV reservoir around a core of less‐differentiated CD4⁺ memory T cells. Nat Commun. 2014;5:5407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Gattinoni L, Lugli E, Ji Y, et al. A human memory T cell subset with stem cell‐like properties. Nature Med. 2011;17(10):1290‐1297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lugli E, Gattinoni L, Roberto A, et al. Identification, isolation and in vitro expansion of human and nonhuman primate T stem cell memory cells. Nat Protoc. 2013;8(1):33‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roberto A, Castagna L, Zanon V, et al. Role of naive‐derived T memory stem cells in T‐cell reconstitution following allogeneic transplantation. Blood. 2015;125(18):2855‐2864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Khamis F, Al Naabi H, Al Lawati A, et al. Randomized controlled open label trial on the use of favipiravir combined with inhaled interferon beta‐1b in hospitalized patients with moderate to severe COVID‐19 pneumonia. Int J Infect Dis. 2021;102:538‐543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vahidi Y, Bagheri M, Ghaderi A, Faghih Z. CD8‐positive memory T cells in tumor‐draining lymph nodes of patients with breast cancer. BMC Cancer. 2020;20(1):257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Mateus J, Lasso P, Pavia P, et al. Low frequency of circulating CD8+ T stem cell memory cells in chronic chagasic patients with severe forms of the disease. PLoS Neglected Trop Dis. 2015;9(1):e3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Klatt NR, Bosinger SE, Peck M, et al. Limited HIV infection of central memory and stem cell memory CD4+ T cells is associated with lack of progression in viremic individuals. PLoS Pathog. 2014;10(8):e1004345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Gattinoni L, Restifo NP. Moving T memory stem cells to the clinic. Blood. 2013;121(4):567‐568. [DOI] [PubMed] [Google Scholar]
- 38. Padgett LE, Dinh HQ, Wu R, et al. Naive CD8(+) T cells expressing CD95 increase human cardiovascular disease severity. Arterioscler Thromb Vasc Biol. 2020;40(12):2845‐2859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pušnik J, Eller MA, Tassaneetrithep B, et al. Expansion of stem cell‐like CD4(+) memory T cells during acute HIV‐1 infection is linked to rapid disease progression. J Virol. 2019;93(14):e00377‐19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bonini C, Lee SP, Riddell SR, Greenberg PD. Targeting antigen in mature dendritic cells for simultaneous stimulation of CD4+ and CD8+ T cells.The Journal of Immunology.2001;166(8):5257. [DOI] [PubMed] [Google Scholar]
- 41. Vignali D, Cantarelli E, Bordignon C, et al. Detection and characterization of CD8(+) autoreactive memory stem T cells in patients with type 1. Diabetes. 2018;67(5):936‐945. [DOI] [PubMed] [Google Scholar]
- 42. Duraiswamy J, Ibegbu CC, Masopust D, et al. Phenotype, function, and gene expression profiles of programmed death‐1(hi) CD8 T cells in healthy human adults. J Immunol. 2011;186(7):4200‐4212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Matteucci E, Ghimenti M, Di Beo S, Giampietro O. Altered proportions of naïve, central memory and terminally differentiated central memory subsets among CD4+ and CD8 + T cells expressing CD26 in patients with type 1 diabetes. J Clin Immunol. 2011;31(6):977‐984. [DOI] [PubMed] [Google Scholar]
- 44. Dubois E, Ruschil C, Bischof F. Low frequencies of central memory CD4 T cells in progressive multifocal leukoencephalopathy. Neurol Neuroimmunol Neuroinflamm. 2015;2(6):e177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Edmunds MR, Durrani OM, Boelaert K, Franklyn JA, Curnow SJ. Decreased CD4+ and CD8+ effector memory T lymphocyte populations in thyroid‐associated ophthalmopathy. Invest Ophthalmol Visual Sci. 2012;53(14):1011. [Google Scholar]
- 46. Wherry EJ, Teichgräber V, Becker TC, et al. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nature Immunol. 2003;4(3):225‐234. [DOI] [PubMed] [Google Scholar]
- 47. van Faassen H, Saldanha M, Gilbertson D, Dudani R, Krishnan L, Sad S. Reducing the stimulation of CD8+ T cells during infection with intracellular bacteria promotes differentiation primarily into a central (CD62LhighCD44high) subset. J Immunol. 2005;174(9):5341‐5350. [DOI] [PubMed] [Google Scholar]
- 48. Wu H, Miao M, Zhang G, Hu Y, Ming Z, Zhang X. Soluble PD‐1 is associated with aberrant regulation of T cells activation in aplastic anemia. Immunol Invest. 2009;38(5):408‐421. [DOI] [PubMed] [Google Scholar]
- 49. Dolff S, Quandt D, Feldkamp T, et al. Increased percentages of PD‐1 on CD4+ T cells is associated with higher INF‐γ production and altered IL‐17 production in patients with systemic lupus erythematosus. Scand J Rheumatol. 2014;43(4):307‐313. [DOI] [PubMed] [Google Scholar]
- 50. Thompson RH, Dong H, Lohse CM, et al. PD‐1 is expressed by tumor‐infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13(6):1757‐1761. [DOI] [PubMed] [Google Scholar]
- 51. Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka‐Akita H, Nishimura M. B7‐H1 expression on non‐small cell lung cancer cells and its relationship with tumor‐infiltrating lymphocytes and their PD‐1 expression. Clin Cancer Res. 2004;10(15):5094‐5100. [DOI] [PubMed] [Google Scholar]
- 52. Xu L, Zhang Y, Luo G, Li Y. The roles of stem cell memory T cells in hematological malignancies. J Hematol Oncol. 2015;8:113. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing is not applicable—no new data is generated, or the article describes entirely theoretical research.


