Abstract
The cyanobacterium Synechococcus sp. strain PCC7942 has three dnaK homologues (dnaK1, dnaK2, and dnaK3), and a gene disruption experiment was carried out for each dnaK gene by inserting an antibiotic resistance marker. Our findings revealed that DnaK1 was not essential for normal growth, whereas DnaK2 and DnaK3 were essential. We also examined the effect of heat shock on the levels of these three DnaK and GroEL proteins and found a varied response to heat shock, with levels depending on each protein. The DnaK2 and GroEL proteins exhibited a typical heat shock response, that is, their synthesis increased upon temperature upshift. In contrast, the synthesis of DnaK1 and DnaK3 did not respond to heat shock; in fact, the level of DnaK1 protein decreased. We also analyzed the effect of overproduction of each DnaK protein in Escherichia coli cells using an inducible expression system. Overproduction of DnaK1 or DnaK2 resulted in defects in cell septation and formation of cell filaments. On the other hand, overproduction of DnaK3 did not result in filamentous cells; rather a swollen and twisted cell morphology was observed. When expressed in an E. coli dnaK756 mutant, dnaK2 could suppress the growth deficiency at the nonpermissive temperature, while dnaK1 and dnaK3 could not suppress this phenotype. On the contrary, overproduction of DnaK1 or DnaK3 resulted in growth inhibition at the permissive temperature. These results suggest that different types of Hsp70 in the same cellular compartment have specific functions in the cell.
All living organisms respond to environmental stresses such as high temperature by synthesizing a set of proteins which have been called heat shock proteins (Hsps) (23). Some of them are highly conserved in the course of evolution, especially the proteins encoded by the groEL (hsp60 or cpn60) and the dnaK (hsp70) genes. They have been identified and characterized in various organisms as well as major cellular compartments, including cytoplasm, nucleus, endoplasmic reticulum, mitochondria, and chloroplasts (11, 14, 23). Although the synthesis of Hsp70 is enhanced under various stress conditions, many Hsp70 proteins are constitutively expressed and have also been shown to be essential under normal growth conditions (11, 14). One of the well-established functions of Hsp70 is the regulation of protein-protein interactions. The folding and assembly of proteins require the action of protein factors termed molecular chaperones (7, 8), and the Hsp70 family is one of the ubiquitous groups of such factors (11, 14).
Cyanobacteria are prokaryotic cells which carry a complete set of genes for oxygenic photosynthesis similar to that found in chloroplasts of higher plants. These organisms are also interesting from the point of view of evolution, since they are associated with prehistoric ages and have survived various environmental conditions. Under natural conditions they inhabit areas with suitable amounts of sunlight, where they are inevitably subject to a variety of stresses such as UV irradiation and high temperature. Therefore, it is interesting to study the mechanism of stress response and the function of stress proteins of cyanobacteria. We have previously identified three dnaK homologue genes, dnaK1, dnaK2, and dnaK3, in the transformable cyanobacterium Synechococcus sp. strain PCC7942 (27, 28).
The genome of another cyanobacterium, Synechocystis sp. strain PCC6803 (17), also disclosed the presence of three dnaK homologues (open reading frame [ORF] designations in the database are sll0058, sll0170, and sll1932). Those three DnaK homologues show high similarity to each of the three DnaKs of Synechococcus sp. strain PCC7942. Synechococcus DnaK3 has a characteristically very long C-terminal region (28), and the corresponding Synechocystis DnaK (sll1932) also contains this region, including the conserved GWDDDDDD/EWF sequence at the termini. The genome of the cyanobacterium Anabaena sp. strain PCC7120 (http://www.kazusa.or.jp/cyano) also revealed the presence of three dnaK homologues, although they have less similarity to each DnaK gene of Synechococcus than those of Synechocystis. Other than the cyanobacteria, the existence of multiple dnaK genes is rare among prokaryotes, only two other examples have been identified so far. One is Escherichia coli, which also has two dnaK homologues (hsc66 and hsc62) other than dnaK (20, 36, 45), and the other is Borrelia burgdorferi B31 (9), which has two dnaK homologues. In most eukaryotes, Hsp70s constitute a multigene family whose members have been shown to be expressed differentially under a variety of physiological conditions (23, 42). Some are expressed constitutively and are not induced by stress, and others are both constitutive and stress inducible. However, it is not clearly understood how these Hsp70 proteins in the same cellular compartment are assigned with their respective functions. In Saccharomyces cerevisiae, most of the cytosolic Hsp70 proteins belong to either the Ssa or Ssb subfamily. Those which belong to the same subfamily have compensatory functions for each other, but those from different subfamilies are not interchangeable (5, 6, 43).
Although chaperone functions are well characterized using certain substrates, it has been a major subject in recent studies to understand how molecular chaperones find their specific target among many substrates in a specific cellular process. It would contribute to this subject to clarify how the different Hsp70s in the same cellular compartment are allocated to their functions. As an initial step for studying the functional distinction among spatially colocalized Hsp70s, we analyzed in vivo functions and gene regulation of the three DnaK proteins in Synechococcus sp. strain PCC7942. Here we report the various properties of these proteins, which suggest that each protein has a specific function(s) in the cell.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
Synechococcus sp. strain PCC7942(R2-spc), which has been cured of its indigenous plasmid pUH24 (19), was obtained from T. Endo (Nagoya University, Nagoya, Japan). Cells were grown photoautotrophically at 30°C in BG-11 medium (4) under bubbling with air and continuous illumination. When necessary, media were supplemented with kanamycin at a final concentration of 10 μg/ml. E. coli MC4100 (3) and NRK156 (18) cells were grown in Luria-Bertani (LB) medium at the indicated temperatures. Media were supplemented with ampicillin at a final concentration of 100 μg/ml if required.
Disruption of dnaK genes.
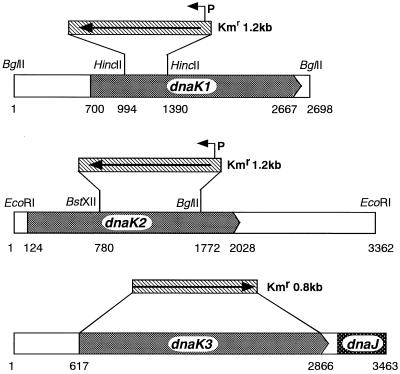
Plasmid pDK1A, which has 2,698-bp DNA fragment including the dnaK1 gene (27), was used for gene disruption of dnaK1. The kanamycin resistance gene was isolated as a BamHI fragment from pUC4K (Pharmacia) and inserted into the HincII site within the dnaK1 gene of pDK1A to make pDK1KM. Similarly, plasmid pDK2KM, which has the kanamycin resistance gene inserted between the BstXII and BglII sites within the dnaK2 gene, was constructed. To avoid single-cross recombination, pDK1KM and pDK2KM were linearized by digestion with EcoRI, and the linearized product was used to transform Synechococcus sp. strain PCC7942 according to the method described by Porter et al. (31). For dnaK3 gene disruption, three DNA fragments upstream and downstream of dnaK3 and promoterless kanamycin resistance cassette were amplified by PCR using primers which have 5′ add-on sequences designed to create overlapping sequences among those fragments and were recombined by a recombinant PCR technique (15), as shown in Fig. 1. The resulting fragment frDK3KM was transformed into Synechococcus. Transformants were grown on BG-11 agar plate containing kanamycin (10 μg/ml).
FIG. 1.
Disruption of dnaK genes from Synechococcus sp. strain PCC7942 by insertion of kanamycin resistance gene. The kanamycin resistance gene (Kmr) from pUC4K was inserted into each dnaK gene as described in Materials and Methods. P, promoter.
Protein purification and preparation of antisera.
DNA fragments encoding the C-terminal domain of each DnaK protein (amino acids [aa] 520 to 655 of DnaK1, aa 515 to 634 of DnaK2, and aa 516 to 685 of DnaK3) were amplified by PCR. Those fragments of dnaK1 and dnaK2 were inserted in the EcoRI and SalI sites of the hexahistidine fusion expression vector pET21b (Novagen). Similarly, the fragment of dnaK3 was inserted in the BamHI and HindIII sites of the hexahistidine fusion expression vector pQE10 (Qiagen). C-terminal segment of each DnaK protein was purified using Ni-nitrilotriacetic acid (NTA) affinity resin (Qiagen) according to the manufacturer's instructions. Purified proteins were injected into mice. The mice were boosted after 3 weeks and bled for serum preparation 4 weeks later.
Western blotting analysis.
Crude extracts of Synechococcus sp. strain PCC7942 cells were prepared as follows. Nine-milliliter aliquots of cell culture were mixed with 1 ml of 100% trichloroacetic acid (TCA). After incubation for 15 min or more on ice, precipitates were harvested by centrifugation at 6,000 × g for 10 min. To remove TCA, the pellet was washed successively with 500 μl of 100% acetone and then 500 μl of 100% ether and then solubilized with 90 μl of 10 mM Tris-HCl (pH 7.5)–2% sodium dodecyl sulfate (SDS)–20 mM NaOH. The debris was removed by centrifugation at 10,000 × g for 1 min, and the supernatant was used as the crude extract. Part of the crude extract was measured for protein concentration and one-fourth volume of 5× modified sample buffer (250 mM Tris-HCl [pH 6.8], 500 mM dithiothreitol, 0.5% bromophenol blue, 2% SDS, and 50% glycerol) for SDS-polyacrylamide gel electrophoresis (PAGE) was added to the extract and boiled for 3 min. Protein concentration was determined by the Lowry method with bovine serum albumin as the standard. The proteins were separated by SDS-PAGE (12.5% polyacrylamide) and transferred onto polyvinylidene difluoride membranes (Immobilon; Millipore). For the detection of DnaK proteins, mouse antiserum against each DnaK protein was used as the primary antibody, and horseradish peroxidase-conjugated anti-mouse immunoglobulin G (IgG; heavy and light chains) antibody (sheep; Amersham) was used as the secondary antibody. Horseradish peroxidase activity was detected by color development using a substrate kit (Bio-Rad). To detect GroEL protein, rabbit antiserum against Bacillus subtilis GroEL (H. Yoshikawa, unpublished) was used as the primary antibody, and alkaline phosphatase-conjugated anti-rabbit IgG antibody (goat; Biomarker) was used as the secondary antibody. Color development associated with alkaline phosphatase was performed using nitro blue tetrazolium (Sigma) and 5-bromo-4-chloro-3-indolyl phosphate (Sigma) as described (33).
Pulse-labeling experiments.
Synechococcus sp. strain PCC7942 cells were grown in BG-11 at 30°C, and at the logarithmic growth phase (optical density at 700 nm [OD700] of 0.5), the culture temperature was shifted to 45°C. At appropriate intervals, 10-ml aliquots of the culture were sampled and pulse labeled with 100 μCi of [35S]methionine (Amersham) at the culture temperature for 30 min. At the end of the labeling, 1.1 ml of 100% TCA (final, 10%) was added and incubated on ice for 15 min. Precipitates were then harvested by centrifugation at 6,000 × g for 10 min, washed with acetone and ether, and dissolved in 200 μl of 50 mM Tris-HCl (pH 7.5)–2% SDS–20 mM NaOH. The solution was mixed with 1 ml of 50 mM Tris-HCl (pH 7.5)–150 mM NaCl–2% Triton X-100–1 mM EDTA, and the debris was removed by centrifugation at 16,000 × g for 5 min. Supernatant was divided in four, and immunoprecipitation was carried out with four kinds of antisera. To each aliquot, 750 μl of 50 mM Tris-HCl (pH 7.5)–150 mM NaCl–2% Triton X-100–1 mM EDTA and 4 μl of each antiserum were added, and the mixture was incubated overnight at 4°C. To each sample, 50 μl of 10% (wt/vol) IgGsorb was added, and the mixture was kept at 4°C for 20 min. After centrifugation at 10,000 × g for 1 min, the pellets were successively washed with 0.5 ml of 50 mM Tris-HCl (pH 7.5)–1 M NaCl–1% Triton X-100 and with 0.5 ml of 50 mM Tris-HCl–0.5 M NaCl–0.05% SDS. The washed pellets were resuspended in 20 μl of 1× sample buffer for SDS-PAGE (50 mM Tris-HCl [pH 6.8], 100 mM dithiothreitol, 0.1% bromophenol blue, 2% SDS, 10% glycerol) and then boiled for 3 min. To seperate proteins from the IgGsorb, the mixture was centrifuged at 10,000 × g for 1 min, and the supernatant was loaded on SDS-PAGE (12.5% polyacrylamide). Bands were detected using Bio-Imaging analyser BAS2000 (Fujix).
Oligonucleotides and construction of plasmids.
Oligodeoxyribonucleotides were synthesized using an ABI392 DNA/RNA synthesizer (Perkin Elmer Applied Biosystems Japan). Sequences of the oligonuculeotides used for construction of dnaK overexpression plasmids are as follows: TRC1-N, GCGAGCTCTAAGGAGGTAATTTATGGGCAAGGTTATC; TRC1-Na, GCGAATTCTAAGGAAAAAATTTATGGGCAAGGTTATC; TRC1-C, GCTCTAGACTACTCAATCGCCTCGTAGTC; TRC2-N, GCGAGCTCTAAGGAACTGGACTATGGCCAAAGTTGTC; TRC2-C, GCTCTAGATTACTTCGACTCAGAGAACTCTGC; TRC3-N, GCGAGCTCTAAGGAGGTGACAGCATGGGACGAGTCGTAG; TRC3-Na, GCGAGCTCTAAGGAAAGACAGCATGGGACGACGAGTCGTAG; and TRC3-C, GCTCTAGACAGCCTGATCCGCCGACTGAG.
The expression vector pTrc99A/X, which carries the lacIq gene and trc promoter, was obtained from T. Endo (Nagoya University) and used to express each dnaK gene under the control of isopropyl-β-d-thiogalactopyranoside (IPTG). This vector contains a ribosome-binding site (RBS) and an initiation codon upstream of multicloning sites. Therefore, we synthesized forward primers (TRC-N series: TRC1-N, TRC2-N, and TRC3-N for dnaK1, dnaK2, and dnaK3, respectively) which contain an in-frame termination codon (TAA) and RBS downstream of the SacI site to produce intact DnaK proteins. Reverse primers (TRC-C series: TRC1-C, TRC2-C, and TRC3-C for dnaK1, dnaK2, and dnaK3, respectively) were designed to contain an XbaI site after the termination codon of each dnaK gene. A DNA fragment harboring each dnaK gene was amplified by PCR with the above primers, digested with SacI and XbaI, and inserted into the SacI and XbaI sites of pTrc99A/X.
Expression of dnaK3 in Synechococcus at the neutral site of the chromosome.
Plasmid pNS1 is a derivative of pTZ18R containing the spectinomycin resistance cassette of pHP45 (32) in the middle of the Synechococcus fragment designated the neutral site (22), which allows homologous recombination between the transforming plasmid DNA and the recipient cyanobacterial chromosome to take place. A fragment containing lacIq, trc promoter, and dnaK3 was isolated from pTrcDK3 and recloned into pNS1 between the separated neutral site segments, next to the spectinomycin resistance cassette. This plasmid was used to transform Synechococcus sp. strain PCC7942, and spectinomycin-resistant transformants were selected on BG-11 medium containing spectinomycin (40 μg/ml). Chromosomal DNA was extracted from one of the transformants, and recombination was confirmed by PCR. This strain, which expresses dnaK3, was designated NSK3.
RESULTS
Disruption of each dnaK gene.
In E. coli, dnaK is not essential for growth, but deletion of the gene confers a temperature-sensitive (ts) phenotype (2, 30). To determine whether each dnaK homologue gene of Synechococcus sp. strain PCC7942 is essential for cell growth, we attempted to disrupt each dnaK gene by inserting an antibiotics resistance marker. By using plasmid pDK1KM (Fig. 1), dnaK1 was disrupted as described in Materials and Methods. Synechococcus is known to contain multiple copies of the chromosome. To confirm if all the chromosomal copies of the dnaK1 gene were changed to the mutant form, we isolated chromosomal DNA of transformants, and the dnaK1 gene region was examined by PCR using primers TRC1-N and TRC1-C. When chromosomal DNA from the wild-type strain was used as the template, a 2.0-kb DNA fragment was detected, and a band of this size was not amplified from the DNA of transformants; instead, a 2.8-kb fragment was observed (data not shown). This increase in size corresponds to the kanamycin resistance gene insertion. Additionally, a 70-kDa protein band was no longer detected in the Western blot analysis for the crude extracts of the transformant cells using anti-DnaK1 antiserum (data not shown). These results confirmed that all chromosomal copies of the dnaK1 genes were totally disrupted. This disruptant, named DK1KM, could grow under normal conditions. Considering that DnaK1 is a heat shock protein homologue, we examined the growth of the mutant at high temperatures and found that DK1KM could grow even at high temperatures, similar to the wild-type strain (data not shown).
In E. coli, mutation in dnaK results in high basal levels of other heat shock proteins at 30°C and failure to turn off the heat shock response at 42°C, suggesting that DnaK functions as a negative regulatory factor in the heat shock response (30, 38, 39). We therefore analyzed the effect of heat shock on the levels of DnaK2, DnaK3, and GroEL proteins in DK1KM by Western blotting. We did not detect any difference in the amount of either DnaK2, DnaK3, or GroEL protein between the wild-type and dnaK1 mutant cells, both at basal levels and after heat shock (data not shown). Therefore, Synechococcus DnaK1 does not seem to function as a regulator in the heat shock response, in contrast to the E. coli DnaK.
We also attempted to disrupt dnaK2 and dnaK3 by inserting a kanamycin resistance marker. Considering that dnaK3 may be cotranscribed with downstream dnaJ7942, frDK3KM, which has an insertion of a promoterless and terminatorless kanamycin resistance casette, was used for the dnaK3 gene disruption (Fig. 1). However, we could not disrupt all copies of either dnaK2 or dnaK3 gene in the cell. Most transformants formed extremely small colonies, and when their dnaK2 and dnaK3 loci were examined by PCR using primers TRC2-N and TRC2-C or TRC3-N and TRC3-C, fragments of two sizes were amplified as a mixture in each case. The size of one band corresponds to the wild-type allele, and the size of the other band represents the allele with the kanamycin resistance cassette insertion (data not shown). These results suggest that DnaK2 and DnaK3 are both essential for growth under normal conditions.
To confirm the essentiality of dnaK3, the same Kmr insertion-carrying dnaK3 fragment was used to transform the NSK3 strain, which carries an IPTG-inducible intact dnaK3 at the neutral site. Kmr transformants appeared as normal-size colonies, and all of the original dnaK3 alleles in these cells were replaced by ones with the Kmr insertion. In these experiments, the transformants were spread onto BG-11 plates containing 0, 0.01, 0.1, and 1.0 mM IPTG to express dnaK3 at various levels. Most of the transformants were viable without IPTG, and the number and size of the colonies decreased with increasing IPTG concentration. These findings indicate that leaky expression from the trc promoter produced a sufficient amount of DnaK3 protein and that a comparative increase in DnaK3 expression had a deleterious effect on the cell.
Differential accumulation and synthesis of DnaK and GroEL proteins after heat shock.
To analyze the changes in the level of each DnaK protein after heat shock, we made specific antisera for each DnaK protein using less-conserved C-terminal regions. We expressed the C-terminal polypeptides of the DnaK proteins with a hexahistidine tag at their C terminus (DnaK1 and DnaK2) or N terminus (DnaK3), purified the polypeptides using Ni-NTA affinity resin, and prepared antisera against them as described in Materials and Methods. We first performed Western blot analyses for crude extracts of Synechococcus sp. strain PCC7942 cells and confirmed that three dnaK genes were actually expressed and that each antiserum was specific to each DnaK protein (Fig. 2). Then we examined the effect of heat shock on the levels of DnaK as well as GroEL protein. Cells were grown at 30°C and shifted to 45°C at the logarithmic growth phase. Crude extracts of the cells at various times were prepared and subjected to Western blot analysis. The levels of DnaK and GroEL proteins in response to heat shock varied (Fig. 3). DnaK2 protein level increased until 30 min after heat shock, and thereafter, the increased level was maintained over the period examined. The GroEL protein level also, but more markedly, increased from 0 to 100 min. Although the levels did not decrease to those before heat shock within 120 min, these two proteins exhibited a typical heat shock response. On the other hand, the level of DnaK3 protein was not affected by heat shock. Characteristically, the amount of DnaK1 protein decreased after heat shock. These results indicate that expression of the dnaK and groEL genes is differentially regulated upon temperature upshift. Since the band intensities of Western blots using purified proteins were almost identical (data not shown), the antibody titers of anti-DnaK antiserum seem to be comparable. Therefore, the weak intensity of the DnaK3 band reflects a relatively small amount of the protein in the cell, compared with DnaK1 and DnaK2.
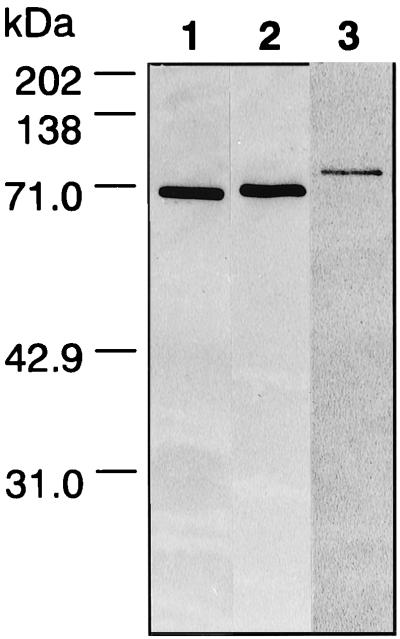
FIG. 2.
Specificity of antiserum raised against DnaK proteins of Synechococcus sp. strain PCC7942. C-terminal regions of three DnaK proteins were purified, and antisera against these polypeptides were prepared as described in Materials and Methods. The specificity of each antiserum was tested by Western blotting of crude extracts (30 μg of protein) from Synechococcus cells. Lane 1, anti-DnaK1; lane 2, anti-DnaK2; lane3, anti-DnaK3.
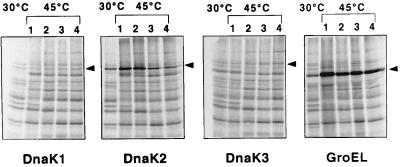
FIG. 3.
Western blot analysis of DnaK and GroEL proteins after heat shock. Synechococcus cells were grown at 30°C and shifted to 45°C at the logarithmic phase (OD700 of 0.5). Crude extracts were prepared from cultures at the indicated times after temperature upshift, and samples (30 μg of protein for DnaK and 10 μg of protein for GroEL) were analyzed by Western blotting using antiserum specific to each DnaK protein or anti-B. subtilis GroEL antiserum as described in Materials and Methods.
In additional experiments, we measured the synthesis rate of DnaK and GroEL proteins by 30-min pulse labeling at various times after temperature upshift. The synthesis rate of DnaK1 protein was not obviously changed (Fig. 4). The results of the Western blot exhibiting a reduction in protein accumulation (Fig. 3) show that DnaK1 seems to be degraded more rapidly during heat shock. DnaK2 and GroEL proteins showed a transient but significant increase of synthesis rate after heat shock, which correlates with the result from the Western analysis. While DnaK3 protein did not show a distinct change in synthesis rate (Fig. 4), its accumulation seems to be kept constant (Fig. 3), suggesting the existence of a mechanism to maintain the level of this protein.
FIG. 4.
De novo synthesis of DnaK and GroEL proteins after heat shock. Logarithmically growing Synechococcus cultures (OD700 of 0.5) were shifted from 30 to 45°C. Aliquots of the cultures were sampled before (control, first lane in each panel) or after temperature upshift at appropriate intervals (lane 1, 0 min; 2, 30 min; 3, 60 min; and 4, 90 min after temperature upshift) and pulse labeled with [35S]methionine at the indicated temperatures for 30 min. Proteins were immunoprecipitated with either DnaK or GroEL antiserum and separated by SDS-PAGE. Bands were detected using Bio-Imaging analyzer BAS2000.
Heat shock response in ΔrpoD mutant.
To discern whether each dnaK or groEL gene is transcribed by RNA polymerase holoenzyme containing any one of four principal sigma factors (RpoD1, RpoD2, RpoD3, and RpoD4), we carried out Western blot analyses on each rpoD mutant. Crude extracts were prepared from wild-type Synechococcus sp. strain PCC7942 cells and from mutant strains D1KM (ΔrpoD1), D2KM (ΔrpoD2), D3KM (ΔrpoD3), and D4KM (ΔrpoD4) (12) both before and after heat shock at 45°C for 60 min. For the non-heat-shocked samples, there was less difference in the amounts of the DnaK and GroEL proteins between wild-type cells and rpoD mutant cells (Fig. 5).
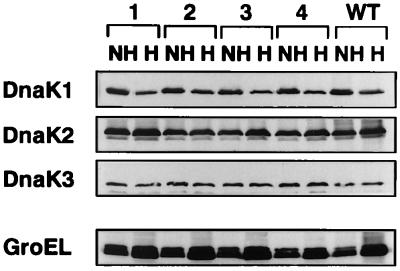
FIG. 5.
Heat shock response in ΔrpoD mutants. Crude extracts were prepared from wild-type Synechococcus cells (WT) and from the rpoD1 (lanes 1), rpoD2 (lanes 2), rpoD3 (lanes 3), and rpoD4 (lanes 4) mutants, which encode each of four principal type sigma factors, both before (NH) and after (H) heat shock at 45°C for 60 min, and subjected to Western blotting. Note that RpoD1 is an essential sigma factor, and the ΔrpoD1 mutant, in which the rpoD1 gene is partially deleted at its N terminus, has residual RpoD1 activity.
The responses of these proteins to heat shock in the rpoD mutants exhibited the same pattern as those of the wild-type cells; the amount of DnaK1 protein decreased, that of DnaK2 and GroEL increased, and that of DnaK3 was constant. RpoD2, RpoD3, and RpoD4 are not responsible for the transcription of these Hsp genes, since these rpoD genes are completely inactivated in strains D2KM, D3KM, and D4KM, respectively. On the other hand, RpoD1 is an essential sigma factor, and strain D1KM, in which rpoD1 is partially deleted at its N terminus, has residual RpoD1 activity (Masuda et al., unpublished results). Therefore, the possibility that RpoD1 controls the transcription of these Hsp genes cannot be ruled out.
Differential effects of Synechococcus DnaK overproduction on E. coli cell morphology.
To examine the effect of Synechococcus sp. strain PCC7942 DnaK production on cell physiology in E. coli and to determine whether the E. coli dnaK756 ts phenotype can be complemented by the Synechococcus dnaK genes, we constructed IPTG-inducible expression plasmids for each dnaK in which Synechococcus sequences are preceded by Shine-Dalgarno sequences of E. coli. The sequences of the junction regions are shown in Table 1. Our initial constructs are those containing sequences TRC1-Na, TRC2-N, and TRC3-Na. Among these, cells harboring a plasmid carrying TRC1-Na and TRC3-Na produced only detectable amounts of DnaK protein (Table 1), whereas pTrcDK2 could produce distinct amounts of DnaK2 (Fig. 6). We therefore reconstructed plasmids with primers in which RBSs and the preceding sequences are modified close to the complementary sequence of the 3′ end of 16SRNA of E. coli (Table 1). Newly constructed pTrcDK1 and pTrcDK3 indeed drove overexpression of DnaK proteins, as shown in Fig. 6.
TABLE 1.
5′ oligonucleotide sequences of primers used to construct each dnaK expression plasmida
| Plasmid | 5′ oligonucleotide | Sequencea | Expressionc |
|---|---|---|---|
| pTrcDK1a | DK1TRC-Na | TAAGGAAAAAATTT (ATG) | ± |
| pTrcDK1 | DK1TRC-N | TAAGGAGGTAATTT (ATG) | +++ |
| pTrcDK2 | DK2TRC-N | TAAGGAACTGGACT (ATG) | +++ |
| pTrcDK3a | DK3TRC-Na | TAAGGAAAGACAGC (ATG) | + |
| pTrcDK3 | DK3TRC-N | TAAGGAGGTGACAGC (ATG) | +++ |
| TAAGGAGGTGATCb |
Nucleotides that are identical to the complementary sequence of the 3′ end of E. coli 16S rRNA are highlighted in boldface.
Complementary sequence of the 3′ end of E. coli 16S rRNA.
Symbols indicate the expression levels of each DnaK protein in the range from detectable (±) to the maximum (+++) which we obtained in Fig. 6.
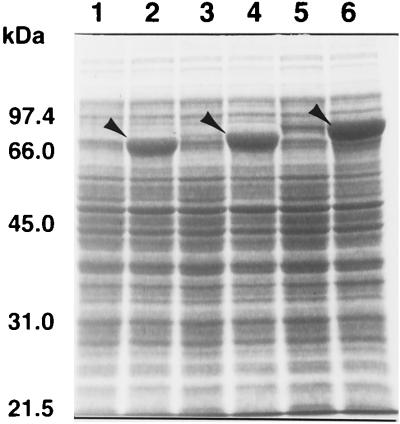
FIG. 6.
Overproduction of DnaK1, DnaK2, and DnaK3 proteins in E. coli. E. coli MC4100 cells harboring the dnaK expression plasmids pTrcDK1 (lanes 1 and 2), pTrcDK2 (lanes 3 and 4), and pTrcDK3 (lanes 5 and 6) were grown at 37°C in the presence (lanes 2, 4, and 6) or absence (lanes 1, 3, and 5) of 1 mM IPTG. After incubation for 5 h, cultures were sampled and cells were harvested by centrifugation. Cells were suspended in SDS sample buffer and subjected to SDS-PAGE on 10% polyacrylamide gels, followed by Coomassie blue staining. The positions of molecular size standards are indicated on the left. Arrows indicate the positions of the DnaK proteins.
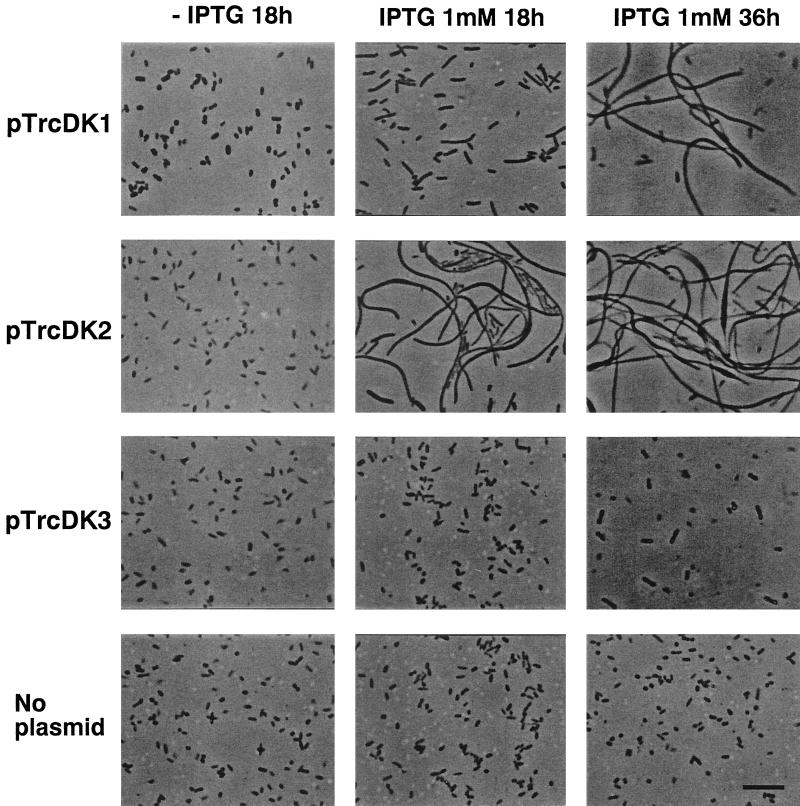
By using the expression plasmids described above, we examined the effect of DnaK overproduction on the cell morphology of E. coli MC4100. Strains expressing any of the Synechoccccus DnaK proteins from these plasmids were found to produce colonies of equal size to those of the control strain harboring no plasmid. We also observed that cells grown on LB plates without IPTG were indistinguishable from those containing no plasmid (Fig. 7). The addition of 1 mM IPTG, which results in overproduction of DnaK proteins (Fig. 6), led cells harboring each dnaK producer to exhibit aberrant morphology. A substantial population of cells producing DnaK1 or DnaK2 became filamentous (Fig. 7). Overproduction of E. coli DnaK has been shown to result in cell filamentation (1). From the viewpoint that overproduction leads to a defect in cell septation, DnaK1 and DnaK2 seem to exhibit a function similar to that of E. coli DnaK.
FIG. 7.
Effects of Synechococcus DnaK overproduction on E. coli cell morphology. E. coli MC4100 cells harboring Synechococcus dnaK expression plasmids were grown on LB agar plates in the presence or absence of 1 mM IPTG. After incubation for 18 and 36 h at 37°C, cells from multiple representative colonies were examined under a microscope (BX60; Olympus) with a U Plan F1 objective lens (×40), and photographs were taken with Neopan 400 Presto films (Fujifilm). In the absence of IPTG, the cells showed no difference in morphology after incubation for 18 and 36 h. As controls, MC4100 cells without plasmid were similarly grown on LB agar plates and examined after incubation for 18 and 36 h. Bar, 10 μm.
Moreover, the toxic effect of DnaK2 protein is more prominent than that of DnaK1, since extremely filamentous cells were observed just 18 h after the addition of IPTG. These two proteins seem to be produced at similar levels (Fig. 6), and therefore, the difference in extent of the toxic effect suggests a functional difference between these proteins. When DnaK3 was overproduced, filamentous cells were not seen; rather, somewhat unusual morphology was observed (Fig. 7). Cells were relatively swollen and sometimes twisted. The appearance of the colonies on an LB plate was also unique, with heterologous regions at their edges. It is possible that the plasmid was instantaneously rearranged and cells no longer overproducing DnaK3 began to grow.
Complementation of E. coli dnaK ts mutant.
To determine whether the three DnaK proteins are functional homologues of the E. coli DnaK, complementation experiments were carried out using the E. coli NRK156 strain, which has the dnaK756(Ts) mutation (Table 2). NRK156 cells harboring each dnaK expression plasmid were grown in LB medium at 37°C, and at the logarithmic growth phase (OD660 of 0.2), 5 × 103 cells were spread on LB agar plates containing various concentrations of IPTG as indicated. Cells were incubated at the permissive temperature (37 or 42°C) or nonpermissive temperature (44.5°C), and colony-forming ability was determined. As summarized in Table 2, DnaK2 could suppress ts growth at 44.5°C, while DnaK1 and DnaK3 could not suppress growth at 44.5°C; rather, overproduction of these proteins resulted in growth inhibition even at the permissive temperature (42°C). This inhibitory effect was not seen in the absence of IPTG and became more severe as the IPTG concentration was increased. Therefore, the effect is likely the consequence of overproduction of DnaK1 or DnaK3. DnaK3 seems to be more toxic, since overproduction of DnaK3 inhibited growth at relatively lower IPTG concentrations than DnaK1. The growth inhibition was not observed at 37°C even in the presence of 1 mM IPTG, indicating that this effect is temperature dependent.
TABLE 2.
Effect of Synechococcus dnaK expression in E. coli dnaK756(Ts) mutant
| IPTG (mM) | Growtha
|
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 37°C
|
42°C
|
44.5°C
|
||||||||||
| No plasmid | pTrcDK1 | pTrcDK2 | pTrcDK3 | No plasmid | pTrcDK1 | pTrcDK2 | pTrcDK3 | No plasmid | pTrcDK1 | pTrcDK2 | pTrcDK3 | |
| 0 | + | + | + | + | + | + | + | + | − | − | − | − |
| 0.1 | NT | NT | NT | NT | NT | + | + | + | NT | − | + | − |
| 0.5 | NT | + | + | + | NT | + | + | ± | NT | − | + | − |
| 1.0 | NT | + | + | + | NT | ± | + | − | NT | − | + | − |
+, growth; −, no growth; NT, not tested.
DISCUSSION
We could not completely disrupt all copies of either dnaK2 or dnaK3 in Synechococcus cells, as our results indicate. This strongly suggests that these two genes are essential for normal growth and that DnaK2 and DnaK3 have some specific function which cannot be compensated for by the remaining two DnaK proteins. There have been no previous reports mentioning that two species of Hsp70 in the same cellular compartment are both essential. In Saccharomyces cerevisiae, 15 genes of the Hsp70 family which encode proteins localized in different cellular compartments have been found. Among them, Ssa and Ssb represent the two classes of abundant cytosolic Hsp70s. The essential Ssa proteins are encoded by four genes, SSA1 to SSA4. The Ssb proteins are encoded by the SSB1 and SSB2 genes. Though Ssbs are not essential for growth (6), the Ssa and Ssb families have been suggested to be functionally distinct; overexpression of an Ssa protein fails to rescue the phenotype of an ssb mutant, and vice versa (5). A chimera consisting of the N-terminal ATPase domain from Ssa1 protein and the remainder from Ssb1 protein was shown to be able to rescue the phenotype of ssb1 ssb2 cells (16). The N-terminal regions of DnaK2 and DnaK3 bearing the ATP-binding domain and the following ca. 100 amino acids are quite similar, and the remaining C-terminal regions are variable. Since DnaK3 is characteristic in that it has a relatively long C-terminal nonconserved region, it would be interesting to discern whether this C-terminal region is responsible for functional specificity.
We have shown here that each DnaK protein exhibits a characteristic accumulation pattern and synthesis rate after heat shock, suggesting that the three dnaK genes are differentially regulated. The different responses after heat shock suggest functional differences between Hsp70 proteins in a multigene family. In our study, the synthesis of DnaK1 was constitutive but the protein was found to be degraded after heat shock. This property, similar to that of Ssb proteins in S. cerevisiae, seems instead to reflect a general response of ordinary proteins after heat shock.
The level of DnaK2 increased in response to heat shock, like that of GroEL and other typical Hsp proteins. In prokaryotic cells, the regulation of heat shock response in E. coli has been well studied and is known to be under the control of the rpoH gene product, a ς32 transcription factor (10, 13, 38, 48). However, ς32 might not be a universal regulator of the heat shock response in prokaryotes, since no ς32-related factor or ς32-specific heat shock promoter has been found in many other bacteria (47). Instead, a novel inverted repeat termed CIRCE has been found between the transcriptional and translational start sites of heat-inducible genes in some gram-positive bacteria, including Bacillus subtilis and cyanobacteria (21, 44, 49). In B. subtilis, groE is transcribed by RNA polymerase holoenzyme containing the principal ςA factor, and its heat-inducible transcriptional start sites are the same as those at low temperature and are preceded exclusively by vegetative promoters (21, 34, 40). From in vivo and in vitro studies, the inverted repeat (CIRCE) has been suggested to serve as an operator, and both dnaK and groE operons have been postulated to be negatively regulated by a repressor encoded by the hrcA gene (24, 35, 46, 49). The Western blot analyses for four principal sigma factor mutants of Synechococcus sp. strain PCC7942 suggested that three dnaK and groEL genes are transcribed by RNA polymerase holoenzyme containing either principal sigma factor RpoD1 or another, if any, minor sigma factor. In Synechococcus sp. strain PCC7942, ς32-related factor has not been found, but CIRCE is conserved upstream of the groESL operon (41). Therefore, it is possible that the dnaK2 gene also contains a CIRCE element in the promoter region and is transcribed by RNA polymerase holoenzyme containing the principal RpoD1 factor. However, no CIRCE element was found in the upstream region of any of the three dnaK genes of Synechocystis sp. strain PCC6803, although it is conserved in both the groESL operon and groEL-2 gene. Alternatively, cyanobacterial dnaK genes may have another regulatory mechanism that dose not depend on CIRCE or ς32.
The synthesis rate of DnaK1 and DnaK3 did not show any distinct change after temperature upshift. After heat shock, the DnaK3 level was kept constant, while DnaK1 seems to be degraded more rapidly, suggesting the existence of a mechanism to maintain the level of DnaK3 protein. Gene disruption experiments indicated that DnaK3 is essential for growth. DnaK3 therefore appears to play an important role in cell physiology under normal conditions other than heat shock response.
Although E. coli dnaK is dispensable under normal growth conditions, mutation in dnaK causes ts growth at both high and low temperatures and defects in septation, which result in cell filamentation (2, 30). E. coli dnaK mutants also survive poorly during carbon starvation and stationary phase (37). Overproduction of DnaK also results in the formation of filamentous cells and reduced cell viability during stationary phase (1). It is interesting to note that both DnaK deficiency and overproduction result in similar physiological defects. Overproduction of the FtsZ protein in dnaK mutants suppressed filamentation, suggesting that DnaK might play a role in cell division via some action on FtsZ (2). Defective septation in strains overproducing DnaK may also result from an interaction(s) between DnaK protein and proteins involved in cell division (1). Since overproduction of Synechococcus DnaK1 or DnaK2 in E. coli resulted in cell filamentation, it is possible that DnaK1 and DnaK2 interact with E. coli proteins involved in cell division. When expressed in the E. coli dnaK756 mutant, dnaK2 could suppress the growth deficiency at the nonpermissive temperature, while dnaK1 and dnaK3 could not. On the contrary, overproduction of DnaK1 or DnaK3 resulted in growth inhibition at the permissive temperature. Taken together, these results suggest that DnaK2 is a functional counterpart of the E. coli DnaK protein.
The E. coli genome project has disclosed two other dnaK homologs in this organism. However, neither of these seems to be structurally related to any dnaK gene of Synechococcus. It should be noted that both DnaK2 and DnaK3 contain several motifs specific to Hsp70 proteins in chloroplasts and significant amounts of DnaK3 are localized to the thylakoid membrane (26). Moreover, a dnaJ homologue gene (dnaJ7942) which is located immediately downstream of dnaK3 has also been identified and characterized (29). dnaJ7942 is essential for growth, and DnaJ7942 is also detected quantitatively in the thylakoid membranes. These facts suggest that DnaK3 and DnaJ7942 cooperatively have some specific function(s) related to photosynthesis. Synechocystis sp. PCC6803 was revealed to have four DnaJ homologues (ORF designations in the database are sll0897, sll1666, sll1933, and slr0093). Among them, dnaJ6803 (sll1933) is similarly located downstream of dnaK6803 (sll1932), which corresponds to Synechococcus dnaK3, and DnaJ6803 (sll1933) shows similarity with DnaJ7942 in that they do not have a Gly/Phe-rich domain or a zinc finger domain, both of which are often identified in DnaJ homologues. These two genes seem to be cotranscribed and to be the only pair which constitute an operon among the three dnaK and four dnaJ genes. It is therefore intriguing to assume that DnaK3/DnaJ7942 is involved in localizing proteins required for photosynthesis to thylakoid membranes.
ACKNOWLEDGMENTS
We are indebted to T. Endo (Nagoya University, Nagoya, Japan) for providing the Synechococcus strain and plasmid pTrc99A/X. We also thank H. Yamamoto (Osaka University, Osaka, Japan) for his help in preparation of mouse antibodies.
This research was supported by a grant-in-aid from the Ministry of Education, Science, Sports and Culture of Japan and by a grant from the Biodesign Research Program of Riken.
REFERENCES
- 1.Blum P, Ory J, Bauernfeind J, Krska J. Physiological consequences of DnaK and DnaJ overproduction in Escherichia coli. J Bacteriol. 1992;174:7436–7444. doi: 10.1128/jb.174.22.7436-7444.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bukau B, Walker G C. Cellular defects caused by deletion of the Escherichia coli dnaK gene indicate roles for heat shock protein in normal metabolism. J Bacteriol. 1989;171:2337–2346. doi: 10.1128/jb.171.5.2337-2346.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–556. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 4.Castenholz R W. Culturing methods for cyanobacteria. Methods Enzymol. 1988;167:68–93. [Google Scholar]
- 5.Craig E A, Jacobsen K. Mutations in cognate gene of Saccharomyces cerevisiae hsp70 result in reduced growth rates at low temperatures. Mol Cell Biol. 1985;5:3517–3524. doi: 10.1128/mcb.5.12.3517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Craig E A, Jacobsen K. Mutations of the heat inducible 70 kilodalton genes of yeast confer temperature-sensitive growth. Cell. 1984;38:841–849. doi: 10.1016/0092-8674(84)90279-4. [DOI] [PubMed] [Google Scholar]
- 7.Ellis R J. Proteins as molecular chaperones. Nature. 1987;328:378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- 8.Ellis R J, van der Vies S M. Molecular chaperones. Annu Rev Biochem. 1991;60:321–347. doi: 10.1146/annurev.bi.60.070191.001541. [DOI] [PubMed] [Google Scholar]
- 9.Fraser C M, Casjens S, Huang W M, Sutton G G, Clayton R, Lathigra R, White O, Ketchum K A, Dodson R, Hickey E K, Gwinn M, Dougherty B, Tomb J F, Fleischmann R D, Richardson D, Peterson J, Kerlavage A R, Quackenbush J, Salzberg S, Hanson M, van Vugt R, Palmer N, Adams M D, Gocayne J, Venter J C, et al. Genomic sequence of a Lyme disease spirochaete, Borrelia burgdorferi. Nature. 1997;390:580–586. doi: 10.1038/37551. [DOI] [PubMed] [Google Scholar]
- 10.Gamer J, Multhaup G, Tomoyasu T, McCarty J S, Rüdiger S, Schönfeld H-J, Schirra C, Bujard H, Bukau B. A cycle of binding and release of the DnaK, DnaJ, and GrpE chaperones regulates activity of the Escherichia coli heat shock transcription factor ς32. EMBO J. 1996;15:607–617. [PMC free article] [PubMed] [Google Scholar]
- 11.Gething M-J, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0. [DOI] [PubMed] [Google Scholar]
- 12.Goto-Seki A, Shirokane M, Masuda S, Tanaka K, Takahashi H. Specificity crosstalk among group 1 and group 2 sigma factors in the cyanobacterium Synechococcus sp. PCC7942: in vitro specificity and phylogenetic analysis. Mol Microbiol. 1999;34:473–484. doi: 10.1046/j.1365-2958.1999.01608.x. [DOI] [PubMed] [Google Scholar]
- 13.Grossman A D, Straus D B, Walter W A, Gross C A. Sigma 32 synthesis can regulate the synthesis of heat shock proteins in Escherichia coli. Genes Dev. 1987;1:179–184. doi: 10.1101/gad.1.2.179. [DOI] [PubMed] [Google Scholar]
- 14.Hartl F U. Molecular chaperones in cellular protein folding. Nature. 1996;381:571–580. doi: 10.1038/381571a0. [DOI] [PubMed] [Google Scholar]
- 15.Higuchi R. Using PCR to engineer DNA. In: Erlich H A, editor. PCR technology. New York, N.Y: Stockton Press; 1989. pp. 61–70. [Google Scholar]
- 16.James P, Pfund C, Craig E A. Functional specificity among Hsp70 molecular chaperones. Science. 1997;275:387–389. doi: 10.1126/science.275.5298.387. [DOI] [PubMed] [Google Scholar]
- 17.Kaneko T, Sato S, Kotani H, Tanaka A, Asamizu E, Nakamura Y, Miyajima N, Hirosawa M, Sugiura M, Sasamoto S, Kimura T, Hosouchi T, Matsuno A, Muraki A, Nakazaki N, Naruo K, Okumura S, Shimpo S, Takeuchi C, Wada T, Watanabe A, Yamada M, Yasuda M, Tabata S. Sequence analysis of the genome of the unicellular cyanobacterium Synechocystis sp. strain PCC6803. II. Sequence determination of the entire genome and assignment of potential protein-coding regions. DNA Res. 1996;3:109–136. doi: 10.1093/dnares/3.3.109. [DOI] [PubMed] [Google Scholar]
- 18.Kawasaki Y, Wada C, Yura T. Roles of Esherichia coli heat shock proteins Dnak, DnaJ, and GrpE in mini-F plasmid replication. Mol Gen Genet. 1990;220:277–282. doi: 10.1007/BF00260494. [DOI] [PubMed] [Google Scholar]
- 19.Kuhlemeier C J, Thomas A A M, van der Ende A, van Leen R W, Borrias W E, van den Hondel C A M J J, van Arkel G A. A host-vector system for gene cloning in the cyanobacterium Anacystis nidulans R2. Plasmid. 1983;10:156–163. doi: 10.1016/0147-619x(83)90068-9. [DOI] [PubMed] [Google Scholar]
- 20.Lelivelt M J, Kawula T H. Hsc66, an Hsp70 homolog in Escherichia coli, is induced by cold shock but not by heat shock. J Bacteriol. 1995;177:4900–4907. doi: 10.1128/jb.177.17.4900-4907.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Wong S-L. Cloning and characterization of the groESL operon from Bacillus subtilis. J Bacteriol. 1992;174:3981–3992. doi: 10.1128/jb.174.12.3981-3992.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li R, Golden S S. Enhancer activity of light-responsive regulatory elements in the untranslated leader regions of cyanobacterial psbA genes. Proc Natl Acad Sci USA. 1993;90:11678–11682. doi: 10.1073/pnas.90.24.11678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lindquist S, Craig E A. The heat-shock proteins. Annu Rev Genet. 1988;22:631–677. doi: 10.1146/annurev.ge.22.120188.003215. [DOI] [PubMed] [Google Scholar]
- 24.Mogk A, Homuth G, Scholz C, Kim L, Schmid F X, Schumann W. The GroE chaperonin machine is a major modulator of the CIRCE heat shock regulon of Bacillus subtilis. EMBO J. 1997;16:4579–4590. doi: 10.1093/emboj/16.15.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Narberhaus F, Bahl H. Cloning, sequencing, and molecular analysis of the groESL operon of Clostridium acetobutylicum. J Bacteriol. 1992;174:3282–3289. doi: 10.1128/jb.174.10.3282-3289.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nimura K, Yoshikawa H, Takahashi H. DnaK3, one of the three DnaK proteins of cyanobacterium Synechococcus sp. PCC7942, is quantitatively detected in the thylakoid membrane. Biochem Biophys Res Commun. 1996;229:334–340. doi: 10.1006/bbrc.1996.1802. [DOI] [PubMed] [Google Scholar]
- 27.Nimura K, Yoshikawa H, Takahashi H. Identification of dnaK multigene family in Synechococcus sp. PCC7942. Biochem Biophys Res Commun. 1994;201:466–471. doi: 10.1006/bbrc.1994.1724. [DOI] [PubMed] [Google Scholar]
- 28.Nimura K, Yoshikawa H, Takahashi H. Sequence analysis of the third dnaK homolog gene in Synechococcus sp. PCC7942. Biochem Biophys Res Commun. 1994;201:848–854. doi: 10.1006/bbrc.1994.1778. [DOI] [PubMed] [Google Scholar]
- 29.Oguchi K, Nimura K, Yoshikawa H, Takahashi H. Sequence and analysis of a dnaJ homologue gene in cyanobacterium Synechococcus sp. PCC7942. Biochem Biophys Res Commun. 1997;236:461–466. doi: 10.1006/bbrc.1997.6992. [DOI] [PubMed] [Google Scholar]
- 30.Paek K-H, Walker G C. Escherichia coli dnaK null mutants are inviable at high temperature. J Bacteriol. 1987;169:283–290. doi: 10.1128/jb.169.1.283-290.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Porter R D. DNA transformation. Methods Enzymol. 1988;167:703–712. doi: 10.1016/0076-6879(88)67081-9. [DOI] [PubMed] [Google Scholar]
- 32.Prentki P, Krisch H M. In vitro insertional mutagenesis with a selectable DNA fragment. Gene. 1984;29:303–313. doi: 10.1016/0378-1119(84)90059-3. [DOI] [PubMed] [Google Scholar]
- 33.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 34.Schmidt A, Schiesswohl M, Völker U, Hecker M, Schumann W. Cloning, sequencing, mapping, and transcriptional analysis of the groESL operon from Bacillus subtilis. J Bacteriol. 1992;174:3993–3999. doi: 10.1128/jb.174.12.3993-3999.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schulz A, Schumann W. hrcA, the first gene of the Bacillus subtilis dnaK operon, encodes a negative regulator of class I heat shock genes. J Bacteriol. 1996;178:1088–1093. doi: 10.1128/jb.178.4.1088-1093.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Seaton B L, Vickery L E. A gene encoding a DnaK/hsp70 homolog in Escherichia coli. Proc Natl Acad Sci USA. 1994;91:2066–2070. doi: 10.1073/pnas.91.6.2066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spence J, Cegielska A, Georgopoulos C. Role of Escherichia coli heat shock proteins DnaK and HtpG(C62.5) in response to nutritional deprivation. J Bacteriol. 1990;172:7157–7166. doi: 10.1128/jb.172.12.7157-7166.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Straus D, Walter W, Gross C. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of ς32. Genes Dev. 1990;4:2202–2209. doi: 10.1101/gad.4.12a.2202. [DOI] [PubMed] [Google Scholar]
- 39.Tilly K, McKittrick N, Zylicz M, Georgopoulos C. The dnaK protein modulates the heat-shock response of Escherichia coli. Cell. 1986;34:641–646. doi: 10.1016/0092-8674(83)90396-3. [DOI] [PubMed] [Google Scholar]
- 40.Tozawa Y, Yoshikawa H, Kawamura F, Itaya M, Takahashi H. Isolation and characterization of the groES and groEL genes of Bacillus subtilis Marburg. Biosci Biotechnol Biochem. 1992;56:1995–2002. doi: 10.1271/bbb.56.1995. [DOI] [PubMed] [Google Scholar]
- 41.Webb R, Reddy K J, Sherman L A. Regulation and sequence of the Synechococcus sp. strain PCC7942 groESL operon, encoding a cyanobacterial chaperonin. J Bacteriol. 1990;172:5079–5088. doi: 10.1128/jb.172.9.5079-5088.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Werner-Washburne M, Becker J, Kosic-Smithers J, Craig E A. Yeast Hsp70 RNA levels vary in response to the physiological status of the cell. J Bacteriol. 1989;171:2680–2688. doi: 10.1128/jb.171.5.2680-2688.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Werner-Washburne M, Stone D E, Craig E A. Complex interactions among members of an essential subfamily of hsp70 genes in Saccharomyces cerevisiae. Mol Cell Biol. 1987;7:2568–2577. doi: 10.1128/mcb.7.7.2568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wetzstein M, Völker U, Dedio J, Löbau S, Zuber U, Schiesswohl M, Herget C, Hecker M, Schumann W. Cloning, sequencing, and molecular analysis of the dnaK locus from Bacillus subtilis. J Bacteriol. 1992;174:3300–3310. doi: 10.1128/jb.174.10.3300-3310.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yoshimune K, Yoshimura T, Esaki N. Hsc62, a new DnaK homolog of Escherichia coli. Biochem Biophys Res Commun. 1998;250:115–118. doi: 10.1006/bbrc.1998.9255. [DOI] [PubMed] [Google Scholar]
- 46.Yuan G, Wong S-L. Regulation of groE expression in Bacillus subtilis: the involvement of the ςA-like promoter and the roles of the inverted repeat sequence (CIRCE) J Bacteriol. 1995;177:5427–5433. doi: 10.1128/jb.177.19.5427-5433.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yura T, Nakahigashi K. Regulation of the heat-shock response. Curr Opin Microbiol. 1999;2:153–158. doi: 10.1016/S1369-5274(99)80027-7. [DOI] [PubMed] [Google Scholar]
- 48.Yura T, Nagai H, Mori H. Regulation of the heat-shock response in bacteria. Annu Rev Microbiol. 1993;47:321–350. doi: 10.1146/annurev.mi.47.100193.001541. [DOI] [PubMed] [Google Scholar]
- 49.Zuber U, Schumann W. CIRCE, a novel heat shock element involved in regulation of heat shock operon dnaK of Bacillus subtilis. J Bacteriol. 1994;176:1359–1363. doi: 10.1128/jb.176.5.1359-1363.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]