Abstract
Intermycelial transfer of Streptomyces plasmid pIJ101 occurs prior to cellular differentiation and is mediated by plasmid functions that are also required for production of zones of growth-inhibited recipient cells (i.e., pocks) that develop around individual donors during mating on agar medium. Several other pIJ101 functions, including that of the kilB gene, whose unregulated expression on pIJ101 is lethal, are required for normal pock size and so have been postulated to mediate intramycelial spread of the plasmid throughout recipient cells. Using antibodies raised against a KilB fusion protein expressed in Escherichia coli, native KilB protein was detected throughout development of pIJ101-containing Streptomyces lividans cells, with the concentration of KilB increasing dramatically and reaching a maximum during the final stages (i.e., sporulation and secondary metabolism) of cellular differentiation. Insertion of the kilB gene of pIJ101 into the S. lividans chromosome in cells lacking the pIJ101 KorB protein, which normally represses kilB gene transcription, resulted in elevated but still temporally increasing amounts of KilB. The increased expression or accumulation of the KilB spread protein throughout cellular differentiation of S. lividans, which leads to maximum KilB concentrations during developmental stages that occur far later than when intermycelial transfer of pIJ101 is mediated, supports the existence of a subsequent intramycelial component to the pIJ101 spread function. The results also suggest that intramycelial spread of pIJ101 molecules within the recipient extends beyond intercompartmental movements within the substrate mycelia and includes undetermined steps within the spore-yielding aerial hyphae as well.
Streptomyces bacteria are gram-positive actinomycete soil organisms that display complex cellular differentiation which involves both morphological and physiological changes (4). Although they persist vegetatively as an infrequently septated, multinucleoid substrate mycelium, this growth pattern ceases as nutrients become scarce, and substrate hyphal compartments begin to differentiate, yielding vertically directed aerial hyphae that appear fuzzy white. Concomitant with this morphological change, Streptomyces bacteria also begin producing a vast array of secondary metabolites, including antibiotics. Growth of aerial hyphae, which is fueled by organic material derived from the dying substrate layer, eventually also stops, and regularly spaced septation then divides the tips of these vertical structures into unigenomic sections that subsequently develop into grayish-colored spores. Submerged cultures of species such as Streptomyces lividans grow in a mycelial form that does not differentiate morphologically but does develop physiologically, with cells producing secondary metabolites as they enter stationary phase (3).
Conjugative plasmids in Streptomyces spp. can be detected when individual spores containing a plasmid germinate within a dense lawn of plasmidless potential recipient mycelia, and subsequent transfer of plasmids from donors to surrounding recipients leads to finite circular regions where aerial hypha development and sporulation are transiently delayed or prevented (1, 10). Since such zones or “pocks” correspond to cells that have received plasmid copies (1), pock formation depends on and is coincident with transmission of streptomycete plasmids (1, 9). Presumably, such developmental inhibition in turn promotes the transfer process, perhaps by prolonging the growth period during which transmission can occur (9).
In marked contrast to plasmids from other bacteria, Streptomyces plasmids encode few transfer functions. Such loci can be divided into those that are essential for plasmid transfer and pock formation and others that are not required for transfer and pocking to occur but affect pock size and thus plasmid “spread” (9, 13). The first set of loci are undoubtedly required for the intermycelial transfer of plasmid molecules between donor and recipient hyphae, while the function of the latter group is less clear. Given the mycelial pattern of streptomycete growth, these loci may mediate intramycelial spread of plasmids within recipient cells, such as movement across infrequent hyphal cross walls that separate the original point of transfer from other connected cell compartments (9, 13). Alternatively, it is possible that plasmid spread functions instead augment the initial intermycelial transfer step, for example, by increasing its efficiency or by extending the transfer period (9, 14).
The transmission properties of pIJ101 (Fig. 1), the 8,830-base pair (bp) (11), high-copy-number (i.e., up to 300 copies per chromosome) (13) S. lividans plasmid, have been among the most studied for a Streptomyces extrachromosomal element. Loci responsible for intermycelial plasmid transfer as well as pock formation include the pIJ101 tra gene (12, 13), which encodes a temporally expressed 70-kDa membrane protein of unknown function that is found only in the substrate mycelium of S. lividans cells (14), as well as clt, a cis-acting locus that participates in the transfer event in an undetermined manner (7, 15). Coincident with expression of the essential Tra protein of pIJ101, intermycelial transfer of the plasmid is also known to be complete by the onset of aerial hypha development (14).
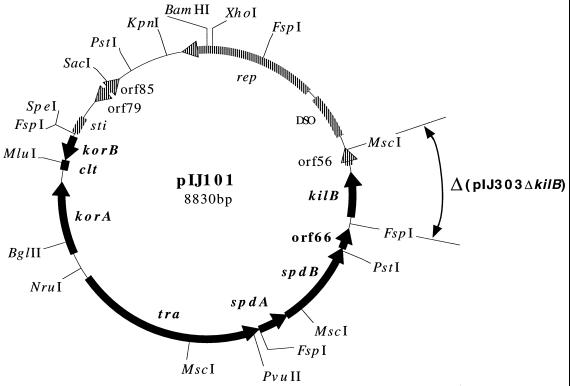
FIG. 1.
Physical map and genetic organization of Streptomyces plasmid pIJ101. The highlighted region includes previously determined genetic functions related to transmission of the plasmid (12, 13, 15), which are shown alongside their respective ORFs (filled arrows) or, in the case of clt, its locus (filled box). The remaining plasmid region includes functions involved in replication of the plasmid (2, 6, 13), and these are also indicated beside their respective ORFs (striped arrows) or loci (striped boxes). Small ORFs (i.e., orf56, orf66, orf79, and orf85) (11) whose functions remain undetermined are indicated in both regions. The sequence that is deleted (Δ) in plasmid pIJ303ΔkilB, which is a derivative of the conjugative, thiostrepton-resistant pIJ101 plasmid pIJ303 (13), is indicated.
Three other pIJ101 genes, namely spdA, spdB, and kilB (Fig. 1), are required for plasmid spread since mutations in any of them dramatically reduce the diameter of pocks resulting from pIJ101 transmission (12). All three genes encode putative membrane proteins (11) which do not appear to be related to any other known proteins. The spdA and spdB genes are included on a transcript (14) that initiates upstream of the tra gene and appears to terminate downstream of orf66, a small open reading frame (ORF) of unknown function (11), while the kilB gene is expressed separately from its own promoter (20).
Interestingly, plasmids consisting of the kilB gene cloned into a minimal pIJ101 replicon (i.e., a pIJ101 replicon that lacks the entire transfer region, as highlighted in Fig. 1) cannot be introduced via transformation into S. lividans. This lethality phenotype (kil-override) is known to be suppressed when either certain pIJ101 loci termed kor (for kil-override) are also present in high copy number or a lower-copy-number non-pIJ101 plasmid containing the cloned kilB gene of pIJ101 is used in the absence of kor loci to transform S. lividans (12). One of these loci, the korB gene of pIJ101 (Fig. 1), encodes a repressor protein (19) that regulates transcription of kilB as well as of the korB gene itself (19, 20). While the korA gene (Fig. 1) can also suppress kilB-associated lethality by an undetermined mechanism (12), its repressor product does not interact with the kilB promoter but instead regulates expression from the korA and tra promoters (19, 20).
There is currently no information available on the expression of proteins that mediate plasmid spreading in Streptomyces bacteria. To begin to elucidate the mechanism of plasmid spread, we have focused on the pIJ101 kilB gene, whose associated lethality function suggests that it may play a unique role in the process. Using antibodies raised against an Escherichia coli fusion protein comprised largely of KilB sequences, we found KilB protein in pIJ101-containing S. lividans cells throughout their differential growth, with concentrations reaching a maximum during the terminal sporulation and antibiotic production stages. A similar pattern of elevated KilB expression also appeared in the absence of KorB in an S. lividans strain containing the chromosomally integrated kilB gene. By demonstrating that the KilB spread protein is present throughout cellular differentiation of S. lividans, including morphological stages that occur considerably later than that during which intermycelial transfer of pIJ101 is completed, our data provide support for a KilB-mediated intramycelial component to spreading of pIJ101, which may be operative during all stages of Streptomyces development.
MATERIALS AND METHODS
Bacterial strains and plasmids.
S. lividans strain TK23 (spc-1) has been described previously (8), while E. coli hosts for cloning were DH10B [F− mcrA Δ(mrr-hsdRMS-mcrBC) φ80dlacZΔM15 ΔlacX74 deoR recA1 endA1 araD139 Δ(ara-leu)7697 galU galK λ− rpsL nupG] (Life Technologies Inc., Gaithersburg, Md.) and BRL2288, which is a recA56 derivative of MC1061 [F− araD139 Δ(ara-leu)7697 ΔlacX74 galU galK hsdR2(rK− mK−) mcrB1 rpsL] (Life Technologies Inc.). For overexpression of recombinant KilB protein, the E. coli host was BL21(DE3) (21). To construct plasmid pIJ303ΔkilB, the 3.5-kb PstI fragment of pIJ101 was first ligated into this site in the E. coli vector pSP72 (Promega, Madison, Wis.) in order to create pGSP304. This plasmid was partially digested with FspI and then digested to completion with MscI, and the vector-containing fragment lacking the 726-bp MscI-FspI (nucleotides 1772 to 2497) region of pIJ101 (11) was ligated to itself. The 2.8-kb PstI insert of the resulting plasmid, pGSP305, was then ligated to the 7.3-kb PstI fragment of pIJ303 in the natural orientation to create pIJ303ΔkilB. A 481-bp DNA fragment containing the 444-bp kilB ORF (11) was amplified following 30 PCR cycles (1 cycle = 94°C for 30 s, 37°C for 1 min, and 72°C for 2 min) using the primers kilB5, 5′-GTGAGCTACGTTCAGGATCCATGGTGACCACGCTCATTGCCGTGA, and kilB3, 5′-CTGTAGCTGCAGTACTCGAGTCAGGCGCCGAACCGGCGGGCGG CG, both at 0.5 μM, 100 ng of the 6.1-kb BglII-BamHI fragment of pIJ101 as a template, and ULTma DNA polymerase (Perkin-Elmer, Branchburg, N.J.) in the presence of 10% dimethyl sulfoxide. Following extraction with phenol-chloroform (50:50) and chloroform, DNA was ethanol precipitated, resuspended, and digested to completion with BamHI and XhoI. The 446-bp kilB-containing BamHI-XhoI digestion product was then isolated on a 1.5% agarose gel, purified using a Geneclean kit (Bio 101, Carlsbad, Calif.), and ligated to similarly digested pET30a(+) (Novagen, Inc., Madison, Wis.) vector DNA in order to create pGSP281. Plasmid pGSP290, a pSP72 derivative that contains the 1.0-kb PstI-BalI region of pIJ101 that includes the kilB gene, has been described previously (16). To create pGSP295, plasmid pGSP290 was digested with BamHI and BglII and the 1.0-kb kilB-containing fragment was ligated to BamHI-digested pBeBal2 (15) in the orientation indicated below. Besides kilB, this pIJ101 fragment also contains orf66, which appears to lacks its own promoter (11, 14) and does not show obvious effects on S. lividans cell growth when present in high copy numbers (12).
Bacteriological methods and molecular biology techniques.
Transformation of S. lividans and E. coli was performed as described previously (8) using R5 agar (8) and Luria-Bertani agar (18), respectively, for plating of transformants. Transformant colonies of S. lividans TK23 were excised with a scalpel, macerated with a pipette tip, and spread in patches onto Streptomyces ipomoeae growth agar (SIGA) (5) containing thiostrepton in order to obtain spores. Nonselective growth of NWR2 isolates was performed by patching spores onto SIGA in the absence of thiostrepton and then plating appropriate dilutions of the resulting spores onto either Luria-Bertani agar or SIGA; upon subsequent growth and sporulation, colonies were replica plated onto the same respective media either containing or lacking thiostrepton in order to score for loss of thiostrepton resistance. Submerged cultures of S. lividans were grown in yeast extract-malt extract medium (8). Thiostrepton was used in agar or liquid medium at the previously described (8) concentrations of 50 and 5 μg/ml, respectively.
Cloning was performed using standard procedures described previously (18). Genomic DNA was extracted and purified from S. lividans NWR2 spores using the method of Rainey et al. (17). Amplification of the 481-bp kilB-containing fragment from 100 ng of purified genomic DNA was performed using the kilB5 and kilB3 primers along with the PCR conditions described above.
Preparation of KilB antiserum and Western blotting of cell extracts.
A 200-ml Luria-Bertani broth culture of E. coli strain BL21(DE3) containing plasmid pGSP281 was grown and induced for expression of recombinant KilB protein as described previously (21), except that induction occurred at an A600 of 0.6 and lasted for 2 h. Insoluble cell fractions containing recombinant protein and prepared according to the Novagen pET system manual (Novagen, Inc.) were subjected to preparative sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) using 1.5-mm-thick 12% polyacrylamide gels. Following subsequent Coomassie blue staining, the recombinant KilB protein band with an Mr of approximately 23,000 was excised and used as antigen (by Animal Pharm Services, Inc., Healdsburg, Calif.) to raise polyclonal antibodies in a rabbit. Lysed TK23 protoplasts from submerged cultures were used to preadsorb antibody-containing serum.
To collect S. lividans surface cultures, spores were heat shocked and cooled as described previously (8) and then plated immediately onto cellophane (Bio-Rad, Hercules, Calif.) placed on R5 agar plates. Extracts of cells collected at the indicated times were prepared and quantified as described previously (14). SDS-PAGE analysis of proteins and Western blotting were also performed as previously described (14), except that proteins were electrophoresed on 15% polyacrylamide gels, unless otherwise indicated, and goat anti-rabbit immunoglobulin G-horseradish peroxidase conjugate was obtained from Bio-Rad and used at a 1:3,000 dilution.
RESULTS
KilB protein concentration increases temporally throughout S. lividans cellular differentiation.
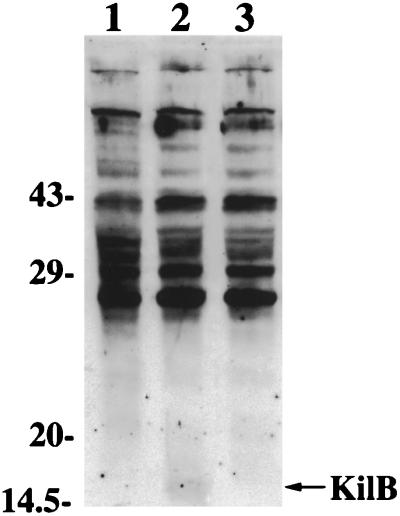
The kilB gene of plasmid pIJ101 encodes a 147-amino-acid protein (11) that has a predicted molecular mass of 15 kDa. Analysis by SDS-PAGE and Coomassie blue staining of extracts of S. lividans strain TK23 substrate mycelia containing conjugative pIJ101 derivatives versus equivalent amounts of extracts from plasmidless TK23 cells failed to reveal any additional protein bands in this molecular mass range that were specific for plasmid-containing cells. However, Western blotting of these same extracts using antibodies raised against a fusion protein expressed in E. coli that was encoded in part by the entire kilB ORF (see Materials and Methods) revealed a rather weakly expressed protein of 15 kDa (Fig. 2, lane 2) for S. lividans strain TK23 containing the conjugative pIJ101 derivative pIJ303 (13). This protein was absent in extracts either from strain TK23 cells alone (Fig. 2, lane 1) or from TK23-containing plasmid pIJ303ΔkilB (Fig. 2, lane 3), a derivative of pIJ303 that has a deletion (Fig. 1) of the entire kilB gene as well as a portion of orf56, a small ORF of undetermined function (11), which may encode a protein of approximately 6 kDa. As expected, the products of pIJ101 genes unaffected by the deletion in pIJ303ΔkilB, including the KorA protein, were present in approximately equivalent amounts (as judged by Western blotting) (22) in cells that contained either pIJ303 or pIJ303ΔkilB (data not shown).
FIG. 2.
Identification of the KilB protein of plasmid pIJ101. Following growth on R5 agar of S. lividans strain TK23, either containing or lacking conjugative pIJ101 derivatives, substrate mycelia were harvested and equivalent amounts of extracts were analyzed by SDS-PAGE on 15% polyacrylamide gels and then by Western blotting using antiserum raised against recombinant KilB protein (see Materials and Methods). The position of the 15-kDa KilB protein is indicated, as are the positions of molecular mass markers. Lanes: 1, TK23; 2, TK23(pIJ303); 3, TK23(pIJ303ΔkilB).
The pIJ101 Tra protein, which mediates intermycelial transfer of the plasmid, was shown by previous Western blotting to be expressed temporally in S. lividans, with its cellular concentration being highest at the earliest point analyzed during growth of substrate mycelia and then rapidly decreasing in amount so that Tra was undetectable by about the time aerial hyphae began to form and antibiotic production began (14). To determine whether the KilB spread protein of pIJ101 shows a similar pattern of expression, spores of strain TK23(pIJ303) were seeded onto R5 agar plates, and following growth for various times, cells were collected and equivalent amounts of extracts were examined by Western blotting (Fig. 3A) as described above for KilB. Extracts from identically grown and harvested strain TK23 alone were also analyzed (Fig. 3A) to provide a control for nonspecific background unrelated to KilB in Western blots.
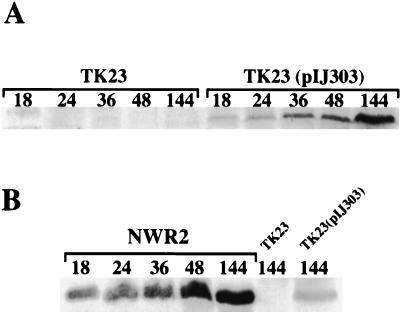
FIG. 3.
Western blot analysis of KilB protein during surface growth of S. lividans. Spores of strain TK23 (A), either containing or lacking pIJ303, or of strain NWR2 (B) were heat shocked and spread onto R5 agar plates, which contained thiostrepton in the case of NWR2, and following growth for the indicated times (in hours), cells were harvested and extracts were prepared and analyzed by SDS-PAGE and Western blotting for the presence of the pIJ101 KilB protein as described in the legend for Fig. 2.
In contrast to the pattern observed for Tra, the 15-kDa KilB protein was present throughout cellular differentiation of S. lividans TK23(pIJ303), with its lowest concentration being found in the substrate mycelium (18 h after inoculation of plates with spores); at subsequent morphologically distinguishable points, including the first approximate indication of aerial hypha production (24 h), later times during aerial hyphal growth (36 and 48 h), and finally following sporulation (144 h), the amount of KilB increased steadily such that by sporulation it had reached approximately 13-fold-higher concentrations than those seen in the initial substrate mycelium.
To examine the temporal pattern of KilB production in S. lividans cells under conditions where morphological changes do not occur (3), we performed the same Western blot analysis on strain TK23(pIJ303) extracts (as well as control TK23 extracts) prepared from submerged cultures grown to different points throughout their exponential and stationary growth phases (Fig. 4A). Similar to the results for surface cultures, KilB was at its lowest concentration at the earliest time analyzed for TK23(pIJ303) during exponential growth (time point E1) and then showed a steady increase in concentration at subsequent time points during both exponential (E2 and E3) and stationary (S1, S2, and S3) growth phases. These results were again in contrast to those shown previously for the pIJ101 Tra protein, which was found only in exponentially growing broth cultures of Tra-producing S. lividans strains (14).
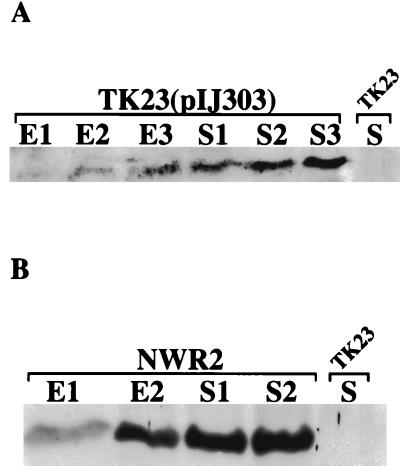
FIG. 4.
Western blot analysis of KilB protein in S. lividans submerged cultures. Spores of TK23 (A), either containing or lacking pIJ303, or of strain NWR2 (B) were used to inoculate yeast extract-malt extract medium (including thiostrepton for NWR2), and at successive time points (indicated numerically) during exponential (E) and stationary (S) growth, cells were harvested. Preparation of extracts and their analysis by SDS-PAGE and Western blotting for KilB protein were performed as indicated in the legend for Fig. 2. Only one time point during stationary growth for strain TK23 is shown, although multiple time points in both exponential and stationary growth of this strain were analyzed (data not shown).
Construction of an S. lividans strain containing the chromosomally inserted pIJ101 kilB gene in the absence of KorB repressor.
Binding of the pIJ101 KorB repressor to the kilB gene promoter controls the transcription of kilB and also suppresses the lethality phenotype associated with unregulated kilB expression on pIJ101 (9). To determine whether this critical regulation is the basis for the temporal increase in KilB protein levels during differentiation of S. lividans, we sought to monitor production of KilB in S. lividans cells that lacked KorB repressor. Since previous results raised the possibility that lower dosages (i.e., five copies or less per chromosome) of unregulated kilB might alleviate its effects on cell viability and thus circumvent the need for KorB (12), we inserted the pIJ101 kilB gene into the S. lividans chromosome, which therefore allowed unregulated kilB expression at a permissibly low gene dosage. As such construction also guaranteed that the copy number of kilB would remain invariant, we were simultaneously able to evaluate whether copy number increases in pIJ101 (and thus in the kilB gene), which are known to occur during the course of streptomycete growth (13), could be responsible for the temporally increasing pattern of KilB protein expression.
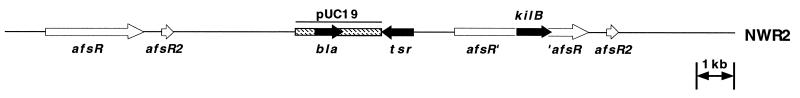
Using a gene replacement method (24) previously employed to integrate numerous exogenous genes, including the tra and korA genes of pIJ101 (15), into the S. lividans chromosome, we inserted the pIJ101 kilB gene into the cloned S. lividans chromosomal afsR locus present on the thiostrepton-resistant pUC19-based vector pBeBal2 (15), which lacks the ability to replicate in Streptomyces bacteria (24). Transformation of S. lividans TK23 using the resulting plasmid, pGSP295, yielded thiostrepton-resistant transformants in which single reciprocal (Campbell-like) recombination between homologous sequences present in the chromosome and on the plasmid led to integration of pGSP295 sequences at the chromosomal afsR locus (Fig. 5). Screening of transformants by PCR analysis of their chromosomal DNA using opposing primers specific for the 5′ and 3′ ends of the kilB gene resulted in an amplified product of the expected size (see Materials and Methods), which confirmed that kilB gene sequences had been retained upon integration of pGSP295 into the S. lividans chromosome (data not shown).
FIG. 5.
Genetic organization at the chromosomal afsR locus of S. lividans strain NWR2. A previously described (24) gene replacement procedure was used to integrate the pIJ101 kilB gene at the S. lividans afsR locus. A 1.0-kb kilB-containing pIJ101 fragment was inserted into the afsR gene contained within the cloned S. lividans chromosomal afsR locus present on the thiostrepton-resistant, pUC19-based E. coli plasmid pBeBal2 (15, 23). Upon transformation of S. lividans strain TK23 with the resulting plasmid, pGSP295, integration of the plasmid at the chromosomal afsR locus by single reciprocal recombination between homologous sequences present on the plasmid and in the chromosome led to the genetic organization shown. The relative location of the afsR2 gene (23) within the afsR locus is also indicated. bla, beta-lactamase gene present within pUC19 sequences (unfilled box); tsr, thiostrepton resistance gene; afsR′ and ′afsR, interrupted portions of the afsR gene that resulted from insertion of the kilB-containing pIJ101 fragment.
Following integration of pBeBal2 derivatives by this method, nonselective growth for two rounds of sporulation typically results in loss of the integrated thiostrepton resistance gene and associated pUC19 sequences by homologous recombination between duplicated afsR locus flanking sequences at a frequency of 1 to 10% (24); of these now thiostrepton-sensitive derivatives, 25% or more still retain a stably integrated copy of the exogenous gene(s) of interest (24). Here, however, thiostrepton-sensitive isolates were obtained following identical nonselective growth at the remarkably high rate of approximately 90% (i.e., 735 thiostrepton-sensitive isolates out of a total of 840 colonies analyzed). Additional screening of genomic DNA from 43 of these thiostrepton-sensitive derivatives by PCR amplification for potential kilB sequences as described above revealed that none of these isolates had retained the integrated kilB gene determinants that were present in the original thiostrepton-resistant strain, which we have named NWR2 (Fig. 5). Thus, due to the apparent high degree of instability of integrated kilB-containing pGSP295 sequences in the absence of thiostrepton selection, we used strain NWR2 itself grown continually in the presence of thiostrepton for subsequent studies on KilB protein expression in cells lacking KorB repressor.
S. lividans strain NWR2 shows elevated yet still temporally increasing concentrations of KilB.
To examine the temporal profile of KilB in strain NWR2, spores were spread onto R5 agar containing thiostrepton, and following growth for various times and analysis of cell extracts by Western blotting (Fig. 3B), we found that, similar to the results seen earlier for strain TK23(pIJ303), KilB showed a steady temporal increase in concentration. Although the pattern of KilB expression or accumulation was similar in strain NWR2 compared to KorB-containing cells, the amount of KilB present at each time point following the plating of NWR2 spores was appreciably higher than the corresponding levels seen in equivalent amounts of TK23(pIJ303) cell extracts (e.g., Fig. 3B, compare NWR2 and TK23 containing pIJ303 at 144 h). The temporal increase in KilB concentration occurred in NWR2 despite the fact that little or no aerial hyphae formed and sporulation was not evident during the course of the experiment. Though the enhanced intracellular levels of KilB may have contributed to the observed inhibited development of strain NWR2, we were unable to rule out growth effects related to the presence of thiostrepton in the medium, since a TK23-based control strain containing a chromosomally integrated copy of the thiostrepton-resistant pBeBal2 integration vector was also somewhat inhibited for its development when grown identically on R5 agar containing thiostrepton (data not shown).
Western blots of NWR2 extracts prepared from submerged cell cultures (Fig. 4B) also showed temporally increasing amounts of KilB throughout exponential (E1 and E2) and stationary (S1 and S2) growth. Aside from elevated KilB concentrations (data not shown), the only detectable difference in profile from that seen for similarly grown TK23(pIJ303) cells was that KilB appeared to reach and maintain maximum levels earlier either in late log phase or just as cells entered stationary growth. We conclude that the overall pattern of KilB protein expression or accumulation seen during streptomycete cellular differentiation is not due to temporal changes in either KorB control of kilB gene expression or copy number of pIJ101 but rather to some additional, previously unknown regulatory mechanism.
DISCUSSION
It has been hypothesized that plasmid functions that contribute to the size of pocks elicited by transmission of Streptomyces plasmids mediate the movement or spread of plasmid molecules within recipient cells (9, 13). Following the initial intermycelial transfer step, plasmid spread through established cross walls that separate hyphal compartments within the substrate mycelial network of the recipient, for example, not only would theoretically increase pock size but also would enhance plasmid dissemination by allowing plasmid copies to reach more cell compartments and therefore more of the occasionally emerging aerial hyphae that ultimately yield dispersible spores. The presence of the KilB spread protein of pIJ101 at stages of streptomycete differentiation that are far subsequent to that when Tra-mediated intersubstrate mycelial transfer is completed and steady-state Tra protein expression ends (14) is consistent with the existence of a subsequent intramycelial component to plasmid spread. If KilB were instead only required for modulating some aspect of intermycelial plasmid transfer, as has been alternatively hypothesized (9), then its presence would no longer be required once growth in the substratum ceases. The intriguing temporal increase in KilB, which reaches its highest concentration following sporulation, raises the possibility that the KilB spread function may in fact be active (perhaps even most active) following the presumed intramycelial movement of plasmid molecules between vegetative substrate compartments and so during the latest stages of Streptomyces development; for example, KilB may somehow promote movement of plasmids within aerial hyphae either prior to or possibly following their systematic septation, a compartmentalization process that eventually leads to the formation of chains of individual spores.
The exact role of KilB protein in the spreading of pIJ101 remains undetermined. Previously, kilB-associated lethality raised the possibility that KilB may function to inhibit cell growth, which may then prolong the period during which intramycelial spread and perhaps additional rounds of intermycelial transfer can occur (12). As shown here, KilB's presence in substrate mycelia may serve, for example, to keep open the initial “transfer window” during which Tra-mediated intermycelial transfer between substrate compartments is known to occur (14), while the appearance of KilB throughout cellular differentiation may indicate that spread-promoting growth inhibition continues during the entire differentiation process, thereby leading to the retarded development of plasmid-containing aerial hyphae and spores that is a hallmark of pock formation. Alternatively, it is possible that KilB is directly required for intramycelial plasmid spread (and possibly contributes to intermycelial transfer as well) and that any associated growth inhibition is instead a consequence of its direct role in pIJ101 transmission.
The elevated concentrations of KilB seen here in nonmating S. lividans NWR2 cells may approach the transient levels thought to occur during transmission of pIJ101 when presumably single copies of the plasmid are transferred into recipients (or subsequently between recipient compartments); upon such transfer events, the absence of Kor proteins in recipient cells may induce temporary derepression of pIJ101 functions such as kilB that either direct plasmid transmission or inhibit recipient growth, and this induction, whose magnitude may be further enhanced as transferred pIJ101 molecules begin to replicate, may in turn stimulate additional plasmid transfer and spreading (12). Should such induction exist for kilB during mating, it will be interesting to determine whether this derepression affects the temporally increasing pattern of KilB protein expression or accumulation seen here under nonmating conditions for both pIJ101-containing cells and strain NWR2.
With further regard to the increased levels of KilB seen in strain NWR2, we have also observed variable reductions in growth rates and maximum cell densities achieved among NWR2 isolates grown in submerged culture (K. Schully and G. Pettis, unpublished results), despite the fact that the overall temporal pattern of elevated KilB protein expression remained invariant. While the basis for this variation in growth effects is currently unknown and under investigation, the results are nevertheless consistent with the notion that the higher KilB concentrations seen in strain NWR2 are growth inhibitory for Streptomyces cells.
We speculate that the instability observed for integrated, thiostrepton-resistant pGSP295 sequences in strain NWR2, which led to abnormally high numbers of thiostrepton-sensitive derivatives following nonselective growth, is another indication of toxic effects related to unregulated KilB protein expression. Upon repeated sporulation cycles, cells that had not undergone additional homologous recombination to remove integrated kilB-containing pGSP295 sequences (this should be the vast majority of cells) (24) apparently became nonviable at a high frequency so that most of the isolates recovered at this point (approximately 90%) were thiostrepton sensitive and had deletions of all of the integrated pGSP295 sequences, including kilB. Consistent with this argument, we found that even under constant selection for thiostrepton NWR2 isolates passaged through multiple rounds of sporulation lost the ability to produce KilB protein as judged by Western blotting (data not shown); thus, unregulated expression of kilB apparently led to nonviability and selection for KilB− derivatives under all growth conditions tested.
It will be interesting to determine the KorB-independent mechanism that temporally regulates KilB protein levels in Streptomyces cells and whether this additional control is implemented during the course of kilB gene transcription or instead occurs posttranscriptionally. In any event, the temporal changes in KilB concentration for both submerged and surface-grown cell cultures indicate that physiological rather than morphological cues are involved in this regulatory process. Previously, the temporal decrease of pIJ101 Tra protein prior to cell differentiation in S. lividans was shown to be controlled by a posttranscriptional mechanism that also operates independently of morphological development (14).
Although temporal increases in KilB are not manifested by changes in KorB regulation per se, KilB concentrations were much greater in strain NWR2 compared to TK23 containing the pIJ101 derivative pIJ303, despite a reduction of some 300-fold in copy number of the kilB gene in NWR2. These data indicate that KorB repression normally results in significantly reduced intracellular steady-state levels of KilB throughout Streptomyces growth. That KilB production is so tightly regulated is not surprising given the associated lethality and otherwise deleterious growth effects seen for this plasmid spread protein.
ACKNOWLEDGMENTS
We thank Sally Murphy for assistance with overexpression of recombinant KilB protein and Kevin Kendall for critical reading of the manuscript.
This work was funded by grant MCB-9604879 from the National Science Foundation (to G.S.P.). K.L.S. is the recipient of a Louisiana State University Board of Supervisors scholarship.
REFERENCES
- 1.Bibb M J, Hopwood D A. Genetic studies of the fertility plasmid SCP2 and its SCP2* variants in Streptomyces coelicolor A3(2) J Gen Microbiol. 1981;126:427–442. [Google Scholar]
- 2.Brasch M A, Cohen S N. Sequences essential for replication of plasmid pIJ101 in Streptomyces lividans. Plasmid. 1995;33:191–197. doi: 10.1006/plas.1995.1020. [DOI] [PubMed] [Google Scholar]
- 3.Champness W C, Chater K F. Regulation and integration of antibiotic production and morphological differentiation in Streptomyces spp. In: Piggot P, Moran C P Jr, Youngman P, editors. Regulation of bacterial differentiation. Washington, D.C.: American Society for Microbiology; 1994. pp. 61–93. [Google Scholar]
- 4.Chater K F. Genetics of differentiation in Streptomyces. Annu Rev Microbiol. 1993;47:685–713. doi: 10.1146/annurev.mi.47.100193.003345. [DOI] [PubMed] [Google Scholar]
- 5.Clark C A, Chen C, Ward-Rainey N, Pettis G S. Diversity within Streptomyces ipomoeae based on inhibitory interactions, rep-PCR, and plasmid profiles. Phytopathology. 1998;88:1179–1186. doi: 10.1094/PHYTO.1998.88.11.1179. [DOI] [PubMed] [Google Scholar]
- 6.Deng Z, Kieser T, Hopwood D A. “Strong incompatibility” between derivatives of the Streptomyces multi-copy plasmid pIJ101. Mol Gen Genet. 1988;214:286–294. doi: 10.1007/BF00337723. [DOI] [PubMed] [Google Scholar]
- 7.Ducote M J, Prakash S, Pettis G S. Minimal and contributing sequence determinants of the cis-acting locus of transfer (clt) of streptomycete plasmid pIJ101 occur within an intrinsically curved plasmid region. J Bacteriol. 2000;182:6834–6841. doi: 10.1128/jb.182.23.6834-6841.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, England: John Innes Foundation; 1985. [Google Scholar]
- 9.Hopwood D A, Kieser T. Conjugative plasmids of Streptomyces. In: Clewell D B, editor. Bacterial conjugation. New York, N.Y: Plenum Press; 1993. pp. 293–311. [Google Scholar]
- 10.Hopwood D A, Lydiate D J, Malpartida F, Wright H M. Conjugative sex plasmids of Streptomyces. In: Helinski D R, Cohen S N, Clewell D B, Jackson D A, Hollaender A, editors. Plasmids in bacteria. New York, N.Y: Plenum Press; 1985. pp. 615–634. [DOI] [PubMed] [Google Scholar]
- 11.Kendall K J, Cohen S N. Complete nucleotide sequence of the Streptomyces lividans plasmid pIJ101 and correlation of the sequence with genetic properties. J Bacteriol. 1988;170:4634–4651. doi: 10.1128/jb.170.10.4634-4651.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kendall K J, Cohen S N. Plasmid transfer in Streptomyces lividans: identification of a kil-kor system associated with the transfer region of pIJ101. J Bacteriol. 1987;169:4177–4183. doi: 10.1128/jb.169.9.4177-4183.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kieser T, Hopwood D A, Wright H M, Thompson C J. pIJ101, a multi-copy broad host-range Streptomyces plasmid: functional analysis and development of DNA cloning vectors. Mol Gen Genet. 1982;185:223–238. doi: 10.1007/BF00330791. [DOI] [PubMed] [Google Scholar]
- 14.Pettis G S, Cohen S N. Plasmid transfer and expression of the transfer (tra) gene product of plasmid pIJ101 are temporally regulated during the Streptomyces lividans life cycle. Mol Microbiol. 1996;19:1127–1135. doi: 10.1046/j.1365-2958.1996.493986.x. [DOI] [PubMed] [Google Scholar]
- 15.Pettis G S, Cohen S N. Transfer of the pIJ101 plasmid in Streptomyces lividans requires a cis-acting function dispensable for chromosomal gene transfer. Mol Microbiol. 1994;13:955–964. doi: 10.1111/j.1365-2958.1994.tb00487.x. . (Erratum, 16:170, 1995.) [DOI] [PubMed] [Google Scholar]
- 16.Pettis G S, Prakash S. Complementation of conjugation functions of Streptomyces lividans plasmid pIJ101 by the related Streptomyces plasmid pSB24.2. J Bacteriol. 1999;181:4680–4685. doi: 10.1128/jb.181.15.4680-4685.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rainey F A, Ward-Rainey N, Kroppenstedt R M, Stackebrandt E. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int J Syst Bacteriol. 1996;46:1088–1092. doi: 10.1099/00207713-46-4-1088. [DOI] [PubMed] [Google Scholar]
- 18.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 19.Stein D S, Cohen S N. Mutational and functional analysis of the korA and korB gene products of Streptomyces plasmid pIJ101. Mol Gen Genet. 1990;222:337–344. doi: 10.1007/BF00633838. [DOI] [PubMed] [Google Scholar]
- 20.Stein D S, Kendall K J, Cohen S N. Identification and analysis of transcriptional regulatory signals for the kil and kor loci of Streptomyces plasmid pIJ101. J Bacteriol. 1989;171:5768–5775. doi: 10.1128/jb.171.11.5768-5775.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Studier F W, Moffatt B A. Use of bacteriophage T7 RNA polymerase to direct selective high-level expression of cloned genes. J Mol Biol. 1986;189:113–130. doi: 10.1016/0022-2836(86)90385-2. [DOI] [PubMed] [Google Scholar]
- 22.Tai J T-N, Cohen S N. Mutations that affect regulation of the korB gene of Streptomyces lividans plasmid pIJ101 alter plasmid transmission. Mol Microbiol. 1994;12:31–39. doi: 10.1111/j.1365-2958.1994.tb00992.x. [DOI] [PubMed] [Google Scholar]
- 23.Vögtli M, Chang P-C, Cohen S N. afsR2: a previously undetected gene encoding a 63-amino-acid protein that stimulates antibiotic production in Streptomyces lividans. Mol Microbiol. 1994;14:643–653. doi: 10.1111/j.1365-2958.1994.tb01303.x. [DOI] [PubMed] [Google Scholar]
- 24.Vögtli M, Cohen S N. The chromosomal integration site for the Streptomyces plasmid SLP1 is a functional tRNATyr gene essential for cell viability. Mol Microbiol. 1992;6:3041–3050. doi: 10.1111/j.1365-2958.1992.tb01762.x. [DOI] [PubMed] [Google Scholar]