Abstract
In recent years, the growing use of ART (assisted reproductive techniques) has led to a progressive improvement of protocols; embryo freezing is certainly one of the most important innovations. This technique is selectively offered as a tailored approach to reduce the incidence of multiple pregnancies and, most importantly, to lower the risk of developing ovarian hyperstimulation syndrome when used in conjunction with an ovulation-triggering GnRH antagonist. The increase in transfer cycles with frozen embryos made it possible to study the effects of the technique in children thus conceived. Particularly noteworthy is the increase in macrosomal and LGA (large for gestational age) newborns, in addition to a decrease in SGA (small for gestational age) and LBW (low birth weight) newborns. The authors aimed to outline a broad-ranging narrative review by summarizing and elaborating on the most important evidence regarding the neonatal outcome of children born from frozen embryos and provide information on the medium and long-term follow- up of these children. However, given the relatively recent large-scale implementation of such techniques, further studies are needed to provide more conclusive evidence on outcomes and implications.
Keywords: fresh embryo transfer, frozen embryo transfer, cryopreservation, vitrification, neonatal outcomes
1. Introduction
Gynecological diseases can lead to severe consequences for the quality of life of those affected [1]. Among these, infertility plays a central role. This is defined as the inability to conceive after at least one year of unprotected intercourse, and it has reached a global prevalence of 15% of couples at reproductive age [2]. The prevalence in the 1990–2017 period grew both for male (by 8.224% from 710.19 per 100,000 to 768.59 per 100,000, i.e., a 0.291% increase on a yearly basis) and female infertility (by 14.962% from 1366.85 per 100, to 1571.35 per 100 in 2017, a 0.370% increase every year) [3]. However, the increase in the prevalence of infertility and subfertility has led to an increasing demand for the use of assisted reproduction techniques. In particular, with the progress of conservation techniques in recent years, frozen embryo transfer has spread rapidly, giving rise to medical as well as ethical debates [4,5,6,7,8]. This technique, especially freezing by vitrification, lowers the likelihood of multiple pregnancies as well as the risk of incurring ovarian hyperstimulation syndrome. No significant differences have been found compared to transfer from fresh embryo in terms of pregnancy rate per cycle (63.1% vs. 60.9%) and the clinical pregnancy rate per cycle (55.4% vs. 58.7%) [9,10]. At the same time, as the technique has spread, several authors have investigated the possible issues related to it, such as the greater risk of hypertension syndrome and the increase in macrosomal and Large for Gestational age (LGA) newborns. The authors aimed to provide a comprehensive picture of the latest evidence regarding the neonatal outcome of babies born from frozen embryos. In addition, the authors have seen fit to try and shed a light on areas where current research data are still inconclusive. The further elaboration of still-indecisive findings (e.g., whether frozen cycles may result in higher live birth rates among patients with ovulatory disorder, or whether fresh cycles may be more designated for younger patients aged ≤ 30) would go a long way towards a higher degree of objectivity in the definition of evidence-based risk factors, which should be reflected in widely acknowledged guidelines and recommendations. Relying on broadly recognized criteria will in fact benefit clinical practice, patient care, and even shield doctors from malpractice claims, which are partly fueled by uncertainty and have increasingly affected healthcare professionals and OB/GYN operators in particular. Part of the review article will focus on the long-term outcome, particularly on the growth and development of children born from this technique. The search conducted by the authors ultimately identifies a total of 32 articles published in the 1987–2021 period, namely: 11 reviews/meta-analyses, 7 cohort studies, 12 retrospective cohort studies, and 2 randomized controlled trials. Most of the papers were published in 2020 (six), three in 2021, five in 2019, and five in 2018. The remaining ones were published in 2017, 2016, 2014, (two for each year, respectively), three in 2013, and only one each for years 2012, 2009, 2000, 1998, 1997, respectively. The selection criteria included papers in which the authors focused on the comparison of frozen embryo transfer births with fresh embryo births in terms of neonatal outcome and/or long-term development. The databases drawn upon by the authors were PubMed/Medline, Cochrane Database of Systematic Reviews, and Scopus and EMBASE; all were searched up to 2 February 2022 via the following search phrases: “Fresh embryo transfer”, “Frozen embryo transfer”, “adverse neonatal outcomes”, “infant birth weight”, “Assisted reproductive technology”, “In Vitro Fertilization (IVF)”, “ovarian stimulation”, “live birth”, ”ovarian hyperstimulation syndrome (OHSS)”, ”obstetric outcomes”. All studies that covered fresh vs. frozen embryo transfer from different perspectives than the ones specified above were excluded. The most significant findings from the various sources were schematized and points of agreement and divergence were discussed. Despite the degree of variability, inconsistency, and heterogeneity of findings between older studies and recent ones, the authors have set out to piece together a historical overview meant to reflect how research has progressed and evolved over the years as cryopreservation techniques have improved, thus offering new opportunities for tailored and targeted approaches. As an elaboration on the theme of cryopreservation, opinions of the authors on different neonatal outcomes between different freezing methods have been reported. Furthermore, six papers focusing on chromosomal abnormalities found in embryos obtained from frozen oocytes have also been included.
2. Neonatal Outcomes
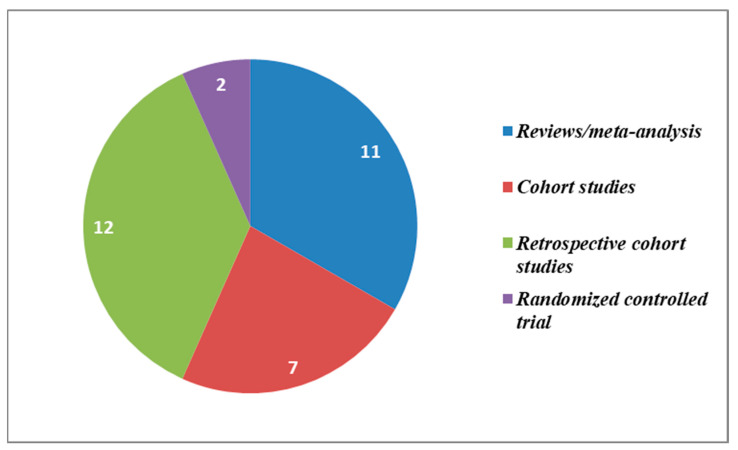
The following figure (Figure 1) sums up the research studies accounted for in this review according to the type of publication.
Figure 1.
Studies distribution according to type of publication.
Table 1 summarizes the evidence reported by the various sources herein analyzed [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42]. In particular, the aspects characterizing children born from frozen embryos compared to children born from fresh embryos have been highlighted. In the table, the section on perinatal outcomes and the section concerning congenital malformations in newborns and/or long-term outcomes of the children have been divided for the sake of clarity.
Table 1.
Studies description regarding to the main findings on neonatal outcomes and child growth and/or development after transferring frozen versus fresh embryos.
| Frozen and Vitrified Embryos vs. Fresh Embryos | ||||
|---|---|---|---|---|
| Authors (et al.) | Type of Study | Year | Neonatal Outcomes | Child Growth and/or Development |
| Terho et al. [11] | Retrospective cohort | 2021 | More LGA newborns and higher birth weight both in boys and girls | Not evaluated |
| Zaat et al. [12] | Review | 2021 | More LGA newborns and higher birth weight | Not evaluated |
| Acet et al. [13] | Retrospective cohort | 2021 | Higher pregnancy rate and live birth rate | Not evaluated |
| Pirtea et al. [14] | Review | 2020 | More LGA and fewer SGA newborns; less premature and more postdate births | Not evaluated |
| Vuong et al. [15] | RCT | 2020 | Not evaluated | Better ASQ-3 score and motor skills scores |
| Orvieto et al. [16] | Review | 2020 | Higher live birth rate; fewer premature and LBW babies; more LGA newborns | Not evaluated |
| Chen et al. [17] | Retrospective cohort | 2020 | Higher birthweight in live- born twins | Not evaluated |
| Djuwantono et al. [18] | Review | 2020 | Not evaluated | Not higher risk of neurodevelopmental disorders |
| Elias et al. [19] | Review and meta-analysis | 2020 | More LGA newborns; fewer SGA and LBW newborns | Not evaluated |
| Ginström Ernstad et al. [20] | Cohort | 2019 | More LGA and macrosomic newborns | Not evaluated |
| Ginström Ernstad et al. [21] | Retrospective cohort | 2019 | More LGA and macrosomic newborns; fewer premature and LBW newborns |
Not evaluated |
| Ainsworth et al. [22] | Cohort | 2019 | Higher birth length, weight, and head circumference | No significant differences in age/sex specific weight and BMI |
| Maris et al. [23] | Retrospective cohort | 2019 | Higher birth weight (even after adjustment) | Not evaluated |
| Hwang et al. [24] | Retrospective cohort | 2019 | More LGA newborns and higher birth weight; fewer SGA newborns | Increased odds of infectious disease, respiratory, and neurologic abnormalities. |
| Maheshwari et al. [25] | Review | 2018 | Higher BW, fewer SGA and preterm babies; not differences in perinatal deaths | No significant differences in congenital malformations |
| Bernsten et al. [26] | Review | 2018 | More LGA and macrosomic newborns; fewer premature and LBW newborns | Not evaluated |
| Sha et al. [27] | Review | 2018 | Fewer LBW, SGA newborns and perinatal deaths |
Not evaluated |
| Zhang et al. [28] | Retrospective cohort | 2018 | More LGA and macrosomic newborns; fewer premature and LBW newborns | No significant differences in congenital malformations |
| Wong et al. [29] | Review | 2017 | Similar cumulative live birth rates | Not evaluated |
| Vidal et al. [30] | Cohort | 2017 | Fewer premature and LBW newborns | Not evaluated |
| Chen et al. [31] | RCT | 2016 | Higher live birth rate; no differences in neonatal complications | Not evaluated |
| Belva et al. [32] | Cohort | 2016 | Higher BW and fewer SGA newborns; no differences in preterm births and perinatal death rate; similar neonatal outcomes if considered twins | No significant differences in congenital malformations both in twins and singletons |
| Pinborg et al. [33] | Cohort | 2014 | More LGA newborns | Not evaluated |
| Li et al. [34] | Cohort | 2014 | Fewer preterm and LBW newborns | Not evaluated |
| Wennerhom et al. [35] | Retrospective cohort | 2013 | More LGA, macrosomic newborns, postdate births and perinatal deaths; fewer premature and LBW newborns | Not evaluated |
| Liu et al. [36] | Cohort | 2013 | Higher birthweight, fewer LBW newborns; no differences in preterm and perinatal death | Not evaluated |
| Check et al. [37] | Retrospective cohort | 2013 | Higher live-delivered pregnancy rate | Not evaluated |
| Check et al. [38] | Retrospective cohort | 2012 | Higher live-delivered pregnancy rate | Not evaluated |
| Wennerhom et al. [39] | Review | 2009 | Fewer preterm and LBW newborns | No significant differences in congenital malformations |
| Wennerhom [40] | Review | 2000 | Not significant differences in perinatal outcome | No significant differences in congenital malformations nor child development |
| Wennerhom et al. [41] | Retrospective cohort | 1998 | Not evaluated | No significant differences in growth and chronic diseases |
| Wennerhom et al. [42] | Retrospective cohort | 1997 | Similar perinatal risk | Not evaluated |
ASQ-3: Ages & Stages Questionnaires ®, Third Edition. BMI: body mass index. LGA: large for gestational age; SGA: small for gestational age; LBW: low birth weight; RCT: randomized controlled trial. BW: birth weight. LBW: low birth weight.
The topic of neonatal outcome is apparently much more broadly covered than congenital malformations and long-term outcome. In particular, 21 out of the 32 studies have focused exclusively on neonatal outcome, three exclusively on malformations and long-term outcome, and six on both aspects. Wennerholm et al. [41], in 1998, found similar gestational age at delivery, birthweight, the incidence of malformations, and the perinatal mortality between the frozen embryo transfer (FET) group and the fresh embryo transfer (ET) group both for singletons and twins, examining the medical records of 270 infants. The number of infants with Apgar score > 7 (calculated at the fifth minute) was similar, as was the percentage of intensive care admissions. Such data apparently point to a similar risk of adverse perinatal events between the two groups. In the following years, the aspect of neonatal outcome has been widely discussed by the authors and, as shown in Table 1, there is a common agreement in attributing a higher birth weight to newborns born from frozen embryos. The consequence is an increased number of macrosomic (>4500 g) and LGA newborns, in addition to a reduced number of LBW and SGA newborns [14,15,16]. Another aspect worth noting is the higher number of post-term infants accompanied by a reduced number of preterm infants. Certainly, the reduction of SGA and preterm infants solves one of the “side effects” associated with ART, though the increase in macrosomic/LGA infants entails different issues such as higher risk of stillbirth, fetal hypoxia, perineal lacerations, shoulder dystocia, cesarean section, postpartum hemorrhage, and neonatal metabolic disturbances at birth. For Belva et al. [32] and Liu et al. [36], the incidence of preterm infants in the two groups is comparable. In a 2016 cohort study, Belva et al. [32] reported that when considering twins instead of singletons, neonatal outcomes between the two groups are ultimately comparable. Ainsworth et al. [22] also reported a higher birth length and larger head circumference in children born from frozen embryos. An interesting retrospective cohort study published by Terho et al. [11] in 2021 differentiated neonatal outcomes based on sex and gestational age. Mean birth weights were significantly higher in the FET group compared to the fresh ET group starting from gestational week (GW) 33 for boys and from GW 34 for girls. In boys, there was a greater number of LGA births between GW 36 and 42, compared to those born from fresh ET. For girls, the same difference was found between GW 37 and 42. The proportion of SGA was significantly lower among boys born after FETs compared to fresh ETs between GW 36 and 42. For girls born after FET, the same difference was seen at GW 38 compared to those born after fresh ET. The percentage of LGA also was found to be significantly higher for boys born after FET between GW 38 and 41 and for girls born after FET between GW 37 and 40, if compared to boys and girls naturally conceived. For Maheshwari et al. [25], Belva et al. [32], Liu et al. [36], and Wennerholm et al. (in the 1997 paper) [42], the rate of perinatal death was also similar between the frozen embryo transfer (ET) group and the fresh ET group. This finding is very important if we consider the increased number of macrosomic infants in the frozen embryo group. Only one paper reported an increased rate of perinatal deaths [35] and only one reported a lower rate [27]. With regard to pregnancy rate and live birth rate, according to Acet et al. [13] and Orvieto et al. [16], the frozen ET groups seemed to have higher scores than fresh ET groups. However, in two cohort studies published in 2012 and 2013, Check et al. [37,38] found a higher rate of pregnancies successfully brought to term in fresh ETs, although the use of FET has been linked to a lower risk of ovarian hyperstimulation syndrome. In a review published in 2017, Wong et al. [27] found similar cumulative live birth rates. Furthermore, LGA newborns are common for patients with insulin resistance and polycystic ovarian syndrome (PCOS) that could be preemptively treated with inositol supplementation [43,44], as could patients with GD (gestational diabetes) to improve birth outcomes [45], as well as young women who decide to preserve their oocytes and have a FET close to menopausal age [46].
Table 2 summarizes the comparison between the two techniques with regard to neonatal outcomes.
Table 2.
Comparison of neonatal outcomes between frozen embryo and fresh embryo methods according to the frequency.
| Compared Neonatal Outcomes between Freezing Methods | ||
|---|---|---|
| Frozen Embryo > Fresh Embryo | Frozen Embryo = Fresh Embryo | Frozen Embryo < Fresh Embryo |
| Live births | Perinatal Deaths | LBW newborns |
| Obtained pregnancies | SGA newborns | |
| Macrosomic newborns | Premature Births | |
| LGA newborns | ||
| Postdate births | ||
LGA: large for gestational age; SGA: small for gestational age.
2.1. Differences in Neonatal Outcomes between Freezing Methods
The recent improvements in freezing techniques has led to a gradual abandonment of the slow-freeze technique with cleavage stage embryos in favor of vitrification at the blastocyst stage. Studies comparing the outcomes of the two techniques are still few and with conflicting conclusions. Ginström Ernstad et al. [21] conducted a cohort study published in 2019 in which they found comparable neonatal outcomes in children born from the two different techniques. However, Liu et al. [36], comparing techniques, found a median birthweight from vitrified embryos (3455.3 g) higher than those from slow freezing (3352.3 g) and fresh (3355.8 g) transfers. The rate of perinatal mortality is instead reported as comparable between the three groups.
Moreover, in a cohort study published in 2014, Li et al. [34] suggested that the freezing method can influence neonatal outcome; in particular, they found an higher clinical pregnancy rate in vitrified blastocyst transfer cycles than in slow frozen blastocyst transfer cycles.
In addition, Alviggi et al. [47] suggested that the freezing method and the time of transfer may influence pregnancy outcomes in terms of preterm birth, very preterm birth, LGA, SGA, and perinatal mortality.
2.2. The Role of Confounding Factors
Although most authors agree on the data regarding birth weight, doubts do arise in some studies. According to Ainsworth et al. [19], in fact, there is no difference in birth weight when adjusting for gestational age, sex, and maternal factors. However, Vidal et al. [30], in a 2017 cohort study, argue that adjusted regression model birthweight is significantly higher in the fresh ET group than the frozen one. In a retrospective cohort study published in 2019, Maris et al. [23] also found a higher birthweight after a multivariate analysis adjusted according to confounding factors such as gestational age, maternal age, maternal body mass index (BMI), tobacco exposure, the number of embryos transferred, and birth order. In a 2014 cohort study, Pinborg et al. [33] argued that the increased risk of LGA newborns could not be related exclusively to intrinsic maternal factors, but must necessarily be related, at least for the most part, to the freezing procedure. According to Pirtea et al. [14], the difference found in neonatal outcomes derives from issues regarding the depth of placentation, possibly being too shallow in the fresh ET group. For Berntsen et al. [26], further studies are needed to define what changes, probably epigenetic, may stem from frozen embryo transfers.
2.3. Congenital Malformations and Long-Term Outcome in Children
In a 1997 study by Wennerholm et al., 255 children from cryopreserved embryos were matched (regard to maternal age, date of delivery, and parity, single or twin pregnancy), with 255 children born after IVF with fresh embryos, and 252 children from spontaneous pregnancies [42]. Growth features were similar for both singletons and twins in the three groups. There were six (2.4%) major malformations in the cryopreserved group, nine in standard IVF group (3.5%), and eight (3.2%) in naturally conceived group.
The prevalence of chronic diseases during infancy and early childhood did not differ between the three groups (18.0%, 15.3%, and 16.7% in the cryopreserved group, standard IVF, and spontaneous groups, respectively). In that paper, occurrences of minor behavioral disturbances, learning difficulties, and attention and perception deficits were not reported because of too young an age of the children involved. In the following years, as mentioned above, few studies focused on the long-term health outcomes not exclusively neonatal of children born from frozen embryos. In an RCT published in 2020, Vuong et al. [15] performed follow-ups of children in the study group (consisting of 391 pairs) until an age of 37 months. Developmental screening was performed using the well-known ASQ-3 questionnaire that covers 5 domains: communication, gross motor, fine motor, problem solving, and personal social behavior. The study reported relevant findings: problem solving scores were found to be higher in the frozen ET group than in the fresh ET group, but not when singletons and twins were analyzed separately. Other data in favor of the frozen ET group concerned the fine motor skills in the overall analysis (p = 0.056 vs. fresh ET) and twins (p = 0.06 vs. fresh ET) but not in singletons. There were no significant differences in the prevalence of abnormal ASQ-3 scores found among the study groups. This finding is important and indicates that there is no difference in the incidence of neurodevelopmental abnormalities, although for some developmental domains, the scores of children born from frozen embryos are even better. The few data available, however, do not allow for a determination as to whether any difference exists when considering singletons separately from twins.
The same aspect has been evaluated by Djuwantono et al. [18] in a 2020 review; in particular, these authors do not report a higher rate of neurodevelopmental abnormalities in children born after frozen embryo transfers. The already-mentioned prospective study by Belva et al. [32] collected data from 960 cycles after frozen embryo transfers and 1644 cycles after fresh embryo transfers, performed between 2008 and 2013. Follow-up was performed in the 3 months after birth with a close focus on congenital malformations. Children’s pediatricians were blinded to the transfer method. Data were adjusted for treatment variables and maternal characteristics. The mothers of the children belonging to the frozen ET group tended to be older and more prone to pregnancy-related hypertension, a finding already known in the literature. As for the frequency of major congenital malformations in live births (i.e., malformations that have both a morphological and functional impact), it was found to be comparable between the vitrified group and the fresh group, both among singletons and twins. Even considering major and minor malformations together, the study groups had similar rates. Zhang et al. [28], in a 2018 retrospective cohort study, and Maheshwari et al. [25] in a 2018 review also reported similar congenital malformation rates between frozen ET groups and fresh ET groups. Ainsworth et al. [22] focused on child growth by including 136 women in the study, 87 of whom underwent a fresh embryo transfer and 49 a frozen embryo transfer. Age- and sex-specific weight and body mass index results, considering percentiles, were comparable between the study groups.
Only one retrospective cohort study published in 2019 reported a comparison regarding other health outcomes. Significantly, such a study found that babies born from frozen embryos had greater odds of infectious disease (AOR = 1.46), respiratory conditions (AOR = 1.23), and neurological (AOR = 1.32) conditions. No statistically significant differences were found for birth defects, cardiovascular, hematologic, and gastrointestinal/feeding conditions. [24]
In light of the limitations due to the dearth of currently available research data, Table 3 summarizes the long-term follow-up of children born from frozen embryos.
Table 3.
Comparison of long-term health aspects in children between frozen embryo and fresh embryo methods.
| Compared Long Term Follow-Up in Children between Freezing Methods | ||
|---|---|---|
| Frozen Embryo > Fresh Embryo | Frozen Embryo = Fresh Embryo | Frozen Embryo < Fresh Embryo |
| Problem solving scores | Congenital malformations rate | |
| Fine motricity scores | ND prevalence | |
| Age- and sex-specific BMI and weight |
||
| Growth and chronic diseases | ||
BMI: body max index; ND: neurodevelopmental disorders.
3. Concluding Remarks
This narrative review has been conceived to elaborate on the latest research data regarding the neonatal and long-term developmental outcomes of children born from frozen embryos, which were collected and weighed against the outcomes of children born from fresh embryo transfers.
The increased presence of macrosomic and LGA newborns certainly reduces the proportion of SGA newborns, but it requires greater attention during childbirth maneuvers and in the monitoring of neonatal problems. LGA newborns have rarely been found in cases of perinatal infections [48].
The increase in pregnancy rate and live birth rate are certainly reassuring aspects as to the use of frozen embryo transfer, as is the lower risk of ovarian hyperstimulation syndrome and multiple pregnancies. Such favorable outcomes may be partly due to the fact that frozen embryo transfers make it possible to wait until the ovary has recovered from the ovarian stimulation and the exposed endometrial lining has shed, thus enabling a “fresh start” for both. In fact, higher OHSS risks and pregnancy loss have been linked to higher estradiol levels following IVF. It is worth noting in that regard that estradiol levels in fresh cycles are considerably higher than in frozen cycles [31,49]. Since frozen embryos would be implanted long after ovulation induction, the mother’s body would have had the chance to get back to normal conditions from the hormonal standpoint. Such newfound normalcy is thought to better reproduce the natural conception path associated with a higher likelihood of success. Moreover, better planning made possible by the use of frozen embryos enables the patient to have the embryo transferred at the ideal time. At any rate, the differences found between the two groups are unlikely to be related to mother-dependent factors. Of the studies herein examined, only one accounts for length and head circumference in addition to birth weight, showing the two parameters to be greater in the FET children. With regard to perinatal morbidity and mortality, the risk appears to be similar between the two groups. Only one study reported a higher perinatal death rate in the FET group and only one reported a lower rate in the FET group compared to the fresh ET group.
As for the rate of congenital malformations, in the five studies that dealt with this topic, all the authors reported comparable malformation rates between the two groups. This finding appears reassuring with regard to the use of freezing techniques.
Another important finding concerns the incidence of neurodevelopmental abnormalities, with apparently no difference between the two groups. This finding, in addition to the most recent scientific evidence regarding the safety of ART on the neuro-psychomotor outcome of newborns, is important and reassuring for all couples with infertility and sub-fertility problems. In a single study, randomized, controlled evidence has emerged reflecting improved cognitive performance in children born from frozen embryos, at least in some specific domains. Although significant, such data must be contextualized because they were not found by separate analyses of twins and singletons. With regard to the growth of children born from frozen embryos, two studies investigating the issue found weight, BMI (normalized on age and sex), and other growth parameters to be similar in the two study groups. This finding is meaningful in that it suggests that a higher incidence of macrosomic newborns in frozen embryo babies does not necessarily result in worse long-term health outcomes in such children.
Only one study of the aforementioned reported a greater risk in newborns of incurring infectious diseases and respiratory or neurological abnormalities. The data reproducibility needs further research. In conclusion, research data on neonatal outcomes from frozen embryos are varied and evidence-based. On the contrary, the lack of data on the long-term follow-up of children requires further in-depth studies. With regard to the study of neurodevelopmental alterations, prospective studies with an adequate number of patients should be prioritized (given the rarity of some disorders), setting up a clinical monitoring framework that should cover not only the first years of life, but also the school age in which mild neurodevelopmental disorders (non-pervasive) may appear that are not easily recognizable in early childhood and preschool age. With regard to congenital malformations, future studies should be focused on individual organs that are possibly affected, e.g., rates of congenital heart disease, renal/urinary malformation, or malformations of the digestive system. Reliance on more objective, factual, and evidence-based data would also go a long way towards enabling doctors to be safe from legal malpractice claims in case of adverse outcomes. Such lawsuits are in fact particularly common and often severely burdensome for OB/GYN professionals. The delineation of broadly acknowledged guidelines and best practices is in fact instrumental in providing a degree of objectivity through which healthcare professionals can abide by and document their compliance with recognized criteria if called to answer for adverse outcomes.
Author Contributions
Conceptualization, G.G., M.S., G.C., V.C., A.P., M.E.G., A.S.L., E.M., G.B. and S.Z.; methodology, G.G., V.C., E.M., G.B. and S.Z.; validation, G.G., E.M., G.B. and S.Z.; investigation, G.G., E.M., G.B. and S.Z.; data curation, G.C., V.C., A.P., M.E.G. and A.S.L.; writing—original draft preparation, A.S.L., E.M., G.B. and S.Z.; writing—review and editing, G.C., V.C., A.P., M.E.G. and A.S.L.; visualization, G.G., E.M., G.B. and S.Z.; supervision, G.G., E.M., G.B. and S.Z. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this case report are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.La Rosa V.L., Valenti G., Sapia F., Gullo G., Rapisarda M.C.R. Psychological impact of gynecological diseases: The importance of a multidisciplinary approach. Ital. J. Gynaecol. Obstet. 2018;30:2. doi: 10.14660/2385-0868-86. [DOI] [Google Scholar]
- 2.Gerrits T., Van Rooij F., Esho T., Ndegwa W., Goossens J., Bilajbegovic A., Jansen A., Kioko B., Koppen L., Migiro S.K., et al. Infertility in the Global South: Raising Awareness and Generating Insights for Policy and Practice. Facts Views Vis. ObGyn. 2017;9:39–44. [PMC free article] [PubMed] [Google Scholar]
- 3.Sun H., Gong T.-T., Jiang Y.-T., Zhang S., Zhao Y.-H., Wu Q.-J. Global, Regional, and National Prevalence and Disability-Adjusted Life-Years for Infertility in 195 Countries and Territories, 1990–2017: Results from a Global Burden of Disease Study, 2017. Aging. 2019;11:10952–10991. doi: 10.18632/aging.102497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vergallo G.M., Marinelli E., di Luca N.M., Zaami S. Gamete Donation: Are Children Entitled to Know Their Genetic Origins? A Comparison of Opposing Views. The Italian State of Affairs. Eur. J. Health Law. 2018;25:322–337. doi: 10.1163/15718093-12530378. [DOI] [Google Scholar]
- 5.Zegers-Hochschild F., Crosby J.A., Salas S.P. Medical and ethical basis for embryo cryopreservation. Rev. Med. Chile. 2014;142:896–902. doi: 10.4067/S0034-98872014000700010. [DOI] [PubMed] [Google Scholar]
- 6.Zaami S. Assisted Heterologous Fertilization and the Right of Donorconceived Children to Know Their Biological Origins. La Clin. Ter. 2018;169:e39–e43. doi: 10.7417/T.2018.2052. [DOI] [PubMed] [Google Scholar]
- 7.Milman L.W., Senapati S., Sammel M.D., Cameron K.D., Gracia C. Assessing Reproductive Choices of Women and the Likelihood of Oocyte Cryopreservation in the Era of Elective Oocyte Freezing. Fertil. Steril. 2017;107:1214–1222.e3. doi: 10.1016/j.fertnstert.2017.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaami S., Busardò F.P. Elective Egg Freezing: Can You Really Turn Back the Clock? Eur. Rev. Med. Pharmacol. Sci. 2015;19:3537–3538. [PubMed] [Google Scholar]
- 9.Papatheodorou A., Vanderzwalmen P., Panagiotidis Y., Petousis S., Gullo G., Kasapi E., Goudakou M., Prapas N., Zikopoulos K., Georgiou I., et al. How Does Closed System Vitrification of Human Oocytes Affect the Clinical Outcome? A Prospective, Observational, Cohort, Noninferiority Trial in an Oocyte Donation Program. Fertil. Steril. 2016;106:1348–1355. doi: 10.1016/j.fertnstert.2016.07.1066. [DOI] [PubMed] [Google Scholar]
- 10.Gullo G., Petousis S., Papatheodorou A., Panagiotidis Y., Margioula-Siarkou C., Prapas N., D’Anna R., Perino A., Cucinella G., Prapas Y. Closed vs. Open Oocyte Vitrification Methods Are Equally Effective for Blastocyst Embryo Transfers: Prospective Study from a Sibling Oocyte Donation Program. Gynecol. Obstet. Investig. 2020;85:206–212. doi: 10.1159/000506803. [DOI] [PubMed] [Google Scholar]
- 11.Terho A.M., Pelkonen S., Opdahl S., Romundstad L.B., Bergh C., Wennerholm U.B., Henningsen A.A., Pinborg A., Gissler M., Tiitinen A. High Birth Weight and Large-for-Gestational-Age in Singletons Born after Frozen Compared to Fresh Embryo Transfer, by Gestational Week: A Nordic Register Study from the CoNARTaS Group. Hum. Reprod. 2021;36:1083–1092. doi: 10.1093/humrep/deaa304. [DOI] [PubMed] [Google Scholar]
- 12.Zaat T., Zagers M., Mol F., Goddijn M., van Wely M., Mastenbroek S. Fresh versus Frozen Embryo Transfers in Assisted Reproduction. Cochrane Database Syst. Rev. 2021;2:CD011184. doi: 10.1002/14651858.CD011184.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Acet F., Hortu I., Sahin G., Goker E.N.T., Tavmergen E. Is Frozen Embryo Transfer Better than Fresh Embryo Transfer in Women Undergoing Intracytoplasmic Sperm Injection over the Age of Thirty-Five? A Single Referral Centre Experience. J. Obstet. Gynaecol. 2022;42:276–280. doi: 10.1080/01443615.2021.1882973. [DOI] [PubMed] [Google Scholar]
- 14.Pirtea P., de Ziegler D., Ayoubi J.M. Children Born from Frozen Embryo Transfers: Is There a Difference? Fertil. Steril. 2020;114:502–503. doi: 10.1016/j.fertnstert.2020.05.012. [DOI] [PubMed] [Google Scholar]
- 15.Vuong L.N., Ly T.T., Nguyen N.A., Nguyen L.M.T., Le X.T.H., Le T.K., Le K.T.Q., Le T.V., Nguyen M.H.N., Dang V.Q., et al. Development of Children Born from Freeze-Only versus Fresh Embryo Transfer: Follow-up of a Randomized Controlled Trial. Fertil. Steril. 2020;114:558–566. doi: 10.1016/j.fertnstert.2020.04.041. [DOI] [PubMed] [Google Scholar]
- 16.Orvieto R., Kirshenbaum M., Gleicher N. Is Embryo Cryopreservation Causing Macrosomia-and What Else? Front. Endocrinol. 2020;11:19. doi: 10.3389/fendo.2020.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen L., Ni X., Xu Z., Fang J., Zhang N., Li D. Effect of Frozen and Fresh Embryo Transfers on the Birthweight of Live-Born Twins. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020;246:50–54. doi: 10.1016/j.ejogrb.2020.01.008. [DOI] [PubMed] [Google Scholar]
- 18.Djuwantono T., Aviani J.K., Permadi W., Achmad T.H., Halim D. Risk of Neurodevelopmental Disorders in Children Born from Different ART Treatments: A Systematic Review and Meta-Analysis. J. Neurodev. Disord. 2020;12:33. doi: 10.1186/s11689-020-09347-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Elias F.T.S., Weber-Adrian D., Pudwell J., Carter J., Walker M., Gaudet L., Smith G., Velez M.P. Neonatal Outcomes in Singleton Pregnancies Conceived by Fresh or Frozen Embryo Transfer Compared to Spontaneous Conceptions: A Systematic Review and Meta-Analysis. Arch. Gynecol. Obstet. 2020;302:31–45. doi: 10.1007/s00404-020-05593-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ernstad E.G., Spangmose A.L., Opdahl S., Henningsen A.-K.A., Romundstad L.B., Tiitinen A., Gissler M., Wennerholm U.-B., Pinborg A., Bergh C., et al. Perinatal and Maternal Outcome after Vitrification of Blastocysts: A Nordic Study in Singletons from the CoNARTaS Group. Hum. Reprod. 2019;34:2282–2289. doi: 10.1093/humrep/dez212. [DOI] [PubMed] [Google Scholar]
- 21.Ernstad E.G., Wennerholm U.-B., Khatibi A., Petzold M., Bergh C. Neonatal and Maternal Outcome after Frozen Embryo Transfer: Increased Risks in Programmed Cycles. Am. J. Obstet. Gynecol. 2019;221:126.e1–126.e18. doi: 10.1016/j.ajog.2019.03.010. [DOI] [PubMed] [Google Scholar]
- 22.Ainsworth A.J., Wyatt M.A., Shenoy C.C., Hathcock M., Coddington C.C. Fresh versus Frozen Embryo Transfer Has No Effect on Childhood Weight. Fertil. Steril. 2019;112:684–690.e1. doi: 10.1016/j.fertnstert.2019.05.020. [DOI] [PubMed] [Google Scholar]
- 23.Maris E., Ferrieres-Hoa A., Gala A., Coffy A., Vintejoux E., Ranisavljevic N., Hamamah S. Comparison of birth weights of children born after slow frozen embryo replacement versus fresh embryo transfer. Gynecol. Obstet. Fertil. Senol. 2019;47:305–310. doi: 10.1016/j.gofs.2019.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Hwang S.S., Dukhovny D., Gopal D., Cabral H., Diop H., Coddington C.C., Stern J.E. Health Outcomes for Massachusetts Infants after Fresh versus Frozen Embryo Transfer. Fertil. Steril. 2019;112:900–907. doi: 10.1016/j.fertnstert.2019.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maheshwari A., Pandey S., Raja E.A., Shetty A., Hamilton M., Bhattacharya S. Is Frozen Embryo Transfer Better for Mothers and Babies? Can Cumulative Meta-Analysis Provide a Definitive Answer? Hum. Reprod. Update. 2018;24:35–58. doi: 10.1093/humupd/dmx031. [DOI] [PubMed] [Google Scholar]
- 26.Berntsen S., Pinborg A. Large for Gestational Age and Macrosomia in Singletons Born after Frozen/Thawed Embryo Transfer (FET) in Assisted Reproductive Technology (ART) Birth Defects Res. 2018;110:630–643. doi: 10.1002/bdr2.1219. [DOI] [PubMed] [Google Scholar]
- 27.Sha T., Yin X., Cheng W., Massey I.Y. Pregnancy-Related Complications and Perinatal Outcomes Resulting from Transfer of Cryopreserved versus Fresh Embryos In Vitro Fertilization: A Meta-Analysis. Fertil. Steril. 2018;109:330–342.e9. doi: 10.1016/j.fertnstert.2017.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Zhang J., Du M., Li Z., Wang L., Hu J., Zhao B., Feng Y., Chen X., Sun L. Fresh versus Frozen Embryo Transfer for Full-Term Singleton Birth: A Retrospective Cohort Study. J. Ovarian Res. 2018;11:59. doi: 10.1186/s13048-018-0432-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wong K.M., van Wely M., Mol F., Repping S., Mastenbroek S. Fresh versus Frozen Embryo Transfers in Assisted Reproduction. Cochrane Database Syst. Rev. 2017;3:CD011184. doi: 10.1002/14651858.CD011184.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vidal M., Vellvé K., González-Comadran M., Robles A., Prat M., Torné M., Carreras R., Checa M.A. Perinatal Outcomes in Children Born after Fresh or Frozen Embryo Transfer: A Catalan Cohort Study Based on 14,262 Newborns. Fertil. Steril. 2017;107:940–947. doi: 10.1016/j.fertnstert.2017.01.021. [DOI] [PubMed] [Google Scholar]
- 31.Chen Z.-J., Shi Y., Sun Y., Zhang B., Liang X., Cao Y., Yang J., Liu J., Wei D., Weng N., et al. Fresh versus Frozen Embryos for Infertility in the Polycystic Ovary Syndrome. N. Engl. J. Med. 2016;375:523–533. doi: 10.1056/NEJMoa1513873. [DOI] [PubMed] [Google Scholar]
- 32.Belva F., Bonduelle M., Roelants M., Verheyen G., Van Landuyt L. Neonatal Health Including Congenital Malformation Risk of 1072 Children Born after Vitrified Embryo Transfer. Hum. Reprod. 2016;31:1610–1620. doi: 10.1093/humrep/dew103. [DOI] [PubMed] [Google Scholar]
- 33.Pinborg A., Henningsen A.A., Loft A., Malchau S.S., Forman J., Andersen A.N. Large Baby Syndrome in Singletons Born after Frozen Embryo Transfer (FET): Is It Due to Maternal Factors or the Cryotechnique? Hum. Reprod. 2014;29:618–627. doi: 10.1093/humrep/det440. [DOI] [PubMed] [Google Scholar]
- 34.Li Z., Wang Y.A., Ledger W., Edgar D.H., Sullivan E.A. Clinical Outcomes Following Cryopreservation of Blastocysts by Vitrification or Slow Freezing: A Population-Based Cohort Study. Hum. Reprod. 2014;29:2794–2801. doi: 10.1093/humrep/deu246. [DOI] [PubMed] [Google Scholar]
- 35.Wennerholm U.-B., Henningsen A.-K.A., Romundstad L.B., Bergh C., Pinborg A., Skjaerven R., Forman J., Gissler M., Nygren K.G., Tiitinen A. Perinatal Outcomes of Children Born after Frozen-Thawed Embryo Transfer: A Nordic Cohort Study from the CoNARTaS Group. Hum. Reprod. 2013;28:2545–2553. doi: 10.1093/humrep/det272. [DOI] [PubMed] [Google Scholar]
- 36.Liu S.Y., Teng B., Fu J., Li X., Zheng Y., Sun X.X. Obstetric and Neonatal Outcomes after Transfer of Vitrified Early Cleavage Embryos. Hum. Reprod. 2013;28:2093–2100. doi: 10.1093/humrep/det104. [DOI] [PubMed] [Google Scholar]
- 37.Check J.H., Summers-Chase D., Yuan W., Horwath D., Garberi-Levito M.C. Pregnancy Rates Following the Exclusive Transfer of Twice Frozen Twice Thawed Embryos Using a Modified Slow Cool Cryopreservation Technique. Clin. Exp. Obstet. Gynecol. 2013;40:20–21. doi: 10.1016/j.fertnstert.2011.01.116. [DOI] [PubMed] [Google Scholar]
- 38.Check J.H., Katsoff B., Wilson C., Choe J.K., Brasile D. Pregnancy Outcome Following Fresh vs Frozen Embryo Transfer into Gestational Carriers Using a Simplified Slow Freeze Protocol. Clin. Exp. Obstet. Gynecol. 2012;39:23–24. [PubMed] [Google Scholar]
- 39.Wennerholm U.-B., Söderström-Anttila V., Bergh C., Aittomäki K., Hazekamp J., Nygren K.-G., Selbing A., Loft A. Children Born after Cryopreservation of Embryos or Oocytes: A Systematic Review of Outcome Data. Hum. Reprod. 2009;24:2158–2172. doi: 10.1093/humrep/dep125. [DOI] [PubMed] [Google Scholar]
- 40.Wennerholm W.B. Cryopreservation of Embryos and Oocytes: Obstetric Outcome and Health in Children. Hum. Reprod. 2000;15((Suppl. 5)):18–25. doi: 10.1093/humrep/15.suppl_5.18. [DOI] [PubMed] [Google Scholar]
- 41.Wennerholm U.B., Albertsson-Wikland K., Bergh C., Hamberger L., Niklasson A., Nilsson L., Thiringer K., Wennergren M., Wikland M., Borres M.P. Postnatal Growth and Health in Children Born after Cryopreservation as Embryos. Lancet. 1998;351:1085–1090. doi: 10.1016/S0140-6736(97)08247-0. [DOI] [PubMed] [Google Scholar]
- 42.Wennerholm U.B., Hamberger L., Nilsson L., Wennergren M., Wikland M., Bergh C. Obstetric and Perinatal Outcome of Children Conceived from Cryopreserved Embryos. Hum. Reprod. 1997;12:1819–1825. doi: 10.1093/humrep/12.8.1819. [DOI] [PubMed] [Google Scholar]
- 43.Gullo G., Carlomagno G., Unfer V., D’Anna R. Myo-Inositol: From Induction of Ovulation to Menopausal Disorder Management. Minerva Ginecol. 2015;67:485–486. [PubMed] [Google Scholar]
- 44.Espinola M.S.B., Laganà A.S., Bilotta G., Gullo G., Aragona C., Unfer V. D-Chiro-Inositol Induces Ovulation in Non-Polycystic Ovary Syndrome (PCOS), Non-Insulin-Resistant Young Women, Likely by Modulating Aromatase Expression: A Report of 2 Cases. Am. J. Case Rep. 2021;22:e932722. doi: 10.12659/AJCR.932722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.D’Anna R., Corrado F., Loddo S., Gullo G., Giunta L., Di Benedetto A. Myoinositol plus α-Lactalbumin Supplementation, Insulin Resistance and Birth Outcomes in Women with Gestational Diabetes Mellitus: A Randomized, Controlled Study. Sci. Rep. 2021;11:8866. doi: 10.1038/s41598-021-88329-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.D’Anna R., Santamaria A., Giorgianni G., Vaiarelli A., Gullo G., Di Bari F., Benvenga S. Myo-Inositol and Melatonin in the Menopausal Transition. Gynecol. Endocrinol. 2017;33:279–282. doi: 10.1080/09513590.2016.1254613. [DOI] [PubMed] [Google Scholar]
- 47.Alviggi C., Conforti A., Carbone I.F., Borrelli R., de Placido G., Guerriero S. Influence of Cryopreservation on Perinatal Outcome after Blastocyst- vs. Cleavage-Stage Embryo Transfer: Systematic Review and Meta-Analysis. Ultrasound Obstet. Gynecol. 2018;51:54–63. doi: 10.1002/uog.18942. [DOI] [PubMed] [Google Scholar]
- 48.Lin L.-T., Sapia F., La Rosa V. Correlation between Maternal Gingivitis/Periodontitis and Preterm Delivery: Fact or Fancy? Ital. J. Gynaecol. Obstet. 2018;30:7–12. doi: 10.14660/2385-0868-96. [DOI] [Google Scholar]
- 49.Wang J.X., Norman R.J., Wilcox A.J. Incidence of Spontaneous Abortion among Pregnancies Produced by Assisted Reproductive Technology. Hum. Reprod. 2004;19:272–277. doi: 10.1093/humrep/deh078. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this case report are available on request from the corresponding author.