Abstract
Pathogen suppression and induced systemic resistance are suitable alternative biocontrol strategies for integrated plant disease management and potentially comprise a sustainable alternative to agrochemicals. The use of Actinobacteria as biocontrol agents is accepted in practical sustainable agriculture, and a short overview on the plant-beneficial members of this phylum and recent updates on their biocontrol efficacies are the two topics of this review. Actinobacteria include a large portion of microbial rhizosphere communities and colonizers of plant tissues that not only produce pest-antagonistic secondary metabolites and enzymes but also stimulate plant growth. Non-pathogenic Actinobacteria can also induce systemic resistance against pathogens, but the mechanisms are still poorly described. In the absence of a pathogen, a mild defense response is elicited under jasmonic acid and salicylic acid signaling that involves pathogenesis-related proteins and secondary plant metabolites. Priming response partly includes the same compounds as the response to a sole actinobacterium, and the additional involvement of ethylene signaling has been suggested. Recent amplicon sequencing studies on bacterial communities suggest that future work may reveal how biocontrol active strains of Actinobacteria can be enriched in plant rhizosphere.
Keywords: actinobacteria, biocontrol, induced systemic resistance, plant defense
1. Introduction
Intensive agricultural practice is accompanied by the leaching of mineral fertilizers and combatting emerging phytopathogens with synthetic agrochemicals, and the necessity of developing complementary methods to improve plant nutrition and to control plant pathogens has been recognized [1]. Biological control uses microbial biocontrol agents to protect plants against pathogens with direct and indirect mechanisms. Direct mechanisms include hyperparasitism, predation and antibiosis, as well as competition for nutrients and space with other microorganisms, but the impacts of single microbial strains on the microbiome assembly and the induction of host resistance are indirect mechanisms for microbial biocontrol agents against pathogens [2]. Damage to plant pathogens and the effect of bacterial biocontrol agents have been proven in several field studies [3,4,5,6,7,8,9].
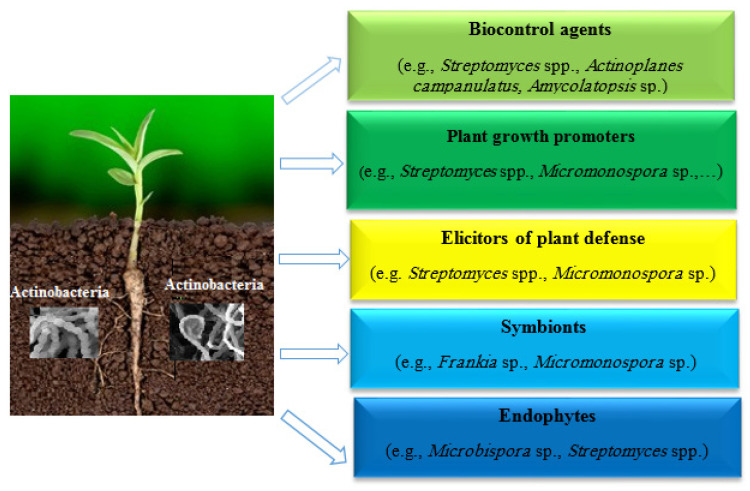
Members of Actinobacteria are engaged in beneficial interactions with plants, stimulating plant growth and disease resistance (Figure 1). Among microbial biocontrol agents, the members of Actinobacteria are particularly interesting due to their widespread abilities to inhibit the growth of a wide range of phytopathogens and the prolific production of antimicrobial compounds [10,11]. Though most studies on biocontrol have involved Streptomyces species, reports also exist on, e.g., isolates from the genera Actinoplanes, Arthrobacter, Microbacterium, Micromonospora and Rhodococcus. Since the members of Actinobacteria are generally versatile in their metabolism and thus competitive for both root exudates and plant litter, they form intimate associations with plant materials and comprise frequent colonizers of rhizospheres and plant tissues [12]. Plant growth promotion by Actinobacteria takes place through the secretion of plant growth regulators [13,14], nitrogen fixation, phosphate solubilization, and iron acquisition [15,16,17,18,19]. Such traits are expressed by, for instance, members of the genera Frankia, Streptomyces, Micrococcus, Micromonospora, Kitasatospora and Thermobifidia. Actinobacteria may also influence symbiosis formation between host plants and their mutualists, nitrogen-fixing bacteria [20] and mycorrhizal fungi [21]. Investigations on plant growth promotion have revealed that the in vitro antagonistic activity against pathogens by Actinobacteria does not necessarily correlate with their biocontrol activity [22]. Instead, plant growth promotion has been associated with biocontrol activity, and this has two important implications. First, the screening for biocontrol strains should not be limited to the results of in vitro bioactivity assays; second, the Actinobacteria may protect host plants in vivo by not only inhibiting the pathogen but also by eliciting plant disease resistance [23].
Figure 1.
Beneficial interactions of Actinobacteria with plants.
Indeed, rhizobacteria can mediate induced systemic resistance (ISR) in plants by priming for plant defense, first revealed with Pseudomonas and Bacillus strains [24,25,26]. Priming brings the plants to an altered state that enables them to more quickly and/or strongly respond to a subsequent pathogen infection [27,28]. The traditional ISR pathways in plants during Pseudomonas- and Bacillus-mediated ISR lead to the faster and stronger expression of marker genes for the salicylic acid, jasmonic acid, and ethylene signaling pathways upon subsequent pathogen infection. ISR by Actinobacteria was identified by Conn et al. [29] as a result of Micromonospora or Streptomyces strain inoculations.
In this review, we focus on recent developments in the area of Actinobacteria-based biocontrol, starting with the compound production against the pests and then moving to the elicitation of plant defenses. We close the review by evaluating the community studies of plant-associated Actinobacteria and discussing the potential to enrich stress releasing members of this phylum by specific treatments. We expect that the appreciation of these thematic areas will be crucial for the development of novel Actinobacteria-based biocontrol approaches.
2. Actinobacteria as Successful Biocontrol Agents
Numerous studies have proven that Actinobacteria are successful biocontrol agents against plant pathogens (Table 1). Biological activity against pathogens has been established for several actinobacterial secondary metabolites. For instance, Cheng et al. [30] reported that azalomycin produced by Streptomyces malaysiensis MJM1968 exhibited antifungal activity on Fusarium oxysporum, Rhizoctonia solani, Cladosporium cladosporioides, Fusarium chlamydosporum, Colletotrichum gloeosporioides, Pestalotia spp. and Alternaria mali. Additionally, prodiginines from S. lividans caused the inhibition of Verticillium dahliae growth [31]. Siderophores are other bioactive compounds produced by Actinobacteria that can promote plant growth and induce resistance in plants against pathogens [32,33]. Siderophores are small molecules with a high affinity for Fe3+. Sadeghi et al. [34] reported that a siderophore-producer Streptomyces strain improved iron acquisition and wheat growth promotion under salinity stress conditions. Actinobacteria isolated from Achillea fragrantissima that produced both chitinases and siderophores showed antimicrobial activity against pathogenic microorganisms [35]. Dimkpa et al. [36] reported that hydroxamate siderophores produced by Streptomyces tendae F4 promoted the growth and improved the cadmium uptake of sunflower plants.
Table 1.
The examples of biocontrol activity of the actinobacterial strains against some phytopathogens.
| Strain | Host | Pathogen | Reference |
|---|---|---|---|
| Streptomyces halstedii AJ-7 | Red pepper | Phytophthora capsici | [52] |
| Streptomyces sp. CA2, AA2 | Tomato | Rhizoctonia solani | [22] |
| S. griseus | Tomato | Fusarium sp. | [53] |
| Streptomyces sp. S2,C | Sugar beet | Rhizoctonia solani | [54] |
| Streptomyces sp. MBCu-56 | Cucurbit | Colletotrichum orbiculare | [55] |
|
S. aurantiogriseus VSMGT1014 |
Rice | Rhizoctonia solani | [56] |
| Streptomyces sp. J-2 | Sugar beet | Sclerotium rolfsii | [57] |
| Streptomyces spp. | Sugar beet | Fusarium spp. | [58] |
|
Actinoplanes campanulatus #2 Micromonospora chalcea #8 S. spiralis #17 |
Cucumber | Pythium aphanidermatum | [41] |
|
Streptomyces sp. strain g10 S. malaysiensis 8ZJF-21 |
Banana | Fusarium oxysporum f.sp. cubense | [59] [60] |
| Streptomyces sp. S160 | Chickpea | Macrophomina phaseolina | [61] |
| Amycolatopsis sp. 521 | Apple | Colletotrichum gloeosporioides | [62] |
| S. albidoflavus | Tomato |
Alternaria solani, A. alternata, Colletotrichum gloeosporioides, Fusarium oxysporum, Fusarium solani, Rhizoctonia solani, and Botrytis cinerea |
[63] |
| Streptomyces sp. A1022 | Pepper, Cherry Tomato |
Colletotrichum gloeosporioides | [64] |
| S. misionensis BH4-1,BH4-3 | Pistachio | Paecilomyces formosus | [65] |
| S. globisporus JK-1 | Rice | Magnaporthe oryzae | [66] |
| Streptomyces sp. MT7 | - | Wood-rotting fungi | [42] |
| S. mutabilis IA1 | Wheat | Fusarium culmorum | [67] |
|
Micromonospora sp. ALFpr18c, ALFb5 |
Tomato | Botrytis cinerea | [68] |
| S. globosus UAE1 | Date Palm | Thielaviopsis punctulata | [69] |
| Streptomyces spp. A20, 5.1, 7.1 | Rice | Burkholderia glumae | [70] |
|
S. angustmyceticus NR8-2 |
Cabbage |
Colletotrichum sp. and Curvularia lunata |
[51] |
| Streptomyces sp. HAAG3-15 | Cucumber | F. oxysporum f.sp. cucumerinum | [71] |
| Streptomyces spp. R7,F8 | Tomato | R. solani | [72] |
| S. laydicus M01 | Cucumber | A. alternata | [73] |
| S. fulvissimus Uts22 | Cucumber Wheat |
Pythium aphanidermatum and Gaeumannomyces graminis var. tritici |
[74] [75] |
| Streptomyces sp. TP199 | Potato |
Pectobacterium carotovorum subsp. Carotovorum, and Pectobacterium atrosepticum |
[76] |
| S. violaceusniger AC12AB | Potato | Streptomyces scabies | [77] |
| Streptomyces sp. AN090126 | Tomato Red Pepper Creeping bentgrass |
Ralstonia solanacearum, Xanthomonas euvesicatoria, and Sclerotinia homoeocarpa |
[78] |
Actinobacteria are also well-known for the release of enzymes that are active against phytopathogens, including chitinases, glucanases, amylases, cellulases, lipases and proteases [37]. Chitinase- and glucanase-producing S. cavourensis SY224 controlled anthracnose disease in pepper [38]. S. halstedii and S. griseus produced highly active antifungal chitinases and are effective biological agents for the protection of crops [39,40]. Glucanase-producing Actinoplanes campanulatus and Micromonospora chalcea protected cucumber from Pythium aphanidermatum under greenhouse conditions [41]. Streptomyces sp. MT7, as a chitinolytic strain, showed antagonistic activity against several wood-rotting fungi including Phanerochaete chrysosporium, Coriolus versicolor, Polystictus versicolor, and Schizophyllum commune, the causal agents of white rot, as well as Gloeophyllum trabeum, Postia placenta, Polyporus agaricans and Polyporus friabilis, the causal agents of brown rot [42]. Gopalakrishnan et al. [43] reported that Streptomyces strains reduced Fusarium wilt in chickpea via the production of several metabolites in concert including not only the enzymes cellulase and protease but also hydrogen cyanide. Dieback caused by the fungus Lasiodiplodia theobromae is an important disease on mango plantations, and the antifungal action of Micromonospora tulbaghiae UAE1 against the fungus was associated with both antibiotic and chitinase production [44]. The quenching of quorum-sensing molecules may also lead to biocontrol by Actinobacteria. The biocontrol agent of soft rot disease in various host plants, Rhodococcus pyridinivorans XN-36, degrades a wide range of N-acyl homoserine lactones and prevents quorum-sensing among plant-pathogenic bacteria [45]. Additionally, in co-cultures between Arthrobacter sp. IBN110 and the plant pathogen Erwinia carotovora, the N-acyl homoserine lactone levels and pectate lyase activity, both important for rot induction, were shown to be significantly reduced in relation to a single culture of E. carotovora [46].
Volatile organic compounds (VOCs) are bioactive molecules produced by many plant-associated Actinobacteria, e.g., Streptomyces strains possessing antifungal activity [47,48,49]. Volatile substances produced by S. platensis F-1 caused resistance in rice, oilseed rape, and strawberry against Rhizoctonia solani, Sclerotinia sclerotiorum, and Botrytis cinerea, respectively [50]. S. angustmyceticus NR8-2 was shown to emit volatile antifungal compounds including alcohols, aldehydes, carboxylic acids and fatty acids. This species also produced β-1,3-glucanase, and controlled Colletotrichum sp. and Curvularia lunata leaf spot on Tokyo Bekana cabbage [51].
Several commercial products derived from Actinobacteria are available for use in crop protection. Table 2 shows the Streptomyces spp.-based products and active substances derived from them registered as commercial products for the control of plant pathogens. Mycostop was the first actinobacterial commercial product derived from S. griseoviridis K61 that is used against some soilborne fungal pathogens [79].
Table 2.
List of Streptomyces spp.-based products and active substances derived from them registered as commercial products to control of plant pathogens (data collected and modified into a table from [80,81,82,83]).
| Product Name | Organism | Targeted Pathogen/Disease |
|---|---|---|
| Mycostop, Verdera Oy, Finland |
S. griseoviridis K61 | Damping off caused by Alternaria and R. solani and Fusarium, Phytophthora, and Pythium wilt and root diseases |
| Actinovate, Novozymes BioAg Inc., USA |
S. lydicus WYEC 108 | Soilborne pathogens, viz. Pythium, Fusarium, Phytophthora, Rhizoctonia, and Verticillium; foliar diseases such as powdery and downy mildew, Botrytis, Alternaria, Postia, Geotrichum, and Sclerotinia |
| Mykocide KIBC Co., Ltd. South Korea |
S. colombiensis | Powdery mildews, grey mold, and brown patch |
| Safegrow KIBC Co., Ltd. South Korea |
S. kasugaensis | Sheath blight and large patch |
| Bactophil | S. albus | Seed germination diseases |
|
Blasticidin-S BLA-S |
S. griseochromogenes | Pyricularia oryzae |
|
Kasugamycin Kasumin, Kasurab |
S. kasugaensis | Leaf spot in sugar beet and celery (Cercospora spp.), scab in pears and apples (Venturia spp.), and soybean root rot (Phytophthora sojae) |
|
Streptomycin Agrimycin, Paushak, Cuprimicin 17, AAstrepto 17, AS-50, Dustret, Cuprimic 100 and 500 |
S. griseus | Bacterial rots, canker, and other bacterial diseases; Xanthomonas oryzae, Xanthomonas citri, and Pseudomonas tabaci of pome fruit, stone fruit, citrus, olives, vegetables, potatoes, tobacco, cotton, and ornamentals |
|
Phytomycin Mycoshield, Cuprimic 100 and 500, Mycoject |
S. rimosus | Fire blight (Erwinia amylovora) and diseases caused by Pseudomonas sp., Xanthomonas sp. and mycoplasma-like organisms |
|
Validamycin Validacin, Valimun, Dantotsupadanvalida, Mycin Hustler, Valida, Sheathmar |
S. hygroscopicus | R. solani and other Rhizoctonia in rice, potatoes, vegetables, strawberries, tobacco, ginger, cotton, sugar beet, etc. |
|
Polyoxorim Endorse, PolyoxinZ, Stopit, Polyoxin AL and Z, Polybelin |
S. cacaoi var. asoensis | Plant-pathogenic fungi, Sphaerotheca spp. and other powdery mildews; Botrytis cinerea, Sclerotinia sclerotiorum, Corynespora melonis, Cochliobolus miyabeanus, Alternaria alternata and other species in vines, apples, pears, vegetables, and ornamentals; rice sheath blight (R. solani), apple, pear canker, and Helminthosporium in rice |
|
Natamycin Delvolan |
S. natalensis and S. chattanoogensis |
Basal rots on daffodils and ornamentals caused by Fusarium oxysporum |
Bold names in the first column indicate biocontrol metabolites as active substances.
Although biocontrol activities by Actinobacteria have been recognized as potentially useful for sustainable agriculture, only few products are currently commercialized [84]. The establishment of suitable and rapid screening for appropriate biocontrol candidates is one of the critical steps towards the development of novel commercial biocontrol products [85]. Additionally, formulation methods and procedures of inoculations play an important role in obtaining satisfactory results of the application of the certain commercial product in the field conditions [86], and their further development is crucial in order to obtain robust actinobacterial formulations.
3. The Potential of Actinobacteria to Induce Systemic Resistance in Plants
3.1. General Mechanisms of Induced Systemic Resistance (ISR)
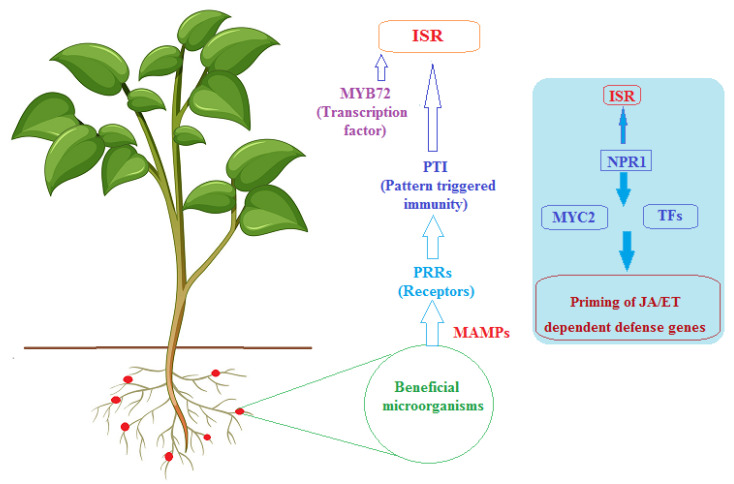
ISR exerts a broad-spectrum response against pathogens, and it can be comparably effective in different plant species [87]. The elicitors of ISR that are produced by or derived from bacteria include lipopolysaccharides (LPS), flagella, siderophores, biosurfactants, volatile organic compounds (VOCs), quorum-sensing molecules and antibiotics [88,89,90]. The perception of some of the beneficial microorganisms involves early responses such as ion fluxes, MAP kinase cascade activation, extracellular medium alkalization, and the production of reactive oxygen species (ROS) followed by the activation of various molecular and cellular host defense responses [91,92,93]. Jasmonic acid (JA) and ethylene (ET) are central players in the priming of plant resistance by bacteria [26,87]. Figure 2 sums up the molecular components and mechanisms involved in ISR by beneficial microbes. Although beneficial microorganisms often trigger ISR through the JA/ET pathway, several plant growth-promoting rhizobacteria and fungi have been shown to trigger ISR through salicylic acid (SA)-dependent mechanisms. For example, Paenibacillus alvei K-165 and P. fluorescens SS101 were found to induce an SA-dependent pathway in Arabidopsis [94,95], and an SA-producing mutant of Pseudomonas aeruginosa 7NSK2 did not induce resistance to Botrytis cinerea in wild-type tomatoes [96].
Figure 2.
Induced systemic resistance (ISR) by beneficial microorganisms. JA and ET are central regulators phytohormones of ISR, and transcription factors (e.g., MYC2) mediate the increased responsiveness of this pathway to stimulation, known as priming. Transcription factor MYB72, as a root-specific transcription factor and early signaling factor, functions as a node of convergence in ISR elicited by beneficial microbes. (ET, ethylene; JA, jasmonic acid; NPR1, NONEXPRESSOR OF PR GENES1; MAMPs, microbe-associated molecular patterns; PRRs, plant recognition receptors; PTI, PAMP-triggered immunity; TFs, transcription factors).
3.2. Actinobacteria Priming Plant Defense
In a pioneering paper, Conn et al. [29] reported priming by wheat endophytic Actinobacteria belonging to Micromonospora and Streptomyces. The priming by these Actinobacteria was associated with upregulating genes in either the SAR and/or JA/ET pathways, depending on the infecting pathogen, and the ISR also occurred after the application of bacterial culture filtrates. Priming by a culture filtrate was also proven with the culture filtrate of S. bikiniensis HD-087. Its application induced resistance in cucumber against Fusarium oxysporum f.sp. cucumerinum and was associated with highly increased activities of peroxidase, β-1,3-glucanase, and phenylalanine ammonia lyase [97]. The induction of cytosolic Ca2+ and biphasic oxidative burst by Streptomyces sp. OE7 in tobacco cells was demonstrated by Baz et al. [98], suggesting that this strain elicits ISR in a similar manner to the Pseudomonas and Bacillus strains. The ability of Streptomyces strains S. toxytricini vh22, S. avidinii vh32, S. tricolor vh85, S. toxytricini vh6 and S. flavotricini vh8 to protect tomato against Rhizoctonia solani under greenhouse conditions was reported by Patil et al. [99]. Phenylalanine ammonia lyase (PAL) activity and total phenolic contents in tomato increased following the inoculation of these four strains compared to an untreated control [99], and they were further enhanced by the presence of the plant pathogen, though Streptomyces strain-specific differences were observed. Whereas the isolates vh6 and vh8 offered the most extensive disease reductions, the highest PAL activities and levels of total phenolic compounds were observed for the strain vh32, suggesting that protection against R. solani involves further determinants of plant phenolics induction [99]. Similarly, biochemical experiments revealed that actinomycetes isolated from vermicompost enhanced defense-related enzyme activities, including those of peroxidase, polyphenol oxidase, and phenylalanine ammonia lyase, in tomato plants challenged by R. solani [100]. Streptomyces sp. strain AcH 505 induced resistance in oak against Microsphaera alphitoides, the causal agent of powdery mildew. RNA-Seq analysis revealed that not only JA but also the ET, SA, and (in part) ABA pathways may play roles in Streptomyces AcH 505-mediated priming in oaks. The study also revealed that Streptomyces sp. strain AcH 505 was able to activate plant defense responses in the absence of pathogen challenge [101]. Furthermore, in accordance with reports discussed earlier, the authors of the study demonstrated the priming-like accumulation of transcripts related to phenylpropanoid biosynthesis and reported enhanced phenylalanine ammonia lyase activity, suggesting that plant secondary metabolism may be involved.
Martinez-Hidalgo et al. [68] demonstrated that Micromonospora strains ALFpr18c and ALFb5 stimulated defense responses of different tomato cultivars upon Botrytis cinerea attack. Their study revealed that the induced systemic resistance in tomato was long lasting and that jasmonates played a key role in the defense priming effect [68]. Singh and Gaur [102] reported that endophytic Streptomyces spp. triggered systemic resistance against Sclerotium rolfsii in chickpeas and mitigated the oxidative stress generated by this pathogen. Their biochemical experiments indicated that S. griseus in challenge with the pathogen caused increases in the amount of defense-related enzymes such as PAL and PPO along with the accumulation of total phenolics and flavonoids. Furthermore, real-time PCR analysis revealed significant enhancements of genes encoding superoxide dismutase (SOD), PAL, peroxidase (PO), ascorbate peroxidase (APX), catalase (CAT), chitinase (CHI), and β-glucanase (GLU) after priming with S. griseus, which corroborated the above-mentioned findings [102].
The grapevine rhizosphere inhabitant Streptomyces anulatus S37 promotes grapevine growth and induces resistance against phytopathogens, including B. cinerea. The local defense events induced in grapevine suspension cells were investigated by Vatsa-Portugal et al. [103]; S. anulatus S37 induced early defense responses including oxidative burst, extracellular alkalization, protein kinase activation, the induction of defense gene expression, and phytoalexin accumulation [103]. Additionally, an early interaction between Streptomyces sp. UPMRS4 and rice plant under Pyricularia oryzae stress [104] has demonstrated increases in chitinase (Cht-1), glucanase (Gns1), pathogenesis-related gene (OsPR1a), and salicylic acid-responsive gene (Oswrky45) transcript abundancies. The ability of S. rochei A-1 in inducing resistance against Botryosphaeria dothidea in apple fruit during storage was reported by Zhang et al. [105], including enhanced POD, CAT, SOD, PAL, GLU and CHI activities and H2O2 generation but decreased lipid peroxidation.
Streptomyces sp. strain NSP3 triggered tomato defense responses against F. oxysporum f.sp. lycopersici [106]. The effects of seed treatment or soil application with the Streptomyces sp. strain NSP3 and the combination of two methods were compared under pathogen challenge. The combination of two above-described methods was more effective for the induction of PR genes including PR-1a, Chi3, Chi9, and CEVI-1 than either alone [106]. In another study, Abbasi et al. [107] demonstrated how Streptomyces strains induced systemic resistance to F. oxysporum f.sp. lycopersici in tomato, and in cucumber, Streptomyces sp. LH4 was shown to mediate JA and SA defenses in response to Sclerotinia sclerotiorum [108]. Inoculations of S. fimicarius and S. laurentii to rice rhizosphere led to resistance against rice bacterial blight, as reported by Saikia and Bora [109]. The application of S. lydicus M01 to rhizospheres promoted cucumber growth via its phosphate solubilization, IAA secretion, siderophore and ACC deaminase production activities and led to higher numbers of potentially plant-beneficial bacteria in cucumber rhizosphere [73]. It alleviated foliar disease caused by Alternaria alternata on cucumber, reduced reactive oxygen species accumulation, and enhanced the activities of antioxidant enfzymes related to ROS scavenging under A. alternata stress [73]. Tomato-root-colonizing Streptomyces strains R7 and F8 inhibited R. solani infection under greenhouse conditions and enhanced the expression of PAL1 and LOXB genes of tomatoes, especially upon pathogen inoculation [72]. Lee et al. [110] showed how plant protection by Streptomyces sp. JCK-6131 takes place via two mechanisms: antibiosis with antimicrobial compounds, streptothricins, and priming. JCK-6131 treatment induced the expression of pathogenesis-related protein genes, suggesting the simultaneous activation of the salicylate and jasmonate signaling pathways. The induction of plant resistance against tobacco mosaic virus infection by S. cellulosae was indicated by the work of Abo-Zaid et al. [111], with a significant increase in the phenylalanine ammonia lyase, chalcone synthase, and pathogenesis-related protein transcripts. Again, the simultaneous activation of the salicylate and jasmonate signaling pathways took place. Finally, Vergnes et al. [112] inoculated Streptomyces sp. AgN23 on Arabidopsis leaves, which resulted in resistance against the Alternaria brassicicola infection of the leaves. The activation of Arabidopsis defense responses by AgN23-induced resistance was partially compromised in salicylate, jasmonate, and ethylene mutants. In conclusion, these insights into the mechanisms of priming by Actinobacteria suggest a capacity to activate plant defense responses in the absence of a pathogen. The common determinants of priming seem to be eliciting both JA/ET- and SA-related signaling, commonly associated with enhanced PR protein and plant secondary metabolism levels. One interesting open question is whether the plant-associated microbiomes modulate the priming process, as their community compositions do change upon the introduction of Actinobacteria to the rhizosphere [73]. According to the studies mentioned above, Actinobacteria can trigger both the SA and JA/ET pathways in plants. That the plant response to the biocontrol agents so commonly leads to the partial elicitation of defense pathways in the absence of the pathogen is intriguing and calls for further investigations into the mechanisms behind Actinobacteria-based priming.
4. Enrichment of Actinobacteria during the Establishment of Suppressive Soils, Pathogen Attacks and Abiotic Stress: A Sign of Their Central Role in Plant Protection?
Amplicon sequencing studies have repeatedly indicated that Actinobacteria in soil and plant microbiomes are associated with the suppression of plant disease and the induction of abiotic stress tolerance. We expect that a greater understanding of the mechanisms that lead to higher abundances of plant-protective Actinobacteria can be used to support plant production [23,110]. There is potential for this idea, since, as described in previous parts of this review, basic knowledge of disease suppression by Actinobacteria is established and plants are capable of building up beneficial rhizosphere communities and inducing disease-suppressive soils [113,114]. Plants accomplish these tasks by modulating their root exudation patterns to support the recruitment of beneficial microorganisms [115,116]. Increasing evidence from amplicon sequencing studies suggests that Actinobacteria form an important part of disease-suppressive microbial consortia [117,118]. For instance, the relative abundance of members of Streptomyces, Gaiella, and Microbacterium increase in suppressive soils [118,119], implying their potential beneficial effects on disease control. Other studies have shown that disease-induced changes in plant microbiome assembly also include the enrichment of, e.g., Streptomyces and Microbacterium species [120], that serve as so-called network hubs with strong interactions with several other taxa in co-occurrence analyses. This suggests that the recruitment of Actinobacteria by plants is one means to ensure the survival of the plant until the next generation [118]. Interestingly, bacterial community analyses also suggest an important role for Actinobacteria as a central phylum of bacteria in plant rhizospheres and endospheres that support plant drought tolerance [121]. Studies on bacterial community responses to drought indicate a central role for Actinobacteria, especially Streptomycetes, in the abiotic stress resistance of plants [122]. A study of the root bacteria of sorghum [123], as well as a survey of thirty different plant species [124], revealed an increase in the relative abundance of sequences affiliated with Actinobacteria in root endosphere communities upon drought. An important mechanism how streptomycetes support the growth of plants during stress is by suppressing ethylene emissions with ACC deaminase activity [125], and Gebauer et al. [126] showed that Actinobacteria strongly contribute to the ACC-deaminase-carrying bacterial community, in particular during water deficits. Thus, although the community composition research on suppressive soil, plant disease and drought tolerance-associated microbiomes does not prove that the enriched Actinobacterial genera are responsible for plant-beneficial activities, they have been largely implicated as the agents responsible for these traits. Community sequencing has strongly contributed to the existing knowledge on Actinobacteria in the rhizospheres and endospheres of plants, as well as their relations in plant microbiomes. We think that reconstructions of soil microbial structures by pathogen pressure or abiotic stress are promising means of how biocontrol and plant-stress-attenuating Actinobacteria can be enriched in future applications. In this context, omics techniques such as metatranscriptomics could be used to tackle their potential activities, e.g., if they may produce antagonistic compounds against pathogens, elicit plant immunity responses, or synthesize plant growth stimulators.
5. Conclusions
The application of microbial biocontrol agents for disease control through the induction of resistance or priming relies on complex consecutive events including the successful establishment of biocontrol agent on the host, the release of specific elicitors that are recognized by the specific receptors of plants, and signaling. Defense priming by Actinobacteria has great potential as a successful strategy for modern plant protection, and the mechanisms behind it involve JA/ET- and SA-mediated signaling. The production of defense compounds often already occurs in the absence of a pathogen, but it is enhanced by its presence. Optimally, antibiosis and the production of lytic enzymes of an Actinobacteria biocontrol strain should be combined with the priming activity of the same strain or another member of a synthetic community. According to plant microbiome studies, the application of stress, the enrichment of plant-protective actinobacterial consortia, and higher numbers of potentially plant-beneficial bacteria may constitute novel and promising avenues for improving plant disease resistance. Amplicon and metagenome and metatranscriptome sequencing will increase the existing knowledge on Actinobacteria during rhizosphere colonization and interactions between these bacteria and other microbial communities in the rhizosphere, as well as create new information on their potential for the production of antagonistic secondary metabolites and priming effectors. As another important issue, further studies are needed on actinobacterial bioinoculant formulation using different additives, carriers, and various methods of inoculation in the field conditions to develop effective commercial products. Ideally, bioinoculants will also promote plant growth in the absence of pathogen pressure, and to reach this goal, future work should combine biocontrol and biofertilizer activity analyses.
Acknowledgments
The authors would like to thank their current and earlier laboratory members.
Author Contributions
Conceptualization, M.E.-Z., R.S.R. and M.T.T.; writing—original draft preparation, M.E.-Z. and R.S.R.; writing—review and editing, M.T.T. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Current grants 403641192 and 466312020 of the German Science Foundation (M.T.T.).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singh B., Trivedi P., Singh S., Macdonald C., Verma J. Emerging microbiome technologies for sustainable increase in farm productivity and environmental security. Microbiol. Aust. 2018;39:17–23. doi: 10.1071/MA18006. [DOI] [Google Scholar]
- 2.Nega A. Review on concepts in biological control of plant pathogens. J. Biol. Agric. Health. 2014;4:33–54. [Google Scholar]
- 3.Jamali F., Sharifi-Tehrani A., Okhovvat M., Zakeri Z., Saberi-Riseh R. Biological control of chickpea Fusarium wilt by antagonistic bacteria under greenhouse condition. Commun. Agric. Appl. Biol. Sci. 2004;69:649–651. [PubMed] [Google Scholar]
- 4.Moradi Pour M., Saberi-Riseh R., Mohammadinejad R., Hosseini A. Investigating the formulation of alginate-gelatin encapsulated Pseudomonas fluorescens (VUPF5 and T17-4 strains) for controlling Fusarium solani on potato. Int. J. Biol. Macromol. 2019;133:603–613. doi: 10.1016/j.ijbiomac.2019.04.071. [DOI] [PubMed] [Google Scholar]
- 5.Fathi F., Saberi-Riseh R., Khodaygan P. Survivability and controlled release of alginate-microencapsulated Pseudomonas fluorescens VUPF506 and their effects on biocontrol of Rhizoctonia solani on potato. Int. J. Biol. Macromol. 2021;183:627–634. doi: 10.1016/j.ijbiomac.2021.04.159. [DOI] [PubMed] [Google Scholar]
- 6.Saberi-Riseh R., Hajieghrari B., Rouhani H., Sharifi-Tehrani A. Effects of inoculum density and substrate type on saprophytic survival of Phytophthora drechsleri, the causal agent of gummosis (crown and root rot) on pistachio in Rafsanjan, Iran. Commun. Agric. Appl. Biol. Sci. 2004;69:653–656. [PubMed] [Google Scholar]
- 7.Saberi Riseh R., Skorik Y.A., Thakur V.K., Moradi Pour M., Tamanadar E., Noghabi S.S. Encapsulation of plant biocontrol bacteria with alginate as a main polymer material. Int. J. Mol. Sci. 2021;22:11165. doi: 10.3390/ijms222011165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saberi Riseh R., Javan-Nikkhah M., Heidarian R., Hosseini S., Soleimani P. Detection of fungal infectous agent of wheat grains in store-pits of Markazi province, Iran. Commun. Agric. Appl. Biol. Sci. 2004;69:541–544. [PubMed] [Google Scholar]
- 9.Morales-Cedeño L.R., Orozco-Mosqueda M.d.C., Loeza-Lara P.D., Parra-Cota F.I., de los Santos-Villalobos S., Santoyo G. Plant growth-promoting bacterial endophytes as biocontrol agents of pre- and post-harvest diseases: Fundamentals, methods of application and future perspectives. Microbiol. Res. 2021;242:126612. doi: 10.1016/j.micres.2020.126612. [DOI] [PubMed] [Google Scholar]
- 10.Ludwig W., Euzéby J., Schumann P., Busse H.-J., Trujillo M., Kämpfer P., Whitman W. Road Map of the Phylum Actinobacteria. In: Goodfellow M., Kämpfer P., Busse H.-J., Trujillo M.E., Suzuki K., Ludwig W., Whitman W.B., editors. Bergey’s Manual of Systematic Bacteriology. 2nd ed. Springer Nature; Cham, Switzerland: 2012. pp. 1–28. [Google Scholar]
- 11.Palaniyandi S.A., Yang S.H., Zhang L., Suh J.-W. Effects of actinobacteria on plant disease suppression and growth promotion. Appl. Microbiol. Biotechnol. 2013;97:9621–9636. doi: 10.1007/s00253-013-5206-1. [DOI] [PubMed] [Google Scholar]
- 12.Viaene T., Langendries S., Beirinckx S., Maes M., Goormachtig S. Streptomyces as a plant’s best friend? FEMS Microbiol. Ecol. 2016;92:fiw119. doi: 10.1093/femsec/fiw119. [DOI] [PubMed] [Google Scholar]
- 13.Khamna S., Yokota A., Peberdy J., Lumyong S. Indole3-acetic acid production by Streptomyces sp. isolated from some Thai medicinal plant rhizosphere soils. EurAsian J. Biosci. 2010;4:23–32. doi: 10.5053/ejobios.2010.4.0.4. [DOI] [Google Scholar]
- 14.Chukwuneme C.F., Babalola O.O., Kutu F.R., Ojuederie O.B. Characterization of actinomycetes isolates for plant growth promoting traits and their effects on drought tolerance in maize. J. Plant Interact. 2020;15:93–105. doi: 10.1080/17429145.2020.1752833. [DOI] [Google Scholar]
- 15.Yamaura M., Uchiumi T., Higashi S., Abe M., Kucho K.-I. Identification by suppression subtractive hybridization of Frankia genes induced under nitrogen-fixing conditions. Appl. Environ. Microbiol. 2010;76:1692–1694. doi: 10.1128/AEM.01813-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hamdali H., Hafidi M., Virolle M.J., Ouhdouch Y. Rock phosphate-solubilizing Actinomycetes: Screening for plant growth-promoting activities. World J. Microbiol. Biotechnol. 2008;24:2565–2575. doi: 10.1007/s11274-008-9817-0. [DOI] [Google Scholar]
- 17.Oliveira C.A., Alves V.M.C., Marriel I.E., Gomes E.A., Scotti M.R., Carneiro N.P., Guimarães C.T., Schaffert R.E., Sá N.M.H. Phosphate solubilizing microorganisms isolated from rhizosphere of maize cultivated in an oxisol of the Brazilian Cerrado Biome. Soil Biol. Biochem. 2009;41:1782–1787. doi: 10.1016/j.soilbio.2008.01.012. [DOI] [Google Scholar]
- 18.Franco-Correa M., Quintana A., Duque C., Suarez C., Rodríguez M.X., Barea J.-M. Evaluation of actinomycete strains for key traits related with plant growth promotion and mycorrhiza helping activities. Appl. Soil Ecol. 2010;45:209–217. doi: 10.1016/j.apsoil.2010.04.007. [DOI] [Google Scholar]
- 19.Boubekri K., Soumare A., Mardad I., Lyamlouli K., Hafidi M., Ouhdouch Y., Kouisni L. The screening of potassium- and phosphate-solubilizing Actinobacteria and the assessment of their ability to promote wheat growth parameters. Microorganisms. 2021;9:470. doi: 10.3390/microorganisms9030470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Solans M. Discaria trinervis—Frankia symbiosis promotion by saprophytic actinomycetes. J. Basic Microbiol. 2007;47:243–250. doi: 10.1002/jobm.200610244. [DOI] [PubMed] [Google Scholar]
- 21.Riedlinger J., Schrey S.D., Tarkka M.T., Hampp R., Kapur M., Fiedler H.P. Auxofuran, a novel metabolite that stimulates the growth of fly agaric, is produced by the mycorrhiza helper bacterium Streptomyces strain AcH 505. Appl. Environ. Microbiol. 2006;72:3550–3557. doi: 10.1128/AEM.72.5.3550-3557.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goudjal Y., Toumatia O., Yekkour A., Sabaou N., Mathieu F., Zitouni A. Biocontrol of Rhizoctonia solani damping-off and promotion of tomato plant growth by endophytic actinomycetes isolated from native plants of Algerian Sahara. Microbiol. Res. 2014;169:59–65. doi: 10.1016/j.micres.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 23.Newitt J.T., Prudence S.M.M., Hutchings M.I., Worsley S.F. Biocontrol of cereal crop diseases using Streptomycetes. Pathogens. 2019;8:78. doi: 10.3390/pathogens8020078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Van Wees S.C., Van der Ent S., Pieterse C.M. Plant immune responses triggered by beneficial microbes. Curr. Opin. Plant Biol. 2008;11:443–448. doi: 10.1016/j.pbi.2008.05.005. [DOI] [PubMed] [Google Scholar]
- 25.De Vleesschauwer D., Djavaheri M., Bakker P.A.H.M., Höfte M. Pseudomonas fluorescens WCS374r-induced systemic resistance in rice against Magnaporthe oryzae is based on pseudobactin-mediated priming for a salicylic acid-repressible multifaceted defense response. Plant Physiol. 2008;148:1996–2012. doi: 10.1104/pp.108.127878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pieterse C.M.J., van Wees S.C.M., van Pelt J.A., Knoester M., Laan R., Gerrits H., Weisbeek P.J., van Loon L.C. A novel signaling pathway controlling induced systemic resistance in Arabidopsis. Plant Cell. 1998;10:1571–1580. doi: 10.1105/tpc.10.9.1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Conrath U., Beckers G.J.M., Langenbach C.J.G., Jaskiewicz M.R. Priming for enhanced defense. Annu. Rev. Phytopathol. 2015;53:97–119. doi: 10.1146/annurev-phyto-080614-120132. [DOI] [PubMed] [Google Scholar]
- 28.Mauch-Mani B., Baccelli I., Luna Diez E., Flors V. Defense priming: An adaptive part of induced resistance. Annu. Rev. Plant Biol. 2017;68:485–512. doi: 10.1146/annurev-arplant-042916-041132. [DOI] [PubMed] [Google Scholar]
- 29.Conn V.M., Walker A.R., Franco C.M. Endophytic actinobacteria induce defense pathways in Arabidopsis thaliana. Mol. Plant Microbe Interact. 2008;21:208–218. doi: 10.1094/MPMI-21-2-0208. [DOI] [PubMed] [Google Scholar]
- 30.Cheng J., Yang S.H., Palaniyandi S.A., Han J.S., Yoon T.-M., Kim T.-J., Suh J.-W. Azalomycin F complex is an antifungal substance produced by Streptomyces malaysiensis MJM1968 isolated from agricultural soil. J. Korean Soc. Appl. Biol. Chem. 2010;53:545–552. doi: 10.3839/jksabc.2010.084. [DOI] [Google Scholar]
- 31.Meschke H., Walter S., Schrempf H. Characterization and localization of prodiginines from Streptomyces lividans suppressing Verticillium dahliae in the absence or presence of Arabidopsis thaliana. Environ. Microbiol. 2012;14:940–952. doi: 10.1111/j.1462-2920.2011.02665.x. [DOI] [PubMed] [Google Scholar]
- 32.Rungin S., Indananda C., Suttiviriya P., Kruasuwan W., Jaemsaeng R., Thamchaipenet A. Plant growth enhancing effects by a siderophore-producing endophytic streptomycete isolated from a Thai jasmine rice plant (Oryza sativa L. cv. KDML105) Antonie Leeuwenhoek. 2012;102:463–472. doi: 10.1007/s10482-012-9778-z. [DOI] [PubMed] [Google Scholar]
- 33.Aznar A., Dellagi A. New insights into the role of siderophores as triggers of plant immunity: What can we learn from animals? J. Exp. Bot. 2015;66:3001–3010. doi: 10.1093/jxb/erv155. [DOI] [PubMed] [Google Scholar]
- 34.Sadeghi A., Koobaz P., Azimi H., Karimi E., Akbari A. Plant growth promotion and suppression of Phytophthora drechsleri damping-off in cucumber by cellulase-producing Streptomyces. BioControl. 2017;62:805–819. doi: 10.1007/s10526-017-9838-4. [DOI] [Google Scholar]
- 35.El-Shatoury S., Elkraly O., El Kazzaz W., Dewedar A. Antimicrobial activities of actinomycetes inhabiting Achillea fragrantissima (Family: Compositae) Egypt. J. Nat. Toxins. 2009;6:1–15. [Google Scholar]
- 36.Dimkpa C.O., Svatos A., Dabrowska P., Schmidt A., Boland W., Kothe E. Involvement of siderophores in the reduction of metal-induced inhibition of auxin synthesis in Streptomyces spp. Chemosphere. 2008;74:19–25. doi: 10.1016/j.chemosphere.2008.09.079. [DOI] [PubMed] [Google Scholar]
- 37.Jog R., Nareshkumar G., Rajkumar S. Enhancing Soil Health and Plant Growth Promotion by Actinomycetes. In: Subramaniam G., Arumugam S., Rajendran V., editors. Plant Growth Promoting Actinobacteria: A New Avenue for Enhancing the Productivity and Soil Fertility of Grain Legumes. Springer; Singapore: 2016. pp. 33–45. [Google Scholar]
- 38.Lee S.Y., Tindwa H., Lee Y.S., Naing K.W., Hong S.H., Nam Y., Kim K.Y. Biocontrol of anthracnose in pepper using chitinase, beta-1,3 glucanase, and 2-furancarboxaldehyde produced by Streptomyces cavourensis SY224. J. Microbiol. Biotechnol. 2012;22:1359–1366. doi: 10.4014/jmb.1203.02056. [DOI] [PubMed] [Google Scholar]
- 39.Joo G.J. Purification and characterization of an extracellular chitinase from the antifungal biocontrol agent Streptomyces halstedii. Biotechnol. Lett. 2005;27:1483–1486. doi: 10.1007/s10529-005-1315-y. [DOI] [PubMed] [Google Scholar]
- 40.Gherbawy Y., Elhariry H., Altalhi A., El-Deeb B., Khiralla G. Molecular screening of Streptomyces isolates for antifungal activity and family 19 chitinase enzymes. J. Microbiol. 2012;50:459–468. doi: 10.1007/s12275-012-2095-4. [DOI] [PubMed] [Google Scholar]
- 41.El-Tarabily K.A., Nassar A.H., Hardy G.E., Sivasithamparam K. Plant growth promotion and biological control of Pythium aphanidermatum, a pathogen of cucumber, by endophytic actinomycetes. J. Appl. Microbiol. 2009;106:13–26. doi: 10.1111/j.1365-2672.2008.03926.x. [DOI] [PubMed] [Google Scholar]
- 42.Nagpure A., Choudhary B., Kumar S., Gupta R.K. Isolation and characterization of chitinolytic Streptomyces sp. MT7 and its antagonism towards wood-rotting fungi. Ann. Microbiol. 2014;64:531–541. doi: 10.1007/s13213-013-0686-x. [DOI] [Google Scholar]
- 43.Gopalakrishnan S., Pande S., Sharma M., Humayun P., Kiran B.K., Sandeep D., Vidya M.S., Deepthi K., Rupela O. Evaluation of actinomycete isolates obtained from herbal vermicompost for the biological control of Fusarium wilt of chickpea. Crop Prot. 2011;30:1070–1078. doi: 10.1016/j.cropro.2011.03.006. [DOI] [Google Scholar]
- 44.Kamil F.H., Saeed E.E., El-Tarabily K.A., AbuQamar S.F. Biological control of mango dieback disease caused by Lasiodiplodia theobromae using Streptomycete and Non-streptomycete Actinobacteria in the United Arab Emirates. Front. Microbiol. 2018;9:829. doi: 10.3389/fmicb.2018.00829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhou Z., Wu X., Li J., Zhang Y., Huang Y., Zhang W., Shi Y., Wang J., Chen S. A novel quorum quencher, Rhodococcus pyridinivorans XN-36, is a powerful agent for the biocontrol of soft rot disease in various host plants. Biol. Control. 2022;169:104889. doi: 10.1016/j.biocontrol.2022.104889. [DOI] [Google Scholar]
- 46.Park S.Y., Lee S.J., Oh T.K., Oh J.W., Koo B.T., Yum D.Y., Lee J.K. AhlD, an N-acylhomoserine lactonase in Arthrobacter sp., and predicted homologues in other bacteria. Microbiology. 2003;149:1541–1550. doi: 10.1099/mic.0.26269-0. [DOI] [PubMed] [Google Scholar]
- 47.Citron C.A., Barra L., Wink J., Dickschat J.S. Volatiles from nineteen recently genome sequenced actinomycetes. Org. Biomol. Chem. 2015;13:2673–2683. doi: 10.1039/C4OB02609H. [DOI] [PubMed] [Google Scholar]
- 48.Cordovez V., Carrion V.J., Etalo D.W., Mumm R., Zhu H., van Wezel G.P., Raaijmakers J.M. Diversity and functions of volatile organic compounds produced by Streptomyces from a disease-suppressive soil. Front. Microbiol. 2015;6:1081. doi: 10.3389/fmicb.2015.01081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schmidt R., Cordovez V., de Boer W., Raaijmakers J., Garbeva P. Volatile affairs in microbial interactions. ISME J. 2015;9:2329–2335. doi: 10.1038/ismej.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wan M., Li G., Zhang J., Jiang D., Huang H.-C. Effect of volatile substances of Streptomyces platensis F-1 on control of plant fungal diseases. Biol. Control. 2008;46:552–559. doi: 10.1016/j.biocontrol.2008.05.015. [DOI] [Google Scholar]
- 51.Wonglom P., Suwannarach N., Lumyong S., Ito S.-i., Matsui K., Sunpapao A. Streptomyces angustmyceticus NR8-2 as a potential microorganism for the biological control of leaf spots of Brassica rapa subsp. pekinensis caused by Colletotrichum sp. and Curvularia lunata. Biol. Control. 2019;138:104046. doi: 10.1016/j.biocontrol.2019.104046. [DOI] [Google Scholar]
- 52.Joo G.-J. Production of an anti-fungal substance for biological control of Phytophthora capsici causing phytophthora blight in red-peppers by Streptomyces halstedii. Biotechnol. Lett. 2005;27:201–205. doi: 10.1007/s10529-004-7879-0. [DOI] [PubMed] [Google Scholar]
- 53.Anitha A., Rabeeth M. Control of Fusarium wilt of tomato by bioformulation of Streptomyces griseus in green house condition. Afr. J. Basic Appl. Sci. 2009;1:9–14. [Google Scholar]
- 54.Sadeghi A., Hessan A.R., Askari H., Aghighi S., Shahidi Bonjar G.H. Biological control potential of two Streptomyces isolates on Rhizoctonia solani, the causal agent of damping-off of sugar beet. Pak. J. Biol. Sci. 2006;9:904–910. doi: 10.3923/pjbs.2006.904.910. [DOI] [Google Scholar]
- 55.Shimizu M., Yazawa S., Ushijima Y. A promising strain of endophytic Streptomyces sp. for biological control of cucumber anthracnose. J. Gen. Plant Pathol. 2009;75:27–36. doi: 10.1007/s10327-008-0138-9. [DOI] [Google Scholar]
- 56.Harikrishnan H., Shanmugaiah V., Balasubramanian N., Sharma M.P., Kotchoni S.O. Antagonistic potential of native strain Streptomyces aurantiogriseus VSMGT1014 against sheath blight of rice disease. World J. Microbiol. Biotechnol. 2014;30:3149–3161. doi: 10.1007/s11274-014-1742-9. [DOI] [PubMed] [Google Scholar]
- 57.Errakhi R., Bouteau F., Lebrihi A., Barakate M. Evidences of biological control capacities of Streptomyces spp. against Sclerotium rolfsii responsible for damping-off disease in sugar beet (Beta vulgaris L.) World J. Microbiol. Biotechnol. 2007;23:1503–1509. doi: 10.1007/s11274-007-9394-7. [DOI] [Google Scholar]
- 58.Aallam Y., Dhiba D., El Rasafi T., Lemriss S., Haddioui A., Tarkka M., Hamdali H. Growth promotion and protection against root rot of sugar beet (Beta vulgaris L.) by two rock phosphate and potassium solubilizing Streptomyces spp. under greenhouse conditions. Plant Soil. 2022;472:407–420. doi: 10.1007/s11104-021-05252-w. [DOI] [Google Scholar]
- 59.Getha K., Vikineswary S., Wong W.H., Seki T., Ward A., Goodfellow M. Evaluation of Streptomyces sp. strain g10 for suppression of Fusarium wilt and rhizosphere colonization in pot-grown banana plantlets. J. Ind. Microbiol. Biotech. 2005;32:24–32. doi: 10.1007/s10295-004-0199-5. [DOI] [PubMed] [Google Scholar]
- 60.Zhang L., Liu Z., Wang Y., Zhang J., Wan S., Huang Y., Yun T., Xie J., Wang W. Biocontrol potential of endophytic Streptomyces malaysiensis 8ZJF-21 from medicinal plant against banana Fusarium wilt caused by Fusarium oxysporum f.sp. cubense tropical race 4. Front. Plant Sci. 2022;13:874819. doi: 10.3389/fpls.2022.874819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Yadav A.K., Yandigeri M.S., Vardhan S., Sivakumar G., Rangeshwaran R., Tripathi C.P.M. Streptomyces sp. S160: A potential antagonist against chickpea charcoal root rot caused by Macrophomina phaseolina (Tassi) Goid. Ann. Microbiol. 2014;64:1113–1122. doi: 10.1007/s13213-013-0750-6. [DOI] [Google Scholar]
- 62.Sadeghian M., Shahidi Bonjar G.H., Sharifi Sirchi G.R. Post harvest biological control of apple bitter rot by soil-borne Actinomycetes and molecular identification of the active antagonist. Postharvest Biol. Technol. 2016;112:46–54. doi: 10.1016/j.postharvbio.2015.09.035. [DOI] [Google Scholar]
- 63.Haggag W.M., Singer S.M., Aly M.D.E.H. Application of broad-spectrum of marine Streptomyces albidoflavus as biofungicide and plant growth promoting of tomato diseases. Res. J. Pharm. Biol. Chem. Sci. 2014;5:142–148. [Google Scholar]
- 64.Kim H., Lee E., Park S., Lee H.-S., Chung N. Biological control of anthracnose (Colletotrichum gloeosporioides) in pepper and cherry tomato by Streptomyces sp. A1022. J. Agric. Sci. 2014;6:54. doi: 10.5539/jas.v6n2p54. [DOI] [Google Scholar]
- 65.Torabi A., Shahidi Bonjar G.H., Abdolshahi R., Pournamdari M., Saadoun I., Barka E.A. Biological control of Paecilomyces formosus, the causal agent of dieback and canker diseases of pistachio by two strains of Streptomyces misionensis. Biol. Control. 2019;137:104029. doi: 10.1016/j.biocontrol.2019.104029. [DOI] [Google Scholar]
- 66.Li Q., Jiang Y., Ning P., Zheng L., Huang J., Li G., Jiang D., Hsiang T. Suppression of Magnaporthe oryzae by culture filtrates of Streptomyces globisporus JK-1. Biol. Control. 2011;58:139–148. doi: 10.1016/j.biocontrol.2011.04.013. [DOI] [Google Scholar]
- 67.Toumatia O., Compant S., Yekkour A., Yacine G., Sabaou N., Mathieu F., Sessitsch A., Zitouni A. Biocontrol and plant growth promoting properties of Streptomyces mutabilis strain IA1 isolated from a Saharan soil on wheat seedlings and visualization of its niches of colonization. S. Afr. J. Bot. 2016;105:234–239. doi: 10.1016/j.sajb.2016.03.020. [DOI] [Google Scholar]
- 68.Martínez-Hidalgo P., García J.M., Pozo M.J. Induced systemic resistance against Botrytis cinerea by Micromonospora strains isolated from root nodules. Front. Microbiol. 2015;6:922. doi: 10.3389/fmicb.2015.00922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saeed E.E., Sham A., Salmin Z., Abdelmowla Y., Iratni R., El-Tarabily K., AbuQamar S. Streptomyces globosus UAE1, a potential effective biocontrol agent for black scorch disease in date palm plantations. Front. Microbiol. 2017;8:1455. doi: 10.3389/fmicb.2017.01455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Suárez-Moreno Z.R., Vinchira-Villarraga D.M., Vergara-Morales D.I., Castellanos L., Ramos F.A., Guarnaccia C., Degrassi G., Venturi V., Moreno-Sarmiento N. Plant-growth promotion and biocontrol properties of three Streptomyces spp. isolates to control bacterial rice pathogens. Front. Microbiol. 2019;10:290. doi: 10.3389/fmicb.2019.00290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Cao P., Li C., Wang H., Yu Z., Xu X., Wang X., Zhao J., Xiang W. Community structures and antifungal activity of root-associated endophytic Actinobacteria in healthy and diseased cucumber plants and Streptomyces sp. HAAG3-15 as a promising biocontrol agent. Microorganisms. 2020;8:236. doi: 10.3390/microorganisms8020236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ebrahimi-Zarandi M., Bonjar G.H., Riseh R.S., El-Shetehy M., Saadoun I., Barka E.A. Exploring two Streptomyces species to control Rhizoctonia solani in tomato. Agronomy. 2021;11:1384. doi: 10.3390/agronomy11071384. [DOI] [Google Scholar]
- 73.Wang M., Xue J., Ma J., Feng X., Ying H., Xu H. Streptomyces lydicus M01 regulates soil microbial community and alleviates foliar disease caused by Alternaria alternata on cucumbers. Front. Microbiol. 2020;11:942. doi: 10.3389/fmicb.2020.00942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Saberi Riseh R., Moradi Pour M., Ait Barka E. A novel route for double-layered encapsulation of Streptomyces fulvissimus Uts22 by alginate and arabic gum for controlling of Pythium aphanidermatum in cucumber. Agronomy. 2022;12:655. doi: 10.3390/agronomy12030655. [DOI] [Google Scholar]
- 75.Saberi-Riseh R., Moradi-Pour M. A novel encapsulation of Streptomyces fulvissimus Uts22 by spray drying and its biocontrol efficiency against Gaeumannomyces graminis, the causal agent of take-all disease in wheat. Pest Manag. Sci. 2021;77:4357–4364. doi: 10.1002/ps.6469. [DOI] [PubMed] [Google Scholar]
- 76.Padilla-Gálvez N., Luengo-Uribe P., Mancilla S., Maurin A., Torres C., Ruiz P., France A., Bravo I., Urrutia H. Antagonistic activity of endophytic actinobacteria from native potatoes (Solanum tuberosum subsp. tuberosum L.) against Pectobacterium carotovorum subsp. carotovorum and Pectobacterium atrosepticum. BMC Microbiol. 2021;21:335. doi: 10.1186/s12866-021-02393-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sarwar A., Latif Z., Zhang S., Hao J., Bechthold A. A potential biocontrol agent Streptomyces violaceusniger AC12AB for managing potato common scab. Front. Microbiol. 2019;10:202. doi: 10.3389/fmicb.2019.00202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Le K.D., Yu N.H., Park A.R., Park D.J., Kim C.J., Kim J.C. Streptomyces sp. AN090126 as a biocontrol agent against bacterial and fungal plant diseases. Microorganisms. 2022;10:791. doi: 10.3390/microorganisms10040791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sabaratnam S., Traquair J.A. Formulation of a Streptomyces biocontrol agent for the suppression of Rhizoctonia damping-off in tomato transplants. Biol. Control. 2002;23:245–253. doi: 10.1006/bcon.2001.1014. [DOI] [Google Scholar]
- 80.Copping L.G., Duke S.O. Natural products that have been used commercially as crop protection agents. Pest Manag. Sci. 2007;63:524–554. doi: 10.1002/ps.1378. [DOI] [PubMed] [Google Scholar]
- 81.Saxena S., Pandey A.K. Microbial metabolites as eco-friendly agrochemicals for the next millennium. Appl. Microbiol. Biotechnol. 2001;55:395–403. doi: 10.1007/s002530000517. [DOI] [PubMed] [Google Scholar]
- 82.Kabaluk J.T., Svircev A.M., Goettel M.S., Woo S.G. The Use and Regulation of Microbial Pesticides in Representative Jurisdiction Worldwide. IOBC Global; Hong Kong, China: 2010. p. 99. [Google Scholar]
- 83.Aggarwal N., Thind S.K., Sharma S. Role of Secondary Metabolites of Actinomycetes in Crop Protection. In: Subramaniam G., Arumugam S., Rajendran V., editors. Plant Growth Promoting Actinobacteria: A New Avenue for Enhancing the Productivity and Soil Fertility of Grain Legumes. Springer; Singapore: 2016. pp. 99–121. [Google Scholar]
- 84.Bailey K.L., Falk S.P. Turning research on microbial bioherbicides into commercial products a Phoma Story. Pest Technol. 2011;5:73–79. [Google Scholar]
- 85.Raymaekers K., Ponet L., Holtappels D., Berckmans B., Cammue B.P.A. Screening for novel biocontrol agents applicable in plant disease management—A review. Biol. Control. 2020;144:104240. doi: 10.1016/j.biocontrol.2020.104240. [DOI] [Google Scholar]
- 86.Bashan Y., de-Bashan L.E., Prabhu S.R., Hernandez J.-P. Advances in plant growth-promoting bacterial inoculant technology: Formulations and practical perspectives (1998–2013) Plant Soil. 2014;378:1–33. doi: 10.1007/s11104-013-1956-x. [DOI] [Google Scholar]
- 87.Kloepper J.W., Ryu C.M., Zhang S. Induced systemic resistance and promotion of plant growth by Bacillus spp. Phytopathology. 2004;94:1259–1266. doi: 10.1094/PHYTO.2004.94.11.1259. [DOI] [PubMed] [Google Scholar]
- 88.De Vleesschauwer D., Höfte M. Advances in Botanical Research. Vol. 51. Academic Press; Cambridge, MA, USA: 2009. Rhizobacteria-Induced Systemic Resistance; pp. 223–281. [Google Scholar]
- 89.Ryu C.M., Farag M.A., Hu C.H., Reddy M.S., Kloepper J.W., Paré P.W. Bacterial volatiles induce systemic resistance in Arabidopsis. Plant Physiol. 2004;134:1017–1026. doi: 10.1104/pp.103.026583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Meziane H., van der Sluis I., van Loon L.C., Höfte M., Bakker P.A.H.M. Determinants of Pseudomonas putida WCS358 involved in inducing systemic resistance in plants. Mol. Plant Pathol. 2005;6:177–185. doi: 10.1111/j.1364-3703.2005.00276.x. [DOI] [PubMed] [Google Scholar]
- 91.van Loon L.C., Bakker P.A.H.M., van der Heijdt W.H.W., Wendehenne D., Pugin A. Early responses of tobacco suspension cells to rhizobacterial elicitors of induced systemic resistance. Mol. Plant Microbe Interact. 2008;21:1609–1621. doi: 10.1094/MPMI-21-12-1609. [DOI] [PubMed] [Google Scholar]
- 92.Verhagen B.W., Trotel-Aziz P., Couderchet M., Höfte M., Aziz A. Pseudomonas spp. induced systemic resistance to Botrytis cinerea is associated with induction and priming of defence responses in grapevine. J. Exp. Bot. 2010;61:249–260. doi: 10.1093/jxb/erp295. [DOI] [PubMed] [Google Scholar]
- 93.Jones J.D.G., Dangl J.L. The plant immune system. Nature. 2006;444:323–329. doi: 10.1038/nature05286. [DOI] [PubMed] [Google Scholar]
- 94.Tjamos S.E., Flemetakis E., Paplomatas E.J., Katinakis P. Induction of resistance to Verticillium dahliae in Arabidopsis thaliana by the biocontrol agent K-165 and pathogenesis-related proteins gene expression. Mol. Plant Microbe Interact. 2005;18:555–561. doi: 10.1094/MPMI-18-0555. [DOI] [PubMed] [Google Scholar]
- 95.van de Mortel J.E., de Vos R.C., Dekkers E., Pineda A., Guillod L., Bouwmeester K., van Loon J.J., Dicke M., Raaijmakers J.M. Metabolic and transcriptomic changes induced in Arabidopsis by the rhizobacterium Pseudomonas fluorescens SS101. Plant Physiol. 2012;160:2173–2188. doi: 10.1104/pp.112.207324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Audenaert K., Pattery T., Cornelis P., Höfte M. Induction of systemic resistance to Botrytis cinerea in tomato by Pseudomonas aeruginosa 7NSK2: Role of salicylic acid, pyochelin, and pyocyanin. Mol. Plant Microbe Interact. 2002;15:1147–1156. doi: 10.1094/MPMI.2002.15.11.1147. [DOI] [PubMed] [Google Scholar]
- 97.Zhao S., Du C.M., Tian C.Y. Suppression of Fusarium oxysporum and induced resistance of plants involved in the biocontrol of cucumber Fusarium wilt by Streptomyces bikiniensis HD-087. World J. Microbiol. Biotechnol. 2012;28:2919–2927. doi: 10.1007/s11274-012-1102-6. [DOI] [PubMed] [Google Scholar]
- 98.Baz M., Tran D., Kettani-Halabi M., Samri S.E., Jamjari A., Biligui B., Meimoun P., El-Maarouf-Bouteau H., Garmier M., Saindrenan P., et al. Calcium- and ROS-mediated defence responses in BY2 tobacco cells by nonpathogenic Streptomyces sp. J. Appl. Microbiol. 2012;112:782–792. doi: 10.1111/j.1365-2672.2012.05248.x. [DOI] [PubMed] [Google Scholar]
- 99.Patil H.J., Srivastava A.K., Singh D.P., Chaudhari B.L., Arora D.K. Actinomycetes mediated biochemical responses in tomato (Solanum lycopersicum) enhances bioprotection against Rhizoctonia solani. Crop Prot. 2011;30:1269–1273. doi: 10.1016/j.cropro.2011.04.008. [DOI] [Google Scholar]
- 100.Singh S., Gupta R., Gaur R., Srivastava A. Antagonistic actinomycetes mediated resistance in Solanum lycopersicon Mill. against Rhizoctonia solani Kühn. Proc. Natl. Acad. Sci. India Sect. B Biol. Sci. 2015;87:789–798. doi: 10.1007/s40011-015-0651-5. [DOI] [Google Scholar]
- 101.Kurth F., Mailänder S., Bönn M., Feldhahn L., Herrmann S., Große I., Buscot F., Schrey S.D., Tarkka M.T. Streptomyces-induced resistance against oak powdery mildew involves host plant responses in defense, photosynthesis, and secondary metabolism pathways. Mol. Plant Microbe Interact. 2014;27:891–900. doi: 10.1094/MPMI-10-13-0296-R. [DOI] [PubMed] [Google Scholar]
- 102.Singh S.P., Gaur R. Endophytic Streptomyces spp. underscore induction of defense regulatory genes and confers resistance against Sclerotium rolfsii in chickpea. Biol. Control. 2017;104:44–56. doi: 10.1016/j.biocontrol.2016.10.011. [DOI] [Google Scholar]
- 103.Vatsa-Portugal P., Aziz A., Rondeau M., Villaume S., Morjani H., Clément C., Ait Barka E. How Streptomyces anulatus primes grapevine defenses to cope with gray mold: A study of the early responses of cell suspensions. Front. Plant Sci. 2017;8:1043. doi: 10.3389/fpls.2017.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Awla H.K., Kadir J., Othman R., Rashid T.S., Hamid S., Wong M.-Y. Plant growth-promoting abilities and biocontrol efficacy of Streptomyces sp. UPMRS4 against Pyricularia oryzae. Biol. Control. 2017;112:55–63. doi: 10.1016/j.biocontrol.2017.05.011. [DOI] [Google Scholar]
- 105.Zhang Q., Yong D., Zhang Y., Shi X., Li B., Li G., Liang W., Wang C. Streptomyces rochei A-1 induces resistance and defense-related responses against Botryosphaeria dothidea in apple fruit during storage. Postharvest Biol. Technol. 2016;115:30–37. doi: 10.1016/j.postharvbio.2015.12.013. [DOI] [Google Scholar]
- 106.Vilasinee S., Toanuna C., McGovern R., Nalumpang S. Expression of pathogenesis-related (PR) genes in tomato against Fusarium wilt by challenge inoculation with Streptomyces NSP3. Int. J. Agric. Technol. 2019;15:157–170. [Google Scholar]
- 107.Abbasi S., Safaie N., Sadeghi A., Shamsbakhsh M. Streptomyces strains induce resistance to Fusarium oxysporum f.sp. lycopersici race 3 in tomato through different molecular mechanisms. Front. Microbiol. 2019;10:1505. doi: 10.3389/fmicb.2019.01505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Mun B.-G., Lee W.-H., Kang S.-M., Lee S.-U., Lee S.-M., Lee D.Y., Shahid M., Yun B.-W., Lee I.-J. Streptomyces sp. LH 4 promotes plant growth and resistance against Sclerotinia sclerotiorum in cucumber via modulation of enzymatic and defense pathways. Plant Soil. 2020;448:87–103. doi: 10.1007/s11104-019-04411-4. [DOI] [Google Scholar]
- 109.Saikia K., Bora L.C. Exploring actinomycetes and endophytes of rice ecosystem for induction of disease resistance against bacterial blight of rice. Eur. J. Plant Pathol. 2021;159:67–79. doi: 10.1007/s10658-020-02141-3. [DOI] [Google Scholar]
- 110.Lee S.-M., Kong H.G., Song G.C., Ryu C.-M. Disruption of Firmicutes and Actinobacteria abundance in tomato rhizosphere causes the incidence of bacterial wilt disease. ISME J. 2021;15:330–347. doi: 10.1038/s41396-020-00785-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Abo-Zaid G.A., Matar S.M., Abdelkhalek A. Induction of plant resistance against tobacco mosaic virus using the biocontrol agent Streptomyces cellulosae isolate Actino 48. Agronomy. 2020;10:1620. doi: 10.3390/agronomy10111620. [DOI] [Google Scholar]
- 112.Vergnes S., Gayrard D., Veyssière M., Toulotte J., Martinez Y., Dumont V., Bouchez O., Rey T., Dumas B. Phyllosphere colonization by a soil Streptomyces sp. promotes plant defense responses against fungal infection. Mol. Plant Microbe Interact. 2020;33:223–234. doi: 10.1094/MPMI-05-19-0142-R. [DOI] [PubMed] [Google Scholar]
- 113.Cha J.-Y., Han S., Hong H.-J., Cho H., Kim D., Kwon Y., Kwon S.-K., Crüsemann M., Bok Lee Y., Kim J.F., et al. Microbial and biochemical basis of a Fusarium wilt-suppressive soil. ISME J. 2016;10:119–129. doi: 10.1038/ismej.2015.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Schlatter D., Kinkel L., Thomashow L., Weller D., Paulitz T. Disease suppressive soils: New insights from the soil microbiome. Phytopathology. 2017;107:1284–1297. doi: 10.1094/PHYTO-03-17-0111-RVW. [DOI] [PubMed] [Google Scholar]
- 115.Yuan J., Zhao J., Wen T., Zhao M., Li R., Goossens P., Huang Q., Bai Y., Vivanco J.M., Kowalchuk G.A., et al. Root exudates drive the soil-borne legacy of aboveground pathogen infection. Microbiome. 2018;6:156. doi: 10.1186/s40168-018-0537-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Zhalnina K., Louie K.B., Hao Z., Mansoori N., da Rocha U.N., Shi S., Cho H., Karaoz U., Loqué D., Bowen B.P., et al. Dynamic root exudate chemistry and microbial substrate preferences drive patterns in rhizosphere microbial community assembly. Nat. Microbiol. 2018;3:470–480. doi: 10.1038/s41564-018-0129-3. [DOI] [PubMed] [Google Scholar]
- 117.Xiong W., Li R., Ren Y., Liu C., Zhao Q., Wu H., Jousset A., Shen Q. Distinct roles for soil fungal and bacterial communities associated with the suppression of vanilla Fusarium wilt disease. Soil Biol. Biochem. 2017;107:198–207. doi: 10.1016/j.soilbio.2017.01.010. [DOI] [Google Scholar]
- 118.Zheng Y., Han X., Zhao D., Wei K., Yuan Y., Li Y., Liu M., Zhang C.-S. Exploring biocontrol agents from microbial keystone taxa associated to suppressive soil: A new attempt for a biocontrol strategy. Front. Plant Sci. 2021;12:655673. doi: 10.3389/fpls.2021.655673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tan H., Zhou S., Deng Z., He M., Cao L. Ribosomal-sequence-directed selection for endophytic streptomycete strains antagonistic to Ralstonia solanacearum to control tomato bacterial wilt. Biol. Control. 2011;59:245–254. doi: 10.1016/j.biocontrol.2011.07.018. [DOI] [Google Scholar]
- 120.Gao M., Xiong C., Gao C., Tsui C.K.M., Wang M.M., Zhou X., Zhang A.M., Cai L. Disease-induced changes in plant microbiome assembly and functional adaptation. Microbiome. 2021;9:187. doi: 10.1186/s40168-021-01138-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Naylor D., DeGraaf S., Purdom E., Coleman-Derr D. Drought and host selection influence bacterial community dynamics in the grass root microbiome. ISME J. 2017;11:2691–2704. doi: 10.1038/ismej.2017.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hartman K., Tringe S.G. Interactions between plants and soil shaping the root microbiome under abiotic stress. Biochem. J. 2019;476:2705–2724. doi: 10.1042/BCJ20180615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Xu L., Naylor D., Dong Z., Simmons T., Pierroz G., Hixson K.K., Kim Y.-M., Zink E.M., Engbrecht K.M., Wang Y., et al. Drought delays development of the sorghum root microbiome and enriches for monoderm bacteria. Proc. Natl. Acad. Sci. USA. 2018;115:E4284–E4293. doi: 10.1073/pnas.1717308115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fitzpatrick C., Copeland J., Wang P., Guttman D., Kotanen P., Johnson M. Assembly and ecological function of the root microbiome across angiosperm plant species. Proc. Natl. Acad. Sci. USA. 2018;115:201717617. doi: 10.1073/pnas.1717617115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Yoolong S., Kruasuwan W., Thanh Phạm H.T., Jaemsaeng R., Jantasuriyarat C., Thamchaipenet A. Modulation of salt tolerance in Thai jasmine rice (Oryza sativa L. cv. KDML105) by Streptomyces venezuelae ATCC 10712 expressing ACC deaminase. Sci. Rep. 2019;9:1275. doi: 10.1038/s41598-018-37987-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Gebauer L., Breitkreuz C., Heintz-Buschart A., Reitz T., Buscot F., Tarkka M., Bouffaud M.-L. Water deficit history selects plant beneficial soil bacteria differently under conventional and organic farming. Front. Microbiol. 2022;13:824437. doi: 10.3389/fmicb.2022.824437. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.