Abstract
Background: Tetrabromobisphenol A (TBBPA) is the most commonly used brominated flame retardant (BFR) in the industry. TBBPA has been determined in environmental samples, food, tap water, dust as well as outdoor and indoor air and in the human body. Studies have also shown the toxic potential of this substance. In search of a better and less toxic BFR, tetrabromobisphenol S (TBBPS) has been developed in order to replace TBBPA in the industry. There is a lack of data on the toxic effects of TBBPS, while no study has explored apoptotic mechanism of action of TBBPA and TBBPS in human leukocytes. Methods: The cells were separated from leucocyte-platelet buffy coat and were incubated with studied compounds in concentrations ranging from 0.01 to 50 µg/mL for 24 h. In order to explore the apoptotic mechanism of action of tested BFRs, phosphatidylserine externalization at cellular membrane (the number of apoptotic cells), cytosolic calcium ion and transmembrane mitochondrial potential levels, caspase-8, -9 and -3 activation, as well as PARP-1 cleavage, DNA fragmentation and chromatin condensation in PBMCs were determined. Results: TBBPA and TBBPS triggered apoptosis in human PBMCs as they changed all tested parameters in the incubated cells. It was also observed that the mitochondrial pathway was mainly involved in the apoptotic action of studied compounds. Conclusions: It was found that TBBPS, and more strongly TBBPA, triggered apoptosis in human PBMCs. Generally, the mitochondrial pathway was involved in the apoptotic action of tested compounds; nevertheless, TBBPS more strongly than TBBPA caused intrinsic pathway activation.
Keywords: tetrabromobisphenol A, tetrabromobisphenol S, peripheral blood mononuclear cells, apoptosis, cytosolic calcium ion level, transmembrane mitochondrial potential, caspase activation, PARP-1 cleavage, chromatin condensation
1. Introduction
Brominated flame retardants (BFRs), including brominated bisphenols, are utilized in the production of various everyday products in order to reduce their flammability [1,2].
The synthesis of tetrabromobisphenol A (TBBPA) accounts for 60% of the total global market for BFRs, making it the most widely used flame retardant worldwide [3]. This substance is utilized in the synthesis of polymers (polycarbonates, epoxy resins) and production of electronic and electric equipment, as well as textiles and furniture [4,5].
Due to the widespread use of TBBPA, this substance has been repeatedly found in the environment [6,7,8], dust of residential and office spaces [9], as well as ambient and indoor air [7,10,11].
TBBPA has also been determined in the human organism. Cariou et al. [12] found TBBPA in samples of mothers milk and serum, as well as fetuses serum in the range of concentrations from 0.06 to 37.34 ng/g of fat, while Fujii et al. [13] detected TBBPA in the plasma of Japanese men in an average concentration of 950 pg/g fresh weight. Some research works have documented the occurrence of significant amounts of TBBPA in the human body. For instance, Lankova et al. [14] determined TBBPA in the concentration of up to 16.2 µg/L in samples of women breast milk.
The ubiquitous presence of TBPPA in the environment and the human body prompted the scientists to assess toxic potential of this substance. The conducted studies have shown pro-oxidative, pro-inflammatory, neurotoxic, nephrotoxic, hepatotoxic, genotoxic and endocrine disrupting potential of TBBPA [15,16,17,18,19,20,21,22,23].
In search of a better and less toxic BFR, tetrabromobisphenol S (TBBPS) has been developed in order to potentially replace TBBPA in the industry [24]. TBBPS is commonly utilized in the synthesis of polycarbonates and epoxy resins, as well as in the production of textiles and electronic devices [25,26,27]. Although TBBPS is still less commonly utilized in the industry than TBBPA, it has been found in the environment, food and indoor air [1,27,28,29,30,31]. Recently, TBBPS was determined in an average concentration of 0.593 μg/L of serum samples collected from pregnant Chinese women [32].
Limited data exist on the effect of TBBPS on living organisms. Ding et al. [33] noticed that TBBPS changed the circadian rhythm network in zebrafish and induced developmental changes in zebrafish embryos. These scientists have considered that TBBPS may not have exhibited lower toxicity, in comparison to TBBPA in humans. Liang et al. [34] noticed that TBBPA and TBBPS revealed comparable toxic effects on human embryonic stem cells. They found that tested substances altered the development of neural ectoderm, influenced the growth of axons and the transmission of neurons, as well as disturbed the WNT and AHR signaling pathways.
Apoptosis is a regulated process that allows the removal of old and damaged cells without inflammatory reactions. However, it has been shown that different stimuli, including toxicants, accelerate apoptosis that may contribute to development of various pathological states [35]. Two main apoptotic pathways are involved in apoptosis of lymphocytes. The intrinsic (mitochondrial) cell death pathway is triggered by various apoptotic stimuli, such as genomic instability, cytokine withdrawal or toxicants. Intrinsic cell death signals generally focus on the cell at the outer membrane of mitochondria, which leads to a loss of mitochondrial membrane integrity, and the subsequent induction of apoptotic pathways, in which various proteases, including essential initiator caspase-9 are activated. The major mediators in the intrinsic cell death pathway are Bcl-2 family proteins, which control mitochondrial membrane integrity [36].
On the other hand, death receptors are involved in the initiation of the extrinsic (receptor) death pathway. All death receptors contain a death domain (DD) in their cytoplasmic tail. There are two major DD-containing adaptor proteins implicated in death receptor signaling, such as Fas-associated death domain (FADD) and tumor necrosis factor (TNF) receptor-associated death domain (TRADD). When ligands, including reactive oxygen species (ROS) and xenobiotics, bind to death receptors, the DD mediates the interaction with other DD-containing adaptor proteins, which activates the extrinsic pathway, in which the induction of other important proteins, such as initiator caspase 8 occurs [37].
PBMCs are directly exposed to toxicants entering the human organism. Those cells play a crucial role in the human body as they are involved in antibodies production, killing virus-infected and cancer cells, as well as regulating the immune system response [38].
It was shown that accelerated apoptosis of PBMCs may be associated with adverse changes in the immune system, such as depleted human immunity [39], which, in consequence, may lead to the development of autoimmune diseases (type 1 diabetes, asthma, allergy) and cancer [40,41,42,43].
Some research works have revealed that TBBPA can disturb the function of the immune system. Feiterio et al. [44] proved that TBBPA altered the tumor killing function of NK cells and changed cytokines production, such as interferon gamma (IFNɣ), interleukin-1β (IL-1β) and tumor necrosis factor (TNF). In another work, Hall et al. [45] noticed that, in rats, TBBPA caused the downregulation of genes involved in the immune response, which could have led to estrogen-mediated immunosuppression in studied animals.
According to our best knowledge, no research work has been performed to assess the mechanism of apoptotic action of TBBPA in human leucocytes, and only Cato et al. [46] noticed that TBBPA activated caspase-3 and mitogen-activated protein kinases (MAPKs) in human NK cells. Moreover, the effect of TBBPS on apoptosis induction has not been assessed in any cell type. Our earlier research works have revealed that TBBPA and TBBPS caused ROS formation and induced damage to lipids, proteins and DNA in human PBMCs [22,23], while it is well known that ROS induction and DNA damage can trigger apoptotic cell death [47].
Taking the above into consideration, we compared apoptotic potential of TBBPA and TBBPS in human PBMCs and determined the mechanism underlying the action of these substances by assessing changes in phosphatidylserine translocation in cellular membrane, alterations in cytosolic calcium ion and transmembrane mitochondrial potential levels, and caspase-8, -9 and -3 activation. Moreover, the effect of tested compounds on the cleavage of PARP-1, DNA fragmentation and condensation of chromatin was evaluated.
The investigations were conducted in the range of concentrations of tested compounds that referred to their levels detected in humans environmentally or/and occupationally exposed. If no effects were observed at the above-mentioned BFRs levels, higher concentrations of TBBPA and TBBPS were used.
2. Results
2.1. Quantitative Analysis of Apoptosis
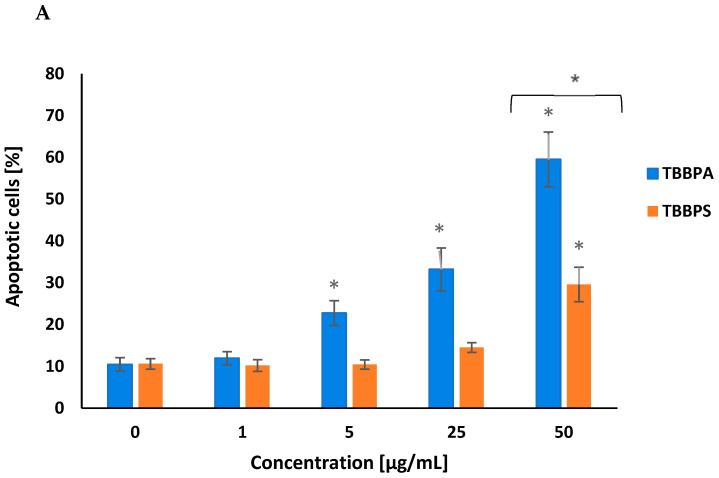
TBBPA and TBBPS increased PS translocation in peripheral blood mononuclear cells (PBMCs), which was assessed by annexin V-FITC and PI staining. After 24 h of treatment, TBBPA at 5 µg/mL, 25 µg/mL and 50 µg/mL caused a concentration-dependent increase in the number of apoptotic cells. TBBPS induced substantially lower alterations in examined parameters, increasing the number of apoptotic cells only at 50 µg/mL (Figure 1A,B).
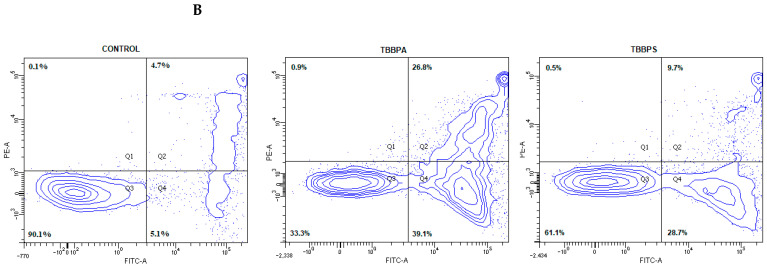
Figure 1.
Apoptotic alterations in human PBMCs treated with TBBPA and TBBPS in the range from 1 to 50 µg/mL (n = 4) for 24 h (A). The cells were stained with Annexin-FITC and PI. Exemplary dot plots showing apoptotic alterations in human PBMCs unexposed (control) and exposed to TBBPA and TBBPS at 50 μg/mL for 24 h, Q3—live cells, Q2 + Q4—apoptotic cells (B). Statistically different from negative control at * p < 0.05. Statistical analysis was conducted using one-way ANOVA and a posteriori Tukey test.
2.2. Cytosolic Calcium Ions Level
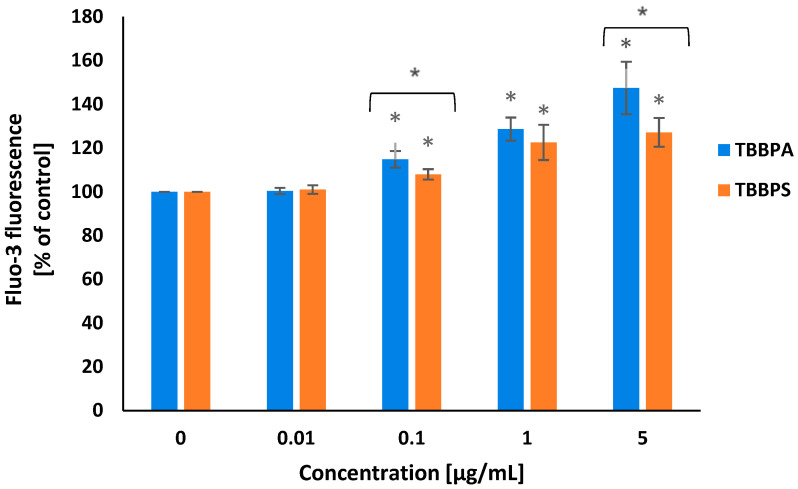
Alteration in the intracellular calcium ion level were determined using Fluo-3/AM, which is hydrolyzed by membrane esterases to Fluo-3. Fluo-3 fluorescence intensity increases with rise of cytosolic Ca2+ level. A concentration-dependent increase in cytosolic Ca2+ level was noted in PBMCs incubated with TBBPA at 0.1 µg/mL, 1 µg/mL and 5 µg/mL for 24 h. TBBPS at the same concentrations caused smaller changes in the studied parameter (Figure 2).
Figure 2.
Alterations in cytosolic calcium ion level in human PBMCs treated with TBBPA and TBBPS in the range from 0.01 to 5 µg/mL (n = 4) for 24 h. Statistically different from negative control at * p < 0.05. Statistical analysis was conducted using one-way ANOVA and a posteriori Tukey test.
2.3. Transmembrane Mitochondrial Potential
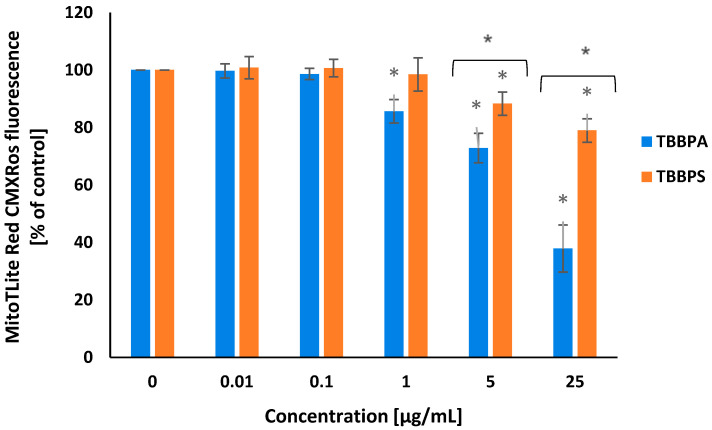
Transmembrane mitochondrial potential (ΔΨm) was evaluated using MitoLite Red CMXRos probe. Alterations in the intensity of marker fluorescence were associated with changes in ΔΨm level. It was revealed that after 24 h of treatment, TBBPA and TBBPS in a concentration-dependent manner reduced ΔΨm in the incubated cells. TBBPA at 1 µg/mL and 5 µg/mL, and, particularly at 25 µg/mL, substantially reduced the tested parameter, while TBBPS at 5 µg/mL and 25 µg/mL induced a smaller depletion of ΔΨm in PBMCs (Figure 3).
Figure 3.
Alterations in transmembrane mitochondrial potential (ΔΨm) in PBMCs treated with TBBPA and TBBPS in the range from 0.01 to 25 µg/mL (n = 4) for 24 h. Statistically different from negative control at * p < 0.05. Statistical analysis was conducted using one-way ANOVA and a posteriori Tukey test.
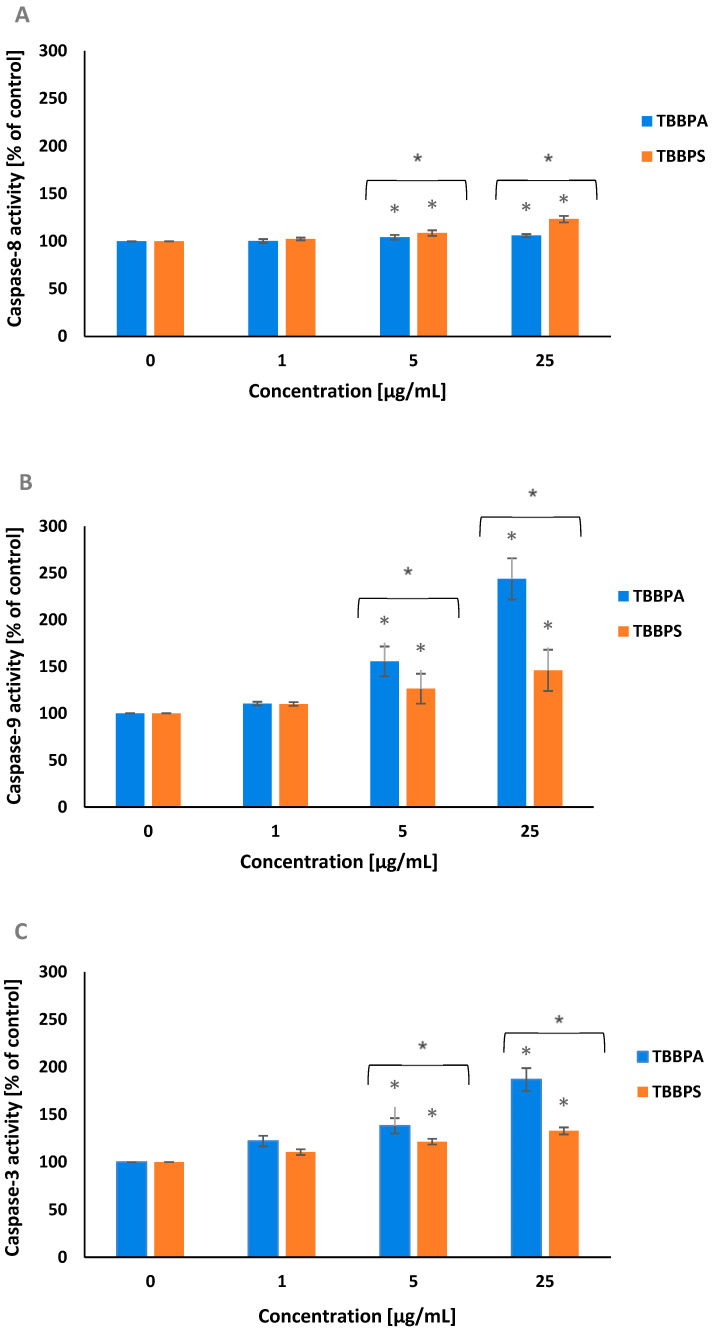
2.4. Caspase-8, -9 and -3 Activation
Caspase-8 and caspase-9 belong to the initiator enzymes of programmed cell death. A small rise in caspase-8 activation was shown in tested cells treated for 24 h with TBBPA and TBBPS at 5 µg/mL and 25 µg/mL, whereas TBBPS caused slightly higher caspase-8 activation than TBBPA in tested cells (Figure 4A).
Figure 4.
Alterations in caspase-8 (A), caspase-9 (B) and caspase-3 (C) activity in PBMCs treated with TBBPA and TBBPS in the range from 1 to 25 µg/mL (n = 4) for 24 h. Statistically different from negative control at * p < 0.05. Statistical analysis was conducted using one-way ANOVA and a posteriori Tukey test.
After 24 h of treatment, TBBPA and TBBPS at 5 µg/mL and 25 µg/mL caused a substantial increase in caspase-9 activation. It was observed that TBBPA, more strongly (particularly at its highest concentration) than TBBPS, increased caspase-9 activation in tested cells (Figure 4B). Caspase-3 is an executory enzyme of apoptosis. It was noticed that after 24 h of incubation, both studied BFRs, and more strongly TBBPA at 5 µg/mL and 25 µg/mL raised caspase-3 activation in PBMCs (Figure 4C).
Caspase-8, caspase-9 and caspase-3 inhibitors were also used in these experiments. The preincubation of the cells with appropriate caspase inhibitor depleted caspase activity up to control value (data not shown).
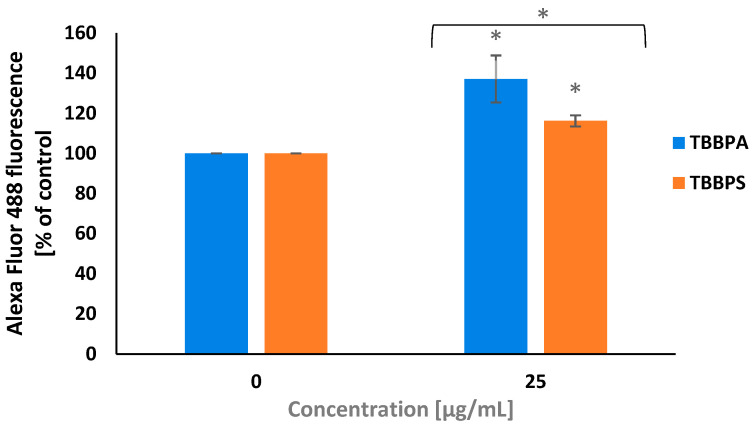
2.5. PARP-1 Cleavage
PARP-1 is a substrate for caspase-3. The cleavage of PARP-1 and its inactivation helps cells undergoing apoptosis. To determine PARP1 cleavage, a concentration of 25 µg/mL of TBBPA and TBBPS was selected, which caused the strongest caspase-3 activation in PBMCs.
It was found that after 24 h of treatment, TBBPS and more strongly TBBPA at 25 µg/mL induced PARP-1 cleavage in tested cells (Figure 5).
Figure 5.
Changes in the level of 85 kDa PARP1 fragments in PBMCs treated with TBBPA and TBBPS at 25 µg/mL (n = 3) for 24 h. Statistically different from negative control at * p < 0.05. Statistical analysis was conducted using Welch’s test.
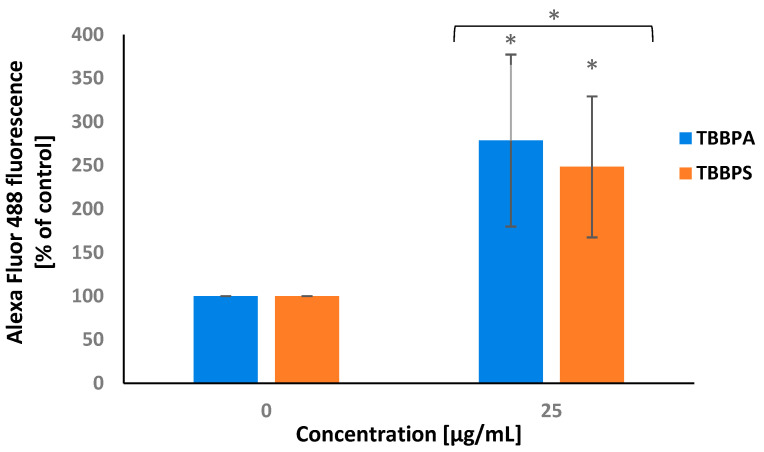
2.6. APO-BrdU TUNEL Assay
APO-BrdU TUNEL assay was used for labeling DNA strand breaks along with total cellular DNA to detect apoptotic cells. It was noticed that after 24 h of treatment, TBBPA and less strongly TBBPS at 25 µg/mL induced substantial DNA fragmentation in PBMCs (Figure 6).
Figure 6.
Alterations in DNA fragmentation in PBMCs incubated with TBBPA and TBBPS at 25 µg/mL (n = 3) for 24 h. Statistically different from negative control at * p < 0.05. Statistical analysis was conducted using Welch’s test.
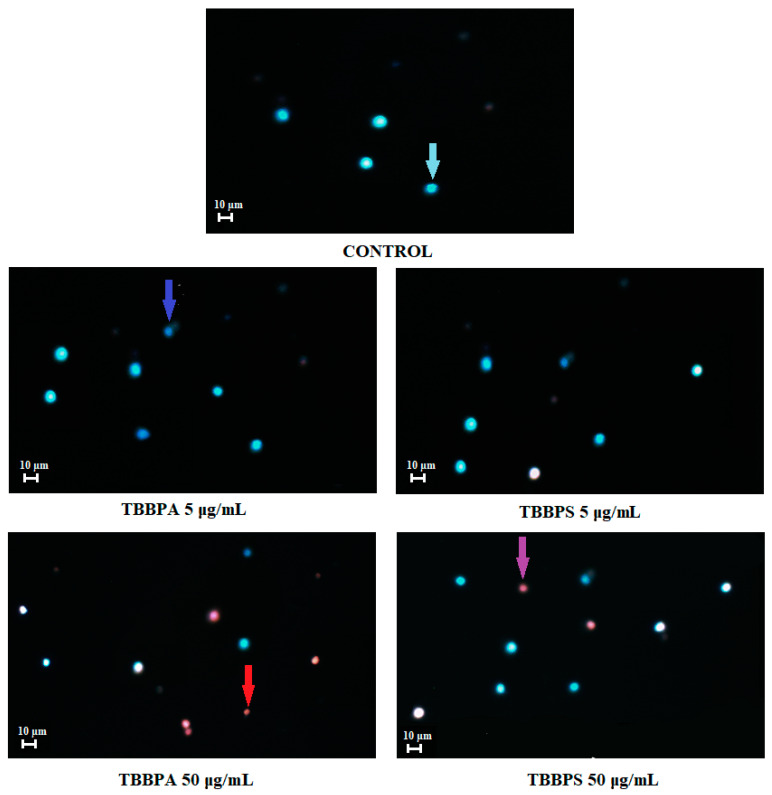
2.7. Apoptosis Detection by Fluorescence Microscopy
Figure 7 presents representative photomicrographs of PBMCs stained with Hoechst 33324/PI. Before staining, the cells had been incubated for 24 h with DMSO at 0.2% (control), as well as with TBBPA or TBBPS at 5 µg/mL and 50 µg/mL. In control samples, only viable PBMCs were noticed. In probes incubated with TBBPA at 5 µg/mL mainly viable, as well as single early apoptotic cells were observed, while in the probes treated with TBBPS at 5 µg/mL, mainly viable PBMCs were found. Probes treated with TBBPA at 50 µg/mL contained mostly late apoptotic and necrotic cells, whereas the probes incubated with TBBPS at 50 µg/mL consisted of mainly viable, as well as some early apoptotic and late apoptotic PBMCs.
Figure 7.
Apoptotic alterations in PBMCs treated with 0.2% DMSO (negative control), as well as with TBBPA and TBBPS at 5 µg/mL and 50 µg/mL. PBMCs were stained with Hoechst 33342 and PI. Viable cells (blue fluorescence), early apoptotic cells (intensive bright blue fluorescence), late apoptotic cells (blue/violet fluorescence) and necrotic cells (red fluorescence) [48].
3. Discussion
Apoptosis is a regulated process, which enables the removal of unwanted (old, damaged) cells from organisms without inflammatory reactions. It has been shown that various xenobiotics can trigger apoptosis [35], while excessive apoptosis may contribute to the development of infections, heart failure, neurodegenerative diseases or myocardial ischemia.
Peripheral blood mononuclear cells (PBMCs) are key cells of the immune system and are directly exposed to toxicants entering the human body. The accelerated apoptosis of lymphocytes (main population of PBMCs) caused by action of xenobiotics, such as BFRs, may lead to disorders in the immune system function and possibly be involved in cancer development. Additionally, excessive apoptosis of PBMCs can contribute to the development of allergy and asthma [49].
In this research work, apoptotic potential and mechanism of action of TBBPA and TBBPS in human PBMCs was assessed by analyzing phosphatidylserine (PS) translocation in cellular membrane, determining cytosolic calcium ion and transmembrane mitochondrial potential levels, as well as evaluation of caspase-8, -9 and -3 activation, PARP-1 degradation, chromatin condensation and DNA fragmentation.
Our study revealed that tested compounds induced apoptosis of PBMCs but exhibited different apoptotic potential in studied cells. Both examined compounds increased the number of cells showing PS exposure, while TBBPA caused greater alteration in this parameter, when compared with TBBPS. It was observed that TBBPA from 5 µg/mL induced apoptosis of PBMCs, whereas TBBPS, only at 50 µg/mL, triggered apoptotic cell death (Figure 1). Similar findings were achieved by Mokra et al. [50] who assessed the apoptotic potential of debrominated analogs of TBBPA and TBBPS in PBMCs. They observed that bisphenol A (BPA) exhibited stronger apoptotic potential than bisphenol S (BPS) in this cell type. Cho et al. [51] proved that TBBPA (25–200 µM) after 24 h of incubation induced apoptosis in mouse astrocytes and neural stem cells. The authors reported that TBBPA increased ROS formation, which caused mitochondrial dysfunction and ultimately led to apoptosis through c-Jun N-terminal kinase-p53 pathway activation. In another study, Zhang et al. [52] reported a substantial rise in the number of apoptotic human liver L02 cells treated for 48 h with TBBPA in the range from 5 to 40 µM. TBBPA at 20 µM was also shown to induce significant apoptosis of insulinoma RIN-m5F rat cells after 48 h of incubation [53], while at a low concentration of 81 nM, this substance increased the number of apoptotic HepG2 cells by increasing ROS formation, decreasing ΔΨm and activating Ras signaling pathway [54]. Moreover, Wu et al. [55] observed an increased number of apoptotic cells in the heart and brain of embryos and larvae of Zebrafish exposed to TBBPA.
A crucial point in the apoptosis is the increase in cytosolic calcium ions level. Calcium ions released from endoplasmic reticulum into cytosol accumulate in mitochondria, which may lead to the depletion of ΔΨm and, finally, to the induction of apoptosis [56,57,58].
We observed that tested substances increased the level of cytosolic calcium ions and depleted ΔΨm level from 0.1 µg/mL and 1 µg/mL, respectively, while TBBPA induced greater changes than TBBPS (Figure 2 and Figure 3). Ogunbayo et al. [59] noticed that TBBPA at 30 µM raised Ca2+ level and reduced ΔΨm in TM4 mouse Sertoli cells. Similarly, Cho et al. [51] reported that TBBPA from 25 µg/mL depleted ΔΨm in mouse astrocytes and neural stem cells, whereas Lu et al. [54] noticed that this substance at 81 nM depleted ΔΨm in HepG2 cells. Moreover, Mokra et al. [50] observed that BPA and BPS were capable of increasing Ca2+ level and decreasing ΔΨm in human PBMCs.
The intrinsic pathway, the so-called mitochondrial pathway, is activated as a result of oxidative stress and DNA damage, as well as by increased cytosolic calcium ion level and disturbances of ΔΨm [60,61]. The main component of the intrinsic pathway is the mitochondria. It has been shown that through the reactions involving Bcl-2 family proteins, mitochondrial permeability transition pores (MTP) are formed that release cytochrome c into mitochondrial intermembrane space. Then, cytochrome c together with procaspase-9, ATP and the cytosolic protein Apaf-1 forms an apoptosome in the cytoplasm that activates initiator caspase-9 [61,62,63]. On the other hand, the extrinsic (receptor) pathway may be activated by ROS, which involves the interactions of ligands with transmembrane receptors leading to the activation of initiator caspase-8 [47,62]. Although, the formation of ROS has not been assessed in this study, our previous work showed that TBBPA and TBBPS at low concentrations increased ROS level in human PBMCs [22].
Generally, our study showed that caspase-9 was more strongly activated than caspase-8 in PBMCs exposed to TBBPA and TBBPS at 5 µg/mL and 25 µg/mL. Moreover, it was observed that TBBPA induced a stronger increase (than TBBPS) in caspase-9 activity, while in the case of caspase-8 activation, the results were opposite (Figure 4A,B). The stronger activation of caspase-9 suggests that mitochondrial pathway was mainly implicated in apoptosis induction of PBMCs exposed to TBBPS and, in particular, TBBPA.
It has been proven that both caspase-8 and caspase-9 can activate caspase-3, which is the executioner enzyme of apoptosis.
Obtained results revealed a substantial rise in caspase-3 activity, which was stronger in cells incubated with TBBPA (Figure 4C). A rise in caspase-8, -9, and -3 activities in human PBMCs exposed to debrominated analogs of tested BFRs, such as BPA and BPS was reported by Mokra et al. [50]. In other studies, Szychowski and Wójtowicz [64] observed that TBBPA (100 nM–100 µM, 6 h of incubation) increased caspase-3 activity in mouse hippocampal neuronal cells, while Al-Mousa and Michelangeli [17] showed that TBBPA in the concentrations from 1 to 30 µM activated caspase-3 in SH-SY5Y neuroblastoma cell line. Caspase-3 activation was also reported by Wu et al. [65] in A549 cells (human lung carcinoma cell line) exposed for 48 h to TBBPA in the concentrations range from 8 to 64 µg/mL. Moreover, Jarosiewicz et al. [66] observed apoptotic changes in human erythrocytes exposed to bromophenolic flame retardants. They demonstrated that TBBPS, and more strongly TBBPA, caused PS externalization (increased the number of apoptotic erythrocytes) and induced caspase-3 activation.
Caspase-3 causes PARP-1 cleavage into fragments of 89 kDa and 24 kDa. Fragments of 24 kDa bind to DNA breaks. In contrast, fragments of 89 kDa with attached PAR polymers are moved from nucleus into cytoplasm and interact with apoptosis-inducing factors (AIF), which, as a consequence, leads to the shrinking of the cell nucleus and apoptosis [67].
In order to determine PARP-1 cleavage, a concentration of 25 µg/mL of TBBPA and TBBPS was chosen, which the most strongly activated caspase-3 in tested cells. It was revealed that TBBPA and less strongly TBBPS at 25 µg/mL caused PARP-1 cleavage in human PBMCs (Figure 5). Similarly, Mokra et al. [50] observed PARP-1 fragmentation in human PBMCs treated with BPA and BPS.
TUNEL method allows the detection of apoptotic DNA fragmentation and is one of the most widely used assays to detect programmed cell death [68]. We used this method to confirm that tested compounds were capable of inducing apoptosis in studied cells.
Our study revealed that TBBPS, and more strongly TBBPA at 25 µg/mL, increased the number of TUNEL-positive cells (Figure 6). Park et al. [69] reported that TBBPA at 125 µg/mL (24 h of incubation) caused caspase-3 activation and increased the number of TUNEL-positive HEI-OC1 cells (mouse auditory cell line). Moreover, an increase in TUNEL-positive cells was noticed by Zatecka et al. [70] in mouse spermatozoa, treated with TBBPA at 200 µg/L dissolved in drinking water.
Staining PBMCs with Hoechst33342/PI and using fluorescence microscopy allowed to visualize differences in apoptotic/necrotic changes induced by tested compounds. We noted that TBBPA, particularly at higher concentration of 50 µg/mL induced greater alterations than TBBPS in studied cells. The samples treated with TBBPA contained mainly late apoptotic and necrotic cells, while the probes exposed to TBBPS showed the presence of mostly early apoptotic and viable PBMCs (Figure 7).
It must be underlined that the determination of the mechanism of proapoptotic action of toxicants, such as TBBPA and TBBPS, is crucial for the recognition of cellular targets, which are affected by studied compounds. It must also be taken into account that alterations in some apoptotic parameters, such as calcium ion level or ΔΨm (that usually occur at relatively low toxicant concentrations), are linked to other cellular processes (e.g., cell signaling, energy state of the cell, etc.), and their disturbance may change cell function before apoptosis occurs.
Summing up, this research work represents a mechanistic approach elucidating the effect of tested BFRs on nucleated blood cells, which brings us closer to understanding the action of these substances in the human organism.
4. Conclusions
In conclusion, (1) TBBPA and TBBPS exhibited different apoptotic potential in human PBMCs. (2) Tested compounds triggered apoptosis by PS externalization on cellular membrane, increasing cytosolic Ca2+ level, decreasing transmembrane mitochondrial potential, activating caspase-8, -9 and -3, as well as increasing PARP-1 cleavage, DNA fragmentation and chromatin condensation. (3) Tested BFRs induced apoptosis mainly by the involvement of mitochondrial pathway; although TBBPA and TBBPS more strongly activated intrinsic and extrinsic mitochondrial pathways, respectively. (4) Similarly, as in our previous studies on prooxidative and genotoxic potential, stronger apoptotic effects were provoked by TBBPA, in comparison to TBBPS.
5. Materials and Methods
5.1. Chemicals
Tetrabromobisphenol A (99%, 2,2-bis[3,5-dibromo-4-hydroxyphenyl]propane) was bought from LGC Standards (Teddington, UK). Tetrabromobisphenol S (4,4′-sulfonylbis[2,6-dibromo-phenol]) (98.8%) was synthetized in the Institute of Industrial Organic Chemistry in Warsaw (Warsaw, Poland). Caspase-3 fluorimetric assay kit, HBSS solution, pluronic F-127, Hoechst 33342, propidium iodide and valinomycin were bought in Sigma-Aldrich (St. Louis, MO, USA). Perm/Wash Buffer and FITC Annexin V Apoptosis Detection Kit were obtained from Becton Dickinson (Franklin Lakes, NJ, USA). MitoLite Red CMXRos was bought in AAT Bioquest (Sunnyvale, CA, USA). Fluo-3 AM was obtained in PromoCell (Heidelberg, Germany). Caspase-8 fluorimetric assay kit and caspase-9 chromogenic substrate and caspase-9 inhibitor were bought in BioVision (San Francisco, CA, USA). PARP1 (cleaved Asp214) Monoclonal Antibody (HLNC4) and APO-BrdU TUNEL Assay Kit were obtained from Thermo-Fisher (Waltham, MA, USA). Separation medium (LSM) (1.077 g/cm3) and RPMI medium with L-glutamine were purchased from Cytogen (Seoul, South Korea). Camptothecin was bought from Pol-Aura (Dywity, Poland). Ionomycin was obtained from Biokom (Janki, Poland). Other chemicals were obtained from Pol-Aura (Dywity, Poland) and Roth (Karlsruhe, Germany).
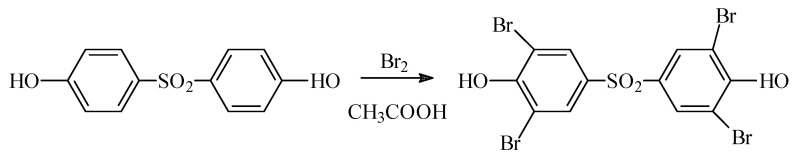
TBBPS was synthesized in a reaction of bisphenol S bromination with liquid bromide in concentrated acetic acid at 80 °C [71], according to Scheme 1:
Scheme 1.
TBBPS synthesized in a reaction of bisphenol S bromination with liquid bromide in concentrated acetic acid at 80 °C.
Crude residue was rinsed with concentrated acetic acid, dissolved in tetrahydrofuran and precipitated out of the solution using hexane. As a result, TBBPS was obtained, which was a colorless solid with melting temperature of 293.3 °C and HPLC purity of 98.8% (internal standardization), efficiency of the reaction was 35%.
Analysis of TBBPS using mass spectrometry - (EI, 70 eV, m/z, int[%]): 570(19), 569(9), 568(67), 567(14), 566(100, M), 565(100, 564(69), 562(18), 502(8), 315(14), 302(120, 301(35), 300(23), 299(64), 298(12), 297(33), 270(18), 269(13), 268(38), 267(22), 266(19), 265(11), 253(10), 252(17), 251(25), 250(14), 249(10), 223(10), 221(10), 172(24), 171(16), 170(25), 169(10), 143(14), 141(13), 91(30), 90(27), 63(33), 62(43), 61(13), 53(13).
5.2. Methods
5.2.1. Cell Isolation and Treatment
The method of peripheral blood mononuclear cells (PBMCs) isolation was described in detail by Włuka et al. [22]. PBMCs were isolated from the leukocyte-platelet buffy coat, which was separated from whole blood in the Blood Bank in Lodz, Poland. Blood was taken from healthy, non-smoking volunteers (aged 18–45) who showed no signs of infection disease symptoms. The study was approved by Bioethical Commission of Scientific Research at the University of Lodz, no. 1/KBBN-UŁ/II/2017.
Tested compounds were dissolved in DMSO. Final concentration of DMSO in untreated samples (negative control) and samples incubated with TBBPA or TBBPS was 0.2%. The above concentration of DMSO was not toxic for PBMCs, as evaluated by all studied parameters.
The cells were exposed to tested substances in the range of concentrations from 0.01 to 50 µg/mL (depending on the method used) for 24 h at 37 °C in 5% CO2 atmosphere in total darkness. The final PBMCs density used in the experiments (after addition of BFR solution) was 4 × 106 cells/mL for caspase analysis and 1 × 106 cells/mL for assessment of other tested parameters. The viability of PBMCs in negative control was over 95%.
The lowest tested BFRs concentration used in this study corresponded to TBBPA concentration determined in humans environmentally exposed to this substance [14]. If no effects were observed at the above-mentioned BFRs levels, higher TBBPA and TBBPS concentrations were used.
5.2.2. Quantitative Determination of Apoptosis (Annexin V-FITC/PI Staining)
During apoptosis phosphatidylserine (PS) is translocated from the inner to the outer leaflet of the plasma membrane. The method is based on the ability of annexin V (labelled with fluorescein isothiocyanate—FITC) to bind to PS located on the outer monolayer of the cellular membrane. Propidium iodide (PI) is used in order to detect necrotic cells as it enters the cell through damaged membrane and binds to DNA. The experiment was conducted according to manufacturer’s procedure, as described by Jarosiewicz et al. [66].
PBMCs were incubated with TBBPA or TBBPS in the range from 1 to 50 µg/mL for 24 h at 37 °C in total darkness. After incubation, the cells were centrifuged (300× g) for 5 min at 4 °C and suspended in RPMI medium. Then, PBMCs were stained with the mixture of Annexin V-FITC and PI (1 µM each) dissolved in Annexin V-binding buffer and incubated for 20 min at room temperature in total darkness. In the cells, apoptosis was induced with camptothecin at 10 μM (positive control). The samples were detected by flow cytometry (LSR II, Becton Dickinson, Franklin Lakes, NJ, USA) (excitation/emission maxima: 488/525 nm for annexin V and 530/620 nm for PI, respectively). FMC gate on PBMCs was established and the data were recorded for a total of 10,000 cells per sample.
5.2.3. Cytosolic Calcium Ion Level
One of the primary parameter of apoptosis is the rise in the level of cytosolic Ca2+, which can be measured using stain Fluo-3/AM. Fluo-3/AM shows very low fluorescence, but after its hydrolysis by membrane esterases (Fluo-3 formation) and complexation with calcium ions, it exhibits about 100-fold increase in green fluorescence intensity. The analysis of calcium ion level in human PBMCs was previously described in detail by Mokra et al. and Barańska et al. [50,72].
The cells were incubated with TBBPA or TBBPS in the range from 0.01 to 5 µg/mL for 24 h at 37 °C in total darkness. Then, PBMCs were centrifuged (300× g) for 5 min at 4 °C, resuspended in solution of Fluo-3 AM (1 µM), and incubated for 20 min at 37 °C in total darkness. In the next step, HBSS consisting of 1% BSA was added to the cells, which were incubated for 40 min at 37 °C in total darkness. PBMCs were rinsed twice using HEPES buffer, and then centrifuged (300× g) for 5 min at 4 °C. Finally, the cells were resuspended in HEPES buffer, and incubated for 10 min at 37 °C in total darkness. Positive control contained the cells treated with ionomycin at 1 µM (calcium ionophore). The samples were detected using flow cytometry (LSR II, Becton Dickinson, Franklin Lakes, NJ, USA) (excitation/emission maxima: 488/525 nm for Fluo-3). FMC gate on PBMCs was established, and the data were recorded for a total of 10,000 cells per sample.
5.2.4. Mitochondrial Transmembrane Potential
A characteristic sign of early apoptosis is a reduction in transmembrane mitochondrial potential (ΔΨm), which can be determined by changes in the level of fluorescence of MitoLite Red CMXRos (excitation/emission maxima: 579/599 nm). This fluorescent stain is a cationic dye that readily penetrates living cells and accumulates in mitochondria, depending on the value of ΔΨm. Due to the presence of thiol-reactive chloromethyl moieties, this stain is retained in the mitochondria [50].
PBMCs were treated with TBBPA or TBBPS in the range of the concentrations from 0.01 to 25 µg/mL for 24 h at 37 °C in total darkness. Nigericin and valinomycin (1 µM), which are capable of increasing and decreasing ΔΨm, respectively, were used as positive controls. After the probes had been incubated, they were centrifuged (300× g) for 5 min at 4 °C. The supernatant was discarded, and the cells were resuspended in PBS solution. PBMCs were stained with MitoLite CMXRos at 1 µM, and then incubated for 20 min at 37 °C in total darkness. The probes were determined in 96-well plates using a microplate reader (Cary Eclipse, Varian, Waltham, MA, USA).
5.2.5. Caspase-3, -8, -9 Activation
The process of apoptosis is regulated directly and indirectly by caspases. Fluorimetric analysis of caspase-3 and caspase-8 activation was conducted according to the manufacturers’ protocols with slight modifications [72].
The methods of caspase-3 and caspase-8 detection are based on the hydrolysis of peptide substrates acetyl-Asp-Glu-Val-Asp-7-amino-4-methylcoumarin (Ac-DEVD-AMC) and acetyl-Ile-GluThr-Asp-7-amino-4-methylcoumarin (Ac-IETD-AMC), respectively. Hydrolysis of the substrates results in the release of fluorescent 7-amino-4-methylcoumarin (AMC) (excitation/emission maxima: 360/460 nm). Colorimetric determination of caspase-9 activity was associated with hydrolysis of the substrate Acetyl-Leu-Glu-His-Asp-p-nitroaniline (Ac-LEHD-pNA), which resulted in a release of p-nitroaniline (pNA) (absorption at 405 nm). In all experiments, positive controls were employed that contained cells suspension treated with camptothecin (10 µM). Preincubation with caspases inhibitors was also executed for all experiments. Determination of caspase-3 and caspase-8 activity was performed using a fluorescent microplate reader (Fluoroskan Ascent FL, Labsystem, Thermo-Fisher, Waltham, MA, USA), whereas detection of caspase-9 activity was done using an absorbance microplate reader (BioTek ELx808, Bio-Tek, Santa Clara, CA, USA).
5.2.6. PARP-1 Cleavage
Human poly (ADP-ribose) polymerase (PARP1) is a 116 kDa nuclear enzyme implicated in DNA repair. During apoptosis, caspase-3 cleaves PARP1 between Asp214 and Gly215, generating two fragments of 85 kDa and 25 kDa. The HLNC4 antibody (conjugated with Alexa fluor 488) added to the sample specifically recognizes the 85 kDa PARP1 fragment formed after the enzyme cleavage.
PBMCs were incubated with TBBPA or TBBPS at 25 ug/mL for 24 h at 37 °C in total darkness. Then, PBMCs were washed and suspended in 1% paraformaldehyde (dissolved in PBS). Then, HLNC4 antibody conjugated with Alexa fluor 488 was added to the probes, which were incubated for 30 min at 37 °C in total darkness. In the cells, apoptosis was induced by camptothecin at 10 μM (positive control). Cytometric analysis of the samples was conducted (LSR II, Becton Dickinson, Franklin Lakes, NJ, USA) at excitation/emission maxima of 494/519 nm for Alexa Fluor 488. FMC gate on PBMCs was established for data acquisition, and the data were recorded for 10,000 cells per sample.
5.2.7. APO-BrdU TUNEL Assay
The TUNEL method detects apoptosis by labelling the 3′-OH ends of single- and double-stranded DNA fragments with labelled Br-dUTP (brominated deoxyuridine triphosphate nucleotides). The reaction is catalyzed by terminal deoxynucleotidyl transferase (TdT) [73].
The cells were incubated with TBBPA or TBBPS at 25 µg/mL for 24 h at 37 °C in total darkness. Then, PBMCs were fixed in 1% paraformaldehyde. The samples were incubated (1 h at 37 °C in total darkness) in DNA labelling solution containing BrdUTP and TdT. Then, anti BrdUTP antibody was added to the probes, which were incubated for 30 min at room temperature in total darkness. In the cells, apoptosis was induced by camptothecin at 10 μM (positive control). The probes were determined by flow cytometry (LSR II, Becton Dickinson, Franklin Lakes, NJ, USA). Maxima of excitation and emission for Alexa Fluor 488 were 494 nm and 519 nm, respectively. FMC gate on PBMCs was established for data acquisition, and the data were recorded for 10,000 cells per sample.
5.2.8. Apoptosis Determination by Fluorescence Microscopy
Apoptotic changes in tested cells incubated with TBBPA or TBBPS were evaluated by their staining with Hoechst 33342 and PI and analyzing by fluorescence microscopy. The method was described by Rogalska et al. [48], while preparation of the samples (PBMCs) was presented in detail by Mokra et al. and Barańska et al. [50,72].
5.2.9. Statistical Analysis
Data are shown as average values with standard deviation. ANOVA (one-way analysis of variance) test and Tukey’s post-hoc test or Welch’s test (analysis of PARP1 cleavage and DNA fragmentation) were employed in order to evaluate statistical significance between examined probes [74]. Statistical significance was p < 0.05. All analyses were done using STATISTICA 13 software (StatSoft, Inc., Tulusa, OK, USA). The experiments were done on blood taken from 4 donors, while for each individual experiment (one blood donor), an experimental point was a mean value of 2–3 replications.
Author Contributions
Conceptualization, J.M.; methodology A.B.; validation, A.B.; investigation, A.B. and B.B.; writing—original draft preparation, A.B. and J.M., writing—review and editing, J.M. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was approved by Bioethical Commission of Scientific Research at the University of Lodz, no. 1/KBBN-UŁ/II/2017.
Informed Consent Statement
PBMCs were isolated from the leucocyte-buffy coat separated from blood bought in the Regional Centre of Blood Donation and Blood Treatment in Lodz, Poland. All the procedures related to blood donation were executed at the Regional Centre of Blood Donation and Blood Treatment in Lodz, Poland. Blood donors recruitment was completed at the Centre, according to national legal procedures and European Union regulations (incl. the regulation (EU) 2016/679 OF THE EUROPEAN PARLIAMENT AND OF THE COUNCIL of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data).
Data Availability Statement
The raw data supporting the conclusions of this paper are deposited in the Department of Biophysics of Environmental Pollution, University of Lodz and will be made available by the authors, without undue reservation.
Conflicts of Interest
The authors declare no conflict of interest.
Sample Availability
Samples of the compounds are not available from authors.
Funding Statement
This work was carried out under the research project PRELUDIUM 17 no. 2019/33/N/NZ7/01202 awarded by the National Science Centre, Poland, and the grant for Young Scientists no. B1911000002126.02 awarded by the Dean of the Faculty of Biology and Environmental Protection, University of Lodz.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Liu A., Shi J., Shen Z., Lin Y., Qu G., Zhao Z., Jiang G. Identification of unknown brominated bisphenol S congeners in contaminated soils as the transformation products of tetrabromobisphenol S derivatives. Environ. Sci. Technol. 2018;52:10480–10489. doi: 10.1021/acs.est.8b03266. [DOI] [PubMed] [Google Scholar]
- 2.Michałowicz J., Włuka A., Bukowska B. A review of environmental occurrence, toxic effects and transformation of man-made bromophenols. Sci. Total Environ. 2022;811:152289. doi: 10.1016/j.scitotenv.2021.152289. [DOI] [PubMed] [Google Scholar]
- 3.Yang H., Luo X.-J., Zeng Y.-H., Mai B.-X. Determination of tetrabromobisphenol-A/S and their eight derivatives in abiotic (soil/dust) samples using ultra-high performance liquid chromatography-tandem mass spectrometry. J. Chromat. A. 2021;1647:462152. doi: 10.1016/j.chroma.2021.462152. [DOI] [PubMed] [Google Scholar]
- 4.Kacew S., Hayes A. Absence of neurotoxicity and lack of neurobehavioral consequences due to exposure to tetrabromobisphenol A (TBBPA) exposure in humans, animals and zebrafish. Arch. Toxicol. 2019;94:59–66. doi: 10.1007/s00204-019-02627-y. [DOI] [PubMed] [Google Scholar]
- 5.Macêdo W., Sánchez F., Zaiat M. What drives tetrabromobisphenol A degradation in biotreatment systems? Rev. Environ. Sci. Bio/Technol. 2021;20:729–750. doi: 10.1007/s11157-021-09579-9. [DOI] [Google Scholar]
- 6.Xiong J.K., An T.C., Zhang C.S., Li G.Y. Pollution profiles and risk assessment of PBDEs and phenolic brominated flame retardants in water environments within a typical electronic waste dismantling region. Environ. Geochem. Health. 2015;37:457–473. doi: 10.1007/s10653-014-9658-8. [DOI] [PubMed] [Google Scholar]
- 7.Liu K., Li J., Yan S., Zghang W., Li Y., Han D. A review of status of tetrabromobisphenol A (TBBPA) in China. Chemosphere. 2016;148:8–20. doi: 10.1016/j.chemosphere.2016.01.023. [DOI] [PubMed] [Google Scholar]
- 8.Rothenbacher K., Pecquet A. Summary of historical terrestrial toxicity data for the brominated flame retardant tetrabromobisphenol A (TBBPA): Effects on soil microorganisms, earthworms, and seedling emergence. Environ. Sci. Pollut. Res. 2018;25:17268–17277. doi: 10.1007/s11356-018-2255-0. [DOI] [PubMed] [Google Scholar]
- 9.Abafe O.A., Martincigh B.S. Determination and human exposure assessment of polybrominated diphenyl ethers and tetrabromobisphenol A in indoor dust in South Africa. Environ. Sci. Pollut. Res. 2016;23:7038–7049. doi: 10.1007/s11356-015-6031-0. [DOI] [PubMed] [Google Scholar]
- 10.Xie Z., Ebinghaus R., Lohmann R., Heemken O., Caba A., Püttmann W. Trace determination of the flame retardant tetrabromobisphenol A in the atmosphere by gas chromatography–mass spectrometry. Anal. Chim. Acta. 2007;584:333–342. doi: 10.1016/j.aca.2006.10.062. [DOI] [PubMed] [Google Scholar]
- 11.Abdallah M.A.E., Harrad S., Covaci A. Hexabromocyclododecanes and tetrabromobisphenol-A in indoor air and dust in Birmingham, UK: Implications for human exposure. Environ. Sci. Technol. 2008;42:6855–6861. doi: 10.1021/es801110a. [DOI] [PubMed] [Google Scholar]
- 12.Cariou R., Antignac J.P., Zalko D., Berrebi A., Cravedi J.P., Maume D., Marchand P., Montenau F., Riu A., Andre F., et al. Exposure assessment of French women and their newborns to tetrabromobisphenol-A: Occurrence measurements in maternal adipose tissue, serum, breast milk and cord serum. Chemosphere. 2008;73:1036–1041. doi: 10.1016/j.chemosphere.2008.07.084. [DOI] [PubMed] [Google Scholar]
- 13.Fujii Y., Harada K.H., Hitomi T., Kobayashi H., Koizumi A., Haraguchi K. Temporal trend and age-dependent serum concentration of phenolic organohalogen contaminants in Japanese men during 1989–2010. Environ. Pollut. 2014;185:228–233. doi: 10.1016/j.envpol.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Lankova D., Lacina O., Pulkrabova J., Majslova J. The determination of perfluoroalkyl substances, brominated flame retardants and their metabolites in human breast milk and infant formula. Talanta. 2013;117:318–325. doi: 10.1016/j.talanta.2013.08.040. [DOI] [PubMed] [Google Scholar]
- 15.Fukuda N., Ito Y., Yamaguchi M., Mitumori K., Koizumi M., Hasegawa R., Kamata E., Ema M. Unexpected nephrotoxicity induced by tetrabromobisphenol A in newborn rats. Toxicol. Lett. 2004;150:145–155. doi: 10.1016/j.toxlet.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 16.Choi E., Suh K., Rhee S., Oh S., Kim S., Pak Y., Choe W., Ha J., Chon S. Exposure to tetrabromobisphenol A induces cellular dysfunction in osteoblastic MC3T3-E1 cells. J. Environ. Sci. Health Part A. 2017;52:561–570. doi: 10.1080/10934529.2017.1284435. [DOI] [PubMed] [Google Scholar]
- 17.Al-Mousa F., Michelangel F., Vavvas D. Some commonly used brominated flame retardants cause Ca2+-ATPase inhibition, beta-amyloid peptide release and apoptosis in SH-SY5Y neuronal cells. PLoS ONE. 2012;7:e33059. doi: 10.1371/journal.pone.0033059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koike E., Yanagisawa R., Takano H. Brominated flame retardants, hexabromocyclododecane and tetrabromobisphenol A, affect proinflammatory protein expression in human bronchial epithelial cells via disruption of intracellular signalling. Toxicol. In Vitro. 2016;32:212–219. doi: 10.1016/j.tiv.2015.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Sanders J., Coulter S., Knudsen G., Dunnick J., Kissling G., Birnbaum L. Disruption of estrogen homeostasis as a mechanism for uterine toxicity in Wistar Han rats treated with tetrabromobisphenol A. Toxicol. Appl. Pharmacol. 2016;298:31–39. doi: 10.1016/j.taap.2016.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yasmin S., Whalen M. Flame retardants, hexabromocyclododecane (HCBD) and tetrabromobisphenol a (TBBPA), alter secretion of tumor necrosis factor alpha (TNFα) from human immune cells. Arch. Toxicol. 2018;92:1483–1494. doi: 10.1007/s00204-018-2156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sharma P., Chadha P., Saini H. Tetrabromobisphenol A induced oxidative stress and genotoxicity in fish Channa punctatus. Drug Chem. Toxicol. 2019;42:559–564. doi: 10.1080/01480545.2018.1441864. [DOI] [PubMed] [Google Scholar]
- 22.Włuka A., Woźniak A., Woźniak E., Michałowicz J. Tetrabromobisphenol A, terabromobisphenol S and other bromophenolic flame retardants cause cytotoxic effects and induce oxidative stress in human peripheral blood mononuclear cells (in vitro study) Chemosphere. 2020;261:127705. doi: 10.1016/j.chemosphere.2020.127705. [DOI] [PubMed] [Google Scholar]
- 23.Barańska A., Woźniak A., Mokra K., Michałowicz J. Genotoxic mechanism of action of TBBPA, TBBPS and selected bromophenols in human peripheral blood mononuclear cells. Front. Immunol. 2022;13:869741. doi: 10.3389/fimmu.2022.869741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xu H., Li Y., Lu J., Lu J., Zhou L., Chovelo J.M., Ji Y. Aqueous photodecomposition of the emerging brominated flame retardant tetrabromobisphenol S (TBBPS) Environ. Pollut. 2021;271:116406. doi: 10.1016/j.envpol.2020.116406. [DOI] [PubMed] [Google Scholar]
- 25.Letcher R., Chu S. High-sensitivity method for determination of tetrabromobisphenol-S and tetrabromobisphenol-A derivative flame retardants in Great Lakes Herring Gull eggs by liquid chromatography−atmospheric pressure photoionization−tandem mass spectrometry. Environ. Sci. Technol. 2010;44:8615–8621. doi: 10.1021/es102135n. [DOI] [PubMed] [Google Scholar]
- 26.Liu K., Lu J., Ji Y. Formation of brominated disinfection by-products and bromate in cobalt catalyzed peroxymonosulfate oxidation of phenol. Water Res. 2015;84:1–7. doi: 10.1016/j.watres.2015.07.015. [DOI] [PubMed] [Google Scholar]
- 27.Qu G., Liu A., Hu A., Liu S., Shi J., Jiang G. Recent advances in the analysis of TBBPA/TBBPS, TBBPA/TBBPS derivatives and their transformation products. TrAC Trends Anal. Chem. 2016;83:14–24. doi: 10.1016/j.trac.2016.06.021. [DOI] [Google Scholar]
- 28.Wang X.L., Luan J., Lu Q.L., Lin H.G., Xu C. Three new metal–organic complexes derived from N,N′-bis(3-pyridinecarboxamide)-1,2-ethane and polycarboxylate ligands: Synthesis, fluorescent and electrochemical properties. J. Organometal. Chem. 2012;719:1–7. doi: 10.1016/j.jorganchem.2012.08.008. [DOI] [Google Scholar]
- 29.Song S., Song M., Zeng L., Wang T., Liu R., Ruan T., Jiang G. Occurrence and profiles of bisphenol analogues in municipal sewage sludge in China. Environ. Pollut. 2014;186:14–19. doi: 10.1016/j.envpol.2013.11.023. [DOI] [PubMed] [Google Scholar]
- 30.Yang B., Ying G.G., Chen Z.F., Zhao J.L., Peng F.Q., Chen X.W. Ferrate(VI) oxidation of tetrabromobisphenol A in comparison with bisphenol A. Water Res. 2014;62:211–219. doi: 10.1016/j.watres.2014.05.056. [DOI] [PubMed] [Google Scholar]
- 31.Malysheva S., Goscinny S., Malarvannan G., Poma G., Andjelkovic M. Development and validation of a quantitative UHPLC-MS/MS method for selected brominated flame retardants in food. Food Addit. Contam. Part A. 2018;35:293–304. doi: 10.1080/19440049.2017.1393110. [DOI] [PubMed] [Google Scholar]
- 32.Li A., Zhuang T., Shi W., Liang Y., Liao C., Song M., Jiang G. Serum concentration of bisphenol analogues in pregnant women in China. Sci. Total Environ. 2020;707:136100. doi: 10.1016/j.scitotenv.2019.136100. [DOI] [PubMed] [Google Scholar]
- 33.Ding Y., Dong X., Feng W., Mao G., Chen Y., Oiu X., Chen K., Xu H. Tetrabromobisphenol S alters the circadian rhythm network in the early life stages of zebrafish. Sci. Total Environ. 2022;806:150543. doi: 10.1016/j.scitotenv.2021.150543. [DOI] [PubMed] [Google Scholar]
- 34.Liang S., Liang S., Yin N., Hu B., Faiola F. Toxicogenomic analyses of the effects of BDE-47/209, TBBPA/S and TCBPA on early neural development with a human embryonic stem cell in vitro differentiation system. Toxicol. Appl. Pharmacol. 2019;379:114685. doi: 10.1016/j.taap.2019.114685. [DOI] [PubMed] [Google Scholar]
- 35.Pallardy M., Biola A., Lebrec H., Breard J. Assessment of apoptosis in xenobiotic-induced immunotoxicity. Methods. 1999;19:36–47. doi: 10.1006/meth.1999.0825. [DOI] [PubMed] [Google Scholar]
- 36.Zhang N., Hartig M., Dzhagalov I., Draper D., He Y.W. The role of apoptosis in the development and function of T lymphocytes. Cell Res. 2005;15:749–769. doi: 10.1038/sj.cr.7290345. [DOI] [PubMed] [Google Scholar]
- 37.Karuma M.M., Tan N.Y., Bennett M.R. Death receptors and their ligands in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2008;28:1694–1702. doi: 10.1161/ATVBAHA.107.155143. [DOI] [PubMed] [Google Scholar]
- 38.LaRosa F.D., Orange S.J. Lymphocytes. J. Allergy Clin. Immunol. 2008;121:364–369. doi: 10.1016/j.jaci.2007.06.016. [DOI] [PubMed] [Google Scholar]
- 39.Weinberg A., Jesser R., Edelstein C., Billy J., Wohl D. Excess apoptosis of mononuclear cells contributes to the depressed cytomegalovirus-specific immunity in HIV-infected patients on HAART. Virology. 2004;330:313–321. doi: 10.1016/j.virol.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 40.Ratomski K., Skotnicka B., Kasprzycka E., Żelazowska-Rutkowska B., Wysocka J., Anisimowicz S. Evaluation of percentage of the CD19+ CD5+ lymphocytes in hypertrophied adenoids at children with otitis media with effusion. Otolaryngol. Pol. 2007;61:962–966. doi: 10.1016/S0030-6657(07)70561-9. [DOI] [PubMed] [Google Scholar]
- 41.Zhou C.-X., Zhou D.-H., Liu G.-X., Suo X., Zhu X.-Q. Transcriptomic analysis of porcine PBMCs infected with Toxoplasma gondii RH strain. Acta Tropica. 2016;154:82–88. doi: 10.1016/j.actatropica.2015.11.009. [DOI] [PubMed] [Google Scholar]
- 42.Halit S., Salameh P. Exposure to toxins during pregnancy and childhood and asthma in children: A pilot study. J. Epidemiol. Global Health. 2017;7:147–154. doi: 10.1016/j.jegh.2017.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sen P., Kemppainen E., Orešič M. Perspectives on Systems Modeling of Human Peripheral Blood Mononuclear Cells. Front. Mol. Biosci. 2017;4:96. doi: 10.3389/fmolb.2017.00096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Feiterio J., Mariana M., Cairrao E. Health toxicity effects of brominated flame retardants: From environmental to human exposure. Environ. Pollut. 2021;285:117475. doi: 10.1016/j.envpol.2021.117475. [DOI] [PubMed] [Google Scholar]
- 45.Hall S.M., Coulter S.J., Knudsen G.A., Sanders J.M., Birnbaum L.S. Gene expression changes in immune response pathways following oral administration of tetrabromobisphenol A (TBBPA) in female Wistar Han rats. Toxicol. Lett. 2017;272:68–74. doi: 10.1016/j.toxlet.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cato A., Celada L., Kibakaya E., Simmons N., Whalen M. Brominated flame retardants, tetrabromobisphenol A and hexabromocyclododecane, activate mitogen-activated protein kinases (MAPKs) in human natural killer cells. Cell. Biol. Toxicol. 2014;30:345–360. doi: 10.1007/s10565-014-9289-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Elmore S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rogalska A., Gajek A., Szwed M., Joźwiak Z., Marczak A. The role of reactive oxygen species in WP 631-induced death of human ovarian cancer cells: A comparison with the effect of doxorubicin. Toxicol. Vitr. 2011;25:1712–1720. doi: 10.1016/j.tiv.2011.08.009. [DOI] [PubMed] [Google Scholar]
- 49.Rutkowska J. Zjawisko apoptozy w chorobach alergicznych. Now. Lek. 2007;76:48–54. [Google Scholar]
- 50.Mokra K., Kocia M., Michałowicz J. Bisphenol A and its analogs exhibit different apoptotic potential in peripheral blood mononuclear cells (in vitro study) Food Chem. Toxicol. 2015;84:79–88. doi: 10.1016/j.fct.2015.08.007. [DOI] [PubMed] [Google Scholar]
- 51.Cho J.-H., Lee S., Jeon H., Kim A., Lee W., Lee Y., Yang S., Yun J., Jung Y.-S., Lee J. Tetrabromobisphenol A-induced apoptosis in neural stem cells through oxidative stress and mitochondrial dysfunction. Neurotox. Res. 2020;38:74–85. doi: 10.1007/s12640-020-00179-z. [DOI] [PubMed] [Google Scholar]
- 52.Zhang Y., Wang X., Chen C., An J., Shang Y., Li H., Xa H., Yu J., Wang C., Liu Y., et al. Regulation of TBBPA-induced oxidative stress on mitochondrial apoptosis in L02 cells through the Nrf2 signaling pathway. Chemosphere. 2019;226:463–471. doi: 10.1016/j.chemosphere.2019.03.167. [DOI] [PubMed] [Google Scholar]
- 53.Suh K., Choi E., Rhee S., Oh S., Kim S., Pak Y., Choe W., Ha J., Chon S. Tetrabromobisphenol A induces cellular damages in pancreatic β-cells in vitro. J. Environ. Sci. Health Part A. 2017;52:624–631. doi: 10.1080/10934529.2017.1294964. [DOI] [PubMed] [Google Scholar]
- 54.Lu L., Hu J., Li G., An T. Low concentration Tetrabromobisphenol A (TBBPA) elevating overall metabolism by inducing activation of the Ras signalling pathway. J. Hazard. Mat. 2021;416:125797. doi: 10.1016/j.jhazmat.2021.125797. [DOI] [PubMed] [Google Scholar]
- 55.Wu S., Ji G., Liu J., Zhang S., Gong Y., Shi L. TBBPA induces developmental toxicity, oxidative stress, and apoptosis in embryos and Zebrafish Larvae (Danio rerio) Environ. Toxicol. 2016;31:1241–1249. doi: 10.1002/tox.22131. [DOI] [PubMed] [Google Scholar]
- 56.Hajnoczky G., Davies E., Madesh M. Calcium signalling and apoptosis. Biochem. Biophys. Res. Commun. 2003;304:445–454. doi: 10.1016/S0006-291X(03)00616-8. [DOI] [PubMed] [Google Scholar]
- 57.Kaufman R.J., Scheuner D., Schröder M. The unfolded protein response in nutrient sensing and differentiation. Nat. Rev. Mol. Cell Biol. 2002;3:411–421. doi: 10.1038/nrm829. [DOI] [PubMed] [Google Scholar]
- 58.Kroemer G., Reed J.C. Mitochondrial control of cell death. Nat. Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- 59.Ogunbayo O., Lai P., Conolly T., Michelangeli A. Tetrabromobisphenol A (TBBPA), induces cell death in TM4 Sertoli cells by modulating Ca2+ transport proteins and causing dysregulation of Ca2+ homeostasis. Toxicol. Vitr. 2008;22:943–952. doi: 10.1016/j.tiv.2008.01.015. [DOI] [PubMed] [Google Scholar]
- 60.Łabędzka K., Grzanka A., Izdebska M. Mitochondrium a śmierć komórki. Post. Hig. Med. Dośw. 2006;60:439–446. [PubMed] [Google Scholar]
- 61.Hongmei Z. Apoptosis and Medicine. Volume 16. IntechOpen; London, UK: 2012. Extrinsic and intrinsic apoptosis signal pathway review; pp. 1–22. [Google Scholar]
- 62.Stępień A., Izdebska M., Grzanka A. Rodzaje śmierci komórki. Post. Hig. Med. Dośw. 2007;61:420–428. [PubMed] [Google Scholar]
- 63.Pihán P., Carreras-Sureda A., Hetz C. BCL-2 family: Integrating stress responses at the ER to control cell demise. Cell Death Different. 2017;24:1478–1487. doi: 10.1038/cdd.2017.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Szychowski K., Wójtowicz A. TBBPA causes neurotoxic and the apoptotic responses in cultured mouse hippocampal neurons in vitro. Pharmacol. Rep. 2015;68:20–26. doi: 10.1016/j.pharep.2015.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Wu S., Wu M., Qi M., Zhong L., Qiu L. Effects of novel brominated flame retardant TBBPA on human airway epithelial cell (A549) in vitro and proteome profiling. Environ. Toxicol. 2018;33:1245–1253. doi: 10.1002/tox.22632. [DOI] [PubMed] [Google Scholar]
- 66.Jarosiewicz M., Michałowicz J., Bukowska B. In vitro assessment of eryptotic potential of tetrabromobisphenol A and other bromophenolic flame retardants. Chemosphere. 2019;215:404–412. doi: 10.1016/j.chemosphere.2018.09.161. [DOI] [PubMed] [Google Scholar]
- 67.Mashimo M., Onishi M., Uno A., Tanimichi A., Nobeyama A., Mori M., Yamada S., Negi S., Bu X., Kato J., et al. The 89-kDa PARP1 cleavage fragment serves as a cytoplasmic PAR carrier to induce AIF-mediated apoptosis. J. Biol. Chem. 2021;296:100046. doi: 10.1074/jbc.RA120.014479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chmielewski M., Linke K., Zabel M. Metody wykrywania zjawiska apoptozy w komórkach wątrobowych in situ. Now. Lek. 2008;77:223–226. [Google Scholar]
- 69.Park C., Se-Jin K., Lee W.K., Moon S.K., Kwak S., Choe S.K., Park R. Tetrabromobisphenol-A induces apoptotic death of auditory cells and hearing loss. Biochem. Biophys. Res. Commun. 2016;478:1667–1673. doi: 10.1016/j.bbrc.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 70.Zatecka E., Castillo J., Elzeinova F., Kubatova A., Ded J., Peknicova J., Oliva R. The effect of tetrabromobisphenol A on protamine content and DNA integrity in mouse spermatozoa. Andrology. 2014;2:910–917. doi: 10.1111/j.2047-2927.2014.00257.x. [DOI] [PubMed] [Google Scholar]
- 71.Lue J., Han L.-W., Lin J.-X., Cao R. Organic complexes built by halogenated molecules: Unexpected in situ C_N bond formation in metal-free solvothermal conditions. Cryst. Growth Des. 2010;10:4217–4220. [Google Scholar]
- 72.Barańska A., Sicińska P., Michałowicz J. Apoptosis-inducing potential of selected bromophenolic flame retardants 2,4,6-tribromophenol and pentabromophenol in human peripheral blood mononuclear cells. Molecules. 2022;27:5056. doi: 10.3390/molecules27165056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Gralewska P., Gajek A., Marczak A., Rogalska A. Metformin affects olaparib sensitivity through induction of apoptosis in epithelial ovarian cancer cell lines. Int. J. Mol. Sci. 2021;22:10557. doi: 10.3390/ijms221910557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Watała C. Biostatystyka—Wykorzystanie Metod Statystycznych w Pracy Badawczej w Naukach Biomedycznych. 2nd ed. Alfa-Medica Press; Bielsko-Biała, Poland: 2002. pp. 54–68. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The raw data supporting the conclusions of this paper are deposited in the Department of Biophysics of Environmental Pollution, University of Lodz and will be made available by the authors, without undue reservation.