Abstract
With the recent outbreak of the COVID-19 pandemic and emergency use authorization of anti-SARS-CoV-2 vaccines, reports of post-vaccine immune thrombocytopenia (ITP) have gained attention. With this systematic review, we aim to analyze the clinical characteristics, therapeutic strategies, and outcomes of patients presenting with ITP after receiving COVID-19 vaccination. Medline, Embase, and Ebsco databases were systematically explored from inception until 1 June 2022. Case reports and case series investigating the association between the anti-SARS-CoV-2 vaccine and ITP were included. We found a total of 66 patients. The mean age of presentation was 63 years with a female preponderance (60.6%). Sixteen patients had pre-existing ITP. The mean time from vaccine administration to symptom onset was 8.4 days. More ITP events were triggered by mRNA vaccines (BNT162b2 (n = 29) > mRNA-1273 (n = 13)) than with adenoviral vaccines (ChAdOx1-S AstraZeneca (n = 15) > Ad26.COV2-S (n = 9)). Most of the patients were treated with steroids or IVIG, or both. The overall outcome was promising, with no reported deaths. Our review attempts to increase awareness among physicians while evaluating patients presenting with thrombocytopenia after receiving the vaccine. In our solicited opinion, the rarity of these events and excellent outcomes for patients should not change views regarding the benefits provided by immunization.
Keywords: COVID-19 vaccine, BNT162b2 vaccine, mRNA-1273 vaccine, Ad26.COV2-S vaccine, ChAdOx1 nCoV-19 vaccine, thrombocytopenia, immune thrombocytopenia, ITP, immune thrombocytopenic purpura, idiopathic thrombocytopenic purpura
1. Introduction
Immune thrombocytopenia (ITP) is an autoimmune disorder characterized by low platelet counts (<100 × 103/μL) unexplainable by an alternative etiology [1]. ITP carries an annual incidence of about 3 cases per 100,000 adults, with a predilection for the female gender in the younger population [2]. Although patients may be asymptomatic upon presentation, typical clinical features include mucocutaneous bleeding, such as petechiae; purpura; ecchymoses; and sometimes, hemorrhage, with intracranial being the most serious [1,3]. While primary ITP is idiopathic in origin, secondary ITP can be caused by other autoimmune disorders, cancer, infection, or medications and accounts for less than a fourth of total ITP cases [4]. Amongst drugs, almost half of the cases are attributed to vaccines, with the measles-mumps-rubella (MMR) vaccine being the most common culprit [5]. With the recent outbreak of the COVID-19 pandemic and emergency use authorization of anti-SARS-CoV2 vaccines, reports of post-vaccine thrombocytopenia have gained attention [6]. Vaccine-induced immune thrombotic thrombocytopenia (VITT) has now been increasingly recognized, predominantly after the administration of adenovirus-vector-based vaccines [7]. It is marked by the formation of widespread thrombi and positive platelet factor 4 antibodies, a lab parameter classically seen in patients with heparin-induced thrombocytopenia. Furthermore, reports of post-vaccine thrombotic thrombocytopenic purpura, defined by low ADAMTS-13 activity and microangiopathic hemolytic anemia, have also emerged [8]. Contrary to these two entities, vaccine-related ITP involves isolated thrombocytopenia and has a relatively favorable prognosis. That said, patients with ITP still carry a higher thromboembolism risk and increased mortality compared to the general population [2]. Therefore, it becomes imperative to understand its epidemiology in relation to the administration of COVID-19 vaccines. With this review, we aim to analyze the clinical characteristics, presenting features, laboratory parameters, therapeutic strategies, and outcomes of patients presenting with ITP after receiving COVID-19 vaccination.
2. Materials and Methods
2.1. Search Strategy and Selection of Studies
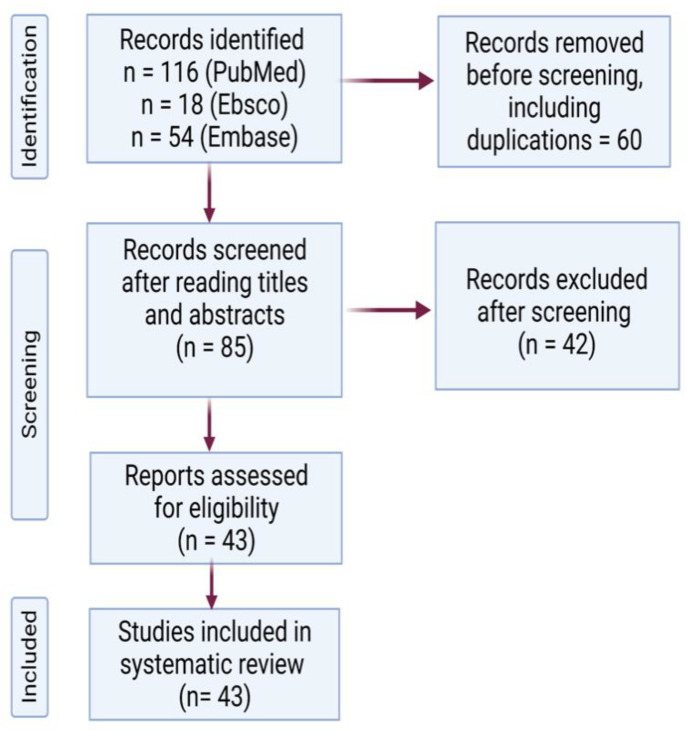
Following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines, Medline, Embase, and Ebsco databases were systematically explored from inception until 1 June 2022, with the following keywords: “Purpura, Thrombocytopenic, Idiopathic” (Mesh) AND “COVID-19 Vaccines” (Mesh) OR “2019-nCoV Vaccine mRNA-1273” (Mesh) OR “BNT162 Vaccine” (Mesh) OR “ChAdOx1 nCoV-19” (Mesh) OR “Ad26COVS1” (Mesh). The search was accomplished by two independent authors (PS and NG). Papers hence identified underwent screening at the title and abstract level. The following inclusion criteria were used: 1. case reports and case series investigating the association between the anti-SARS-CoV-2 vaccine and ITP; 2. presence of isolated thrombocytopenia in the absence of thrombosis; 3. ITP is diagnosed after ruling out other causes of thrombocytopenia. Duplicate articles, articles in languages other than English, and studies reporting other causes of thrombocytopenia were excluded. Cohort studies, data from surveillance systems, and review articles were excluded as detailed information on demographics, treatment, and outcome was required for each patient to synthesize the results of this analysis. After reviewing the full text of the eligible articles and overcoming disagreements through discussion, a total of 43 case reports (66 patients) were included in this study (Figure 1).
Figure 1.
PRISMA flow diagram for selected studies.
2.2. Data Extraction
We extracted the following data: 1. author name and year of publication; 2. gender of the patient and age at presentation; 3. comorbidities; 4. presenting features; 5. platelet counts at presentation, or nadir if admission counts were not reported; 6. type and dose of the vaccine received; 7. time to symptom onset or presentation post-vaccine, whichever came early; 8. therapies received; 9. outcomes; and 10. whether the second dose, if applicable, was administered or not.
3. Results
We found a total of 66 patients with ITP following COVID-19 vaccination, as listed in Table 1. The median age of presentation was 52 years (range: 19–86 years) with a female preponderance (60.6%, n = 40). Twenty-four patients had a pre-existing autoimmune disease (17 had pre-existing ITP), one was nine weeks pregnant, and one was receiving immunotherapy (durvalumab) for refractory lung adenocarcinoma. One patient with chronic ITP had a history of flare-up post-Shingrix vaccine. On presentation, two patients had concurrent active Hepatitis C and HIV viral infection, one had autoimmune hemolytic anemia (Evans syndrome), and one patient had weakly positive platelet factor 4 antibodies. Most of the patients (85%) presented with spontaneous mucocutaneous bleeding (gums > nose) or petechiae. Two patients presented with hemoptysis, and none with life-threatening intracranial hemorrhage. The mean time from vaccine administration to symptom onset was 8.4 days, with 73% of patients (n = 48) presenting after the first dose and 27% of patients (n = 18) after the second dose. More ITP events were triggered by mRNA vaccines (BNT162b2 (n = 29) > mRNA-1273 (n = 13)) than with adenoviral vaccines (ChAdOx1-S AstraZeneca (n = 15) > Ad26.COV2-S Johnson & Johnson (n = 9)). On laboratory workup, two patients had positive SS-A antibodies, one had positive GPIb IgG, two had positive lupus anticoagulant, and three had positive GPIIb/IIIa antibodies. A total of 71% of patients (n = 47) had thrombocytopenia of ≤10 × 103/μL. Most patients were treated with steroids or IVIG, or both. Escalation of therapy with rituximab and thrombopoietin receptor agonists (TPO-RA) (eltrombopag or romiplostim) was needed in 22 patients (four patients had pre-existing ITP while 18 were newly diagnosed), out of which, in addition, two received vinca alkaloids, two received aminocaproic acid, one received danazol, one received Rho IgG, and one received fostamatinib. Seven patients, all with platelet counts of >30 × 103/μL, were not treated. The overall outcome was promising, with no reported deaths. Ten patients had a relapse, either during hospitalization or post-discharge. One patient had an emergency room visit due to iatrogenic thrombocytosis from treatment (platelet transfusion, IVIG, steroids, TPO-RA, and vincristine during hospitalization). Four patients who developed ITP after the first dose received the second dose of mRNA-based vaccines with no further relapse. A comparison of new cases of ITP versus relapse post-vaccination is illustrated in Table 2.
Table 1.
Characteristics of included studies.
| Author, Year | Age | Sex | Presenting Features | Time to Symptom Onset or Presentation after Vaccine (in Days) | Vaccine Dose | Comorbidities | Admission Platelet Counts or Nadir (Whichever Is Reported First) | Treatment Received | Outcomes | Second Dose, If Applicable |
|---|---|---|---|---|---|---|---|---|---|---|
| Cases due to Ad26.COV2-S (Johnson & Johnson vaccine) | ||||||||||
| Shah et al., 2021 [4] | 59 | F | Abdominal cramps, diarrhea | 2 | - | Chronic ITP and SLE (prior flare-up 2 years ago after Shingrix vaccine) | 64 × 103/μL | Dexamethasone | Discharged | No comment |
| Banerjee et al., 2021 [9] | 63 | F | Bleeding gums | 14 | - | Cervical cancer s/p hysterectomy | 2 × 103/μL | Platelets, Prednisone, IVIG, and dexamethasone | Discharged on day 5 | - |
| Cases due to ChAdOx1 nCoV-19 vaccine | ||||||||||
| Scanvion et al., 2021 [10] | 62 | F | Asymptomatic | 6 | First dose | ITP, overweight, HLD, HTN | 60 × 103/μL | None | No hospitalization | No comment |
| Scanvion et al., 2021 [10] | 45 | F | Asymptomatic | 3 | First dose | ITP | 53 × 103/μL | None | No hospitalization | No comment |
| Candelli et al., 2021 [11] | 28 | M | Petechiae, oral bleed, fatigue, headache, fever | 1 | First dose | None | 4 × 103/μL (Lupus anticoagulant positive) | Four-day Dexamethasone course, once during hospitalization and once 10 days after discharge | Discharged after 4 days | No comment |
| Paulsen et al., 2021 [12] | 72 | M | Petechiae, hematomas | 11 | First dose | Radioiodine-treated autoimmune thyroiditis | <5 × 103/μL | Prednisolone, IVIG | Discharged | No comment |
| Paulsen et al., 2021 [12] | 71 | F | Petechiae, headache | 11 | First dose | Latent hyperthyroidism, breast cancer, stroke | <5 × 103/μL | Prednisolone followed by dexamethasone, IVIG, TPO-RA | Discharged but readmitted 7 days after | No comment |
| Paulsen et al., 2021 [12] | 66 | M | Petechiae, hyposphagma | 1 | First dose | HTN, mild thrombocytopenia | < 5 × 103/μL | Prednisolone | Discharged | No comment |
| Paulsen et al., 2021 [12] | 64 | F | Petechiae, epistaxis | 15 | First dose | HTN, COPD, hepatic steatosis | 6 × 103/μL | Prednisolone | Discharged | No comment |
| Gardellini et al., 2021 [13] | 63 | M | Hematomas, epistaxis | 14 | First dose | DM, HTN, HLD | 2 × 103/μL | Prednisone | Discharged | Second dose given after 9 weeks |
| Kim et al., 2021 [14] | 66 | F | Bruising, gum bleeding | 2 | First dose | None | 4 × 103/μL | Dexamethasone and IVIG | Discharged | No comment |
| Sivaramakrishnan et al., 2022 [15] | - (middle-aged) | F | Hemoptysis and menorrhagia | 11 (had been evaluated 30 days after first dose for similar complaints and was given platelets) | First and second doses | None | 10 × 103/μL | Prednisolone | Discharged with relapse after 3 weeks | - |
| Al-Ahmad et al., 2022 [16] | 56 | M | Admitted for partial small bowel obstruction | 14 | First dose | Primary ITP s/p splenectomy | 9 × 103/μL | IVIG and TPO-RA (refused steroids) | Discharged with a repeat dose of TPO-RA for worsening counts | No comment |
| Al-Ahmad et al., 2022 [16] | 63 | F | Petechiae | 10 | Second dose | Chronic ITP | 35 × 103/μL | None | Not admitted | No comment |
| Al-Ahmad et al., 2022 [16] | 28 | F | Petechiae, gum, and nosebleed | 10 | First dose | Chronic ITP (maintained on romiplostim and prednisolone) | 30 × 103/μL | Romiplostim was continued and prednisolone dose was increased | Not admitted | No comment |
| Al-Ahmad et al., 2022 [16] | 54 | F | Ecchymoses | 13 | First dose | None | 10 × 103/μL | Prednisolone and IVIG | Discharged after 6 days – readmitted 3 days later with counts of 10 × 103/μL | No comment |
| Al-Ahmad et al., 2022 [16] | 33 | F | Ecchymoses | 21 | First dose | None | 3 × 103/μL | IVIG, Prednisolone, and Romiplostim | Discharged after 7 days, was readmitted 26 days later with recurrence | No comment |
| Wong et al., 2022 [17] | 86 | M | Gingival bleeding, ecchymosis, and tongue blisters | 2 | First dose | NA | 4 × 103/μL | Dexamethasone, platelets, IVIG and Rituximab | Discharged | NA |
| Wong et al., 2022 [17] | 38 | F | Petechiae and purpura | 10 | First dose | NA | 3 × 103/μL | Prednisone and IVIG | Discharged | NA |
| Razzaq et al., 2021 [18] | 26 | M | Asymptomatic | 2 | First dose | Mild thrombocytopenia | 64 × 103/μL | Methylprednisolone and IVIG | Discharged | No comment |
| Uaprasert et al., 2022 [19] | 80 | M | Bleeding from bitten tongue | 19 | First dose | None | 14 × 103/μL | Dexamethasone, prednisolone, IVIG, TPO-RA | Improved | No comment |
| Uaprasert et al., 2022 [19] | 84 | M | Dizziness | 9 | First dose | Adrenal insufficiency due to adrenal histoplasmosis, cirrhosis, past HBV infection | 36 × 103/μL (HCV and HIV positive) | None | Improved | No comment |
| Uaprasert et al., 2022 [19] | 55 | F | Purpura and oral bleeding | 24 | First dose | HLD | 41 × 103/μL | None | Improved | No comment |
| Liao et al., 2021 [20] | 79 | M | Asymptomatic | 8 | First dose | Stroke | 2 × 103/μL | Hydrocortisone followed by prednisolone | Discharged after 12 days | No comment |
| Cases due to BNT162b2 vaccine | ||||||||||
| Ganzel et al., 2021 [21] | 53 | M | Epistaxis, purpura, petechiae | 14 | First dose | Obesity, DM, HTN | 1 × 103/μL | Dexamethasone and IVIG | Improved | Second dose not given |
| Tarawneh et al., 2021 [22] | 22 | M | Petechiae, gum bleeding | 3 | First dose | None | 2 × 103/μL (mild transaminitis, SSA-antibody which later normalized) | Dexamethasone, platelet transfusion, and IVIG | Discharged after 5 days | No comment |
| Fueyo-Rodriguez et al., 2021 [23] | 41 | F | Fever, tachycardia, nausea | 1 | First dose | HTN, hypothyroidism, pre-DM | 65 × 103/μL (elevated IgE and CRP) | Methylprednisolone, dexamethasone, and IVIG | Discharge after 5 days | No comment |
| Shah et al.,2021 [4] | 53 | M | Fever, chills, myalgia, petechiae | 8 | Second dose | Crohn’s disease | 2 × 103/μL | Dexamethasone and IVIG | Discharged | No comment |
| Shah et al.,2021 [4] | 67 | M | Generalized weakness, melena, petechiae | 2 | First dose | Seizures, atrial fibrillation, chronic ITP in remission | 2 × 103/μL | Platelet, IVIG and dexamethasone | Discharged | Second dose not advised |
| Jawed et al., 2021 [24] | 47 | F | Gum bleeding, epistaxis | 18 | First dose | Chronic ITP, Hypothyroidism secondary to Hashimoto’s thyroiditis | 1 × 103/μL | Platelet, IVIG | Discharged | No comment |
| King et al., 2021 [25] | 39 | F | Petechiae | 1 | Second dose | PCOS | 1 × 103/μL (elevated ESR) | Platelet, methylprednisolone, and IVIG | Discharged after 3 days | No comment |
| Gardellini et al., 2021 [13] | 27 | M | Hematomas, epistaxis | 10 | First dose | None | 1 × 103/μL | IVIG, prednisolon, dexamethasone | Discharged | No comment |
| Gardellini et al., 2021 [13] | 39 | F | Petechiae, ecchymosis | 6 | Second dose | Chronic ITP | 1 × 103/μL | IVIG-prednisone, TPO-RA | Not reported | No comment |
| Qasim et al., 2021 [26] | 28 | M | Petechiae and epistaxis | 2 | Second dose | ITP | 1 × 103/μL | IVIG and dexamethasone | Discharged on prednisolone taper | Not reporter |
| Shonai et al., 2021 [27] | 69 | M | Oral bleeding and hemoptysis | 10 | Second dose (had asymptomatic thrombocytopenia after first dose) | Well-controlled postoperative intestinal obstruction and hypopharyngeal cancers s/p permanent tracheal fistula surgery | 6 × 103/μL (H pylori antibody positive) | Prednisolone | Improved (refused hospitalization) | No comment |
| Krajewski et a., 2021 [28] | 74 | M | Hemorrhagic mucosal blisters and purpura | 1 | First dose | HTN | 2 × 103/μL | Platelet and Dexamethasone | No comment | No comment |
| Al-Ahmad et al., 2022 [16] | 19 | M | Mouth and nosebleed | 4 | Second dose | Chronic ITP (maintained on eltrombopag) | 4 × 103/μL | Methylprednisolone, prednisolone, and increased dose of eltrombopag | Left against medical advice | No comment |
| Idogun et al., 2021 [29] | 54 | F | Petechiae, ecchymosis, mucosal bleeding | 7 days after first dose but presented 5 days after second dose (21 days after symptom onset) | First and second doses | HTN, congenital epidermal dysplasia, overactive bladder, mild cognitive impairment, CKD and anxiety | 0 | Platelet, dexamethasone, IVIG | Discharged but was readmitted after 4 days | - |
| Hidaka et al., 2022 [30] | 53 | F | Shortness of breath | 14 days after second dose but had transient wheezing and purpura after first dose | First and second doses | Asthma, Vogt-Koyanagi-Harada disease, Hashimoto disease | 39 × 103/μL (Also had AIHA, lupus anticoagulant and ANA positive, hypocomplementemia, COVID IgG positive) | Prednisolone, blood transfusion (for Evans syndrome associated with SLE post-COVID vaccination) | Discharged | - |
| Al-Ahmad et al., 2022 [16] | 30 | F | Petechiae | 7 | First dose | Chronic migraine, depression, chronic ITP | 40 × 103/μL | None | Not admitted | No comment |
| Saito et al., 2022 [31] | 66 | F | Malaise, lymphadenopathy, fever, hematuria, oral bleeding, and purpura | 2 | First dose | None | <1 × 103/μL (positive antiplatelet glycoprotein IIb/IIIa antibodies, elevated inflammatory markers) | Platelet, IVIG, prednisolone, pulsed methylprednisolone, TPO-RA, danazol and vincristine | Discharged on day 22 | No comment |
| Pasin et al., 2022 [32] | 84 | M | Petechiae, gum bleeding | 5 | First dose | Localized bladder cancer, tremors, mild CKD, Atrial fibrillation on apixaban | 3 × 103/μL (SARS-CoV-2 negative; positive antiplatelet glycoprotein IIb/IIIa antibodies) | Platelet, IVIG and prednisone | Improved | None |
| Nakamura et al., 2022 [33] | 32 | F | Petechiae, oral bleeding | 5 | Second dose | None | <1 × 103/μL (Platelet associated GPIbα IgG) | Prednisolone | Discharged on day 12 | No comment |
| Al-Ahmad et al., 2022 [16] | 37 | F | Petechiae | 10 | Second dose | Primary ITP | 25 × 103/μL | Prednisolone | Improved | No comment |
| Al-Ahmad et al., 2022 [16] | 30 | M | Fatigue, petechiae, gum bleeding, epistaxis | 7 | First dose | Primary ITP (on eltrombopag) | 11 × 103/μL | Prednisolone, IVIG and eltrombopag | Discharged on day 2 | Allowed to take second dose with close follow-up |
| Al-Ahmad et al., 2022 [16] | 56 | F | Gum and nose bleeding | 7 | Second dose | HTN, DM | 2 × 103/μL | IVIG, prednisolone and TPO-RA | Discharged after 3 days but readmitted 2 weeks later | No comment |
| Ogai et al., 2021 [34] | 64 | F | Oral bleeding and petechiae | 2 | First dose | Chronic ITP | 1 × 103/μL | Prednisolone and IVIG | Improvement | No comment |
| Ogai et al., 2021 [34] | 61 | F | Petechiae | 17 | Second dose | Chronic ITP, Scleroderma and Sjogren syndrome | 1 × 103/μL | Platelet, Prednisolone and TPO-RA | Improvement | No comment |
| Battegay et al., 2021 [35] | 77 | M | Asymptomatic; petechiae on buccal mucosa | 8 | First dose | CAD, Atrial fibrillation, HTN (Had mild thrombocytopenia pre-vaccination) | 28 × 103/μL | IVIG, prednisone and TPO-RA | Improvement | Second dose under eltrombopag taper |
| Ghosh et al., 2022 [36] | 63 | F | Rash and easy bruising | 1 | Second dose | COPD, HTN, DM | 0 (positive for SS-A and scleroderma antibodies) | Dexamethasone, IVIG, TPO-RA, rituximab | Discharged | - |
| Akiyama et al., 2021 [37] | 20 | F | Subcutaneous hemorrhage | 12 | First dose | None | 16 × 103/μL | Prednisolone | Improved | No comment |
| Jasaraj et al., 2021 [38] | 67 | F | Petechiae, gum bleeding, epistaxis, subconjunctival hemorrhage | 2 | Second dose (also had symptoms 14 days after the first dose) | HTN, DM, hypothyroidism, depression, b12 deficiency, headaches | 3 × 103/μL | Prednisone, IVIG, platelet, ACA, rituximab, TPO-RA | Discharged on day 14 (received rituximab and TPO-RA outpatient) | - |
| Ghous et al., 2021 [39] | 69 | F | Bruising and gum bleeding | 14 | First dose | Cataract, SCC, BCC | 5 × 103/μL (AST and LDH were high) | Platelet, IVIG, dexamethasone, TPO-RA, vincristine, prednisone | Discharged with return to ER for iatrogenic thrombocytosis | No comment |
| Cases due to mRNA-1273 vaccine | ||||||||||
| Abuhelwa et al., 2021 [40] | 65 | F | Epistaxis and rash | 1 | First dose | None | 3 × 103/μL | Platelets, IVIG, dexamethasone, Rho immunoglobulins and TPO-RA | Discharged on day 14 | No comment |
| Prasad et al., 2021 [41] | 58 | M | Mucosal bleed, petechiae | 21 | First dose | HTN, DM | 3 × 103/μL (PF4 antibody was weakly positive but SRA negative) | Dexamethasone, platelets, IVIG, second relapse treated with platelets, IVIG, TPO-RA and fostamatinib | Discharged in 6 days but presented 5 days later with recurrence, and then again 10 days later | Refused |
| Ogai et al., 2021 [34] | 73 | F | Petechiae | 11 | First dose | HTN, HLD | 2 × 103/μL | Prednisolone, IVIG, and TPO-RA | Improvement | No comment |
| Chanut et al., 2022 [42] | 73 | F | Epistaxis, intra-buccal hemorrhage, and bruises | 7 | First dose | IgA monoclonal gammopathy of undetermined significance, HTN, HLD, hypothyroidism, glaucoma | 2 × 103/μL | IVIG | Discharged | Rechallenged with BNT162b2 vaccine with no relapse |
| Helms et al., 2021 [43] | 74 | M | Epistaxis and diffuse cutaneous purpura | 1 | First dose | HTN, Gout, HLD and nonischemic cardiomyopathy | 10 × 103/μL | Dexamethasone, IVIG, rituximab, TPO-RA | Discharged on day 5; readmitted on day 13 | No comment |
| Chong et al., 2022 [44] | 75 | F | Hemoptysis | 3 | First dose | Refractory lung adenocarcinoma on durvalumab | 7 × 103/μL (prior hepatitis B infection) | Platelets, prednisolone | Discharged on day 5 | Second dose not advised |
| Malayala et al., 2021 [45] | 60 | M | Purpura, nausea, vomiting, shortness of breath, leg edema, chest and abdominal pain | 1 | First dose | Hepatitis C infection, CKD-stage IV, HTN, HFrEF, smoker | 84 × 103/μL (With deranged LFTs – heavy hepatitis C viral load and cirrhosis) | None | Left against medical advice on day 3 of hospitalization | No comment |
| Gardellini et al., 2021 [13] | 24 | M | Petechiae | 21 | Second dose | None | 2 × 103/μL | IVIG and prednisone | Discharged on steroid taper | No comment |
| Julian et al., 2021 [46] | 72 | F | Rash, spontaneous oral bleeding, headache | 1 | First dose | DM, seasonal contact dermatitis, gout | 12 × 103/μL (prior parvovirus infection) | Dexamethasone, IVIG, ACA, rituximab, and platelets | No comment | No comment |
| Toom et al., 2021 [47] | 36 | F | Petechiae, easy bruising, bleeding gum, headache | 7 | First dose | Familial ITP | 3 × 103/μL (unlikely due to vaginal estrogen ring) | Dexamethasone and IVIG | Discharged | No comment |
| Shonai et al., 2021 [27] | 34 | F | Purpura | 21 | Second dose | None | 11 × 103/μL | Initially not treated, however, on 1 week follow- up, had worsened platelet count for which prednisolone and TPO-RA were given | Improved | - |
| Hines et al., 2021 [48] | 26 | F | Petechiae | 7 | First dose o | Irregular menses on OCPs | 19 × 103/μL (transaminitis present) | Prednisone, dexamethasone, and IVIG | Discharged on day 5 | No comment |
| Bennett et al., 2021 [49] | 32 | F | Petechiae and bruising | 11 | First dose | None – was currently pregnant at 9 weeks | 1 × 103/μL | Prednisone | Discharged on day 3 | No comment |
ACA: aminocaproic acid; AIHA: autoimmune hemolytic anemia; BCC: basal cell carcinoma; CAD: coronary artery disease; COPD: chronic obstructive pulmonary disease; CKD: chronic kidney disease; DM: diabetes; ER: emergency room; F: female; HFrEF: heart failure with reduced ejection fraction; HLD: hyperlipidemia; HTN: hypertension; HBV: hepatitis B virus; ITP: immune thrombocytopenic purpura; IVIG: intravenous immunoglobulins; M: male; OCP: oral contraceptive pills; PCOS: polycystic ovarian syndrome; SCC: squamous cell carcinoma; SLE: systemic lupus erythematosus; SRA: serotonin release assay; TPO-RA: thrombopoietin receptor agonists, e.g., eltrombopag or romiplostim.
Table 2.
Comparison of new cases of ITP versus relapse, post-vaccination.
| Variables | Post-Vaccination ITP Flare-Up (n = 29) | Post-Vaccination New ITP Diagnosis (n = 49) |
|---|---|---|
| Median age in years at presentation [42] | 43 (19–67) | 53 (20–86) |
| Females (%) | 41% | 57% |
| Most common symptom | Mucocutaneous bleed | Mucocutaneous bleed |
| Number of asymptomatic individuals | 3 | 3 |
| Median days to presentation | 7.5 | 12.5 |
| Number of cases/vaccine | 1—Ad26.COV2-S (Johnson & Johnson vaccine) 5—ChAdO × 1 nCoV-19 vaccine 10—BNT162b2 vaccine 1—mRNA-1273 vaccine |
1—Ad26.COV2-S (Johnson & Johnson) vaccine 18—ChAdO × 1 nCoV-19 vaccine 18—BNT162b2 vaccine 12—mRNA-1273 vaccine |
| Median platelet counts | 32.5 × 103/μL | 42 × 103/μL |
| % of patients needing escalation of treatment with second-line agents | 10% | 32.6% |
4. Discussion
As of July 2022, over 500 million doses of the COVID-19 vaccine have been delivered across the United States [50]. While there have been around 300,000 reports of adverse outcomes following mRNA vaccination, more than 90% of those were non-serious [51]. Some major adverse events, such as myopericarditis, Guillain-Barre syndrome, and coagulopathy, including ITP, have prompted the need for closer surveillance in the peri-vaccination period [51]. The concept of vaccine-related ITP is not new and has been documented in relation to various other vaccines, such as MMR, influenza, hepatitis B, polio, pneumococcal vaccines, etc. [52]. Most studies reported the occurrence of thrombocytopenia within six weeks of receiving the vaccine and more than 90% of these cases were self-limiting, with only a few progressing to chronic thrombocytopenia [53,54]. With the advent of COVID-19 vaccines, it has become challenging to monitor such cases owing to the emergent need to countermeasure the pandemic, ushering in expedited manufacturing of the vaccines.
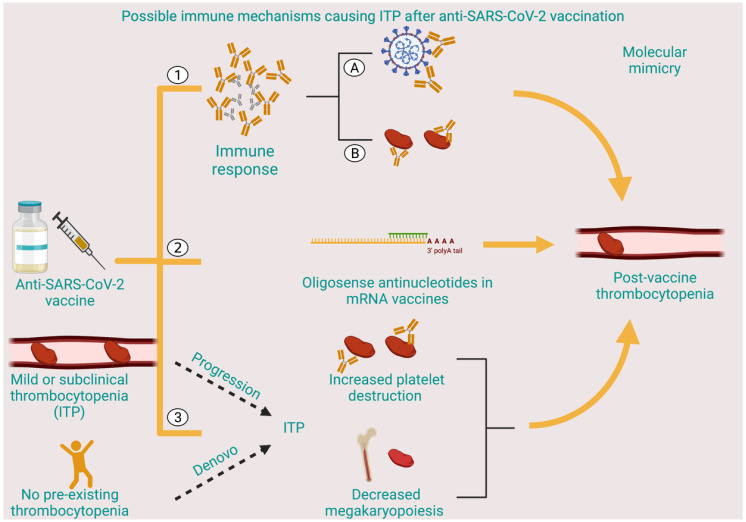
Diagnosis of ITP is one of exclusion, involving a thorough history taking to rule out other causes of thrombocytopenia and screening for secondary etiologies of ITP, along with the demonstration of mere thrombocytopenia on peripheral smear without any other hematologic abnormalities [3]. Antiplatelet antibodies are positive in less than two-thirds of patients with ITP, with poor specificity, and are therefore not recommended for diagnosis [55]. In all the cases described above, the authors came to the diagnosis of ITP based on the temporal sequence of events and the absence of any other inciting factors for thrombocytopenia. Despite the question of causality, it is worthwhile to underscore the possible pathophysiological mechanisms by which vaccine-associated ITP might occur (Figure 2). Molecular mimicry, epitope spreading, polyclonal activation, superantigen, and bystander activation are some suggested mechanisms. Analogous to natural infection-causing autoimmunity, both vaccines and their adjuvants carry the structural potential to generate and enhance self-reactivity, respectively [56]. This dysregulated immune response can also lead to the formation of immune complexes, which can additionally perpetuate platelet damage. Furthermore, antisense oligonucleotides, a constituent of the mRNA vaccines, have an inherent ability to cause thrombocytopenia, albeit with a need for a much higher dose than is delivered by a single injection [57]. Possible mechanisms proposed for this effect are platelet consumption through binding of receptors, formation of antibodies on repeated exposure, or an electrostatic platelet-binding effect similar to heparin [57]. Alternatively, a subclinical ITP can manifest as full-blown ITP post-vaccination [6]. Lastly, de novo ITP remains a distinct possibility, particularly in patients developing symptoms some days after vaccination, and is further affirmed by a positive response to traditional ITP-directed therapies, suggesting immune-mediated platelet destruction [6]. The potential mechanism involves vaccine-mediated polyclonal B- and T-cell activation causing both peripheral and bone marrow platelet destruction.
Figure 2.
Possible immune mechanisms causing ITP after anti-SARS-CoV-2 vaccination: (1) Immune response causing production of protective antibodies against SARS-CoV-2 (A) with possible molecular mimicry against platelet antigens (B); (2) oligosense antinucleotides in mRNA vaccines causing post-vaccine thrombocytopenia; (3) development of ITP, either de novo or progression from sub-clinical ITP after anti-SARS-CoV-2 vaccine.
Consistent with natural ITP demographics in patients younger than 65, women were more likely to have vaccine-related ITP, in our review. ITP has long been associated with autoimmune diseases such as systemic lupus erythematosus, thyroid, and inflammatory bowel diseases [58]. A total of 13% of patients in our review had an established prior diagnosis of autoimmune disease, with thyroid disorders being the most common. It is noteworthy that 4.5% (n = 3) of patients with no history of autoimmune disorders had evidence of positive autoantibodies on laboratory workup. Given the short follow-up period, the inference of whether these patients had an undiagnosed autoimmune disease, or if antibodies were elevated as a consequence of ITP, is challenging to make. Tarawneh et al. [22] reported the resolution of SSA-antibodies on follow-up, thereby favoring the latter hypothesis. Two-thirds of the post-vaccine ITP cases were seen after mRNA vaccines (64%), and whether this is due to the upregulation of toll-like receptors by mRNA vaccines leading to further immune activation is still unknown [59]. A formal diagnosis of ITP was present in 24% of patients before presentation, highlighting the possible risk of relapse post-vaccination. The mean time to presentation was around eight days, which is consistent with other epidemiological studies [14,60]. A total of 86% of patients presented with thrombocytopenia of less than or equal to 30 × 103/μL, the commonly accepted threshold of ITP patients presenting with major bleeding [61]. It is essential to highlight that there might have been other cases of subclinical ITP that have gone undetected, given the absence of symptoms.
Standard treatment guidelines for ITP favor a short course of steroids as the first-line therapy, with the addition of intravenous immunoglobulin (IVIG) to rapidly increase counts [62]. In our review, most patients showed an improvement in their platelet count and bleeding manifestations with the use of steroids and/or IVIG. Second-line agents were employed in around 33% of patients, which is higher than the use of such agents in a report of over 200,000 ITP patients, wherein only 5% of patients were on such therapeutic modalities [63]. Some patients (n = 7) with counts of >30 × 103/μL were not treated per the 2019 recommended guidelines [62]. Except for one reported death from intracranial bleed due to suspected vaccine-related ITP [64], our review showed an excellent short-term prognosis in terms of zero fatalities and safe discharge if hospitalized, for all 66 patients. While data on long-term outcomes of such patients is lacking, prior studies with other vaccine types have shown patients to have a better prognosis than for viral-associated ITP, which is more likely to progress to a chronic state (28% versus 10% following vaccination) [65]. More prospective data are needed to guide patients on the long-term prognosis and chronicity (if any) of anti-SARS-CoV-2 vaccine-related ITP.
Conflicting evidence exists on whether the aforementioned cases were caused by vaccines or were a mere coincidence. As per some reports, the background incidence rates of ITP remained similar in the pre-and post-vaccination periods [6,66]. On the contrary, the administration of the AstraZeneca vaccine in Scotland, Australia, and England showed a higher-than-expected ITP rate [60,67,68]. More prospective data are needed to adjudicate this relationship accurately and identify predictive biomarkers. Whether the next dose should be advised in such patients and whether of the same vaccine type are other potential areas that need exploring. While only four patients in our review received the next dose, reports of safely immunizing chronic ITP patients, in some cases with an alternate vaccine, have been reassuring [67]. Monitoring of platelet counts in the peri-vaccination period, along with sharing the knowledge to present to the hospital emergently upon the first bleeding symptom, are a few of the potential steps that can be taken while administering the next dose.
The main limitations of our study are the absence of confirmatory tests or a standard definition for anti-SARS-CoV-2 vaccine-associated ITP. Even with a decent sample size, comments on the type of association cannot be made as the included studies were case reports. Furthermore, there exists a likelihood of bias in reporting subclinical cases with an emphasis on reporting instances of severe thrombocytopenia. Lastly, there is always a potential of missing germane articles with any review, despite employing a robust search strategy.
5. Conclusions
Although vaccine-related ITP is rarely a cause of death, it significantly hampers patients’ quality of life owing to the fatigue and adverse effects of therapeutic interventions, making it a critical pathology to understand in the context of accelerated global vaccination efforts. Our review attempts to make physicians conscious of ruling out this entity while evaluating patients presenting with thrombocytopenia after receiving the anti-SARS-CoV-2 vaccine. In our solicited opinion, the rarity of these events and excellent outcomes for patients should not change views regarding the benefits provided by immunization to combat this global crisis. Instead, it should raise awareness about the need for an in-depth anamnesis before vaccination.
Author Contributions
Conceptualization, P.S. and H.G.; methodology, P.S. and N.G.; formal analysis, P.S. and F.A.; writing—original draft preparation, P.S..; writing—review and editing, P.S., F.A. and N.G.; supervision, H.G. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Onisâi M., Vlădăreanu A.-M., Spînu A., Găman M., Bumbea H. Idiopathic thrombocytopenic purpura (ITP)—New era for an old disease. Rom. J. Intern. Med. 2019;57:273–283. doi: 10.2478/rjim-2019-0014. [DOI] [PubMed] [Google Scholar]
- 2.Lambert M.P., Gernsheimer T.B. Clinical updates in adult immune thrombocytopenia. Blood. 2017;129:2829–2835. doi: 10.1182/blood-2017-03-754119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper N., Ghanima W. Immune Thrombocytopenia. N. Engl. J. Med. 2019;381:945–955. doi: 10.1056/NEJMcp1810479. [DOI] [PubMed] [Google Scholar]
- 4.Shah S.R.A., Dolkar S., Mathew J., Vishnu P. COVID-19 vaccination associated severe immune thrombocytopenia. Exp. Hematol. Oncol. 2021;10:42. doi: 10.1186/s40164-021-00235-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moulis G., Sommet A., Sailler L., Lapeyre-Mestre M., Montastruc J.-L., the French Association of Regional. The French Association of Regional Pharmacovigilance Centers Drug-induced immune thrombocytopenia: A descriptive survey in the French PharmacoVigilance database. Platelets. 2011;23:490–494. doi: 10.3109/09537104.2011.633179. [DOI] [PubMed] [Google Scholar]
- 6.Lee E.J., Cines D.B., Gernsheimer T., Kessler C., Michel M., Tarantino M.D., Semple J.W., Arnold D.M., Godeau B., Lambert M.P., et al. Thrombocytopenia following Pfizer and Moderna SARS-CoV-2 vaccination. Am. J. Hematol. 2021;96:534–537. doi: 10.1002/ajh.26132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Long B., Bridwell R., Gottlieb M. Thrombosis with thrombocytopenia syndrome associated with COVID-19 vaccines. Am. J. Emerg. Med. 2021;49:58–61. doi: 10.1016/j.ajem.2021.05.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Saluja P., Gautam N., Yadala S., Venkata A.N. Thrombotic thrombocytopenic purpura (TTP) after COVID-19 vaccination: A systematic review of reported cases. Thromb. Res. 2022;214:115–121. doi: 10.1016/j.thromres.2022.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Banerjee S., Sandhu M., Tonzi E., Tambe A., Gambhir H.S. Immune-Mediated Thrombocytopenia Associated With Ad26.COV2.S (Janssen; Johnson & Johnson) Vaccine. Am. J. Ther. 2021;28:e604–e606. doi: 10.1097/MJT.0000000000001431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Scanvion Q., Lambert M., Hachulla E., Terriou L. Correspondence in reference to the previously published Epub manuscript: Immune thrombocytopenic purpura after SARS-CoV-2 vaccine. Br. J. Haematol. 2021;194:e93–e95. doi: 10.1111/bjh.17628. [DOI] [PubMed] [Google Scholar]
- 11.Candelli M., Rossi E., Valletta F., De Stefano V., Franceschi F. Immune thrombocytopenic purpura after SARS-CoV-2 vaccine. Br. J. Haematol. 2021;194:547–549. doi: 10.1111/bjh.17508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paulsen F.-O., Schaefers C., Langer F., Frenzel C., Wenzel U., Hengel F.E., Bokemeyer C., Seidel C. Immune Thrombocytopenic Purpura after vaccination with COVID-19 Vaccine (ChAdOx1 nCov-19) Blood. 2021;138:996–999. doi: 10.1182/blood.2021012790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gardellini A., Guidotti F., Maino E., Steffanoni S., Zancanella M., Turrini M. Severe immune thrombocytopenia after COVID-19 vaccination: Report of four cases and review of the literature. Blood Cells Mol. Dis. 2021;92:102615. doi: 10.1016/j.bcmd.2021.102615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kim G., Choi E.-J., Park H.-S., Lee J.-H., Lee J.-H., Lee K.-H. A Case Report of Immune Thrombocytopenia after ChAdOx1 nCoV-19 Vaccination. J. Korean Med. Sci. 2021;36:e306. doi: 10.3346/jkms.2021.36.e306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sivaramakrishnan P., Mishra M. Vaccination-associated immune thrombocytopenia possibly due to ChAdOx1 nCoV-19 (Covishield) coronavirus vaccine. BMJ Case Rep. 2022;15:e249237. doi: 10.1136/bcr-2022-249237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Al-Ahmad M., Al Rasheed M., Shalaby N., Rodriguez-Bouza T., Altourah L. Immune Thrombocytopenia (ITP): Relapse Versus de novo After COVID-19 Vaccination. Clin. Appl. Thromb. Hemost. 2022;28:1–3. doi: 10.1177/10760296211073920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong J.S.Y., Kang J.H.-E., Maw K.Z. Acute immune thrombocytopenic purpura post first dose of COVID-19 vaccination. Postgrad. Med. J. 2022;98:e129–e130. doi: 10.1136/postgradmedj-2021-140947. [DOI] [PubMed] [Google Scholar]
- 18.Razzaq A.K., Al-Jasim A. Oxford-AstraZeneca Coronavirus Disease-2019 Vaccine-Induced Immune Thrombocytopenia on Day Two. Case Rep. Hematol. 2021;2021:2580832. doi: 10.1155/2021/2580832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Uaprasert N., Panrong K., Tungjitviboonkun S., Dussadee K., Decharatanachart P., Kaveevorayan P., Shoosanglertwijit R., Watanaboonyongcharoen P., Bunworasate U., Rojnuckarin P. ChAdOx1 nCoV-19 vaccine-associated thrombocytopenia: Three cases of immune thrombocytopenia after 107 720 doses of ChAdOx1 vaccination in Thailand. Blood Coagul. Fibrinolysis. 2022;33:67–70. doi: 10.1097/MBC.0000000000001082. [DOI] [PubMed] [Google Scholar]
- 20.Liao P.-W., Teng C.-L.J., Chou C.-W. Immune Thrombocytopenia Induced by the Chimpanzee Adenovirus-Vectored Vaccine against SARS-CoV-2 Infection. Vaccines. 2021;9:1486. doi: 10.3390/vaccines9121486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ganzel C., Ben-Chetrit E. Immune Thrombocytopenia Following the Pfizer-BioNTech BNT162b2 mRNA COVID-19 Vaccine. Isr. Med. Assoc. J. 2021;23:341. [PubMed] [Google Scholar]
- 22.Tarawneh O., Tarawneh H. Immune thrombocytopenia in a 22-year-old post COVID-19 vaccine. Am. J. Hematol. 2021;96:E133–E134. doi: 10.1002/ajh.26106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fueyo-Rodriguez O., Valente-Acosta B., Jimenez-Soto R., Neme-Yunes Y., Inclán-Alarcón S.I., Trejo-Gonzalez R., García-Salcido M. Secondary immune thrombocytopenia supposedly attributable to COVID-19 vaccination. BMJ Case Rep. 2021;14:e242220. doi: 10.1136/bcr-2021-242220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jawed M., Khalid A., Rubin M., Shafiq R., Cemalovic N. Acute Immune Thrombocytopenia (ITP) Following COVID-19 Vaccination in a Patient with Previously Stable ITP. Open Forum Infect. Dis. 2021;8:ofab343. doi: 10.1093/ofid/ofab343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.King E.R., Towner E. A Case of Immune Thrombocytopenia After BNT162b2 mRNA COVID-19 Vaccination. Am. J. Case Rep. 2021;22:e931478. doi: 10.12659/AJCR.931478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Qasim H., Ali E., Yassin M.A. Immune thrombocytopenia relapse post COVID-19 vaccine in young male patient. IDCases. 2021;26:e01344. doi: 10.1016/j.idcr.2021.e01344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shonai T., Kimura F., Watanabe J. Severe Immune Thrombocytopenia after COVID-19 Vaccination: Two Case Reports and a Literature Review. Intern. Med. 2022;61:1581–1585. doi: 10.2169/internalmedicine.9177-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krajewski P.K., Szepietowski J.C. Immune thrombocytopenic purpura associated with COVID-19 Pfizer-BioNTech BNT16B2b2 mRNA vaccine. J. Eur. Acad. Dermatol. Venereol. 2021;35:e626–e627. doi: 10.1111/jdv.17444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Idogun P.O., Ward M.C., Teklie Y., Wiese-Rometsch W., Baker J. Newly Diagnosed Idiopathic Thrombocytopenia Post COVID-19 Vaccine Administration. Cureus. 2021;13:e14853. doi: 10.7759/cureus.14853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hidaka D., Ogasawara R., Sugimura S., Fujii F., Kojima K., Nagai J., Ebata K., Okada K., Kobayashi N., Ogasawara M., et al. New-onset Evans syndrome associated with systemic lupus erythematosus after BNT162b2 mRNA COVID-19 vaccination. Int. J. Hematol. 2022;115:424–427. doi: 10.1007/s12185-021-03243-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Saito K., Ichikawa S., Hatta S., Katsuoka Y., Harigae H., Izumi T. Vincristine therapy for severe and refractory immune thrombocytopenia following COVID-19 vaccination. Int. J. Hematol. 2022;115:424–427. doi: 10.1007/s00277-021-04666-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pasin F., Calabrese A., Pelagatti L. Immune thrombocytopenia following COVID-19 mRNA vaccine: Casuality or causality? Intern. Emerg. Med. 2022;17:295–297. doi: 10.1007/s11739-021-02778-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nakamura T., Morodomi Y., Kanaji S., Okamura T., Nagafuji K., Kanaji T. Detection of anti-GPIbα autoantibodies in a case of immune thrombocytopenia following COVID-19 vaccination. Thromb. Res. 2022;209:80–83. doi: 10.1016/j.thromres.2021.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ogai A., Yoshida R., Yuasa C., Chin K., Fujimaki K., Nakajima H. Acute immune thrombocytopenia following SARS-CoV-2 vaccination in chronic ITP patients and a healthy individual. Int. J. Hematol. 2022;115:293–295. doi: 10.1007/s12185-021-03235-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Battegay R., Istampoulouoglou I., Holbro A., Buser A., Hirsiger J.R., Eckstein J., Bergerg C.T., Koechlinbh S., Leuppi-Taegtmeyerbch A.B. Immune thrombocytopenia associated with COVID-19 mRNA vaccine tozinameran—A clinical case and global pharmacovigilance data. Swiss Med. Wkly. 2021;151:w30084. doi: 10.4414/smw.2021.w30084. [DOI] [PubMed] [Google Scholar]
- 36.Ghosh A.K., Bhushan S., Lopez L.D.R., Sampat D., Salah Z., Hatoum C.A. BNT162b2 COVID-19 Vaccine Induced Immune Thrombocytopenic Purpura. Case Rep. Med. 2022;2022:5603919. doi: 10.1155/2022/5603919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Akiyama H., Kakiuchi S., Rikitake J., Matsuba H., Sekinada D., Kozuki Y., Iwata N. Immune thrombocytopenia associated with Pfizer-BioNTech’s BNT162b2 mRNA COVID-19 vaccine. IDCases. 2021;25:e01245. doi: 10.1016/j.idcr.2021.e01245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jasaraj R.B., Shrestha D.B., Gaire S., Kassem M. Immune Thrombocytopenic Purpura Following Pfizer-BioNTech COVID-19 Vaccine in an Elderly Female. Cureus. 2021;13:e16871. doi: 10.7759/cureus.16871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ghous G., Allahyar, Zafar M.U., Tarar Z.I., Shoukat H.M.H. Immune Thrombocytopenic Purpura Associated With Pfizer-BioNTech COVID-19 Vaccine Refractory to Conventional Treatment. Am. J. Ther. 2021;28:e521–e522. doi: 10.1097/MJT.0000000000001393. [DOI] [Google Scholar]
- 40.Abuhelwa Z., Ning Y., Abdulsattar W., Ghazaleh S., Kahlon N., Elsayed A. Romiplostim for SARS-CoV-2 Vaccine Induced Immune Thrombocytopenia. Am. J. Ther. 2021;28:e685–e687. doi: 10.1097/MJT.0000000000001447. [DOI] [PubMed] [Google Scholar]
- 41.Prasad S., Jariwal R., Adebayo M., Jaka S., Petersen G., Cobos E. Immune Thrombocytopenia following COVID-19 Vaccine. Case Rep. Hematol. 2022;2022:6013321. doi: 10.1155/2022/6013321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chanut M., Jaidi R., Kohn M., Grange T., Brones C., Lombion N., Rousselot P., Longval T. Successful mRNA SARS-Cov-2 vaccine rechallenge after a first episode of immune thrombocytopenic purpura. Platelets. 2022;33:652–653. doi: 10.1080/09537104.2022.2044463. [DOI] [PubMed] [Google Scholar]
- 43.Helms J.M., Ansteatt K.T., Roberts J.C., Kamatam S., Foong K.S., Labayog J.-M.S., Tarantino M.D. Severe, Refractory Immune Thrombocytopenia Occurring After SARS-CoV-2 Vaccine. J. Blood Med. 2021;12:221–224. doi: 10.2147/JBM.S307047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chong K.-M., Yang C.-Y., Lin C.-C., Lien W.-C. Severe immune thrombocytopenia following COVID-19 vaccination (Moderna) and immune checkpoint inhibitor. Am. J. Emerg. Med. 2022;56:395.e1–395.e3. doi: 10.1016/j.ajem.2022.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Malayala S.V., Mohan G., Vasireddy D., Atluri P. Purpuric Rash and Thrombocytopenia After the mRNA-1273 (Moderna) COVID-19 Vaccine. Cureus. 2021;13:e14099. doi: 10.7759/cureus.14099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Julian J.A., Mathern D.R., Fernando D. Idiopathic Thrombocytopenic Purpura and the Moderna Covid-19 Vaccine. Ann. Emerg. Med. 2021;77:654–656. doi: 10.1016/j.annemergmed.2021.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Toom S., Wolf B., Avula A., Peeke S., Becker K. Familial thrombocytopenia flare-up following the first dose of mRNA-1273 Covid-19 vaccine. Am. J. Hematol. 2021;96:E134–E135. doi: 10.1002/ajh.26128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hines A., Shen J.G., Olazagasti C., Shams S. Immune thrombocytopenic purpura and acute liver injury after COVID-19 vaccine. BMJ Case Rep. 2021;14:e242678. doi: 10.1136/bcr-2021-242678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bennett C., Chambers L.M., Son J., Goje O. Newly diagnosed immune thrombocytopenia in a pregnant patient after coronavirus disease 2019 vaccination. J. Obstet. Gynaecol. Res. 2021;47:4077–4080. doi: 10.1111/jog.14978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Centers for Disease Control and Prevention . COVID Data Tracker. US Department of Health and Human Services, CDC; Atlanta, GA, USA: 2022. [(accessed on 5 July 2022)]. Available online: https://covid.cdc.gov/covid-data-tracker. [Google Scholar]
- 51.Rosenblum H.G., Gee J., Liu R., Marquez P.L., Zhang B., Strid P., E Abara W., McNeil M.M., Myers T.R., Hause A.M., et al. Safety of mRNA vaccines administered during the initial 6 months of the US COVID-19 vaccination programme: An observational study of reports to the Vaccine Adverse Event Reporting System and v-safe. Lancet Infect. Dis. 2022;22:802–812. doi: 10.1016/S1473-3099(22)00054-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perricone C., Ceccarelli F., Nesher G., Borella E., Odeh Q., Conti F., Shoenfeld Y., Valesini G. Immune thrombocytopenic purpura (ITP) associated with vaccinations: A review of reported cases. Immunol. Res. 2014;60:226–235. doi: 10.1007/s12026-014-8597-x. [DOI] [PubMed] [Google Scholar]
- 53.Jefferson T., Price D., Demicheli V., Bianco E. Unintended events following immunization with MMR: A systematic review. Vaccine. 2003;21:3954–3960. doi: 10.1016/S0264-410X(03)00271-8. [DOI] [PubMed] [Google Scholar]
- 54.Cecinati V., Principi N., Brescia L., Giordano P., Esposito S. Vaccine administration and the development of immune thrombocytopenic purpura in children. Hum. Vaccines Immunother. 2013;9:1158–1162. doi: 10.4161/hv.23601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mithoowani S., Gregory-Miller K., Goy J., Miller M.C., Wang G., Noroozi N., Kelton J.G., Arnold D.M. High-dose dexamethasone compared with prednisone for previously untreated primary immune thrombocytopenia: A systematic review and meta-analysis. Lancet Haematol. 2016;3:e489–e496. doi: 10.1016/S2352-3026(16)30109-0. [DOI] [PubMed] [Google Scholar]
- 56.Watad A., Sharif K., Shoenfeld Y. The ASIA syndrome: Basic concepts. Mediterr. J. Rheumatol. 2017;28:64–69. doi: 10.31138/mjr.28.2.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chi X., Gatti P., Papoian T. Safety of antisense oligonucleotide and siRNA-based therapeutics. Drug Discov. Today. 2017;22:823–833. doi: 10.1016/j.drudis.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 58.Lizbeth-Estefan D.-P., Pujol-Moix N., Jiménez B., Canals C., Barranco-Charris E., Muñiz-Díaz4 E., Souto J.-C. Shared Autoimmunity: A Case Series of 56 Patients with Immune Thrombocytopenia (ITP) Associated with Other Autoimmune Disorders. Open Access Libr. J. 2016;3:8. [Google Scholar]
- 59.Kuter D.J. Exacerbation of immune thrombocytopenia following COVID-19 vaccination. Br. J. Haematol. 2021;195:365–370. doi: 10.1111/bjh.17645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Simpson C.R., Shi T., Vasileiou E., Katikireddi S.V., Kerr S., Moore E., McCowan C., Agrawal U., Shah S.A., Ritchie L.D., et al. First-dose ChAdOx1 and BNT162b2 COVID-19 vaccines and thrombocytopenic, thromboembolic and hemorrhagic events in Scotland. Nat. Med. 2021;27:1290–1297. doi: 10.1038/s41591-021-01408-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cohen Y.C., Djulbegovic B., Shamai-Lubovitz O., Mozes B. The Bleeding Risk and Natural History of Idiopathic Thrombocytopenic Purpura in Patients with Persistent Low Platelet Counts. Arch. Intern. Med. 2000;160:1630–1638. doi: 10.1001/archinte.160.11.1630. [DOI] [PubMed] [Google Scholar]
- 62.Neunert C., Terrell D.R., Arnold D.M., Buchanan G., Cines D.B., Cooper N., Cuker A., Despotovic J.M., George J.N., Grace R.F., et al. American Society of Hematology 2019 guidelines for immune thrombocytopenia. Blood Adv. 2019;3:3829–3866. doi: 10.1182/bloodadvances.2019000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lal L.S., Said Q., Andrade K., Cuker A. Second-line treatments and outcomes for immune thrombocytopenia: A retrospective study with electronic health records. Res. Pract. Thromb. Haemostasis. 2020;4:1131–1140. doi: 10.1002/rth2.12423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Grady D.M. Death of a doctor who got Covid shot is being investigated. New York Times. Jan 12 , 2021.
- 65.Rodeghiero F., Stasi R., Gernsheimer T., Michel M., Provan D., Arnold D.M., Bussel J.B., Cines D.B., Chong B.H., Cooper N., et al. Standardization of terminology, definitions and outcome criteria in immune thrombocytopenic purpura of adults and children: Report from an international working group. Blood. 2009;113:2386–2393. doi: 10.1182/blood-2008-07-162503. [DOI] [PubMed] [Google Scholar]
- 66.Welsh K.J., Baumblatt J., Chege W., Goud R., Nair N. Thrombocytopenia including immune thrombocytopenia after receipt of mRNA COVID-19 vaccines reported to the Vaccine Adverse Event Reporting System (VAERS) Vaccine. 2021;39:3329–3332. doi: 10.1016/j.vaccine.2021.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gordon S.F., Clothier H.J., Morgan H., Buttery J.P., Phuong L.K., Monagle P., Chunilal S., Wood E.M., Tran H., Szer J., et al. Immune thrombocytopenia following immunisation with Vaxzevria ChadOx1-S (AstraZeneca) vaccine, Victoria, Australia. Vaccine. 2021;39:7052–7057. doi: 10.1016/j.vaccine.2021.10.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hippisley-Cox J., Patone M., Mei X.W., Saatci D., Dixon S., Khunti K., Watkinson P., Shankar-Hari M., Harrison D.A., Sheikh A. Risk of thrombocytopenia and thromboembolism after COVID-19 vaccination and SARS-CoV-2 positive testing: Self-controlled case series study. BMJ. 2021;374:n1931. doi: 10.1136/bmj.n1931. [DOI] [PMC free article] [PubMed] [Google Scholar]