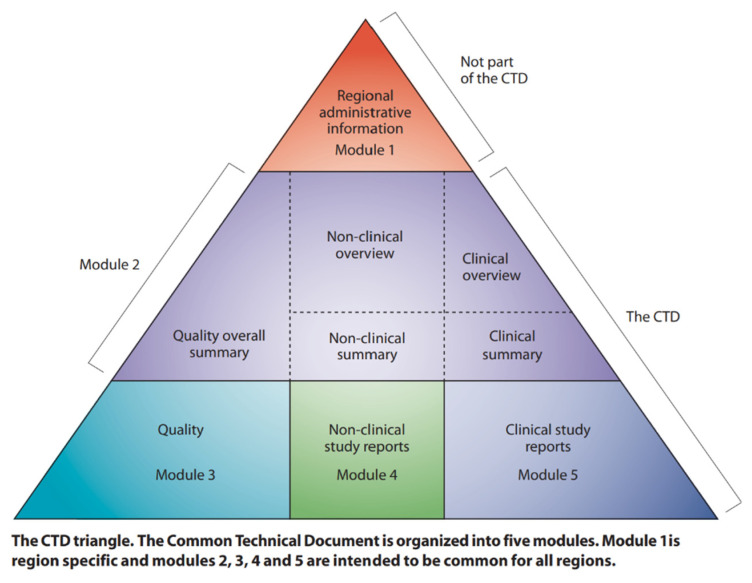
Figure 8.
The Common Technical Document (Reference: [114]). The eCTD has five modules: 1. Administrative information and prescribing information. This is a country specific regional module, i.e., different for each region or country; 2. Common technical document summaries. This is a common module in all regions; 3. Quality; 4. Nonclinical study reports; 5. Clinical study reports. Image source: https://www.ich.org/page/ctd © International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use, https://www.ich.org/page/legal-mentions (accessed on 1 August 2022).