Abstract
Molecularly imprinted polymers (MIPs) continue to gain increasing attention as functional materials due to their unique characteristics such as higher stability, simple preparation, robustness, better binding capacity, and low cost. In particular, MIP-coated inorganic nanoparticles have emerged as a promising platform for various biomedical applications ranging from drug delivery to bioimaging. The integration of MIPs with inorganic nanomaterials such as silica (SiO2), iron oxide (Fe3O4), gold (Au), silver (Ag), and quantum dots (QDs) combines several attributes from both components to yield highly multifunctional materials. These materials with a multicomponent hierarchical structure composed of an inorganic core and an imprinted polymer shell exhibit enhanced properties and new functionalities. This review aims to provide a general overview of key recent advances in the fabrication of MIPs-coated inorganic nanoparticles and highlight their biomedical applications, including drug delivery, biosensor, bioimaging, and bioseparation.
Keywords: molecularly imprinted polymers, inorganic nanoparticles, biomedical applications
1. Introduction
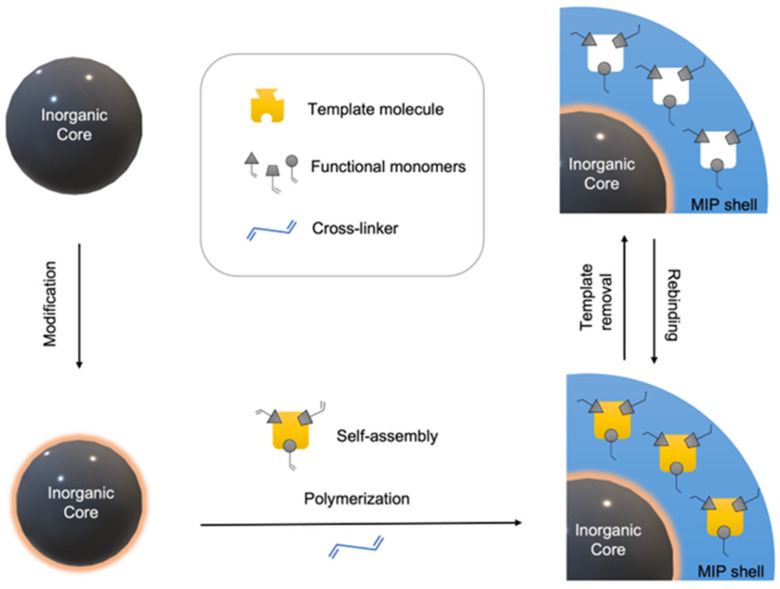
Many diagnostic and therapeutic platforms require selective recognition of a target molecule. Nature employs biological receptors such as enzymes [1], antibodies [2], or histones [3] as tools to recognize the respective antigens. However, using these biological components in clinical applications remains limited due to their physical and chemical instability and high cost. New synthetic materials have recently been proposed to provide more stable, durable, and cheaper alternatives [4]. Molecular imprinting is an approach that entails creating artificial recognition sites by polymerizing functional and cross-linking monomers in the presence of a target molecule (Figure 1) [5,6]. The preparation of MIPs first requires the formation of a pre-assembly between the template molecule and functional monomers via either covalent or non-covalent interactions. Then, cross-linkers arrest the temporary structure into a permanent one during the polymerization process to yield a three-dimensional polymeric network. After the polymerization, the template molecule is removed from the matrix to obtain the complimentary cavities. Herewith, the polymer carries the memory that can selectively recognize the targeted molecule.
Figure 1.
Illustration of molecularly imprinted polymer shell coating onto an inorganic core.
Molecular imprinting has emerged as an attractive technology due to its remarkable features. These materials are highly stable, straightforward to synthesize, and possess superior mechanical properties. Furthermore, the method can be applied to a diverse range of target molecules, including amino acids [7], pharmaceuticals [8], pesticides [9], nucleotides [10], viruses [11], and cells [12]. Importantly, even when a biological receptor is unknown, imprinting technology can allow the generation of a tailor-made receptor-like material. These attractive features have expanded the application areas of MIPs from chemical separation [13,14], selective extraction [15], and catalysis [16,17] to molecular sensing [6,18,19,20,21,22], bioimaging [4], and drug delivery [23,24,25]. Given the ever-increasing importance of point-of-care testing devices [26,27], MIPs with microfluidic systems creates new devices and enables their utilization in point-of-care applications [28].
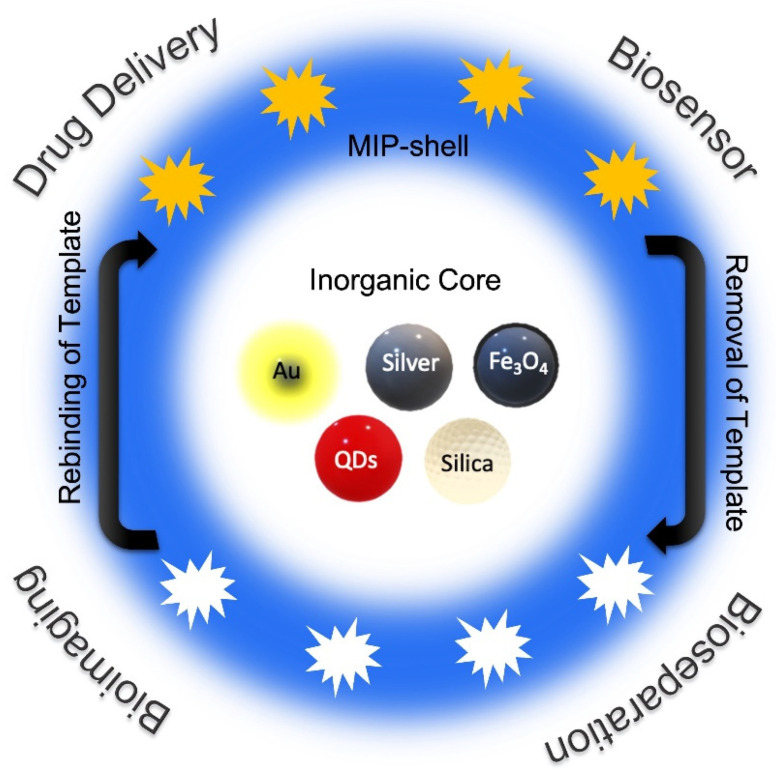
Decades of research have demonstrated the superior performance of micro- and nanosized MIPs compared to their bulk macroscopic counterparts [29,30,31]. MIPs can be fabricated in various formats such as films [32], membranes [33], hydrogels [34], microparticles [35], multi-walled carbon nanotubes (MWCNTs) [36], or nanoparticles [37]. The overall performance of large and macroscopic bulk materials-based MIPs is compromised due to the heterogeneity of their analyte binding site and the poor accessibility of interior binding sites. Furthermore, removal of the template to obtain effective MIPs is also difficult. The large monolithic structures may also be difficult to integrate with a device or a signal transducer in a sensor. These problems are generally addressed by converting the bulk monolithic materials to microparticles by mechanical methods such as grinding. Compared to the bulk and microscale MIPs, nano-MIPs have several advantages, including a higher surface-to-volume ratio, accessibility of imprinted cavities due to easier template removal, improved recognition capabilities, and lower mass-transfer resistance [38]. Nano-MIPs can be produced by combining imprinted polymeric interface with inorganic nanoparticles or directly synthesizing MIP nanoparticles. Integration of MIPs with inorganic nanoparticles yields materials with predetermined particle sizes and distributions and provides flexibility in functionalization by choosing the appropriate nanomaterials. Furthermore, it was demonstrated that the sensitivity and selectivity obtained with MIP-coated inorganic nanoparticles are enhanced compared to the average response of its constituents. [39,40]. Gold (Au), silver (Ag), silica (SiO2), QDs, and iron oxide (Fe3O4) nanoparticles have been extensively used as core materials to support the surface imprinting of the template molecule on their polymeric shell [41]. Such a combination harnesses the intrinsic properties of the inorganic core and the tailored characteristics of the polymeric shell to obtain materials with unique functional characteristics suited for various biomedical applications (Figure 2).
Figure 2.
Illustration of various types of inorganic nanoparticles combined with MIP-shell and their possible biomedical applications.
This review aims to overview the leading fabrication methods for MIP-coated inorganic nanoparticles and highlight their biomedical applications. Recent examples in this field are presented by focusing on preparing MIP shell-coated inorganic core systems and their essential biomedical applications. First, a brief introduction of the synthetic approaches for the fabrication of MIPs is provided. This section is followed by a survey of the specific methodologies employed for obtaining MIPs using various inorganic nanoparticle cores. The methodology to obtain the MIP-based polymeric shell depends on the chemical composition of the inorganic core since the surface chemistry determines approaches for robust attachment of the polymeric shell. Finally, various examples of MIP-coated inorganic nanoparticles are discussed to highlight their biomedical applications, such as biosensing, drug delivery, imaging, and bioseparations. The overall aim of the review is to highlight the potential of MIP-coated nanoparticles and thus attract readers to further the design and implementation of such materials in addressing challenges in various areas of biomedical sciences.
2. MIP Synthesis
In the 1970s, Wulff and coworkers synthesized an organic polymeric material that was able to separate racemates. It was the primary demonstration of copolymerization of derivatives of a template molecule and a cross-linking monomer [42]. The interaction between the template molecule and the functional monomers classifies the imprinting approaches into two main classes: covalent and non-covalent. Covalent bonds between the template molecule and the suitable monomers require chemical cleavage that can reform during the rebinding process [43]. This method, however, remains limited due to the inability to perform reversible bonding and relatively harsh conditions for template removal. Mosbach and coworkers widely explored the non-covalent approach and successfully attempted to form a pre-polymerization complex between the template and functional monomers through non-covalent interactions [44,45]. In this bio-inspired approach, non-covalent interactions drive the self-assembly of monomers around the template to create the specific recognition site, as witnessed in various biomolecular recognition events in nature. While the method offers operational simplicity, this method tends to provide less homogenous rebinding sites than covalent ones.
Traditionally, MIPs were synthesized in bulk forms that require mechanical grinding to obtain polymeric particles with a larger surface area. The time-consuming procedure, irregular sizes within the range of 5–100 μm, and heterogeneous binding sites make this method undesirable. These shortcomings have directed the researchers to create monodisperse polymeric nanoparticles [46]. Due to their uniform geometric features, nanoparticles offer improved binding kinetics and easier template removal with reduced template leaching. In particular, MIPs with controlled size and physical forms are preferred for analytical applications. For example, regular shapes favor chromatographic and separation applications and homogenous binding sites are more suitable for binding assays.
Direct access to homogenous polymeric nanoparticles can be obtained using precipitation polymerization. Mosbach and coworkers cleverly employed this technique to synthesize MIP nanoparticles for the targets of theophylline and 17β-estradiol [47]. Later, many successful attempts were shown for various biomedical applications. For instance, Ruela et al. [48] demonstrated in vitro nicotine release and its permeability through the skin by using MIPs synthesized by precipitation polymerization, and Pardeshi et al. [49] explained how advantageous nano-MIPs prepared by the precipitation polymerization method is in chromatographic applications. While the method offers potential advantages, it requires the utilization of excess solvent. Due to the dilution factor during fabrication, MIPs with decreased selectivity is obtained. Emulsion polymerization offers another approach to synthesizing imprinted nanoparticles, usually in an oil-in-water system. This method provides better control over particle size distribution than precipitation polymerization. Tovar and coworkers [50] presented the direct non-covalent imprinting of L- or D-Boc-phenylalanine anilid using emulsion polymerization. They obtained nanoparticles with an approximate size of 200 nm in high yield. Nevertheless, surfactants or cosurfactants must be used for this imprinting method to stabilize the emulsion, which may result in interference with molecular recognition and contamination.
As an alternative approach, the MIP can be grown as a shell on a nanoparticle core. Such an approach yields MIP-coated core-shell nanoparticles, which will provide MIPs with uniform and predetermined particle sizes and create systems with increased multifunctionality like magnetic, optical, or semi-conductive, the later attributes arising from the inorganic core. Due to the aforementioned attractive characteristics, increasing interest in designing and fabricating MIPs based on inorganic nanomaterials has emerged. Combining traditional MIPs with nanostructures to create core-shell MIPs offers an opportunity to engineer materials with excellent properties by judicious choice of the nanoparticle core and the polymeric shell. Some of these advantages of core-shell MIPs over bulk MIPs are listed in Table 1. To date, silica (SiO2), QDs, gold (Au), and magnetic nanoparticles have been investigated as core materials. The imprinted polymeric shells have been prepared using precipitation, emulsion polymerization, or surface grafting methods.
Table 1.
Comparison of bulk MIPs and core-shell MIP nanoparticles.
| Properties | Bulk MIPs | Core-Shell MIP NPs |
|---|---|---|
| Synthesis method | Easy | Complex |
| Morphology | Irregular | Uniform |
| Surface area | Smaller | Larger |
| Adsorption capacity | Lower | Higher |
| Binding sites distribution | Heterogeneous | Homogeneous |
| Template leakage | More | Less |
| Yield | Lower | Higher |
3. Fabrication of Core-Shell Nanoparticles
Nanoparticles used as solid support needs surface anchoring of the polymeric materials to provide robust adherence to the MIP shell. Different approaches for ensuring such integration of the polymeric component are required depending on the chemical composition of the inorganic core materials. One such approach involves surface modification of the inorganic core with heterobifunctional molecules as an adlayer that chemically binds to the inorganic surface from one end and has the other end available for attachment with the organic polymeric component. Alternatively, the polymeric component may contain functional groups that chemically attach to the inorganic surface. The sections below provide illustrative examples of approaches employed for different inorganic cores.
It should be noted that thorough characterization of core-shell nanoparticles is an important requirement to ensure understanding and validation of the recognition process, as well as warrant reproducibility of the performance of obtained MIP-coated nanoparticles. Different techniques can characterize the physical properties of fabricated nanoparticles. While dynamic light scattering (DLS), electron microscopy, or X-ray diffraction methods provide information about the size of nanoparticles, scanning electron microscopy (SEM) gives information about the surface topography of the nanoparticles. Transmission electron microscopy (TEM) is perhaps the suitable tool to visualize the presence of the polymeric shell around the inorganic core. Additionally, the surface composition of the nanoparticles was characterized by ultraviolet-visible spectroscopy (UV-vis), X-ray photoelectron spectroscopy (XPS), or Fourier transform infrared spectroscopy (FTIR).
3.1. Silica (SiO2) NPs
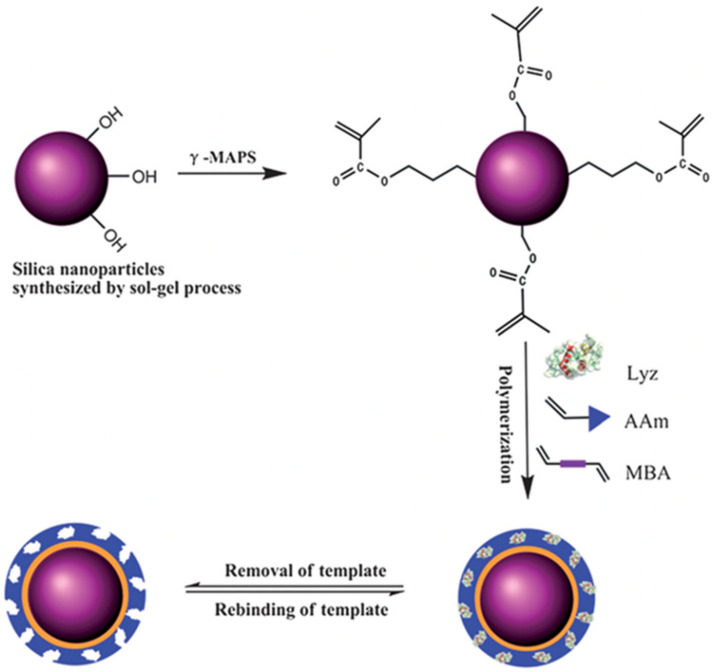
Silica nanoparticles are widely used core material due to their well-established synthesis process (mainly the Stöber method), flexible chemistry, stability under certain conditions, and apparent biocompatibility [51]. Silica nanoparticles possess hydroxyl groups on their surfaces, which react with alkoxysilane-containing molecules to provide grafting sites for the polymer shell. Using appropriately functionalized alkoxysilane derivatives, silica nanoparticles can be functionalized with vinylic, isocyanate, or amine groups. After that, the imprinting of the target analyte is undertaken using the polymerization of functional monomers and cross-linkers. For example, Chen and coworkers [52] used silica nanoparticles with a diameter of 400 nm as a core substrate for lysozyme (Lyz) recognition. Tetraethoxysilane (TEOS) was used for the synthesis of nanoparticles, followed by their modification with γ-methacryloxypropyltrimethoxysilane (γ-MAPS) to introduce the vinyl groups on the surface. A thin film MIP layer was fabricated on these vinyl-modified nanoparticles by the surface imprinting of lysozyme using acrylamide (AAm) and N,N’-methylenebisacrylamide (MBA) as monomer and cross-linker, respectively (Figure 3).
Figure 3.
General scheme of surface Lyz imprinting over the surface of silica nanoparticles. Reproduced with permission from [52].
Zhang and coworkers presented another study on the extraction of sulfonamides [53]. Silica nanoparticles of diameter 80 nm were first functionalized with 3-aminopropyltriethoxysilane (APTES) to install amine groups on the surface. Subsequently, vinyl groups were introduced using acryloyl chloride (CH2CHCOCl)-based modification. The vinyl end groups were co-polymerized with the functional monomer (acrylamide) and the cross-linker (EGDMA) in the presence of the target molecule (sulfamethoxazole). The resulting nanoparticle with a MIP shell demonstrated highly accessible binding sites.
In 2010, Chang et al. reported the preparation of core-shell MIPs by combining the reversible addition-fragmentation chain transfer polymerization (RAFT) and click chemistry [54]. Azide-modified silica nanoparticles and alkyne terminated RAFT chain transfer agent (CTA) were synthesized separately following click reaction between silica and RAFT agent. A thin MIP film with a thickness of 2.27 nm was grafted onto the silica surface to create recognition sites for 2,4-dichlorophenol. This hybrid design showed high affinity, better rebinding kinetics, and selectivity toward target molecule.
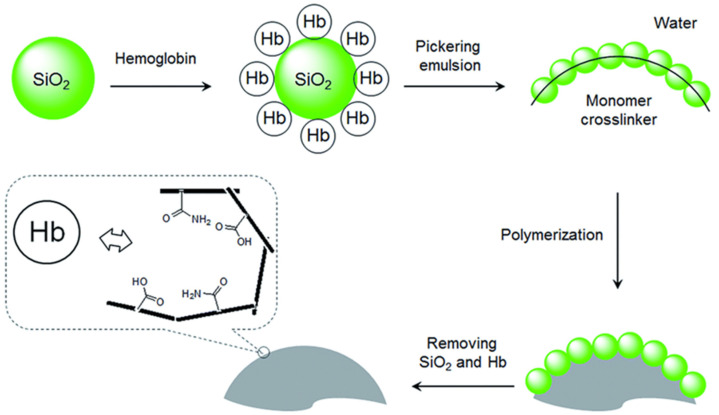
As a different approach, Pickering emulsion polymerization using protein-modified silica beads was reported by Ye and coworkers [55]. Silica particles were coated with the target protein hemoglobin (Hb) and utilized to stabilize a Pickering emulsion. The oil phase comprised the cross-linker and monomers, which were polymerized through free radical polymerization. To obtain template imprinted recognition sites into the polymeric matrix, Hb-coated silica nanoparticles were removed. This approach for the MIP particles creates a well-controlled structure with larger pore size. High selectivity with faster recognition time was obtained since the recognition sites are accessible on the surface, as illustrated in Figure 4.
Figure 4.
Demonstration of Pickering emulsion procedure for protein imprinting. Reproduced with permission from [55].
3.2. Magnetic NPs
Magnetic fields can manipulate magnetic nanoparticles composed of iron, cobalt, nickel, or alloy. Iron oxide (Fe3O4) nanoparticles are favorably used in many biological applications since they have low toxicity and a low-cost profile [56]. Most of the studies in the field of MIP-coated magnetic nanoparticles utilize Fe3O4 nanoparticles. The external magnetic field easily separates the MIP-coated Fe3O4 from the solution, thus eliminating centrifugation and filtration procedures in recognition, extraction, or separation applications.
A primary approach for obtaining a MIP-coated Fe3O4 nanoparticle utilizes the “grafting-from” method, where imprinted polymers are grown from the nanoparticle’s surface using contemporary polymerization methods such as atom transfer radical polymerization (ATRP) and reversible addition-fragmentation chain transfer polymerization (RAFT). Apart from being tolerant to a wide range of functional groups, these polymerization techniques are well-controlled, thus allowing homogenous chain growth to yield functional coatings with uniform thickness. Thus, the grafting-from technique offers a powerful method for obtaining polymer-coated nanoparticles [57,58], and its utilization in obtaining MIP-coated nanomaterials continues to grow.
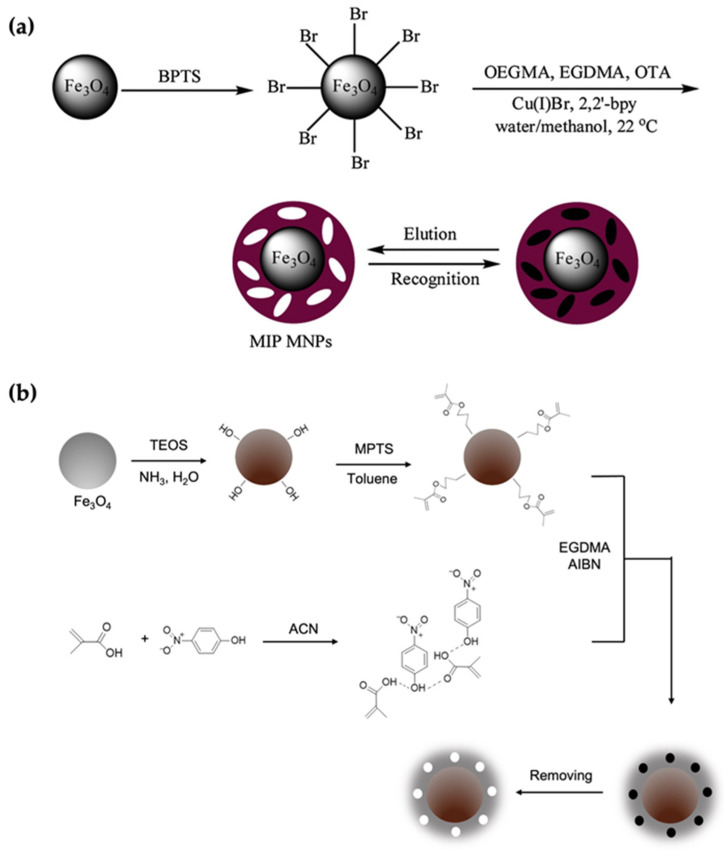
In a recent example, Sahin and coworkers used the “grafting-from” approach to extract the carcinogenic mycotoxin ochratoxin-A [59] selectively. First, Fe3O4 nanoparticles were functionalized with (3-bromopropyl) trimethoxy silane (BPTS) to install -Br groups as radical initiators on the surface (Figure 5a). The MIP synthesis was performed in the presence of oligo (ethylene glycol) monomethyl ether methacrylate as a monomer, ethylene glycol dimetachrylate as a cross-linker, and the template molecule via surface-initiated ATRP. A polymerized MIP shell with an average thickness of 18 nm was obtained. According to their experimental results, the MIP-coated magnetic nanoparticles indicated fast adsorption, perfect selectivity, and high affinity toward the target molecule.
Figure 5.
(a,b) The formation mechanisms of the surface imprinting process use the “grafting-from” technique. Reproduced with permission from [59,60], respectively.
Generally, the inorganic nanoparticles are modified with a silica layer to provide facile and robust surface modifications, increase biocompatibility, avoid the leakage of metal ions, and prevent their surface oxidation. This approach has been extensively used for modifying magnetic nanoparticles before their utilization as a core. It should be noted that the additional silica layer leads to a decreased magnetization of Fe3O4, but it is still sufficient for applications [61]. For example, Liu et al. proposed a novel Fe3O4@SiO2 @NH2 nanoparticle with a pefloxacin mesylate (PEF-M) imprinted shell [62]. The fabrication of this novel nanoparticle started with TEOS functionalization in the presence of ammonium hydroxide to obtain a silica layer onto the Fe3O4 nanoparticles. Later, the amino groups were introduced onto the Fe3O4@SiO2 by treatment with ammonium hydroxide and APTES. This step was followed by the preparation of imprinted polymer shells by ATRP. Another example was reported by Mehdinia et al. [60], where authors utilized the grafting method for the selective recognition of 4-nitrophenol (4-NP). They first modified the magnetic nanoparticles with tetraethyl orthosilicate (TEOS) and 3-methacryloxypropyl trimethoxy silane (MPTS). Then the surface was used for grafting a polymeric layer composed of the functional monomer methacrylic acid (MAA) and cross-linker ethylene glycol dimethacrylate (EGDMA). They investigated the nanoparticles’ adsorption capacity and selectivity performance by comparison with non-imprinted polymers (Figure 5b).
3.3. Quantum Dots (QDs)
Quantum dots (QDs) are fluorescent semiconductor nanomaterials that possess unique optoelectronic characteristics [63]. QDs have been widely used in bioimaging applications for disease diagnosis due to their distinctive optical features. Additionally, they have been utilized as luminescent probes in bioassays or as bio/chemosensors [64]. Hence, incorporating a MIP shell on QDs has grabbed the attention of researchers to explore new sensing strategies. The fabrication methods for preparing MIP@QDs can be divided into three categories [65].
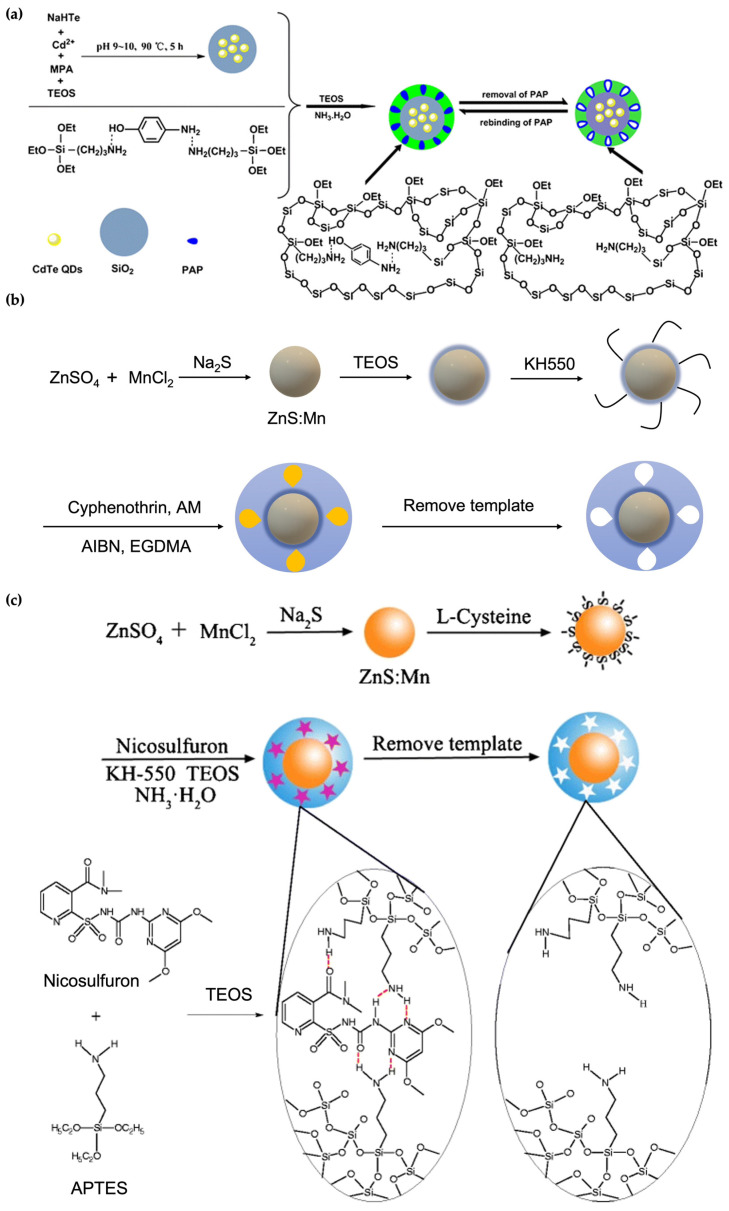
In the first approach, a silica coating is introduced onto the QDs. This method takes advantage of silica’s stability, nontoxicity, and biocompatibility. Using such a modification, Lu et al. designed a sensor system for recognizing p-aminophenol (PAP) by grafting a MIP layer onto silica-coated CdTe QDs [66]. They used a one-pot approach [67] for the synthesis of CdTe@SiO2, which allows first the CdTe QDs formation, followed by the silica adhering in the presence of TEOS in the same reactor (Figure 6a). The introduction of the silica shell creates hydroxyl groups onto the nanoparticle surface, which are subsequently utilized in the imprinting step. APTES is used as a functional monomer, and a silanization reaction was employed for the production of the imprinted polysiloxane shell.
Figure 6.
Demonstration of different strategies for preparing MIP@QDs. (a) Silica coating onto QDs, (b) inorganic–organic MIP@QDs, and (c) organic MIP@QDs. Reproduced with permission from [66,68,69] respectively.
The second approach addresses overcoming the drawback of silica’s limited modifiable chemical functionalities. Since organic polymers hold a more extensive selection of functional groups, combining inorganic and organic compounds for the MIP@QDs is desirable. In these hybrid systems, QDs can act as either a sensing material or a supporting material in the core of the final core-shell nanoparticles. For example, a cyphenothrin imprinted polymer was synthesized onto a QD which acts as a fluorescence probe for pesticide detection [68]. As illustrated in Figure 6b, the first step involved the synthesis of Mn-doped ZnS QDs, with subsequent stabilization with a silica layer by hydrolysis of TEOS. Amine groups were introduced onto the surface by silanization with APTES. Surface imprinting on the amino-modified QDs was done in the presence of acrylamide (AM) as the functional monomer, ethylene glycol dimetachrylate (EGDMA) as the cross-linker, and the target molecule (cyphenothrin). Quenching of the fluorescence of the MIP-coated QDs upon interaction with cyphenothrin enables selective sensing of this pesticide.
The third strategy generally employed for the fabrication of MIP@QDs involves directly coating the polymeric layer on the inorganic core. Even though silica is mainly used in this field and the QDs@organicMIPs are reported less, the approach offers certain advantages. For example, Chen and coworkers compared QD-based MIPs consisting of imprinted silica versus an imprinted organic polymer-based shell. Experimental results showed that the acrylate-based MIP@QDs exhibited better recognition than the silica-based nanomaterial [70]. In addition, the direct incorporation of polymeric shell decreases the number of preparation steps and, as a result, shortens the fabrication time. Later on, another organic MIP@QDs example was demonstrated by Ren et al. [69]. They presented the combination of inorganic (Mn-doped ZnS QDs)-organic MIPs to determine nicosulfuron in water samples. The synthesis process is comprised of first, the fabrication of Mn-doped ZnS QDs, and second, the direct surface imprinting of the polymers onto the nanoparticle Figure 6c. The resulting QDs nanoparticles had a high selectivity toward nicosulfuron and a substantial fluorescence property.
3.4. Gold (Au) and Silver (Ag) NPs
Gold (Au) nanoparticles are among the most widely used core materials for the fabrication of inorganic nanoparticle-based MIPs since they possess a larger surface area, conductivity, high surface energy, and a characteristic surface plasmon resonance [71]. These features make Au nanoparticles desirable in the applications of biosensing and diagnostics. Hence, various sensor designs have been investigated by combining Au nanoparticles with MIPs to obtain analytical platforms.
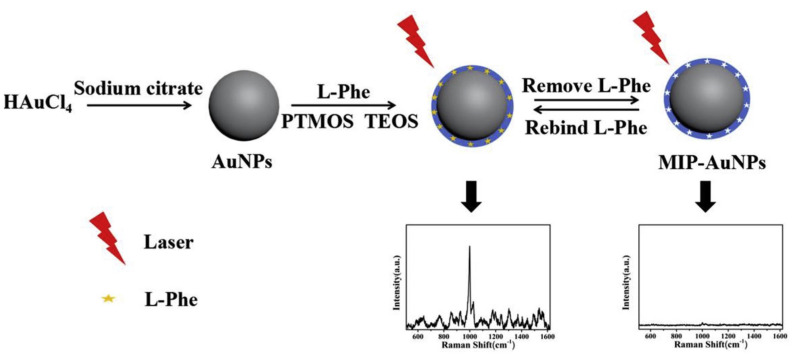
For example, in 2015, Yang et al. designed a MIP-coated Au nanoparticle system that was covered onto an electrode to build an electrochemical sensor [72]. First, the Au nanoparticles were synthesized by reducing HAuCl4. Then, an ionic liquid (IL) was self-assembled onto the nanoparticle surface to be further used as a monomer to synthesize MIPs in the presence of dimetridazole as the template, EGDMA as the cross-linker, and AIBN as the initiator. The resultant core-shell nanoparticle system was coated onto the carbon and graphene-modified glassy carbon electrode surface to build a sensor. Recently, another example of the MIP-coated Au nanoparticles for the selective recognition and quantification of L-phenylalanine (L-Phe) was demonstrated by Zhou et al. [73]. MIP@Au nanoparticles were utilized as a surface-enhanced Raman scattering (SERS) interface. After preparing the Au nanoparticles, the authors constructed the imprinted polymer layer onto the nanoparticles by sol-gel and surface imprinting techniques (Figure 7). The polymerization was performed with tetraethyl orthosilicate as the cross-linker and phenyltrimethoxysilane as the functional monomer. When removal of the template molecule from the MIP shell was completed, rebinding was observed according to the obtained SERS signals.
Figure 7.
General scheme for the synthesis of the MIP@AuNPs, and SERS signal of MIP@AuNPs before and after the elution of the target molecule. Reproduced with permission from [73].
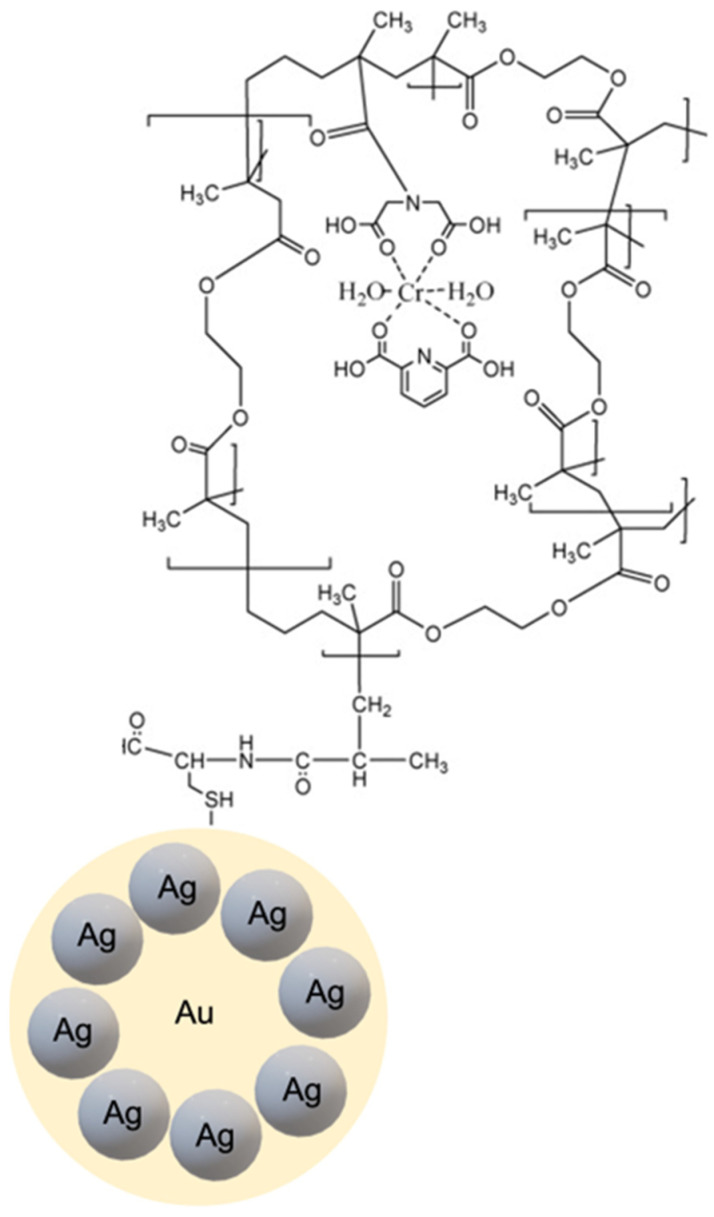
In a different approach, Gultekin et al. [74] employed a nanocluster system composed of Au and silver (Ag) nanoparticles for the detection of Bacillus cereus spores through recognition of dipicolinic acid (DPA), a characteristic compound present in the Bacillus spores. A thiol-exchange capping method was utilized to synthesize gold-silver nanocomposite-based core material. Polymerizable methacryloylamidocysteine (MAC) was attached to the gold nanoparticle surface, followed by the addition of silver salt (AgNO3) to obtain the Au-Ag nanocluster. To construct the MIP shell, methacryloyl-activated nanoclusters were subjected to photopolymerization after adding the metal-chelate (MAIDA-Cr(III)/DPA) monomer, EDMA cross-linking, and initiator. The Au-Ag nanocluster-based MIPs were obtained after removing the template molecules using acidic conditions (Figure 8). Fluorescence quenching of the Au-Ag nanocluster MIP was used as a modality for detecting DPA.
Figure 8.
Representation of Au-Ag nanoclusters with shell based on DPA template imprinting. Reproduced with permission from [74].
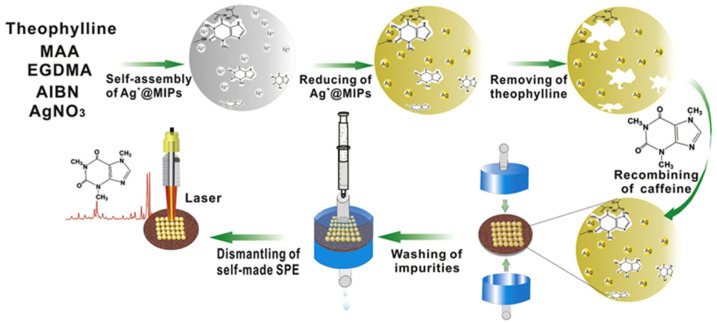
The utilization of Ag nanoparticles plays a vital role in biomedical applications among metallic nanoparticles. Using them as a SERS substrate is widely used and well-established in the literature [30]. For the fabrication of a MIP-based SERS sensor, Hu et al. developed theophylline-imprinted polymer-loaded Ag nanoparticles [75]. They used precipitation polymerization to form the Ag@MIP system to be further used as a solid phase extraction (SPE) agent and SERS substrate for the single-step extraction and the quantification of caffeine. Theophylline was chosen as the dummy template. The polymeric layer was formed in excess acetonitrile with MAA as the functional monomer and EGDMA as the cross-linker (Figure 9). The overall time for the detection and separation of caffeine was determined as 23 min with a detection limit of 100 ng/L.
Figure 9.
Synthesis of theophylline imprinted AgNPs, and subsequent SPE of caffeine before SERS detection. Reproduced with permission from [75].
4. Biomedical Applications of Core-Shell MIPs
4.1. Biosensors
Selective recognition of a target molecule is a requisite for a biosensing system. Recognition of a specific biomarker, protein, or antibody is required in diagnostic applications, and such recognition is accomplished using biomolecules such as proteins and antibodies. As discussed in the introduction, MIPs offer a synthetic alternative to these natural detection elements. A literature survey indicates that core-shell MIPs containing an inorganic nanoparticle core enable the fabrication of a wide range of analytical sensors. The output detection signal is sorted as an electrochemical, optical, or piezoelectric response depending on the core material.
Magnetic nanoparticles for electrochemical sensing have been widely employed for diagnostic applications. In the context of molecular imprinting-based sensing, Cao and coworkers proposed an approach for hemoglobin detection in clinical diagnostics [76]. Hemoglobin (Hb) is an important biomarker that gives information regarding many clinical diseases such as hypertension, pulmonary diseases, and leukemia. Thus, developing methods for rapid and accurate quantification is essential. Their sensor design was based on Fe3O4@SiO2 composite nanoparticles with a MIP layer. The Hb oxidation measurements at the glassy carbon electrode surface allowed the determination of Hb with a response time of 7 min. The authors also tested the sensor using whole blood samples with recoveries between 95.7% and 105%.
As another application, magnetic core-shell nanoparticles were prepared for the selective determination of microorganisms to address antimicrobial resistance by Mishra and coworkers [77]. They used Fe3O4 nanoparticles as the core material, which was surface modified with 3-methacryloxypropyltrimethoxysilane (MPS). The imprinted polymer was synthesized onto the core material using pyocyanin as the template molecule, which is directly related to Pseudomonas infections. Authors utilized the core-shell design to detect bacteria in complex biological samples. The binding capacity and the imprinting factor of pyocyanin to the MIP-coated nanoparticle was found to be 2.5 mg/g and 5, respectively.
Recently, another electrochemical sensor example for detecting tumor necrosis factor-alpha (TNF-α) was reported [78]. Neonatal sepsis is a bloodstream infection encountered in newborn infants and causes neonatal deaths [79]. The TNF-α level is used as an indication of this condition. Previously used methods for neonatal sepsis diagnosis have drawbacks such as higher cost, time-consuming features, or difficulty in operations [80,81]. The proposed platform used a Fe3O4@SiO2 -modified screen-printed electrode that requires less sample volume from 50 to 100 μL to analyze the sample. The instant detection of the biomarker was achieved with a low detection limit of 0.01 pM.
MIP@QDs nanomaterials offer unique optical superiority, which makes them attractive components in the fabrication of devices for sensing applications. Mehrzad-Samarin et al. designed MIP-based graphene QDs for fluorescence-based detection of metronidazole [82]. Metronidazole is an antibacterial drug that is generally used for the treatment of acne diseases. This sensor system was proposed to monitor drug resistance caused by long-term and uncontrolled usage. A proportional fluorescence quenching in the presence of the drug was observed.
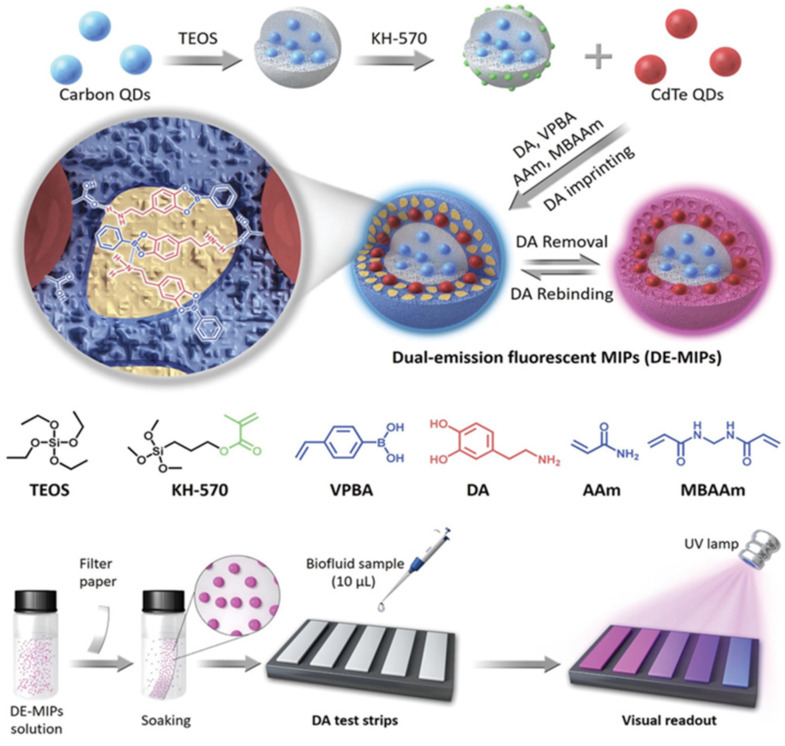
The fluorescence sensor systems use an optical readout method. It is desirable to have a handheld readout device to give faster results and provide portability, as Wang et al. presented [83]. They introduced a MIP-based-QDs approach for dopamine detection in tiny amounts of fluids. Dopamine is a neurotransmitter correlated to several pathological disorders such as Parkinson’s, schizophrenia, and hyperactivity disorders [84]. Their system is a paper-based assay that enables dopamine detection in 10 μL of serum within the linear range of 0–1.2 × 10−6 M. MIP shell fabricated using a boronic acid unit which has a high affinity for dopamine was coated onto the dual emission QDs to achieve visual verification of the presence of dopamine using exposure to UV light, and the detection limit was calculated as (100–150) × 10−9 M (Figure 10).
Figure 10.
General scheme of generation and operation of the QDs@MIP. Reproduced with permission from [83].
Another dopamine detection system was introduced by Shi and coworkers [85]. A MIP-based voltammetric sensor was fabricated using Au nanoparticles covered with biocompatible porous SiO2. A MIP polymer layer was assembled onto the nanoparticle through the hydrogen bonding and π–π interactions between dopamine molecules and the silane-based monomers. Cyclic voltammetry measurements were carried out using the glassy carbon electrode for the detection of dopamine in the range from 4 × 10−8 to 5 × 10−5 M within the detection limit of 2 × 10−8 M. They tested the sensor design in human urine samples which performed as a versatile tool for DA determination.
Gold nanoparticles are among the most widely used substrates in SERS-based sensing. Using gold nanoparticle-based MIP, Li and coworkers designed an antibody-free immunoassay [86]. The sensor system was used to precisely determine carcinoembryonic antigen (CEA), a protein typically found in deficient levels in the blood, and increased level is associated with certain types of cancer. MIP preparation was done using 4-vinylbenzeneboronic acid (VPBA) as the functional monomer and ethylene glycol dimetachrylate (EGDMA) as the cross-linking agent. The resulting sensor, also called SERS-tag, was used to quantify CEA in spiked serum within the detection limit of 0.1 ng/mL. For a quick overview, the above examples are summarized in Table 2.
Table 2.
Summary of the presented works.
| Target | Core Material | Linear Range | LOD | Ref. |
|---|---|---|---|---|
| Hemoglobin | Fe3O4@SiO2 | 0.005–0.1 mg/mL | 0.0010 mg/mL | [76] |
| Pyocyanin | Fe3O4@SiO2 | - | - | [77] |
| TNF-α | Fe3O4@SiO2 | 0.01 pM–100 nM | 0.01 pM | [78] |
| Metronidazole | GQDs@SiO2 | 0.2–15 μM | 0.15 μM | [82] |
| Dopamine | CdTe QDs@SiO2 | 0–1.2 × 10−6 M | (100–50) × 10−9 M | [83] |
| Dopamine | Au@SiO2 | 4 × 10−8–5 × 10−5 M | 2 × 10−8 M | [85] |
| Carcinoembryonic Antigen | Au | - | 0.1 ng/mL | [86] |
4.2. Drug Delivery
The imprinting technology is of interest in pharmaceuticals because the polymer-nanoparticle composites can act as versatile drug delivery vehicles. Polymer based nanocarriers have been extensively employed in delivery of imaging agents and therapeutically active molecules [87]. It has been demonstrated that the MIP-based drug delivery systems enhanced drug efficiency with desired controlled release and decreased side effects due to better drug encapsulation [23,88,89]. Surface properties of the inorganic material have an important role in determining the overall biocompatibility; therefore, surface modifications can deliver MIP-based core-shell systems suitable for in vivo studies.
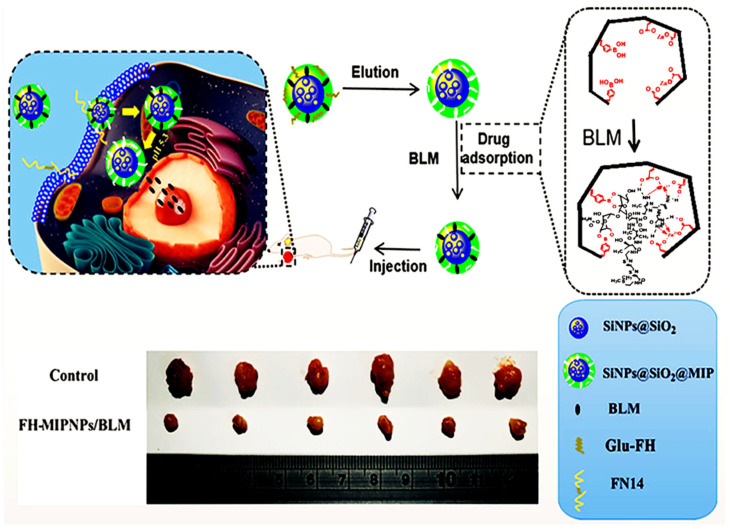
Jia et al. [90] presented a MIP@nanoparticle design for the dual templates; bleomycin (BLM) and 71–80 peptide of human fibroblast growth-factor-inducible 14 modified with glucose (Glu-FH). They first synthesized the SiO2 nanoparticles and modified them by silanization. Dual templates-imprinted polymers covered the surface of nanoparticles to be used for both targeting and the drug delivery for pancreatic cancer BxPC-3 cells. Targeting and the drug release performances of the design were first confirmed by in vitro experiments, followed by the in vivo tumor treatment. The results proved these MIP-coated nanoparticles’ low toxicity and tumor growth inhibition capacity (Figure 11).
Figure 11.
General illustration of preparation and application of silane-modified MIP@SiO2. Reproduced with permission from [90].
Zavareh, Hashemi-Moghaddam, and coworkers investigated the usage of Fe3O4 nanoparticles in the drug delivery system in two parallel studies [91,92]. They worked with the breast tumor-induced mouse models and delivered the doxorubicin (DOX) and 5-fluorouracil (5-FU) drugs to the tumor sites. Both studies used dopamine, which can be self-polymerized, to adhere the polydopamine layer onto the Fe3O4 core. The mouse models used an external magnetic field to deliver the drugs. Recently, Nerantzaki et al. proposed the DOX-loaded molecularly imprinted polymer adhering with Fe3O4 nanoparticles [93]. They designed by using a biotinylated surface since biotin divides the cancer cells and thus, increases the drug uptake.
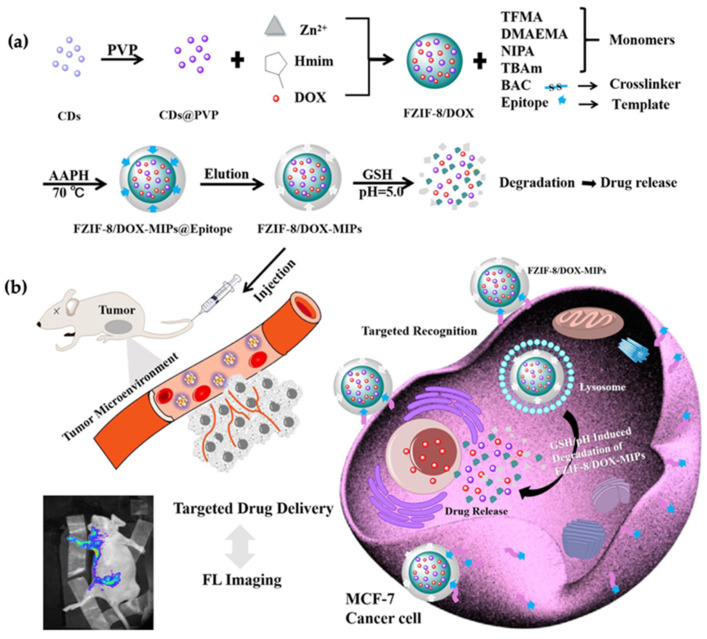
In another example, a dual stimulative drug delivery system was developed using carbon-based QDs (CQDs) [94]. Zhang and coworkers synthesized the tumor-sensitive biodegradable DOX-loaded MIP particles. The particles targeted the MCF-7 cancer cells, and the particle degradation was induced by low pH and high glutathione levels. The preparation of MIPs@nanoparticles was done with an epitope of the CD59 cell membrane glycoprotein and DOX as templates, and the high concentration of glutathione and weak acidic environment due to the cancer cells triggered DOX release (Figure 12a). They employed fluorescent zeolitic imidazolate framework-8 (FZIF-8) as the framework since its fully biodegradable nature in an acidic environment. The fluorescence intensity of MIP@nanoparticles was found ~4 times higher than the control group, which indicated enhanced adsorption and strong selectivity. Additionally, the viability of MCF-7 cells was dramatically lower after the treatment with the FZIF-8/DOX MIP nanoparticles, with 20% survival after 72 h compared to the control trials. In vivo experiments showed that tumor volume in mice was found to be smaller after being treated with FZIF-8/DOX MIP nanoparticles (Figure 12b). Most importantly, they did not observe significant apoptosis in normal cells, which showed successful selective targeting.
Figure 12.
(a) Synthesis of tumor-sensitive degradable DOX-loaded MIP nanoparticles, (b) illustration of targeted imaging and drug delivery. Reproduced with permission from [94].
4.3. Bioimaging
Bioimaging plays a crucial role in life-science studies since it enables scientists to visualize the biological activities to analyze molecules, cells, or tissues. Bioimaging involves the determination of the targeted biological entities selectively but at the same time with the information related to their spatial localization. Integrating inorganic nanoparticles and MIPs elevate the bioimaging performances of functional nanomaterials. The approach is attractive since the preparation method is usually adaptable for any targeting, the biocompatibility of core-nanoparticles can be enhanced with surface modifications, and the desired core material can be employed.
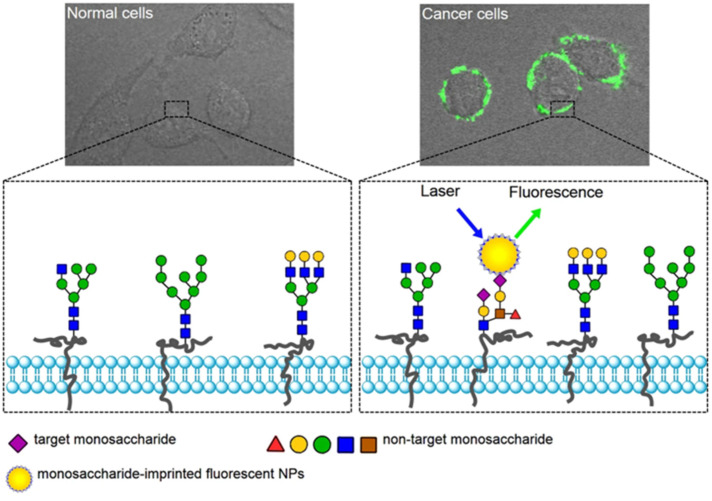
When the bioimaging and drug delivery modalities are combined, the platform becomes much more desirable since additional information such as how long MIP-coated nanoparticles stay in the bloodstream and if they target the site of interest can be obtained. Such an approach was demonstrated by Liu and coworkers, where authors designed monosaccharide-imprinted fluorescence nanoparticles for both the targeting and imaging of human hepatoma carcinoma cells (HepG-2) and mammary cancer cells (MCF-7) (Figure 13) [95]. They used fluorescein isothiocyanate (FITC)-doped SiO2 nanoparticles with an imprinted shell. The SiO2 nanoparticles were functionalized with boronic acid to create boronate affinity. It was confirmed that boronate affinity-based approaches enable efficient and controllable preparation of antibody mimics for monosaccharides. The same boronate-affinity system was also used in another study of the same group [96]. This time, Ag@ SiO2 nanoparticles were functionalized with boronic acid and investigated in SERS imaging. Sialic acid imprinted polymers recognized the cancer cells and tissues where the sialic acid overexpressed.
Figure 13.
Illustration of the targeting and imaging of cancer cells with MIP@FTC@SiO2 nanoparticles. Reproduced with permission from [95].
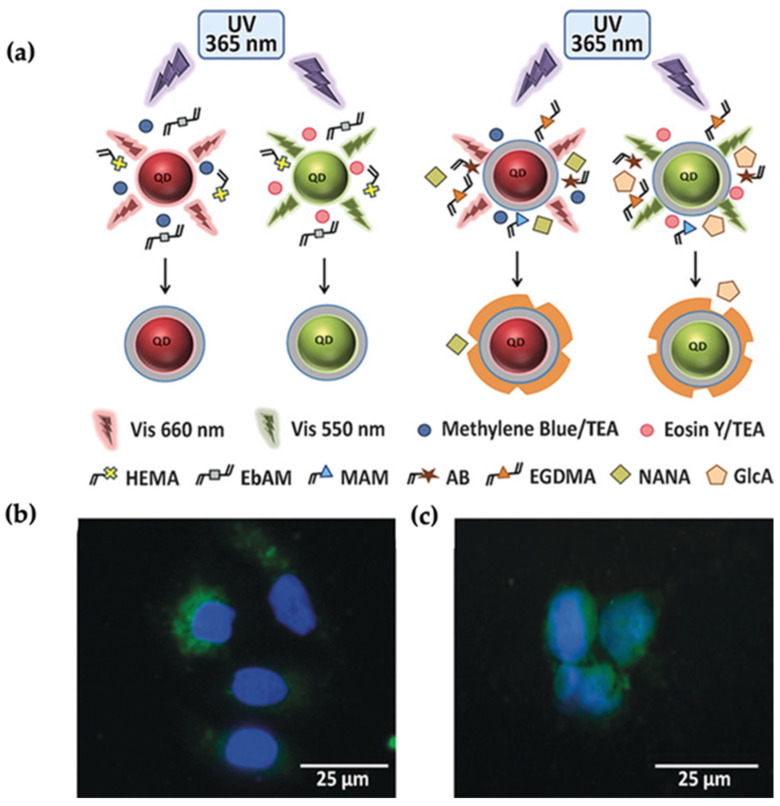
Apart from the tumor glycan targeting, Haupt and coworkers designed fluorescent nanoMIPs to target and image the normal cell and tissues for the first time [97]. They demonstrated the fluorescents nanoMIPs prepared by precipitation polymerization in the presence of polymerizable rhodamine dye. Fluorescently labeled polymers imprinted with glucuronic acid (GlcA) to be used for the identification of hyaluronic acid of keratinocytes; therefore, the red-fluorescent nanoMIPs were localized on the cell surface and used as an imaging tool for human keratinocyte cells and adult skin tissue. Later the same group proposed a core-shell design (MIP-coated QDs) for the imaging of multiple targets; cell membrane glycans, glucuronic acid (GlcA), and N-acetylneuraminic acid (NANA) [98]. A MIP shell was photopolymerized onto the QDs by using methacrylamide (MAM), ethylene glycol dimethacrylate (EGDMA), and eosin/TEA in the presence of target molecules (Figure 14a). Since the QDs possess tunable light emission and broad excitation features, they used the different light emissions for multiplexed cell imaging. Green (550 nm) and red (660 nm) light-emitting QDs were excited in the presence of a UV source. In vivo cell imaging studies showed that MIP-coated QDs provided better binding to human keratinocytes and can be used as a versatile multiplexed imaging tool (Figure 14b).
Figure 14.
(a) Demonstration of red and green light emitting QDs excited by UV and MIP shell around the particles. (b,c) Staining of keratinocytes, (b) KU812 cells, (c) with MIP@QDs Reproduce with permission from [98].
4.4. Bioseparation
Novel bioseparation techniques are in increasing demand, given the importance of identifying and efficiently removing proteins or biological compounds of interest from bodily fluids for disease diagnostics and treatment. Efficient separation of biomolecules, especially highly abundant proteins in proteomics, is required. Some inheriting gene mutations are responsible for producing abnormal types of proteins in the body. In this case, separating these highly abundant proteins from the potential biomarkers helps determine a disorder. MIP-based platforms are attractive materials for separation studies due to their easy fabrication, scale-up, and high stability [14,99,100].
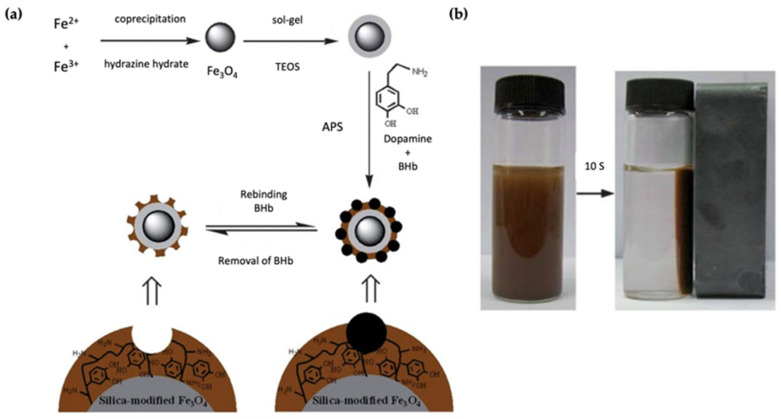
Polymer-coated magnetic nanoparticles are the perfect candidate for separation processes due to their facile separation by applying an external magnetic field [101]. Jia et al. demonstrated silica modified-Fe3O4 nanoparticles to separate bovine hemoglobin (BHb) [102]. Removal of the high abundance of BHb is essential to enrich the low abundance biomarkers and proteins. For this purpose, they coated polydopamine onto the SiO2@Fe3O4, which avoided protein diffusion (Figure 15a). Surface imprinting was performed in the presence of the template molecule, dopamine, and ammonium persulfate (APS). Since the adsorption sites are close to the surface, the imprinted nanoparticles reached the adsorption equilibrium in a short time, and quick separation was achieved using a magnetic field (Figure 15b). BHb separation with polydopamine-coated inorganic nanoparticles was also demonstrated by Xia et al. [103]. They used SiO2 nanoparticles as the core material, which differed from the previously reported work. The protein recognition and separation profiles were determined by a single protein, BHb, or rebinding experiments in the presence of competitive compounds.
Figure 15.
(a) General preparation of BHb imprinted SiO2@Fe3O4 nanoparticles, (b) magnetic response of the nanoparticles in the presence of an external magnetic field. Reproduce with permission from [102].
In another example, MIP core-shell superparamagnetic nanoparticles were prepared to separate cholesterol [104]. A high cholesterol level is associated with atherosclerosis, a chronic inflammatory disease within the artery wall [105]. The current treatments are either risky or relatively limited. Therefore, removing cholesterol using organic–inorganic hybrid systems is desirable as the reported design. A cholesterol imprinted core-shell nanoparticles were fabricated for this purpose. First, oleic acid-coated Fe3O4 nanoparticles were modified using a ligand exchange reaction with 3-(benzylsulfanylthiocarbonylsulfanyl) propionic acid (BSPA) to anchor BSPA onto the surface. Surface-mediated RAFT polymerization was employed for obtaining a cholesterol-imprinted polymer shell. The hybrid nanocomposites were obtained 20 nm in size with a 6 nm MIP shell. The final product was tested using human serum samples and successfully separated cholesterol with a recovery of 91.6%. The approach here can be modified to establish a separation system for any target steroid.
Recently, a multi-template imprinted polymer was combined with a hybrid nanocomposite system [106]. The selective separation and determination of nonsteroidal anti-inflammatory drugs, naproxen (NPX), methocarbamol (MTH), and omeprazole (OMZ) from biological and pharmaceutical samples was demonstrated. They utilized the composition of multiple nanomaterials to reinforce the final product’s performance. The imprinted polymer layer was constructed onto the Fe3O4/ZnO/CuO/MWCNT nanocomposite core. The presence of MWCNT provides high contact surface and electrical conductivity. Carbon paste electrodes were employed to obtain satisfactory analytical results for separating and detecting NPX, MTH, and OMZ.
5. Conclusions and Future Perspectives
In this review, we presented a general overview of the recent progress in the design and fabrication of MIP-coated inorganic nanoparticles and highlighted their applications in several areas of biomedical sciences. While in the past, MIPs were generally used in sensing applications because of their cheaper cost and higher stability as compared to their biological counterparts such as natural antibodies, advances in their synthesis and integration with functional nanomaterials have led to diverse applications.
Core-shell MIPs have displayed great potential in biomedical science due to their excellent characteristics, such as biocompatibility, stability, controlled size, and the efficient accessibility of the binding sites on the imprinted layer. Using inorganic nanomaterials such as Au, Ag, or Fe3O4 as a core material, helps the organic–inorganic system to have the capacity to respond to external physical stimuli. Therefore, Au, Ag, and Fe3O4 nanomaterials are perfect candidates for bioimaging, bio/chemosensors, and bioseparation applications. Examples illustrate that combining MIPs with QDs opens new doors in the bioimaging area. On the other hand, cytotoxicity is the main challenge for inorganic nanoparticles to be further used in in vivo studies. Even though many examples have been presented for in vitro and short-term in vivo studies, there is still a demand for more investigations to demonstrate lower toxicity in clinical uses. Since SiO2 nanoparticles indicate excellent biocompatibility, they can be employed for in vivo biological applications. SiO2-based coatings can be either directly imprinted or provide a layer to increase the biocompatibility of the inorganic core. Few investigations have been undertaken so far, suggesting that MIP-coated inorganic nanoparticles are suitable candidates for in vivo studies, thus indicating that they may be amenable for translation to clinical settings. The advantages and flexibility of the molecular imprinting technology, and rapid developments in the functionalization and modifications of core inorganic nanoparticles, convincingly suggest that MIP-coated inorganic nanoparticles will continue to play an increasingly important role in the fabrication of diagnostic tools and drug delivery devices.
Author Contributions
Conceptualization, A.S.; writing—original draft preparation, S.O.; writing—review and editing, S.O., O.K., R.S. and A.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kemp E., Palomäki T., Ruuth I.A., Boeva Z.A., Nurminen T.A., Vänskä R.T., Zschaechner L.K., Pérez A.G., Hakala T.A., Wardale M., et al. Influence of Enzyme Immobilization and Skin-Sensor Interface on Non-Invasive Glucose Determination from Interstitial Fluid Obtained by Magnetohydrodynamic Extraction. Biosens. Bioelectron. 2022;206:114123. doi: 10.1016/j.bios.2022.114123. [DOI] [PubMed] [Google Scholar]
- 2.Makaraviciute A., Ramanaviciene A. Site-Directed Antibody Immobilization Techniques for Immunosensors. Biosens. Bioelectron. 2013;50:460–471. doi: 10.1016/j.bios.2013.06.060. [DOI] [PubMed] [Google Scholar]
- 3.Numnuam A., Kanatharana P., Mattiasson B., Asawatreratanakul P., Wongkittisuksa B., Limsakul C., Thavarungkul P. Capacitive Biosensor for Quantification of Trace Amounts of DNA. Biosens. Bioelectron. 2009;24:2559–2565. doi: 10.1016/j.bios.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 4.Haupt K., Medina Rangel P.X., Bui B.T.S. Molecularly Imprinted Polymers: Antibody Mimics for Bioimaging and Therapy. Chem. Rev. 2020;120:9554–9582. doi: 10.1021/acs.chemrev.0c00428. [DOI] [PubMed] [Google Scholar]
- 5.Haupt K. Peer Reviewed: Molecularly Imprinted Polymers: The Next Generation. Anal. Chem. 2003;75:376A–383A. doi: 10.1021/ac031385h. [DOI] [PubMed] [Google Scholar]
- 6.Haupt K., Mosbach K. Molecularly Imprinted Polymers and Their Use in Biomimetic Sensors. Chem. Rev. 2000;100:2495–2504. doi: 10.1021/cr990099w. [DOI] [PubMed] [Google Scholar]
- 7.Dinu A., Apetrei C. A Review of Sensors and Biosensors Modified with Conducting Polymers and Molecularly Imprinted Polymers Used in Electrochemical Detection of Amino Acids: Phenylalanine, Tyrosine, and Tryptophan. Int. J. Mol. Sci. 2022;23:1218. doi: 10.3390/ijms23031218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Che Lah N.F., Ahmad A.L., Mohd Amri M.H., Chin J.Y. Configuration of Molecularly Imprinted Polymers for Specific Uptake of Pharmaceutical in Aqueous Media through Radical Polymerization Method. J. Polym. Res. 2022;29:41. doi: 10.1007/s10965-022-02908-8. [DOI] [Google Scholar]
- 9.Saylan Y., Akgönüllü S., Çimen D., Derazshamshir A., Bereli N., Yılmaz F., Denizli A. Development of Surface Plasmon Resonance Sensors Based on Molecularly Imprinted Nanofilms for Sensitive and Selective Detection of Pesticides. Sens. Actuators B Chem. 2017;241:446–454. doi: 10.1016/j.snb.2016.10.017. [DOI] [Google Scholar]
- 10.Huang Y.-C., Lin C.-C., Liu C.-Y. Preparation and Evaluation of Molecularly Imprinted Polymers Based on 9-Ethyladenine for the Recognition of Nucleotide Bases in Capillary Electrochromatography. Electrophoresis. 2004;25:554–561. doi: 10.1002/elps.200305735. [DOI] [PubMed] [Google Scholar]
- 11.Amorim M.S., Sales M.G.F., Frasco M.F. Recent Advances in Virus Imprinted Polymers. Biosens. Bioelectron. X. 2022;10:100131. doi: 10.1016/j.biosx.2022.100131. [DOI] [Google Scholar]
- 12.Lieberzeit P.A., Bajwa S., Mustafa G., Wangchareansak T., Dickert F.L. From Metal Ions to Biospecies: Template-Assisted Synthesis as a Strategy to Generate Artificial Receptor Materials. Adv. Mater. Lett. 2011;2:319–321. doi: 10.5185/amlett.2011.1024am2011. [DOI] [Google Scholar]
- 13.Zhang H., Ye L., Mosbach K. Non-Covalent Molecular Imprinting with Emphasis on Its Application in Separation and Drug Development. J. Mol. Recognit. 2006;19:248–259. doi: 10.1002/jmr.793. [DOI] [PubMed] [Google Scholar]
- 14.Cheong W.J., Yang S.H., Ali F. Molecular Imprinted Polymers for Separation Science: A Review of Reviews: Other Techniques. J. Sep. Sci. 2013;36:609–628. doi: 10.1002/jssc.201200784. [DOI] [PubMed] [Google Scholar]
- 15.Saad E.M., El Gohary N.A., Abdel-Halim M., Handoussa H., Mohamed El Nashar R., Mizaikoff B. Molecularly Imprinted Polymers for Selective Extraction of Rosmarinic Acid from Rosmarinus officinalis L. Food Chem. 2021;335:127644. doi: 10.1016/j.foodchem.2020.127644. [DOI] [PubMed] [Google Scholar]
- 16.Wulff G., Liu J. Design of Biomimetic Catalysts by Molecular Imprinting in Synthetic Polymers: The Role of Transition State Stabilization. Acc. Chem. Res. 2012;45:239–247. doi: 10.1021/ar200146m. [DOI] [PubMed] [Google Scholar]
- 17.Mirata F., Resmini M. Molecularly Imprinted Polymers for Catalysis and Synthesis. In: Mattiasson B., Ye L., editors. Molecularly Imprinted Polymers in Biotechnology. Volume 150. Springer International Publishing; Cham, Switzerland: 2015. pp. 107–129. Advances in Biochemical Engineering/Biotechnology. [DOI] [PubMed] [Google Scholar]
- 18.Leibl N., Haupt K., Gonzato C., Duma L. Molecularly Imprinted Polymers for Chemical Sensing: A Tutorial Review. Chemosensors. 2021;9:123. doi: 10.3390/chemosensors9060123. [DOI] [Google Scholar]
- 19.Ramanavicius S., Jagminas A., Ramanavicius A. Advances in Molecularly Imprinted Polymers Based Affinity Sensors (Review) Polymers. 2021;13:974. doi: 10.3390/polym13060974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saylan Y., Akgönüllü S., Yavuz H., Ünal S., Denizli A. Molecularly Imprinted Polymer Based Sensors for Medical Applications. Sensors. 2019;19:1279. doi: 10.3390/s19061279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jahangiri-Manesh A., Mousazadeh M., Nikkhah M., Abbasian S., Moshaii A., Masroor M.J., Norouzi P. Molecularly Imprinted Polymer-Based Chemiresistive Sensor for Detection of Nonanal as a Cancer Related Biomarker. Microchem. J. 2022;173:106988. doi: 10.1016/j.microc.2021.106988. [DOI] [Google Scholar]
- 22.Fernández-Puig S., Lazo-Fraga A.R., Korgel B.A., Oza G., Dutt A., Vallejo-Becerra V., Valdés-González A.C., Chávez-Ramírez A.U. Molecularly Imprinted Polymer-Silica Nanocomposite Based Potentiometric Sensor for Early Prostate Cancer Detection. Mater. Lett. 2022;309:131324. doi: 10.1016/j.matlet.2021.131324. [DOI] [Google Scholar]
- 23.He S., Zhang L., Bai S., Yang H., Cui Z., Zhang X., Li Y. Advances of Molecularly Imprinted Polymers (MIP) and the Application in Drug Delivery. Eur. Polym. J. 2021;143:110179. doi: 10.1016/j.eurpolymj.2020.110179. [DOI] [Google Scholar]
- 24.Han S., Song Y., Liu S., Zhao L., Sun R. Dual Responsive Molecularly Imprinted Polymers Based on UiO-66-DOX for Selective Targeting Tumor Cells and Controlled Drug Release. Eur. Polym. J. 2022;171:111219. doi: 10.1016/j.eurpolymj.2022.111219. [DOI] [Google Scholar]
- 25.Ali Z., Sajid M., Manzoor S., Ahmad M.M., Khan M.I., Elboughdiri N., Kashif M., Shanableh A., Rajhi W., Mersni W., et al. Biodegradable Magnetic Molecularly Imprinted Anticancer Drug Carrier for the Targeted Delivery of Docetaxel. ACS Omega. 2022;7:28516–28524. doi: 10.1021/acsomega.2c03299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Z., Ma P., Ahmed R., Wang J., Akin D., Soto F., Liu B., Li P., Demirci U. Advanced Point-of-Care Testing Technologies for Human Acute Respiratory Virus Detection. Adv. Mater. 2022;34:2103646. doi: 10.1002/adma.202103646. [DOI] [PubMed] [Google Scholar]
- 27.Hu X., Zhang P., Wang D., Jiang J., Chen X., Liu Y., Zhang Z., Tang B.Z., Li P. AIEgens Enabled Ultrasensitive Point-of-Care Test for Multiple Targets of Food Safety: Aflatoxin B1 and Cyclopiazonic Acid as an Example. Biosens. Bioelectron. 2021;182:113188. doi: 10.1016/j.bios.2021.113188. [DOI] [PubMed] [Google Scholar]
- 28.Saylan Y., Denizli A. Molecularly Imprinted Polymer-Based Microfluidic Systems for Point-of-Care Applications. Micromachines. 2019;10:766. doi: 10.3390/mi10110766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.González G.P., Hernando P.F., Alegría J.S.D. A Morphological Study of Molecularly Imprinted Polymers Using the Scanning Electron Microscope. Anal. Chim. Acta. 2006;557:179–183. doi: 10.1016/j.aca.2005.10.034. [DOI] [Google Scholar]
- 30.Shen X.S., Wang G.Z., Hong X., Zhu W. Nanospheres of Silver Nanoparticles: Agglomeration, Surface Morphology Control and Application as SERS Substrates. Phys. Chem. Chem. Phys. 2009;11:7450. doi: 10.1039/b904712c. [DOI] [PubMed] [Google Scholar]
- 31.Lu H., Tian H., Wang C., Xu S. Designing and Controlling the Morphology of Spherical Molecularly Imprinted Polymers. Mater. Adv. 2020;1:2182–2201. doi: 10.1039/D0MA00415D. [DOI] [Google Scholar]
- 32.Sagawa T., Oishi M., Yataka Y., Sato R., Iijima K., Hashizume M. Control of the Molecular Permeability of Polysaccharide Composite Films Utilizing a Molecular Imprinting Approach. Polym. J. 2022;54:571–579. doi: 10.1038/s41428-021-00605-9. [DOI] [Google Scholar]
- 33.Chen M., Lu J., Gao J., Yu C., Xing W., Dai J., Meng M., Yan Y., Wu Y. Design of Self-Cleaning Molecularly Imprinted Membrane with Antibacterial Ability for High-Selectively Separation of Ribavirin. J. Membr. Sci. 2022;642:119994. doi: 10.1016/j.memsci.2021.119994. [DOI] [Google Scholar]
- 34.Cao Y., Liu G., Qin X., Li H., Liu C. Preparation and Application of 2-Chlorophenol Molecularly Imprinted Photonic Crystal Hydrogel Sensor. J. Macromol. Sci. Part A. 2021;58:336–343. doi: 10.1080/10601325.2020.1854046. [DOI] [Google Scholar]
- 35.Takimoto K., Takano E., Kitayama Y., Takeuchi T. Synthesis of Monodispersed Submillimeter-Sized Molecularly Imprinted Particles Selective for Human Serum Albumin Using Inverse Suspension Polymerization in Water-in-Oil Emulsion Prepared Using Microfluidics. Langmuir. 2015;31:4981–4987. doi: 10.1021/acs.langmuir.5b00769. [DOI] [PubMed] [Google Scholar]
- 36.Aravind A., Mathew B. Nano Layered Ion Imprinted Polymer Based Electrochemical Sensor and Sorbent for Mn (II) Ions from Real Samples. J. Macromol. Sci. Part A. 2020;57:256–265. doi: 10.1080/10601325.2019.1691451. [DOI] [Google Scholar]
- 37.Pu H., Xu L. Molecularly Imprinted Nanoparticles Synthesized by Electrochemically Mediated Atom Transfer Radical Precipitation Polymerization. Macro Chem. Phys. 2022;223:2100478. doi: 10.1002/macp.202100478. [DOI] [Google Scholar]
- 38.Canfarotta F., Cecchini A., Piletsky S. Chapter 1. Nano-Sized Molecularly Imprinted Polymers as Artificial Antibodies. In: Kutner W., Sharma P.S., editors. Polymer Chemistry Series. Royal Society of Chemistry; Cambridge, UK: 2018. pp. 1–27. [Google Scholar]
- 39.Ansell R.J., Mosbach K. Magnetic Molecularly Imprinted Polymer Beads for Drug Radioligand Binding Assay. Analyst. 1998;123:1611–1616. doi: 10.1039/a801903g. [DOI] [PubMed] [Google Scholar]
- 40.Mustafa G., Lieberzeit P.A. Molecularly Imprinted Polymer–Ag 2 S Nanoparticle Composites for Sensing Volatile Organics. RSC Adv. 2014;4:12723–12728. doi: 10.1039/C3RA44208J. [DOI] [Google Scholar]
- 41.Chiozzi V., Rossi F. Inorganic–Organic Core/Shell Nanoparticles: Progress and Applications. Nanoscale Adv. 2020;2:5090–5105. doi: 10.1039/D0NA00411A. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wulff G., Sarhan A. The Use of Polymers with Enzyme-Analogous Structures for the Resolution of Racemates. Angew. Chem. Int. Ed. 1972;11:341–344. [Google Scholar]
- 43.Wulff G. Molecular Imprinting in Cross-Linked Materials with the Aid of Molecular Templates—A Way towards Artificial Antibodies. Angew. Chem. Int. Ed. Engl. 1995;34:1812–1832. doi: 10.1002/anie.199518121. [DOI] [Google Scholar]
- 44.Arshady R., Mosbach K. Synthesis of Substrate Selective Polymers by Host-Guest Polymerization. Makromol. Chem. 1981;182:687–692. doi: 10.1002/macp.1981.021820240. [DOI] [Google Scholar]
- 45.Sellergren B., Lepistoe M., Mosbach K. Highly Enantioselective and Substrate-Selective Polymers Obtained by Molecular Imprinting Utilizing Noncovalent Interactions. NMR and Chromatographic Studies on the Nature of Recognition. J. Am. Chem. Soc. 1988;110:5853–5860. doi: 10.1021/ja00225a041. [DOI] [Google Scholar]
- 46.Yoshimatsu K., Reimhult K., Krozer A., Mosbach K., Sode K., Ye L. Uniform Molecularly Imprinted Microspheres and Nanoparticles Prepared by Precipitation Polymerization: The Control of Particle Size Suitable for Different Analytical Applications. Anal. Chim. Acta. 2007;584:112–121. doi: 10.1016/j.aca.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 47.Ye L., Cormack P.A.G., Mosbach K. Molecularly Imprinted Monodisperse Microspheres for Competitive Radioassay. Anal. Commun. 1999;36:35–38. doi: 10.1039/a809014i. [DOI] [Google Scholar]
- 48.Ruela A.L.M., de Figueiredo E.C., Carvalho F.C., de Araújo M.B., Pereira G.R. Adsorption and Release of Nicotine from Imprinted Particles Synthesised by Precipitation Polymerisation: Optimising Transdermal Formulations. Eur. Polym. J. 2018;100:67–76. doi: 10.1016/j.eurpolymj.2018.01.021. [DOI] [Google Scholar]
- 49.Pardeshi S., Singh S.K. Precipitation Polymerization: A Versatile Tool for Preparing Molecularly Imprinted Polymer Beads for Chromatography Applications. RSC Adv. 2016;6:23525–23536. doi: 10.1039/C6RA02784A. [DOI] [Google Scholar]
- 50.Vaihinger D., Landfester K., Kräuter I., Brunner H., Tovar G.E.M. Molecularly Imprinted Polymer Nanospheres as Synthetic Affinity Receptors Obtained by Miniemulsion Polymerisation. Macromol. Chem. Phys. 2002;203:1965–1973. doi: 10.1002/1521-3935(200209)203:13<1965::AID-MACP1965>3.0.CO;2-C. [DOI] [Google Scholar]
- 51.Mehmood A., Ghafar H., Yaqoob S., Gohar U.F., Ahmad B. Mesoporous Silica Nanoparticles: A Review. J. Dev. Drugs. 2017;06:1000174. doi: 10.4172/2329-6631.1000174. [DOI] [Google Scholar]
- 52.Lin Z., Xia Z., Zheng J., Zheng D., Zhang L., Yang H., Chen G. Synthesis of Uniformly Sized Molecularly Imprinted Polymer-Coated Silica Nanoparticles for Selective Recognition and Enrichment of Lysozyme. J. Mater. Chem. 2012;22:17914. doi: 10.1039/c2jm32734a. [DOI] [Google Scholar]
- 53.Gao R., Zhang J., He X., Chen L., Zhang Y. Selective Extraction of Sulfonamides from Food by Use of Silica-Coated Molecularly Imprinted Polymer Nanospheres. Anal. Bioanal Chem. 2010;398:451–461. doi: 10.1007/s00216-010-3909-z. [DOI] [PubMed] [Google Scholar]
- 54.Chang L., Li Y., Chu J., Qi J., Li X. Preparation of Core-Shell Molecularly Imprinted Polymer via the Combination of Reversible Addition-Fragmentation Chain Transfer Polymerization and Click Reaction. Anal. Chim. Acta. 2010;680:65–71. doi: 10.1016/j.aca.2010.09.017. [DOI] [PubMed] [Google Scholar]
- 55.Zhou T., Zhang K., Kamra T., Bülow L., Ye L. Preparation of Protein Imprinted Polymer Beads by Pickering Emulsion Polymerization. J. Mater. Chem. B. 2015;3:1254–1260. doi: 10.1039/C4TB01605J. [DOI] [PubMed] [Google Scholar]
- 56.Dadfar S.M., Roemhild K., Drude N.I., von Stillfried S., Knüchel R., Kiessling F., Lammers T. Iron Oxide Nanoparticles: Diagnostic, Therapeutic and Theranostic Applications. Adv. Drug Deliv. Rev. 2019;138:302–325. doi: 10.1016/j.addr.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Oz Y., Arslan M., Gevrek T.N., Sanyal R., Sanyal A. Modular Fabrication of Polymer Brush Coated Magnetic Nanoparticles: Engineering the Interface for Targeted Cellular Imaging. ACS Appl. Mater. Interfaces. 2016;8:19813–19826. doi: 10.1021/acsami.6b04664. [DOI] [PubMed] [Google Scholar]
- 58.Oz Y., Abdouni Y., Yilmaz G., Becer C.R., Sanyal A. Magnetic Glyconanoparticles for Selective Lectin Separation and Purification. Polym. Chem. 2019;10:3351–3361. doi: 10.1039/C8PY01748D. [DOI] [Google Scholar]
- 59.Turan E., Şahin F. Molecularly Imprinted Biocompatible Magnetic Nanoparticles for Specific Recognition of Ochratoxin A. Sens. Actuators B Chem. 2016;227:668–676. doi: 10.1016/j.snb.2015.12.087. [DOI] [Google Scholar]
- 60.Mehdinia A., Baradaran Kayyal T., Jabbari A., Aziz-Zanjani M.O., Ziaei E. Magnetic Molecularly Imprinted Nanoparticles Based on Grafting Polymerization for Selective Detection of 4-Nitrophenol in Aqueous Samples. J. Chromatogr. A. 2013;1283:82–88. doi: 10.1016/j.chroma.2013.01.093. [DOI] [PubMed] [Google Scholar]
- 61.Ramimoghadam D., Bagheri S., Hamid S.B.A. Progress in Electrochemical Synthesis of Magnetic Iron Oxide Nanoparticles. J. Magn. Magn. Mater. 2014;368:207–229. doi: 10.1016/j.jmmm.2014.05.015. [DOI] [Google Scholar]
- 62.Liu Y., Huang Y., Liu J., Wang W., Liu G., Zhao R. Superparamagnetic Surface Molecularly Imprinted Nanoparticles for Water-Soluble Pefloxacin Mesylate Prepared via Surface Initiated Atom Transfer Radical Polymerization and Its Application in Egg Sample Analysis. J. Chromatogr. A. 2012;1246:15–21. doi: 10.1016/j.chroma.2012.01.045. [DOI] [PubMed] [Google Scholar]
- 63.Zora A., Triberis G.P., Simserides C. Near-Field Optical Properties of Quantum Dots, Applications and Perspectives. Recent Pat. Nanotechnol. 2011;5:188–224. doi: 10.2174/1872210511105030188. [DOI] [PubMed] [Google Scholar]
- 64.Abbasi E., Kafshdooz T., Bakhtiary M., Nikzamir N., Nikzamir N., Nikzamir M., Mohammadian M., Akbarzadeh A. Biomedical and Biological Applications of Quantum Dots. Artif. Cells Nanomed. Biotechnol. 2016;44:885–891. doi: 10.3109/21691401.2014.998826. [DOI] [PubMed] [Google Scholar]
- 65.Díaz-Álvarez M., Martín-Esteban A. Molecularly Imprinted Polymer-Quantum Dot Materials in Optical Sensors: An Overview of Their Synthesis and Applications. Biosensors. 2021;11:79. doi: 10.3390/bios11030079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lu X., Wei F., Xu G., Wu Y., Yang J., Hu Q. Surface Molecular Imprinting on Silica-Coated CdTe Quantum Dots for Selective and Sensitive Fluorescence Detection of p-Aminophenol in Water. J. Fluoresc. 2017;27:181–189. doi: 10.1007/s10895-016-1944-7. [DOI] [PubMed] [Google Scholar]
- 67.Zhou L., Gao C., Hu X., Xu W. One-Pot Large-Scale Synthesis of Robust Ultrafine Silica-Hybridized CdTe Quantum Dots. ACS Appl. Mater. Interfaces. 2010;2:1211–1219. doi: 10.1021/am9009296. [DOI] [PubMed] [Google Scholar]
- 68.Ren X., Chen L. Quantum Dots Coated with Molecularly Imprinted Polymer as Fluorescence Probe for Detection of Cyphenothrin. Biosens. Bioelectron. 2015;64:182–188. doi: 10.1016/j.bios.2014.08.086. [DOI] [PubMed] [Google Scholar]
- 69.Ren X., Chen L. Preparation of Molecularly Imprinted Polymer Coated Quantum Dots to Detect Nicosulfuron in Water Samples. Anal. Bioanal Chem. 2015;407:8087–8095. doi: 10.1007/s00216-015-8982-x. [DOI] [PubMed] [Google Scholar]
- 70.Chao M.-R., Hu C.-W., Chen J.-L. Comparative Syntheses of Tetracycline-Imprinted Polymeric Silicate and Acrylate on CdTe Quantum Dots as Fluorescent Sensors. Biosens. Bioelectron. 2014;61:471–477. doi: 10.1016/j.bios.2014.05.058. [DOI] [PubMed] [Google Scholar]
- 71.Yeh Y.-C., Creran B., Rotello V.M. Gold Nanoparticles: Preparation, Properties, and Applications in Bionanotechnology. Nanoscale. 2012;4:1871–1880. doi: 10.1039/C1NR11188D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang G., Zhao F. Electrochemical Sensor for Dimetridazole Based on Novel Gold Nanoparticles@molecularly Imprinted Polymer. Sens. Actuators B Chem. 2015;220:1017–1022. doi: 10.1016/j.snb.2015.06.051. [DOI] [Google Scholar]
- 73.Zhou J., Sheth S., Zhou H., Song Q. Highly Selective Detection of L-Phenylalanine by Molecularly Imprinted Polymers Coated Au Nanoparticles via Surface-Enhanced Raman Scattering. Talanta. 2020;211:120745. doi: 10.1016/j.talanta.2020.120745. [DOI] [PubMed] [Google Scholar]
- 74.Gültekin A., Diltemiz S.E., Ersöz A., Sarıözlü N.Y., Denizli A., Say R. Gold–Silver Nanoclusters Having Dipicolinic Acid Imprinted Nanoshell for Bacillus Cereus Spores Recognition. Talanta. 2009;78:1332–1338. doi: 10.1016/j.talanta.2009.02.007. [DOI] [PubMed] [Google Scholar]
- 75.Hu R., Tang R., Xu J., Lu F. Chemical Nanosensors Based on Molecularly-Imprinted Polymers Doped with Silver Nanoparticles for the Rapid Detection of Caffeine in Wastewater. Anal. Chim. Acta. 2018;1034:176–183. doi: 10.1016/j.aca.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 76.Sun B., Ni X., Cao Y., Cao G. Electrochemical Sensor Based on Magnetic Molecularly Imprinted Nanoparticles Modified Magnetic Electrode for Determination of Hb. Biosens. Bioelectron. 2017;91:354–358. doi: 10.1016/j.bios.2016.12.056. [DOI] [PubMed] [Google Scholar]
- 77.Rajpal S., Bhakta S., Mishra P. Biomarker Imprinted Magnetic Core–Shell Nanoparticles for Rapid, Culture Free Detection of Pathogenic Bacteria. J. Mater. Chem. B. 2021;9:2436–2446. doi: 10.1039/D0TB02842H. [DOI] [PubMed] [Google Scholar]
- 78.Balayan S., Chauhan N., Kumar P., Chandra R., Jain U. Fabrication of a Sensing Platform for Identification of Tumor Necrosis Factor-Alpha: A Biomarker for Neonatal Sepsis. 3 Biotech. 2022;12:37. doi: 10.1007/s13205-021-03083-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shane A.L., Sánchez P.J., Stoll B.J. Neonatal Sepsis. Lancet. 2017;390:1770–1780. doi: 10.1016/S0140-6736(17)31002-4. [DOI] [PubMed] [Google Scholar]
- 80.Hosseini Ghalehno M., Mirzaei M., Torkzadeh-Mahani M. Electrochemical Aptasensor for Tumor Necrosis Factor α Using Aptamer–Antibody Sandwich Structure and Cobalt Hexacyanoferrate for Signal Amplification. J. Iran. Chem. Soc. 2019;16:1783–1791. doi: 10.1007/s13738-019-01650-1. [DOI] [Google Scholar]
- 81.Ben Halima H., Bellagambi F.G., Alcacer A., Pfeiffer N., Heuberger A., Hangouët M., Zine N., Bausells J., Elaissari A., Errachid A. A Silicon Nitride ISFET Based Immunosensor for Tumor Necrosis Factor-Alpha Detection in Saliva. A Promising Tool for Heart Failure Monitoring. Anal. Chim. Acta. 2021;1161:338468. doi: 10.1016/j.aca.2021.338468. [DOI] [PubMed] [Google Scholar]
- 82.Mehrzad-Samarin M., Faridbod F., Dezfuli A.S., Ganjali M.R. A Novel Metronidazole Fluorescent Nanosensor Based on Graphene Quantum Dots Embedded Silica Molecularly Imprinted Polymer. Biosens. Bioelectron. 2017;92:618–623. doi: 10.1016/j.bios.2016.10.047. [DOI] [PubMed] [Google Scholar]
- 83.Wang J., Dai J., Xu Y., Dai X., Zhang Y., Shi W., Sellergren B., Pan G. Molecularly Imprinted Fluorescent Test Strip for Direct, Rapid, and Visual Dopamine Detection in Tiny Amount of Biofluid. Small. 2019;15:1803913. doi: 10.1002/smll.201803913. [DOI] [PubMed] [Google Scholar]
- 84.Franco R., Reyes-Resina I., Navarro G. Dopamine in Health and Disease: Much More Than a Neurotransmitter. Biomedicines. 2021;9:109. doi: 10.3390/biomedicines9020109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yu D., Zeng Y., Qi Y., Zhou T., Shi G. A Novel Electrochemical Sensor for Determination of Dopamine Based on AuNPs@SiO2 Core-Shell Imprinted Composite. Biosens. Bioelectron. 2012;38:270–277. doi: 10.1016/j.bios.2012.05.045. [DOI] [PubMed] [Google Scholar]
- 86.Feng J., Li X., Cheng H., Huang W., Kong H., Li Y., Li L. A Boronate-Modified Molecularly Imprinted Polymer Labeled with a SERS-Tag for Use in an Antibody-Free Immunoassay for the Carcinoembryonic Antigen. Microchim. Acta. 2019;186:774. doi: 10.1007/s00604-019-3972-x. [DOI] [PubMed] [Google Scholar]
- 87.Das S.S., Bharadwaj P., Bilal M., Barani M., Rahdar A., Taboada P., Bungau S., Kyzas G.Z. Stimuli-Responsive Polymeric Nanocarriers for Drug Delivery, Imaging, and Theragnosis. Polymers. 2020;12:1397. doi: 10.3390/polym12061397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Chambre L., Degirmenci A., Sanyal R., Sanyal A. Multi-Functional Nanogels as Theranostic Platforms: Exploiting Reversible and Nonreversible Linkages for Targeting, Imaging, and Drug Delivery. Bioconjugate Chem. 2018;29:1885–1896. doi: 10.1021/acs.bioconjchem.8b00085. [DOI] [PubMed] [Google Scholar]
- 89.Feyzioğlu Demir E., Akgöl S. Synthesis and Characterization of Double Molecular Imprinted Nanoparticles and Investigation to Adsorption of Respiratory Drugs. Polym.-Plast. Technol. Mater. 2022;61:384–389. doi: 10.1080/25740881.2021.1991949. [DOI] [Google Scholar]
- 90.Jia C., Zhang M., Zhang Y., Ma Z.-B., Xiao N.-N., He X.-W., Li W.-Y., Zhang Y.-K. Preparation of Dual-Template Epitope Imprinted Polymers for Targeted Fluorescence Imaging and Targeted Drug Delivery to Pancreatic Cancer BxPC-3 Cells. ACS Appl. Mater. Interfaces. 2019;11:32431–32440. doi: 10.1021/acsami.9b11533. [DOI] [PubMed] [Google Scholar]
- 91.Zavareh S., Mahdi M., Erfanian S., Hashemi-Moghaddam H. Synthesis of Polydopamine as a New and Biocompatible Coating of Magnetic Nanoparticles for Delivery of Doxorubicin in Mouse Breast Adenocarcinoma. Cancer Chemother. Pharmacol. 2016;78:1073–1084. doi: 10.1007/s00280-016-3169-5. [DOI] [PubMed] [Google Scholar]
- 92.Hashemi-Moghaddam H., Kazemi-Bagsangani S., Jamili M., Zavareh S. Evaluation of Magnetic Nanoparticles Coated by 5-Fluorouracil Imprinted Polymer for Controlled Drug Delivery in Mouse Breast Cancer Model. Int. J. Pharm. 2016;497:228–238. doi: 10.1016/j.ijpharm.2015.11.040. [DOI] [PubMed] [Google Scholar]
- 93.Nerantzaki M., Michel A., Petit L., Garnier M., Ménager C., Griffete N. Biotinylated Magnetic Molecularly Imprinted Polymer Nanoparticles for Cancer Cell Targeting and Controlled Drug Delivery. Chem. Commun. 2022;58:5642–5645. doi: 10.1039/D2CC00740A. [DOI] [PubMed] [Google Scholar]
- 94.Qin Y.-T., Feng Y.-S., Ma Y.-J., He X.-W., Li W.-Y., Zhang Y.-K. Tumor-Sensitive Biodegradable Nanoparticles of Molecularly Imprinted Polymer-Stabilized Fluorescent Zeolitic Imidazolate Framework-8 for Targeted Imaging and Drug Delivery. ACS Appl. Mater. Interfaces. 2020;12:24585–24598. doi: 10.1021/acsami.0c05154. [DOI] [PubMed] [Google Scholar]
- 95.Wang S., Yin D., Wang W., Shen X., Zhu J.-J., Chen H.-Y., Liu Z. Targeting and Imaging of Cancer Cells via Monosaccharide-Imprinted Fluorescent Nanoparticles. Sci. Rep. 2016;6:22757. doi: 10.1038/srep22757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yin D., Wang S., He Y., Liu J., Zhou M., Ouyang J., Liu B., Chen H.-Y., Liu Z. Surface-Enhanced Raman Scattering Imaging of Cancer Cells and Tissues via Sialic Acid-Imprinted Nanotags. Chem. Commun. 2015;51:17696–17699. doi: 10.1039/C5CC05174F. [DOI] [PubMed] [Google Scholar]
- 97.Kunath S., Panagiotopoulou M., Maximilien J., Marchyk N., Sänger J., Haupt K. Cell and Tissue Imaging with Molecularly Imprinted Polymers as Plastic Antibody Mimics. Adv. Healthc. Mater. 2015;4:1322–1326. doi: 10.1002/adhm.201500145. [DOI] [PubMed] [Google Scholar]
- 98.Panagiotopoulou M., Salinas Y., Beyazit S., Kunath S., Duma L., Prost E., Mayes A.G., Resmini M., Tse Sum Bui B., Haupt K. Molecularly Imprinted Polymer Coated Quantum Dots for Multiplexed Cell Targeting and Imaging. Angew. Chem. Int. Ed. 2016;55:8244–8248. doi: 10.1002/anie.201601122. [DOI] [PubMed] [Google Scholar]
- 99.Bagheri H.F., Arvand M., Habibi M.F. An Ultra-Sensitive Tailor-Made Sensor for Specific Adsorption and Separation of Rutin Based on Imprinted Cavities on Magnetic Sensing Platform. Microchem. J. 2022;181:107712. doi: 10.1016/j.microc.2022.107712. [DOI] [Google Scholar]
- 100.Lie K.R., Samuel A.O., Hasanah A.N. Molecularly Imprinted Mesoporous Silica: Potential of the Materials, Synthesis and Application in the Active Compound Separation from Natural Product. Chem. Pap. 2022;76:2595–2613. doi: 10.1007/s11696-022-02074-7. [DOI] [Google Scholar]
- 101.Akbulut Söylemez M., Kemaloğulları B.Ö. Surface Modification of Magnetic Nanoparticles via Admicellar Polymerization for Selective Removal of Tetracycline from Real Water Samples. New J. Chem. 2021;45:6415–6423. doi: 10.1039/D1NJ00494H. [DOI] [Google Scholar]
- 102.Jia X., Xu M., Wang Y., Ran D., Yang S., Zhang M. Polydopamine-Based Molecular Imprinting on Silica-Modified Magnetic Nanoparticles for Recognition and Separation of Bovine Hemoglobin. Analyst. 2013;138:651–658. doi: 10.1039/C2AN36313E. [DOI] [PubMed] [Google Scholar]
- 103.Xia Z., Lin Z., Xiao Y., Wang L., Zheng J., Yang H., Chen G. Facile Synthesis of Polydopamine-Coated Molecularly Imprinted Silica Nanoparticles for Protein Recognition and Separation. Biosens. Bioelectron. 2013;47:120–126. doi: 10.1016/j.bios.2013.03.024. [DOI] [PubMed] [Google Scholar]
- 104.Zengin A., Yildirim E., Tamer U., Caykara T. Molecularly Imprinted Superparamagnetic Iron Oxide Nanoparticles for Rapid Enrichment and Separation of Cholesterol. Analyst. 2013;138:7238. doi: 10.1039/c3an01458d. [DOI] [PubMed] [Google Scholar]
- 105.Polyakova I., Borovikova L., Osipenko A., Vlasova E., Volchek B., Pisarev O. Surface Molecularly Imprinted Organic-Inorganic Polymers Having Affinity Sites for Cholesterol. React. Funct. Polym. 2016;109:88–98. doi: 10.1016/j.reactfunctpolym.2016.10.010. [DOI] [Google Scholar]
- 106.Vahidifar M., Es’haghi Z., Oghaz N.M., Mohammadi A.A., Kazemi M.S. Multi-Template Molecularly Imprinted Polymer Hybrid Nanoparticles for Selective Analysis of Nonsteroidal Anti-Inflammatory Drugs and Analgesics in Biological and Pharmaceutical Samples. Environ. Sci. Pollut. Res. 2022;29:47416–47435. doi: 10.1007/s11356-021-18308-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.