Abstract
Chronic kidney disease (CKD) is projected to become the fifth global cause of death by 2040 as a result of key shortcomings in the current methods available to diagnose and treat kidney diseases. In this regard, the novel holobiont concept, used to describe an individual host and its microbial community, may pave the way towards a better understanding of kidney disease pathogenesis and progression. Microbiota-modulating or -derived interventions include probiotics, prebiotics, synbiotics and postbiotics. As of 2019, the concept of postbiotics was updated by the International Scientific Association of Probiotics and Prebiotics (ISAPP) to refer to preparations of inanimate microorganisms and/or their components that confer a health benefit to the host. By explicitly excluding purified metabolites without a cellular biomass, any literature making use of such term is potentially rendered obsolete. We now review the revised concept of postbiotics concerning their potential clinical applications and research in kidney disease, by discussing in detail several formulations that are undergoing preclinical development such as GABA-salt for diet-induced hypertension and kidney injury, sonicated Lactobacillus paracasei in high fat diet-induced kidney injury, GABA-salt, lacto-GABA-salt and postbiotic-GABA-salt in acute kidney injury, and O. formigenes lysates for hyperoxaluria. Furthermore, we provide a roadmap for postbiotics research in kidney disease to expedite clinical translation.
Keywords: chronic kidney disease, postbiotics, prebiotics, probiotics, hyperoxaluria, Oxalobacter formigenes, GABA-salt
1. The Global Burden of Kidney Disease
Chronic kidney disease (CKD) is to date defined by the measurement of the estimated glomerular filtration rate (eGFR, i.e., a measure of kidney function) and albuminuria (i.e., a measure of kidney injury), which are associated with a combined risk of premature all-cause and cardiovascular death, CKD progression and acute kidney injury (AKI) [1]. Despite recent advances in the field, CKD is estimated to become the fifth global cause of death by 2040 and the second cause of death by the end of this century in countries with a long-life expectancy [2]. These projections illustrate key shortcomings in the current methods used to diagnose and treat kidney diseases. As such, an improved understanding of the underlying pathogenesis of CKD may help us identifying novel diagnostic clues and therapeutic targets. In this regard, the holobiont concept, a term used to describe an individual host and its microbial community, including viruses and cellular microorganisms [3], may pave the way to a better understanding of the pathogenesis of CKD. There is indeed increasing evidence suggesting that health and disease may be the result of a bilateral interaction between a host and its microbiota, particularly the gut microbiota. Such interaction may be affected by several factors: disease condition in the host, diet, drugs and antibiotics [4]. Attempts to modulate the host-microbiota interactions have resulted in the design and prescription of prebiotics, probiotics, synbiotics and postbiotics. As a result of a recent consensus on the definition of postbiotics [5], the pre-2019 literature on postbiotics and kidney disease has now become potentially obsolete. Accordingly, we here reviewed the concept and updated the literature on postbiotics in the field of kidney disease, by focusing on these interventions in line with the revised consensus on postbiotics concept.
2. The Gut Microbiota: A Key Modifier of Kidney Disease and Health
In recent years, a key role of the gut microbiota as a homeostasis modulator, influencing both health and disease, has emerged in the context of CKD [6,7]. Indeed, there is a close connection between intestinal dysbiosis, hypervolemia, systemic inflammation, myocardial stunning and the malnutrition-inflammation syndrome in CKD populations [8]. Kidney disease may influence the gut microbiota through several mechanisms, ranging from the influence of the uremic milieu to the impact of prescribed diets (e.g., low potassium diets are often low in dietary fiber), as well as the frequent use of antibiotics and polypharmacy which may adversely modulate the gut microbiota [9,10,11]. Conversely, an altered gut microbiota may contribute to CKD development and progression by mediating increased inflammation and/or generating uremic toxins and their precursors [12]. The increase in intestinal permeability resulting from the degradation of the barrier integrity, as a consequence of an altered microbiota, allows for the transition of endotoxins and bacterial products to the blood. This potentially leads to an increase in oxidative stress and inflammation, contributing to CKD complications, such as cardiovascular disease and mineral metabolism disorders. The gut microbiota may also generate indoles, amines and phenols from dietary components, mainly proteins. These gut-derived metabolites can be metabolized further into the uremic toxins p-cresyl sulphate (pCS), indoxyl sulphate (IS) and trimethylamine N-Oxide (TMAO) [9,11,13]. The presence of uremic toxins in the circulation may lead to proinflammatory events, promoting the expression of transforming factor-β (TGF-β1) and the production of reactive oxygen species (ROS) in kidney tubular epithelial cells [14]. IS and pCS also inhibit Klotho expression via gene hypermethylation [15]. Klotho has antiaging and nephroprotective effect. All these factors can lead to an acceleration of CKD progression and of its associated cardiovascular disorders. The recognition of the interplay between the kidneys and the microbiota has led to increased efforts in the development of novel therapeutic approaches focused on targeting the microbiota.
3. Prebiotics, Probiotics, Synbiotics and Postbiotics
Diet has a large impact on the composition of the microbiota as both humans and their gut microbiota use dietary components as nutrients. As a result, microbiota-modulating or -derived interventions have emerged, including probiotics, prebiotics, synbiotics and postbiotics, that could be available as drugs, diet or food supplements [16,17] (Figure 1). The concepts of probiotics, prebiotics and synbiotics have all been well described in the scientific literature.
Figure 1.
Prebiotics, probiotics, synbiotics and postbiotics. Prebiotics are molecules that can be metabolized by the microbiota while probiotics are live strains microorganisms, i.e., individual live components of the microbiota. When prebiotics and probiotics are used in combination these are named synbiotics. In contrast, postbiotics are the result of specific bacterial inactivation procedures. Figure created with Biorender.com (license number 9E1540F2).
The term “probiotic” is of Greek origin and means “for life”. It was probably first used in 1954 although its meaning has changed over time. The current definition was formulated in 2002 by the Food and Agriculture Organization of United Nations (FAO) and World Health Organization (WHO): “live strains of strictly selected microorganisms which, when administered in adequate amounts, confer a health benefit on the host”. The definition was adopted by the International Scientific Association for Probiotics and Prebiotics (ISAPP) in 2013 [18,19]. Prebiotics were defined in 2007 by FAO/WHO as “nonviable food component that confers a health benefit on the host associated with modulation of the microbiota”. Prebiotics are molecules that can be metabolized by bacteria in the gastrointestinal tract and include dietary fibers that are metabolized by bacterial enzymes to produce beneficial metabolites such as short chain fatty acids (SCFAs), including butyrate, acetate and propionate [20]. Prebiotics could be used either as an alternative to probiotics or combined with them [19]. In 1995, the term “synbiotic” was introduced to define the synergistic action of pro- and prebiotics. The main objective of synbiotics is to improve the survival rate of probiotics inside the intestinal tract, and an appropriate combination should provide an enhanced effectiveness in comparison to the use of each one individually.
Finally, another strategy able to promote health by reproducing the benefits of the gut microbiota is the use of postbiotics, an emerging microorganism-derived tool which we discuss in greater depth below.
4. The 2019 Concept of Postbiotic: What Is and What Is Not a Postbiotic
Although probiotics are live microorganisms, by the end of their shelf life they might get injured and die. Little attention has been paid to the contribution of these dead microorganisms and of the products of fermentation with regards to any biological impact of probiotics [5]. In 2019, the International Scientific Association of Probiotics and Prebiotics (ISAPP) defined the concept of postbiotic from a scientific, commercial, and regulatory point of view as a “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” [5]. “Preparation” indicates that it should have a specific formulation achieved through certain inactivation methods to confer any beneficial effects. “Inanimate” refers to the microorganisms that were alive and once they have been killed, they retain their beneficial effect. The word “components” focuses on the beneficial effect that might be played by microbial components, such as cell wall components and pili. While microbial metabolites might be present in postbiotic preparations and might play an essential role, the definition does not include the purified metabolites lacking cellular biomass. However, until 2019, the literature has frequently used the term incorrectly by referring to vaccines and purified metabolites, such as SCFAs, proteins and peptides as postbiotics [21,22,23]. This potentially renders obsolete any older literature using the term postbiotic and calls for an urgent reset.
The preparation of a postbiotic should follow defined criteria: firstly, the composition of the microorganism must be characterized prior to inactivation, for example through genome sequencing. Secondly, the process of inactivation should be described and verified. Thirdly, the postbiotic preparation must be reported [5].
The main advantages of postbiotics compared to probiotics lies in their stability. Their preparation and composition render them extremely stable for several years at room temperature, thus making them particularly suitable for those areas where the maintenance of the cold chain for transport and storage are still a limitation. In addition, they may have a better safety profile compared to probiotics as they cannot replicate and cause bacteremia or fungaemia. Nevertheless, safety should still be assessed for any kind of postbiotic [5].
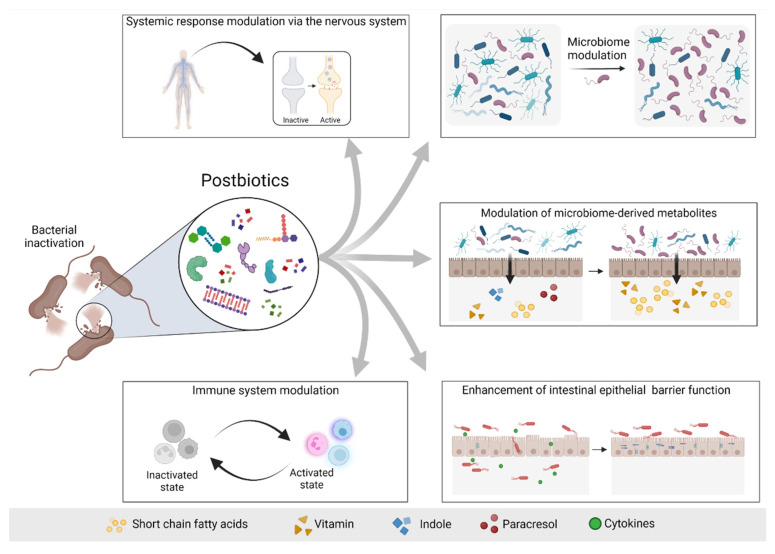
The possible mechanisms of actions of postbiotics include the modulation of resident microbiota, enhancement of epithelial barrier function, modulation of systemic or local immune responses, modulation of systemic metabolic responses and system signaling via the nervous system [5] (Figure 2).
Figure 2.
Possible mechanisms of actions of postbiotics. Postbiotics mediate their beneficial effects to the host via different mechanisms, including modulation of the systemic response via the nervous system (e.g., GABA), modulation of the microbiome and consequently modulation of the microbiome-derived metabolites, enhancement of the intestinal epithelial barrier and modulation of the immune system response. Based on [5]. Figure created with Biorender.com (license number 9E1540F2).
5. Postbiotics in Non-Kidney Disease
Data from postbiotics in human studies are limited. Salminen et al. recently discussed the clinical postbiotic studies in adults and pediatric cohorts identified in the Cochrane central registration of controlled trials and in a MEDLINE database search for randomized controlled trials (RCTs), cohort studies and meta-analysis in adults and children [5] (Table 1 and Table 2) [24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54]. They identified fifteen clinical trials with postbiotics. Three studies tested postbiotics in gut diseases, these being two in irritable bowel syndrome (IBS) and one in chronic diarrhea. In five studies, postbiotics were used to treat pulmonary and respiratory diseases. The remaining others involved patients with cancer, obstructive jaundice, tuberculosis and helicobacter pylori. Three of them aimed to treat chronic stress or improve inflammatory response and performance during training [31]. Of these studies, eleven made use of inactivated bacteria and four of bacterial lysates.
Table 1.
Intervention studies of postbiotics in adult humans identified by Salminen et al. [5]. Although no specific date for the search was provided, references up to 2020 were cited.
| Intervention Group | Postbiotic | Reference |
|---|---|---|
| Helicobacter pylori | Inactivated culture of Lactobacillus | [24] |
| Irritable bowel syndrome and diarrhea | Lacteol, inactivated L. acidophilus LB plus fermented culture medium | [25] |
| Irritable bowel syndrome | Non-viable, heat-inactivated Bifidobacterium bifidum MIMBb75 | [26] |
| Chronic diarrhea | Heat-killed L. acidophilus LB (Lacteol Fort) | [27] |
| Obstructive jaundice | Inactivated Lactiplantibacillus plantarum | [28] |
| Stress response in undergraduate medical students | Heat-inactivated L. gasseri strain CP2305 | [29] |
| Chronic responses in medical students | Heat-inactivated L. gasseri strain CP2305 | [30] |
| Response (endocrine, inflammation, performance) during self-defense training in soldiers | Inactivated Bacillus coagulans | [31] |
| Latent tuberculosis | Heat-killed Mycobacterium manresensis | [32] |
| Asthma | Inactivated Mycobacterium phlei | [33] |
| Chronic obstructive pulmonary disease | Inactivated, non-typable H. influenzae | [34] |
| Bacterial colonization of nose and throat | Lysate containing S. aureus, Streptococcus mitis, S. pyogenes, S. pneumoniae, K. pneumoniae, M. catarrhalis, H. influenzae | [35] |
| Chronic obstructive pulmonary disease | Lyophilized bacterial fragments derived from S. aureus, Streptococcus viridans, S. Pneumoniae, Klebsiella ozaenae, M. catarrhalis, H. influenzae | [36] |
| Recurrent respiratory tract infectious | Lantigen B, suspension of bacterial antigens obtained from S. pneumoniae type 3, S. pyrogenes group A, B. catarrhalis, S. aureus, H. influenzae type B and K. pneumoniae | [37] |
| Cancer and leukopenia following chemotherapy | DEODAN, lysozyme lysates of Lactobacillus bulgaricus | [38] |
Table 2.
Intervention studies of postbiotics in pediatric patients identified by Salminen et al. [5]. Although no specific date for the search was provided, references up to 2020 were cited.
| Intervention Group | Postbiotic | References |
|---|---|---|
| Fermented formula (healthy infants) | Fermented formula with BB C50 and ST065 | [39,40,41,42,43] |
| Fermented formula in preterm infants | Heat-inactivated fermented formula with BB C50 and ST 065 | [44] |
| Acute gastroenteritis | Heat-killed Lactobacillus LB | [45,46,47,48] |
| Prevention of common infectious diseases | Heat-inactivated L. paracasei CBA L74 or L. acidophilus | [49,50,51] |
| Atopic eczema and cow’s milk allergy | Live or heat-inactivated L. rhamnosus | [52] |
| Allergic rhinitis | Live or heat-killed L. paracasei 33 | [53] |
| Lactose malabsorption | Killed and live Lactobacillus helveticus R-52 and L. rhamnosus R-11 | [54] |
Several studies reported efficacy for oral administration. Inactivated Lactobacillus acidophilus in Helicobacter pylori-positive patients treated with rabesprazole, clarithromycin and amoxicillin resulted in a higher eradication rate than antibiotics alone (p = 0.02) [24]. In patients with IBS, a heat-inactivated Bifidobacterium bifidum MIMBb75 decreased pain over the placebo group [26]. Patients with chronic diarrhea treated with heat-killed L. acidophilus LB (Lacteol Fort) also showed improved symptoms [27]. Medical students treated with heat-inactivated L. gasseri strain CP2305 showed a significant reduction in anxiety and sleep disturbance (p < 0.05) [30]. In pre-term infants, one RCT observed a reduced incidence in abdominal distention and lower fecal calprotectin (p = 0.001) when treated with formula fermented by Bifidobacterium breve and S. thermophilus [44]. A systematic review that considered four studies in healthy infants showed that fermented formula could provide benefits for gastrointestinal symptoms [55]. A meta-analysis of four RCT that involved children with acute gastroenteritis reported that heat-inactivated Lactobacillus acidophilus LB reduced the duration of diarrhea in hospitalized patients but not outpatients, compared to placebo [45,46,47]. In a postbiotic trial, the heat inactivated Lacticaseibacillus paracasei CBA L74 prevented common infectious diseases in children who were attending daycare probably by stimulating innate or acquired immunity [49]. Another clinical trial confirmed that supplementation with cow’s skim milk fermented with L. paracasei CBA L74 could be a valid approach in preventing common infectious diseases in children [50]. Finally, one study investigated supplementation of infant formula with viable or heat-inactivated L. ramnosus GG and found that only viable L. ramnosus GG might be an efficient strategy to treat cow’s milk allergy and atopic eczema [52].
Overall, there is limited evidence suggesting that postbiotics may have beneficial effects in the treatment of diseases and this must be investigated in detail in well-designed controlled clinical trials.
6. Postbiotics in Kidney Disease
To our knowledge, there are no human studies conducted so far that investigated the use of postbiotics in kidney disease. However, a PubMed search performed in May 2022 identified several preclinical studies that examined the role and function of postbiotics in kidney-related diseases in animal models (Supplementary materials). In this search, we also found manuscripts published between 2020–2022 that used the term “postbiotic” to refer to compounds that would not be considered postbiotics according to the 2019 consensus definition [5]. In this regard, the short-chain fatty acid (SCFA) butyric acid and its derivative N-[2-(2-Butyrylamino-ethoxy)-ethyl]-butyramide (BA-NH-NH-BA) are produced by Cutibacterium acnes and reported to solubilize calcium phosphate [56]. A study that applied BA-NH-NH-BA topically in a murine model of uremic itching, considered this compound as a postbiotic [56]. However, this does not comply with the novel definition proposed by the ISAAP panel since a purified microbial metabolite itself cannot be considered a postbiotic [56].
Several studies on postbiotics and kidney disease were not very informative as they studied healthy animals or were too preliminary and did not address in vivo and functional consequences following administration. In aged or adult mice, treatment with probiotics or probiotics and postbiotics mix (Lactobacillus and Bifidobacterium strains and their postbiotics compounds selected for potential antioxidative activity) decreased oxidative stress as assessed by MDA (malondialdehyde) in the kidneys [57]. However, an impact on kidney function was not assessed and whether or not the combination of postbiotics with probiotics added up to the impact of probiotics alone was not formally assessed, although a trend towards a greater impact was observed in the higher dose groups.
Fifteen weeks of a diet supplemented by a postbiotic based on lactic acid bacteria in healthy male rabbits was not associated with differences in kidney function parameters, including serum urea and creatinine [58]. Based on the design, this study should be considered a safety study, as the impact on a disease condition was not assessed.
The postbiotic OM-85 is a standardized lysate of 21 bacterial strains, often found in human airways, that is undergoing clinical trials for diverse respiratory conditions and it has already been authorized in several European countries [59]. The EMA limits its use to the prevention of recurrent respiratory infections [60]. A clinical trial investigating children following the first episode of idiopathic nephrotic syndrome is not yet recruiting (NCT05044169), but plans to enroll 83 patients to whom OM-85 will be administered for 6 months after remission with a primary endpoint of one year relapse-free survival rate. Since nephrotic syndrome relapse is frequently preceded by infections, OM-85 is hypothesized to reduce the incidence of bacterial respiratory infections and, thus, reduce infection-related relapses. Unfortunately, a comparison to placebo was not considered, making the results of the trial difficult to interpret. In cultured epithelial cells, including kidney-derived Vero E6 monkey cells, OM-85 downregulated ACE2 and TMPRSS2 and, as a result, inhibited SARS-CoV-2 cell infection [61]. Whilst these results are promising, the absence of in vivo and clinical studies hampers the translatability and applicability of these observations. Despite the generally weak and/or preliminary data on postbiotics and kidney disease, promising, mainly preclinical, results were reported for postbiotics in hyperoxaluria, AKI, high fat diet-induced kidney disease and hypertension, as discussed below.
7. Postbiotics in Hyperoxaluria: Oxalobacter formigenes Lysates
In hyperoxaluria, increased oxalate absorption from diet or endogenous oxalate production result in increased urinary oxalate excretion potentially leading to calcium oxalate (CaOx) urolithiasis and CaOx crystal formation in kidney tissue, that can lead to renal calcification and, eventually, to kidney failure and systemic CaOx deposition or oxalosis [62]. CaOx crystals may cause kidney injury, inflammation and tubular obstruction that drive the progressive loss of kidney function, eventually leading to a need of kidney replacement therapy in the most severe cases [63,64,65]. Hyperoxaluria results from either a hepatic oxalate overproduction caused by genetic disorders of glyoxylate metabolism (primary hyperoxaluria) or ingestion of oxalate precursors, or from an elevated intestinal oxalate absorption (secondary hyperoxaluria). Secondary hyperoxaluria is more common and usually milder than primary hyperoxaluria and treatable with diet (low oxalate, calcium-containing diet). However, hyperoxaluria may cause AKI if oxalate ingestion is suddenly excessive (e.g., juicing) especially if this is linked to decreased gut calcium availability (e.g., during fat malabsorption as fat chelates calcium) as gut calcium oxalate crystal are not absorbed but excreted in feces.
Primary hyperoxaluria type 1 (PH1) is a rare genetic disease caused by a deficient liver alanine-glyoxylate transaminase enzyme activity. Being the most severe form of hyperoxaluria, considerable efforts have been made to develop novel therapies. Current treatment options for PH1 are suboptimal. To date, supportive treatments focus on high fluid intake and crystallization inhibitors as well as pyridoxine treatments [66]. Eventual development of kidney failure is, however, associated with oxalosis and premature death. Liver transplantation restores hepatic alanine-glyoxylate transaminase enzyme activity. Novel therapies based on RNA interference (RNAi) can target enzymes upstream and reduce or prevent oxalate production. For this, lumasiran, targeting liver glycolate oxidase (GO) is already approved by EMA and FDA, while nedosiran, targeting liver lactate dehydrogenase A (LDH-A) is currently undergoing RCTs [67].
Probiotics and, more recently, postbiotics have been studied for therapy of preclinical hyperoxaluria and human PH. O. formigenes is an anaerobic bacterium found in the gut that might help reducing the risks of developing urinary oxalate stones [68,69]. O. formigenes relies solely on oxalate for its growth and is a key oxalate-degrading bacterium that prevents kidney toxicity in animals fed on an oxalate-rich plants diet [69]. Clinical studies suggest an association between the absence of O. formigenes in the gut and the development of oxalate stone disease and hyperoxaluria [70,71,72]. Interestingly, treatment with either whole O. formigenes to colonize the gut (i.e., probiotics) or encapsulated O. formigenes lysates (i.e., postbiotics) reduced urinary oxalate excretion in rats [73]. Artificial or natural colonization of control Sprague-Dawley rats with O. formigenes promoted oxalate degradation and there is also evidence for physical interaction with the mucosa initiating colonic oxalate secretion. Urinary oxalate excretion was also decreased. In longer term studies, nephrocalcinosis was reduced [74]. Interestingly, dietary calcium influenced the ability to maintain O. formigenes colonization, which was persistent only when dietary calcium was low, i.e., when the amount of available calcium to bind to oxalate was low [73]. This would create a problem for the efficacy of live O. formigenes therapy since the potential benefits of O. formigenes on oxalate absorption in the gut could be offset by the need to maintain a low calcium diet. The benefits of probiotic could be reproduced by using postbiotic enteric-coated encapsulated O. formigenes freeze-dried lysates twice daily for five days that also reduced urinary oxalate excretion by 50% and supported colonic oxalate secretion in hyperoxaluric rats with renal insufficiency [73]. The O. formigenes lysate was hypothesized to have both a secretagogue function and an enzymatic degradation effect on luminal oxalate. The gelatin capsules used in the study contained freeze-dried lysate of the O. formigenes strain, oxalyl CoA, and thiamine pyrophosphate (8:1:1) and thus fits the current ISAAP definition of a postbiotic. Unfortunately, comparing the results obtained with the probiotic (live O. formigenes) and postbiotic (freeze-dead O. formigenes) is not possible, since dead bacteria were only tested in rats with renal insufficiency induced by unilateral nephrectomy and not in healthy rats [73]. These results support the idea that the O. formigenes postbiotic could contribute to the maintenance of the balance between renal and enteric oxalate [73], however the efficacy of the postbiotic should be confirmed in clinical studies. If efficacious to reduced oxalate load in vivo in humans, postbiotic O. formigenes may address several of the issues associated with postbiotic O. formigenes: the difficulty to grow and maintain alive a strict anaerobe, the potentially negative impact of calcium-containing diets (a current recommendation to prevent oxalate absorption) on maintaining O. formigenes colonization in vivo and the negative impact of antibiotic courses on O. formigenes colonization [75,76]. As an additional potential barrier to the success of prebiotic O. formigenes therapy, colonization is associated with a more complex microbiota (higher alpha-diversity) and the association of O. formigenes with other multiple taxa known to also be stimulated by oxalate in rodent models better differentiated gut microbiota from patients with and live-in individuals without urinary stone disease [75,77,78].These findings suggest that O. formigenes may better protect from oxalate associated diseases in conjunction with other components of the microbiota. Eventually, postbiotics may be designed that promote this associated microbiota.
More recently, O. formigenes culture conditioned medium was found to increase oxalate uptake (>2.4 fold) in human intestinal Caco-2-BEE cells when compared to control medium [68]. In contrast, conditioned medium from Lactobacillus did not stimulate oxalate uptake. The observed increase in oxalate transport might involve signaling via protein kinase A (PKA), as this was inhibited by H89 and required transport by a 4,4′-diisothiocyanostilbene-2,2′-disulfonic acid (DIDS)-sensitive anionic exchanger. There are two well-known DIDS-sensitive anionic exchangers: SLC26A2 (also known as Sulfate Anion Transporter 1 and diastrophic dysplasia sulfate transporter, DTDST) and SLC26A6 (also known as CFEX and PAT1). SLC26A6 knockout using siRNA led to a 50% decrease of oxalate transport in Caco-2-BEE treated with conditioned medium [68]. These results were not reproduced by others [79], however it should be pointed out that both groups tested different strains of O. formigenes that had been previously shown to promote oxalate transport in colonized mouse gut: sheep rumen strain of Oxalobacter (OxB, ATTC #35274) [68] and a human strain of Oxalobacter (HC-1) [79]. In vivo, in PH1 mice treated with O. formigenes conditioned medium, a postbiotic, (rectal administration), the urinary oxalate excretion was significantly reduced (32.5%) and the distal colonic oxalate secretion increased (42%) [68] (Figure 3). Thus, the postbiotic O. formigenes OxB, ATTC #35274 conditioned medium modulates oxalate transport in both in vitro cultured human intestinal epithelial cells and in vivo in murine colon. Nonetheless, these observations may not be applicable to other Oxalobacter strains. A more recent study observed that the increase in oxalate flux across the colon of mice colonized with live Oxalobacter was still observed in mice deficient for the apical oxalate transporters Slc26a6 and Slc26a3/Dra [80], suggesting that other oxalate transporters might be involved as well [79].
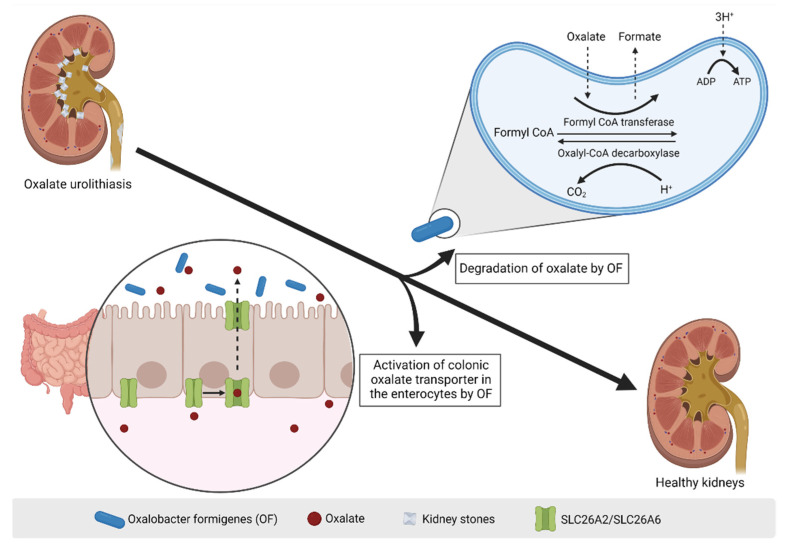
Figure 3.
O. formigenes and hyperoxaluria. The activity of the Oxalobacter formigenes bacteria resident in the gut has been proposed as a potential mechanism for the treatment of kidney stones via two defined mechanisms: 1. Degradation of oxalate. Oxalate is taken up by the bacteria and transformed into formate via the enzymatic activity of Formyl CoA transferase; 2. Activation of colonic oxalate transporter in the enterocytes. Circulating oxalate is transported by SLC26A2 and SLC26A6 transporters located at the basolateral side of intestinal epithelial cells and gets extracted into the intestinal lumen. Figure created with Biorender.com (license number 9E1540F2).
Postbiotic preparations of O. formigenes should not be confused with Oxabact™, a lyophilized O. formigenes formulation that aims at colonizing the gut with live O. formigenes. Oxabact™ is a capsule containing lyophilized O. formigenes, strain HC-1 (≥ 109 to < 510 colony forming units per dose). Since lyophilization does not kill bacteria, Oxabact™ is considered a probiotic. However, Oxabact will be discussed in certain detail as, similar to other prebiotics, the ratio of live/dead bacteria could change during the shelf life, resulting in a variable postbiotics contents whose contribution to any efficacy result remains understudied.
Oxabact™ has been tested in various RCTs: in a phase II, open-label trial aimed at PH1 patients on dialysis, Oxabact™ administration for 24 months decreased plasma oxalate levels and improved or stabilized cardiac function as well as clinical status when compared with placebo [81]. Oxabact™ also improved clinical disease progression in a female infant with severe PH1 [82]. However, placebo-controlled trials were not that successful. The most recent phase III, double-blind, placebo-controlled randomized trial investigated the effectiveness of Oxabact™, orally administered for 1 year, in reducing oxalate levels in PH patients, but failed to find a significant difference in plasma oxalate as compared to placebo (p = 0.06) [83]. Other studies with Oxabact™ also did not observe differences versus placebo, including two randomized, placebo-controlled, double-blind studies assessing urinary oxalate in PH patients treated with Oxabact™ for 24 weeks [84,85]. In this regard, no active Oxabact™ trials are listed in clinicaltrials.gov as of 16 June 2022 and a phase 3 extension study to evaluate the long-term efficacy and safety of Oxabact™ in patients with PH (NCT03938272) was terminated in July 2021 when the parent trial failed to meet the primary endpoint. At the time of termination, no advantage of Oxabact™ was observed for the primary endpoint investigating eGFR. Thus, attempts at human colonization by O. formigenes cannot be considered successful so far. Whether this may be the result of the probiotics bioavailability issues or the viability of O. formigenes in the formulations, it is currently unclear. Maintaining O. formigenes alive proved to be challenging due to its anaerobic need. Its stability could also be a limitation, as this can be affected during both industrial processing and storage. Moreover, the ratio of live/dead bacteria could substantially change during the shelf life, affecting its overall efficacy. Accordingly, to overcome such challenges, a postbiotic approach should be considered by taking into consideration different dosing and administration strategies.
8. Diet-Induced Kidney Injury
Postbiotics have been studied in preclinical, but not in clinical diet-induced hypertension and kidney disease. A postbiotic derived from sonicated Lactobacillus paracasei isolated from Egyptian cheese was administered to adult Wistar albino male rats fed with a high fat diet for 9 weeks and compared to atorvastatin treated or placebo to assess the impact on weight and lipids [86]. The postbiotic contained several enzymes, including proteases, lipases and antioxidant enzymes (superoxide dismutase, catalase, and glutathione peroxidase) and displayed antibacterial activity against pathogenic bacteria. Both the postbiotic and atorvastatin reduced total serum lipids, serum triglycerides and total serum cholesterol and prevented an increase in body weight. However, the overall body weight of atorvastatin- and postbiotic-treated rats was lower than that of rats fed on a normal diet, which may be a cause for concern. In this regard, there was evidence for atorvastatin toxicity such as increased liver enzymes and bilirubin levels, whereas this was not observed for the postbiotic treatment. A high fat diet results in kidney disease characterized by an increase in serum creatinine, uric acid and urea levels, and, surprisingly, this was exacerbated by atorvastatin. In postbiotic-treated rats instead, serum creatinine, uric acid and urea levels remained in the normal range and were lower than in high fat diet rats with or without atorvastatin treatment [86]. Although the molecular mechanisms or underlying kidney pathohistology were not explored, the postbiotic treatment appeared to have a protective effect from a high fat diet-induced kidney dysfunction.
A potential postbiotic that has also been studied in a preclinical diet-induced hypertension and kidney injury model is GABA-salt. For preclinical studies, GABA-salt was prepared by culturing Lactobacillus brevis BJ20 for 24 h and then further fermented with 40% (w/w) refined seawater salt for 6 h and then filtered and spray dried to yield GABA-salt. GABA-salt contained 0.76% ± 0.01% GABA, as well as other unmeasured postbiotics components [87]. Based on the information provided in the manuscript, no purification step appears to have been performed to eliminate other bacterial metabolites. Oral GABA-salt and salt treatment were compared in a murine model of hypertension induced by a high salt and high cholesterol diet. Replacing salt for GABA-salt attenuated the diet-induced increase in serum creatinine and urea, blood pressure, intima-media thickness, and other changes in aorta, such as M1 polarization (CD86 expression), cell death (TUNEL staining) and TNF-α and inducible nitric oxide synthase (NOS) levels. Furthermore, GABA-salt induced changes consistent with the in vivo observations in cultured macrophages, endothelial cells, and vascular smooth muscle cells. While the authors hypothesized that any impact of GABA-salt was due to the GABA content, the GABA-salt preparation would be expected to contain other bacterial products, i.e., to be a postbiotic, and from the experiments performed, it remains unclear which individual or combination of postbiotic components was responsible for the observed effect. Indeed, it was not tested whether GABA itself added to control salt resulted in the same effects as the postbiotic GABA-salt.
GABA-salt studies followed prior description of a fermented milk containing GABA by using Lactobacillus casei strain Shirota and Lactococcus lactis YIT 2027 that lowered blood pressure in humans and rats [88,89]. The Lb. casei strain hydrolyzes milk protein into glutamic acid, and the Lc. lactis converts glutamic acid into GABA (GABA 10–12 mg/dL). However, from the manuscripts, it is unclear whether the fermented milk contained probiotics (i.e., live bacteria) and/or postbiotics (i.e., dead bacteria and their products) [89]. The human study was a pilot study in which 39 mildly hypertensive patients received daily GABA fermented milk or placebo (non-fermented milk) for 12-weeks. Systolic blood pressure decreased by 17.4 ± 4.3 mmHg in the intervention group and this was reported to be statistically significantly different from the placebo group, in which blood pressure also decreased [89]. In SHR hypertensive rats, a diet containing freeze-dried GABA fermented milk (100 g/kg, final concentration of GABA 0.1 g/kg) prevented the progressive increase in systolic blood pressure observed between 7 and 10 weeks of age [88].
9. Postbiotics in Nephrotoxic AKI
Postbiotics have been studied in preclinical, but not in clinical AKI. In preclinical studies, AKI was induced by cisplatin administration to mice and the severity of injury was magnified by the oral administration of salt from 1 h prior to cisplatin up to 48 h post induction of AKI, with a 72 h readout [90]. GABA-salt was prepared as for the hypertension studies [87] with slight modifications involving further fermentation of Lactobacillus. plantarum BJ21, to yield GABA-salt, and two other compounds that were termed lacto-GABA-salt and postbiotic-GABA-salt. The manuscript does not provide further details on the differential processes performed/used to generate the three compounds or their full composition other than indicating that they contained 93, 112, and 97 mg/g GABA, and that 108, 175 and 109 mg/g were neither sodium chloride nor GABA, respectively. The sodium chloride content of the parent seawater salt was not reported. The results are not very clearly expressed, but it appears that salt administration increased the severity of cisplatin-induced AKI as assessed by serum creatinine and urea, histological injury and expression of inflammatory mediators, while the different GABA-salt was protective, the results for lacto-GABA-salt and postbiotic-GABA-salt being unclear [90].
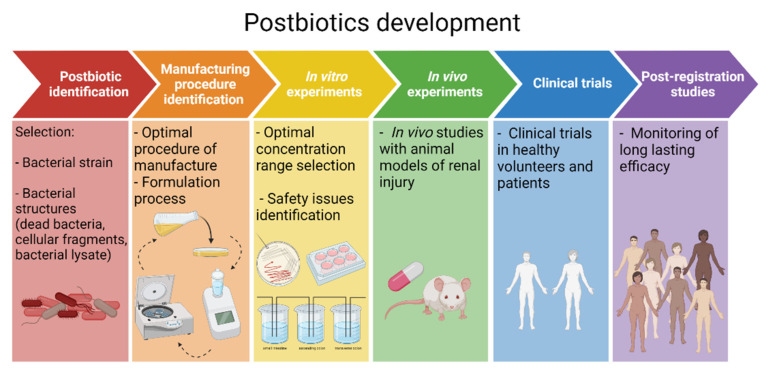
10. A Roadmap towards Postbiotic Therapy for Kidney Disease
The ultimate aim of preclinical research is to develop products that could improve the diagnosis and treatment of diseases. Postbiotics are not well-known and postbiotics research is still in its early stages and has not yet led to products for clinical use. Indeed, as recently as July 2022 postbiotics were still erroneously defined as therapeutic strategies that target downstream signaling pathways of microbiome [91]. We suggest a roadmap for the clinical development of postbiotics in the field of kidney disease (Figure 4). First, it would be necessary to identify the key bacterial strains that play an important role in the development and progression of kidney disease and define the mechanisms underlying their beneficial function as it might be that not all the bacterial components might be involved. Recognizing which fragments have a functional role and which do not would be of extreme importance in the development of postbiotics. A postbiotic approach would overcome limitations faced with probiotics related products, this mainly being their instability. Furthermore, it is necessary to characterize the optimal procedures of manufacturing, formulation and administration, and in vitro experiments are required to establish the optimal concentration range and to identify early any safety issues. In vitro studies should address any positive or negative impact of the postbiotic on cultured mammal cells, including human gut, kidney and immune cells, as well as on complex bacterial communities, using technologies such as the Dynamic Gastrointestinal Simulator (SIMGI®) [92]. Tubular cells are the key kidney cell types to study, given that they represent most of the kidney mass and have an array of transporters and receptors, although glomerular and endothelial cells may also be of interest. Subsequently, in vivo experiments with animal models of kidney injury are required. Given the short timelines, preclinical AKI models may be of interest. Moreover, some of them result in features of CKD within a reasonable time frame (the so-called AKI-to-CKD transition), so insight into chronicity may be gained from these studies. However, no AKI therapy identified in preclinical models has ever reached routine clinical use, pointing to potentially large differences in pathophysiology and/or timing of the intervention between preclinical models and human disease. In this regard, models representing clinical situations in which the inventio may be applied prophylactically in the clinic may offer advantages (e.g., kidney ischemia during surgery, exposure to nephrotoxic chemotherapeutic agents). In order to be effective, postbiotics should be prepared in such a way that it makes them resistant to degradation whilst reaching the targeted segment of the gastrointestinal tract. Manufacturing procedures should ensure low variability of the finished product and scalability to produce large batches. In this regard, several postbiotics possibilities are available, ranging from bacterial lysates to conditioned cultured medium to extracellular vesicles as recently demonstrated for O. formigenes [93]. Moreover, more complex postbiotcs consisting of diverse bacteria encoding oxalate degradation pathways may be explored [72,94]. Finally, RCTs in patients with kidney diseases should be planned. Short-term trials should establish their safety in humans and explore biomarkers of their biological activity (e.g., oxaluria in patients with hyperoxaluria). Larger efficacy trials focusing on broader endpoints will be more difficult to design and fund but are required to gather the dataset necessary for approval by the medicine’s regulatory agencies. In this regard, PH1 is a rare disease which has severe, potentially life-threatening complications and treatment of PH1 represents both an unmet clinical need and an attractive target from the human and regulatory point of view. However, being a severe condition in which oxalic acid is produced endogenously, PH1 may be less amenable to interventions acting at the level of the gut lumen. A shortcut to postbiotics as a drug treatment would be to consider them as nutritional supplements or medical nutrition. This would increase the feasibility factor for clinical studies, but would decrease the trustworthiness of any claim of health benefit. O. formigenes postbiotics may be suitable for assessment as nutritional supplements in hyperoxaluria of enteric origin. This condition is more common and less severe than PH1, facilitating clinical trials or nutritional research; the via of entry of oxalic acid in the body is by ingestion, similar to the postbiotics, which may increase efficacy and finally; there is a subset of health-conscious but ill-advised persons with hyperoxaluria causing kidney injury resulting from fashionable trends or diets (e.g., juicing) who would may also be prone to taking supplements such as O. formigenes postbiotics, especially if this would allow then to pursue their diets more safely. Another potential scenario of O. formigenes postbiotics use if prevention of antibiotic-associated urolithiasis, especially if long-term antibiotics are needed, as while on antibiotics, attempts at colonization by O. formigenes probiotics are expected to be futile [95]. Once on the market, post-registration studies will monitor the long-lasting safety and efficacy effects. In the case of postbiotics for enteric hyperoxaluria, the whole process from research to marketing and post marketing experience may allow to learn enough about the intervention to re-design it for more severe conditions such as PH1.
Figure 4.
A roadmap towards postbiotic therapy for kidney disease. Roadmap displaying the postbiotic development steps from initial postbiotics identification up to the final post-registration studies. In the specific case of kidney disease, initial candidates for this roadmap include O. formigenes postbiotics for enteric hyperoxaluria-related kidney injury and urolithiasis. Figure created with Biorender.com (license number 9E1540F2).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/toxins14090623/s1, Search strategy and results.
Author Contributions
Conceptualization, C.F., M.D.S.-N. and A.O.; methodology, all; validation, all; writing—original draft preparation, C.F., M.D.S.-N. and A.O.; writing—review and editing, all; visualization, all; supervision, R.M., M.D.S.-N. and A.O.; funding acquisition, R.M., M.D.S.-N. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
AO has received grants from Sanofi and consultancy or speaker fees or travel support from Advicciene, Astellas, Astrazeneca, Amicus, Amgen, Fresenius Medical Care, GSK, Bayer, Sanofi-Genzyme, Menarini, Mundipharma, Kyowa Kirin, Alexion, Freeline, Idorsia, Chiesi, Otsuka, Novo-Nordisk, Sysmex and Vifor Fresenius Medical Care Renal Pharma and is Director of the Catedra Mundipharma-UAM of diabetic kidney disease and the Catedra Astrazeneca-UAM of chronic kidney disease and electrolytes.
Key Contribution
This is the first overview of postbiotics and kidney diseases that has adopted the 2019 ISAPP definition of postbiotics. Furthermore, we provide a roadmap for postbiotics research in kidney disease to expedite clinical translation.
Funding Statement
European Union′s Horizon 2020 Research and Innovation Program (860329 Marie-Curie ITN “STRATEGY-CKD”). FIS/Fondos FEDER (PI18/01366, PI19/00815, PI21/00251), ERA-PerMed-JTC2018 (KIDNEY ATTACK AC18/00064), Comunidad de Madrid en Biomedicina B2017/BMD-3686 CIFRA2-CM. Instituto de Salud Carlos III (ISCIII) RICORS program to RI-CORS2040 (RD21/0005/0001) and SPACKDc PMP21/00109, funded by European Union—NextGe-nerationEU, Mecanismo para la Recuperación y la Resiliencia (MRR). MDSN were supported by MICINN Ramon y Cajal program RYC2018-024461-I.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Perez-Gomez M.V., Bartsch L.-A., Castillo-Rodriguez E., Fernandez-Prado R., Fernandez-Fernandez B., Martin-Cleary C., Gracia-Iguacel C., Ortiz A. Clarifying the Concept of Chronic Kidney Disease for Non-Nephrologists. Clin. Kidney J. 2019;12:258–261. doi: 10.1093/ckj/sfz007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ortiz A., Roger M., Jiménez V.M., Perez J.C.R., Furlano M., Atxer L.S., Zurro D.G., Casabona C.M.R., Gómez C.G., Bermúdez P.P., et al. RICORS2040: The need for collaborative research in chronic kidney disease. Clin. Kidney J. 2021;15:372–387. doi: 10.1093/ckj/sfab170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Douglas A.E., Werren J.H. Holes in the Hologenome: Why Host-Microbe Symbioses Are Not Holobionts. mBio. 2016;7:e02099. doi: 10.1128/mBio.02099-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maier L., Pruteanu M., Kuhn M., Zeller G., Telzerow A., Anderson E.E., Brochado A.R., Fernandez K.C., Dose H., Mori H., et al. Extensive Impact of Non-Antibiotic Drugs on Human Gut Bacteria. Nature. 2018;555:623–628. doi: 10.1038/nature25979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Salminen S., Collado M.C., Endo A., Hill C., Lebeer S., Quigley E.M.M., Sanders M.E., Shamir R., Swann J.R., Szajewska H., et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021;18:649–667. doi: 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang P., Zou J.-Z., Chen J., Tan X., Xiang F.-F., Shen B., Hu J.-C., Wang J.-L., Wang Y.-Q., Yu J.-B., et al. Association of Trimethylamine N-Oxide with Cardiovascular and All-Cause Mortality in Hemodialysis Patients. Ren. Fail. 2020;42:1004–1014. doi: 10.1080/0886022X.2020.1822868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhao J., Ning X., Liu B., Dong R., Bai M., Sun S. Specific Alterations in Gut Microbiota in Patients with Chronic Kidney Disease: An Updated Systematic Review. Ren. Fail. 2021;43:102–112. doi: 10.1080/0886022X.2020.1864404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zsom L., Zsom M., Salim S.A., Fülöp T. Estimated Glomerular Filtration Rate in Chronic Kidney Disease: A Critical Review of Estimate-Based Predictions of Individual Outcomes in Kidney Disease. Toxins. 2022;14:127. doi: 10.3390/toxins14020127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cigarran Guldris S., González Parra E., Cases Amenós A. Gut Microbiota in Chronic Kidney Disease. Nefrologia. 2017;37:9–19. doi: 10.1016/j.nefro.2016.05.008. [DOI] [PubMed] [Google Scholar]
- 10.Favero C., Carriazo S., Cuarental L., Fernandez-Prado R., Gomá-Garcés E., Perez-Gomez M.V., Ortiz A., Fernandez-Fernandez B., Sanchez-Niño M.D. Phosphate, Microbiota and CKD. Nutrients. 2021;13:1273. doi: 10.3390/nu13041273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chi M., Ma K., Wang J., Ding Z., Li Y., Zhu S., Liang X., Zhang Q., Song L., Liu C. The Immunomodulatory Effect of the Gut Microbiota in Kidney Disease. J. Immunol. Res. 2021;2021:5516035. doi: 10.1155/2021/5516035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Castillo-Rodriguez E., Fernandez-Prado R., Esteras R., Perez-Gomez M.V., Gracia-Iguacel C., Fernandez-Fernandez B., Kanbay M., Tejedor A., Lazaro A., Ruiz-Ortega M., et al. Impact of Altered Intestinal Microbiota on Chronic Kidney Disease Progression. Toxins. 2018;10:300. doi: 10.3390/toxins10070300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fernandez-Prado R., Esteras R., Perez-Gomez M.V., Gracia-Iguacel C., Gonzalez-Parra E., Sanz A.B., Ortiz A., Sanchez-Niño M.D. Nutrients Turned into Toxins: Microbiota Modulation of Nutrient Properties in Chronic Kidney Disease. Nutrients. 2017;9:489. doi: 10.3390/nu9050489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Poveda J., Sanchez-Niño M.D., Glorieux G., Sanz A.B., Egido J., Vanholder R., Ortiz A. P-Cresyl Sulphate Has pro-Inflammatory and Cytotoxic Actions on Human Proximal Tubular Epithelial Cells. Nephrol. Dial. Transplant. 2014;29:56–64. doi: 10.1093/ndt/gft367. [DOI] [PubMed] [Google Scholar]
- 15.Sun C.-Y., Chang S.-C., Wu M.-S. Suppression of Klotho Expression by Protein-Bound Uremic Toxins Is Associated with Increased DNA Methyltransferase Expression and DNA Hypermethylation. Kidney Int. 2012;81:640–650. doi: 10.1038/ki.2011.445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gut Microbiota in Chronic Kidney Disease—PubMed. [(accessed on 11 July 2022)]; Available online: https://pubmed.ncbi.nlm.nih.gov/27553986/
- 17.Mills S., Stanton C., Lane J.A., Smith G.J., Ross R.P. Precision Nutrition and the Microbiome, Part I: Current State of the Science. Nutrients. 2019;11:923. doi: 10.3390/nu11040923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kim S.-K., Guevarra R.B., Kim Y.-T., Kwon J., Kim H., Cho J.H., Kim H.B., Lee J.-H. Role of Probiotics in Human Gut Microbiome-Associated Diseases. J. Microbiol. Biotechnol. 2019;29:1335–1340. doi: 10.4014/jmb.1906.06064. [DOI] [PubMed] [Google Scholar]
- 19.Markowiak P., Śliżewska K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients. 2017;9:1021. doi: 10.3390/nu9091021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holscher H.D. Dietary Fiber and Prebiotics and the Gastrointestinal Microbiota. Gut Microbes. 2017;8:172–184. doi: 10.1080/19490976.2017.1290756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Muralitharan R.R., Jama H.A., Xie L., Peh A., Snelson M., Marques F.Z. Microbial Peer Pressure: The Role of the Gut Microbiota in Hypertension and Its Complications. Hypertension. 2020;76:1674–1687. doi: 10.1161/HYPERTENSIONAHA.120.14473. [DOI] [PubMed] [Google Scholar]
- 22.Vrzáčková N., Ruml T., Zelenka J. Postbiotics, Metabolic Signaling, and Cancer. Molecules. 2021;26:1528. doi: 10.3390/molecules26061528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rhys-Jones D., Climie R.E., Gill P.A., Jama H.A., Head G.A., Gibson P.R., Kaye D.M., Muir J.G., Marques F.Z. Microbial Interventions to Control and Reduce Blood Pressure in Australia (MICRoBIA): Rationale and Design of a Double-Blinded Randomised Cross-over Placebo Controlled Trial. Trials. 2021;22:496. doi: 10.1186/s13063-021-05468-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Canducci F., Armuzzi A., Cremonini F., Cammarota G., Bartolozzi F., Pola P., Gasbarrini G., Gasbarrini A. A Lyophilized and Inactivated Culture of Lactobacillus Acidophilus Increases Helicobacter Pylori Eradication Rates. Aliment. Pharmacol. Ther. 2000;14:1625–1629. doi: 10.1046/j.1365-2036.2000.00885.x. [DOI] [PubMed] [Google Scholar]
- 25.Tarrerias A.L., Costil V., Vicari F., Létard J.C., Adenis-Lamarre P., Aisène A., Batistelli D., Bonnaud G., Carpentier S., Dalbiès P., et al. The Effect of Inactivated Lactobacillus LB Fermented Culture Medium on Symptom Severity: Observational Investigation in 297 Patients with Diarrhea-Predominant Irritable Bowel Syndrome. Dig. Dis. 2011;29:588–591. doi: 10.1159/000332987. [DOI] [PubMed] [Google Scholar]
- 26.Andresen V., Gschossmann J., Layer P. Heat-Inactivated Bifidobacterium Bifidum MIMBb75 (SYN-HI-001) in the Treatment of Irritable Bowel Syndrome: A Multicentre, Randomised, Double-Blind, Placebo-Controlled Clinical Trial. Lancet Gastroenterol. Hepatol. 2020;5:658–666. doi: 10.1016/S2468-1253(20)30056-X. [DOI] [PubMed] [Google Scholar]
- 27.Xiao S.-D., Zhang D.Z., Lu H., Jiang S.H., Liu H.Y., Wang G.S., Xu G.M., Zhang Z.B., Lin G.J., Wang G.L. Multicenter, Randomized, Controlled Trial of Heat-Killed Lactobacillus Acidophilus LB in Patients with Chronic Diarrhea. Adv. Ther. 2003;20:253–260. doi: 10.1007/BF02849854. [DOI] [PubMed] [Google Scholar]
- 28.Jones C., Badger S.A., Regan M., Clements B.W., Diamond T., Parks R.W., Taylor M.A. Modulation of Gut Barrier Function in Patients with Obstructive Jaundice Using Probiotic LP299v. Eur. J. Gastroenterol. Hepatol. 2013;25:1424–1430. doi: 10.1097/MEG.0b013e328363e26e. [DOI] [PubMed] [Google Scholar]
- 29.Takiishi T., Korf H., Van Belle T.L., Robert S., Grieco F.A., Caluwaerts S., Galleri L., Spagnuolo I., Steidler L., Van Huynegem K., et al. Reversal of Autoimmune Diabetes by Restoration of Antigen-Specific Tolerance Using Genetically Modified Lactococcus Lactis in Mice. J. Clin. Investig. 2012;122:1717–1725. doi: 10.1172/JCI60530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishida K., Sawada D., Kuwano Y., Tanaka H., Rokutan K. Health Benefits of Lactobacillus Gasseri CP2305 Tablets in Young Adults Exposed to Chronic Stress: A Randomized, Double-Blind, Placebo-Controlled Study. Nutrients. 2019;11:1859. doi: 10.3390/nu11081859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffman J.R., Hoffman M.W., Zelicha H., Gepner Y., Willoughby D.S., Feinstein U., Ostfeld I. The Effect of 2 Weeks of Inactivated Probiotic Bacillus Coagulans on Endocrine, Inflammatory, and Performance Responses During Self-Defense Training in Soldiers. J. Strength Cond. Res. 2019;33:2330–2337. doi: 10.1519/JSC.0000000000003265. [DOI] [PubMed] [Google Scholar]
- 32.Montané E., Barriocanal A.M., Arellano A.L., Valderrama A., Sanz Y., Perez-Alvarez N., Cardona P., Vilaplana C., Cardona P.-J. Pilot, Double-Blind, Randomized, Placebo-Controlled Clinical Trial of the Supplement Food Nyaditum Resae® in Adults with or without Latent TB Infection: Safety and Immunogenicity. PLoS ONE. 2017;12:e0171294. doi: 10.1371/journal.pone.0171294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang J., Guo S., Li C., Jiang X. Therapeutic Effects of Inhaled Inactivated Mycobacterium Phlei in Adult Patients with Moderate Persistent Asthma. Immunotherapy. 2012;4:383–387. doi: 10.2217/imt.12.25. [DOI] [PubMed] [Google Scholar]
- 34.Tandon M.K., Phillips M., Waterer G., Dunkley M., Comans P., Clancy R. Oral Immunotherapy with Inactivated Nontypeable Haemophilus Influenzae Reduces Severity of Acute Exacerbations in Severe COPD. Chest. 2010;137:805–811. doi: 10.1378/chest.09-1382. [DOI] [PubMed] [Google Scholar]
- 35.Zagólski O., Stręk P., Kasprowicz A., Białecka A. Effectiveness of Polyvalent Bacterial Lysate and Autovaccines Against Upper Respiratory Tract Bacterial Colonization by Potential Pathogens: A Randomized Study. Med. Sci. Monit. 2015;21:2997–3002. doi: 10.12659/MSM.893779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Braido F., Melioli G., Cazzola M., Fabbri L., Blasi F., Moretta L., Canonica G.W., AIACE Study Group Sub-Lingual Administration of a Polyvalent Mechanical Bacterial Lysate (PMBL) in Patients with Moderate, Severe, or Very Severe Chronic Obstructive Pulmonary Disease (COPD) According to the GOLD Spirometric Classification: A Multicentre, Double-Blind, Randomised, Controlled, Phase IV Study (AIACE Study: Advanced Immunological Approach in COPD Exacerbation) Pulm. Pharmacol. Ther. 2015;33:75–80. doi: 10.1016/j.pupt.2015.03.006. [DOI] [PubMed] [Google Scholar]
- 37.Braido F., Melioli G., Candoli P., Cavalot A., Di Gioacchino M., Ferrero V., Incorvaia C., Mereu C., Ridolo E., Rolla G., et al. The Bacterial Lysate Lantigen B Reduces the Number of Acute Episodes in Patients with Recurrent Infections of the Respiratory Tract: The Results of a Double Blind, Placebo Controlled, Multicenter Clinical Trial. Immunol. Lett. 2014;162:185–193. doi: 10.1016/j.imlet.2014.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Krusteva E., Hristova S., Damyanov D., Bogdanov A., Altaparmakov I., Pacelli E. Clinical Study of the Effect of the Preparation DEODAN on Leukopenia, Induced by Cytostatics. Int. J. Immunopharmacol. 1997;19:487–492. doi: 10.1016/S0192-0561(97)00080-5. [DOI] [PubMed] [Google Scholar]
- 39.Indrio F., Ladisa G., Mautone A., Montagna O. Effect of a Fermented Formula on Thymus Size and Stool PH in Healthy Term Infants. Pediatr. Res. 2007;62:98–100. doi: 10.1203/pdr.0b013e31806772d3. [DOI] [PubMed] [Google Scholar]
- 40.Morisset M., Aubert-Jacquin C., Soulaines P., Moneret-Vautrin D.-A., Dupont C. A Non-Hydrolyzed, Fermented Milk Formula Reduces Digestive and Respiratory Events in Infants at High Risk of Allergy. Eur. J. Clin. Nutr. 2011;65:175–183. doi: 10.1038/ejcn.2010.250. [DOI] [PubMed] [Google Scholar]
- 41.Mullié C., Yazourh A., Thibault H., Odou M.-F., Singer E., Kalach N., Kremp O., Romond M.-B. Increased Poliovirus-Specific Intestinal Antibody Response Coincides with Promotion of Bifidobacterium Longum-Infantis and Bifidobacterium Breve in Infants: A Randomized, Double-Blind, Placebo-Controlled Trial. Pediatr. Res. 2004;56:791–795. doi: 10.1203/01.PDR.0000141955.47550.A0. [DOI] [PubMed] [Google Scholar]
- 42.Thibault H., Aubert-Jacquin C., Goulet O. Effects of Long-Term Consumption of a Fermented Infant Formula (with Bifidobacterium Breve C50 and Streptococcus Thermophilus 065) on Acute Diarrhea in Healthy Infants. J. Pediatr. Gastroenterol. Nutr. 2004;39:147–152. doi: 10.1097/00005176-200408000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Roy P., Aubert-Jacquin C., Avart C., Gontier C. Benefits of a thickened infant formula with lactase activity in the management of benign digestive disorders in newborns. Arch. Pediatr. 2004;11:1546–1554. doi: 10.1016/j.arcped.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 44.Campeotto F., Suau A., Kapel N., Magne F., Viallon V., Ferraris L., Waligora-Dupriet A.-J., Soulaines P., Leroux B., Kalach N., et al. A Fermented Formula in Pre-Term Infants: Clinical Tolerance, Gut Microbiota, down-Regulation of Faecal Calprotectin and up-Regulation of Faecal Secretory IgA. Br. J. Nutr. 2011;105:1843–1851. doi: 10.1017/S0007114510005702. [DOI] [PubMed] [Google Scholar]
- 45.Liévin-Le Moal V., Sarrazin-Davila L.E., Servin A.L. An Experimental Study and a Randomized, Double-Blind, Placebo-Controlled Clinical Trial to Evaluate the Antisecretory Activity of Lactobacillus Acidophilus Strain LB against Nonrotavirus Diarrhea. Pediatrics. 2007;120:e795–e803. doi: 10.1542/peds.2006-2930. [DOI] [PubMed] [Google Scholar]
- 46.Salazar-Lindo E., Figueroa-Quintanilla D., Caciano M.I., Reto-Valiente V., Chauviere G., Colin P., Lacteol Study Group Effectiveness and Safety of Lactobacillus LB in the Treatment of Mild Acute Diarrhea in Children. J. Pediatr. Gastroenterol. Nutr. 2007;44:571–576. doi: 10.1097/MPG.0b013e3180375594. [DOI] [PubMed] [Google Scholar]
- 47.Simakachorn N., Pichaipat V., Rithipornpaisarn P., Kongkaew C., Tongpradit P., Varavithya W. Clinical Evaluation of the Addition of Lyophilized, Heat-Killed Lactobacillus Acidophilus LB to Oral Rehydration Therapy in the Treatment of Acute Diarrhea in Children. J. Pediatr. Gastroenterol. Nutr. 2000;30:68–72. doi: 10.1097/00005176-200001000-00020. [DOI] [PubMed] [Google Scholar]
- 48.Kaila M., Isolauri E., Saxelin M., Arvilommi H., Vesikari T. Viable versus Inactivated Lactobacillus Strain GG in Acute Rotavirus Diarrhoea. Arch. Dis. Child. 1995;72:51–53. doi: 10.1136/adc.72.1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nocerino R., Paparo L., Terrin G., Pezzella V., Amoroso A., Cosenza L., Cecere G., De Marco G., Micillo M., Albano F., et al. Cow’s Milk and Rice Fermented with Lactobacillus Paracasei CBA L74 Prevent Infectious Diseases in Children: A Randomized Controlled Trial. Clin. Nutr. 2017;36:118–125. doi: 10.1016/j.clnu.2015.12.004. [DOI] [PubMed] [Google Scholar]
- 50.Corsello G., Carta M., Marinello R., Picca M., De Marco G., Micillo M., Ferrara D., Vigneri P., Cecere G., Ferri P., et al. Preventive Effect of Cow’s Milk Fermented with Lactobacillus Paracasei CBA L74 on Common Infectious Diseases in Children: A Multicenter Randomized Controlled Trial. Nutrients. 2017;9:669. doi: 10.3390/nu9070669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sharieff W., Bhutta Z., Schauer C., Tomlinson G., Zlotkin S. Micronutrients (Including Zinc) Reduce Diarrhoea in Children: The Pakistan Sprinkles Diarrhoea Study. Arch. Dis. Child. 2006;91:573–579. doi: 10.1136/adc.2005.086199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirjavainen P.V., Salminen S.J., Isolauri E. Probiotic Bacteria in the Management of Atopic Disease: Underscoring the Importance of Viability. J. Pediatr. Gastroenterol. Nutr. 2003;36:223–227. doi: 10.1097/00005176-200302000-00012. [DOI] [PubMed] [Google Scholar]
- 53.Peng G.-C., Hsu C.-H. The Efficacy and Safety of Heat-Killed Lactobacillus Paracasei for Treatment of Perennial Allergic Rhinitis Induced by House-Dust Mite. Pediatr. Allergy Immunol. 2005;16:433–438. doi: 10.1111/j.1399-3038.2005.00284.x. [DOI] [PubMed] [Google Scholar]
- 54.Rampengan N.H., Manoppo J., Warouw S.M. Comparison of Efficacies between Live and Killed Probiotics in Children with Lactose Malabsorption. Southeast Asian J. Trop. Med. Public Health. 2010;41:474–481. [PubMed] [Google Scholar]
- 55.Szajewska H., Skórka A., Pieścik-Lech M. Fermented Infant Formulas without Live Bacteria: A Systematic Review. Eur. J. Pediatr. 2015;174:1413–1420. doi: 10.1007/s00431-015-2629-y. [DOI] [PubMed] [Google Scholar]
- 56.Keshari S., Wang Y., Herr D.R., Wang S.-M., Yang W.-C., Chuang T.-H., Chen C.-L., Huang C.-M. Skin Cutibacterium Acnes Mediates Fermentation to Suppress the Calcium Phosphate-Induced Itching: A Butyric Acid Derivative with Potential for Uremic Pruritus. J. Clin. Med. 2020;9:312. doi: 10.3390/jcm9020312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lin W.-Y., Lin J.-H., Kuo Y.-W., Chiang P.-F.R., Ho H.-H. Probiotics and Their Metabolites Reduce Oxidative Stress in Middle-Aged Mice. Curr. Microbiol. 2022;79:104. doi: 10.1007/s00284-022-02783-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Díaz Cano J.V., Argente M.-J., García M.-L. Effect of Postbiotic Based on Lactic Acid Bacteria on Semen Quality and Health of Male Rabbits. Animals. 2021;11:1007. doi: 10.3390/ani11041007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Notification to the CHMP/EMA Secretariat of a Referral under Article 31 of Directive 2001/83/EC. [(accessed on 5 June 2022)]. Available online: https://www.ema.europa.eu/en/documents/referral/bacterial-lysate-medicines-article-31-referral-notification_en.pdf.
- 60.EMA/351772/2019 Bacterial Lysate Medicines for Respiratory Conditions to Be Used Only for Prevention of Recurrent Infections. [(accessed on 5 June 2022)]. Available online: https://www.ema.europa.eu/en/documents/press-release/bacterial-lysate-medicines-respiratory-conditions-be-used-only-prevention-recurrent-infections_en.pdf.
- 61.Pivniouk V., Pivniouk O., DeVries A., Uhrlaub J.L., Michael A., Pivniouk D., VanLinden S.R., Conway M.Y., Hahn S., Malone S.P., et al. The OM-85 Bacterial Lysate Inhibits SARS-CoV-2 Infection of Epithelial Cells by Downregulating SARS-CoV-2 Receptor Expression. J. Allergy Clin. Immunol. 2022;149:923–933.e6. doi: 10.1016/j.jaci.2021.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bacchetta J., Wood K.D. Primary Hyperoxaluria Type 1: Time for Prime Time? Clin. Kidney J. 2022;15:i1–i3. doi: 10.1093/ckj/sfab233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Robijn S., Hoppe B., Vervaet B.A., D’Haese P.C., Verhulst A. Hyperoxaluria: A Gut-Kidney Axis? Kidney Int. 2011;80:1146–1158. doi: 10.1038/ki.2011.287. [DOI] [PubMed] [Google Scholar]
- 64.Witting C., Langman C.B., Assimos D., Baum M.A., Kausz A., Milliner D., Tasian G., Worcester E., Allain M., West M., et al. Pathophysiology and Treatment of Enteric Hyperoxaluria. Clin. J. Am. Soc. Nephrol. 2021;16:487–495. doi: 10.2215/CJN.08000520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Demoulin N., Aydin S., Gillion V., Morelle J., Jadoul M. Pathophysiology and Management of Hyperoxaluria and Oxalate Nephropathy: A Review. Am. J. Kidney Dis. 2022;79:717–727. doi: 10.1053/j.ajkd.2021.07.018. [DOI] [PubMed] [Google Scholar]
- 66.Gupta A., Somers M.J.G., Baum M.A. Treatment of Primary Hyperoxaluria Type 1. Clin. Kidney J. 2022;15:i9–i13. doi: 10.1093/ckj/sfab232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Dejban P., Lieske J.C. New Therapeutics for Primary Hyperoxaluria Type 1. Curr. Opin. Nephrol. Hypertens. 2022;31:344–350. doi: 10.1097/MNH.0000000000000790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Arvans D., Jung Y.-C., Antonopoulos D., Koval J., Granja I., Bashir M., Karrar E., Roy-Chowdhury J., Musch M., Asplin J., et al. Oxalobacter Formigenes-Derived Bioactive Factors Stimulate Oxalate Transport by Intestinal Epithelial Cells. J. Am. Soc. Nephrol. 2017;28:876–887. doi: 10.1681/ASN.2016020132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Daniel S.L., Moradi L., Paiste H., Wood K.D., Assimos D.G., Holmes R.P., Nazzal L., Hatch M., Knight J. Forty Years of Oxalobacter Formigenes, a Gutsy Oxalate-Degrading Specialist. Appl. Environ. Microbiol. 2021;87:e0054421. doi: 10.1128/AEM.00544-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sidhu H., Schmidt M.E., Cornelius J.G., Thamilselvan S., Khan S.R., Hesse A., Peck A.B. Direct Correlation between Hyperoxaluria/Oxalate Stone Disease and the Absence of the Gastrointestinal Tract-Dwelling Bacterium Oxalobacter Formigenes: Possible Prevention by Gut Recolonization or Enzyme Replacement Therapy. J. Am. Soc. Nephrol. 1999;10:S334–S340. [PubMed] [Google Scholar]
- 71.Kleinschmidt K., Mahlmann A., Hautmann R. Microbial Degradation of Dietary Oxalate in the Human Gut and Urinary Oxalate Concentrations in Patients with Calcium Oxalate Urolithiasis and Control Persons. Investig. Urol. 1994;5:222–224. [PubMed] [Google Scholar]
- 72.Liu M., Devlin J.C., Hu J., Volkova A., Battaglia T.W., Ho M., Asplin J.R., Byrd A., Loke P., Li H., et al. Microbial Genetic and Transcriptional Contributions to Oxalate Degradation by the Gut Microbiota in Health and Disease. Elife. 2021;10:e63642. doi: 10.7554/eLife.63642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hatch M., Cornelius J., Allison M., Sidhu H., Peck A., Freel R.W. Oxalobacter Sp. Reduces Urinary Oxalate Excretion by Promoting Enteric Oxalate Secretion. Kidney Int. 2006;69:691–698. doi: 10.1038/sj.ki.5000162. [DOI] [PubMed] [Google Scholar]
- 74.Verhulst A., Dehmel B., Lindner E., Akerman M.E., D’Haese P.C. Oxalobacter Formigenes Treatment Confers Protective Effects in a Rat Model of Primary Hyperoxaluria by Preventing Renal Calcium Oxalate Deposition. Urolithiasis. 2022;50:119–130. doi: 10.1007/s00240-022-01310-9. [DOI] [PubMed] [Google Scholar]
- 75.Nazzal L., Francois F., Henderson N., Liu M., Li H., Koh H., Wang C., Gao Z., Perez G.P., Asplin J.R., et al. Effect of Antibiotic Treatment on Oxalobacter Formigenes Colonization of the Gut Microbiome and Urinary Oxalate Excretion. Sci. Rep. 2021;11:16428. doi: 10.1038/s41598-021-95992-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hiremath S., Viswanathan P. Oxalobacter Formigenes: A New Hope as a Live Biotherapeutic Agent in the Management of Calcium Oxalate Renal Stones. Anaerobe. 2022;75:102572. doi: 10.1016/j.anaerobe.2022.102572. [DOI] [PubMed] [Google Scholar]
- 77.Miller A.W., Choy D., Penniston K.L., Lange D. Inhibition of Urinary Stone Disease by a Multi-Species Bacterial Network Ensures Healthy Oxalate Homeostasis. Kidney Int. 2019;96:180–188. doi: 10.1016/j.kint.2019.02.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ticinesi A., Milani C., Guerra A., Allegri F., Lauretani F., Nouvenne A., Mancabelli L., Lugli G.A., Turroni F., Duranti S., et al. Understanding the Gut-Kidney Axis in Nephrolithiasis: An Analysis of the Gut Microbiota Composition and Functionality of Stone Formers. Gut. 2018;67:2097–2106. doi: 10.1136/gutjnl-2017-315734. [DOI] [PubMed] [Google Scholar]
- 79.Whittamore J.M., Hatch M. The Role of Intestinal Oxalate Transport in Hyperoxaluria and the Formation of Kidney Stones in Animals and Man. Urolithiasis. 2017;45:89–108. doi: 10.1007/s00240-016-0952-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Hatch M. Induction of Enteric Oxalate Secretion by Oxalobacter Formigenes in Mice Does Not Require the Presence of Either Apical Oxalate Transport Proteins Slc26A3 or Slc26A6. Urolithiasis. 2020;48:1–8. doi: 10.1007/s00240-019-01144-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hoppe B., Pellikka P.A., Dehmel B., Banos A., Lindner E., Herberg U. Effects of Oxalobacter Formigenes in Subjects with Primary Hyperoxaluria Type 1 and End-Stage Renal Disease: A Phase II Study. Nephrol. Dial. Transplant. 2021;36:1464–1473. doi: 10.1093/ndt/gfaa135. [DOI] [PubMed] [Google Scholar]
- 82.Pape L., Ahlenstiel-Grunow T., Birtel J., Krohne T.U., Hoppe B. Oxalobacter Formigenes Treatment Combined with Intensive Dialysis Lowers Plasma Oxalate and Halts Disease Progression in a Patient with Severe Infantile Oxalosis. Pediatr. Nephrol. 2020;35:1121–1124. doi: 10.1007/s00467-019-04463-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ariceta G., Collard L., Abroug S., Moochhala S.H., Gould E., Boussetta A., Ben Hmida M., De S., Hunley T.E., Jarraya F., et al. EPHex: A Phase 3, Double-Blind, Placebo-Controlled, Randomized Study to Evaluate Long-Term Efficacy and Safety of Oxalobacter Formigenes in Patients with Primary Hyperoxaluria. Pediatr. Nephrol. 2022 doi: 10.1007/s00467-022-05591-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Milliner D., Hoppe B., Groothoff J. A Randomised Phase II/III Study to Evaluate the Efficacy and Safety of Orally Administered Oxalobacter Formigenes to Treat Primary Hyperoxaluria. Urolithiasis. 2018;46:313–323. doi: 10.1007/s00240-017-0998-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hoppe B., Groothoff J.W., Hulton S.-A., Cochat P., Niaudet P., Kemper M.J., Deschênes G., Unwin R., Milliner D. Efficacy and Safety of Oxalobacter Formigenes to Reduce Urinary Oxalate in Primary Hyperoxaluria. Nephrol. Dial. Transplant. 2011;26:3609–3615. doi: 10.1093/ndt/gfr107. [DOI] [PubMed] [Google Scholar]
- 86.Osman A., El-Gazzar N., Almanaa T.N., El-Hadary A., Sitohy M. Lipolytic Postbiotic from Lactobacillus Paracasei Manages Metabolic Syndrome in Albino Wistar Rats. Molecules. 2021;26:472. doi: 10.3390/molecules26020472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Son M., Oh S., Lee H.S., Choi J., Lee B.-J., Park J.-H., Park C.H., Son K.H., Byun K. Gamma-Aminobutyric Acid-Salt Attenuated High Cholesterol/High Salt Diet Induced Hypertension in Mice. Korean J. Physiol. Pharmacol. 2021;25:27–38. doi: 10.4196/kjpp.2021.25.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hayakawa K., Kimura M., Kasaha K., Matsumoto K., Sansawa H., Yamori Y. Effect of a Gamma-Aminobutyric Acid-Enriched Dairy Product on the Blood Pressure of Spontaneously Hypertensive and Normotensive Wistar-Kyoto Rats. Br. J. Nutr. 2004;92:411–417. doi: 10.1079/BJN20041221. [DOI] [PubMed] [Google Scholar]
- 89.Inoue K., Shirai T., Ochiai H., Kasao M., Hayakawa K., Kimura M., Sansawa H. Blood-Pressure-Lowering Effect of a Novel Fermented Milk Containing Gamma-Aminobutyric Acid (GABA) in Mild Hypertensives. Eur. J. Clin. Nutr. 2003;57:490–495. doi: 10.1038/sj.ejcn.1601555. [DOI] [PubMed] [Google Scholar]
- 90.Lee H., Ji S.Y., Hwangbo H., Kim M.Y., Kim D.H., Park B.S., Park J.-H., Lee B.-J., Kim G.-Y., Jeon Y.-J., et al. Protective Effect of Gamma Aminobutyric Acid against Aggravation of Renal Injury Caused by High Salt Intake in Cisplatin-Induced Nephrotoxicity. Int. J. Mol. Sci. 2022;23:502. doi: 10.3390/ijms23010502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Shankaranarayanan D., Raj D.S. Gut Microbiome and Kidney Disease: Reconciling Optimism and Skepticism. Clin. J. Am. Soc. Nephrol. 2022 doi: 10.2215/CJN.04480422. online ahead of print . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Aguilera-Correa J.-J., Madrazo-Clemente P., Martínez-Cuesta M.D.C., Peláez C., Ortiz A., Sánchez-Niño M.D., Esteban J., Requena T. Lyso-Gb3 Modulates the Gut Microbiota and Decreases Butyrate Production. Sci. Rep. 2019;9:12010. doi: 10.1038/s41598-019-48426-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chamberlain C.A., Hatch M., Garrett T.J. Extracellular Vesicle Analysis by Paper Spray Ionization Mass Spectrometry. Metabolites. 2021;11:308. doi: 10.3390/metabo11050308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Chamberlain C.A., Hatch M., Garrett T.J. Oxalobacter Formigenes Produces Metabolites and Lipids Undetectable in Oxalotrophic Bifidobacterium Animalis. Metabolomics. 2020;16:122. doi: 10.1007/s11306-020-01747-2. [DOI] [PubMed] [Google Scholar]
- 95.Joshi S., Goldfarb D.S. The Use of Antibiotics and Risk of Kidney Stones. Curr. Opin. Nephrol. Hypertens. 2019;28:311–315. doi: 10.1097/MNH.0000000000000510. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.