Abstract
Lycium barbarum L. (LB) fruits have high nutritive values and therapeutic effects. The aim of this study was to comprehensively evaluate the differences in phenolic composition of LB fruits from different geographical regions. Different methods of characterization and statistical analysis of data showed that different geographic sources of China could be significantly separated from each other. The highest total phenolic compound (TPC) content was observed in LB fruits from Ningxia (LBN), followed by those from Gansu (LBG) and Qinghai (LBQ). The Fourier transform infrared (FTIR) spectra of LB fruits revealed that LBQ had a peak at 2972 cm−1 whereas there was no similar peak in LBG and LBQ. A new HPLC method was established for the simultaneous determination of 8 phenolic compounds by quantitative analysis of multiple components by a single marker (QAMS), including 4 phenolic acids (chlorogenic acid, caffeic acid, 4-hydroxycinnamic acid, and ferulic acid), 1 coumarin (scopoletin), and 3 flavonoids (kaempferol-3-O-rutinoside, rutin, and narcissoside). It was showed that rutin was the most dominant phenolic compound in LBQ, although the average content of 4 phenolic acids was also high in LBQ, and scopoletin was the richest in LBG. UHPLC-Q-TOF-MS was used to qualitatively analyze the phenolics, which showed LBN was abundant in phenolic acids, LBQ was rich in flavonoids, and coumarins were the most plentiful in LBG. In conclusion, this study can provide references for the quality control and evaluation of phenolics in LB fruits and their by-products.
Keywords: Lycium barbarum L. fruits, phenolic compounds, profile, classification
1. Introduction
The plant genus Lycium (Solanaceae) is distributed widely in the world with high nutritional and medicinal values, and occurs in America, Africa, and Eurasia [1,2]. One of the most widely used species is Lycium barbarum L. (LB), which has been utilized as a commodity worldwide and has become a super food [2]. This species grows primarily in Asia and has also been cultivated in Europe and the Mediterranean. The main producing district of LB is China, particularly northwest China, where cultivation has a history of over 2000 years. LB is the most widely distributed cultivar in China [3]. The fruits of LB are used as a medicine named Goqizi, which can nourish the liver and kidney and replenish vital essence to improve eyesight [4]. Modern pharmacological studies on LB indicate that it has the beneficial effects of immune modulation, anti-aging effects, improving osteoblastic proliferation, radiological protection, ameliorating hepatic or brain injuries and impaired locomotor activities, neuroprotective effects, prevention of benign prostatic hyperplasia, delay of retinopathy, antioxidant and enzyme inhibitory effects, anti-inflammatory properties, anti-diabetes effects, anti-cancer properties, anti-hypertension effects, and cardioprotective effects [5,6,7,8,9,10,11,12,13,14,15,16,17]. LB has high nutritive value and therapeutic effects relevant to bioactive components, including polysaccharides, phenolics, carotenoids, alkaloids, vitamins, amino acids, and fatty acids [18,19,20,21].
In recent years, studies on phenolic compounds in LB have been second only to those on polysaccharides. Phenolics are aromatic rings with one or more hydroxyl groups that consist of simple phenols, polyphenols, benzoic and cinnamic acids, coumarins, tannins, lignins, lignans, and flavonoids [22,23]. These compounds are known as antioxidant and bioactive agents, with great benefits to health and in the prevention and treatment of diseases [24]. Therefore, researchers have paid increasing attention to the qualitative and quantitative analysis of phenolic compounds in LB, which mainly contain phenolic acids, flavonoids, phenolic amides, lignans, lignins, stilbenes, alkylphenols, curcuminoids, and terpenes. Among these compounds, phenolic acids and flavonoids are the best-studied constituents in LB fruits. The principal analysis methods utilize LC-MS, followed by HPLC and UHPLC. Thirty-five polyphenolic compounds were detected and quantified in the fresh and dried Lycium fruits, including five phenolic acids, 11 anthocyanins, and 19 phenolamides, using UHPLC-ESI-Q-TOF-MS [25]. After extraction by condensation reflux, hydrochloric acid acidification, and ethyl acetate, nine phenolic acids were determined in LB fruits by HPLC [26]. The isolation and purification of polyphenols from LB fruits were carried out by ultrasound-assisted extraction and solid-phase extraction, and then 10 phenolic acids and 11 flavonoids were identified and quantified by UHPLC-UV [27]. Furthermore, the quantitative analysis of complex components in herbs or foods is difficult in the absence of reference standards or the expensive cost of reference standards [28]. Consequently, a quantitative method with wide applicability should be established. Within the scope of a certain linearity range, the amount (weight or concentration) of one component is proportionate to the response values of the detector [29]. Quantitative analysis of multiple components by a single marker (QAMS) is a simple and economical method that only requires one standard reference, and all analytes in the sample can be identified simultaneously [30,31]. It was essential to select a suitable internal reference (IR) in order to establish the relative correction factor (RCF) between IR and other effective ingredients, and RCF can be influenced by many factors, such as laboratories, chromatographic instrument systems, packing, and the models of chromatographic columns [29,32]. The method of QAMS has been widely accepted and applied in the quality control of herbal medicine, which has been adopted by the Chinese Pharmacopoeia, the United States Pharmacopoeia, and the European Pharmacopoeia Standards. The study of simultaneous determination of phenolic compounds in LB fruits using HPLC-QAMS has been reported scarcely.
Ecological factors had a significant effect on fruit morphology and bioactive constituents. High soil, air temperatures, low altitude, light intensity, and moderate soil moisture were shown to be suitable conditions to produce Lycium fruits with a high content of nutritious metabolites [33]. Nzeuwa et al. [34] found that there was a slight difference in the contents of nutrients and phytochemicals among Lycium fruits from different areas, and the total phenol content of fruits grown in Nepal was higher than that of China. Lu et al. [35] reported that there are distinct differences in the functional components and antioxidant activity of Lycium barbarum L. fruits from different regions in China. Geographical factors had a great influence on phenolic compositions. Nevertheless, little is known about the overall distinction of phenolic compounds in LB fruits from different geographical sources.
In the present study, LB fruits coming from three major regions of China (Ningxia, Gansu, and Qinghai) were analyzed in multiple methods. The objective of this study was to evaluate the phenolic profile of LB fruits from different regions using qualitative and quantitative methods in order to gain a profound understanding of the phenolic diversity. We established a new method for quantifying eight phenolic compounds in LB fruits from different regions in China by HPLC combined with QAMS. The qualitative analysis of the phenolic profile was determined by UHPLC-Q-TOF-MS.
2. Results and Discussion
2.1. Physical Characteristics of LB Fruits
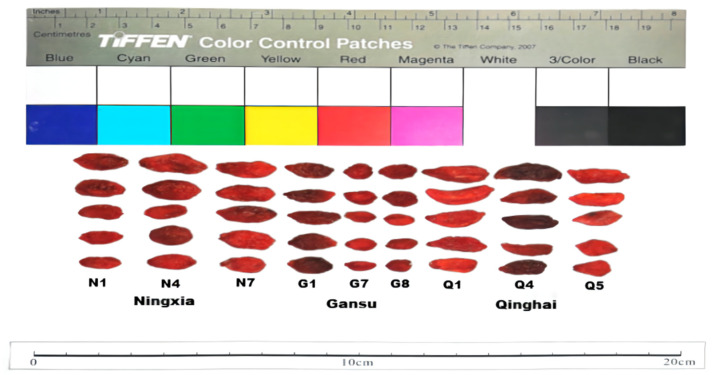
LB is widely cultivated in China, particularly in northwest districts. Generally, there were quite some different distinctions in fruit sizes and tastes of LB from different habitats [36]. A total of nine batches of samples were observed, including their color, shape, diameter and length (Figure 1). The appearance of all LB fruits was mostly red and fusiform. The highest values of diameter and length were observed for LBQ, which were 6.93 ± 0.18 and 17.10 ± 2.58 mm, respectively, while those of LBN were fractionally behind, with values of 6.67 ± 0.63 and 14.19 ± 1.24 mm. In the LBG fruits, the diameter was 5.66 ± 0.16 mm, and the length was 11.43 ± 2.09 mm. In a recent study, LB fruit morphological traits were also recorded from three regions, and the results showed that fruits from Qinghai were the largest, followed by those from Xinjiang and Ningxia. While the detailed morphological characteristics of LBN were smaller than those of LB fruits from other regions, it was traditionally an authentic (Daodi) herb in China [35].
Figure 1.
The characteristics of LB fruits from different regions.
2.2. TPC Content
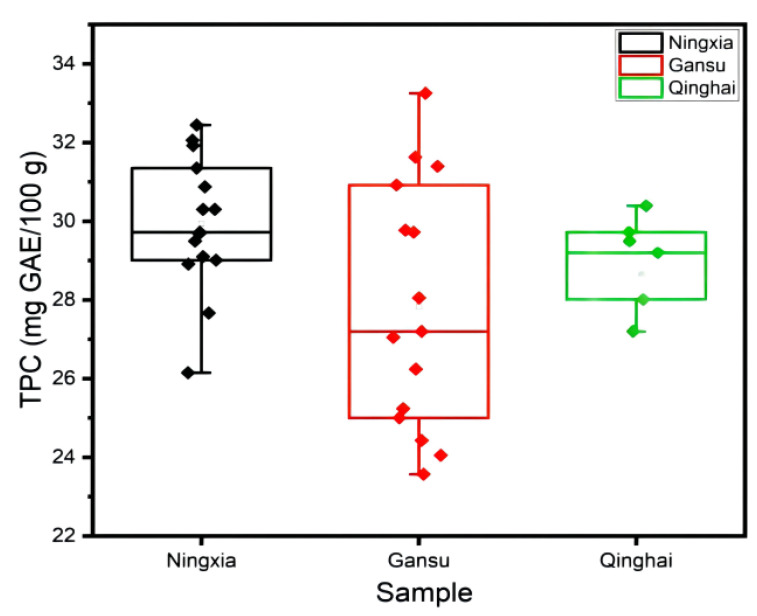
The Folin–Ciocalteu assay was used for TPC content determinations. There were obvious differences in TPC contents in LB fruits from different regions. The highest TPC content was observed for LBN (29.931 ± 1.70 mg GAE/100 g), followed by LBQ (29.080 ± 1.08 mg GAE/100 g) and LBG (27.835 ± 3.11 mg GAE/100 g). According to the following scatter diagram (Figure 2), the TPC contents in LBN were higher than in LBG and LBQ, and the TPC contents of LBN and LBQ were more stable and consistent than LBG. The TPC content is influenced by many factors, including geographical, environmental, and cultivation methods [37]. Lu et al. also reported that the highest TPC contents were in LB fruits from Zhongning of Ningxia, which was greater than in samples from Gansu and Xinjiang [35].
Figure 2.
Total phenolic compound (TPC) content of LB fruits from different regions.
2.3. FTIR-ATR
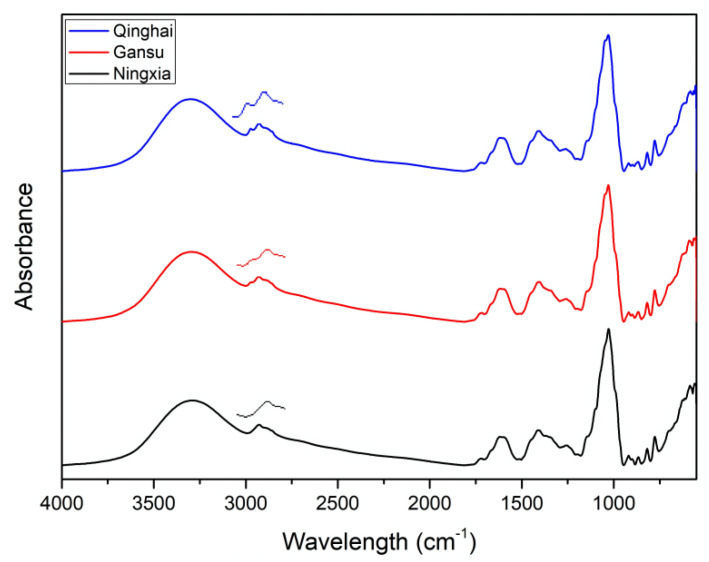
Fourier transform infrared spectroscopy is a widespread technique in the analysis of food components and can be a tool for rapid evaluation of foods and their by-products [38]. The spectra of the phenolic extracts of LBN, LBG and LBQ are shown in Figure 3. The spectra were dominated by typical vibrations in the OH region (3400–3200 cm−1) and aromatics (1500–1300 cm−1) related to phenolic compounds [39]. Peaks at 2923 and 2853 cm−1 were mainly associated with the hydrocarbon chains of the lipids or lignins [40]. The results showed that there was some difference in the 3000–2800 cm−1 region. Between them, the spectra of LBQ had two peaks at 2972 cm−1 (C-H stretching of the methylene bridges) and 2927 cm−1 (C-H stretching vibration) [41,42,43], and the absorbance of LBG and LBN was only at 2928 or 2927 cm−1, respectively. The absorption intensity of LBQ was higher than that of LBG and LBN at 2972 cm−1. A study from Peng et al. identified seven species and three variations of genus Lycium in China by FTIR, based on the additive infrared spectroscopy absorption of the chemical components and the differences of their relative contents in various Gouqi [44]. This method could provide a new way for the identification of LB fruits.
Figure 3.
Spectra of phenolic extracts in LB fruits from different regions.
2.4. Method Validation and the Relative Correction Factor of HPLC-QAMS
All of the calibration curves and their linear regression equations of eight quantitative phenolic compounds including chlorogenic acid, caffeic acid, 4-hydroxycinnamic acid, ferulic acid, scopoletin, kaempferol-3-O-rutinoside, rutin, and narcissoside, are displayed in Table 1. The correlation coefficients (r) ranged from 0.9993 to 0.9999. The limit of detection (LOD) ranged from 0.0007 to 0.0094 μg/mL, and the limit of quantification (LOQ) from 0.0025 to 0.0314 μg/mL. The precision, repeatability, stability, and recovery of the eight analytes were presented in Table 1. The relative standard deviations (RSD) values of precision ranged from 0.07% to 2.38%. Six samples from the same batch were analyzed by an identical method, and the RSD values of repeatability were all lower than 2.96%. The RSD values of stability were less than 2.48. The mean recoveries of the eight analytes ranged from 95.94% to 104.36%, and the RSD values of recovery were under 3.00%.
Table 1.
Method validation data of 8 phenolic compounds by HPLC.
| Reference Substance | Linearity | LOD (μg/mL) |
LOQ (μg/mL) |
Precision RSD (%) |
Repeatability RSD (%) |
Stability RSD (%) |
Recovery | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Regression Equation | Range (μg/mL) | r | Mean (%) |
RSD (%) |
||||||
| Chlorogenic acid | Y = 14333X − 3436.9 | 0.39–24.95 | 0.9994 | 0.0019 | 0.0065 | 0.28 | 0.95 | 0.70 | 97.87 | 2.18 |
| Caffeic acid | Y = 17526X − 2502.4 | 0.81–25.90 | 0.9999 | 0.0040 | 0.0135 | 0.15 | 1.80 | 0.22 | 100.25 | 2.20 |
| 4-Hydroxycinnamic acid | Y = 2081.6X − 615.94 | 1.88–30.15 | 0.9998 | 0.0094 | 0.0314 | 2.38 | 1.10 | 2.28 | 103.56 | 3.00 |
| Scopoletin | Y = 48845X − 4441.1 | 0.42–27.10 | 0.9999 | 0.0021 | 0.0071 | 0.86 | 2.10 | 0.86 | 103.72 | 2.26 |
| Ferulic acid | Y = 13278X − 2424.1 | 0.64–10.16 | 0.9993 | 0.0032 | 0.0106 | 1.95 | 2.96 | 2.48 | 104.36 | 2.34 |
| Rutin | Y = 32673X − 6917.5 | 1.68–215.20 | 0.9999 | 0.0021 | 0.0070 | 0.07 | 1.13 | 0.49 | 100.23 | 1.25 |
| Kaempferol-3-O- rutinoside |
Y = 35325X − 1081.2 | 0.15–9.72 | 0.9999 | 0.0008 | 0.0025 | 0.71 | 2.81 | 0.84 | 95.94 | 0.99 |
| Narcissoside | Y = 39925X − 1172.1 | 0.15–9.46 | 0.9999 | 0.0007 | 0.0025 | 1.28 | 2.14 | 1.29 | 101.63 | 1.03 |
To establish a new HPLC-QAMS method, some factors, such as columns and instruments, were required for detection. Other variables, such as wavelength, temperature, flow rate, and injection volume were also considered in order to gain an appropriate gradient elution method. During the research, it was found that these factors had effects on peak number, peak shape, and retention time. In this study, the influences of different instruments and chromatographic columns on relative correction factor (RCF) were investigated (Table 2). The results proved that different instruments and columns had no significant effects on the RCF value.
Table 2.
The value of RCF of each component in different influence factors.
| Instrument | Column | fa/d | fb/d | fc/d | fe/d | ff/d | fg/d | fh/d |
|---|---|---|---|---|---|---|---|---|
| SHIMADZU-LC-20AD | Shim-pack GIST C18-AQ | 0.285 | 0.343 | 0.041 | 0.258 | 0.658 | 0.664 | 0.760 |
| Welch Ulimate® AQ-C18 | 0.287 | 0.341 | 0.041 | 0.261 | 0.647 | 0.657 | 0.754 | |
| Kromasil | 0.286 | 0.342 | 0.041 | 0.260 | 0.653 | 0.660 | 0.757 | |
| Waters2695 | Shim-pack GIST C18-AQ | 0.286 | 0.336 | 0.041 | 0.260 | 0.654 | 0.672 | 0.775 |
| Welch Ulimate® AQ-C18 | 0.277 | 0.322 | 0.039 | 0.256 | 0.646 | 0.658 | 0.767 | |
| Kromasil | 0.282 | 0.325 | 0.039 | 0.262 | 0.649 | 0.657 | 0.757 | |
| Mean | 0.284 | 0.335 | 0.041 | 0.260 | 0.651 | 0.661 | 0.761 | |
| RSD (%) | 1.365 | 2.766 | 2.936 | 0.814 | 0.718 | 0.860 | 1.023 | |
a. Chlorogenic acid; b. Caffeic acid; c. 4-Hydroxycinnamic acid; d. Scopoletin; e. Ferulic acid; f. Rutin; g. Kaempferol-3-O-rutinoside; h. Narcissoside.
2.5. Quantitative Determination of Phenolic Compounds in LB Fruits from Different Regions
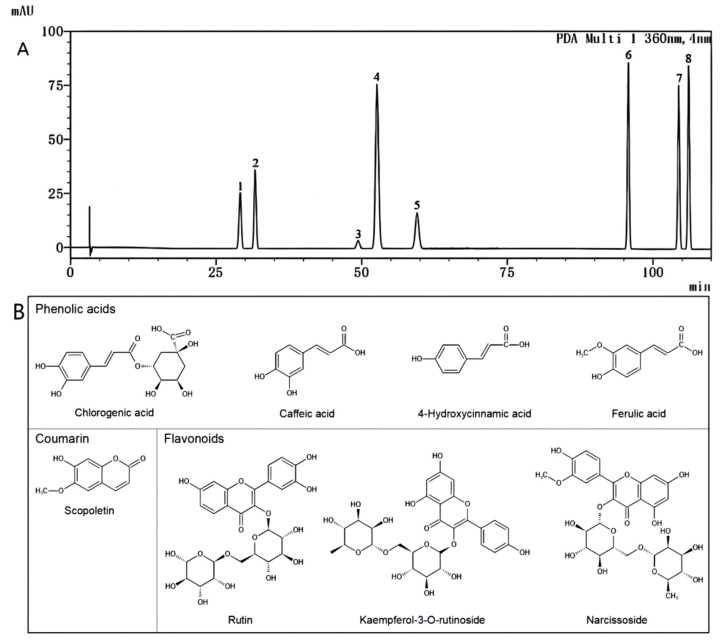
Generally, quantitative methods usually use multiple standards to determine analytes. QAMS only requires the use of an IR to detect all analytes [28]. In a recent study, a method for simultaneous determination of four carotenoids in Lycium barbarum was built by using QAMS [45]. In this study, a new HPLC-QAMS method was established (Figure 4A) that could be used to determine eight phenolic compounds (Figure 4B) in LB fruits. The analytes included four phenolic acids (chlorogenic acid, caffeic acid, 4-hydroxycinnamic acid, and ferulic acid), one coumarin (scopoletin), and three flavonoids (kaempferol-3-O-rutinoside, rutin, and narcissoside). Among them, scopoletin was selected as the IR with its moderate retention time, stable property, low price, and its peak shape that were presented well. Compared with external standard methods (ESM) that were used for comparison, the contents of the other seven analytes by the QAMS method showed a narrow gap. Their average RSD values were less than 5.0% (Table 3). The results showed that there was no significant difference between the results of the ESM and QAMS methods, and it was indicated that the establishment of HPLC-QAMS was feasible for the determination of eight phenolic compounds in LB fruits by using scopoletin as IR.
Figure 4.
(A) HPLC chromatogram of mixed standard solutions (1: chlorogenic acid; 2: caffeic acid; 3: 4-hydroxycinnamic acid; 4: scopoletin; 5: ferulic acid; 6: rutin; 7: kaempferol-3-O-rutinoside; 8: narcissoside). (B) Chemical structures of eight phenolic compounds that were quantified in LB fruits.
Table 3.
The average content of eight phenolic compounds in LB fruits from three different regions (μg/g).
| Compounds | LBN | LBG | LBQ | ||||||
|---|---|---|---|---|---|---|---|---|---|
| ESM | QAMS | RSD (%) | ESM | QAMS | RSD (%) | ESM | QAMS | RSD (%) | |
| Chlorogenic acid | 0.0041 | 0.0039 | 2.79 | 0.0025 | 0.0026 | 3.68 | 0.0068 | 0.0071 | 2.89 |
| Caffeic acid | 0.0041 | 0.0040 | 1.60 | 0.0048 | 0.0047 | 1.02 | 0.0071 | 0.0070 | 1.26 |
| 4-Hydroxycinnamic acid | 0.0043 | 0.0045 | 3.12 | 0.0071 | 0.0068 | 3.27 | 0.0097 | 0.0093 | 2.85 |
| Scopoletin | 0.0022 | - | - | 0.0037 | - | - | 0.0022 | - | - |
| Ferulic acid | 0.0034 | 0.0036 | 3.31 | 0.0017 | 0.0018 | 2.52 | 0.0043 | 0.0046 | 4.19 |
| Rutin | 0.0126 | 0.0127 | 0.39 | 0.0132 | 0.0131 | 0.42 | 0.0196 | 0.0196 | 0.05 |
| Kaempferol-3-O-rutinoside | 0.0008 | 0.0008 | 0.43 | 0.0009 | 0.0009 | 0.39 | 0.0009 | 0.0009 | 0.38 |
| Narcissoside | 0.0009 | 0.0009 | 0.46 | 0.0011 | 0.0011 | 0.28 | 0.0010 | 0.0010 | 0.45 |
The highest mean contents of chlorogenic acid, caffeic acid, 4-hydroxycinnamic acid, ferulic acid, and rutin were in LBQ, which were 0.0068 mg/g, 0.0071 μg/g, 0.0097 μg/g, 0.0043 μg/g and 0.0196 μg/g, respectively. The highest mean contents of scopoletin, narcissoside, and kaempferol-3-O-rutinoside were 0.0035, 0.0011 and 0.0009 μg/g in LBG, respectively. There was a significant difference among the regions.
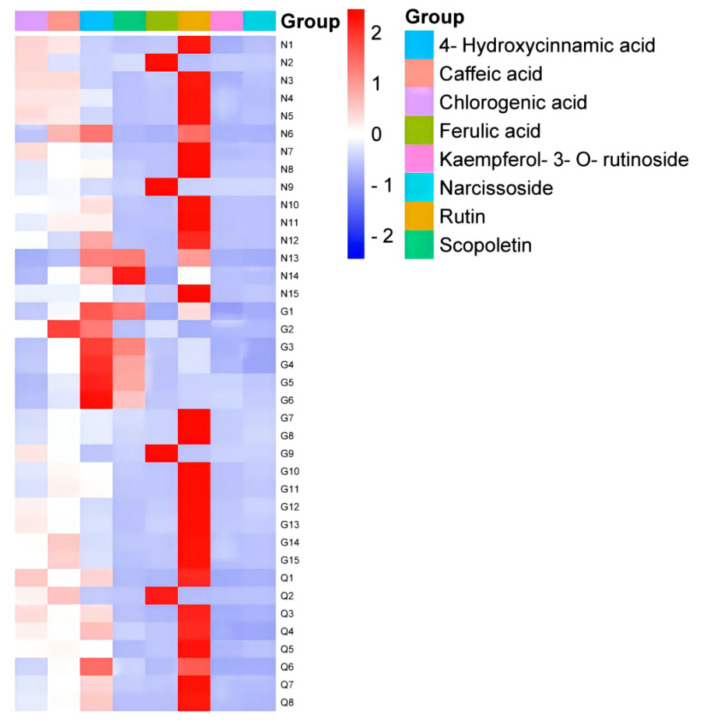
For phenolic acids, 4-hydroxycinnamic acid was the main phenolic acid in LBQ, which accounted for 0.0097 μg/g. The next was caffeic acid with a content of 0.0071 μg/g, and the lowest was ferulic acid with 0.0043 μg/g. These differences were observed in the heatmap (Figure 5). The changes in phenolic acids in LBN were the same as those in LBQ, and the highest content of 4-hydroxycinnamic acid was 0.0043 μg/g, and the lowest was 0.0034 μg/g. The conditions were particularly clear in LBG. The minimum and maximum contents were 0.0071 μg/g of 4-hydroxycinnamic acid and 0.0017 μg/g of ferulic acid. The greatest total phenolic acid content was 0.0279 μg/g in LBQ, which was 1.75 times and 1.73 times higher than that in LBN and LBG, respectively.
Figure 5.
Heatmap of phenolic content in LB fruits from different regions.
For flavonoids, rutin had the highest content in all samples, which was 0.0196 μg/g in LBQ, 1.56 times and 1.48 times compared with LBN and LBQ, respectively. There were nearly no differences in the contents of narcissoside and kaempferol-3-O-rutinoside applied to all samples. The sum of the flavonoid contents was 0.0143, 0.0152 and 0.0215 μg/g in LBN, LBG and LBQ, respectively. LBG has the greatest content of scopoletin at 0.0037 μg/g, and that in LBN was similar to that in LBQ.
In general, the total content of four phenolic acids was less than that of three flavonoids. The highest total content of the eight analytes was observed for LBQ, followed by LBG and LBN. The maximum contents of scopoletin, kaempferol-3-O-rutinoside and narcissoside were observed in LBG. The contents of other analytes were the highest in LBQ.
2.6. Qualitative Analysis of Phenolic Compounds in LB Fruits by UPLC-Q-TOF-MS
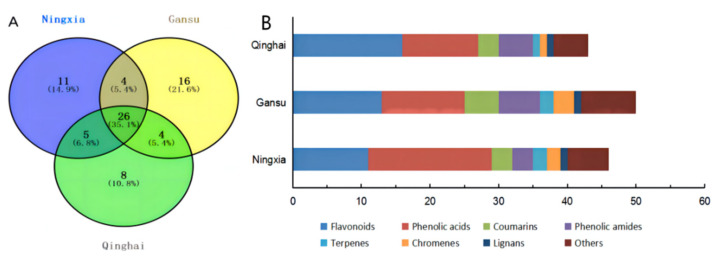
By analyzing mass data from previous literature and studies [46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67], 74 phenolic constituents were identified in total in our samples, including 18 flavonoids, 19 phenolic acids, seven phenolic amides, six coumarins, three terpenes, three chromenes, two lignans and 16 other phenolics. The data were presented in Table 4. There were 46, 50 and 43 phenolic compounds identified in this study in LBN, LBG and LBQ fruits, respectively (Figure 6A). Among them, 26 phenolic substances were found in all LB fruits from three different regions. In particular, 11 phenolic compounds were unique in LBN, including six phenolic acids, one terpene and four other phenolics. In addition, 16 phenolic compounds were only identified in LBG, including one flavonoid, one phenolic acid, two coumarins, two phenolic amides, one terpene, one chromene, one lignan and seven other phenolics. Meanwhile, eight phenolic compounds barely existed in LBQ, including three flavonoids, one coumarin, one phenolic amide and three other phenolics.
Table 4.
The qualitative analysis of phenolics in LB fruits from different regions in China.
| No. | tR (min) | Identification | Formula | Mass (m/z) |
Cacl. Mass (m/z) | mDa | Fragements (MS2) | LBN | LBG | LBQ |
|---|---|---|---|---|---|---|---|---|---|---|
| Flavonoids | ||||||||||
| 1 | 2.54 | 2’-hydroxyflavanone | C15H12O3 | 239.0662 | 239.0708 | −4.6 | 191.0190; 130.0862; 124.0392 | √ | √ | √ |
| 2 | 3.13 | 7-methoxy-2-phenyl-3,4-dihydro-2H-1-benzopyran-4-one | C16H14O3 | 253.0823 | 253.0865 | −4.2 | 218.0653; 194.9445; 137.0233; 128.0341 | √ | √ | |
| 3 | 4.16 | 3-(2,4-dihydroxybenzoyl)-4,5- dimethyl-5-[4-methyl-5-(4-methyl-5- (4-methyl-2-furyl)-3(E)-penten -1-l-yl]tetrahydro-2-furanone |
C24H28O6 | 411.177 | 411.1808 | −3.8 | 249.1241; 135.0446 | √ | √ | |
| 4 | 5.58 | 4-(2-Carboxyethenyl)-2-(3,4-di hydroxy phenyl)-2,3-dihydro-7- hydroxy-3-methylester, [2α, 3β, 4(E)-3-benzofuran carboxylic acid | C16H20O10 | 371.0973 | 371.0978 | −0.5 | 163.0396; 119.0497 | √ | √ | |
| 5 | 8.61 | Quercetin-rhamno-tri-hexoside | C39H50O26 | 933.2519 | 933.2512 | 0.7 | 470.2283 | √ | ||
| 6 | 9.95 | Quecetin 3-O-galactosylrutinoside | C33H40O21 | 771.2003 | 771.1984 | 1.9 | 609.1465; 301.0349 | √ | √ | √ |
| 7 | 11.43 | Quercetin3-O-α-L-rhamno pyranosyl-(1→6)-β-D-galacto pyranosyl-7-O-β-D-sophoroside |
C39H50O26 | 933.2511 | 933.2115 | −0.1 | 609.1454; 301.0342 | √ | √ | √ |
| 8 | 12.61 | Quercetin deoxyhexose -hexose- deoxyhexose | C33H40O20 | 755.2018 | 755.2035 | −1.7 | 593.1491 | √ | ||
| 9 | 13.77 | 5,4′-dihydroxy-3′-methoxyflavonol-3-O-glucosyl-(1→6)-glucosyl-7-O rhamnoside | C34H42O21 | 785.2155 | 785.214 | 1.5 | 623.1630; 315.0510 | √ | √ | |
| 10 | 15.71 | Chakaflavonoside A | C39H50O25 | 917.2571 | 917.2563 | 0.8 | 194.9445 | √ | ||
| 11 | 16.15 | Qucercetin 3-O-glucosylrutinoside | C33H40O21 | 771.1995 | 771.1984 | 1.1 | 609.1457; 301.0345 | √ | √ | |
| 12 | 17.78 | Quercetin 3-O-rutinoside-(1-2)-O- rhamnoside | C33H40O20 | 755.2018 | 755.2035 | −1.7 | 300.0262; 194.9411 | √ | √ | √ |
| 13 | 20.42 | Parviside A | C39H50O26 | 933.2516 | 933.2512 | 0.4 | 771.1987; 292.9211 | √ | √ | |
| 14 | 21.43 | Sachaloside IV | C33H40O21 | 771.1985 | 771.1984 | 0.1 | 301.0340; 194.9416 | √ | √ | √ |
| 15 | 23.46 | Rutin | C27H30O16 | 609.1459 | 609.1456 | 0.3 | 300.0270; 101.0231 | √ | √ | √ |
| 16 | 30.71 | Kaempferol 3-O-rutinoside | C27H30O15 | 593.1505 | 593.1506 | −0.1 | 285.0395; 194.9424 | √ | √ | √ |
| 17 | 32.92 | Isorhamnetin 3-O-rutinoside | C28H32O16 | 623.1620 | 623.1612 | 0.8 | 315.0498; 194.9424 | √ | √ | √ |
| 18 | 38.27 | Swertianolin | C20H20O11 | 435.0919 | 435.0927 | −0.8 | 216.9271; 194.9447 | √ | ||
| Phenolic acids | ||||||||||
| 19 | 2.61 | Quinic acid derivate | C11H22O9 | 297.1182 | 297.1186 | −0.4 | 239.0646; 191.0183; 163.0382; 124.0394 | √ | ||
| 20 | 4.43 | Caffeic acid derivative | C13H32O14 | 411.1747 | 411.1714 | 3.3 | 179.0345; 161.0244; 135.0441 | √ | ||
| 21 | 4.56 | Dicaffeoylquinic acid derivative | C22H30O15 | 533.1498 | 533.1506 | −0.8 | 515.1372; 191.0541; 163.0391; 135.0437; 109.0285 | √ | √ | |
| 22 | 4.90 | Caffeoylquinic acid derivative 1 | C34H36O19 | 747.1754 | 747.1773 | −1.9 | 191.0555; 179.0542; 163.0398; 161.0452 |
√ | √ | |
| 23 | 5.15 | Coumarinylquinic acid derivative 1 | C34H36O19 | 747.1756 | 747.1773 | −1.7 | 191.0555; 163.0399; 145.0294; 119.0498 | √ | ||
| 24 | 5.39 | 3-O-(4’-O-Caffeoyl glucosyl)quinic acid | C22H28O14 | 515.1401 | 515.1401 | 0 | 353.0868; 191.0559; 163.0396; 135.0441 | √ | √ | √ |
| 25 | 5.53 | Caffeoylquinic acid derivative 2 | C18H24O14 | 463.1114 | 463.1088 | 2.6 | 203.0826; 191.0560 | √ | √ | √ |
| 26 | 5.92 | Coumarinylquinic acid derivative 2 | C34H36O19 | 747.1749 | 747.1773 | −2.4 | 163.0397; 145.0290; 119.0492 | √ | √ | √ |
| 27 | 6.03 | Coumarinylquinic acid derivative 3 | C35H38O20 | 777.1855 | 777.1878 | −2.3 | 461.1659; 193.0501; 113.0236 | √ | √ | √ |
| 28 | 6.20 | Feruloylquinic acid derivative 1 | C39H64O20 | 851.3942 | 851.3913 | 2.9 | 337.0762; 216.9280; 193.0505; 191.0553; 163.0391 | √ | √ | √ |
| 29 | 6.92 | Feruloylquinic acid derivative 2 | C28H38O20 | 693.187 | 693.1878 | −0.8 | 337.0762; 216.9270; 191.0348; 163.0393 | √ | ||
| 30 | 7.09 | Coumarinylquinic acid derivative 4 | C20H38O22 | 629.1824 | 629.1776 | 4.8 | 337.0756; 179.0342; 163.0390; 161.0237 | √ | ||
| 31 | 7.22 | 5-O-(3’-O-Caffeoyl glucosyl)quinic acid | C22H28O14 | 515.14 | 515.1401 | −0.1 | 323.0767; 191.0557; 179.0348; 161.0241; 108.0201 | √ | √ | √ |
| 32 | 7.40 | Caffeoylquinic acid derivative 3 | C33H54O15 | 689.3394 | 689.3384 | 1 | 191.0556; 179.0349; 163.0395; 135.0445 | √ | ||
| 33 | 7.64 | Chlorogenic acid | C16H18O9 | 353.087 | 353.0873 | −0.3 | 191.0555 | √ | √ | √ |
| 34 | 9.46 | Caffeic acid | C9H8O4 | 179.1572 | 179.157 | 0.2 | 135.0447 | √ | √ | √ |
| 35 | 11.3 | p-hydroxycinnamic acid | C9H8O3 | 163.0396 | 163.0395 | 0.1 | 135.0441; 119.0496 | √ | √ | √ |
| 36 | 13.89 | Ferulic acid | C10H10O4 | 193.1847 | 193.184 | 0.7 | 145.0321 | √ | √ | √ |
| 37 | 18.11 | Clinopodic acid Q | C33H32O17 | 699.1534 | 699.1561 | −2.7 | 194.9423 | √ | ||
| Coumarins | ||||||||||
| 38 | 0.72 | Cephalosol | C16H14O8 | 333.0583 | 333.061 | −2.7 | 260.8785; 128.9590; 112.9853 | √ | ||
| 39 | 7.26 | Umbelliferone | C9H6O3 | 163.0402 | 163.0395 | 0.7 | 127.0398 | √ | ||
| 40 | 7.36 | Esculetin | C9H6O4 | 177.0191 | 177.0188 | 0.3 | 163.0393; 135.0443; 119.0494 | √ | ||
| 41 | 8.96 | (R)-6-hydroxymellein diglycoside | C21H28O13 | 487.1449 | 487.1452 | −0.3 | 470.2279; 163.0393; 145.0290 | √ | √ | √ |
| 42 | 11.77 | Scopoletin | C10H8O4 | 193.0506 | 193.0501 | 0.5 | 163.0400; 133.0293 | √ | √ | √ |
| 43 | 15.77 | 6,7-di-O-(2′, 3′, 4′, 6′-tetra-O- acetyl-β-D-galactopyranosyl)-4- methylcoumarin |
C38H44O22 | 851.2272 | 851.2246 | 2.6 | 623.1628; 292.9214; 194.9420; 191.0555 | √ | √ | |
| Phenolic amides | ||||||||||
| 44 | 31.60 | N-feruloyltiramine | C18H19NO4 | 312.1234 | 312.1236 | −0.2 | 292.9217; 194.9418; 178.0499; 148.0522; 135.0443 | √ | √ | √ |
| 45 | 7.65 | Caffeoyl (dihydrocaffeoyl) spermidine-tri-hexose | C43H63N3O21 | 956.3878 | 956.3876 | 0.2 | 677.1931; 470.2284; 191.0549 | √ | ||
| 46 | 8.21 | Lycibarbarspermidine S | C37H53N3O16 | 794.3357 | 794.3348 | 0.9 | 632.2822; 470.2292; 334.1768 | √ | √ | √ |
| 47 | 9.88 | Lycibarbarspermidine P | C31H43N3O11 | 632.2831 | 632.2819 | 1.2 | 470.2290; 334.1764 | √ | √ | √ |
| 48 | 10.25 | Lycibarbarspermidine R | C31H43N3O11 | 632.2823 | 632.2819 | 0.4 | 540.2342; 470.2287; 334.1765; 135.0446 | √ | √ | |
| 49 | 11.04 | (E)-3-(3,4-dihydroxyphenyl)-N- ethylacrylamide |
C11H13NO3 | 206.082 | 206.0817 | 0.3 | 194.9426; 135.0445 | √ | ||
| 50 | 11.18 | N,N’-dicaffeoylspermidine | C25H31N3O6 | 470.2281 | 470.2291 | −1 | 220.0976; 163.0396 | √ | ||
| Terpenes | ||||||||||
| 51 | 0.82 | Vaccihein A | C18H18O9 | 377.085 | 377.0873 | −2.3 | 341.1085; 191.0560; 179.0556; 101.0238 | √ | ||
| 52 | 5.08 | Mudanpioside J | C31H34O14 | 629.1833 | 629.187 | −3.7 | 163.0397; 135.0444 | √ | √ | √ |
| 53 | 6.80 | Mudanpioside J isomer | C31H34O14 | 629.1836 | 629.187 | −3.4 | 529.3028 | √ | ||
| Chromenes | ||||||||||
| 54 | 13.02 | 2-(2-hydroxy-benzylidene)-3,3a dihydrocyclopenta [b] chromen -1(2H)-one | C19H14O3 | 291.099 | 291.1021 | −3.1 | 159.0926; 130.0659 | √ | √ | √ |
| 55 | 16.73 | 4,7-Dihydroxy-2-oxo-2H- chromene-3-acetyle derivative |
C25H31N3O5 | 454.2336 | 454.2342 | −0.6 | 163.0396 | √ | ||
| 56 | 22.33 | 4H-1-benzopyran-4-one,2-(3,4 -dimeth oxyphenyl)-6,8-di -β-D -glucopyranosyl-5,7-dihydroxy | C27H30O16 | 609.1445 | 609.1456 | −1.1 | 300.0260; 194.9420 | √ | √ | |
| Lignans | ||||||||||
| 57 | 37.47 | Pharsyringaresinol | C30H39O14 | 623.2389 | 623.234 | 4.9 | 460.1757 | √ | ||
| 58 | 38.61 | Terminaloside G | C30H40O14 | 623.2384 | 623.234 | 4.4 | 460.1749; 216.9263; 194.9442 | √ | √ | |
| Others | ||||||||||
| 59 | 0.76 | Pentacenehydroquinone | C22H14O | 293.0984 | 293.0966 | 1.8 | 215.0323; 131.0457 | √ | ||
| 60 | 2.77 | 2-(4-methoxyphenyl)-3,4-dihydro-2H-1-benzopyran-4-one | C16H14O3 | 253.0826 | 253.0865 | −3.9 | 231.0291; 128.0351 | √ | ||
| 61 | 4.71 | Caffeoyl derivative | C34H36O19 | 747.1744 | 747.1773 | −2.9 | 629.1830; 487.1455; 163.0396 | √ | ||
| 62 | 6.56 | Levodopa | C9H11NO4 | 196.0612 | 196.061 | 0.2 | 161.0246; 122.0608 | √ | √ | |
| 63 | 7.09 | Juglanoside D | C16H20O9 | 355.1024 | 355.1029 | −0.5 | 193.0500; 134.0366 | √ | ||
| 64 | 7.51 | 1-O-(E)-caffeoyl-β-D-glucopyranosyl-(1→2)-[β-D-glucopyranosyl-(1→6)]-β-D-glucopyranose | C27H38O19 | 665.1922 | 665.1929 | −0.7 | 503.1395; 341.0870; 179.0348; 161.0241 | √ | ||
| 65 | 11.12 | Rhinacanthin | C25H30O5 | 411.2125 | 411.2171 | −4.6 | 163.0393 | √ | ||
| 66 | 11.21 | Kankanoside F | C26H40O17 | 623.2191 | 623.2187 | 0.4 | 468.2128; 332.1606 | √ | ||
| 67 | 12.54 | 2,3-diphenylphenol | C18H14O | 245.0927 | 245.0966 | −3.9 | 203.0822; 135.0447; 116.0498 | √ | ||
| 68 | 12.61 | 3-O-β-D-Apiofuranosyl(1→2)-β-D-glucopyranosyl Rhamnocitrin 4′-O -β-D-Glucopyranoside | C33H40O20 | 755.2043 | 755.2035 | 0.8 | 593.1512 | √ | ||
| 69 | 13.34 | 3,5-diphenylphenol | C18H14O | 245.0934 | 245.0966 | −3.2 | 203.0821; 135.0442 | √ | √ | √ |
| 70 | 14.22 | Verbascoside | C29H36O15 | 623.1973 | 623.1976 | −0.3 | 461.1648; 194.9418; 161.0241 | √ | ||
| 71 | 15.25 | 1-O-[(5-O-syringoyl)-β-D-apiofuranosyl]-(1→2)-β-D-glucopyranosie | C28H34O17 | 641.1713 | 641.1718 | −0.5 | 479.1167; 194.9416; 167.0342 | √ | ||
| 72 | 15.32 | Lamiuside C | C35H46O20 | 785.2505 | 785.2504 | 0.1 | 771.1986; 194.9423; 161.0239 | √ | ||
| 73 | 19.61 | (E)-2-({[2-(1,3-dioxan-2-yl) phenyl]imino}methyl)phenol |
C17H17NO3 | 282.1133 | 282.113 | 0.3 | 194.9414 | √ | ||
| 74 | 37.45 | Dihydroxy-3:5:3’:5’-tetra-2”- hydroxybenzyl-diphenylmethane |
C41H36O6 | 623.2391 | 623.2343 | −4.3 | 196.8947 | √ | ||
Figure 6.
Qualitative analysis of phenolic compounds in LB fruits by UPLC-Q-TOF-MS. (A) The Venn diagram of phenolic compounds in LB fruits from different regions. (B) The types of phenolic compounds in LB fruits from different regions.
The main phenolic compounds in LB fruits are phenolic acids and flavonoids, and it is also crucial to learn about their amounts and varieties in these medicinal fruits [27,68]. As shown in Figure 6B, there were 18, 12, and 11 and 11, 13, and 16 phenolic acids and flavonoids in LBN, LBG and LBQ fruits, respectively. LBN was rich in phenolic acids, and LBQ was rich in flavonoids. The amounts of coumarins and phenolic amides in LBG were greater than those in LBQ and LBN.
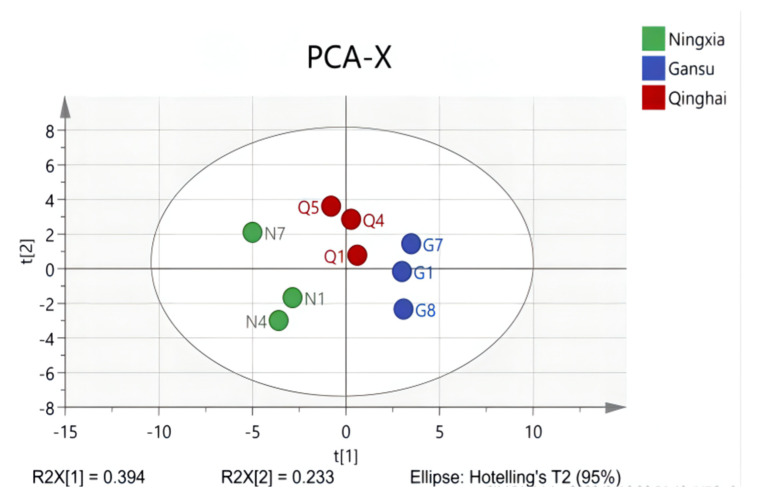
Principal component analysis (PCA) is a mathematical tool that aims to represent the variation present in the dataset using a small number of factors [69]. It is used to identify how one sample differs from another, which variables contribute most to the difference, and whether these variables are correlated [70]. Cossignani et al. found that the geographic origin of goji samples could be discriminated against using PCA for fatty acids and sterol percent compositions [71]. In a recent study by Gong et al., samples of Lycium barbarum L. from the same place could be partially discriminated by PCA using stable isotopes, earth elements, free amino acids, and saccharides [72]. To obtain the overall characteristics and similarities of phenolic compounds in LB fruits from three different regions, a PCA test based on identified 74 phenolic compounds was performed in this study. The two main principal components accounted for approximately 62.7% of the total variance. The results showed that in the PCA model (Figure 7), the LB fruits could be differentiated into three groups which contained LBN, LBQ, and LBG respectively.
Figure 7.
The PCA−X plot of LB fruits from different regions on the basis of 74 phenolic compounds identified by UPLC-Q-TOF-MS.
In a study from Poland [73], it was observed that Goji fruit (Lycium barbarum L.) from China showed a wide variety of available phenolic acids using chromatographic analysis (LC-ESI-MS/MS). Phenolic acids, coumaric, isoferulic, and caffeic acids, and their derivatives, were found to be the dominant ones of Lycium barbarum cultivated in Greece [74]. Phenolic acids were determined as the most abundant compounds of Lycium barbarum L. cultivated in Italy, followed by flavanols [75]. There were significant differences in the numbers and types of phenolics in LB fruits from three different regions in China, indicating that regions were important factors in the quality of LB fruits. The results also showed that LB fruits were abundant in phenolic compounds and had great potential as natural functional foods and nutritional pharmaceutic.
3. Materials and Methods
3.1. Materials and Chemicals
LB fruits (Cultivar: Ningqi 7) from three different regions (15 batches of Ningxia, 15 batches of Gansu and 8 batches of Qinghai) in China were collected at harvest from places of origin (Ningxia: 35°14′–39°23′N, 104°17′–107°39′ E, Gansu: 32°31′–42°57′ N, 92°13′–108°46′ E, Qinghai: 31°4′–39°19′ N, 89°35′–103°03′ E). The samples were preserved at −40 °C and then freeze-dried under vacuum. Whole LB fruits including LBN, LBG, and LBQ were ground into a fine powder and stored at −40 °C.
Chlorogenic acid (≥96.1%), caffeic acid (≥99.7%), 4-hydroxycinnamic acid (≥99.7%), scopoletin (≥99.7%), ferulic acid (≥99.4%), kaempferol-3-O-rutinoside (≥94.0%) and rutin (≥91.6%) were obtained from the National Institutes for Food and Drug Control (Beijing, China). Narcissoside (≥98%) was purchased from Shenzhen Botaier Biotechnology Company (Shenzhen, China). HPLC-grade methanol and formic acid were purchased from Fisher and Roe Scientific Inc. Pure water was obtained from Wahaha Group Co., Ltd. (Hangzhou, China).
3.2. The Appearance Character of LB Fruits
The main physical characteristics of LB fruits from three different regions in China were observed. Due to the limited quantity of some sample batches, three batches of samples were selected for each region because of their abundant quantities. The color and shape of LB fruits were recorded, and the values of length and diameter were analyzed.
3.3. Extraction of Phenolic Compounds
Briefly, 1.5 g of dried powder of LB fruits was extracted with 10 mL of methanol/water solution (80:20, v/v) and subjected to ultrasound-assisted extraction for 30 min. The supernatant was obtained after filtration, and then the solutions were filtered through 0.22 μm microporous membranes and stored at −40 °C. Each sample was analyzed in duplicate. The filtrates were concentrated in a rotary evaporator at 45 °C and dried by vacuum freeze-drying for FTIR analysis. All 38 batches of samples were extracted and further analyzed for 3.4, 3.5, and 3.6.
3.4. Determination of the Total Phenolic Content
The TPC content of the extracts was determined by the Folin–Ciocalteu method [76], with slight modifications. A total of 1 mL of diluted extract was transferred to a 25 mL volumetric flask and mixed with 1 mL of Folin–Ciocalteu reagent and 2 mL of sodium carbonate (1 mol/L). Then, the solution was diluted with pure water to volume. Subsequently, the mixtures were incubated in darkness for 1 h. The absorbance was measured utilizing a UV-vis spectrophotometer (T6, Persee) at 760 nm against a blank. Each sample was tested in triplicate. Gallic acid was used as a standard to prepare the calibration curve. The results were expressed as milligram equivalents of gallic acid per 100 g (mg GAE·100 g−1) dry weight.
3.5. FTIR-ATR Analysis
The FTIR spectra were recorded on an FTIR spectrometer (PerkinElmer Frontier) equipped with a ZnSe crystal cell for attenuated total reflection (ATR) operation. The spectra were acquired (three scans per sample) in the midinfrared region of 4000–550 cm−1 at a resolution of 4 cm−1.
3.6. Analysis of Phenolic Composition by HPLC-QAMS
3.6.1. Investigation of the Instrumental Conditions
HPLC analysis was performed on a Shimadzu HPLC-DAD (SIL-20A, SPD-M20A, CTO-20A) system and a Waters 2695 system, by using a Shimadzu GIST C18-AQ column (4.6 mm × 250 mm, 5 µm), a Welch Ulimate® AQ-C18 column (4.6 mm × 250 mm, 5 µm), and a Kromasil column (4.6 mm × 250 mm, 5 µm). The mobile phases were A (methanol) and B (0.5% formic acid) at a flow rate of 1.0 mL/min. The gradient elution was as follows: 0–10 min, 2–20% A; 10–55 min, 20–25% A; 55–80 min, 25–30% A; 80–90 min, 30–40% A; 90–100 min, 40–45% A; and 100–110 min, 45–50% A. The injection volume was 20 μL. The column temperature was 35 °C, and the detection wavelength was 360 nm.
3.6.2. Method Validation and Calculation of the Relative Correction Factor
Eight standards, including chlorogenic acid, caffeic acid, 4-hydroxycinnamic acid, scopoletin, ferulic acid, rutin, kaempferol-3-O-rutinoside and narcissoside, were prepared by dissolving them in methanol. The mixed standard solution was diluted to different concentrations and stored at 4 °C. The HPLC-QAMS method was validated in terms of precision, stability, reproducibility, linearity, LOD, LOQ and recovery. The linearity was established with the peak areas of six different concentrations for each phenolic compound. The LOD and LOQ were calculated at the signal-to-noise ratio of 3:1 and 10:1, respectively. The inter-day precision was evaluated by RSD under six repeated injections, which was assessed by repeatability. The repeatability was determined by analyzing six prepared repeated samples from the same batch. Recovery tests were measured by spiking six samples with known content which were determined in the repeatability tests from the same batch, with known amounts of each analyte. Scopoletin was applied as the internal reference (IR). The RCF was calculated according to the following formulas:
| fs/i = (As × Ci)/(Ai × Cs) |
where As and Cs represented the peak areas and concentrations of the IR, respectively, and Ai and Ci represented the peak areas and concentrations of analytes, respectively.
3.7. Qualitative Analysis of Phenolic Compounds by UHPLC-Q-TOF-MS
A total of 9 batches of samples were determined, which were the same as 2.1. Separation was performed on an Acquity UPLC BEH C18 column (2.1 mm×100 mm, 1.7 μm, Waters) using an Acquity UPLC system (Waters) with a column temperature of 35 °C. A volume of 2 μL was injected at a flow rate of 0.3 mL/min. The mobile phases were 0.1% formic acid (A) and methanol (B), and the gradient program was as follows: 0–0.5 min, 5% B; 0.5–2.0 min, 5–10% B; 2.0–4.5 min, 10–15% B; 4.5–7.0 min, 15–20% B; 7.0–20.0 min, 20–25% B; 20.0–32.0 min, 25–30% B; 32.0–35.0 min, 30–40% B; and 35.0–40.0 min, 40–50% B.
Analysis was performed on a UHPLC-Q-TOF-MSE (Waters) system equipped with a Xevo G2-S Q-TOF mass spectrometer, a lock-spray interface and an electrospray ionization (ESI) source operated in both positive and negative ionization modes. The capillary and cone voltages were 2.2 kV and 40 V, respectively. The temperature of the ionization source was 120 °C, and the ion collision energy was 20–50 eV. The mass range was set at 100–1800 m/z. The data were collected and processed by MassLynx4.1 software.
3.8. Statistical Analysis
All results were expressed as the mean, standard deviation (SD) and relative standard deviation (RSD). The data were analyzed using Microsoft Excel 2016, Origin 2021, SPSS Statistics 17.0, and SIMCA 14.1. The heatmap was generated using a bioinformatics network (http://www.bioinformatics.com.cn, accessed on 7 April 2022).
4. Conclusions
In this study, multiple analytical methods were applied to reveal the differences between LB fruits from the three different regions. While LB fruits from Qinghai were the largest in the size, the highest TPC content was observed in LBN. In the FTIR spectra, a similar trend was found in LBN and LBG, whereas there was a special peak in LBQ. It was shown that rutin was the main constituent in LB fruits with a new HPLC-QAMS method, especially in LBQ. The distribution of phenolic compounds was determined by UHPLC-Q-TOF-MS analysis. The most amounts of phenolic acids were in LBN, flavonoids in LBQ, and coumarins in LBG. In our study, the qualitative and quantitative phenolic compound profiles of LB fruits from different regions in China were established. The results will be helpful for the quality control and evaluation of LB fruits and their by-products as functional foods.
Abbreviations
| LB | Lycium barbarum L. |
| TPC | total phenolic compound |
| LBN | Lycium barbarum L. fruits from Ningxia |
| LBG | Lycium barbarum L. fruits from Gansu |
| LBQ | Lycium barbarum L. fruits from Qinghai |
| QAMS | quantitative analysis of multiple components by a single marker |
| UV-vis | ultraviolet-visible spectroscopy |
| LOD | limit of detection |
| LOQ | limit of quantification |
| RSD | relative standard deviations |
| RCF | relative correction factor |
| ATR | attenuated total reflection |
| IR | internal reference |
| PCA | principal component analysis |
Author Contributions
Data curation and analysis: W.D. and F.T. Methodology: W.D., R.L., J.Y. and Z.Z. Resources and Software: W.D., J.Z., D.Z., D.Q., Y.Z. and F.C. Roles/Writing—original draft: W.D., Z.Z. and J.Y. Writing—review & editing: W.D. and R.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by grants from the Scientific and Technological Innovation Project of the China Academy of Chinese Medical Sciences (No. CI 2021A03707), and the Key Research and Development Project of Ningxia Hui Autonomous Region (No. 2020BFG02016).
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chang R.C.-C., So K.-F. Use of Anti-aging Herbal Medicine, Lycium barbarum, against Aging-associated Diseases. What Do We Know So Far? Cell. Mol. Neurobiol. 2008;28:643–652. doi: 10.1007/s10571-007-9181-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao R., Heinrich M., Weckerle C.S. The genus Lycium as food and medicine: A botanical, ethnobotanical and historical review. J. Ethnopharmacol. 2018;212:50–66. doi: 10.1016/j.jep.2017.10.010. [DOI] [PubMed] [Google Scholar]
- 3.Yimin W., Baolin Z. Species Resources of Lycium L. in China and Their Development Strategies. World For. Res. 2021;34:107–111. [Google Scholar]
- 4.Chinese Pharmacopoeia Commission . Chinese Pharmacopoeia (Volume 1) China Medical Science Press; Beijing, China: 2020. pp. 128–260. [Google Scholar]
- 5.Ding Y., Yan Y., Chen D., Ran L., Mi J., Lu L., Jing B., Li X., Zeng X., Cao Y. Modulating effects of polysaccharides from the fruits of Lycium barbarum on the immune response and gut microbiota in cyclophosphamide-treated mice. Food Funct. 2019;10:3671–3683. doi: 10.1039/C9FO00638A. [DOI] [PubMed] [Google Scholar]
- 6.Zhang Z., Zhou Y., Fan H., Billy K.J., Zhao Y., Zhan X., Yang L., Jia Y. Effects of Lycium barbarum Polysaccharides on Health and Aging of C. elegans Depend on daf-12/daf-16. Oxidative Med. Cell. Longev. 2019;2019:6379493. doi: 10.1155/2019/6379493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang W., Yue Z. RETRACTED: Lycium barbarum polysaccharides promote osteoblasts viability by regulating microRNA-17/PTEN. Life Sci. 2019;225:72–78. doi: 10.1016/j.lfs.2019.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Zheng Y., Pang X., Zhu X., Meng Z., Chen X., Zhang J., Ding Q., Li Q., Dou G., Ma B. Lycium barbarum mitigates radiation injury via regulation of the immune function, gut microbiota, and related metabolites. Biomed. Pharmacother. 2021;139:111654. doi: 10.1016/j.biopha.2021.111654. [DOI] [PubMed] [Google Scholar]
- 9.Sun X., Lv Y., Huang L., Gao H., Ren C., Li J., Bie M., Li W., Koike K., So K.-F., et al. Pro-inflammatory cytokines serve as communicating molecules between the liver and brain for hepatic encephalopathy pathogenesis and Lycium barbarum polysaccharides protection. J. Ethnopharmacol. 2020;248:112357. doi: 10.1016/j.jep.2019.112357. [DOI] [PubMed] [Google Scholar]
- 10.Tang L., Bao S., Du Y., Jiang Z., Wuliji A., Ren X., Zhang C., Chu H., Kong L., Ma H. Antioxidant effects of Lycium barbarum polysaccharides on photoreceptor degeneration in the light-exposed mouse retina. Biomed. Pharmacother. 2018;103:829–837. doi: 10.1016/j.biopha.2018.04.104. [DOI] [PubMed] [Google Scholar]
- 11.Liu W., Xia M., Bai J., Yang L., Wang Z., Wang R., Shi Y. Chemical characterization and 5α-reductase inhibitory activity of phenolic compounds in goji berries. J. Pharm. Biomed. Anal. 2021;201:114119. doi: 10.1016/j.jpba.2021.114119. [DOI] [PubMed] [Google Scholar]
- 12.Chan H.H.-L., Lam H.-I., Choi K.-Y., Li S.Z.-C., Lakshmanan Y., Yu W.-Y., Chang R.C.-C., Lai J.S.-M., So K.-F. Delay of cone degeneration in retinitis pigmentosa using a 12-month treatment with Lycium barbarum supplement. J. Ethnopharmacol. 2019;236:336–344. doi: 10.1016/j.jep.2019.03.023. [DOI] [PubMed] [Google Scholar]
- 13.Mocan A., Moldovan C., Zengin G., Bender O., Locatelli M., Simirgiotis M., Atalay A., Vodnar D.C., Rohn S., Crișan G. UHPLC-QTOF-MS analysis of bioactive constituents from two Romanian Goji (Lycium barbarum L.) berries cultivars and their antioxidant, enzyme inhibitory, and real-time cytotoxicological evaluation. Food Chem. Toxicol. 2018;115:414–424. doi: 10.1016/j.fct.2018.01.054. [DOI] [PubMed] [Google Scholar]
- 14.Wang S., Suh J.H., Hung W.-L., Zheng X., Wang Y., Chi-Tang H. Use of UHPLC-TripleQ with synthetic standards to profile anti-inflammatory hydroxycinnamic acid amides in root barks and leaves of Lycium barbarum. J. Food Drug Anal. 2018;26:572–582. doi: 10.1016/j.jfda.2017.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao X.-Q., Guo S., Lu Y.-Y., Hua Y., Zhang F., Yan H., Shang E.-X., Wang H.-Q., Zhang W.-H., Duan J.-A. Lycium barbarum L. leaves ameliorate type 2 diabetes in rats by modulating metabolic profiles and gut microbiota composition. Biomed. Pharmacother. 2020;121:109559. doi: 10.1016/j.biopha.2019.109559. [DOI] [PubMed] [Google Scholar]
- 16.Gong G., Liu Q., Deng Y., Dang T., Dai W., Liu T., Liu Y., Sun J., Wang L., Liu Y., et al. Arabinogalactan derived from Lycium barbarum fruit inhibits cancer cell growth via cell cycle arrest and apoptosis. Int. J. Biol. Macromol. 2020;149:639–650. doi: 10.1016/j.ijbiomac.2020.01.251. [DOI] [PubMed] [Google Scholar]
- 17.Xie M., Tao W., Wu F., Wu K., Huang X., Ling G., Zhao C., Lv Q., Wang Q., Zhou X., et al. Anti-hypertensive and cardioprotective activities of traditional Chinese medicine-derived polysaccharides: A review. Int. J. Biol. Macromol. 2021;185:917–934. doi: 10.1016/j.ijbiomac.2021.07.008. [DOI] [PubMed] [Google Scholar]
- 18.Chen D., Guo S., Zhou J., Zhu Y., Zhang F., Zeng F., Duan R., Xu M., Duan J.-A. Chemical constituents from Lycium barbarum (Solanaceae) and their chemophenetic significance. Biochem. Syst. Ecol. 2021;97:104292. doi: 10.1016/j.bse.2021.104292. [DOI] [Google Scholar]
- 19.Li G., You J., Suo Y., Song C., Sun Z., Xia L., Zhao X., Shi J. A developed pre-column derivatization method for the determination of free fatty acids in edible oils by reversed-phase HPLC with fluorescence detection and its application to Lycium barbarum seed oil. Food Chem. 2011;125:1365–1372. doi: 10.1016/j.foodchem.2010.10.007. [DOI] [Google Scholar]
- 20.Guo M., Shi T., Duan Y., Zhu J., Li J., Cao Y. Investigation of amino acids in wolfberry fruit (Lycium barbarum) by solid-phase extraction and liquid chromatography with precolumn derivatization. J. Food Compos. Anal. 2015;42:84–90. doi: 10.1016/j.jfca.2015.03.004. [DOI] [Google Scholar]
- 21.Kan X., Yan Y., Ran L., Lu L., Mi J., Zhang Z., Li X., Zeng X., Cao Y. Ultrasonic-assisted extraction and high-speed counter-current chromatography purification of zeaxanthin dipalmitate from the fruits of Lycium barbarum L. Food Chem. 2020;310:125854. doi: 10.1016/j.foodchem.2019.125854. [DOI] [PubMed] [Google Scholar]
- 22.Khoddami A., Wilkes M.A., Roberts T.H. Techniques for Analysis of Plant Phenolic Compounds. Molecules. 2013;18:2328–2375. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chirinos R., Betalleluz-Pallardel I., Huamán A., Arbizu C., Pedreschi R., Campos D. HPLC-DAD characterisation of phenolic compounds from Andean oca (Oxalis tuberosa Mol.) tubers and their contribution to the antioxidant capacity. Food Chem. 2009;113:1243–1251. doi: 10.1016/j.foodchem.2008.08.015. [DOI] [Google Scholar]
- 24.Tungmunnithum D., Thongboonyou A., Pholboon A., Yangsabai A. Flavonoids and Other Phenolic Compounds from Medicinal Plants for Pharmaceutical and Medical Aspects: An Overview. Medicines. 2018;5:93. doi: 10.3390/medicines5030093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qi W., Huiru T. Composition differences of polyphenolic compounds in fruits of Lycium ruthenicum Murr. and Lycium barbarum L. J. Shanghai Jiao Tong Univ. (Med.Sci.) 2019;39:1–8. doi: 10.3969/j.issn.1674-8115.2019.01.008. [DOI] [Google Scholar]
- 26.Chunxia Y. Determination of Nine Kinds of Phenolic Acids in Lycium barbarum L. Using High-performance Liquid Chromatography. Food Res. Dev. 2021;42:148–153. [Google Scholar]
- 27.Magiera S., Zaręba M. Chromatographic Determination of Phenolic Acids and Flavonoids in Lycium barbarum L. and Evaluation of Antioxidant Activity. Food Anal. Methods. 2015;8:2665–2674. doi: 10.1007/s12161-015-0166-y. [DOI] [Google Scholar]
- 28.Su C., Li C., Sun K., Li W., Liu R. Quantitative analysis of bioactive components in walnut leaves by UHPLC-Q-Orbitrap HRMS combined with QAMS. Food Chem. 2020;331:127180. doi: 10.1016/j.foodchem.2020.127180. [DOI] [PubMed] [Google Scholar]
- 29.Zhimin W., Zhongzhi Q., Qiwei Z., Jingjing Z., Huimin G., Zhengtao W. A technical guideline of the establishment of quantitative analysis of multi-components by singlemarker. China J. Chin. Mater. Med. 2011;36:657–658. [Google Scholar]
- 30.Dong Y., Guo Q., Liu J., Ma X. Simultaneous determination of seven phenylethanoid glycosides in Cistanches Herba by a single marker using a new calculation of relative correction factor. J. Sep. Sci. 2018;41:1913–1922. doi: 10.1002/jssc.201701219. [DOI] [PubMed] [Google Scholar]
- 31.Ren R., Li Y., Chen H., Wang Y., Yang L., Su C., Zhao X., Chen J., Ma X. Carotenoid Contents of Lycium barbarum: A Novel QAMS Analyses, Geographical Origins Discriminant Evaluation, and Storage Stability Assessment. Molecules. 2021;26:5374. doi: 10.3390/molecules26175374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chunsheng Z., Xiaoping L., Bing Z., Zhijian L. Quantitative analysis of multi-components by single marker—A rational method for the internal quality of Chinese herbal medicine. Integr. Med. Res. 2017;6:1–11. doi: 10.1016/j.imr.2017.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang Y., Liang X., Li Y., Fan Y., Li Y., Cao Y., An W., Shi Z., Zhao J., Guo S. Changes in Metabolome and Nutritional Quality of Lycium barbarum Fruits from Three Typical Growing Areas of China as Revealed by Widely Targeted Metabolomics. Metabolites. 2020;10:46. doi: 10.3390/metabo10020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nzeuwa I.B.Y., Guo B., Zhang T., Wang L., Ji Q., Xia H., Sun G. Comparative Metabolic Profiling of Lycium Fruits (Lycium barbarum and Lycium chinense) from Different Areas in China and from Nepal. J. Food Qual. 2019;2019:4396027. doi: 10.1155/2019/4396027. [DOI] [Google Scholar]
- 35.Lu Y., Guo S., Zhang F., Yan H., Qian D.-W., Wang H.-Q., Jin L., Duan J.-A. Comparison of Functional Components and Antioxidant Activity of Lycium barbarum L. Fruits from Different Regions in China. Molecules. 2019;24:2228. doi: 10.3390/molecules24122228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Y., Jin H., Dong X., Yang S., Ma S., Ni J. Quality evaluation of Lycium barbarum (wolfberry) from different regions in China based on polysaccharide structure, yield and bioactivities. Chin. Med. 2019;14:49. doi: 10.1186/s13020-019-0273-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhang Q., Chen W., Zhao J., Xi W. Functional constituents and antioxidant activities of eight Chinese native goji genotypes. Food Chem. 2016;200:230–236. doi: 10.1016/j.foodchem.2016.01.046. [DOI] [PubMed] [Google Scholar]
- 38.Sandra D.S., Rodrigo P.F., Luís V.B., Maria R.B. Application of FTIR-ATR to Moscatel dessert wines for prediction of total phenolic and flavonoid contents and antioxidant capacity. Food Chem. 2014;150:489–493. doi: 10.1016/j.foodchem.2013.11.028. [DOI] [PubMed] [Google Scholar]
- 39.Lucarini M., Durazzo A., Kiefer J., Santini A., Lombardi-Boccia G., Souto E.B., Romani A., Lampe A., Nicoli S.F., Gabrielli P., et al. Grape Seeds: Chromatographic Profile of Fatty Acids and Phenolic Compounds and Qualitative Analysis by FTIR-ATR Spectroscopy. Foods. 2020;9:10. doi: 10.3390/foods9010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kennedy J.A., Troup G.J., Pilbrow J.R., Hutton D.R., Hewitt D., Hunter C.R., Ristic R., Iland P.G., Jones G.P. Development of seed polyphenols in berries from Vitis vinifera L. cv. Shiraz. Aust. J. Grape Wine Res. 2000;6:244–254. doi: 10.1111/j.1755-0238.2000.tb00185.x. [DOI] [Google Scholar]
- 41.Li W., Zhao Z., Hu W., Cheng Q., Yang L., Hu Z., Liu Y.A., Wen K., Yang H. Design of Thiazolo[5,4-d]thiazole-Bridged Ionic Covalent Organic Polymer for Highly Selective Oxygen Reduction to H2O2. Chem. Mater. 2020;32:8553–8560. doi: 10.1021/acs.chemmater.0c02843. [DOI] [Google Scholar]
- 42.Leela J.S.P.P., Hemamalini R., Muthu S., Al-Saadi A.A. Spectroscopic investigation (FTIR spectrum), NBO, HOMO–LUMO energies, NLO and thermodynamic properties of 8-Methyl-N-vanillyl-6-nonenamideby DFT methods. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015;146:177–186. doi: 10.1016/j.saa.2015.03.027. [DOI] [PubMed] [Google Scholar]
- 43.Sun B., Lin Y., Wu P., Siesler H.W. A FTIR and 2D-IR Spectroscopic Study on the Microdynamics Phase Separation Mechanism of the Poly(N-isopropylacrylamide) Aqueous Solution. Macromolecules. 2008;41:1512–1520. doi: 10.1021/ma702062h. [DOI] [Google Scholar]
- 44.Peng X., Sun S.Q., Zhao Z.Z., Leung H.W. Structural characterization of the glycan part of glycoconjugate LbGp2 from Lycium barbarum L. Carbohydr. Res. 2001;331:95–99. doi: 10.1016/S0008-6215(00)00321-9. [DOI] [PubMed] [Google Scholar]
- 45.Huan C., Ling M., Xiaojuan S., Yanting L., Xia M., Xueqin M. Establishment of quantitative analysis of mul-ti-components by singer marker for content determination of 4 carotenoids in Lycium barbarum. China Pharm. 2022;33:957–961. [Google Scholar]
- 46.Ishmetova R.I., Ignatenko N.K., Belyaninova I.A., Tolshchina S., Korotina A.V., Slepukhin P.A., Evstigneeva N.P., Zil’Berberg N.V., Amineva P.G., Kungurov N.V., et al. Synthesis and antifungal activity of 3-substituted imidazo[1,2-b][1,2,4,5]tetrazines. Bull. Acad. Sci. USSR Div. Chem. Sci. 2015;64:2100–2105. doi: 10.1007/s11172-015-1124-y. [DOI] [Google Scholar]
- 47.Aziz B., Skender D., Bahrije D., Ilir S. Synthesis, Characterization and Antibacterial Studies of 2-Amino-5-{4-(4-amino-4-carboxy-butylamino)-3-[1-(2-hydroxy -phenylimino)-ethyl]-2-oxo-2H-chromen-7-ylamino}-pentanoic acid and its Metal (II) Complexes. Int. J. Pharm. Sci. Rev. Res. 2013;20:1–10. [Google Scholar]
- 48.Barakat A., Al-Majid A.M., Mabkhot Y.N., Ghabbour H.A., Fun H.-K. Crystal structure of (E)-3-mesityl-1-phenylprop-2-en-1-one, C18H18O. Z. Kristallogr. NCS. 2014;229:235–236. doi: 10.1515/ncrs-2014-0119. [DOI] [Google Scholar]
- 49.Sun X., Tang J., Hu W., Xu N. Antioxidant Flavonol Compounds from the Marine Cordgrass Spartina anglica. Food Sci. Technol. Res. 2013;19:1093–1097. doi: 10.3136/fstr.19.1093. [DOI] [Google Scholar]
- 50.Kamoto T., Xinwen L., Yoshino J., Hayashi N. Preparation of solid solution and crystal-glass composite consisting of stable phenoxyl radical and its phenol analogue. Arkivoc. 2020;2020:58–69. doi: 10.24820/ark.5550190.p011.346. [DOI] [Google Scholar]
- 51.Tsutomu W., Yoshimi N., Tadataka N. Constituents from the bark of Tabebuia impetiginosa. Phytochemistry. 2004;65:2003–2011. doi: 10.1016/j.phytochem.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 52.Le T.N., Cho W.-J. Total Synthesis of Oxyfagaronine, Phenolic Benzo[c]phenanthridine and General Synthetic Way of 2,3,7,8- and 2,3,8,9-Tetrasubstituted Benzo[c]phenanthridine Alkaloids. Chem. Pharm. Bull. 2006;54:476–480. doi: 10.1248/cpb.54.476. [DOI] [PubMed] [Google Scholar]
- 53.Ito N., Nihei T., Kakuda R., Yaoita Y., Kikuchi M. Five New Phenylethanoid Glycosides from the Whole Plants of Lamium purpureum L. Chem. Pharm. Bull. 2006;54:1705–1708. doi: 10.1248/cpb.54.1705. [DOI] [PubMed] [Google Scholar]
- 54.Yoshikawa M., Sugimoto S., Nakamura S., Matsuda H. Medicinal Flowers. XXII. Structures of Chakasaponins V and VI, Chakanoside I, and Chakaflavonoside A from Flower Buds of Chinese Tea Plant (Camellia sinensis) Chem. Pharm. Bull. 2008;56:1297–1303. doi: 10.1248/cpb.56.1297. [DOI] [PubMed] [Google Scholar]
- 55.Zhou Z.-Q., Fan H.-X., He R.-R., Sun W.-Y., Xiao J., Bao X.-F., So K.-F., Yao X.-S., Gao H. China Four New Dicaffeoylspermidine Derivatives From Lycium barbarum. World J. Tradit. Chin. Med. 2016;2:1–5. doi: 10.15806/j.issn.2311-8571.2016.0028. [DOI] [Google Scholar]
- 56.Qunqun Z., Xin D., Xinguang L., Wen G., Ping L., Hua Y. Rapid separation and identification of multiple constituents in Danhong Injection by ultra-high performance liquid chromatography coupled to electrospray ionization quadrupole time-of-flight tandem mass spectrometry. Chin. J. Nat. Med. 2016;14:147–160. doi: 10.15806/10.3724/SP.J.1009.2016.00147. [DOI] [PubMed] [Google Scholar]
- 57.Huo F.-J., Yin C.-X., Jin X.-L., Yang P. 3,3a-Dihydrocyclopenta[b]chromen-1(2H)-ones from the reaction of salicylaldehyde and 2-cyclopenten-1-one. Acta Crystallogr. Sect. C Cryst. Struct. Commun. 2005;C61:o332–o335. doi: 10.1107/S0108270105008528. [DOI] [PubMed] [Google Scholar]
- 58.Shoja M., Samuel K., Athanasopoulos D. Crystal structure of 6-hydroxyflavanone, C15H12O3. Z. Kristallogr. NCS. 1998;213:373–374. doi: 10.1524/ncrs.1998.213.14.387. [DOI] [Google Scholar]
- 59.Małecka M., Chęcińska L., Kusz J., Biernacka M., Kupcewicz B. Interactions in flavanone and chalcone derivatives: Hirshfeld surface analysis, energy frameworks and global reactivity descriptors. Acta Crystallogr. Sect. C Struct. Chem. 2020;C76:212–224. doi: 10.1107/S2053229620001503. [DOI] [PubMed] [Google Scholar]
- 60.Kramberger K., Barlič-Maganja D., Bandelj D., Arbeiter A.B., Peeters K., Višnjevec A.M., Pražnikar Z.J. HPLC-DAD-ESI-QTOF-MS Determination of Bioactive Compounds and Antioxidant Activity Comparison of the Hydroalcoholic and Water Extracts from Two Helichrysum italicum Species. Metabolites. 2020;10:403. doi: 10.3390/metabo10100403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chenqin Q., Youwang Y., Yang M., Shihua D., Jun T., Hong D. Chemical Composition Analysis and Quality Identification of Gastrodia Tuber in Tibet Based on HPLC-MS. Prog. Mod. Biomed. 2014;14:611–649. doi: 10.13241/j.cnki.pmb.2014.04.003. [DOI] [Google Scholar]
- 62.Sun J., Chen P., Lin L.-Z., Harnly J.M. A Non-targeted Approach to Chemical Discrimination between Green Tea Dietary Supplements and Green Tea Leaves by HPLC/MS. J. AOAC Int. 2011;94:487–497. doi: 10.1093/jaoac/94.2.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Haiyang L., Cencan X., Mengdi G., Fang F., Jixia L., Donghui W., Fengzhong W. Characterization pf Polyphenols from Lycium barbarum L. Fruit by Q-TOF/MSE and its antioxidant activity in Cell Culture Cystems. J. Nucl. Agric. Sci. 2017;31:298–306. doi: 10.11869/j.issn.100-8551.2017.02.0298. [DOI] [Google Scholar]
- 64.Joo Y.-H., Shreeve J.M. Functionalized Tetrazoles from Cyanogen Azide with Secondary Amines. Eur. J. Org. Chem. 2009;2009:3573–3578. doi: 10.1002/ejoc.200900389. [DOI] [Google Scholar]
- 65.Urbain A., Marston A., Marsden-Edwards E., Hostettmann K. Ultra-performance liquid chromatography/time-of-flight mass spectrometry as a chemotaxonomic tool for the analysis of Gentianaceae species. Phytochem. Anal. 2009;20:134–138. doi: 10.1002/pca.1107. [DOI] [PubMed] [Google Scholar]
- 66.Zhao Y.-X., Li C.-S., Luo X.-D., Wang Y.-F., Zhou J. Palaeophytochemical Constituents of Cretaceous Ginkgo coriacea Florin Leaves. J. Integr. Plant Biol. 2006;48:983–990. doi: 10.1111/j.1744-7909.2006.00283.x. [DOI] [Google Scholar]
- 67.Baoliang C., Motoyuki N., Junei K., Toshihiro N. Chemical Constituents of Astragali Semen. Chem. Pharm. Bull. 1993;41:178–182. [Google Scholar]
- 68.Scalbert A., Williamson G. Dietary Intake and Bioavailability of Polyphenols. J. Nutr. 2000;130:2073S–2085S. doi: 10.1093/jn/130.8.2073S. [DOI] [PubMed] [Google Scholar]
- 69.Granato D., Santos J.S., Escher G.B., Ferreira B.L., Maggio R.M. Use of principal component analysis (PCA) and hierarchical cluster analysis (HCA) for multivariate association between bioactive compounds and functional properties in foods: A critical perspective. Trends Food Sci. Technol. 2018;72:83–90. doi: 10.1016/j.tifs.2017.12.006. [DOI] [Google Scholar]
- 70.Soo-Yun P., War T.P., Young C.P., Jung I.J., Sang U.N., Jae K.K. Metabolomics for the quality assessment of Lycium chinense fruits. Biosci. Biotechnol. Biochem. 2012;76:2188–2194. doi: 10.1271/bbb.120453. [DOI] [PubMed] [Google Scholar]
- 71.Cossignani L., Blasi F., Simonetti M.S., Montesano D. Fatty Acids and Phytosterols to Discriminate Geographic Origin of Lycium barbarum Berry. Food Anal. Methods. 2017;11:1180–1188. doi: 10.1007/s12161-017-1098-5. [DOI] [Google Scholar]
- 72.Gong H., Rehman F., Li Z., Liu J., Yang T., Liu J., Li H., Hu Z., Ma Q., Wu Z., et al. Discrimination of Geographical Origins of Wolfberry (Lycium barbarum L.) Fruits Using Stable Isotopes, Earth Elements, Free Amino Acids, and Saccharides. J. Agric. Food Chem. 2022;70:2984–2997. doi: 10.1021/acs.jafc.1c06207. [DOI] [PubMed] [Google Scholar]
- 73.Olech M., Kasprzak K., Wójtowicz A., Oniszczuk T., Nowak R., Waksmundzka-Hajnos M., Combrzyński M., Gancarz M., Kowalska I., Krajewska A., et al. Polyphenol Composition and Antioxidant Potential of Instant Gruels Enriched with Lycium barbarum L. Fruit. Molecules. 2020;25:4538. doi: 10.3390/molecules25194538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Benchennouf A., Grigorakis S., Loupassaki S., Kokkalou E. Phytochemical analysis and antioxidant activity of Lycium barbarum (Goji) cultivated in Greece. Pharm. Biol. 2016;55:596–602. doi: 10.1080/13880209.2016.1265987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Montesano D., Rocchetti G., Cossignani L., Lucini L., Simonetti M.S., Blasia F. Italian Lycium barbarum L. Berry: Chemical Characterization and Nutraceutical Value. Nat. Prod. Commun. 2018;13:1934578X1801300. doi: 10.1177/1934578X1801300913. [DOI] [Google Scholar]
- 76.John B., Sulaiman C.T., Satheesh G., Reddy VR K. Total phenolics and flavonoids in selected medicinal plants from Kerala. Int. J. Pharm. Pharm. Sci. 2014;6:406–408. [Google Scholar]