Abstract
The molecular evolution of the leukotoxin structural gene (lktA) of Mannheimia (Pasteurella) haemolytica was investigated by nucleotide sequence comparison of lktA in 31 bovine and ovine strains representing the various evolutionary lineages and serotypes of the species. Eight major allelic variants (1.4 to 15.7% nucleotide divergence) were identified; these have mosaic structures of varying degrees of complexity reflecting a history of horizontal gene transfer and extensive intragenic recombination. The presence of identical alleles in strains of different genetic backgrounds suggests that assortative (entire gene) recombination has also contributed to strain diversification in M. haemolytica. Five allelic variants occur only in ovine strains and consist of recombinant segments derived from as many as four different sources. Four of these alleles consist of DNA (52.8 to 96.7%) derived from the lktA gene of the two related species Mannheimia glucosida and Pasteurella trehalosi, and four contain recombinant segments derived from an allele that is associated exclusively with bovine or bovine-like serotype A2 strains. The two major lineages of ovine serotype A2 strains possess lktA alleles that have very different evolutionary histories and encode divergent leukotoxins (5.3% amino acid divergence), but both contain segments derived from the bovine allele. Homologous segments of donor and recipient alleles are identical or nearly identical, indicating that the recombination events are relatively recent and probably postdate the domestication of cattle and sheep. Our findings suggest that host switching of bovine strains from cattle to sheep, together with inter- and intraspecies recombinational exchanges, has played an important role in generating leukotoxin diversity in ovine strains. In contrast, there is limited allelic diversity of lktA in bovine strains, suggesting that transmission of strains from sheep to cattle has been less important in leukotoxin evolution.
Mannheimia haemolytica is the etiological agent of bovine and ovine pneumonic pasteurellosis, a disease that causes considerable economic losses to the cattle and sheep industries (17, 20). Capsular serotyping provides the primary basis for the classification of strains and epidemiological typing of M. haemolytica, which has traditionally been subdivided into 13 serotypes (1, 49). The association of different serotypes with infections of cattle and sheep (17, 20) suggests that serotype-related strain differences occur in host specificity and virulence. For example, serotype A1 and A6 strains account for almost all cases of bovine pneumonic pasteurellosis, whereas serotype A2 and A7 isolates are the major causes of disease in sheep. It has also been shown that bovine and ovine isolates of the same serotype, e.g., A1, A2, or A6, can be distinguished by differences in chromosomal genotype (13) or outer membrane protein (OMP) profiles (10). The inference is that natural populations of M. haemolytica consist of distinct evolutionary lineages that are differentially adapted to either cattle or sheep (13).
Leukotoxin is a key virulence factor in the pathogenesis of pneumonic pasteurellosis (6, 32, 35, 46, 47). It is a member of the RTX (repeats in toxin) family of gram-negative bacterial cytotoxins, which includes the alpha-hemolysin of Escherichia coli (30, 45). Most RTX toxins interact with different cell types from a variety of species, but the cytotoxins produced by M. haemolytica, as well as those from Actinobacillus actinomycetemcomitans and Actinobacillus pleuropneumoniae (ApxIIIA), have both cell type- and species-specific effects. The leukotoxin of M. haemolytica interacts only with the alveolar macrophages, neutrophils, and lymphocytes of ruminants and is believed to promote bacterial proliferation by killing or incapacitating these cells (4, 7, 23, 40). It has been postulated that leukotoxin target cell specificity underlies the host specificity of M. haemolytica infections, and it has recently been demonstrated that β2 integrins are the putative leukotoxin receptors (2, 22, 28). Restriction endonuclease analysis (5), together with studies on the neutralizing activity of monoclonal antibodies (19), has demonstrated interserotypic variation of the M. haemolytica leukotoxin determinants, but the significance of allelic diversity for the pathogenesis of pneumonic pasteurellosis and for host specificity is not known.
The purpose of the present study was to investigate nucleotide sequence variation in the M. haemolytica leukotoxins and to determine how this variation relates to differences in virulence and host specialization. Horizontal DNA transfer and recombination are now recognized as important evolutionary mechanisms, complementing mutation, in the diversification of molecules involved in virulence, such as those encoding cell surface structures and other macromolecules for which there is an adaptive advantage in structural diversity (8, 15, 24, 29, 36, 44). Here we used an established framework of evolutionary relationships among strains of M. haemolytica (13) to study the molecular evolution of the leukotoxin (lktA) gene and to determine the role of horizontal DNA transfer and recombination in leukotoxin evolution.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
The complete lktA gene was sequenced in 31 M. haemolytica, 6 Mannheimia glucosida, and 4 Pasteurella trehalosi isolates. M. glucosida represents serotype A11 strains of P. haemolytica, which have recently been reclassified as a separate species (3), and P. trehalosi was recognized as the T biotype of P. haemolytica until its reclassification (43). Partial lktA sequences were obtained for a further 13 serotype A2 M. haemolytica isolates of bovine and ovine origin. The 54 isolates have been well characterized in previous studies (10–14) and were chosen to represent selected evolutionary lineages, serotypes, and hosts of origin. Properties of these isolates are presented in Table 1.
TABLE 1.
Properties of 44 M. haemolytica, 6 M. glucosida, and 4 P. trehalosi isolates
| Isolate | ETa | Capsular serotype | Host species | lktA alleleb | GenBank accession no. |
|---|---|---|---|---|---|
| M. haemolytica | |||||
| PH2 | 1 | A1 | Bovine | lktA1.1 | AF314503 |
| PH30 | 1 | A1 | Bovine | lktA1.1 | |
| PH376 | 1 | A6 | Bovine | lktA1.1 | |
| PH346 | 1 | A12 | Ovine | lktA1.2 | |
| PH540 | 2 | A1 | Bovine | lktA1.1 | |
| PH338 | 3 | A9 | Ovine | lktA1.2 | |
| PH388 | 4 | A7 | Ovine | lktA1.3 | AF314504 |
| PH50 | 5 | A5 | Ovine | lktA1.2 | |
| PH56 | 5 | A8 | Ovine | lktA1.4 | AF314505 |
| PH238 | 5 | A9 | Ovine | lktA1.4 | |
| PH8 | 6 | A1 | Ovine | lktA1.5 | AF314506 |
| PH398 | 7 | A1 | Ovine | lktA1.5 | |
| PH284 | 8 | A6 | Ovine | lktA1.2 | AF314507 |
| PH232 | 9 | A6 | Ovine | lktA1.5 | |
| PH66 | 10 | A14 | Ovine | lktA9 | AF314508 |
| PH706 | 11 | A16 | Ovine | lktA7 | AF314509 |
| PH296 | 12 | A7 | Ovine | lktA8.1 | |
| PH396 | 13 | A7 | Ovine | lktA8.1 | |
| PH484 | 14 | A7 | Ovine | lktA8.1 | |
| PH588 | 15 | A13 | Ovine | lktA6 | AF314510 |
| PH494 | 16 | A2 | Ovine | lktA2.1 | AF314511 |
| PH672 | 16 | A2 | Ovine | lktA2* | |
| PH550 | 17 | A2 | Bovine | lktA2.1 | |
| PH294 | 17 | A2 | Bovine | lktA2* | |
| PH758 | 17 | A2 | Bovine | lktA2* | |
| PH196 | 18 | A2 | Bovine | lktA3 | AF314512 |
| PH786 | 18 | A2 | Bovine | lktA3 | |
| PH526 | 19 | A2 | Ovine | lktA8.1 | |
| PH598 | 20 | A2 | Ovine | lktA8.1 | |
| PH202 | 21 | A2 | Bovine | lktA2.2 | AF314513 |
| PH210 | 21 | A2 | Bovine | lktA2* | |
| PH470 | 21 | A2 | Bovine | lktA2* | |
| PH546 | 21 | A2 | Bovine | lktA2* | |
| PH278 | 21 | A2 | Ovine | lktA10.1 | AF314514 |
| PH372 | 21 | A2 | Ovine | lktA10.1 | |
| PH380 | 21 | A2 | Ovine | lktA10* | |
| PH486 | 21 | A2 | Ovine | lktA10* | |
| PH536 | 21 | A2 | Ovine | lktA10* | |
| PH576 | 21 | A2 | Ovine | lktA10* | |
| PH714 | 21 | A2 | Ovine | lktA10* | |
| PH292 | 22 | A2 | Ovine | lktA8.1 | AF314515 |
| PH392 | 22 | A2 | Ovine | lktA8.2 | AF314516 |
| PH358 | 22 | A2 | Ovine | lktA8* | |
| PH384 | 22 | A2 | Ovine | lktA8* | |
| M. glucosida | |||||
| PH344 | 1 | A11 | Ovine | lktA4.1 | AF314517 |
| PH498 | 3 | A11 | Ovine | lktA4.2 | AF314518 |
| PH240 | 5 | A11 | Ovine | lktA4.3 | AF314519 |
| PH496 | 7 | UG3 | Ovine | lktA4.4 | AF314520 |
| PH574 | 10 | UG3 | Ovine | lktA4.5 | AF314521 |
| PH290 | 16 | UG3 | Ovine | lktA4.6 | AF314522 |
| P. trehalosi | |||||
| PH246 | 2 | T4 | Ovine | lktA5.1 | AF314523 |
| PH252 | 4 | T10 | Ovine | lktA5.2 | AF314524 |
| PH254 | 15 | T15 | Ovine | lktA5.3 | AF314525 |
| PH68 | 19 | T3 | Ovine | lktA5.4 | AF314526 |
Bacteria that had been stored at −70°C in 50% (vol/vol) glycerol in brain heart infusion broth (BHIB) were subcultured on blood agar (brain heart infusion agar containing 5% [vol/vol] sheep's blood) and incubated aerobically overnight at 37°C. For preparation of DNA, a few colonies were inoculated into 5-ml volumes of BHIB and grown overnight at 37°C at 120 rpm.
Preparation of DNA.
Cells from 0.5 ml of overnight cultures were harvested by centrifugation for 1 min at 13,000 × g and washed once in sterile distilled H2O. DNA was prepared with the InstaGene Matrix (Bio-Rad) according to the manufacturer's instructions and stored at −20°C.
PCR amplification and DNA sequence analysis.
M. haemolytica strains have been shown to possess only one lktA gene (5), and a direct PCR approach was adopted. The complete coding and flanking regions of the lktA gene were amplified with a Taq DNA polymerase kit (Boehringer Mannheim) according to the manufacturer's instructions. PCR error rates were shown to be insignificant by the complete sequence identity of duplicate amplifications of the lktA gene in isolate PH278. The lktA gene was amplified from the chromosomal DNA with the 5′ primer lktA9 (5′-TCAAGAAGAGCTGGCAAC-3′) and the 3′ primer lktA7 (5′-AGTGAGGGCAACTAAACC-3′). The primers were designed from the published sequences for the lktA genes of serotype A1 (21, 30) and A11 (5) isolates. Primer lktA9 corresponds to residues 53 to 70 upstream of the lktA initiation codon; primer lktA7 corresponds to residues 105 to 122 downstream of the lktA termination codon. Primers were designed using the Primer Designer (version 2.0) computer program and synthesized with a Beckman Oligo 1000 DNA synthesizer. PCRs were carried out in a Perkin-Elmer 480 DNA thermal cycler using the following amplification parameters: denaturation at 94°C for 45 s, annealing at 62°C for 45 s, and extension at 72°C for 2 min. Thirty cycles were performed, and a final extension step of 72°C for 10 min was used. Production of a PCR amplicon of the expected size (∼3 kbp) was confirmed by agarose gel electrophoresis, and the DNA was purified with a QIAquick PCR purification kit (Qiagen, Chatsworth, Calif.). The DNA was finally eluted in 30 μl of sterile distilled H2O and stored at −20°C. Sequence reactions were performed with the ABI Prism Dye Terminator Cycle Sequencing Kit (Perkin-Elmer), and sequence analysis was performed with an Applied Biosystems 373A DNA Sequencer. Both strands of the lktA gene were sequenced with seven internal pairs of primers designed as sequence data became available.
Analysis of nucleotide and protein sequence data.
Nucleotide sequence data were analyzed and edited with the SEQED (version 1.0.3) computer program (Perkin-Elmer Applied Biosystems). Statistical and phylogenetic analyses were carried out with MEGA (25) in conjunction with alignment programs written by T.S.W. Statistical analyses for clustering of polymorphic sites were carried out by the maximum chi-square method (42) with a program written by T.S.W. Predictions of hydrophilicity, hydrophobicity, antigenic index, and surface probability of protein sequences were performed with the PROTEAN program (DNASTAR Inc.).
Nucleotide sequence accession numbers.
The GenBank accession numbers for the lktA gene sequences obtained in this study are given in Table 1.
RESULTS
Nucleotide and amino acid sequence variation.
The complete nucleotide sequence of the lktA gene was determined for 31 isolates of M. haemolytica representing 12 capsular serotypes and the 22 electrophoretic types (ETs) previously defined by multilocus enzyme electrophoresis (MLEE) (13). The lktA gene was also sequenced for six isolates representing different ETs of M. glucosida (13) and for four isolates that each represent one of the capsular serotypes (T3, T4, T10, and T15) of P. trehalosi (14). Part of the lktA gene was also sequenced for an additional 13 serotype A2 isolates of M. haemolytica recovered from cattle and sheep in order to identify the allele type. In this case, the first 700 nucleotides at both the 5′ and 3′ ends of the gene were analyzed. Properties of these isolates are shown in Table 1.
With the exception of four strains, the lktA genes of the M. haemolytica and M. glucosida isolates were 2,859 bp in length (953 amino acids); the lktA genes of M. haemolytica isolates PH202, PH494, and PH550 were 2,862 bp in length due to an additional amino acid (lysine) at position 885, whereas that of the M. glucosida isolate PH274 was 2,838 bp in length due to the deletion of 7 amino acids at positions 29 to 35. The lktA genes of the four P. trehalosi isolates were 2,865 bp in length (955 amino acids) due to two amino acid insertions between positions 7 and 23. The total aligned length (including gaps) of the 43 sequences was 2,868 nucleotides.
Twenty-four unique lktA sequences, representing distinct alleles, were identified among the 41 isolates for which complete sequences were obtained, but, based on overall sequence similarity, their characteristic mosaic structures (see below), and species of origin, these were assigned to one of 10 groups of allelic variants designated lktA1 to lktA10. M. haemolytica was represented by allelic groups lktA1 to lktA3 and lktA6 to lktA10, M. glucosida by lktA4, and P. trehalosi by lktA5. There were 740 (25.8%) polymorphic nucleotide sites and 177 (18.5%) variable inferred amino acid positions among the 24 sequences. Pairwise differences in nucleotide and inferred amino acid sequences between representative pairs of the 10 lktA allele types of M. haemolytica, M. glucosida, and P. trehalosi ranged from 39 to 485 nucleotide sites (1.4 to 17.0%) and 3 to 122 amino acid positions (0.3 to 12.7%) (Table 2).
TABLE 2.
Percent differences in nucleotide and amino acid sequences between representative pairs of the 10 lktA allele types of M. haemolytica, M. glucosida, and P. trehalosi
| Allele | % Pairwise differences in nucleotide and amino acid sequencesa
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| lktA1.1 | lktA2.1 | lktA3 | lktA4.1 | lktA5.1 | lktA6 | lktA7 | lktA8.1 | lktA9 | lktA10.1 | |
| lktA1.1 | 14.5 | 7.5 | 7.9 | 15.4 | 13.9 | 12.7 | 13.4 | 12.7 | 1.5 | |
| lktA2.1 | 11.6 | 14.6 | 10.9 | 17.0 | 11.8 | 11.4 | 7.0 | 10.1 | 13.0 | |
| lktA3 | 3.4 | 7.8 | 13.0 | 16.8 | 15.7 | 15.4 | 15.4 | 15.4 | 9.0 | |
| lktA4.1 | 6.9 | 6.9 | 9.5 | 16.0 | 8.1 | 6.7 | 8.8 | 6.6 | 7.0 | |
| lktA5.1 | 11.1 | 12.0 | 11.6 | 11.4 | 9.7 | 10.6 | 11.1 | 11.2 | 15.9 | |
| lktA6 | 11.4 | 7.1 | 12.0 | 5.7 | 7.1 | 1.8 | 6.0 | 3.1 | 12.5 | |
| lktA7 | 10.5 | 6.7 | 12.2 | 4.5 | 8.0 | 1.5 | 4.5 | 1.4 | 11.8 | |
| lktA8.1 | 10.8 | 5.4 | 12.7 | 5.4 | 8.1 | 2.8 | 1.4 | 3.1 | 12.4 | |
| lktA9 | 10.5 | 6.4 | 12.4 | 4.5 | 8.1 | 1.8 | 0.3 | 1.0 | 11.8 | |
| lktA10.1 | 1.7 | 10.1 | 5.5 | 5.7 | 11.8 | 9.9 | 9.4 | 9.8 | 9.4 | |
Values on the upper right represent pairwise differences in nucleotide sequences (percent polymorphic nucleotide sites), and values on the lower left represent pairwise differences in inferred amino acid sequences (percent variable amino acids).
Whereas there was a relatively high degree of nucleotide variation between most of the allelic groups lktA1 to lktA10 (Table 2), there was, in contrast, a low degree of variation among alleles representing each group, particularly the M. haemolytica groups. For example, lktA1 was represented by 14 sequences that could be divided into five subgroups, alleles lktA1.1 to lktA1.5, on the basis of variation at just two synonymous and two nonsynonymous sites (nucleotides 993, 1263, 1967, and 2521); lktA2 was represented by alleles lktA2.1 and lktA2.2, which differed at a single synonymous site (nucleotide 729); and lktA8 was represented by alleles lktA8.1 and lktA8.2, which differed at a single nonsynonymous site (nucleotide 425). The lktA gene of the six M. glucosida isolates was represented by alleles lktA4.1 to lktA4.6, which had 58 polymorphic nucleotide sites, and the lktA gene of the four P. trehalosi isolates was represented by alleles lktA5.1 to lktA5.4, which had 20 polymorphic nucleotide sites. Most of the variation in the M. glucosida and P. trehalosi isolates occurred in alleles lktA4.3, lktA4.4, and lktA5.4.
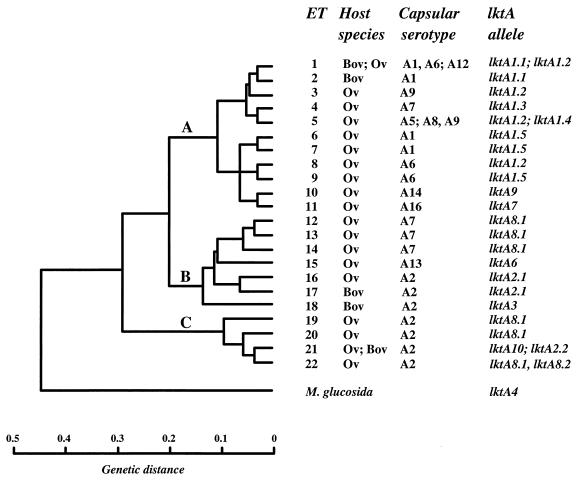
Association of lktA alleles with evolutionary lineages and serotypes of M. haemolytica and the host species of origin.
The association of lktA alleles with evolutionary lineages (represented by ETs) and serotypes of M. haemolytica, together with the host species of origin, is shown in Fig. 1. There were three principal findings, which are summarized below. First, lktA1 type alleles were associated exclusively with strains representing ETs of lineage A. However, allele lktA1.1 occurred only in bovine serotype A1 and A6 strains of ETs 1 and 2, whereas alleles lktA1.2 to lktA1.5 were present in ovine isolates of seven serotypes representing eight ETs. Second, lktA2 type alleles occurred in serotype A2 strains of ETs 16, 17, and 21. With the exception of two strains of ET 16, all isolates possessing this allele type were of bovine origin. The uncommon allele lktA3 was similarly associated only with bovine strains of ET 18. Third, the recombinant alleles lktA6 to lktA10 (see below) were associated with ovine strains having a wide range of genetic diversity. Alleles lktA6, lktA7, and lktA9 occurred in serotype A13, A16, and A14 strains representing ETs 15, 11, and 10, respectively; lktA8 type alleles were more widely distributed among serotype A2 isolates of ETs 19, 20, and 22 and among serotype A7 isolates of ETs 12 to 14; and allele lktA10 was associated with serotype A2 isolates representing ET 21.
FIG. 1.
Association of lktA alleles with evolutionary lineage, capsular serotype, and host species of origin. The dendrogram shows the genetic relationships of ETs of M. haemolytica and was generated by the UPGMA method of clustering from a matrix of coefficients of pairwise genetic distances based on 18 enzyme loci (11). For MLEE data, genetic distance is defined as the number of detectable codon changes per locus (39). Three lineages, identified at a genetic distance of 0.28, are indicated by the letters A, B, and C. Bov, bovine; Ov, ovine.
Intragenic recombination.
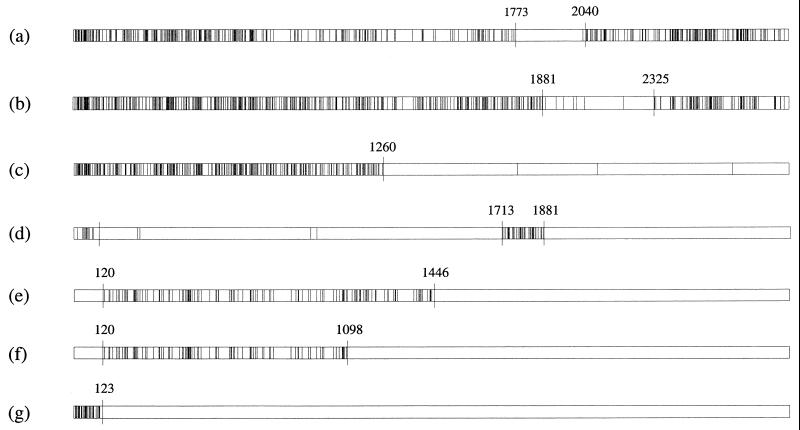
Comparison of the distribution of polymorphic nucleotide sites among lktA alleles representing each of the 10 allelic groups revealed the presence of mosaic structures of varying degrees of complexity. Thus, for pairs of alleles, certain regions of the gene were identical, or nearly so, in sequence whereas adjacent regions of the same alleles were very different. The maximum chi-square method (42) was used to make pairwise comparisons of sequences representing each allelic group, identify statistically significant clusters of polymorphic nucleotide sites, and determine the endpoints of recombinant segments (Fig. 2). On the basis of these analyses, the mosaic structures of single alleles representative of each group are illustrated schematically in Fig. 3.
FIG. 2.
Maximum chi-square comparisons for the following pairs of alleles: (a) lktA1.1 versus lktA2.1, (b) lktA4.1 versus lktA5.1, (c) lktA5.1 versus lktA6, (d) lktA6 versus lktA7, (e) lktA7 versus lktA8.1, (f) lktA8.1 versus lktA9, and (g) lktA1.3 versus lktA10. Vertical lines represent polymorphic nucleotide sites, and numbered nucleotides indicate positions where the chi-square value was a maximum (i.e., kmax) with respect to the partitions on the left and right. These positions mark the endpoints of partitions that represent recombinant segments. The probability that the expected maximum chi-square value in 1,000 randomly generated data sets was greater than the observed value for the real data was <0.05.
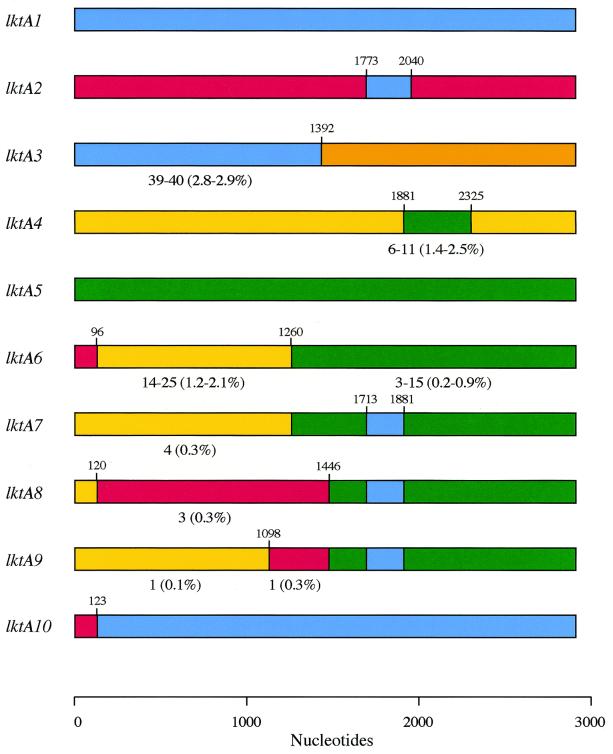
FIG. 3.
Schematic representation of the mosaic structures of alleles representative of the major allelic groups lktA1 to lktA10. The different colors indicate sequence identity and the likely origins of recombinant segments. The number of sites different from those in the corresponding region of the likely donor allele(s) (and the degree of divergence) are indicated below certain recombinant segments (see the text). All other segments exhibited 100% sequence identity to the corresponding regions of the donor alleles. Numbers above the proposed recombination sites indicate the position of the last nucleotide at the downstream end of the recombinant segment.
lktA1 and lktA2 type alleles are 14.4 to 14.5% divergent but have a common 267-bp recombinant segment (nucleotides 1774 to 2040 [Fig. 2]) that is identical in sequence in all alleles (except lktA1.5, which differs at one nucleotide site). Allele lktA3 consists of an upstream 1,392-bp segment (nucleotides 1 to 1392) that is most similar to the corresponding region of lktA1 type alleles (2.8 to 2.9% divergence) and a downstream segment (nucleotides 1393 to 2868) that differs from the corresponding region of lktA1 type alleles at ∼12.0% of the nucleotides. The downstream section also differs substantially from the corresponding regions of lktA2 (13.7% divergence), lktA4 (11.6 to 12.5%), and lktA5 (16.1 to 16.5%) alleles.
lktA4 and lktA5 type alleles are 15.2 to 15.9% divergent overall but have a common 444-bp segment (nucleotides 1882 to 2325 [Fig. 2]) that differs at only ∼1.0 to 2.0% of the sites; because it is present in all alleles, this segment presumably represents an early recombinational exchange (see Discussion). The M. glucosida lktA4.3 allele is 15.4% divergent with respect to the P. trehalosi alleles lktA5.1 to lktA5.3 but has a 111-bp segment (nucleotides 2599 to 2709 [data not shown]) that exhibits 100% identity (lktA5.3 differs at one site) with the corresponding region of the P. trehalosi alleles. This segment accounts for most of the diversity of the M. glucosida lktA4.3 allele and probably represents a recombinant segment derived from P. trehalosi. The maximum chi-square analysis also identified a significant partition between lktA1 and lktA4 alleles at nucleotide position 732 (data not shown). The upstream segments vary at 168 nucleotide sites (23.0% divergence), whereas the downstream regions differ at only 51 to 69 sites (2.4 to 3.2% divergence), between these two groups of alleles. These data clearly indicate different evolutionary origins for the upstream and downstream segments of one or both allele types.
Alleles lktA6 to lktA10 have mosaic structures and consist of two to four distinct segments that have complete, or almost complete, identity with the corresponding regions of lktA1, lktA2, lktA4, and lktA5 type alleles (Fig. 3). The most probable explanation for the structures of alleles lktA6 to lktA10 is that they have been derived from alleles of types lktA1, lktA2, lktA4, and lktA5 in a series of sequential intragenic recombinational exchanges. A model for the sequence of events leading to the formation of these alleles is proposed and discussed in further detail below. Comparison of pairs of alleles by the maximum chi-square method (Fig. 2) clearly demonstrates the complete, or almost complete, identity of homologous regions of donor and recipient alleles as well as the endpoints of recombinant segments.
Synonymous and nonsynonymous substitutions.
Visual inspection of the aligned amino acid sequences representing alleles lktA1, lktA2, lktA4, and lktA5 indicated that the distribution of polymorphic amino acid sites is nonrandom, because well-defined regions of amino acid conservation and heterogeneity occur throughout the leukotoxin molecule (Table 3). The numbers of synonymous substitutions per synonymous site (dS) and nonsynonymous substitutions per nonsynonymous site (dN) were estimated for the combined variable and conserved regions, and the dS/dN ratios were calculated. The dS values for the variable (0.8269 ± 0.0800) and conserved (0.5724 ± 0.0322) regions were not significantly different, but the dN value for the variable regions (0.1887 ± 0.0150) was an order of magnitude larger than that for the conserved regions (0.0214 ± 0.0026). The dS/dN ratios for the variable and conserved regions were 4.4 and 26.7, respectively, and provide evidence of strong selective constraint against amino acid replacement in the conserved regions of the gene and relaxed constraint in the variable regions.
TABLE 3.
Amino acid variation in variable and conserved domains of leukotoxins encoded by alleles lktA1.1, lktA2.1, lktA4.1, and lktA5.1
| Variable domains
|
Conserved domains
|
||
|---|---|---|---|
| Codons | No. of variable sites/total no. of sites (%) | Codons | No. of variable sites/total no. of sites (%) |
| 1–38 | 21/38 (55.3) | 39–51 | 1/13 (7.7) |
| 52–60 | 6/9 (66.7) | 61–79 | 0/19 (0) |
| 80–100 | 8/21 (38.1) | 101–120 | 1/20 (5.0) |
| 121–139 | 12/19 (63.2) | 140–165 | 1/26 (3.8) |
| 166–172 | 6/7 (85.7) | 173–196 | 1/24 (4.2) |
| 197–238 | 17/42 (40.5) | 239–791 | 43/553 (7.8) |
| 792–807 | 7/16 (43.8) | 808–823 | 0/16 (0) |
| 824–861 | 14/38 (36.8) | 862–880 | 0/19 (0) |
| 881–913 | 18/33 (54.5) | 914–956 | 6/43 (14.0) |
| Total | 109/223 (48.9) | Total | 53/733 (7.2) |
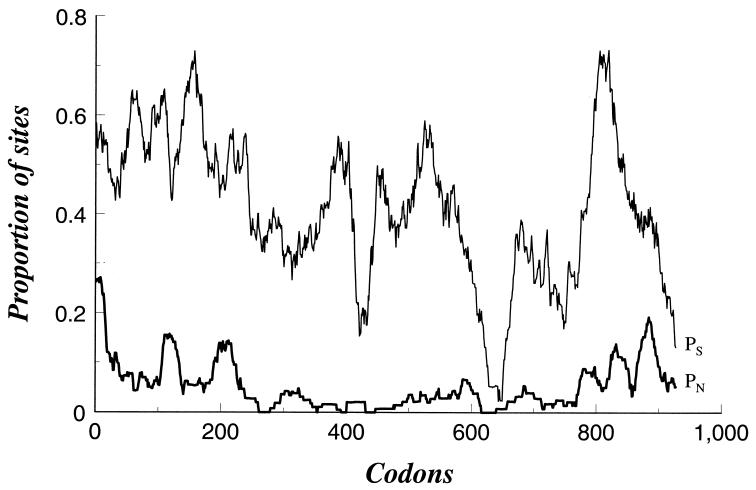
To examine in further detail how the level of selective constraint varies along the leukotoxin molecule, the proportions of synonymous substitutions per synonymous site (pS) and nonsynonymous substitutions per nonsynonymous site (pN) were calculated for subsets of 30 codons in a sliding window for the length of the gene (Fig. 4). The synonymous substitution rate was, overall, much higher than the nonsynonymous substitution rate, indicating evolutionary constraint of amino acid replacement. However, synonymous substitution rates were very low in the regions representing codons 433 to 452 and 628 to 668. In the case of codons 628 to 668, this represents the overlapping region (nucleotides 1882 to 2040) of the recombinant segments of alleles lktA1 and lktA2 (nucleotides 1774 to 2040) and lktA4 and lktA5 (nucleotides 1882 to 2325) (see Fig. 3). Six distinct peaks in pN towards the N- and C-terminal ends of the molecule, corresponding to codons 1 to 38, 121 to 139, 197 to 238, 792 to 807, 824 to 861, and 881 to 913 (Table 3), indicate regions of relaxed constraint of amino acid replacement.
FIG. 4.
Variation in frequency of synonymous and nonsynonymous nucleotide substitutions along the length of the lktA gene among alleles lktA1, lktA2, lktA4, and lktA5. pS and pN were calculated for subsets of 30 codons in a sliding window for the length of the gene.
Relationship between degree of amino acid variation and hydrophilicity-hydrophobicity.
Kyte-Doolittle hydrophilicity-hydrophobicity profiles for leukotoxins encoded by lktA1, lktA2, lktA4, and lktA5 type alleles were remarkably similar (data not shown). The N-terminal half of the molecule (amino acids 1 to 400) consists of a series of hydrophobic and hydrophilic domains, and comparison of the plots with the data given in Table 3 revealed that hydrophobic domains representing codons 41 to 48, 65 to 72, 141 to 158, 172 to 190, 227 to 251, 262 to 318, and 355 to 401 correspond to regions of conserved amino acid sequence, whereas hydrophilic domains representing codons 1 to 40, 49 to 64, 73 to 86, 113 to 140, 159 to 171, and 191 to 226 correspond to regions of variable amino acid sequence. The hydrophobic domains also have low predicted antigenic indices and surface probabilities (data not shown).
DISCUSSION
The lktA gene of M. haemolytica is highly diverse and is represented by at least eight major allelic variants with a complex evolutionary history. These allelic variants have mosaic structures of varying degrees of complexity that reflect a history of extensive intragenic recombination. The frequency and sites of the recombinational exchanges are possibly related to the presence of chi sequences represented by “hot spots” of recombination at nucleotide positions 96 to 123 (alleles lktA6, lktA8, and lktA10), 1446 (lktA8 and lktA9), and 1881 (lktA4 and lktA7). The complete, or nearly complete, identity of homologous segments in donor and recipient alleles suggests that most of these recombination events occurred relatively recently and probably postdate the domestication of cattle and sheep; this applies to alleles lktA6 to lktA10 in particular. Additional evidence for a recent origin is provided by the fact that each allelic variant is represented by only a small number of alleles which exhibit very little interallelic diversity.
In addition to intragenic recombination, assortative (entire-gene) recombination has also contributed to genetic diversity in M. haemolytica. For example, the presence of lktA8 alleles in genetically diverse strains representing six ETs (Fig. 1) is clearly due to horizontal gene transfer, because the formation of identical recombinant alleles by convergent evolution in different lineages is highly unlikely. The pattern of sequence diversity described here clearly accounts for the seven variants of the lktA gene previously identified by restriction endonuclease analysis (5).
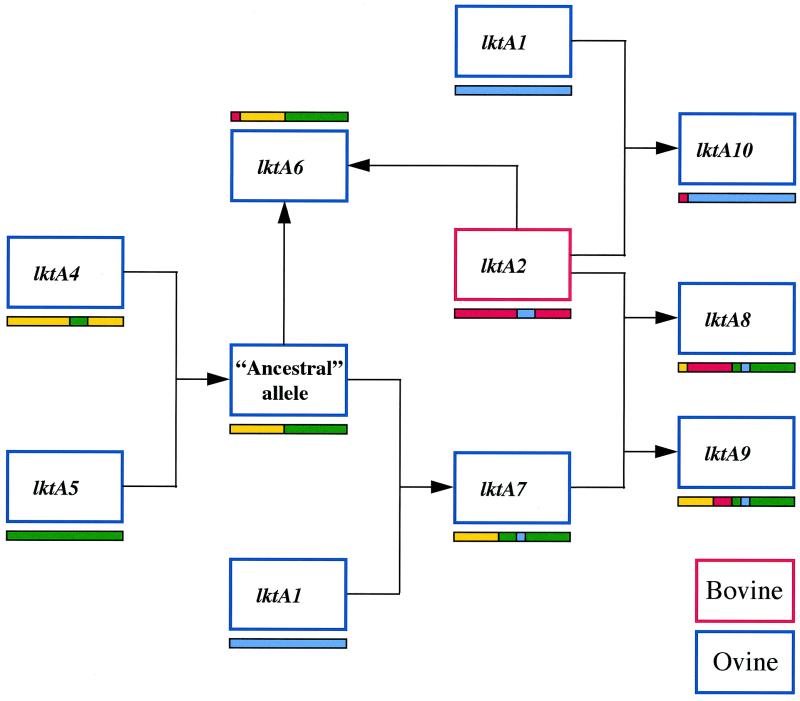
Origin of the mosaic alleles lktA6 to lktA10.
The majority (5 of 8) of the M. haemolytica alleles, namely lktA6 to lktA10, consist of two to four distinct segments that are identical or nearly identical to segments from lktA1, lktA2, lktA4, and lktA5 type alleles (Fig. 3). To account for the complex mosaic structure of lktA alleles, we propose an evolutionary model that posits that new alleles have been created by a series of gene transfers and recombination events (Fig. 5). We hypothesize that these alleles have been formed by a sequential series of intragenic recombinational exchanges culminating in the formation of alleles lktA8 and lktA9. A model representing the proposed sequence of events leading to the formation of alleles lktA6 to lktA10 is shown in Fig. 5. High percentages of the DNA contents of alleles lktA6 to lktA9 (96.7, 94.1, 52.8, and 87.8%, respectively) originate from M. glucosida and P. trehalosi alleles of types lktA4 and lktA5 (Fig. 3). Strong support for a common origin of alleles lktA6 to lktA9 is provided by the fact that homologous segments have identical, or nearly identical, nucleotide sequences. The simplest explanation for this finding is that alleles lktA6 to lktA9 are derived from an ancestral allele that itself was formed by a recombination event involving lktA4 and lktA5 type alleles (Fig. 5). Such recombination events between these two species are not uncommon, because two examples of intragenic recombinational exchanges were discovered in the present study.
FIG. 5.
Proposed sequence of recombination events in the evolution of lktA leading to the formation of lktA8 and lktA10 type alleles in the ovine-specific lineages represented by ETs 12 to 14 and 19 to 22. The central role of the bovine lktA2 allele in the evolution of ovine alleles lktA6, lktA8, lktA9, and lktA10 is clearly seen. The mosaic structures of the alleles are as shown in Fig. 3.
Allele lktA6 could subsequently have been formed by the incorporation of a 96-bp segment (nucleotides 1 to 96) from a lktA2 type allele into the ancestral allele, and allele lktA7 could have been formed by the independent incorporation of a 168-bp segment (nucleotides 1714 to 1881) from a lktA1 type allele (Fig. 5). lktA8 could have been formed by the incorporation of a 1,326-bp segment (nucleotides 121 to 1446) from a lktA2 type allele into lktA7 (Fig. 5); in lktA8.1 this segment differs from the corresponding region of lktA2.1 at only three nucleotide sites, whereas the rest of lktA8.1 is identical to lktA7. There are two possible explanations for the formation of lktA9. Either the 348-bp segment (nucleotides 1099 to 1446) could have been incorporated into allele lktA7 from a lktA2 type allele, or the 1,098-bp segment (nucleotides 1 to 1098) could have been incorporated into a lktA8 type allele from allele lktA7 or a lktA4 type allele. We favor the first event (Fig. 5) because the genetic backgrounds of strains carrying lktA7 and lktA9 alleles are very similar, whereas the genetic backgrounds of strains carrying lktA8 and lktA9 alleles are different (Fig. 1). The 348-bp segment (nucleotides 1099 to 1446) of allele lktA9 differs from the corresponding region of lktA2.1 at a single nucleotide position; the remainder of lktA9 differs from allele lktA7 also at only a single nucleotide position. Finally, lktA10 consists of a 123-bp upstream segment derived from a lktA2 type allele and a 2,745-bp downstream segment (nucleotides 124 to 2868) that is identical to the corresponding region of allele lktA1.3. Since the genetic background of strains containing lktA10 is similar to that of strains possessing allele lktA2.2 (Fig. 1), it is most likely that the donor strain was a serotype A7 strain containing allele lktA1.3 and that the recipient was a serotype A2 strain containing lktA2.2.
The finding that large proportions of M. haemolytica alleles have been derived from M. glucosida and P. trehalosi alleles was unexpected because, as well as being different species, M. glucosida and P. trehalosi differ from M. haemolytica in their pathobiology. M. glucosida represents a genetically diverse group of opportunistic sheep pathogens of low virulence (3, 13), whereas P. trehalosi is responsible for a systemic infection of sheep that is pathologically distinct from pneumonic pasteurellosis (20). It has also been shown that both of these species have low leukotoxic activities (37), suggesting that leukotoxin is likely to be a less important virulence determinant in M. glucosida and P. trehalosi than it is in M. haemolytica. Thus, recombinant leukotoxins have evolved in pathogenic ovine lineages of M. haemolytica from the lktA genes of two species in which leukotoxin probably has a less important role in infection.
It is clear that lktA2-type alleles have played a central role in leukotoxin evolution (Fig. 5). However, comparison of the nucleotide sequence of lktA2 alleles with those of lktA1, lktA4, and lktA5 alleles (Table 2) indicates that lktA2 alleles are more divergent from M. haemolytica lktA1 alleles (14.5% divergence) than are M. glucosida lktA4 alleles (7.9%) and are almost as divergent as P. trehalosi lktA5 alleles (15.4%). These data suggest that the bovine lktA2 alleles were originally acquired by horizontal DNA transfer from a species more distantly related to M. haemolytica than is M. glucosida. Therefore, the recombinant alleles lktA6 to lktA10 have been derived from as many as three, and possibly four, different species, namely, M. haemolytica (lktA1), M. glucosida (lktA4), P. trehalosi (lktA5), and an unknown species (lktA2).
Host switching of bovine serotype A2 strains to sheep has led to the evolution of new recombinant alleles in ovine strains.
Recombinational exchanges involving lktA2 alleles have played a significant role in leukotoxin evolution, as evidenced by the fact that lktA2-derived segments are present in lktA6, lktA8, lktA9, and lktA10 alleles (Fig. 5). The following evidence suggests that the latter four alleles have evolved as a consequence of host switching of serotype A2 strains from cattle to sheep. First, lktA2 alleles are associated only with bovine or bovine-like serotype A2 strains (Fig. 1). Partial sequence analysis of the lktA gene from a wider range of serotype A2 strains confirmed that, with two exceptions, lktA2 type alleles occur only in bovine strains of ETs 17 and 21 (Table 1). Although two exceptional lktA2-possessing serotype A2 isolates (ET 16 [Fig. 1]) were isolated from sheep, these strains possess OMP profiles characteristic of bovine isolates (10). Furthermore, recent sequence analysis of the OMP pomA gene (50) from bovine and ovine isolates (R. L. Davies, unpublished data) has confirmed that these two isolates are related to bovine and not ovine serotype A2 strains. Therefore, we suspect that the two ET 16 isolates are not true ovine-adapted strains but instead represent a bovine-adapted clone that recently spread to sheep. Second, lktA4 and lktA5 type alleles are present in the species M. glucosida and P. trehalosi, respectively, bacteria that occur only in sheep (3, 13, 20). In support of this, recombinant segments derived from lktA4 and lktA5 type alleles were not identified in any of the alleles associated with bovine strains. Third, the recombinant alleles lktA6 to lktA10 are associated only with ovine strains of serotypes A2, A7, A13, A14, and A16; with the exception of A2, none of these serotypes are known to occur in cattle (17). Partial sequence analysis of the lktA gene from additional serotype A2 strains confirmed that lktA8 and lktA10 alleles occur only in ovine isolates of ETs 19 to 22 (Table 1).
The distribution of alleles among bovine and ovine strains suggests that the recombinational exchanges leading to the formation of lktA6 to lktA10 alleles could not have occurred in cattle. It follows that the formation of lktA6, lktA8, lktA9, and lktA10 alleles can be satisfactorily explained only by host switching of lktA2-containing serotype A2 strains from cattle to sheep and subsequent recombinational exchanges involving ovine strains. If sheep, rather than cattle, were the ancestral hosts of lktA2-containing serotype A2 strains, we would expect to isolate a higher number of such strains from sheep and, assuming random transmission, to recover lktA8- and lktA10-containing serotype A2 strains from cattle—but this is not the case. Therefore, transmission of strains from cattle to sheep, together with horizontal DNA transfer and recombination, has resulted in the evolution of new ovine lktA alleles and an increase in leukotoxin diversity. In contrast, leukotoxin diversity is much lower in bovine strains than in ovine isolates, a finding that parallels the greater overall diversity of ovine strains and suggests limited transmission of strains from sheep to cattle.
Molecular evolution and leukotoxin structure.
The leukotoxin molecule of M. haemolytica consists of well-defined regions of conservation and heterogeneity which correspond to different functional domains (9, 16, 26, 31, 33, 45, 48). In particular, there are three highly conserved regions that are common to all RTX toxins (45, 48). The N-terminal half of the molecule consists of a series of hydrophobic putative membrane-spanning domains, separated by hydrophilic regions, that are involved in pore formation; the hydrophobic domains are followed by a second region of approximately 200 amino acids, rich in β-turns, that is involved in cell binding and LktC-mediated toxin activation; the third conserved region consists of amino acids 733 to 786 in M. haemolytica and forms six glycine-rich tandem repeat domains that are involved in Ca2+ binding.
The synonymous substitution rate of the lktA gene is, overall, much higher than the nonsynonymous substitution rate, suggesting that amino acid replacement is subject to selective constraint. However, the patterns of synonymous and nonsynonymous substitution rates vary throughout the gene, indicating that differing selective pressures are operating on different regions of the protein. For example, amino acid replacement is highly constrained within the conserved regions, but amino acid constraint is more relaxed in the variable regions, particularly in the six domains located towards the C- and N-terminal ends of the molecule (Fig. 4). In the pore-forming N-terminal half of the molecule (amino acids 1 to 400), the degree of evolutionary constraint on amino acid replacement correlates with leukotoxin structure inasmuch as hydrophobic domains are generally conserved in amino acid sequence whereas hydrophilic domains exhibit heterogeneity in amino acid sequence. The conserved, hydrophobic domains have a low surface probability and, most likely, represent membrane-spanning regions involved in pore formation (16, 31, 45), whereas the variable, hydrophilic domains probably represent surface-exposed regions of lesser structural importance. Similarly, in the central part of the molecule that corresponds to the cell binding, toxin activation, and Ca2+-binding domains (9, 33, 48), there is a high degree of evolutionary constraint on amino acid replacement that is reflected in a low nonsynonymous substitution rate compared to the synonymous substitution rate (Fig. 4). Despite the presence of substantial allelic diversity and extensive amino acid variation (Table 2) among different leukotoxins, the remarkable similarity in hydrophilicity and hydrophobicity profiles suggests that selective pressure is operating to maintain overall leukotoxin structure.
Although single-site mutational changes are uncommon among M. haemolytica alleles, indirect evidence suggests that a single amino acid substitution could be involved in leukotoxin adaptation to the bovine host. Replacement of G at nucleotide position 2521 in alleles lktA1.2 to lktA1.5 with A in allele lktA1.1 has resulted in an amino acid change from aspartic acid to asparagine at position 841. lktA1.1-encoded leukotoxin is associated exclusively with genetically related bovine serotype A1 and A6 strains of ETs 1 and 2, whereas alleles lktA1.2 to lktA1.5 are present only in ovine strains (Fig. 1). In addition, asparagine is present at position 841 in the lktA2-encoded leukotoxin of bovine A2 strains. These findings suggest that asparagine at position 841 provides a selective advantage to leukotoxin function in the bovine host and that lktA1.1 emerged in bovine strains by mutation and selection for this amino acid. Although this amino acid substitution occurs in that part of the molecule known to be involved in receptor binding and specificity, i.e., flanking the glycine-rich repeat region (27), the precise effect and significance of the change remain to be determined.
Recombination has been shown to play a role in the generation of antigenic variation in different surface antigens of a number of pathogens (8, 15, 29, 38) and is thought to be an adaptation to the host immune response. Since leukotoxin is an important virulence determinant and is involved in host immunity (18, 34, 41), it is likely that recombination of the lktA gene provides an adaptive advantage against the host antibody response by generating antigenic variation. Therefore, recombination produces new variants that may have an advantage in fitness either within hosts (cattle or sheep), with an enhanced ability to avoid the immune response, or between hosts, with an increased chance of spreading against the effects of herd immunity. The most antigenically diverse leukotoxins are those encoded by alleles lktA6 and lktA8 in that they contain variable domains from three different sources, including segments from the bovine lktA2 alleles. It is reasonable to assume that the occurrence of lktA8 type alleles in different lineages and their association with a high proportion of ovine disease isolates might be due to a selective advantage resulting from the antigenic diversity of the encoded leukotoxin.
lktA8 and lktA10 type alleles are representative of the two major lineages of ovine serotype A2 strains, ETs 22 and 21, respectively, which are responsible for a high proportion of ovine disease (13). These alleles have very different evolutionary histories and encode divergent leukotoxins (5.3% amino acid divergence), but both contain segments derived from bovine lktA2 alleles. The occurrence of two different leukotoxin types in A2 strains has important implications for vaccine design and disease prevention in sheep because leukotoxin is a key component of some vaccines (26). Furthermore, the recent evolutionary origin of these two leukotoxins suggests that new, immunologically distinct molecules could evolve in the future. Finally, the presence of lktA10 alleles in ovine A2 strains of the same genetic background as lktA2-containing bovine A2 strains provides a clue about the possible origins of the ovine A2 lineages. It is interesting to speculate that these evolved from bovine A2 strains as a consequence of host switching and acquisition of specific genes necessary for adaptation to the ovine environment. Our findings for the lktA gene have wider implications for our understanding of the role of host switching in the evolution of virulence genes and in the emergence of new pathogens not only in M. haemolytica but also in other members of the Pasteurellaceae.
ACKNOWLEDGMENTS
This study was supported by a Wellcome Trust Biodiversity Fellowship to R. L. Davies (038464/Z/93/Z/REH/MW) and by grant AI22144 from the NIH.
We thank S. Plock and S. O'Bryan (PSU) and Susan Campbell (Glasgow) for excellent technical assistance.
REFERENCES
- 1.Adlam C. The structure, function and properties of cellular and extracellular components of Pasteurella haemolytica. In: Adlam C F, Rutter J M, editors. Pasteurella and pasteurellosis. London, England: Academic Press; 1989. pp. 75–92. [Google Scholar]
- 2.Ambagala T C, Aruna P N, Ambagala S S. The leukotoxin of Pasteurella haemolytica binds to β2 integrins on bovine leukocytes. FEMS Microbiol Lett. 1999;179:161–167. doi: 10.1111/j.1574-6968.1999.tb08722.x. [DOI] [PubMed] [Google Scholar]
- 3.Angen O, Mutters R, Caugant D A, Olsen J E, Bisgaard M. Taxonomic relationships of the [Pasteurella] haemolytica complex as evaluated by DNA-DNA hybridizations and 16S rRNA sequencing with proposal of Mannheimia haemolytica gen. nov., comb. nov., Mannheimia granulomatis comb. nov., Mannheimia glucosida sp. nov., Mannheimia ruminalis sp. nov. and Mannheimia varigena sp. nov. Int J Syst Bacteriol. 1999;49:67–86. doi: 10.1099/00207713-49-1-67. [DOI] [PubMed] [Google Scholar]
- 4.Berggren C A, Baluyut C S, Simonson R R, Bemrick W J, Maheswaran S K. Cytotoxic effects of Pasteurella haemolytica on bovine neutrophils. Am J Vet Res. 1981;42:1383–1388. [PubMed] [Google Scholar]
- 5.Burrows L L, Olah-Winfield E, Lo R Y C. Molecular analysis of the leukotoxin determinants from Pasteurella haemolytica serotypes 1 to 16. Infect Immun. 1993;61:5001–5007. doi: 10.1128/iai.61.12.5001-5007.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang Y F, Prost D, Struck D K. Identification and characterization of the Pasteurella haemolytica leukotoxin. Infect Immun. 1987;55:2348–2354. doi: 10.1128/iai.55.10.2348-2354.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clinkenbeard K D, Mosier D A, Timko A L, Confer A W. Effects of Pasteurella haemolytica leukotoxin on cultured bovine lymphoma cells. Am J Vet Res. 1989;50:271–275. [PubMed] [Google Scholar]
- 8.Coffey T J, Enright M C, Daniels M, Morona J K, Morona R, Hryniewicz W, Paton J C, Spratt B G. Recombinational exchanges at the capsular polysaccharide biosynthetic locus lead to frequent serotype changes among natural isolates of Streptococcus pneumoniae. Mol Microbiol. 1998;27:73–83. doi: 10.1046/j.1365-2958.1998.00658.x. [DOI] [PubMed] [Google Scholar]
- 9.Cruz W T, Young R, Chang Y F, Struck D K. Deletion analysis resolves cell-binding and lytic domains of the Pasteurella leukotoxin. Mol Microbiol. 1990;4:1933–1939. doi: 10.1111/j.1365-2958.1990.tb02042.x. [DOI] [PubMed] [Google Scholar]
- 10.Davies R L, Donachie W. Intra-specific diversity and host specificity within Pasteurella haemolytica based on variation of capsular polysaccharide, lipopolysaccharide and outer-membrane proteins. Microbiology. 1996;142:1895–1907. doi: 10.1099/13500872-142-7-1895. [DOI] [PubMed] [Google Scholar]
- 11.Davies R L, Quirie M. Intra-specific diversity within Pasteurella trehalosi based on variation of capsular polysaccharide, lipopolysaccharide and outer-membrane proteins. Microbiology. 1996;142:551–560. doi: 10.1099/13500872-142-3-551. [DOI] [PubMed] [Google Scholar]
- 12.Davies R L, Paster B J, Dewhirst F E. Phylogenetic relationships and diversity within the Pasteurella haemolytica complex based on 16S rRNA sequence comparison and outer membrane protein and lipopolysaccharide analysis. Int J Syst Bacteriol. 1996;46:736–744. doi: 10.1099/00207713-46-3-736. [DOI] [PubMed] [Google Scholar]
- 13.Davies R L, Arkinsaw S, Selander R K. Evolutionary genetics of Pasteurella haemolytica isolates recovered from cattle and sheep. Infect Immun. 1997;65:3585–3593. doi: 10.1128/iai.65.9.3585-3593.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies R L, Arkinsaw S, Selander R K. Genetic relationships among Pasteurella trehalosi based on multilocus enzyme electrophoresis. Microbiology. 1997;143:2841–2849. doi: 10.1099/00221287-143-8-2841. [DOI] [PubMed] [Google Scholar]
- 15.Feavers I M, Heath I A, Bygraves J A, Maiden M C J. Role of horizontal genetic exchange in the antigenic variation of the class 1 outer membrane protein of Neisseria meningitidis. Mol Microbiol. 1992;6:489–495. doi: 10.1111/j.1365-2958.1992.tb01493.x. [DOI] [PubMed] [Google Scholar]
- 16.Forestier C, Welch R A. Identification of RTX toxin target cell specificity domains by use of hybrid genes. Infect Immun. 1991;59:4212–4220. doi: 10.1128/iai.59.11.4212-4220.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Frank G H. Pasteurellosis of cattle. In: Adlam C F, Rutter J M, editors. Pasteurella and pasteurellosis. London, England: Academic Press; 1989. pp. 197–222. [Google Scholar]
- 18.Gentry M J, Confer A W, Panciera R J. Serum neutralization of cytotoxin from Pasteurella haemolytica serotype 1 and resistance to experimental bovine pneumonic pasteurellosis. Vet Immunol Immunopathol. 1985;9:239–250. doi: 10.1016/0165-2427(85)90074-1. [DOI] [PubMed] [Google Scholar]
- 19.Gerbig D G, Cameron M R, Struck D K, Moore R N. Characterization of a neutralizing monoclonal antibody to Pasteurella haemolytica leukotoxin. Infect Immun. 1992;60:1734–1739. doi: 10.1128/iai.60.5.1734-1739.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gilmour N J L, Gilmour J S. Pasteurellosis of sheep. In: Adlam C F, Rutter J M, editors. Pasteurella and pasteurellosis. London, England: Academic Press; 1989. pp. 223–261. [Google Scholar]
- 21.Highlander S K, Chidambaram M, Engler M J, Weinstock G M. DNA sequence of the Pasteurella haemolytica leukotoxin gene cluster. DNA Cell Biol. 1989;8:15–28. doi: 10.1089/dna.1.1989.8.15. [DOI] [PubMed] [Google Scholar]
- 22.Jeyaseelan S, Hsuan S L, Kannan M S, Walcheck B, Wang J F, Kehrli M E, Lally E T, Sieck G C, Maheswaran S K. Lymphocyte function-associated antigen 1 is a receptor for Pasteurella haemolytica leukotoxin in bovine leukocytes. Infect Immun. 2000;68:72–79. doi: 10.1128/iai.68.1.72-79.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaehler K L, Markham R J F, Muscoplat C C, Johnson D W. Evidence of species specificity in the cytocidal effects of Pasteurella haemolytica. Infect Immun. 1980;30:615–616. doi: 10.1128/iai.30.2.615-616.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kapur V, Kanjilal S, Hamrick M R, Li L-L, Whittam T S, Sawyer S A, Musser J M. Molecular population genetic analysis of the streptokinase gene of Streptococcus pyogenes: mosaic alleles generated by recombination. Mol Microbiol. 1995;16:509–519. doi: 10.1111/j.1365-2958.1995.tb02415.x. [DOI] [PubMed] [Google Scholar]
- 25.Kumar S, Tamura K, Nei M. MEGA: Molecular Evolutionary Genetics Analysis software for microcomputers. Comput Appl Biosci. 1993;10:189–191. doi: 10.1093/bioinformatics/10.2.189. [DOI] [PubMed] [Google Scholar]
- 26.Lainson F A, Murray J, Davies R C, Donachie W. Characterization of epitopes involved in the neutralization of Pasteurella haemolytica serotype A1 leukotoxin. Microbiology. 1996;142:2499–2507. doi: 10.1099/00221287-142-9-2499. [DOI] [PubMed] [Google Scholar]
- 27.Lally E T, Golub E E, Kieba I R. Identification and immunological characterization of the domain of Actinobacillus actinomycetemcomitans leukotoxin that determines its specificity for human target cells. J Biol Chem. 1994;269:31289–31295. [PubMed] [Google Scholar]
- 28.Li J, Clinkenbeard K D, Ritchey J W. Bovine CD18 identified as a species-specific receptor for Pasteurella haemolytica leukotoxin. Vet Microbiol. 1999;67:91–97. doi: 10.1016/s0378-1135(99)00040-1. [DOI] [PubMed] [Google Scholar]
- 29.Li J, Nelson K, McWhorter A C, Whittam T S, Selander R K. Recombinational basis of serovar diversity in Salmonella enterica. Proc Natl Acad Sci USA. 1994;91:2552–2556. doi: 10.1073/pnas.91.7.2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lo R Y C, Strathdee C A, Shewen P E. Nucleotide sequence of the leukotoxin genes of Pasteurella haemolytica A1. Infect Immun. 1987;55:1987–1996. doi: 10.1128/iai.55.9.1987-1996.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ludwig A, Schmid A, Benz R, Goebel W. Mutations affecting pore formation by haemolysin from Escherichia coli. Mol Gen Genet. 1991;226:198–208. doi: 10.1007/BF00273604. [DOI] [PubMed] [Google Scholar]
- 32.Maheswaran S K, Kannan M S, Weiss D J, Reddy K R, Townsend E L, Yoo H S, Lee B W, Whitely L O. Enhancement of neutrophil-mediated injury to bovine pulmonary endothelial cells by Pasteurella haemolytica leukotoxin. Infect Immun. 1993;61:2618–2625. doi: 10.1128/iai.61.6.2618-2625.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McWhinney D R, Chang Y F, Young R, Struck D K. Separable domains define target cell specificities of an RTX hemolysin from Actinobacillus pleuropneumoniae. J Bacteriol. 1992;174:291–297. doi: 10.1128/jb.174.1.291-297.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Moore R N, Walker R D, Shaw G A, Hopkins F M, Shull E P. Antileukotoxin antibody produced in the bovine lung after aerosol exposure to viable Pasteurella haemolytica. Am J Vet Res. 1985;46:1949–1952. [PubMed] [Google Scholar]
- 35.Petras S M, Chidambaram M, Illyes E F, Froshauer S, Weinstock G M, Reese C P. Antigenic and virulence properties of Pasteurella haemolytica leukotoxin mutants. Infect Immun. 1995;63:1033–1039. doi: 10.1128/iai.63.3.1033-1039.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reeves P R. Evolution of Salmonella O antigen variation by interspecific gene transfer on a large scale. Trends Genet. 1993;9:17–22. doi: 10.1016/0168-9525(93)90067-R. [DOI] [PubMed] [Google Scholar]
- 37.Saadati M, Gibbs H A, Parton R, Coote J G. Characterization of the leukotoxin produced by different strains of Pasteurella haemolytica. J Med Microbiol. 1997;46:276–284. doi: 10.1099/00222615-46-4-276. [DOI] [PubMed] [Google Scholar]
- 38.Seifert H S, Ajioka R S, Marchal C, Sparling P F, So M. DNA transformation leads to pilin antigenic variation in Neisseria gonorrhoeae. Nature. 1988;336:392–395. doi: 10.1038/336392a0. [DOI] [PubMed] [Google Scholar]
- 39.Selander R K, Caugant D A, Ochman H, Musser J M, Gilmour M N, Whittam T S. Methods of multilocus enzyme electrophoresis for bacterial population genetics and systematics. Appl Environ Microbiol. 1986;51:873–884. doi: 10.1128/aem.51.5.873-884.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shewen P E, Wilkie B N. Cytotoxin of Pasteurella haemolytica acting on bovine leukocytes. Infect Immun. 1982;35:91–94. doi: 10.1128/iai.35.1.91-94.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shewen P E, Wilkie B N. Evidence for the Pasteurella haemolytica cytotoxin as a product of actively growing bacteria. Am J Vet Res. 1983;46:1212–1214. [PubMed] [Google Scholar]
- 42.Smith J M. Analyzing the mosaic structure of genes. J Mol Evol. 1992;34:126–129. doi: 10.1007/BF00182389. [DOI] [PubMed] [Google Scholar]
- 43.Sneath P H A, Stevens M. Actinobacillus rossii sp. nov., Actinobacillus seminis sp. nov., nom. rev., Pasteurella bettii sp. nov., Pasteurella lymphangitidis sp. nov., Pasteurella mairi sp. nov., and Pasteurella trehalosi sp. nov. Int J Syst Bacteriol. 1990;40:148–153. doi: 10.1099/00207713-40-2-148. [DOI] [PubMed] [Google Scholar]
- 44.Spratt B G, Bowler L D, Zhang Q Y, Zhou J, Smith J M. Role of interspecies transfer of chromosomal genes in the evolution of penicillin resistance in pathogenic and commensal Neisseria species. J Mol Evol. 1992;34:115–125. doi: 10.1007/BF00182388. [DOI] [PubMed] [Google Scholar]
- 45.Strathdee C A, Lo R Y C. Extensive homology between the leukotoxin of Pasteurella haemolytica A1 and the alpha-hemolysin of Escherichia coli. Infect Immun. 1987;55:3233–3236. doi: 10.1128/iai.55.12.3233-3236.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sutherland A D. Effects of Pasteurella haemolytica cytotoxin on ovine peripheral blood leucocytes and lymphocytes obtained from gastric lymph. Vet Microbiol. 1985;10:431–438. doi: 10.1016/0378-1135(85)90025-2. [DOI] [PubMed] [Google Scholar]
- 47.Sutherland A D, Donachie W. Cytotoxic effect of serotypes of Pasteurella haemolytica on sheep bronchoalveolar macrophages. Vet Microbiol. 1986;11:331–336. doi: 10.1016/0378-1135(86)90063-5. [DOI] [PubMed] [Google Scholar]
- 48.Welch R A. Pore-forming cytolysins of Gram-negative bacteria. Mol Microbiol. 1991;5:521–528. doi: 10.1111/j.1365-2958.1991.tb00723.x. [DOI] [PubMed] [Google Scholar]
- 49.Younan M, Fodor I. Characterization of a new Pasteurella haemolytica serotype (A17) Res Vet Sci. 1995;58:98. doi: 10.1016/0034-5288(95)90097-7. [DOI] [PubMed] [Google Scholar]
- 50.Zeng H, Pandher K, Murphy G L. Molecular cloning of the Pasteurella haemolytica pomA gene and identification of bovine antibodies against PomA surface domains. Infect Immun. 1999;67:4968–4973. doi: 10.1128/iai.67.9.4968-4973.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]