Abstract
(1) Background: Autoimmune diseases, including autoimmune endocrine diseases (AIED), are thought to develop following environmental exposure in patients with genetic predisposition. The vaccines against severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) could represent a new environmental trigger for AIED, including Graves’ disease (GD). (2) Methods: We performed a literature search of MEDLINE/PubMed databases regarding thyroid dysfunction after SARS-CoV-2 vaccination since 1 January 2020 to 31 July 2022, considering only cases of thyrotoxicosis that meet the 2016 American Thyroid Association guidelines criteria for the diagnosis of GD and arising after administration of the anti-SARS-CoV-2 vaccine, regardless of the number of doses. (3) Results: A total of 27 articles were identified, consisting of case reports or case series, of which 24 describe the appearance of 48 new diagnoses of GD and 12 GD recurrences arising after the administration of the anti-SARS-CoV-2 vaccine, and 3 papers that instead report only 3 cases of GD relapse following vaccination. (4) Conclusions: physicians should be aware of the possibility of developing GD and other autoimmune sequelae following SARS-CoV-2 vaccination. Regardless of the underlying pathogenetic mechanisms (autoimmune/inflammatory syndrome induced by adjuvants (ASIA syndrome), cytokines induction, molecular mimicry, and cross-reactivity), an individual predisposition seems to be decisive for their development.
Keywords: Graves’ disease, SARS-CoV-2, COVID-19, vaccine, hyperthyroidism, ASIA syndrome, autoimmune thyroid diseases
1. Introduction
As of the end of July 2022, the COVID-19 pandemic caused by SARS-CoV-2 has spread around the world with nearly 600 million cases and more than 6 million confirmed deaths, according to the WHO databases [1]. SARS-CoV-2 infection can run asymptomatically or provoke mild upper respiratory tract symptoms such as dry cough, headache, fever, and loss of smell and taste, or it can induce an interstitial pneumonia that can result in ARDS (Acute Respiratory Distress Syndrome) with the need for mechanical ventilation [2]. To date, the most effective weapon to fight SARS-CoV-2 infection is represented by primary prophylaxis with vaccines. In a short time, numerous sera have been released [3], including, for the first time, mRNA-technology-based vaccines (Pfizer-Biontech’s BNT162b2 and Moderna’s mRNA-1273) [4,5]. As with other vaccinations, the anti-SARS-CoV-2 vaccination campaign immediately highlighted the possibility of developing side effects, ranging from mild local reactions (pain at the insertion point) to systemic phenomena (fever, headache, asthenia, muscle aches) [6]. However, beyond these expected events, numerous more severe autoimmune phenomena such as myocarditis [7,8] and other manifestations have been documented soon thereafter, especially among those subjects already suffering from other forms of autoimmunity or with a familiarity for it [9]. There are several reports of auto-inflammatory and autoimmune reactions also affecting the endocrine system and the thyroid, especially in the form of subacute thyroiditis [10] and GD [11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38]. GD is an autoimmune thyroid disease characterized by the presence of autoantibodies against the TSH receptor (TRAb) expressed on thyrocytes, which cause thyroid gland growth and thyroid hormone synthesis and release with consequent hyperthyroidism [39,40]. Given the remarkable diffusion of anti-SARS-CoV-2 vaccination and its impact on public health, in this review, we report all the postvaccine cases of GD documented in the literature up to 31 July 2022, more than a year after the beginning of the immunization campaign, focusing on their main epidemiological and clinics features. Furthermore, although a causality relationship cannot be proven yet, we summarize the potential pathogenetic mechanisms that could explain the onset of autoimmunity following the anti-SARS-CoV-2 vaccination to provide up-to-date information about this emerging topic.
2. Materials and Methods
We performed a literature search of MEDLINE/PubMed databases regarding thyroid dysfunction after SARS-CoV-2 vaccination from 1 January 2020 to 31 July 2022. We included original articles, reviews, viewpoints, commentaries, case series and case reports, and both published and unpublished articles. The search terms, used both separately and in combination, included: “SARS-CoV-2”, “COVID19”, “thyroid”, “Graves’ disease”, “hyperthyroidism”, “autoimmune thyroid disease”, “vaccine”, “vaccination”, “thyrotoxicosis”, and “thyroiditis”.
In accordance with the 2016 guidelines of the American Thyroid Association [41], only cases of thyrotoxicosis that meet the following criteria for the diagnosis of GD and arising after administration of the anti-SARS-CoV-2 vaccine, regardless of the number of doses, were considered: (a) thyroid function tests consistent with hyperthyroidism; (b) TRAb or TSI positivity; (c) presence of thyroid scan showing high radioactive iodine uptake (RAIU); or (d) thyroid ultrasound picture of the glandular parenchyma with diffuse hypervascularization (“thyroid inferno” pattern). The findings of this review are reported in accordance with PRISMA guidelines [42].
Univariate descriptive statistics were performed. Categorical variables were analyzed using frequencies, and quantitative continuous variables were expressed the median and interquartile range (IQR).
3. Results
3.1. General Characteristics
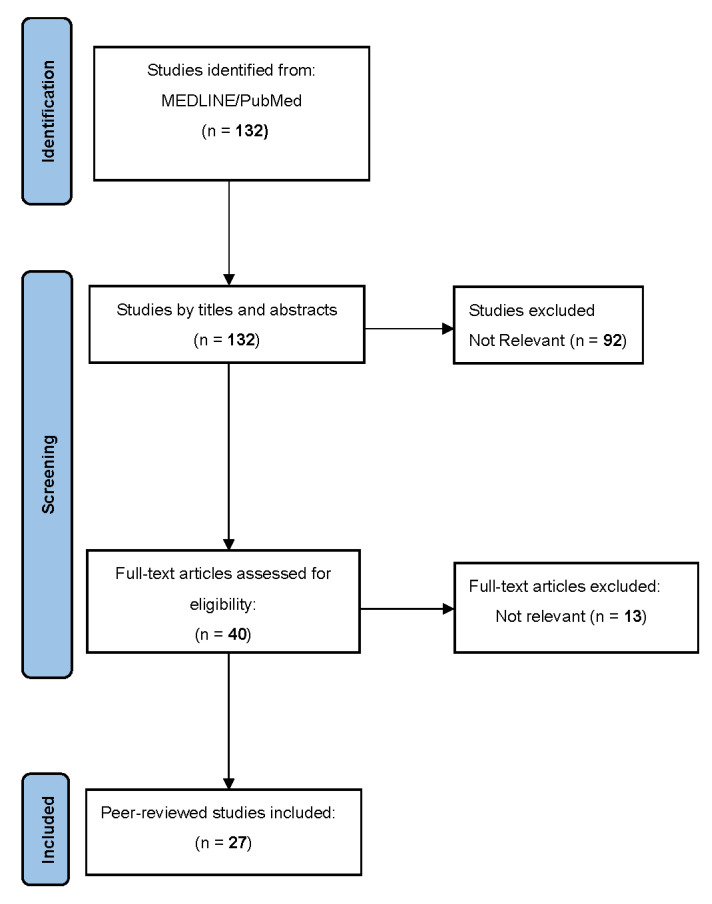
A total of 27 articles were identified (Figure 1) consisting of case reports or case series, of which 24 describe the appearance of 48 new diagnoses of GD and 12 GD recurrences arising after the administration of the anti-SARS-CoV-2 vaccine, and 3 papers that instead report only 3 cases of GD relapse following vaccination. All these cases have been summarized in Table 1 and Table 2 and compared in Table 3.
Figure 1.
PRISMA flow diagram of study search and selection.
Table 1.
Summary of demographic, clinical, and laboratory characteristics of new Graves’s disease cases following SARS-CoV-2 vaccination in the literature.
| Gender | Age | Country | Vaccine | History of COVID | Dose | Personal/Family History of AITD | Medical History | Symptoms | Days until Symptoms | TSH (mIU/L) | fT3 (ng/L) | fT4 (ng/dL) | TgAb (IU/mL) | TPOAb (IU/mL) | TRAb (IU/L) | Thyroid US | Thyroid Scan | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| M | 52 | Italy | BNT162b2 | No | 2nd | None | Type 2 Diabetes, Vitiligo | Weight loss, asthenia, insomnia | 20 | <0.004 (N: 0.4–4) | 15 (N: 2.7–5.7) | 5.56 (N: 0.7–1.7) | 30 (N: 0–30) | 21 (N: 0–10) | 6.48 (N: 0–1.49) | Enlargement and hypervascularity | N/A | Patrizio [13] |
| F | 40 | Mexico | BNT162b2 | Yes | 1st | None | None | Nausea, vomiting, fatigue, insomnia, and palpitations | 2 | <0.001 (N: 0.27–4.4) | 10.5 (N: 2.04–4.4) | 3.57 (N: 0.93–1.71) | 210 (N: 0–44) | 3450 (N: 0–5.6) | 16.56 (N: 0–1.75) | Enlargement and hypervascularity | N/A | V.Lastra [11] |
| F | 28 | Mexico | BNT162b2 | No | 1st | None | None | Anxiety, insomnia, palpitations, distal tremors | 3 | <0.001 (N: 0.27–4.4) | 9.2 (N: 2.04–4.4) | 1.84 (N: 0.93–1.71) | 33 (N: 0–44) | 833 (N: 0–5.6) | 5.85 (N: 0–1.75) | N/A | Diffuse toxic goiter | V.Lastra [11] |
| M | 46 | Austria | BNT162b2 | No | 1st | None | None | None | 15 | N/A | 5.18 (N: 2.15–4.12) | 1.63 (N: 0.7–1.7) | N/A | N/A | 2.9 (N: 0–1.5) | Hypoechogenic parenchyma, large anechogenic areas with increased vascularization |
Patchy, inhomogenous, normal uptake |
Zettining [12] |
| F | 71 | Spain | BNT162b2 | No | 2nd | None | N/A | Weight loss, asthenia, afib | 60 | <0.005 (N: 0.38–5.33) | N/A | 2.3 (N: 0.54–1.24) | <0.9 (N: 0–4) | 30 (N: 0–9) | 3.6 (N: 0–1.75) | Enlargement and hypervascularity | Diffuse toxic goiter | Pla Peris [14] |
| F | 42 | Spain | BNT162b2 | No | 1st | None | N/A | Weight loss, palpitations | 14 | <0.005 (N: 0.38–5.33) | N/A | 2.9 (N: 0.54–1.24) | N/A | 2.5 (N: 0–9) | 4.39 (N: 0–1.75) | Enlargement and hypervascularity | Diffuse toxic goiter | Pla Peris [14] |
| F | 54 | Spain | mRNA-1273 | No | 2nd | None | N/A | Weight loss, asthenia, palpitations | 14 | <0.005 (N: 0.38–5.33) | N/A | 4.7 (N: 0.54–1.24) | 55 (N: 0–4) | 30 (N: 0–9) | 5.1 (N: 0–1.75) | Enlargement and hypervascularity | N/A | Pla Peris [14] |
| F | 46 | Spain | BNT162b2 | No | 1st | None | N/A | Weight loss, palpitations, irritability | 50 | <0.005 (N: 0.38–5.33) | N/A | 3.2 (N: 0.54–1.24) | 90 (N: 0–4) | 60 (N: 0–9) | 3.2 (N: 0–1.75) | Enlargement and hypervascularity | N/A | Pla Peris [14] |
| M | 32 | Italy | ChAdOx1 | No | 2nd | None | None | Anxiety, tachycardia, palpitations | 10 | 0.005 (N: N/A) | 7.9 (N: 2–4.4) | 2.96 (N: 0.6–1.12) | N/A | N/A | 7.98 (N: 0–2.9) | Enlargement and hypervascularity | N/A | Di Filippo [15] |
| M | 35 | Italy | ChAdOx1 | No | 1st | None | None | Nausea, headache, tachycardia, palpitations, asthenia | 5 | <0.004 (N: N/A) | N/A | 4,96 (N: 0.6–1.12) | N/A | N/A | 3.2 (N: 0–2.9) | Enlargement and hypervascularity | N/A | Di Filippo [15] |
| F | 71 | USA | BNT162b2 | No | 2nd | GMN/None | Stage IV breast cancer in remission | Tachycardia, palpitations, fever, dizziness, distal tremors | 14 | <0.02 (N: 0.3–2) | N/A | 7.2 (N: 0.9–1.7) | N/A | 8.9 (N: 0–9) | N/A(TSI +ve) | Multinodular Goiter | N/A | Goblirsch [16] |
| M | 32 | USA | BNT162b2 | Yes | 1st | None | None | Palpitations, insomnia, tremors, irritability, sweating, dyspnea | 10 | <0.005 (N: 0.282–4) | N/A | 5.41 (N: 0.84–1.62) | 53 (N: 0–40) | 119 (N: 0–35) | N/A (TSI +ve) | Heterogeneous thyroid with micronodules | Diffuse uptake | Hamouche [17] |
| M | 70 | Thailand | ChAdOx1 | No | 2nd | None | N/A | Myalgia, palpitations, exertional dyspnea | 2 | <0.0036 (N: 0.35–4.94) | >20 (N: 1.88–3.18) | 3.19 (N: 0.7–1.48) | N/A | N/A | 3.23 (N: 0–1.75) | N/A | N/A | Sriphrapradang [21] |
| F | 38 | Spain | BNT162b2 | No | 1st | None | Schizophrenia | Behavioral disturbance, insomnia, sweating | 12 | <0.008 (N: 0.35–4.95) | 7.46 (N: 0.7–1.48) | 2.01 (N: 0.7–1.48) | 36.57 (N: 0–5.6) | 3303.71 (N: 0–5.6) | 12.54 (N: 0–0.7) | Reduced echogenicity, echogenic septa, hypervascularity | Diffuse toxic goiter | Pujol [19] |
| F | 38 | USA | BNT162b2 | No | 1st | None | None | Fever, tachycardia, GI symptoms (thyroid storm) | 5 | <0.008 (N: 0.45–4.5) | N/A | 8.39 (N: 0.82–1.77) | N/A | 1730 (N: 0–9) | 32 (N: 0–1.75) | Enlargement and hypervascularity | N/A | Weintraub [23] |
| F | 63 | USA | mRNA-1273 | No | 1st | None | N/A (sister with LES) | Pruritic rash upper chest and neck | 7 | 0.011 (N: 0.55–4.78) | N/A | 2.4 (N: 0.9–1.8) | N/A | 1149 (N: 0–9) | 22 (N: 0–1.75) | Heterogeneous thyroid and hypervascularity | Diffuse uptake | Weintraub [23] |
| M | 30 | USA | BNT162b2 | No | 2nd | None | None (mother post- partum GD) |
Weight loss, irritability, palpitations, tremors, restless sleep | 28 | <0.005 (N: 0.45–4.5) | N/A | 1.77 (N: 0.82–1.77) | N/A | 15 (N: 0–34) | N/A (TSI +ve) | N/A | N/A | Weintraub [23] |
| F | 40 | China | BNT162b2 | No | 2nd | Hypothyroidism/None | None | Palpitations and tachycardia | 35 | <0.02 (N: 0.47–4.68) | 19.8 (N: 2.77–5.29) | 5.17 (N: 0.7–2.19) | 7.2 (N: 0–4) | 239.2 (N: 0–5) | N/A (TSI +ve) | Heterogeneous thyroid and hypervascularity | Diffuse uptake | Wai Lu [22] |
| F | 35 | Australia | ChAdOx1 | No | 1st | None/Hyperthyroidism | None | Palpitations, hyperphagia, heat intolerance and tremors | 5 | <0.02 (N: 0.5–4) | >19.5 (N: 2.2–3.9) | 4.97 (N: 0.77–1.55) | 33 (N: 0–4.5) | >1300 (N: 0–4.5) | 24 (N: 0–0.55) | Heterogeneous thyroid and hypervascularity/A | Raven [20] | |
| F | 46 | South Korea | ChAdOx1 | N/A | 1st | None | N/A | Chest pain, dyspnea | 1 | 0.01 (N: 0.55–4.78) | N/A | 2.63 (N: 0.89–1.76) | 137.5 (N: 0–115) | 77.72 (N: 0–34) | 6.42 (N: 0–1.75) | Diffuse Hypervascularity | Diffuse uptake | Lee [18] |
| F | 73 | South Korea | ChAdOx1 | N/A | 2nd | None | N/A | Weight loss, dyspnea | 14 | <0.008 (N: 0.55–4.78) | N/A | 5.7 (N: 0.89–1.76) | N/A | 41.03 (N: 0–34) | 6.30 (N: 0–1.75) | Diffuse Hypervascularity | Diffuse uptake | Lee [18] |
| M | 20 | India | ChAdOx1 | N/A | 1st | None | None | Weight Loss, tremors | 7 | 0.002 (N: 0.34–5.60) | N/A | N/A | N/A | N/A | 2.6 (<1.22) | N/A | N/A | Chaudhary [27] |
| F | 46 | India | ChAdOx1 | N/A | 1st | None/AITD | None | Weight loss | 10 | <0.01 (N: 0.34–5.60) | N/A | N/A | N/A | N/A | >40 (<1.22) | N/A | N/A | Chaudhary [27] |
| F | 19 | India | ChAdOx1 | N/A | 1st | None/AITD | None | Weight loss, palpitations, hair loss | 28 | <0.01 (N: 0.34–5.60) | N/A | N/A | N/A | N/A | 7.32 (<1.22) | N/A | N/A | Chaudhary [27] |
| F | 37 | India | ChAdOx1 | N/A | 1st | None/AITD | None | Weight loss, palpitations, increased defecation | 14 | <0.01 (N: 0.34–5.60) | N/A | N/A | N/A | N/A | 4.37 (<1.22) | N/A | N/A | Chaudhary [27] |
| F | 31 | Japan | BNT162b2 | N/A | 2nd | None | Type 1 Diabetes | Dyspnea, sweating, diarrhea | 7 | <0.005 (N: 0.61–4.23) | 32.5 (N: 2.3–4) | >7.77 (N: 0.9–1.7) | 82 (<28) | 481 (<16) | 11.9 (<2.0) | Diffuse Hypervascularity | N/A | Sakai [28] |
| M | 22 | Belgium | BNT162b2 | Yes | 1st | None | Ulcerative Colitis and Nephrotic Syndrome | Tremors | 14 | <0.01 (N: 0.27–4.20) | 17.7 (N: 3.10–6.8) | 3.17 (N: 0.9–1.7) | N/A | N/A | 3.76 (<0.55) | Heterogeneous thyroid and hypervascularity | Diffuse uptake | Manta [29] |
| F | 44 | France | BNT162b2 | N/A | 1st | AITD/None | N/A | None | 5 | <0.01 (N: N/A) | N/A | N/A | N/A | N/A | N/A(+ve) | Diffuse Hypervascularity | N/A | Bres [30] |
| F | 45 | Singapore | BNT162b2 | N/A | 1st | None | None | Chest pain, palpitations | 4 | <0.005 (N: 0.7–4.28) | N/A | 3.5 (N: 0.98–1.57) | N/A | 0.3 (N: N/A) | 5.75 (N: <1.76) | Heterogeneous thyroid and hypervascularity | N/A | Chua [31] |
| F | 43 | Tunisia | BNT162b2 | No | 1st | None | None | Palpitations, sleep disorders, heat intolerance, asthenia | 3 | <0.002 (N: 0.38–5.33) | N/A | 5.12 (N: 0.93–1.7) | N/A | N/A | 3.1 (N: <1) | N/A | Diffuse uptake | Taieb [32] |
| M | 57 | Mexico | ChAdOx1 | No | 1st | None | None | Tremor, palpitations, weight loss, asthenia | 7 | <0.005 (N: 0.3–3) | N/A | 4 (N: 0.6–1.2) | N/A(+ve) | N/A(+ve) | N/A | Enlargement and hypervascularity | Diffuse uptake | Cuenca [33] |
| F | 39 | Taiwan | mRNA-1273 | N/A | 1st | AITD/none | None | Palpitations, tremors | 14 | <0.0038 (N: 0.35–4.94) | N/A | 1.54 (N: 0.7–1.48) | <3.0 (N: <14.4) | 64.58 (N: <5.61) | 42.4 (N: <10) | N/A | N/A | Shih [34] |
| F | 59 | Taiwan | ChAdOx1 | N/A | 1st | None/AITD | None | Dyspnea, palpitations, dizziness | 14 | <0.0038 (N: 0.35–4.94) | N/A | 2.28 (N: 0.7–1.48) | 1494.78 (N: <14.4) | <0.3 (N: <5.61) | 68.7 (N: <10) | N/A | N/A | Shih [34] |
| F | 44 | Taiwan | ChAdOx1 | N/A | 1st | None | None | Tremors, weight loss, heat intolerance | 4 | <0.0038 (N: 0.35–4.94) | N/A | 2.74 (N: 0.7–1.48) | 2904.39 (N: <14.4) | 206.64 (N: <5.61) | 80.9 (N: <10) | N/A | N/A | Shih [34] |
| M | 42 | USA | mRNA-1273 | N/A | 3rd | None | None | Dyspnea, sleep disturbance, weight loss, asthenia, nausea, headache | 2 | <0.015 (N: 0.45–4.5) | N/A | 5.96 (N: 0.78–2.19) | N/A | 70.25 (N: <5.6) | 16.1 (N: <1.75) | Heterogeneous thyroid and hypervascularity | Diffuse uptake | Singh [35] |
| F | 68 | USA | Ad26. COV2.S |
N/A | 1st | None | None | Atrial fibrillation | 30 | <0.01 (N: 0.45–4.5) | 13.8 (N: 2.5–3.9) | 3.6 (N: 0.78–0.6–1.3) | N/A | 5.84 (N: <5.6) | 14.3 (N: <1.75) | N/A | Diffuse uptake | Singh [35] |
| M | 50 | Italy | BNT162b2 | N/A | 1st | None/AITD | None | Asthenia, palpitations, tremors, sleep disturbance | 14 | 0.001 (N: 0.25–0.4) | 10.47 (N: 2–4) | 2 (N: 0.7–1.48) | 385.49 (N: <40) | 529.5 (N: <10) | 5 (N: <1) | Enlargement and hypervascularity | Diffuse uptake | Ruggeri [36] |
| M | 50 | Italy | BNT162b2 | N/A | 1st | None/AITD | None | Asthenia, palpitations, tremors, sleep disturbance | 14 | 0.001 (N: 0.25–0.4) | 10.47 (N: 2–4) | 2 (N: 0.7–1.48) | 385.49 (N: <40) | 529.5 (N: <10) | 5 (N: <1) | Enlargement and hypervascularity | Diffuse uptake | Ruggeri [36] |
| F | 47 | Turkey | BNT162b2 | No | 1st | None | None | Sweating, palpitations | 5 | <0.01 (N: 0.27–4.2) | 11 (N: 2.4.4) | 3.32 (N: 0.93–1.7) | 320 (N: <115) | 11.2 (N: <34) | 22.7 (N: <1.5) | Heterogeneous thyroid and hypervascularity | N/A | Bostan [37] |
| M | 46 | Turkey | BNT162b2 | No | 2nd | None | None | Sweating, palpitations, weight loss | 21 | <0.01 (N: 0.27–4.2) | 25.3(N: 2.4.4) | >7.77 (N: 0.93–1.7) | 334 (N: <115) | 146 (N: <34) | 9.10 (N: <1.5) | Enlargement and hypervascularity | N/A | Bostan [37] |
| F | 51 | Turkey | BNT162b2 | N/A | 2nd | None | Type 2 Diabetes, Hypertension | Sweating, palpitations, fever | 4 | <0.01 (N: 0.27–4.2) | 12.6 (N: 2.4.4) | 3.72 (N: 0.93–1.7) | 18.2 (N: <115) | 12.4 (N: <34) | 5.04 (N: <1.5) | Enlargement and hypervascularity | Diffuse toxic goiter | Bostan [37] |
| F | 53 | Turkey | BNT162b2 | Yes | 2nd | AITD/none | None | Sweating, palpitations, weight loss | 7 | <0.01 (N: 0.27–4.2) | 8.83 (N: 2.4.4) | 4.01 (N: 0.93–1.7) | 1197 (N: <115) | 55 (N: <34) | 17.8 (N: <1.5) | Heterogeneous thyroid and hypervascularity | Diffuse uptake | Bostan [37] |
| F | 33 | China | N/A (mRNA) |
No | 1st | None/AITD | N/A | N/A | 7 | 0.01 (N:N/A) | N/A | 3.4 (N: 0.62–1.24) | N/A | N/A | 7.3 (N: <1) | N/A | N/A | Chee [38] |
| F | 37 | China | N/A (mRNA) |
No | 1st | None | N/A | N/A | 7 | <0.01 (N:N/A) | N/A | 4.6 (N: 0.62–1.24) | N/A | N/A | 3.8 (N: <1) | N/A | N/A | Chee [38] |
| F | 37 | China | N/A (mRNA) |
No | 2nd | None | N/A | N/A | 21 | <0.01 (N:N/A) | N/A | 5.5 (N: 0.62–1.24) | N/A | N/A | 11.2 (N: <1) | N/A | N/A | Chee [38] |
| F | 34 | China | N/A (mRNA) |
No | 1st | None/AITD | N/A | N/A | 26 | 0.01 (N:N/A) | 23.8 (N: 3.5–6) | 5.28 (N: 0.62–1.24) | N/A | N/A | 32 (N: <1) | N/A | N/A | Chee [38] |
| F | 33 | China | N/A (mRNA) |
No | 2nd | None | N/A | N/A | 9 | <0.01 (N:N/A) | N/A | 2.25 (N: 0.62–1.24) | N/A | N/A | 4.6 (N: <1) | N/A | N/A | Chee [38] |
| F | 43 | China | N/A (mRNA) |
No | 2nd | None | N/A | N/A | 13 | <0.01 (N:N/A) | >40 (N: 3.5–6) | 5.4 (N: 0.62–1.24) | N/A | N/A | 6.2 (N: <1) | N/A | N/A | Chee [38] |
Abbreviations: F: female; M: male; COVID-19: coronavirus disease 2019; AITD: autoimmune thyroid disease; SLE: Systemic lupus erythematosus; GD: Graves’ disease; DM: diabetes mellitus; AFib: atrial fibrillation; GI: gastro-intestinal; MMI: methimazole; N/A: not available; +ve: positive; TSH: thyrotropin; fT3: free triiodothyronine; fT4: free tiroxine; TPOAb: anti-thyroid peroxidase antibody; TgAb: anti-thyroglobuline antibody; TRAb: TSH receptor antibody; US: ultrasound.
Table 2.
Summary of demographic, clinical, and laboratory characteristics of Graves’s disease relapses following SARS-CoV-2 vaccination in the literature.
| Gender | Age | Country | Vaccine | History of COVID | Dose | Personal/Family History of AITD | Medical History | Symptoms | Days until Symptoms | TSH (mIU/L) | fT3 (ng/L) | fT4 (ng/dL) | TgAb (IU/mL) | TPOAb (IU/mL) | TRAb (IU/L) | Thyroid US | Thyroid Scan | Reference |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| F | 71 | Austria | BNT162b2 | No | 2nd | GD/none | N/A | Palpitations and sweating | 30 | N/A | 11.1 (N: 2.15–4.12) | 3.56 (N: 0.7–1.7) | N/A | N/A | 4.2 (N: 0–1.5) | Heterogeneous thyroid and hypervascularization | Mild increased uptake | Zettining [12] |
| F | 64 | Japan | BNT162b2 | No | 1st | Subclinical Hyperthyroidism/none | colorectal cancer, DM, obesity | Palpitations, dyspnea, fever, legs edema | 6 | <0.008 (N: N/A) | 23.2 (N: N/A) | 3.32 (N: N/A) | N/A | N/A | 33.8 (N: N/A) | Enlargement and hypervascularity | N/A | Yamamoto [26] |
| F | 34 | Belgium | BNT162b2 | No | 1st | GD/none | None | Tremors, sweating, weight loss, swelling of eyelids | 10 | <0.01 (N: 0.4–2.75) | 14.3 (N: 1.95–4.23) | 2.54 (N: 0.75–1.6) | N/A | N/A | >40 (N: 0–0.55) | N/A | N/A | Pierman [25] |
| F | 30 | Thailand | CoronaVac+ChAdOx1 | No | 3rd (ChAdOx1) |
GD on MTZ/none | None | Palpitations, weight loss, increased appetite | 4 | 0.006 (N: 0.35–4.94) | 3.21 (N: 1.88–3.18) | 1.29 (N: 0.7–1.48 | N/A | N/A | 13.4 (N: 0–1,75) | N/A | N/A | Sriphrapradang [24] |
| M | 34 | South Korea | Ad26. COV2.S |
N/A | 1st | GD/none | N/A | Weight loss, palpitations | 14 | <0.008 (N: 0.55–4.78) | N/A | 2.06 (N: 0.89–1.76) | N/A | N/A | 4.24 (N: 0–1.75) | Diffuse Hypervascularity | N/A | Lee [18] |
| M | 41 | Singapore | mRNA-1273 | N/A | 1st | GD/none | N/A | Tremors, palpitations | 5 | <0.001 (N: 0.7–4.28) | N/A | 3.74 (N: 0.98–1.57) | N/A | N/A | 3.85 (N: <1.76) | N/A | N/A | Chua [31] |
| F | 44 | Turkey | CoronaVac | No | 1st | GD/none | None | Sweating, palpitations, asthenia | 7 | <0.01 (N: 0.27–4.2) | 9.65 (N: 2.4.4) | 2.67 (N: 0.93–1.7) | 119 (N: <115) | 284 (N: <34) | 12.18 (N: <1.5) | Heterogeneous thyroid and hypervascularity | N/A | Bostan [37] |
| M | 49 | Turkey | BNT162b2 | No | 2nd | GD/none | None | Sweating, palpitations, tremors | 30 | <0.01 (N: 0.27–4.2) | 13.50 (N: 2.4.4) | 3.86 (N: 0.93–1.7) | 236 (N: <115) | 435 (N: <34) | 3.01 (N: <1.5) | Diffuse Hypervascularity | N/A | Bostan [37] |
| F | 31 | Turkey | BNT162b2 | No | 1st | GD/none | Breast cancer | Sweating, asthenia | 21 | <0.01 (N: 0.27–4.2) | 21.7 (N: 2.4.4) | >7.77 (N: 0.93–1.7) | 11 (N: <115) | 325 (N: <34) | 19.3 (N: <1.5) | Diffuse Hypervascularity | N/A | Bostan [37] |
| M | 59 | China | N/A (mRNA) |
No | 1st | GD/AITD | N/A | N/A | 21 | <0.01 (N:N/A) | N/A | 3.8 (N:0.62–1.24) | N/A | N/A | 12.8 (N: <1) | N/A | N/A | Chee [38] |
| F | 74 | China | N/A (mRNA) |
No | 2nd | GD/AITD | N/A | N/A | 11 | 0.02 (N:N/A) | N/A | 1.08 (N:0.62–1.24) | N/A | N/A | 6.2 (N: <1) | N/A | N/A | Chee [38] |
| F | 25 | China | N/A (mRNA) |
No | 2nd | GD/AITD | N/A | N/A | 11 | 0.01 (N:N/A) | 6.3 (N: 3.5–6) | 1.16 (N:0.62–1.24) | N/A | N/A | 2.9 (N: <1) | N/A | N/A | Chee [38] |
| F | 41 | China | N/A (mRNA) |
No | 2nd | GD/none | N/A | N/A | 28 | <0.01 (N:N/A) | N/A | 3.88 (N:0.62–1.24) | N/A | N/A | 3.9 (N: <1) | N/A | N/A | Chee [38] |
| F | 24 | China | N/A (mRNA) |
No | 2nd | GD/none | N/A | N/A | 63 | 0.01 (N:N/A) | N/A | 1.55 (N:0.62–1.24) | N/A | N/A | 2.4 (N: <1) | N/A | N/A | Chee [38] |
| F | 22 | China | N/A (mRNA) |
No | 1st | GD/none | N/A | N/A | 5 | 0.01 (N:N/A) | >40 (N: 3.5–6) | 5.43 (N:0.62–1.24) | N/A | N/A | 5.8 (N: <1) | N/A | N/A | Chee [38] |
Abbreviations: F: female; M: male; COVID-19: coronavirus disease 2019; AITD: autoimmune thyroid disease; GD: Graves’ disease; DM: diabetes mellitus; MMI: methimazole; N/A: not available; TSH: thyrotropin; fT3: free triiodothyronin. fT4: free tiroxine; TPOAb: anti-thyroid peroxidase antibody; TgAb: anti-thyroglobuline antibody; TRAb: TSH receptor antibody; US: ultrasound; Ref: reference.
Table 3.
Overview of new cases and relapses of Graves’ disease following SARS-CoV-2 vaccination reported in the literature.
| New Onset GD | GD Recurrence | |
|---|---|---|
| Number of Cases | 48 | 15 |
| Sex | F (70.8%)—M (29.2%) | F (73.3%)—M (26.7%) |
| Age (years), median [IQR] | 43 [IQR 35–50.5] | 41 [IQR 30–59] |
| Type of SARS-Cov-2 vaccine | BNT162b2 50% ChAdOx1 27% mRNA-1273 8.3% Ad26.COV2.S 2.2% Not specified (mRNA) 12.5% |
BNT162b2 33.3% ChAdOx1 + CoronaVac 6.6% Ad26.COV2.S 6.6% CoronaVac 6.6% mRNA-1273 6.6% Not specified (mRNA) 40% |
| Days to symptoms onset, median [IQR] | 10 [IQR 5–14] | 11 [IQR 6–28] |
| Major symptoms | palpitations (61.9%) weight loss (35.7%) distal tremor (28.6%) behavioral/sleep disorders (26.1%) asthenia (21.4%) GI symptoms (14.3%) |
palpitations (77.8%) sweating (55.5%) weight loss (33.3%) |
| TSH (IU/mL), median [IQR] | 0.008 [0.004–0.01] | 0.01 [0.008–0.01] |
| fT3 (ng/L), median [IQR] | 13.2 [9.83–19.9] | 13.5 [7.97–22.45] |
| fT4 (ng/dL), median [IQR] | 3.58 [254–5.34] | 3.32 [1.55–3.86] |
| TRAb (IU/L), median [IQR] | 6.45 [4.39–16-56] | 5.8 [3.85–13.4] |
Abbreviations: GD, Graves’ disease; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; IQR, interquartile range; GI, gastrointestinal; TSH, thyrotropin; fT3, free triiodothyronine; fT4, free tiroxine; TRAb, TSH receptor antibody.
Among the new cases of GD, 34 are female (70.8%) and 14 men (29.2%) with a median age of 43 years [IQR 35–50.5]. Eleven women (73.3%) and four men (26.7%) with a median age of 41 [IQR 30–59] had a GD relapse. Data were collected from patients living in Europe, North America, Asia, Africa, and Oceania. Of the patients, 29/63 (46%) received the BNT162b2 vaccine (24/48 (50%) in the newly diagnosed group and 5/15 (33.3%) among relapses); 14/63 (22.2%) (13/48 (27%), and 1/15 (6.7%), respectively) received the AstraZeneca’s ChAdOx1; 5/63 (7.9%) received Moderna’s mRNA-1273; and only 2/63 (3.2%) received Janssen’s Ad26.COV2.S and CoronaVac. In 12 cases, the brand of the vaccine was not specified, but only the type (mRNA based) [38]. All cases who received more than one shot received a homologous vaccination, except for one patient who received the first two doses of Sinovac’s CoronaVac and a third booster dose of ChAdOx1 (24). One patient complained of symptoms after the third dose of mRNA-1273 [35]. Only four patients had a documented previous COVID-19 infection and in all of these patients, a new diagnosis of GD was established.
3.2. Clinical Features
Among those with newly diagnosed GD, only 5 of 48 patients (10.4%) reported a history of thyroid disease: 1 patient was suffering from multinodular goiter [16], 1 from hypothyroidism of unspecified etiology but on hormone replacement therapy [22], and 3 from non-GD AITD [30,34,36]. On the other hand, family history for thyroid autoimmunity was inconsistently specified. A total of 31 out of 48 patients (64.6%) in the new diagnosis group, and 8 out of 15 (53.3%) in the relapse group, developed autoimmune hyperthyroidism after a single dose of vaccine; the median time from immunization to the onset of hyperthyroidism clinical features was 10 days [IQR 5–14] and 11 days [IQR 6–28], respectively.
In patients with new-onset GD following COVID vaccination, when reported (42/48), the most frequent symptoms were palpitations (26/42, 61.9%), weight loss (15/42, 35.7%), distal tremor (12/42, 28.6%), behavioral disturbances and sleep disturbances (11/42, 26.1%), asthenia (9/42, 21.4%), gastrointestinal disturbances (6/42, 14.3%), and finally, with lower frequency, fever, exertional dyspnea, sweating, heat intolerance, and headache. The median value of measured TSH was 0.008 IU/mL (0.4–4.00) [IQR 0.004–0.01], median fT3 13.2 ng/L (2.7–5.7) [IQR 9.83–19.9], and median fT4 3.58 ng/dL (0.7–1.7) [IQR 2.5–5.34], while increased levels of TRAb were reported in 43/48 cases (89.5%) with a median value of 6.45 IU/L (0–1.5) [IQR 4.39–16.56]. A total of 4/48 (8.3%) patients were investigated only for thyroid-stimulating immunoglobulins (TSI), which turned frankly positive in all cases.
Patients affected by GD relapse, when reported (9/15), complained mainly of palpitations (77.8%), sweating (55.5%), and weight loss (33.3%); the thyroid profile showed a median TSH value of 0.01 mIU/L (0.4–4.00) [IQR 0.008–0.01], median fT3 of 13.5 ng/L (2.7–5.7) [IQR 7.97–22.45], median fT4 of 3.32 ng/dL (0.7–1.7) [IQR 1.55–3.86], and TRAb level above the normal range in all cases (100%).
In both groups, antibodies directed against thyroid antigens (thyroglobulin antibodies (TgAb) and thyroperoxidase antibodies (TPOAb)) were occasionally measured, just as an imaging examination (thyroid ultrasound or RAIU) was not always performed (Table 1 and Table 2). When available, the neck ultrasonography showed a picture of a widespread increase in gland size and vascularity, while the RAIU was high.
Given the short period of observation of these patients, all the authors reported the administration of antithyroid drugs (Thionamides) and beta-blockers as an initial therapy and symptom-control strategy for hyperthyroidism.
4. Discussion
Previous studies have already reported that vaccines, including those against human papillomavirus (HPV), hepatitis B (HBV), and influenza, can trigger the development or recurrence of autoimmune diseases, including chronic lymphocytic thyroiditis [43,44,45]. To respond quickly and efficaciously to the global health emergency represented by the SARS-CoV-2 pandemic, several vaccines have been approved in a short time: some of them use existing technologies, such as viral vectors (ChAdOx1, Ad26.COV2.S) [46,47] or inactivated viruses (CoronaVac) [48], but others have been based on platforms never used before, such as those based on mRNA: BNT162b2 and mRNA-1273. The latter uses a carrier system for the nucleic acid consisting of lipid nanoparticles that transfer the mRNA encoding for the antigen (SARS-CoV-2 spike protein) inside the host cells, where it is translated by the ribosomes and stimulates a robust immune response mediated by CD4 +and CD8 + T-cells [49].
The GD cases are largely collected following the administration of mRNA-based vaccines (46/63, 73%). However, it must be mentioned that these types of vaccine are the most widely administered globally. In fact, as of 11 August 2022, in the European Union, more than 1 billion shots out of approximately 1,2 billion doses administered were produced by Pfizer-Biontech and Moderna [50].
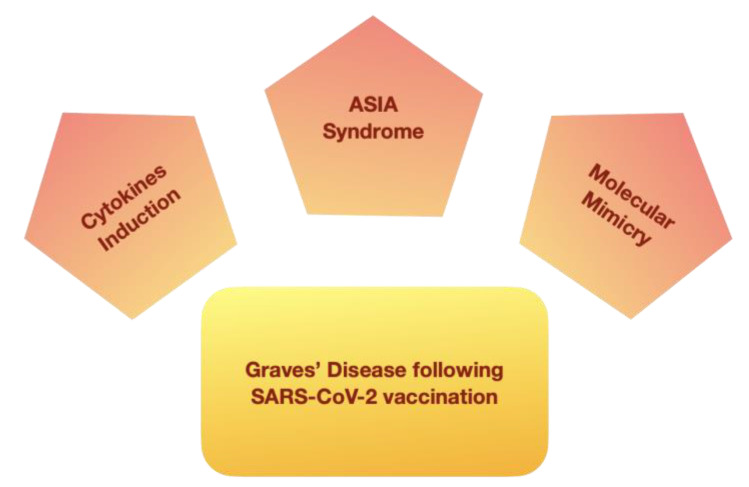
Several pathogenetic mechanisms have been considered to explain the development of thyroid autoimmune reactions after SARS-CoV-2 vaccination (Figure 2).
Figure 2.
Potential pathogenetic mechanisms underlying the development of GD following SARS-CoV-2 vaccination.
Many authors agree that these manifestations are the result of the “autoimmune/inflammatory syndrome induced by adjuvants” (ASIA), defined by Shoenfeld in 2011 [51]. According to this theory, the adjuvants contained in the vaccine with the aim of increasing their immunogenicity can activate an immunological cascade capable, in predisposed subjects, of breaking the immunological tolerance towards self-antigens. In adenovirus-based vaccines (ChAdOx1), this role could be played by buffer/oxidation inhibitor molecules (histidine) and non-ionic surfactant (polysorbate 80), while in inactivated virus vaccines (CoronaVAc), this role is played by aluminum salts [52]. Of mRNA-based vaccines (BNT162b2 and mRNA-1273), both the nucleic acid molecule itself, capable of inducing the so-called self-adjuvant effect [53], and the lipid conjugates of polyethylene glycole (PEG) [54], which stabilize the transport nanoparticles, are believed to be responsible. Moreover, PEGs have also been considered to be the culprit of hypersensitivity and anaphylaxis reactions [55]. Regarding the ASIA syndrome hypothesis, in animal models, inflammatory responses induced by the lipid nanoparticles have been described, and they were characterized by a significant neutrophil infiltrate and by the production of numerous cytokines and chemokines, including IL-1beta/IL-6 and the macrophage inflammatory protein-α and macrophage inflammatory protein-β, which in turn could trigger a sustained inflammatory response [56].
According to Sprent and King [57], the adverse effects of anti-COVID-19 vaccines are nothing more than the epiphenomenon of an important production of interferon (IFN) type 1 and thus of a concomitant activation of the immune response. Precisely these cytokines, such as IFN-alpha, IFN-gamma, and CXCL10/IP10, peculiar of Th1-type immune response, play a crucial role in the pathogenesis of autoimmune thyroid diseases, including GD and Graves’ ophthalmopathy (GO) [58,59,60,61,62].
In addition, Poma et al. recently demonstrated that thyrocytes with direct evidence of SARS-CoV-2 genome and antigens taken from patients who died of COVID-19 carry transcriptional variations of the immunity genes, resulting in an important activation of IFN type 1 (IFN alpha) and type 2 (IFN gamma) pathways, which in turn are able to induce or reactivate thyroid autoimmunity [63]. Therefore, although the above data were obtained following natural infection, it could be speculated that the initial burst in IFN-1 release induced by vaccination could also contribute to triggering autoimmune reactions in predisposed subjects, similarly to what seems to be possible after the virus entry into the cells.
A further mechanism considered plausible for the development of autoimmune reactions from the anti-SARS-CoV-2 vaccine is represented by the “molecular mimicry” and by the cross-reactivity between some SARS-CoV-2 proteins and a variety of host antigens. In fact, it has been shown that the spike protein, the nucleoprotein, and the membrane protein of SARS-CoV-2 all cross-react with thyroid peroxidase (TPO) due to the similarity and homology of peptide sequences between this thyroid enzyme and the viral proteins [64,65]. Therefore, the SARS-CoV-2 spike protein produced within the host cells to stimulate the immune response against it could induce autoimmune reactions through the molecular mimicry mechanism. However, as observed for other vaccines (HPV, influenza and HBV) [43,44,45], the development of cross reactivity between exogenous and endogenous antigens seems limited to a minority of vaccinated subjects, demonstrating that even “molecular mimicry”, such as ASIA syndrome, requires an individual predisposition, probably of genetic nature. Nevertheless, to date, no data capable of explaining or predicting this susceptibility are available.
5. Conclusions
The risk of developing autoimmune sequelae after vaccination remains to be defined and there are no universally accepted criteria for their diagnosis yet. In addition, the management of these phenomena is not well defined, and the standard therapies for the “sporadic” counterparts are generally adopted. Moreover, it is becoming challenging for healthcare workers to establish the pertinence to inject the next scheduled shot in patients who have suffered from debilitating autoimmune sequelae such as in some cases of severe hyperthyroidism.
After all, we support and encourage the COVID-19 vaccination campaign as a major weapon in the fight against the pandemic, but at the same time, we want to underline the importance of being vigilant for the development of any autoimmune adverse events, including GD and hyperthyroidism, in order to have a rapid diagnosis along with the proper management of affected patients.
The small number of available papers and cases does not allow a statistical interpretation of the results, and the proposed pathogenetic mechanisms are only attempts to describe the development of this immune event without proving a causal relationship. Despite these limitations, our paper provides the most updated insight into the topic.
More than a year after the beginning of the immunization campaign, in this paper, we review the new cases and relapses of GD arising after the anti-SASR-CoV-2 vaccination, and we discuss the main features of the affected patients and the most plausible pathogenetic mechanisms (ASIA syndrome, cytokines induction, molecular mimicry, and cross-reactivity): considering the small number of cases compared to millions of vaccinated, an individual predisposition seems required; furthermore, the entire literature considered here consisted of case reports or series, proving only a temporal association.
The aim of this review is to raise awareness of healthcare workers about the possibility of developing GD and other autoimmune sequelae after SARS-CoV-2 vaccination, with the hope that future studies will allow us to identify the exact underlying pathogenetic mechanisms and subjects at highest risk.
Author Contributions
All the authors contributed to this paper. Writing, A.P., S.M.F., A.A. and P.F.; Review and editing, V.M., S.R.P., G.E. and F.R. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.World Health Organization (WHO) Coronavirus (COVID-19) Dashboard. [(accessed on 31 July 2022)]. Available online: https://covid19.who.int.
- 2.Guan W.J., Ni Z.Y., Hu Y., Liang W.H., Ou C., He J., Liu L., Shan H., Lei C., Hui D.S.C., et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mathieu E., Ritchie H., Ortiz-Ospina E., Rosr M., Hasell J., Appel C., Giattin C., Rodes-Guirao L. A global database of COVID-19 vaccinations. Nat. Hum. Behav. 2021;5:947–953. doi: 10.1038/s41562-021-01122-8. [DOI] [PubMed] [Google Scholar]
- 4.Baden L.R., El Sahly H.M., Essink B., Kotloff K., Frey S., Novak R., Ciembert D., Spector S.A., Rouphael N., Creech C.B., et al. Efficacy and Safety of the mRNA-1273 SARS-CoV-2 Vaccine. N. Engl. J. Med. 2021;384:403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Polack F.P., Thomas S.J., Kitchin N., Absalon J., Gurtman A., Lockhart S., Perez J.L., Perez Marc G., Moreira E.D., Zerbini C., et al. Safety and Efficacy of the BNT162b2 mRNA Covid-19 Vaccine. N. Engl. J. Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castells M.C., Phillips E.J. Maintaining Safety with SARS-CoV-2. N. Engl. J. Med. 2021;384:643–649. doi: 10.1056/NEJMra2035343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mevorach D., Anis E., Cedar N., Bromber M., Haas E.J., Nadir E., Olsha-Castell S., Arad D., Hasin T., Levi N., et al. Myocarditis after BNT162b2 mRNA Vaccine against COVID-19 in Israel. N. Engl. J. Med. 2021;385:2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Arepally G.M., Ortel T.L. Vaccine-induced immune thrombotic thrombocytopenia: What we know and do not know. Blood. 2021;138:293–298. doi: 10.1182/blood.2021012152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y., Xu Z., Wang P., Li X., Shuai Z., Ye D., Pan H. New-onset autoimmune phenomena post-COVID-19 vaccination. Immunology. 2022;165:386–401. doi: 10.1111/imm.13443. [DOI] [PubMed] [Google Scholar]
- 10.Iremli B.G., Sendur S.N., Unluturk U. Three Cases of Subacute Thyroiditis Following SARS-CoV-2 Vaccine: Postvaccination ASIA Syndrome. J. Clin. Endocrinol. Metab. 2021;106:2600–2605. doi: 10.1210/clinem/dgab373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vera-Lastra O., Ordinola Navarro A., Pilar Cruz Domigiez M., Medina G., Sanchez Valadez T.I., Jara L.J. Two cases of Graves’ disease following SARS-CoV-2 vaccination: An aoutoimmune/inflammatory syndrome induced by adjuvants. Thyroid. 2021;136:168–186. doi: 10.1089/thy.2021.0142. [DOI] [PubMed] [Google Scholar]
- 12.Zettining G., Krebs M. Two further cases of Graves’ disease following SARS-CoV-2 vaccination. J. Endocrinol. Investig. 2022;45:227–228. doi: 10.1007/s40618-021-01650-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patrizio A., Ferrari S.M., Antonelli A., Fallahi P. A case of Graves’ disease and type 1 diabetes mellitus following SARS-CoV-2 vaccination. J. Autoimmun. 2021;125:102738. doi: 10.1016/j.jaut.2021.102738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pla Peris B., Merchante Alfaro A.A., Maravall Royo F.J., Abellan Galiana P., Perez Naranjo S., Gonzalez Boillos M. Thyrotoxicosis following SARS-CoV-2 vaccination: A case series and discussion. J. Endocrinol. Investig. 2021;5:1071–1077. doi: 10.1007/s40618-022-01739-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Di Filippo L., Castellino L., Giustina A. Occurrence and response to treatment of Graves’ disease after COVID vaccination in two male patients. Endocrine. 2022;75:19–21. doi: 10.1007/s12020-021-02919-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goblirsch T.J., Paulson A.E., Tashko G., Mekonnen A.J. Graves’ disease following administration of second dose of SARS-CoV-2 vaccine. BMJ Case Rep. 2021;14:e246432. doi: 10.1136/bcr-2021-246432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hamouche W., El Soufi Y., Alzaraq S., Okafor B.V., Zhang F., Paras C. A case report of new onset graves’ disease induced by SARS-CoV-2 infection or vaccine? J. Clin. Transl. Endocrinol. Case Rep. 2022;23:100104. doi: 10.1016/j.jecr.2021.100104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee K.A., Kim Y.J., Jin H.Y. Thyrotoxicosis after COVID-19 vaccinations: Seven case reports and a literature review. Endocrine. 2021;74:470–472. doi: 10.1007/s12020-021-02898-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pujol A., Gomez L.A., Gallegos C., Nicolau J., Sanchis P., Gonzalez-Freire M., Lopez-Gonzalez A.A., Dotres K., Masmiquel L. Thyroid as a target of adjuvant autoimmunity/inflammatory syndrome due to mRNA-based SARS-CoV-2 vaccination: From Graves’ disease to silent thyroiditis. J. Endocrinol. Investig. 2022;4:875–882. doi: 10.1007/s40618-021-01707-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raven L.M., McCormack A.I., Greenfield J.R. Letter to the Editor From Raven et al: “Three Cases of Subacute Thyroiditis Following SARS-CoV-2 Vaccine”. J. Clin. Endocrinol. Metab. 2022;107:1767–1768. doi: 10.1210/clinem/dgab822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sriphrapradang C., Shantavasinkul P.C. Graves’ disease following SARS-CoV-2 vaccination. Endocrine. 2021;74:473–474. doi: 10.1007/s12020-021-02902-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wai Lui D.T., Lee K.K., Lee C.H., Lee A.C.H., Hung I.F.N., Beng Tan K.C. Development of Graves’ Disease After SARS-CoV-2 mRNA Vaccination: A Case Report and Literature Review. Front. Public Health. 2021;9:778964. doi: 10.3389/fpubh.2021.778964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Weintraub M.A., Ameer B., Gregory N.S. Graves Disease following SARS-CoV-2 Vaccine: Case Series. J. Investig. Med. High Impact Case Rep. 2021;9:23247096211063356. doi: 10.1177/23247096211063356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sriphrapradang C. Aggravation of hyperthyroidism after heterologous prime-boost immunization with inactivated and adenovirus-vectored SARS-CoV-2 vaccine in a patient with Graves’ disease. Endocrine. 2021;74:226–227. doi: 10.1007/s12020-021-02879-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pierman G., Delgrange E., Jonas C. Recurrence of Graves’ Disease (a Th1-type Cytokine Disease) Following SARS-CoV-2 mRNA Vaccine Administration: A Simple Coincidence? Eur. J. Case Rep. Intern. Med. 2021;8:002807. doi: 10.12890/2021_002807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamamoto K., Mashiba T., Takano K., Suzuki T., Kami M., Takita M., Kusumi E., Mizuno Y., Hamaki T. A Case of Exacerbation of Subclinical Hyperthyroidism after First Administration of BNT162b2 mRNA COVID-19 Vaccine. Vaccines. 2021;9:1108. doi: 10.3390/vaccines9101108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chaudhary S., Dogra V., Walia R. Four cases of Graves’ disease following viral vector severe acute respiratory syndrome corona virus-2 (SARS-CoV-2) vaccine. Endocr. J. 2022;2022:EJ22-0208. doi: 10.1507/endocrj.EJ22-0208. [DOI] [PubMed] [Google Scholar]
- 28.Sakai M., Takao K., Kato T., Ito K., Kubota S., Hirose T., Liu Y., Mizuno M., Hirota T., Suwa T., et al. Graves’ disease after administration of severe respiratoru coronavirus (SARS-CoV-2) vaccine in a type 1 diabetes patient. Intern. Med. 2022;61:1561–1565. doi: 10.2169/internalmedicine.9231-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Manta R., Martin C., Muls V., Poppe K.G. New-onset Graves’ disease following SARS-CoV-2 vaccination: A case report. Eur. Thyroid J. 2022;11:e220049. doi: 10.1530/ETJ-22-0049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bres F., Joyeux M., Delemer B., Vitellius G., Barraud S. Three cases of thyroiditis after COVID-19 RNA-vaccine. Ann. Endocrinol. 2022;83:262–264. doi: 10.1016/j.ando.2022.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chua M.W.J. Graves’ disease after COVID-19 vaccination. Ann. Acad. Med. Singap. 2022;51:127–128. doi: 10.47102/annals-acadmedsg.2021398. [DOI] [PubMed] [Google Scholar]
- 32.Taieb A., Sawsen N., Asma B.A., Ghada S., Hamza E., Yosra H., Amel M., Molka C., Maha K., Koussay A. A rare case of grave’s disease after SARS-CoV-2 vaccine: Is it an adjuvant effect? Eur. Rev. Med. Pharmacol. Sci. 2022;26:2627–2630. doi: 10.26355/eurrev_202204_28500. [DOI] [PubMed] [Google Scholar]
- 33.Cuenca D., Aguilar-Soto M., Mercado M. A case of Graves’ disease following vaccination with the Oxford-AstraZeneca SARS-CoV-2 Vaccine: Case report and review of the literature. Eur. J. Case Rep. Intern. Med. 2022;9:003275. doi: 10.12890/2022_003275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shih S., Wang C. SARS-CoV-2 vaccination related hyperthyroidism of Graves’ disease. J. Formos. Med. Assoc. 2022;121:1881–1882. doi: 10.1016/j.jfma.2022.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Singh G., Howland T. Graves’ disease following COVID-19 vaccination. Cureus. 2022;14:e24418. doi: 10.7759/cureus.24418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ruggeri R.M., Giovanella L., Campennì A. SARS-CoV-2 vaccine may trigger thyroid autoimmunity: Real-life experience and review of the literature. J. Endocrinol. Investig. 2022:1–7. doi: 10.1007/s40618-022-01863-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bostan H., Ucan B., Kizilgul M., Calapkulu M., Hepsen S., Gul U., Unsal I., Cakal E. Relapsed and newly diagnosed Graves’ disease due to immunization against COVID-19: A case series and review of the literature. J. Autoimmun. 2022;128:102809. doi: 10.1016/j.jaut.2022.102809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chee Y.J., Liew H., Han Hoi W., Lee Y., Lim B., Chin H.X., Rui Lai R.T., Koh Y., Tham M., Seow C.J., et al. SARS-CoV-2 mRNA vaccination and Graves’ diseae: A report of 12 cases and review f the literature. J. Clin. Endocrinol. Metab. 2022;107:2324–2330. doi: 10.1210/clinem/dgac119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Antonelli A., Fallahi P., Elia G., Ragusa F., Paparo S.R., Ruffili I., Patrizio A., Gonnella D., Giusti C., Virili C., et al. Graves’ disease: Clinical manifestations, immune pathogenesis (cytokines and chemokines) and therapy. Best Pract. Res. Clin. Endocrinol. Metabol. 2020;34:101388. doi: 10.1016/j.beem.2020.101388. [DOI] [PubMed] [Google Scholar]
- 40.Antonelli A., Ferrari S.M., Ragusa F., Elia G., Paparo S.R., Ruffili I., Patrizio A., Giusti C., Gonnella D., Cristaudo A., et al. Graves’ disease: Epidemiology, genetic and environmental risk factors and viruses. Best Pract. Res. Clin. Endocrinol. Metabol. 2020;34:101387. doi: 10.1016/j.beem.2020.101387. [DOI] [PubMed] [Google Scholar]
- 41.Ross D.S., Burch H.B., Copper D.S., Greenlee M.C., Laurberg P., Maia A.L., Rivkees S.A., Samuels M., Sosa J.A., Stan M.N., et al. 2016 American Thyroid Association Guidelines for Diagnosis and Management of Hyperthyroidism and Other Causes of Thyrotoxicosis. Thyroid. 2016;26:1343–1421. doi: 10.1089/thy.2016.0229. [DOI] [PubMed] [Google Scholar]
- 42.Paje M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 statement: An uptdate guidelin for reporting systematic reviews. BMJ. 2021;372:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pelelgrino P., Radice S., Clementi E. Immunogenicity and safety of the human papillomavirus vaccine in patients with autoimmune diseases: A systematic review. Vaccine. 2015;33:3444–3449. doi: 10.1016/j.vaccine.2015.05.041. [DOI] [PubMed] [Google Scholar]
- 44.Watad A., David P., Brown S., Shoenfeld Y. Autoimmune/inflammatory Syndrome Induced by Adjuvants and Thyroid Autoimmunity. Front. Endocrinol. 2017;7:150. doi: 10.3389/fendo.2016.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bragazzi N.L., Hejly A., Watad A., Adawi M., Amital H., Shoenfeld Y. ASIA syndrome and endocrine autoimmune disorders. Best Pract. Res. Clin. Endocrinol. Metabol. 2020;34:101412. doi: 10.1016/j.beem.2020.101412. [DOI] [PubMed] [Google Scholar]
- 46.Ramasamy M.N., Minassian A.M., Ewer K.J., Flaxman A.L., Folegatti P.M., Owens D.R., Voysey M., Aley P.K., Angus B., Babbage G., et al. Safety and immunogenicity of ChAdOx1 nCoV-19 vaccine administered in a prime-boost regimen in young and old adults (COV002): A single-blind, randomised, controlled, phase 2/3 trial. Lancet. 2021;396:1979–1993. doi: 10.1016/S0140-6736(20)32466-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sadoff J., Gray G., Vandebosch A., Cardenas V., Shukarev G., Grinsztejn B., Goepefert P.A., Truyers C., Fennema H., Spiessens B., et al. Safety and Efficacy of Single-Dose Ad26.COV2.S Vaccine against Covid-19. N. Engl. J. Med. 2021;384:2187–2201. doi: 10.1056/NEJMoa2101544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wu Z., Hu Y., Xu M., Chen Z., Yang W., Jiang Z., Li M., Jin H., Cui G., Chen P., et al. Safety, tolerability, and immunogenicity of an inactivated SARS-CoV-2 vaccine (CoronaVac) in healthy adults aged 60 years and older: A randomised, double-blind, placebo-controlled, phase 1/2 clinical trial. Lancet Infect. Dis. 2021;21:803–812. doi: 10.1016/S1473-3099(20)30987-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cagigi A., Lorè K. Immune response induced by mRNA Vaccination in Mice, Monkeys and Humans. Vaccines. 2021;9:61. doi: 10.3390/vaccines9010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.European Centre for Disease Prevention and Control (ECDC) COVID-19 Vaccine Tracker. [(accessed on 26 August 2022)]. Available online: https://vaccinetracker.ecdc.europa.eu/public/extensions/COVID19/vaccinetracker.html#uptake-tab)
- 51.Shoenfeld Y., Agmon-Levin N. ‘ASIA’—Autoimmune/inflammatory syndrome induced by adjuvants. J. Autoimmun. 2011;36:4–8. doi: 10.1016/j.jaut.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 52.Borgsteede S.D., Geersing T.H., Tempels-Pavlica Z. Other excipients than PEG might cause serious hypersensitivity reactions in COVID-19 vaccines. Allergy. 2021;76:1941–1942. doi: 10.1111/all.14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xu S., Yang K., Li R., Zhang L. mRNA Vaccine Era-Mechanisms, Drug Platform and Clinical Prospection. Int. J. Mol. Sci. 2020;21:6582. doi: 10.3390/ijms21186582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Klimek L., Novak N., Cabanillas B., Jutel M., Bousquet J., Akdis C.A. Allergenic components of the mRNA-1273 vaccine for COVID-19: Possible involvement of polyethylene glycol and IgG-mediated complement activation. Allergy. 2021;76:3307–3313. doi: 10.1111/all.14794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Garvey L.H., Nassr S. Anaphylaxis to the first COVID-19 vaccine: Is polyethylene glycol (PEG) the culprit. Br. J. Anaesth. 2021;126:106–108. doi: 10.1016/j.bja.2020.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ndeupen S., Qin Z., Jacobsen S., Bouteau A., Estanbouli H., Igyarto B. The mRNA-LNP platform’s lipid nanoparticle component used in preclinical vaccine studies is highly inflammatory. iScience. 2021;24:103479. doi: 10.1016/j.isci.2021.103479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sprent J., King C. COVID-19 vaccine side effects: The positives about feeling bad. Sci. Immunol. 2021;6:eabj9256. doi: 10.1126/sciimmunol.abj9256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Antonelli A., Ferrari S.M., Corrado A., Di Domenicantonio A., Fallahi P. Autoimmune thyroid disorders. Autoimmun. Rev. 2015;14:174–180. doi: 10.1016/j.autrev.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 59.Antonelli A., Fallahi P., Rotondi M., Ferrari S.M., Romagnani P., Grosso M., Ferrannini E., Serio M. Increased serum CXCL10 in Graves’ disease or autoimmune thyroiditis is not associated with hyper- or hypothyroidism per se, but is specifically sustained by the autoimmune, inflammatory process. Eur. J. Endocrinol. 2006;154:651–658. doi: 10.1530/eje.1.02137. [DOI] [PubMed] [Google Scholar]
- 60.Antonelli A., Rotondi M., Fallahi P., Grosso M., Boni G., Ferrari S.M., Romagnani P., Serio M., Mariani G., Ferrannini E. Iodine-131 given for therapeutic purposes modulates differently interferon-gamma-inducible alpha-chemokine CXCL10 serum levels in patients with active Graves’ disease or toxic nodular goiter. J. Clin. Endocrinol. Metab. 2007;92:1485–1490. doi: 10.1210/jc.2006-1571. [DOI] [PubMed] [Google Scholar]
- 61.Fallahi P., Ferrari S.M., Ragusa F., Ruffili I., Elia G., Paparo S.R., Antonelli A. Th1 Chemokines in Autoimmune Endocrine Disorders. J. Clin. Endocrinol. Metab. 2020;105:1046–1060. doi: 10.1210/clinem/dgz289. [DOI] [PubMed] [Google Scholar]
- 62.Ferrari S.M., Ruffilli I., Elia G., Ragusa F., Paparo S.R., Patrizio A., Mazzi V., Antonelli A., Fallahi P. Chemokines in hyperthyroidism. J. Clin. Transl. Endocrinol. 2019;16:100196. doi: 10.1016/j.jcte.2019.100196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Poma M., Basolo A., Bonuccelli D., Proietti A., Macerola E., Ugolini C., Torregrossa L., Alì G., Giannini R., Vignali P., et al. Activation of Type I and Type II Interferon Signaling in SARS-CoV-2-Positive Thyroid Tissue of Patients Dying from COVID-19. Thyroid. 2021;31:1766–1775. doi: 10.1089/thy.2021.0345. [DOI] [PubMed] [Google Scholar]
- 64.Vojdani A., Vojdani E., Kharrazian D. Reaction of Human Monoclonal Antibodies to SARS-CoV-2 Proteins with Tissue Antigens: Implications for Autoimmune Diseases. Front. Immunol. 2021;11:3679. doi: 10.3389/fimmu.2020.617089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kanduc D., Shoenfeld Y. Molecular mimicry between SARS-CoV-2 spike glycoprotein and mammalian proteomes: Implications for the vaccine. Immunol. Res. 2020;68:310–313. doi: 10.1007/s12026-020-09152-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that the data supporting the findings of this study are available within the article.