Abstract
Oral nutrition interventions are commonly applied as an assistant therapeutic approach, which could affect the balance of the immunological response but with mixed evidence. The objective of this study is to identify the potential of different oral nutrition interventions for blood immune cell parameters in cancer patients. Randomized controlled trials, which were published in peer-reviewed journals in the language of English, and which identified the effects of different oral nutrition interventions on cancer patients, were screened and included in the databases of PubMed, Medline, Embase, and Web of Science. White blood cell count (WBC), lymphocyte count, CD4/CD8, and neutrophil count were selected as outcome measures. For the result, 11 trials were included. The agreement between authors reached a kappa value of 0.78. Beta-carotene supplementation has a high potential in inducing a positive effect on blood immune cell parameters for cancer patients (first positive for WBC and CD4/CD8, second positive for lymphocyte count), as well as a combination of physical exercise and hypocaloric healthy eating intervention (first positive for lymphocyte and neutrophil count, second positive for WBC). Oral nutrition supplementations with a single substance have less potential to provide a positive effect on blood immune cell parameters for cancer patients (glutamine: 0.30 and 0.28 to be the last selection for WBCs and lymphocytes; Omega 3: 0.37 to be the last selection for WBCs; Protein: 0.44 to be the last selection for lymphocytes; Zinc: 0.60 to be the last selection for neutrophils). In conclusion, the programs of immunonutrition therapy for different cancer patients might be different. The past perception that mixed oral nutritional supplementations are superior to oral nutritional supplements with a single substance might be wrong and the selection of oral nutritional supplementation need cautiousness. A combination of physical exercise might have a positive effect but also needs a higher level of evidence. Registration Number: CRD42021286396.
Keywords: nutrition, supplementation, immune, systematic review, network meta-analysis
1. Introduction
1.1. Rationale
Since one of the major characteristics of cancer is immune escape, monitoring the functioning of the immune system has a significant meaning during the treatments of cancers [1,2]. In recent years, immunonutrition has gradually become one of the hotspots in the related academic circle. Understanding the effect of nutrition and energy intake strategies on the functioning of the immune system in individuals with cancers has significant value in both the treatment and prevention processes of cancer. Under this situation, oral nutrition intervention is becoming one of the common assistant treatment protocols in cancer treatment; much evidence has identified that the nutrition intake, diet, and energy consumption would indirectly affect the body’s immune function of cancer patients because the metabolic processes could regulate immune cell responses [3,4,5,6]. For example, recent discoveries support a growing appreciation that microbial metabolites derived from bioactive foods are also important regulators of host immune and metabolic functions [7,8]. Moreover, a previous study has found that, in tumor-bearing mice, cyclic fasting or fasting-mimicking diets (FMDs) could enhance the activity of antineoplastic treatments by modulating systemic metabolism and boosting antitumor immunity and be safe, feasible, and resulting in a consistent decrease of blood glucose and growth factor concentration [9].
However, despite immunonutritional therapies seeming to have finally found their role in a wide range of tumors, several questions remain unanswered. Among these questions, the lack of validated biomarkers of response represents an important issue since only a proportion of cancer patients could benefit from immunotherapy. Based on these premises, a greater understanding of the role of potential biomarkers, including programmed death ligand 1 (PD-L1) expression, tumor mutational burden (TMB), microsatellite instability (MSI) status, gut microbiota, and several others, is necessary [10]. In addition, clinical trials on immunotherapy have widely differed in terms of drugs, patients, designs, terms of study phases, and inconsistent clinical outcomes [11].
When it comes to humans, as an important part of nutrition treatment protocols, oral nutrition interventions not only have a huge potential to provide a positive effect on the immune system function of cancer patients but also are very convenient in clinical practice. Oral nutrition interventions could be conducted at home so they are cheaper and easier to operate than enteral nutrition and injection, which could only be applied in hospitals and cost more money [12,13,14,15,16]. Some evidence has been provided to support the application of oral nutrition supplementation, but some have not. For example, the representative formula of Yanghe decoction in TCM was considered an important prophylactic and therapeutic treatment for breast cancer [17], which inhibits proliferation, reduces metastasis, and induces the apoptosis of breast cancer cells; its mechanism may be related to its inhibition of the activation of PI3K/Akt/NF-kB signaling pathway [18,19,20]. However, a study by Szefel’s team in the same year demonstrated that L-arginine supplementation did not support the hypothesis that L-arginine supplementation in colorectal cancer patients could reduce immunosuppression by decreasing the frequency of suppressor cells and increasing the frequency of effector CD4(+) T cells. It was not beneficial to the frequency of myeloid-derived suppressor cells and T lymphocytes in tumors and blood [21]. The potential mechanism of the heterogeneity might be that the integrated immune responses are correlated with dietary intake, energy utilization, and storage to immune regulation of tissue function [22]. At the same time, many trials have found paradoxical results of changes in nutrition and energy intake in the immune system function of both human and animal models [23,24].
At present, the best oral nutrition supplementation protocol for cancer patients is still unknown. The reasons were from many perspectives. Firstly, there are huge differences between different kinds of cancers [25]. Second, the function of the human immune system could be affected by many objective factors such as age, life habits, the gravity of the disease, comorbidities, etc. For example, the function of the human immune system will not change linearly with age; individuals with physical exercise habits may have a more frequent and lasting window of immune system function stress due to high-intensity exercise than sedentary ones [26,27]. Second, research published so far have mainly focusing on the correlation between nutrition and the functioning or state of the immune system in some special populations that need to pay attention to their immunometabolism, such as athletes [28], the elderly [29], infants [30], and pregnant females [31], as well as in individuals with metabolic or immune dysfunction such as type II diabetes, metabolic syndrome, or innate immunodeficiency [32,33,34]. Third, most of the present trials have focused on the correlations between different nutrition intake and diet strategies on the risk and mortality of cancer [35,36,37,38,39,40]. Last but not the least, the heterogeneity, which is created by the different designs and protocols of trials, results in vague and low-quality evidence for clinical practice.
The vagueness and heterogeneity of the evidence indicate the necessity of further comprehensive synthesis with a higher evidence level. According to the principle of evidence-based medicine (EBM), a registered systematic review with meta-analysis has the highest level in its evidence pyramid, and a network meta-analysis could compare more than two interventions synchronously, quantize and pool the effects of different treatment protocols together, and then rank these protocols according to a certain outcome measure. Therefore, a new systematic review with network meta-analysis is needed to make a mixed treatment comparison for different oral nutrition supplementation for cancer patients.
In addition, the results of the network meta-analysis provide the rank probabilities of interventions based on Bayes’ theorem and help clinical decision makers choose the optimal treatment protocols. Strictly speaking, the process of network meta-analysis is more in line with the spirit of EBM since its calculation is based on prior probabilities [41].
1.2. Objective
The objective of this systematic review is to identify the potential of different oral nutrition interventions for the blood immune cell parameters in cancer patients. It is the first network meta-analysis to identify the effects of different oral nutrition interventions on the blood immune cell parameters in cancer patients and could provide clinicians with high-level medical evidence for the control of immune indicators in the treatment of cancer patients. On the other hand, an adjusted and indirect comparison could compare more than two intervention protocols at the same time, bringing out information with more comprehensiveness for relevant clinical decisions.
2. Methods
2.1. Protocol and Registration
This systematic review was conducted according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) extension statement for reporting systematic reviews incorporating network meta-analyses of health care intervention guidelines [42]. Literature eligibility and exclusion criteria and the search strategy were proposed and agreed on by two authors (Yufei Fang and Yuting Zhang) with a priori to minimize bias. The PROSPERO registration number of this review is CRD42021286396.
2.2. Eligibility Criteria
2.2.1. Participants (P)
This systematic review included trials in which participants were patients (1) over 18 years old; (2) clinically diagnosed with non-digestive-tract cancer at all stages by oncologists; (3) clinically diagnosed with digestive tract cancer at all stages by oncologists; (4) without metastatic diseases.
2.2.2. Interventions (I)
This systematic review included trials in which participants in experimental groups were provided oral nutritional supplementations or asked to have energy restriction eating strategies as interventions. All the included interventions were reclassified according to the following protocols: (1) Oral nutrition supplementations would be reclassified according to their nutrition substances, for example, an intervention in which patients were asked to take oral syrups of zinc sulfate at mealtimes was reclassified as “Zinc” group, whereas interventions in which patients were taking standard amino acids was reclassified as “Protein” group; (2) interventions in which patients were taking oral nutrition supplementations with more than one substance were reclassified as “Mixed” group; (3) interventions in which patients were asked to follow a plan that combined physical exercise and energy restriction were reclassified as “Lifestyle” group.
It needed to be emphasized that, since the absorption process of enteral nutrition treatments and injection treatments were different from that of the oral nutrition supplementation (as had been mentioned in the introduction), enteral nutrition treatments and injection treatments must be conducted at a hospital whereas oral nutrition supplementation could be conducted at home. For the consideration of minimizing the inconsistency and heterogeneities within trials, as well as the application in clinical practice, enteral nutrition treatments and injection treatments were excluded from this systematic review.
2.2.3. Comparators (C)
This systematic review included trials in which participants in control groups were asked to maintain regular diets, conduct placebo intake protocols, or provide just patient education.
2.2.4. Outcomes (O)
In clinical practice for cancer patients, blood immune cell parameters are the most used indicators to monitor the functioning of the immune system and the “Golden Standard” in the diagnosis of many immune-related diseases such as viral infection, inflammation, and stress response, and are also one of the important risk assessment factors in the process of cancer treatment. In the cancer treatment process, clinicians draw blood from their patients regularly, assess their blood immune cell parameters, and then choose the assistant treatment protocols or adjust the parameters in the treatment process such as the timing, type, or dose of drug administration according to the assessment results [43].
In this systematic review, white blood cell count (WBC), lymphocyte count, the ratio of CD4 and CD8 (CD4/CD8), and neutrophil count were selected as outcomes. Only studies with cancer patients whose blood immune cell parameters were in the normal range according to the clinical standard at the baseline were included. The normal range of blood immune cell parameters was: (1) WBC—from 4.0 × 109/L to 10.0 × 109/L; (2) lymphocyte count—from 800/mm3 to 4000/mm3; (3) CD4/CD8—from 1.4 to 2.0; (4) neutrophil count—from 1800/mm3 to 6300/mm3.
2.2.5. Study Design (S)
Only randomized controlled trials were included in this systematic review.
2.2.6. Exclusion Criteria
Trials were excluded if: (1) They applied non-oral nutrition interventions such as injections and enteral nutrition interventions; (2) participants were patients with different types of cancer, or the type of cancer was not specified; (3) the study was a published abstract without full text or lacked data; (4) outcome measures did not correspond with those in the eligibility criteria.
2.3. Information Sources
A comprehensive, reproducible search strategy had been performed on the databases of PubMed, Medline, Embase, and Web of Science from January 1990 to May 2022. Reference lists were also searched in all screened trials for identifying grey literature that might be potentially eligible. When the data of any eligible trials were insufficient, the authors were contacted and the missing data were requested.
2.4. Search
The search terms used in each database were as follows: (1) in PubMed and Embase, the search term was “((cancer) OR (tumor) [Titile/Abstract]) AND ((immun*) [Title/Abstract]) AND ((randomized) OR (randomised) [Title/Abstract])”; (2) in Medline and Web of Science, the search term was “(AB cancer OR tumor) AND (AB randomized OR randomised) AND (AB immun*) NOT (TI design or protocol or review)”. The search terms for eligible interventions were not limited in the database searching process since many terms could refer to oral nutrition supplementations.
2.5. Study Selection
The screening of the eligibility of intervention was conducted in the abstract and full-text screening process to guarantee that all the potentially eligible studies could be included in this systematic review.
Trials that were searched from the database were imported into EndNote 20 (Thomson Reuters, Carlsbad, CA, USA) to further screen and remove duplicates. Since there were no uniform keywords about oral nutrition interventions in the titles of trials searched from the databases, two independent authors (Yining Xu and Yuting Zhang) screened all the titles of the searched trials to identify all the potential trials before the abstract screening.
2.6. Data Collection Process
Data were extracted by two independent authors (Yufei Fang and Feng Ren).
2.7. Data Items
Details of trials were summarized and information such as population characteristics (age, gender, nationality, and type of cancer) and intervention protocols with their classification were collected and put into an extraction sheet which summarized the included trials. The data of each trial, which involved the sample size (N), mean value (Mean) with its standard deviation (SD) of each outcome of each group in baseline, and every data recording point, were recorded in an independent extraction sheet for the data preprocessing.
2.8. Geometry of the Network
The network geometry was made by the Aggregate Data Drug Information System (Version 1.16.8, http://drugis.org/software/addis/index, accessed on 1 July 2022) to display all kinds of interventions and key information, such as the type of intervention represented by each node, direct comparisons between each pair of interventions represented by the edges, and the arms of each comparison, which are represented by the number on every edge. Only interventions could be included in an adjusted indirect comparison, or a mixed treatment comparison would be analyzed in a network meta-analysis.
2.9. Risk of Bias within Individual Studies
The risk of bias within individual studies was assessed by two independent authors (Yining Xu and Yufei Fang) by applying the Cochrane Collaboration Risk of Bias Assessment Tool [44] in the Cochrane Library Review Manager software (Version 5.3, Wiley, Chichester, UK). An independent arbitrator (Ee-chon Teo) was invited when a disagreement occurred. The agreement between authors was represented by Cohen’s kappa value.
A study which had no items with high risk and which had less than 3 (contain) items with unclear risk were regarded as overall low risk; a study which had no item with high risk, but had more than 3 items with unclear risk, were regarded as an overall moderate risk; a study which had one item with high risk was also regarded as overall moderate risk, while a study which had more than one item with high risk was regarded as overall high risk.
2.10. Summary Measures
The effect size of the network meta-analysis was presented in the form of mean differences (MD).
The results under the consistency model were shown in the rank probability plot. The sum of all rank probabilities is 1, both within a rank over treatments and within a treatment over ranks. Moreover, a league table was provided after the model of data analysis had been determined, reporting results that represented the mean difference in the column-defining treatment compared with the row-defining treatment.
The results under the inconsistency model were shown in a league table [41].
2.11. Planned Methods of Analysis
Data preprocessing and analysis were conducted by two independent authors (Yining Xu and Feng Ren). Microsoft Office Excel (Version 16.0, Microsoft Corporation, Redmond, WA, USA) was used to preprocess the original data by transferring all the outcomes into a uniform unit according to the clinical criteria. In this review, the WBC data was transferred into the unit of 109/L, the lymphocyte and neutrophil count were transferred into the unit of/mm3, and the CD4/CD8 was transferred into the standard decimal form that reserved two decimal fractions.
The Aggregate Data Drug Information System was used to pool data into the network meta-analysis and the Cochrane Library Review Manager (Version 5.3, Wiley, Chichester, UK) was applied to make the pair-wise meta-analysis.
In clinical practice, the medical nutrition treatment of most cancers aims to prevent the extreme increase of relevant immune cells induced by cancer and control the relevant immune cell count within the normal range. Therefore, in this review, the lower the blood immune cell parameters of WBC, lymphocyte, and neutrophil counts, the better. At the same time, CD4 mainly represents helper T cells and suppressor T cells, while CD8 represents killer T cells and cancer usually lowers CD4/CD8; therefore, in this review, the higher the CD4/CD8, the better.
2.12. Assessment of Inconsistency
The random-effects standard deviations were calculated under both consistency and inconsistency models and were compared with each other to identify if there was inconsistency within interventions. If there were closed loops in the intervention structure, the inconsistency of the evidence must be assessed. Moreover, while the results are easier to interpret, it requires a separate model to be run for each node to be split. The node-splitting analysis is an alternative method to assess inconsistency in network meta-analysis, which assesses whether direct and indirect evidence on a specific node (the split node) agree [45].
The consistency model was used if there was neither closed-loop nor split node in the intervention structure, the random-effects standard deviations in the consistency and inconsistency models were identical, or the identified discrepancy could be determined by examining the calculating a respective Bayesian p-value in the node-splitting analysis was statistically insignificant (p > 0.05). Otherwise, the inconsistency model should be applied [41].
2.13. Risk of Bias across Studies
The risk of bias across studies was assessed by two independent authors (Yining Xu and Yufei Fang) by applying the Cochrane Collaboration Risk of Bias Assessment Tool [44] in the Cochrane Library Review Manager software (Version 5.3, Wiley, Chichester, UK).
2.14. Additional Analyses
The Confidence in Network Meta-Analysis (CINeMA https://cinema.ispm.unibe.ch, assessed on 1 July 2022) was used to evaluate the confidence and assess the reporting bias in the findings from the network meta-analysis. According to the method research of CINeMA, if the item “within-study bias” was a “Major concern”, the confidence should be downgraded by one level. If other items were “Some concern”, the confidence would be downgraded by one level and if they were “Major concern”, the confidence would be downgraded by two levels [46,47].
The summarizing risk of bias assessments, which were set at “Average RoB”, applied a weighted average score for each relative effect estimate according to the percentage contribution of studies at each bias level. For example, studies of a direct comparison, which had low (arbitrarily assigned a score of 1), moderate (score 2), and high (score 3) risk of bias, had 40%, 25%, and 35% rate of contribution, and the total risk of bias score would be 0.40 × 1 + 0.25 × 2 + 0.35 × 3 = 1.95, which rounded to 2 and lead to “Some concerns” [46,47].
3. Results
3.1. Study Selection
Eleven trials were included in the final analysis [48,49,50,51,52,53,54,55,56,57,58]. The identification process was shown by a flow diagram, as in Figure 1.
Figure 1.
The PRISMA 2009 flow diagram of search and study selection.
There were nine categories of interventions included in this review, which were “Arginine”, “Beta-carotene”, “Glutamine”, “Omega 3”, “Protein”, “Zinc”, “Mixed”, “Lifestyle”, and “Control”. The information of all included trials is presented in Table 1. All the original data are provided in the Supplementary File.
Table 1.
Study Characteristics.
| Study | Participants | Interventions | Outcome Measures | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Age | Gender (F/M) | Cancer | Nationality | Protocol | Process | Classification | Therapy Form | ||
| Kazi 1997 [54] | 67.47 | 3/16 | Colon cancer |
American | Beta-carotene capsules | 30 mg/day, 3 months | Beta-carotene | After surgery | WBC Lymphocyte CD4/CD8 |
| Placebo capsules | 30 mg/day, 3 months | Control | |||||||
| Yoshida 1998 [51] | 61.18 | 2/11 | Esophageal cancer |
Japanese | Oral glutamine | 30 g/day, 28 days, | Glutamine | Irradiation and chemotherapy | WBC Lymphocyte |
| Standard amino acid solution | Isonitrogenous aminos, 28 days | Protein | |||||||
| de Luis 2005 [56] | 61.8 | 5/68 | Head and Neck cancer |
Spanish | Omega 3-enhanced oral immunonutrition | Omega 3-enhanced supplementation with a basal oral diet, 12 weeks | Omega 3 | After surgery | Lymphocyte |
| Arginine-enhanced oral immunonutrition | Arginine-enhanced supplementation with a basal oral diet, 12 weeks | Arginine | |||||||
| Saxton 2014 [49] | 55.56 | 85/0 | Early-stage breast cancer |
British | An exercise and hypocaloric healthy eating intervention |
600 kcal below their calculated energy requirements/day + 3 supervised exercise sessions (30 min aerobic exercise + 10 to 15 min of muscle-strengthening exercises)/week | Lifestyle | After surgery | WBC Lymphocyte CD4/CD8 Neutrophil |
| Blank | A healthy eating booklet | Control | |||||||
| Sangthawan 2015 [53] | 61.00 | 8/64 | Head and Neck cancer |
Thai | Zinc sulfate supplementation | Oral syrups zinc sulfate, 5 mg/cc, 50 mg (10 cc)/meal, 3 times/day at mealtimes | Zinc | Radiation Therapy after surgery | WBC Lymphocyte CD4/CD8 Neutrophil |
| Placebo | Oral syrups of a placebo, 3 times/day at mealtimes | Control | |||||||
| Paixao 2017 [50] | 51.06 | 37/0 | Breast cancer |
Brazilians | EPA and DHA-enriched fish oil | 2 g/day of fish oil concentrate containing 1.8 g of n-3 fatty acids for 30 days | Omega-3 | Perioperative period | WBC |
| Placebo | 2 g/day of mineral oil for 30 days | Control | |||||||
| Feijo 2019 [52] | 58.00 | 22/44 | Gastric cancer |
Brazilians | Omega-3 supplementation | 600 kcal, 24 g protein, and 3.2 g of omega 3/day, 200 mL/day, 30 days | Mixed | Before Surgery | CD4/CD8 |
| Standard formula without Omega-3 | 560 kcal and 29 g protein/day, 30 days | Protein | |||||||
| Wierdak 2021 [55] | 64.26 | 14/12 | Colorectal cancer |
Polish | Standard oral nutritional | 2 times Nutricia Nutridrink Protein/day, 2/day, 2 weeks | Protein | Perioperative period | WBC Lymphocyte Neutrophil |
| Immunonutrition | 2 times Arginine + Glutamine + Omega-3 + Nucleotides + Zinc, 2 weeks | Mixed | |||||||
| Wang 2021 [58] | 55.4 | 36/0 | Breast cancer |
Chinese | Spleen amino-peptide oral lyophilized powder |
4 mg on the first day of chemotherapy for two cycles. | Mixed | Unlimited | CD4/CD8 |
| Placebo | 4 mg on the first day of chemotherapy for two cycles. | Control | |||||||
| Bumrungpert 2018 [57] | 52.92 | 32/10 | Cancer without metastatic diseases |
Thai | Whey Protein Supplementation | 40 g Whey protein isolate with Zn (2.64 mg/day) and Se (0.76 mg/day) | Mixed | During chemotherapy | WBC |
| Maltodextrin oral snack | 40 g of maltodextrin as a daytime snack | Control | |||||||
| Homkham 2021 [48] | 56.00 | 35/49 | Cancer | Thai | Regular diet | 1500 kcal, 60 g protein/day (esophageal cancer patients, 2000 kcal, 75 g/day via feeding tube) | Control | During chemo-therapy | Lymphocyte |
| Immune-enhanced nutritional supplementation | A regular diet + 500 kcal/day of supplementation containing arginine 6.16 g, L-glutamine 3.07 g, and fish oil 2.73 g that prepared in sachet form, 2 times/day | Mixed | |||||||
WBC: white blood cell count (Leukocytes).
3.2. Presentation of Network Structure
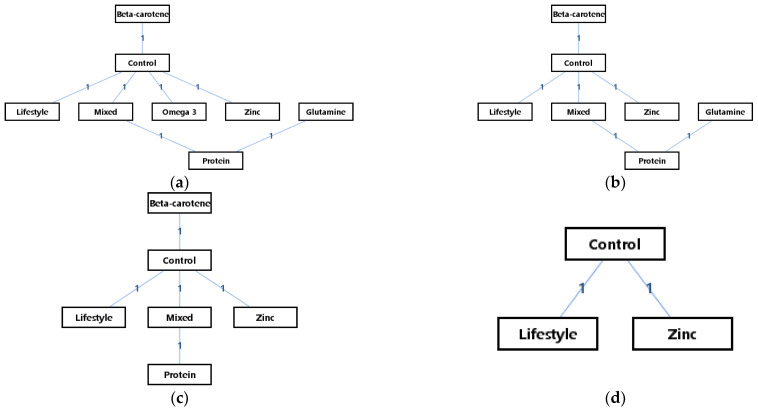
There were eight interventions in the network meta-analysis of WBC, seven interventions in the network meta-analysis of lymphocytes, four interventions in the network meta-analysis of CD4/CD8, and three interventions in the network meta-analysis of neutrophils. The network geometries displayed all kinds of treatments, providing key information such as the type of treatment represented by each node, the available direct comparisons between each pair of interventions (which is represented by the lines), and the arms of each trial (which are represented by the number on the edges). The network geometries of the interventions are presented in Figure 2. It can be seen that there was no closed loop in the outcome measures of the WBCs, lymphocyte count, CD4/CD8, and neutrophil count. Therefore, to determine whether to use the consistency or inconsistency model, what only needed to be conducted was the comparison of the random-effects standard deviation in each result of outcome measures.
Figure 2.
The network geometry of the interventions: (a) WBCs; (b) lymphocyte count; (c) CD4/CD8; (d) neutrophil count.
3.3. Study Characteristics
Characteristics of included studies are provided in Table 1.
3.4. Risk of Bias within Studies
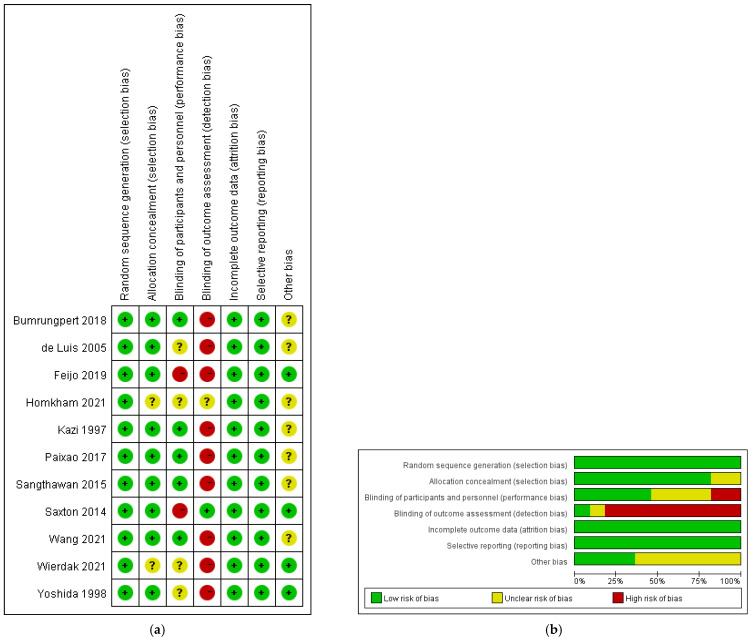
A consensus was reached for all items with a kappa value of 0.78. The results of the risk of bias assessment are shown in Figure 3. It can be seen that two trials had a high risk of bias, seven trials had a moderate risk of bias, and two trials had a low risk of bias. The risk of performance bias (blinding of participants and personnel) was moderate (high in two trials and unclear in four trials); (2) the risk of detection bias (blinding of outcome assessors) was high (high in nine trials and unclear in one trial); (3) the risk of attrition bias (incomplete outcome data) was low (low in all trials); (4) the risk of selection bias (random sequence generation and allocation concealment) was low (low in all trials); (5) the risk of reporting bias (selective reporting of outcomes) was low (low in all trials).
Figure 3.
The result of the risk of bias assessment. (a) Risk of bias summary [48,49,50,51,52,53,54,55,56,57,58]; (b) risk of bias graph.
3.5. Results of Individual Studies
The results of individual studies are summarized and provided in Table 2.
Table 2.
Results of Individual Studies.
| Study | Duration | Reporting Time | Main Results of Blood Immune Cell Parameters |
|---|---|---|---|
| Kazi 1997 [54] | 12 weeks | Pre treatment 12 weeks |
A significant increase in lymphocytes and CD4. |
| Yoshida 1998 [51] | 4 weeks | Pre treatment 4 weeks |
|
| de Luis 2005 [56] | 12 weeks | Pre treatment 12 weeks |
No significant intergroup differences in the trend of the three serum proteins and lymphocytes were detected. |
| Saxton 2014 [49] | 24 weeks | Pre treatment 24 weeks |
Women in the control group had higher total leukocyte, neutrophil, and lymphocyte counts in comparison to the intervention group at the 6-month follow-up. |
| Sangthawan 2015 [53] | Unlimited | Pre treatment 5 weeks Post treatment |
|
| Paixao 2017 [50] | 30 days | Pre treatment 30 days |
|
| Feijo 2019 [52] | 30 days | Pre treatment 30 days |
There was the maintenance of the immune profile in both groups; |
| Wierdak 2021 [55] | 2 weeks | Pre treatment 2 weeks |
In both groups, a decrease in superficial neutrophil infiltration was observed, but this was only statistically significant in the immune group; |
| Wang 2021 [58] | 12 weeks | Pre treatment 3 weeks 6 weeks 12 weeks |
On day 84, the number of CD3, CD4, and CD8 cells was significantly higher in the experimental group; |
| Bumrungpert 2018 [57] | 12 weeks | Pre treatment 6 weeks 12 weeks |
|
| Homkham 2021 [48] | 4 weeks | Pre treatment 2 weeks 4 weeks |
|
3.6. Synthesis of Results
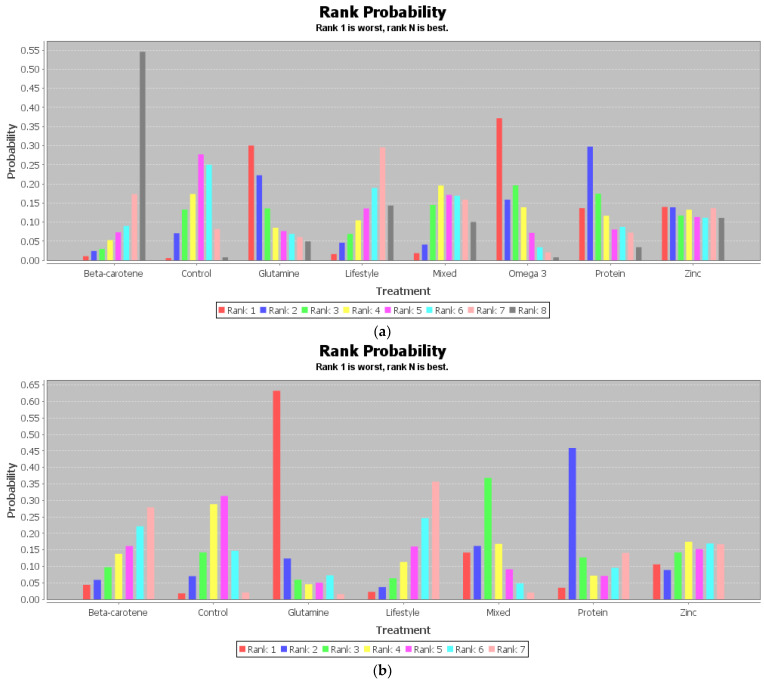
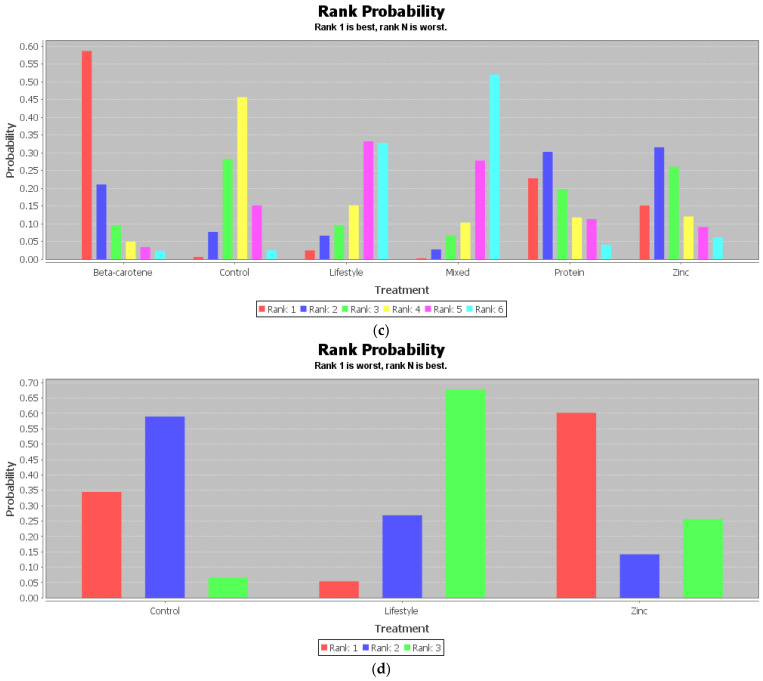
Table 3 is the league table of the network geometries, and the ranking of measures and probabilities are provided in Table 4 and Figure 4. What should be paid attention to is that in the probabilities ranking figure of CD4/CD8, as in Figure 4c, the rank N was the worst one, and rank 1 was the best one, whereas in those of WBC, lymphocyte, and neutrophil, the rank N was the best one, and rank 1 was the worst one.
Table 3.
The league tables of the network geometries.
| WBC | Beta-carotene | 1.26 | 2.23 | 0.81 | 1.20 | 2.37 | 2.04 | 1.45 |
| Control | 0.99 | −0.44 | −0.06 | 1.10 | 0.84 | 0.22 | ||
| Glutamine | −1.42 | −1.05 | 0.13 | −0.16 | −0.76 | |||
| Lifestyle | 0.37 | 1.55 | 1.25 | 0.64 | ||||
| Mixed | 1.16 | 0.88 | 0.25 | |||||
| Omega 3 | −0.28 | −0.89 | ||||||
| Protein | −0.63 | |||||||
| Zinc | ||||||||
| Lymphocyte | Beta-carotene | 58.54 | 318.67 | −29.41 | 149.02 | 186.55 | 57.62 | |
| Control | 260.49 | −87.11 | 89.32 | 129.54 | −1.97 | |||
| Glutamine | −344.58 | −171.71 | −132.38 | −263.43 | ||||
| Lifestyle | 176.71 | 215.26 | 84.98 | |||||
| Mixed | 39.20 | −89.82 | ||||||
| Protein | −132.57 | |||||||
| Zinc | ||||||||
| CD4/CD8 | Beta-carotene | −0.62 | −0.84 | −0.96 | −0.35 | −0.40 | ||
| Control | −0.22 | −0.33 | 0.26 | 0.22 | ||||
| Lifestyle | −0.11 | 0.49 | 0.44 | |||||
| Mixed | 0.60 | 0.55 | ||||||
| Protein | −0.04 | |||||||
| Zinc | ||||||||
| Neutrophil | Control | −376.33 | 285.34 | |||||
| Lifestyle | 650.94 | |||||||
| Zinc | ||||||||
WBC: white blood cell count (Leukocytes); Lifestyle: a combination of physical exercise and hypocaloric healthy eating.
Table 4.
Ranking of measures and probabilities.
| Outcome | Intervention | Rank 1 | Rank 2 | Rank 3 | Rank 4 | Rank 5 | Rank 6 | Rank 7 | Rank 8 |
|---|---|---|---|---|---|---|---|---|---|
| WBC | Beta-carotene | 0.01 | 0.02 | 0.03 | 0.05 | 0.07 | 0.09 | 0.17 | 0.55 |
| Control | 0.01 | 0.07 | 0.13 | 0.17 | 0.28 | 0.25 | 0.08 | 0.01 | |
| Glutamine | 0.30 | 0.22 | 0.14 | 0.09 | 0.08 | 0.07 | 0.06 | 0.05 | |
| Lifestyle | 0.02 | 0.05 | 0.07 | 0.10 | 0.14 | 0.19 | 0.30 | 0.14 | |
| Mixed | 0.02 | 0.04 | 0.14 | 0.20 | 0.17 | 0.17 | 0.16 | 0.1 | |
| Omega 3 | 0.37 | 0.16 | 0.20 | 0.14 | 0.07 | 0.03 | 0.02 | 0.01 | |
| Protein | 0.14 | 0.30 | 0.17 | 0.12 | 0.08 | 0.09 | 0.07 | 0.03 | |
| Zinc | 0.14 | 0.14 | 0.12 | 0.13 | 0.11 | 0.11 | 0.14 | 0.11 | |
| Lymphocyte | Beta-carotene | 0.04 | 0.06 | 0.10 | 0.13 | 0.16 | 0.24 | 0.27 | |
| Control | 0.00 | 0.03 | 0.14 | 0.32 | 0.35 | 0.14 | 0.02 | ||
| Glutamine | 0.28 | 0.32 | 0.22 | 0.11 | 0.04 | 0.02 | 0.01 | ||
| Lifestyle | 0.02 | 0.03 | 0.06 | 0.10 | 0.15 | 0.28 | 0.36 | ||
| Mixed | 0.13 | 0.30 | 0.26 | 0.14 | 0.09 | 0.05 | 0.02 | ||
| Protein | 0.44 | 0.11 | 0.08 | 0.06 | 0.07 | 0.08 | 0.16 | ||
| Zinc | 0.09 | 0.13 | 0.14 | 0.14 | 0.15 | 0.18 | 0.16 | ||
| CD4/CD8 | Beta-carotene | 0.59 | 0.21 | 0.1 | 0.05 | 0.03 | 0.02 | ||
| Control | 0.01 | 0.08 | 0.28 | 0.46 | 0.15 | 0.03 | |||
| Lifestyle | 0.02 | 0.07 | 0.1 | 0.15 | 0.33 | 0.33 | |||
| Mixed | 0 | 0.03 | 0.07 | 0.1 | 0.28 | 0.52 | |||
| Protein | 0.23 | 0.3 | 0.2 | 0.12 | 0.11 | 0.04 | |||
| Zinc | 0.15 | 0.32 | 0.26 | 0.12 | 0.09 | 0.06 | |||
| Neutrophil | Control | 0.34 | 0.59 | 0.07 | |||||
| Lifestyle | 0.05 | 0.27 | 0.68 | ||||||
| Zinc | 0.60 | 0.14 | 0.26 |
WBC: white blood cell count (Leukocytes); Lifestyle: a combination of physical exercise and hypocaloric healthy eating.
Figure 4.
Ranking of measures and probabilities: (a) WBCs; (b) lymphocyte count; (c) CD4/CD8; (d) neutrophil count.
It can be seen that beta-carotene supplementation had a 0.55 probability to be the best intervention for WBCs, a 0.59 probability to be the best intervention for CD4/CD8, and a 0.27 probability to be the sub-best intervention for lymphocytes. Changing lifestyle, which referred to a daily calorie intake of 600 kcal below the energy requirements with additional supervised exercise sessions, had a 0.36 probability to be the best intervention for lymphocytes and a 0.68 probability to be the best intervention for the neutrophil count.
3.7. Explanation for Inconsistency
The results of the random-effects standard deviation calculations in both the consistency model and inconsistency model of each outcome measure are provided in Table 5 in the form of the mean value and its 95% confidence intervals. According to the results, the random-effects standard deviations of the consistency model and that of the inconsistency model in the network structure of each outcome measure were well identical (p > 0.05). It means that the analysis under the consistency model had good validity.
Table 5.
The results of the random-effects standard deviation calculations.
| Outcome | Model | Inference Samples | Random-Effects Standard Deviation | t | Sig. |
|---|---|---|---|---|---|
| WBC | Consistency | 10,000 | 0.64 (0.06, 1.23) | 0.042 | 0.97 |
| Inconsistency | 20,000 | 0.62 (0.04, 1.23) | |||
| Lymphocyte | Consistency | 40,000 | 67.09 (5.76, 127.80) | 0.001 | 0.99 |
| Inconsistency | 40,000 | 64.07 (3.34, 127.67) | |||
| CD4/CD8 | Consistency | 10,000 | 0.32 (0.02, 0.62) | 0.031 | 0.98 |
| Inconsistency | 40,000 | 0.33 (0.02, 0.63) | |||
| Neutrophil | Consistency | 10,000 | 193.51 (12.39, 369.40) | 0.017 | 0.99 |
| Inconsistency | 20,000 | 191.02 (10.13, 369.41) |
WBC: white blood cell count (Leukocytes).
3.8. Results of Additional Analyses
Table 6 provides the results of the confidence assessment made by CINeMA. According to Table 6, except for the mixed comparison of glutamine and protein, and the indirect comparisons of beta-carotene and glutamine, in terms of the effect on leukocytes (WBC) in blood for cancer patients, control treatment and glutamine, control treatment and protein, glutamine and lifestyle change, glutamine and mixed supplementation protocol, glutamine and omega 3, and glutamine and zinc all had low confidence ratings; all other indirect and mixed evidences had a very low confidence rating.
Table 6.
Results of the confidence rating.
| Outcome | Structure | Comparison | Arms | Within-Study Bias | Reporting Bias | Indirectness | Imprecision | Heterogeneity | Incoherence | Confidence Rating | Reason(s) for Downgrading |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Leukocytes (WBC) | Mixed | Beta-carotene:Control | 1 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence |
| Control:Lifestyle | 1 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Control:Mixed | 2 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Control:Omega 3 | 1 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Control:Zinc | 2 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Glutamine:Protein | 3 | Some concerns | Low risk | No concerns | No concerns | No concerns | Major concerns | Low | Incoherence | ||
| Mixed:Protein | 1 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Indirect | Beta-carotene:Glutamine | 0 | Some concerns | Low risk | No concerns | No concerns | No concerns | Major concerns | Low | Incoherence | |
| Beta-carotene:Lifestyle | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Beta-carotene:Mixed | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Beta-carotene:Omega 3 | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Beta-carotene:Protein | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Beta-carotene:Zinc | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Control:Glutamine | 0 | Some concerns | Low risk | No concerns | No concerns | No concerns | Major concerns | Low | Incoherence | ||
| Control:Protein | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Glutamine:Lifestyle | 0 | Some concerns | Low risk | No concerns | No concerns | No concerns | Major concerns | Low | Incoherence | ||
| Glutamine:Mixed | 0 | Some concerns | Low risk | No concerns | No concerns | No concerns | Major concerns | Low | Incoherence | ||
| Glutamine:Omega 3 | 0 | Some concerns | Low risk | No concerns | No concerns | No concerns | Major concerns | Low | Incoherence | ||
| Glutamine:Zinc | 0 | Some concerns | Low risk | No concerns | No concerns | No concerns | Major concerns | Low | Incoherence | ||
| Lifestyle:Mixed | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Lifestyle:Omega 3 | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Lifestyle:Protein | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Lifestyle:Zinc | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Mixed:Omega 3 | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Mixed:Zinc | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Omega 3:Protein | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Omega 3:Zinc | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Protein:Zinc | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Lymphocyte | Mixed | Beta-carotene:Control | 1 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence |
| Control:Lifestyle | 1 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Control:Mixed | 2 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Control:Zinc | 2 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Glutamine:Protein | 3 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Mixed:Protein | 1 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Indirect | Beta-carotene:Glutamine | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | |
| Beta-carotene:Lifestyle | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Beta-carotene:Mixed | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Beta-carotene:Protein | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Beta-carotene:Zinc | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Control:Glutamine | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Control:Protein | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Glutamine:Lifestyle | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Glutamine:Mixed | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Glutamine:Zinc | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Lifestyle:Mixed | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Lifestyle:Protein | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Lifestyle:Zinc | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Mixed:Zinc | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Protein:Zinc | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| CD4/CD8 | Mixed | Beta-carotene:Control | 1 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence |
| Control:Lifestyle | 1 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Control:Mixed | 3 | Some concerns | Low risk | No concerns | No concerns | Major concerns | Major concerns | Very low | Heterogeneity and Incoherence | ||
| Control:Zinc | 2 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Mixed:Protein | 1 | Major concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Indirect | Beta-carotene:Lifestyle | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | |
| Beta-carotene:Mixed | 0 | Some concerns | Low risk | No concerns | No concerns | Major concerns | Major concerns | Very low | Heterogeneity and Incoherence | ||
| Beta-carotene:Protein | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Beta-carotene:Zinc | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Control:Protein | 0 | Major concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Lifestyle:Mixed | 0 | Some concerns | Low risk | No concerns | No concerns | Major concerns | Major concerns | Very low | Heterogeneity and Incoherence | ||
| Lifestyle:Protein | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Lifestyle:Zinc | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Mixed:Zinc | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Very low | Imprecision and Incoherence | ||
| Protein:Zinc | 0 | Some concerns | Low risk | No concerns | Major concerns | No concerns | Major concerns | Low | Imprecision and Incoherence | ||
| Neutrophil | Mixed | Control:Lifestyle | 1 | Some concerns | Low risk | No concerns | None | Unclear | Unclear | Unclear | Unclear |
| Control:Zinc | 2 | Some concerns | Low risk | No concerns | None | Unclear | Unclear | Unclear | Unclear | ||
| Indirect | Lifestyle:Zinc | 0 | Some concerns | Low risk | No concerns | None | Unclear | Unclear | Unclear | Unclear |
4. Discussion
4.1. Summary of Evidence
The objective of this systematic review was to identify the potential of different oral nutrition interventions for the blood immune cell parameters in cancer patients. The main findings are as follows. First, according to the results of the network meta-analysis, for cancer patients and without considering the effect size, intaking beta-carotene seems to be the supplementation protocol with the most potential for inducing a positive effect on the blood immune cell parameters, whereas the change in lifestyle—which was a low daily calorie intake with additional supervised exercise sessions—seems to be another potential protocol to induce a positive effect on the blood immune cell parameters. Second, other oral nutrition supplementation protocols, such as glutamine, protein (or amino acids), zinc, and mixed substance, seemed not effective as hoped. Third, although the effect sizes were statistically insignificant from very small to small, the overall results of the pair-wise meta-analysis supported the advantage of oral nutrition interventions over the interventions used in control groups, which were regular diets, placebo intake protocols, and patient education. Last but not the least, different nationalities of patients might affect the effect of oral nutrition intervention on blood immune cell parameters.
Part of these results corresponds with the demonstration of some previous trials. First, according to the results of the pair-wise meta-analysis, giving oral nutrition supplementations had a statistically insignificant small but positive effect on the blood immune cell parameters for patients with cancer. This finding corresponded with the results of a systematic review that assessed the effects of glutamine, arginine, and omega-3 supplementation on the tolerance to treatment, nutritional status, and immune function of head and neck cancer patients undergoing chemoradiotherapy, claiming that the glutamine supplementation could significantly reduce the risk of mucositis [59]. Second, the results of the network meta-analysis of this systematic review identified the high potential of beta-carotene supplementation protocols in preventing the immune cell parameters from extremely increasing (ranked first positive for WBC and CD4/CD8, second positive for lymphocyte count). Some previous trials presented similar results to that of this systematic review. For instance, a study published in 2016 claimed that beta-carotene might have an immune-enhancing effect through the production of Th1 cytokines by activation of splenocytes and macrophages [60]; a study whose participants were workers engaged in the copper-smelting industry found that preventive use of beta-carotene could prevent negative changes in immunological parameters for the participants [61]; and a randomized, double-blind controlled trial in 2010 showed that maternal supplementation including beta-carotene would affect the newborn’s immune development in specific ways [62]. Third, another important finding of this review is the large potential of the combination of physical exercise and hypocaloric healthy eating, which was allocated in the classification of “Lifestyle”, to provide a positive effect on the immune cell parameters for cancer patients. Some previous trials have verified the positive effect of energy restriction strategies on the functioning of the human immune system; a study of an animal model conducted in 2004 demonstrated that energy restriction could restore the impaired immune response in overweight rats [23], and another animal trial in 1994 found that energy restriction could prevent and reverse immune thrombocytopenic purpura and increases the life span of mice [63]. An important human study published in 1998 claimed that energy restriction was associated with a significant decrease in mitogen-stimulated lymphocyte proliferation, but no change in natural killer cell activity, monocyte and granulocyte phagocytosis and oxidative burst, or symptoms of upper respiratory tract infection [24]. A narrative review published in 2008 supported the role of physical exercise and energy restriction in the treatment process of cancer, demonstrating that some key biological mechanisms were providing important metabolic links between nutrition, physical activity, and cancer, including insulin resistance and reduced glucose tolerance, increased activation of the growth hormone/IGF-I axis, alterations in sex-steroid synthesis and/or bioavailability, and low-grade chronic inflammation through the effects of adipokines and cytokines [64]. Last, the pair-wise meta-analysis found that oral nutrition interventions had a small and insignificant advantage over regular diets, placebo intake protocols, and patient education with the heterogeneities potentially coming from patients’ nationalities or differences in treatment protocols [65,66,67,68].
However, some previous trials and reviews hold different viewpoints. For example, a study published in 2014 claimed that the evidence to recommend routine use of immune nutrition in patients undergoing esophageal cancer surgery was still insufficient [69], and a review published in 2014 declared that there was not enough evidence in malnourished urological study cohorts to establish a consensus on immune-nutrition and the role of immune-nutrition should be considered investigational in patients with bladder cancer until there are more well-controlled comparative effective trials or randomized trials [70]. Another systematic review published in 2006 that included randomized controlled trials examined the effects of nutritional interventions on patients with cancer or preinvasive lesions and demonstrated that there was no evidence that dietary modification by cancer patients could improve survival and benefit disease prognosis because of the limited number of high-quality trials [71]. Moreover, the evidence to support applying beta-carotene in cancer patients is still weak. A randomized controlled trial of Dunstan’s team identified that supplementation with beta-carotene did not affect the antioxidant status and immune responses in allergic adults [72], and a randomized prospective study conducted in 2000 found that beta-carotene could only enhance the cytotoxicity of NK cells but could not affect phenotypic expression of T cell subsets [73]. Moreover, one should be careful to interpret this result since the causal relationship between the energy restriction and the improvement of blood immune cell parameters is still unclear. On one hand, malnutrition is commonly reported in cancer patients. On the other hand, there are various ways to create energy deficiency and many different energetic balance equation hypotheses. Therefore, caution should be paid when planning to apply the energy restriction strategies in the process of oral nutrition treatment for cancer patients. Additionally, the evidence from this perspective is also vague since some other previous trials demonstrated the positive effect provided by certain oral nutrition supplements. An animal study finished in 2021 claimed that dietary palmitic acid could promote metastasis in oral carcinomas and melanoma in mice; tumors from mice that were fed a short-term palm-oil-rich diet, or tumor cells that were briefly exposed to PA in vitro, remained highly metastatic even after being serially transplanted [74]. A systematic review and meta-analysis suggest that parenteral omega-3 fatty acid supplementation was beneficial for gastrointestinal cancer patients, and was accompanied by improved postoperative immune function and satisfactory clinical outcomes [75].
What should be paid more attention is that the controversy surrounding beta-carotene is not limited to its effect on the function of the immune system. As has been mentioned in the introduction, clinical practice is more concerned with the safety indicators, such as mortality and morbidity, when it comes to cancer treatments. However, the results of some previous studies have raised concerns about the safety of beta-carotene for cancer patients. For example, a randomized trial conducted by Bairati’s team in 2006 found increased mortality in head and neck cancer patients who were supplemented with alpha-tocopherol and beta-carotene [76]. Similar results were reported in a systematic review with a broader population included as participants. A Cochrane systematic review published in 2012 assessed the beneficial and harmful effects of antioxidant supplements for the prevention of mortality in 269,707 adults, claiming that eta-carotene seemed to increase mortality and should be considered as medicinal products and should undergo sufficient evaluation before marketing [77]. Moreover, Bjelakovic’s team examined the association between beta-carotene and mortality based on their 2012 Cochrane systematic review to assess whether different doses of beta-carotene affected mortality in primary and secondary prevention randomized clinical trials with low risk of bias by using meta-analyses, meta-regression, and trial sequential analyses. Eventually, Bjelakovic’s team concluded that beta-carotene in doses higher than the recommended daily allowances seemed to significantly increase mortality [78]. Considering all the information above, beta-carotene supplementation has the potential to improve the immunometabolism of cancer patients, but their chances of mortality might be significantly higher. The heterogeneity between trials might come from their different intervention protocols and different populations of participants. For example, the detailed physiological mechanics of beta-carotene’s functioning in the human body is still lacking exploration. A cross-sectional study published in 2000 claimed that plasma beta-carotene lacked association with the immune response to the influenza vaccine in the healthy elderly [79]; however, a double-blind, placebo-controlled, crossover study, whose subjects were adult male nonsmokers, found that after dietary supplementation of beta-carotene, there were significant increases in plasma levels of beta-carotene and the percentages of monocytes expressing the major histocompatibility complex class II molecule HLA-DR, the adhesion molecules intercellular adhesion molecule-1, the leukocyte function-associated antigen-3, and the ex vivo TNF-alpha secretion by blood monocytes were significantly increased [80]. Considering that all the trials related to the beta-carotene supplementation included in this review were single-arm and their results were not statistically significant, more high-quality research is needed in the future to clarify the effects of beta-carotene, explain its mechanism, and provide the best guideline by comparing different intake protocols. When it comes to the patient population, the results of subgroup analysis in the pair-wise meta-analysis identified the potential that the nationalities might become one of the heterogeneity sources since the I2s between subgroups were larger than for those within the overall effects. The heterogeneity that came from the nationalities also indicated the possibility of publication bias, which was mainly induced by the lack of trials with participants from East Asia.
To sum up, oral nutrition intervention for cancer patients is a complex issue. Although this review and other previous trials failed to verify a significant positive effect of any oral nutrition supplementation protocol on immune cell parameters of cancer patients, the result of this review could still provide important enlightenment for future research because of two main strengths. On one hand, the network meta-analysis based on the Bayesian approach indicated that the preconception that oral nutrition supplementation must have a positive effect on cancer patients should be avoided and the importance of lifestyle interventions and overall energy intake control could not be neglected. On the other hand, the results of subgroup analysis and publication bias assessment in the pair-wise meta-analysis indicated that further trials should focus on the comparison of cancer patients of different races or nationalities to identify the different effects of oral nutrition interventions.
4.2. Limitations
First, the meta-analysis in this review did not include clinical outcomes such as mortality, morbidity, or adverse events such as malnutrition and immunological stress reaction [81,82,83]. It could not be ignored that the surrogate outcome measures, especially laboratory indices—which were very often unreliable substitutes in clinical practice—were applied instead of clinical outcomes, inducing potential dangers when assessing new treatment protocols. The ideal primary outcomes should be relevant to the patient’s quality of life or the course of the disease; a significant correlation between a surrogate and a clinical outcome could not explicitly mean that the observed beneficial effect of an intervention on the surrogate outcome will be the same on the clinical outcome.
Second, since the monitoring of blood immune cell parameters in cancer treatment is usually continuous, the outcome measured at baseline and at each endpoint could only represent the current status [75]. Unfortunately, since there were only a few studies included in these comparisons, the publication bias in the comparisons of some outcome measures, such as CD4/CD8 and the neutrophil count, could not be quantitatively evaluated, and the confidence of evidence for CD4/CD8 and the neutrophil count is also unknown.
Third, there was a lot of variation between the studies in terms of gender, tumor type, treatment, and stage of the disease, and there was very small number of studies representing a given type of intervention. Moreover, the different tumor types would affect the outcomes and results. For example, digestive tract cancers, such as colon cancer, which could block the absorption of nutrition, may derive maximum immunonutrition support from enteral nutrition regimens [84,85,86].
Last, the race and nationality of the participants were not limited in the eligibility criteria of the participants. However, different races differ in the risk of different types of cancer, nutritional needs, and dietary habits. There is a lack of relevant high-quality evidence.
4.3. Conclusions
According to the change in blood immune cell parameters, it could be inferred that the programs of immunonutrition therapy for different cancer patients might be different. Moreover, the past perception that mixed oral nutritional supplementations are superior to oral nutritional supplements with a single substance might be wrong, at least from the mathematical perspective in this review. Therefore, the selection of oral nutritional supplementation needs cautiousness. Finally, a combination of physical exercise might have a positive effect on the immune function of cancer patients, and more relevant high-quality studies should be conducted in the future.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/metabo12090868/s1, All the original data are provided in the Supplementary File.
Author Contributions
Conceptualization, Y.F. and F.R.; methodology, Y.F., Y.Z. and F.R.; formal analysis, Y.X., F.R., J.S.B., Y.Z. and Y.F.; investigation, Y.X., Y.Z. and F.R.; resources, Y.Z., F.R., J.S.B. and Y.F.; data curation, Y.X., Y.Z. and Y.F.; funding acquisition, J.S.B. and F.R. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study was supported by the Zhejiang Province Medical and Health Science and Technology Plan Project (No. 2018KY710), the Ningbo Public Welfare Science and Technology Plan Project (No. 2019C50095), the Health Youth Technical Talent Cultivation Special Fund Project (2020SWSQNGG-01), the Ningbo Medical Science and Technology Plan (2020Y14), the Young Cultivation Fund Project of The Affiliated of School of Medicine of Ningbo University (FYQM-KY-202003), the Open Fund Project of Institute of Human Biomechanics of Ningbo University (CJ-HBIO202112), and the K.C. Wong Magna Fund in Ningbo University.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Mathis D., Shoelson S.E. Immunometabolism: An emerging frontier. Nat. Rev. Immunol. 2011;11:81–83. doi: 10.1038/nri2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rena G., Lang C.C. Repurposing Metformin for Cardiovascular Disease. Circulation. 2018;137:422–424. doi: 10.1161/CIRCULATIONAHA.117.031735. [DOI] [PubMed] [Google Scholar]
- 3.Adiamah A., Rollins K.E., Kapeleris A., Welch N.T., Iftikhar S.Y., Allison S.P., Lobo D.N. Postoperative arginine-enriched immune modulating nutrition: Long-term survival results from a randomised clinical trial in patients with oesophagogastric and pancreaticobiliary cancer. Clin. Nutr. 2021;40:5482–5485. doi: 10.1016/j.clnu.2021.09.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dechaphunkul T., Arundon T., Raungkhajon P., Jiratrachu R., Geater S.L., Dechaphunkul A. Benefits of immunonutrition in patients with head and neck cancer receiving chemoradiation: A phase II randomized, double-blind study. Clin. Nutr. 2021;41:433–440. doi: 10.1016/j.clnu.2021.12.035. [DOI] [PubMed] [Google Scholar]
- 5.Kilgore K. An Invitation to Live: Insights from an Older, Long-Term Practitioner of Tai Chi. Phys. Act. Health. 2019;3:11–22. doi: 10.5334/paah.31. [DOI] [Google Scholar]
- 6.Lavanya M., Muthu Kannan P., Arivalagan M. Lung cancer diagnosis and staging using firefly algorithm fuzzy C-means segmentation and support vector machine classification of lung nodules. Int. J. Biomed. Eng. Technol. 2021;37:185–200. doi: 10.1504/IJBET.2021.119504. [DOI] [Google Scholar]
- 7.Kumar A., Smith C., Jobin C., Trinchieri G., Howcroft T.K., Seifried H., Espey M.G., Flores R., Kim Y.S., Daschner P.J. Workshop Report: Modulation of Antitumor Immune Responses by Dietary and Microbial Metabolites. JNCI J. Natl. Cancer Inst. 2017;109:djx040. doi: 10.1093/jnci/djx040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Caccialanza R., De Lorenzo F., Gianotti L., Zagonel V., Gavazzi C., Farina G., Cotogni P., Cinieri S., Cereda E., Marchetti P., et al. Nutritional support for cancer patients: Still a neglected right? Support. Care Cancer. 2017;25:3001–3004. doi: 10.1007/s00520-017-3826-1. [DOI] [PubMed] [Google Scholar]
- 9.Vernieri C., Fucà G., Ligorio F., Huber V., Vingiani A., Iannelli F., Raimondi A., Rinchai D., Frigè G., Belfiore A., et al. Fasting-Mimicking Diet Is Safe and Reshapes Metabolism and Antitumor Immunity in Patients with Cancer. Cancer Discov. 2021;12:90–107. doi: 10.1158/2159-8290.CD-21-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rizzo A., Ricci A.D. PD-L1, TMB, and other potential predictors of response to immunotherapy for hepatocellular carcinoma: How can they assist drug clinical trials? Expert Opin. Investig. Drugs. 2021;31:415–423. doi: 10.1080/13543784.2021.1972969. [DOI] [PubMed] [Google Scholar]
- 11.Westheim A.J.F., Stoffels L.M., Dubois L.J., van Bergenhenegouwen J., van Helvoort A., Langen R.C.J., Shiri-Sverdlov R., Theys J. Fatty Acids as a Tool to Boost Cancer Immunotherapy Efficacy. Front. Nutr. 2022;9:868436. doi: 10.3389/fnut.2022.868436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu H., Ling W., Shen Z.Y., Jin X., Cao H. Clinical application of immune-enhanced enteral nutrition in patients with advanced gastric cancer after total gastrectomy. J. Dig. Dis. 2012;13:401–406. doi: 10.1111/j.1751-2980.2012.00596.x. [DOI] [PubMed] [Google Scholar]
- 13.Nagano T., Fujita H., Tanaka T., Matono S., Murata K., Ishibashi N., Shirouzu K., Yanagawa T. A randomized controlled trial comparing antioxidant-enriched enteral nutrition with immune-enhancing enteral nutrition after esophagectomy for cancer: A pilot study. Surg. Today. 2012;43:1240–1249. doi: 10.1007/s00595-012-0424-1. [DOI] [PubMed] [Google Scholar]
- 14.Rocha K.C., Vieira M.L.D.S., Beltrame R.L., Cartum J., Alves S.I.P.M.D.N., Azzalis L.A., Junqueira V.B.C., Pereira E.C., Fonseca F.L.A. Impact of Selenium Supplementation in Neutropenia and Immunoglobulin Production in Childhood Cancer Patients. J. Med. Food. 2016;19:560–568. doi: 10.1089/jmf.2015.0145. [DOI] [PubMed] [Google Scholar]
- 15.Duan P., Wang Z.M. Clinical study on effect of Astragalus in efficacy enhancing and toxicity reducing of chemotherapy in patients of malignant tumor. Zhongguo Zhong Xi Yi Jie He Za Zhi. 2002;22:515–517. [PubMed] [Google Scholar]
- 16.Zhang J.W., Du P., Chen D.W., Cui L., Ying C.M. Effect of viable Bifidobacterium supplement on the immune status and inflammatory response in patients undergoing resection for colorectal cancer. Zhonghua Wei Chang Wai Ke Za Zhi. 2010;13:40–43. [PubMed] [Google Scholar]
- 17.Chunbo X.U., Shen M., Shanming R. Research Situation of Yang-warming Method for Tumor. J. New Chin. Med. 2019;8:51–53. [Google Scholar]
- 18.Dou J., Huang Q., Zhao T., Pharmacy S.O. Effect of Yanghe Decoction on NF-κB and IL-8 Expression of MDA-MB-231 Cells for Breast Cancer. J. Sichuan Tradit. Chin. Med. 2017;35:44–47. [Google Scholar]
- 19.Huang Q., Zhang F., Li X. Effect of Yanghe Decoction on Human Breast Cancer MCF-7 Cells and Its PI3K/Akt Signaling Pathway. J. Sichuan Tradit. Chin. Med. 2019;2:47–50. [Google Scholar]
- 20.Kangle L.I., Peng P., Zhang X., University X.J. Effect of Yanghe Decoction on Apoptosis of MDA-MB-231 Cells for Human Breast Cancer. J. Sichuan Tradit. Chin. Med. 2018;12:41–42. [Google Scholar]
- 21.Szefel J., Ślebioda T., Walczak J., Kruszewski W.J., Szajewski M., Ciesielski M., Stanisławowski M., Buczek T., Małgorzewicz S., Owczarzak A., et al. The effect of l-arginine supplementation and surgical trauma on the frequency of myeloid-derived suppressor cells and T lymphocytes in tumour and blood of colorectal cancer patients. Adv. Med. Sci. 2022;67:66–78. doi: 10.1016/j.advms.2021.12.005. [DOI] [PubMed] [Google Scholar]
- 22.Lee A.H., Dixit V.D. Dietary Regulation of Immunity. Immunity. 2020;53:510–523. doi: 10.1016/j.immuni.2020.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamas O., Martinez J.A., Marti A. Energy restriction restores the impaired immune response in overweight (cafeteria) rats. J. Nutr. Biochem. 2004;15:418–425. doi: 10.1016/j.jnutbio.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 24.Nieman D.C., Nehlsen-Cannarella S.L., Henson D.A., Koch A.J., Butterworth D.E., Fagoaga O.R., Utter A. Immune response to exercise training and/or energy restriction in obese women. Med. Sci. Sports Exerc. 1998;30:679–686. doi: 10.1097/00005768-199805000-00006. [DOI] [PubMed] [Google Scholar]
- 25.Afaq S., Ali M., Ahmad M., Hussain S., Ali W., Munir I. Metabolic Health Profile of Employees in a Printing Press in Peshawar, Pakistan. Phys. Act. Health. 2022;6:55–63. doi: 10.5334/paah.176. [DOI] [Google Scholar]
- 26.Methnani J., Amor D., Yousfi N., Bouslama A., Omezzine A., Bouhlel E. Sedentary behavior, exercise and COVID-19: Immune and metabolic implications in obesity and its comorbidities. J. Sports Med. Phys. Fit. 2021;61:1538–1547. doi: 10.23736/S0022-4707.20.11898-X. [DOI] [PubMed] [Google Scholar]
- 27.Nieman D.C., Buckley K.S., Henson D.A., Warren B.J., Suttles J., Ahle J.C., Simandle S., Fagoaga O.R., Nehlsen-Cannarella S.L. Immune function in marathon runners versus sedentary controls. Med. Sci. Sports Exerc. 1995;27:986–992. doi: 10.1249/00005768-199507000-00006. [DOI] [PubMed] [Google Scholar]
- 28.Nieman D.C., Lila M.A., Gillitt N.D. Immunometabolism: A Multi-Omics Approach to Interpreting the Influence of Exercise and Diet on the Immune System. Annu. Rev. Food Sci. Technol. 2019;10:341–363. doi: 10.1146/annurev-food-032818-121316. [DOI] [PubMed] [Google Scholar]
- 29.Ramzan F., Mitchell C.J., Milan A.M., Schierding W., Zeng N., Sharma P., Mitchell S.M., D’Souza R.F., Knowles S.O., Roy N.C., et al. Comprehensive Profiling of the Circulatory miRNAome Response to a High Protein Diet in Elderly Men: A Potential Role in Inflammatory Response Modulation. Mol. Nutr. Food Res. 2019;63:e1800811. doi: 10.1002/mnfr.201800811. [DOI] [PubMed] [Google Scholar]
- 30.Okala S.G., Darboe M.K., Sosseh F., Sonko B., Faye-Joof T., Prentice A.M., Moore S.E. Impact of nutritional supplementation during pregnancy on antibody responses to diphtheria-tetanus-pertussis vaccination in infants: A randomised trial in The Gambia. PLoS Med. 2019;16:e1002854. doi: 10.1371/journal.pmed.1002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kang C.M., Chiang B.L., Wang L.C. Maternal Nutritional Status and Development of Atopic Dermatitis in Their Offspring. Clin. Rev. Allergy Immunol. 2021;61:128–155. doi: 10.1007/s12016-020-08780-y. [DOI] [PubMed] [Google Scholar]
- 32.Hu F.B. Nutrient supplementation no substitute for healthy diets. Nat. Rev. Cardiol. 2019;16:77–79. doi: 10.1038/s41569-018-0143-4. [DOI] [PubMed] [Google Scholar]
- 33.van Daal M.T., Folkerts G., Garssen J., Braber S. Pharmacological Modulation of Immune Responses by Nutritional Components. Pharmacol. Rev. 2021;73:198–232. doi: 10.1124/pharmrev.120.000063. [DOI] [PubMed] [Google Scholar]
- 34.Nobs S.P., Zmora N., Elinav E. Nutrition Regulates Innate Immunity in Health and Disease. Annu. Rev. Nutr. 2020;40:189–219. doi: 10.1146/annurev-nutr-120919-094440. [DOI] [PubMed] [Google Scholar]
- 35.Bargetzi L., Brack C., Herrmann J., Bargetzi A., Hersberger L., Bargetzi M., Kaegi-Braun N., Tribolet P., Gomes F., Hoess C., et al. Nutritional support during the hospital stay reduces mortality in patients with different types of cancers: Secondary analysis of a prospective randomized trial. Ann. Oncol. 2021;32:1025–1033. doi: 10.1016/j.annonc.2021.05.793. [DOI] [PubMed] [Google Scholar]
- 36.Boretti A. Nutrition, lipidic parameters, and cancer risk and progress. Nutrition. 2020;69:110538. doi: 10.1016/j.nut.2019.06.019. [DOI] [PubMed] [Google Scholar]
- 37.Maumy L., Harrissart G., Dewaele P., Aljaber A., Bonneau C., Rouzier R., Elies A. Impact of nutrition on breast cancer mortality and risk of recurrence, a review of the evidence. Bull. Cancer. 2020;107:61–71. doi: 10.1016/j.bulcan.2019.08.009. [DOI] [PubMed] [Google Scholar]
- 38.Tanaka Y., Shimizu S., Shirotani M., Yorozu K., Kitamura K., Oehorumu M., Kawai Y., Fukuzawa Y. Nutrition and Cancer Risk from the Viewpoint of the Intestinal Microbiome. Nutrients. 2021;13:3326. doi: 10.3390/nu13103326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zitvogel L., Pietrocola F., Kroemer G. Nutrition, inflammation and cancer. Nat. Immunol. 2017;18:843–850. doi: 10.1038/ni.3754. [DOI] [PubMed] [Google Scholar]
- 40.Reglero C., Reglero G. Precision Nutrition and Cancer Relapse Prevention: A Systematic Literature Review. Nutrients. 2019;11:2799. doi: 10.3390/nu11112799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Catala-Lopez F., Tobias A., Cameron C., Moher D., Hutton B. Network meta-analysis for comparing treatment effects of multiple interventions: An introduction. Rheumatol. Int. 2014;34:1489–1496. doi: 10.1007/s00296-014-2994-2. [DOI] [PubMed] [Google Scholar]
- 42.Hutton B., Salanti G., Caldwell D.M., Chaimani A., Schmid C.H., Cameron C., Ioannidis J.P., Straus S., Thorlund K., Jansen J.P., et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015;162:777–784. doi: 10.7326/M14-2385. [DOI] [PubMed] [Google Scholar]
- 43.Andrejeva G., Rathmell J.C. Similarities and Distinctions of Cancer and Immune Metabolism in Inflammation and Tumors. Cell Metab. 2017;26:49–70. doi: 10.1016/j.cmet.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Armijo-Olivo S., Stiles C.R., Hagen N.A., Biondo P.D., Cummings G.G. Assessment of study quality for systematic reviews: A comparison of the Cochrane Collaboration Risk of Bias Tool and the Effective Public Health Practice Project Quality Assessment Tool: Methodological research. J. Eval. Clin. Pr. 2012;18:12–18. doi: 10.1111/j.1365-2753.2010.01516.x. [DOI] [PubMed] [Google Scholar]
- 45.Rouse B., Chaimani A., Li T. Network meta-analysis: An introduction for clinicians. Intern. Emerg. Med. 2017;12:103–111. doi: 10.1007/s11739-016-1583-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nikolakopoulou A., Higgins J.P.T., Papakonstantinou T., Chaimani A., Del Giovane C., Egger M., Salanti G. CINeMA: An approach for assessing confidence in the results of a network meta-analysis. PLoS Med. 2020;17:e1003082. doi: 10.1371/journal.pmed.1003082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Papakonstantinou T., Nikolakopoulou A., Higgins J.P.T., Egger M., Salanti G. CINeMA: Software for semiautomated assessment of the confidence in the results of network meta-analysis. Campbell Syst. Rev. 2020;16:e1080. doi: 10.1002/cl2.1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Homkham N., Muangwong P., Pisprasert V., Traisathit P., Jiratrachu R., Chottaweesak P., Chitapanarux I. Dynamic changes in practical inflammation and immunity markers in cancer patients receiving immune-enhancing nutritional supplementation during concurrent chemoradiotherapy. Cancer Biomark. 2021;32:281–291. doi: 10.3233/CBM-210086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Saxton J.M., Scott E.J., Daley A.J., Woodroofe M., Mutrie N., Crank H., Powers H.J., Coleman R.E. Effects of an exercise and hypocaloric healthy eating intervention on indices of psychological health status, hypothalamic-pituitary-adrenal axis regulation and immune function after early-stage breast cancer: A randomised controlled trial. Breast Cancer Res. 2014;16:R39. doi: 10.1186/bcr3643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paixao E., Oliveira A.C.M., Pizato N., Muniz-Junqueira M.I., Magalhaes K.G., Nakano E.Y., Ito M.K. The effects of EPA and DHA enriched fish oil on nutritional and immunological markers of treatment naive breast cancer patients: A randomized double-blind controlled trial. Nutr. J. 2017;16:71. doi: 10.1186/s12937-017-0295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshida S., Matsui M., Shirouzu Y., Fujita H., Yamana H., Shirouzu K. Effects of glutamine supplements and radiochemotherapy on systemic immune and gut barrier function in patients with advanced esophageal cancer. Ann. Surg. 1998;227:485–491. doi: 10.1097/00000658-199804000-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Feijo P.M., Rodrigues V.D., Viana M.S., Dos Santos M.P., Abdelhay E., Viola J.P., de Pinho N.B., Martucci R.B. Effects of omega-3 supplementation on the nutritional status, immune, and inflammatory profiles of gastric cancer patients: A randomized controlled trial. Nutrition. 2019;61:125–131. doi: 10.1016/j.nut.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 53.Sangthawan D., Phungrassami T., Sinkitjarurnchai W. Effects of zinc sulfate supplementation on cell-mediated immune response in head and neck cancer patients treated with radiation therapy. Nutr. Cancer. 2015;67:449–456. doi: 10.1080/01635581.2015.1004735. [DOI] [PubMed] [Google Scholar]
- 54.Kazi N., Radvany R., Oldham T., Keshavarzian A., Frommel T.O., Libertin C., Mobarhan S. Immunomodulatory effect of beta-carotene on T lymphocyte subsets in patients with resected colonic polyps and cancer. Nutr. Cancer. 1997;28:140–145. doi: 10.1080/01635589709514566. [DOI] [PubMed] [Google Scholar]
- 55.Wierdak M., Surmiak M., Milian-Ciesielska K., Rubinkiewicz M., Rzepa A., Wysocki M., Major P., Klek S., Pedziwiatr M. Immunonutrition Changes Inflammatory Response in Colorectal Cancer: Results from a Pilot Randomized Clinical Trial. Cancers. 2021;13:1444. doi: 10.3390/cancers13061444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Luis D.A., Izaola O., Aller R., Cuellar L., Terroba M.C. A randomized clinical trial with oral Immunonutrition (omega3-enhanced formula vs. arginine-enhanced formula) in ambulatory head and neck cancer patients. Ann. Nutr. Metab. 2005;49:95–99. doi: 10.1159/000084742. [DOI] [PubMed] [Google Scholar]
- 57.Bumrungpert A., Pavadhgul P., Nunthanawanich P., Sirikanchanarod A., Adulbhan A. Whey Protein Supplementation Improves Nutritional Status, Glutathione Levels, and Immune Function in Cancer Patients: A Randomized, Double-Blind Controlled Trial. J. Med. Food. 2018;21:612–616. doi: 10.1089/jmf.2017.4080. [DOI] [PubMed] [Google Scholar]
- 58.Wang J., Ma X., Shang K., Wu S., Ma Y., Ma Z., Cao B. Safety and efficacy of spleen aminopeptide oral lyophilized powder for improving quality of life and immune response in patients with advanced breast cancer: A multicenter, randomized, double-blind, placebo-controlled clinical trial. Anti-Cancer Drugs. 2021;32:1067–1075. doi: 10.1097/CAD.0000000000001109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lyra M.M.F., Meira J.E.C., Guedes G.D.S., Bueno N.B. Immunonutrition in head and neck cancer: Systematic review and metanalysis of its clinical and nutritional effects. Clin. Nutr. ESPEN. 2021;41:30–41. doi: 10.1016/j.clnesp.2020.12.014. [DOI] [PubMed] [Google Scholar]
- 60.Kim H.Y., Nam S.Y., Yang S.Y., Kim H.M., Jeong H.J. Cucurbita moschata Duch. and its active component, beta-carotene effectively promote the immune responses through the activation of splenocytes and macrophages. Immunopharmacol. Immunotoxicol. 2016;38:319–326. doi: 10.1080/08923973.2016.1202960. [DOI] [PubMed] [Google Scholar]
- 61.Vlasova I.A., Kazantseva S.V., Adrianovskii V.I., Eremin Iu N. Beta-carotene prevention of immune disorders in workers engaged in the fire copper refining. Gig. Sanit. 2002:28–32. [PubMed] [Google Scholar]
- 62.Wieringa F.T., Dijkhuizen M.A., Muhilal, Van der Meer J.W. Maternal micronutrient supplementation with zinc and beta-carotene affects morbidity and immune function of infants during the first 6 months of life. Eur. J. Clin. Nutr. 2010;64:1072–1079. doi: 10.1038/ejcn.2010.115. [DOI] [PubMed] [Google Scholar]
- 63.Mizutani H., Engelman R.W., Kurata Y., Ikehara S., Good R.A. Energy restriction prevents and reverses immune thrombocytopenic purpura (ITP) and increases life span of ITP-prone (NZW × BXSB) F1 mice. J. Nutr. 1994;124:2016–2023. doi: 10.1093/jn/124.10.2016. [DOI] [PubMed] [Google Scholar]
- 64.Dossus L., Kaaks R. Nutrition, metabolic factors and cancer risk. Best Pr. Res. Clin. Endocrinol. Metab. 2008;22:551–571. doi: 10.1016/j.beem.2008.08.003. [DOI] [PubMed] [Google Scholar]
- 65.Davoodi S.H., Yousefinejad V., Ghaderi B., Akbari M.E., Darvishi S., Mehrabi Y., Darvishi N. Oral Propolis, Nutritional Status and Quality of Life with Chemotherapy for Breast Cancer: A Randomized, Double-Blind Clinical Trial. Nutr. Cancer. 2022;74:2029–2037. doi: 10.1080/01635581.2021.1988118. [DOI] [PubMed] [Google Scholar]
- 66.Dhruva A., Wu C., Miaskowski C., Hartogensis W., Rugo H.S., Adler S.R., Kaptchuk T.J., Kelkar R., Agarawal S., Vadodaria A., et al. A 4-Month Whole-Systems Ayurvedic Medicine Nutrition and Lifestyle Intervention Is Feasible and Acceptable for Breast Cancer Survivors: Results of a Single-Arm Pilot Clinical Trial. Glob. Adv. Health Med. 2020;9:2164956120964712. doi: 10.1177/2164956120964712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Schmoranzer F., Fuchs N., Markolin G., Carlin E., Sakr L., Sommeregger U. Influence of a complex micronutrient supplement on the immune status of elderly individuals. Int. J. Vitam. Nutr. Res. 2009;79:308–318. doi: 10.1024/0300-9831.79.56.308. [DOI] [PubMed] [Google Scholar]
- 68.Romero F.G., Lira F.S., Marques F.A., Muzy P.C., Peres R.A., Caperuto E.C. PAKs supplement improves immune status and body composition but not muscle strength in resistance trained individuals. J. Int. Soc. Sports Nutr. 2010;7:36. doi: 10.1186/1550-2783-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mudge L., Isenring E., Jamieson G.G. Immunonutrition in patients undergoing esophageal cancer resection. Dis. Esophagus. 2011;24:160–165. doi: 10.1111/j.1442-2050.2010.01117.x. [DOI] [PubMed] [Google Scholar]
- 70.Munbauhal G., Drouin S.J., Mozer P., Colin P., Phe V., Cussenot O., Roupret M. Malnourishment in bladder cancer and the role of immunonutrition at the time of cystectomy: An overview for urologists. BJU Int. 2014;114:177–184. doi: 10.1111/bju.12529. [DOI] [PubMed] [Google Scholar]
- 71.Davies A.A., Davey Smith G., Harbord R., Bekkering G.E., Sterne J.A., Beynon R., Thomas S. Nutritional interventions and outcome in patients with cancer or preinvasive lesions: Systematic review. J. Natl. Cancer Inst. 2006;98:961–973. doi: 10.1093/jnci/djj263. [DOI] [PubMed] [Google Scholar]
- 72.Dunstan J.A., Breckler L., Hale J., Lehmann H., Franklin P., Lyons G., Ching S.Y., Mori T.A., Barden A., Prescott S.L. Supplementation with vitamins C, E, beta-carotene and selenium has no effect on anti-oxidant status and immune responses in allergic adults: A randomized controlled trial. Clin. Exp. Allergy. 2007;37:180–187. doi: 10.1111/j.1365-2222.2007.02657.x. [DOI] [PubMed] [Google Scholar]
- 73.Wood S.M., Beckham C., Yosioka A., Darban H., Watson R.R. beta-Carotene and selenium supplementation enhances immune response in aged humans. Integr. Med. 2000;2:85–92. doi: 10.1016/S1096-2190(00)00009-3. [DOI] [PubMed] [Google Scholar]
- 74.Pascual G., Dominguez D., Elosua-Bayes M., Beckedorff F., Laudanna C., Bigas C., Douillet D., Greco C., Symeonidi A., Hernandez I., et al. Dietary palmitic acid promotes a prometastatic memory via Schwann cells. Nature. 2021;599:485–490. doi: 10.1038/s41586-021-04075-0. [DOI] [PubMed] [Google Scholar]
- 75.Bai H., Li Z., Meng Y., Yu Y., Zhang H., Shen D., Chen L. Effects of parenteral omega-3 fatty acid supplementation in postoperative gastrointestinal cancer on immune function and length of hospital stay: A systematic review and meta-analysis. Asia Pac. J. Clin. Nutr. 2018;27:121–128. doi: 10.6133/apjcn.022017.19. [DOI] [PubMed] [Google Scholar]
- 76.Bairati I., Meyer F., Jobin E., Gelinas M., Fortin A., Nabid A., Brochet F., Tetu B. Antioxidant vitamins supplementation and mortality: A randomized trial in head and neck cancer patients. Int. J. Cancer. 2006;119:2221–2224. doi: 10.1002/ijc.22042. [DOI] [PubMed] [Google Scholar]
- 77.Bjelakovic G., Nikolova D., Gluud L.L., Simonetti R.G., Gluud C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012;2012:CD007176. doi: 10.1002/14651858.CD007176.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Bjelakovic G., Nikolova D., Gluud C. Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: Do we have evidence for lack of harm? PLoS ONE. 2013;8:e74558. doi: 10.1371/journal.pone.0074558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gardner E.M., Bernstein E.D., Popoff K.A., Abrutyn E., Gross P., Murasko D.M. Immune response to influenza vaccine in healthy elderly: Lack of association with plasma beta-carotene, retinol, alpha-tocopherol, or zinc. Mech. Ageing Dev. 2000;117:29–45. doi: 10.1016/S0047-6374(00)00134-2. [DOI] [PubMed] [Google Scholar]
- 80.Hughes D.A., Wright A.J., Finglas P.M., Peerless A.C., Bailey A.L., Astley S.B., Pinder A.C., Southon S. The effect of beta-carotene supplementation on the immune function of blood monocytes from healthy male nonsmokers. J. Lab. Clin. Med. 1997;129:309–317. doi: 10.1016/S0022-2143(97)90179-7. [DOI] [PubMed] [Google Scholar]
- 81.Zhang F., Jin Y., Qiang W. The effects of dietary advice on malnutrition in Cancer patients: A systematic review and meta-analysis. Support. Care Cancer. 2020;28:1579–1585. doi: 10.1007/s00520-019-05222-0. [DOI] [PubMed] [Google Scholar]
- 82.Ying J., Cen X., Yu P. Effects of Eccentric Exercise on Skeletal Muscle Injury: From An Ultrastructure Aspect: A Review. Phys. Act. Health. 2021;5:15–20. doi: 10.5334/paah.67. [DOI] [Google Scholar]
- 83.Nicolini A., Ferrari P., Masoni M.C., Fini M., Pagani S., Giampietro O., Carpi A. Malnutrition, anorexia and cachexia in cancer patients: A mini-review on pathogenesis and treatment. Biomed. Pharmacother. 2013;67:807–817. doi: 10.1016/j.biopha.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 84.Ryan A.M., Reynolds J.V., Healy L., Byrne M., Moore J., Brannelly N., McHugh A., McCormack D., Flood P. Enteral nutrition enriched with eicosapentaenoic acid (EPA) preserves lean body mass following esophageal cancer surgery: Results of a double-blinded randomized controlled trial. Ann. Surg. 2009;249:355–363. doi: 10.1097/SLA.0b013e31819a4789. [DOI] [PubMed] [Google Scholar]
- 85.Owen M., Au C. The development of a brain controlled interface employing electroencephalography to control a hand prostheses. Int. J. Biomed. Eng. Technol. 2021;35:173–190. doi: 10.1504/IJBET.2021.113332. [DOI] [Google Scholar]
- 86.Heys S.D., Walker L.G., Smith I., Eremin O. Enteral nutritional supplementation with key nutrients in patients with critical illness and cancer: A meta-analysis of randomized controlled clinical trials. Ann. Surg. 1999;229:467–477. doi: 10.1097/00000658-199904000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.